Note: Descriptions are shown in the official language in which they were submitted.
~ WO 95/~691 21 67 1 52 PCT~iL94/~164
Title: PRODUCTION AND APPLICATION OF TRANSGENIC MUSHROOM MYCELIUM AND
FRUITBODIES.
FIELD OF THE INVENTION
The invention involves different methods to modify genetic charac-
teristics of homobasidiomycetes in particular commercial homobasidio-
mycetes such as the common or button mushroom Agaricus bisporus via
treatment with donor DNA or fusions using protoplasts and via matings
between strains. The methods may be used for the i ~ t of com-
mercial characteristics and for the commercial production of enzymes and
metabolites.
BACKGROUND OF THE INVENTION
Fruitbodies from the common or button mushroom A.bisporus (LAnge
Imbach), also denoted A.brunnescens be~on~ing to the class of
basi~i~ ycetes (order Agarica~es), constitute an important crop in the
Netherlands with a production of l90 million kilos in 1992. Almost 75% of
this crop is exported. The United States of America, China and France are
other important mushroom producing nations. General problems associated
with the production and keeping of mushrooms involve e.g. infections by
pathogens like Pseudomonas to~aasii or P.agarici, dsRNA viruses and
browning caused by the action of endogenous poly-phenol-oxidases (PPO,
like tyrosinase). To further improve product quality, conventional
breeding pro~L- a already carried out have been only moderately
successful and may appear not to be sufficient on the long run, because
this procedure is highly time consuming and because the genetic variation
in commercially available strains is limited (Horgen et al. l99l).
The main problem for effective breeding strategies is caused by the
rather abnormal life-cycle of A.bisporus, which involves the usual simul-
taneous segregation of either parental nucleus into one basidiospore.
After outgrowth of this basidiospore heterokaryotic mycelium is formed
contAining nuclei and genetic characteristics that do not differ from
those present in the parental mycelium. In addition, only little recombi-
national activity is observed during meiosis (Summerbell et al. 1989).
- 35 For this reason investigators all over the world have attempted for
quite some years to develop a transformation system for commercial mush-
rooms such as A. bisporus for the introduction of novel characteristics.
In other organisms, especially in plants the application of gene transfer
technology is quite common and has already resulted in the first commer-
W O 95/02691 2 1 ~ 2 PCT~YL94/00164
cial applications, but the absence of a transformation system generally
applicable in a wild-type background in many basidiomycetes has strongly
hampered molecular-biological research of such organisms, especially that
of edible mushrooms.
Contrary to the situation in many ascomycetes, the application of
antibiotic resistance markers for ~,- nAnt selection of transformants of
basidiomycetes has to date only been moderately successful. As a repre-
sentative for heterobasidiomycetes, the phytopathogenic fungus VstiZago
maydis has been reported to be transformable with both auxotrophic and
antibiotic-resistance markers (Wang et al. 1988).
Within the class of homobasidiomycetes to which also edible mush-
rooms like A.bisporus and P~eurotus ostreatus (oyster mushroom) belong,
SchizophyZ~um commune is considered a model organism to study genetics
and developmental biology (Raper 1988). S. commune is in fact one of the
first representatives of this class for which a transformation system was
developed. This system is based on complementation of a trpl auxotroph
with the h ~logous TRPl gene (Munoz-Rivas et al. 1986). However, it does
not offer the possibility to transform non-auxotrophic homobasidio-
mycetes. So far, donor DNA comprising a prokaryotic ~, inAnt selection
marker has not yet been found both integrated and expressed at a level
which allows direct selection and stable maint~nAnce of transformed
homobasidiomycetes.
Successful transformations with auxotrophic markers have been
described for S.commune (Munoz-Rivas et al. 1986), Coprinus cinereus
(Binninger et al. 1987) and Phanerochaete chrysosporium (Alic et al
1989). In P. chrysosporium (~An~Al 1 and Reddy 1992) and P. ostreatus (Peng
et al. 1992) selection with antibiotic resistance markers was recently
described, however the donor DNA sequences were not found to be
integrated into the recipient genome and were subject to methylation.
Peng et al describe the use of recl~ h;nAnt plasmid pAN7-1 for
transformation with resistance to hy~L-~ ycin B as selectable marker in
P.ostreatus.
Similar processing of donor DNA was also observed in S. commune where
the heterologous E. co~i hpt (hy~L-I yCin B phosphotransferase~ gene was
introduced as antibiotic selectable marker by co-transformation in an
auxotrophic strain of S. commune (Mooibroek et al. 1987). The heterologous
sequences appeared to be heavily methylated, which may explain the low
level of their expression (Mooibroek et al. 1990) and thus the difficulty
to retrieve transformants by direct hy~r~ ycin B selection. It was
~wo gS/~2691 ~ ~ 6 7 ~ ~ ~ PCTA~L94100164
necessary to first select transformants for their ability to overcome the
auxotrophic deficiency and subsequently to detect amon~st those trans-
formants a subset having hy~l1- ycin B resistance. The reverse selection,
i.e. first for hy~ ycin B resistance did not produce results presumably
due to low expression of the heterologous DNA and the consistent genera-
tion of false positive colonies that escaped the selective pressure but
were not transformed. Si lArly it was also suggested for A. bisporus that
methylation of donor DNA or the low level of rec~ ~;nAtion in this orga-
nism might be causative for its resistance to genetic transformation
(Royer and Horgen l99l). Despite n1 --ous world-wide attempts, successful
transformation of A. bisporus with heterologous DNA (Challen et al. l99l,
Royer and Horgen l99l) leading to transformants comprising said DNA
stably integrated and/or expressing it sufficiently to be detectable
still has not been reported. The numerous unsuccessful attempts have been
carried out with various strains (among them the A.bzsporus strain Ul)
with the hpt-marker already mentioned as well as with other heterologous
markers (ChAl 1 en et al. l99l, Royer and Horgen l99l). It has been sug-
gested that other markers should perhaps be included in the transforma-
tion experiments, e.g. ~-glucuronidase (GUS) gene for transient expres-
sion (Royer and Horgen l99l).
All data to date suggest that hf ~bA~idiomycetes are reluctant to
genetic transformation with heterologous especially prokaryotic ~,~ nAnt
selection markers. The results of attempting genetic manipulation of
hf -bA~idiomycetes include the perception that this reluctance may be due
to insufficient expression and subsequently difficulties in detection of
transformants.
One method currently used to obtain genetic variance in mushrooms is
protoplast fusion. Teikoku-Pharm's Japanese patent e.g. discloses the
preparation of a new strain of mushroom by protoplast fusion of Lentinus
edodes and P. ostreatus exhibiting the taste of L. edodes and also exhibit-
ing rapid growth. This method does not however lead to controlled manipu-
lation of the characteristics of the resulting hybrid.
In Campbell Soup's US patent 4996390 commercial mushrooms of the
genus Agaricus are disclosed contA;ning genetic material from more than
- 35 one species obtained by protoplast fusion. In particular the auxotrophic
A.bitorquis (auxotrophic for nicotinic acid and resistant to cyclo-
heximide) and a h~ Aryotic strain of A.b~sporus (auxotrophic for
A~fn;ne and uracil and resistant to cycloh~xi ;de) is described. This
hybrid cannot form fruiting bodies, but can be crossed to form another
wo gS/0269~ 7 1 5 2 PCT/NLg4/OUl64 ~
hybrid. Yet again this is not a method for selectively incorporating one
or a few selected genes but is a method for random transfer of genetic
material. Furthermore it suggests that auxotrophic species and species
already comprising naturally occurring or mutagen in~uce~ resistance
markers can only be used for efficient selection of hybrids.
DESCRIPTION OF THE INVENTION
Completely surprisingly during work on an A.bisporus strain 'Abade'
to develop a transformation system based on complementation with the
corresponding wild-type gene with a view to later applications in co-
transformation systems along the lines of the previously mentioned
approach for S. commune (Mooibroek et al. 1990) and A. bisporus
transformation (Royer and Horgen 1991) a novel and efficient method of
transformation of homobasidiomycetes was developed.
As the nature of the deficiency resulting in an A~Pnine requirement
and the mutation in the correspon~ing DNA sequences are unknown and in
view of the large amount of work required to isolate the putative comple-
menting sequence, transformation experiments were first initiated with
available vectors contAining the E.co~i hpt-gene. The efficient
transformation of this strain was completely unexpected in view of the
fact that despite occA~ionAl satisfactory yields of protoplasts from
A.bisporus Ul having been achieved, no transformants had previously been
detected when using the same vector.
It was even more surprising to ascertain that successful use could
be made of the heterologous hpt-marker for transformation of UlmplO, a
new protoclone isolated from strain Ul via protoplasting and regenera-
tion. This strain was selected for its similarity with 'Abade' with
respect to early growth characteristics and colony morphology. Although
UlmplO yielded fewer protoplasts than 'Abade', strain UlmplO also
appeared to be transfo~ ~hle with the hpt-gene, contrary to strain Ul or
other protoclones thusfar. These data indicate that the devel_, t of
the transformation systems now available for 'Abade' and UlmplO could not
be anticipated on the basis of current knowledge of A. bisporus
transformation.
The subject invention is directed at a method for obtAinlng a
selectable stable transformant of a homobasidiomycete capable of expres-
sing integrated donor DNA comprising at least a ~: lnAnt selectable
marker at a detectable level, wherein said host is opti~nAlly non-auxo-
trophic and can be transformed without cotransformation with said
~ WO 9S/02691 2 1 6 7 1 5 2 PCT~L94/Uo164
~, nAnt selectable marker and said host is transformed with said donor
DNA. The method according to the invention can successfully be used on
c~ cial strains of homobasidiomycetes, such as strains belnng;ng to
the genus Agaricus. It has thus become possible to produce transgenic
mycelium and fruitbodies by the introduction of foreign and/or homologous
DNA sequences and the expression thereof resulting in new genetic and/or
phenotypic characteristics in said mushroom mycelium and/or fruitbodies.
The trf~nsgenic mushroom material may also be applied for the transfer of
transformed nuclei to mushroom mycelium of the same strain, another
strain of the same species, another species of the same family or another
species of another family. The transgenic organism or any part thereof
may further be used for the production of foreign and/or homologous
proteins, (poly)peptides and/or metabolites. The proteins, (poly)peptides
and/or metabolites may be recovered from the organism's tissues and/or
from the medium. The method may be used for the production of strains
with ; ruved quality aspects, such as reduced levels of browning (e.g.
PP0-activity), for the production of pathogen resistant strains and for
the genetic marking of commercially interesting strains of mushrooms to
estAhli~h proprietary rights. It has now become possible to integrate
specific desired heterologous nucleic acid sequences in a
homobasidiomycete and maintain said nucleic acid sequence in the
transformed mycelium and the fruiting body resulting therefrom, without
the need for sustained selective pressure.
For the subject specification a df nAnt selectable marker is meant
to be a marker that is selectable in a wild type of the host to be trans-
formed i.e. a host without auxotrophic deficiencies. It is therefore now
possible to transform homobasidiomycetes strains that are not auxotrophic
with a selectable marker.
The method of transformation according to the invention in the
embodiments just described can in particular be very successfully carried
out on a host obtained by subjecting homobasidiomycete material to a
transformation procedure, wherein the host to be transformed exhibits
delayed differentiation in comparison either to non-protocloned
homobasidiomycete material and/or the wild type strain Ul as obtainable
from ATCC, said delayed differentiation being macroscopically visible in
the form of r ~ed morphology due to a change in the number and!or
height of aerial hyphae, preferably by the absence of aerial hyphae
and/or by a ~; n;che~ hyphae aggregate formation preferably by the
W O 95/02691 2 1 6 7 1 5 2 PcTn~Lg4l00l64 ~
absence of hyphae aggregates. In particular a method wherein the host
belongs to the strain 'Abade' is an embodiment that provides good
results. 'Abade' exhibits delayed differentation when compared to Ul as
obtained from ATCC. A strain exhibiting ~ ~n~e~ morphology of the
required type for use in a method of transformation according to the
invention can be suitably obtained by subjecting homobasidiomycete
material to protocloning followed by selection of resulting
homobasidiomycete material exhibiting the desired delayed
differentiation. A suitable host can also be obtained by subjecting
homobasidiomycete material to a rejuvenation procedure followed by
selection of resulting homobasidiomycete material exhibiting the desired
delayed differentiation. Preferably the transformation procedure
C~ ri ng with the protoplast formation is carried out as soon as
possible after detection of the occurrence of delayed differentiation.
