Note: Descriptions are shown in the official language in which they were submitted.
~6632a
WO 95/01342 PCT/IP,94/00160
.
ANSAMYCIN DERIVATIVES AS ANTIONCOGENE AND ANTICANCER AGENTS
Background of the Invention
This invention relates to derivatives of geldanamycin, pharmaceutically
acceptable salts and prodrugs of said derivatives, processes for their preparation and
antitumor and oncogene product inhibiting compositions containing said derivatives,
10 salts and prodrugs as the active i"~edier~la.
Oncogene products are proti- ,s generated by cancer genes and are involved
in the transformation of normal cells into cancer cells.
Geldanamycin is an antibiotic whose preparation and uses were described in
United States Patent 3,595,955 (incorporated herein by reference).
Co-pending United States Patent application serial number 07/817,235, filed
January 6, 1992 and assigned to Pfizer Inc. describes fermentation processes forpreparing 4,5-dihydrogeldanamycin and its 18,21-hydroquinone.
Other derivatives of geld&namycin, and their use as antitumor agents are
described in United States Patent 4,261,989.
Summary of the Invention
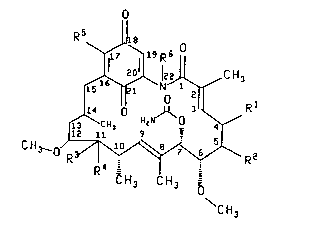
The presenl invention relates to compounds of the formula
C
~ ~ H3
1112 '.1 9
C H3_o/ 3~ \ R2
CH3 CH3
CH
el 3
35 and pharmaceutically acceptable salts and prodrugs thereof herei"a~ler, also, ~efer,ed
to as the active compounds;
wherein Rt and R2 are both hydrogen or Rl and R2 together form a single bond;
WO 95/01342 PCT/lB94/00160 --
21~632~
-2-
wherein Rl and R2 are both hydrogen or R' and R2 together form a single bond;
R3 is hydrogen and R4 is selected from the group consisting of
-ORl, -NHR8 and halo;
wherein Rl is selected from the group consisting of hydrogen, Rl1C(=O)-,
5 Rl lSO2- and Rl2Rl3NSO2NHC(=O)-;
wherein Rll is selected from the group consisting of amino, (Cl-C8)alkyl,
amino(Cl-C8)alkyl, hydroxy(Cl-C8)alkyl, protected amino(Cl-C8)alkyl, prote-,1ed
hydroxy(Cl-C8)alkyl, phenyl and naphthyl; and
Rl2 and Rl3 are each independently selected from the group
10 consisli"g of hydrogen, (Cl-C8)alkyl, amino(Cl-C8)alkyl, dimethylamino(Cl-C8)alkyl,
cyclo(C3-C8)alkyl, phenyl and naphthyl; or Rl2 and Rl3 together with the nitrogen to
which they are attached form a heterocyclic residue scl~ied from the group consi~li"g
of aziridinyl, az~lidi"yl, py"olidi.,yl, piperidinyl, thiazolidinyl, ox~oli~;rlyl, morpholino,
piperæinyl, ~(Cl-C4)alkylpiperidinyl and N-(Cl-C4)piperazinyl;
and said alkyl, phenyl and naphthyl groups may be substituted
with one or more residues selected from the group consisting of (Cl-C8)alkyl, halo,
nitro, amino, azido and (Cl-C8)alkoxyl;
or R3 and R4 together form a group of the formula
= J
20 wherein J is selected from O and NOH;
R5 is NR9R9 wherein R3 and R9 are each independently selected from the group
consiali"g of hydrogen, (Cl-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl; wherein said alkyl, alkenyl and alkynyl are optionally suhstituted wherein
said substituents are selected from the group consixli"g of halo, cyano, mercapto, (Cl-
25 C8)alkylthio, optionally substituted amino, hydroxyl, (Cl-C8)alkoxyl, carboxyl, amidino,
acylamino, and (C2-C6)heterocycloalkyl and (Cz-C6)heterocycloaryl groups selected from
the group compl isi"g imidizaloly, furyl, tetrahydrofuryl; and if comprising more than two
carbon atoms may be branched, cyclic or unbranched or combinations of branched,
cyclic and unbranched groups; or R9 and R9 together with the nitrogen to which they
30 are attached form a heterocyclic residue selected from the group consisting of
aziridinyl, azetidinyl and pyrrolidinyl;
or R5 is Rl4O wherein Rl4 is hydrogen or (Cl-C4)alkyl;
and R5 is hydrogen or a group of the formula
WO 95/01342 ~16 6 3 2 ~ PCT/I~394/00160
( R 7 ) m
O
(~C-CH2-
R
wherein m is 0 or an integer from 1-5 and each R7 is independently selected from halo,
P7ido, nitro, (Cl-C8)alkyl, Cl-C8alkoxyl, phenyl and naphthyl, cyano and NR3R9 wherein
R3 and R9 are as defined above; with the proviso that when Rl and R2 together form a
10 single bond R3 is hydrogen and R4 is ORl wherein R' is hydrogen then R5 cannot be
oR14, wherein R14 is hydrogen or methyl, or NR8R9 wherein HNR8R9 is selected from the
group cGIlsialill9 of &r"l"onia, methylamine, ethylamine, propylamine, butylamine,
pentylamine, hexylamine, heptylamine, octylamine, allylamine, 13-hydroxyethylamine, 13-
chloroethylamine, B-glycoxyethylamine, aminobutylamine, adamantylmethylamine,
15 cyclopropylamine, cyclopentylamine, cyclohexylamine, cycloheptylamine,
cyclooctylamine, benzylamine, phenethylarnine, ethyleneamine, pyrrolidine, piperidine,
dimethylamine, aminoethylamine, diglycolamine, 13-morpholinoethylamine, 13-
piperidinoethylamine, picolylamine, 13-pyrrolidinoethylamine, 13-pyridinylethylamine, 13-
methoxyethylamine, and 13-N-methylaminoethylamine; and when R5 is OR14 and Rl is
20 R11C(=O), R11 cannot be methyl.
Preferably, the compounds of the invention are compounds of formula I
wherein
1. R1 and R2 are each hydrogen;
a. R3 and R~ are each hydrogen and R4 is fluoro or OR10 wherein Rl
25 is selected from hydrogen, Rl lC(=O)- and Rl2Rl3NSO2NHC(o)- wherein Rll is selected
frorn amino, (Cl-C8)alkyl, amino(C1-C8)alkyl, prol~cted amino(Cl-C8)alkyl phenyl and
naphthyl; and Rl2 and Rl3 are each independently selected from the group consisting
of hydrogen, (Cl-C8)alkyl, amino(Cl-C8)alkyl, dimethylamino(Cl-C8)alkyl, cyclo(C3-
C8)alkyl, phenyl and naphthyl; or Rl2 and Rl3 together with the nitrogen to which they
30 are attached form a heterocyclic residue selected from the group consisting of
aziridinyl, a_etidinyl, pyrrolidonyl, piperidinyl, thi~oli~ yl, ox~olidinyl, morpholino,
pipera_inyl, 4-(C1-C4)alkylpiperidinyl, N-(C1-C4)piperazinyl; wherein is as defined above;
and R5 is ORl4 or NR8R9 wherein
WO 95/01342 . PCT/IB94/00160 --
~66~
i. when R5 is R8RgN, R8 is hydrogen and R9 is sPiected from
the group consiali"g of (C1-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
wherein said alkyl, alkenyl and alkynyl are optionally substituted wherein said
substituents are selected from the group consisting of halo, cyano, mercapto, (Cl-
5 C8)alkylthio, optionally s~ ~hstih~ted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino,
acylamino, (C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl and if comprising more
than two carbon atoms may be branched, cyclic or unbranched or combinations of
branched, cyclic and unbranched groups;
ii. when R5 is R8R9N, R8 and R9 together with the nitrogen to
10 which they are attached form a heterocyclic residue selected from the group consisting
of optionally substituted aziridinyl, azetidinyl and pyrrolidinyl wherein said substituents
are selected from the group con5i~1i"9 of halo, cyano, mercapto, (Cl-C8)alkylthio,
substituted or uns~hstituted amino, hydroxyl, (Cl-C8)alkoxyl, carboxyl, amidino and
acylamino;
and iii. when R5 is Rl4O, Rl4 is hydrogen or (C1-C~)alkyl; with the
proviso that when R3 and R6 are hydrogen and R4 is OR10 wherein R' is hydrogen, R'4
is not methyl; or
b. R3 is hydrogen and R4 is selected from the group consi~li"g of
fluoro and OR10 wherein Rl is selected from hydrogen, R"C~=O)- and
20 R'2R'3NSO2NHC(O)- wherein R11 is selected from amino, (C1-C8)alkyl, amino(C1-C8)alkyl, protected amino(C1-C8)alkyl, phenyl and naphthyl; and R12 and R13 are each
independently selected from the group consisting of hydrogen, (C1-C8)alkyl, amino(Cl-
C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyl; or R12 and
R13 together with the nitrogen to which they are attached form a heterocyclic residue
25 sel~_ted from the group consisting of aziridinyl, azetidinyl, pyrrolidinyl, piperidinyl,
~ 'S'91i l'-lyl, oxazolidinyl, morpholino, piperazinyl, 4-(C1-C4)alkylpiperidinyl, N-(C1-
C4)piperazinyl; R3 is a group of the formula A wherein m is defined as above and R5 is
OR14 or NR8R9 wherein
i. when R5 is R8R9N, R8 is hydrogen and R9 is selected from
30 the group consi ,Li"g of (Cl-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
wherein said alkyl, alkenyl and alkynyl are optionally substituted wherein said
suhstihlents are selected from the group consisting of halo, cyano, mercapto, (C1-
C8)alkylthio, optionally substituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino,
WO 95101342 216 6 3 2 0 PCT/IB94100160
acylamino, (C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl and if comprising more
than two carbon atoms may be branched, cyclic or unbranched or combinations of
branched, cyclic and unbranched groups;
ii. when R5is R8R9N, R8 and R9 together with the nitrogen to
5 which they are attached form a heterocyclic residue selected from the group consi~li"g
of optionally substituted aziridinyl, azetidinyl and py" olidii ,yl wherein said substituents
are selected from the group consisting of halo, cyano, mercapto, (C1-C8)alkylthio,
substituted or unsubstituted amino, hydroxyl, (Cl-C8)alkoxyl, carboxyl, amidino and
acylamino;
and iii. when R5is Rl40, Rl4is hydrogen or (C1-C8)alkyl; or
c. R6 is hydrogen, R3 and R4 together form a group of the formula
=J
wherein J is O or NOH; and R5is ORl4 or R8R9N wherein
i. when R5is R3R9N, R8is hydrogen and R9is selected from
15 the group consisting of (C1-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
wherein said alkyl, alkenyl and alkynyl are optionally substituted wherein said
s~hstitl~ents are s~lected from the group consi~li"g of halo, cyano, mercapto, (Ct-
C8)alkylthio, oplior,ally suhstituted amino, hydroxyl, (Cl-C8)alkoxyl, carboxyl, amidino,
acylamino, (C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl and if comprising more
20 than two carbon atoms may be branched, cyclic or unbranched or combinations of
branched, cyclic and unbranched groups; or R8 and R9 together with the nitrogen to
which they are attached form a heterocyclic residue selected from the group consi lil .g
of ~ilidi,lyl, azetidinyl and pyrrolidinyl;
ii. when R5is R8R9N, R8 and R9 together with the nitrogen to
which they are attached form a heterocyclic residue selected from the group consi~ling
of optionally substituted aziridinyl, azetidinyl and pyrrolidinyl wherein said substituents
are selected from the group consisting of halo, cyano, mercapto, (C1-C8)alkylthio,
substituted or unsubstituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino and
acylamino;
and iii. when R5is R140, Rl4is hydrogen or (Cl-C8)alkyl; or
d. R3 and R4 together form a group of the formula
= J
wherein J is O or NOH;
WO 95/01342 PCT/IB94/00160 --
2 ~ 2 ~ `
-6-
R~is a group of the formula A wherein m and R' are as defined above;
and R5is R'40 or R8R9N wherein
i. when R5is R8R9N, R8is hydrogen and R9is selected from
the group consisting of (Cl-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
5 wherein said alkyl, alkenyl and alkynyl are optionally substituted wherein said
s~hstih~Pnts are selected from the group consisting of halo, cyano, mercapto, (Cl-
C8)alkylthio, optionally s~hstihlted amino, hydroxyl, (C,-C8)alkoxyl, carboxyl, a~"i-li"o,
acylamino, (C2-C6)heterocycloalkyl and (C2-C~)heterocycloaryl and if comprising more
than two carbon atoms may be branched, cyclic or unbranched or combinations of
10 branched, cyclic and unbranched groups;
ii. when R5is R8R9N, R9 and R9 together with the nitrogen to
which they are attached form a heterocyclic residue selected from the group consi j~ 9
of optionally substituted aziridinyl, azetidinyl and py"oliJi"yl wherein said substituents
ue selected from the group cot,si;jli"g of halo, cyano, mercapto, (C,-C8)alkylthio,
15 s~hstitl~ted or unsubstituted amino, hydroxyl, (C,-C8)alkoxyl, carboxyl, amidino and
acylamino;
and iii. when R5is Rl40, R'4is hydrogen or (C,-C8)alkyl; or
or 2. R' and R2 together form a single bond and
a. R3 and R~ are each hydrogen, R4 is selected from the group
20 consi~li"g of fluoro and ORl wherein R'is selected from hydrogen, R"(C = 0)- and
R'2R'3NS02NHC(0)- wherein R" is selected from amino, (C,-C8)alkyl, amino(C,-
C8)alkyl, protected amino(C,-C8)alkyl, phenyl and naphthyl; and R12 and R'3 are each
independently sele 1ed from the group consi~ 9 of hydrogen, (C,-C8)alkyl, amino(C,-
C8)alkyl, dimethylamino(C,-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyi; or RlZ and
25 R'3 together with the nitrogen to which they are a~tached form a heterocyclic residue
sol~ed from the group consisli"g of aziridinyl, ~ ,yl, pyrrolidinyl, piperidinyl,
~1 ,i~olidinyl, oxazc!iJL Iyl, morpholino, piperazinyl, ~(C, -C4)alkylpiperidinyl, N-(C,-
C4)piperazinyl; and R5is oRl4 or NR8R9 wherein
i. when Rs is R8R9N, R8 is hydrogen and R9is selected from
30 the group consisting of (C1-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
wherein said alkyl, alkenyl and alkynyl are optionally substituted wherein said
sl~hstihlents are selected from the group consi~lir,g of halo, cyano, mercapto, (C,-
C8)alkylthio, optionally substituted amino, hydroxyl, (Cl-C8)alkoxyl, carboxyl, amidino,
WO 95/01342 ~16 6 3 2 ~ PCT/IB94/U11160
acylamino, (C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl and if comprising more
- than two carbon atoms may be branched, cyclic or unbranched or combinations of
branched, cyclic and unbranched groups;
ii. when R5 is R8R9N, R8 and R9 together with the nitrogen to
which they are attached form a heterocyclic residue selected from the group consisting
of optionally s~hstituted ~i,idi,lyl, azetidinyl and pyrrolidinyl wherein said s~hstit~ents
are cele 1e~1 from the group consisting of halo, cyano, mercapto, (Cl-C8)alkylthio,
optionally substituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino and acylamino;
and iii. when R5 is R14O, R14 is hydrogen or (C1-C8)alkyl; with the
proviso that when R10 is hydrogen then R5 cannot be OR14 wherein R14 is hydrogen or
mel:hyl or NR8R9 wherein HNR8R9 is selected from the group consisting of ammonia,
mel:hylamine, ethylamine, propylamine, butylamine, pentylamine, hexylamine,
heptylamine, octylamine, allylamine, B-hydroxyethylamine, 13-chloroethylamine, 13-
glycoxyethylamine, aminobutylamine, adamantylmethylamine, cyclopropylamine,
cyclopentylamine, cyclohexylamine, cycloheptylamine, cyclooctylamine, benzylamine,
ph~nethylamine, ethyleneamine, pyrrolidine, piperidinyl, dimethylamine,
aminoethylamine, diglycoamine, B-morpholinonethylamine, 13-piperidinoethylamine,picolylamine, 13-pyrrolidinoethylamine, 13-pyridinylethylamine, 13-methoxyethylamine, and
13-N-methylaminoethylamine; or
b. R3 is hydrogen and R4 is selected from the group consisting of
fluoro and OR10 wherein R10 is selected from hydrogen, R11C(=O)- and
R12R13NSO2NHC(O)- wherein R11 is selected from amino, (C1-C8)alkyl, amino(C1-
C8)alkyl, protected amino(C1-C8)alkyl, phenyl and naphthyl; and R12 and R13 are each
ind0pendently selected from the group consisli"g of hydrogen, (C1-C8)alkyl, amino(C1-
C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyl; or R12 and
R13 together with the nitrogen to which they are attached form a heterocyclic residue
selected from the group consi~ling of æiridinyl, ~ttidi,lyl, pyrrolidinyl, piperidinyl,
tl ,i~ ~oli~ ,yl, oxP~olidinyl, morpholino, pipera_inyl, 4-(Cl-C4)alkylpiperidinyl, N-(Cl-
C")piperæinyl; R~ is a group of the formula A wherein m is defined as above and R5 is
OR14 or NR8R9 wherein
i. when R5 is R8R9N, R8 is hydrogen and R9 is selected from
the group consi jli, Ig of (C,-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
wherein said alkyl, alkenyl and alkynyl are optionally substituted wherein said
WO 95/01342 PCT/IB94/00160 --
-8-
s~lhstitllPnts are sPlected from the group consi~Lir,g of halo, cyano, mercapto, (C1-
C8)alkylthio, optionally substituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino,
acylamino, (C2-C~)heterocycloalkyl and (C2-C,3)heterocycloaryl and if comprising more
than two carbon atoms may be branched, cyclic or unbranched or combinations of
branched, cyclic and unbranched groups;
ii. when R5 is R8R9N, R8 and R9 together with the nitrogen to
which they are attached form a heterocyclic residue selected from the group consisting
of optionally s~ Ihstitutecl aziridinyl, az~li.l;"yl and pyrrolidinyl wherein said substituents
are selected from the group consisting of halo, cyano, mercapto, (C1-C8)alkylthio,
substituted or unsubstituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino and
acylamino;
and iii. when R5 is R14O, R14 is hydrogen or (C1-C8)alkyl; or
c. R5 is hydrogen, R3 and R4 together form a group of the formula
=J
wherein J is O or NOH; and Rs is OR14 or NR8R9 wherein
i. when R5 is R8R9N, R8 is hydrogen and R9 is selected from
the group consiali. ,g of (C1-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
wherein said alkyl, alkenyl and alkynyl are optionally s~ Ihstituted wherein said
substituents are selected from the group consisting of halo, cyano, mercapto, (C1-
C8)alkylthio, optionally substituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino,
acylamino, (C2-C~)heterocycloalkyl and (C2-C~)heterocycloaryl and if comprising more
than two carbon atoms may be branched, cyclic or unbranched or combinations of
branched, cyclic and unbranched groups;
ii. when R5 is R8R9N, R8 and R9 together with the nitrogen to
which they are attached form a heterocyclic residue selected from the group consi;,li"g
of optionally substituted aziridinyl, azetidinyl and pyrrolidinyl wherein said substituents
are sPlectecl from the group consi~li"g of halo, cyano, mercapto, (Cl-C8)alkylthio,
substituted or unsubstituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino and
acylamino;
and iii. when R5 is R14O, R14 is hydrogen or (C1-C8)alkyl; or
d. R3 and R4 together form a group of the formula =O or =NOH; R~
is a group of the formula A wherein m and R7 are defined as above; and R5 is R14O or
R8R9N wherein
t WO 95/01342 PCT/IB94/00160
2 0
g
i. when R5 is R8R9N, R8 is hydrogen and R9 is selected from
the group consi~li"g of (C1-C8)alkyl, (C3-C8)cycloalkyl, (C2-C8)alkenyl and (C2-C8)alkynyl;
wherein said alkyl, alkenyl and alkynyl are optionally substituted wherein said
substituents are Sf~lECtæCI from the group consisting of halo, cyano, mercapto, (C,-
5 C8)alkylthio, optionally substituted amino, hydroxyl, (Cl-C8)alkoxyl, carboxyl, amidino,
acylamino, (C2-C~)heterocycloalkyl and (C2-C6)heterocycloaryl and if comprising more
than two carbon atoms may be branched, cyclic or unbranched or combinations of
branched, cyclic and unbranched groups;
ii. when R5 is R8R9N, R8 and R9 together with the nitrogen to
10 which they are attached form a heterocyclic residue selected from the group consi~li"g
of optionally substituted ~i-idillyl, a_elidinyl and pyrrolidinyl wherein said substituents
are selected from the group consisting of halo, cyano, mercaplo, (Cl-C8)alkylthio,
s~hstitllted or unsubstituted amino, hydroxyl, (C1-C8)alkoxyl, carboxyl, amidino and
acylamino;
and iii. when R5 is R14O, R14 is hydrogen or (C1-C8)alkyl.
More pre~er,~d co",pounds of the invention are selected from the group
consi~li"g of compounds of the formula I wherein
1. R1, R2, R3 and R are each hydrogen, R4 is fluoro or OR10 wherein R10 is
selected from hy.llogen, RllC(=O)- and R12R13NSO2NHC(o)- wherein Rll is selected20 from amino, (Cl-C8)alkyl, amino(Cl-C8)alkyl, protected amino(C1-C8)alkyl, phenyl and
naphthyl; and R12 and R13 are each independently selected from the group consisting
of hy.l, ogen, (Cl-C8)alkyl, amino(Cl-C8)alkyl, dimethylamino(Cl-C8)alkyl, cyclo(C3-
C8)alkyl, phenyl and na,cl,ll,yl; wherein is as defined above; or Rl2 and Rl3 together
with the nitrogen to which they are attached form a heterocyclic residue selected from
25 the group consi;,li"g of a~iridinyl, a_etidinyl, pyrrolidinyl, piperidinyl, thi~oli~ yl,
ox~ inyl, morpholino, pipera_inyl, 4-(Cl-C4)alkylpi,ueridinyl, and N-(Cl-C4)piper~7inyl;
and R5 is R8R9N wherein R8 is hydrogen and R9 is selected from hydrogen, (C1-C6)alkyl,
- (C2 C8)alkenyl and (C2-C8)alkynyl; wherein said alkyl, alkenyl and alkynyl groups are
optionally substituted and said substituents are selected from the group consisting of
30 halo, cyano, mercapto, (Cl-C8)alkylthio, optionally substituted amino, hydroxyl, (Cl-
C8)alkoxyl, carboxyl, amidino, acylamino, (C2-C~)heterocycloalkyl and (C2-
C~,)heterocycloaryl and if con,pri~i"g more than two carbon atoms may be branched,
cyclic or unbranched or combinations of branched, cyclic and unbranched groups;
WO 95/01342 PCT/IB94/00160 ~
3 2 ~
-10-
2. R1, R2, R3 and R6 are each hydrogen, R4 is fluoro or -ORl wherein Rl
is selected from hydrogen, Rl1C(=O)- and R12R13NSO2NHC(o)- wherein R11 is selected
from amino, (C1-C8)alkyl, amino(C1-C8)alkyl, protected amino(C1-C8)alkyl, phenyl and
naphthyl; and R12 and R13 are each independently selected from the group consisting
of hydrogen, (C1-C8)alkyl, amino(C1-C8)alkyl, dimethylamino(Cl-C8)alkyl, cyclo(C3-
C8)alkyl, phenyl and naphthyl; wherein is as defined above; or RtZ and Rl3 together
with the nitrogen to which they are attached form a heterocyclic residue selected from
the group cons6li"9 of aziridinyl, azetidinyl, pyrrolidinyl, piperidinyl, thiazolidinyl,
oxazolidinyl, morpholino, piperæinyl, 4-(Cl -C4)alkylpiperidinyl, N-(Cl -C4)piperazinyl; and
R5 Ts R8R9N wherein R8 and R9 together with the nitrogen to which they are attached
form a 3 to 6 membered heterocycloalkyl or heterocycloaryl ring which is optionally
substituted with one or more groups selected from hydroxyl, halo, cyano, (Cl-
C~)alkoxyl, (C1-C~)alkylthio, (C2-C~)heterocycloalkyl and (C2-C~)heterocycloaryl;
3. R1, R2, R3 and R~ are each hydrogen, R4 is fluoro or OR10 wherein Rl is
selectecl from hyd~ogen, R11C(=O)- and R12R13NSO2NHC(o)- wherein Rl1 is selectedfrom amino, (C1-C8)alkyl, amino(C1-C8)alkyl, protected amino(C1-C8)alkyl, phenyl and
naphthyl; and R12 and R'3 are each independently selected from the group consi~li"g
of hydrogen, (C1-C8)alkyl, amino(C1-C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-
C8)alkyl, phenyl and naphthyl; wherein is as defined above; or R12 and R13 together
with the nitrogen to which they are attached form a heterocyclic residue selected from
the group consiali, lg of a_iridinyl, a_etidinyl, pyrrolidinyl, piperidinyl, thi~oli~ yl,
oxazolidinyl, morpholino, pipera_inyl, 4-(C1-C4)alkylpiperidinyl and N-(C1-C4)piperazinyl;
and R5 is R14O wherein R14 is (C1-C8)alkyl with the proviso that when R10 is hydrogen
R14 is not methyl;
4. R1 and R2 together form a single bond, R3 and R6 are each hydrogen,
R4 is fluoro or OR' wherein R' is selected from hydrogen, R'1C(=O)- and
R12R13NSO2NHC(O)- wherein R11 is selected from amino, (C1-C8)alkyl, amino(Cl-
C8)alkyl, protected amino(C,-C8)alkyl, phenyl and naphthyl; and R12 and R13 are each
independently sPlected from the group consi~Lil ,9 of hydrogen, (C1-C8)alkyl, amino(Cl-
C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyl; wherein
is as defined above; or R12 and R13 together with the nitrogen to which they areattached form a heterocyclic residue selected from the group consisli"g of aziridine,
azetidine, pyrrolidone, piperidinyl, thiazolidinyl, oxazolidinyl, morpholino, piperazinyl, 4-
WO 95/0134Z PCT/IB94/00160
3 2 ~
(Cl-C4)alkylpiperidinyl and N-(C1-C4)piperazinyl; and R5 is R8R9N wherein R8 is hydrogen
and R9 is selected from hydrogen, optionally substituted (Cl-C6)alkyl, optionally
s~ ~hstitl ~ted (C3-C~)cycloalkyl, optionally substituted (C2-C~)alkenyl and optionally
substituted (C2-C~)alkynyl wherein the substituents of said alkyl and alkenyl and alkynyl
5 groups are selected from hydroxyl, halo, cyano, (C1-C~3)alkoxyl, (Cl-C~)alkylthio,
(C2-C~)heterocycloalkyl and (C2-C~)heterocycloaryl; with the proviso that when Rl is
hydrogen then Rs cannot be NR8R9 wherein HNR8R9 is selected from the group
consisting of ammonia, methylamine, ethylamine, propylamine, butylamine,
pentylamine, hexylamine, heptylamine, octylamine, allylamine, 13-hydroxyethylamine, 13-
10 ch'-rwtl,ylamine, 13glycoxyethylamine, aminobutylamine, benzylamine, phenethylamine,
dimethylamine, aminoethylamine, diglycolamine, 13-morpholinoethylamine, 13-
piperidinoethylamine, 13-pyrrolidinoethylamine, 13-pyridinylethylamine, 13-
methoxyethylamine, and 13-N-methylaminoethylamine;
5. R1 and R2 together form a single bond, R3 and R8 are each hydrogen,
15 R4 is fluoro or ORl wherein Rl is selected from hydrogen, RllC(=O)- and
Rl2P~l3NSO2NHC(O)- wherein Rll is selected from amino, (Cl-C8)alkyl, amino(Cl-
C8)alkyl, prote~ted amino(Cl-C8)alkyl, phenyl and naphthyl; and Rl2 and Rl3 are each
independer,lly s~lectæ~ from the group consi~Li"g of hydrogen, (Cl-C8)alkyl, amino(Cl-
C8)alkyl, dimethylamino(C,-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyl; wherein
20 is as defined above; or Rl2 and R13 together with the nitrogen to which they are
attached form a heterocyclic residue selected from the group consisting of aziridinyl,
~elidinyl, py"olidinyl, piperidinyl, this~sli~inyl, ~ sli~inyl, morpholino, piperazinyl, ~
(Cl-C4)alkylpiperidinyl and N-(Cl-C4)piperazinyl; and R5 is R8R9N wherein R8 and R9
tog0ther with the nitrogen to which they are attached form a 3 to 6 membered
25 heterocycloalkyl or heterocycloaryl ring which is optionally s~hstituted with a group
selected from hydroxyl, halo, cyano, (Cl-C6)alkoxyl, (Cl-C~)alkylthio,
(C3-C8)heterocycloalkyl and (C4-C8)heterocycloaryl; with the proviso that when Rl is
hydrogen then NR8R9 cannot be derived from ethyleneamine, pyrrolidine or piperidine;
6. Rl and R2 together form a single bond, R3 and R~ are each hydrogen,
30 R4 is fluoro or ORl wherein Rl is selected from hydrogen, RllC(=O)- and
Rl2Rl3NSO2NHC(O)- wherein Rll is selected from amino, (Cl-C8)alkyl, amino(C1-
C8)alkyl, protected amino(C1-C8)alkyl, phenyl and naphthyl; and R12 and R13 are each
independently selected from the group consisting of hydrogen, (Cl-C8)alkyl, amino(Cl-
WO 95/01342 PCT/IB94/00160 ~
~6~
-12-
C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyl; wherein
is as defined above; or R12 and Rl3 together with the nitrogen to which they areattached form a heterocylic residue selected from the group consisting of aziridinyl,
azetidinyl, pyrrolidinyl, piperidinyl, thiazolidinyl, ox~oli~inyl, morpholino, piperæinyl, 4-
(C1-C4)alkylpiperidinyl and N-(C,-C4)piperazinyl; and R5 is R14O wherein R14 is hydrogen
or (C1-C8)alkyl; with the proviso that when R10 is hydrogen R14 cannot be hydrogen or
methyl and when R10 is R11C(=O), R11 cannot be methyl;
7. Rl, R2 and R8 are each hydrogen, R3 and are R4 together form a group
s~lectecl from =O and =NOH and R5 is R8R9N wherein R8 is hydrogen and R9 is
selected from hydrogen, optionally substituted (Cl-C6)alkyl or optionally s~ Ihstihlted (C3-
C6)cycloalkyl (C2-C6)alkenyl and (C2-C6)alkynyl wherein the substituents of said alkyl,
alkenyl and alkynyl groups are selected from hydroxyl, halo, cyano, (Cl-C6)alkoxyl, (Cl-
C~,)alkylthio, (C2-C~)heterocycloalkyl and (C2-C~)heterocycloaryl;
8. Rl, R2 and R~ are each hydrogen, R3 and R4 together form a group
sEl~ecl from =O and =NOH and R5 is R8R9N wherein R8 and R9 together with the
nitrogen to which they are attached form a 3 to 6 membered heterocycloalkyl or
heterocycloaryl ring which is optionally substituted with one or more groups selected
from hydroxyl, halo, cyano, (Cl-C~)alkoxyl, (Cl-C6)alkylthio, (C2-C6)heterocycloalkyl and
(C2-C6)heterocycloaryl;
9. R1, R2 and R6 are each hydrogen, R3 and R4 together form a group
selected from =O and =NOH and R5 is R14O wherein R14 is hydrogen or (Cl-C8)alkyl;.
