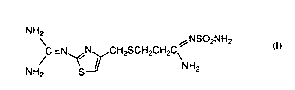Note: Descriptions are shown in the official language in which they were submitted.
- wo 95,0l7g5 2 ~ 6 ~- 7 3 ~ PCT/US94/07519
TITLE OF T~F. ~IVENTION
H2 ANTAGONIST-ALGINATE-AN~ACID COMBINATIONS
.
BACKGROUND OF THE ~VENTION
H2 antagonisls are c~mmrnly prescAbed to treat and
prevent ulcers in the walls of the stom~rh, duo~3c~ or esophagus. H2
antagonists are alsb used to treat non-ulcerative con~lition~. Damage to
the mucus lining ~ul~v~ lin~ these tissues en~bles destructive action of
stom~rh acids which erodes the underlying tissue. ~ommonly known
H2 antagonists for the tre~tmPnt of ulcers include cim~Ptlin~.? r~niti-lin~,
nizatidine, rox~ti~line and famoti~lin~q
Combinations of ~l~in~tes with certain H2 antagonists have
been disclosed. See U.S. Pat No. 5,007,790 which discloses a solid state
drug c~--t~ g (cimetidine)/polymer (sodium ~lgin~t~); GB 2æ2772
which discloses the H2 antagonist r~niti~line and ~lginir acid. GB
2,207,865 discloses a wound h~lin~ agent co~lising H-2 antagonist
(famotidine) with carrier such as an ~ in~te wherein the composition is
used to treat wounds rather than as a gastric acid inhibitor. EP-
290,229-B discloses an H2-antagonist (cim~oti~line) plus an antacid and/or
~l~in~te. See also U.S. Pat. No. 4,996,222. It is known that with
certain H2 antagonists, an ~lgin~t~ added to treat gastroesophageal
reflux can promote oxi~l~tion of the H2 antagonist to a biological
inactive form and additional ingre~i~nts have to be added to prevent this
re~ction Combin~tion~ of antacids and ~l~in~tes have been used to
provide ~y~ omatic relief of gastroesophageal reflu~. See
Martindale's Extra Pharmacopoeia at page 1432. Combinations of H2
antagonists and antacids have been disclosed: See FR2648710,
GB2219940, EP- 294933-A, EP-286,781-A, SU 1,362,477-A, U.S.
c 4,824,664, EP 233,853 and WO 9209286 Al. There is a need,
however, to employ a drug combin~tion with the advantages of an
~lpin~te or ~l inic acid and an antacid to prevent gastroesoph~e~
reflux (GER) in cQmbin~tion with an H2 antagonist selected from
famotidine or its salts, hydrates, stereoisomers or polymorphs to treat
and prevent the discol~foll associated wi~ indigestion, sour ætQrn~Cll,
WO 95/0179~ 2 i 6 6 7 3 I PCT/US94107S19
hcall~ or other gaslr~ al disorders including GER.
Additional ~nto~ nt~ may be added to the cl~ime~l famotidine/
in~te/antacid co.~ ;on to prevent o~ t on of famotidine to a less
active metabolite.There is a need to employ a combination v~l~il~ an
5 advantage is that the overall ~y~ toms of ga~lr~ s~ l distress can be
effectively treated with a combin~tion of the most powerful H2
antagonist av~ ble with an ~lgin~t~ and an ~nt~ci~ such as a c~l,.~nate
salt or m~gn~sjllm or ~1...";..1.... hydroxide wherein the combination
simlllt~nP,ously relieves and prevents s~ln~toms associated with excess
gastric acid secretion or evolution in the stom~rh and esophagus
respectively.
The present illv~ ion th~.~role provides an effective
synergistic tre~tm~nt of ga~lr~ estin~l disorders using the combin~tion
of famotidine and its salts, hydrates, or ph~rm~cologically active
stereoisomers or polymorphs ~,vith an ~ ,in~t~ and an antacid. The
c!~ime~l combin~ti~ is particularly useful for treating gastro-
esophageal reflux disorder at ni~h*me since famotidine or the
biologically active forms of ~mot~ n~ has a long-l~*n~ effect (9
hours) thereby aiding in the prevention of he~ ~ l and other
gasL..,i,llestinal distress while the ~l~in~te aids in elimin~tin~ the rafting
effect and the ~ntar,i~l provides rapid 1, lrrelillg relief in the stomach.
Other H2 antagonists that may be employed in this illvelltion include
cimP-litine, r~niti~line, ni7~tit1inP, and roY~ti~linP.
