Note: Descriptions are shown in the official language in which they were submitted.
WO 94125403 PCT/US94/04758
2166402
A DEVICE FOR AIDING THE SOLUHILIZATION
OF GASES IN LIQUIDS
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention pertains to the dissolution of gases in
liquids and more specifically to a device for introducing
large numbers of microbubbles of the gas into the liquid to
greatly increase the gas/liquid contact, facilitating rapid
dissolution.
2. Description of the Prior Art.
The ability to rapidly and efficiently dissolve gases and
liquids is required in several different, fields in several
different applications. Soluble gases are relatively easily
and rapidly dissolved in liquids, especially under the
application of elevated pressure. However, less soluble the
gases are more difficult to dissolve in liquid economically
and efficiently.
Additional difficulties are encountered when attempting
to dissolve one gas of a gas mixture, such as dissolving
i
j oxygen in water from an air mixture that is approximately
20%
oxygen and 80~ nitrogen. It is well known that the rate of
solubility of the gases in the liquid is directly proportional
to the concentration of the gases in the mixture. Thus, it
will take oxygen in air about five times as long to dissolve
in water than it would take a 100% oxygen gas to dissolve
in
water. However, many times it is desirable to dissolve oxygen
.
in a fluid and typically air is the desired source of oxygen
due to the availability.
i
I
W'O 94125403 PCTIUS94I04758
~16fi402
Frequently, it is desirable to dissolve oxygen in water.
Oxygen, however, is relatively insoluble in water. For
example, at 32C the solubility of oxygen in water in contact
with air at one atmosphere of pressure is only 7.3 mg./liter
or 7.3 parts per million (p.p.m). Solubility increases with
the decrease in temperature - at OC the solubility is
approximately 14.6 p.p.m.
Many applications exist which require the dissolution of
large amounts of oxygen into a large volume of liquid. For
example, it is necessary to oxygenate commercial fish ponds
to
enhance production and to oxygenate treated sewage or process
water from industrial plants and mills to purify the liquid.
Oxygenation of commercial fish ponds is necessary for the
following reasons. For example, aquatic organisms, including
both animals and plants require at least a minimum amount
of
dissolved oxygen in water to survive. The amount of required
dissolved oicygen varies between different aquatic organisms.
For example, cold water fish such as trout and salmon require
much more dissolved oxygen than warm water organisms, such
as
catfish or crawfish. Currently, aquatic animals such as
crawfish, shrimp, catfish, trout, salmon, and abalone are
being raised in horticultural ponds. In order to sustain
maximum production in these ponds, a minimum amount of
dissolved oxygen is required. The more oxygen dissolved in
the pond water, the more animals that can be raised.
j Oxygen is introduced into commercial fish ponds by a
1 variety of mechanisms where the natural air-to-water contact
i
is insufficient to reach the desired oxygen level. As
previously stated, when water is in contact with air, the
maximum concentration or saturation point of oxygen in water
i
i at 32C and one atmosphere of pressure is approximately 7.3
p.p.m. Typically, it is desirable to maintain the oxygen
concentration in the fish pond as close to saturation point
as
possible to enhance production. The rate of solution
increases as wind and wave action increase because of
2
PATENT
increased air-to-water contact. However, even on windy
days the rate of solution is slow.
One source of oxygen in the outdoor commercial ponds
is green plants. Any green plant that engages in
photosynthesis utilizes some of the dissolved oxygen, but
normally produces significantly more photosynthetic oxygen
than it uses. However, in darkness no photosynthetic
oxygen is produced, yet the plant organism is using some of
the dissolved oxygen. Therefore, typically in a fish pond
the dissolved oxygen decreases during the night to its
lowest value at daybreak, unless there is considerable
night wave action. On the other hand, many commercial fish
ponds are inside, requiring photosynthesis producing light
sources.
