Note: Descriptions are shown in the official language in which they were submitted.
W094/28425 2 16 ~ PCT~S94/05692
-1-
E~Y~lOXIC FACTORS
Government Support
Support for this work was provided by grants HD00815,
HD23547 and HD23775 from the National Institutes of Health.
Field of the Invention
This invention relates to methods and compositions for
diagnosing and treating immunologic reproductive failure.
The methods include the administration of an immunomodulating
agent for suppressing a cellular immune response.
Backqround of the Invention
Pregnancy is a unique immunologic state in which a
natural homeostasis exists between antigenically different
tissues (Feinberg, B. and Gonik, B., Clinical Obstetrics and
Gynecology 34(1) 3-16 (1991). In one out of five
pregnancies, this homeostatic state is disrupted and the
fetus is prematurely aborted. In one out of 300 pregnancies,
this homeostatic state is repeatedly disrupted, i.e., two or
more pregnancy losses before 20 weeks gestation, and the
fetus is prematurely aborted. Such recurrent spontaneous
abortior~s are attributed to a variety of causes.
Chromosomal defects are found in 3-7 % of couples with a
history of recurrent spontaneous abortion and are the only
well-established cause of recurrent abortion. Non-genetic
causes of recurrent abortion are difficult to ascribe,
although numerous associations have been made with anatomic,
endocrine, infectious, and humoral immune abnormalities. To
date, the basis for recurrent spontaneous abortion remains
unexplained in 40% to 60% of couples, although immunologic
mechanisms have been proposed in many unexplained cases of
recurrent spontaneous abortion.
W094/~ 216 3 ~ ~ O - 2- PCT~S94/0569~ ~
The human immune system has two effector arms: humoral
(antibody mediated) immunity that is highly effective at
neutralizing pathogens, and cellular (T cell mediated)
immunity that principally functions to destroy foreign and
infected cells. This dichotomous nature of the immune system
is at least in part determined by regulatory CD4 T
helper/inducer lymphocytes. Following activation by antigen,
CD4 T lymphocytes produce one of two distinctive cytokine
profiles which has led to their classification as T helper 1
(THl) and T helper 2 (TH2) cells (Mosmann T. et al.,
1986, J. Immunol. 136:2348; Cherwinski, H. et al., 1987, J.
Exp. Med. 166:1229; Kurt-Jones, E. et al., 1987, 166:1774).
The different cytokines produced by these cells lead to
differences in immune function (Cher, D. et al., 1987, J.
Immunol. 138:3688; Mosmann, T. et al., 1989, Ann. Rev.
Immunol. 7:145). THl cells primarily secrete
interferon-gamma (IFN-gamma), but also interleukin-2 (IL-2)
and tumor necrosis factor-beta (TNF-beta, Lymphotoxin), and
induce cellular immunity. TH2 cells primarily secrete
interleukin-4 (IL-4), but also IL-5 and IL-10, and
downregulate cellular immunity while playing a major role in
the induction of antibody responses mediated by plasma cells
(Kurt-Jones, E. et al., 1987, 166:1774; Cher, D. et al.,
1987, J. Immunol. 138:3688; Mosmann, T. et al., 1989, Ann.
Rev. Immunol. 7:145; Mosmann, T. et al., 1987, Immunol. Today
8:223; Abbas, A. et al., 1990, J. Immunol. 144:2031; Powrie,
F. et al., 1993, Immunol. Today 14:270). The cytokine
TNF-~ can be produced during both THl and TH2 immune
responses, although levels are higher in THl responses and
this cytokine is known for cytolytic effects that contribute
to the efficacy of cellular immunity (Mosmann, T. et al.,
1989, Adv. Immunol. 46:11; Romagnani, S. et al., 1992, Int.
Arch. Allergy Immunol. 4:279). Most of the original work on
THl and TH2 lymphocyte subsets was performed with mouse
helper T cell clones, however, it is now recognized that
humar. CD4 T cells secrete similar cytokine profiles and can
wog4n8425 ~ 5 ~ PCT~S94/05692
also be classified into THl and TH2 subsets (Powrie, F.
et al., 1993, Immunol. Today 14:270; Romagnani, S. et al.,
1992, Int. Arch. Allergy Immunol. 4:279; Romagnani, S. et
al., 1991, Immunol. Today 8:256).
The precise nature and extent of immunologic mechanisms
for recurrent abortion are not well understood. Although it
is generally known that the cellular mediators of the
cellular immune response, T lymphocytes and macrophages, are
present in the uterine endometrium in the secretory phase of
the menstrual cycle (the time of implantation) and throughout
pregnancy, the role of these cells in mediating spontaneous
abortion has not been precisely defined. As a consequence,
therapies for treating immunologic spontaneous abortion have
not been adequately explored.
Summary of the Invention
The present invention provides methods and compositions
for diagnosing and preventing immunologic reproductive
failure.
According to one aspect of the invention, a method for
diagnosing a predisposition to immunologic reproductive
failure is provided. The method involves contacting a sample
containing a plurality of leukocytes derived from the mammal
with a reproductive antigen under conditions to stimalate
release by the plurality of leukocytes of an extracellular
embryotoxic factor, determining the concentration of the
embryotoxic factor in the sample and diagnosing a
predisposition to immunologic reproductive failure in the
mammal by comparing the sample embryotoxic factor
concentration to the concentration of embryotoxic factor
present in at least one standard. The standard can be a
"positive" standard (which contains a concentration of the
embryotoxic factor indicative of a predisposition to
immunologic reproductive failure) or a "negative" standard
(which contains a concentration of the embryotoxic factor
indicative of the absence of a predisposition to immunologic
21~3~
W094/28425 PCT~S94/05692
reproductive failure). A diagnosis of a predisposition to
immunologic reproductive failure is made if the sample
embryotoxic factor concentration is elevated compared to the
negative standard or is substantially the same as the
positive standard.
According to another aspect of the invention, a method
for diagnosing a predisposition to immunologic reproductive
failure in a non-pregnant mammal is provided. The method
involves obtaining from the mammal a leukocyte
secretion-containing sample, determining the concentration of
an embryotoxic factor in the sample and diagnosing a
predisposition to immunologic reproductive failure by
comparing the sample embryotoxic factor concentration to the
concentration of embryotoxic factor present in at least one
standard as described above. The sample concentration of
embryotoxic factor is determined by, for example, an
immunoassay that specifically recognizes the embryotoxic
factor, an embryo development bioassay, a trophoblast assay
or by a lymphocyte proliferation assay.
According to yet another aspect of the invention, a
third method for diagnosing a predisposition to immunologic
reproductive failure is provided. The method involves
stimulating a sample containing a plurality of leukocytes as
described above to release a cytokine, determining the
concentration of the cytokine to obtain a sample cytokine
concentration and comparing the sample cytokine concentration
to the concentration of cytokine present in at least one
standard. The standard can be a positive standard (which
contains a concentration of cytokine indicative of a
predisposition to immunologic reproductive failure) or a
negative standard (which contains a concentration of cytokine
indicative of the absence of a predisposition to immunologic
reproductive failure). For a cytokine that is a TH-l
cytokine, a diagnosis of immunologic reproductive failure is
made if the sample cytokine concentration is elevated
compared to the negative cytokine standard or is
W094/28425 ~16 3 4 ~ a PCT~S94/05692
substantially the same as the positive cytokine standard.
For a cytokine that is a TH-2 cytokine, a diagnosis of
immunologic reproductive failure is made if the sample
cytokine conce~tration is reduced compared to the negative
cytokine standard or is substantially the same as the
positive cytokine standard. Thus, a diagnosis of immunologic
reproductive failure can be made by observing an increase in
the concentration of TH-l cytokines, e.g.,
interferon-gamma, tumor necrosis factor-alpha, tumor necrosis
factor-beta or by observing a reduction in the level of
certain cytokines, e.g., TH-2 cytokines such as
interleukin-4 and interleukin-10, in stimulated leukocyte- or
leukocyte secretion-containing samples. Accordingly, a
determination of the ratio of embryotoxic factor
concentration to the concentration of, for example,
interleukin-4 or interleukin-10, also can be used to predict
a woman's predisposition to immunologic reproductive failure.
According to another aspect of the invention, a method
for preventing immunologic reproductive failure is provided.
The method includes administering a therapeutically effective
dose of an immunomodulating agent to a mammal diagnosed as
having a predisposition to immunologic reproductive failure.
According to one embodiment, the method further includes
isolating from the mammal a leukocyte secretion-containing
sample; determining the concentration of embryotoxic factor
present in the sample; and administering one or more
subsequent doses of the immunomodulating agent.
Alternatively, a leukocyte-containing sample can be isolated
from the mammal following initial administration of the
immunomodulating agent. The leukocyte-containing sample then
is "stimulated", i.e., contacted with a plurality of
reproductive antigens to release embryotoxic factor(s) in
vitro. The amount of released factor then is determined
using one or more of the above-mentioned assays. The
W094/~425 2 ~ 5 ~ PCT~S94/05692
-6-
subsequent dose of the immunomodulating agent is selected to
cause a reduction in the amount of the embryotoxic factor
released in vivo by the leukocytes.
The immunomodulating agent can be an agent that
downregulates a TH-l immune response (e.g., a TH-2
cytokine or an antibody which specifically binds to or
otherwise regulates a TH-l cytokine, thereby abrogating the
cytokine's biological activity) or an agent that upregulates
a TH-2 immune response or otherwise increases the
concentration of a TH-2 cytokine in vivo. Preferably, the
immunomodulating agent specifically modulates a cellular
immune response to a reproductive antigen. Accordingly, the
preferred immunomodulating agent is a vaccine containing a
reproductive antigen (or an antigen structurally-related
thereto) in an adjuvant that downregulates a TH-l immune
response or in an adjuvant that upregulates a TH-2,
response. Nonspecific immunomodulating agents also are
useful for the purposes of the invention, e.g., progesterone,
glucocorticoids, cyclosporins, nifidipine, pentoxiphylline
transforming growth factor-beta, intravenous immunoglobulin,
anticytokines, cytokine receptor blockers, and antibodies to
cytokine producing cells. Progesterone is a preferred
nonspecific immunomodulating agent.
Also provided are kits for determining the concentration
of an extracellular embryotoxic factor in a sample. The kits
include a first vial containing an antibody to the
embryotoxic factor; a second vial containing a positive or
negative standard; and instructions for using the antibody to
determine the concentration of embryotoxic facto- in the
sample and for comparing the sample embryotoxic factor
concentration to the standard embryotoxic factor
concentration to determine whether the mammal has a
predisposition to recurrent immunologic reproductive
failure. Preferably, the first vial includes a plurality of
antibodies, each antibody being directed against a different
embryotoxic factor. In this manner, the kits can be used to
W094/28425 ~16 3 1~ ~ PCT~S94/05692
-7-
detect a plurality of different embryotoxic factors.
According to one embodiment, the second vial contains a
positive standard containing an amount of the embryotoxic
factor indicative of a predisposition to immu--~ologic
reproductive failure and the kit further inc a~es a third
vial containing a negative standard. The ne~.ive standard
contains an amount of the embryotoxic factor indicative of
the absence of a predisposition to immunologic reproductive
failure. Kits that are to be used to determine the amount of
embryotoxic factor present in a leukocyte-containing sample
further contain an additional vial including a plurality of
reproductive antigens for stimulating the leukocytes to
release the embryotoxic factor(s) in vitro.
According to yet another aspect of the invention, a vial
containing an amount of one or more isolated reproductive
antigens capable of stimulating a plurality of leukocytes to
release an embryotoxic factor also is provided. Exemplary
reproductive antigens include sperm antigens, rophoblast
antigens and choriocarcinoma cell line-derived antigens.
Preferably, the vial containing the reproductive antigens
targeted for administration to a mammalian recipient (i.e.,
for stimulating leukocytes in vivo) further includes a
pharmaceutically acceptable carrier. It is believed that
administration of such reproductive antigens (or
structurally-related antigens) in the appropriate type of
adjuvant (e.g., an adjuvant that downregulates a TH-l
cytokine immune response or that upregulates a TH-2
cytokine response to the reproductive antigen) to a patient
can reduce a TH-l cytokine immune response, thereby
preventing immunologic pregnancy loss.
These and other aspects of the invention as well as
various advantages and utilities will be more apparent with
reference to the detailed description of the preferred
embodiments.
W094/28425 ~ 3 ~ 8- PCT~S94/05692
Brief Description of the Drawinqs
Fig. 1 shows the results of an active immunization
experiment with various control groups indicating reduced
fertility and increased fetal~resorptions in sperm-immunized
mice.
Fig. 2 shows the effects of passive transfer of T
lymphocytes from immunized mice (from experiment depic~ed in
Fig. 1), on fertility of recipient mice.
Fig. 3 shows the mean numbers + S.D. of T lymphocyte
subpopulations and macrophages in uterine sections of mice
after immunizations (from exper:ment depicted in Fig. 1).
Fig. 4 shows the mean numbers + S.D. of T lymphocyte
subpopulations and macrophages in uterine sections of mice
after passive transfer of T lymphocytes from immunized mice
(same experiment as depicted in Figs. 1-3).
Fig. 5 shows preimplantation embryo recovery in mice
following allogenic trophoblast immunization. Immunization
groups: f, saline; g, adjuvant; adjuvant plus trophoblast.
Fig. 6 shows the number of viable offspring (1) and
fetal resorption (2) in mice following allogenic trophoblast
immunization. The groups are as described in Fig. 4.
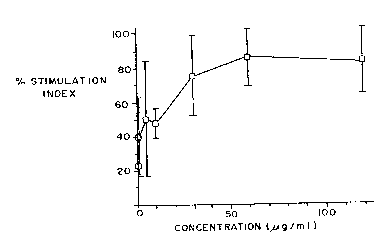
Fig. 7 shows the dose response curve of Jeg-3 antigen in
a lymphocyte proliferation assay.
Fig. 8 shows the trophoblast antigen fractions
responsible for lymphocyte proliferation (SI>3).
Fig. 9 shows the correlation between lymphocyte
proliferation (SI) and embryotoxic factors (% blastocysts) in
response to trophoblast antigen stimulation.
Detailed Description of the Invention
The prior art reveals the difficulties of diagnosing
non-genetic causes of recurrent spontaneous abortion. The
present invention provides a solution to this problem by
disclosing a method for diagnosing in a patient a
predisposition to immunologic reproductive failure. The
phrase "immunologic reproductive failure" refers to those
W094/z842~ 2 ~ ~ 3 ~ S ~ PCT~S94/05692
_g_
reproductive failures (abortions occurring at any time
post-conception) that are attributable to a cell-mediated
immune response. Accordingly, the accurate diagnosis of a
predisposition to immunologic reproductive failure is
essential for designing effective therapies for preventing or
reducing the occurrence of such abortions.
According to one aspect of the invention, a method is
provided for diagnosing in a mammal a predisposition to
immunologic reproductive failure. The method involves
contacting a sample containing a plurality of leukocytes
derived from the mammal with a reproductive antigen under
conditions to stimulate release by the plurality of
leukocytes of an extracellular embryotoxic factor,
determining the concentration of the embryotoxic factor in
the sample to obtain a sample embryotoxic factor
concentration, and diagnosing a predisposition to immunologic
reprodu.ctive failure in the mammal by comparing the sample
embryotoxic factor concentration to the concentration of
embryotoxic factor present in at least one standard
(described below).
As used herein, "leukocytes" embraces lymphocytes (e.g.,
T-lymphocytes and natural killer cells) and macrophages,
i.e., the cellular mediators of the cellular immune
response). As used herein, the term "leukocyte
secretion-containing sample" refers to a preparation
containing soluble factors that have been released by
lymphocytes or macrophages, in vivo or in vitro. These
extracellular factors are released by the leukocytes in
response to stimulation by reproductive antigens. Exemplary
leukocyte and leukocyte secretion-containing samples include
peripheral blood and serum, peritoneal fluid, endometrial
tissue, vaginal secretions and saliva. Methods for isolating
the above-described samples from a patient are known to one
of ordinary skill in the art. The preferred leukocyte (e.g.,
lymphocyte/macrophage) and leukocyte secretion-containing
sample is peripheral blood. The sample containing a
W094/28425 2 ~ ~ 3 ~ ~ ~ PCT~S94/05692 ~
-10-
plurality of leukocytes derived (i.e., obtained) from the
mammal can be used in the methods of the invention with or
without prior culturing. Preferably, the
leukocyte-containing sample is isolated from the mammal,
cultured and stimulated in vitro (by contacting the
leukocytes with a plurality of reproductive antigens) to
release one or more embryotoxic factor(s). By contacting, it
is meant that the leukocytes are exposed to the plurality of
reproductive antigens, under conditions such that the
leukocytes are stimulated to release soluble factors.