With a view to preventing loss of the transformability of the rejuvenated
homobasi~i~ ycete material it is preferable to c~ ce the
transformation procedure with the formation of protoplasts using material
which has not been subjected to more than 25 outgrowth phases. An
outgrowth phase in this respect comprises selecting a colony, plating out
the colony and growing the colony on a 9 cm agar plate until the plate is
full and a person skilled in the art will recognise that equivalents to
the number and length of outgrowth phases thus defined also fall within
the scope of the invention. Extremely good results were achieved when
less than five outgrowth phases took place between selection of the
desired rejuvenated host homobasi~ir ycete material and protoplast
formation as part of the transformation procedure. It is supposed that
the transformability can be lost if the number of outgrowth phases is too
high.
The good results achieved are in particular illustrated by the
results of transformation of the strains 'Abade' and UlmplO with donor
DNA comprising a ~ n~nt selectable marker in a manner according to the
invention. In the description of the experiments a detailed method of
transformation of these strains with plasmid DNA comprising the hpt gene
as ~: 'n~nt selectable marker is given. To our knowledge no
investigations were conducted or attempts undertaken for the description
of the 'Abade' flat or thin type of morphology. The morphology of 'Abade'
colonies was never observed in any other commercial or non-commercial
strain of A. bisporus, except shortly after protoplasting and regeneration
on CMPS-medium. On different media tested, including MMP, CMP, DT80, MS
~WO 95/02691 2 1 6 7 1 5 2 PCT/NLg4/00164
and B5, it has a brownish to light-brown, translucent appearance, lacking
any macroscopically visible aerial hyphae emerging from the main colony
which is attached to the agar medium, nor does it show any aggregation of
hyphae. The different morphology was apparent directly after purchase
from the ATCC strain collection. The 'Abade' morphology shows resemblance
with the phenotypes described e.g. for S.commune thn (thin) mutants
(Wessels et al., l991a). In the thn mutant described the expression of
the Sc3-hydrophobin gene is blocked as well as the generation of aerial
hyphae. It has been suggested that hydrophobins accumulate in the cell
walls of the hyphae that excrete them and contribute to the formation of
hydrophobic crosslinked structures within the cell wall. The S. commune
thn mutation is analogous to the Streptomyces b~d mutation. In
A.nidu~ans, disruption of the analogous rodA gene encoding a hydrophobin-
like protein resulted in a decreased hydrophobicity of aerial mycelium
(Stringer et al., 1991, for review see Chater, 1991). In addition to the
formation of aerial hyphae, hydrophobin genes are also involved in
fruitbody formation in S. commune (Wessels et al., l991b). The possible
reduced levels or even absence of these or other similar crosslinked
structures in the cell walls of "Abade' might contribute to better
digestibility of the cell walls by lytic enzymes resulting in higher
yields of protoplasts observed by us. It is difficult to i ~gine that the
known A~enine-auxotrophic mutation in 'Abade' would have such a dramatic
effect on cell wall synthesis. Therefore, we assume that other defects
may be present in 'Abade'. However, we cannot rule out the possibility
that some key functions would be defective resulting in pleiotropic
effects on both ~n;n~-requirement and cell wall assembly. It may be
significant to note that the ade mutation in 'Abade' does not result in
absolute A~nine-auxotrophy. After transfer of mycelial inocula on media
withou~ ni ne and longterm incubation, we have observed some slow
growth~ perhaps feeding on the inner parts of the colony. This ph~n~ ~non
of slow growth has been associated by others with instability of the ade
mutation.
In addition, the known ~nin~-requirement of 'Abade' cannot solely
explain its better transformability.
Strain UlmplO was isolated (with other similar ~colonies) after
protoplasting and regeneration on CMPS-medium of strain U1, which was
also purchased from the ATCC-collection (ATCC62462). In addition to a
number of normal appearing regenerates, the UlmplO-type of colonies were
isolated because of their resemblance with the 'Abade'-phenotype. The
W O 95/02691 2 1 6 7 1 5 ~ PCT~L94/00164 ~
frequency of their generation was about O. o8 . This phenotype was still
retained after the regeneration plate was fully covered with outgrown
regenerates. At the moment of the isolation of inocula for further
propagation, the colonies touched neighbouring colonies (mp stands for
mating protoclones). In addition to the flatter type of growth the
UlmplO-type of colony also revealed densely packed hyphae, especially in
the middle of the colonies. Another form of altered morphology was
apparent i.e. the marked reduction of the presence of hyphal aggregates
as is visible by absence of a thread-like morphology. After further
propagation it appeared that the aberrant morphology was transiently
retained on CMP and MMP agar medium, with a later tPndQncy to form aerial
hyphae. For the production of cultures on MMP t cellophane and subsequent
cultivation in liquid medium the outer differentiating parts of the
colonies were avoided. From the later experiments, including a-exterase
isozyme-analysis and fruitbody induction from UlmplO-derived
transformants, it was concluded that the UlmplO-type of colony was not a
h~ Aryon~ but a special type of heterokaryon. The primary UlmplO-
transformants either had the Ul phenotype (5O%) or the UlmplO phenotype
(5O%). After prolonged cultivation all primary transformants formed
sectors with the Ul phenotype.
It is well-known that protoclones derived from a heterokaryotic parental
strain may demonstrate a ~ ous variation in morphologies. In plant
biotechnology this phPn~ on is denoted s~ ~rl nnAl variation. These
f;n~ing~ are best explained with the assumption that imbalances have
occurred during protoplast formation and/or regeneration with respect to
the presence of cellular organelles and/or nuclei. ~lese phPnr ~ may
well represent a stress to the organism which is accompanied by the
development of competence for transformation. Numerous examples exist of
i roving transformation effic;enriQ~ by pretreatment of the host or the
donor DNA with ( W)-radiation or carcinogens. This was in fact the reason
why we attempted the use of linear DNA's which provides numerous double-
strand breaks. It is well-known from other systems that these double-
strand breaks may induce repair merhAn;! and recl binAtion ~rhineries.
It is anticipated that in "Abade', which only yields one type of
regenerates, this stress may be constantly present if not only the ade
mutation is present, but also other undefined mutations.
The efficiency of protoplast formation cannot be the only reason for
transformability of A. bisporus, because from UlmplO successful
transformation experiments were conducted with 2.7 x 1O6 protoplasts.
~ WO 95/02691 2 1 6 7 1 5 2 PCT~L94/00164
Therefore, we assume that some elements involved in the morphological
aberrations, possibly by the action of pleiotropic effects, may improve
the competence for genetic transformation, e.g. by the induction of
S.O.S.-systems.
In a further preferred embodiment of the invention the donor DNA is
linearized prior to transformation as this leads to a more positive
effect on the transformation efficiency. This positive effect of the use
of DNA that is linearized prior to transformation on transformation
efficiency is in line with observations in other organisms than homo-
basi~;( V,cetes such as the yeast HansenuZa po~ymorpha (Faber et al. 1992)
and some filamentous fungi (Banks et al. 1992, Liou et al. 1992, Tsai et
al. 1992).
Any c~ ly acceptable method for transformation of protoplasts can
be used in the method according to the invention, such as electropora-
tion, use of PEG or particle bombardment. A person skilled in the art
will be able to determine which method best suits the homobasi~ cete
material to be transformed, whether it has been derived by protoplasting
or notO The use of electroporation to mediate uptake of donor DNA is a
good choice due to the cell type specific controllAhility of parameters.
Furthermore the use of electroporation eli in~tes the risk of aggregate
formation of protoplasts by PEG and thus prevents potential segregational
instability of the donor DNA. This effect may be even more realistic for
multinucleate A. bisporus.
In another preferred embodiment of the method according to the
invention the efficiency of transformation can be significantly increased
by taking measures to ensure a higher yield in the number of protoplasts
per unit time prior to the actual transformation step. Methods are known
to a person skilled in the art for increasing protoplast yield and
regeneration efficiency (.Snnnenherg et al 1988). It is pointed out here
that the known methods for increasing protoplast formation as such are
insufficient to obtain transformability of the host homobasidiomycete
material. This was illustrated by the non transformability of Ul. As
however the chances of finding transformants are increased by such
measures the inclusion of such measures in the method according to the
invention is preferred.
As disclosed the rejuvenation is an important aspect of the subject
method. Rejuvenation as such is a known procedure (Fritsche 1991) and can
occur in the form of natural rejuvenation or artificial rejuvenation.
Natural rejuvenation occurs in the form of mating h: ~kAryotic spore
W O 9S/02691 2 1 6 7 1 5 2 PcTn~Lg4l00l64 ~
cultures leA~ing to mating products which are considered to be
rejuvenated offspring. In the method according to the invention further
h~ Aryotes can be derived from the rejuvenated offspring for
transformation. The artificial method of rejuvenation comprises formation
of protoplasts, regeneration and selection of hl ~lkAryons therefrom.
In particular the invention is thus directed at a method for
obtAining a Id nAnt selectable ætable transformant of a
h~ .ci ~i f ycete capable of expressing stably integrated donor DNA
comprising at least a ~ nflnt selectable marker at a detectable level,
said method comprising
a) subjecting the mycelium of the host to be transformed to at least:
1) protoplast formation, followed by
2) an outgrowth phase to colonies, followed by
3) isolation of individual protoclones resulting from step 2,
followed by
4) an outgrowth phase to colonies followed by
5) a selection of a protoclone resulting from step 4 on the basis
of exhibiting a delayed differentiation in comparison either to
non-protocloned homobasi~i ycete material and/or the wild type
strain U1 as obtainable from ATCC, said delayed differentiation
being macroscopically visible in the form of - ~e~ morphology
due to a change in the number and/or height of aerial hyphae,
preferably by the absence of aerial hyphae and/or by a
~i inifihed hyphae aggregate formation preferably by the absence
of hyphae aggregates.
6) optionally at least one cycle of further propagation of a
selected clone, including a subsequent outgrowth phase to
colonies and cultivation, preferably in liquid medium
7) at least one pr~toplast formation step from such a colony
and subsequently
b) subjecting protoplasts resulting from step 7 to transformation with
donor DNA. With the method according to the invention the host to be
transformed can optionAlly be non-auxotrophic and can also be
transformed with said ~: inAnt selectable marker without
cotransformation.
The indicated pretreatment of protocloning the host material to be
transformed prior to the actual transformation step appears to improve
the success rate of transformations. Parallel transformation experiments
~ W O 95/02691 2 1 6 7 1 5 2 PCT~L94100164
in which Ul and UlmplO were compared yielded only transformants from
UlmplO, but not from Ul indicating the preferred use of protocloning.
In a more preferred embodiment of this method according to the
invention the material to be used in step b) for transformation has not
been subjected to more than 5 successive outgrowth phases in step 6. The
use Or recently protocloning material for the transformation apparently
~nhAnces the success rate of obtA;ning transformants from a commercial
strain, which was previously not transfoL- ~hle.
In a further preferred embodiment of the subject method of
protocl nn; ng and transformation of such protoplasts with donor DNA
comprising a ~s ;nAnt selectable marker the protoplast formation step can
be further improved by growth of fungal mycelium in plant medium for
cultivation and regeneration of plant cells e.g. MS medium (Murashige and
Skoog 1962) i ~ tely preceding step 7. This additional measure has
been found to be particularly effective for a host bel~nging to the genus
Agaricus. It seems that the application of MS-medium during cultivation
in liquid medium causes a less densely packed type of mycelium and
perhaps also prevents the deposition of extracellular or cell wall
specific metabolites that might interfere with the activity of the lytic
enzyme used for protoplasting. On the other hand MS-medium lacks some
essential components (which are present in DT80) neede~ for long term
cultivation of Agarcus. The shift from a rich medium to a poorer medium
for the induction of competence for genetic transformation is also well
known for other transformation systems e.g. BacfZZus subtZis and E.coZ.
For the transformation methods according to the invention in the
various embodiments described above the transformation can be
successfully carried out using donor DNA comprising at least a ~ ;nAnt
selectable marker. The selectable marker can for example encode resis-
tance to an antibiotic and/or a fungicide. The resistance ~nco~ ng
sequence can suitably encode for resistance against hy~L-~ ycin B. Such
resistance can be provided by the hpt gene. The gene sequence is known
for hpt from both E. coZ and Streptomyces. The sequence from E.coZ is
used in the Examples and is eminently suitable for use in a method
according to the invention.
The donor DNA can further comprise at least one nucleotide sequence
homologous to a part of the DNA of the non-transformed host. The presence
of such a homologous sequence can imply the presence of a sequence at
which integration is desirable in the host chromosome, i.e. in order for
site specific homologous rec: b;nAtion to occur. It can also imply the
.
W O 95102691 2 1 6 7 1 5 2 PCT~L94/00164 ~
12
presence of homologous control sequences in order to ensure optimal
processing of the donor DNA by the host. Preferably the donor DNA further
comprises a promoter and optionally a terminator sequence homologous to
the host to be transformed.
In general no critical ~ 9n~ other than the usual ones on shuttle
vector composition generally applicable and well known to a person
~kille~ in the art of transformation of other organisms, in particular
bas;~ cetes beside the already mentioned presence of the ~ inAnt
selectable marker are made on the vector to be used.
