Note: Descriptions are shown in the official language in which they were submitted.
W094/29272 ~ h~ ~ ~ 9 PCT/SE94/00~8
l-Sub~tituted I~atin and Oxindole Deri~ati~es aR
Inhibitor~ of AcetYlcholineRteraRe
Field of the invention
The preRent invention relateR to novel compounds having
therapeutic activity, intermediate~ for their
~reparation, proce~ReR for their preparation,
~harmaceutical formulation~ contA;n;ng ~aid compound~ and
medicinal u~e of Raid compound~.
sack~round of the invention
A major characteristic of Alzheimer~s Disease (Senile
Dementia, SDAT) i~ a marked central cholinergic
dyRfunction. ThiR cholinergic deficit haR been reported
to correlate with cognitive impairment (P.T. Francis et
al, New Engl. J. Med., 1985, 313, 7). VariouR attempta
to increaRe central cholinergic activity and thereby
re~erQe the cogniti~e deficit~ have, to date, met with
only limited ~ucce~.
There i~ ~ome e~idence that uRe of the alkaloid
~hy~o~tigmine can, in ~ome case~, be marginally
beneficial, but the uRe of thiR compound in the clinic i~
compromiRed by a low therapeutic ratio, a Rhort half-life
and ~oor bioavailability. The cholineRtera~e inhibitor,
9-amino-1,2,3,4-tetrahydroacridine (THA) ha~ been
reported to be of therapeutic ~alue in the treatment of
a ~mall group of ~atientR with SDAT (W.R. ~ummer~ et al,
New Engl. J. Med., 1986, 315, 1241). Further clinical
trial~ of THA ha~e ~roduced ~ome encouragi~g re~ult~ but
have been hampered by the a~sociation of thi~ drug with
certain toxic ~ide effect~.
Other com~ound~ ~tructurally related to either
phy~o~tigmine or THA have been reported and are the
W094/29272 PCT/SE94/00
9 2
~ub~ect of ongoing inve~tigations.
There remain~ an urgent need for a ~afe and clinically
effective drug for the sym~tomatic treatment of
Alzheimer's Disease and related conditionR.
The ~resent invention
A ~rimary objective of the ~resent invention is to
~rovide structurally novel com~ound~ which by virtue of
their pharmacological ~rofile enh~nce central cholinergic
function and are of value in the treatment of the
cognitive dy~functions which may be a~ociated with
ageing or with conditionn ~uch a~ Alzheimer's Disease,
Senile and related Dementias, Parkinson~s Disea~e, Down~a
Syndrome and Huntington's Chorea. This utility i~
manife~ted, for exam~le, by the ability of these
compounds to inhibit the enzyme acetylcholinesterase.
Further, the com~ounds of this invention are, in general,
highly ~otent and selective, have an improved duration of
action and are, in general, less toxic than hitherto
known compounds.
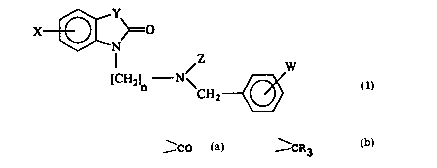
The ~re~ent invention relates to a com~ound having the
general formula (1)
X~ ~0
N/Z W (1)
[ 2]n \CH
wherein:
n i~ 3, 4, 5, 6 or 7;
X re~resents one or more substituent~ independently
selected from hydrogen, lower alkyl, aryl, lower alkoxy,
W094/29272 2~ 6 ~ PCT/SE94/00448
halogen, trifluoromethyl, nitro, -NHCOR where R i~ lower
alkyl or aryl, -NR1R2 where R1 and R2 are inde~endently
hydrogen or lower alkyl or together form a ring, or
cycloalkyl, cycloalkenyl or bicycloalkyl either
optionally further ~ubstituted by lower alkyl;
Y i~ CO or _ CR3R4 where R3 and R4 are independently
hydrogen, lower alkyl, lower alkoxy or together form a
cyclic acetal;
Z i~ lower alkyl;
and W re~re~ent~ one or more ~ub~tituents inde~endently
~elected from hydrogen, lower alkyl, lower alkoxy or
halogen.
Stereo and o~tical i~omer~ and racemateQ thereof where
~uch isomer~ exi~t, aQ well aR ~harmaceutically
acceptable acid addition ~alt~ thereof and ~olvate~
thereof are al~o ~art of the invention.
Preferred embodiment~ of thi~ invention relate to
com~ound~ having the general formula (2)
O
X~O
/ W (2)
[ 2]n \CH ~
wherein n, X, W and Z are a~ ~reviou~ly defined above;
or to com~ound~ having the general formula (3)
W094/29272 5 4 ll~ PCT/SE94/00448
,,
X ~ W (3)
[CH ] N/ ~
wherein n, X, W and Z are a~ ~reviously defined above.
Throughout the ~ecification and the a~ended claim~, a
given chemical formula or name ~hall encom~a~ all ~tereo
and optical i~omer~ and racemate~ thereof where ~uch
i~omer~ exi~t, a~ well a~ ~harmaceutically acce~table
acid addition salt~ thereof and ~olvate~ thereof ~uch as
for in~tance hydrates.
The following definition~ ~hall a~ly throughout the
~pecification and the a~ended claim~.
~nle~s otherwi~e ~tated or indicated, the term "lower
alkyl" denote~ a ~traight or brAnche~ alkyl grou~ having
from 1 to 6 carbon atoms. Exam~le~ of ~aid lower alkyl
include methyl, ethyl, n-~ropyl, i~o-propyl, n-butyl,
i~o-butyl, sec-butyl, t-butyl and ~traight- and brAn~h~-
chain ~entyl and hexyl.
~nle~s otherwi~e stated or indicated, the term"cycloalkyl" denote~ a cyclic alkyl group having a ring
~ize from C3 to C7, o~tionally additionally ~ub~tituted
by lower alkyl. Example~ of ~aid cycloalkyl include
cyclopro~yl, cyclobutyl, cyclo~entyl, cyclohexyl,
methylcyclohexyl and cyclohe~tyl.
~nle~ otherwi~e ~tated or indicated, the term
"cycloalkenyl" denote~ a cyclic alkenyl grou~ having a
ring ~ize from C3 to C7, o~tionally additionally
substituted by lower alkyl. Example~ of aid cycloalkenyl
W094/29272 PCT/SE94/00448
21 64~ ~
include cyclopropenyl, cyclobutenyl, cyclopentenyl,
cyclohexenyl, methylcyclohexenyl and cycloheptenyl.
~ nle~ otherwi~e ~tated or indicated, the term "lower
alkoxy~ denote~ a Qtraight or brAnc~e~ alkoxy group
ha~ing from 1 to 6 carbon atomQ. ~xample~ of ~aid lower
alkoxy include methoxy, ethoxy, n-propoxy, i~o-propoxy,
n-butoxy, i~o-butoxy, ~ec-butoxy, t-butoxy and ~traight-
and brAnc~e~-chain pentoxy and hexoxy.
~nle~ otherwi~e ~tated or indicated, the term ~halogen~
~hall mean fluorine, chlorine, bromine or iodine.
~ nle~ otherwi~e stated or indicated, the term ~aryl"
denote~ a phenyl, furyl or thienyl group in which the
ring i~ optionally further ~ubQtituted by lower alkyl,
lower alkoxy or halogen.
~ nleQ~ otherwi~e Qtated or indicated, the term
"bicycloalkyl" denoteQ a bicyclic alkyl group having a
~ize from C6 to Cg, optionally additionally ~ubQtituted
by lower alkyl. Example~ of ~aid bicycloalkyl include
bicyclo[2.2.1]heptyl, bicyclo12.2.2]octyl and
bicyclo[2.2.3]nonyl.
~nle~ otherwi~e ~tated or indicated, the term ~cyclic
acetal" denote~ a cyclic acetal group having a ring ~ize
from C5 to C7. Example~ of ~aid cyclic acetal include
1,3-dioxolanyl and 1,3-dioxanyl.