10. Rl and R2 together form a single bond, R6 is hydrogen, R3 and R4
together form a group selected from =O and =NOH;
and R5 is R8R9N wherein R8 is hydrogen and R9 is selected from hydrogen,
optionally substituted (Cl-C6)alkyl or optionally substituted (C3-C6)cycloalkyl, (C2-
C6)alkenyl and (C2-C6)alkynyl wherein the substituents of said alkyl, alkenyl and alkynyl
groups are selected from hydroxyl, halo, cyano, (Cl-CG)alkoxyl, (Cl-C6)alkylthio,
(C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl;
11. Rl and R2 together form a single bond, R6 is hydrogen, R3 and R4
together form a group selected from =O and =NOH and R5 is R8R9N wherein R8 and
R9 together with the nitrogen to which they are attached form a 3 to 6 membered
heterocycloalkyl or heterocycloaryl ring which is optionally substituted with one or more
groups selected from hydroxyl, halo, cyano, (Cl-C6)alkoxyl, (Cl-C6)alkylthio,
~ WO 95/01342 PCT/IB94/00160
2 0
(C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl;
12. R1 and R2 together form a single bond, R6 is hydrogen, R3 and R4
together form a group selected from =O and =NOH and R5 is Rl40 wherein R14 is
hydrogen or (Cl-C8)alkyl;
13. Rl, R2 and R3 are each hydrogen, R6is a group of the formula A wherein
m and R7 are as defined above, R4 fluoro or OR10; wherein R10 is selected from the
group consi~Ling of hydrogen, RllC(=O)-, RllSO2- and R12Rl3NSO2NHC(=O)-; whereinR11 is selected from the group consisting of amino, (C1-C8)alkyl, amino(C1-C8)alkyl,
protected amino(C1-C8)alkyl, phenyl and naphthyl; and R12 and R13 are each
independer,lly selected from the group consisting of hydrogen, (Cl-C8)alkyl, amino(Cl
C8)alkyl, dimethylamino(Cl-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyl; and said
alkyl, phenyl and naphthyl groups may be s~hstituted with one or more residues
selected from the group consi~li"g of (Cl-C8)alkyl, halo, nitro, amino, azido and (C1-
C8)alkoxyl; and R5 is R8R9N wherein R8 is hydrogen and R9is selected from hydrogen,
optionally substituted (C1-C6)alkyl, optionally substituted (C3-C6)cycloalkyl, optionally
sl Ihstituted (C2-C6)alkenyl and optionally substituted (C2-C6)alkynyl wherein the
s~hstihlents of said alkyl, alkenyl and alkynyl groups are selected from hydroxyl, halo,
cyano, (C,-C~)alkoxyl, (Cl-C~)alkylthio, (C2-C6)heterocycloalkyl and
(c2-c~)heterocycloaryl;
14. Rl, R2 and R3 are each hydrogen, R6is a group of the formula A wherein
m and R7 are as defined above, R4 is fluoro or OR10; wherein R10 is selected from the
group consi~li"g of hydrogen, R1lC(=O)-, RllSO2- and Rl2Rl3NSO2NHC(=O)-; whereinR1l is selected from the group consisting of amino, (Cl-C8)alkyl, amino(Cl-C8)alkyl,
protected amino(Cl-C8)alkyl, phenyl and naphthyl; and Rl2 and Rl3 are each
independently selected from the group consisting of hydrogen, (Cl-C8)alkyl, amino(Cl-
C8)alkyl, dimethylamino(Cl-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and naphthyl; and said
alkylp phenyl and naphthyl groups may be substituted with at least one residue selected
from the group consisting of (Cl-C8)alkyl, halo, nitro, amino, azido and (Cl-C8)alkoxyl;
and R5 is R9R9N wherein R8 and R9 together with the nitrogen to which they are
attached form a 3 to 6 membered heterocycloalkyl or heterocycloaryl ring which is
optionally substituted with at least one group selected from hydroxyl, halo, cyano, (Cl-
C6)alkoxyl, (Cl-C6)alkylthio, (C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl;
15. Rl, R2 and R3 are each hydrogen, R6is a group of the formula A wherein
WO 95/01342 PCT/IB94/00160
-14-
m and R7 are as defined above, R4is OR10 or fluoro; wherein Rl is selected from the
group consi~li"g of hydrogen, R11C(=O)-, R11SO2- and R12R13NSO2NHC(=O)-; whereinRll is selE~,1ed from the group consisting of amino, (C1-C8)alkyl, amino(Cl-C8)alkyl,
prote-,ted amino(C3-C8)alkyl, phenyl and naphthyl; Rl2 and Rl3 are each independently
5 sele_ted from the group consisting of hydrogen, (C1-C8)alkyl, amino(C1-C8)alkyl,
dimethylamino(Cl-C8)alkyl, cyclo(C3-C8)alkyl, morpholino, N-methylpiperazinyl, phenyl
and naphthyl; and said alkyl, phenyl and naphthyl groups may be substituted with one
or more residues selected from the group consisting of (Cl-C8)alkyl, halo, nitro, amino,
azido and (Cl-C8)alkoxyl; and R5is R14O wherein R14 is hydrogen or (C1-C8)alkyl;16. Rl and R2 together form a single bond, R3is hydrogen, R6is a group of
the formula A wherein m and R' are as defined above, R4is OR10 or fluoro; wherein R10
is selected from the group consisting of hydrogen, Rl1C(=O)-, R11SO2- and
R12R13NSO2NHC(=O)-; wherein R11 is selected from the group consisting of amino, (Cl-
C8)alkyl, amino(Cl-C8)alkyl, protected amino(Cl-C8)alkyl, phenyl and naphthyl; and Rl2
and Rl3 are each independently selected from the group consisting of hydrogen, (C1-
C8)alkyl, amino(C1-C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and
naphthyl and said alkyl, phenyl and naphthyl groups may be substituted with one or
more s~ ~hstituents selected from the group consi~lir,g of (C1-C8)alkyl, halo, nitro, amino,
azido and (C1-C8)alkoxyl;
and R5 is R8R9N wherein R3 is hydrogen and R9 is selected from hydrogen,
optionally substituted (Cl-C~)alkyl, optionally substituted (C3-C~j)cycloalkyl, optionally
substituted (C2-C6)alkenyl and optionally substituted (C2-C6)alkynyl wherein thesubstituents of said alkyl and alkenyl and alkynyl groups are selected from hydroxyl,
halo, cyano, (C1-C6)alkoxyl, (C1-C6)alkylthio, (C2-C6)heterocycloalkyl and
(C2-C~)heterocycloaryl;
17. R1 and R2 togetherform a single bond, R3is hydrogen, R6 is a group of
the formula A wherein m and R7 are as defined above, R4is OR10 or fluoro; wherein R10
is selected from the group consisting of hydrogen, R11C(=O)-, R11SO2- and
R12R13NSO2NHC(=O)-; wherein R11 is selected from the group consisting of amino, (C1-
Cg)alkyl, amino(C1-C8)alkyl, protected amino(C1-C8)alkyl, phenyl and naphthyl; and R12
and R13 are each independently selected from the group consi~li"g of hydrogen, (C1-
C8)alkyl, amino(C1-C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and
naphthyl; and said alkyl, phenyl and naphthyl groups may be substituted with one or
~I WO 95/0134:~ PCT/IB94/00160
~6~
-15-
more residues selected from the group consi~;li"g of (Cl-C8)alkyl, halo, nitro, amino,
- azicio and (C1-C8)alkoxyl; and R5is R8R9N wherein R8 and R9 together with the nitrogen
to which they are attached form a 3 to 6 membered heterocycloalkyl or heterocycloaryl
ring which is opliGnally substituted with at least one group selected from hydroxyl,
halo, cyano, (C1-C6)alkoxyl, (C1-C6)alkylthio, (C2-C6)heterocycloalkyl and
(C2- CB)heterocycloaryl;
18. R1 and R2 together form a single bond, R3 is hydrogen, R6 is a group of
the formula A wherein m and R' are as defined above, R4 is OR10 or fluoro; wherein R10
is !selected from the group consisting of hydrogen, R11C(=O)-, R11SO2- and
R12R13NSo2NHC(=o)-; wherein R11is selected from the group consisting of amino, (C1-
C8)alkyl, amino(C1-C8)alkyl, protected amino(C1-C8)alkyl, phenyl and naphthyl; and R12
and R13 are each independently selected from the group consi~li"g of hydrogen, (C1-
C8)alkyl, amino(C1-C8)alkyl, dimethylamino(C1-C8)alkyl, cyclo(C3-C8)alkyl, phenyl and
naplhthyl; and said alkyl, phenyl and naphthyl groups may be sllhstitllted with one or
more residues selected from the group consi~li"g of (C1-C8)alkyl, halo, nitro, amino,
azido and (C1-C8)alkoxyl; and R5 is R14O wherein R14 is hydrogen or (C1-C8)alkyl;
19. R1 and R2 are each hydrogen, R~is a group of the formula A wherein m
is and R7 are defined above, R3 and are R4 together form a group selected from =O
and =NOH and R5 is R8R9N wherein R8 is hydrogen and R9is selecteri from hydrogen,
optionally substituted (C1-C6)alkyl, optionally substituted (C3-C6)cycloalkyl, optionally
s~ ~hstihlted (C2-C6)alkenyl and optionally substituted (C2-C6)alkynyl wherein the
substituents of said alkyl, alkenyl and alkynyl groups are selected from hydroxyl, halo,
cy~no, (C1-C6)alkoxyl, (C1-C~)alkylthio, (C2-C6)heterocycloalkyl and
(C2- C~)heterocycloaryl;
20. R1 and R2 are each hydrogen, R~ is a group of the formula A wherein m
and R7 are as defined above, R3 and R4 together form a group selected from =O and
=NOH and R5is R8R9N wherein R8 and R9 together with the nitrogen to which they are
- attached form a 3 to 6 membered heterocycloalkyl or heterocycloaryl ring which is
optionally s~ ~hstituted with one or more groups selected from hydroxyl, halo, cyano, (C1-
C6)alkoxyl, (C1-C~)alkylthio, (C2-C6)heterocycloalkyl and (C2-C~)heterocycloaryl;
21. R1 and R2 are each hydrogen, R~ is a group of the formula A wherein m
and R7 are as defined above, R3 and R4 together form a group selected from =O and
=NOH and R5 is R14O wherein Rl4 is hydrogen or (Cl-C8)alkyl;
WO 95/01342 PCT/IB94/00160 f
2 ~
-16-
22. R' and R2 together form a bond, R~ is a group of the formula A wherein
m and R' are as defined above, R3 and R4 together form a group selected from =O and
=NOH and R5 is R8R9N wherein R8 is hydrogen and R9 is selected from hydrogen,
optionally substituted (C,-C~)alkyl or optionally substituted (C3-C6)cycloalkyl, (C2-
C~)alkenyl and (C2-C0)alkynyl wherein the substituents of said alkyl and alkenyl and
alkynyl groups are selected from hydroxyl, halo, cyano, (Cl-C6)alkoxyl, (C,-C")alkylthio,
(C2-C~)heterocycloalkyl and (C2-CB)heterocycloaryl;
23. R1 and R2 together form a bond, R~ is a group of the formula A wherein
m and R' are as defined above, R3 and R4 together form a group selected from =O and
=NOH and R5 is R8R9N wherein R8 and R9 together with the nitrogen to which they are
attached form a 3 to 6 membered heterocycloalkyl or heterocycloaryl ring which is
optionally substituted with one or more groups selected from hydroxyl, halo, cyano, (Cl-
C~3)alkoxyl, (Cl-C6)alkylthio, (C2-C6)heterocycloalkyl and (C2-C6)heterocycloaryl;
or 24. R' and R2 together form a bond, R~ is a group of the formula A wherein
m and R7 are as defined above, R3 and R4 together form a group selected from =O and
=NOH and R~ is R14O wherein R14 is hydrogen or (Cl-C8)alkyl.
Most prefe, - ed compounds of the invention are selected from ths group
col~si~li"g of
17-Amino-4,5-dihydro-17-demethoxy-geldanamycin;
17-Methylamino-4,5-dihydro-17-demethoxygeldanamycin;
17-Cyclopropylamino~,5-dihydro-17-demethoxygeldanamycin;
17-(2'-Hydroxyethylamino)-4,5-dihydro-17-demethoxygeldanamycin;
17-(2-Methoxyethylamino)4,5-dihydro-17-demethoxygeldanamycin;
17-(2'-Fluoroethylamino)4,5-dihydro-17-demethoxygeldanamycin;
17-[s-(+)-2-Hydroxypropylamino]~,5-dihydro-17-demethoxygeldanamycin;
17-Azetidin-1 -yl-4,5-dihydro-17-demethoxygeldanamycin;
17-(3-Hydroxyazetidin-1 -yl)-4,5-dihydro-17-demethoxygeldanamycin;
17-Azetidin-1 -yl-4,5-dihydro-11 -a-fluoro-17-demethoxygeldanamycin;
17-Azetidin-1 -yl-17-demethoxygeldanamycin;
17-(2'-Cyanoethylamino)-17-demethoxygeldanamycin;
17-(2'-Fluoroethylamino)-17-demethoxygeldanamycin;
17-Amino-22-(2'-methoxyphenacyl)-17-demethoxygeldanamycin;
17-Amino-22-(3'-methoxyphenacyl)-17-demethoxygeldanamycin;
WO 95/0134Z ~ 3 ~ ~ PCT/Is94/00160
17-Amino-22-(4'-chlorophenacyl)-17-demethoxygeldanamycin;
- 17-Amino-22-(3',4'-dichlorophenacyl)-17-demethoxygeldanamycin;
17-Amino-22-(4'-amino-3'-iodophenacyl)-17-demethoxygeldanamycin;
17-Amino-22-(4'-azido-3'-iodophenacyl)-17-demethoxygeldanamycin;
17-Amino-11 -a-fluoro-17-demethoxygeldanamycin;
17-Aliylamino-11 -a-fluoro-17-demethoxygeldanamycin;
17-Propargylamino-11 -a-fluoro-17-demethoxygeldanamycin;
17-(2'-Fluoroethylamino)-11 -a-fluoro--17-demethoxygeldanamycin;
17-Azetidin-1 -yl-11 -(4'-~idophenyl)sulfamylcarbonyl-17-demethoxygelclan~ "ycin;
17-(2'-Fluoroethylamino)-11 -keto-17-demethoxygeldanamycin;
17-Azetidin-1 -yl-11 -keto-17-demethoxygeldanamycin; and
17-(3'-Hydroxy~lidi, l-1 -yl)-11 -keto-17-demethoxygeldanar"ycin.
This invention also relates to a pharmaceutical composition cGmprisi"g
an antitumor or oncogene product inhibiting or cancer preventing or treating eflective
15 arncunt of a compound of the formula ll or a pharmaceutically acceptable salt or
prodrug thereof, and a pharmaceutically acceptable carrier.
This invention also relates to a method of inhibiting an oncogene product in a
l"~r"al, including a human, comprising admi"i ,leri"g to said mammal an oncogeneprocluct inhibiting effective amount of a compound of the formula I or a
20 pharmaceutically acceptable salt or prodrug thereof.
This invention also relates to a method of inhibiting an ErbB-2, src, lckl fyn or
abl oncogene product in a mammall including a human, comprising administering tosaid mammal an ErbB-21 src, Ick, fyn or abl oncogene product inhibiting effective
amount of a compound of the formula I or a pharmaceutically accept~ble salt or
25 prodlrug thereof.
This invention also relates to a method of treating or preventing cancer in a
ma"~r"al, including a human, comprising administering to said mammal an antitumor
or oncogene product inhibiting effective amount of a compound of the formula I or a
phar",P~ceutic~y acceptable salt or prodrug thereof.
30 This invention also relates to a method of preventing or inhibiting the growth of
a tumor in a mammall including a human, comprising administering to said mammal
an antitumor effective amount of a compound of the formula I or a pharmaceutically
acceptable salt or prodrug thereof.
WO 95/01342 f~ 63~2 ~ PCT/~B94/00160
This invention also relates to a method of inhibiting growth factors that play an
important role in uncontrolled cell proliferation such as the EGF l~ceptor, the NGF
receptor, the PDGF receptor and the insulin receptor in a mammal, including a human,
comprising administering to said mammal a growth factor inhibiting effective amount
5 of a compound of the formula I or a pharmaceutically acceptable salt or prodrug
thereof.
The pharmaceutically acceptable salts of the present invention are those which
are non-toxic at the dosages ad",i"isteled. Since compounds of the invention maycontain basic groups, acid addition salts are possi~le. Pharmaceutically acceptable
10 acid addition salts include, for example, the hydrochloride, hydrobromide, hydroiodide,
sulfate, bisulfate, phosphate, acid phosphate, acetate, lactate, maleate, mesylate,
fumarate, citrate, acid citrate, tartrate, bitartrate, succinate, gluconate and saccharate
salts.
Typical pharmAceutic~"y acceptable anions include the ~et~tP;
15 benzenesulfonate; benzocLle; bicarbonate; bitartrate; bromide; calcium edetate;
camsylate; carbonate; chloride; citrate; dihydrochloride; edetate; edisylate; estolD.te;
esylate; fumarate; gluceplcle; gluconate; glutamate; glycollylarsnilate; hexylresorcinate;
hydroxynaphthoate; iodide; isothionate; lactate; lactobionate; malate; maleate;
mandelate; mesylate; methylbromide; methylnitrate; methylslllf~t~; mucate; napsylate;
20 nitrate; pamoate (embonate); pantothenate; phosphate; polyg~ t~ronate; salicylate;
stearate; sub~et~te; succinate; sulfate; tannate; tartrate; and teoclate.
Unless in~ic~ted otherwise, the alkyl, alkoxy, and alkenyl moieties r~rled to
herein may cor"prise linear, branched and cyclic moieties and combinations thereof
and the term ~halo" includes fluoro, chloro, bromo and iodo. It will be understood,
25 however that a group comprising only 1 or 2 atoms cannot be branched or cyclic.
i xamples of alkyl groups are methyl, ethyl, propyl, cyclopropyl, isopropyl, butyl, t-butyl,
cyclobutyl, pentyl, isopentyl, cyclopentyl, hexyl and cyclohexyl.
Furthermore, unless otherwise indicated optionally substituted means
comprising from zero to the maximum number of s~hstituents, e.g., 3 for a methyl30 group, 1 for a hexyl group and 5 for a phenyl group.
The active compounds of the invention may be administered orally, topically,
parenler~lly, by inhalation spray or rectally in dosage unit formulations containing
conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles.
WO 95/0134~, PCT/IB94/00160
~16~32~
-1 9-
The term parer,l~rdl as used herein includes subcutaneous injections,
- intravenous, intramusclJ~r, i"l,~ler"al injection or infusion techniques.
Detailed Descri~lion of the Invention
The following reaction schemes illustrate the preparation of the compounds of
5 the formula 1. In the reaction schemes and rliscussiQn that follow, except where
otherwise indicated, R1, R2, R4, R5, R~, R7, R8, R9 and R' are defined as for formula I
above.
wo 95/0l3n ~,~ 66~ PCT/IB94/00160 --
-20-
SCHEME 1
o o
CH30~J~ R9R3N~J¦~
Rl ~ ~H3
~ o~ R2 oJ;;~'`' ~ \R2
CH3 CH3 ~ CH3 CH3 ,
o
Rs 11
~)~1
CHI R2
CH3 CH3 OCH3
O O
CH3 R2 CH3
CH3 CH3 OCH3 CH3 CH3 OCH3
4 5
WO 95/OL342 ~16 6 3 2 0 PCT/IB94/00160
--21--
r~
O
~ Z ~""'11llo
o =Ij~o ",.,. <I~
fu zI
a~ / ~ ~ ¦
~< <J~ ~
~ ~ ~ ~.. """~)
'O
C~ l
~,' ~0 1~}~
~ 1 o =~ """ ~
~ZI ~ I
- 1 o~o ~ ~""""~
`~ I ~ Y
</-""0
I
~, ZI ~--o
~ ~ ~ ~>""""'1~
~0
1~ 0 Il^) O
WO 95/01342 PCT/IB94/00160 --
3~
-22-
As shown in Scheme 1 compound 2 is formed by condensation of the
geldanamyacin or 4,5-dihydrogeldanamyacin 1 with an amine R8R~NH. This reaction
is generally carried out by mixing the amine and the ansamycin in an inert solvent such
as chloroform, methylene chloride, N,N-dimethylformamide (DMF), pyridine, acetonitrile,
5 tetrahydrofuran (THF) or a lower alcohol, preferably chloroform or methylene chloride,
at a temperature from about ambient temperature to the reflux temperature of thesolvent, preferably from about ambient temperature to about 65C.
The conversion of 1 or 2 to 3 is generally carried out by oxidizing 2 with
standard oxidizing reagents such as pyridinium chlorochromate in methylene chloride,
10 pyridinium dichromate in DMF, oxalyl chloride/dimethyl sulfoxide (DMSO) in methylene
chloride, Dess-Martin periodinane in chloroform, and Jones reagent in acetone,
preferably Dess-Martin periodinane in chloroform at reflux. Those skilled in the art will
recGy"i~e that these reagents can be used with additional, inert solvents and attemperatures ranging from -60C to the reflux temperature of the solvent.
The conversion of 3 to 4 is generally carried out by reacting 3 with
hydroxylamine hydrochloride in the presence of a base (e.g., sodium acetate, pyridine,
sodium carbonate, sodium hydroxide, potassium carbonate, and triethylamine) in water
or a lower alcohol solvent at about 0C to about 100C. Preferably, 3 is combined
with hydroxylamine hydrochloride in the presence of triethylamine in ethanol at room
20 temperature.
The conversion of 3 to 5 is generally carried out under standard reductive
amination conditions such as combining the amine and 3 in an inert solvent (e.g.,
halogenated (Cl-C6) alkanes and (C1-C6)alcohols) with a suitable reducing agent (e.g.,
sodium borohydride, sodium cyanoborohydride, sodium triacetoxyborohydride and
formic acid), optionally in the presence of a dehydrating agent (e.g., sodium sulfate,
molecular sieves, and calcium sulfate), at temperatures ranging from about ambient
temperature to the reflux temperature of the solvent. Preferably, the reaction is carried
out by combining 3, the amine, sodium triacetoxyborohydride and sodium sulfate in
1,2-dichloroethane at ambient temperature.
As shown in Scheme 2 compound 1 or 2 can be selectively 22-N-alkylated to
afford 6 by treatment with a base, such as a (Cl-C6) alkoxide, in a polar solvent, for
instance dimethylformamide or dimethyl sulfoxide, followed by reaction with an
appropriate alkylating agent, for example, an alkyl halide. Reaction temperatures are
WO 95/01342 21~ 6 3 2 ~ PCT/IB94/00160
-23-
maintained between about 5 and about 65C, optimally from about 5 to about 25C.- Alternatively, the compound 1 or 2 can be reacted with anhydrous potassium carbonate
and the alkyl halide in acetone at reflux.
Compound 7 can be prepared by treating compound 1 or 2 with
5 diethylaminosulfurtrifluoride (DAST). This reaction is performed in an inert solvent (e.g.,
methyiene chloride, chloroform and dichloroethane) at low temperature of about -78 to
abolJt 0C, preferably from about -78 to about -50C. Optimally, the reaction isquenched at low temperature with dilute aqueous base, for example 5% sodium
bicarbonate.
10Compound 1 or 2 can be converted to 11-O-acyl or 1 1-O-sulfonyl derivatives bytreatment with acylating or sulfonating agents in the presence of non-nucleophilic
bases. The acylating agents include acid anhydrides, halides and isocyanates.
Sulfonating agents include sulfonyl halides and anhydrides.
Solvents used in these reactions include a wide variety of aprotic polar and non-
15polar media, for example, acetone, chloroform, ethyl acetate, DMF, pyridine,
tetrahydrofuran. Bases used include 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU),
triethylamine and 4-dimethylaminopyridine. If desired, compounds 3-7 wherein R5 is
RI4O may be converted to compounds 3-7 wherein R5 is R8R9N by the method for
converting 1 to 2 in Scheme 1.
20The preparation of other compounds of the formula I not specifically describedabove can be accomplished using combinations of the above reactions that will beclear to those skilled in the art in view of the foregoing r~isclos~ ~re.
The compounds of formula I and their pharmaceutically acceptable salts are
useful as antitumor agents (including, but not limited to anticancer agents) and25oncogene product inhibitors. They are useful, for example, in inhibiting the ErbB-2, src,
Ick, fyn and abl oncogene products. They are also useful in inhibiting certain growth
factors that play an important role in uncontrolled cell proliferation such as the EGF
eceptor, the NGF receptor, the PDGF receptor and the insulin receptor.
The ability of the active compounds to inhibit the ErbB-2 oncogene product may
30be determined by the following method for determining the p185 concentrations in
SKBr3 cells.
SKBr3 human breast cancer cells, obtained from the ATCC, Rockville, Maryland
were seeded in 8 well tissue culture plates (9.5 cm2/well, Falcon, Becton Dickenson,
WO 95/01342 PCT/IB94/00160 --
~fi~32~ ~
-24-
Lincoln Park, NJ) at 5 x 105 cells/well in 2 ml McCoys medium, supplemented with 10%
fetal calf serum and glutamine. Cells were allowed to attach overnight at 37C in a 5%
CO2 atmosphere.
The compounds are dissolved in DMSO and tested over a range of
5 conce, Ill ~lions by addition to the medium, followed by incubation at 37C for 6 hours.
At the end of the incubation, the medium is aspirated from the well, and the cells are
washed twice with 2 ml of TNK buffer (50 mM tris (hydroxymethyl)aminomethane
hydrochloride,140 mM NaCI, 3.3 mM KCI, 0.5 mM sodium orthovanadate, adjusted to
pH 7.4). The cells are then Iysed by addition of 250,ul boiling Laemmli sample buffer
10 (140 mM tris(hydroxymethyl)aminomethane hydrochloric acid, pH 6.8, 5.7% sodium
dodecyl sulfate,29% glycerol) with shaking. The cell Iysate is transferred to a tube and
then placed in a boiling water bath for 5 mins. The Iysates are then sonicated with a
probe sonicator and stored at -70C until analysis.
The p185 concer,l, ~lion of each sample may be determined by standard
15 immunoblotting procedures essentially as described by Harlow and Lane (Antibodies:
A Laboratory Manual. Cold Spring Harbor Laboratory, 1988). A standard portion ofeach sample is mixed with dithiothreitol (10% added of a 1M solution), and then a
portion corresponding to--10 IJ9 of protein is blotted onto a nitrocellulose membrane
(BA-S, Schleicher and Schuell, Keene, New Hampshire) equilibrated with rinse buffer
20 (10 mM Tris hydrochloric acid pH 7.4, 150 mM NaCI) by use of a dot blot apparatus
(Mini-fold, Schleicher and Schuell, Keene, New Hampshile) with an underlayer of filter
paper. The wells are rinsed with 200,ul of a rinse buffer, blocked by incubation with a
blocking buffer (5% bovine serum albumin, 1% ovalbumin in rinse buffer), and then
inc~hAted for 4 to 12 hours with a 1:1000 dilution of NT1, an affinity purified rabbit
25 polyclonal antibody raised by standard methods (Harlow and Lane, Antibodies, A
Labor~lo~ Manual, Cold Spring Harbor Laboratory, 1988) against a peptide
representing the C-terminal domain of human p186 (sequence, TAENPEYLGLDVPV, by
the standard 1 letter amino acid code). The membrane is then rinsed twice for 10minutes with rinse buffer and once for 10 minutes in rinse buffer with 0.05% Triton X-
30 100, and then twice more for 10 minutes in rinse buffer. The membrane is thenincuhAtecl with a 1 :3000 dilution of horseradish peroxidase labeled donkey anti-rabbit
antibody (Amersham, Arlington Heights, lllinois) in a rinse buffer with shaking for 20-45
minutes. The membrane is then again rinsed twice for 10 minutes in the rinse buffer,
WO 95/01342 ~ ~ 6 6 3 2 ~ PCT/IB94/00160
-25-
once for 10 minutes in the rinse buffer with 0.05% Triton X-100, and then twice more
- for 110 minutes in the rinse buffer. The pl85 is then visuAIi~ed with the ECL Detection
Kit (,~mersham, Ariington I lei~l ,ls, Illinois) and recorded with Hyperfilm-ECL (Amersham,
Arlington I laiyhl~a, Illinois). The pl 85 is then estimated by densitometric analysis of the
5 film. IC50 values are determined by reference to the pl 85 content of samples of cells
exposed only to vehicle (DMSO) and measured as described.
The ability of the active compounds to inhibit the ErbB-2 oncogene product may
be determined byfollowing the method of Kamps et al., Oncogene, 2, 305-315 (1988)
for determining the phosphorylation of pl85 in SKBR3 and other ErbB-2 transformed
10 cell lines.
The ability of the active compounds to inhibit the growth of certain human
carcinoma cells may be determined by the methods of Alley et al., Cancer Research
48, 589-601 (1988) using SKBr3 and MCF7 cell lines. This le~elence is incorporated
herein in its entirety.
When the compounds of the formula I and their pharmAceuticAIly acceptable
salts are used as antiproliferative agents, such as anticancer agents, they can be
administered to a mamr"alian subject either alone or, preferably, in combination with
phal",~ceutically acceplable carriers or diluents in a pharmaceutical co"~posilion
according to standard phar"~aceutical practice. The compounds can be ad" ,i"ialered
orally or parer,le,Glly. Parer,lelal administration includes intracenous, intramusc~ r,
intraperitoneal, subcutaneous and topical.
In general, the active compounds are administered in dosages ranging from
about 0.1 mg to about 20 mg per kg of body weight as needed (e.g., every 4 to 6
hours), preferably from about O.1 to about 15 mg per kg of body weight; variations will
necessArily occur depending upon the condition of the subject being treated and the
particular compound and dosage form being admir-ialered. It is to be noted that these
compounds may be administered in combination with pharmaceutically acceptable
carriers by either of the routes previously indicated, and that such administration can
be carried out in both single and multiple dosages.
The active compounds of the invention may be administered orally, topically,
par~r,ler~lly, by inhalation spray or rectally in dosage unit formulations containing
conventional non-toxic pharmaceutically acceptable carriers, adjuvants and vehicles.
The term parer,le,cll as used herein includes subcutaneous injections, intravenous,
WO 9S/01342 PCT/IB94/00160 --
2 ~
-26-
intramuscular, il,l,asler"al injection or infusion techniques.
