Note: Descriptions are shown in the official language in which they were submitted.
W094/26274 ~ ~ 6 3 0 9 5 PCT~S94/03661
Tit:Le: TREATMENT PROCESS WITH BIOLOGICALLY ACTIVE
TROPANE DERIVATIVES
GRANT R~K~C~:
This invention was made with government su~po.
under R01-DA-6301-02 and P50-DA06634 awarded by the
Nat~ on~ 1 Institute on Drug Abuse. The government has
certain rights in the invention.
8AC~ROUND OF THE lNv~N~lON
The tropane skeleton is a basic structural unit
that can lead to compounds with diverse Central
Nervous System (CNS) activity. Due to the rigid
natl~re of the structure, the possibility exists for
the preparation of highly selective ~o...~ounds. This
application describes the syntheci~ of tropane
derivatives that selectively bind to mo~ 1 ne
neu.u~Lansmitters and thus have the potential for the
treatment of major depression, Parkinson's ~ Q and
attention-deficit hyperactivity disorder (ADD).
Two important central nervous system
neu-oL.ansmitters are serotonin (5-HT) and dopamine
(DA). Together with norep;neph~ine and epinephrine,
these neurotransmitters comprise the group of agents
known as the monoamines. Either 5-HT or DA have been
implicated in a variety of disorders, including
depression, Parkinsons disease, ADD, obesity and
co~ addiction.
Major depression represents one of the most
.o ~ mental illness, affecting between 5-10~ of the
population. The disease is characterized by extreme
changes in mood which may also be associated with
psychoses. It has generally been found that most
W094/26274 PCT~S94/03661
~63095 2 -
ant~pressant agents exert significant effects on the
regulation of ~onoA~~ne neuloL ansmitters, including
DA, 5-HT and norep~ephrine. The tricyclic
antidepressants, such as imipramine, are the most
~ormonly used drugs for the treatment of depression.
Their ab$1ity to ~ nh~ h~ t the neuronal uptake of
norep;nephrine is believed to be a ma~or factor behind
their efficacy.
A number of new types of antidepressants have
been developed in recent years. Two such compounds
that are marketed in the U.S. are tr~7o~ne and
fluoxetine. Both of these compounds interact with the
regulation of S-HT. Tr~oAone potentiates the actions
of 5-HT while fluoxetine is a potent and selective
~ nh~ h~ tor of 5-HT reuptake. 3-Chloroimipramine which
1 nh~ h~ ts both 5-HT and norep~n~phrine reuptake has
been extensively used as an antidepr~cAnt in Europe
and ~n~A. Other compounds which are of ~ e-lt
interest or have been examined as ant~pressants
include flUVOxAm~ n~, citalopram, zimeldine, bupropion
and nomifensine. All of these drugs inhibit mo~oA~ ne
uptake mech~n~sms, but differ in selectivity between
the dop~n~-, 5-HT and norep~neFhrine tran~o~ers.
Other syndromes also respond to antidepressant
drugs. These include (1) severe anxiety syndromes
characterized by panic reactions, and (2) obseccive-
compulsive disorder, both of which seem most likely to
respond to 5-HT selective agents. Monoamine uptake
blockers have also been useful in treatment of chronic
pain, neuralgias, migraine, sleep apnea, fibromyalgia,
and irritable bowel syndrome.
Parkinson's ~;S~Ace effects about 1% of the
population over the age of 65 and leads to serious
W094l26274 ~ ~ 6 3 0 9 5 PCT~S94/03661
neurological disorders. The main ~l~n~c~l features of
the ~lsQ~e are centered around disruption of motor
function, such as w~lk~ng~ speech, eating and other
skilled acts. It has been recognized that the ~ se
is the result of dop~1~e deficiency in the basal
ganglia. Thus, drugs that can increase the levels of
dopamine have the potential to be effective
medications for the treatment of Park~ncon's ~1s~se.
The most effective drug in this regard has been
levodopa which acts as a biogenic precursor to
dopamine.