Preferred compounds according to the invention are thoQe
of general formula (2) or general formula (3) in which:
n ia 4, 5 or 6
W iQ hydrogen or F, e~pecially 4-F,
and X i~ lower alkyl, e~pecially methyl or ethyl, lower
alkoxy, especially methoxy or ethoxy, cycloalkyl,
especially C5 to C7 cycloalkyl, F, aryl, especially
W094/29272 PCT/SE94/00
6~19 6
~henyl, or -NRlR2, e~pecially l-~yrrolidinyl or 1-
~i~eridinyl. More ~referred com~ounds according to the
invention are those of general formula (2) or general
formula (3) in which the X ~ubstituent is at the 5-
~o~ition.
Among the most ~referred compounds of formula (1)
according to the ~re~ent invention are:
1,3-dihydro-1-~4-(N-ethyl-N-phenylmethylamino)butyl)-2H-
indol-2-one;
5-cyclohexyl-1,3-dihydro-1-(5-(N-ethyl-N-phenylmethyl-
amino)pentyl)-2H-indol-2-one;
5-cyclohexyl-1-(5-(N-ethyl-N-~henylmethylA~ino)pentyl)-
lH-indole-2,3-dione;
1,3-dihydro-1-(5-(N-ethyl-N-(4-fluorophenyl)
methyl A m ; n o)pentyl-2H-indol-2-one;
5-cyclohexyl-1-(4-(N-ethyl-N-~henylmethylamino)butyl)-lH-
indole-2,3-dione;
1-(4-(N-ethyl-N-~henylmethylAm;no)butyl)-5-phenyl-lH-
indole-2,3-dione;
1-(5-(N-ethyl-N-phenylmethylamino)pentyl)-5-(1-
~i~eridinyl)-lH-indole-2,3-dione;
5-cyclohexyl-1,3-dihydro-1-(4-(N-ethyl-N-~henylmethyl-
amino)butyl)-2H-indol-2-one;
1,3-dihydro-1-(5-(N-ethyl-N-~henylmethylamino)~entyl)-5-
~henyl-2H-indol-2-one;
1,3-dihydro-1-(5-(N-ethyl-N-~henylmethylamino)~entyl)-5-
methoxy-2H-indol-2-one;
and ~harmaceutically acce~table acid addition ~alts or
solvates thereof.
The ~re~ent invention al~o relate~ to proces~e~ for
~re~aring the compound having formula (1). Said compound
may be pre~ared by treating a com~ound of the general
formula (4)
W094t29272 PCT/SE94/00~
~6~1~9
X ~ ~ O (4)
wherein X and Y are a~ defined above,
with a 1,n-dihalo~l~Ane to obtain a compound of the
general formula (5)
X ~ ~ O (5)
[CHJn H~
wherein X, Y and n are a~ defined above and Hal i~
halogen,
whereafter the com~ound of the general formula (5) i~
reacted with a com~ound of the ~eneral formula (6)
H - N/ ~ (6)
wherein W and Z are a~ defined abo~e.
The ~roce~ can be achieved, for exam~le, by treating a
compound of ~tructure (4) with a 1,n-dihaloAl~Ane, in a
suitable ~olvent ~uch a~ toluene or 3-methyl-2-butAno~
or acetonitrile or acetone or dimethyl~ulphoxide or
W094/29272 ~ 9 PCT/SE94/00448
dimethylformamide in the ~reRence of a ba~e ~uch a~
triethylamine or anhydrou~ ~otas~ium cArhon~te. Such
reaction should be conducted at a ~uitable tem~erature,
normally between 0C and 100C, optionally in an inert
atmo~here. Some com~ound~ of ty~e (5) are known in the
literature. The intermediate (5) may either be i~olated
and ~urified and characteri~ed u~ing ~tAn~Ard techniques
or elae may be reacted in a crude form with a compound of
~tructure (6). Such reaction i~ preferably conducted in
a ~uitable ~olvent ~uch a~ dichloromethane or
dimethylformamide in the ~reQence of a ba~e ~uch a~
triethylAm;ne or anhydrou~ ~ota~ium carbonate or an
exce~ of com~ound (6), o~tionally with the addition of
a catalytic amount of ~otaasium iodide. The reaction
~hould be conducted at a ~uitable temperature, normally
between 0C and 100C, o~tionally in an inert atmo~phere.
The required ~roduct (1) may then be i~olated and
~urified and characteriQed using ~t~n~rd technique~. In
the ca~e of ~roduct~ wherein Y represent~ an acetal or
cyclic acetal grou~, the corre~o~;ng ~roduct~ wherein
Y i~ ~ CO can be ~ub~equently ~re~ared by acid-cataly~ed
hydroly~i~ in a - -nner that will be readily a~reciated
by one ~killed in the art of or~anic ~ynthe~iR.
Com~ound~ of ~tructure (4) wherein Y i~ _ CO are known
a~ iRatins (~y~tematic name lH-indole-2,3-dioneR). The
i~atin~ of ~tructure (4) are, depen~;ng on the nature of
the Rub~tituent ( R ) X, either com~ound~ which have been
~reviou~ly described in the literature, or com~oundR
which can be ~re~ared by the ~traightforward ap~lication
of known method~. The Sandmeyer procedure (Or~anic
Synthe~e~, Coll. Vol. I., ~ 327), in which an aniline,
chloral hydrate and hydroxylamine are reacted to~ether to
give an intermediate iRonitro~oacetanilide which i~ then
.:
W094/29272 ~ 641 1 ~ PCT/SE94/00448
cycli~ed to the i~atin on treatment with ~trong acid, i~
a ~articularly useful method.
Com~ound~ of ~tructure (4) in which Y i~ = CH2 are known
aR oxindole~ (~y~tematic name 1,3-dihydro-2H-indol-2-
one~). The oxindole~ of ~tructure (4) are, depen~;ng on
the nature of the ~ub~tituent(~) X, either known
compound~ or compoundR which can be ~re~ared u~ing known
method~. The GaRQman reaction (P.G. Ga~man et al,
J.Amer.Chem.Soc., 1974, 96, 5508 and 5512) con~titute~ a
well-known and general ~ynthe~i~ of oxindoles.
Compound~ of Rtructure (4) wherein Y re~re~ent~ an acetal
or cyclic acetal can be prepared from com~ound~ of
~tructure (4) wherein Y iR - CO by the Rtraightforward
a~lication of known method~ in a manner that will be
readily under~tood by tho~e ~killed in the art.
Thu~, the preRent invention al~o refers to ~ome new
intermediateR of formula (5), namely:
X ~ ~ O (5)
n
wherein n i~ 5, 6 or 7 and X, Y and Hal are aR defined
above, with the ~ro~i~o that when n i~ 5 and Y i~ ~CO,
X i~ not H.
In certain circum~tances it i~ ad~antageou~ to ~repare
oxindole~ from the corre~o~ng i~atins. Thi~
W094t29272 PCT/SE94/00448
~6~
tran~formation may be achieved using ~uch known methods
a~:
a)catalytic hydrogenation/hydrogenoly~
b)formation of the corresponA;ng 3-hydrazone followed by
reductive elimination under basic condition~ (Wolff-
~i~chner procedure);
or
c)formation of the corre~po~A;ng 3-dithioacetal followed
by reduction using Raney nickel or nickel boride.
Method (c) repre~ents a preferred proce~ for the
conver~ion of certain i~atin~ (l;Y is ~CO) or (4;Y is
~ O) into the corresponA; ng oxindole~ (l;Y i~ = CH2)
or
(4;Y i~ = CH2) respectively.
The pre~ent invention al~o relate~ to pharmaceutical
formulationR containing a compound according to claim 1.
Another object of the pre~ent invention i~ a compound
according to claim 1 for u~e in therapy.