It has however been found that a specific new vector can quite
successfully be used for transformation purposes of the hpt gene as
~r ;nA~t selectable marker. The vector is obtainable by at least the
following essential steps:
1) Introduction of an NcoI-site comprising the methion;ne-encoding
translation initiation codon of the hpt gene e.g. via the PCR
method using the wild-type E. coZi hpt-gene or plasmids pHRC or
pAN7-1 as templates and c~ b~n~tions of the following primers
having seq. id 1, 2 as illustrated in the sequence listing.
2) Removal of the unique NcoI- and EcoRI-sites from the wild-type
hpt coding region by in vitro mutagenesis e.g. via PCR using
primers having sequence id 3 and 4.
3) Cloning of the fragment altered in steps 1 and 2 in a proper
E. co Z ~ ( e.g. pUC-based) vector.
4) Introduction of EcoRI/NcoI genomic fragments preferably from
A.bisporus and opt;~nAlly comprising promoter activity.
5) Introduction of BamHI-HindIII genomic fragments preferably from
A.bisporus and opt;on~lly comprising terminator activity.
6) Propagation of this plasmid DNA in a proper host strain
= preferably lA~k; ng or mutated for the capacity to restrict
and/or modify cloned homobasidiomycete DNA.
The invention covers this vector and also use thereof in any of the
transformation methods according to the invention. In particular a vector
according to the invention comprises a promoter controlling the do ;nAnt
selectable marker of the donor DNA, said promotor being derived from the
host to be transformed. For transformation of A. bIsporus a vector
comprising an A. bisporus promoter is for example preferred. Suitably a
pAN7-1 vector can be modified when e.g. an A. bisporus promoter replaces
the A. niduZans promoter. Preferably a strong promoter sequence will be
used to control expression of the ~ nAnt selectable marker in a vector
~ W O 9~/02691 2 1 6 7 1 5 2 PCT~Lg4/00164
13
according to the invention. The strength of the promoter can generally be
derived from the level of expression of a gene 60 that any promoter of a
gene encoding a product that is present in large amounts will be
suitable. An eminently suitable promoter for transformation vectors for
A.bisporus is the GPD-2 promoter sequence. A pAN7-l modified vector
comprising the A.bisporus GPD-2 promoter æequence instead of the
A.nidu~ans promoter sequence controlling expression of the hpt-gene is
comprised within the invention.
Also termination sequences that can be recognised both by A.niduZans
and A.bisporus are preferably absent in a vector according to the
invention. In the case of the pAN7-l vector being modified for use in
A.bisporus as a vector according to the invention the termination
sequences such as present in the TRPC terminator sequence of A.niduZans
are preferably removed.
A further i~L'o~ ~rt of a vector according to the invention, in
particular a pAN7-l modified vector comprises the presence of a mutated
E.coZi hpt gene as gene Pnco~ing the d: inAnt selectable marker. The
mutated E.co~i hpt gene comprises a CG duplet at position 799 from the
5'-NcoI-site comprising the ATG start codon instead of the native GC
duplet. Consequently the mutant hpt-gene encodes Ile-Val instead of Met-
Leu. The mutated sequence driven by proper expression signal sequences
resulted in a higher resistance level of E. co~i transformants. A vector
comprising any other mutation in a hpt gene resulting in a higher
resistance level of host transformants is included in the scope of the
invention.
For homologous integration it is desirable to include a sequence of
nucleic acid h: -logous to a part of the chromosome of the host to be
transformed. A suitable sequence for A.bisporus comprises the AbGH3
sequence, a sequence that can be isolated through hybridisation with
putative N crassa tyrosinase sequences obtained by PCR with degenerate
primers and published sequences (Lerch 1992) in a manner known to a
person skilled in the art. Any number of alternatives will be apparent to
a person with knowledge of homologous integration and with access to the
known DNA sequences of the host to be transformed.
- 35 A preferred vector according to the invention for transforming
A.bisporus will comprise the homologous strong promoter GPD-2 controlling
the domin~nt selectable marker, the E.co~i hpt-gene with the mutated
duplet disclosed above and will also comprise a sequence of homologous
W O 95/02691 2 1 6 ~ ~ ~ 2 PCT~L94/00164 ~
14
nucleic acid for A.bisporus such as AbGH3 if homologous integration is
desired.
It is possible to also carry out cotransformation~with the trans-
formation method in the various embodiments of the invention disclosed.
Besides the transformation with the donor DNA comprising the ~, ;n~nt
selectable marker as primary marker as described it is possible to
introduce by co-transformation further DNA comprising a desirable
sequence to addit;onA11y transform the host. The technique of co-
transformation allows the introduction and stable integration of any DNA
sequence together with the primary (e.g. hpt) selectable marker. In order
to increase co-transformation effic;~ncie , in particular in A.bisporus
which exhibits low (~10%) cotransformation efficiencies, transformation
vectors can be constructed comprising both the primary selectable marker
and the cotransforming sequence (figure 19). The resulting transformants
comprising the cotransformed DNA can be demonstrated by Southern blot
analysis. The cotransforming DNA may code for any homologous or hetero-
logous polypeptide or protein which may or may not be excreted, thus
affecting metabolic and biochemical potential of the transformant when
expressed in the proper tissue and at the proper growth stage. Specific
homologous or heterologous genes may also be over-expressed by using a
strong (e.g. GPD-2) promoter or by insertion of higher copynumbers of the
same gene. GPD which is a constitutive enzyme, may constitute about 5% of
total cellular protein. The addition of rDNA sequences might also favour
the integration of multiple copies. Alternatively, a specific gene may be
repressed by different techniques. In the yeast Saccharomyces cerev~siae
and some fungi, like A.nidu~ans and Neurospora crassa, where homologous
integration may occur relatively frequently, gene-disruption may be the
best technique to silence a specific gene (Fincham 1989). The probability
of the occurrence of homologous (site-specific) integration has been
correlated with the length of the homologous insert of the donor DNA
plasmid with linearization of the donor DNA plasmid within the homologous
insert sequence. We have applied this system by cloning the AbGH3-
~in~III-fragment into the unique HindIII site of pAN7-1 generating pHAG3-
1, followed by digestion of the unique KpnI-site within the AbGH3-
sequence. phenl ~ like antisense RNA inhibition and co-suppression are
common in plant genetic engi neering~ but may not be applicable in
heterokaryotic fungal transformants cont~;ning only one (co)transformed
nucleus, unless both nuclei have been transformed directly or cf ~ine~ by
mating or protoplast fusion. Co-transformation with sequences coding for
2167152
W O 95/02691 , PCT~L94/00164
specific antibodies (in plants denoted 'plantibodies' Hiatt l99O),
however, may find general applicability. It appeared that also parts of
antibodies comprising the variant (Fv) chains and expressed in E. cozi may
have sufficient bin~;ng capacities (Pl~ckthun l99O), possibly also for
application in fungi (fungibodies).
Desirable sequences for example suitable for cotransformation using
the transformation procedure according to the invention are the putative
A.bfsporus tyrosinase genes (isolated at our institute) cloned sense or
antisense, the A.bisporus mannitol-dehydrogenase gene or the glucose-6-
phosphate dehydrogenase gene (Wood et al. l99l), the A.bisporus methallo-
thionein genes (Nishiyama et al. l99O), e.g. the barley ~-th;onin gene
(~.All~ing 1987) or resistance to dsRNA viruses through cross-protection
using a gene coding for e.g. a coat protein (Harmsen et al. 1989, Harmsen
et al. l99l). In particular these sequences can be introduced into
A.bisporus protoplasts by cotransformation according to the invention. It
is in fact possible to insert multiple nucleic acid sequences from the
cotransforming vectors at the same site in the chromosome. This is
probably due to in vivo ligation of nucleic acid from the various vectors
after linearization of the vectors has occurred such that compatible
sticky ends or blunt ends are created prior to the integration event.
Compatible sticky ends can be created simply e.g. by digestion of the
vectors with the same restriction enzyme(s). It is also poss;hle to
insert sequences in tandem using one vector.
With the above mentioned methods for transformation that have now
become available it is possible to produce stable transgenic fruitbodies
directly from transgenic heterokaryons, like UlmplO primary
transformants, or by matings or protoplast fusions between two compatible
strains wherein at least one of the mating strains is a transformant
obtainable from such a transformation method according to the invention.
For example a suitable transformant to be used for such a method is an
'Abade' transformant comprising resistance to hy~l~ ycin B as transgenic
selectable marker and comprising an A~enine deficiency as does the non-
transformed strain 'Abade'. This transformant can advantageously be mated
with another mating strain that is not deficient for A~nine and is also
- 35 sensitive to hy~L~ ycin B, resulting in selectability of the product of
said mating on both lack of ~en;ne deficiency and resistance to
hy~L-~~ ycin B. Such mating can take place by generally -known techniques
such as naturally occurring anastomosis or artificial protoplast fusion.
W O 95/02691 ~ 1 6 7 ~ ~ 2 PCT~L94/00164
16
It is also possible to produce h~ -kAryotic material from trans-
formed heterokaryotic material obtainable through the transformation
method according to the invention. These h- ~kAryons can be used for
matings. As already indicated a method of providing a genetic fingerprint
specific for a transformed homobasidiomycete comprising DNA analysis of a
transformant or a transgenic fruitbody obtAi~Ahle through a
transformation method according to the invention also falls within the
scope of the invention. In particular such a method is described, wherein
a genetic fingerprint specific for heterokaryotic material resulting from
a method of mating using a transformant obtAinAhle through the
transformation method according to the invention as at least one of the
mating strains, can be determined distin~li~hing sai-d heterokaryotic
material from the h~ ~kAryotic transformant used as mating strain by
analysing for the presence of more or different genetic material in the
heterokaryotic material than in the h~ -,kAryotic transformant. This can
be carried out for example by counting the number of nuclei per cell or
using protoplasts analysed in a cell sorter on the basis of the presence
of about twice the amount of genetic material in the heterokaryotic
mating product than in the h~ ~kAryotic transformant. Even more
elegantly, use can be made e.g. of 'Abade' transformant C25-1 which
contains the donor DNA integrated at the h~ ogous AbGH3-sequence.
Mating products with this strain as one of the two mating partners
contain the native 3.5 kb AbGH3-CtaI-fragment and the C25-1/ClaI fragment
which has a much higher molecular weight (depending on the number of
plasmid copies integrated). When the second mating strain comprises
different genetic material than the transformant mating strain the
genetic fingerprint can be further completed with an analysis of the
RFLP, RAPD or isozyme band pattern of the resulting heterokaryotic
material and comparison thereof to the starting material or any other
known strains can be used to ascertain proprietary rights.
The invention is also directed at non-auxotrophic transgenic homo-
basidiomycete material derived from a non-auxotrophic homobasi~ir~ycete,
said transgenic material comprising stably integrated donor DNA compris-
ing a ~ 'nAnt selectable marker such as a resistance to antibiotic and
said transgenic material further being capable of expressing said donor
DNA in an amount sufficient to ensure selectability over the correspond-
ing non transgenic material.
Certain aspects of the invention just described are further eluci-
dated in the following detailed disclosure of the invention.
2167152
W O 95/02691 PCT~L94/00164
17
DETAILED DISCLOSURE OF THE INVENTION
GENERAL METHODS
1- Source and growth of mycelia
Strains from A. bisporus were purchased from the American Type
Culture Collection (ATCC 24663, denoted 'Abade', and ATCC 62462,
commercial strain U1), inoculated on MMP agar medium contA;ning Malt
Extract (1%, Oxoid), Mycological Peptone (0.5%, Oxoid) and agar (1.5%)
and propagated at 24C for 1-2 weeks for 3 generations each. Thus, the
avAilAhility of inoculation material of identical generations was
guaranteed for all individual experiments. Except mycelia neede~ directly
for further processing, stocks were kept at 8C. In order to prepare
liquid cultures for the production of protoplasts, MMP plates contAining
a cellophane sheet were loaded with 5-10 inocula each and grown for 5-10
days at 24C. Colonies were subsequently scraped off the cellophane and
macerated for 20 seconds in a Waring Blender, contAining 50 mL of MSG20
(Murashige and Skoog 1962, contAining 20 g.L~1 glucose) medium. For
'Abade' 20 ,ug.mL~1 A~enine was added. The amount of macerated mycelium
that corresponded with the material derived from two plates was
inoculated in each Fernbach flask contAining a final volume of 150 mL.
Depending on the strain used growth was allowed while stAn~ing for 3-7
days at 24C. Protoplasts were usually isolated from the mycelium grown
in 3-4 Fernbach flasks.