Cons~e~able attention has recently been directed
to the condition known as attention-deficit
hyperactivity disorder. Children with this condition
tend to be very active physically but have great
difficulty with situations requiring long periods of
a~tention. Co~ce~uently, they tend to underachieve
academically and can be very disruptive. Furthermore,
these behavioral problems often persist in modified
forms into adulthood. The condition appears to be
ACCO~.~ ated to the effect of monoamines in the cerebral
cortex, which are involved with control of attention.
A number of stimulant drugs such as dextroamphet ~ne-,
methylphenidate as well as the tricyclic
antidepressants, antipsychotic agents and clonidine
have been used as medications to control the disorder.
Many of these drugs interact with the monoamine uptake
tran~o~Lers.
Another disorder for which inhibitors of
monoamine transport are useful therapeutic agents is
obesity. In general, sympathomimetic drugs (i.e.,
those which increase synaptic levels of monoamines)
promote weight loss by suppressing appetite. Drugs
W094/26274 PCT~S94103661 ~
216309~ 4
like m~n~ol, which act as sympathomimetic agents by
blo~k~ng monoamine uptake, have been useful in the
treatment of obesity.
C~c~ine has the following formula:
MeN COOCH3
~COO~>
The basic ring structure of coc~i ne. is a tropane ring
xy x l a,-- .
It has previously been shown that coc~ne and
related compounds are potent inhibitors of dopamine
reuptake and this may lead to ~ ,ounds with
reinfo~ n~ properties. In recent years a number of
new e~ ~ ly potent coc~ n~ ~n~ 1 ogs have been
prepared based on the tropane structure (Abraham et
al., Journal of Medtc~nal Chemtstry 1992, 35, 141;
Bo;a et al., European Journal of Phar_acology, 1990,
183,329; Bo;a et al., European Journal of
Pharmacology, l99l, 194, 133; Carroll et al., Journal
of Med~cinal Chem~-~try, 1992, 35, 969; Carroll et al.,
JournaL of Med~c~nal Chem~stry, 1992, 35, 1813;
Carroll et al., Journal of Me~ç~n~7 Chem~stry, 1992,
35, 2497, Cline et al., Journal of Pharmacology and
Expertmental Therapeut~cs, 1992, 260, 1174; Cline et
al., Synapse, 1992 12, 37; Kozikowski et al.,
Medicinal ~hemistry Research, 1991, 1, 312, Kozikowski
et al., Journal of Medic~nal Chem~stry, 1992, 35,
4764; Lewin et al., Journal of Med~c~nal Che~l~try~
1992, 35, 135: Madras et al., Molecular Pharmacology,
1989, 36, 518). All of these compounds are based on
the tropane skeleton and tend to selectively bind to
W094/26274
216 ~095 PCT~S94/03661
-
-- 5 --
the dor~ ne tranx~o,~er. Certain structural
variations can lead to compounds that bind with very
hi~h selectivity to the dopr ~nQ reuptake site
(Carroll et al, Journal of Med~c~nal Chem~st~y, 1992,
35, 2497). However, all of these tropane derivatives
are very similar to each other because they are all
derived from r,o~n~ as starting material.
It has now been discovered that if the tropane
ring ~y~L~- is modified, particularly at the aryl
moiety as hereinafter described, compounds can be
produced which are more selective in binding to 5-HT
transporters as co~r~ed to DA transporters. Since
these modified tropanes (as described below) bind
preferentially to the 5-HT transporter, they may
preferentially block 5-HT transport, thus, incr~c~ng
synaptic levels of 5-HT. This may be helpful in
treating ~eAses related to 5-HT function.
Similarly, tropane analogs can be synthesized which
selectively block DA tranX~ol~ers and selectively
increase synaptic levels of DA.
In principle, the tropane skeleton is l~e~lly
suited to prepare highly selective compounds because
it is a rigid structure and tropane derivatives will
have rather limited conformational flexibility. Such
derivatives may be altered by a~plo~liate structural
changes so that analogs favoring b~n~ing to either the
5-HT or DA reuptake site could be prepared. The novel
ch~m1qtry that has been developed, as referred to in
our parent application, has enabled preparation of a
much wider range of tropane analogs than was
previously ~cc~qible, l~ng to novel structures
with selective biological activity.