The pharmaceuticai compositions containing the active ingredient may be in a
form suitable for oral use, for example, as tablets, troches, lozenges, aqueous or oily
suspensions, dispersible powders or granules, emulsions, hard or soft capsules, or
5 syrups or elixirs. Compositions intended for oral use may be prepared according to
any method known to the art for the manufacture of pharmaceutical compositions and
such compositions may contain one or more agents selected from the group col~si~lil ,9
of sw,eetening agents, flavoring agents, coloring agents and preserving agents in order
to provide pha",~aceutically elegant and palatable preparations. Tablets contain the
10 active ingredient in admixture with non-toxic pharmaceutically acceptable excipients
which are suitP~Ie for the manufacture of tablets. These excipients may be, for
example, inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium
pllosphate or sodium phosphate; granulating and di~illleylalillg agents, for example,
corn starch, or alginic acid; binding agents, for example, starch, gelatin or acacia, and
15 lubricating agents, for example magnesium stearate, stearic acid or talc. The tablets
may be uncoated or they may be coated by known techniques to delay disintegration
and absorption in the gastrointestinal tract and thereby provide a sustained action over
a longer period. For example, a time delay material such as glyceryl mono:,learala or
glyceryl d;~tearale may be employed. They may also be coated by the techniques
20 described in, e. g., the U.S. Pat. Nos. 4,256,108; 4,166,452; and 4,266,874 to form
osmotic therapeutic tablets for control release.
The hard capsules for oral use may also be presenled as gelatin capsules
wherein the active ingredient is mixed with an inert solid diluent, for example, calcium
carbonate, calcium phosphate or kaolin, or whereas the soft capsules may be
25 preser,led as gelatin c~ps~ ~'es wherein the active ingredient is mixed with water or an
oil medium, for example peanut oil, liquid paraffin, or olive oil.
Aqueous suspensions contain the active materials in admixture with ex~ Jier,l~
suitable for the manufacture of aqueous suspensions. Such excipients are suspending
agents, for example, sodium carboxymethylcellulose, methylcellulose, hydroxy-
30 propylmethylcell~ ~'cse, sodium alginate, polyvinyl pyrrolidone, gum tragacanth and gumacacia; dispersing or wetting agents may be a naturally-occurring phosphatide, for
example lecithin, or condensation products of an alkylene oxide with fatty acids, for
example polyoxyethylene stearate, or condensation products of ethylene oxide with
~ WO 95/01342 PCT/IB94/00160
~16~32~
-27-
long chain aliphatic alcohols, for example heptadecaethyleneoxycetanol, or
condensation products of ethylene oxide with partial esters derived from fatty acids and
a hexitol such as polyoxyethylene sorbitol monooleate, or condensation products of
ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides, for
5 example polyethylene sorbitan monooleate. The aqueous suspensions may also
contain one or more preservatives, for example ethyl, or n-propyl, p-hydroxybenzoate,
one or more coloring agents, one or more flavoring agents, and one or more
sweetening agents, such as sucrose or saccharin.
Oiiy suspensions may be formulated by suspending the active ingredient in a
10 vege.table oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a mineral
oil such as liquid paraffin. The oily suspensions may contain a thickening agent, for
exarr ple beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as those set
forth above, and flavoring agents may be added to provide a palatable oral preparation.
These compositions may be preserved by the addition of an anti-oxidant such as
15 ascorbic acid.
Dispersible powders and granules suitable for preparation of an aqueous
suspension by the addition of water provide the active ingredient in admixture with a
disper:,i"g or wetting agent, suspending agent and one or more preservatives. Suitable
dispersing or wetting agents and suspending agents are exemplified by those already
20 mentioned above. Additional excipients, for example sweetening, flavoring and coloring
agents may also be present.
The pharmaceutical compositions of the invention may also be in the form of oil-in water emulsions. The oily phase may be a vegetable oil, for example, olive oil or
arachis oil; a mineral oil such as liquid paraffin or mixtures of these. Suitable
25 emulsifying agents may be naturally occurring gums, for example, gum acacia or gum
tragacanth; naturally-occurring phosphatides such as example soy bean and lecithin;
and esters or partial esters derived from fatty acids and hexitol anhydrides, for example,
sorbitan monooleate, and condensation products of the said partial esters with ethylene
oxide, for example polyoxyethylene sorbitan monooleate. The emulsions may also
30 contain swet:leni"g and flavoring agents.
Syrups and elixirs may be formulated with sweetening agents, for example
glycerol, propylene glycol, sorbitol or sucrose. Such formulation may also contain a
demulcent, a preservative and flavoring and coloring agents. The pharmaceutical
WO 95/01342 PCT/IB94/00160 ~
2~66~
-28-
compositions may be in the form of a sterile injectable aqueous or oleaginous
suspension. This suspension may be formulated according to the known art using
those suitable dispersing or wetting agents and suspending agents which have been
mentioned above. The sterile injectable preparation may also be a sterile injectable
solution or suspension in a non-toxic pare"lt:rGlly-acceptable diluent or solvent, for
example as a solution in 1 ,3-butane diol. Among the acceptable vehicles and solvents
that may be employed are water, Ringe~s solution and isotonic sodium chloride
solution. In addition, sterile, fixed oils are conventionally employed as a solvent or
suspending medium. For this purpose any bland fixed oil may be employed including
synthetic mono or diglycerides. In addition, fatty acids such as oleic acid find use in
the preparation of injectables.
The active compounds of the invention may also be administered in the form of
suppositories for rectal administration of the drug. These compositions can be
prepaled by mixing the drug with a s~itAhle non-irritating excipient which is solid at
ol di"ary temperatures but liquid at the rectal temperature and will therefore melt in the
rectum to release the drug. Such materials include cocoa butter and polyethyleneglycols.
For topical use, creams, ointments, Jellies, solutions or suspensions, etc.,
containing the active compounds of the invention are employed.
For administration by inhalation, the active compounds of the invention are
conveniently delivered in the form of an aerosol spray presentation from pressurized
packs or a nebulizer. The pr~t,r,ed composition for inhalation is a powder which may
be formulated as a cartridge from which the powder composition may be inhaled with
the aid of a suitable device. In the case of a pressurized aerosol, the dosage unit may
be determined by providing a valve to deliver a metered amount.
One or more other active compounds may be added to the formulations
described above to provide formulations for combination therapy. Such compounds
include cytostatic, cytotoxic and antiemetic agents conventionally used in cancer
chemotherapy, such as adriamycin.
The following examples illustrate the invention but are not to be construed as
limiting the same. All melting points are uncorrected. In the Examples, HBOC" refers
to -butoxycarbonyl .
WO 95/01342 PCT/IB94/00160
~632~
-29-
EXAMPLES
General Methods
High pressure liquid chromatography (HPLC) was performed at 1.0 mLlminute
with 254 nm detection on a 250 x 4.6 mm Dupont Zorbax Sil (trademark) column eluted
isocratically by a two-pump/mixer system supplying the in~ic~ted mixture of 1%
methanol in ethyl acetate and hexanes respectively. Samples to be thus analyzed are
lissolved in an HPLC eluent. The HPLC retention times are reported followed by the
ethyl~cet~t~/hexane ratio in parentheses. The terms ~concer,llGted in vacuo" and~coevaporated" refer to removal of solvent at water aspirator pressure on a rotary
evaporator with a bath temperature of less than 40C.
EXAMPLE 1
17-lsopropylamino4,5-dihvdro-17-demethoxyqeldanamvcin
To 4,5-dihydro-geldanamycin (75 mg, 0.13 mmol) in CHCI3 was added
isopropylamine (68,uL, 0.80 mmol) and the reaction stirred at room temperature for 24
hours at which time TLC analysis indicated the reaction was not complete. The
reac~ion mixture was then heated at reflux for 3 hours. The solvent was removed by
rotary evaporation and the purple residue partitioned between ethyl acetate and 1 M
hydrochloric acid. The organic layer was dried, the solvent removed by rotary
evaporation, and the crude material purified by column chromatography (silica gel, 9:1
CH2CI2:methanol) to give the title compound as a purple solid; Yield 56 mg (72%), mp
114-115C; 1H-NMR (300 MHz, CDCI3) ~ 0.95 (d, 3 H, J = 7 Hz), 1.00 (d, 3 H, J = 7
Hz), 1.2 (d, 3 H, J = 7 Hz), 1.35 (d, 3 H, J = 7 Hz), 1.7 (s, 3 H), 1.9 (s, 3 H), 2.2 (dd,
1 H,J=14,7),2.4(dd,1 H,J=14Hz,7Hz),2.5-2.8(m,1 H),3.3(m,1 H),3.35(s,
3 H), 3.4 (s, 3 H), 3.4-3.5 (m, 1 H), 3.6 (d, 1 H, J = 9 Hz), 3.7 (br s, 1 H), 4.1 (m, 1 H),
4.95(s,2H),5.2(d,1 H,J=7HzHz),5.75(d,1 H,J=9Hz),6.2(t,1 H,J=9Hz),
7.1 (s, 1 H), 9.3 (br s, 1 H); mass spectrum m/z 612 (M + Na).
The compounds of Examples 2-14 were prepared from 4,5-dihydro-
geldanamycin and the appropriate amines using the conditions described above.
EXAMPLE 2
17-Amino-4.5-dihydro-17-demethoxyqeldanamvcin
1H-NMR (300 MHz, CDCI3) ~ 0.95 (m, 6 H), 1.5-1.8 (m, 8 H, contains methyl
singlet), 1.85 (s, 3 H), 2.0 (m,1 H), 2.35 (m, 2 H), 2.6 (m, 1 H), 2.7 (m, 1 H), 3.2-3.5 (m,
8 H, contains 2 methyl singlets), 3.8 (m, 2 H), 5.0 (br s, 2 H), 5.1 (d, 1 H, J = 4 Hz), 5.6
WO 95/01342 PCT/IB94/00160 ~
~63~
-30-
(br s, 2 H), 5.7 (d, 1 H, J = 10 Hz), 6.2 (t, 1 H, J = 7 Hz), 7.05 (s, 1 H), 9.15 (s, 1 H);
mass spectrum m/z 547 (M+); Analysis calculated for C28H4lN3O8-0.5 H2O: C, 60.42;
H, 7.60; N, 7.55%. Found: C, 60.42; H, 7.45; N, 7.51%.
EXAMPLE 3
1 7-Methylamino4.5-dihvdro-1 7-demethoxvqeldanamycin
Mp 181C; 'H-NMR (300 MHz, CDCI3) ~ 1.0 (m, 6 H), 1.5-1.8 (m, contains
methyl singlet, 9 H), 1.9 (s, 3 H), 2.3-2.8 (m, 5 H), 3.2 (d, 3 H, J = 7 Hz), 3.3-3.45 (m,
contains 2 methyl singlets, 8 H), 3.45-3.55 (m, 1 H), 3.6 (br d, 1 H, J = 7 Hz), 3.75 (br
s, 1 H), 4.7 (br s, 2 H), 5.2 (d, 1 H, J = 7 Hz), 5.8 (d, 1 H, J = 10 Hz), 6.25 (t, 1 H, J
= 7 Hz), 6.4-6.55 (m, 1 H), 7.15 (s, 1 H), 9.3 (br s, 1 H); mass spectrum m/z 561 (M+);
Analysis c~lcul~ted for C29H43N3O8: C, 62.01; H, 7.72; N, 7.48%. Found: C, 61.60; H,
7.73; N, 7.16%.
EXAMPLE 4
1 7-Cyclo~oropylamino-4,5-dihvdro-1 7-demethoxvgeldanamycin
Mp 14~147C; lH-NMR (300 MHz, CDCI3) ~ 0.6-0.9 (m, 4 H), 0.9-1.1 (m, 6 H),
1.5-1.8 (m, co"ltli"s methyl singlet, 6 H), 1.9 (s, 3 H), 2.3-2.5 (m, 2 H), 2.5-2.8 (m, 2 H),
2.8-3.0 (m, 2 H), 3.2-3.4 (m, corllai"s 2 methyl singlets, 8 H), 3.4-3.5 (m, 1 H), 3.5-3.7
(br d, 2 H), 4.9 (br s, 2 H), 5.15 (d, 1 H, J = 7 Hz), 5.75 (d, 1 H, J = 10 Hz), 6.2 (t, 1
H, J = 7 Hz), 6.35 (d, 1 H, J = 3), 7.1 (s, 1 H), 9.25 (br s, 1 H); mass spectrum m/z 587
(M+); Analysis c~lc~ t~d for C3l H45N3O8-0.5 H2O: C, 62.40; H, 7.77; N, 7.04%. Found:
C, 62.55; H, 7.61; N, 6.83%.
EXAMPLE 5
1 7-Allvlamino-4.5-dihydro-1 7-demethoxvqeldanamvcin
Mp 205C; lH-NMR (300 MHz, CDCI3) ~1.0 ( m, 6 H, 2 methyl doublets), 1.5-1.8
(m, 8 H, contains methyl singlet), 1.9 (s, 3 H), 2.2-2.5 (m, 3 H), 2.5-2.8 (m, 2 H), 3.2-3.5
(m, 8 H, contains 2 methyl groups), 3.6 (d, 1 H, J = 7 Hz), 4.1 (m, 2 H), 4.8 (s, 2 H),
5.2 (d, 1 H, J = 7 Hz), 5.25 (d, 1 H, J = 10 Hz), 5.3 (s, 1 H), 5.75 (d, 1 H, J = 10 Hz),
5.9 (m, 1 H), 6.25 (t, 1 H, J = 7 Hz), 6.4 (br t, 1 H), 7.25 (s, 1 H), 9.25 (br s, 1 H); mass
spectrum m/z 610 (M + Na); Analysis calculated for C31H45N3O8-0.5 H2O: C, 62.40; H,
30 7.77; N, 7.04%. Found: C, 62.26; H, 7.83; N, 6.75%.
~ WO 95/01342 PCT/IB94/00160
216~3~0
-31 -
E)CAMPLE 6
1 7-(2'-Hydroxyethvlamino)4,5-dihydro-1 7-demethoxyqeldanamvcin
Mp 129C ffoam); 1H-NMR (300 MHz, CDC13) ~ 0.9-1.1 (m, 6 H), 1.6-1.8 (m,
cor,l~, 15 methyl singlet, 8 H), 1.9 (s, 3 H), 2.3-2.5 (m, 3 H), 2.7-2.8 (m, 2 H), 3.2-3.5 (m,
5 contains 2 methyi singlets, 8 H), 3.55-3.65 (d, 1 H, J = 10 Hz), 3.65-3.8 (m, 2 H), 3.8-
4.0 (m, 1 H), 4.85 (br s, 2 H), 5.15 (d, 1 H, J = 4 Hz), 5.78 (d, 1 H, J = 10 Hz), 6.2 (t,
1 H, J = 7 Hz), 7.1 (s, 1 H), 9.21 (s, 1 H); mass spectrum m/z 591 (M+); Analysis
c~c~ ted for C30H45N309: C, 60.90; H, 7.67; N, 7.10%. Found: C, 60.40; H, 7.89; N,
6.63%.
EXAMPLE 7
1 7-r2'-Methoxyethylamino)-4,5-dihydro-1 7-demethoxyqeldanamycin
Mp 115C (dec); lH-NMR (300 MHz, CDCI3) ~ 0.8-1.0 (m, 6 H), 1.5-1.8 (m,
con~i"s broad methyl singlet, 7 H), 1.85 (br s, 3 H), 2.2-2.5 (m, 3 H), 2.5-2.8 (m, 2 H),
3.2-3.5 (m, contains 3 methy singlets, 12 H), 3.5-3.8 (m, 5 H), 5.0 (br s, 2 H), 5.15 (d,
15 1 H, J = 7 Hz), 5.7 (d, 1 H, J = 10 Hz), 6.2 (br t, 1 H, J = 7 Hz), 6.55 (br s, 1 H), 7.1
(s, 1 H), 9.25 (br s, 1 H); mass spectrum m/z 605 (M+); Analysis c~lclJl~ted forC3lH47N309: C, 61.47; H, 7.82; N, 6.94%. Found: C, 61.0; H, 7.58; N, 6.7196.
E)CAMPLE 8
1 7-(2'-FluoroethYlamino)-4,5-dihYdro-1 7-demethoxvqeldanamYcin
Mp 157C ffoam); 1H-NMR (300 MHz, CDCI3) ~ 1.0 (m, 6 H), 1.6-1.8 (m,
contains methyl singlet, 8 H), 1.9 (s, 3 H), 2.0-2.1 (br s, 1 H), 2.2-2.5 (m, 3 H), 2.6-2.8
(m, 2 H), 3.2-3.7 (m, contains 2 methyl singlets, 8 H), 3.7-4.0 (m, 2 H), 4.55 (t, 1 H, J
=7Hz),4.74.85(m,3H),5.2(d,1 H,J=7Hz),5.8(d,1H,J=lOHz),6.25(t,1 H,
J = 7 Hz), 6.4 (t, 1 H, J = 7 Hz), 7.15 (s, 1 H), 9.2 (s, 1 H); mass spectrum m/z 593
25 (M+)-
E)(AMPLE 9
1 7-(2'-Methvlthioethylamino)-4.5-dihvdro-1 7-demethoxyqeldanamvcin
Mp 110C ffoam); lH-NMR (300 MHz, CDCI3) ~1.0 (m, 6 H), 1.5-1.75 (m,
contains methyl singlet, 8 H), 1.85 (s, 3 H), 2.1 (s, 3 H), 2.2-2.4 (m, 3 H), 2.5-2.8 (m, 4
30 H), 3.2-3.5 (m, contains 2 methyl singlets, 8 H), 3.5-3.8 (m, 4 H), 5.0 (s, 2 H), 5.15 (d,
1 H, J = 4 Hz), 5.75 (d, 1 H, J = 10 Hz), 6.2 (t, 1 H, J = 7 Hz), 6.6 (t, 1 H, J = 4 Hz),
7.1 (s, 1 H), 9.2 (s, 1 H); mass spectrum m/z 644 (M + Na); Analysis c~lc~lls.tPd for
WO 95/01342 PCTI~94/00160 ~
2~ -32-
C31H47N308s-0.5 H20: C, 59.03; H, 7.67; N, 6.66%. Found: C, 58.87; H, 7.67; N,
6.60%
EXAMPLE 10
1 7-rs-(+)-2'-HvdroxyproPvlaminol4~5-dihvdro-l 7-demethoxvqeldanamycin
lH-NMR (300 MHz, CDCI3) ~ 0.95 (d, 3 H, J = 7 Hz), 1.0 (d, 3 H, J = 7 Hz), 1.3
(d, 3 H, J = 7 Hz), 1.5-1.75 (m, contains methyl singlet, 6 H), 1.85 (s, 3 H), 2.25-2.45
(m, 2 H), 2.6-2.7 (m, 1 H), 3.2-3.5 (m, contains 2 methyl singlets, 8 H), 3.5-3.7 (m, 2 H),
4.05 (m, 1 H), 4.9 ts, 2 H), 5.15 (d, 1 H, J = 7 Hz), 5.8 (d, 1 H, J = 7 Hz), 6.2 (t, 1 H,
J = 7 Hz), 6.7 (br s, 1 H), 7.05 (s, 1 H), 9.2 (br s, 1 H); mass spectrum m/z 605.3 (M+).
EXAMPLE 11
17-(2'-Cyanoethvlamino~4,5-dihvdro-17-demethoxvqeldanamycin
Mp 130-140C ffoam); 1H-NMR (300 MHz, CDCI3) ~ 1.0 (m, 6 H), 1.5-1.8 (m,
cor,~ni"s methyl singlet, 8 H), 1.9 (s, 3 H), 2.0-2.2 (m, 2 H), 2.4 (q, 2 H, J = 7 Hz), 2.6-
2.8 (m, 4 H), 3.2-3.5 (m, contains 2 methyl singlets, 8 H), 3.6 (br d, 1 H, J = 7 Hz), 3.85
(q,2H,J=7Hz),4.75(brs,2H),5.2(d,1 H,J=7Hz),5.75(d,1 H,J=lOHz),6.2
(q, 2 H, J = 7 Hz), 7.15 (s, 1 H), 9.15 (s, 1 H); mass spectrum m/z600 (M+); Analysis
c~lcl~ tecl for C3lH44N408: C, 61.98; H, 7.38; N, 9.33%. Found: C, 61.30; H, 7.31; N,
9.12%.
EXAMPLE 12
17-Azetidin-1 -yl4,5-dihYdro-17-demethoxvqeldanamycin
Mp 110C ffoam); 1H-NMR (300 MHz, CDCI3) ~0.95 (d, 3 H, J = 7 Hz), 1.0 (d,
3 H, J = 7 Hz), 1.7 (s, 3 H), 1.6-1.8 (m, 9 H), 1.9 (s, 3 H), 2.2 (dd, 1 H, J = 14 Hz, 7
Hz), 2.3-2.55 (m, 4 H), 2.65 (d, 1 H, J = 14 Hz), 2.75 (m, 1 H), 3.35 (m, 1 H), 3.38 (s,
3 H), 3.4 (s, 3 H), 3.5 (m, 1 H), 3.6 (d, 1 H, J = 10 Hz), 4.54.8 (m, 6 H), 5.2 (d, 1 H,
J = 7 Hz), 5.7 (d, 1 H, J = 10 Hz), 6.25 (t, 1 H, J = 10 Hz), 7.0 (s, 1 H), 9.3 (br s, 1 H);
mass spectrum m/z 587 (M+).
EXAMPLE 13
1 7-(3'-HydroxYazetidin-1 -vl)-4.5-dihydro-1 7-demethoxvqeldanamycin
Mp (amorphous); 1H-NMR (300 MHz, CDCI3) ~ 1.0 (m, 6 H),1.5-1.8 (m, contains
1 methyl singlet, 8 H), 1.9 (s, 3 H), 2.2 (m, 1 H), 2.35 (m, 2 H), 2.6 (br d, 1 H, J = 14
Hz), 2.75 (m, 1 H), 3.2-3.5 (m, contains 2 methyl singlets, 8 H), 3.6 (d,1 H, J = 10 Hz),
4.34.6 (m, 2 H), 4.7 (m, 1 H), 4.75-5.0 (m, 4 H), 5.15 (d, 1 H, J = 4 Hz), 5.8 (d, 1 H,
J = 10 Hz), 6.2 (brt, 1 H, J = 7 Hz), 6.95 (s, 1 H), 9.2 (s, 1 H); mass spectrum mlz 626
~ WO 9S/01342 216 6 3 ~ O PCT/IB94/00160
(M t- Na); Analysis calcul~t~d for C3lH45N3Og: C, 61.68; H, 7.51; N, 6.96%. Found: C,
61.21; H, 7.51; N, 6.75%.
EXAMPLE 14
1 7-(3'-Methoxvazetidin-1 -yl)4,5-dihvdro-1 7-demethoxvqeldanamycin
Mp 118C ffoam); lH-NMR (300 MHz, CDCI3) ~ 1.0 (m, 6 H, two methyl
doublets), 1.5-1.8 (m, 8 H, contains methyl singlet), 1.9 (s, 3 H), 2.0-2.3 (m, 1 H), 2.4
(m, :2 H), 2.5-2.8 (m, 2 H), 3.2-3.5 (m, 11 H, contains 3 methyl singlets), 3.6 (m, 1 H),
4.0(d,1 H,J=7Hz),4.2(m,1 H),4.34.6(m,2H),4.64.9(m,4H),5.2(d,1 H,J=
4 Hz), 5.8 (d, 1 H, J = 10 Hz), 6.25 (brt, 1 H, J = 7 Hz), 7.0 (s, 1 H), 9.25 (s, 1 H);
mass spectrum m/z 640 (M+ + Na); Analysis calculated for C32H47N3Og-HzO C, 60.84;
H, 718; N, 6.65%. Found: C, 60.74; H, 7.54; N, 6.75%.
EXAMPLE 15
1 7-Azetidin-1 -vl4.5-dihvdro-11 -a-fluoro-1 7-demethoxyqeldanamycin
A solution of diethylaminosulfurtrifluoride (DAST) (0.154 9, 0.960 mmol, 0.127
mL) in 3 mL of methylene chloride was added to a flame dried flask under nitrogen and
cooled to -68 C with an external dry ice/acetone bath. 1 7-Azetidine-1 -yl4,5-dihydro-17-
demethoxygeldana"~ycin (0.188 9, 0.320 mmol) dissolved in 15 mL of methylene
chloride was added dropwise. After 0.5 hour 5 mL of 5% ~lueous NaHCO3 was added
slowly at about -68C. After warming to room temperature the product was extracted
into 100 mL of methylene chloride. The organic layer was washed with 3 x 50 mL of
water and 2 x 50 mL of brine, dried with MgSO4, filtered and concentrated to a purple
solid which was purified by flash column chromatography using 5:95
methanol:chloroform. Material of Rf = .42 (1:9 methanol:chloroform), the desiredproduct was disolved in a minimal amount of ethyl acetate and ple.,i~ led with
hexanes; Yield 0.096 g (51%), mp 104C (dec); 1H-NMR (300 MHz, CDCI3) ~1.00(d,
J = 8 Hz, 3H, 14-CH3), 1.06(d, J = 8 Hz, 3H, 10-CH3), 1.4 (br m, 2H, H-13), 1.56 (s, 3H,
8-CH3), 1.75 (m, 1H, H-14), 1.89 (s, 3H, 2-CH3), 2.20 (dd, J = 8.5 Hz, 16 Hz, 1H, H-15),
2.4 (br m, 3H, 3' azetidine CH2 and H-5), 2.66 (dd, J = 7 Hz, 16 Hz, 1 H, H-15), 2.75 (br
d, J = 26 Hz, 1H, H-10), 3.25 (m, 1H, H4), 3.4 (br s, 7H, 6-OCH3, 12-OCH3 and H4),
3.60 (br m, 1 H, H-12), 4.40 (br d, J = 44 Hz, 1 H, H-11), 4.65 (br m, 7H, NH2 and 2' and
4' azetidine CH2 and H-6), 5.06 (d, J = 8 Hz, lH H-7), 5.62 ( d, J = 9 Hz, lH, H-9), 6.13
(brt, lH, H-3), 6.96 (s, lH, H-19), 9.27 (s, lH, NH-22); m/z 612. (M+ + Na); IR (KBr,
WO 95/01342 ~ PCT/IB94/00160
-34-
cm-') 1735, 1695, 1650; Analysis calculated for C3lH44FN307-0.25H20: C, 62.66; H,
7.96; N, 7.07%. Found: C, 62.38; H, 7.53; N, 6.97%.
EXAMPLE 16
17-Allvlamino-4,5-dihydro-11 -a-fluoro-17-demethoxyqeldanamycin
The title compound was made by the method of Example 15 from 1 7-allylamino-
4,5-dihydro-17-demethoxygeldanamycin. Yield 0.079 g (44%), mp 84C (dec); 1H-NMR(300 MHz, CDCI3) ~ 1.02(d, 3H, J = 8Hz, 14-CH3), 1.07(d, 3H, J = 8Hz, 10-CH3), 1.45
(br m, 2H, H-13), 1.60 (s, 3H, 8-CH3), 1.7 (m, 2H, H-5), 1.83 (br m, 1 H, H-14), 1.90 (s,
3H, 2-CH3), 2.30 (dd, J = 8.5 Hz, 16 Hz, lH, H-15), 2.4 (m, 2H, H4), 2.70 (dd, J = 7
Hz, 16 Hz, lH, H-15), 2.75 (brd, J = 26 Hz, lH, H-10), 3.26 (m, lH, H-6), 3.40 and 3.43
(br s, 6H, OCH3), 3.57 (br m, 1 H, H-12), 4.08 (br t, 2H, allylic CH2), 4.35 (br d, J = 47
Hz, 1 H, H-11), 4.65 (br m, 2H, NH2), 5.07 (s, 1 H H-7), 5.25 (br d, 2H, vinylic CH2), 5.61
(d, J = 9 Hz, lH, H-9), 5.9 (m, 2H, H-5 and vinylic CH), 6.15 (brt, lH, H-3), 6.32 (brt,
lH, NH), 7.15 (s, lH, H-19), 9.25 (s, lH, NH-22); m/z 612. (M+ + Na); IR (KBr, cm l)
1730, 1695, 1655; Analysis cpllc~ ted for C31H44FN307-0.25H20: C, 62.66; H, 7.96; N,
7.07%. Found: C, 62.53; H, 7.32; N, 6.97%.
EXAMPLE 17
17-Azetidin-1 -yl4,5-dihydro-11 -keto-17-demethoxygeldanamYcin
Prepared from 17-azetidin-1-yl-4,5-dihydro-17-demethoxygeldanamycin by the
method of Example 76; 1H-NMR (300 MHz, CDCI3) ~ 1.0 (d, 3 H, J = 6), 1.3 (d, 3 H, J
= 6), 1.35-1.5 (m, 2 H), 1.5-1.8 (rr~, 6 H, contains methyl singlet), 1.9 (s, 3 H), 2.15-2.3
(m, 2 H), 2.45-2.5 (m, 2 H), 2.6 (m, 1 H), 3.15 (m, 1 H), 3.35 (s, 3 H), 3.4 (s, 3 H), 3.55
(m, 1 H), 4.0 (m, 1 H), 4.65 (m, 4 H), 4.8 (br s, 2 H), 5.0 (d, 1 H, J = 6 Hz), 5.55 (d, 1
H, J = 8 Hz), 6.25 (m, 1 H), 6.92 (s, 1 H), 9.2 (s, 1H); mass spectrum m/z 608 (M
Na);
EXAMPLE 18
17-Azetidin-1 -Yl-17-demethoxvqeldanamycin
Geldanamycin (14.0 gm, 25.0 mmol) was added to a flame dried flask under
nitrogen and slurried in 350 mL of methylene chloride. Azetidine (2.85g, 49.9 mmol,
3.36 mL) in 10 mL of methylene chloride was added dropwise. The yellow suspension
turned purple during the addition. A~ter 1 hour the reaction mixture was evaporated to
dryness and the residue dissolved in 50 mL of chloroform and precipitated with 600 mL
of hexanes. Filtration and vacuum drying at 70C afforded pure product, yield 14.2 gm
WO 9~/0134~, PCT/IB94/00160
~6~2~
-35-
(97%); 225 C; lH-NMR (300 MHz, CDCI3) ~ 0.94 (brt, 6H,10-CH3 and 14-CH3),1.2 (m, lH, H-13), 1.65 (m, lH, H-13), 1.73 (m, lH, H-14), 1.76 (s, 3H, 8-CH3), 2.0 (s, 3H, 2-
CH3), 2.17 (dd, J = 12 Hz, 16 Hz, lH, H-15), 2.40 (p, J = 8 Hz, 2H, 3' azetidine CH2),
2.56 (d, J = 16 Hz, lH, H-15), 2.67 (m, lH, H-10), 3.20(s, 3H, OCH3), 3.30 (s, 3H,
5 OCH3), 3.40 (m, lH, H-12), 3.50 (m, lH, H-11), 4.25 (d, J = 10.5 Hz, 1H, H-6), 4.5-4.9
(m, 6H, 2'and 4' azetidine CH2 and NH2), 5.13 (s, lH H-7), 5.79 (t, J = 9 Hz, lH, H-5),
5.87 (d, J = 9 Hz, lH, H-9), 6.53 (t, J = 9 Hz, lH, H4), 6.88 (d, J = 9 Hz, lH, H-3),
7.06 (s,1 H, H-19), 9.13 (s,1 H, NH-22); m/z 608. (M+ + Na); IR (KBr, cm~l) 1730,1680,
1645; Analysis calc~ t~d for C3l H43N3O8: C,63.54; H, 7.40; N, 7.17%; Found: C,63.09;
10 H, 7.33; N, 6.85%.
EXAMPLE 19
17-Propargylamino-17-demethoxYqeldanamycin
r, oparyylamine hydrochloride (0.200 gm,2.180 mmol) and triethylamine (0.2206
gm, 2.180 mmol, 0.303 mL) were added to a flame dried flask under nitrogen and
15 slurried in 5mL chloroform. After 10 minutes geldanamycin (0.200 gm, 0.3567 mmol)
was added to the mixture and the reaction was stirred at room temperature overnight.