2- PreParation and regeneration of ProtoPlasts
The Fernbach cultures were rinsed thoroughly over cheese-cloth with
sterile milliQ water and finally with 0.6 M sucrose. The mycelium was
then transferred to an Erlenmeyer flask contAining 0.6 M sucrose and 10
mg.mL~1 Novozym 234 (Sigma or Interspex Products Inc.) and incubated for
2-3 hours at 24C. The formation of protoplasts was monitored micro-
scopically with regular intervals. Their number amounted usually 108-109
protoplasts per experiment for 'Abade' and 106-107 for strains U1 and
UlmplO. Protoplasts were purified by sequential filtration through
cheese-cloth and 50 mL-syringes contAinin~ about 2 g of glass-wool,
previously rinsed extensively with 0.6 M sucrose and pelleted for 30 min
at 3000 rpm at 8C using a Heraeus Christ centrifuge accommodating 4-6
100 mL tubes. Pellets were further purified by 2 washes with 0.6 M
sucrose and 1 wash with SEH-electroporation buffer contAining 0.6 M
sucrose, 1 mM EDTA, 1 mM HEPES, pH 7.0, each time by centrifugation in 35
W O 95/02691 ~ l 6 7 1 5~ PCT~L94/00164
18
mL Corex tubes for 5 min at 3500 rpm at 4C using a Beckman RC-5C
centrifuge and the B 4 swinging bucket rotor. Protoplasts were finally
resuspended in 100 ~uL ice-cold SEH-buffer per parallel (usually 3-4)
experiment. Alternatively, protoplasts can also be produced by growing
colonies on membranes (e.g. Gene Screen, Dupont de Nemours and Co. Inc.
NEN Products) layered on top of agar media contAin;ng a cellophane
'r~le. After growth, the membrane is incubated upside-down in the
protoplasting solution for 1-2 h at 24C. Protoplasts can be recovered
from the liquid phase and washed as described above. Regeneration of
protoplasts was accomplished in two ways: 1- by plating directly onto
CMPS agar medium (for the isolation of protoclones) or 2- by incubating
in liquid CMPS for 3-5 days (for transformation experiments, see 4-
transformation procedure).
~~ Clonin~ ~rocedures. ~reParation of ~lasmid DNA and Southern blot
AnAl~sis of transformants
Cloning procedures were carried out essentially according to
Maniatis et al. (1982). Transforming plasmid DNA was isolated using CsCl
density gradient centrifugation or according to the Qiagen maxiprep
extraction protocol. Transforming plA~ were pAN7-1 (Punt et al. 1987)
and pHAG3-1 (a derivative of pAN7-1 contA;n;ng a 3 kb random A.bisporus
genomic HindIII-fragment (which weakly hybridised to a N. crassa laccase
specific oligonucleotide [Lerch 1982] cloned in the unique HindIII
restriction site of pAN7-1). Plasmid pAN7-1 was linearized with HindIII,
pHAG3-1 with KpnI, which is located in the A.bisporus insert sequence,
thus yielding two A.bispo~us DNA termini. Restriction enzymes were used
according to the suppliers rec ~Ations (PhaL- ~c;~). The DNA was then
dialyzed for 30 min on Millipore VM membranes floating in a solution
contAining 10% glycerol and lmM EDTA or the DNA was phenol/chloroform-
purified.
For the identification of transformants Southern blot analysis was
performed on genomic DNA isolated essentially according to Raeder and
Broda (1985), and comprising restriction enzyme digestions using condi-
tions rec ~e~ by the supplier (Pharmacia, with 10-fold excess of
restriction enzymes), electrophoresis, blotting and digoxigenin-
dUTP/AMPPD (DIG) autoluminescence detection (Boehringer ~Annh~i ).
A new set of multipurpose fungal transformation vectors has also
been constructed, which allow the convenient ~x~hAnge of EcoRI-NcoI
promoter fragments, the exchAnge of NcoI-BamHI structural gene fragments
2167152
W O 95/02691 PCT~L94/00164
.
19
and/or the ex~hAnge of BamHI-H~ndIII terminator sequences. The construc-
tion of these novel vectors is described in Example 2.
4- Transformation ~rocedure
Isolated protoplasts were resuspended in ice-cold SEH-electro-
poration buffer and i ~ tely electroporated with about 10 ~ug of donor
DNA using the BioRad Gene Pulser (parameters, electrode gap: 0.2 cm;
voltage: 0.45 kV; capacity: 25~F; shunt resistance: 200 Q), mixed
~ iAtely with 10 mL of CMPS medium (Compost extract 25% v/v, Myco-
logical peptone 0.5% w/v, plus sucrose, 0.6 M) contAining 100 ~g.mL~1
cefotaxim (Duchefa) and incubated for 3-5 days at 24C to regenerate cell
walls. Then the suspension of regenerates was warmed briefly at 38C and
mixed with 1 volume of 2% SeaPlaque low melting point agarose plus
sucrose 0.6M (38C) and poured as overlays (5 mL per plate) on DT80
(Dijkstra Tween 80) medium (Dijkstra 1976, Sonnenberg et al. 1988) con-
tAining 10, 25 or 50 ~ug.mL~1 hy~L-~ ycin B (Duchefa) plus A~nine (20
,ug.mL~1) for 'Abade', or on DT80 or B5 (plus glucose, 2%) medium
contAinine 50 or 100 ug.ml~1 hy~l-l- ycin B for UlmplO. Viability was tested
before and after pulse delivery with serial dilutions on CMPS agar
medium. Plates were incubated at 24C. Regenerates became visible by
microscopy after about 3 days. Transformants arose macroscopically after
1 to several weeks.
~- Cotransformation
As an example of a method of cotransformation according to the
invention co-transformation with pUT720, comprising the phleo~ycin (k~
resistance gene from StreptoaZZoteichus hindustanus is described here. In
addition, experiments with the Phot~nus pyra~is (firefly) luciferase gene
as reporter system were carried out.
Cotransformations were performed after linearizing both plasmids
(e.g. pAN7-1 and pUT720 by digestion with HindIII) after mixing equal
amounts of DNA contAining the primary selectable marker and the
cotransforming DNA (10-25 ,ug). Hereby, the native and/or modified ble
and/or mutated LUC genes (see below) were used as reporter genes for the
- 35 determination of expression levels after integration into the recipient
genome.
The hpt gene constructs described in example 2 have been used for
promoter probing and promoter trapping (promoter fishing) experiments in
a manner known per se to find promoters that are activated at specific
W O 95/02691 2~ ? PCTn~L94/00164
growth stages or under specific conditions. For this purpose A. nid~Zans
promoter sequences were removed by digestion followed by electrophoresis
and isolation of the proper vector-contA;ning band from the gel by the
Qiaex extraction protocol. For promotor-probing experiments random
genomic A. bisporus fragments were ligated into the promoterless vector
for transformation of E. coZi . From all E. coZi transformants obtained the
plasmid DNA ~now contAlning a variety of genomic A.bisporus fragments)
was re-extracted and used for A. bisporus transformation as linear
molecules. In case lUC or bZe genes were used as reporter genes, the
constructs were introduced with the hpt gene as primary selectable
marker, by co-transformation. The promoter sequences may be recovered by
techniques like marker or plasmid rescue, inverse PCR or PCR with one
specific anchor-primer Anne~l ;ng to the donor DNA plus a random (RAPD)
primer. For plasmid or marker rescue the genomic DNA of transformants is
digested with a restriction enzyme, which does not cut the donor DNA
introduced. Several restriction enzymes may be applicable separately. The
total genomic DNA is then ligated to make the donor DNA plus the fl Anking
genomic sequences circular, which can then be used to transform
appropriate E. coZi strains. The E. coZi strain may be propagated to
isolate the new rec '~inAnt plasmid for further analysis, including
retransformation of A. bisporus . In case of tandem integrations the PCR-
mediated techniques may be more suitable.
6- Marking of transformants
Southern blot analysis of 'Abade' and UlmplO transformants obtained
according to the invention revealed specific banding patterns upon
restriction of the total DNA with BgZII, BamHI or EcoRI (or their combi-
nation), which cut the original vectors and upon hybridization with the
hpt-probe. For each transformant analyzed the unique position of either
single or multiple integrations was determined by the flanking genomic
BgZII, BamHI or EcoRI restriction sites. Thus for any transformant the
number of hybridizing fragments generated and their specific sizes after
single or multiple digestions is unique and may therefore be regarded as
a transformant-specific and thus strain-specific fingerprint. In addi-
tion, the unique flAnking DNA sequences for each individual transformant
can be deteL- 'ned following inverse PCR and sequ~nc~ng.
7- StabilitY of transformants
2167152
WO 95/02691 21 PCT~L94/00164
The mitotic stability of 'Abade' transformants was assessed in two
ways. Primary hygromycin B resistant colonies were grown on non-selective
MMP agar medium covered with a sheet of cellophane for several (10)
'generations', each time by transferring inocula from the edge of the
colony. Phenotypic expression of the hpt-gene was checked for growth on
DT80 medium contA;n;ng 10 or 25 ,ug.mL~l h~L~ ycin B and 20 ,ug.mL~l
A~n;ne. The non-transformed strain and a colony that grew on a selective
plate without having received any donor DNA (false positive) were taken
as controls. It may be significant to note that with the experimental
protocols now developed no or hardly any false positive colonies appear.
UlmplO transformants demonstrated a sectored type of growth resembling
the parental Ul strain. Sectors were investigated separately for
retention of the hpt-gene and the hy~L-~ ycin B resistant phenotype in the
same way as for the 'Abade' transformants.
The stability of transformants was also investigated by Southern
blot analysis after growth of selected colonies on MMP agar medium. The
position of autoluminescent si gn~l S detected after hybridization with the
hpt-probe were compared with the positions of ethidium bromide-fluores-
cent bands from the corresponding gel. This procedure shows whether the
donor DNA has been integrated into the recipient genome or is present
within the cells as free plasmids. By selecting PrOPer restriction
enzymes conclusions can also be drawn with respect to the integrated
nature of the donor DNA.
8- Production of hr ~k~ryotic transformants from heterokarvotic primar~
tr~n~formants
Primary UlmplO hygromycin B resistant transformants were inoculated
with up to five inocula per plate on GeneScreen (Dupont de Nemours and
Co. Inc, NEN Products) hybridization membranes layered onto MMP agar
medium and grown at 24C for about 7 days. M~ es contA;nin~ the
colonies were then transferred upside down to petridishes cont~;n;ng 10
mL of o.6 M suc~ose cont~;n;ng Nu~u~y ~ 234 (10 mg.mL~l). After incubation
for 2-3 hours at 24C protoplasts produced were recovered from the proto-
plasting solution by centrifugation for 10 min at 3500 rpm and washed
three times with o.6 M sucrose by repeated centrifugations. Protoplasts
were incubated for 3 days at 24C in petr;~;~hes contA;n;ng 10 mL of
liquid CMPS medium to regenerate cell walls. Serial dilutions were then
plated onto DT80 agar medium cont~;n;n~ 10, 25 or 50 ~ug.mL~l hy~L-~ ycin B.
Similar procedures have been described earlier for the production of
W O 95/02691 2Z 2 1 6 7 1 5 2 PCT~Lg4~00l64 ~
h( ~kAryotic breeding material from heterokaryotic parental strains.
TT -lAryons were generated with an efficiency of approximately 10%
(Snnn~nherg et al. 1988). The nuclear constitutions were assessed e.g.
using ~-esterase isozyme analysis (Sonnenherg et al. 1988) and/or RAPD-
mapping (Khush et al. 1991).
9- Mating of transformants
For the production of transgenic fruitbodies from A. bisporus, a
heterokaryotic constitution of the mycelium is required, which should
contain at least two compatible nuclei with opposite mating types. Unless
the initial transformant is already heterokaryotic (e.g. from UlmplO),
heterokaryosis between hl ~kAryotic strains or even between a
h~ ~kAryotic and a heterokaryotic strain (socalled Buller ph~n~ nn,
Raper et al. 1972) may be accomplished by naturally occurring
anastomosis, or artificially by protoplast fusions (see below).
For the initiation of anastomosis, inocula of about 2-5 mm2 were
placed upside down onto non-selective MMP agar medium, about 1-5 mm
apart. Inoculated plates were incubated at 24C for 1-4 weeks. Withln the
zone where outgrowing colonies touch, compatible interactions may be
observed resulting in pigmentation, in an aberrant mycelial morphology
and the frequent excretion of intracellular material resulting in the
formation of brown droplets in e.g. P.ostreatus (Kay and Vilgalys 1992).
Similar phen~ a were also observed for matings described here. Inocula
from the interaction zones that also included some parental mycelium were
transferred to new agar media for further analyses described below in
example 5.