WOg4/26274 ~ 1 ~ 3 0 ~ 5 PCT~S94/03661
Accordingly, it is a primary ob~ective of the
present inventlon to provide a process for preparing
tropane analogs which are selective inhibitors of 4
either 5-HT or DA reuptake.
Another primary ob;ective of the present
invention is to prepare a range of tropane An~lo~s
which can be investigated as drugs for the treatment
of chronic depression.
A still further ob;ective of the present
invention is to provide a wide range of tropane
derivatives which can be systematically used and
tested to determine structure-activity relat~o~h~ps
for ~n~ng at dopamine, 5-HT and norep~n~ph~ine
transporters.
A further objective is to provide a treatment
system for diseases whose course can be altered by
patient treatment with compounds that selectively bind
to either the 5-HT or DA reuptake site and therefore
ylevellt neurotrAne~~ ons at this site.
SUMMARY OF THE lN V ~ ~ lON
Biologically active derivatives of the tropane
ring ~ys~e-l- are provided which selectively bind either
to the 5-HT or DA reuptake site ~ leA~ i n~ to ~o...~unds
which have use for treatment of clinical depression,
Parkinson's Disease, ADD and obesity.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures l, 2, 3, & 4, show the potencies of
various analogs of the present invention 5-HT and DA
in b;n~i ng to transporters. These results demonstrate
analogs with three different categories of
selectivity: DA-selective, 5-HT selective and non-
selective.
WO 94126274
PCTIUS94tO3661
DE'rATT-~P DESCRIPTION OF T~EE lNVI~ lON
~ he focus of this application will be on uses
of tropane derivatives of the general formula:
Wherein R1 is an aromatic moeity and may be any 1-
naphthyl, 2-naphthyl, phenyl, C1 to C8 alkylaryl or
indole moiety. Preferred are isopropylphenyl and
naphthyl. R2 and R3 may be as follows: Only one of
R2 and R3 can be hydrogen at the same time and each
of R2 and R3 can be a ketone moiety, (c R ) an
Y~ (c-OR2_3) a phosponate, a sulfone
moiety, a cyano, an oxazole, or a imidazole. It is
preferred that R2 and R3 be selected from ketone
moieties or ester moieties, preferably C1 to Cg
alkyl or alkoxy. If desired the Me group may be
more generally described as R4 which may be hydrogen
or lower (C1 to Cg) alkyl.
r~he very most preferred compounds for use in
the present process are:
MeN Q~R
H
Wherein R is Cl to Cg and Ar is an aryl moiety as
earlier defined.
The synthesis of the tropane derivatives was
achieved by the general scheme shown below. The
experimental procedure for the final step has been
SUBs 11 1 IJTE SHEE~ (RULE 26)
W094/26274 PCT~S94/03661
21 ~30~ 5 _ 8 -
described in detail in the original patent. The
details of the earlier steps have been le~o ~ed
(Davies, et al., Journal of O~ganic ~ try, 1991,
56, 5696).
Br COR COR ,~\
RCN ~ SO2N3 N2~ ~/NBOC
,~ Zn ~ NEt3 ,~ Rh2(00ct)~
E ~C l.(PPh3)3RhCl/H2 MeN COR ArMq~r
~ s COR 2.TFA b b~ Oul
b~ ~.CH20/Na(CN)BH3
MeN~, + ~,le~ ,OR
Basically, in the process of that case, 3-
aryltropane derivatives are prepared by reacting 8-
azabicyclo[3.2.1]oct-2-ene with an aryl Grignard
reagent in the presence of catalytically effective
amounts of copper (I) and/or copper (II) salts. The
3-aryl-tropane derivative starting material can be
conveniently prepared by ~e~- ,o~ing function~ ed
vinyldiazometh~e~ in the precence of certain
pyrroles, preferably in substantial ~e~s of the
stoichiometric amount, using a ~ osition catalyst,
preferably a rhodium catalyst. The catalyst may also
be a copper, palladium or silver salt catalyst. This
provides a bicyclic intermediate cont~; n; ng the basic
W094/26274
216 3 09 5 PCT~S94/03661
g
tropane ring ~y~L which is thereafter converted to
an 8-azabicyclo [3.2.1]oct-2-ene, which ~tself may be
used as a starting material to react with an aryl
Grignard reagent in providing the synth~cl~ route to
the unique coc~ne analogs of the present invention.