The solution changed from a pale yellow color to a dark orange/red color. The reaction
mixture was diluted with 50 mL chloroform and washed with 3 x 25 mL 1 N hydrochloric
acid. The or~ic layer was then dried over magnesium sulfate, filtered and evaporated
20 to dryness to yield a crude purple residue. The crude product was then purified by
flash column chl Jl"otography using 200 gm silica gel and eluting with 3:97 isopropyl
alcohol:methylene chloride to afford pure purple product, 0.015 gm (7%) mp 172C;
H-NMR (300 MHz, CDCI3) ~ 0.96-1.10 (m, lH, H-13), 1.00 (d, J = 8 Hz, 3H, 10-CH3),
1.01 ~d, J = 8 Hz, 3H, 14-CH3), 1.62-1.90 (br m, 2H, H-13, H-14), 1.76 (s, 3H, 8-CH3),
25 2.04 (s, 3H, 2-CH3), 2.34-2.47 (br m, 1 H, H-15), 2.42 (s, 1 H, acetylene CH), 2.68-2.81
(br m, 2H, H-10, H-15), 3.28 (s, 3H, OCH3), 3.47 (s, 3H, OCH3), 3.49 (br m, 1H, H-12),
3.61 ~brm, 1H, H-11), 4.02 (d, J = 6 Hz, 1H, H-6), 4.31 (s, 1H, 11-OH), 4.32 (m, 2H,
propargyl CH2), 4.77 (br m, 2H, NH2), 5.21 (s, 1H, H-7), 5.87 (t, J = 9 Hz, 1H, H-5),
5.90 ~d, J = 9 Hz, 1 H, H-9), 6.32 (br t, 1 H, NH), 6.60 (t, J = 9 Hz, 1H, H-4), 6.97 (d, J
30 = 9 Hz, 1H, H-3), 7.32 (s, 1H, H-19), 9.09 (s, 1H, NH-22); m/z 606.3 (M+ + Na); IR
(KBr, cm-1) 2120, 1730, 1695, 1645; Analysis calculated for C3lH4lN308-3.50H20: C,
57.55; H, 7.47; N, 6.49%. Found: C, 57.55; H, 6.14; N, 6.23%.
WO 95101342 ~ PCT/IB94/00160
-36-
EXAMPLE 20
17-(2'-Cyanoethylamino)-17-demethoxyqeldanamycin
Geldanamycin (0.200 gm, 0.3567 mmol) was added to a flame dried flask under
nitrogen and slurried in 5 mL chloroform. 3-Aminopropionitrile 0.153 gm (2.18 mmol,
5 0.161 mL) was added and the reaction mixture was stirred at room temperature
overnight. The reaction mixture went from a pale yellow color to a dark red/orange
color. An additional 0.161 mL of 3-aminopropionitrile was added and the reactionmixture was refluxed for 8 hours. The cooled reaction mixture was diluted with 75 mL
chloroform and washed with 3 x 50 mL water. The organic layer was dried over
10 magnesium sulfate, filtered and evaporated to dryness. The purple residue wasrecrystA~ ed from a minimal amount of hot ethyl acetate affording pure purple product,
0.160 gm (75%) mp 152C; lH-NMR (300 MHz, CDCI3) ~ 0.74-0.91 (br m, lH, H-13),
0.82 (d, J = 7 Hz, 3H, 10-CH3), 0.82 (d, J = 7 Hz, 3H, 14-CH3), 1.45-1.7 (br m, 2H, H-
13, H-14),1.61 (s, 3H, 8-CH3),1.83 (s, 3H, 2-CH3), 1.93-2.09 (brm,1H, H-15), 2.49-2.64
15 (m, 4H"~-ethyl CH2, H-10, H15), 3.08 (s, 3H, OCH3), 3.19(s, 3H, OCH3), 3.28 (brm,1H,
H-12), 3.39 (m, lH, 11-H), 3.62-3.79 (brm, 2H, a-ethyl CH2), 4.12 (d, J = 9 Hz, 1H, H-
6), 4.62 (br m, 2H, NH2), 5.00 (s, 1 H, H-7), 5.62-5.72 (br m, 2H, H-5, H-9), 5.96 (br t,1 H,
NH), 6.40 (t, J = 9 Hz, 1H, H4), 6.78 (d, J = 9 Hz, 1H, H-3), 7.12 (s, lH, H-19), 8.82
(s, 1H, NH-22); mtz 621.3 (M+ + Na); IR (KBr, cm-l) 1730, 1690, 1650, 1585, 1480;
20 Analysis calculated for C3lH42N4O8: C, 62.19; H, 7.07; N, 9.35%. Found: C, 62.02; H,
6.63; N, 9.09%.
EXAMPLE 21
17-(2'-Fluoroethvlamino~-17-demethoxvqeldanamycin
17-(2'-Fluoroethylamino)-17-demethoxygeldanamycin was prepared by the
25 method of Example 19. Pure purple product was obtained from the crude residue after
flash column chro",atoy"~ hy using 200 gm silica gel eluting with 20% acetone inmethylene chloride; yield 0.060 gm (28%) mp 176C; lH-NMR (300 MHz, CDCI3) ~ 0.87
(d, J = 7 Hz, 3H, 10-CH3), 0.91 (d, J = 7 Hz, 3H, 14-CH3), 1.55-1.73 (br m, 3H, H-13,
H-14), 1.70 (s, 3H, 8-CH3), 1.92 (s, 3H, 2-CH3), 2.21 (dd, J = 8 Hz, 16 Hz, lH, H-15),
30 2.62 (br m, 2H, H-10, H-15), 3.14 (s, 3H, OCH3), 3.24 (s, 3H, OCH3), 3.35 (br m,1 H, H-
12), 3.48 (br m, 1 H, H-11), 3.59-3.91 (br m, 2H, o-ethyl CH2), 4.19 (d, J = 9 Hz, 1 H, H-
6), 4.65 (two br t, J = 46 Hz, 5 Hz, 2H, ,B-ethyl CH2), 4.524.79 (br m, 2H, NH2), 5.07
(s, 1 H, H-7), 5.72 (br m, 2H, H-5,H-9), 6.29 (br t, 1 H, NH), 6.48 (t, J = 9 Hz, 1 H, H-4),
WO 95/01342 ~ ~ 6 6 3 2 0 PCT/IB94/00160
-37-
6.85 (d, J = 9 Hz, 1H, H-3), 7.14 (s, 1H, H-19), 8.97 (s, 1H, NH-22); m/z 591.3. (M+);
IR (KBr, cm~') 1742,1655,1585; Analysis calculated for C30H4zN3O8: C, 60.90; H, 7.15;
N, 7.10%. Found: C, 60.65; H, 6.90; N, 6.92%.
EXAMPLE 22
17-tert-Butylamino-17-demethoxy-geldanamvcin
Geldanamycin (0.200 gm, 0.3567 mmol) was slurried in 5 mL of tert -
butylamine, in a flame dried flask under nitrogen, at reflux overnight. The reaction color
went from yellow to dark purple. The reaction mixture was evaporated to dryness. The
residue was dissolved into 50 mL chloroform. The chloroform solution was washed
with 3 x 25 mL brine and 3 x 25 mL water. The organic layer was dried over
magnesium sulfate, filtered and evaporated to dryness. The crude product was purified
by flash column chromotagraphy with 200 gm silica gel eluted with 10%
acetone/methylene chloride; yield 0.053gm (25%) mp 102C (dec); 1H-NMR (300 MHz,CDCI3) ~ 0.89 (d, J = 5 Hz, 3H, 10-CH3), 0.94 (d, J = 5 Hz, 3H, 14-CH3), 1.39 (s, 9H,
t-butyl CH3), 1.49-1.80 (br m, 3H, H-13, H-14),1.74 (s, 3H, 8-CH3),1.98 (s, 3H, 2-CH3),
2.27 (br m, 1H, H-15), 2.65 (br m, 2H, H-10, H-15), 3.21 (s, 3H, OCH3), 3.30 (s, 3H,
OCH3), 3.99 (br m, 1H, H-12), 3.45 (br m, 1H, H-11), 4.17 (br m, 1H, 11-OH), 4.23 (d,
J = 7 Hz, 1H, H-6), 4.72~.91 (br m, 2H, NH2), 5.13 (s, 1H, H-7), 5.82 (br m, 3H, H-5,
H-9, NH), 6.51 (t, J = 7 Hz, 1H, H-4), 6.87 (d, J = 7 Hz, 1H, H-3), 7.18 (s, 1H, H-19),
8.96 (s, 1H, NH-22); m/z 601.4 (M+); 1R (KBr, cm~') 1720, 1685, 1645, 1585, 1560;
Analysis calculated for C32H47N30e: C, 63.87; H, 7.87; N, 6.98%. Found: C, 63.91; H,
7.95; N, 6.03%.
EXAMPLE 23
17-(2'-Mercaploethvlamino)-17-demethoxYqeldanamvcin
Geldanamycin (0.200 gm, 0.3567 mmol) was added to a flame dried flask under
nitros~en and slurried in 5 mL pyridine. Thiazolidine (0.194 gm, 2.18 mmol, 0.171 mL)
was added and the reaction mixture was heated at 70C for 2 hours. The reaction
mixture was evaporated to dryness in vacuo. The crude product was purified by flash
column chromatography using 200 gm silica gel eluting with 4% methanol in methylene
chloride to afford pure purple product, 0.041gm (20%) mp 156C; lH-NMR (300 MHz,CDCI3) ~ 0.99 (d, J = 5 Hz,6H,10-CH3,14-CH3),1.63-1.81 (br m, 3H, H-13, H-14),1.78
(s,3H, 8-CH3),2.01 (s,3H, 2-CH3),2.47(m,1 H, H-15), 2.64-2.79 (br m,2H, H-10, H-15),
2.83-3.00 (br m, 2H, 2'-CHz), 3.25 (s, 3H, OCH3), 3.35 (s, 3H, OCH3), 3.42 (m, lH, H-
WO 95/0L342 PCTtIB94/00160 ~
3 ~ ~
-38-
12), 3.55 (m, lH, H-11), 3.87 (m, 2H, 1'-CH2), 4.10 (br m,1H, 11-OH), 4.29 (d, J = 9 Hz,
lH, H-6), 4.92 (br m, 2H, NH2), 5.16 (s, 1H, H-7), 5.81-5.89 (br m, 2H, H-5, H-9), 6.48
(brt, lH, NH), 6.55 (t, J = 9 Hz, lH, H4), 6.94 (d, J = 9 Hz, lH, H-3), 7.24 (s, lH, H-
19), 9.12(s,1H, NH-22); m/z 642.3(M+ +Na); IR (CHCI3, cm-l) 1730,1690,1655, 1575;
5 Analysis cz~lo~'oted for C30H44N308S-H20; C, 57.67; H,7.41; N, 6.72%. Found: C,
57.44; H, 6.37; N, 6.72%.
EXAMPLE 24
17-~2'Methvlthio)ethylaminol-17-demethoxy~eldanamycin
Geldanamycin (0.200 gm, 0.3567 mmol) was added to a flame dried flask under
10 nitrogen and slurried in 5 mL chloroform. 2-(Methylthio)ethylamine (0.199 gm, 2.18
mmol,) was added and the reaction was stirred at room ter"peralure for 36 hours. The
leaction mixture was diluted with 75 mL chloroform and washed with 2 x 50 mL lN
hydrochloric acid, 2 x 50 mL brine and 3 x 50 mL water. The organic layer was dried
over magnesium sulfate, filtered and evaporated to dryness. The crude product was
15 purified by flash column chromatography using 200 gm silica gel and eluting with 3%
acetoni~,ile in ethyl etherto afford pure purple product, 0.095gm (43%) mp 157C; 'H-
NMR (300 MHz, CDCI3) ~ 1.09(d, J = 7 Hz, 3H, 14-CH3), 1.14 (d, J = 7 Hz, 3H, 10-CH3), 1.71-1.89 (br m, 3H, H-13, H-14), 1.89 (s, 3H, 8-CH3), 2.13 (s, 3H, 2-CH3), 2.22
(s, 3H, SCH3), 2.46 (dd, J = 7Hz, 13Hz, 1H, H-15), 2.79 (d, J = 13 Hz, lH, H-15), 2.72-
20 2.92 (br m, 3H, 2'-CH2, H-10), 3.36 (s, 3H, OCH3), 3.46 (s, 3H, OCH3), 3.54 (m, 1H, H-
12), 3.68 (t, J = 7 Hz, 1H, H-11), 3.72-3.86 (br m, 1H, 1'CH2), 3.86-3.95 (br m, 1H,
1'CH2), 4.41 (d, J = 7 Hz, 2H, H-6, 11-OH), 4.93(br m, 2H, NH2), 5.28 (s,1 H, H-7), 5.91-
6.04 (br m, 2H, H-5, H-9), 6.62-6.74 (br m, 2H, H-4, NH), 7.03(d, J = 7 Hz, 1 H, H-3),
7.37 (s, lH, H-19), 9.22 (s, 1H, NH-22); m/z 642.3 (M+ + Na); IR (CHCI3, cm~l) 1735,
25 1685, 1650, 1570; Analysis calculated for C3tH45N308S-1.51120: C, 57.56; H, 7.48; N,
6.49%. Found: C, 57.30; H, 6.87; N, 6.21%.
E)CAMPLE 25
1 7-r(S)-2'-Azetidinecarboxvlicacidl-1 7-demethoxyqeldanamvcin
Geldanamycin (0.200 gm, 0.3567 mmol) was added to a flame dried flask under
30 nitrogen and slurried in 5 mL chloroform. (S)-2-Azetidine carboxylic acid (0.200 gm,
1.978 mmol) in 2 mL of pyridine, and triethylamine (0.200 gm, 1.978 mmol, 0.275 mL)
were added. The reaction mixture was stirred at room temperature for 36 hours,
refluxed for 4 hours, cooled to room temperature, diluted with 75 mL chloroform and
WO 9~;/01342 PCT/IB94/00160
~ 1663~ d
-39-
washed with 2 x 50 mL 1 N hydrochloric acid. The organic layer was extracted with 3
- x 50 mL lN NaOH. The pooled basic phases were ~Qri~lifiecl to pH = 6 with lN
hydrochloric acid and extracted with 3 x 75 mL chloroform. The combined chloroform
layers were dried over magnesium sulfate, filtered, and evaporated to dryness. The
residue was d;ssolv0d in a minimal amount of ethyl acetate and precipitated withhexane to afford pure purple product, 0.056gm (25%) mp 210C (dec); 'H-NMR
(C5D5N) ~1.29-1.47 (br m, 6H, 10-CH3, 14-CH3), 1 .9~2.22(br m, 3H, H-13, H-14), 2.09
(s, 3H, 8-CH3), 2.23 (s, 3H, 2-CH3), 2.49 (br m, 1 H, H-15), 2.81 (t, J = 7 Hz, 1 H, H-10),
2.9'3 (br m, 1 H, 4'azetidine-CH), 3.28(br m, 1 H, 4'azetidine-CH), 3.42 (s, 3H, OCH3), 3.50
(s, 3H, OCH3) 3.81 (m, 1H, H-12), 4.14 (m, lH, H-11), 4.34 (br m, 1H, 3'azetidine-CH),
4.89 (br m, lH, 3'azetidine-CH), 5.01 (d, J = 7 Hz, lH, H-6), 5.79 (m, lH, 2'azetidine-
CH~, 5.91 (s, lH, H-7 ), 6.21 (t, J = 7 Hz, lH, H-5), 6.47 (d, J = 7 Hz, lH, H-9), 6.60
(t, J = 7 Hz, 1 H, H4), 7.41-7.54 (br m, 2H, H-3, H-19), 9.57 (s, 1 H, NH-22); m/z 652.3
(M~- + Na); IR (CHCI3, cm-l) 1730, 1695, 1645, 1585; Analysis c-lcl~QtPd for
C321143N3O,o-3H20: C, 56.21; H, 7.22; N, 6.14%. Found: C, 56.16; H, 6.13; N, 6.04%.
The compounds of Examples 26-31 were prepared from geldanamycin and the
approp~ e amines by the method of Example 1 above.
EXAMPLE 26
17 1 lislal"i,lo-17-demethoxyqeldanamvcin
Mp 150C (decomp); 'H-NMR (300 MHz, CDCI3) ~ 0.98 (overlapping d, 6 H),
1.68 (m, 1 H), 1.77 (s, 3 H)~ 2.02 (s, 3 H), 2.43 (t, 1 H, J = 7 Hz), 2.6-2.8 (m, 2 H), 2.94
(t, 2H, J = 5 Hz), 3.23 (s, 3 H), 3.39 (s, 3 H), 3.40 (m, 1 H), 3.52 (m, 1 H~, 3.7-3.9 (m,
2 H~, 4.27 (d, 1 H, J = 9 Hz), 4.88 (br exchangeable, 2 H), 5.13 (s, 1 H), 5.85 (m, 2 H),
6.56(t, 1 H,J= 12Hz),6.75(m, 1 H),6.92(m,2H),7.19(s, 1 H),7.61 (s, 1 H),9.17
(s, I H); mass spectrum m/z 640 (M+); Analysis C~QIcl~lQtP~i for C35H45N508-0.5(ethyl
acetate): C, 61.48; H, 7.22; N, 10.24%. Found: C, 61.06; H, 7.35; N, 10.32%.
EXAMPLE 27
1 7-Furfurylamino-1 7-demethoxv~ldanar"vcin
Mp 122-130C (dec); 'H-NMR (300 MHz, CDCI3) ~1.09 (overlapping d, 6 H),
1.75(m,1 H),1.79(s,3H),1.98(s,3H),2.42(dd,1 H,J=7Hz,6Hz),2.72(m,2H),
3.28 (s, 3 H), 3.37 (s, 3 H), 3.48 (m, 1 H), 3.59 (m, 1 H), 4.18 (br exchang~-~le, 1 H),
4.29 (d, 1 H, J = 9 Hz), 4.67 (dd, 1 H, J = 9 Hz, 5 Hz), 4.73 (dd, 1 H, J = 9 Hz, 5 Hz),
4.88 (br exchangeable, 2 H), 5.18 (s, 1 H), 5.89 (m, 2 H), 6.28 (m, 1 H), 6.34 (m, 1 H),
WO 95/0L342 PCT/IB94/00160 ~
2~GG3~
40-
6.42 (m, 1 H), 6.57 (t, 1 H, J = 12 Hz), 6.94 (d, 1 H, J = 12 Hz), 7.28 (s, 1 H), 7.41 (s,
H), 9.11 (s, 1 H); mass spectrum m/z 626 (M+); Analysis calGu'ated for
C33H43N309-0.33CHCI3: C, 62.31; H, 6.90; N, 6.54%. Found: C, 62.28; H, 6.78; N,
6.63%~
EXAMPLE 28
17-Tetrahvdrofurfurylamino-17-demethoxygeldanamvcin
Mp 157-166C (dec); lH-NMR (300 MHz, CDCI3) ~ 1.05 (overlapping d, 6 H),1.7
(m, 2 H), 1.87 (s, 3 H), 1.9-2.1 (m, 3 H), 2.06 (s, 3 H), 2.38 (m, 1 H), 2.70 (m, 2 H), 3.27
(s, 3 H), 3.34 (s, 3 H), 3.45 (m, 1 H), 3.53 (m, 2 H), 3.82 (m, 1 H), 3.93 (m, 1 H), 4.09
(m, 1 H), 4.27 (d, 1 H, J = 8 Hz), 4.88 (br exhangeable, 2 H), 5.18 (s, 1 H), 5.7-6.0 (m,
2 H), 6.56 (m, 2 H), 6.93 (d, 1 H, J = 11 Hz), 7.26 (s, 1 H), 9.17 (s, 1 H); mass
spectrum m/z 632 (M+ + 2); Analysis coic~ ot~cl for C33H4,N309: C, 62.94; H, 7.52; N,
6.69%. Found: C, 62.92; H, 7.57; N, 6.39%.
EXAMPLE 29
17-Tetramethylguanidino-17-demethoxvqeldanamycin
Mp 140-145C (dec); lH-NMR (300 MHz, CDCI3) ~ 1.0 (overlapping d, 6 H),1.70
(m,1 H),1.77(s,3H),2.02(s,3H),2.51 (m,2H),2.76(brs,6H),2.83(s,6H),3.26
(s, 3 H), 3.38 (s, 3 H), 3.42 (m, 1 H), 3.60 (m, 1 H), 4.28 (d, 1 H, J = 9 Hz), 5.15 (s, 1
H), 5.83 (t, 1 H, J = 7 Hz), 5.95 (m, 1 H), 6.57 (t, 1 H, J = 12 Hz), 6.97 (br d, 1 H, J =
12 Hz), 7.07 (s, 1 H), 9.36 (br s, 1 H); mass spectrum m/z 646 (M + 2); Analysisc-o'cu'otPd for C33H49N5O8-0.2CHCI3: C, 60.55; H, 7.56; N, 10.64%. Found: C, 60.57;
H, 7.67; N, 10.59%.
EXAMPLE 30
17-beta-Alanvl-17-demethoxyqeldanamycin
Mp 143-147C (dec); lH-NMR (300 MHz, CDCI3) ~ 0.99 (overlapping d, 6 H),
1.67 (m, 1 H), 1.79 (s, 3 H), 2.03 (s, 3 H), 2.32 (m, 1 H), 2.69 (m, 3 H), 3.22 (s, 3 H),
3.32(s,3H),3.42(m,1 H),3.55(m,1 H),3.82(m,2H),4.28(d,1 H,J=9),5.08(br
s,2H),5.19(s,1 H),5.83(m,2H),6.58(m,2H),6.91 (d,1 H,J=12Hz),7.25(s,1
H), 9.09 (s, 1 H); mass spectrum m/z 640 (M + Na); Analysis co~c~ ~oted for
C3lH43N3Olo-H2O: C, 58.66; H, 6.99; N, 6.62%. Found: C, 58.98; H, 7.03; N, 6.60%.
EXAMPLE 31
17-Homohistamino-17-demethoxy~eldanamycin
Mp 128-136C (dec); lH-NMR (300 MHz, CDC13) ~0.87 (d, 3 H, J = 7 Hz), 0.98
~ WO 95tO134~, PCT/IB94/00160
~6~3~
-41 -
(d,3H,J =7Hz), 1.7(m,3H), 1.76(s,3H), 1.98(s,3H),2.12(m,4H),2.5-2.7(m,
3 H)l 3.21 (s, 3 H), 3.32 (s, 3 H), 3.4-3.55 (m, 4 H), 4.05 (m, 2 H), 4.27 (d, 1 H, J = 9
Hz), 4.85 (br exchangeabie, 2 H), 5.13 (s, 1 H), 5.85 (m, 2 H), 6.22 (m, 1 H), 6.57 (t, 1
H, J = 11 Hz), 6.88 (m, 2 H), 7.08 (s, 1 H), 7.26 (s, 1 H), 7.53 (s, 1 H), 9.09 (s, 1 H);
5 mass spectrum m/z 654 (M + 1); Analysis calculated for C34H47N508-0.1CH2CI2: C,
61.84; H, 7.18; N, 10.57%. Found: C, 61.90; H, 7.49; N, 10.29%.
E)CAMPLE 32
1 7-Amino-22-(4'-fluoroPhenacvl)-l 7-demethoxvqeldanamycin
17-Amino-17-demethoxygeldanamycin (0.254 g, 0.465 mmol) was dissolved in
10 5 ml anhydrous dimethyl sulfoxide in flame dried ~lassware. Bolassium -butoxide
(0.064 9, 0.468 mmol) was added and the solution stirred at room temperature under
nitro~en for 30 minutes. p-Fluorophenacyl bromide (0.102 g, 0.479 mmol) was added
and the solution stirred at room temperature for 1.5 hours. This solution was diluted
with ~ethyl acetate, washed with water and brine, dried over magnesium sulfate, filtered
15 and ev~ ordled in vacuo. The resulting purple residue was flash chromatographed with
silica gel eluted with 2% methanol in chloroform to give a purple solid; Yield 0.270 g
(66%): mp 183-185C(dec); 1H NMR (300 MHz, CDCI3) ~ 0.61 (d, J = 7 Hz, 3H, 14-
CH3), 0.78 (br m, 1 H, H-13), 0.93 (d, J = 7 Hz, 3H, 10-CH3), 1.30 (s, 3H, 8-CH3), 1.49
(br m, lH, H-13), 1.82 (dd, J = 15 Hz and 4 Hz, lH, H-15), 1.92 (s, 3H, 2-CH3), 2.02
20 (brsl lH, 11-OH), 2.20 (br m, 2H, H-10 and H-14), 2.77 (dd, J = 15 Hz and 4 Hz, lH,
H-15), 2.84 (br d, J = 10 Hz, lH, H-12), 3.23 (s, 3H, OCH3), 3.27 (s, 3H, OCH3), 3.54
(d, J = 9 Hz, lH, H-11), 4.19 (t, J = 9 Hz, lH, H-6), 4.41 (d, J = 16 Hz, lH, a-CH2),
4.95(d,J=9Hz,lH,H-7),4.97(s,2H,NH2),5.12(d,J=8Hz,lH,H-9),5.20(t,J=
9 Hzr lH, H-5), 5.40 (br s, 2H, NH2), 5.84 (s, lH, H-19), 5.96 (d, J = 17 Hz, lH, ~-CH2),
25 6.35 (t, J = 10 Hz, lH, H-4), 7.03 (d, J = 13 Hz, lH, H-3), 7.12 (m, 2H, aromatic), 7.99
(m, 2H, aromatic); m/z 704 (M+ + Na); IR (KBr, cm-1) 1722, 1691, 1675, 1659, 1580,
1504; Anal. for C36H"4N3O9F: C, 63.42; H, 6.51; N, 6.16%. Found C, 63.40; H, 6.65; N,
5.84%.
The compounds of Examples 33 to 45 were prepared by the method of Example
30 32 from the appropriately substituted phenacyl bromides.
EXAMPLE 33
1 7-Amino-22-(2'.4'-dimethvlPhenacvl~-1 7-demethoxyqeldanamvcin
The residue obtained upon evaporation of the ethyl acetate was purified by
WO 95/01342 PCT/IB94/00160
42-
Chromatotron (trademark) using 2% methanol in chloroform and again with 1%
methanol in chloroform to give fractions containing esser,lially pure product which were
ev~porc~ed in vacuo to yield a red solid which was further purified by preparative HPLC
using aZorbax (trademark) column eluted with 54:1:45 ethyl acetate:methanol:hexanes.
Fractions containing pure material were evaporated in vacuo then dissolved in a
minimum amount of chloroform and precipitated with hexanes to give a dark rose-
colored solid; Yield 0.002 gm, (0.6%) mp 154C (dec); 'H-NMR (300 MHz, CDCI3)
0.55(d, J = 6 Hz, 3H, 14-CH3), 0.89 (d, J = 6 Hz, 3H, 10-CH3), 2.76 (m, 2H, H-10, H-
15), 3.04-3.19 (m, lH, H-12), 3.11 (s, 3H, OCH3), 3.17 (s, 3H, OCH3), 3.47 (m, 1H, H-
11), 4.12(t, J = 7 Hz, 1H, H-6), 4.21 (d, J = 15 Hz, 1H, o-CH), 4.43(brs, 2H, NH2), 4.83
(d, J = 7 Hz, 1H, H-7), 4.89-5,07 (m, 2H, NH2), 5.04(d, J = 7 Hz, lH, H-9), 5.10(t, J =
7 Hz, 1H, H-5), 5.71 (d, J = 15 Hz, lH, o-CH), 5.80(s, lH, H-19),6.22(t, J = 7 Hz, lH,
H-4), 6.81-7.03(m, 3H, H-3, Aromatic), 7.5 (d, J = 7 Hz, lH, Aromatic). m/z 714 (M+
+ Na); IR (KBr, cm 1) 1720, 1670, 1655, 1580; Analysis c~Icu'qted for
C38H49N3Og-0.5H2O:C, 65.12; H, 7.19; N, 6.07%. Found C, 55.38; H, 6.89; N, 5.99%.
EXAMPLE 34
1 7-Amino-22-(2'-methoxyPhenacyl)-1 7-demethoxyqeldanamycin
Yield 0.110 9 (34%): mp 165-168C; 'H NMR (300 MHz, CDCI3) ~ 0.72 (d, J =
7 Hz, 3H, 14-CH3), 0.84 (m, lH, H-13), 1.03 (d, J = 7 Hz, 3H, 10-CH3), 1.40 (s, 3H, 8-
CH3), 1.49 (m, 1 H, H-13), 1.90 (dd, J = 12 Hz and 4 Hz, 1 H, H-15), 2.03 (s, 3H, 2-CH3),
2.24 (br m, 2H, H-10 and H-14), 2.41 (s, 1 H, 1 1-OH), 2.94 (m, 2H, H-12 and H-15), 3.30
(s, 3H, OCH3), 3.34 (s, 3H, OCH3), 3.62 (d, J = 8 Hz, lH, H-11), 3.97 (s, 3H, OCH3),
4.28 (t, J = 10 Hz, lH, H-6), 4.51 (d, J = 19 Hz, lH, o-CH2), 4.60 (br s, 2H, NH2), 5.02
(d, J = 10 Hz, lH, H-7), 5.10 (br s, 2H, NH2), 5.20 (d, J = 9 Hz, lH, H-9), 5.27 (t, J =
12 Hz, lH, H-5), 5.86 (d, J = 17 Hz, lH, o-CH2), 5.98 (s, lH, H-19), 6.40 (t, J = 13 Hz,
lH, H-4), 7.03 (m, 2H, aromatic), 7.14(d, J = 12 Hz, lH, H-3), 7.56 (m, lH, aromatic),
7.90 (m, lH, aromatic); m/z 694 (M+); IR (KBr, cm~') 1732, 1678, 1661, 1584.