When a h: ~kAryotic transformant, which is marked via the presence
of a known Southern blot banding pattern produced after digestion of the
genomic DNA with said or other restriction enzymes followed by Southern
blot analysis with appropriate probes (described in section 'Marking of
strains'), is mated with another preferably h~ Anyotic strain with a
different RFLP-, RAPD- and/or isozyme banding pattern, the resulting
heterokaryotic strain yields the same bAn~ing pattern as the original
transformed 'Abade' h -kAryon used as mating strain. This method is
referred to as indirect marking of strains. The parental h~ ~kAryotiC
transformed strain can be distinguished from the new heterokaryon by
additional counting of the number of nuclei per cell, by cell sorting of
protoplasts or by RFLP-, RAPD-techniques and/or by isozyme analyses. An
elegant example is given by using 'Abade' C25-1 as one of the mating
2167152
W O 9~/02691 PCT/~ 94100164
23
partners. In true heterokaryons the presence of two different AbGH3-
fragments can be demonstrated, due to the modified size of the AbGH3-ClaI
fragment through homologous integration of pHAG3-l.
lQ- StAbility of mated transformants
Heterokaryotic transformants that were generated by matings between
'Abade' hy~L-~ ycin B resistant transformants and Ul protoclones, were
transferred to non-selective MMP-medium covered with a sheet of cello-
phane and propagated for several 'generations', each time by taking one
inoculum from the edge of a colony of about 5 cm in diameter to new MMP-
plates plus cellophane, and another inoculum to test the ability to grow
on double selective SD-medium cont~;n;ng hygromycin B (5O ,ug.mL~l). After
further propagation the r~ ~;n;ng colony was removed from the cellophane
and subjected to Southern blot analysis to verify the presence of the
donor 1)NA.
ll- Proto~last fusions with transformed ~roto~lasts
HY~LI ycin B or phleomycin resistant transformants from A.bisporus
can be used for the production of intra- or interspecies hybrids by
protoplast fusions follo~ed by ~: ;n~nt selection of fusion products.
Transformants, preferably (made) h~ ryotic~ derived e.g. from
UlmplO and expressing the hpt or bte gene, may be fused to one another
and fusion products may be ~1 ;nAntly selected by the simultaneous
application of hy~L-I ycin B and phleomycin contained in the growth
medium. They may also be fused to other organisms, cont~;n;ng another
endogenous or donated d: ;n~nt selection marker.
'Abade' transformants expressing the hpt or bte gene may be used for
the dnm, nAnt selection of fusion products with protoplasts expressing the
complementing ADE gene, from any wild-type organism, but preferably from
A.bisporus, A.bitorquis, A.arvensis or other Agaricus spp. Fusions of
protoplasts have already been accomplished between A.bisporus and
A.bitorquis (Patent number US4996390; 91-302364/41), between P.ostreatus
and Lentinus edodes (Patent number JP4173034; 92-255378/31) and between a
variety of different fungal species (Patent number J02245179; 90-
339235/45). However, none of these fusions relied upon the presence and
~1 ;n~nt selection of a donor DNA marker. Similarly, other fungal proto-
plasts e.g. from P.ostreatus or L.edodes or even from plants e.g. SoZanum
tuberosum, which are sensitive to a hy~r~- ycin B concentration of about
50 ,ug.mL~l or a phleomycin concentration of about 7.5 ~g.mL~1 may be used
-
W O 95/02691 2 1 6 7 1 5 2 PCT~*Lg4l00l64 ~
24
for the ~. ;n~nt selection of hybrids. In each case the transformed
'Abade' transformant donates the antibiotic resistance, whereas the fused
counterpart donates the functional, complementing ADE gene.
Protoplasts from Ag~ricus spp (or e.g. P.ostreatus) were prepared as
described above for 'Abade' and A. arvensis or according to ~onnenherg et
al. (1988) and finally resuspended in o.6 M sucrose. Then about equal
amounts (100 uL, contAin;ng 107-108 protoplasts) of either protoplast type
were mixed in a 15 mL pointed centrifuge tube (Corex). One volume of 40%
PEG6000 was added by pipetting carefully onto the centrifuge tube wall.
By this way the PEG solution formed the lower layer at the interphase,
covered by the protoplast suspension. Protoplasts were then centrifuged
for 5 min at 800 rpm in a swinging bucket rotor using a table centrifuge
(Beckman) onto the interphase, which allowed the formation of fusions.
After st~n~ing for 5 min at room temperature 5 mL of 0.6 M sucrose were
added and carefully mixed by inversion. Subsequently, the protoplast
suspension was washed three times by centrifugation for 5 min at 2500 rpm
in a table centrifuge and resuspended in CMPS for 3 days to regenerate
cell walls and plated onto DT80 agar medium cont~;ning the proper anti-
biotic. Serial dilutions were plated onto CMP agar medium to determine
the efficiency of regeneration.
12- Production of trans~enic fruitbodies
MMP agar plates covered with cellophane were inoculated with 5
inocula each of the desired transformed, mated or fused strain. Plates
were incubated at 24C for 10-15 days. Liquid cultures were prepared in
Fernbach flasks contA;ning 150 mL MSG20 (supplemented with 100 ,ug.mL~l
Cefotaxim) and the macerated material of 2 plates for each flask. The
Fernbach flasks were incubated at 24C for 3-5 days. Then for spawning
100 grams of wheat-grains were sterilized in the presence of 50 ml of
~e~ineralized water by autoclaving for 20 min. The sterilized wheat
grains were added to the liquid Fernbach cultures and incubated at 24C
for about 2-3 weeks, dependent on the strain used. The total contents of
overgrown wheat-grains plus r~- ~i n; ng MSG20 medium were mixed with about
750 grams of pasteurized ready to use compost (CNC, Milsbeek, The Nether-
lands) and incubated in a 5-10 L polystyrene box. The box was incubated
closed, at 24C for 2-3 weeks. Subsequently, a layer of casing soil of
about 3 cm (CNC, Milsbeek, The Netherlands) was added on top of the
compost now overgrown by the mycelium. The box was incubated closed at
24C for another 5-8 days and then transferred to 16C and a relative
W 0 95/02691 2 ~ PCT~L94/00164
humidity of 75-90%. The lid was then placed on 4 wooden pins in the
corners of the box leaving a ventilation gap of 2-3 cm. The surface of
the casing soil was moistened daily. After several days fruitbodies start
to emerge.
Fruitbodies can also be produced in jars essentially as described
but with adapted amounts of mycelial inocula, grain kernels, compost and
casing soil. Details are described in the legends to figure 17.
F- ,le 1: Protoplasting and regeneration o~ 'Abade' and A..J~,~fs
Mycelia of 'Abade' and A.arvensZs were grown in principle as
described earlier (~nnn~nherg et al. 1988) except for a growth phase in
liquid MSG20-medium (Murashige and Skoog 1962, cont~ining 20 g.L~1 of
glucose as a carbohydrate source) instead of DT80. With this medium we
obtained extremely high yields of 'Abade' protoplasts of-over 5.7x108 per
4 Fernbach flask cultures. Under similar conditions from P. ostreatus
l.lxlO9 protoplasts were produced, suggesting the general applicability
of this medium for the production of protoplasts from edible fungi.
F le 2: Construction of multipurpose transfonmation vectors
A novel convenient set of multipurpose transformation vectors has
been constructed based on pUT720 purchased from Cayla, Toulouse, which
contains pUC19 vector sequences, the AspergiZZus niduZans GPDA promoter
plus the Trichoderma reesei cellobiohydrolase I (SSA) excretion signal
sequences, contained in a 2.2 kb EcoRI-NcoI fragment, the StreptoaZZo-
teichus hindustanus phleomycin-resistance (bZe) gene cloned as a 0.44 kb
NcoI-BamHI fragment and the A.niduZans 0.76 kb BamHI-HindIII TRPC-termi-
nator fragment. After removal of the A. niduZans GPDA EcoRI-NcoI promoter
fragment this vector can be used for the insertion of EcoRI-NcoI promoter
fragments from any organism, but preferably from A.bisporus. Before or
after exchAnge of the promoter fragments, the NcoI-BamHI bZe gene frag-
ment may be replaced by any structural gene, but preferably by the modi-
fied E.co~ 1.0 kb hpt NcoI-BamHI fragment or the Phot~nus pyraZis ( fire-
fly) 1.9 kb lVC (luciferase)-gene also contained in an adapted NcoI-BamHI
fragment. The modifications are described below. The latter lUC-contain-
ing construct may be used for transient expression assays, preferably in
fungal protoplasts. Finally, the A.niduZans TRPC terminator sequence may
be ~xrh~nged by any, but preferably A.bisporus terminator sequence, con-
tained in a BamHI-HindIII fragment. The vectors thus constructed may be
W O 95/02691 2 1 6 7 1 5 2 PCT~L94/00164 ~
26
used for the isolation and in vivo selection of random genomic sequences
with promoter or terminator activity from any organism, but preferably
from A. bisporus . This technique is called promoter or terminator fishing,
respectively.
Modified hpt construct.
The E.colI hpt 1.0 kb BamHI fragment (Gritz and Davies 1983), cloned
into pAN7-1 was used to introduce a 5'-NcoI site encompassing the ATG
translational initiation codon by PCR overhang-extension. The hpt gene
and 5'-adjoining sequences of plasmid pAN7-1, which is able to transform
a number of different fungi (Punt et al. 1987) and which also was used
for transformation of the basidiomycetes SchZzophy~um commune (Mooibroek
et al. 1990) and PZeurotus ostreatus (Peng et al. 1992), was used as a
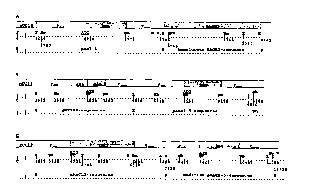
template for PCR reactions. Figure 3 shows the schematic reconstruction
f the hpt gene, cont~;n;ng a 5'-NcoI site, and with deleted NcoI and
EcoRI-sites within the coding region. The PCR primers used for different
steps in vector constructions are shown in table 1.
Primer Nco-HPT5 introduces an NcoI-site encompassing the translation
initiation codon (ATG) of the hpt gene. Furthermore, introduction of this
NcoI-site changes the second codon of the hpt gene in pAN7-1 from proline
to Al ~nine. AnTRPC3C primes at the 3' end of the TRPC terminator of pAN7-
1. The PCR reaction yielded a 1.7 kb hpt-TRPC terminator fragment, which
was isolated and digested with BamHI, thereby separating the hpt gene
from the TRPC terminator. Subsequently, partial digestion with NcoI was
performed and a 1.0 kb fragment corresponding to the hpt gene was
isolated and ligated into NcoI/BamHI-digested vector pMTL23, yielding
plasmid pMHN.
In order to remove the internal EcoRI- and NcoI-sites of the hpt
gene in this construct (located at positions 244 and 352 relative to the
ATG), two phosphorylated primers were designed which mutate the EcoRI-
and NcoI-sites without ~hAng;ng the amino acid sequence (primers HPT-E2
and HPT-N2C, respectively). With these primers a 0.1 kb subfragment of
the hpt gene was synthesized on template pAN7-1. Plasmid pMHN was
digested with EcoRI, followed by partial digestion with NcoI at position
352, and treatment with Mungbean nuclease and CIP (calf-intestine alka-
line phosphatase) to remove protruding ends and 5' phosphate groups. This
vector was used to ligate the 0.1 kb PCR fragment, resulting in plasmid
pMHMut carrying the hpt gene as a NcoI/BamHI fragment in pMTL23.