DETA~ED DESCRIPTION OF THE INVENTION
This invention claims ~ ce~llir~l composidons for use
in the tr~tmP-nt of mild stom~h and esophagus disorders including the
prevention and l-e~t~ -t of L~lL..Ill.. The composition colrll~lises:
(i) an amPunt erre.,live in the relief of ga~o;.. ~estin~l or ''
esophagus disorders of an H2 antagonist selected
from a colllp~,ulld of the form~
*rB
WO 95/01795 . 2 1 ~ 6 7 ~ T PCTIUS94/07519
NH2` NSO2NH2
~C=Ny~N~CH2SCH2CH2C\
NH2 S - b NH2
and its ph~rm~celltic~lly accephble salts, hydrates,
stereoi~ m~.rs or polymorphs and
(ii) an amount effective in relief of gaslrol~l~esffn~l or
o esophagus disorders of at least one of an ~l~in~te and
(iii) an amount effective in relief of ga~LIo;.,~s~
distress of an arltacid wherein the ~n~cid provides a
;.~g effect and generates carbon dioxide to
aerate the ~l~in~te and optionally
(iv) an ~ntifl~tlll~.nt amount of s;~lle~ cone~
This invention is also directed to a method of preventing
20 and treating indigestion, sour stomach, he~~ "l, overindulgence,
gastroesoph~e~l reflux and other ga~lr~ estinal disorders in
m~mm~ls, inchldinp 1.~ , in need of tre~tmtont thereof, conlplising
ini~tering to such or~ni~m:
(i) an amount effective in the relief of ga~,.Jillt~stinal or
esoph~ls disorders of an H2 antagonist selected
from a compound of the formnl~.
NH2 ~NSO2NH2
~C=Ny~N~CH2SCH2CH2C\
3 o NH2 S--Y NH2
and its ph~ celltic~lly acceptable salts, hydrates,
stereoisomers or polymorphs and
wo 95,0l7g5 2 1 6 6 7 3 t PCT/US94/07519
(ii) an amount effective in relief of gasllo;..l~s(;~-~l or
eso~h~ls disorders of at least one of an ~l~in~te
(iii) an amount effective in relief of ga~ro;..~.stin~l
distress of an antacid ~11~l~ the antacid provides a
l~urre~ g effect and generates carbon dio~ide to
aerate the ~l~in~t~. and optionally
o (iv) an ~ntifl~hll~P.nt amount of simPthicone.
The te~n m~mm~l~ or m~mm~ n or~ni~m includes but is
not limited to hllm~n~, dogs, cats, horses and cows.
The term tre~SmPnt çnromr~cses the complete range of
therapelltir.~lly positive effects associated with ~.h~ ell~
me-lic~tion including reduction of, alleviation of and relief from the
s~m~oms or illness which affect the org~ n~
Famotidine may be purchq~e.~ in buLk or ot~er suitable
~l~ntitiçs as it is cu~lly available on the ma~ket and formlll~tr-3 via
typical form~ tiQn processes with ~l~n~tes selecte~3 from ~1 inir, acid
which is suitable for tablet forml~l~tion~ or ~o~ m ~l~in~tP. which is
suitable for liquid form~ tions of the cl~imP,~ combination and antacids
which are also known including calcium c~l,onate and other carbonate
salts as well as ~ -l--n and m~ npsillm hydroxides. In ~dition,
simethicone, a known ~ntifl~tl~lent~ may be added to the above
25 combination to provide ma~ and broad relief of gasll~stinal
diblulb~..res and distress. Famotidine as a prescription drug product is
sold in the United States under the tr~-iPm~rk PEPCID~).
The ph~rm~cel)hc.~l co~ ositions of the present invention
are useful in the tre~tmP,nt of various mild ga~lr~i..tes~ l disorders
including indigestion, sour stomach, overindulgence and heall~ul~. In
particular, an ~lgin~te and ~nt~cid coml~inP~ with an H2 antagonist
selected from famotidine, a coll~ound of the fo~
WO 95/01795 ;~ l 6 6 7 3 1 PCI/US94/07519
NH2~ ~NSO2NH2
~C =Ny~NycH2scH2cH2c\
NH2 s_b NH2
or its rh~rm^^~ c~lly acceptable salts, hydrates, stereoisomers or
polymorphs is useful for the prevention and tr~ of various
ga~lloi~ stinal disorders such as indigestion,- sour stom~rh, or
h~ . The llhli7~tion of the ~ lly known biologically active
o forms and/or salts or hydrates of famotidine in combination with an
~1 in~te selected from ~1 inic acid or sodinm ~l~in~te or other
ph~rm~ceutically acceptable ~ tç salts or hydrates and an antacid is
advantageously used to treat mild ga~lruilltestinal disorders.