The starting material of the process is, namely
the 8-azabicyclo[3.2.1]oct-2-ene, and has the
following formula:
H3CNb~s,COR
In the above formula R is selected from the group
consisting of Cl to C8 alkyl and C, to C8 oxyalkyl. In
other words, the two position moiety may be
f~nctionally substituted by ketone groups or ester
groups.
One of the present inventors, namely Dr. Huw M.
L. Davies, has previously published ~onc~ning the
general syn~ c used for the starting material of
the parent case, namely synthesizing 8-azabicyclo
[3.2.1]oct-2-ene of the above formula. In this regard
see, Davies, et al., "Novel Entry to the Tropane
System ~y Reaction of Rhodium (II) Acetate Stabilized
Vinylcarbenoides with Pyrroles," Tetrahedron Letters,
vol. 30, no.35, pp. 4653-4656, (1989) a December 1990
abstract of a regional ACS meeting held in New
Orleans, entitled Davies, et al., "Chemistry of
Vinylcarbenoids with a Single Electron Withdrawing
Group, an Approach to Tropane Alkaloids", American
Chemical Society, Dec. 5-7, 1990, pp. 181-182; Davies,
et al., "Synthesis of ~ Ferruginine and Anhydro-
ecgonine Methyl Ester by a Tandem Cyclopropanation/
W094/26274
PCT~S94/03661
2~3~5 lo-
Cope Rearrangement", Journal of Organic Chemistry,
1991, Vol. 56, pp. 5696-5700. The sub~ect matter of
each of these publications of Davies et al is
incorporated herein by reference and therefore need
not be described in full detail. However, certain
preferred process operations, not specifically
mentioned in the above articles, are described herein
for sake of complet~es~.
Preparation of the starting material for the
Grignard addition of the present invention, namely,
preparation of 8-azabicyclo[3.2.1]oct-2-ene as above
described employs in its first step a process of
decomposing of a functionAl17ed vinyldiazomethane of
the formula:
COR
N2~
in the presence of at least a stoi~-h~o-~tric amount of
a pyrrole of the formula:
lCOO~
wherein Z is a functional group protector, and also in
the presence of a small but effective amount of a
decomposition catalyst selected from the group
consisting of rhodium, copper, palladium and silver
salts, to provide an intermediate bicyclic compound.
wo g4l26274
~1 6 3 09~ PCT~S94103661
R as shown above represents a Cl to C8 alkyl or C
to C8 oxyalkyl. Preferably R is an alkyl and
therefore as expl A~ neA herein after, the resulting
a~alog of cocAine ultimately prepared will have a
ketone group at the two position. In the pyrrole, Z
represents a functional group protector such as
trimethylsilylethyl, although it is unde ~oo~ that
other classic protecting groups such as tertiarybutyl
group may also be employed.
The amount of the pyrrole for this first reaction
scheme needs to be at least a stoich$ometric amount in
comparison with the ~inyldiazomethane and preferably
is in excess of the stolchiometric amount, perhaps
within the range of a two-fold to a five-fold ~xc~ss.
An ~r~cc iS preferred in terms of achieving the
desired high yields of the bicyclic intermediate
because the vinyl~i~7omethane is decomroc~ to a very
reactive intermediate, namely a vinylcarbenoid which
will, unless it is trapped by use of stoichiometric
e~ of the pyrrole, rapldly ~
The pyrroles above described can be ~o1.~e,1tion-
ally prepared using well known chemistry as described
i~ the Journal of Organic Chemistry, l99l, vol. 56
article, of the author earlier cited. ~he reaction is
preferably run at a temperature of within the range of
from 25C to about 100C, preferably at about 80C.
The reaction can be run at 25C if there is slow
addition of the vinyldiazomethane to the pyrrole. The
pressure is not critical in this reaction step.