Many attempts have been made at trying to raise the
oxygen content of the water in commercial fish ponds. Most
techniques are targeted at improving the gas-to-water
contact, including: pumping pond water over rocky
waterfalls; squirting water from fountains in the air;
turning paddle wheels on surface of the ponds; and pumping
water/air mixtures at very high pressures and velocities
into the pond surfaces at various angles. All of these
techniques require large amounts of energy primarily
because of the large amount of energy required to lift 8.3
lb./gallons (1 kilogram/liter) of water above the pond
surface.
French Patent No. 2,466,271 ("Brandin") discloses a
different type of aeration device. Specifically, the
device is comprised of a vertical tube, an injector joined
to the interior part of the tube, a centrifugal pump wheel
that turns in the interior of a diffuser located below the
injector, and an electric motor for turning the centrifugal
pump wheel. The entire device is submerged into the liquid
to be aerated. Although not as much energy is required to
aerate the liquid, the aeration process is relatively
3
c~166402
P~a'1'EidT
inefficient. In addition, the device is structurally
comulex.
An alternate means of introducing more oxygen into the
water is the use of a simple stack of wire screens placed
in the water with the screen mesh fines decreasing from
bottom to top. A stream of air is then pumped into the
water below the screen stack.- This technique requires
little energy, but the bubbles coming through the screens
are still relatively large and at shallow depths a-rWthe
to efficiency is very poor.
Another oxygen introducing means is the use of
spinning air nozzles beneath the water. The nozzles are
somewhat more efficient than the other schemes, because
they are capable of producing small bubbles. However, the
devices are unsymmetrical and require considerable energy
to spin. Venturi tubes and porous diffuser stones are also
used, but are not efficient, particularly at shallow
depths.
U. S. Patent No. ~,~22,766 ("Sunada") discloses a gas
liquid contacting device that includes a rotor consisting
of a hollow inverted closed bottomed cone. The rotor is
either partially or entirely submerged in a liquid and then
rotated so that the liquid adheres to the outer periphery
of the rotor and is projected outward in a substantially
continuous thin film to be spread in the form of a film
. comprising fine particles. This device does not require a
great deal of energy to rotate. However, this device is
inefficient in that only the amount of water that adheres
to. the side of the rotor will be projected outwardly.
Oxygen is also used in water treatment applications.
For example, government regulation requires sewage
treatment plants to dissolve oxygen in effluent prior to
releasing the effluent into rivers, lakes, or oceans. The
spillage of any organic substance into a body of water
3~ causes the fairly raid loss of dissolved oxygen, because
of the oxidative destruction of the organic material. The
4
AMEI~DcD Si-#E~I
.-
1 s64a2
pl~.'1'ErIT
amount of dissolved oxv en required to decompose the
or gap is sewage by bac ter a~ xida tion is grea tly i~ excess
of the amount of dissolved oxygen that must be added to the
effluent prior to release. Reduction of oxygen causes
death in fish and aquatic plants. The devices used in the
sewage treatment plants are very costly and require a great
deal of power to run, just as those described above.
Oxygen can also be used to clean process water fron
industrial plants, such as chemical plants, paper mills,
and many other similar operations.. However, again the
dissolution process is very costly.
Just as oxygen is used to remove undesirable products
in water, so too is ozone. ozone is a form of oxygen
having three oxygen atoms per molecule rather than twc.
Ozone is a much better oxidizing agent than oxygen because
ozone is a much more energetic molecule. The ozone is used
for oxidatively destroying organic compounds in the liquid.
Organic destruction using ozone requires only seconds to
minutes, as opposed to the hours to days reauired to
destroy the organic compound using oxygen. an acrueous
solution of ozone decomposes within about 15 minutes,
leaving no undesirable product. Ozone is very fast acting
at very low concentration which makes it invaluable for
removing undesirable bacteria, viruses, and contaminating
organic matter from drinking water, spas, swimming pool
. water, and industrial water. However, there are very few
efficient means for producing ozone.