Exemplary conditions are provided in the Examples.
The term "reproductive antigens" refers to sperm
antigens, trophoblast antigens, and/or choriocarcinoma cell
line-derived antigens. The sperm antigens are isolated from
mammalian (e.g., human) motile sperm from fertile donors.
Antigen isolation from tissue or cells involves subjecting
the tissue or cells to homogenization, followed by washing
and dividing the preparation into aliquots containing about
300 ug/ml protein. (see also Examples 1, 3 and 5).
Endometrial antigens may be isolated from eutopic
(intrauterine) or ectopic (endometriosis) endometrium. The
preferred reproductive antigens are derived from a
trophoblast cell line, such as choriocarcinoma cell lines
BeWo or Jeg-3.
As disclosed in Example 1 ("Diagnosis of Immunologic
Recurrent Spontaneous Abortion"), both sperm and trophoblast
antigens stimulate peripheral blood lymphocytes and
macrophages from women diagnosed with recurrent reproductive
failure to secrete embryotoxic factors in vitro.
Embryotoxicity was measured by bioassay, e.g., observing the
adverse effect of the released factors on embryo development
and trophoblast proliferation in vitro.
The trophoblast antigens used in Example 1 were derived
from the choriocarcinoma cells lines BeWo and Jeg-3. These
cell lines were chosen as the trophoblast antigen model for
three reasons: (1) they are homogeneous trophoblast cel
W094/28425 ~i fi 3 ~ ~ ~ PCT~S94/05692
lines uncontaminated by other cellular constituents such as
stromal mesenchymal cells or lymphoid and myeloid populations
that are always associated with normal placental trophoblast;
(2) like normal trophoblast, they are devoid of classic ma~or
histocompatability complex class I and II antigens; and (3)
they are established antigenic models of early normal
trophoblast because of their invasive capabilities and cell
surface antigen profiles that are similar to those of early
normal human trophoblast. There were no observed differences
between BeWo and Jeg-3 antigen preparations in terms of their
ability to stimulate embryotoxic factor release from
activated leukocytes of susceptible individuals.
Accordingly, these two trophoblast antigen sources were used
interchangeably for leukocyte stimulation in Examples 1-3.
The reproductive antigen(s) derived from the Jeg-3 cell
line have been fractionated to determine their cellular
location (see Example 5). Further purification of the Jeg-3
antigen is within the ordinary skill in the art, using
conventional purification and characterization techniques.
Thus, for example, gel filtration chromatography is used
initially to fractionate a preparation based upon differences
in molecular size and various additional chromatographic
separation techniques (e.g., ion-exchange HPLC) are used to
isolate reproductive antigenic proteins that are capable of
stimulating leukocytes to release an embryotoxic factor in
vitro. The Jeg-3 reproductive antigen proteins are isolated
and cloned using procedures known to the artisan of ordinary
skill in the art. The isolation and cloning of a trophoblast
membrane protein antigen from human placenta has recently
been reported (see International Patent Application having
publication number WO 93/06857 and publication date April 15,
1993, the contents of which application are incorporated
herein by reference, for disclosure of a trophoblast membrane
expressed protein derived from normal human placenta).
W094l28425 2 ~ PCT~S94/05692
-12-
The relative amount or absolute concentration of
embryotoxic factor present in a reproductive
antigen-stimulated leukocyte-containing sample or in a
leukocyte secretion-containing sample is determined using one
or more of the following exemplary assays: an immunoassay
that specifically detects the embryotoxic factor, an embryo
development assay, a trophoblast proliferation assay and/or a
lymphocyte proliferation assay (see Examples 1, 4 and 5 for
assay protocols). To diagnose a predisposition to
immunologic reproductive failure, the "sample embryotoxic
factor concentration" (i.e., the concentration of embryotoxic
factor in the sample) is compared to the concentration of
embryotoxic -actor present in at least one standard. As used
herein, a "positive standard" refers to a standard which
contains a concentration of the embryotoxic factor that is
indicative of a predisposition to immunologic reproductive
failure. Conversely, a "negative standard" refers to a
standard which contains a concentration of the embryotoxic
factor that is indicative of the absence of a predisposition
to immunologic reproductive failure.
Although various assays can be used to determine the
concentration of embryotoxic factor, the preferred assays
are immunoassays which specifically determine the amount of
an embryotoxic factor present in the sample (see e.g.,
Example 4) and the lymphocyte proliferation assay (see, e.g.,
Example 5). In addition to the specific immunoassays
disclosed in Example 4, the preferred immunoassays for the
purposes of the invention are those which specifically
recognize the following antigens: gamma-interferon, tumor
necrosis factor-alpha, interleukin-l, interleukin-2,
interleukin-4, interleukin-6, interleukin 10 and transforming
growth factor-beta. As discussed in more detail below, in
some instances, elevated levels of cytokines (e.g., TH-l
cytokines) are indicative of immunologic reproductive
W094/28425 PCT~S94/05692
~ 2~634SU
-13-
failure. In yet other instances, reduced levels of some
cytokin~es (e.g., TH-2 cytokines such as interleukins 4 and
10), are indicative of this condition.
As used herein, the phrase "elevated concentration" in
reference to an embryotoxic factor or cytokine refers to a
concentration which is elevated with respect to the
concentration of the embryotoxic factor or cytokine in the
negative standard. In general, diagnosis of a predisposition
to immunologic reproductive failure is made by an immunoassay
specific for the embryo~oxic factor if the ratio of the
sample embryotoxic factor concentration to the negative
standard embryotoxic factor concentration is at least about
2:1, i.e., the sample embryotoxic factor concentration is
elevated two-fold compared to the concentration of
embryotoxic factor in the negative standard. Alternatively,
or additionally, a diagnosis of a predisposition to
immunologic reproductive failure is made if the sample
embryotoxic factor concentration is substantially the same as
the concentration of embryotoxic factor present in the
positive standard. By "substantially the same" it is meant
that the sample embryotoxic factor concentration falls within
the margin of error of the concentration of embryotoxic
factor in the positive standard for the particular assay
performed.
Immunoassays have been used to determine the
concentrations of three different embryotoxic factors:
interferon-gamma (IFN-gamma), tumor necrosis factor-alpha
(T~-alpha) and tumor necrosis factor-beta (TNF-beta)
(Example 4). IFN-gamma was detected in reproductive
antigen-stimulate~ leukocyte supernatants from 125 of 255
recurrent abortion patients and significantly correlated with
embryotoxicity. TNF-alpha and TNF-beta also were detected.
Thus, Applicants have discovered that a majority of women
with unexplained recurrent abortion release TH-l cytokines
following stimulation with trophoblast extract reproductive
antigens. In contrast, antigen-stimulated lymphocytes from
W094/28425 2 ~ ~ 3 4 ~ ~ - 14- PCT~S94/05692
parous women with normal reproductive histories and men
release TH-2 cytokines (Example 4). Although not intending
to be bound to a particular theory, Applicants believe that
TH-l cytokines play a role in reproductive failure by
mediating a cellular immune response to reproductive
antigens, whereas TH-2 cytokines are associated with a
successful normal pregnancy and presumably play a role in
downregulating a cellular immune response to reproductive
antigens in vivo.
As used herein, "TH-l cytokines" refers to the
cytokines that are released by leukocytes in vivo as part of
a TH-l type immune response. Exemplary TH-l cytokines
include interferon-gamma, TNF-beta, IL-2, TNF-alpha, and
potentially IL-12. As used herein "TH-2 cytokines" refers
to the cytokines released by leukocytes in vivo as part of a
TH-2 type immune response. Exemplary TH-2 cytokines
include IL-4, IL-5, IL-6, IL-10 and potentially TGF-beta and
colony stimulating factors. Accordingly, the invention is
not limited in scope to cytokines that are presently known to
be TH-l or TH-2 cytokines but rather, embraces cytokines
that are identified in future to fall within these categories.
Alternatively, the diagnosis of a predisposition to
immunologic reproductive failure can be made by performing a
lymphocyte proliferation assay and observing lymphocyte
proliferation in response to stimulating the lymphocytes with
the above-described trophoblast antigen. Example 5
demonstrates that antigen-stimulated lymphocyte proliferation
was significantly higher in women with recurrent abortion of
unknown etiology than in fertile controls and that lymphocyte
proliferation significantly correlated with embryotoxic
factor activity in stimulated culture supernatants. A
diagnosis of a predisposition to immunologic reproductive
failure was made if the stimulated leukocyte supernatant had
a Stimulation Index greater than about 3Ø Fertile control
subjects were observed to have a stimulation index that was
less than 3Ø As used herein in reference to the lymphocyte
W094/28425 21 ~ 3 ~ ~ ~ PCT~S94/05692
-15-
proliferation assay, the term "Stimulation Index" refers to
the ratio of counts per minute (cpm) observed for
unstimulated cultures to the cpm observed for
antigen-stimulated cultures.
The term "embryotoxic factor" refers to an
extracellular factor that is released by leukocytes (i.e.,
lymphocytes and/or macrophages) in response to stimulation by
a reproductive antigen. The embryotoxic factor is further
characterized as being toxic to developing mouse embryos and
human placental cell lines. Toxicity is determined using the
above-recited bioassays. Applicants disclose herein at least
three cytokines that are embryotoxic factors.
A preparation containing one or more embryotoxic factors
has been partially purified and characterized (see Example
1). The preparation is heat-sensitive and, based upon
molecular weight dialysis experiments, contains a factor(s)
having a molecular weight between about 10 and about 30 kD.
By "heat-sensitive", it is meant that the ability of the
embryotoxic factor to inhibit mouse embryo development is
abrogated following exposure of the factor to 56C for 1
hour. Passage of the embryotoxic factor preparation through
an affinity column containing anti-interferon gamma-coated
beads (Endogen, Boston, MA) abrogated approximately 80 ~ of
the embryotoxic factor activity, as measured in the
above-mentioned embryo mouse development bioassay (Example
1), suggesting that IFN-gamma is indeed an embryotoxic
factor. An immunoassay which specifically detects
interferon-gamma has been used to definitively demonstrate
the presence of interferon-gamma in trophoblast
antigen-stimulated leukocytes derived from women having a
history of recurrent spontaneous abortion (Example 4).
Stimulated leukocytes from fertile parous controls did not
release IFN-gamma in response to trophcblast antigen
stimulation.
W094/2842s 21~ 3 4 ~ ~ PCT~S94/05692
-16-
Immunoassays also have been used to detect an elevated
concentration of two other TH-l cytokines (TNF-alpha,
TNF-beta) in stimulated leukocytes from women having a
history of recurrent spontaneous abortion compared to
stimulated leukocytes from fertile controls (Example 4).
Accordingly, at least gamma-interferon (one form of which has
a molecular weight of 17,000) or a soluble molecule which
closely associates (or works in synergy) with
gamma-interferon, TNF-alpha and TNF-beta have been identified
as embryotoxic factors. Soluble products of activated
macrophages (e.g., tumor necrosis factor (TNF)) also have
been reported to have toxic effects on preimplantation
embryos and trophoblast cell lines in vitro. Accordingly,
the term embryotoxic factors is not limited to
gamma-interferon, but rather embraces any and all soluble
factors including cytokines (e.g., TH-l cytokines) that are
involved in a cell mediated immune response to a reproductive
antigen. Exemplary cytokines that modulate a cellular immune
response are shown in Table I.
Example 4 also demonstrates that trophoblast
antigen-stimulated leukocytes from women with recurrent
reproductive failure do not release TH-2 cytokines such as
IL-2, IL-4 and IL-10 but that trophoblast antigen-stimulated
leukocytes obtained from fertile parous controls do release
these TH-2 cytokines, as measured in immunoassays which
specifically detect each of the above-mentioned cytokines.
The implications of this discovery with respect to methods
and compositions for diagnosing and/or preventing immuno:ogic
reproductive failure are discussed below.
According to another aspect of the invention, yet
another method for diagnosing a predisposition to immunologic
reproductive failure is provided. The method involves
stimulating a sample containing a plurality of leukocytes as
described above to release at least one cytokine, determining
the concentration of the cytokine to obtain a sample cytokine
concentration and comparing the sample cytokine concentration
W O 94/28425 2 1 6 3 4 S O PCTrUS94/05692
-17-
TABLE 1
Immunorequlatory Cytokines
Cytokine Function
Cytokines involved in Cell Mediated Immune Responses
Interleukin-2 Proliferation of activated B and T cells
Migration Inhibitory Factor Inhibition of macrophage migration
Cytotoxic and Cytostatic Kill or inhibit growth of susceptible
Factors target cells
Leukocyte Inhibitory Factor Inhibits migration of neutrophils
Tissue Factor Procoagulant activity
Macrophage-Activating Activates macrophages against tumor
Factors (GM-CSF, cells and intracellular organisms
Interferon-~,
Interleukin-3
Interleukin 4)
Chemotactic Factors Selectively mobilize and attract
monocytes, neutrophils, eosinophils,
or basophils to inflammatory site
Cytokines involved in B cell regulation and antibody production
Interleukin-4 Activates resting B and T cells
Inhibits antibody class switching
from IgM
Interleukin-5 B cell growth factor
Induces eosinophil differentiation
Interleukin-6 Induces terminal maturation of
B cells
Activates mitogen stimulated T cells
Induces T cell proliferation
Interleukin-7 Induces proliferation, but not
maturation, of early B cells
Stimulates proliferation of early
T cells
W O 94/28425 2 ~ 3 ~ PCTrUS94105692
Miscellaneous Cytokines
Colony-Stimulating Factors Stimulates granulocyte and monocyte
(Gm-CSF, Interleukin-3) differentiation
Activates mature macrophages to kill
tumor cells and certain
microorganisms
Interleukin-l Aids in s.timulation of T cell IL-2
production
Endogenous pryogen, glucocorticoid
synthesis, prostaglandin release,
collagenase production, synthesis
of acute phase reactants, chemotaxic
activity
Tumor Necrosis Induction of hemorrhagic necrosis
Factor/Cachectin of certain tumors
Causes cachexia, shock
Enhances eosinophil ADCC
Enhances neutrophil adhesion to
endothelial cells
Highly suppressive of B and T cell
proliferation
Potent chemoattractant for
macrophages
Render cells resistant to viral
infection
Promotes B cell differentiation
W094/28425 PCT~S94/05692
21 ~34~0
-19-
to the concentration of cytokine present in at least one
standard. The standard can be a positive standard (which
contains a concentration of cytokine indicative of a
predisposition to immunologic reproductive failure) or a
negative standard (which contains a concentration of cytokine
indicative of the absence of a predisposition to immunologic
reproductive failure). For a cytokine that is a TH-l
cytokine, a diagnosis of immunologic reproductive failure is
made if the sample cytokine concentration is elevated
compared to the negative cytokine standard or is
substantially the same as the positive cytokine standard.
For a cytokine that is a TH-2 cytokine, a diagnosis of
immunologic reproductive failure is made if the sample
cytokine concentration is reduced compared to the negative
cytokine standard or is substantially the same as the
positive cytokine standard. Thus, a diagnosis of immunologic
reproductive failure can be made by observing an increase in
the concentration of TH-l cytokines, e.g., interleukin-2,
interferon-gamma, tumor necrosis factor-alpha, tumor necrosis
factor-beta or by observ'ng a reduction in the level of
certain cytokines, e.g., TH-2 cytokines such as
interleukin-4 and interleukin-10, in stimulated leukocyte- or
leukocyte secretion containing samples.
The invention also provides a method~for preventing
immunologic reproductive failure (see, e.g., Example 2
"Immunosuppressive Therapy in Pregnant Women for Prevention
of Immunologic Spontaneous Abortion"). The method involves
selecting a mammal diagnosed as having a predisposition tc
immunologic reproductive failure and administering to the
mammal a therapeutically effective dose of an
immunomodulating agent to prevent an immunologic reproductive
failure. Preferably, the method for preventing immunologic
reproductive failure further includes performing one or more
of the above-described methods for diagnosing the condition
for a leukocyte- or leukocyte secretion-containing sample as
described above and administering to tne mammal a subsequen~
WOg4/28425 21~ 3 ~ ~ ~ PCT~S94/05692
-20-
dose of the immunomodulating agent to cause a reduction in
the amount of embryotoxic factor released in vitro or in vivo
by the mammal's leukocytes.
In contrast to the time-consuming bioassays of the
prior art, immunoassay quantitation of the embryotoxic
factors (see e.g., Example 4) permits the rapid e--aluation of
the efficacy of an immunomodulating treatment regimen.
