Note: Descriptions are shown in the official language in which they were submitted.
WO95tO1184 216 5 2 9 4 PCTtUS93/06170
AN IODINATED NEUROPROBE FOR MAPPING
MONOAMINE REUPTAKE SITES
FIELD OF I~v~NllON
This invention relates to neuroprobes for mapping
monoamine reuptake sites in the brain, and particularly to
a neuroprobe that can also serve as a radiotracer for use in
single-photon emission computed tomography (SPECT) and
positron emission tomography (PET) for imaging of such
reuptake sites.
BACKGROUND OF THE INVENTION
A brain consists of a plurality of neurons that interact
by exchanging chemical messengers. Each neuron generates
neurochemicals, referred to as neurotransmitters;
neurotransmitters act at sites on the cellular membrane of
a neuron, the sites being referred to as receptors.
Receptors are associated with either ion channels through the
cellular membrane or secondary neurochemical messenger
systems. By contrast, reuptake sites are molecular complexes
which transport chemicals across the cellular membrane of a
neuron. When a neurotransmitter has served its function, it
is removed from the vicinity of the receptor by being bound
to a reuptake site which transports the neurotransmitter to
the interior of the neuron.
Just as there are many specialized neurons in the brain,
there are also a variety of neurotransmitters, associated
receptors, and reuptake sites. The distribution of
specialized neurons depends upon the particular organism
under study, and the state of health of that organism.
A neuron can be classified according to the type of
neurotransmitter that it uses to communicate with other
neurons. Certain types of neurons can be found predominantly
in particular regions of the brain. For example, the
striatal region of a mammalian brain is innervated by neurons
using dopamine as a neurotransmitter. The striatum also
WO95/01184 PCT~S93/06170
2l 6~2g4
contains a large number of non-dopaminergic neurons that have
dopamine receptors. Certain compounds, such as cocaine, have
a preferential affinity for dopamine reuptake sites, and
therefore tend to bind to such reuptake sites. The effect
of a molecule such as cocaine upon a dopamine reuptake site
is to inhibit reuptake of the neurotransmitter dopamine,
leaving more dopamine available in the vicinity of the
dopamine receptors.
In certain neurological diseases, such as Parkinson's
disease, distinct groups of neurons lose their normal
physiological functioning. Consequently, the abnormal
neurons may behave differently in the presence of some
neurotransmitters, and may also produce neurotransmitters in
a manner that differs from a healthy neuron.
The major neurotransmitters, dopamine, norepinephrine,
and serotonin, are referred to collectively as the monoamine
neurotransmitters. Many neurons have receptors adapted to
receive at least one of these neurotransmitters. Parkinson's
disease is caused by the degeneration of some of the
dopaminergic neurons in the brain. The neurons lost in
Parkinson's disease have a large number of dopamine reuptake
sites; cocaine and chemical analogs of cocaine have an
affinity for such reuptake sites.
A radioisotope is commonly incorporated in molecules
that have a demonstrated binding affinity for a particular
type of neuroreceptor, and such molecules are commonly used
as neuroprobes. The localization of neuroprobes can be used
to find specialized neurons within particular regions of the
brain. It is also known that a neurological disease can be
detected by observing abnormal binding distributions of a
neuroprobe. Such abnormal binding distributions can be
observed by incorporating a radionuclide within each molecule
of the neuroprobe with a high binding affinity for the
particular reuptake sites of interest. Then, an imaging
technique can be used to obtain a representation of the in
vivo spatial distribution of the reuptake sites of interest.
~ WO95/01184 PCT~S93/06170
2165291
In single photon emission computed tomography (SPECT)
imaging, the most commonly used radionuclides are heavy
metals, such as ~mTc. Heavy metals are very difficult to
incorporate into the molecular structure of neuroprobes
because such probes are relatively small molecules (molecular
weight less than 400).
In positron emission tomography (PET), the radiohalide
F (fluorine) is commonly used as a substitute for H
(hydrogen) in radiopharmaceuticals because it is similar in
size. Not all halogens will work, however. For example, I
(iodine) is much larger than both H and F, being
approximately half the size of a benzene ring. However, due
to the small size of typical radiopharmaceuticals for use as
neuroprobes, the presence of iodine markedly changes the size
of the compound, thereby altering or destroying its
biological activity.
In addition, the presence of iodine in a neuroprobe
tends to increase its lipophilicity, and therefore increases
the tendency of the neuroprobe to engage in non-specific
binding. For example, paroxetine is a drug with high
affinity and selectivity for serotonin reuptake sites, and
[3H]paroxetine has been shown in rodents to be a useful in
vivo label (Scheffel, U. and Hartig, PR. J. Neurochem., 52:
1605-1612, 1989). However, several iodinated analogs of this
compound with iodine attached at several different positions
had unacceptably low affinity, in fact being one tenth of the
affinity of the parent compound. Furthermore, when the
iodinated compound was used as an in vivo radiolabled
neuroprobe, non-specific binding activity was found to be so
high that no measurable portion of the brain uptake appeared
to be specifically bound to the serotonin reuptake site.