The problems of producing ozone from oxygen and the
inefficient methods currently available for dissolving it
make the ozone purification of water more expensive than
chlorine treatment. Even so, it is now being recognized as
superior because any excess ozone decomposes within about
25 minutes, leaving no bad taste, bad odor or toxic
products, as is true for chlorine. Chlorine does not
destroy organic contaminants but does react with them to
produce substances that are now recognized as carcinogens.
5
AMEt~DcD Sk-;EET
-~ 1 s~ ~ 4 0 2
uATENT
In spite of the current greater cost of the ozone
purification of water, the drinking water in at least one
major United States city is purified with ozone, as is
virtually all the drinking water in Europe. The water in
virtually all European swimming pools and spas and
increasing number of pools and spas in the United States is
purified with ozone. Yet, the available methods for
dissolving ozone in a liquid are relatively inefficient.
The dissolution of gases in liquids is required in
other areas as well. A gas-to-liquid reaction can be used
in any chemical process which requires the dissolution of
a slightly soluble gas. For example, cleaning and
disinfecting agents, like bleach and related products, are
produced by dissolving the slightly soluble chlorine gas in
a water slurry of lime. Carbon monoxide is a valuable gas
for reacting with many organic liquids for producing
products of great value such as different types of polymers
and pharmaceuticals.
European Patent Application No. 0,151,434 ("Venas")
discloses a device for treating and breaking up a liquid,
primarily molten metal, with the help of "centripetal
force." The device is a rotor having a cylindrical hollow
body with holes around the perimeter of the device and.a
hole in the bottom of the device. As the rotor is rotated
in a liquid, the liquid rises inside the rotor as a result
of centripetal force, creating a centripetal pump. A
centripetal pump is a very poor pump for liquids and even
worse for gases, because the pumping action is caused by
the friction that occurs between the liquid and the inside
of the cylinder. In order to throw molten metal, the
liquid used in the example described in the Venas
specification, out of the holes in the side wall and above
the molten metal surface, a significant amount of energy
must be expended to overcome the forces of gravity and tie
high density of the molten metal.
5a
A~~~NDcO Si-3EET
'.
a ~ PATENT
The devices described above are directed to some, but
not ail, of the scientific principles involving dissolving
soluble gases in liquids. An example of one principle is
that the rate of solubility of a gas in a liquid is
directly proportional to the area of liquid-gas contact.
Accordingly, a related principle is that the smaller the
gas or liquid bubbles that are in contact wi ~.h the other
medium, the faster the gas will dissolve. Even though most
of the patents :aentioned above attempted to meet these
principals, they did so inefficiently.
P.nother scientif is principal is that a liquid has a
density that is about 700 times greater than that of
gaseous oxygen and, therefore, requires much more energy to
lift and pump than is required to lift or pump a gas. This
1~ a major deficiency of the surface aeration devices that
spray water into the air mentioned above. For this reason,
it is much cheaper and more efficient. to pump and subdivide
a gas than a liquid. In addition, a gallon of water, in
water, "weighs" nothing, but raising a gallon. of water
above tile SLLT'faCe of the water requires the lifting of 8.3
/' pounds (~3~~ilogram) and requires the expenditure of
significant energy, depending on how high the water is
lifted. Lifting the same volume of gas in the same manner
would require only a small fraction of such energy. Thus,
2~ the choice is to move gas rather than liquid and to not
move it out of its own medium, if possible. Clearly, the
Sunada device is contrary to this principle.
Yet another principal is that air is 53 times less
viscous than water. Therefore, it takes less energy to
subdivide air than to subdivide water. The Sunada device
fails to utilize this principle, because it subdivides
water rather than gas. The Brandin device also fails under
this principal, because its pumping of large amounts of
water causes the gas to be pumped and subdivided along with
the water.