Accordingly, the instant invention provides a method for
preventing immunologic reproductive failure, which method
includes ~min; stering at least one subsequent dose of the
immunomodulating agent to the patient. The instant invention
is not limited to administration of a single immunomodulating
agent, but rather embraces the administration of one or more
agents, depending upon the particular immunologic response of
the patient to immunomodulating agent drug therapy. This
iterative treatment process, i.e., administration of an
immunomodulating agent followed by determination c~ the
concentration of embryotoxic factor released by patient
leukocytes in response to immunomodulating agent drug
therapy, allows the tailoring of treatment to the cellular
immune response for each patient.
As used herein, "immunomodulating agent" refers to an
agent capable of modulating a cellular immune response and
includes agents which directly or indirectly modulate the
effective embryotoxic factor concentration in vivo. The
immunomodulating agent can be a nonspecific immunomodulating
agent (i.e., not targeted to modulating an immune response to
a particular target antigen) that downregulates a TH-l
immune response or that upregulates a TH-2 immune
response. Exemplary nonspecific immunomodulating agents
include glucocorticoids, cyclosporins, nifidipine,
pentoxiphylline and progesterone. Alternatively, the
immunomodulating agent can be a specific immunomudulating
agent that modulates the cellular immune response to a
spec~fic reproductive antigen. According to a particularly
pre.erred e~bodiment, the specific immunomodulating aqent .s
W094/28425 2 1 ~ 3 ~ ~ PCT~S94/05692
-21-
a vaccine including a reproductive antigen contained in an
adjuvant. An adjuvant is selected that downregulates a
r TH-l type immune response or that upregulates a TH-2 type
immune response to the reproductive antigen in vivo. Oral
vaccines for modulating a cellular immune response to a
specific antigen have been described (see, e.g., PNAS, USA
91:437-438 (1994); Immunology Today 12:383-385 (1991);
Cellular Immunology 131:302 (1990); PNAS, USA 89:421-425
(1992); Science 259:1321-1324 (1993); and Science
261:1727-1730 (1993), the contents of which references are
incorporated herein by references). Accordingly, a
particularly preferred cellular vaccine of the invention is
an oral vaccine prepared by placing the Jeg-3 antigen in
adjuvants such as those described in the above-identified
references. Adjuvants which downregulate a TH-l type
response and/or upregulate a TH-2 type response can be
selected by determining the TH-l and/or TH-2 cytokine
profile following immunization of, for example, an animal
with a ~accine containing a test antigen (e.g., BSA,
reproductive antigen) contained in the test adjuvant.
Exemplary adjuvants that upregulate a TH-2 type response
(and thereby downregulate a TH-l response) include alum and
squalene in oil. Additional adjuvants which can be screened
for their ability to downregulate a TH-l type response
and/or to upregulate a TH-2 type response include the
ISCOMS (Morein, B., et al., Nature (Lond.). 308:457-459
(1984)), cholera toxin adjuvants (Quiding, M., et al., J.
Clin. Invest. 88:143-148 (1991)) and complete Freunds
adjuvant. The ISCOMS are prepared by removing detergent in a
controlled fashion from a mixture of choiesterol, protein,
phospholipid, detergent and Quil-A (e.g., by dialysis and
centrifugation on a Quil-A-containing sucrose gradient).
ISCOM formation is confirmed by negative contrast electron
microscopy and by their distinctive sedimentation constant
(19S) in a sucrose gradient. Quil-A is a saponin extracted
from the bark of the tree Quillaja saponaria. Purification
wo 94,28425 2 ~ ~ 3 ~5 ~ PCT~S94/05692
-22-
of these saponins and their use as adjuvants is described in
U.S. Patent No. 5,057,540, issued to Kensil et al., the
contents of which patent are incorporated herein by reference.
As used herein, "effective cytokine concentration"
refers to the concentration of cytokine that is available for
binding to cytokine receptors, i.e., the concentration of
cytokine that is capable of tr-ggering a cellular immune
response. Thus, immunomodulating agents embrace agents which
function by (1) reducing TH-l cytokine release from
leukocytes; (2) reducing the concentration of receptors
capable of binding to the TH-l cytokines; (3) binding
directly to TH-l cytokines, thereby preventing cytokine
binding to receptors; (4) competing with the TH-l cytokines
for binding to cytokine receptors; as well as (5) agents
which modulate the concentration of any of the above (e.g.,
TH-2 cytokines). Accordingly, immunomodulating agents
include agents which are kno~n in the art for their ability
to suppress an immune response (e.g., progesterone), as well
as TGF-beta and antibodies to the embryotoxic cytokines
and/or antibodies to the embryotoxic cytokine receptors
(e.g., antibodies to gamma-interferon, tumor necrosis
factor-alpha, interleukin-2 and interleukin-6 or antibodies
to cytokine producing cells such as CD-3 and CD56 cells or to
their receptors). In a preferred embodiment, the
immunomodulating agent is capable of modulating the cellular
immune response in a localized area, i.e., the area in fluid
or tissue communication with fetal cells, as distinguished
from a humoral immune response.
The immunomodulating agents are administered in
therapeutically effect amounts. A thera~eutically effective
amount is that amount which is sufficient to reduce the level
of embryotoxic factor(s) to an amount(s) that is insufficient
to cause a reproductive failure. The effective amount of
agent will depend upon the clinical condition of the subjec_
being treated. A therapeutically effective amount can be
determined in a number of ways using medicai tech:~iques
W094/28425 PCT~S94/05692
2i~3~5~
-23-
customary to one of ordinary skill in the art. For example,
different amounts of immunomodulating agent are administered
to mammals predisposed to reproductive failure, followed by
assaying leukocyte- and/or leukocyte secretion-containing
samples obtained from the subject. Exemplary assays for
determi.ning the level of embryotoxic factor in such samples
are provided in the Examples. The therapeutically effective
dose of immunomodulating agent is selected which reduces the
level of extracellular embryotoxic factor to that level
observed in the samples of mammals not having a
predisposition to reproductive failure (i.e., a "normal"
level). Such levels are considered non-toxic. Likewise,
embryotoxic levels that are above normal levels, but which
are insufficient to cause reproductive failure, also are
considered non-toxic.
The selection of a therapeutically effective dose of
the i~munomodulating agent to prevent immunologic
reproductive failure is made in accordance with standard
Erocedures known to one of ordinary skill in the art, taking
into consideration the patient's clinical condition. Such
amounts will depend, of course, on the particular condition
being treated, the severity of the condition, and individual
patient parameters including age, physical condition, size,
weight and concurrent treatment. These factors are well
known to those of ordinary skill in the art and can be
addressed with no more than routine experimentatic~. It is
preferred generally that a maximum dose be used, that is, the
highest safe does according to sound medical judgment. It
will be understood by those of ordinary skill in the art,
however, that a patient may insist upon a lower dose or
tolerable dose for medical reasons, psychological reasons or
for virtually any other reasons.
Optionally, a mouse model (see Example 3) is used to
screen various immunomodulating agents (and/or the method cf
administration) for the ability to prevent immunologic
reproductive failure. Such screening is performed, for
W094/2842s 2 ~ ~ 3 4 5 ~ PCT~S94/05692
. ~
-24-
example, by beginning administration of the immunomodulating
agent to the mouse prior to, or shortly after conception, and
determining whether (1) the incidence of reproductive failure
is reduced and/or (2) the level of embryotoxic factor present
in mouse samples (i.e., leukocyte and/or leukocyte secretion
containing) is reduced.
~ mini stration of the immunomodulating agent is
performed in accordance with methods known to one of ordinary
skill in the art. Accordingly, a variety of administration
routes are available. The particular mode selected will
depend, of course, upon the particular drug selected, the
particular condition being treated and the dosage required
for therapeutic efficacy. The methods of this invention,
generally speaking, may be practiced using any mode of
administration that is medically acceptable, meaning any mode
that produces therapeutic levels of the agents of the
invention without causing clinically unacceptable adverse
effects. Such modes of administration include oral, rectal,
vaginal, topical, transdermal or parenteral (e.g.
subcutaneous, intramuscular and intravenous) routes.
Formulations for oral administration include discrete units
such as capsules, tablets, suppositories, patches, lozenges
and the like. For an example of a vaginal suppository
containing an immunomodulating agent (progesterone), see U.S.
Patent No. 5,084,277, the contents of which patent are
incorporated herein by reference.
The compositions may conveniently be presented in unit
dosage form, including oral vaccine form, and may be prepared
by any of tre methods well known in the art of pharmacy.
Such methods include the step of bringing the active agents
into association with a carrier which constitutes one or more
accessory ingredients. In general, the compositions are
prepared by uniformly and intimately bringing the agents into
association with a liquid carrier, a finely divided solid
carrier, or both, and then, if necessary, shaping the product.
W094l28425 PCT~S94/05692
~.634~
-25-
Compositions of the present invention suitable for oral
administration may be presented as discrete units such as
capsules, cachets, tablets or lozenges, each containing a
predetermined amount of the agents. Compositions suitable
for parenteral administration conveniently comprise a sterile
aqueous preparation of the agents, which is preferably
isotonic with the blood of the recipient. This aqueous
preparation may be formulated according to known methods
using those suitable dispersing or wetting agents and
suspending agents. The sterile injectable preparation may
also be a sterile injectable solution or suspension in a
non-toxic parenterally-acceptable diluent or solvent, for
example as a solution in polyethylene glycol and lactic
acid. Among the acceptable vehicles and solvents that may be
employed are water, Ringer's solution and isotonic sodium
chloride solution. In addition, sterile, fixed oils are
conventionally employed as a solvent or suspending medium.
For this purpose any bland fixed oil may be employed
:ncluding synthetic mono- or diglycerides. In addition, fatt~
acids such as oleic acid find use in the preparation of
injectibles.
Other delivery systems can include sustained release
delivery systems. Preferred sustained release delivery
systems are those which can provide for release of the agents
of the invention in sustained release pellets or capsules.
Many types of sustained release delivery systems are
available. These include, but are not limited to: (a)
erosional systems in which the agents is contained in a form
within a matrix, found in U.S. Patent Nos. 4,452,775 (Kent),
4,667,014 (Nestor et al.); 4,748,024 and 5,239,660 (Leonari)
and (b) diffusional systems in which an active component
permeates at a controlled rate through a polymer, found in
U.S. Patent Nos. 3,832,252 (Higuchi et al.) and 3,854,480
(Zaffaroni). In addition, a pump-based hardware delivery
system can be used, some of which are adapted for
implantation.
W094/28425 PCT~S94/05692
2~3~5~ ~
-26-
Generally, daily oral doses of active compound will be
from about 0.01 milligrams/kg per day to 1000 milligrams/kg
per day. Small doses (0.01 - 1 mg) may be administered
initially, followed by increasing doses up to about 1000
mg/kg per day. In the event that the response in a subject
is insufficient at such doses, even higher doses (or
effective higher doses by a different, more localized
delivery route) may be employed to the extent patient
tolerance permits. Multiple doses per day are contempla~ed
to achieve appropriate systemic levels of compounds.
An exemplary therapeutic dose for administration of
progesterone in suppository form is provided in Example 2.
The therapeutic dose of progesterone (a 50 mg progesterone
suppository) was administered twice a day (beginning three
days after ovulation in a subsequent conception cycle) to
patients having a history of recurrent reproductive
failure). In the preferred embodiments, the therapeutically
effective dose of immunomodulating agent is initially
administered in the luteal phase prior to implantation or
within one week post-conception. Preferably,
immunomodulating agent drug therapy is continued until the
risk of immunologic reproductive failure is eliminated (about
20 weeks).
Also within the scope of the invention are kits for
determining the presence of an elevated concentration of an
extracellular embryotoxic factor in a sample. In its
simplest form, the kit contains a first vial including ,an
antibody to the embrytoxic factor, a second vial containing
a standard including a concentration of embryotoxic factor
indicative of the absence of a predisposition to immunologic
reproductive failure ("negative" standard) or including a
concentration of embryotoxic factor indica~ive of a
predisposition to immunologic reproductive failure
("positive" standard); and instructions for using the
antibody to determine the concentration of the embryotoxic
factcr in the sample and to compare the sample embryo~oxic
W094128425 PCT~S94/05692
2~6~4~1~
-27-
factor concentration to the standard embryotoxic factor
concentration to determine whether the mammal has a
predisposition to immunologic reproductive failure. The
instructions delineate the assay protocol and the statistical
analysis necessary for determining the relative amount or
absolute concentration of embryotoxic factor present in the-
sample. Such instructions are tailored to the specific type
of immunoassay being performed, e.g., a competitive assay, a
sandwich assay, according to the standard procedures.
The antibody to the embryotoxic factor is specifically
reactive with the embryotoxic factor. By "specifically
reactive", it is meant that the antibody recognizes and binds
to an epitope on the embryotoxic factor and does not bind to
extraneous molecules present in the sample preparation, which
molecules do not include the embryotoxic factor epitope.
Tests for determinin~ the specificity of an antibody for a
particular protein are known to those of ordinary skill in
the art. For example, to demonstrate antibody specificity in
an immunoassay in which the signal measured in the
immunoassay is generated by virtue of a label attached to the
antigen (e.g., a radiolabel, fluorescent tag, or enzyme
label), the immunoassay is performed in the presence of an
increasing amount of unlabeled antigen. If the antibody
specifically reacts with the antigen, then a reduction in
signal is observed, the signal reduction being proportiona'
to the amount of unlabeled antigen present in the assay.
Other methods for evaluating the specificity and sensi~ivity
of immunoassays are known to those of ordinary skill in the
art without the need for undue experimentation.
According to one embodiment, the second vial includes
an amount of embryotoxic factor indicative of the absence
of a predisposition to immunologic reproductive failure
(negative standard). The amount of factor which satisfies
this criterion is established by assaying (e.g., bioassay and
immunoassay) sample preparations (i.e., leukocyte - and/or
leukocyte secretion- containing samples) obtained from
W094/28425 PCT~S94/05692
~3~ Q -28-
fertile women who have no propensity to reproductive failure
(see, e.g., the Examples). Accordingly, the second vial
serves as a s~andard to establish a reference value to which
the amount of embryotoxic factor quantitated in the sample is
compared. Preferably, the amount of embryotoxic factor
present in the second vial is selected such that a ratio of
sample embryotoxic factor concentration to standard
embrytoxic factor greater than about 2:1 is indicative of a
predisposition to immunologic reproductive failure.
In the preferred embodiments, the kits further contain a
third vial including a positive control, i.e., a vial
including an amount of embryotoxic factor indicative of a
predisposition to ;mmllnologic reproductive failure. The
amount of embryotoxic factor contained in the positive
control vial is determined by assaying samples obtained from
women diagnosed with immunologic reproductive failure as
described above. (see also the Examples).
To determine the amount of embryotoxic factor in
leukocyte-containing samples, the kits further contain a vial
including a plurality of reproductive antigens for
stimulating the leukocytes to release embryotoxic factors.
Preferably, the reproductive antigens are sperm antigens,
trophoblast antigens and/or antigens derived from a human
choriocarcinoma cell line. In the most preferred
embodiments, the reproductive antigens are purified from
choriocarcinoma cell lines BeWo or Jeg-3. It will be
understood that the above-described kits for determining an
elevated concentration of the embryotoxic factor can easily
be adapted for determining the concentration of a particular
TH-l or a TH-2 cytokine(s) in the sample (e.g., by
substituting an antibody to the cytokine for the
above-mentioned antibody to the embryotoxic 'actor) and
including instructions which describe comparing the sample
cytokine concentration to a positive or negative cytokine
standard to determine whether the mammal has a predisposition
to immunologic reproductive failure.
W094/2842~ 2 ~ 6 3 ~ 5 ~ PCT~S94/05692
-29-
Also provided within the scope of the invention are
vials containing various isolated reproductive antigens
and/or embryotoxic factors. By isolated, it is meant that
the antigen or factor has been removed from its natural
matrix (e.g., blood, tissue) and exists in a substantially
purified form (e.g., greater than about 80 % pure as assessed
by gel electrophoresis). The vial can include one or more
isolated antigens. Preferably, the isolated antigen has a
purity exceeding 90%. A higher degree of purity is needed
for the in vivo than is required for in vitro applications.
According to one aspect of the invention, a vial is
provided which includes an amount of isolated reproductive
antigen capable of stimulating a plurality of leukocytes to
release an embryotoxic factor. In the preferred embodiments,
the vial containing the reproductive antigens for stimulating
leukocytes in vivo further includes a pharmaceutically
acceptable carrier. Such carriers are known to one of
ordinary skill in the art and include, for example, an
isotonic saline solution. To modulate a cellular immune
response to a reproductive antigen in vivo, a vaccine is
prepared which includes the reproductive antigen in the
above-described adjuvants for downregulating a TH-l type
response or for upregulating a TH-2 type response.