As explained above, the reaction is conducted in
the presence of a ~r,omrosition catalyst selected from
the group consisting of rhodium, copper, p~ um and
silver salts. Preferably the catalyst is a rhodium
W094/26274
PCT~S94103661
216309~ 12 -
salt catalyst and may be a rhodium (II) a~e~a~e,
m~n~ te, trifluoroacetate, he~no~te, pivalate or
octanoate. The presently most preferred catalyst is
rhodium octanoate which seems to allow h~he~ yields
of desired product. The amount of catalyst may vary
from 0.25 mole per cent to about 2.0 mole per cent of
the vinyl~ o.-thane, and is preferably about l.O
mole per cent of the amount of the vinyl~A7omethane
reactant.
Reaction time does not appear to be critical and
the time may vary from a few minutes up to several
hours if drop wise addition is accompl~hed. The
other carbon atoms of the 8-azabicyclo[3.2.1]oct-2-ene
can include substituents other than hydrogen (e.g. one
or more of the other carbon atoms of the bicyclic
~y~L~ can include a lower alkyl substituent group)
because a more highly substituted pyrrole or
vinyldiazomethane may be used as starting material.
This first step reaction produces an intermediate
bicyclic co...~ound which upon hydrogenating, removal of
the de~L~ective group and reductive methylation is
~o-~veL~ed to the earlier described 8-~h~yclo
[3.2.1]oct-2-ene. The l,y~Logenation, depL~Le~Ling and
reductive methylation are all well known steps and
need not be described herein.
Where R equals methyl and the protecting group
used is trimethylsilyl the intermediate is methyl
8-(2-(trimethyl-silyl)ethoxycarbonyl)-8-azabicyclo
[3.2.1]octa-2,6-dien-2-oate.
This reaction is preferably co~llrted in the
presence of a solvent and the solvent is preferably a
non-polar solvent. Suitable non-polar solvents for
conducting this reaction may be pentane, heY~ne, and
W094/26274 PCT~S94/03661
~6309~
- 13 -
benzene. Other suitable non-polar solvents, cApAhle
of dissolving the basic reactants may also be
employed, with the precise solvent not being critical,
as long as it is in fact non-polar.
For details of the hydrogenating, deprotecting
and reductive methylation see, the previously
in~oL~ated by reference 1991 vol. 56, Journal of
Or~anic Chemistry article. There it is h~c~nAlly
described that the catalytic h~dloyenation is a
process employing a Wilkinson's catalyst and that
dep oLection occurs with, for example, tertiarybutyl
ammonium flouride to give the desired 8-azabicyclo
[3 2.1]oct-2-ene at yields as high as 95%. As
exp~ e~ in the earlier referenced article, the
composition is purified by c~l~cA gel column
chroma~oyl~hy.
The 8-azabicyclo[3.2.1]oct-2-ene is then used as
a starting material for the process of the present
invention. It has been found that the 8-azabicyclo
[3~2.1]oct-2-ene fo~ earlier described, can be
~onv~l~ed to biologically active ~o~-~1n~ analogs
having a wide variety of active analog structures by
reacting with a aryl Grignard reagent in the prece
of a catalytically effective amount of a copper salt
ca~alyst. The copper salt catalyst may be a copper
(I) or copper (II) catalyst.
As previously described, it is preferred that the
R group of the 8-azabicyclo~3.2.1~oct-2-ene be C1 to C8
al}cyl, rather than an oxyalkyl since it is preferred
that the two substituent be a ketone substitution
ra~her than an ester substitution. The ketones behave
better in the copper catalysed reaction, and as
explained later in the biological activity section of
W094/26274
PCT~S94/03661
~16~0~
- 14 -
the specification, should have higher met~hol~c
stability and have equivalent h~ n~ ~ ng site activity.
The Grignard addition reaction is run in a suitable
non-polar organic solvent, preferably ether or
tetrahydrofuran.