SimP,thirone or another anti-fl~ lent such as alpha-galactosid~ee (ADG)
15 may be added to this ~l~f~ d co~nbin~tion to provide anti-fl~t~ t
relief. In particular, the cl~imed combin~tion is used to treat the
~y~ ullle associated with ~t ic acid secretion while simlllt~neously
treating the Sylll~tOlllS of gastroesoph~ge~l reflux and fl~t~llenr~. The
- ~nim~l, p~ti~nt, or org~niem in need of tre~ -nt thereof the,e~o~e
20 bençfite from the Gl~ime~l ph~rm - elltic~l compositi~n-
H2 antagonists are well known in the tre~nent of ulcers
and other ga~llo;nlestin~l disorders and may be used, according to the
present inv~ntion, in combination with an ~lgin~te and an antacid and
optional anti-fl~ t H2 antagonists used for ulcer ~lel~y fall into
25 four major structural cl~ses imi~l~701e denvalives; s~lbsl ;l~e~l furans,
~mino~lkylrh.ono~y de~ivalives and ~li...;.l;l.othiazole compounds.
Famotidine (N-(~minoslllfonyl)-3-[[[2-[~i~...;.-nln~thylene)~minQ]4-
thiazolyl]methyl]thio] yloy~ mi~le)~ a m~mber of the latter class,
is a colllye~iliv-e inhibitor of hist~min~ H2-rec~tol;, and its primary
3 0 y~ cQlogical activity is the inhibition of gastric acid secretion.
Famotidine su~l~sses both the acid co--cæ-~ alion and the volume of
gastric acid secretion. Famotidine is well tolerated and has ~I;t~it~ side
effects and thus advantageously may be used in the present invention in
combin~tion with an ~1 in~te and an antacid. Famotidine is also the
most potent and selective H2 antagonist.
WO 95tO1795 2 1 6 6 7 3 t PCT/US94/07519
The combination of famotidine or its ph~ celltie~lly
effective salts, hydrates, ~lereoi~omPrs or polymorphs with an antacid
and an ~l~in~te and optionally simP-thirone provides a combin~tir~n
which simnlt~neously and selectively provides relief from and
prevention of disco nfoll and injury to the stom~h, esophagus, or
dllo~lenllm from excess production of gastric acid. I;,llll.P-I...ore,
famotidine in combination with an ~l~in~te and an antacid may not
interact with ~lr,ohol so that it may be ~lmini~tered prior to or during
ingestion of meals or beverages which Cû~ lcQhol and, thef~fol~, a
p~tiPnt in need of rapid tre~tment of ga~ l distress may take
the drug combination at an &~pf~ll&te time which may be during a
meal in which alcohol was c~ ecl The combin~ti~ n of an alginate
and antacid with famotidine provides relief of gastroesoph~ge~l reflux
while also providing long acting relief from and tre~tmP-nt of
ga~ 'estin~l disorders associ~te~l with gastric acid secretion. The
~nt~rid ~ min;~tered in the cl~imP~ comkinqtion provides both a
l~urrt~ g effect and sim~11t~nPously generates c~l,ull dioxide to aerate
the ~ n~te raft formed from the ~1 in~te The raft has a lower density
after this aeration effect and floats on the stom~r~ co~ s;
A ~l~"~eulically active stereoisomer or polymorph of
famotidine may be employed sllbst~nti~lly free of other stereoisomeric
forms of polymorphs, sllbst~nti~lly free should be taken to mean at least
90% of one ~ tinct stereoisomer or polymorph.
The combin~tion of famotidine which is a highly potent H2
antagonist with an ~l~in~te and an ~nt~cid re-lllces the size and weight of
all pl-~fm~r,eutical delivery forms or combination form~ tions and
lllelefole i~ wes p~ti~ t compliance or tolerance. The tablet or
capsule form of this co~ tion is more readily swallowable by
p~tirnt~ in need of tre~tmpnt thereof.
F~moti-line or its ~rm~r,elltiG~lly acceptable salts,
hydrates, stereoisomers or polymorphs is adv~nt~eously used in the
present invention in combination with ~l~inir acid or sodium ~1 in~te
and calcium c~l~ . Of course, other suitable and known antacids
such as the ~1--...;..--.~. hydroxide or m~esillm hydroxide salts or
WO 95/0179~ ~ 1 6 6 7 3 1 PCTtUS94/07519
~ ,s or com~in~tions thereof may be used in the cl~imP(l
form~ tion. For example, a one to one ratio of m~gnP,,cium hydroxide
to ~11... ~ hydroxide salts may be ntili7~jcl in the p~,se-ll invention.