Thus, the iodinated form of paroxetine is not useful as an
in vivo probe.
The addition of iodine to a neuroprobe can unfavorably
alter its biological properties. For example, tomoxetine has
high affinity and selectivity for norepinephrine reuptake
WO95/01184 PCT~S93/06170
216~
sites. However, when tomoxetine is iodinated, e.g. to form
R-4-iodotomoxetine, the resulting labeled compound has low
affinity for such reuptake sites, and relatively high
affinity for serotonin reuptake sites. In vivo labeling
studies have shown that it is an unacceptably poor probe even
for the serotonin reuptake sites because it exhibits low
total brain uptake and immeasurably low specific uptake.
An iodinated compound can be useful as an in vitro
probe, but may be useless as an in vivo probe, because an in
vivo probe must meet the requirements associated with
intravenous administration of the probe to a living subject.
Reasons for the loss of in vivo utility include the fact that
the compound may be metabolized too quickly, that it may not
cross the blood-brain-barrier, and that it may have high non-
specific uptake into the lipid stores of the brain. In vitro
homogenate binding studies remove these obstacles by
isolating the brain tissue from hepatic metabolic enzymes,
by homogenizing the brain tissue so as to destroy the blood-
brain-barrier, and by diluting the brain tissue so as to
decrease the concentration of lipids in the assay tube.
Accordingly, it cannot be assumed that a probe will be useful
in both in vivo and in vitro modalities.
An in vivo SPECT probe was developed by iodinating
cocaine. However, this probe shows a binding affinity and
specificity no better than cocaine itself, which is
inadequate for purposes of SPECT imaging.
SUMMARY OF THE INVENTION
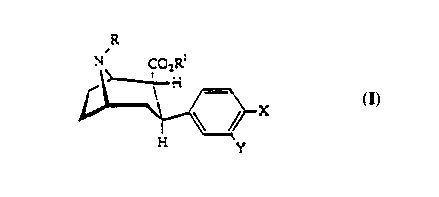
An iodinated neuroprobe is provided for mapping
monoamine reuptake sites. The iodinated neuroprobe is of the
formula:
N~ CO2R
X
WO95/01184 PCT~S93/06170
~16529~
wherein R can be a CnH2n+~ group, where n=0-6, an alkenyl
group, a monofluoroalkyl group including DF where n=18 or 19,
or a 'nCnH2n+l group where n=1-6 and where m=11 or 14 for at
least one mc. Also R' can be a CnH2n+l group where n=0-6, a p-
iodophenylmethyl group, a p-iodophenylethyl group, a
phenylmethyl group, or a phenylethyl group. X can be an
isotope of F, an isotope of Cl, an isotope of Br, an isotope
of I, CH3, or Sn(R"~R"2R"3). R"~ can be a CnH2n+l group where
n=1-6, or an aryl group. R"2 can be a C8H2n+~ group where n=1-
6, or an aryl group. R"3 can be a CnH2n+~ group where n=1-6,
or an aryl group. Y can be H only if X is an isotope of I,
or R' is a p-iodophenylmethyl group, or R~ is a
p-iodophenylethyl group. Otherwise Y must be an isotope
of I. Also provided is a diastereomer of this embodiment
wherein the carboxyl-R' group is in the alpha position.
In a further embodiment, the iodinated neuroprobe for
mapping monoamine reuptake sites of the invention is of the
formula: R
~j~ C~R
~w ~
wherein R can be a CnH2n+l group where n=0-6, an alkenyl group,
a monofluoroalkyl group including nF where n=18 or 19, or a
'nCDH2n+~ group where n=1-6 and where m=11 or 14 for at least
one mC. R' can be a CnH2~+l group where n=0-6, a p-
iodophenylmethyl group, a p-iodophenylethyl group, a
phenylmethyl group, or a phenylethyl group. X can be an
isotope of F, an isotope of Cl, an isotope of Br, an isotope
of I, CH3, or Sn(R''IR''2R''3). R"~ can be a CDH2D+I group where
n=1-6, or an aryl group. R"2 can be a CnH2n+l group where n=1-
6, or an aryl group. R"3 can be a CnH2D+I group where n=1-6,
or an aryl group. Y can be H only if X is an isotope of I,
or R' is a p-iodophenylmethyl group, or R' is a
p-iodophenylethyl group. Otherwise, Y must be an isotope
WO95/011~ PCT~S93/06170
2165294
of I. Further, W can be O, S, (CH2)n, O(CH2)n where n=1-6,
wherein X resides on a benzene ring of the formula at an
ortho, meta, or para position with respect to W, and Y
resides at any remaining position on the benzene ring. Also
provided is a further embodiment which is a diastereomer of
this embodiment wherein the carboxyl-R' group is in the alpha
position.
For each of the foregoing embodiments there is provided
a precursor of the radiolabled neuroprobe that lacks a
radiotracer atom, and a kit for preparing an associated
iodinated neuroprobe.