Still another object of the present invention is the u~e
of a compound having the general formula (1)
X~ ~0
N/Z W (1)
[ 2]n \CH
wherein:
W094/29272 PCT/SE94/00448
~ 6 ~ 9
11
n i8 3, 4, 5, 6 or 7;
X re~resents one or more substituents inde~endently
selected from hydrogen, lower alkyl, aryl, lower alkoxy,
halogen, trifluoromethyl, nitro,
-NHCOR where R is lower alkyl or aryl,
-NR1R2 where R1 and R2 are inde~en~ntly hydrogen or
lower alkyl or together form a ring,
or cycloalkyl, cyclo~lkenyl or bicycloalkyl either
o~tionally further substituted by lower alkyl;
Y is CO or CR3R4 where R3 and R4 are inde~endently
hydrogen, lower alkyl, lower alkoxy or together form a
cyclic acetal;
Z is lower alkyl;
and W re~resents one or more substituents independently
selected from hydrogen, lower alkyl, lower alkoxy or
halogen;
stereo and o~tical isomers and rac~m~tes thereof where
such isomers exist, as well as pharmaceutically
acceptable acid addition salts thereof and solvates
thereof, for the manufacture of a medicament for the
treatment of conditions such as glaucoma and myasthenia
gravis and, more particularly, for the ~revention or
treatment of cognitive dysfunctions which may be
a8aociated with ageing or with condition~ ~uch a~
Alzheimer's Disease, Senile and related Dementias,
Parkinson's Disease, Down's Syndrome and Huntington's
Chorea.
Moreover, the ~resent in~ention relates to a method for
the treatment of central cholinergic dysfunction whereby
a pharmacologically effecti~e amount of a compound
W094/29272 PCT/SE94/00
~ ~ 12
according to claim 1 iR A~;n~Btered to a hoRt in need of
Raid treatment.
Pharmacolooy
The compoundR of general formula (1) of the ~reRent
invention are u~eful in the treatment of variouR
cognitive dysfunctionR, Ruch a~ thoRe occurring in
Alzheimer~ R diReaRe . ThiR utility iR manifeRted by the
ability of theRe compoundR to inhibit the enzyme
acetylcholinesteraRe.
AcetylcholineRteraRe Inhibition A~Ray
The ability of com~oundR in general to inhibit the
acetylcholineRteraRe activity of rat brain homogenate was
determined uRing the s~ectrophotometric method of Ellman
et al, Biochem.Pharmacol., 1961, 7, 88. ReRultR are
expreRRed aR IC50 nanomolar (i.e. the nanomolar
concentration of teRt com~ound required to inhibit enzyme
activity by 50%).
Further the compounds of thiR invention ~otentiate
cholinergic function in the brain Ruch that when
A~; n; stered to rodents theRe com~oundR induce marked
cholinergic effectR Ruch aR tremor. TheRe utilitie~ are
further demonstrated by the ability of the~e compoundR to
reRtore cholinergically deficient memory in a delayed
non-matched to Rample taRk.
Delayed Non-Matched to Sam~le A~Ray
RatQ were trained on a delayed non-matched to Ram~le taRk
R imilar to that de~cribed by Murray et al,
PRycho~harmacology, 1991, 105, 134-136. Sco~olamine, an
anticholinergic that iR known to cauRe memory impairment,
induces an im~airment in ~erformance of thi~ taRk. ThiR
impairment iR reverRed by com~oundR of the ty~e deRcribed
in the ~reRent invention.
W094l29272 PCT/SE94/00~
~15~
13
Pharmaceutieal formulation~
The admini~tration in the novel method of treatment of
this invention may conveniently be oral, rectal, or
~arenteral at a dosage level of, for exam~le, about
0.0001 to 10 mg/kg, preferably about 0.001 to 1.0 mg/kg
and es~ecially about 0.01 to 0.2 mg/kg and may be
administered on a regimen of 1 to 4 doses or treatments
per day. The dose will depend on the route of
administration, a preferred route being by oral
admini~tration. It will be a~preciated that the severity
of the disease, the age of the ~atient and other factors
normally considered by the atten~;ng physician will
influence the individual regimen and aosage most
a~ro~riate for a ~articular patient.
The ~harmaceutical formulations com~rising the com~ound
of this invention may conveniently be tablets, ~ills,
ca~sules, syru~, powders or granules for oral
A~;n;stration; sterile parenteral solutions or
suspension~ for parenteral administration; or as
su~positories for rectal administration; or as suitable
to~ical formulation~. Co~-ve~tional ~rocedures for the
selection and ~reparation of suitable pharmaceutical
formulations are described, for example, in
~Pharmaceuticals - The Science of Do~age Form DeQign",
M.E. Aulton, Churchill ~ivingstone, 1988.
To produce pharmaceutical formulations containing a
compound according to the ~resent invention in the form
of dosage units for oral ap~lieation the aetive substance
may be A~m~Yed with an adjuvant/a earrier e.g. laeto~e,
saccharose, sorbitol, mannitol, ~tarches such an ~otato
~tarch, corn starch or amylo~ectin, cellulose
derivatives, a binder such as gelatine or
~olyvinyl~yrrolidone, and a lubricant such as ma~nesium
stearate, calcium stearate, polyethylene glycol, waxes,
~araffin, and the like, and then compres~ed into tablets.
WO 94129272 n ~ PCTISE94/00448
14
If coated tablets are required, the cores, ~re~ared as
described above, may be coated with a concen~rated sugar
solution which may contain e.g. gum arabic, gelatine,
talcum, titanium dioxide, and the like. Alternatively,
the tablet can be coated with a ~olymer known to the man
skilled in the art, dissolved in a readily volatile
organic solvent or mixture of organic solvents. Dyestuffs
may be added to these coatings in order to readily
distinguish between tablets containing different active
substances or different amounts of the active com~ounds.
For the ~re~aration of soft gelatine ca~sules, the active
substance may be admixed with e.g. a vegetable oil or
polyethylene glycol. Hard gelatine ca~sules may contain
granules of the active substance using either the above-
mentioned exci~ients for tablets e.g. lactose,
saccharose, sorbitol, mannitol, starches (e.g. ~otato
starch, corn starch or amylo~ectin), cellulose
derivatives or gelatine. Also liquids or semisolids of
the drug can be filled into hard gelatine ca~sules.
Dosage units for rectal a~lication can be solutions or
sus~ensions or can be ~re~ared in the form of su~osi-
tories com~rising the active substance in admixture with
a neutral fatty base, or gelatine rectal ca~sules
comprising the active substance in admixture with
~egetable oil or ~araffin oil.
Liquid preparations for oral a~lication may be in the
form of syrups or sus~ensions, for exam~le ~olution~
containing from about 0.02% to about 20% by weight of the
active substance herein described, the h~lAnce being
sugar and mixture of ethanol, water, glycerol and
~ro~ylene glycol. Optionally such liquid ~reparations may
contain colouring agents, flavouring agents, saccharine
and carboxymethylcellulose as a thicke~;n~ agent or other
exci~ients known to the man in the art.
W094/29272 2~ 6 111~ PCT/SE94/00~8
SolutionR for parenteral a~plicationR by in~ection can be
~re~ared in an aqueou~ ~olution of a water-Roluble
~harmaceutically acce~table Ralt of the active Qub~tance,
~referably in a concentration of from about 0.5% to about
10% by we~ght. TheRe ~olutionR may alRo contA~n ~tabili-
zing agentQ and/or buffering agent~ and may conveniently
be ~rovided in variou~ doRage unit am~oule~.
R~
5-CYclohexYl-1,3-dihydro-2H-indol-2-one
5-Cyclohexyl-lH-indole-2,3-dione (3.4 g) in methanol (lO0
ml) wa~ treated with 1,2-eth~neA;thiol (1.5 g) and boron
trifluoride diethyletherate (2 ml). The mixture was
~tirred at room tem~erature o~ernight and then eva~orated
to drynesR under reduced ~reRRure. The reRidue waR
~urified by flaRh chromatogra~hy to yield the
correQ~on~ng dithioacetal. This material in ethanol
(100 ml) waR treated with Raney nickel (50% Rlurry in
water, 40 g) and the mixture waR heated under reflux
overnight. The mixture wa~ filtered through Celite and
the re~idue~ wa~hed thoroughly with ethanol. The
combined filtrate~ were e~a~orated to give the title
com~ound a~ a white solid (2.9 g, 88%), m.~. 153-155C.