EXAMPLE 35
1 7-Amino-22-t3'-methoxyPhenacvl)-1 7-demethoxygeldanamvcin
The residue obtained upon evaporation of the ethyl acetate was dissolved in 1
mL chloroform and precipitated with hexanes to give a salmon-colored solid; Yield
0.151 g (48%): mp 165-168C; 'H NMR (300 MHz, CDCI3) ~0.67 (d, J = 9 Hz, 3H, 14-CH3), 0.78 (m, 1 H, H-13), 0.98 (d, J = 9 Hz, 3H, 10-CH3), 1.32 (s, 3H, 8-CH3), 1.43 (m,
WO 95/0134:~ PCT/IB94100160
2~63~0
43-
1H, H-13), 1.83 (dd, J = 15 Hz and 5 Hz, lH, H-15), 1.92 (s, 3H, 2-CH3), 2.10-2.27 (br
m, 2H, H-10 and H-14), 2.42 (s,1 H,11-OH), 2.82-2.89 (m, 2H, H-12 and H-15), 3.22 (s,
3H, OCH3), 3.28 (s, 3H, OCH3), 3.58 (d, J = 11 Hz,1 H, H-11), 3.81 (s, 3H, OCH3), 4.22
(t, J = 10 Hz, 1 H, H-6), 4.40 (d, J = 18 Hz, 1 H, a-CH2), 4.68 (br s, 2H, NH2), 4.99 (d,
5 J = 9 Hz, 1H, H-7), 5.15 (brs, 2H, NH2), 5.16 (d, J = 11 Hz, 1H, H-9), 5.21 (t, J = 11
Hz, IH, H-5), 5.90 ~s,1H, H-19), 5.97 (d, J = 17 Hz,1H, a-CH2), 6.35 (t, J = 12 Hz,1H,
H4), 7.02 (d, J = 11 Hz, 1H, H-3), 7.12 (dd, J = 8 Hz and 5 Hz, 1H, aro",alic), 7.38 (t,
J = 10 Hz, 1H, aromatic), 7.42 (d, J = 6 Hz, 1H, aromatic), 7.49 (dd, J = 8 Hz and 5
Hz, 1H, aromatic); m/z 716 (M+ + Na); IR (CH2CI2, cm-1) 1733, 1672, 1661, 1585.
EXAMPLE 36
17-Amino-22-(4'-methoxYphenacyl)-17-demethoxygeldanamycin
The residue obtained upon evaporation of the ethyl acetate was dissolved in 1
mL chloroform and precipitated with hexanes to yield a salmon-colored solid; Yield
0.205 g (64%): mp 175-178C; lH NMR (300 MHz, CDCI3) ~ 0.65 (d, J = 9 Hz, 3H, 14-
CH3,~, 0.76 (m, 1 H, H-13), 0.98 (d, J = 8 Hz, 3H,10-CH3),1.32 (s, 3H, 8-CH3),1.43 (m,
1H,1~-13), 1.82 (dd, J = 15 Hz and 5 Hz, 1H, H-15), 1.92 (s, 3H, 2-CH3), 2.10-2.28 (br
m, 2H, H-10 and H-14), 2.40 (s,1 H,11-OH), 2.81-2.89 (m, 2H, H-12 and H-15), 3.22 (s,
3H, OCH3),3.28 (s, 3H, OCH3),3.57 (d, J = 13 Hz,1 H, H-11), 3.83 (s, 3H, -OCH3), 4.21
(t, J = 11 Hz, 1H, H-6), 4.36 (d, J = 17 Hz, 1H, o-CH2), 4.67 (br s, 2H, NH2), 4.98 (d,
J = 10 Hz,1H, H-7), 5.11 (br s, 2H, NH2), 5.13 (d, J = 12 Hz, 1H, H-9), 5.21 (t, J = 12
Hz 1H, H-5), 5.89 (s,1H, H-19), 5.94 (d, J = 18 Hz,1H, a-CH2), 6.33 (t, J = 13 Hz,1H,
H4), 6.91 (m, 2H, alo",hlic), 7.08 (d, J = 13 Hz,1H, H-3), 7.89 (m, 2H, aromatic); m/z
691 ~(M~) and 713 (M+ + Na); IR (CH2CI2, cm-l) 1732, 1673, 1661, 1586, 1507.
EXAMPLE 37
17-Amino-22-(2'-chlorophenacyl)-17-demethoxygeldanamycin
The residue obtained upon evaporation of the ethyl Acet~te was purified by
preparative centrifugally accelerated radial thin-layer chromatography (Chro",atol,onR)
using a gradient of 24% methanol in chloroform to give fractions containing pureproduct which were evaporated in vacuo to afford a red solid; Yield 0.138 g (43%): lH
NM~ (300 MHz, CDCI3) ~ 0.72 (d, J = 7 Hz, 3H,14-CH3), 0.83 (m, 1H, H-13), 1.03 (d,
J = 6 Hz, 3H,10-CH3),1.40 (s, 3H, 8-CH3),1.49 (br m,1H, H-13), 1.91 (dd, J = 13 Hz
and 6 Hz,1 H, H-15),1.99 (s, 3H, 2-CH3), 2.18-2.31 (br m, 2H, H-10 and H-14), 2.43 (s,
1 H,11-OH), 2.38-2.46 (m, 2H, H-12 and H-15), 3.25 (s, 3H, OCH3), 3.33 (s, 3H, OCH3),
WO 95/01342 , PCT/IB94/00160
6~
44-
3.63 (d, J = 8 Hz, 1H, H-11), 4.22 (t, J = 10 Hz, 1H, H-6), 4.49 (d, J = 19 Hz, 1H, o-
CH2), 4.62 (br s, 2H, NH2), 5.02 (d, J = 9 Hz, 1 H, H-7), 5.19 (s, 2H, NH2), 5.21 (d, J =
12 Hz, 1H, H-9), 5.30 (t, J = 11 Hz, 1H, H-5), 5.40 (brs, 2H, NH2), 5.83 (d, J = 18 Hz,
lH,a-CH2),6.15(s,1H,H-19),6.39(t,J=12Hz,lH,H4),6.96(d,J=12Hz,lH,H-
5 3), 7.38-7.43 (m,1 H, aromatic), 7.48 (m, 2H, aromatic), 7.67 (m,1 H, aromatic); m/z 720
(M+ + Na); IR (KBr, cm-l) 1722, 1680, 1661, 1590; Analysis cr'~ tPd for.
C3~H44CIN309: C, 61.93; H, 6.35; N, 6.02%. Found C, 60.59; H, 6.18; N, 5.54%.
EXAMPLE 38
17-Amino-22-(4'-chlorophenacyl)-17-demethoxyqeldanamycin
The residue obtained upon evaporation of the ethyl acetate was dissolved in 2
mL chloroform and precipit~t~d with hexanes to give a salmon-colored solid; Yield
0.087 9 (27%): mp 175-178C; lH NMR (300 MHz, CDCI3) ~ 0.66 (d, J = 7 Hz, 3H, 14-
CH3), 0.76 (m, 1H, H-13), 0.98 (d, J = 6 Hz, 3H, 10-CH3), 1.33 (s, 3H, 8-CH3), 1.43 (m,
lH, H-13), 1.83 (dd, J = 15 Hz and 4 Hz, 1H, H-15), 1.95 (s, 3H, 2-CH3), 2.10-2.27 (br
15 m, 2H, H-10 and H-14), 2.41 (s, 1 H, 11 -OH), 2.81 -2.89 (m, 2H, H-12 and H-15), 3.22 (s,
3H, OCH3), 3.30 (s, 3H, OCH3), 3.58 (d, J = 11 Hz, 1 H, H-11), 3.81 (s, 3H, OCH3), 4.21
(t, J = 10 Hz, 1H, H-6), 4.36 (d, J = 17 Hz, 1H, a-CH2), 4.54 (br s, 2H, NH2), 4.99 (d,
J=10Hz,1H,H-7),5.13(brs,2H,NH2),5.15(d,J=10Hz,lH,H-9),5.22(t,J=12
Hz 1H, H-5), 5.89 (s, 1H, H-19), 5.97 (d, J = 17 Hz, 1H, a-CH2), 6.34 (t, J = 12 Hz, 1H,
20 H4),7.01 (d,J= 12Hz,1H,H-3),7.43(d,J=9Hz,2H,aromatic),7.87(d,J=9Hz,
2H, aromatic); m/z 720 (M+ + Na); IR (CH2CI2, cm-l) 1733, 1677, 1662, 1584.
EXAMPLE 39
1 7-Amino-22-Phenacvl-1 7-demethoxyqeldanamvcin
The residue obtained upon evaporation of the ethyl acetate was purified by
25 Chrol"al~,l,on (trademark) using 2% methanol in chloroformto givefractions containing
pure product which were evaporated in vacuo to yield a red solid, 0.161 gm (53%) mp
188-91 C (dec); lH-NMR (300 MHz, CDCI3) ~ 0.68 (d, J = 5 Hz, 3H, 14-CH3), 0.80 (m,
1H, H-13), 0.98 (d, J = 5 Hz, 3H, 10-CH3), 1.35 (s, 3H, 8-CH3), 1.43 (m,1H, H-13), 1.85
(dd, J = 3 Hz, J = 10 Hz, 1H, H-15), 1.96 (s, 3H, 2-CH3), 2.11-2.29 (br m, 2H, H-10, H-
30 14), 2.39 (s, 1H, 11-OH), 2.85 (m, 2H, H-12, H-15), 3.21(s, 3H, OCH3), 3.26 (s, 3H,
OCH3), 3.60 (dd, J = 3 Hz, J = 7 Hz, 1H, H-11), 4.21 (t, J = 9 Hz, 1H, H-6), 4.39 (d,
J = 15 Hz, 1H, a-CH), 4.61 (br s, 2H, NH2), 5.03-5.19 (m, 2H, NH2), 5.16 (d, J = 8 Hz,
1H, H-9), 5.71 (t, J = 8 Hz, 1H, H-5), 5.89 (s, 1H, H-19), 6.00 (d, J = 15 Hz, 1H, a-CH),
WO 95/01342 ~16 6 3 ~ O PCT/IB94/00160
-45-
6.36 (t, J = 8 Hz, 1H, H4), 7.05 (d, J = 8 Hz, lH, H-3), 7.46 (t, J = 6 Hz, 2H,
- LroI"alic), 7.60 (t, J = 6 Hz, 1H, aromatic), 7.93 (d, J = 6 Hz, 2H, aromatic); m/z 686
(M~ + Na), 664 (M+ ~H); IR (KBr, cm-l) 1720, 1670, 1655, 1580; Analysis c~Ic~IAted
for C3~H45N3O0-0.5H2O: C, 64.27; H, 6.89; N, 6.27%. Found C, 64.13; H, 6.33; N,
5 6.19%.
EXAMPLE 40
1 7-Amino-22-(3'.4'-dichloroPhenacvl)-1 7-demethoxvqeldanamycin
The residue obtained upon evaporation of the ethyl ~cetAtP was further purified
by preparative HP~C using a Zorbax column eluted with 59:1:40 ethyl Acet~te
methanol:hexanes. The fractions containing pure material were evaporated in vacuo
then dissolved in a minimum amount of chloroform and precipitated with hexanes to
give a dark rose-colored solid; Yield 0.134 g (40%): mp 176-178C; lH NMR (300 MHz,
CDCI3) ô 0.63 (d, J = 7 Hz, 3H,14-CH3), 0.74 (m,1 H, H-13), 0.98 (d, J = 8 Hz, 3H, 10-
CH3~,1.31 (s, 3H, 8-CH3),1.42 (m,1 H, H-13), 1.82 (dd, J = 15 Hz and 5 Hz,1 H, H-15),
1.92 (s, 3H, 2-CH3),2.09-2.24 (br m,2H, H-10 and H-14),2.42 (s,1 H,11 -OH),2.78-2.88
(m, 2H, H-12 and H-15), 3.21 (s, 3H, OCH3), 3.27 (s, 3H, OCH3), 3.57 (d, J = 10 Hz,
lH, H-11), 4.18 (t, J = 11 Hz, lH, H-6), 4.36 (d, J = 19 Hz, lH, o-CH2), 4.69 (brs, 2H,
NHz),4.98 (d, J = 11 Hz, lH, H-7), 5.12 (d, J = 11 Hz, lH, H-9), 5.16 (brs, 2H, NH2),
5.21 (t, J = 11 Hz, lH, H-5), 5.85 (s, lH, H-19), 5.92 (d, J = 18 Hz, lH, a-CH2), 6.34
(t, J = 12 Hz, lH, H-4), 6.98 (d, J = 12 Hz, lH, H-3), 7.57 (d, J = 9 Hz, 1H, aromatic),
7.76 (dd, J = 9 Hz and 4 Hz, 1 H, aromatic), 8.01 (d, J = 4 Hz, 1 H, aromatic); m/z 732
(M+); IR (CH2CI2, cm~l) 1731, 1704, 1673, 1661, 1583.
EXAMPLE 41
1 7-Amino-22-(4'-aminoPhenacyl)-l 7-demethoxyqeidanamycin
From 4-aminophenacyl chloride: the product was a pink solid. Yield 0.066 g
(21%): mp 188C (dec); lH-NMR (300 MHz, CDC13) ~ 0.71 (d, J = 8 Hz, 3H, 14-CH3),0.86 (br m, 1 H, H-13), 1.04 (d, J = 8 Hz, 3H, 10-CH3), 1.39 (s, 3H, 8-CH3), 1.49 (br m,
lH, H-13), 1.90 (dd, J = 13 Hz, 5 Hz, 1H, H-15), 2.00 (s, 3H, 2-CH3), 2.22 (br m, 2H,
H-10 H-14),2.48 (br s,1 H, -OH), 2.92 (m, 2H, H-12 and H-15),3.29 (s,3H, OCH3),3.33
(s, 3~-i, OCH3), 3.62 (d, J = 10 Hz, lH, H-11), 4.28 (s, 2H, NH2), 4.29 (t, J = 9 Hz, lH,
H-6), 4.37 (d, J = 18 Hz, 1 H, a-CH2), 4.75 (br s, 2H, NH2), 5.03 (d, J = 9 Hz,1 H, H-7),
5.18 I(br s, 2H, NH2), 5.21 (d, J = 9 Hz, lH, H-9), 5.26 (t, J = 13 Hz, lH, H-5), 5.95 (d,
J = 18 Hz, 1H, a-CH~), 5.96 ts, lH, H-19), 6.49 (t, J = 10 Hz, lH, H-4), 6.66 (d, J= 9
_
WO 95/01342 2 ~ ~ ~ 3 2 ~ PCT/lB94/00160
-46-
Hz, 2H, aromatic), 7.18 (d, J=13 Hz, lH, H-3), 7.78 (d, J = 9 Hz, 2H, aromatic); m/z
701 (M+ + Na); IR (KBr, cm-l) 1718, 1708, 1655, 1619, 1580.
EXAMPLE 42
1 7-Amino-22-(4'-cvanoPhenacYI)-1 7-demethoxyqeldanamvcin
The residue obtained upon evaporation of the ethyl acetate was further purified
by pr~ arali~/e HPLC using a Zorbax column eluted with a mixture comprising 59:1:40
ethyl ~cetP~te:methanoI:hexanes. Fractions containing pure material were evaporated
in vacuo then dissolved in a minimum amount of chloroform and precipitated with
hexanes to give a dark rose-colored solid; yield 0.0296 g (8.4%): mp 186C (dec); lH-
NMR (300 MHz, CDCI3) ~ 0.71 (d, J = 6 Hz, 3H, 14-CH3), 0.83 (br m, 1 H, H-13), 1.04
(d, J = 6 Hz, 3H, 10-CH3), 1.39 (s, 3H, 8-CH3), 1.48 (m, lH, H-13), 1.90 (dd, J = 11 Hz
and 5 Hz, lH, H-15), 1.99 (s, 3H, 2-CH3), 2.25 (br m, 2H, H-10 and H-14), 2.41 (s, lH, -
OH), 2.92 (m, 2H, H-12 and H-15), 3.27 (s, 3H, OCH3), 3.34 (s, 3H, OCH3), 3.64 (d, J
= 11 Hz, lH, H-ll), 4.23 (t, J = 10 Hz, lH, H-6), 4.45 (d, J = 18 Hz, lH, a-CH2), 4.61
(brs, 2H, NH2), 5.05 (d, J = 10 Hz, lH, H-7), 5.20 (brs, 2H, NH2), 5.21 (d, J = 11 Hz,
lH, H9), 5.29 (t, J = 11 Hz, lH, H-5), 5.96 (s, lH, H-l9), 6.02 (d, J = 18 Hz, lH, a-
CH2), 6.40 (t, J = 11 Hz, lH, H-4), 7.00 (d, J = 11 Hz, lH, H-3), 7.84 (d, J = 8 Hz, 2H,
aromatic), 8.08 (d, J = 8 Hz, 2H, aromatic); m/z 712 (M+ + Na); IR (KBr, cm-l) 2220,
1721, 1701, 1672, 1655, 1580; Analysis c~Ic~ tPd for C3~H44N4Oll: C, 64.52; H, 6.44;
N, 8.13%. Found C, 58.56; H, 5.81; N, 7.33%.
EXAMPLE 43
1 7-Amino-22-t2'-nitrophenacYi)-l 7-demethoxvqeldal ,~mycin
Yield 0.0689 g (21%): mp 165C (dec); lH NMR (300 MHz, CDCI3) ~ 0.70 (d, J
= 7 Hz, 3H, 14-CH3), 0.82 (m, lH, H-13), 1.03 (d, J = 7 Hz, 3H, 10-CH3), 1.40 (s, 3H,
8-CH3), 1.52 (m, lH, H-13), 1.90 (dd, J = 14 Hz and 4 Hz, lH, H-15), 2.00 (s, 3H, 2-
CH3), 2.08 (s, lH, ll-OH), 2.26 (br m, 2H, H-10 and H-14), 2.90 (m, 2H, H-12 and H-
15), 3.19 (s, 3H, OCH3), 3.33 (s, 3H, OCH3), 3.64 (d, J = 10 Hz, lH, H-ll), 4.15 (t, J
= 10 Hz, lH, H-6), 4.42 (d, J = 18 Hz, lH, a-CH2), 5.01 (d, J = 10 Hz, lH, H-7), 5.10
(br s, 2H, NH2), 5.20 (d, J = 9 Hz, lH, H-9), 5.23 (br s, 2H, NH2), 5.26 (t, J = 13 Hz,
lH, H-5), 5.63 (d, J = 18 Hz, lH, a-CH2), 6.38 (t, J = 12 Hz, lH, H-7), 6.52 (s, lH, H-
19), 6.87 (d, J = 12 Hz, 1 H, H-3), 7.63 (m, 1 H, aromatic), 7.69 (m, 1 H, aromatic), 7.80
(m, lH, aromatic), 8.20 (m, lH, aromatic); m/z 731 (M+ + Na); IR (KBr, cm~l) 1718,
1671, 1657, 1580, 1521; Analysis c~IcuI~ted for (C36H44N4Oll): C, 61.00; H, 6.26; N,
WO 95/01342 PCT/IB94/00160
~1~632~
-47-
7.91%. Found C, 59.92; H, 6.10; N, 7.71%.
EXAMPLE 44
17-Amino-22-(3'-nitroPhenacvI)-17-demethoxv~eIdanamycin
Yield 0.0225 g (6.9%):; 1H-NMR (300 MHz, CDCI3) ~ 0.71 (d, J = 8 Hz, 3H, 14-
5 CH3), 0.88 (m, lH, H-13),1.04 (d, J = 8 Hz, 3H,10-CH3),1.34 (s, 3H, 8-CH3), 1.42 (m,
1H, H-13), 1.90 (dd, J = 13 Hz and 5 Hz, 1H, H-15), 2.00 (s, 3H, 2-CH3), 2.25 (br m,
2H, H-10 and H-14), 2.42 (br s, 1H, -OH), 2.93 (m, 2H, H-12 and H-15), 3.29 (s, 3H,
OCH3), 3.34 (s, 3H, OCH3), 3.64 (d, J = 10 Hz, 1H, H-11), 4.23 (t, J = 9 Hz, 1H, H-6),
4.52 (d, J = 18 Hz, 1 H, o-CH2), 4.62 (br s, 2H, NH2), 5.06 (d, J = 9 Hz, 1 H, H-7), 5.15
10 (br s, 2H, NH2), 5.21 (d, J = 9 Hz, lH, H-9), 5.30 (t, J = 13 Hz, lH, H-5), 5.98 (s, 1H,
H-19), 6.06 (d, J = 18 Hz, 1H, a-CH2), 6.41 (t, J = 9 Hz, lH, H-4), 7.00 (d, J = 13 Hz,
1H, H-3), 7.76 (t, J = 8 Hz, 1H, aromatic), 8.32 (d, J = 8 Hz, lH, aromatic), 8.52 (d, J
= 8 Hz,1 H, aromatic), 8.83 (s,1 H, aromatic); m/z 731 (M+ + Na); IR (KBr, cm-l) 1719,
1664, 1652, 1580, 1521.
EXAMPLE 45
1 7-Amino-22-(4'-nitroPhenacvl)-1 7-demethoxyqeldanamycin
The residue obtained upon evaporation of the ethyl aceta~e was further purified
by preparative HPLC using a Zorbax column eluted with 59: 1 :40 ethyl ~cet~te:methanoI:
hexanes. Fractions co"L"i"g pure material were evaporated in vacuo then dissolved
20 in a minimum amount of chloroform and precipitated with hexanes to give a dark rose-
colored solid; Yield 0.0175 g (1.3%): mp; lH NMR (300 MHz, CDCI3) ~ 0.70 (d, J = 7Hz, 3H, 14-CH3), 0.82 (m, 1H, H-13), 1.02 (d, J = 7 Hz, 3H, 10-CH3), 1.38 (s, 3H, 8-
CH3),1.50 (m,1 H, H-13),1.90 (dd, J = 16 Hz and 4 Hz,1 H, H-15), 2.00 (s, 3H, 2-CH3),
2.25 (br m, 2H, H-10 and H-14), 2.50 (br s, 1 H, 11-OH), 2.89 (m, 2H, H-12 and H-15),
25 3.27 (s, 3H, OCH3), 3.33 (s, 3H, OCH3), 3.63 (d, J = 10 Hz, 1 H, H-11), 4.23 (t, J = 10
Hz, lH, H-6),4.50 (d, J = 18 Hz, lH, a-CH2), 4.77 (s, 2H, NH2), 5.03 (d, J = 10 Hz, lH,
H-7), 5.20 (d, J = 11 Hz, lH, H-9), 5.25 (br s, 2H, NH2), 5.28 (t, J = 11 Hz, lH, H-5),
5.96 (s, lH, H-19), 6.03 (d, J = 18 Hz, lH, a-CH2), 6.40 (t, J = 12 Hz, lH, H-4), 6.99
(d, J = 12 Hz, lH, H-3), 8.15 (d, J = 9 Hz, 2H, aru",alic)~ 8.37 (d, J = 9 Hz, lH,
30 aromatic); m/z 731 (M+ + Na); IR (KBr, cm~l) 1660, 1590.
EXAMPLE 46
17-Amino-22-(4'-azidoPhenacyI)-17-demethoxygeldanamycin
17-Amino-17-demethoxygeldanamycin (0.500 g,0.92 mmol) was dissolved in 25
WO 95/01342 PCT/IB94/00160 --
-48-
mL of acetone (stored over potassium carbonate) and 1.27 g potassium carbonate was
added (9.17 mmol, 10 eq). The reaction was stirred in the dark for 24 hours, filtered,
concer,l-aled in vacuo to a residue which was flash chromatographed through silica gel
using 69:1:30 ethyl acetate:methanol:hexanes. The product was dissolved in 1 mL
5 chlorofor"l and precipit~ted with hexanes to give a rose-colored solid; Yield 0.095 g
(15%): mp 165-167C; 1H-NMR (300 MHz, CDCI3) ~ 0.65 (d, J = 8 Hz, 3H, 14-CH3),
0.76 (m, lH, H-13), 0.97 (d, J = 8 Hz, 3H, 10-CH3), 1.32 (s, 3H, 8-CH3), 1.42 (m, lH,
H-13), 1.82 (dd, J = 15 Hz and 5 Hz, lH, H-15), 1.91 (s, 3H, 2-CH3), 2.09-2.27 (br m,
2H, H-10 and H-14), 2.41 (br s, 1 H, 11 -OH), 2.79-2.90 (m, 2H, H-12 and H-15), 3.21 (s,
10 3H, OCH3), 3.27 (s, 3H, OCH3), 3.58 (d, J = 11 Hz, 1H, H-11), 4.20 (t, J = 10 Hz, 1H,
H-6), 4.36 (d, J = 17 Hz, 1H, a-CH2), 4.65 (brs, 2H, NH2), 4.98 (d, J = 10 Hz, 1H, H-7),
5.11 (br s, 2H, NH2), 5.14 (d, J = 10 Hz, 1 H, H-9), 5.20 (t, J = 12 Hz, 1 H, H-5), 5.89 (s,
lH, H-19), 5.95 (d, J = 17 Hz, 1H, a-CH), 6.33 (t, J = 13 Hz, 1H, H~), 7.02 (d, J = 13
Hz, 1 H, H-3), 7.08 (d, J = 9 Hz, 2H, aromatic), 7.91 (d, J = 9 Hz, 2H, aromatic); m/z
15 705 (M+ + H); Analysis calculated for C3~H44N~303-0.2H20: C, 58.37; H, 6.53; N,
11.34%; Found C, 58.37:, H, 5.85;N, 11.08%.
EXAMPLE 47
17-Amino-22-(4'-amino-3'-iodophenacyl)-17-demethoxvqeldanamycin
Potassium chloride (0.022 g, 0.29 mmol) was dissolved in 1 mL water and
20 poured into iodine monochloride (0.043 g, .27 mmol). This mixture was poured into a
solution of the title compound of Example 41 (0.139 g, 0.2 mmol) in 24 mL 0.1N
hydrochloric acid and 3 mL methanol. After stirring for 2 hours at room temperature
the reaction was quenched with sodium bisulfite. The reaction mixture was extracted
with ethyl ~cet~te The organic layer was washed with 3N hydrochloric acid, saturated
25 sodium bicarbonate, water and brine, dried over magnesium sulfate and filtered. The
residue obtained upon removal of the solvent was dissolved in 1 mL chloroform and
precipitated with hexanes to give a salmon-colored solid; Yield 0.099 g (60%): 'H NMR
(300 MHz, CDCI3) ~ 0.66 (d, J = 7 Hz, 3H, 14-CH3), 0.75 (m, lH, H-13), 0.98 (d, J =
6 Hz, 3H, 10-CH3), 1.34 (s, 3H, 8-CH3), 1.47 (m, lH, H-13), 1.91 (m, lH, H-15), 1.95 (s,
30 3H, 2-CH3), 2.10-2.29 (br m, 2H, H-10 and H-14), 2.59 (s,1 H,11 -OH), 2.78-2.92 (m, 2H,
H-12 and H-15), 3.25 (s, 3H, OCH3), 3.29 (s, 3H, OCH3), 3.59 (d, J = 10 Hz, lH, H-11),
4.24 (t, J = 10 Hz, lH, H-6), 4.32 (d, J = 18 Hz, lH, a-CH2), 4.56 (s, 2H, NH2), 4.95 (d,
J=10Hz,1H,H-7),5.04(brs,2H,NH2),5.15(d,J=11 Hz,1H,H-9),5.22(t,J=11
WO 95/OL34~, , PCT/IB94/00160
~1663~
49-
Hz, lH, H-5), 5.58 (br s, 2H, NH2), 5.88 (d, J = 18 Hz, lH, a-CH2), 5.89 (s, lH, H-19),
- 6.37 (t, J = 12 Hz, lH, H-4), 6.60 (dd, J = 9 Hz and 3 Hz, lH, aromatic), 7.06 (d, J =
12 Hz, lH, H-3), 7.71 (dd, J = 9 Hz and 2 Hz, lH, aromatic), 8.22 (dd, J = 2 Hz and
3 Hz, lH, alolllalic) IR (KBr, cm~') 1715, 1660, 1610, 1580.
The acid washes were neutralized with sodium bicarbonate and extracted with
ethyl ~cet~tP. The ethyl acetate extracts were washed with water and brine, dried over
magnesium sulfate, filtered and stripped to recover the title compound of Example 41,
Yield 0.045 g (27%).
EXAMPLE 48
17-Amino-22-(4'-azido-3'-iodophenacyl)-17-demethoxvqeldanamycin
17-Amino-22-(4'-amino-3'-iodophenacyl)-17-demethoxygeldanamycin, from
Example 47, (0.036 g,0.44 mmol) was dissolved in 89 mL methanol, cooled to 0 C and
shielded from light. To this solution was added 45 mL 1 N hydrochloric acid and 45 mL
0.5N sodium nitrite. After 15 minutes of stirring, an additional 45 mL 0.5N sodium azide
was added and the reaction mixture stirred another 15 minutes at 0C. The resulting
solution was ~xllacted with ethyl acetate. The ethyl acetate e,.l.acts were washed with
water and brine, dried over magnesium sulfate, filtered and stripped to give a red glass
which was dissolved in 1 mL ethyl acetate and precipitated with hexanes. Yield 0.027
g p:2%): lH NMR (300 MHz, CDCI3) ~ 0.69 (d, J = 7 Hz, 3H, 14-CH3), 0.79 (m, lH, H-
13), 1.03 (d, J = 8 Hz, 3H, 10-CH3), 1.50 (s, 3H, 8-CH3), 1.89 (m, 2H, H-13 and H-15),
1.99 (s, 3H, 2-CHJ, 2.11-2.32 (br m, 2H, H-10 and H-14), 2.53 (br s, 1 H,11-OH), 2.81-
2.95 (m, 2H, H-12 and H-15), 3.28 (s, 3H, OCH3), 3.32 (s, 3H, OCH3), 3.62 (d, J = 9 Hz,
lH, H-11), 4.22 (t, J = 12 Hz, lH, H-6), 4.41 (d, J = 17 Hz, lH, o-CH2), 4.80 (br s, 2H,
NH2~, 5.02 (d, J = 9 Hz,1 H, H-7), 5.16-5.31 (br m, 4H, NH2, H-9, and H-5), 5.92 (s,1 H,
H-1g), 5.99 (d, J = 18 Hz, lH, a-CH2), 6.40 (t, J = 14 Hz, lH, H-4), 7.03 (d, J = 14 Hz,
lH, H-3), 7.12 (m, lH, aromatic), 8.00 (m, 2H, aromatic), 8.40 (m, lH, aromatic); IR
(KBr, cm-') 1722, 1680, 1661, 1580.
The compounds of Examples 49 and 50 were prepared according to the method
of Exarnple 32.