5b
AltClln~-r, "..__
PATENT
Another principle is that the gas-to-liquid contact
must be accomplished with a minimum expenditure of energy
for the amount of gas dissolved. The major problem with
most of the devices described above is that they expend a
great deal of energy to overcome the excessive friction
produced during operation due to the complex designs. It
is desirable to use a mechanism that is simple, as
symmetrical as possible, with no sharp corners (e. g.,
flanges, paddles) , and with the smoothest possible surfaces
to reduce the amount of energy required to overcome
friction. The blades, vanes, and paddles of the devices
described above create a great deal of friction. For
example, the Brandin device is complicated and difficult to
construct. The Venas device is designed to function by
friction and, thus, as stated above, is very inefficient.
Finally, the mechanism should be of the simplest
design so that it might be most easily constructed from
cheap and readily available materials and at the lowest
possible cost. None of the devices mentioned above
successfully meet this principle.
Therefore, it is a feature of the present invention to
provide an improved apparatus for inexpensively and
efficiently dissolving a gas in a liquid.
It is another feature of the present invention to
provide an improved process for oxygenating horticulture
ponds to enhance the productions of the ponds.
It is yet another feature of the present invention to
provide an improved mechanism for oxygenating sewage water.
5c
WO 94/25403 PCT/US94/04758
It is another feature of the present invention to provide
an improved process for removing impurities from liquid.
SUMMARY
These and other features and advantages are accomplished
by an apparatus including a hollow frustum having a closed top
and a bottom opening with the closed top being larger than the
bottom opening. A plurality of side openings are located
around the circumference of the frustum and preferably nearer
to the closed top rather than the bottom opening. The frustum
is rotated at sufficient speed to create a pumping action to
draw water up through the bottom opening and out through the
plurality of openings.
Preferably, the total surface area of the plurality of
openings is greater than 20% of surface area of the bottom
opening. The hollow frustum is either conical or pyramidal.
This apparatus is used in the processes of oxygenating of
horticultural ponds, waste water treatment, and impurity
removal from water and an application that requires
dissolution of a gas in a liquid.
BRIEF DESCRIPTION OF
THE DRAWINGS
So that the manner in which the above-cited features,
advantages and objects of the invention, as well as others
which will become apparent, are obtained and can be understood
in detail, more particularly a description of the invention
briefing summarized above may be had by reference to the
embodiments thereof that are illustrated in the drawings,
which drawings form a part of the specification. It is to be
noted, however, that the impended drawings illustrate only
preferred embodiments of the invention and are, therefore, not
to be considered limiting of its scope for the invention may
admit to other equally effective embodiments.
6
PATENT
~1 ~~64a~ _
In the Drawings:
FIG. 1 is a side view of a diffuser illustrating a
preferred embodiment of the invention.
FIG. 2 is a perspective view of a system used to
dissolve gases in a liquid in accordance with this
invention.
FIG. 3 is a side view of a diffuser illustrating an
alternate embodiment of the invention.
FIG. 4 is a perspective view of an system used to
dissolve gases in a liquid in accordance with an alternate
embodiment of this invention.
DESCRIPTION OF THE
PREFERRED EMBODIMENTS
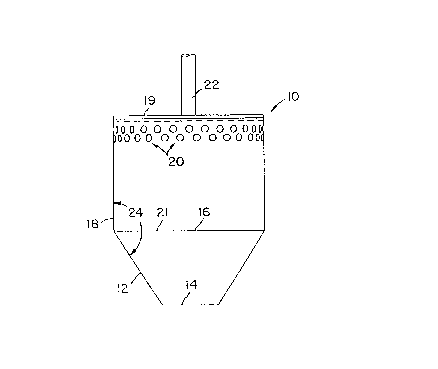
Now referring to the drawings and first to FIG. 1, a
typical preferred embodiment of the present invention is
illustrated. Diffuser 10 includes a hollow frustum 12 and
attached to hollow cylindrical member 18. Frustum 12
includes bottom opening 14 and top opening 16.