Seqll~nc; ng of the new h~t gene revealed a change in the nucleotides at
~ W O 95/02691 2 1 6 7 1 5 2 PCT~L94/00164
27
position 799 from the ATG start codon (see below). This change improved
the resistance level of recombinant E. coIi and the transformation
efficiency of A. niger. The hpt gene from pMHMut was cloned as an
NcoI/BamHI fragment into vectors with different promoters and the
A. niduZans TRPC terminator (see below).
Table 1: PCR primers used for vector constructions
Code Sequence
Nco-HPT~ Sequence id 1
5' GAC ATC ACC ATG GCT GAA CTC 3'
ATG, translation initiation site of hpt gene
AnTRPC~C Sequence id 2
5' CCG CTC GAG TGG AGA TGT GGA 3'
complement
HPT-E2 Sequence id 3
5' p_G TTC AGC GAG AGC CTG ACC 3'
p, primer phosphorylated; A, position 244 relative to ATG of
hpt gene
HPT-N2C Sequence id 4
5' pCAT AGC CTC CGC GAC CGG CT 3'
complement; p, primer phosphorylated; C, position 356 relative
to ATG of hpt gene
EN-LUC-1 Sequence id 5
5' GGG AAT TCC ATG CC ATG GAA GAC GCC AAA AAC ATA 3'
r ATG, translation initiation site of lUC gene
Sequence id 6
5' TAA TAC GAC TCA CTA TAG GG 3'
complement
W O 95/02691 2 1 6 7 1 5 2 PCT~L94100164 ~
28
AbGPDl Sequence id 7
5' G GAA TTC GTT GTC ATC ACC GCT CCT GGG AG 3'
G, position -1074 relative to ATG of GPDAg2 gene
AbGPD~ Sequence id 8
5' AA GAA GAA TTC AGA GGT CCG CAA GT 3'
G, position -290 relative to ATG of GPDA~2 gene
AbGPD2c Sequence id 9
5' GCT TAT CGC CAT GGT TTG TCT CTC 3'
complement; CAT, complement of ATG of GPDAe2 gene
PR-HPTl Sequence id 10
5'-ATG.AAA.AAG.CCT.GAA.CTC.ACC.GCG.ACG.TCT-3'
(çnC ,~aSSing ATG at position 1)
PR-HPT2c Sequence id 11
3'-GGG.TCG.TGA.GCA.GGC.TCC.CGT.TTC.CTT.ATC-5'
(complement, enc~ ,assing TAG around position 1050)
PR-LUC-l Sequence id 12
5'-ATG.GAA.GAC.GCC.AAA. M C.ATA.AAG.AAA.GGC-3'
(~n- ,~assing ATG at position 304)
PR-LUC-2c Sequence id 13
3'-CAC.CGG.GGC.CGA.CTT.AAC.CTT.AGC.TAT.AAC-5'
(complement, ~ncl~ ,o~sing position 1896)
Modified luciferase construct.
In order to introduce an NcoI-site Pnc( ,o~sing the ATG of the lUC
gene from P. pyraZis, primer EN-LUC-l was designed. This primer was used
together with the (standard) T7 primer and plasmid pT3T7-LUC (Promega) in
a PCR reaction which yielded a 1.8 kb LUC fragment. After digestion with
NcoI and BamHI the LUC gene was ligated into vector pMTL23 digested with
NcoI and BamHI, which yielded plasmid pLUC-N. The LUC gene was cloned as
an NcoI/BamHI fragment into vectors with different promoters and the
A. nidulans TRPC terminator (see below). The integrity of the mutated hpt-
and LUC-genes was assessed by positive expression in E. co~i after cloning
~ W O 95/02691 2 1 6 7 1 5 2 PCT~L94/00164
29
in appropriate expression vectors. The modified h~t gene also resulted in
a higher tr_nsformation efficiency in A.niger.
P.~ -t~l construct~.
In A.bisporus two GPD genes have been detected which are separated
by a 0.29 kb intergenic region. Only the downstream GPDAg2 gene is active
in mycelium (Harmsen et al. 1991). The promoter region of this gene is
used in new transformation vectors because of its high level of
expression and because it normalizes the generation of mRNA when fused to
the h~t gene (see below). Two different sized promoter fragments were
isolated via PCR. A 0.29 kb fragment, representing the intergenic region
of the two GPD genes, was isolated using primers AbGPD3 and AbGPD2c.
Primers AbGPD1 and AbGPD2c were employed to obtain a 1.0 kb fragment
which extends into the coding region of the upstream GPDAgl gene. Both
fragments have the NcoI-site, encompassing the translation initiation
site of the GDPAg2 gene, as the 3' end. The two fragments were isolated by
PCR on genomic DNA from both A.bisporus Ul and 'Abade'.
Primers AbGPD1 and AbGPD3 introduced an EcoRI-site at the 5' end of
the PCR fragments. Thus, the fragments were cloned as EcoRI/NcoI frag-
ments into EcoRI/NcoI-digested pUT720, thereby replacing the A.nidu~ans
GPD promoter by the A.bisporus GPD promoter fragments. This procedure
yielded plasmids pFAAG1 and pFUG1 (contAini~g l.O kb GPD promoter frag-
ments from 'Abade' and U1, respectively) and pFAAG2 and pFUG2 (ContA;n;ng
0.29 kb GPD promoter fragments from 'Abade' and U1, respectively). These
plasmids therefore, contain GPD promoter sequences fused to the S. hindu-
stanus b~e gene and the A.nidu~ans TRPC terminator.
These plasmids were digested with NcoI and BamHI to replace the b~e
gene by the LUC gene which was isolated after NcoI/BamHI digestion of
plasmid pLUC-N (see 'Modified luciferase construct'). The resulting con-
structs were named pAbAGL1 and pAbUGL1 (contA;n;ng 1.0 kb GPD promoter
fragments from 'Abade' and U1, respectively) and pAbAGL2 and pAbUGL2
(contA;n;ng 0.29 kb GPD promoter fragments from 'Abade' and Ul, respec-
tively).
Furthermore, the LVC gene was ligated directly into NcoI/BamHI-
digested pUT720, creating construct pAnGLl, and into pUT720, in which the
EcoRI/NcoI A.nidu~ans GPD promoter fragment including the signal sequence
from the SSA cellobiohydrolase I gene from T. reese~ was replaced by the
EcoRI/NcoI A.nidu~ans GPD promoter fragment from pAN8-1, yielding pAnGL2.
wo gs/026gl 2 1 6 7 1 5 2 PCT/NLg4/00164 ~
3o
Analogous to the construction of the LUC-cont~;n~ng pl~
described above, constructs were made with the modified hpt gene Prom
plasmid pMHMut. These constructs are: pAlH (analogous to pAbAGLl), pUlH
(analogous to pAbUGLl), pA2H (analogous to pAbAGL2), pU2H (analogous to
pAbUGL2), pAnHl-5 (analogous to pAnGLl) and pAnH2-5 (analogous to
pAnGL2).
The methods described here may be applied for the construction of
transformation vectors with any, but preferably A.b~sporus promoter
sequence and/or with any, but preferably A. bisporus transit signal
sequence and/or with any homologous or heterologous structural gene or
fusions thereof and/or with any, but preferably A. b~sporus terminator
sequence.
F le 3: Transformation of 'Abade' protoplasts through elec~ o,-ation
with linear pl~ DNA.
Figure 1 shows hybridization si gn~ from linearized plasmids pAN7-1
(6.5kb) and pHAG3-1 (9.5kb), lanes 1 and 2, respectively, from genomic
'Abade' DNA, lane 3 and from the genomic DNA isolated from colonies all
recovered from selective plates (DT80 plus hy~L-~ ycin B and A~nine).
Strains A10-1 and A25-1 had not been exposed to any donor DNA, thus
representing the well known false positives which were only detected
during early transformation attempts. Strains B10-1 and B25-2 had
origin~lly been exposed to native (non-digested) pAN7-1. Strain B25-2,
lane 7, is apparently also a false positive, whereas B10-1 is a true
transformant characterized by the hpt-specific hybridizing signal at the
position of the non-digested chromosomal DNA, indicating that the donor
DNA had been integrated into the 'Abade' genome. Integration was also
observed after electroporation with linear pAN7-1 (C25-1 and C25-2, lanes
8 and 9, respectively) and with linear pHAG3-1 (D10-1, D10-2, D10-3 and
D25-1, lanes 10, 11, 12 and 13, respectively). No free plasmid migrating
at the approximate position of the linear plasmids has been observed at
any occasion. These data also support the stable nature of the hy~L-~~ ycin
B resistant transformants.
Figure 2 shows a Southern blot analysis of the genomic DNAs from
four 'Abade' hy~L-~ ycin B transformants after digestion with different
restriction enzymes. In each transformant bands were detectable at the
same position as the linearized pAN7-1 or pHAG3-1 plasmids, indicating
that within the transformants tandem repeats had been formed during
processing of the donor DNA. In addition, bands migrating at other
~wo 95/02691 2 1 6 7 1 5 2 PCT~L94/00164
31
positions are detectable as well (except in lanes 5 and 6, from transfor-
mant C25-2), indicating that double- or multiple integration events had
occurred, which is quite common in fungal transformants. In addition,
bordering fragments from the recipient also contribute to the generation
of new bands. Figure 2 also shows that the individual transformants can
be distinguished on the basis of their unique banding patterns, which may
be considered a strain specific fingerprint.
The same procedure here described for transformations with the hpt
gene was also successfully applied with the phleomycin resistance (bZe)
gene present in pUT720 and pAN8-1. These vectors contain the A.niduZans
GPD promoter region, the S.hfndustanus bZe gene and the A.niduZans TRPC
terminator region. Plasmid pUT720 also contains T. reese i cellobiohydro-
lase I (SSA) excretion signal sequence (see example 2). The donor DNA was
linearized via HindIII digestion. After electroporation and regeneration
(as described for the hpt gene), regenerates were plated onto DT80 plates
contA;n;ng 5 or 7.5 ~g.mL~l phleomycin (Cayla, Toulouse).
Exampl~ 4: Transformation of UlmplO, a derivative of --eial strain
Ul.
Protoplasts from strain UlmplO, which was isolated as described were
transformed by electroporation with HindIII linearized pAN7-1. The total
number of protoplasts used for each individual experiment amounted
2.7x106. A number of 2.3x104 regenerates was counted after plating serial
dilutions and incubation at 24C for about 7 days. From a DT80 plate
cont~;n;ng 25 ~g.mL~l hygromycin B one transformant was obtained and from
plant B5 medium (Duchefa, Netherlands) cont~;n;ng 50 or 100 ~g.mL~l
hy~ ycin B in total 7 transformants were recovered within 3 weeks
without any background growth. Figures 4 A, B and C show Southern blot
analyses of all UlmplO transformants isolated in this experiment. The
same insertional characteristics were observed as for 'Abade' transfor-
mants described above, which is support for the stability of integrated
donor DNA (figure 4A).
- The primary transformants had either the Ul-phenotype (50%) or the
UlmplO--phenotype (50%), immediately after isolation from the selective
plate and further propagation on MMP. One major difference observed in
UlmplO and 'Abade' hy~l-l- ycin B resistant transformants was the
consistent occurrence of differentiating sectors in those derived from
UlmplO upon transfer to non-selective MMP-medium. In order to investigate
the segregational stability of the donor DNA, inocula from individual
WO 95/02691 2 1 6 7 1 5 2 PCT~YLg4/00164 ~
sectors were transferred to MMP-medium plus cellophane. Different sectors
had a different growth rate. Between 1-4 weeks after inoculation the
material was freeze-dried and subjected to Southern blot analysis. Figure
4B shows that with one exception in sector 3 from transformant
UlmplO/BblOO-2 all sectors had retained the donor DNA. In the same
transformant sector 1 and 2 had slightly different banding patterns.
Figure 4C shows that transformant specific fingerprints were generated
which allows discrimination between one another but also between 'Abade'
transformants (figure 2).
F ,le 5: Production and ~: n~nt selection of A.b~sporus heterokaryons
con~in;ng an ade/hyg~ and a wild-type nucleus
New strains were produced after mating h,~ nk~ryotic hy~L-~ ycin B-
resistant transformants from 'Abade' and preferably h: -Ik~ryotic
protoclones from commercial strain Ul as described in the General
procedures section 'Mating of transformants'. Figure 5 shows a
representative result of experiments in which inocula from said
interaction zones and both parental mycelia were grown on double
selective SD-medium (Yeast Nitrogen Base without amino acids, prepared
according to the suppliers instructions with glucose as a carbohydrate
source, Difco Laboratories, USA) thus la~ki ng adenin and supplemented
with hy~ ycin B (50 ,ug.mL~l). Only inocula from said interaction zones
exhibited growth contrary to either parental strain. When fresh matings
were attempted between either parental strain directly onto said double
selective agar medium, no growth was observed, indicating that the mating
procedure described was essential to yield ADE/hy~ colonie,5.
To rule out the possibility that double selected colonies were just
mixtures of parental mycelia, protoplasts were produced, regenerated in
liquid CMPS-medium and plated onto double selective SD-medium. The
results demonstrate that with a high efficiency of about 90% again
ADE/hy~ colonies were formed. In about 10% of the regenerates no growth
was observed by microscopy. These results confirm the heterokaryotic
status of double selected crosses.
F , le 6: Analysis of transformant DNA using PCR.
Total DNAs were extracted according to the protocol described
earlier. Samples (100 ng) of template DNAs were subjected to PCR analysis
with primers indicated in the legend to figure 6. PCR was performed using
Taq-polymerase in 30 cycles of 1 min 94C. 1 min 55C and 1 min 72C. PCR-
wo 95,026gl 2 1 6 7 1 5 2 PCT~L94100164
33
products were electrophoresed in l.O% agarose in TAE (Tris-HCl, acetate,
EDTA)-buffer stained with ethidium-bromide.