Both the radiostable and radioactive variants of the
iodinated neuroprobe of the invention are useful for human
and non-human research. For example, in vivo and in vitro
experiments can be performed using the compounds of the
invention to study dopamine reuptake sites generally, and
cocaine binding sites in particular.
DESCRIPTION OF THE DRAWING
The invention will be more fully understood from the
following detailed description, in conjunction with the
accompanying figures in which:
Fig. 1 shows prior art compounds compared to compounds
of the invention;
Fig. 2 shows regional activity in a baboon brain
following injection of a compound of the invention;
Fig. 3 shows a synthesis route for a compound of the
nvention;
Fig. 4 shows regional areas of brain uptake of a
compound of the invention;
Fig. 5A shows regional activity in a baboon brain
following injection of compound of the invention; and
Fig. 5B shows regional activity in a baboon brain
following injection of a compound of the invention.
WO95/01184 ~1 6 5 2 9 4 PCT~S93/06170
DETAILED DESCRIPTION OF THE INVENTION
Metabolically stable cocaine analogs such as
2~-carbomethoxy-3~-(4-iodophenyl)-tropane), an iodine-
containing analog of ~-CIT (also designated RTI-55), as shown
in Fig. 1, compound 3, have high affinities for dopamine and
serotonin reuptake sites in brain. As will be discussed
below, [l23I]-~-CIT is shown to be a SPECT (single photon
emission computed tomography) radiotracer for dopamine and
serotonin reuptake sites.
[l23I]-~-CIT was prepared by reaction of the
corresponding tributyltin precursor with no-carrier added
Na[l23I] in the presence of peracetic acid, followed by
preparative HPLC on a C-18 column with
methanol/water/triethylamine (75/25/0.2) at a flow rate of
1.0 ml/min. The final product was formulated in 6 ml sterile
saline containing 5-10% ethanol.
Six SPECT experiments were performed in four female
baboons (10 kg Papio anubis) under isoflurane anesthesia.
The animals were injected with 10.6 + 1.4 mCi[l23I]-~-CIT and
scanned for 333 + 25 min in either the 810X Brain Imager
(Strichman Medical Equipment; five experiments) or the ASPECT
device (Digital Sintigraphics, Cambridge, MA; one
experiment), with these and subsequent data expressed as
means + S.E.M. Serial 2-6 min images were reconstructed
assuming uniform attenuation equal to that of water in an
ellipse drawn around the brain. Data were decay-corrected
to the time of injection.
The highest activities were found in the striatal region
and reached peak levels at 179+9 min (n=6) post injection
(p.i.)(Fig. 2). Striatal activity was monitored in two
animals for an additional 190 and 260 min post peak values.
In one animal, striatal activity was virtually unchanged for
the remaining 190 min of the experiment. With reference to
Fig. 2, in the second animal, washout of striatal activity
wo 95/oll~ 215 5 2 9 4 PCT~S93/06170
was fit to an exponential function and had Tl~2 = 27 h (r =
0.92).
The brain region which approximately overlay the
mesencephalon or midbrain area had the second highest levels
of activity. Midbrain values peaked earlier (45+16 min p.i.;
n=6) and washed out more rapidly (Tl~2 = 294+59 min;
r=0.98+0.01; n=3) than that in the striatum.
At the time of peak striatal uptake, the ratios of
regional brain activities were: striatum (100%); hypothalamus
(38.1+5.2%); occipital lobe (13.5+0.8%); temporo-parietal
lobes (14.3+2.0%); frontal lobe (10.3+1.0%); and cerebellum
(10.0+1.5%), all measured with n=6.
(-)Cocaine (Fig. 1, compound l) and CFT (Fig. l,
compound 2), both potent dopamine and serotonin reuptake
inhibitors, induced rapid and dose-dependant displacement of
both striatal and midbrain activity. (-)Cocaine
(2.9 ~mol/kg) administered at 200 min p.i. caused
displacement of 17% of striatal and 49% of midbrain levels
within 30-65 min. At 14.7 ~mol/kg administered at 230 min
p.i., the corresponding cumulative displacements were 62% and
77%, respectively, within the same period of time.
CFT (0.4 ~mol/kg) administered i.v. at 180 min p.i.
caused displacement of 57% of striatal and 72% of midbrain
levels within 60-120 min. At 2.0 ~mol/kg administered at 298
min p.i., the corresponding cumulative displacements were 83%
and 91%, respectively, within the same period of time.
In contrast, citalopram (a selective serotonin reuptake
inhibitor) caused greater displacement of midbrain than
striatal activity. At a dose of 8.3 ~mol/kg i.v. at 190 min
p.i., midbrain levels decreased by 57~ during the following
110 min, compared to only 5% decrease in striatal activity
during the same period.