1H Nmr (d6-DMSO) 1.2-1.5 (5H, m), 1.7-2.0 (5H, m), 2.5
(lH, m), 3.5 (2H, ~), 6.8 (lH, d), 7.08 (lH, dd) and 7.15
(lH, d) ~m.
EXA~PL~ 2
1-(5-Bromo~entyl)-1,3-dihydro-2H-indol-2-one
1,3-Dihydro-2H-indol-2-one (13.3g), 1,5-dibromopentane
(46g) and anhydrou~ ~ota~ium carbonate (17g) in
acetonitrile (200ml) were heated under reflux for 24
hour~. The mixture wa~ filtered. The filtrate wa~
eva~orated to dryneRR and the reRidue thu~ obtained was
~urified by fla~h chromatogra~hy to give the title
compound aR an oil.
W094l29272 PCT/SE94/004
16
lH Nmr (CDC13) 1.51, 1.7 and 1.9 (each 2H, m), 3.37 (2H,
t), 3.5 (2H, ~), 3.7 (2H, t), 6.82 (lH, d), 7.03 (lH, t)
and 7.25 (2H, m) ~m.
13C Nmr (CDC13) 25.3, 26.7, 32.1, 33.0, 35.5, 39.4,
108.0, 122.0, 124.3, 124.5, 127.6, 144.3 and 174.7 Dpm.
~ing the a~ro~riate ~tarting material~ and following
the general method of Exam~le 2 the com~ound~ of Exam~le~
3 to 5 were ~re~ared.
~XAXP~ 3
1-(4-BromobutYl)-1,3-dihydro-2H-indol-2-one
lH Nmr (CDC13) 1.9 (4H, m), 3.42 (2H, t), 3.5 (2H, ~),
3.74 (2H, t), 6.83 (lH, d), 7.02 (lH, t) and 7.25 (2H, m)
p~m.
E8AMPh~ 4
1-(6-Bromohexyl)-1,3-dihydro-2H-indol-2-one
13C Nmr (CDC13) 25.6, 26.8, 27.3, 32.1, 33.2, 35.2, 39.3,
107.8, 121.6, 124.0, 124.2, 127.3, 144.0 and 174.4 ~pm.
EXAMPL~ 5
1-(5-Bromo~entyl)-1~3-dihydro-5-cyclohexyl-2H-indol-2-
one
13C Nmr (CDCl3) 25.5, 26.1, 26.7, 26.9, 32.3, 33.3, 34.8,
35.9, 39.7, 44.3, 107.9, 123.2, 124.7, 125.9, 142.4 and
175.0 ~m.
Rlr~PI~ 6
5'-Cyclohexyl-~irol1,3-dioxolane-2,3'-[3H~-indol]-
2'(1'H)-one
5-Cyclohexyl-lH-indole-2,3-dione (1 equi~alent), ethane-
1,2-diol (5 equivalent~) and ~-toluene~ul~honic acid
(0.02 equivalent~) in drY toluene were heated under
reflux overnight with azeotropic removal of water. The
reaction mixture wa~ cooled, wa~hed with ~aturated ~odium
W094/29272 2 i I g 11 9 PCT/SE94/00448
bicarbonate Polution, and then worked up in the u~ual
mAn~er to afford the title compound.
M.~. 178-180C.
13C Nmr (CDCl3) 175.8, 143.4, 139.6, 129.9, 124.1, 123.4,
110.5, 102.6, 65.7, 44.1, 34.5, 26.8 and 26.0 p~m.
EXA~PL~ 7
1'-(6-BromoheYyl)-~iro[1,3-dioxolane-2,3'-[3Hl-indol]-
2'(1'H)one
S~irotl,3-dioxolane-2,3~-t3H]-indol]-2~ H)-one (5.12g),
1,6-dibromoheYAne (12.2g) and anhydrou~ pota~ium
carbonate (6.9g) in acetone (200ml) were heated under
reflux for 24 hour~. The mixture wa~ filtered. The
filtrate wa~ eva~orated to dryne~ and the re~idue thu~
obtained wa~ ~urified by fla~h chromatography to give the
title com~ound a~ an oil.
13C Nmr (CDCl3) 25.7, 26.8, 27.5, 32.3, 33.4, 39.2, 65.6,
101.9, 108.6, 122.8, 124.0, 124.7, 131.4, 143.8 and 173.0
~m.
~ing the a~pro~riate ~tarting material~ and following
the general method of Exam~le 7 the compound~ of Exam~le~
8 to 10 were pre~ared.
R~ R 8
1'-(4-Bromobutyl)-~irotl,3-dioxolane-2,3'-t3Hl-indoll-
2'(1'H)-one
13C Nmr (CDCl3) 25.4, 29.3, 32.7, 38.2, 65.5, 101.7,
108.5, 122.8, 123.9, 124.6, 131.3, 143.5 and 173.0 ~pm.
R~PI~ 9
1'-(5-Bromo~entyl)-~pirol1,3-dioxolane-2,3'-l3H]-indol]-
2'(1'H)-one
13C Nmr (CDCl3) 25.1, 26.2, 32.0, 33.1, 39.1, 65.6,
101.8, 108.6, 122.9, 123.9, 124.7, 131.4, 143.7 and 173.0
~m.
W094/29272 PCT/SE94/00448
~64~l~
18
~A~ 10
1'-(5-BromopentYl)-5'-cyclohexyl-~iro[1,3-dioxolane-
2,3'-[3H]-indol]-2'(1'H)-one
13C Nmr (CDCl3) 25.2, 25.9, 26.3, 26.7, 32.1, 33.0, 34.4,
39.2, 44.0, 65.6, 102.2, 108.4, 123.3, 123.8, 129.6,
141.7, 143.2 and 173.2 ~m.
~CA~PLE 11
1,3-Dihydro-1-(5-(N-ethyl-N-Dhenylmethylamino)~entyl)-2H-
indol-2-one
l-(5-Bromo~entyl)-1,3-dihydro-2H-indol-2-one (8g) and N-
ethyl-N-~henylmethylamine (12.15g) in dichloromethane
(150ml) were heated under reflux for 24 hour~. The
mixture wa~ eva~orated to dryne~ and the re~idue wa~
purified by fla~h chromatogra~hy to give the title
com~ound.
13C Mmr (CDCl3) 11.6, 24.6, 26.6, 27.2, 35.6, 39.8, 47.2,
52.8, 58.0, 108.1, 121.9, 124.2, 124.5, 126.5, 127.6,
127.9, 128.6, 140.0, 144.5 and 174.7 ~m.
m/z 337 (M + H )
The corres~on~;ng fumarate wa~ ~repared using fumaric
acid in methanol.
13C Nmr (CDCl3) 9.0, 23.4, 24.1, 26.9, 35.7, 39.4, 45.9,
50.8, 55.7, 108.4, 122.2, 124.4, 124.5, 127.9, 128.9,
130.5, 131.4, 135.3, 144.3, 170.4 and 175.1 ~pm.
~ing the a~ro~riate starting material and following the
general method of Example 11 the com~ound~ of Examples 12
to 14 were pre~ared.
W094/29272 PCTtSE94/004~
2~4119
19
RY~ 12
1~3-Dihydro-1-(4-(N-ethyl-N-~henylmethYlamino)butyl)-2H
indol-2-one
13C Nmr (CDCl3) 11.6, 24.4, 25.0, 35.5, 39.6, 47.1, 52.5,
57.9, 108.1, 121.8, 124.2, 124.4, 126.5, 127.5, 127.9,
128.6, 139.8, 144.4 and 174.6 ~pm.
m/ 323 (M + H )
Fumarate, 13C Nmr (CDC13) 9.6, 22.1, 25.0, 35.7, 39.3,
46.2, 51.2, 56.4, 108.4, 122.3, 124.4, 124.5, 127.9,
128.4, 128.7, 130.1, 133.3, 135.3, 144.2, 170.4 and 175.1
~m.