EXAMPLE 49
17-Arnino-22-(4'-phenyIphenacyI)-17-demethoxy~eldanar"vcin
Yield 0.119 g (35%): mp 193-195C (dec); 1H NMR (300 MHz, CDCI3) ~ 0.72 (d,
J = 7 Hz, 3H,14-CH3), 0.83 (m, lH, H-13),1.04 (d, J = 7 Hz, 3H,10-CH3),1.40 (s, 3H,
WO95/01342 ~ 3~ ~ PCT/~94/00160 -
-50-
8-CH3), 1.49 (m, 1H, H-13), 1.90 (dd, J= 16 Hz and 5 Hz, 1H, H-15), 2.00 (s, 3H, 2-
CH3), 2.25 (br m, 2H, H-10 and H-14), 2.47 (s, 1H, 11-OH), 2.91 (m, 2H, H-12 and H-
15), 3.31 (s, 3H, OCH3), 3.33 (s, 3H, OCH3), 3.64 (d, J=10 Hz, 1H, H-11), 4.29 (t, J
= 10 Hz, 1H, H-6), 4.49 (d, J= 18 Hz, 1H, o-CH2), 4.72 (br s, 2H, NH2), 5.04 (d, J=
5 10 Hz, 1H, H-7), 5.17 (brs, 2H, NH2), 5.21 (d, J=11 Hz, 1H, H-9), 5.28 (t, J=11 Hz,
1H, H-5), 5.99 (s, lH, H-19), 6.09 (d, J = 18 Hz, 1H, a-CH2), 6.41 (t, J = 12 Hz, 1H, H-
4), 7.13 (d, J = 12 Hz, 1H, H-3), 7.47 (m, 3H, aromatic), 7.12 (m, 3H, aromatic), 7.74
(d, J= 8 Hz, 2H, aromatic), 8.05 (d, J= 8 Hz, 2H, aromatic); m/z 731 (M++Na+).
EXAMPLE 50
1 7-Amino-22-(2-acetonaphthvl)-1 7-demethoxvqeldanamycin
The crude title compound, prepared from 2-(2'-bromoacetyl)naphthalene was
dissolved in 1 mL chloroform and precipitated with hexanes to give a salmon-colored
solid; Yield 0.163 g (50%): mp 185-187C; tH NMR (300 MHz, CDCI3) ~ 0.71 (d, J=
8 Hz, 3H, 14-CH3), 0.82 (m, 1H, H-13), 1.02 (d, J= 7 Hz, 3H, 10-CH3), 1.40 (s, 3H, 8-
CH3), 1.49 (m, 1 H, H-13), 1.89 (dd, J= 15 Hz and 5 Hz, 1 H, H-15), 2.00 (s, 3H, 2-CH3),
2.22 (br m, 2H, H-10 and H-14), 2.44 (s, 1 H, 11-OH), 2.91 (m, 2H, H-12 and H-15), 3.30
(s, 3H, OCH3), 3.33 (s, 3H, OCH3), 3.51 (d, J=11 Hz, 1H, H-11), 4.30 (t, J=11 Hz,
1H, H-6), 4.59 (d, J= 19 Hz, 1H, a-CH2), 4.68 (brs, 2H, NH2), 5.02 (d, J=10 Hz, 1H,
H-7), 5.15 (br s, 2H, NH2), 5.20 (d, J= 13 Hz, 1H, H-9), 5.28 (t, J = 13 Hz, 1H, H-5),
6.00 (s, 1H, H-19), 6.20 (d, J= 17 Hz, 1H, a-CH2), 6.40 (t, J= 12 Hz, 1H, H-4), 7.13
(d, J= 13 Hz, 1H, H-3), 7.54-7.68 (m, 2H, aromatic), 7.87-8.02 (m, 2H, aromatic), 8.51 .
(s, 1H, aromatic); m/z 714 (M+) and 736 (M+ + Na); IR (CH2CI2, cm~1) 1733, 1677,1662, 1585.
EXAMPLE 51
1 7-Azetidin-1 -v1-1 1 -a-fluoro-1 7-demethoxvqeldanar"ycin
1 7-Azetidin-1 -yl-1 7-demethoxygeldanamycin, the title compound of Example 18,
(0.200 9, 0.342 mmol) was added to a flame dried flask under nitrogen and dissolved
in 15 mL of methylene chloride. The mixture was cooled to -68C with an exLer"al dry
ice/acetone bath and then a solution of DAST (0.0559, 0.342 mmol, 0.045 mL) in 2.5
mL of methylene chloride was added dropwise. After 1 hour 5 mL of 596 aqueous
NaHCO3 was ~ddded slowly and the product extracted into 100 mL of methylene
chloride. The organic layer was washed with 3 x 50 mL of water and 2 x 50 mL of brine,
dried with MgSO4, filtered and stripped of solvent to afford a purple solid. This was
WO 95/0134Z, 21~ 6 3 2 ~ PCT/IB94/00160
-51 -
purif3ed by flash column chromatography using 5% methanol in chloroform. Material of
Rf = .42 (1 :9 methanol: chloroform), the desired product, (0.058 g 29%) was disolved
in a minimal amount of ethyl acetate and precipitated with hexanes; Yield 0.042 9
(219~), mp 128C (dec); 1H-NMR (300 MHz, CDCI3) ~ 1.05(m, 6H,10-CH3 and 14-CH3),1.25 (brt, J = 15 Hz, lH, H-13), 1.55 (brt, J = 15 Hz, lH, H-13), 1.78 (s, 3H, 8-CH3),
1.96 (br m, lH, H-14), 2.03 (s, 3H, 2-CH3), 2.23 (dd, J = 8.5 Hz, 16 Hz, lH, H-15), 2.40
(br m, 2H, 3' azetidine CH2), 2.55 (dd, J = 7 Hz, 16 Hz, 1 H, H-15), 2.80 (br d, J = 26
Hz, lH, H-10), 3.35 (s, 3H, OCH3), 3.37 (s, 3H, OCH3), 3.53 (br m, lH, H-12), 4.39 (d,
J = 9 Hz,1 H, H-6), 4.0 (br m, 7H, NH2 and 2' and 4' azetidine CH2 and H-11), 5.60 (d,
J = 9 Hz, lH, H-9), 5.70 (s, lH, H-7), 5.88 (t, J = 9 Hz, lH, H-5), 6.55 (t, J = 9 Hz, lH,
H-4), 6.96 (d, J = 9 Hz, lH, H-3), 7.05 (s, lH, H-19), 9.33 (s, lH, NH-22); m/z610. (M+
+ Na); IR (KBr, cm-l) 1735,1690, 1650; Analysis CP~Ic~ t~cl for C3lH42FN307-5H20: C,
54.93; H, 7.73; N, 6.20%. Found: C, 55.07; H, 6.23; N, 6.07%.
The compounds of Examples 52-58 were prepared by the method of Example
51 from the appru~ leIy substituted 17-amino-17-demethoxygeldanamycin derivatives.
EXAMPLE 52
17-Amino-11 -o-fIuoro-17-demethoxvqeIdanamvcin
Yield 0.155 g (44%), mp >250C (dec); lH-NMR (300 MHz, CDCI3) ~ 0.80 (d,
3H, J = 8Hz, 10-CH3), 0.90 (d, 3H, J = 8Hz, 14-CH3), 0.93 (br m, 1 H, H-13), 1.35 (br
t, lH, H-13),1.60 (s, 3H, 8-CH3),1.85 (s, 3H,2-CH3),1.85 (brm, lH, H-14),1.85-2.1 (br
m, 2H, H-15), 2.63 (brd, J = 26 Hz, lH, H-10), 3.16 (s, 6H, OCH3), 3.26 (brm, lH, H-
12), ~.28 (d, J = 9 Hz, lH, H-6), 4.45 (br d, J = 47 Hz, lH, H-11), 4.60 (br m, 2H, NH2),
4.95 (s, lH H-7), 5.07 (br s, 2H, NH2), 5.45 (d, J = 9 Hz, 1 H, H-9), 5.70 (t, J = 9 Hz,
lH, H-5), 6.35 (t, J = 9 Hz, lH, H~), 6.80 (d, J = 9 Hz, lH, H-3), 7.05 (s, lH, H-19),
9.0 (s, lH, NH-22); m/z 570. (M+ + Na); IR (KBr, cm~l) 1715, 1685, 1670; Analysis
c~Ic~ ted for C28H38FN3O7-0.25H2O: C, 60.91; H, 7.03; N, 7.61%. Found: C, 60.78;H, 6.87; N, 7.43%.
EXAMPLE 53
17-lsoproPvIamino-11 -a-fluoro-17-demethoxygeldanamvcin
Yield 0.035 g (16%), mp 132C; lH-NMR (300 MHz, CDCI3) ~ 0.85 (m, 6H, 10-
CH3, 14-CH3), 0.85 (m, lH, H-13), 1.07 and 1.11 (br d, J = 8 Hz, 6H, isopropyl CH3),
1.38 (br m, lH, H-13), 1.60 (s, 3H, 8-CH3), 1.71 (br m, 1 H, H-14), 1.75 (s, 3H, 2-CH3),
2.15 ~dd, J = 8.5 Hz, 16 Hz, lH, H-15), 2.45 (dd, J = 7 Hz, 16 Hz, lH, H-15), 2.65 (br
WO 95/01342 PCT/IB94/00160
-52-
d, J = 26 Hz, lH, H-10), 3.19 and 3.20 (br s, 6H, OCH3), 3.35 (br m, lH, H-12), 3.95
(m, 1H, isopropyl CH), 4.22 (d, J = 9 Hz, 1 H, H-6), 4.30 (br d, J = 47 Hz, 1H, H-11),
4.65 (brm, 2H, NH2), 5.04 (s, 1H H-7), 5.46 (d, J = 9 Hz, 1H, H-9), 5.73 (t, J = 9 Hz,
1H, H-5), 5.94 (br d, J = 10.5 Hz, 1H, NH), 6.40 (t, J = 9 Hz, 1H, H4), 6.80 (d, J = 9
5 Hz, 1H, H-3), 7.05 (s, 1H, H-19), 9.15 (s, lH, NH-22); m/z 612. (M+ + Na); IR (KBr,
cm-1) 1740,1705,1655;AnaIysiscaIc~ tedforC31H44FN3O7-0.5H2O: C, 62.19; H,7.57;
N, 7.01%. Found: C, 62.36; H, 7.48; N, 6.81%.
EXAMPLE 54
1 7-CycloproPvIamino-11 -a-fluoro-1 7-demethoxyqeIdanamycin
Yield 0.056 g (25%), mp 119C (dec); 1H-NMR (300 MHz, CDCI3) ~0.70 (m, 2H,
cyclopropyl CH2), 0.86 (m, 3H, cyclopropyl CH2 and H-13), 0.92 (d, 3H, J = 8Hz, 10-
CH3), 1.00 (d, 3H, J = 8Hz, 14-CH3), 1.53 (br m, 1H, H-13), 1.70 (s, 3H, 8-CH3), 1.95
(s, 3H, 2-CH3), 2.00 (br m, 1 H, H-14), 2.62 (dd, J = 8.5 Hz, 16 Hz, 1 H, H-15), 2.70 (br
d, J = 26 Hz, 1H, H-10), 2.80 (m, lH, cyclopropyl CH), 2.90 (dd, J = 7 Hz, 16 Hz, lH,
15 H-15), 3.33 (s, 6H, OCH3), 3.45 (br m, lH, H-12), 4.33 (d, J = 9 Hz, lH, H-6), 4.36 (br
d, J = 47 Hz, lH, H-11), 4.7 (br m, 2H, NH2), 5.10 (s, lH H-7), 5.55 (d, J = 9 Hz, lH,
H-9), 5.75 (t, J = 9 Hz, lH, H-5), 6.20 (brt, lH, NH), 6.46 (t, J = 9 Hz, lH, H-4), 6.90
(d, J = 9 Hz, lH, H-3), 7.15 (s, lH, H-19), 9.15 (s, lH, NH-22); m/z 610. (M+ + Na);
IR (KBr, cm-1) 1740, 1690, 1630; Analysis c~lcIII~ted forC31H42FN3O7-1 .5H2C: C, 60.57;
20 H, 7.37; N, 6.83%. Found: C, 60.43; H, 6.79; N, 6.83%.
EXAMPLE 55
1 7-Allvlamino-1 1 -a-fluoro-1 7-demethoxvqeIdanamvcin
Yield 0.049 g (25%), mp 110-112C; 1H-NMR (300 MHz, CDCI3) ~ 0.85(d, 3H,
J = 8Hz, 10-CH3), 0.88(d, 3H, J = 8Hz, 14-CH3), 1.45 (br m, 2H, H-13), 1.63 (s, 3H, 8-
25 CH3), 1.86 (s, 3H, 2-CH3), 1.88 (br m, lH, H-14), 2.20 (dd, J = 8.5 Hz, 16 Hz, lH, H-
15), 2.55 (dd, J = 7 Hz, 16 Hz, lH, H-15), 2.80 (br d, J = 26 Hz, lH, H-10), 3.20 (s, 6H,
OCH3), 3.33 (br m, lH, H-12~, 3.95 (brt, 2H, allylic CH2), 4.23 (d, J = 9 Hz, lH, H-6),
4.26 (brd, J = 49 Hz, lH, H-11), 4.57 (brm, 2H, NH2), 5.03 (s, lH, H-7), 5.15 (brd, 2H,
vinylic CH2), 5.45 (d, J = 9 Hz, 1H, H-9), 5.55 (m, 2H, H-5 and vinylic CH), 6.10 (brt,
30 1H, NH), 6.40 (t, J = 9 Hz, 1H, H-4), 6.80 (d, J = 9 Hz, 1H, H-3), 7.07 (s, 1H, H-19),
9.13 (s, 1H, NH-22); m/z 608. (M+ + Na); IR (KBr, cm-1) 1740, 1700, 1655; Analysis
c~Icll~ted for C31H42FN3O7-0.75H2O: C, 61.93; H, 7.24; N, 6.98%. Found: C, 61.87;
H, 6.93; N, 7.00%.
_ WO 95/01342 2 ~ 6 6 3 2 ~ PCT/IB94/00160
EXAMPLE 56
17-Propar~vlamino-11 -a-fluoro-17-demethoxy~eldanamycin
Yield 0.051 g (27%), mp 111 C (dec); 1H-NMR (300 MHz, CDCI3) ~ 0.83 (br m,
lH, H-13), 0.95 (d, 3H, J = 8Hz, 10-CH3), 0.98 (d, 3H, J = 8Hz, 14-CH3), 1.53 (br m,
5 1H, H-13), 1.73 (s, 3H, 8-CH3), 1.95 (s, 3H, 2-CH3), 1.94 (brm, 1H, H-14), 2.35 (s, 1H,
acetylene CH), 2.35 (dd, J = 8.5 Hz, 16 Hz, lH, H-15), 2.58 (dd, J = 7 Hz, 16 Hz, 1H,
H-15), 2.65 (br d, J = 26 Hz,1 H, H-10), 3.3 (s, 6H, OCH3), 3.45 (br m, 1 H, H-12), 4.20
(br s 2H, propargyl CH2), 4.33 (d, J = 9 Hz, 1 H, H-6), 4.36 (br d, J = 47 Hz,1 H, H-11),
4.8(brm,2H,NH2),5.13(s,1HH-7),5.57(d,J=9Hz,lH,H-9),5.85(t,J=9Hz,lH,
10 H-5), 6.13 (brt, lH, NH), 6.50 (t, J = 9 Hz, lH, H4), 6.90 (d, J = 9 Hz, lH, H-3), 7.20
(s, 111, H-19), 9.13 (s, 1H, NH-22); m/z 608. (M+ + Na); IR (KBr, cm~l) 2120, 1735,
1695,1635; Analysis c~lc~ t~d for C37H40FN307-0.75H~O: C, 62.14; H,6.98; N,7.01%.
Found: C, 61.99; H, 6.71; N, 6.90%.
EXAMPLE 57
17-(2'-Cyanoethvlamino)-11 -a-fluoro-17-demethoxygeldanamvcin
Yield 0.026 g (14%), mp 122-24C (dec); lH-NMR (300 MHz, CDCI3) ~ 1.15 (br
m, 1H, H-13), 1.12 (d, 3H, J = 8Hz, 10-CH3), 1.17 (d, 3H, J = 8Hz, 14-CH3), 1.65 (br
t, lH, H-13), 1.75 (s, 3H, 8-CH3), 1.90 (s, 3H, 2-CH3), 2.1 (br m, 1H, H-14), 2.40 (dd, J
=8.5Hz,16Hz,1H,H-15),2.65(dd,J=7Hz,16Hz,1H,H-15),2.83(t,J=8Hz,2H,
20 ~-ethyl CH2), 2.93 (br d, J = 26 Hz, 1 H, H-10), 3.46 (s, 6H, OCH3), 3.45 (br m, 1 H, H-
12), 3.56 (q, J = 8 Hz, 2H, o-ethyl CH2), 4.46 (d, J = 9 Hz, 1 H, H-6), 4.55 (br d, J = 47
Hz,1 H, H-11), 4.85 (br m, 2H, NH2), 5.26 (s,1 H, H-7), 5.73 (d, J = 9 Hz,1 H, H-9), 6.00
(t, J = 9 Hz, 1H, H-5), 6.07 (br t, 1 H, NH), 6.65 (t, J = 9 Hz, 1 H, H-4), 7.07 (d, J = 9
Hz, 1H, H-3), 7.35 (s, 1H, H-19), 9.25 (s, 1H, NH-22); m/z 623. (M+ + Na); IR (KBr,
25 cm~') 2350, 1730, 1695, 1630; Analysis calculated for C3,H4lFN4O7: C, 61.99; H, 6.88;
N, 9.33%. Found: C, 61.52; H, 6.91; N, 9.25%.
EXAMPLE 58
17-(2'-Fluoroethvlamino)-11 -a-fluoro 17-demethoxyqeldanamvcin
Yield 0.064 g (32%), mp 134C (dec); lH-NMR (300 MHz, CDCI3) ~ 1.05 (br m,
30 1H, H-13),1.03 (d, 3H, J = 8Hz,10-CH3),1.07 (d, 3H, J = 8Hz,14-CH3),1.57 (brt,1H,
H-13), 1.77 (s, 3H, 8-CH3), 2.0 (br m, 1H, H-14), 2.05 (s, 3H, 2-CH3), 2.35 (dd, J = 8.5
Hz, 1 ~ Hz, 1 H, H-15), 2.63 (dd, J = 7 Hz, 16 Hz,1 H, H-15), 2.83 (br d, J = 26 Hz, 1 H,
H-10), 3.4 (s, 6H, OCH3), 3.53 tbr m,1 H, H-12), 3.85 (two brd m, J = 27 Hz, 2H, a-ethyl
WO 95101342 PCT/IB94/00160
-54-
CHz), 4.4 (d, J = 9 Hz, lH, H-6), 4.45 (br d, J = 47 Hz, 1H, H-11), 4.65 (two brd t, J =
46 Hz 7 Hz, 2H, ~-ethyl CH2), 4.75 (br m, 2H, NH2), 5.23 (s, 1 H H-7), 5.63 (d, J = 9 Hz,
1H, H-9), 5.90 (t, J = 9 Hz, 1H, H-5), 6.25 (brt, 1H, NH), 6.57 (t, J = 9 Hz, 1H, H-4),
6.97 (d, J = 9 Hz, 1H, H-3), 7.25 (s, 1H, H-19), 9.25 (s, 1H, NH-22); m/z 596. (M+ ~
5 Na); IR (KBr, cm~l) 1740, 1700, 1630; Analysis calculated for C30H4lF2N3O7: C, 60.69;
H, 6.96; N, 7.07%. Found: C, 60.23; H, 6.99; N, 7.02%.
E)CAMPLE 59
11-a-Fluoro-qeldanamvcin
The title compound was prepared from geldanamycin bythe method of Example
10 51; Yield 0.064 g (32%), mp 232C (dec); lH-NMR (300 MHz, CDCI3) ~ 0.87 (d, 3H, J
= 8Hz, 10-CH3), 1.00 (d, 3H, J = 8Hz, 14-CH3), 1.0 (br m, lH, H-13), 1.4 (br t, lH, H-
13), 1.73 (s, 3H, 8-CH3), 1.95 (s, 3H, 2-CH3), 1.9 (br m, lH, H-14), 2.25 (dd, lH, J = 7
Hz, 16 Hz, lH, H-15), 2.45 (dd, J = 8.5 Hz,16 Hz, lH, H-15), 2.75 (brd, J = 26 Hz, lH,
H-10), 3.3 (s, 3H, OCH3), 3.34 (s, 3H, OCH3), 3.4 (br m, lH, H-12), 4.05 (s, 3H, 17-
15 OCH3), 4.27 (d, J = 9 Hz, lH, H-6), 4.57 (br d, J = 47 Hz, lH, H-11), 4.7 (br m, 2H,
NH2), 5.05 (s, lH, H-7), 5.53 (d, J = 9 Hz, lH, H-9), 5.85 (t, J = 9 Hz, lH, H-5), 6.50
(t, J = 9 Hz, lH, H-4), 6.85 (d, J = 9 Hz, lH, H-3), 7.20 (s, lH, H-19), 8.65 (s, 1H, NH-
22); m/z 585. (M+ + Na); IR (KBr, cm~l) 1740, 1705, 1655; Analysis cE~C~ tpcl for
C28H39FN2O8-0.25H2O: C, 61.41; H, 7.02; N, 4.93%. Found: C, 61.03; H, 6.65;
20 N, 4.92%.
EXAMPLE 60
17-(S)-2'-HydroxvProPYiamino-11 -a-fluoro-17-demethoxYqeldanamycin
The title compound of Example 59 (0.307 9, 0.546 mmol) was slurried in 6 mL
of chloloform and treated with (S)-2-hydroxypropylamine (0.205 g, 2.73 mmol) at 22C
25 for 16 hours. The reaction mixture was diluted with 50 mL of chloroform and washed
with 3 x 50 mL of brine and 3 x 50 mL of water. The organic layer was dried withMgSO4, filtered and vacuum evaporated to a purple solid. Flash column
chromatography on silica gel eluted with 3% methanol in chloroform afforded pureproduct; Yield 0.070 9 (21%), mp 119C (dec); 1H-NMR (300 MHz, CDCI3) ~ 0.95 (br30 t, 7H, H-13 and 10-CH3 and y-propyl CH3), 1.25 (d, 3H, J = 8Hz, 14-CH3), 1.50 (br t,
1 H, H-13), 1.70 (s, 3H, 8-CH3), 1.9 (br m, 1 H, H-14), 1.97 (s, 3H, 2-CH3), 2.33 (dd, J =
8.5Hz,16Hz,1H,H-15),2.53(dd,J=7Hz,16Hz,1H,H-15),2.75(brd,J=26Hz,
lH, H-10), 3.30 (s, 6H, OCH3), 3.30 (m, lH, a-propyl CH), 3.45 (br m, lH, H-12), 3.57
WO 95/01342 PCT/IB94/00160
~ 1 6 ~
(m,1 H, a-propyl CH), 4.0 (m,1 H"6-propyl CH), 4.33 (d, J = 9 Hz, 1 H, H-6), 4.37 (br d,
- J = 27 Hz, lH, H-11), 4.75 (br m, 2H, NH2), 5.15 (s, 1H H-7), 5.55 (d, J = 9 Hz, lH, H-
9), 5.85 (t, J = 9 Hz, lH, H-5), 6.43 (brt, lH, NH), 6.50 (t, J = 9 Hz, lH, H4), 6.90 (d,
J = 9 Hz, lH, H-3), 7.15 (s, lH, H-19), 9.23 (s, lH, NH-22); m/z 628. (M+ + Na); IR
(KBr, cm-l) 1735,1695,1655; Analysis c~lcul~tecl for C3lH44FN308-0.25H20: C, 61.01;
H, 7.35; N, 6.88%. Found: C, 60.90; H, 7.40; N, 6.74%.
EXAMPLE 61
17-Allvlamino-11 -aminocarbonyl-17-demethoxyqeldanamvcin
17-Allylamino-17-demethoxygeldanamycin (0.200 g,0.341 mmol) was dissolved
in 5 InL of methylene chloride and cooled to 0C in a flame dried flask under nitrogen.
Sodi~lm isocyanate (0.311 9,0.4.78 mmol) and trifluoroacetic acid (0.545 g,4.78 mmol
0.368 mL) were added during 10 minutes. After stirring for 16 hours at room
terllperal~re~ the reaction mixture was diluted with 200 mL of water and extracted with
3 x 150 mL of chloroform. The combined organic layers were washed with 2 x 100 mL
of water, dried with sodium sulfate, filtered and evaporated in vacuo to yield a residue,
0.236 g, which was flash chromatographed on 80 g of silica gel eluted with 69:1:30
ethyl AcetAte:methanol:hexanes. Fractions containing pure product were evaporated,
taken up in 2 mL of chlGrof~r"~ and then prec;~ led with hexanes; 0.062 g (29%) mp
214-216C; 'H-NMR (300 MHz, CDCI3) ~ 0.93(d, J = 8Hz, 3H, 10-CH3), 0.98(d, J =
8Hz, 3H, 14-CH3), 1.26 (br m, 1H, H-13), 1.50 (m, 1H, H-13), 1.69 (s, 3H, 8-CH3),1.77
(br m, 1H, H-14), 1.96 (s, 3H, 2-CH3), 2.24 (dd, J = 16 Hz and 7 Hz, 1H, H-15), 2.56
(m, 1 H, H-15), 2.85 (m, 1 H, H-10), 3.31 (s, 3H, OCH3), 3.32 (s, 3H, OCH3), 3.46 (br m,
1H, H-12), 4.05 (br t, 2H, allylic CH2), 4.39 (br m, 2H, NH2), 4.43 (br d, J = 9 Hz, 1H,
H-6), 4.71 (v br s, 2H, NH2) 4.75 (m, 1 H, H-11), 5.15-5.3 (m, 4H, vinylic CH2, H-7, H-9),
5.73-5.9 (br m, 2H, H-5, vinylic CH), 6.15 (br t, 1H, NH), 6.48 (t, J = 9 Hz, 1H, H4),
7.11 (s, 1H, H-19), 7.23 (br m, 1H, H-3), 9.20 (s, 1H, NH-22); m/z 651. (M+ + Na); IR
(KBr,
- cm-l) 1740, 1725, 1705, 1680, 1645 1585, 1470; Analysis cAlc~lAted for
C32H44N4O9-1.5H2O: C,58.61; H, 7.22; N, 8.54%. Found: C, 58.50; H, 6.51; N, 8.48%.
EXAMPLE 62
17-Azetidin-1 -yl-11 -N-BOC-~-alanvl-17-demethoxvqeldanamvcin
17-Azetidin-1 -yl-17-demethoxygeldanamycin, the title compound of Example 18,
(0.200 g,0.341 mmol) was dissolved in 6 mL of dry methylene chloride and treated with
WO 95/01342 PCT/IB94/00160 --
2 ~
-56-
N-BOC-,B-alanine (0.077 g, 0.409 mmol), dicyclohexylcarbodiimide (0.084 g, 0.409mmol) and dimethylaminopyridine (DMAP) (0.050 9, 0.409 mmol). After 24 hours themixture was filtered to remove dicyclohexylurea and concen~l~ted in vacuo to a residue
which was ~;~solved in 200 mL of ethyi acetate and washed with 2 x 100 mL each of
5 lN hydrochloric acid, water and brine. The organic layer was dried with Na2SO4,
filtered and evaporated in vacuo to yield a purple residue (0.240 9). This was flash
chroi"~lographed on silica gel eluted with 69:1:30 ethyl acetate:methanol:hexanes.
Fractions containing pure product were evaporated to yield a residue which was
dissolved in 1 mL of chloroform and precipitated with 100 mL of hexane; yield 0.098 g
10 (38%) mp 122-125C; lH-NMR (300 MHz, CDCI3) ~ 0.87 (d, J = 8 Hz, 3H, 10-CH3), 0.95
(d, J = 8 Hz, 3H, 14-CH3), 1.17 (m, 1H, H-13), 1.6 (m, lH, H-14), 1.63 (s, 3H, 8-CH3),
1.90 (m, 1H, H-15), 1.95 (s, 3H, 2-CH3), 2.35 (pent, J = 8 Hz, 2H, 3'- azetidine CH2),
2.46 (m, 2H, o-alanyl CH2), 2.6-2.8 (m, 2H, H-15, H-10), 3.25 (s, 3H, OCH3), 3.27 (s, 3H,
OCH3), 3.35 (m, 2H"~-alanyl CH2), 3.66 (m, 1H, H-12), 4.46 (d, J = 8 Hz, lH, H-6), 4.55
15 (t, J = 8 Hz, 4H, 2'and 4' azetidine CH2), 4.4-4.9 (br s, 2H, NH2), 5.06 (d, J = 12 Hz,
1H, H-11), 5.15 (brt, lH, alanyl NH), 5.27 (d, J = 11 Hz, 1H, H-9), 5.53 (brs, 1H, H-7),
5.80 (t, J = 9, 1 H, H-5), 6.45 (t, J = 9 Hz, 1 H, H4), 6.90 (s, 1 H, H-19), 7.00 (m, J = 9
Hz, 1 H, H-3), 9.40 (s, 1 H, NH-22); m/z 759. (M+ + Na); IR (KBr, cm-l) 1732, 1645 1583,
1541, 1477; Analysis cplcul~ted for C39H5~N4Oll-2H2O: C, 59.08; H, 7.63; N, 7.07%.
20 Found: C, 59.20; H, 7.11; N, 7.16%.
EXAMPLE 63
1 7-Azetidin-1 -yl-1 1 -B-alanvl-1 7-demethoxygeldanamvcin
The product of Example 62 (0.050 g, 0.066 mmol) was dissolved in 1 mL of
trifluoroac~lic acid at 0C. After 10 minutes the reaction mixture was evaporated in
25 vacuo to a residue which was dissolved in 0.3 mL of methanol and precipitated with 20
mL of isopropylether; 0.35 9 (71%) mp 138-142C; 1H-NMR (300 MHz, CDCI3) ~ 0.84
(d, J = 8 Hz, 3H, 10-CH3), 0.96 (d, J = 8 Hz, 3H, 14-CH3), 1.24 (m, lH, H-13), 1.55 (m,
1H, H-13), 1.61 (s, 3H, 8-CH3), 1.94 (s, 3H, 2-CH3), 1.96 (m, 1H, H-15), 2.35 (pent, J =
8 Hz, 2H, 3' az~lidi.,e CH2), 2.6-2.8 (m, 4H, a-alanyl CH2 and H-15 and H-10), 3.18 (m,
30 2H"B-alanyl CH2), 3.25 (s, 6H, OCH3), 3.65 (m, 1 H, H-12), 4.43 (br s, 1 H, H-6), 4.57 (m,
4H, 2'and 4' ~lidi"e CH2), 5.0-5.5 (br s, 2H, NH2), 5.15 (d, J = 12 Hz, 1H, H-11), 5.3
(br m, lH, H-9), 5.4 (br s, lH, H-7), 5.78 (t, J = 9 Hz, 1H, H-5), 6.44 (t, J = 9 Hz, 1H,
H4), 6.85-7.00 (m, J = 9 Hz, 1H, H-3), 6.93 (s, 1H, H-19), 8.16 (br s, 2H, alanyl NH2),
WO 95/01342 ~ ~ o PCT/IB94/00160
-57-
9.35 (s, lH, NH-22); m/z 657. (M+ + H); IR (KBr, cm-1) 1731, 1688, 1647 1583, 1601,
1541, 1474; Analysis calc~ te~l for C34H48N409-3H20: C, 52.42; H, 6.72; N, 6.79%.
Found: C, 52.23; H, 6.22; N, 6.61%.