[l23I]-~-CIT appears to be a useful SPECT tracer of the
dopamine and serotonin reuptake sites. Brain uptake and
washout are relatively slow in comparison to cocaine itself
and are consistent with the metabolically resistant chemical
WO95/011~ ~16 ~ 2 9 1 PCT~S93/06170
structure of ~-CIT and the location of the radioiodine in a
chemically stable position. Striatal uptake appears to
largely represent labeling of the dopamine reuptake site,
whereas that in the midbrain is largely associated with the
serotonin reuptake site. The high ratios of striatal to
cerebellar activity of [l23I]-~-CIT are consistent with low
non-specific uptake of the tracer, and suggest that tl23I~
CIT may be a useful clinical marker of dopaminergic
deficiencies in Parkinson's disease.
Referring again to Fig. 1, in a second study (Neumeyer,
J.L. et al., J. Med. Chem., 34: 3144-3146, 1991), the potent
cocaine analog 2~-carbomethoxy-3~-(4-fluorophenyl)tropane
(compound 2) (also referred to as CFT or WIN 35,428 (Clarke,
R.L., et al., 1973; Madras, B.K. et al., 1989)) when
tritiated or labeled with IlCH3 was found to be superior to
[3H]cocaine or [IlC]cocaine (Fowler, J.S. et al., Synapse 4:
371-377, 1989) as a radioligand probe for cocaine receptors
in terms of higher affinity and larger residence time on the
dopamine reuptake site. For further development of analogues
suitable for PET and SPECT imaging, 2~-carbomethoxy-3~-(4-
iodophenyl)tropane were synthesized and characterized
(compound 3a; designated as ~-CIT in analogy to CFT, its
corresponding, N-demethylated derivative (compound 4;
designated as nor-CIT), and the C2~ isomer (compound 3b), as
shown in Fig. 1.
Referring to Fig. 3, a synthesis protocol for [l23I]-~-
CIT is described. Ecgonidine methyl ester (compound 5) was
prepared from cocaine by the procedure of Clarke et al.
(1973.) Treatment of compound 5 with phenylmagnesium bromide
and subsequent workup with trifluoroacetic acid at low
temperature gave a mixture of C2 epimers (compound 6) (45%)
and (compound 7) (31%), which were separated by flash
chromatography (silica; CH2Cl2/CH3OH, 25:1). Direct
iodination of compound 6 with I2/HNO3/H2SO4 gave the para-
substituted compound 3a (~-CIT) as an oil; 62%; [~]25D-2.0
(c= 0.85, CHC13). D-Tartrate salt; mp 72-74C; [~]25D -87.7
WO 95/01184 PCT/US93/06170 -- -
216529~
-- 10 --
(c= 1.5, CH30H). Iodination of compound 7 by the same
procedure gave compound 3b (a~-CIT) as an oil; 39% []25D +44
(c= 2.5, CHCl3). 1,5-naphthalenedisulfonate salt; mp 139-140
C. N-Demethylation of compound 6 was accomplished by
conversion to its 2,2,2,-trichloroethyl carbamate followed
by reduction (Zn/acetic acid) to yield compound 8 by the
procedure previously described by Milius, R.A., et al., J.
Med. Chem. Vol. 34, No. 5, 1728-1731, 1991, herein
incorporated by reference, followed by iodination to yield
nor-CIT (compound 4), which was isolated as a yellow
crystalline solid (free base 48% from compound 6): mp 149-151
C; [cr]25D -67.4 (c = 1, CHCl3).
[l23I]-,~-CIT (comp~und ~23I-3a) was synthesized from
nonradioactive ~-CIT (compound 3a) by conversion to the
corresponding tributyltin derivative (compound 9). Treatment
of compound 3a with bis (tributyltin),
tetrakis(triphenylphosphate)palladium(0), and palladium(II)
acetate in refluxing tetrahydrofuran gave compound 9 as a
colorless waxy solid after flash chromatography (silica,
stepwise gradient, hexane to hexane/ether, 75:25) in 26%
yield from 3a. The 300-MH3 NMR (CDCl3) of compound 9 was
consistent with the assigned structure. Reaction of compound
9 with no-carrier-added Nal23I in the presence of peracetic
acid gave compound [l23I]-3a. The radioiodinated product
compound [~23I]-3a was purified by preparative HPLC (Novapak
C~8, MeOH/H2O/Et3N, 75:25:0.2, 1.0 mL/min; tR 6.7 min) and
formulated in normal saline containing 5% ethanol an 1%
ascorbic acid. Compound [l23I]-3a was obtained in average
overall yield of 60.0 +13.4% and with radiochemical purity
of 97.6+1.69~. The tributyltin precursor used in
radiolabeling contained about 7 mol% CIT carrier, resulting
in an l23I product having a specific activity of about 2000
ci/mmol.
The affinities of cocaine (compound 1), ~-CIT (compound
3b), ,~-CIT (compound 3a), and ,B-CFT (compound 2) for the
dopamine and serotonin reuptake sites were determined from
WO 95/01184 21 6 5 2 9 4 PCT/US93/06170
radioligand displacement studies using tissue homogenates
prepared from baboon and rat brain, shown in Table 1 below.