~AMPLE 13
1,3-Dihydro-1-(6-(N-ethyl-N-phenylmethylamino)hexyl)-2H-
indol-2-one
13C Nmr (CDC13) 11.7, 26.8, 26.9, 27.0, 27.4, 35.7, 39.9,
47.2, 53.0, 58.0, 108.2, 121.9, 124.3, 124.6, 126.5,
127.7, 128.0, 128.7, 140.1, 144.6 and 174.8 ~m.
m/z 351 (M I Hl)
Fumarate, 13C Nmr (CDC13) 8.7, 23.1, 26.3, 27.1, 29.5,
35.7, 39.6, 45.7, 50.3, 55.4, 108.3, 122.1, 124.3, 124.5,
127.8, 128.9, 129.1, 130.5, 130.6, 135.2, 144.4, 170.3
and 175.1 ~m.
RY~ 14
5-CYclohexyl-l~3-dihydro-l-(5-(N-ethyl-N-~henylmeth
amino)~entyl)-2H-indol-2-one
13C Nmr (CDC13) 11.7, 24.7, 26.0, 26.7, 26.8, 27.3, 34.7,
35.8, 39.9, 44.2, 47.2, 52.9, 58.0, 108.0, 123.0, 124.6,
125.8, 126.5, 128.0, 128.7, 140.0, 142.1, 142.5 and 174.8
~m.
m/ 419 (M I H )
3~
W094/29272 PCT/SE94/00~
~ 6 ~ 20
Fumarate, 13C Nmr (d6-DMSO) 10.6, 23.8, 25.1, 25.5, 26.3,
26.7, 34.2, 35.1, 43.4, 46.5, 51.8, 56.8, 107.9, 122.6,
124.6, 125.4, 127.1, 128.1, 128.9, 134.2, 137.4, 141.1,
142.2, 166.5 and 174.1 ppm.
R~PI~ 15
1~-(4-(N-Ethyl-N-~henylmethylamino)butyl)-~iro[1~3-
dioxolane-2,3'-[3H]-indoll-2'(1'H)-one
1'-(4-Bromobutyl)-~piro[1,3-dioxolane-2,3'-13H]-indol]-
2~ H)-one (6g), N-ethyl-N-phenylmethylamine (5.13g) and
anhydrou~ pota~sium carbonate (10.5g) in acetonitrile
(150ml) were heated under reflux for 24 hourR. The
mixture waR filtered. The filtrate wa~ evaporated to
dryne~ and the reR~due waR ~urified by fla~h
chromatography to afford the title compound.
3C Nmr (CDC13) 11.7, 24.3, 24.9, 39.5, 47.2, 52.4, 58.0,
65.7, 102.1, 108.9, 122.9, 124.1, 124.8, 126.6, 128.0,
128.7, 131.5, 140.0, 144.1 and 173.2 ppm.
~ing the appropriate ~tarting material and following the
general method of Example 15 the com~ound~ of Examples 16
to 18 were prepared.
R~ ~ 16
1~-(5-(N-~thyl-N-phenylmethylamino)~entyl)-spiro[1,3-
dioxolane-2,3'-13H]-indol]-2'(1'H)-one
13C Nmr (CDC13) 11.7, 24.6, 26.6, 27.1, 39.6, 47.2, 52.9,
58.1, 65.7, 102.1, 108.8, 122.9, 124.1, 124.8, 126.6,
128.0, 128.7, 131.5, 140.0, 144.1 and 173.2 ~m.
Rlrl~PLl~ 17
1'-(6-(N-Ethyl-N-~henylmethyl~ ; no ) hexyl)-~pirol1,3-
dioxolane-2,3'-l3H]-indol]-2'(1'H)-one
13C Nmr (CDC13) 11.6, 26.6, 26.7, 26.9, 27.0, 39.5, 47.1,
52.9, 57.9, 65.6, 102.0, 108.7, 122.8, 124.0, 124.7,
126.4, 127.9, 128.6, 131.4, 140.0, 144.0 and 173.0 ppm.
W094/29272 2~ 9 PCT/SE94/00448
RY~L~ 18
5~-cyclohexyl-l~-(5-(N-ethyl-N-Dhenylmethylamino)
Denty~ Diro[l~3-dioxolane-2~3~-l3H]-indol]-2~ H)-one
~Y~PL~ 19
1-(4-(N-Ethyl-N-Dhenylmethylamino)butyl)-lH-indole-2,3-
dione
1~-(4-(N-Ethyl-N-Dhenylmethylamino)butyl)-~Diro[l~3-
dioxolane-2,3'-l3H]-indol]-2'(1'H)-one (5.8g) in
tetrahydrofuran (150ml) containing conc hydrochloric acid
(4ml) and water (16ml) wa~ heated under reflux overni~ht.
The organic ~olvent waQ l~oved. The re~idue was
basified by the addition of Qodium carbonate solution and
then extracted with dichloromethane. The extract~ were
dried and evaDorated and the re~idue was Durified by
flanh chromatogra-Dhy to give the title comDound.
13C Nmr (CDCl3) 11.6, 24.4, 24.8, 39.9, 47.3, 52.3, 58.0,
110.1, 117.4, 123.4, 125.1, 126.6, 128.0, 128.6, 138.2,
139.7, 150.9, 158.0 and 183.4 DDm.
m/ 337 (M + H )
Fumarate, 13C Nmr (CDC13) 9.3, 22.1, 24.8, 39.6, 46.3,
51.1, 56.2, 110.4, 117.5, 123.8, 125.3, 128.9, 130.3,
135.3, 138.6, 150.6, 158.3, 170.4 and 183.4 DDm.
~ing the aDproDriate Qtarting material and following the
general method of ExamDle 19 the com~oundQ of ExamDle~ 20
to 22 were -DreDared.
Rlrl~PL~Z 2 0
1-(5-(N-Ethyl-N-~henylmethylamino)~entYl)-lH-indole-2,3-
dione
13C Nmr (CDCl3) 11.7, 24.6, 26.7, 27.1, 40.1, 47.3, 52.8,
58.1, 110.1, 117.5, 123.5, 125.3, 126.6, 128.0, 128.7,
W094/29272 ~ 22 PCT/SE94/00448
138.2, 140.0, 151.0, 158.1 and 183.5 ~m.
m/ 351 (M + H )
Fumarate, 13C Nmr (CDC13) 9.6, 24.2, 24.4, 26.7, 39.8,
46.1, 51.3, 56.3, 110.2, 117.4, 123.6, 125.3, 128.2,
128.6, 130.0, 133.9, 135.5, 138.4, 150.8, 158.1, 170.9
and 183.5 ~pm.
E8AMPLE 21
1-(6-(N-Ethyl-N-Dhenylmethylamino)hexyl)-lH-indole-2,3-
dione
13C Nmr (CDC13) 11.6, 26.6, 26.7, 26.8, 27.1, 40.0, 47.1,
52.8, 57.9, 110.0, 117.4, 123.4, 125.1, 126.5, 127.9,
128.6, 138.2, 140.0, 150.9, 157.9 and 183.4 ~m.
Fumarate, 13C Nmr (d6-DNSO) 11.0, 25.6, 26.2, 26.5, 26.9,
46.8, 52.1, 55.0, 57.1, 110.9, 117.6, 123.3 124.6, 127.4,
128.4, 129.2, 134.6, 137.8, 138.4, 151.0, 158.2, 167.0
and 183.7 ~m.