EXAMPLE 64
17-Azetidin-1 -Yl-11 -rN-(4-azidobenzovl)-~-alanvl1-17-demethoxygeldanamycin
The title compound of Example 63 (0.020 9, 0.026mmol) was dissolved in 0.5
mL of anhydrous dimethylformamide and treated with 4-azidobenzoic acid N-
hydroxysucc;.,i",ide ester (0.007 g, 0.025 mmol) and triethylamine (0.0025 9, 0.025
mmol, 0.0034 mL). After three hours the reaction mixture was diluted with 200 mL of
ethyl acetate and washed with 2 x 100 mL of water, 1 N hydrochloric acid, and brine.
The or~an c layer was dried with sodium sulfate, filtered and evaporated in vacuo to a
residue. The residue was dissolved in 0.5 mL of chloroform and precipitated with 70
mL of hexanes, filtered and dried in vacuo; 0.012 9 (61%) mp 123-6C; 1H-NMR (300
MHz, CDCI3) ~0.85 (d, J = 8 Hz, 3H,10-CH3), 0.94 (d, J = 8 Hz, 3H, 14-CH3), 1.15 (m,
1H, H-13), 1.63 (s, 3H, 8-CH3), 1.85 (m, 1H, H-15), 1.95 (s, 3H, 2-CH3), 2.35 (pent, J =
8 Hz, 2H, 3' azetidine CH2), 2.6 (t, J = 8 Hz, 2H, o-alanyl CH2), 2.7-2.9 (m, 2H, H-15
and H-10), 3.26 (s, 3H, OCH3), 3.29 (s, 3H, OCH3), 3.56 (m, 1H"B-alanyl CH2), 3.70 (m,
1H, H-12), 3.82 (m, 1H,,6-alanyl CH2), 4.44.9 (m, 7H, H-6, 2' and 4' azetidine CH2,
NH2), 5.10 (d, J = 12 Hz, 1H, H-11), 5.3 (brm, 1H, H-9), 5.53 (brs, 1H, H-7), 5.79 (t,
J = 9, 1H, H-5), 6.45 (t, J = 9, 1H, H~), 6.91 (s, 1H, H-19), 6.9-7.0 (m, 2H, H-3, NH),
6.98 (d of ABq, J = 10 Hz, 2H, aromatic CH), 7.75 (d of ABq, J = 10 Hz, 2H, aromatic
CH), 8.16 (br s, 2H, alanyl NH2), 9.38 (s, 1 H, NH-22).
EXAMPLE 65
17-Azetidin-1 -v1-11 -acetyl-17-demethoxyqeldanamycin
17-Azetidin-1 -yl-17-demethoxygeldanamycin, the title compound of Example 18,
(0.200 9, 0.341 mmol) was dissolved in 5 mL of methylene chloride in a flame dried
flask under nitrogen and treated with acetic anhydride (0.070 9, 0.683 mmol, 0.064 mL),
DMAP (0.042 9, 0.341 mmol) and triethylamine 0.105 g (1.04 mmol, 0.145 mL) at room
temperature. After 3 hours the mixture was diluted with 200 mL of methylene chloride
and washed with 100 mL of water and 2 x 100 mL of brine. The organic layer was dried
with sodium sulfate, filtered and evaporated in vacuo to a residue; 0.30 9. The residue
was flash chromatographed on 120 9 of silica gel with 2.5% methanol in chloroform to
afford pure product, 0.120 9, which was recrystallized from 10 mL of toluene; 0.080 9
WO 95/01342 PCT/IB94/00160
2~ 32~, ,
-58-
(37%) mp 195C(dec); 'H-NMR (300 MHz, CDCI3) ~ 0.93 (m, 6H, 14-CH3 and 10-CH3),
1.1-1.3 (m, 2H, H-13), 1.55 (m, 1H, H-14), 1.65 (s, 3H, 8-CH3), 1.95 (s, 3H, 2-CH3), 1.96
(s, 3H, acetyl CH3), 2.0 (m, 1 H, H-15), 2.35 (pent, J = 8 Hz, 2H, 3'-azetidine CH2), 2.6-
2.85 (m, 2H, H-15, H-10), 3.29 (s, 3H, OCH3), 3.31 (s, 3H, OCH3), 3.60 (sept, J = 8 Hz,
5 1H, isopropyl CH), 3.63 (m, 1H, H-12), 4.45 (d, J = 8 Hz, 1H, H-6), 4.55 (t, J = 8 Hz,
4H, 2'and 4' azetidine CH2), 4.73 (br s, 2H, NH2), 5.0 (m, 1H, H-11), 5.75 (d, J = 11 Hz,
1H, H-9), 5.41 (br s, 1H, H-7), 5.78 (t, J = 9 Hz, 1 H, H-5), 6.46 (t, J = 9 Hz, 1 H, H4),
6.91 (s, 1H, H-19), 7.10 (m, J = 9 Hz, 1H, H-3), 9.34 (s, lH, NH-22); m/z 650. (M+ +
Na); IR (KBr, cm-1) 1735, 1685, 1645; Analysis c~lc~ ted for C33H4sN3Og: C, 63.14; H,
10 7.26; N, 6.69%. Found: C, 63.36; H, 6.94; N, 6.55%.
EXAMPLE 66
1 7-Azetidin-1 -v1-1 1 -aminocarbonyl-1 7-demethoxv~geldanamycin
Prepared in the manner of Example 61 from 1 7-az~tidi, 1-1 -yl-17-
demethoxygeldanamycin, the title compound of Example 18, (0.200 g, 0.341 mmol);
15 yield 0.083 g (39%) mp 168-171 C; 'H-NMR (300 MHz, CDCI3) ~ 0.98(d, J = 8Hz, 3H,
10-CH3), 1.01 (d, J = 8Hz, 3H, 14-CH3), 1.3 (br m, 2H, H-13), 1.73 (s, 3H, 8-CH3), 1.75
(br m, 1 H, H-14), 2.02 (s, 3H, 2-CH3), 2.15 (m, 1 H, H-15), 2.40 (pent, J = 8 Hz, 2H, 3'-
azetidine CH2), 2.67 (m, lH, H-15), 2.87 (m, lH, H-10), 3.36 (s, 6H, OCH3), 3.60 (m, 1H,
H-12), 4.39 (br m, 2H, NH2), 4.5 (br d, J = 9 Hz, 3H, H-6, NH2), 4.64 (br t, J = 8 Hz,
20 6H, 2'and 4' azetidine CH2, NH2), 4.86 (m, 1H, H-11), 5.35 (d, J = 12 Hz, 1H, H-9), 5.40
(br s, 1H, H-7), 5.83 (br t, J = 9 Hz, lH, H-5), 6.54 (t, J = 9 Hz, lH, H-4), 6.97 (s, 1H,
H-19), 7.21 (br m, 1H, H-3), 9.36 (s, 1H, NH-22); m/z 629. (M+); IR (KBr, cm ') 1720,
1686, 1648 1533, 1475; Analysis c~lc~ tesl for C32H44N40~-H20: C, 59.42; H, 7.61; N,
8.66%. Found: C, 59.67; H, 6.81; N, 8.38%.
EXAMPLE 67
1 7-Allvlamino-1 1 -isoproPylsulfamylcarbonvl-1 7-demethoxygeldanamycin
1 7-Allylamino-1 7-demethoxygeldanamycin (0.200 9, 0.341 mmol) was dissolved
in 5 mL of methylene chloride and cooled to 0C in a flame dried flask under nitrogen.
Chlorosulfonylisocyanide (0.080 mg, 0.564 mmol, 0.049 mL) was added dropwise
30 during 10 minutes. After stirring for one hour in the cold, isopropyl amine (0.066 g,
1.13 mmol, 0.096 mL) was added and the reaction mixture allowed to warm to room
temperature during one hour. The reaction mixture was diluted with 100 mL of
chloroform and extracted with 10 mL of water. The aqueous layer was back extracted
~ WO 95/01342 PCT/IB94/00160
~ 6B32~
-59-
with 3 x 100 mL of chlGrofc r",. The pooled organic layers were extracted with 3 x 75
mL of 1N NaOH. The combined basic layers were washed with 3 x 100 mL of
chlolo~or",. The aqueous layer was acidified to pH 3 with 1N hydrochloric acid and
extracted with 3 x 100 ml of chloroform. These latter organic extracts were pooled,
5 washed with 2 x 100 mL of brine, dried with sodium sulfate, filtered and evaporated in
vacuo to a solid, 0.213 9. Flash column chromatography on silica gel eluted with 5%
methanol in chloroform yielded pure title compound which was dissolved in 1 mL of
chloroform and p,e..i~,ilcled with hexanes, filtered and dried in vacuo; yield 0.061 9
(25~) mp 137-139C; 1H-NMR (300 MHz, CDCI3) ~0.94 (d, 3H, J = 8 Hz,10-CH3), 0.98
(d, 3H, J = 8Hz, 14-CH3), 1.1(m, 6H, isopropyl CH3), 1.3-1.55 (brm, 2H, H-13), 1.65 (s,
3H, B-CH3), 1.70 (br m, 1 H, H-14), 1.95 (s, 3H, 2-CH3), 2.13 (m, 1 H, H-15), 2.27 (dd, J
= 7 Hz, 16 Hz, 1H, H-15), 3.00 (m, 1H, H-10), 3.25 and 3.27 (br s, 6H, OCH3), 3.5 (m,
1 H, isopropyl CH), 3.57 (br m, 1 H, H-12), 4.05 (br t, 2H, allylic CH2), 4.43 (br m, 1 H, H-
6), 4.7 (br m, 2H, NH2), 4.9 (br s, 1 H, NH) 5.02 (br d, J = 11 Hz, 1 H, H-11), 5.2 (br d,
2H, ~Jinylic CH2), 5.38 (br m, 2H, H-7 and H-9), 5.75 (t, J = 9 Hz, 1 H, H-5), 5.85 (m, 1 H,
vinylic CH), 6.27 (brt, lH, NH), 6.45 (t, J = 9 Hz, 1H, H4), 7.03 (br m, lH, H-3), 7.10
(s, 1H, H-19), 9.30 (s, 1H, NH-22); m/z 772. (M+ + Na); IR (KBr, cm~1) 1737, 1690,
1645; Analysis c~lc~lAt~d for C35H5lN5OllS-0.5H2O: C, 55.39; H, 6.91; N, 9.23%.
Found: C, 55.36; H, 6.95; N, 9.19%.
The compounds of Examples 68 and 69 were prepared by the method of
Example 67 from the appropriate 17-demethoxy-geldanamycin.
EXAMPLE 68
17-~-Fluoroethylamino-11 -i~opr~ ylsulfamvlcarbonyl-17-demethoxvqelda~ ,ycin
Yield 0.122 g (38%) mp 142-146C (dec); 1H-NMR (300 MHz, CDCI3) ~ 0.93 (d,
3H, J = 8Hz, 10-CH3), 0.97 (d, 3H, J = 8Hz, 14-CH3), 1.07 (d, J = 8 Hz, 6H, isopropyl
CH3), 1.36 (br m, 1H, H-13), 1.46 (br m, 1H, H-13), 1.63 (br s, 4H, 8-CH3 and H-14),
1.94 (s, 3H, 2-CH3), 2.1 (br m, lH, H-15), 2.82 (dd, J = 7 Hz, 16 Hz, 1 H, H-15), 2.95 (br
m, lH, H-10), 3.24 (s, 3H, OCH3), 3.26 (s, 3H, OCH3), 3.49 (sept, J = 8 Hz, lH,
isopropyl CH), 3.59 (br m, lH, H-12), 3.77 (two brd m, J = 23 Hz, 2H, o-ethyl CH2),
4.43 (br s, 1 H, H-6), 4.56 (two brd t, J = 47 Hz 7 Hz, 2H"B-ethyl CH2), 4.8 (br m, 2H,
NHz), 5.02 (br d, 2H, H-11 and NH), 5.75 (t, J = 9 Hz, 1 H, H-5), 5.86 (br d, 2H, H-7 and
H-9), 6.25 (br t, 1 H, NH), 6.45 (t, J = 9 Hz, 1 H, H4), 7.00 (br s, 1 H, H-3), 7.10 (s, 1 H,
H-19), 7.55 (br s, lH, NH), 9.25 (s, lH, NH-22); m/z 778. (M+ ~ Na); IR (KBr, cm-1)
WO 95/01342 ~ PCT/IB94/00160
-60-
1735, 1690, 1645, 1590, 1480; Analysis calculated for C34H50FN5O, l S: C, 60.69; H, 6.96;
N, 7.07%. Found: C, 60.23; H, 6.99; N, 7.02%.
EXAMPLE 69
1 7-~-Cyanoethylamino-11 -isoPr~,pylsulfamvlcarbonyl-1 7-demethoxvqelclal-~ "vcin
Yield 0.037 9 (11%) mp 150-154C (dec); lH-NMR (300 MHz, CDCI3) ~ 0.98 (d,
6H, J = 8 Hz, 1 0-Me and 1 4-Me), 1.08 (d, J = 8 Hz, 6H, isopropyl CH3), 1 .37 (br m, 1 H,
H-13), 1.5 (brt, lH, H-13), 1.65 (s, 3H, 8-Me), 1.75 (brm, lH, H-14), 1.95 (s, 3H, 2-Me),
2.04 (m, 1 H, H-15), 2.66 (t, J = 8 Hz, 2H"~-ethyl CH2), 2.78 (m, 1 H, H-15), 3.00 (br m,
lH, H-10), 3.26 (s, 3H, OCH3), 3.28 (s, 3H, OCH3), 3.50 (sept, J = 8 Hz, lH, isopropyl
CH), 3.56 (br m, 1 H, H-12), 3.77 (br m, 2H, o-ethyl CH2), 4.40 (br d, J = 9 Hz, 1 H, H-6),
4.75 (br m, 2H, NH2), 4.93 (br s, 1 H, NH), 4.98 (br d, 1 H, H-11), 5.34 (br m, 2H, H-7 and
H-9), 5.76 (t, J = 9 Hz, lH, H-5), 5.96 (brt, lH, NH), 6.45 (t, J = 9 Hz, lH, H4), 7.00
(br s, lH, H-3), 7.13 (s, lH, H-19), 7.38 (br s, lH, NH), 9.15 (s, lH, NH-22); m/z 785.
(M+ + Na); IR (KBr, cm l) 2320, 1730, 1690, 1640, 1580, 1480; Analysis c~lc~ ted for
C35H50N~O11S-1.25H2O: C, 54.78; H, 6.63; N, 10.95%. Found: C, 54.75; H, 6.16; N,10.71~.
EXAMPLE 70
1 7-Azetidin-1 -vl-1 1 -isopropvlsulfamylcarbonvl-1 7-demethoxvqeldanamvcin
1 7-Azetidin-1 -yl-1 7-demethoxygeldanamycin (0.200 g, 0.341 mmol) was
dissolved in 5 mL of methylene chloride and cooled to 0C in a flame dried flask under
nitrogen. Chlorosulfonylisocyanide (0.053 mg, 0.376 mmol, 0.033 mL) was added
dropwise during 10 minutes. After stirring for two hours in the cold, isopropylamine
(0.044 9, 0.75 mmol, 0.064 mL) was added and the reaction mixture allowed to warm
to room temperature during one hour. The reaction mixture was diluted with 100 mL
of methylene chloride and extracted with 2 x 100 mL of 1 N NaOH. The combined basic
layers were washed with 3 x 150 mL of methylene chloride and then acidified to pH 3
with 1 N hydrochloric acid. The acidic aqueous layer was extracted with 3 x 150 mL of
methylene chloride. These latter organic extracts were pooled, dried with sodiumsulfate, filtered and evaporated in vacuo to a solid, 0.121 g which was dissolved in 1
mL of methylene chloride and precipitated with hexanes, filtered and dried in vacuo;
0.110 g (43%) mp 14548C; 'H-NMR (300 MHz, CDCI3) ~ 0.90(d, J = 8 Hz, 3H, 14-
CH3), 0.96 (d, J = 8 Hz, 3H, 10-CH3), 1.14 (d, J = 8 Hz, 6H, isopropyl CH3), 1.3 (m, lH,
H-13), 1.5 (m, 1 H, H-13), 1.6 (m, 1 H, H-14), 1.64 (s, 3H, 8-CH3), 1.94 (s, 3H, 2-CH3), 2.0
WO 95/0L~42 ~16 6 3 2 0 PCT/IB94/00160
-61 -
(m, 1 H, H-15), 2.36 (p, J = 8 Hz, 2H, 3' æetidine CH2), 2.73 (dd, J = 8 Hz and 16 Hz,
1H, H-15), 2.9 (m, lH, H-10), 3.25 (s, 3H, OCH3), 3.27 (s, 3H, OCH3), 3.52 (sept, J =
8 Hz, 1H, isopropyl CH), 3.63 (m, 1H, H-12), 4.43 (m, 1H, H-6), 4.57 (t, J = 8 Hz, 4H,
2'and 4' azetidine CH2), 4.78 (br s, 2H, NH2), 5.0 (br s, 1 H, H-11), 5.86 (m, 2H H-7 and
5 H-9)~ 5.75 (t, J = 9 Hz, 1H, H-5), 6.45 (t, J = 9 Hz, lH, H-4), 6.9 (s, lH, H-19), 6.95 (m,
J = 9 Hz, 1H, H-3), 7.45 (m, 1H, NH), 9.35 (s, 1H, NH-22); m/z 772. (M+ + Na); IR
(KBr, cm-') 1735, 1685, 1645; Analysis c~lc~ ted for C35H5,N5O"S-1.25H2O: C, 54.43;
H, 6~98; N, 9.07%. Found: C, 54.42; H, 6.54; N, 8.73%.
EXAMPLE 71
101 7-~-Cvanoethvlamino-4.5-dihydro-11 -isopropYlsulfamvlcarbonyl-17-
demethoxvqeldanamvcin
The title compound was prepared by the method of Example 70 from the
compound of Example 11.
Yield 0.087 g (46%) mp 128-132C (dec); 'H-NMR (300 MHz, CDCI3) ~ 0.92 (d,
15 J = 8 Hz, 3H, 10-CH3), 0.93 (d, J = 8 Hz, 3H, 14-CH3), 1.07 (d, J = 8 Hz, 3H, isopropyl
CH3~, 1.09 (d, J = 8 Hz, 3H, isopropyl CH3), 1.17 (brm, 1H, H-13), 1.36 (brt, 1H, H-13),
1.43 (s, 3H, 8-CH3), 1.46 (br m, 1 H, H-14), 1.58 (m, 2H, H-5), 1.75 (s, 3H, 2-CH3), 2.00
(dd, J = 14 Hz and 6 Hz, lH, H-15), 2.23 (m, 2H, H~), 2.56 (t, J = 8 Hz, 2H, ~-ethyl
CH2,1, 2.77 (m, 1 H, H-15), 3.06 (br m, 1 H, H-10), 3.25 (s, 3H, OCH3), 3.27 (s, 3H, OCH3),
20 3.35 (br m, 1H, H-12), 3.47 (sept, J = 8 Hz, 1H, isopropyl CH), 3.66 (br m, 2H, a-ethyl
CH2,~l, 4.5-4.63 (br m, 3H, H-6, NH2 and NH), 4.85 (d, J = 6 Hz, 1 H, H-7), 5.05 (br s, 1 H
H-7), 5.74 (d, J = 9 Hz, 1H, H-9), 5.87 (brt, 1H, NH), 6.26 (t, J = 7 Hz, 1H, H-3), 7.05
(s, 1H, H-19), 7.40 (br s, 1H, NH), 9.00 (s, 1H, NH-22); m/z 787. (M+ + Na); IR (KBr,
cm-' ) 2320, 1730, 1690, 1645, 1580, 1480; Analysis c~lc~ ted for
25 C35H52N~O"S-0.25H2O: C, 54.71; H, 6.75; N, 10.93%. Found: C, 54.48; H, 6.88; N,
1 0.6B%.
EXAMPLE 72
1 7-Azetidin-1 -vl-1 1 -(4'-azidoPhenvl~sulfamvlcarbonyl-1 7-demethoxy~elcl~ ~amycin
1 7-Azetidin-1 -yl-1 7-demethoxygeldanamycin, the title compound of Example 18,
30 (0.260 g, 0.427 mmol) was dissolved in 5 mL of methylene chloride and cooled to 0C
in allame dried flask under nitrogen. Chlorosulfonylisocyanide (0.099 mg, 0.704 mmol,
0.061 mL) was added dropwise during 10 minutes. After stirring for one hour in the
cold, 4-azidoaniline (0.126 g, 0.938 mmol) was added and the reaction mixture allowed
WO 95/0L~42 PCT/l[B94100160
-62-
to warm to room temperature during one hour. The reaction mixture was evaporatedto dryness and the residue flash chromatographed on 120 g silica gel with 3% methanol
in chloroform affording pure product which was dissolved in 1 mL of chloroform and
preci,uilaled with hexanes; Yield 0.059 9 (17%), mp 152-154C; 'H-NMR (300 MHz,
5 CDCI3) ~ 0.85 (d, J = 8 Hz, 3H, 14-CH3), 0.94 (d, J = 8 Hz, 3H, 10-CH3), 1.2 (m, lH,
H-13), 1.36-1.7 (m, 2H, H-13 and H-14), 1.6 (s, 3H, 8-CH3), 1.94 (br s, 4H, 2-CH3 and
H-15), 2.35 (pent, J = 8 Hz, 2H, 3'-azetidine CH2), 2.65-2.9 (br m, 2H, H-10 and H-15),
3.24 (br s, 6H, OCH3), 3.64 (m, 1 H, H-12), 4.46 (br s, 1 H, H-6), 4.55 (t, J = 8 Hz, 4H,
2'and 4' azetidine CH2), 4.85-5.1 (br s, 2H, NH2 and H-7), 5.85 (br s, 1 H H-11), 5.48 (br
10 s, lH, H-9), 5.77 (brt, J = 9 Hz, lH, H-5), 6.45 (t, J = 9 Hz, lH, H-4), 6.86 (s, lH, H-
19), 6.8-7.15 (m, 5H, H-3 and aromatic CH), 7.8 (v brd s, lH, NH), 9.35 (s, lH, NH-22);
m/z 847. (M+ + Na); IR (KBr, cm-l 2108, 1737, 1689, 1647, 1584, 1481; Analysis
~-'.,u~oted for C38H48N8Ol1S-1.5H20: C, 53.57; H, 5.86; N, 13.15%. Found: C, 53.70;
., ~ r7. .~, ~ ., ~,.~
n, ~.~ .vc~
EXAMPLE 73
17-Allylamino-11 -azetidin-1 -vlsulfamylcarbonvl-17-demethoxvqeldanamycin
17-Allylamino-17-demethoxygeldanamycin (0.200 g, 0.341 mmol) was ~issolved
in 5 mL of methylene chloride and cooled to 0C in a flame dried flask under nitrogen.
Chlorosulfonylisocyanide (0.053 mg, 0.376 mmol, 0.033 mL) was added dropwise
20 during 10 minutes. A~ter stirring for one hour in the cold, azetidine (0.043 9, 0.75 mmol,
0.051 mL) was added and the reaction mixture allowed to warm to room temperature during one hour. The reaction mixture was evaporated to a residue and flash column
chromcloylaphed on 60 9 silica gel eluted with 69:1 :30 ethyl acetate:methanol:hexanes
to yield pure target compound which was dissolved in 1 mL of chlonJfc,r" " prec"Jildled
25 with hexanes and dried in vacuo; Yield 0.102 g (40%) mp 134-137C; 1H-NMR (300
MHz, CDCI3) ~ 0.92 (d, 3H, J = 8Hz, 10-CH3), 1.01 (d, 3H, J = 8Hz, 14-CH3), 1.4 (br
m, lH, H-13), 1.5 (br m, 2H, H-13 and H-14), 1.65 (s, 3H, 8-CH3), 1.95 (s, 3H, 2-CH3),
2.13 (m, 1 H, H-15), 2.05-2.2 (m, 3H, H-15 and azetidine 3'-CH2), 2.78 (dd, J = 6 Hz and
15 Hz, lH, H-15), 2.93 (m, lH, H-10), 3.26 (s, 3H, OCH3), 3.28 (s, 3H, OCH3), 3.63 (br
30 m, lH, H-12), 3.95-4.05 (br m, 6H, allylic CH2 and az~li.li.,e 2' and 4' CH2), 4.45 (br s,
1 H, H-6), 4.7 (br m, 2H, NH2), 5.02 (br d, J = 11 Hz, 1 H, H-11), 5.2 (m, 2H, vinylic CH2),
5.4 (br m, 2H, H-7 and H-9), 5.73-6.93 (m, 2H, H-5 and vinylic CH), 6.25 (br t, 1 H, NH),
6.45 (t, J = 9 Hz, lH, H~), 7.03 (br m, lH, H-3), 7.10 (s, lH, H-19), 9.32 (s, lH, NH-
~ WO 95/0134~, PCT/IB94/00160
3 2 ~
-63-
22); m/z 769. (M+ + Na); iR (KBr, cm~1) 1734, 1691, 1645 1579, 1474; Analysis
c~cu~ated for C35H49N5Ol1S-0.75H2O: C, 55.21; H, 6.69; N, 9.19%. Found: C, 55.19;
H, 6.18; N, 9.20%.
EXAMPLE 74
17-Azetidin-1 -vl-11 -piperazinYlsulfamylcarbonyl-17-demethoxyqeldanamycin
17-Azetidin-1 -yl-17-demethoxygeldanamycin, the title compound of Example 18,
(0.50 g, 0.854 mmol) was dissolved in 5 mL of methylene chloride and cooled to 0C
in aflame dried flask under nitrogen. Chlorosulfonylisocyanide (0.133 mg, 0.939 mmol,
0.082 mL) was added dropwise during 10 minutes. After stirring for one hour in the
cold, piperazine (0.162 g, 1.88 mmol) was added and the reaction mixture allowed to
warrn to room temperature during one hour. The reaction mixture was evaporated to
dryness and the residue flash chromatographed on 200 g silica gel with 20% methanol
in chlorlJfor.n affording pure product which was dissolved in 5 mL of chloroform and
precirit~ted with 150 mL of hexanes; Yield 0.161 g (24%), mp 180-182C; 1 H-NMR (300
MHz, CDCI3) ~ 0.86(m, 3H, 14-CH3), 0.9 (m, 3H, 10-CH3), 1.6 (s, 3H, 8-CH3), 1.94 (br
s, 2-CH3), 2.35 (pent, J = 8 Hz, 2H, 3'-azetidine CH2), 3.25 (br s, 6H, OCH3), 3.64 (m,
lH, H-12), 4.46 (br s, 1H, H-6), 4.6 (t, J = 8 Hz, 4H, 2' and 4' ~idi"e CH2), 6.43 (br
t, 1 H, H4), 6.9 (s, 1 H, H-19), 9.35 (s, 1 H, NH-22), other protons observed but not well
defined or assiy"able; m/z 799. (M+ + Na); IR (KBr, cm~1) 1734, 1689, 1646, 1600,
1471; Analysis c~lc~ ted for C33H52N~O11S-2H2O: C, 53.19; H, 6.94; N, 10.34%.
Found: C, 52.90; H, 6.81; N, 10.13%.
EXAMPLE 75
17-Azetidin-1 -yl-11 -(4'-methyl-1 '-piperazinvl)-sulfamvlcarbonyl-1 7-demethoxy-
qeldanamycin
17-Azetidin-1 -yl-17-demethoxygeldanamycin, the title compound of Example 18,
(0.200 9, 0.341 mmol) was dissolved in 5 mL of methylene chloride and cooled to 0C
in aYlame dried flask under nitrogen. Chlorosulfonylisocyanide (0.053 mg, 0.376 mmol,
0.033 mL) was added dropwise during 10 minutes. After stirring for one hour in the
cold, N-methylpiperazine (0.075 g, 0.75 mmol, 0.083 mL) was added and the reaction
mixture allowed to warm to room temperature during one hour. The reaction mixture
was diluted with 100 mL of chloroform and extracted with 100 mL of water and 2 x 100
mL of brine. The organic layer was dried with sodium sulfate, filtered and evapo,aled
in vacuo to a solid, 0.280 g. This was flash chromatographed on silica gel with 10%
WO 9S/0L342 ~ i 3 ~ ~ PCT/IB94/00160 ~'
-64-
methanol in chloroform affording pure product: yield 0.114 g (42%) mp 14749C; 1H-
NMR (300 MHz, CDC13) ~ 1.11 (d, J = 8 Hz, 3H, 14-CH3), 1.21 (d, J = 8 Hz, 3H, 10-
CH3), 1.55 (m, lH, H-13), 1.72 (m, lH, H-13), 1.83 (m, lH, H-14), 1.86 (s, 3H, 8-CH3),
2.2 (br s, 4H, 2-CH3 and H-15), 2.53 (s, 3H, N-CH3), 2.60 (br t, J = 8 Hz, 2H, 3'-
5 a2~tid;"e CH2), 2.70 (br s, 4H, piperazinyl CH2), 2.9-3.1 (m, 2H, H-10 and H-15), 3.53
(s, 6H, OCH3), 3.86 (m, lH, H-12), 4.69 (br s, lH, H-6), 4.82 (t, J = 8 Hz, 4H, 2'and 4'
azetidine CHz), 5.15 (br s, 2H, NH2), 5.72 (br s, 1 H, H-7), 5.57 (br d, 1 H H-11), 5.66 (br
s, lH, H-9), 6.00 (t, J = 9 Hz, lH, H-5), 6.68 (t, J = 9 Hz, lH, H-4), 7.15 (s, lH, H-19),
7.24 (br s, lH, H-3), 7.45 (s, lH, NH), 9.60 (s, lH, NH-22); mlz 813. (M+ + Na); IR
10 (KBr, cm l) 1738, 1688, 1646, 1583, 1471; Analysis calculated for C37H54N~OllS-H2O:
C, 54.94; H, 6.97; N, 10.39%. Found: C, 54.92; H, 6.87; N, 10.25%.
EXAMPLE 76
17-Allylamino-11 -keto-17-demethoxvgeldanamycin
17-Allylamino-17-demethoxygeldanamycin (90 mg, 0.15 mmol) was dissolved in
15 CHC13 (4 mL) to which was added the Dess-Martin periodinane (382 mg, 0.90 mmol)
and the reaction heated to reflux. After 1 hour the reaction was complete and the
reaction mixture diluted with CHCI3. The organic layer was washed with aqueous
Na2S2O3, saturated aqueous sodium bicarbonate and dried over sodium sulfate. Thesolvent was removed by evaporation and the residue recrystallized from ethyl
20 AcePt~/hexanes to give 17-allylamino-11 -keto-17-demethoxygeldanamycin, yield 84 mg
(96%), as light red crystals, mp 112-118~C (dec); lH-NMR (300 MHz, CDCI3) ~ 1.02 (d,
3H,J=7Hz),1.25(d,3H,J=7Hz),1.48(m,2H),1.75(m,1 H),1.80(s,3H),1.98
(s, 3 H), 2.32 (dd, 1 H, J = 14 Hz, 5 Hz), 2.58 (dd, 1 H, J = 14 Hz, 7 Hz), 3.29(overlapping s, 6 H), 3.66 (m, 1 ), 4.08 (m, 3 H), 4.28 (d, 1 H, J = 8 Hz), 4.82 (br
25 exchangeable, 2 H), 5.18-5.3 (m, 3 H), 5.55 (d, 1 H, J = 9 Hz), 5.8-6.0 (m, 3 H), 6.83
(br exchangeable, 1 H), 6.49 (t, 1 H, J = 11 Hz), 6.92 (d, 1 H, J = 11 Hz), 7.19 (s,1 H),
9.22 (s, 1 H); mass spectrum m/z 585 (M + 2); Analysis calculated for C31H4,N3O8-0.5
(ethyl acetate): C, 63.14; H, 7.23; N, 6.69%. Found: C, 63.19; H, 7.06; N, 6.92%.