The following non-limiting examples illustrate
representative utilities of the instant invention. Example 1
demonstrates that the preimplantation embryo is a vulnerable
target of embryotoxic factors produced by activated
leukocytes from many women with recurrent abortion.
Example 1 further demonstrates that determination of an
elevated concentration o- embryotoxic factor is indicative of
a predisposition to immunologic reproductive failure.
Example 2 illustrates the use of immunomodulating agent drug
therapy for the treatment of women previously diagnosed w tr
immunologic reproductive failure. Example 3 illustrates the
anti-fertility effects of antisperm cell-mediated immunity -r
mice. Example 4 demonstrates that lymphocytes from a
W094/28425 ~ ~ 4 ~ ~ PCT~S94/05692
-30-
majority of women with unexplained recurrent abortion make
THl-type cyto~ines when exposed to trophoblast extracts,
whereas lymphocytes from parous women with normal
reproductive histories and men make TH2-type cytokines.
Example 5 demonstrates that lymphocyte proliferation in
response to trophoblast antigen stimulation was significantly
higher in women with recurrent abortion of unknown etiology
than in fertile controls and significantly correlated with
embryotoxic factor activity in culture supernatants.
EXAMPLES
Example 1: Immunomodulatinq Aqent Drug Therapy in Pregnant
Women For Prevention of Spontaneous Abortion
(1) Study Patients
The subjects in this study were 300 reproductive-aged,
non-pregnant women undergoing evaluation for recurrent
abortion at the Brigham and Women's Hospital Reproductive
Endocrinology and Infertility Clinic between July 1, 1986 and
June 31, 1989. The women were between 22 and 42 years old
and had a history of at least three prior spontaneous
abortions with or without a stillbirth, ectopic pregnancy, or
live birth. These women were grouped by clinical history as
follows: women with primary abortion (no live births), women
with secondary abortion (abortions subsequent to live births
or stillbirths), women with previous ectopic pregnancy, or
women with other criteria (abortions interspersed with live
births). After a complete medical, surgical, and social
history was obtained from the couple and a thorough physical
examination was performed on the woman, all couples had
peripheral blood chromosome assessment by standard banding
techniques. All women underwent hysterosalpingography
followed by hysteroscopy-laparoscopy when indicated.
Ovulation was assessed by a late luteal-phase endometrial
biopsy with concomitant serum progesterone determination.
When the endometrial biopsy specimen was out of phase
according to the criteria of Noyes et al. (Fertil.
W094/28425 PCT~S94105692
~ 21~3~50
-31-
Steril.1:3-25 (1950), the suspected ovulation disorder was
confirmed by repeating the biopsy in a subsequent cycle. In
addition, all women had cervical cultures for mycoplasma and
ureaplasma, and blood was obtained for thyroid-stimulating
hormone, thyroxine, prolactin, anticardiolipin antibody, and
lupus anticoagulant (Russell viper venom time)
determinations. Cervical mucus and serum were also obtained
for antisperm antibody assessment. After the preceding
evaluation, study patients were categorized as having either
a genetic, anatomic, endocrine, infectious, or humoral immune
cause for their reproductive failure. Peripheral blood was
obtained from all women on their initial visit for embryo-
ar.d trophoblast-toxic factor(s) testing.
Peripheral blood was also obtained from a comparison
group of 30 non-pregnant, paid volunteer women who were
randomly selected from the Obstetrical Nursing Service.
Women in ~his control group were of reproductive age (23 to
41) with a history of at least three prior uncomplicated term
deliveries and no history of either spontaneous abortion,
ectopic pregnancy, or stillbirth. None of the women in the
study were taking medication at the time of blood collection.
(2) Assays
(a) Embryotoxic factor assay
(i) Reproductive antiqen preparation. Trophoblas
antigen extracts were prepared from the human gestational
choriocarcinoma cell lines BeWo and Jeg-3 (American Tissue
Type Collection, Bethesda, Md.) These cells were grown in
culture flasks by standard culture techniques until
confluence. After trypsinization, the cells were washed
twice in Hanks' balanced salt solution without calcium or
magnesium (Gibco, Grand Island, N.Y.), and cells excluding
trypan blue were counted on a hemocytometer (viability always
> 90%). The cells were diluted to 107 cells per millilite-
in tissue culture media comprising Roswell Park Memorial
Institute 1640 medium (RPMI;Gibco), 10% fetal calf serum, 0.2
mmol/L glutamine, 100 IU/ml benzylpenicillin potassium, anc
W094l28425 PCT~S94/05692
2~ ~3~Q ~
.,q
~,
100 mg/ml streptomycin sulfate (Sigma, St. Louis). Cells
were disrupted in a dounce homogenizer, and after
centrifugation at 400g for 10 minutes, the membrane-enriched
trophoblast antigen supernatant was divided into aliquots and
stored at -70C until use.
Semen was obtained from fertile donors by masturbation
into sterile containers, and motile sperm were isolated by
percoll density gradient centrifugation. Aliquots of 107
sperm per milliliter in tissue culture media were dounce
homogenized. The suspension was then centrifuged at 400g for
10 minutes and the membrane-enriched sperm antigen
supernatant was divided into aliquots and stored at -70C
until use.
(ii) Preparation of Leukocyte-containing samples.
Mononuclear leukocytes were separated from blood obtained
from the study patients by Ficoll-Hypaque (Pharmacia,
Uppsala) centrifugation. Isolated cells were washed twice
and resuspended in RPMI medium. Viable mononuclear cells
ex~luding trypan blue were counted on a hemocytometer
(viability alway~ > 90%) and diluted in tissue culture medium
to a concentration of 106 cells per milliliter. Leukocyte
suspensions were then divided into three 50 ml tissue culture
flasks (Becton Dickinson, Lincoln Park, N.J.) containing
107 leukocytes per 10 ml of medium in each loosely capped
flask. One milliliter of either trophoblast antigen
suspension, sperm antigen suspension, or tissue culture
medium alone was added to each flask. After a 72 hour
incubation at 37C in 3% carbon dioxide and 95% air, the cel'
cultures were transferred to separate 15 X 95 mm polystyrene
round-bottom tubes with caps (Becton Dickinson) and pelleted
by centrifugation at 400g for 10 minutes. The cells were
next washed once in RPMI medium and once in Whitten's medi1~m
and then resuspended in 1 ml of Whitten's medium supplemen~ec
with antibiotic-antimycotic solution (Gibco) that had been
previously passed througn a 0.45 mm filter (Corning Glass,
Corn ng, N.Y.). The pH of the medium was adjusted before use
W094l28425 PCT~S94/05692
~lG3~
-33-
to 7.3 with carbon dioxide gas and supplemented with 0.3%
bovine serum albumin (Miles Scientific, Naperville, ILL).
After an additional 12-hour incubation at 37C in 5% carbon
dioxide and 95% air, the three suspensions from each original
sample were centrifuged at 100g for 10 minutes. The three
supernatants were each filtered through a 0.22 m Millex-GS
filter unit (Millipore, Bedford, MA) and stored at -70C
until use.
(iii) Preparation of Leukocyte secretion-
containinq samples. Peripheral blood, peripheral serum,
peritoneal fluid, endometrial tissue (extracted or
homogenized), vaginal secretions and saliva can be used
directly or are processed to remove cellular components
(e.g., leukocytes). The preparation of peripheral blood
mononuclear cell (PBMC) supernatants is described in Example
4. The leukocyte-containing samples are stimulated and
assayed as described above.
(iv) Preparation of Trophoblasts. Trophoblasts were
prepared from a choriocarcinoma cell line as described above
(Example 1).
(b) Embryo development assay
Embryo culture was performed as previously described
(Hill, J.A., et al., J. Immunol. 139:2250-2254 (1987)).
Briefly, two-cell embryos were harvested from CD-l female
mice (Charles River, Kingston, Ontario) 44 hours after human
chorionic gonadotropin administration. Embryos were cultured
in 20 ml drops containing a 1:1 dilution of study supernatant
in Whitten's medium -0.3% bovine serum albumin under carbon
dioxide - equilibrated paraffin oil in Falcon tissue culture
dishes(Becton Dickinson). Media and supernatants were
e~1ilibrated overnight in 5% carbon dioxide and 95% air
before the addition of embryos. Embryos cultured in
Whitten's medium - 0.3% bovine serum albumin alone served as
an additional control. At least 11 embryos were cultured -n
each 20 ml drop. Embryos were assessed for normal
development after 4 days in culture (100 hours after huma~
W094/28~ ~ 3 ~ ~ ~ PCT~S94/05692
-34-
chorionic gonadotropin administration) by the criteria
described by Ducibella (Dev. Biol. 79:356-66 (1980)). The
experimental end point was the percentage of normal
blastocysts in each culture after 4 days of development in
vitro. The sensitivity and specificity of the assay were
maximal when the median percentage of embryos advancing to
the blastocysts stage of development was < 50% of control
values. The intraassay and interassay coefficients of
variation were 8% and 12%, respectively, for the embryo-toxic
bloassay .
(c) Trophoblast proliferation assay
Human gestational choriocarcinoma cells (107 cells per
o.l ml tissue culture medium) were plated in triplicate intG
96-well, flat-bottom microtiter trays (Becton Dickinson).
Two hours after cell plating, 0.1 ml of leukocyte supernatzrt
or media alone was added (four wells per test solution).
After a 2-day incubation at 37C in 5% carbon dioxide and 95
air, 0.5 mCi of tritiated thymidine z(13.1 Ci/mmol; New
England Nuclear, Boston) was added to each well. Cells were
harvested at 72 hours after trypsinization onto glass fiber
filter paper with a MASH II automatic sample harvester
(Microbiological Assoc. Los Angeles). Viability of control
cultures was assessed before harvesting by means of trypan
blue exclusion (viability always > 90%). Filter paper disks
corresponding to each well were air dried, transferred to
scintillation counting vials, and suspended in 2 ml of
Betafluor scintillation cocktail (National Diagnostics,
Summerville, N.J.). Radioactivity (counts per minute) was
measured in a liquid scintillation counter (Beckman
Inslruments Inc., Fullerton, Calif) (efficiency of the syste~.
45%), and the mean = SD of each set of triplicate cultures
was calculated. The data are presented as percentages of
culture medium control. Trophoblast toxicity was assumed
when the median percentage of trophoblast proliferation was ~.
50% of control values.
W094/28425 PCT~S94/05692
21~3~;~jQ
-35-
(d) Leukocyte Proliferation assay
The leukocyte proliferation assay is performed according
to procedures known to one of ordinary skill in the art (see
Example 5 and e.g., Immunol. Rev. 75:61-85 (1983) and J.
Reprod. Immunol. 6:377-391 (1984), the contents of which
references are incorporated herein by reference). Briefly,
the above-described preparation of reproductive antigen~s) is
added to 2.5 x 10(5) leukocytes in culture, followed by
assessment of tritiated thymidine incorporation to determine
the extent of DNA proliferation. All assays are performed in
triplicate. A diagnosis of a predisposition to immunologic
reproductive failure is made if the lymphocyte
secretion-containing sample has a Stimulation Index that is
greater than about 3Ø Fertile control subjects have a
stimulation index that is less than 3Ø As used herein, the
term "Stimulation Index" refers to the ratio of counts per
minute (cpm) for unstimulated cultures to the cpm for
antigen-stimulated cultures.
(e) Gamma-Interferon ELISA
An exemplary IFN-gamma ELISA, as well as other cytok ne
immunoassays useful for the purposes of the invention are
provided in Example 4. A commercially-available
enzyme-linked immunoassay (e.g., Genzyme Corp., Cambridge,
MA) has been used to determine the amount of an embryotoxic
factor present in a reproductive antigen-stimulated
leukocyte-containing samples (see Example 4). These assavs
also are useful for determining the amount of an embryotoxic
factor in a leukocyte-secretion containing samples.
(f) Statistical analysis
We compared the mean age distribution between the 300
women with recurrent abortion and the 30 control subjects
with a two-sample test for independent samples and assumed
equal or unequal variances based on the F distribution.
Differences in mean ages by different categories of abortion
and reproductive failure causes were assessed through
analysis of variance. The percer.tages of mouse blastocys-
W094/2842S PCT~S94/05692
2 ~
-36-
development or trophoblast proliferation in supernatants
failed the test of normality with the Shapiro-Wilk W
statistic, therefore median percentages were compared between
the 300 women with recurrent abortion and the 30 controls
overall and within each of the categories of abortion and
reproductive failure causes with the use of non-parametric
tests of medians and the Wilcoxon rank sum test. All
directional p values were two tailed.
(3) Embryotoxic Factor Characterization
Trophoblast antigen-stimulated leukocyte supernatants
from 15 patients that adversely affected embryo development
were subjected to three different methods, to characterize
the factor(s) responsible for embryo toxicity; molecular
weight dialysis, heat inactivation, and affinity column
purification. The supernatants were dialyzed for 12 hours
against three changes of Whitten's medium - 0.3% bovine serum
albumin in Spectrapor membrane tubing (Spectrum Medical
Industries, Los Angeles) with molecular weight limits of
3500, 10,000 or 30,000 and then tested in the mouse embryo
development assay. In separate experiments aliquots of these
supernatants were heat treated at 56C for 1 hour or passed
through an affinity column containing anti-interferon
gamma-coated beads (Endogen, Boston) before testing in the
mouse embryo development assay. Five leukocyte supernatants
not exhibiting an adverse effect and medium alone were used
as controls in each of these experiments.
(4) Results: The leukocytes of women havinq a predisposi~ion
to immunoloqic spontaneous abortion released Embryotoxic
Factors in response to stimulation with reproductive
antiqens in vitro.
Women with recurrent abortion were 80% white, 10%
Hispanic, 8% black, 1% Native American, and 1% Asian. The 30
fertile controls consisted of 25 white, 3 Hispanic, and 2
black women. The 300 women evaluated for recurrent abortion
had experienced 13~7 prior presnancies, 1213 of which
resulted in reproductive failure (median ~, range 3 to 25).
W094128425 PCT~S94/05692
2i63~5~
-37-
Fetal loss occurred during the first trimester in 88%,
whereas 12% of the abortions occurred during the second
trimester (before 17 weeks of gestation).
The control group of women without a history of abor-ion
were sl.ightly older than women with a history of reproductive
failure (37.3 + 3.1 vs. 35.9 + 3.4, Table II). Among the 300
women with recurrent abortion, 215 women (72%) had primary
abortions, 50 women (17%) had secondary abortions, and the
remaini.ng 35 women (11%) could not be identified as having
either primary or secondary abortions. Seven of these women
had a total of 9 ectopic pregnancies (all tubal gestations),
and the other 28 women experienced recurrent abortions
interspersed with live births.
The cause of recurrent abortion was unexplained in 180
(60%) women. The remaining 120 women (40%) had recurrent
abortion presumably as a result of endocrine abnormalities
(17%), anatomic anomalies (10%), infections (5%), humoral
immune disturbances (4%), or chromosomal aberrations (5%).
There was no correlation between the average age of the women
and etiologic categories, except in women with an infectious
cause (positive cervical culture for mycoplasma or
ureaplasma), who were significantly younger than the other
women with a history of reproductive failure (32.9 + 4.1 vs.
35.7 + 1.0, p < 0.01).
There was no difference between effects of supernatants
derived from unstimulated peripheral blood mononuclear cells
from women in any category and control medium in either the
embryo development or trophoblast proliferation assays (data
not shown). Embryo development and trophoblast prolife:ation
in response to trophoblast antigen-activated leukocyte
supernatants and sperm antigen-activated leu:Yocyte
supernatants have previously been reported (see Hill, J. et
al., A-m. J. Obstet. Gynecol. 166:1044-52 (1992)). Neither
trophoblast antigen-activated nor sperm antigen-activated
leukocyte supernatants from the 30 fertile women adverseiy
affected embryo development or trophcblast proliferation.
W094/2842s PCT~S94/05692
2~3~5~ -38-
Trophoblast and sperm antigen extracts alone had no effect on
either embryo development or trophoblast proliferation.
Neither race nor ethnicity was associated with either the
production of toxic factors or the various causes of
recurrent abortion. Both embryo development and trophoblast
proliferation in the presence of trophoblast
antigen-activated and sperm antigen-activated leukocyte
supernatants were decreased in women with recurrent abortion
as compared with fertile controls. In the 300 patients with
recurrent abortion, embryo development was more often and
more severely affected than trophoblast proliferation in the
presence of both sperm antigen-activated and trophoblast
antigen-activated leukocyte supernatants (effects of
trophoblast and sperm antigen-activated leukocyte
supernatants on embryo development versus trophoblast
proliferation p < O.01). Embryo development was more
adversely af~ected by trophoblast antigen-activated leukocyte
supernatants than by sperm antigen-activated leukocyte
supernatants, except in those women with a prior ectopic
pregnancy.