EXAMP~E 22
5-Cyclohexyl-1-(5-(N-ethyl-N-~henylmethylamino)~entyl)-
lH-indole-2,3-dione
13C Nmr (CDCl3) 11.7, 24.7, 25.9, 26.6, 26.7, 27.2, 34.3,
40.2, 43.7, 47.4, 52.9, 58.1, 109.9, 117.7, 123.6, 126.7,
128.1, 128.7, 136.8, 140.0, 143.8, 149.1, 158.3 and 183.9
~m.
m/ 433 (M + H )
EXAMPLB 23
1,3-Dihydro-1-(5-(N-methyl-N-~henylmethylA~;ns)pentyl)-
2H-indol-2-one
The title compound was ~re~ared using the general method
of Exam~le 11 but em~loying N-methyl-N-phenylmethylamine.
13C Nmr (CDC13) 24.5, 26.8, 27.1, 35.5, 39.7, 42.0, 56.9,
W094/29272 ~ 6 ~ ~ 1 g PCT/SE94/00448
62.2, 108.0, 121.8, 124.2, 124.4, 126.6, 127.5, 127.9,
128.6, 139.1, 144.4 and 174.6 ~m.
ESA~PLE 24
1,3-Dihydro-1-(5-(N-ethYl-N-(4-fluoro~henyl)
methylamino)~entyl-2H-indol-2-one
The title com~ound wa~ ~re~ared u~ing the general method
of Example 11 but employing N-ethyl-N-(4-fluoro~henyl)
methylamine.
13C Nmr (CDC13) 11.5, 24.5, 26.5, 27.0, 35.4, 39.6, 47.0,
52.6, 57.1, 107.8, 114.9(d), 121.8, 123.5, 124.3, 127.4,
129.8(d), 135.5(d), 144.3, 159.7 and 163.3(d), and 174.5
~m.
RY~P~ 2 5
1,3-Dihydro-1-(5-(N-ethyl-N-phenylmethylamino)~entyl)-
5-methyl-2H-indol-2-one
1,3-Dihydro-5-methyl-2H-indol-2-one and 1,5-
dibromo~entane were reacted together according to the
general method of Exam~le 2. The crude 1-(5-bromo~entyl)-
1,3-dihydro-5-methyl-2H-indol-2-one thu~ obtained wa~
then reacted with N-ethyl-N-~henylmethylamine according
to the method of Exam~le 11 to give title compound.
13C Nmr (CDC13) 11.6, 20.9, 24.7, 26.6, 27.3, 35.7, 39.9,
47.2, 52.9, 58.0, 107.9, 124.7, 125.2, 126.7, 127.8,
128.0, 128.8, 131.5, 139.6, 142.2 and 174.8 ~m.
EXAMPLE 26
1-(5-Bromo~entYl)-5-(1-methylethyl)-lH-indole-2,3-dione
5-(1-Methylethyl)-lH-indole-2,3-dione (3.8g), 1,5-
dibromopentane (9.2g) and anhydrouQ potaR~ium carbonate
(5.5g) were heated under reflux in acetonitrile
overnight. The mixture wa~ filtered. The filtrate wa~
evaporated to dryne~ and the re~idue thu~ obtA; neA was
purified by fla~h chromatogra~hy to gi~e the title
W094/29272 ~ ~ 4 ~ ~ ~ PCT/SE94/00448
24
com~ound a~ a red oil.
3C Nmr (CDC13) 23.6, 2S.3, 26.4, 32.0, 32.9, 33.4, 39.8,
109.9, 117.7, 123.1, 136.4, 144.7, 148.9, 158.3 and 183.5
p~m.
~ing the a~pro~riate ~tarting material~ and following
the general method of Example 26, the compound~ of
Exam~le~ 27 to 31 were ~re~ared.
~SAMPLE 27
1-(5-Bromopentyl)-5-methoxy-lH-indole-2,3-dione
1H Nmr (CDC13) 1.45-1.6, 1.65-1.8 and 1.85-2.0 (each 2H,
m), 3.4 (2H, t) 3.7 (2H, t), 3.82 (3H, ~), 6.85 (lH, d)
and 7.1-7.2 (2H, m) ppm.
~PLE 28
5-Bromo-1-(5-bromo~entYl)-lH-indole-2,3-dione
lH Nmr (CDC13) 1.45-1.6, 1.65-1.8 and 1.85-2.0 (each 2H,
m) 3.42 (2H, t) 3.75 (2H, t), 6.84 (lH, d) and 7.67-7.74
(2H, m) ~pm.
~XAKPLE 29
1-(4-Bromobutyl)-5-cyclohexyl-lH-indole-2,3-dione
M.~. 70-71C.
13C Nmr (CDC13) 25.8, 25.9, 26.6, 29.6, 32.7, 34.3, 39.1,
43.7, 109.9, 117.7, 123.7, 136.9, 144.1, 148.7, 158.4 and
183.5 ~m.
R~PLE 30
1-(5-Bromopentyl)-5-~henyl-lH-indole-2~3-dione
13C Nmr (CDC13) 25.4, 26.9, 32.1, 33.0, 40.1, 110.3,
118.1, 123.9, 126.5, 127.9, 129.0, 136.7, 137.3, 139.0,
149.9, 158.3 and 183.4 ~m.
W094/2g272 PCT/SE94/00~8
~16~119
BXA~P~ 31
1-(4-Bromobutyl)-5-phenyl-lH-indole-2,3-dione
13C Nmr (CDC13) 25.8, 29.6, 32.6, 39.3, 110.4, 118.0,
123.9, 126.5, 127.9, 129.0, 136.8, 137.3, 138.9, 149.7,
158.3 and 183.4 ~m.
~SA~PLæ 32
5-Cyclohexyl-1-(4-(N-ethyl-N-~henylmethy~ no)butyl)-lH-
indole-2,3-dione
1-(4-Bromobutyl)-5-cyclohexyl-lH-indole-2,3-dione (4.6g),
N-ethyl-N-~henylmethylamine (1.9g) and triethylamine
(1.3g) were heated under reflux in acetonitrile
overnight. The mixture was eva~orated to drYne~s and the
residue was ~urified by flash chromatogra~hy on silica
gel to gi~e the title com~ound aR a red oil.
13C Nmr (CDC13) 11-7, 24.6, 25.0, 25.9, 26.6, 34.3,
40.0, 43.7, 47.4, 52.3, 58.2, 110.0, 117.7, 123.5, 126.7,
128.1, 128.7, 136.8, 140.0, 143.8, 149.1, 158.3 and 183.9
~m.
Fumarate, M.p. 116-120C.
13C Nmr (d6-DMSO) 12.0, 24.2, 25.4, 26.2, 27.0, 34.5,
39.3, 43.6, 47.5, 52.8, 58.0, 111.4, 118.2, 123.2, 127.6,
128.9, 129.5, 135.0, 137.3, 139.7, 143.6, 149.7, 158.9,
167.1 and 184.5 ppm.
~sing the ap~ropriately substituted lH-indole-2,3-dione
and the ap~ro~riate amine and following the general
method of Example 32, the com~ounds of Exam~les 33 to 38
were ~re~ared.
~MPLE 33
1-(5-(N-Ethyl-N-Dhenylmethylamino)~entyl)-5-(
methylethyl)-lH-indole-2,3-dione
13C Nmr (CDC13) 11.7, 23.6, 24.5, 26.7, 27.0, 33.3, 40.1,
W094/29272 ~ ~ 6 ~ ~ " PCT/SE94/00448
26
47.3, 52.8, 58.1, 109.9, 117.7, 123.0, 126.5, 127.9,
128.6, 136.3, 140.0, 144.4, 149.1, 158.2 and 183.6 ~m.
R~-~PI~E 34
1-(5-(N-Ethyl-N-~henYlmethYlamino)~entyl)-5-methoxy-lH-
indole-2,3-dione
3C Nmr (CDC13) 11.6, 24.4, 26.6, 27.1, 39.8, 47.2, 52.7,
55.7, 58.0, 109.5, 110.9, 117.9, 124.2, 126.4, 127.8,
128.2, 140.0, 144.7, 156.2, 157.9 and 183.6 ~m.