Cylindrical member 18 is of the same diameter as top
opening 16 and has closed top 19 and a bottom opening 21
that aligns with top opening 16, such that when frustum 12
and cylindrical member I8 are attached, one homogeneous
unit is created. A plurality of side openings are spaced
about the top of the perimeter or circumference of
cylindrical member 18. Rotating shaft 22 is positioned in
the middle of the closed top 19 of the cylindrical member
18 for rotating diffuser 10 when submerged in a liquid to
create a centrifuge-pump.
In the preferred embodiment of this invention, the
diameter of cylindrical member 18 and top opening 16 is
2-3/4 inches (70 mm). The overall vertical dimension of
diffuser 10 is 3-15/32 inches (88 mm), with the vertical
dimension of cylindrical member being 2-3/32 inches (53
mm). The diameter of bottom opening 14 is 1 inch (25 mm),
and internal cone angle 24 is 147.5°. Frustum 12 is conical.
7
PATENT
~,1664fl~
Figure 2 shows a diffuser, such as the one shown in
FIG. 1, submerged in reservoir 28 containing liquid 30.
Diffuser 10 is suspended by rotating shaft 22 of motor 34.
Motor 34 spins rotating shaft 22, and thus diffuser 10 at
high velocities, at which point the diffuser becomes a
centrifuge-pump, wherein liquid is drawn up through bottom
opening 14, thrown in an upward direction and out plurality
of openings 20.
Pump 36 injects a stream of gas through tubing 38 into
bottom opening 14, forming a liquid-gas-mixture that is
drawn into the diffuser 10 and ejected through plurality of
openings 20. The injections of the gas into diffuser 10 is
typically only required when diffuser l0 is submerged
substantially below the surface of liquid 30.
When oxygenating a liquid, no gas injection is
required if diffuser 10 is submerged in liquid only a small
distance below the surface. Preliminary results have shown
that the rate of oxygen dissolution into the liquid is much
higher when diffuser 10 is located near the surface of the
water without the injection of the gas, as opposed to the
diffuser being located well below the surface of the liquid
with the injection of the gas. When a diffuser of the
dimensions described above is placed within three inches of
the surface of the liquid in a 40 gallon (151.4 liters)
reservoir containing approximately 35 gallons (132.5
liters) of liquid and rotated at approximately 3,450
revolutions per minute (rpm), a violent surface action is
created generating significant cavitation and a
concentrated water-air-mixture. The diffuser operating
under these conditions produces a greater rate of gas
dissolution than when the diffuser is places within 12
inches (30.5 cm) of the surface with air being introduced
into the bottom opening at rates from 200-2000 milliliters
per minute. The lower rates produce high percentage oxygen
solution, while the higher rates produce poorer percentage
oxygen solution, but achieve a greater total rate of solution.
8
v1
PATENT
The slower rates might be ideal for dissolving gases
like ozone, where the high percentage oxygen solution would
be desirable, but large quantities are not required. For
oxygenating a fish pond or sewage plant effluent, rotating
a diffuser near the surface without introducing additional
gases is a more efficient means of oxygenating, primarily,
because the energy required to pump the gases is not
required.
FIG. 4 shows an alternate embodiment of the present
invention. Diffuser 200 is constructed of PVC plastic
water pipe fittings, including a top cap and a reducing
adapter glued together with PVC cement. The top cap is
approximately 66 millimeters (mm) in diameter. The bottom
opening is approximately 34 mm in diameter and
approximately 94 mm in height. The internal cone angle of
the reducing adapter is approximately 143° and the overall
height diffuser 200 is approximately 94 mm, creating an
internal volume of approximately 22G ml.
Diffuser 200 is connected to hollow shaft 222, which
is constructed of 1 inch (2.54 cm) PVC hollow water pipe
and is approximately a 16.5 inches (43 cm) in length, with
pvc cement. A metal fitting is attached to the top of
hollow shaft 222 to allow for connection to motor 34. Four
3/16 inch (5 mm) holes were drilled two inches below the
top of hollow shaft 222 as air holes. Forty 3/8 inch (9.5
mm) holes were drilled into the top cap of diffuser 200.