Embryo development in the presence of trophoblast
antigen-activated or sperm antigen-activated leukocyte
supernatants from women with an unknown cause of abortion was
significantly less than embryo development in comparable
supernatants from women with other causes (p > 0.01). Embryo
development was also observed to be lower in trophoblast
antigen-activated and sperm antigen-activated leukocyte
sup~rnatants from women with recurrent abortion caused by an
endocrine or humoral immune abnormality (p ~ 0.001) but not
in those from the two groups of women with an anatomic or
genetic ~iology of recurrent abortions. In the two women
with antisperm antibodies, embryo development was unaffected
by sperm antigen-activated leukocyte supernatants but was
adversely affected by trophoblast antigen-activated leukocyte
supernatants. However, within the anatomlc group
embryo-toxic factors were produced in women with a diagnosis
W094/28425 PCT~S94105692
~ 2163~0
-39-
of endometriosis as their only abnormal finding in response
to stimulation by both trophoblast and sperm antigens
(p c 0.05). In the same group trophoblast proliferation was
also significantly inhibited in response to trophoblast
antigen-activated leukocyte supernatants from women with
endometriosis (p c 0.05). A woman with a pericentric
inversi.on of chromosome 9 had low embryo development in
response to both trophoblast antigen-activated and sperm
antigen-activated leukocyte supernatants and low trophoblast
proliferation in response to trophoblast antigen-activated
leukocyte supernatants. Otherwise, cytogenetic abnormalities
were not associated with either embryo or trophoblast
toxicity.
The sensitivity and specificity of the bioassays were
greatest when embryo development and trophoblast
proliferation was < 50% of control values (Table II).
Only one woman in the fertile control population had a
value c 50%. This woman had 45% blastocyst development in
the presence of supernatant from her sperm antigen-activated
leukocyte culture. Using 50% of control values as our
cutoff, we detected women with recurrent abortion as fOllowa:
for embryo development assays, 63% using trophoblast
antigen-act~vated and 51% using sperm antigen-activated
leukocyte supernatants; for trophoblast proliferation, 32%
using trophoblast antigen-activated and only 13~ using sperm
antigen-activated leukocyte supernatants. Only one (3%) of
the fertile controls would have been falsely classified as
having recurrent abortion with sperm antigen-activated
leukocyte supernatant in the embryo development assay, and
none would have been falsely classified with the other
assay-supernatant combinations.
The embryo development assay of reproductive
antigen-stimulated leukocyte preparations was more sensitive
than the trophoblast proliferation assay in predicting the
propensity for abortion. Only four of 300 women had > 50%
W094/28425 PCT~S94/05692
~3~5~ -40-
TABLE II
Sensitivity and specificity for recurrent abortion
by assay and reproductive antigenic factor
.
Women Women
with Without
abortions abortions Sensitivity Specificity
(No.) (No.) (%) (%)
ED-T
Pos.(<50%) 188 0 -
63 100
Neg.(>50%) 112 30
ED-S
Pos.(<50%) 153
51 97
Neg.(>50%) 147 29
TP-T
Pos.(<50%) 95 o
32 10
Neg.(>50%) 205 30
TP-S
Pos.(<50%) 40 0
13 100
Neg.(>50%) 260 30
W094/2842~ 2 1 ~ 3 ~ $ ~ PCT~S94/05692
-41-
TABLE III
Sensitivity and specificity of the embryo development
assay for predicting reproductive failure in women
with known and unknown causes of their abortions
Women Women
with Without
abortions abortions Sensitivity Specificity
(No.) (No.) (%) (%)
All women
ED-T o r
ED-S < 50% (pos.) 236
79% 97
ED-T and
ED-S > 50% (neg.) 64 29
Cause Unknown
ED-T o r
ED-S < 50% (pos.) 161
89% 97
ED-T and
ED-S > 30% (neg.) 19 29
Cause known
ED-T o r
ED-S ~ 50% (pos.) 75
65% 97%
ED-T and
ED-S > 50% (neg.) 45 29
embryo development when trophoblast proliferation was < 50~.
If only trophoblast proliferation had been used to assess
toxic factor production, 127 women would have been falsely
screened as negative. Only 113 women produced toxic factors
in response to reproductive antigens in the trophoblast
proliferation assay compared with 236 women who tested
positive with the embryo de-.-elopment bioassay (see Table -_ ,
Hill, J., et al., Am. J. Obstet. Gynecol. 166:1044-52 (1992
for data~.
W094/28425 PCT~S94/05692
'2 ~ ~ 3 ~
-42-
In Table III we considered a positive test for recurrent
reproductive failure to be c 50% of embryo development in
response to either trophoblast or sperm antigen-activated
leukocyte supernatants. This allowed us to detect 79% of
women with recurrent abortion while still maintaining an
extremely low number of false-positive results (3%). This
propensity to generate Embryotoxic Factors in response to
either trophoblast or sperm antigen stimulation was more
pronounced in women with an unknown cause as compared with a
known cause for their abortion. The presence of embryo- and
trophoblast-toxic factors was not influenced by a change in
partners because six women in whom toxic factors were
produced in response to sperm-antigen stimulation (n = 4) and
trophoblast antigen stimulation (n = 2) experienced recurrent
abortions with both their prior and current husbands.
The effects of embryotoxic factor-containing samples on
embryo development after molecular weight dialysis indicated
that the factor(s) resEonsible for embryo toxicity (in all 15
supernatants tested) was between about 10 and 30 kd. The
embryo-toxic effect of the supernatants was abrogated
followi.g heat treatment at 56C for 1 hour. In 12 of 15
embryo-toxic supernatants (from reproductive
antigen-stimulated leukocyte preparations) the factor(s) was
absorbed on affinity columns containing anti-interferon gamma
beads. Passage of control media through the anti-interferon
gamma column had no effect on embryo development. Further
evidence identifying IFN-gamma as an embryotoxic factor is
provided in Example 4.
Example 2- Immunomodulatinq Aqent Druq Therapy For
Preventinq Immunoloqic Spontaneous Abortion
(1) Study Patients.
We reviewed the records of all women evaluated for
recurrent abortion at the Fertility and Endocrine Unit of
Brigham and Women's Hospital between July 1987 and June
1991. For the purposes of this study, recurre~ abortion w~s
W094/28425 PCT~S94/05692
i 21~3~d
-43-
defined as two or more pregnancy losses before 20 week's
gestation. A11 women were evaluated according to a standard
protocol that included the following: (1) a thorough history
and physical examination; (2) chromosome assessment of the
women and her partner using standard banding techniques; (3)
hysterosalpingogram followed by laparoscopy/hysteroscopy when
indicated; (4) cervical cultures for mycoplasma and
ureaplasma, with treatment if positive; (5) serum assay for
TSH, thyroxine, prolactin, anticardiolipin antibody, and
lupus anticoagulant (Russell viper venom time); (6)
evaluation of anti-sperm antibodies in the woman's serum and
cervical mucus; and (7) evaluation for luteal-phase
insufficiency by an endometrial biopsy during the late luteal
phase. When an endometrial biopsy was out of phase according
to established criteria, the defect was confirmed on a
subsequent biopsy and treated with ovulation induction until
a repeat biopsy demonstrated an in-phase endometrium. At the
conclusion of the evaluation, the patients were categorized
as having either a genetic, anatomical, endocrine,
infectious, or humoral immune etiology for their reproductive
failure. Women in whom no presumed etiology could be
dete~ted were diagnosed with unexplained recurrent abortion.
(2) Sample Preparation. Leukocyte and Leukocyte-secretion
containing samples were prepared as described above from
whole blood and serum. To prepare leukocyte-containing
samples, lymphocytes and macrophages from the patients' blood
were isolated and maintained in tissue culture in the
presence of antigens obtained from a human trophoblast cell
line and human sperm (described above). Supernatants from
these cultures were collected and added to two-cell mouse
embryos in culture, with assessment of subsequent blastocys~
development. A toxic effect was assigned when blastocyst
development was less than 50% of control values as previously
described. All samples were coded and the assays performed
by the same technician, who was not aware of the sample
source or patient status.
W094/28425 PCT~S94/05692
~ ~ ~ 3 ~ 44-
(3) Immunomodulatinq Aqent Drug Therapy. - Preliminary
Results.
(a) Methods 't,,
Women found to be positive for Embryotoxic
Factor(s) upon initial evaluation were given progesterone
(50-mg suppository twice a day) beginni~g 3 days after
ovulation in a subsequent conception cycle. Progesterone was
chosen for its potential immunosuppr~ssive effects. The
women were closely followed by the same investigator and
asked to return weekly for hCG assessment and then at 5
weeks' gestation for pelvic ultrasound and reassessment of
embryotoxic factors. Fetal viability was assessed by pelvic
ultrasound every 2 weeks until 12 weeks' gestation. Women
with a viable intrauterine pregnancy documented by ultrasound
at 12 weeks' gestation were referred to an obstetrician for
continued care.
Patients and their referring physicians were asked to
obtain karyotypes of any subsequent abortions. In some
cases, obstetric care was continued at our hospital and
outcomes were easily obtained from the medical record. In
all cases, we mailed a follow-up questionnaire to the
patients that asked for the outcomes of all pregnancies after
the initial evaluation at our center.
Data were statistically analyzed using x2 with Yates
correction.
(b) Results
We evaluated 450 non-pregnant women in our unit for
a history of recurrent abortion (mean of four previous
losses, range 2 to 25). Of these, 346 had a normal
chromo~omal analysis, normal hysterosalpingogram, normal or
corrected luteal-phase endome'rial biopsy, negative or
treated cervical cultures, normal thyroid function, and
negative anticardiolipin antibody and lupus anticoagulant.
Embryotoxic factors were identified in 328 (95~) of these
women.
W094/28425 PCT~S94/05692
* 2I63450
. -45-
After this evaluation, there were a total of 208 known
pregnancies in 166 women. Rerpeat embryotoxic factor and
outcome data were available ih 141 pregnancies in 117 women.
Of the 67 pregnancies (61 women) for which outcomes were not
known, 31 were positive and 36 were negative for embryotoxic
factors when reassayed in the first trimester.
The embryotoxic factor assay was predictive of pregnancy
outcome (p < .01). Of 56 women still positive for
embryotoxic factors early in pregnancy, 40 (71%) miscarried
(37 in the first 10 weeks of pregnancy and three between
12-16 weeks) and 16 (29%) delivered a viable infant. Of the
85 women who were found to be negative for embryotoxic
factors on repeat testing, only 11 (13%) miscarried (all in
the first trimester), whereas 74 (87%) delivered a viable
infant. The positive and negative predictive values of the
embryotoxic factor assay were 0.76 and 0.86, respectively.
Chromosomal analyses of the aborted gestations were
requested, but not performed in every case. Abnormalities
were found in at least four of 11 karyotyped miscarriages in
women negative for embryotoxic factors early in pregnancy,
including one complete mole, two triploidic partial moles,
and one autosomal trisomy 18. Chromosomal analysis was
available in nine subsequent abortions of women who were
still positive for embryotoxic factors early in pregnancy.
Abnormal karyotypes (two triploidic partial moles and one
autosomal trisomy 21) were found in three of these nine cases.
Among the 21 women with more than one subsequent
pregnancy after their initial evaluation (19 with two
pregnancies, one with three, and one with four), there were
14 pati.ents in whom first-trimester embryotoxic factor values
were di.scordant when compared in subsequent gestations. Ten
of these 14 women miscarried during the pregnancy when their
embryotoxic factors were positive and carried another
pregnancy to term when the~r embryotoxic factors were
negative. Two of the 14 spontaneously aborted all subsequent
pregnancies (three pregnancies in one patient and four in thC
W O 94/28425 PCT~US94/05692
-4 6-
other). The embryotoxic factor assay predicted all
subsequent reproductive failures in these two women except
for two cases (one in each).~n which the embryotoxic factors
were negative yet the pregnancies still spontaneously
aborted. The remaining two of these 14 women each carried
two pregnancies to viability even though the embryotoxic
factor assay predicted that one pregnancy in each would be
aborted. All of these pregnancy losses occurred during the
first trimester after documentation of fetal cardiac activity
at 5 weeks' gestation.
(4) Immunomodulatinq Aqent Druq Therapy: Selection of
Immunotherapeutic Agent Doses For Preventinq Immunoloqic
Reproductive Failure
Leukocyte-containing and/or leukocyte secretion-
containing samples are prepared as described above and are
tested for the presence of an elevated concentration of
Embryotoxic ~actor(s) using the above-described assays.
Preferably, samples are tested using an assay such as an
immunoassay for the Embryotoxic Factor to allow for rapid
assessment of efficacy of the immunomodulating agent
therapy. Accordingly, patient samples (containing leukocytes
and/or leukocyte secretion products) are assayed for the
presence of an elevated concentration of Embryotoxic
Factor~s) prior to and during the immunomodulating agent
therapy regimen.
An immunomodulating agent, such as progesterone, is
administered to a patient previously diagnosed (as above) as
having recurrent reproductive failure. Leukocyte and/or
leukocyte secretion-containing samples are prepared and
assayed as described above to determine the amount of
Embryotoxic factor present in the sample. A subsequent dose
of the immunomodulating agent is administered to the patient,
the subsequent dose being selected to cause a reduction in
the amount of embryotoxic factor released in vivo by the
leukocytes. In this manner, the immunomodulating agent
therapy is precisely tailored to the cellular immune response
w094l2842~ PCT~S94/05692
,~ 2i634~0
-47-
of each patient. This iterative process is continued until
the concentration of extracellular embryotoxic factor in vivo
is sufficiently low (as determined by assay and comparison
with a standard concentration indicative of the absence of a
predisposition to reproductive failure) to preclude abortion
of the fetus.
Example 3: Antifertility Effects Of Antisperm Cell-Mediated
Immunity In Mice
(1) Materials and Methods
(a) Preparation of Cells to be Used as Antiqens
(i) Sperm. DBA/2 retired breeder males (Jackson
Laboratories, Bar Harbor, ME) were killed by cervical
di.slocation and the cauda epididymides were removed and
mi.nced with fine scissors. Sperm were suspended in
Krebs-Ringer solution, passed through a fine screen and
collected from the cell pellet following centrifugation
at 400 X g for 5 min. Motile sperm were further
isolated from immotile sperm and somatic cells by
centrifugation through a discontinuous gradient of
Percoll (Pharmacia, Piscataway, NJ) ~47~/90%) for 30
min. at 500 X g. The cell pellet containing motile
sperm was washed 3 times and resuspended at a final
concentration of 1 X 10 /ml in saline. The cell
suspension was mixed 1:1 with CFA or IFA to form a
homogenous emulsion, aliquoted and stored at -70C until
use.
(ii) Spleen Mononuclear Leukocytes. Splenic
mononuclear leukocytes from DBA/2 retired breeders were
isolated by centrifugation through Lympholyte-M
(Accurate Chemical & Scientific Corp., Westbury, NY) for
30 min. at 500 X g. Leukocytes removed from the
interface above the Lymphocyte-M were washed 3 times,
resuspended at a concentration of 1 X 107 in saline,
mixed 1:1 with CFA or IFA to form a homogenous emulsicr
and aliquoted and stored at -70C until use.
W094/28425 PCT~S94/05692
.
21~3~
-48-
(iii) Red Blood Cells. Human red blood cells were
isolated from heparinized peripheral blood by
centrifugation through Ficoll-Hypaque (Pharmacia LKB
Biotechnology Inc., Piscataway, NJ) for 30 min. at a
500 X g. The buffy coat on the red blood cell pellet
containing granulocytes was discarded. The bottom layer
containing < 99% red blood cells (`confirmed by
microscopic evaluation) was col`lècted, washed,
resuspended in saline at a concentration of 1 X
107/ml, mixed 1:1 with adjuvant, aliquoted and stored
at -70C.
(b) Active Immunization Protocols
C57BL/6 female mice (Jackson Laboratories, Bar
Harbor, ME) maintained on a 12-h light/dark cycle were
immunized with 1 X 10 allogeneic (DBA/2) sperm, in
Complete Freund's Adjuvant (CFA; Cappel, Organon Teknika
Corp., West Chester, PA). Control female mice of the same
age and strain were injected with: (1) saline (0.9% NaCl in
distilled H2O) as a control for stress, (2) saline-plus-CFA
as a control for the effects of the CFA alone, and (3) human
red blood cells and (4) paternal (DBA/2) mouse lymphocytes in
CFA to assess the sperm-specificity of the antifertility
effect. Booster injections of 1 X 10 sperm or control
cells, or saline alone in Incomplete Freund's Adjuvant (IFA;
Cappel, Organon Teknika Corp.) were given on day 14 and 21.