The Grignard reagent (ArMgX) may be any sultable
aryl magnesium halide. The aryl group may be phenyl,
substituted phenyl, C1 to C8 alkylaryl, polyaryl such
as naphthyl, anthracyl or alkylpolyaryl. Alkyl
~~esium h~ (C1 to C~) may also be used. The "X"
moiety represents a hAl~e group and is preferably
bromide. The copper salt may be a copper (I) or (II)
salt and can be, for example, copper bromide dimethyl
sulfide. The amount of the Grignard reagent is
preferably an ~Ynes~ of the sto~ Ch~ ometric amount $n
order to assure completion of the reaction. Suitable
high yields are obtA1ne~ when an excess of up to four-
fold of the Grignard reagent is employed. The amount
of the copper salt catalyst can be from 5% (molar) to
20% (molar) of the Grignard reagent, and is preferably
15 mole percent of the amount of the Grignard reagent.
The reaction to ~lo~e the desired ketone is
represented by the following equation reaction:
Me~ ~ ~R Ar~gBr Me O~R Me
~ Cu~rDMS ~+
As seen the reaction product is a mixture of two
structural isomers, one with the 2-moiety position
upwardly (a) and the second with the 2-moiety position
downwardly. (b) Those analogs that are most preferred
are the analogs wherein R is alkyl and therefore the
W094l26274
PCT~S94/03661
0 9 5
- 15 -
two position moiety is a ketone moiety, and that the
structural isomer is with the ketone groups in an up
position. These are far more active in b~nA~ng
assays, than the downward structural isomers and in
some inst~nn~ as much as 200 times more active in
site-b~n~ng~
Certain other process conditions are wo ~h~ of
mention. The reaction is not temperature critical and
may be run at anything from 0C or lower up to room
temperature, or even higher. The reaction is
preferably run under an inert gas atmosphere. The
reaction is substantially immediate and therefore may
be run from a few minutes to as much as twelve hours.
Preferably the reaction occurs under stirring in order
to assure complet~neRs. After completion the reaction
can be qll~n~e~ with for ~mple HCl/ice, with the
desired compound extracted with ether. It may be
purified as illustrated in the examples by
velltional silica gel chromatography.
The ~: , unds may be administered orally,
parenterally or intravenously. The preferred route of
~ n~ ctration iS oral. The dose levels may be from 4
micrograms per kilogram of body weight up to 50
mi 1 1; grams/kg of body weight and more typically from
20 micrograms/kg up to 15 mg/kg.
The novel tropane analogs synth~R~ by the
vinylcarbeonoid scheme listed above were tested for
their ability to interact with 5-HT and dopamine
transporters by two assays: displacement of
radioligand b;~; ng to transporter sites, and direct
inhibition of 5-HT or dopamine uptake in acutely
dissociated fetal and adult rat neurons. These assays
are known to correlate with transport sites in h~lm~n
wo g4l26274 ~
21~ 3 ~ ~ 5 PCT~S94/03661
- 16 -
brain. In bi n~ ~ ng studies, low ~oncentrations (10-20
pM) of [~I~RTI-55, the potent tropane ~nAl~ recently
synthesized by Carroll's group (Bo~a et al., European
Journal of Pharmaco70gy, 1991, 184, 329), was used to
label dopamine tran~ ers in rat striatal membranes,
while [~]paroxetine (Harbert et al., European Journal
of Pharmacology, 1985, 118, 107) was used to label 5-
HT transporter sites in rat frontal cortex. Up to the
present time, 34 analogs have been tested for b~n~ng;
their potencies in binding and uptake assays are
summarized in Table 1 below.