The ~mount of famotidine used in the ~ t invention in hllm~nc may
range from 2.5 mg/day to 80 mg/day. Adv~nt~g-P,ously, 2.5 to 40
mgs/day is ~llrnini~tered in co~l,;- .~l ;c!n with 200-500 mgs/day mg of an
~l~n~te and 250-750 mgs/day of calcium carbonate. The qll~ntitieS of
each of the active ingre-lientc may vary depen~1in~ upon the severity of
the con~lition and the particular biocl~f ~ and need of the p~tipnt or
other or~ni.cm in need of tre~tn~-nt thereof. A physician or clinician
or veterinarian of ordinary skill in ~e art may readily detel~ine
suitable dosages of any prescription mP~lic~tiQn C~ 'E the cl~ime~l
invPntion- The combination cl~imPi-l in the ;..~ invention is
advantageously ~rlmini~ctered orally. The antacid employed herein may
15 be selec~ed from any of the co~ -~ially available or known antacids
or com~in~tions thereof such as ~l.. ;.. ~ hydro~cide, calcium
c~bollate, m~3yl~s~ l hydroxide or so~ m bicarbonate. The
simP-thicone optionally employed herein is also available con~lelically
and the ~t1mini~tered oral dosage may range in hllm~n~ from 10-1,000
mgs/day. This ~mollnt varies depen~lin~ upon the severity of the
con~ition and typical dosages are described in the Physicians Desk
Reference at pages 1155-56 (1992). ADG may be employed as an anti-
fl~ in doses of 290 to 31,000 ('J~ tosidase ~ ;o~l Units
(GaIU) particularly 675 to 2250 GaIU.
The present composition may be ~ministered to a p~l;.ont
in need of ~e~ ..t thereof in ~e form of tablets, caplets, gelcaps,
capsules, elixirs, lozenges, wafers, effervescent formnl~tioll.c, chewable
tablets, syrups, or s~lepeneioI c or via other known and effective delivery
methods. For oral ~lminictr~tion~ the acfive ingredients may be
30 ~-lmise~ with a ph~ celltir~lly acceptable dilnPnt such as lactose,
sucrose, cellulose, dicalcium phosph~te, c~l~illm slllf~te, ..~,..ilol, and,
in a liquid composit-io~ ethyl alcohol. Acceptable e-ml~leifying or
sllepen-lin~ agents such as PVP, gelatin, natural sugars, com swe~teners,
natural and synthetic gums such as ~ ci~, sodium ~1 in~te, guar gum,
wOgS/0l79!i 2 1 ~7 ~ PCT/US94/07519
agar, I~.to~ e, ca,l~ymethylcellulose so~ m) polyethylene glycol r
and waxes, may also be qtlmixe~l with the active cG~ Jo~?~ . Where
n~ce~ssqry~ lubricants such as m-q~sillm stearic acid talc or mqgn~sillm
st~qr-qte, and ~ inte~atol~ or supe~;~intçg~dtol~ such as starch, sodium
starch glycolate or cross-linked PVP may also be in~ etl~ Electrolytes
such as lic--q-lcillm ~os~ e, so~lillm ~ c~te, sodium qcet~q~te and
so~ m chloride may also be used. The inactive ingreflients may also
include m~psillm or ~ ;n~ tricilirqt~, sodium or potq..~sillm salts
including c~l,o late salts, -q~ .. hydroxide gel, lqr,tose, sorbitol,
as~al~lle or sodium sqcrh~ri~
The active comrQn~-nts may also be form~ ted in sust~in
release or effervescent forml~lqtio~ . The sust-q-in~ release
form~ tions also include layered formnl-qtiQn~ which provide for
tinct release ratios and thus may be more er~c~ive in allowing for
short and long term relief.
The following eY-qmrles illustrate tbe compositions of the
~.~se.ll invention which may be readily ~pa~d and as such are not to
be considered as limiting the lll./e~lion set forth in the claims.