Injections (0.1 ml) were administered: (1) subcutaneously
into Ihe neck (s.c.), (2) intraperitoneally (i.p.), or (3)
intrauterine (i.u.) through the cervix which was dilated by
injection 12 h earlier with 1 IU of Pregnant Mare's Serum
(PMS; Sigma Chemical Co., St. Louis, MO). For intrauterine
injections, mice were anesthetized with 0.2 ml of Advertine
solution (2.5 ml tert-amyl alcohol) Fisher Labs, Medford,
MA), 1.25 g 2,2,2, tribromoethanol 99% (Aldrich Chemical
Company, Milwaukee, WI) in 100 ml distilled water) and the
cervix was visualized through a glass speculum. A catheter
fitted with a l-ml syringe and a 27 1/2-gauge needle (Becton
W094/28425 PCT~S94/05692
~ 2163450
-49-
Dickinson, Rutherford, NJ) was threaded through the cervical
opening and 0.1 ml of cells or saline emulsified with
adjuvant was introduced into the uterine lumen.
Twelve hours before the final booster injection, mice in
all immunization groups were primed with 1 IU of PMS to cycle
the ani.mals but not to induce hyperstimulation. Thirty-six
hours after the booster mice were injected with 1 IU human
chorionic gonadotropin (hCG; Sigma) and placed with breeder
males from our stud colony; one female per male in a single
cage. Half of the mice were assessed for number of ova and
2-cell embryos 40 h later and the other half were killed at
day 15 of pregnancy and the numbers of viable fetuses,
non-viable fetuses and resorption sites were assessed. Blood
was drawn by retroorbital puncture for assessment of
antisperm antibodies and uteri were snap frozen and stored at
-70C for subsequent immunohistologic examination. Each
experimental group consisted of 10 mice and each experiment
was repeated at least three times.
(c) Passive Immunization Studies
Spleens and lymph nodes were harvested from
s.c./s.c/i.u. immunized C57BL/6 female mice one week after
final immunization and from age and strain-matched
non-immunized mice. The lymphoid organs were minced with
fine scissors and cells were passed through a fine nylon
screen. Red blood cells were lysed according to Maruyama et
al. (1985), J. Androl. 6, 127-135 and mononuclear leukocytes
were separated by gradient centrifugation on Lympholyte-M at
500 X g for 30 min. and washed three times in RPMI-1640
medium (GIBCO, Grand Island, NY).
T lymphocytes were obtained by nylon wool
separation following a standard procedure (Trizio and
Cudgowicz (1974), J. Immunol. 113:1093-1097). Non-adheren'
cells (T cell enriched) were resuspended in RPMI 1640 at 10 X
107/ml and p.l ml was injected intravenously into the tail
vein of C57BL/6 female mice. PMS (1 IU) was injected i.p. a-
the same time. Forty-eight hours later, mice were given 1 U
W094/28425 PCT~S94/05692
2163~ 0 _50_
hCG i.p. and mated. On day 15 of pregnancy, mice were killed
by cervical dislocation and the numbers of viable fetuses,
non-viable fetuses and resorption sites were recorded. Uteri
were frozen in OCT gel (Baxter McGaw, Park, IL) on dry ice
and stored at -70C for subsequent immunohistological
assessment. Blood was collected by retroorbital puncture
prior to termination and serum was stored at -70C for
antisperm antibody assay.
(d) Immunohistoloqic Staininq of Mononuclear Cells
T lymphocytes used for the passive study were
suspended in PBS at a concentration of 1 X 107; 5 ~1 of
the cell suspension was applied to individual spots of
teflon-coated, eight-spot slides, air dried, fixed in acetone
for 10 min. and stored at -70C until use.
Uteri from actively immunized, passively immunized
and non-immunized mice were embedded in groups of 5 in OCT
gel on dry ice and stored at -70C until use. Before
sectioning the uteri were equilibrated in the cryostat for 1
h to reach -20C. Tissue slices (4-6 ~m) were placed on
3-spot teflon slides (Roboz Surgical Instrument Co.,
Washington, DC) and were air dried overnight and fixed in 2%
paraformaldehyde. Macrophages and lymphocyte subpopulaticns
were detected in cell smears and tissue sections by
immunoperoxidase technique. A prewash in 0.02 N sodium
azide/phosphate-buffered saline (PBS) solution was performed
to destroy endogenous peroxidase before the primary rat
anti-mouse monoclonal antibodies were applied (Helomy et ai.,
(1988), J. Immunol. Methods 111:101-106). All antibodies
were diluted to optimal working concentration in PBS/1% BSA.
For the monoclonal antibodies Thy 1 (1:20), L3T4 (1:20), B
(1:20) and Ml/70, 15 (1:50) (Sera Lab, Accurate Chemical
Scientific Corp., Westbury, NY), the second
immunoperoxidase-labelled antibody was a peroxidase-labeled
F(ab')2 fragment mouse anti-rat IgG (Jackson Immunoresearch
Laboratories, Inc., West Grove, PA) diluted 1:100 in PBS
containing 1% BSA. For the slides stained with bio~inylated
W094/28425 216 3 ~ 0 PCT~594/05692
-51-
Lyt 2 antibody (1:20) (Becton Dickinson, Mountain View, CA),
peroxidase labeled streptavidin (Kirkegaard & Perry
Laboratories Inc., Gaithesburg, MD) was used diluted 1:200 in
PBS/1% BSA. The substrate was 0.2 g of 3-amino-9-ethyl
carbasol (AEC) dissolved in 50 ml dimethyl formamyl and
diluted in acetate buffer (pH 4.9). The slides were
counterstained with hematoxylin and mounted with an aqueous
mounting solution (GVA, Zymed Laboratories, South San
Francisco, CA). Spleen was used as a positive tissue control
and PBS instead of the first antibody as a negative control.
(e~ Evaluation of Immunoperoxidase-Stained
Uterine Sections
For each group of mice (5 uteri), three
cross-sections from different areas of the block were
evaluated. Localization of macrophages and lymphocyte
subpopulations was noted descriptively. For quantitation,
positi~e cells were counted in five random fields from each
section using a light microscope (X 500 magnification). The
mean numbers and standard deviations of leukocyte
subpopulations per mm2 was calculated. For statistical
evaluation, Student's t-test was used.
(f) Detection of Antisperm Antibodies by Indirect
Immunofluorescence
The method used has been described in detail
elsewhere (Madrigal et al., 1986, Immunol. 9, 175-186).
Epididymal sperm from DBA/2 retired breeders was washed and
resuspended in PBS to 1 X 108 cells/ml and 5 ~1 of this
suspension was applied to each spot of teflon-coated,
eight-spot microscope slides (diameter 8 mm, Roboz
Surgical). After drying, the slides were fixed in acetone
for 15 min. and stored frozen at -70C until use. ~n the day
of assay, slides were thawed, rinsed in PBS, then sera from
immunized and non-immunized mice were added in four-fold
serial dilutions and incubated for 1 h. Following a wash
step, fluorescein conjugated F(ab)2 fragment rabbit anti
mouse IgG (Cappel) diluted 1:40 was applied for 30 min.
W094/28425 PCT~S94/OS692
2163~5~ --
-52-
Following a final series of washes, coverslips were mounted
on the slides with a solution of pheneylene diamine/glycerol
and fluorescence patterns were analyzed on a Zeiss
fluorescence microscope.
(2) Results
Figure Summary: Fig. 1. shows the results of a single
representative s.c./s.c./i.u. active immunization experiment
with various control groups indicating reduced fertility and
increased fetal resorptions in sperm-immunized mice.
Immunization groups: a, non-immunized; b, saline + adjuvant;
c, DBA/2 lymphocytes + adjuvant; d, human RBC + adjuvant;
e, DBA/2 sperm + adjuvant. Fig. 2. shows the effects of
passive transfer of T lymphocytes from s.c./s.c./i.u.
immunized mice (from experiment depicted in Fig. 1), on
fertility of recipient mice. T lymphocytes were from the
groups (a,b,c,d,e) identified in the description of Fig. 1.
Fig. 3. shows the mean numbers + S.D. of T lymphocyte
subpopulations and macrophages in uterine sections of mice
after s.c./s.c./i.u. immunizations (from experiment depicted
in Fig. 1). Immunization groups were as described above
(Fig. 1). Fig. 4. shows the mean numbers + S.D. of T
lymphocyte subpopulations and macrophages in uterine sections
of mice after passive transfer of T lymphocytes from
s.c./s.c./i.u. immunized mice (same experiment as depicted in
Figs. 1-3). Fig. 5 shows preimplantation embryo recovery in
mice following allogeneic trophoblast immunization.
Immunization groups: f, saline; g, adjuvant; adjuvant plus
trophoblast. Fig. 6 shows the number of viable offspring and
fetal resorption in mice following allogeneic trophoblast
immunization. The groups are as described in Fig. 5.
(a) Comparison of Active Immunization Protocols
Different routes of immunization were compared to
determine the most effective way to induce immunologic
infertility with sperm antigens. No significant difference
was found between saline and sperm-immunized mice for any
fertility parameter when either s.c. or i.u. immunization
W094/2842~ 21~ 3 4 ~ O PCT~S94/05692
-53-
approaches were used exclusively (Table IV). Intraperitoneal
immunizations were discontinued after the first experiment
because Freund's adjuvant alone produced inflammation and
adhesions that affected fertility. A combination of systemic
(s.c.) and local (i.u.) immunizations with sperm in adjuvant
did no~ cause a significant decrease in the percentage of
mice that become pregnant as compared with the adjuvant
control group (Table IV). However, a number of individual
fertility parameters were reduced in s.c./s.c./i.u.
sperm-immunized animals as compared to adjuvant controls: on
day 3 after mating the number of unfertilized oocytes per
pregnant mouse was significantly increased (P < 0.01) and the
number of 2-cell embryos per mouse was significantly
decreased (P < 0.001); on day 15 of pregnancy the number of
viable fetuses per pregnant mouse was significantly reduced
(P ~ 0.005) and the number of fetal resorption sites was
significantly increased (P < 0.01) (Table IV, Fig. 1).
(b) Passive Immunization Study
In the passive immunization study, mice were
administered T cell-enriched lymphoid cells from
sperm-immunized, non-immunized and control-immunized
syngeneic mice via the tail vein. The transferred cells were
primarily CD4+ lymphocytes; less than 15% of the cells were
macrophages or CD8+ T cells. Numbers of viable fetuses and
resorption sites were stored on day 15 of pregnancy. There
was no significant difference in any of the fertility
parameters between mice receiving T cells from non-immunized
mice and control immunized mice (Fig. 2). However, the
fertility rate was reduced in mice receiving lymphocytes from
sperm-immunized animals (Table IV). Furthermore, the number
of fetuses per pregnant mouse was significantly decreased in
the group that received T cells from sperm-immunized mice (P
< 0.001) and the number of resorption sites was significantly
increased (P < 0.005) (Table IV).
W O 94/28425 PCTrUS94/05692
2~345 ~ 54
Table IV (i)
Effects of different sperm immunization protocols on fertility parameters.
Active immunization*
s.c/s.c~s.c. i.u./i.u./i.u. s.c/s.c/i.u.
Saline Sperm Saline Sperm Saline Sperm
Fertility
rate 77 76 94 75 80 78
(~) (n=18) (n=17) (n=17) ;~ (n=20) (n=30) (n=30)
Unfertilized 0.7+1.1 0.7+1.2 1.0+1.3 1.7+2.0 0.3+0.8 1.3+1.0*
oocytes (n=10) (n=10) (n=10) (n=10) (n=30) (n=30)
2-Cell 4.3+2.1 3.1+1.5 4.5+2.0 4.8+2.2 6.0+3.2 3.6+2.7***
embryos (n=10) (n=10) (n=10) (n=10) (n=30) (n=30)
Fetuses per 7.8+4.2 9.16+2.3 6.8+2.2 8.7+4.2 8.7+4.6 1.81+2.6**
pregnant (n=20) (n=20) (n=18) (n=20) (n=30) (n=30)
mouse
Resorption per 0.6+0.8 0.3+0.5 0.5+0.7 0.6+0.1 0.1+0.4 4.2-3.5*
pregnant (n=20) (n=20) (n=18) (n=20) (n=30) (n=30)
mouse
Table IV (ii)
Effects of different sperm immunization protocols on fertility parameters.
Passive immunization
T-cell transfter from
s.c/s.c/i.u.
Saline Sperm
Fertility
rate 86 55
(~) (n=27) (n=28)
Unfertilized NA NA
oocytes
2-Cell embryos NA NA
Fetuses per 6.2+4.0 2.6+2.0***
pregnant mouse (n=27) (n=28)
Resorption per 0.4+0.1 4.4+2.2**
r,regnar.t mouse (n=27) (n=28)
a~3Oth saline and sperm immunizations were performed with Freunds's
adjuvant
b~ertility rate = (No. of pregnant mice/Total No. of mice)~
*~0.01, **~cO.305, ***P~0.001
W094/28425 216 3 4 5 ~ PCT~S94/05692
(c) Leukocyte Subpopulations in Uteri From Actively and
Passively Immunized Mice
Uteri from pregnant and non-pregnant cycled animals
(day 15 after hCG and mating) were studied. Sections of
uteri from pregnant animals in both sperm-immunized and
control groups contained numerous lymphocytes and macrophages
and the patterns were indistinguishable from each other. In
non-pregnant s.c./s c./i.u. sperm-immunized mice, the number
of T lymphocytes/mm of uterine section was significantly
higher than that observed in any control group (P ~ 0.001).
Stage of estrus was not correlated with numbers of T cells in
uterine horns of control mice or of sperm-immunized mice.
Both CD4+ and CD8+ subpopulations were observed in uterine
tissue from sperm-immunized mice, but CD8+ cells
predominated. Most of the CD8+ cells were located in the
periglandular space, while CD4+ cells were evenly spread
throughout the epithelium and periglandular region. In ut-ri
from sperm-immunized mice, macrophages were increased
four-fold (P < 0.001) and were evenly distributed between
epithelial, periglandular and interstitial areas. Data from
a representative experiment are presented in Figs. 3 and 4.
When uteri from infertile passively immunized mice were
studied, a significant increase in total T cells was found in
the group which received T lymphocytes from sperm-immunized
animals compared with controls (P < 0.05). A significant
increase in CD4+ cells was observed (P < 0.005); there was
not a significant increase in the number of CD8+ cells.
Macrophages also were more prevalent in uterine tissues from
the sperm passive-immunization group (P < 0.01). Both CD4+
lymphocytes and macrophages were evenly distributed
throughout the uterine tissue. Very few B cells were found
in any of the uterine sections.
(d) Antisperm Antibody Evaluation
Titers of antisperm antibodies in pooled sera from
s.c./s.c./s.c/ or i.u/i.u./i.u. sperm-immunized mice were
1/256 and 1/1014, respective'y. Both groups showed a
W094/28425 PCT~S94/05692
21~3~50 ~
-56-
predominantly acrosomal staining pattern. Pooled sera from
s.c./s.c./i.u. sperm-immunized mice had a titer of 1/1014 and
reactivity directed against both the sperm acrosome and tail
regions. Sera from mice which were passively immunized with
lymphocytes from actively sperm-immunized (s.c./s.c./i.u.)
mice were negative for antisperm antibodies. Normal mouse
sera (negative control), sera from mice immunized with saline
and from mice injected with lymphocytes from non-immunized
mice were also negative.