WO 94/26274 PCT/US94/03661
~163095
1 7
~ ~¦ N 2 o ~o o
N ~ o ~ tl o 'U U ~H ~
_ ~ _ N ~_ N _ ~ æ
o f~i¦ _ N N E~ Z 01> _ N ~ ~ æ ~ N
~Y ~ ~ A A 3 ~ N ~ ~ A ~ A ----~ ~ ~ æ ~ æ ~ ~ O ~
e ~ ~ ~ ~ o o ~ ~ N ~ N ~ ~ ~ ~ O -- O N ~ r
_ '~_~ N r~ --
o T ~I ~ ~~I~~ ~ ~ ~ I ~~ ~~
Q ~ ¦ T C~ ~ c~ T T <,~ T T T T I ~ T I C~ I I T I tJ I T T I I T I T C.:~ I
~n
"T ~) _ _ ~ N N N __ N_____~ t~_--
T T I C~ t~ C~ T T I T ~ ~ T C~ T C~ ~ C ~ ~ c~
h ~ 7 ~ T ~ ~ ~
t
c O _ ~) ~t ID CD 1~ CO 0 ~ 0 ~
SUB~ I I I lJTE SHEET ~RULE 26)
W094/26274 PCT~S94/03661
~30~5
- 18 -
In Table 1, the general formula is that depicted
in the earlier part of this application, ~ust at the
beg~ nn~ n~ of the h~AA~ ng Detailed Description of the
Inventlon. Code names, as presented, WF-l through WF-
35, are internal names of the assignee and simply
stand for "Wake Forest-l" etc. Two AnAl~gS have been
~s~1~neA trivial abbreviations: WF-ll is PTT, and WF-
31 is PIT.
The b~nA~ng affinity of tropane derivatives at
the dopamine transporter was the basis of the original
patent on drugs for the treatment of ~O~,A ~ n~
addiction. In the original application, the binAlng
affinities for WF 1-5, 7-9, 11 were e~ol~ed as
background evidence. Since then, a publication with
the h~n~in~ affinities for WF 1-5, 7-9, 11, 13, 18,
19, 22, 23, 25 has appeared (Davies, et al., European
Journal of Pha~macology - Molecular Pharmacology
Sectfon, 1993, 244,93).
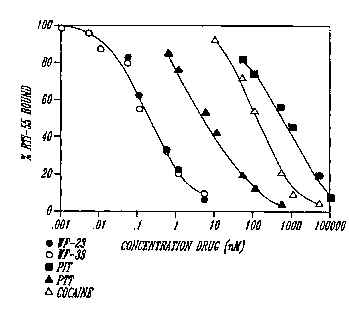
Figure 1 l_- rAreS the disp~ ? t of [~I]RTI-55
h~ n~ ng by cocaine with four tropane analogs, PTT,
PIT, WF-23, and WF-33. This leads to information on
how these compounds bind to the dopamine tran~o Ler.
These data showed that the two 2-naphthyl analogs, WF-
23 and WF-33, were the most potent of these compounds,
followed by PTT. PIT, in contrast, was less potent in
displacing [~I]RTI-55 than cocA~ne. Comparison of
IC~ values (Table 1) showed that WF-23 and WF-33 were
900 to 1300 times, while PTT was 20 times, more potent
than co~Aine in b;nAi ng to dopAr;ne transporters. In
contrast, PIT was 2.5 times less potent than coc~ine
at dopAr~ne transporters.
Figure 2 shows how the selectivities of these
analogs were determined in [~]paroxetine binA~ng
W094/26274
PCT~S94/03661
~16309~
- 19 -
experiments. This leads to information on how these
~ ounds bind to the 5-HT trans~ ~er. Agaln, the 2-
naphthyl analogs, WF-23 and WF-33, were the most
po~ent compounds in disp~ n~ ~3H]paroxetine b~n~1n~,
as they were vs. [~I]RTI-55 b1nA~n~ (Fig. l).