Table I. In Vitro ~ Binding Data for Cocaine and 3-(4-Hsl~Fh~nyl) Analogues
r~ of [3HlCFT .1~ of pH]paroxetine
analogue IC5D(nM) Hill slope (nH) IC50(oM) Hill slope (nH)
S 1 (cocune) 221 i 14 0.69 i 0.06 (3) 207 i 66 0.73 i 0.12 ( 5)
2 (B-C~-l) 15.3 i 1.2 0.75 i 0.01 (3) 479 i 59 1.34 i 0.22 (3)
3b (cY-CIT) 87.6 i 2.9 0.70 i 0.07 (2) 210 i 86 0.73 i 0.04
3a ((~-CrI-) 1.6 i 0.15 0.79 i 0.04 (3) 3.78 i 0.53 0.82 i 0.08 (6)
TABLE 1
The data in Table 1 represent radioligand binding of
[3H]CFT (0.5 nM) to dopamine reuptake sites in tissue
homogenates prepared from primate striatum and binding of
[3H]paroxetine to serotonin reuptake sites in homogenates
prepared from rat cortical membranes. The IC50 value is the
concentration of displacing analogue required to decrease
specific radioligand binding by 50%. Values represent means
+SEM (of n experiments).
With reference to Fig. 4, five SPECT (single photon
emission computer tomography) experiments were performed with
four female baboons (Papio anubis, 10-12 kg) under isoflurane
anesthesia. Animals were injected i.v. with 8.1+1.4 mCi
[l23I]-~-CIT (with these and subsequent data expressed as mean
+ SEM) and scanned for 300+41 min with the 810X Brain Imager
(Strichman Medical Equipment, Medfield, MA). Serial 1-2 min
images were reconstructed assuming uniform attenuation equal
to that of water in an ellipse drawn around the brain. Data
were decay corrected to time of injection.
Highest brain uptake overlay the striatal region and
peaked at 154 + 19 min postinjection (pi) of the radioligand
and showed striatal to cerebellar ratios at that time of 9.8
+ 1.6. Washout of striatal activity was followed for an
additional 200 and 260 min in two of three control animals
and showed 0% and 12 % decreases, respectively, from time of
striatal peak to end of the experiment.
WO95/011~ PCT~S93/06170
2165294 12 -
With reference to Figs. 5A and 5B, the brain area with
second highest activities approximately overlays the midbrain
and showed peak levels at 43+5 min pi (n=5) and had a faster
washout than striatal activity.
The pharmacological specificity of the in vivo labeling
of [l23I]-~-CIT was examined with displacement of brain
activity by indatraline (also designated Lu 19-005), a potent
agent for the dopamine and serotonin reuptake sites, and
citalopram, an agent selective for the serotonin reuptake
site. Indatraline (3 ~mol/kg iv) injected at 200 min pi
radioligand caused significant decrease of both striatal and
midbrain activity, as shown in Fig. 5A. During the loo min
period after injection of Lu 19-005, striatal activity
decreased by 65% compared to a mean decrease of 2% during the
same period in the two control animals followed for that
length of time. In contrast, citalopram (7.4 ~mol/kg iv)
injected 60 min pi radioligand showed a selective decrease
of midbrain activity, as shown in Fig. 5B. Citalopram caused
a 48% decrease of midbrain activity during the 60-min period
after injection, in comparison to 16 ~ 3% decrease (n=3) of
midbrain activity in control animals followed during this
same period.
These results showed that [l23I]-~-CIT was a useful SPECT
probe of monoamine reuptake sites in primates. The majority
of striatal activity was associated with dopamine reuptake
sites, and the majority of midbrain activity was associated
with serotonin reuptake sites, which is consistent with the
densities of these monoamine transporters measured in
postmortem primate brains. Brain washout of activity was
relatively slow, in part because of the high affinities of
~-CIT for the monoamine transporters. In addition, the
iodine atom appears to be in a relatively metabolically
resistant position, since whole body scanning showed low
thyroid uptake, which is indicative of a slow in vivo rate
of deiodination. [l23I]-~-CIT and [IlC]-~-CIT may be useful
clinical markers of dopaminergic and serotonergic innervation
WO95/01184 PCT~S93/06170
2 1 6 ~ 2 r9 4
- 13 -
in human disorders such as Parkinson's disease and
depression, which are thought to have abnormalities in these
neuro-transmitter systems.