(e) Trophoblast antiqen Results
The results of the trophoblast antigen
immunizations are shown in figures 5 and 6. Immunizations
were conducted using the above-described protocols for the
immunization of mice with sperm. Specifically, C57BL/6
female mice were immunized with allogeneic trophoblast in
complete ~reund's adjuvant given subcutaneously,
intraperitoneally and transcervically (intrauterine). These
experiments demonstrate that trophoblast immunization results
in a decreased number of recovered preimplantation embryos
(Fig. 5), a significant reduction in the number of viable
offspring and an increased number of fetal resorptions in the
trophoblast plus adjuvant immunized group as compared to the
adjuvant alone and saline control groups (Fig. 6). We have
also observed that complete Freund's adjuvant injected
between the implantation sites of day 11 viable C57BL/6 x
DBA/2 fetuses will not only disrupt adjacent pregnancies, but
will also cause recurrent abortion after two subsequent
matings. These data provide further evidence of immur.ologic
recurrent abortion involving trophoblast antigen activation
(3) Summary
C57BL/6 female mice were immunized with allogeneic
(D~A/2) sperm in Freund's adjuvant either subcutaneously
(s.c.), transcervically into the uterine lumen (i.u.), or
with a combination of s.c. and i.u. immunization approaches
Control mice received DBA/2 lymphocytes, human ery~hrocytes
or saline in adjuvant using the same immunization protocols
W09412842~ 2 16 3 4 ~ 0 PCT~S94/05692
-57-
Immunization with sperm or control cells in adjuvant
exclusively by s.c. or i.u. approaches did not affect
subsequent fertility, although sperm-injected mice from both
protocols had high titers of circulating antisperm
- antibodies. In contrast, mice that were immunized with sperm
in adjuvant by a combination of s.c. and i.u. injections
demonstrated significant reductions in fertilization rate and
number of viable fetuses and an increased rate of fetal
resorption when compared with non-immunized and
control-immunized mice. Mice receiving sperm by the
s.c./i.u. protocol had high titers of antisperm antibodies
and a marked infiltration of T lymphocytes and macrophages
into the uterine endometrium. To determine whether cellular
immune mechanisms contributed to the infertility effect, T
lymphocytes from spleens and pelvic lymph nodes of s.c./i.u.
sperm-immunized mice and non-immunized mice were passively
transferred to naive syngeneic female recipients which were
subsequently mated. The total number of fetuses on day 15 of
pregnancy was significantly reduced in mice receiving
T-lymphocytes from sperm-immunized mice and a significant
increase in fetal resorption sites was also observed. These
mice did not have detectable titers of circulating antisperm
antibodies, but had a significant infiltration of CD4+T
lymphocytes and macrophages in the uterine epithelium and
endometrium. These data indicate that intrauterine antisperm
cell-mediated immunity can be induced in mice by a
combination of systemic and intrauterine immunizations and
provide evidence for the existence of reproductive tract
mucosal antisperm cellular immune resEonses that adversely
a_fect fertility and pregnancy.
wO94e8425 ~ PCT~S94/05692 ~
21~34a ~
-58-
Example 4: T Helper l-Type Cellular Immunity to Trophoblast
Antiqens in Women with Recurrent Spontaneous
Abortion
(l) Patients
Peripheral blood was obtained from 244 non-pregnant
women with a history of unexplained recurrent reproductive
failure w;-o were referred for evaluati.on to the Recurrent
Miscarriage Center, Brigham & Women's Hospital. The women
were between 26 and 40 years of age and had a history of at
least three prior first trimester spontaneous abortions with
or without a prior ectopic gestation or live birth. The
etiology of prior pregnancy losses was unexplained by
conventional criteria (normal parental chromosomes,
hysterosalpingography, luteal phase endometrial biopsy,
hormonal analysis, cervical cultures and antiphospholipid
antibodies). The time of blood collection relative to the
last pregnancy loss was not constant, but all women had
experienced at least one spontaneous abortion within one year
of testing. Peripheral blood was also obtained from a
control group of 13 paid volunteer non-pregnant women between
27 and 41 years of age with a history of at least two
uncomplicated term deliveries with their last birth occurring
within one year of blood collection and no history of
spontaneous abort on, ectopic pregnancy or still-birth. For
an additional control, blood was obtained from 10 men between
26 and 47 years of age. All of the individuals in this study
were in excellent health, had no history of allergy or atopv,
and were taking no medication at the time of blood collection.
(2) Assays
(a) Trophoblast Antiqen Preparation
A trophoblast antigen extract was prepared from the
human choriocarcinoma cell line Jeg-3 (American Tissue Type
Collection, Bethesda, MD) as described above (see also Hill,
J. et al., 1992, Am. J. Obstet. Gynecoi. 166:1044). Briefly,
mycoplasma-free Jeg-3 cells were cultured in flasks until 80
confluence in Minimum Essential Medium (MEM; Gi~co, Grand
~ W094/2842~ 21 6 3 4 5 0 PCT~S94/05692
-59-
Island, NY) supplemented with 10% fetal bovine serum. The
cells were harvested with a rubber cell scraper (Coaster,
Cambridge, MA) and washed three times in Hank's Balanced Salt
Solution (GIBCO). The cells were then disrupted by Dounce
homogenization (100 strokes) and the supernatant saved after
centrifugation at 400g for 10 minutes. Protein concentration
was determined by BCA reagent kit (Pierce, Rockford, IL) and
antigen extracts were adjusted to 333ug/mL. Fetal calf serum
(GTBCO) was added to a final concentration of 10%, and 1 mL
aliquots containing approximately 300ug of trophoblast
extract each, were stored at -70C until use.
(b) Peripheral Blood Mononuclear Cell (PBMC)
Supernatants
Peripheral venous blood was collected into tubes
containing pyrogen-free sodium heparin (Riches, P. et al.,
1992, J. Immunol. Method 153:125), and mononuclear cells were
isolated by Ficoll-Hypaque centrifugation as described above
(see also Hill, J. et al., 1992, Am. J. Obstet. Gynecol.
166:1044). Briefly, washed cells were resuspended to a
concentration of 10 cells/mL in Roswell Park Memorial
Institute RPMI medium Gibco supplemented with 0.3 mmol/L
glutamine, 100 IU/mL benzylpenicillin potassium and 100 ug/mL
streptomycin sulfate (Sigma Chemical Co., St. Louis, MO) and
10% fetal bovine serum. Ten milliliters of cell suspension
and 1 mL of antigen extract and 1 mL of additional media for
cc~trol were added separately to 50 mL tissue culture flasks
(Becton Dickinson, Lincoln Park, NJ) and cultured for 72
hours at 37C in 5% C02, 95% air. The cells were then
washed and resuspended in 1 mL of Whitten's medium (Whitte:,
W.K., et al., J. Reprod. Fertil. 17:399-402 (1968))
supplemented with 0.3% bovine serum albumin (Miles
Scientific, Naperville, IL). After an additional 24 hour
incubation, cell suspensions were centrifuged and the
supernatants were filtered through a 0.22um Millex GS filter
unit (Miiiipore, Bedford, MA) and stored at -70C until use.
W094/28425 PCT~S94/05692
2163~
-60-
(c) Embryotoxic Factor Assay
This assay was performed as described in Example 1.
(d) Cytokine Determination
Cytokines were measured in trophoblast-activated PBMC
supernatants by ELISA kits according to the manufacturer's
instructions. TNF-a, TNF-n, and IFN-gamma kits were
obtained from Endogen (Boston, MA; lower limit of
sensitivity, 10 pg/mL for TNF-a and IFN-gamma and 31pg/mL
for TNF-~), IL-2 kits from R~D systems (Minneapolis, MN;
lowest limit of sensitivity, 31 pg/mL), IL-4 kits, from
Amersham (International Place, UK; lowest limit of
sensitivity, 31 pg/mL), and IL-10 kits from The Biosource
CytoTM (Biosource International, Camarillo, CA; lowest limit
of sensitivity, 15 pg/mL).
A11 ELISA assays were solid phase sandwich ELISAs in
which specific monoclonal antibodies were attached to wells
in 96 well plates, and a secondary enzyme-conjugated specific
antibody was added fo~ cytokine detection. Following
incubation with substrate, the colored product was measured
in each well on a Dupont microplate reader (Dupont, Dover,
DE) using a wavelength appropriate for the substrate used.
Cytokine concentrations were calculated from a standard curve
generated with specific cytokine standards provided with each
kit.
All 244 culture supernatants from women with recurrent
a~ortion and all 13 supernatants from parous controls were
assayed for IFN-gamma. Analysis of the other cytokines was
performed on supernatants from a subgroup of 20 women chosen
at random from the group with recurrent abortion and
embryotoxic factor activity, and on all supernatants from the
13 women in the control group.
(e) Statistical Analysis
Nonparametric methods using Fisher's exact test were
used to analyze data.
~ W094/28425 216 3 4 ~ O PCT~S94/05692
-61-
(3) Results: Lymphocytes from a majority of women with
unexplained recurrent abortion make TI~l-type cytokines
when exposed to trophoblast extracts, whereas
lymphocytes from parous women with normal reproductive
histories and men take TII2-type cytokines.
Embryotoxic factor activity was detected in
trophoblast-stimulated mononuclear cell supernatants from 160
(65.5%) of 244 women with unexplained recurrent abortion.
None of the supernatants from parous women with normal
reproductive histories or from men had embryotoxic activity.
The THl cytokine IFN-gamma was found in 125 (51.2%) of 244
supernatants from women with unexplained recurrent
reproductive failure and significantly correlated with
embryotoxic factor activity (75.6%) of 160 supernatants with
embryotoxic activity vs 4 (4.8%) of 84 supernatants without
embryotoxic activity contained IFN-gamma (p<0.0001). The
range of IFN-gamma levels in these supernatants was 10.3 to
2295 pg/mL with a mean of 209 + 34 pg/mL.
All supernatants from the subgroup of 20 women with
recurrent reproductive failure and embryotoxic factor
activity chosen for further study contained THl-associated
cytokines ~Table V). IFN-gamma (range 10.95-2,295 pg/mL;
mean 517+141 pg/mL) and TNF-alpha (range 11.75-5646 pg/mL;
mean ll9g+388 pg/mL) were detected in all 20 cases. TNF-
~was detected in 17 of the 20 supernatants (range 47.6-578.1
pg/mL; mean 287 + 46.1 pg/mL). IL-2 was initially detected
(within the first 24 hours) but was not detected not detected
after four days in any of the 20 supernatants. Three
supernatants from the 20 women in this group contained
detectable levels of TH2-type cytokines: two contained low
levels of IL-10 (17.19 and 20.34 pg/mL), and one contained
IL-4 (59.14 pg/mL). Supernatants from unstimulated cultures
of women with recurrent abortion contained neither TH nor
TH2 type cytokines. In contrast, none of the
troEhoblast-activated PBMC culture supernatants from the 13
parous women with normal reproductive histories or from the
WO 94/2842S ` ~ PCT~US94105692 ~
2 1 g 3 4~ ~ -62-
Table V. THl and TH2 Cytokines in Supernatants of cultured
Trophoblast-Antigen-Activated Peripheral Blood Mononuclear Cells from
Nonpregnant Women with a History of Recurrent Spontaneous Abortion*
THl TH2
IFN-qamma TNF-beta IL-2 IL-4 IL-lo TNF-alpha
1. 27.6 549.4 o 0 o 102.3
2. 10.9 215.9 0 0 0 3849.0
3. 889.9 151.8 0 0 0 1082.0
4,828.0 194.1 o 0 20.3 65.9
5. 2295.0 168.7 0 0 0 36.4
6. 22.89 0 0 0 0 19.8
7. 1949.0 50.4 o 0 o 436.9
8. 792.9 0 0 0 0 1145.0
9. 607.0 0 0 0 o 3185.0
lo . 15.11 294.4 o o o 96.9
11. 14.9 415.1 o o o 132.6
12. 605.6 556.0 o 0 o 1139.0
13. 700.7 578.1 o o 0 15.0
14. 105.0 548.5 0 0 0 5645.0
15. 299.0 420.8 0 0 0 11.7
1~. 294.9 329.0 0 0 0 32.8
17, 32.0 88.7 0 0 0 15.0
18. 138.7 47.6 0 0 0 32.9
15. 286.7 99.6 0 0 17.24211.0
20. 425.0 170.3 o 59.1 0 2722.0
*Data expressed as pq/mL
~ WO 94128425 21 6 3 4 5 0 PCT/U594/05692
Table VI. THl and TH2 Cytokines in Supernatants of Cultured
Trophoblast Antigen Activated Mononuclear cells from Nonpregnant Women with
Normal Reproductive Histories~
THl TH2
IFN-gamma IL-2 IL-4 IL-l0 TNF-alpha
1. 0 0 0283.0 0
2. 0 0 022.2 0
3. 0 0 40.620.4 0
4. 0 0 31.1169.7 0
5. 0 0 023.9 0
6. 0 0 023.6 0
7. 0 0 81.621.9 0
8, 0 0 0 29. 0
9. o o 022.8 0
10.~ 0 0 023.1 0
11. 0 0 018.4 0
12. 0 0 033.6 0
13. 0 0 0157.6 0
*Data expressed as pg/mL
W O 94l2842~ PCTrUS94/05692 ~
2 1 ~ 3 ~ J O -64-
Table VII. THl and TH2 cytokines in supernatants of cultured
trophoblast antigen mononuclear celis from men*
THl TH2
TFN-gamma TNF-beta IL-2 IL-4 IL-10 TNF-alpha
1. 0 0 0 0 46.9 0
2. 0 0 0 0 50.2'` 0
3. 0 0 0 0 0 0
4. 0 0 0 0 22.4 0
5, o 0 0 0 38.8 0
6. 0 0 0 0 45.1 0
7, o 0 0 0 25.8 0
8. 0 0 0 0 25.1 0
9, o o O 0 109.0 0
10. 0 0 0 050~7
*Data expressed as pg/mL
~ W094/28425 21 6 3 ~ ~ ~ PCT~S94/05692
-65-
10 men had detectable levels of THl-associated cytokines
(INF-gamma, IL-2, TNF-~, or TNF-a); however, TH2
cytokines were detected in all 13 supernatants from parous
women Table VI) and 9 of 10 supernatants from men (Table
VII). IL-10 was detected in all 13 supernatants from parous
controls (range 18.2-283 pg/mL; mean 65+23 pg/mL), and IL-4
was detected in three supernatants (31.10, 40.58 and 81.65
pg/mL). In men, IL-10 was identified in 9 of 10 trophoblast
activated cell culture supernatants (range 22.4-10.90 pg/mL;
mean 46 + 8.7 pg/mL), IL-4 was not demonstrable, while
unstimulated cell culture supernatants from all parous women
contained IL-10 (range 29.1-279.2 pg/mL; mean 76.8 + 19.2
pg/mL). IL-10 was detected in 5 of 10 unstimulated cell
culture supernatants from men (range 20.3-478.1 pg/mL; mean
260.2 ~- 75.4 pg/mL).
(4) Discussion
This study provides evidence that T lymphocytes from
gravid women respond to trophoblast antigens with a
dichotomous T helper immune response. Lymphocytes from many
women (51.2%) with a history of unexplained recurrent
reproductive failure produced THl-type cytokines following
exposure to trophoblast cell extracts in vitro, whereas
lymphocytes from women with a history of normal pregnancies
and men when placed under identical conditions produced
TH2-type cytokines. These data provide further evidence
that trophoblast induces a TH2-type cytokine response that
benefits normal pregnancy, and of an abnormal THl response
to trophoblast antigens in women with recurrent pregnancy
loss that plays a role in their reproductive dysfunction.
In this study peripheral blood mononuclear cells were
obtained from 244 non-pregnant women with a history of
unexplained recurrent reproductive failure by conventional
testin~ criteria, 13 non-pregnant women with normal
reproductive histories, and 10 men. IFN-gamma was detected
in 51.2% of the trophoblast-activated mononuclear cell
supernatants from 244 women with otherwise unexplained
W094/28425 PCT~S94/05692
21~3k~0
-66-
recurrent reproductive failure and in none of the parous
controls or men. This data supports our hypothesis for a new
etiology for recurrent abortion based on an aberrant cellular
immune response to trophoblast involving the THl pathway.
To further define the cytokines produced by lymphocytes
of women with recurrent abortion following exposure to
trophoblast, and to investigate the possibility of TH2
immunity in normal pregnancy, supernatants from a subgroup of
20 recurrent abortion patients with embryotoxic activity, and
supernatants from 13 parous women and 10 men, none of which
had embryotoxic activity, were studied in detail. TNF-a
and TNF-B was detected in 20 and 17 supernatants respectively
from the recurrent abortion group, and in none from the
parous control group or from men. These data provide further
evidence of a THl response to trophoblast extracts in women
with recurrent abortion since TNF-B is a THl-type cytokine
and because TNF-a is produced in higher amounts in a THl
response than in TH2 immunity (Romagnani, S. et al., 1992,
Int. Arch. Allergy Immunol. 4:279; Del Prete, G. et al.,
1993, J. Immunol. 150:353). The cytotoxic effect exerted in
THl immunity involves IFN-gamma, TNF-B and TNF-a (Tite,
J. et al., 1984, J. Immunol. 135:25). Our finding of
elevated TNF-a levels in trophoblast-activated mononuclear
cell supernatants from women with recurrent abortion suggests
that these cytokines, in addition to IFN-gamma, are part of
an adverse THl immune response to trophoblast, thereby
providing a new mechanism for reproductive failure.
Accordingly, these results support our hypothesis that these
cytokines are indeed, embryotoxic factors.