EXAMPLE 1
al~in~t~lantacid/famotidine Tablet
~lginic acid 500 mg
famotidine 40 mg
PVP 15 mg
Avicel PHlOl 40 mg
.e~ St~ate 4 mg
c~lcillm c~l~ale 500 mg
m~ lm tri~ c~te 25 mg
soflillm bic~l,o~ e 170 mg
~l.. ;... hydroxide gel 100 mg
WO 95/~17~5 2 1 ~ 6 7 } ~ ~/US94107519
EXAMPLE 2
t~/antacid/f~mc)tidine Tablet
~lpinir acid 500 mg
famotidine 20 mg
PVP 15 mg
Avicel PH101 40 mg
.jllm Stearate 4 mg
0 C~lcjnm c~l~l~ate 500 mg
EXAMp~ .F. 3
in~te/antacid/f~motidine Tablet
~lpiniG acid 500 mg
famotidine 15 mg
PVP 15 mg
Avicel PH101 40 mg
2 Ma~siul~ Stearate 4 mg
calcium c~l,ol.ate 500 mg
- EXAMPLE 4
25 al~inate/antacid/famotidine Tablet
~l inicacid g
famotidine 10 mg
PVP 15 mg
3 Avicel PH101 . 40 mg
~gneSjllm Stearate 4 mg
c.~lci~m c~o-~ate 500 mg
wo 95/0l7g5 2 1 6 ~ 7 3 1 PCT/USg4l075lg
- 10-
EXA~IP! .F. 5
~in~tt~./antacid/famotidine Tablet
~1 iniC acid 500mg
famotidine 5 mg
PVP 1~ mg
Avicel PH101 40 mg
Magnesium Stearate 4 mg
calcium c~l~ollat~ 500 mg
EXAMPLE 6
15 ~l~in~te/antacid/f~m~ tidine Sustained Release
~1 inir.acid 600mg
famotidine 40 mg
PVP 30 mg
Avicel PH101 80 mg
Magnesium Stearate 8 mg
Me~hocel ElOMCR 66 mg
Meth~cel KlOOMLV 200 mg
calcinm c~l~ale 600 mg
EXAMP! P 7
~l~inate/famotidine/antacid Sus~lled Release
~1 inicacid 600mg
famotidine 20 mg
PVP 30 mg
AvicelPH101 80 mg
M~esil~mStearate 8 mg
WO 95/017g5 2 1 6 6 7 3 1PCT/US94/07S19
MethooelElOMCR 66 mg
Methocel KlOOMLV 200 mg
calcium c&ll~nale 600 mg
EXAl~IPLE 8
in~tP/antacidtfamotidine Solut;on
sodium ~ n~te 500 mg
o famotidine 10 mg
g.s. syrup 5 ml
c~ -m c~l,o~le 500mg
EXAMP~.F, g
al~inate/~nt~id~f~motidine Solution
sodium ~l~in~te 500 mg
famotidine 20 mg
g.s. syrup 5 ml
c~lcillm carbonate 500 mg
Simethirone may be added to each of the above
form~ hions or examples to provide anti-fl~hllent relief. The ~u~lily
Of ~imethic ?ne ~lminictered to a p~ti~nt in need of tre~tm~-*t thereof is
25 the typical known dosage range to treat fl~tnlPnre. The dose may be,
for example 2~40 mgs of ~imethiconP per 5 mls of a liquid form
cl~ime~ c~ mbin~tion or per chewable tablet wherein the other active
ingre~ P-nt~ inrl~ P famoti~line (20~0 mgs), m~eSi~lm hydroxide (200
mg), ~ll.. li.. ~. hydroxide (dried gel, 200 mg). The inactive
30 ingreflient~ in the tablet form may fur~her include dextrates, m~nnitol,
m~ ea.ate, Yellow 10, collodial silicondioxide andBlue 1 or
Red 27 while the liquid form(s) may ru~ r incl~fle inactives such as
butylparaben, c&lbo~ymethylcel11-lose sodium, flavors, hyd~ypr~yl
methylcellulose, microcryst~lline cellulose, propylparaben, and purified
wo gS/01795 2 1 6 6 7 3 ~ PCT~S94/07519
- 12-
water. Tbe previous examples are to be construed as non-limi*ng and
~di*on~l dosages and ~los~ge forms or routes of ~ nini~tration may be
varied lepen~1in upon tbe individual ~ t being treated for ei~er the
primary (e~cess acid I~P~r1;n~ to ga~ estinal or esophap~e~l
dislu~ lce or tl~m~ge) or secondary (infections) sylllptoms of
gasllu~lestinal disorders. ~ ~d~lition, known ~ "~e~?l*r~lly
acceptab}e exci~k,~s or agents may be added as inactive ingre~lipnt~ to
the cl~ime~ active combination in a variety of forms including tablets,
capsules, or time-release me~lic~m~nts