Tests have shown that submerging diffuser 200 into
reservoir 28 to a depth of 12 3/8 inches (30.5 cm) below
the surface of the water and turning the diffuser at
approximately 3500 rpm pulls air down into the hollow shaft
and expels it at high velocities into diffuser 200,
producing violent gas-water mixing. When diffuser 200 is
rotated at high speed, it acts like a centrifuge-pump and
pumps water through the side holes creating a vacuum inside
the cone that pulls air down through the hollow shaft. The
air-water mixture inside the spinning cone is thrown out
9
.,
PATENT
through the side holes. Thus, this embodiment of the
invention pumps its own air into the cone without requiring
an external air or gas source.
Tests have Shawn that relatively large internal
volumes are required for good results using this embodiment
of the invention, primarily because the water in the hollow
shaft must be displaced by air, before the air can mix with
the water inside the diffuser. The greater the height of
water in the hollow shaft, the greater the vacuum must be
to displace the water. For example, for the shaft length
described above, a diffuser of approximate volume of 182 ml
with twenty 5/16 inch (8 mm) holes in the top cap is not
capable of pumping air down the shaft. A larger volume
inside the cone appears to produce a greater vacuum for
displacing the water in the cone in the shaft. It also
appears that if the total number and/or size of holes on
the parallel portion of the cone is decreased, the solution
efficiency is reduced.
Variations of the parameters of the embodiments
described above produce very similar results without
departing from the heart of the invention. For example,
FIG. 3 shows an alternate embodiment of the diffuser, a
single hollow frustum 100 with bottom opening 114 and
closed top 119 to which rotating shaft 22 is attached.
Plurality of openings 120 are positioned near the top of
frustum 100. Thus, cylindrical member 18 of FIG. 1 is not
necessary.
Preliminary test results have shown that the only
essential parameter of the diffuser is that it must be
conical in nature and have a closed top. The smoothness of
the frustum wall is not critical. For example, the frustum
can be pyramidal. Also, it is not critical that the
diffuser be hollow. For example, deflecting flanges may be
included, but they reduce the efficiency of the diffuser.
Other parameters, such as the size, shape, number, and
location of openings are not critical.
PATENT
The area of the plurality of openings relative to the
area of the bottom opening is an influential factor, but
not a critical factor. A diffuser having opening area of
at least 20% or less than the area of the bottom opening,
produces better results than other commercial devices, but
the results are much poorer than when the area of the
plurality of the openings is more than 20% or greater than
that of the bottom opening. Good results are achieved when
the area of the plurality of openings is 100 - 450% larger
than the area of the bottom opening.
Since the ratio of plurality of opening area to bottom
opening area appear to be the influential parameter, the
number and size of the holes can vary. Good results have
been achieved with both small and large openings, with the
number of openings depending on the size. For a diffuser
of the dimensions described above the maximum whole size
that will produce good results is in the range from 9/32
inch (7 mm) to 1/2 inch (12.5 mmj. The maximum whole size
dimension varies with the size of the diffuser.
In the preferred embodiment of the invention, round
openings were used. However, there is no indication that
the opening must be limited to a round shape.
The location of the holes on the vertical dimension of
the diffuser is not critical. However, better results are
obtained when the openings are concentrated at the closed
top. Also the direction that the holes are drilled into
the diffuser influences the results, but only slightly.
The selection of diffuser material is not critical.
The diffuser of the preferred embodiment is aluminum;
however, any substantially rigid material can be used,
including hard plastic. Diffusers made of only tough
plastic are good for dissolving relatively unreactive
oxygen in water. For dissolving the more reactive gases,
such as ozone, the diffuser should be made of certain
stainless steel alloys, unreactive plastics, or possibly
11
PATENT
aluminum. The diffuser can also be made of two materials
including plastic and aluminum.
12