EXAMPLES OF SYNl~SES
Example 1. 2-beta-Carbomethoxy-3-beta-(4-iodoPhenyl)tro~ane
A mixture of 2-beta-carbomethoxy-3-beta-phenyltropane
(See Example lA below and Milius et al. J. Med. Chem., 1991,
34, 1728) (2.9g, 11.5 mmol) and I2 (3g- 11.8 mmol) in 25 ml
of glacial acetic acid was stirred and treated dropwise with
a mixture of 4.7 mL of concentrated nitric acid and 4.7 mL
of concentrated sulfuric acid. The reaction mixture was
heated to 55OC and stirred for 2 hours, then cooled to room
temperature and poured onto ice (100g) and filtered. The pH
of the filtrate was adjusted to 9.5 by the addition of
concentrated ammonium hydroxide at 0-5C. The resulting
precipitate was removed by filtration and dissolved in
methylene chloride (250ml). The filtrate was extracted with
two 50 mL portions of methylene chloride. The extracts and
solution of precipitate were combined, washed with brine
(50ml) and dried over magnesium sulfate. After the removal
of the solvent, 3.9 g (90.4%) of 2-beta-carbomethoxy-3-beta-
4-iodophenyltropane free base was obtained as an oil.
The free base was dissolved in methanol (20 ml) and
combined with 1.5 g of D-(-)tartaric acid in 20 ml of
methanol. After the removal of methanol under reduced
pressure, the residue was recrystallized from methanol ether
(3:1) to give 2-beta-carbomethoxy-3-beta-(4-
iodophenyl)tropane D-tartrate salt as white crystals, m.p.
72-74C. C~6H2~NO2I.C4H6O6. Calculated: C: 44.88, H: 4.89, N:
2.62. Found: C: 44.70, H: 4.94, N: 2.57. talpha]D22 =
-87.7(c=0.3, CH30H).
Example lA. 2-beta-CarbomethoxY-3-beta-phenyltro~ane
A 2 M ethereal solution of phenylmagnesium bromide (83
mL, 166 mmol) in a 500-mL 3-neck round-bottom flask equipped
with mechanical stirrer, addition funnel, and nitrogen inlet
WO9S/011~ PCT~S93/06170
~165294
- 14 -
tube was diluted with 83 mL of anhydrous diethyl ether and
cooled to -20C under an atmosphere of dry nitrogen. A
solution of anhydroecgonine methyl ester, prepared from
cocaine (1) (15 g, 82.8 mmol) in anhydrous ether (75 mL) was
added dropwise. The heterogeneous mixture was stirred for
1 h at -20C, then poured into an equal volume of ice and
water, and acidified by the dropwise addition of 2 M HCl.
The aqueous layer was made basic by the addition of
concentrated ammonium hydroxide, saturated with NaCl, and
extracted with diethyl ether. The combined extracts were
dried (Na2S04) and concentrated in vacuo to give a brown oil.
Bulb to bulb distillation (70C, 0.9 Torr) of the crude
product gave a pale yellow oil (16 g, 70%). TLC analysis of
the oil (silica, pentane/diethyl ether/2-propylamine,
15:5:0.8) showed it to be a mixture of the C-2 alpha and beta
epimers. The beta isomer was isolated by silica gel
chromatography (pentane: diethyl ether: isopropyl amine,
70:30:3). m.p. 63-66C (lit: 62-64,5C: Clarke et al. J.
Med. Chem. 16: 1260 (1973)).
ExamPle 2. 2-alpha-Carbomethoxy-3-beta-iodoPhenyltropane
The mixture of alpha and beta-2-carbomethoxy-3-beta-
iodophenyltropanes prepared as described in Example 1 were
separated by silica gel chromatography as described in
Example 1. Fractions containing the alpha-2-carbomethoxy-3-
beta-iodophenyltropane were pooled and concentrated in vacuo.
The free base thus obtained was treated with naphthalene-1,5-
disulfonic acid. The crude salt was recrystallized from
acetonitrile to give the 2-alpha-carbomethoxy-3-beta-
iodophenyltropanenaphthalene-1,5-disulfonatesalt,m.p. 166-
1680C. Cl6H20NO2I CloH6(SO3H)2 2H20. Calculated: C:40.01,
H:4:55, N:1.97, I:17.90; Found: C:43.94, H:4.55, N:1.91,
I:17.99.
WO 95/01184 PCT/US93/06170
21652Y~
Example 3 . 2-beta-Carbomethoxy-3-beta- (4-
iodoPhenyl)nortropane.
A solution of 2-beta-carbomethoxy-3-beta-(4-
iodophenyl)tropane (410 mg, 1.5 mmol) in toluene (20 mL) was
treated with of 2,2,2-trichloroethyl chloroformate (1 mL, 7.3
mmol). The mixture was heated at 120C for 1 hour, cooled
to room temperature, and evaporated to dryness in vacuo. The
residue was partitioned between methylene chloride and water.
The organic layer was separated, dried (Na2SO4), and
concentrated in vacuo to give the trichloroethyl
chloroformate as a dry foam. The crude carbamate was
dissolved in 50% aqueous acetic acid, treated with 200 mg
(0.0067 g-atom) of zinc dust, and stirred at room temperature
for 16 hours. The reaction mixture was filtered adjusted to
pH 7 with concentrated ammonium hydroxide, saturated with
NaCl, and extracted with diethyl ether. The extracts were
combined, dried (Na2SO4), and concentrated in vacuo. The
residue was purified by flash chromatography (silica,
pentane/diethyl ether/isopropylamine, 3:7:0.7) to afford 2-
beta-carbomethoxy-3-beta-(4-iodophenyl)nortropane, which was
isolated as a yellow crystalline solid, m.p. 149-151C;
[alpha]25D -67.4 (c = 1, CHCl3).