Figure 6 shows clear banding patterns of specific PCR-fragments
comprising parts of the tandem GPDl and GPD2 genes of A.bisporus (lanes
2, 3, 4 and 5), indicating that both Abade and Ul have the same, or at
least compatible, template DNA sequence of GPD-genes, which is support
for the proper classification of Abade as an A . bisporus ~species, despite
its aberrant colony morphology. From two putative (pAN7-l)-transformants
in lanes 7 and 8, PCR-fragments with the same size were generated as in
lane 9, which contained plasmid pAN7-l as a template, contrary to the
non-transformed Abade control in lane 6. The results s~ow that (stably or
transiently) transformed Abade strains (C25-l and DlO-l) can be obtained
using the hpt-gene and procedures described and that the transformed
status can be assessed using PCR and the primers listed above.
Conclusions regarding the integrated nature of donor DNA sequences
is shown by Southern blot analysis in a number of other examples.
Exampl~ 7: Identification of transformed ~ruitbodies derived from UlmplO.
Fruitbodies were produced as described in the general methods. The
Southern blot analysis shown in figure 7 clearly demonstrates the
presence of donor DNA sequences in each fruitbody sample analyzed except
for the primary transformant (UlmplO/BblOO-2) that had lost the donor DNA
in one of the three sectors observed (figure 4B). This result indicates
that the donor DNA has been stably maintained throughout fruitbody
development, despite the absence of any selective pressure in favour of
the hpt-cont~ining nuclei. There is no sign of differential segregation
of any one of the transformed nuclei. In at least three transformants
identical hybridization patterns were obtained in the starting mycelium
and the derived fruitbodies. In cases of doubt, additional digestions
with other restriction enzymes and/or the determination of adjacent
genomic sequences may provide conclusive discrimination. It can be
concluded that donor DNA sequences are transmitted to A. bisporus
fruitbodies with high frequency and usually with hybridization patterns
identical to the mycelial pattern. These patterns can suitably be used to
assess proprietary rights.
Example 8: Southern blot of transformant DNA derived from 'Abade'.
Total DNA was isolated and Southern blot analysis was carried out as
described in the general methods section.
W O 95/02691 2 ~ b ~ PCT~L94/00164 ~
34
All mating products shown in figure 8 contain the two bands which
are also visible in the transformed Abade parents (D10-1 and C25-1, lanes
1 and 2) and the pAN7-1 control (lane 19). This indicates the presence of
pAN7-1 sequences in the genome of the mating products. As expected, the
parental U1 protoclones do not contain any sequences hybridizing to the
hpt-probe. The faint signal present in lane 12, panel C, is most likely
due to overflow of DNA from the neighbouring slots. The hybridizing bands
remain present for three generations in all mating products. However,
additional bands appear in later generations. Whether these are caused by
partial digestion or by rearrangements of the transforming DNA, or other
donor DNA processing events, l~ 9i n~ to be investigated. It can therefore
be concluded that the mating products between Abade transformants and U1
protoclones stably maintain pAN7-1 sequences in the genome for at least
three generations.
F le 9: Cotransformation with pAN7-1 and pUT720.
Before transformation both plasmids pAN7-1 and pUT720 were
linearized with H~ndIII. After electroporation of Abade protoplasts as
described in the general methods section, transformants were selected on
hy~L-~ ycin-contAining medium. DNA from the hy~L-~ y~in-resistant colony
D20-1 was isolated and subjected to Southern blot analysis as described
in the general methods section.
The restriction enzymes used for Southern blot analysis shown in
figure 9, all have only one recognition site in both pAN7-1 and pUT720.
Thus, the bands of approximately 6 kb in panel A corresponding to the
size of plasmid pAN7-1 indicate the presence of t~n~C ly integrated
copies of pAN7-1 in the genome. NcoI and BamHI digests (figure 9A, lanes
5 and 6) show additional fragments hybridizing to the hpt-probe, which
probably represent border fragments cont~ining neighbouring genomic DNA.
Such border fragments were not observed when a bZe-probe was used tfigure
9B, lanes 5 and 6). This suggests that one or more copies of pUT720 have
co-integrated with and are surrounded by pAN7-1 sequences. The patterns
suggest that all pAN7-1 and pUT720 copies are arranged in the same
orientation. Since the HindIII digests also show plasmid-sized bands
(figure 9A and 9B, lanes 7), the tandem copies were probably obtained by
ligation of the linearized plasmids before integration into the genome.
Figure 9C (hpt- and b~e-probes) shows a co~hinfltion of the patterns
obtained in panels A and B. As pUT720 and pAN7-1 have somewhat different
sizes (6.o4 and 6.55 kb, respectively) double bands appear around 6 kb.
2167152
_ W O 95/02691 PCT~L94/00164
The HindIII-linearized pAN7-1 and pUT720 plasmid molecules have co-
integrated into the genome after ligation ~n vivo. Thus, co-integration
is possible by linearizing both plasmids with the same restriction
enzyme.
Example 10: Production of h~ ryotic protoclones from primary
heterokaryotic transfonmants.
Inocula from fertile, primary transformant UlmplO/BblOO-l which
included tissues from all three sectors observed, were grown on
GeneScreenPlus (DuPont) hybridization membranes layered on top of
cellophane sheets in petri dishes cont~ining MMP agar medium. The plates
were incubated at 24C for 2 weeks. Then the membranes accommodating the
colonies were removed, washed in 0.6 M sucrose and incubated upside down
in a petri dish contAining 10 mL of 0.6 M sucrose plus Novozyme 234 (10
,ug.mL~l) at 24C for 1 hour to release protoplasts. Protoplasts were
purified from the supernatant by sequential centrifugations with 0.6 M
sucrose, resuspended in 5 mL of 0.6 M sucrose plus 1% Low Melting Point
agaros~e (37C) and plated onto CMPS (compost extract, Mycological
Peptone, sucrose)-agar medium. Petri dishes were incubated at 24C and
individual colonies isolated, aided by microscopy, before touching their
neighbours. Nineteen colonies were isolated with equal viability. From
these colonies new inocula were tested for growth on DT80-medium (not
shown) or DT80-medium contAining 50 ~g.mL~l hygromycin. Non-transformed
UlmplO was included as a control (colony A4).
The results in figure 10 show that different types of protoclones
have been produced with different pigmenting (and different levels of
pigments excreted into the medium surrounding the colonies, which is not
visible on the photograph). Control A4 did not grow, nor did colony B3
(UlmplO/BblOO-lpl4), which shows that by the procedure of protoplasting
and dilute regeneration protoclones may be isolated which lost the
nucleus cont~A~ining the integrated hpt-sequences. Thus, colony B3
exclusively contains the non-transformed nuclear type, which m_kes this
strain a h~ -kAryon. Support for protoclonal segregation instead of
deleterious recf hinAtion events is provided by the frequency obtained (1
h~ Aryon out of 19 protoclones tested). This frequency is expected if
from heterokaryons as the starting material hl ~kAryons of either nuclear
type have been generated with an efficiency of 10%, which is the
pllhliShed efficiency. Further support was obtained by -esterase isozyme-
analysis (not shown). With similar frequency hf-QkAryotic protoclones
W O 95/02691 21 67 1 52 PCT~L94/00164 ~
36
were also identified by a-esterase analysis that still contained the hpt-
gene. These protoclones can be used as mating partners with other
h~ okAryons to introduce the donor DNA into a new heterokaryon.
By the method of protoplasting and dilute regeneration of primary
UlmplO (hpt)- transformants h~ ryotic protoclones of two nuclear types
can be isolated. One type, which lost the capability to grow on
hy~L-~ ycin-contAin;n~ medium and the other type which still contained the
hpt-gene.
10 F ,le 11: PCR-analysis of donor DNA selectable markers into
protoplasts.
Protoplasts (3 x 107) were isolated and extensively washed in EB
(electroporation buffer) as described in the general methods section.
Then 30 ,ug of plasmid pAN7-1 was added, the mixture of protoplasts plus
DNA was divided into three portions (107 each) and incubated on ice. To
the first portion (1) of 107 protoplasts plus pAN7-1 (10 ~ug), plasmid
pT3T7-luc (10 ,ug) was added directly. Portions 2 and 3 were subjected to
electroporation according to the parameters described in the general
methods section, incubated at 30C for 30 min to allow sealing of pores
generated by the electroporation procedure, and then transferred back to
ice. To these two portions plasmid pT3T7-luc (10 ,ug to each portion of
protoplasts) was also added followed by extensive washing with 0.6 M
sucrose contAining MgC12 (20 ,ug.mL~l). Then DNAse I was added to portion 2
only (final concentration of 20 ~g.mL~1). Portions 2 and 3 were incubated
for 30 min at 30C, then put back on ice and lysed with phenol/chloroform
simultaneously with portion 1. Total DNA was then extracted according to
the method described and subjected to PCR for 15 or 20 cycles using
~;gox;genin-ll-duTp in the dNTP nucleotide mixture and the sets of
primers indicated in the legend to figure 11. After electrophoresis the
PCR-products were blotted onto a nylon membrane, treated with
antidigoxigenine-AB, Fab-fragment, and AMPPD solution, exposed to FUJI
medical X-ray film and developed. Portions 1, 2 and 3 correspond to fig.
11, lanes 1, 2 and 3, respectively.
Using PCR and the primer sets indicated in the legend to figure 11
it is possible to determine the entry of donor DNA-into A.bisporus
protoplasts by electroporation parameters described in the general
methods section. Additional higher molecular weight bands only occurring
in portions 2 and 3 possibly represent early processing of the donor DNA
after entry.
216715~
W O 95/02691 PCT~L94/00164
37
F. le 12: Northern blot of transformed fruiting body RNA
UlmplO/Bb50-1 fruitbodies were produced in jars essentially as
described in the general methods section with adapted amounts of mycelial
inocula, grain kernels, compost and casing æoil. Details are described in
the legends to figure 17. Fruitbodies were harvested at different
t; .oints. RNA was isolated from freeze-dried material and Northern
blotting was carried out by standard procedures. The blot was hybridized
to a 32P-labelled hpt-probe.
Figure 12 shows a Northern blot analysis of RNA extracted from
transgenic fruitbodies. After isolation the RNA showed considerable
degradation on an ethidium bromide-stained agarose gel. Therefore, the
signAl~ on the Northern blot are weak and vague. However, the hpt-gene
clearly appears to be expressed in transgenic fruitbodies.
F , le 13: Southern blot analysis of Abade transformants derived with
pHAG3-:L
Abade transformants were obtained with plasmid pHAG3-l,linearized
with KpnI within the A. b~sporus AbGH3 insert sequence. DNA isolation and
Southern blot analysis were carried out as described in the general
methods section.
A Southern blot analysis of total DNA from pHAG3-1-derived Abade
transformants is shown in figure 13. Restriction enzyme CZaI does not
have a recognition site in pHAG3-1. Digestion of non-transformed Abade
DNA with C~aI and hybridization with the AbGH3-probe yields a 3.5 kb
band, corresponding to the endogenous AbGH3 sequence plus fl~nking
genomic sequences (Figure 13A, lane 3). In transformant C25-1.15/3 this
band has shifted to a higher position, indicating that the pHAG3-1 DNA
has integrated into the homologous AbGH3 sequence in C25-1 (Figure 13A,
lane 11). This is not the case for transformant C10-1.15/3, which still
shows the endogenous 3.5 kb band, in addition to a higher band, which
corresponds to the pHAG3-1 sequences integrated ectopically (Figure 13A,
lane 7). Both high-position bands in lanes 7 and 11 also hybridize to the
hpt-probe (Figure 13B), confirming that they contain pHAG3-1 sequences.
Both KpnI and Bg~II have only one recognition site in pHAG3-1 and the
former was used to linearize the plasmid before transformation. Digestion
of C10-1 and C25-1 DNA with these enzymes yields 9.8 kb bands hybridizing
to the hpt-probe (Figure 13B, lanes 8, 9, 12 and 13), plus additional
bands representing border fragments cont~in;ng adjacent genomic DNA. The
plasmid sized bands of 9.8 kb indicate that in both C10-1 and C25-1
wo 95/02691 2 1 67 ~ 5~ PCT~L94/00164 ~
38
tandem copies of pHAG3-1 are present. After EcoRI digestion of ClO-1 and
C25-1 DNA two hybri~i~ing bands of expected length appear with the hpt-
probe, which indicate the intactness of the GPD-promoter region and the
hpt-gene in the transformants (Figure 13B, lanes 10 and 14).
Hybridization with the AbGH3 probe yields two different bands of 3.2 kb,
corresponding to the endogenous AbGH3 sequence (Figure 13A, lane 6), and
of 5.9 kb in the transformants (Figure 13A, lanes 10 and 14). The
presence of the 5.9 kb band provides further evidence for the integration
of tandem copies of pHAG3-1 in both transformants. ~r -logous integration
has occurred with high efficiency (in 2 out of four transformants
analyzed) when driven by the AbGH3-sequence. The merhAni r S involved
during integration (of pHAG3-1) are not yet fully understood, but both in
vivo ligation and double strand break repair seem to be involved.