The 11-keto compounds of Examples 77-87 were prepared by oxidation of the
30 appropri~le17-aminosubstituted17-demethoxygeldanamycinsaccordingtothemethod
of Example 76.
WO 95/0134:~ PCT/~94/00160
2~ ~'32~
-65-
EXAMPLE 77
17-CycioproPvlamino-11 -keto-17-demethoxyqeldanamycin
Mp 110-115C (dec); 'H-NMR (300 MHz, CDCI3) ~ 0.75-0.95 (m, 4 H), 1.03 (d,
3H,J=7Hz),1.24(d,3H,J=7Hz),1.72(m,1 H),1.79(s,3H),1.98(s,3H),2.78
5 (m,3H),3.32(s,3H),3.4-3.5(m,4H),4.08(m,2H),4.28(d,1 H,J=8Hz),4.81 (br
exchangeable, 2 H), 5.15 (s, 1 H), 5.57 (d, 1 H, J = 10 Hz), 5.71 (t, 1 H, J = 7 Hz), 6.26
(bræ, 1 H), 6.51 (t, 1 H, J = 12 Hz), 6.92 (d, 1 H, J = 12 Hz), 7.15 (s, 1 H), 9.22 (s, 1
H); mass spectrum m/z 606 (M + Na)
EXAMPLE 78
17-lsoProPvlamino-11 -keto-17-demethoxvqeldanamycin
Mp 105-111 C (dec); 1H-NMR (300 MHz, CDCI3) ~ 1.02 (d, 3 H, J = 7 Hz), 1.25
(overlapping douhletc, 9 H), 1.48 (m, 2 H), 1.75 (m, 1 H), 1.80 (s, 3 H), 1.98 (s, 3 H),
2.27 (dd, 1 H, J = 14 Hz, 5 Hz), 2.66 (dd, 1 H, J = 14 Hz, 7 Hz), 3.32 (overlapping s,
6 H), 3.67 (m, 1 H), 3.98 (m, 1 H), 4.09 (t, 1 H, J = 5 Hz), 4.38 (d, 1 H, J = 9 Hz), 4.82
15 (br ~ ;chal)ge~hlQ, 2 H), 5.17 (s, 1 H), 5.54 (d, 1 H, J = 9 Hz), 5.83 (t, 1 H, J = 7 Hz),
6.17 (d, 1 H, J = 9 Hz), 6.49 (t, 1 H, J = 11 Hz), 6.92 (d, 1 H, J = 11 Hz), 7.17 (s, 1
H), 9.27 (s, 1 H); mass spectrum m/z 587 (M + 2); Analysis c-'cu'~ted for
C3lH43N3O8-0.2CHzC12: C, 62.22; H, 7.20; N,6.98%. Found: C, 62.16; H, 7.0; N, 6.75%.
EXAMPLE 79
17-Methylamino-11 -keto-17-demethoxvqeldanamycin
Mp 108-120C (dec); 1H-NMR (300 MHz, CDCI3) ~ 1.06 (d, 3 H,
J=7Hz),1.23(d,3H,J=7Hz),1.48(m,2H),1.80(s,3H),1.97(s,3H),2.43(dd,
1 H, J = 14 Hz, 5 Hz), 2.68 (dd, 1 H, J = 14 Hz, 7 Hz), 3.17 (s, 3 H), 3.30 (overlapping
s, 6 H), 3.68 (m, 1 H), 4.11 (t, 1 H, J = 5 Hz), 4.32 (d, 1 H, J = 8 Hz), 4.80 (br
25 exch~~eable, 2 H), 5.21 (s, 1 H), 5.53 (d, 1 H, J = 10 Hz), 5.83 (t, 1 H, J = 7 Hz), 6.51
(t, 1 H, J = 12 Hz), 6.92 (d, 1 H, J = 10 Hz), 7.19 (s, 1 H), 9.28 (s, 1 H); mass
spet:trum m/z 580 (M + Na).
EXAMPLE 80
17-(2'-Hydroxyethylamino)-11 -keto-17-demethoxyqeldanamvcin
Mp 108-111 C (dec); 1H-NMR (300 MHz, CDCI3) ~ 1.07 (d, 3 H, J = 7 Hz), 1.25
(d,3H,J=7Hz),1.51 (m,2H),1.76(m,1 H), 1.81 (s,3H),1.98(s,3H),2.32(dd,
1 H, J = 14 Hz, 4 Hz), 2.63 (dd, 1 H, J = 14 Hz, 7 Hz), 3.34 (overlapping s, 6 H), 3.62
(m, 3 H), 3.88 (t, 2 H, J = 5 Hz), 4.09 (t, 1 H, J = 5 Hz), 4.28 (d, 1 H, J = 9 Hz), 4.8
WO 95/0L342 PCT/~B94/00160 --
3~
(br exchangeable, 2 H), 5.18 (s, 1 H), 5.53 (d, 1 H, J = 13 Hz), 5.79 (d, 1 H, J = 8 Hz),
6.48 (t, 1 H, J = 14 Hz), 6.92 (d, 1 H, J = 14 Hz), 7.18 (s, 1 H), 9.25 (s, 1 H); mass
spectrum m/z 589 (M + 2); Analysis calculated for C30H4lN3Og: C, 61.32; H, 7.03; N,
7.15%. Found: C, 60.96; H, 7.12; N, 6.90%.
EXAMPLE 81
1 7-(2'-MethoxYethYlamino)-1 1 -keto-1 7-demethoxyqeldarlar"ycin
Mp 130-134C (dec); lH-NMR (300 MHz, CDCI3) ~1.05 (d, 3 H, J = 7 Hz), 1.24
(d,3H,J=7Hz),1.82(m,2H),1.85(s,3H),2.00(s,3H),2.34(dd,1 H,J=14Hz,
5 Hz), 2.62 (dd, 1 H, J = 14 Hz, 7 Hz), 3.33 (overlapping s, 6 H), 3.40 (s, 3 H), 3.6-3.7
(m, 5 H), 4.10 (m, 2 H), 4.31 (d, 1 H, J = 9 Hz), 4.8 (br exchangeable, 2 H), 5.22 (s, 1
H), 5.55 (d, 1 H, J = 10 Hz), 5.82 (t, 1 H, J = 7 Hz), 6.49 (t, 1 H, J = 12 Hz), 6.92 (d,
1 H, J = 10 Hz), 7.18 (s, 1 H), 9.24 (s, 1 H); mass spectrum m/z 603 (M + 2); Analysis
c~ tPd for C3lH43N3O9: C, 61.79; H, 7.20; N, 6.98%. Found: C, 61.75; H, 7.02;
N, 6.86%.
EXAMPLE 82
1 7-(2'-Methvlthioethylamino)-1 1 -keto-1 7-demethoxyqeldanamycin
Mp 95-100C (dec); lH-NMR (300 MHz, CDCI3) ~1.05 (d, 3 H, J = 7 Hz), 1.23
(d,3H,J=7Hz), 1.48(m,2H), 1.80(m, 1 H), 1.81 (s,3H), 1.95(s,3H),2.11 (s,3
H), 2.32 (dd, 1 H, J = 14 Hz, 5 Hz), 2.63 (dd, 1 H, J = 14 Hz, 5 Hz~, 2.76 (t, 2 H, J =
20 7 Hz), 3.32 (overlapp,g s, 6 H), 3.67 (t, 2 H, J = 7 Hz), 3.67 (single proton under
triplet), 4.08 (t, 1 H, J = 5 Hz), 4.37 (d, 1 H, J = 7 Hz), 4.8 (br s, 2 H), 5.18 (s, 1 H),
5.52 (d, 1 H, J = 9 Hz), 5.83 (apparent t, 1 H, J = 9 Hz), 6.50 (t, 1 H, J = 10 Hz), 6.92
(br d, 1 H, J = 12 Hz), 7.19 (s, 1 H), 9.21 (s, 1 H); mass spectrum m/z 620 (M + 2);
Analysis c~lGulAtQd for C3lH43N3O8S: C, 60.27; H, 7.02; N, 6.80%. Found: C, 60.16; H,
6.82; N, 6.67%.
EXAMPLE 83
1 7-(2'-Fluoroethvlamino)-1 1 -keto-1 7-demethoxyqeldanamycin
Mp 99-105C (dec); lH-NMR (300 MHz, CDCI3) ~1.04 (d, 3 H, J = 7 Hz), 1.25
(d, 3 H, J = 7 Hz), 1.50 (m, 2 H), 1.80 (m, 1 H), 1.81 (s, 3 H), 1.99 (s, 3 H), 2.31 (dd,
1 H, J = 14 Hz, 5 Hz), 2.62 (dd, 1 H, J = 14 Hz, 9 Hz), 3.31 (overlappi.,g s, 6 H), 3.68
(dd,1 H,J=9Hz,7Hz),3.77(m,1 H),3.84(m,1 H),4.10(t,1 H,J=6Hz),4.30(d,
1 H, J = 8 Hz), 4.54 (t, 1 H, J = 5 Hz), 4.70 (t, 1 H, J = 5 Hz), 5.19 (s, 1 H), 5.54 (d,
1 H, J = 9 Hz), 5.83 (t, 1 H, J = 9 Hz), 6.28 (t, 1 H, J = 6 Hz), 6.50 (t, 1 H, J = 12 Hz),
~ WO 95/0134Z ~ ~ ~ 6 ~ ~ ~ PCT/IB94/00160
-67-
6.93 (d, 1 H, J = 12 Hz), 7.20 (s, 1 H), 9.17 (s, 1 H); mass spectrum m/z 591 (M + 2);
Analysis calculated for C30H40N308-1/12CHCI3: C, 60.26; H, 6.84; N, 7.01%. Found: C,
60.57; H, 6.54; N, 6.87%.
EXAMPLE 84
17-(2'-Cyanoethvlamino)-11 -keto-17-demethoxvqeldanamycin
Mp 102-107C (dec); 'H-NMR (300 MHz, CDCI3) ~ 1.07 (d, 2 H, J = 7 Hz), 1.25
(d, 3 H, J = 7 Hz), 1.53 (m, 2 H), 1.80 (m, 1 H), 1.81 (s, 3H), 2.00 (s, 3 H), 2.27 (dd,
1 H,J=14Hz,5Hz),2.58(dd,1 H,J=14Hz,6Hz),2.69(t,2H,J=8Hz),3.32(s,
3 H), 3.33 (s, 3 H), 3.71 (dd, 1 H, J = 9 Hz, 7 Hz), 3.80 (q, 2 H, J = 7 Hz), 4.07 (m, 1
H), 4.29 (d, 1 H, J = 8 Hz), 4.8 (brs, 2 H), 5.17 (s, 1 H), 5.55 (d, 1 H, J = 9 Hz), 5.84
(apparent t, 1 H, J--9 Hz), 6.02 (t, 1 H, J = 6 Hz), 6.51 (t, 1 H, J = 11 Hz), 6.94 (br
d, 1 H, J = 12 Hz), 7.22 (s, 1 H), 9.09 (s, 1 H); mass spectrum m/z 619 (M + Na);
Analysis calc~ ted for C3,H40N408: C, 62.40; H, 6.76; N, 9.39%. Found: C, 61.81; H,
6.45; N, 9.06%.
EXAMPLE 85
17-Azetidin-1 -yl-11 -keto-17-demethoxyqeldanamvcin
Mp 112-116C (dec); 'H-NMR (300 MHz, CDCI3) ~ 1.02 (d, 3 H,
J=7Hz),1.37(d,3H,J=7Hz),1.48(m,2H),1.67(m,1 H),1.82(s,3H),1.97(s,
3 H), 2.22 (dd, 1 H, J = 14 Hz, 5 Hz), 2.42 (m, 2 H), 2.58 (dd, 1 H, J = 14 Hz, 7 Hz),
3.30 (overlapping s, 6 H), 3.61 (m, 1 H), 5.15 (t, 1 H, J = 5 Hz), 4.17 (d, 1 H, J = 8 Hz),
4.62(t,4H,J=7Hz),4.80(brs,2H),5.19(s,1 H),5.51 (d,1 H,J=lOHz),5.76(t,
1 H, J = 10 Hz), 6.48 (t, 1 H, J = 12 Hz), 6.90 (br d, 1 H, J = 12 Hz), 6.97 (s, 1 H),
9.25 (s, 1 H); mass spectrum m/z 583 (M+); Analysis c~lcu~ted for C3,H4,N308: C,63.79; H, 7.08; N, 7.20%. Found: C, 63.83; H, 7.11; N, 6.84%.
EXAMPLE 86
17-(3'-Hydroxyazetidin-1 -vl)-11 -keto-17-demethoxvqeldanamycin
Mp 145C ffoam); 'H-NMR (300 MHz, CDCI3) ~ 1.0 (d, 3 H, J = 4 Hz), 1.2-1.3
(m, 6 H), 1.4 (m, 2 H), 1.7 (m, 1 H), 1.8 (s, 3 H), 2.15 (s, 2 H), 2.2 (d, 1 H, J = 4 Hz),
2.5 (m, 1 H), 3.3 (2 singlets, 6 H), 3.6 (m, 1 H), 3.8 (br s, 1 H), 4.15 (m, 1 H), 4.3 (d,
1 H,J=7Hz),4.354.5(m,2H),4.5-4.9(m,3H),5.0-5.2(m,3H),5.5(d,1 H,J=7
Hz), 5.8 (m, 1 H), 6.5 (t, 1 H, J = 10 Hz), 6.9 (d, 1 H, J = 14 Hz), 7.0 (s, 1 H), 9.2 (s,
1 H); mass spectrum m/z 622 (M + 2); Analysis calculated for C3lH43N30~-H20: C,
60.28; H, 7.02; N, 6.80%. Found: C, 60.76; H, 7.10; N, 6.36%.
WO 95/01342 PCT/IB94/00160
3 2 ~
-68-
E)~AMPLE 87
17-(3'-Methoxvazetidin-1 -yl)-11 -keto-17-demethoxvqeldanamycin
Mp 128C ffoam); 1H-NMR (300 MHz, CDCI3) ~ 1.0 (d, 3 H, J = 7 Hz), 1.25 (m,
6 H),1.45 (m, 2 H), 1.6-1.85 (m, 4 H, contains methyl singlet), 1.9-2.1 (m, 4 H, contains
5 methyl singlet), 2.1-2.3 (m, 1 H), 2.95-3.25 (m, 1 H), 3.3 (m, 9 H, contains 3 methyl
singlets), 3.6 (m, 1 H), 4.04.3 (m, 3 H), 4.35-4.5 (m, 2 H), 4.6-4.8 (m, 2 H), 5.1 (br s,
2H),5.2(s,1H),5.5(d,1H,J=10Hz),5.8(m,1H),6.5(t,1H,J=12Hz),6.9(d,
1 H, J = 12 Hz), 7.0 (s,1 H), 9.25 (s, 1 H); mass spectrum m/z 636 (M + Na); Analysis
c~lc~ ted for Cs2H43N3O9: C, 62.63; H, 7.06; N, 6.85%. Found: C, 62.23; H, 7.19; N,
10 6.70%.
EXAMPLE 88
17-Methvlamino-11 -12'-morPholinoethylamino)-17-demethoxygeldanamycin
In a dry flask, sodium triacetoxyborohydride (152 mg, 0.72 mmol) in
dichloroethane (4 mL) was sonicated until a fine suspension was formed. The mixture
15 was removed from the sonicator and treated with N-aminoethylmorpholine (47 ~L, 0.36
mmol) and a few crystals of sodium sulfate. 11 -Keto-17-methylamino-17-
demethoxygeldanfi."ycin (100 mg, 0.18 mmol) was then added and the mixture stirred
at room temperature for 24 hours. The reaction mixture was washed with saturatedsodium carbonate and brine and then dried over sodium sulfate. The solvent was
20 removed by rotary evaporation and the crude product purified by column
chromatography (silica gel, 10% methanol in methylene chloride) to give the title
compound as a purple solid; Yield 87 mg, (71%), mp 119-120C; 1H-NMR (300 MHz,
CDCI3)~0.90(d,3H,J=7Hz),1.00(d,3H,J=7Hz),1.42(m,2H),1.59(m,1 H),
1.65 (s, 3 H), 1.95 (s, 3 H), 2.03 (br s 1 H), 2.25 (dd, 1 H, J = 8 Hz), 2.68 (m, 3 H), 2.81
25 (m, 2 H), 3.15 (d, 3 H, J = 7 Hz), 3.22 (s, 3 H), 3.28 (s, 3 H), 3.52 (m, 1 H), 3.62 (t, 4
H, J = 4 Hz), 4.44 (brd, 1 H, J = 10 Hz), 4.75 (brs, 1 H), 5.40 (s, 1 H), 5.55 (d, 1 H,
J = 10 Hz), 5.78 (dd, 1 H, J = 8 Hz), 6.28 (d, 1 H, J = 7 Hz), 6.45 (t, 1 H, J = 13 Hz),
7.00 (m, 1 H), 7.05 (s, 1 H), 9.40 (s, 1 H); mass spectrum m/z 672 (M+).
The compounds of Examples 89-95 were prepared from 11 -keto-17-
30 (methylamino)-17-demethoxygeldanamycin and the appropriate amines using the
reductive amination method of Example 88.
~ WO 95/01342 PCT/IB94/00160
21~6320
-69-
EXAMPLE 89
11 -Benzvlamino-1 7-methvlamino-1 7-demethoxyqeldanamycin
Mp 123-126C; 1H-NMR (300 MHz, CDCI3) ~ 0.91 (d, 3 H, J = 8 Hz), 0.98 (d,
3H,J=8Hz),1.35(m,1 H),1.58(m,2H),1.66(m,1 H),1.70(s,3H),1.95(s,3H),
5 2.32 (dd, 1 H, J = 8 Hz), 2.75 (dd, 1 H, J = 8 Hz, 12 Hz), 2.93 (m, 1 H), 3.08 (s, 4 H),
3.12(d,3H,J=8Hz),3.15(s,6H),3.46(d, 1 H,J=8Hz),3.61 (d,1 H,J=24Hz),
3.78 (d, 1 H, J = 24 Hz), 4.30 (d, 1 H, J = 8 Hz), 4.75 (br s, 2 H), 5.35 (s, 1 H), 5.70
(m, ~ H), 6.28 (d, 1 H, J = 8 Hz), 6.43 (t, 1 H, J = 12 Hz), 7.01 (brd, 1 H, J = 16 Hz),
7.08 (s, 1 H), 7.20 (m, 5 H), 9.42 (s, 1 H); mass spectrum m/z 649 (M + 1).
EXAMPLE 90
11 -Cyclopropylamino-1 7-methylamino-1 7-demethoxvqeldanamvcin
Mp 120-123C; 1H-NMR (300 MHz, CDCI3) ~ 0.30 (m, 4 H), 0.93 (d, 3 H, J = 8
Hz), 1.02 (d, 3 H, J = 8 Hz), 1.40 (m, 2 H), 1.72 (s, 3 H), 1.80 (br s, 1 H), 1.99 (s, 3 H),
2.15 (s, 1 H), 2.32 (dd, 1 H, J = 8 Hz, 13 Hz), 3.00 (m, 1 H), 3.18 (d, 3 H, J = 7 Hz),
3.28(s,3H),3.30(s,3H),3.57(m,1 H),4.39(d,1 H,J=8Hz),4.84(brs,2H),5.63
(d,1 H,J=8Hz),5.80(dd,1 H,J=8Hz,8Hz),6.32(d,1 H,J=7Hz),6.51 (t,1
H, J = 13 Hz), 7.01 (br d, 1 H, J = 17 Hz), 7.09 (s, 1 H), 9.44 (s, 1 H); mass spectrum
m/z 600 (M + 1).
EXAMPLE 91
1 1 -Isoamylamino-1 7-methylamino-1 7-demethoxyqeldanamycin
Mp 108-110C; 1H-NMR (300 MHz, CDCI3) ~ 0.90 (d, 3 H, J = 8 Hz), 1.00 (d,
3 H, J = 8 Hz), 1.22-1.60 (m, 5 H), 1.63 (m, 1 H), 1.65 (s, 3 H), 1.95 (s, 3 H), 2.29 (dd,
1 H, J = 7 Hz), 2.53 (m, 1 H), 2.75 (m, 2 H), 2.90 (m, 1 H), 3.13 (d, 3 H, J = 7 Hz),
3.23 (s, 3 H), 3.28 (s, 3 H), 3.52 (m, 1 H), 4.90 (br s, 2 H), 5.35 (s, 1 H), 5.60 (br d, 1
H, J = 8), 5.75 (dd, 1 H, J = 8 Hz), 6.30 (d, 1 H, J = 7 Hz), 6.43 (t, 1 H, J = 12 Hz),
7.02 (s, 1 H), 7.09 (br s, 1 H), 9.38 (s, 1 H); mass spectrum m/z 629 (M + 1)
EXAMPLE 92
11 -(2'-Hvdroxyethylamino)-1 7-methvlamino-1 7-demethoxvqeldanamvcin
Mp (oil); 1H-NMR (300 MHz, CDC13) ~ 0.95 (d, 3 H, J = 7 Hz), 1.09 (d, 3 H, J =
7 Hz), 1.45 (m, 1 H), 1.58 (m, 2 H), 1.72 (s, 3 H), 2.00 (s, 3 H), 2.30 (dd, 1 H, J = 8
Hz), 3.02 (m, 9 H), 3.20 (d, 3 H, J = 7 Hz), 3.28 (s, 3 H), 3.35 (s, 3 H), 3.62 (m, 3 H),
4.55 (br s, 1 H), 4.90 (br s, 2 H), 5.40 (br s, 1 H), 5.63 (d, 1 H, J = 10 Hz), 5.81 (dd, 1
H, J = 8 Hz), 6.36 (d, 1 H, J = 7 Hz), 6.50 (t, 1 H, J = 13 Hz), 7.08 (m, 1 H), 7.12 (s,
WO 95/0L342 PCT/IB94/00160 1~
3 2 ~
-70-
1 H), 9.40 (s, 1 H); mass spectrum m/z 603 (M + 1).
EXAMPLE 93
11 -(3'-DimethYIaminoPropylamino)-1 7-methvlamino-1 7-demethoxy~eldanamYcin
Mp 105-108C; 1H-NMR (300 MHz, CDCI3) ~ 0.95 (d, 3 H, J = 7 Hz), 1.03 (d,
3 H, J = 7 Hz), 1.48 (m, 1 H), 1.65 (m, 3 H), 1.70 (s, 3 H), 1.98 (s, 3 H), 2.20 (s, 6 H),
2.28 (m, 3 H), 2.65 (m, 1 H), 2.70 (m, 2 H), 2.84 (dd, 1 H, J = 7 Hz), 2.90 (m, 1 H), 3.20
(d,3H,J=7Hz),3.29(s,3H),3.33(s,3H),3.57(m,1 H),4.48(d,1 H,J=13Hz),
4.80 (br s, 2 H), 5.41 (br s, 1 H), 5.62 (br d, 1 H, J = 13 Hz), 5.81 (dd, 1 H, J = 8 Hz),
6.32 (d, 1 H, J = 7 Hz), 6.49 (t, 1 H, J = 13 Hz), 7.09 (m, 1 H), 7.10 (s, 1 H), 9.43 (s,
1 H); mass spectrum m/z 644 (M+ + 1).
EXAMPLE 94
1 1 -Allylamino-1 7-methylamino-1 7-demethoxYgeldanamycin
Mp 123-125C; 1H-NMR (300 MHz, CDCI3) ~ 0.95 (d, 3 H, J = 7 Hz), 1.03 (d,
3 H, J = 7 Hz), 1.41 (m, 1 H), 1.52 (m, 1 H), 1.72 (s, 3 H), 1.84 (m, 2 H), 2.00 (s, 3 H),
2.36 (dd, 1 H, J = 7 Hz), 2.80 (m, 2 H), 3.00 (m, 1 H), 3.20 (d, 3 H, J = 7 Hz), 3.25 (s,
3 H), 3.32 (s, 3 H), 3.58 (m, 1 H), 4.42 (d, 1 H, J = 10 Hz), 4.80 (brs, 2 H), 5.08 (dd,
2 H, J = 16 Hz), 5.40 (s, 1 H), 5.65 (d, 1 H, J = 13 Hz), 5.83 (m, 2 H), 6.32 (d, 1 H, J
= 7 Hz), 6.50 (t, 1 H, J = 13 Hz), 7.05 (m, 1 H), 7.10 (s, 1 H), 9.45 (s, 1 H); mass
spectrum m/z 599 (M+ + 1).
E3(AMPLE 95
1 7-Azetidin-1 -yl-1 1 -oximino-1 7-demethoxyqeldanamvcin
To a solution of 17-azetidin-1-yl-11-keto-17-demethoxygeldanamycin (0.10 g,
0.17 mmol) in ethanol was added a solution of hydroxylamine hydrochloride (0.10 g,
1.42 mmol) and triethylamine (0.2 mL) in ethanol. The reaction mixture was stirred at
room temperature for 2.5 hours and there~rler the solvent was removed by rotary
evaporation and the residue dissolved in CHCI3. The chloroform solution was washed
with water, dried over sodium sulfate and the solvent removed by rotary evaporation.
The residue was purified by column chromatography (silica gel, 15% acetone in CHCI3)
to give 1 7-azetidino-11 -oximino-1 7-demethoxygeldanamycin (70 mg, 68 %), as a purple
powder. Mp 130-145C (dec); lH-NMR (300 MHz, CDCI3) ~ 0.98 (d, 3 H, J = 7 Hz),
1.17 (d, 3 H, J = 7 Hz), 1.37 (m, 2 H), 1.54 (m, 1 H), 1.81 (s, 2 H), 1.95 (s, 3 H), 2.25
(dd, 1 H, J = 14 Hz, 4 Hz), 2.41 (m, 2 H), 2.61 (dd, 1 H, J = 14 Hz, 3 Hz), 3.20 (s, 3
H), 3.28 (s, 3 H), 4.02 (m, 2 H), 4.13 (d, 1 H, J = 8 Hz), 4.65 (t, 1 H, J = 8 Hz), 5.01
~ WO 95/01342 PCT/IB94/00160
2~66~2~
-71 -
(br exchangeable, 2 H), 5.09 (s, 1 H), 5.37 (br d, 1 H, J = 10 Hz), 5.74 (t, 1 H, J = 10
Hz)l 6.45 (t, 1 H, J = 12 Hz), 6.89 (br s, 1 H), 6.97 (s, 1 H), 9.27 (s, 1 H); mass
spectrum m/z 586 (M - 2); Analysis calculated for C31H42N408: C, 62.19; H, 7.07; N,
9.36%. Found: C, 61.76; H, 6.88; N, 9.14%.
The compounds of Examples 96-99 were prepared according to the method of
Example 95 from the appropriate 11-keto-17-demethoxygeldanamycin.
EXAMPLE 96
17-(2'-Methoxyethyiamino)-11 -oximino-17-demethoxvqeldanamvcin
Mp 119-127C (dec); lH-NMR (300 MHz, CDCI3) ~ 1.03 (d, 3 H, J = 7 Hz),1.18
(d, 3 H, J = 7 Hz), 1.39 (m, 1 H), 1.84 (s, 3 H), 1.86 (m, 1 H), 1.98 (s, 3 H), 2.32 (dd,
1 H,J=14Hz,4Hz),2.68(dd,1 H,J=14Hz,7Hz),3.21 (s,3H),3.27(s,3H),3.38
(s, 3 H), 3.58 (m, 2 H), 3.62 (m, 2 H), 3.95 (m, 2 H), 4.12 (d, 1 H, J = 8 Hz), 4.90 (br
s,1 H),5.11 (s,1 H),5.38(brd,1 H,J=8Hz),5.75(t,1 H,J=lOHz),6.50(m,2H),
6.9 (br s, 1 H), 7.17 (s, 1 H), 9.25 (s, 1 H); mass spectrum m/z 616 (M+).
EXAMPLE 97
17-CycloPropylamino-11 -oximino-17-demethoxygeldanamvcin
Mp (amorphous); 1H-NMR (300 MHz, CDCI3) ~ 0.7-0.9 (m, 4 H), 1.0 (d, 3 H, J
= 7 Hz), 1.2 (d, 3 H, J = 7 Hz), 1.3-1.6 (m, 3 H), 1.8 (s, 3 H), 1.95 (m, 1 H), 2.0 (s, 3
H), 2.7 (m, 1 H), 2.9 (m, 2 H), 3.2 (s, 3 H), 3.3 (s, 3 H), 4.05 (br s, 2 H), 4.15 (d, 1 H,
20 J = 7 Hz), 5.13 (d, 2 H, J = 10 Hz), 5.45 (d, 1 H, J = 10 Hz), 5.8 (t, 1 H, J = 10 Hz),
6.3 l~d, 1 H, J = 3 Hz), 6.48 (t, 1 H, J = 10 Hz), 6.9 (br d, 1 H, J = 10 Hz), 7.08 (m, 1
H), 7.15 (s, 1 H), 9.28 (br s, 1 H); mass spectrum m/z 621 (M + Na); Analysis
calc~ t~d for C3lH42N408-0.5 H20: C, 61.27; H, 7.13; N, 9.22%. Found: C, 61.74; H,
7.25; N, 8.71%.
EXAMPLE 98
17-lsoproPvlamino-11 -oximino-17-demethoxyqeldanamycin
Mp 158-160C; lH-NMR (300 MHz, CDCI3) ~ 1.0 (d, 3 H, J = 7 Hz), 1.15 (d, 3
H, J = 7 Hz), 1.2 (d, 3 H, J = 7 Hz),1.3 (d, 3 H, J = 7 Hz),1.45-1.6 (br t,1 H), 1.7-1.9
(m, 4 H), 2.0 (s, 3 H), 2.2 (br d, 1 H, J = 14), 2.75 (t, 1 H, J = 14 Hz), 3.15 (s, 3 H),
3.25 (s, 3 H), 3.84.05 (m, 3 H), 4.1 (d, 1 H, J =10), 5.1 (s, 1 H), 5.25-5.5 (br d, 3 H),
5.7(t,1 H,J=10Hz),6.2(d,1 H,J= 10Hz),6.4(t,1 H,J= 10Hz),6.75(brd,1 H),
7.1 (s,1 H), 9.25 (br s,1 H); mass spectrum m/z 623 (M + Na); Analysis c~'cl~l~tecl for
C3lH44N408-1.5 H20: C, 59.32; H,7.55; N,8.93%. Found: C,59.27; H,7.07; N, 8.66%.
WO 95/01342 ~ 6 ~ 32 ~ PCT/Is94/00160
-72-
EXAMPLE 99
1 7-Allylamino-1 1 -oximino-1 7-demethoxvqeldanamvcin
Mp 135C; mass spectrum m/z 621 (M + Na).