Our finding of IL-10 in every trophoblast-activated
mononuclear cell supernatant from previously successful
pregnant women without history of reproductive dysfunction
provides evidence that normal human pregnancy is associated
with the induction of a TH2-type immune response, and that
TL-10 or other TH2-type cytokines are involved in the
maintenance of normal pregnancy, perhaps by suppressing
W094/2842~ PCT~S94/05692
21~34~
IFN-gamma and TNF-~ production by THl cells (Mosmann, T.
et al., 1991, Immunol. Today 12:49; Maggi, E. et al., 1992,
J. Immunol. 148:2142). Our finding of IL-10 in 9 of 10
trophoblast-activated cell supernatants from men provides
further evidence that mononuclear cells normally respond to
trophoblast with TH2-type cytokine production. The fact
that IL-10 was detected in all unstimulated supernatants from
individuals not experiencing reproductive failure suggests
that IL-10 is a natural occurrence.
The findings of this study, together with data
indicating that THl and TH2 cells utilize distinct T-cell
receptor-associated signal transduction pathways (Gajewski,
T. et al., 1990, J. Immunol. 144:4110), and that high
concentrations of anti-CD3 monoclonal antibody inhibit THl
but not TH2 responses (Williams, M. et al., 1990, J.
Immunol. 144:1208) enables the development of new
immunotherapeutic regimens for preventing human reproductive
failure due to THl mediated events. Additional therapies
involving the administration of an immunomodulating agent (to
modulate the concentration the above-identified cytokines
that play a role in immunologic reproductive failure),
antibodies to THl cytokines that abrogate the biological
activity of these cytokines, or administration of intravenous
immunoglobulin through anti-idiotype binding to T-cell
receptor idiotypes, should also prove beneficial in
preventing reproductive failure due to THl immunity.
Example 5: Diaqnosis of Immunoloqic Reproductive Failure
usinq a Lymphocyte Proliferation Assay
(1) Subjects
Blood was obtained from 57 non-pregnant women with a
history of recurrent abortion at the Recurrent Miscarriage
Center, Brigham and Women's Hospital between February and
June, 1993 in accordance with Human Subjects' Committee
approval. The women, were between 29 and 41 years of age and
had a history of at least three prior first trimester
W094/28425 PCT~S94/05692 ~
21~3~5 ~ -68-
spontaneous abortions, with or without a prior ectopic
gestation or live birth. All women were evaluated according
to a standard protocol by a single investigator (JAH) as
previously described. Briefly, after obtaining a thorough
history, all woman had a physical examination,
hysterosalpingography, peripheral blood chromosome assessment
of both partners, luteal phase endometrial biopsy, hormonal
analysis, and antiphospholipid antibody determinations.
Women who were normal by these criteria were designated as
having recurrent abortion of unknown etiology.
Peripheral blood was also obtained from a control group of 10
non-pregnant, paid volunteer women who were randomly selected
from the Obstetrical Nursing Service. Women in this control
group were between 27 and 43 years-of age with a history of
at least two uncomplicated term deliveries and no history of
either spontaneous abortion, ectopic pregnancy, or
still-birth. None of the women in this study were taking
medication at the time of blood collection.
(2) Assays
(a) Antiqen Preparation
Trophoblast antigens were prepared from the human
choriocarcinoma cell lines Jeg-3 and JAR (American Tissue
Type Collection, Bethesda, MD). These cells were cultured in
flasks until 80% confluence in Minimum Essential Medium (MEM;
GIBCO, Grand Island, NY) supplemented with 10% fetal bovine
serum (FBS). The cells were harvested without trypsinization
with a rubber cell scraper (Coaster, Cambridge, MA) and
washed three times in RPMI 1640 media (GIBCO). The cells
were then disrupted by dounce homogenization (greater than
100 strokes) and the supernatant was saved after
centrifugation at 400g for 10 minutes.
For fractionated trophoblast antigens, Jeg-3 cells were
harvested without trypsinization as above and washed three
times in ice-cold phosphate buffered saline (PBS). The cell
pellet was resuspended in ice-cold hypotonic buffer (10m~l
Tris-HCl pH 7.6, 0.5 ~ MgC12) for 10 minutes and the cell
W094/28425 PCT~S94/05692
21634~0
-69-
suspension was added to a chilled Dounce homogenizer and
delivered 35 strokes. Tonicity restoration buffer (10mM
Tris-HCl, pH 7.6, 0.5mM MgC12, 0.15M NaCI), was then added
to the homogenized cells, and the mixture was centrifuged at
500g at 4C for 5 minutes to obtain a crude nuclear pellet
(designated as Nuclear Fraction). The supernatant was then
centrifuged at 20,000g, 4C for 30 minutes to isolate
mitochondria, Golgi, microsomes and cell debris (designated
the Organelle Fraction). A membrane-enriched fraction
(Membrane Fraction) was separated from the Cytosol Fraction
after an additional ultra-centrifugation at 150,000g, 4C,
for 45 minutes. Nuclear, Organelle and Membrane antigen
pellets were solubilized by incubation at 60C for 5 minutes
in 20uL of 0.2% sodium dodecyl sulphate in 10 mM Tris-HCl, pH
7.6 and protein concentrations were determined by BCA reagent
kit (Pierce, Rockford, IL). All trophoblast antigen sources
were sterilized by gamma-irradiation (150 Gy) and stored at
-70C until use. A variety of concentrations of Jeg-3
extracts and Cytosol Fraction and Membrane Fraction antigens
were tested to establish dose-dependent stimulation curves.
Percent stimulation index (% SI) was calculated according to
the following formula: % SI - cpm at the individual antigen
concentration used/the highest cpm at any antigen
concentration x 100. Additionally, the Jeg-3 antigen extract
was trypsinized with insoluble trypsin (GIBCO) at 37C for 90
min or heat-inactivated at 70C for 90 min, and tested for
antigenicity to determine whether the antigens were proteins.
Red blood cells (RBC) were separated from donors' white
blood cells after Ficoll-hypaque gradient centrifugation and
removal of buffy coat containing neutrophils. RBC were
incubated in hypotonic solution and washed three times at
500g, 4C for 10 minutes to remove hemoglobin. RBC membrane
control antigen was then prepared after dounce homogenization
and centrifugation as described above.
W094/28425 PCT~S94/05692 ~
21~34~ ~
-70-
(b) Lymphocyte proliferation assay
Peripheral blood mononuclear cells were isolated from
heparinized blood by Ficoll-hypaque gradient centrifugation.
Washed cells (2 x 105) were cultured in 96-well
round-bottom microtiter plates (Corning, Corning, NY)
containing RPMI 1640 media, 10% human serum (type AB, GIBCO)
and antibiotics in a final volume of 200 uL. Twenty
microtiters of antigen extract or medium alone were added to
triplicate wells. After a 6 day incubation at 37C, 5%
CO2, 95% air, the cultures were pulsed with 0.5 uCi of
[ H]-thymidine (New England Nuclear, Boston, MA) and
harvested 16 hours later on a glass fiber filter. Liquid
scintillation in a beta counter (Beckman, Palo Alto, CA) was
used to determine lymphocyte proliferation expressed as
counts per minute (cpm). The stimulation index (SI) was
calculated according to the formula; SI - cpm in presence of
antigen / cpm in absence of antigen. A SI greater than 3 was
considered positive (significant lymphocyte proliferation in
response to antigen). All experiments were performed by the
same investigator without knowledge of sample source or
embryotoxic factor results.
(c) Embryotoxic Factor Assay
This assay was performed as described above (Example
1). Briefly, peripheral blood mononuclear cells were
isolated from heparinized blood by Ficoll-Hypaque gradient
centrifugation as described for the lymphocyte proliferation
assay. Washed cells were resuspended in lOmL of RP~I medium
supplemented with 10% human serum to a concentration of 106
cells/mL. The cells were cultured with or without Jeg-3
antigen (30ug/mL) for 96 hours at 37C in 5% CO2, 95% air.
After washing, the cell pellet was resuspended in 2mL of
Whitten's media supplemented with 0.3~ bovine serum albumin.
After an additional 24 hour incubation, cell suspensions were
centrifuged and the supernatants were filter-sterilized and
stored at -70 until use. Two-cell embryos were harvested
from superovulated CF-l female mice that had been bred to
w094/~4~s ~1 ~ 3 ~ 0 PCT~594/05692
C57BL6 male mice. All media and supernatants were
equilibrated overnight in 5% Co2 before the addition of
- embryos. Embryos were cultured in 20uL drops containing a
1:1 dilution of study supernatant in Whitten's medium
supplemented with 0.3% bovine serum albumin under
C02-equilibrated paraffin oil in tissue culture dishes
(Falcon, Oxnard, CA). At least 11 embryos were cultured in
each 20uL drop. The embryos were assessed for normal
development after 4 days in culture by the criteria described
by Ducibella (Ducibella T., 1980, Dev. Biol., 79:356).
Embryotoxic factors were considered present when the
percentage of embryos advancing to the blastocyst stage of
development was less than 50% of control values as previously
described (Hill J. et al., 1992, Am. J. Obstet. Gynecol.
166:1044; Ecker J. et al., 1993, Obstet. Gynecol. 84). The
embryotoxic factor assay was performed by a single
investigator without knowledge of sample source or results of
the lymphocyte proliferation assay.
(d) Statistical analysis
All data were analyzed by one factor analysis of
variance. Fisher's PLSD (Protected Least Significant
Difference) tests were used for post hoc pairwise
comparison. Regression analysis was used to correlate
lymphocyte proliferation with embryotoxic factor production.
(3) Results: Lymphocyte proliferation in response to
trophoblast antiqen stimulation was siqnificantly hiqher in
women with recurrent abortion of unknown etioloqy than in
fertile controls, and siqnificantly correlated with
embryotoxic factor activity in culture supernatants.
The optimal concentration of trophoblast antigen derived
from Jeg-3 extracts (30 ug/mL) used in the lymphocyte
proliferation assay was determined from dose response curves
from six women responsive to Jeg-3 out of 12 women initially
tested (Fig. 7).
~ 5~ PCT~S94105692 ~
~ .. . ~ ,
-72-
Only one of the 57 women referred for evaluation of
recurrent abortion was found to have a presumed etiology for
reproductive loss. This individual had a septate uterus and
underwent hysteroscopic resection. She did not respond to
trophoblast antigen stimulation in either the lymphocyte
proliferation assay (SI - 1.4) or the embryotoxic factor
assay (embryo development greater than 50~).
Thirty out of 57 (52.6%) women with recurrent abortion
had an SI greater than 3 to either Jeg-3 or JAR; 14 (46.7%)
responded to antigens derived from both Jeg-3 and JAR; while
13 of the woman (43.3%) responded to Jeg-3 only, and three
(10.0%) to JAR only. Because more women responded to Jeg-3
than JAR, antigen fractionation studies were performed with
the Jeg-3 cell line.
Following stimulation with the optimal concentration of
trophoblast antigen extract (30 ug/ml) the mean SI of the 57
women with a history of recurrent abortion was significantly
higher than that of the control group (3.99+2.83 vs.
1.64+0.34, pc0.01). The mean SI in response to RBC membrane
antigen was not significantly different between groups
(1.33+0.63 vs 1.10+0.29). When the lymphocyte proliferation
responses to trophoblast antigen were divided into two
groups, positive (SI>3) and negative (SI<3), 52.6% of women
(n=30) with recurrent abortion were positive (mean SI =
5.89+2.72) and 47.4% (n=27) were negative (mean SI = 1.89+
0.58). Forty-four out of 57 women had an SI>2 in response to
trophoblast antigen while all fertile controls had a SI less
than 2. The difference between women with recurrent abortion
and fertile controls was statistically significant by Fisher
exact tests (two tailed, p<0.002).
To determine which cellular fraction(s) were responsible
for lymphocyte stimulation in recurrent abortion patients,
lymphocytes from 27 women that had responded to the crude
Jeg-3 antigen extract (30ug/mL) were tested against antigen
extracts made from Jeg-3 derived Nuclear, Organelle, Cytosol
and Membrane fractions. The optimal concentration of each
W094/28425 PCT~594/05692
~ 2163~50
-73-
antige~ extract was lOug/mL, as determined by dose response
studies in the 6 positive women who had originally responded
to Jeg-3 (data not shown). As shown in Fig. 8, trophoblast
antigen derived from cytosol and membrane components were
responsible for lymphocyte proliferation in the majority of
individuals (55.6% and 37.0%, respectively). Antigenicity
was completely abrogated after trophoblast antigen treatment
with either trypsin or heat (data not shown).
Supernatants from two women with recurrent abortion had
to be discarded due to bacterial contamination of the
cultures. Therefore, embryotoxic factor data were only
available from 55 of the original 57 study women.
Embryotoxic factor activity was detected in lymphocyte
supernatants from 36 of 55 women with recurrent abortion
(66%) following stimulation with crude trophoblast antigen
extract. None of the supernatants from fertile control women
contained embryotoxic factor activity, and supernatants from
cells cultured without trophoblast antigen from both
recurrent abortion and control groups did not have
embryotoxic factor activity. Lymphocyte proliferation
responses significantly correlated with the production of
embryotoxic factor (r=0.61, Fig. 9). Embryotoxic factor
production in response to trophoblast antigen stimulation as
determined by impaired embryo development was found in 27
(90.0%) out of 30 women with recurrent abortion who had a
SI>3 in their lymphocyte proliferation assay in response to
trophoblast antigen stimulation, while 9 (36-0%) out of 25
woman with recurrent abortion had evidence of embryotoxic
factors despite a S.I.<3. The sensitivity and specificity
for the lymphocyte proliferation assay compared to the
embryotoxic factor assay was 75% and 84%, respectively, with
a positive and negative predictive value of 90% and 64%.
~4) Discussion
In the present study we provide further evidence that
cellul.ar immunity to trophoblast is involved in the
pathogenesis of immunologic reproductive failure, since 52.6%
W094l28425 - PCT~S94/05692
21634SO
-74-
"
of our patients had evidence of activated lymphocyte
responses to trophoblast antigen(s) and 90.0% Pf these
patients produced embryotoxic factors.
Many studies using maternal lymphocytes in proliferation
assays have reported depressed cellular immunity during
pregnancy in response to the T-cell mitogen,
phytohemagglutinin, although not all investigators have
corroborated these results (reviewed in Goodfellow C., 1983,
Immunol. Rev. 75:61). Few studies have used lymphocytes from
women with recurrent abortion and none have tested lymphocyte
proliferation in response to trophoblast antigen(s) free of
human leukocyte antigen (HLA) expressing cells. Other
investigators have used placental cells derived from
chorionic membranes or normal human placenta as antigenic
sources with conflicting results reported (Goodfellow C.,
1983, Immunol. Rev. 75:61; Hunt J. et al., 1984, J. Reprod.
Immunol. 6:377). Stimulation may be due to contamination by
nontrophoblast cells such as fetal or maternal white blood
cells expressing HLA. In our study, we used as our
trophoblast antigen-source choriocarcinoma cell lines to
provide a consistent trophoblast antigen without
contamination by other cells expressing HLA molecules.
Because the response to trophoblast antigen was so strong in
women with recurrent abortion, we used a more conservative SI
(greater than 3) in contrast to the more conventional SI of
greater than 2 to define significant lymphocyte proliferation
in response to antigen. Jeg-3 and JAR cells were found to be
suitable antigenic sources, with Jeg-3 cells being more
antigenic than JAR in stimulating lymphocyte proliferation in
women with unexplained recurrent abortion. The trophoblast
antigen(s) responsible for activated lymphocyte responses
were abundant in both cytosol and membrane fractions.
Whether cell-mediated immunity was due to a single antigen
which located in cytosol and membranes, or due to a variety
of antigens distributed in both fractions remains as yet
unknown. However, our data provide evidence that responsible
~ wo94n842~ 21~ 3 ~15 n PCT~S94/05692
-75-
antigenic epitopes contained a peptide/protein component
since antigenicity was abrogated by trypsinization or heat
treatment. Lymphocyte proliferation was not observed in any
of the samples following incubation with RBC membrane
antigens. RBC membranes were chosen for the control antigen
because, like villous trophoblast, RBC do not express HLA.
The lymphocyte proliferation assay is an effective
method to distinguish women with activated cellular immunity
to trophoblast which may contribute to their recurrent
abortion. This technique, in addition to the assays
described in Example 4, is useful in the diagnostic
evaluation of women with immunologic reproductive failure and
has applicability regarding the testing of potential
therapeutics such as the above-described immunomodulating
agent drug therapies.
Equivalents
Each of the above-identified references is incorporated
herein by reference.
Those skilled in the art will be able to ascertain,
using no more than routine experimentation, many equivalents
of the specific embodiments of the invention described
herein. These and all other equivalents are intended to be
encompassed by the following claims.
What is claimed is:
.7