BXA~PL~ 35
1-(4-(N-Ethyl-N-~henylmethylamino)butyl)-5-~henyl-lH-
indole-2,3-dione
13C Nmr (CDCl3) 11.6, 24.4, 24.9, 40.0, 47.3, 52.4, 58.0,
110.5, 117.9, 123.5, 126.3, 126.6, 127.7, 128.0, 128.6,
128.9, 136.5, 136.8, 138.8, 139.8, 149.9, 158.0 and
183.5 ~m.
EXAMPhE 36
1-(5-(N-Methyl-N-~henylmethYl~m;no)~entyl)-lH-indole-2~3
dione
13C Nmr (CDCl3) 24.3, 26.6, 26.8, 39.8, 41.8, 56.6, 62.1,
109.9, 117.2, 123.2, 124.8, 126.5, 127.8, 128.5, 138.0,
139.0, 150.7, 157.8 and 183.2 ~m.
Fumarate, M.~. 134-136C.
13C Nmr (d6-DMSO) 23.8, 25.3, 26.4, 38.7, 40.8, 55.8,
60.6, 110.6, 117.3, 123.0, 124.3, 127.3, 128.1, 129.1,
134.1, 136.7, 138.1, 150.7, 157.9, 166.3 and 183.4 p~m.
E~AMPL~ 37
1-(5-(N-Ethyl-N-~henylmethylamino)pentyl)-5-phenyl-lH-
indole-2,3-dione
13C Nmr (CDCl3) 11.8, 24.7, 26.8, 27.2, 40.3, 47.5, 52.9,
58.2, 110.4, 117.5, 123.7, 126.5, 126.6, 127.8, 128.0,
128.7, 129.0, 136.6, 137.1, 139.2, 140.5, 150.1, 158.2
and 183.6 ~m.
W094/29272 2 ~ 9 PCT/SE94/00448
27
Fumarate
Found: C, 68.2; H, 6-2; N, 4-9- C28H30N22 C4H4 2 2
require~ C, 68.6; H, 6.5; N, 5.0%
E~AMPLB 38
5-Bromo-1-(5-N-ethyl-N-~henYlmethylamino)Dentyl)-lH-
indole-2,3-dione
13C Nmr (CDC13) 11.7, 24.6, 26.8, 26.9, 40.3, 47.4, 52.8,
58.2, 111.8, 116.2, 118.8, 126.6, 127.8, 128.0, 128.6,
140.1, 140.3, 149.7, 157.3 and 182.3 ppm.
EXA~PL~ 39
5'-(1-Pi~eridinyl)-~iro-l1,3-dioxolane-2,3'-[3Hl-indol~-
2'(1'H)-one
5-Amino-Rpiro [1,3-dioxolane-2,3'-[3H]-indol~-2'(1'H)-one
(2.04g) and Rodium borohydride (1.5g) in dimethoxyethane
(20ml) were cooled and glutaric dialdehyde (25% ~olution
in water, 6ml) in a mixture of dimethoxyethane (30ml),
methanol (20ml) and 3M ~ulphuric acid (15ml) waR added
~uch that the temperature was maintA;ne~ in the range -5
to 0C. More ~odium borohydride (1.5g) waR then added,
maintA;n;ng the Rame temperature range. The mixture wa~
allowed to warm to room temperature and after 2 hour~ wa~
neutrali~ed and then extracted with dichloromethane.
Fla~h chromatography of the material thuR obtA;ne~ ga~e
the title compound.
m/z 275 (M I Hl)
3C Nmr (CDC13) 24.1, 25.9, 51.9, 65.7, 102.7, 110.9,
115.0, 120.3, 124.9, 134.3, 149.5 and 175.5 ppm.
E~A~PL~ 40
1'-(5-Bromopentyl)-5'-(1-~i~eridinyl)-~iro[1,3-
aioxolane-2,3'-[3H]-indol]-2'(1'H)-one
The product from Bxample 39 waR treated by the general
method of Example 7 to give the title compound.
13C Nmr (CDC13) 23.9, 25.1, 25.7, 26.2, 31.9, 33.0, 39.1,
W094/29272 ~ PCT/SE94/00~8
28
51.5, 65.5, 102.3, 108.9, 114.8, 119.4, 124.6, 136.1,
149.2 and 172.9 ~m.
EXAXPL~ 41
1'-(5-(N-Bthyl-N-Dhenylmethylamino)~entyl)-5'-(1-
Di~eridiny~ Diro[l~3-dioxolane-2~3~-~3H]-indol]
2'(1'H)-one
The ~roduct from Exam~le 40 wa~ treated by the general
method of Exam~le lS to give the title compound.
13C Nmr (CDC13) 11.6, 23.9, 24.4, 25.8, 26.5, 26.9, 39.4,
47.1, 51.6, 52.8, 57.9, 65.4, 102.3, 108.9, 114.8, 119.4,
124.5, 126.3, 127.8, 128.5, 136.4, 140.0, 149.1 and 172.9
~m.
E$A~PLæ 42
1-(5-(N-EthYl-N-~henylmethylamino)~entYl)-5-(1-
~i~eridinYl)-lH-indole-2,3-dione
The ~roduct from Exam~le 41 wa~ treated by the general
method of Exam~le 19 to give the title com~ound.
13C Nmr (CDC13) 11.7, 23.9, 24.7, 25.7, 26.8, 27.2, 40.1,
47.4, 51.2, 52.9, 58.2, 110.6, 113.6, 118.1, 126.3,
126.6, 128.1, 128.7, 140.1, 143.5, 149.3, 158.3 and 183.8
~m.
~MPL~ 43
5~-Iodo-~iro[l~3-dioxane-2~3~-[3H]-indol]-2~ H)-one
5-Iodo-lH-indole-2,3-dione and ~ropane-1,3-diol were
treated according to the general method of Example 6 to
give the title com~ound.
3C Nmr (CDC13) 25.2, 61.2, 85.5, 93.4, 112.1, 129.8,
133.3, 139.6, 139.7 and 173.3 p~m.
WO 94/29272 ~ 1 6 9 I 1 9 PCT/SE94/00~8
29
RY~ 3 44
1~-(5-BromoDentyl)-5~-iodo-s~irorl,3-dioxane-2,3'-[3H]-
indoll-2'(1'H)-one
The ~roduct from Exam~le 43 and 1,5-dibromopentane were
treated according to the general method of Exam~le 7 to
give the title com~ound.
13C Nmr (CDCl3) 25.1, 25.2, 26.2, 32.0, 33.2, 39.0, 61.2,
85.3, 93.0, 110.4, 129.3, 133.0, 139.4, 141.9 and 170.8
~m.
~PLE 45
1'-(5-(N-Ethyl-N-~henylmethylamino)~entyl)-5'-iodo-
s~iro[1,3-dioxane-2,3~-[3H]-indol]-2'(1'H)-one
The product from Exam~le 44 was treated according to the
general method of Exam~le 15 to gi~e the title com~ound.
13C Nmr (CDCl3) 11.7, 24.4, 25.1, 26.5, 26.8, 39.1, 47.2,
52.7, 58.0, 60.9, 85.0, 92.9, 110.3, 126.4, 127.9, 128.5,
129.3, 132.8, 139.2, 140.0, 142.0 and 170.6 ~pm.
~SA~PL~ 46
1'-(5-Bromo~entyl)-5'-nitro-spiro[1,3-dioxolane-2,3'-
13H]-indol]-2~ H)-one
5'-Nitro-s~iro~1,3-dioxolane-2,3'-[3H]-indol]-2'(1'H)-one
and 1,5-dibromopentane were treated according to the
general method of Exam~le 7 to give the title com~ound.
13C Nmr (CDC13) 25.1, 26.2, 31.9, 33.0, 39.7, 66.0,
100.7, 108.5, 120.9, 125.2, 128.2, 143.6, 149.4 and 173.2
~m.