However, whereas these An~1ogs were equipotent in
displacing ~ I]RTI-55 h~ n~ ng, WF-33 was 4 times less
potent than WF-23 in disp1~n~ ~H]paroxetine
h~nA~ng, Furthermore, the two phenyl AnA1o~s, PTT and
PIT, exchanged places in diSplA~n~ ~3H]paroxetine
comrA~ed to [~I]RTI-55: PIT was significantly more
potent in disp1~ ~g t3H]~a~ae~ine than co~A~ne~
whlle PTT was a~lo~imately equipotent with cocaine in
displacing ~3H]paroxetine h~nA~ng (Fig. 2). IC~ values
(Table 1) showed that WF-23 and WF-33 were 480 times
and 140 times, respectively, more potent than ~o~ ne
at 5-HT tran~olLer sites, while PIT was 8 times more
po~ent than COCA ~ n~ and PTT was twice as potent as
cocaine. Table ~ shows the potency ratios of all
analogs in b~nA~ng assays dopamine and 5-HT
transporters (higher numbers demonstrate relatively
greater dopr ~ne tran~ Lel ~vLelloy). These data
su~gested that PIT was relatively selective for 5-HT
transporters, while PTT and PIT were relatively more
selective for dopamine tran~ Lers. In contrast, WF-
23 was like ~o~ n~, with little selectivity between
the two tran~po Lers. However, it was 500-800 times
more potent than co~1 ne at both tran~ol~e
The synthetic scheme that was used to produce
tropane analogs from vinylcarbenoid precu~ ~Gl ~
generated racemic compounds; therefore, all the
binding studies discussed above were conducted with
racemic compounds. When WF-23 was separated into two
W094/26274 PCT~S94/03661
~63~g~
- 20 -
stereoisomers by a chiral HPLC column, the active
isomer displayed an est1mated IC~value of 0.03 nM vs.
[~I]RTI-55, while the inactive isomer demonstrated an
IC~value of 113 nM (see values for WF-23(1) and WF-
23(2) in Table 1). These results not only
demonstrated that ~Le eoisomers can be separated by
the chiral HPLC, but also shows that the active lsomer
is extremely potent. The active isomer of WF-23 was
also potent vs. [~3paroxetine b~n~n~, and the
selectivity of the active and inactive isomers were
the same as the racemic mixture of WF-23. Generally
those isomers with R2 in the up position were more
active, and as well, those compounds where R2 was a
ke~one were more active.
Uptake studies have been con~l~cted on ~ev~ al
selected analogs to confirm the results of the b1 n~ ~ ng
studies. These experiments ut~ A ~ ~ ~co~.~ Ated cells ~
from fetal and adult rat brain, using the striatum for
dopamine uptake assays, and the frontal ~G~ Lex for 5-
HT uptake assays. Fig. 3 shows the inhibition of
[3H]dop~1ne uptake into striatal cells by cocaine and
selected tropane ~Alogs. These results were
comparable to the b~nA~ng assays: while WF-23 was the
most potent analog in inhibiting dop~ uptake
followed by WF-ll, WF-31 was considerably less potent
in blocking [3H]dopamine uptake. Experiments with
[~]5-HT uptake in cortical cells (Fig. 4) also
SU~Ol ~ed the results of the b~nAi~g assays, showing
that WF-23 and WF-31 were both significantly more
potent than cocaine in blocking tH]5-HT uptake. Thus
the uptake assays confirmed the selectivities of these
tropane analogs as determined in b; n~ ~ ng assays. For
example, WF-11 was 140 times more potent in inhibiting
~ W094/26274 PCT~S94/03661
2~3Qg5
- 21 -
dopamine uptake than 5-HT uptake, while WF-31 was 120
tlmes more potent in ~ nh~ h~ ting 5-HT uptake than
dopamine uptake, both somewhat greater than the ratios
detel ~ne~ by h~ ng studies (Table 1). In contrast,
WF-23, whether assayed as a racemic mixture or as its
active stereoisomer, provided a dopamine: 5-HT ratio
of only 3-4 regardless of the assay used.
In addition to the iso~u~ylphenyl derivative WF-
31v it is also clear that the ethylphenyl der$vative
WF--9, and the 1-(4-methylnapthyl) der$vative WF-27
also display considerable selectivity towards the 5-HT
tranx~olLer in terms of h~ ng and inhibition. The
co~mon feature of these derivatives is that they
contain a funct~ O~A lity that may lie to some extent in
the ~el~endicular plane to the aromatic ring. This
stru~Lulal variation leads to a novel type of
biological activity for compounds in the tropane
series with potential for the development of a novel
class of antidepressant drugs. The general activity
of the group of analogs at monoamine transporter sites
strates that they have potential for the
treatment of other diseases associated with monoamine
imbAl~eR such as Parkinson's Disease, attention-
deficit hyperactivity disorder, and obesity.