Example 4. 2-beta-Carbomethoxy-3-beta-(4-iodoPhenyl)-8-
(3-fluoroproPYl)-nortroPane
A solution of 2-beta-carbomethoxy-3-beta-(4-iodophenyl)-
nortropane (371 mg, 1.0 mmol), 1-bromo-3-fluoropropane (155
mg, 1.1 mmol), and triethylamine (0.5 mL) in dry toluene (20
mL) was stirred under an atmosphere of dry nitrogen and
heated to reflux. After four hours, the reaction mixture was
cooled to room temperature and filtered. The filtrate was
concentrated under reduced pressure, and the residue
chromatographed on a silica column (eluant: diethyl ether).
Concentration of product-containing fractions gave 2-beta-
carbomethoxy-3-beta- ( 4 -iodophenyl ) -8- ( 3 -
WO95/011~ PCT~S93/06170
2~5~9~
fluoropropyl)nortropane as a white solid, m.p. 78.5-79.5C
C~8H23N02FI, Calculated: C: 50.13, H:5.34, N: 3.25; Found: C:
50.27, H: 5.26, N:3.15.
Example 5. 2-beta-CarbomethoxY-3-beta-(3-fluoro-4-
iodophenyl)tropane
A mixture of 2-beta-carbomethoxy-3-beta-(3-fluorophenyl)
tropane (400 mg, 1.44 mmol), silver sulfate (400 mg, 1.3
mmol), iodine (600mg, 2.36 mmol) and 80% sulfuric acid (9 Ml)
was stirred for five days at room temperature. The reaction
mixture was poured into 150 mL of ice and water, made basic
by the addition of concentrated ammonium hydroxide, and
extracted with three 60 mL portions of chloroform. The
combined extracts were washed sequentially with solutions of
10% sodium bisulfite, 5% sodium carbonate and water, then
dried over sodium sulfate, and filtered. The filtrate was
concentrated in vacuo and the oily residue was redissolved
in chloroform and treated with a solution of p-toluene
sulfonyl chloride in chloroform. The resulting solid was
repeatedly recrystallized from water and ethanol to give 2-
beta-carbomethoxy-3-beta-(3-fluoro-4-iodophenyl)tropane
tosylate salt as a white crystalline solid, m.p. 68-70C
(soften, 45C), CloHI9FINO2 C7H8S03 H2O: Calculated: C:
46.55,H: 4.93, N: 2.36; Found: C: 46.34, H: 4.86, N:l.99.
ExamPle 6. 2-beta-Carboxy-3-beta-(4-iodophenYl)troPane
A suspension of 2-beta-carbomethoxy-3-beta-(4-
iodophenyl)tropane (100 mg, 0.26 mmol) in 2 mL of H2O was
heated at reflux for 10 hours. The resulting solution was
cooled to room temperature, and the resulting precipitate was
collected by filtration and dried under vacuum overnight to
give 70 mg (70%) of 2-beta-carboxy-3-beta-(4-
iodophenyl)tropane m.p. 299-300C. ClsHI8NO2I . 0.5 H2O:
Calculated C: 47.51, H:5.05, N: 3.69: Found: C: 47.28, H:
4.84, N: 3.69.
WO 95/01184 ~ 1 6 5 ~ 9 4 PCT/US93/06170
ExamPle 7. 2-beta-Carbomethoxy-3-beta-benzYloxYtroPane
A stirred suspension of benzyl bromide (3.0 g, 0.015
mol) and potassium iodide (3.0 g, 0.021 mol) in acetone (20
mL) was treated dropwise with a solution of ecgonine methyl
ester (2.6 g, 0.014 mol) in acetone (10 mL) at room
temperature. The mixture was stirred at room temperature for
70 hours, then heated to reflux and stirred for an additional
8 hours. The reaction mixture was cooled to room temperature
and filtered. The filtrate was concentrated in vacuo, the
residue dissolved in chloroform (200 mL) and extracted with
four 50 mL portions of 2 N hydrochloric acid. The combined
extracts were made basic by the addition of concentrated
ammonium hydroxide. The resulting mixture was extracted with
four 20 mL portions of chloroform. The extracts were dried
over sodium sulfate and concentrated in vacuo to give 1.7 g
of 2-beta-carbomethoxy-3-beta-benzyloxytropane as an oil.
The product was dissolved in acetonitrile (20 mL) and
treated with a solution of naphthalene-1,5-disulfonic acid
(2.2 g) in acetonitrile (20 mL). The solution was
concentrated in vacuo to a syrup, which was diluted with
diethyl ether. The resulting precipitate was collected by
filtration and dried to give 1.6 g of 2-beta-carbomethoxy-3-
beta-benzyloxytropane naphthalene-1,5-disulfonate salt, m.p.