E~A~P~e 47
1'-(5-(N-Ethyl-N-Dhenylmethylamino)pentYl)-5'-nitro-
spiro[1,3-dioxolane-2,3'-r3H]-indol]-2'(1'H)-one
The ~roduct from Example 46 was treated according to the
general method of Exam~le 15 to give the title com~ound.
W094/29272 PCT/SE94/004
13C Nmr (CDC13) 11.6, 24.3, 26.5, 26.8, 39.9, 47.2, 52.6,
58.0, 65.9, 100.7, 108.4, 120.7, 125.1, 126.4, 127.8,
128.1, 128.5, 140.0, 143.4, 149.6 and 173.1 Dpm.
R~MPL~ 48
5~-Amino-1'-(5-(N-ethyl-N-~henYlmethylamino)~entyl)-
~iro[1,3-dioxolane-2,3'-13H]-indol~-2'(1~H)-one
The ~roduct from ~xam~le 47 and 10% palladium on
acti~ated carbon in ethanol were ~hAken under an
atmo~here of hydrogen at room tem~erature overnight.
The catalyst was remo~ed by filtration and the filtrate
wa~ evaDorated to dryness. The residue thus obtained was
~urified by fla~h chromatography to yield the title
com~ound.
13C Nmr (CDC13) 11.5, 24.5, 26.5, 27.0, 39.5, 47.1, 52.8,
57.9, 65.6, 102.3, 109.4, 112.6, 116.9, 125.1, 126.5,
127.9, 128.6, 135.5, 139.8, 142.7 and 172.7 ppm.
BXA~PLE 49
5~-Acetamido-1'-(5-(N-ethyl-N-~henylmethylamino)~entyl)-
~iro[1,3-dioxolane-2,3'-13H]-indol]-2'(1'H)-one
The ~roduct from Exam~le 48 (4.9g), acetyl chloride
(1.9g) and triethylamine (4.8g) in dichloromethane were
stirred o~ernight at room temperature. The reaction
mixture was washed with ~odium hydrogen carbonate
solution, dried and eva~orated to dryness. The residue
was purified by flash chromatogra~hy on silica gel to
give the title com~ound.
M.~. 137-139C.
13C Nmr (d6-DMSO) 11.5, 23.7, 23.9, 26.0, 26.4, 38.9,
46.5, 52.2, 57.4, 65.4, 101.3, 109.3, 116.3, 122.0,
124.0, 126.3, 127.8, 128.3, 134.8, 138.8, 139.9, 167.9
and 172.3 ~m.
W094/29272 ~ PCT/SE94/00448
~2AMPLE 50
5-Cyclohexyl-1,3-dihydro-1-(4-(N-ethyl-N-~henYlmethyl-
amino)butyl)-2H-indol-2-one
The product from Exam~le 32 (2.4g), 1,2-eth~ne~thiol
(0.6ml) and ~-toluene~ul~honic acid (2.2g) were ~tirred
overnight at room temperature in glacial acetic acid.
The mixture wa~ evaporated to dryne~ and the reRidue wa~
further ~roce~ed a~ in Exam~le 1 to give the
intermediate dithioacetal and thence the title compound.
13C Nmr (CDC13) 11.7, 24.5, 25.1, 26.0, 27.0, 34.8, 35.8,
40.0, 44.3, 47.2, 52.7, 58.0, 108.1, 123.1, 124.7, 125.9,
126.7, 128.1, 128.8, 140.0, 142.2, 142.6 and 175.0 p~m.
E~A~PL~ 51
1,3-Dihydro-1-(5-(N-ethyl-N-~henylmethYlamino)~entyl)-5-
(l-methylethyl)-2H-indol-2-one
~sing the general method of Exam~le 50, the ~roduct of
Example 33 wa~ converted into the title compound.
3C Nmr (CDC13) 11.6, 24.1, 24.7, 26.7, 27.3, 33.7, 35.8,
39.9, 47.2, 52.9, 58.0, 107.9, 122.5, 124.6, 125.3,
126.5, 127.9, 128.6, 140.0, 142.5, 142.8 and 174.8 ppm.
~A~PLE 52
1,3-Dihydro-1-(5-(N-ethylamino)~entyl)-5-phenyl-2H-indol-
2-one
~ing the general method of Example 50 but u~ing tert-
butanol rather than ethanol a~ the ~olvent for the ~econd
~tep, the ~roduct of Exam~le 37 gave the title com~ound.
M.~. 214-215C.
13C Nmr (d6-DMSO) 10.8, 23.2, 25.0, 26.4, 35.1, 41.6,
45.9, 108.6, 122.7, 125.4, 125.9, 126.1, 126.6, 128.7,
133.9, 140.1, 143.7 and 174.2 p~m.
W094/29272 ; PCT/SE94/00~8
32
E~AMP~ 53
1,3-Dihydro-1-(5-(N-ethyl-N-~henylmethylA~no)~entyl)-5-
~henyl-2H-$ndol-2-one
The product from Exam~le 52 (500mg), benzyl bromide
(300mg) and anhydrou~ ~ota~ium carbonate (660mg) were
~tirred in dry dimethylformamide at room tem~erature to
give the title com~ound.
3C Nmr (CDC13) 11.8, 24.8, 27.1, 27.4, 35.8, 40.1, 47.4,
53.0, 58.2, 108.4, 123.4, 125.2, 126.5, 126.6, 126.8,
128.0, 128.6, 128.7, 135.6, 140.1, 140.9, 144.1 and 174.8
~m.
RY~LE 54
1,3-Dihydro-1-(5-(N-ethyl-N-~henylmethylamino)Dentyl)-5-
methoxy-2H-indol-2-one
~ing the general method of Exam~le 50, the ~roduct of
Exam~le 34 wa~ converted into the title com~ound.
13C Nmr (CDC13) 11.7, 24.7, 26.7, 27.3, 36.1, 40.0, 47.3,
53.0, 55.8, 58.1, 108.4, 111.9, 112.1, 125.9, 126.6,
128.0, 128.7, 138.2, 140.1, 155.6 and 174.4 ~m.
P~ARMACY ~PLES
The following example~ illustrate uitable ~harmaceutical
com~o~ition~ to be used in the method of the invention.
Com~o~ition 1 - Tablet~
Com~ound of ExamDle 14 2g
Lacto~e 98g
Microcry~talline celluloRe 90g
Polyvinyl~yrrolidone 8g
MAg~e~ium atearate 2g
The compound of Example 14, lacto~e, cellulo~e and
~oly~inyl~yrrolidone are ~ieved and blended. The
magne~ium ~tearate i~ ~ie~ed and then blended into the
abo~e mixture. Com~re~ion uaing ~uitable ~unche~ then
W094/29272 2 ~ PCT/SE94/004~
yields 1000 tablet~ each cont~n;ng 2mg of the active
ingredient. If de~ired, the obtained tablet~ can then be
film coated.
Com~oqition 2 - Tablet~
Co~vo~nd of Example 42 20g
Lacto~e 90g
Microcryqtalline cellulo~e30g
Potato ~tarch 50g
Polyvinylpyrrolidone 8g
Magne~ium ~tearate 2g
The com~ound of Exam~le 42, lacto~e, cellulo~e and part
of the ~tarch are mixed and granulated with 10% ~tarch
pa~te. The re~ulting mixture iq dried and blended with
the remaining Rtarch, the ~olyvinylpyrrolidone and the
~ieved magne~ium ~tearate. The re~ulting blend i~ then
compre~ed to give 1000 tablet~ each containing 20mg of
the active ingredient.
Com~o~ition 3 - Cap~ule~
Com~ound of Example 53 lOg
Pregelatiniqed ~tarch 188g
Magne~ium ~tearate 2g
The compound of Example 53 and the ~tarch are ~ieved,
blended together and then lubricated with the ~ieved
magne~ium ~tearate. The blend i~ u~ed to fill 1000 hard
gelatine cap~uleQ of a ~uitable ~ize. Each cap~ule
eontain~ lOmg of the aetive ingredient.