126-130C, Cl7H23N03.CIoH6(SO3H)2.2.5 H2O. Elemental analysis:
Calculated, C: 52.08, H: 5.83, N: 2.25. Found, C: 52.02, H:
5.69, N: 2.72. [alpha]D24= -25.4(c=1, CH30H).
Example 8. 2-beta-CarbomethoxY-3-beta- (4-
tributylstannylphenYl)troPane
A mixture of 2-beta-carbomethoxy-3-beta-(4-
iodophenyl) tropane (250 mg, 0 . 65 mmol),
bis(tributyl)distannane (522 mg, 0.9 mmol),
tetrakis(triphenylphosphine)palladium(0) (3 mg) and anhydrous
toluene (10 mL) was heated to reflux under an atmosphere of
dry nitrogen and stirred for 28 hours. The mixture was
filtered, and the filtrate concentrated in vacuo. The
WO 95/01184 PCT/US93/06170 --
216a29~
-- 18 --
residue was applied to a silica gel column and eluted with
a mixture of hexane: diethyl ether: isopropyl amine
(70:30:3). The fractions containing product were pooled,
concentrated in vacuo and treated with pentane to precipitate
2-beta-carbomethoxy-3-beta-
(4-tributylstannylphenyl)tropane as a solid. The 300 MHz NMR
spectrum was consistent with the assigned structure.
[alpha]D22= -8.9(c=0.4, CHCl3).
Example 9. rl23Il-2-beta-Carbomethoxy-3-beta-r4-
iodophenyl)tropane
To a vial containing 50 ,~Lg (0.094 ~mol) of 2-beta-
carbomethoxy-3-beta-(4-tributylstannylphenyl)tropane was
added 50 ~L ethanol, 150 ~L 0.5M H3PO4, 125-500 ~LL (20-30 mCi)
[~23I]NaI solution, and 100 ,uL (4.2 ~mol) 0.042M peracetic
acid. After 20-30 minutes, 50 ~L of lOOmg/mL aqueous NaHS03
solution was added. Saturated NaHCO3 solution was added, and
the mixture extracted with ethyl acetate. The combined
extracts were dried (Na2SO4) and concentrated to dryness. The
residue was redissolved in methanol and purified by HPLC (C-
18 column, eluant: CH30H: H20: triethylamine; 75:25:0.2).
The fraction eluting at the retention time of 2-beta-
carbomethoxy-3-beta-(4-iodophenyl)tropane was collected
evaporated to dryness and reconstituted in 5% ethanol and 0.1
nM ascorbic acid.
In SPECT applications, the radiostable iodinated
neuroprobe of the invention is useful as a reference
standard, and can also be used as a dilutant for the
radioactive form of the neuroprobe. The radioiodinated
compound is generally identified by its chromatographic
mobility as compared with a fully characterized reference
standard. Thus, preparation of the radioiodinated compound
requires the non-radioactive iodinated compound.
To avoid the necessity of storing a radioactive
neuroprobe, it is useful to provide a kit containing the non-
radioactive iodinated compound and an appropriate oxidizing
~ W09~101184 ~16 ~ 2 9 4 PCT~S93/06170
-- 19 --
agent, such as perchloric acid, performic acid, peracetic
acid, hydrogen peroxide, hydrogen peroxide with
lactoperoxidase, 1,3,4,6-tetrachloro-3~,6~-diphenylglycouril,
or a N-chloro-4-methylbenzenesulfonamide sodium salt. Then,
the non-radioactive precursor compound can be oxidized in the
presence of a suitable radioactive compound, such as the
carrier free Na[l23I] shown in the synthesis route described
herein, any other radioisotope source, such as any solution
of a salt of a radioactive isotope of iodine, a reagent
containing mCnH2n+~X, where n=0-6 and X is a leaving group, or
a reagent containing '8F of the formula FCnH2nX, where n=0-6
and X is a leaving group, to prepare the iodinated neuroprobe
at its time and place of use.
Radiolabled neuroprobes of the invention are also useful
in other imaging procedures. For example, an l25I-labled
neuroprobe can be used in autoradiography or therapy, and an
3lI-labled neuroprobe is useful as a multiple photon emitter
for use in animal studies. Also, IlC-, l4C-, and l8F-labeled
neuroprobes can be used in PET imaging.
Both the radiostable and radioactive variants of the
iodinated neuroprobe of the invention are useful for human
and non-human research. For example, in vivo and in vitro
experiments can be performed using the compounds of the
invention to study the dopamine transporter generally, and
cocaine binding sites in particular.
Additionally, the radiostable version of the neuroprobe
of the invention can be used as a drug for influencing
dopamine reuptake.
Other modifications and implementations will occur to
those skilled in the art without departing from the spirit
and the scope of the invention as claimed. Accordingly, the
above-description is not intended to limit the invention
except as indicated in the following claims.