Transformation of Abade using plasmid pHAG3-1, linearized with KpnI
inside the region of homology with A.bispor~s DNA, can give rise to
homologous integration of the plasmid into the endogenous AbGH3 region.
This ph~nt on occurs with high efficiency (50%). In the cases of both
homologous and ectopic integration investigated here, tandem copies of
the integrated plasmid were found. Furthermore, in both instances the
KpnI-site was restored. MPrhAni! ~ res '-ling double strand break repair
and ~n v~vo ligation are involved.
F , le 14: Southern blot analysis of total DNA from pHAG3-1 derived
Abade transformants derived ~rom pHAG3-1 and mated with U1 derived
protocl~ne~
Forced mating products were produced from strains indicated in the
legend to figure 14 as described in the general methods section, in
example 5 and figures 5A and 5B. The mating products were further
propagated with no selective pressure on MMP agar medium (plus a sheet of
cellophane) to isolate the total DNA for Southern blot analysis as
described.
The luminograph of figure 14 shows the aberrant position of the
AbGH3/C~aI-fragment of transformant C25-1/4/12 (lane 6). Lanes 12 - 15
comprise the DNA from different mating products with this transformed
strain. Either nuclear type was clearly present in the c- hin~tions with
Ulp6 and Ulp8, although the Ulp8 nucleus seems to be under-represented in
C25-1/Ulp8. Over-exposure (not shown) also revealed weakly hybridizing
material at the 3.5 kb position in lanes 14 and 15. These results
demonstrate that by the forced mating procedure described, in two out of
2167152
_ W O 95/02691 PCT~L94/00164
-
39
four mating products analyzed the presence of both participating nuclei
can be identified. It may be significant to note that the colony
morphologies of mating products between Abade transformants and Ul-
derived protoclones was intermediate between the two parental strains
with no t~n~ncy to form hyphal aggregates or sectors. The same type of
morphology was also obtained after fusion of protoplasts from Abade
transformants and from Ul, followed by double selection (SD agar medium
with no ~dpn;ne~ with hy~ ycin~ 50 ,ug.mL~l). Abade transformants D10-1
and C25-1 were also mated with protoclones from strain B131 (T.J. Elliot;
ATCC36974) which is characterized by the frequent formation of 4-spored
basidia. The combination with B131-protoclones allows the isolation of
transgenic h- ~k~ryotic spores. A variety of new morphologies was
observed after forced matings between B131-protoclones and Abade
transformants on double selective medium and further propagation on MMP
agar medium, including the formation of hyphal aggregates, which is also
observed during early fruitbody formation. Fruitbody initiation has been
started with these mating products.
The forced mating procedure described here can yield mating products
in which the presence of either ~ucl e~r type can be -demonstrated. In
addition, unequal distribution or segregation of either one of the
parental nuclei can be followed in time.
Exampl~ 15: Southern blot analysis of Abade transformants of pAlH or
pUlH.
Plasmids pUlH and pAlH were constructed as described and linearized
with EcoRI before electroporation of Abade protoplasts. Southern blot
analysis was carried out as described in the general methods section.
A prel; ; n~ry comparison with the transformation vectors described
before, showed that the new pAlH and pUlH constructs, both cont~;n;ng
A. b~sporus GPD2-promoter sequences resulted in a 2 - 10 fold increase of
the transformation efficiency of Abade protoplasts. Figure 15 shows that
also the new constructs have been integrated into the recipient DNA. In
the four transformants shown hybridizing bands of 2.1 kb were present,
representing the EcoRI/BamHI-fragments of the new ~constructs that
comprise the 1.05 kb GPD2- plus 1.04 kb hpt-fragments.
In five transformants analyzed in more detail (results not shown) no
homologous integration had occurred through the homologous GPD2-sequence
(1.05 kb), indicating that additional factors specify the occurrence or
lack of homologous integration, e.g. the nature and/or the length of the
WO 95/02691 2 1 6 7 1 5 2 PCT~L94tO0164 ~
homologous insert and/or the presence of a new EcoRI-site introduced at
the 5'-end of the GPD2-promoter region by PCR (in order to allow cloning
and later linearization before electroporation).
Figure 15 shows a Southern blot analysis of DNA from Abade
transformants, obtained after transformation with plasmids pAlH or pUlH
cont~;ning A.bisporus GPD2-promoter sequences. The new pAlH and pUlH
transformation constructs allow the direct selection of hy~L-~ ycin-
resistant transformants from Abade with a somewhat increased efficiency.
The GPD2-sequence cont~;n;ng vector does not integrate at the homologous
position as efficiently as the vector that comprises the AbGH3-sequence
(pHAG3-1).
F ~le 16: Southern blot analysis o~ an Abade cotransformant
Transformation of Abade was performed as described in the general
methods section with a mixture of plasmids pAN7-1 and pUT720, both
linearized with HindIII. Transformant D20-1.14/6 was selected by growth
on hy~L-I~ ycin-cont~;n;ng medium. DNA was isolated and Southern blot
analysis was performed as described in the general methods section.
Figure 16 shows a Southern blot analysis of total DNA from Abade co-
transformant D20-1.14/6. Digests with NcoI/BamHI and EcoRI/BamHI of the
DNA from D20-1.14/6 (lanes 15 and 16) show the same patterns as the
plasmid controls pAN7-1 (lanes 2 and 3) and pUT720 (lanes 5 and 6) after
hybridization with the hpt-probe (figure 16A) and the bZe-probe (figure
16B), respectively. This indicates the presence of both hpt- and bZe-
sequences in D20-1. This is further demonstrated by the combination of
patterns visible in figure 16C (hpt- and bZe-probes). Digestion with
HindIII (lane 17) yields plasmid sized bands with both probes, indicating
that the HZndIII-sites of the linearized plasmids were restored upon
integration in the Agaricus genome. Digestion with ~coRV (lane 18), which
does not cut either plasmid, yields one band hybridizing to both probes
(figure 16C), suggesting co-integration of pAN7-1 and pUT720 at one
genomic site.
Co-transformation of A. bisporus is possible. In the co-transformant
studied in detail, it appears that the HindIII-sites, which were used for
linearization of the plasmids prior to transformation, are restored e.g.
by ligation of the pl~ before integration. Furthermore, the
hybridization patterns observed are in agreement with integration of both
plasmids at the same site in the genome.
~ W O 95/02691 2 1 6 7 1 5 2 PCTn~L94/00164
41
Example 17: Effect of substrate ~ ition on fruitbody formation.
Fruitbodies were produced from commercial strain Ul according to the
protocol described in the general methods section. This time the
experiments were performed in 500 mL jars, now with 25 grams of grain
kernels that were inoculated with the amount of mycelium from one
cellophane-covered MMP agar plate and, after colonization, with 50 grams
of compost or hemp core tissue. Other hAn~l in~ were in principle the
same as described before.
Figure 17 shows the effect of substrate composition on the
efficiency of small scale fruitbody formation. With this protocol for
A. bisporus fruitbody production from strain Ul, normally one fruitbody
emerged and developed while primordia already present did not develop
until the older fruitbody was removed (jar 1). Replacing the compost by
hemp tissue resulted in the same type of fruitbody development (jar 2).
However, with increasing amounts of added freeze-dried and finely ground
compost (1% and 10% in jars 3 and 4, respectively) increasing numbers of
primordia developed into fruitbodies. These results indicate that by
mechanically rel eAc; ng compounds from commercially available compost,
which are present but not (easily) accessible to the growing mycelium,
fruitbody initiation and/or development can be improved. This implies
that providing A. b~sporus with more or better enzyme activities suitable
for the degradation of essential compost constituents (e.g. by co-
transformation) may increase fruitbody yields.
Moreover, these results suggest that the production of mushroom
fruitbodies can be applied for the bio-degradation of agro-waste
materials (such as hemp core tissue). This system may be improved by the
production of A. bisporus transformants that comprise suitable added
genes.
Mushroom fruitbodies can be produced on alternative substrates. The
system can be improved by the addition of compost-borne components that
can be released mechanically (or perhaps enzymatically).
- Example 18: Northern blot analysis of Abade transformants of pHAG3-1,
pAlH and pUlH
Transformant C25-1.4/12 was obtained from KpnI-digested plasmid
pHAG3-1. Transformants E10-1.28/3, E20-1&2.28/3 and F10-1&2.28/3, were
obtained from pl ~ C pAlH and pUlH, respectively, both digested with
EcoRI. Total RNA was isolated from freeze-dried mycelium and Northern
blot analysis was performed using standard procedures.
W O 9S/02691 2 1 6 7 1 5 2 PCT~L94/00164 ~
42
Total RNA was extracted and electrophoresed as described in the
general methods section.
The results shown in figure 18 indicate that in A. bfsporus
transformants obtained with a pAN7-1-derived construct, two transcripts
are generated which are full-length (arrows a and b, also occurring in
A.niger strains transformed with pAN7-1, data not shown), whereas other
transcripts are too short to yield full-length translation products
(arrows c and d). The generation of two full-length mRNA products (a and
b) may be explained by the two different termination sl gn~l ~, separated
by about 250 bp, which are present in the A.nfdutans TRPC-terminator
sequence. Apparently, these are similarly recognized in A.bisporus. After
long term propagation band c has disappeared for unknown reasons. Banding
patterns from lanes 1 and 2 are representative for all transformants
obtained with pAN7-1 or its direct derivative (pHAG3-l).~On the contrary,
Abade transformants cont~;ning vectors which comprise the A.bfsporus
GPD2-promoter sequence (and the modified hpt-gene) yield the normal
transcripts also observed in A.niger pAN7-1 transformants.
Pr~li in~ry results indicate that with the new transformation
construct a 2 - 10 -fold increase of the transformation efficiency was
achieved in A.bfsporus.
Using novel transformation vectors comprising the A.bfsporus GPD2-
promoter sequence (and the modified hpt-gene), the efficiency of
A.bfsporus transformation can be increased and the nature of hpt-specific
transcripts normalized.
~wo 95,026gl 2 1 6 7 1 ~ 2 PCTn~L94/00164
43
FIGURE LEGEND
Fig.1: Southern blot of undigested total DNA from eight 'Abade'
transformants and controls. Lane 1, BomHI-digested plasmid
pAN7-1. Lane 2, KpnI-digested plasmid pHAG3-1. Lanes 3-13,
undigested total DNA extracted from untransformed 'Abade' (lane
3) and colonies A10-1, A25-1, B10-1, B25-2, C25-1, C25-2, D10-
1, D10-2, D10-3, D25-1 (lanes 4-13, respectively). The blot was
hybridized to a DIG-labelled hpt-probe.
Fig.2: Southern blot of digested total DNA from four 'Abade' trans-formants. Lane 1, Bg~II-digested plasmid pHAG3-1. Lane 2,
BamHI-digested plasmid pHAG3-1. Lane 11, BamHI-digested plasmid
pAN7-1. Lane 12, EcoRI-digested plasmid pAN7-1. Lanes 3-10,
digested total DNA from transformants C25-1 (lanes 3&4), C25-2
(lanes 5&6), D10-1 (lanes 7&8) and D10-2 (lanes 9&10). DNA's
were digested with BgZII (lanes 3&5), BomHI (lanes 4, 6, 7, 9)
and EcoRI (lanes 8 & 10). The blot was hybridized to a DIG-
labelled hpt-probe.
Fig.3: Schematic representation of the reconstruction of the wild-type
E. coZ~ hpt gene.
Fig.4A: Southern blot analysis of A . bisporus UlmplO-derived primary
hy~lo~ycin B resistant transformants using a DIG-labelled hpt
probe. Lanes 3-11 contain undigested total DNA and lanes 12-20
contain EcoRV-digested DNA from non-transformed control UlmplO
(lanes 3, 12) and transformants Bd25-1 (lanes 4, 13), Bb50-1
(lanes 5, 14), Bb50-2 (lanes 6, 15), Bb50-3 (lanes 7, 16),
BblOO-1 (lanes 8, 17), BblOO-2 (lanes 9, 18), BblOO-3 (lanes
10, 19), BblOO-4 (lanes 11, 20). Lanes 1, 22: pAN7-1 digested
with BomHI, lanes 2, 21: size marker, phage Lambda DNA digested
with EcoRI and H~ndIII.
Fig. 4B: Southern blot analysis of A. b~sporus UlmplO-derived hy~ nycin
B resistant sectoring transformants using a DIG-labelled hpt
probe. Total DNAs were digested with EcoRI and BomHI. Symbols,
a,b,c: inocula taken from sectors that developed at the centre,
W O 95/02691 2 1 6 7 1 5 2 PCT~YL94/00164 ~
44
halfway or at the edge of the colony, respectively; p: plasmid
pAN7-1 digested with EcoRI and ~amHI.
Fig. 4C: Strain-æpecific fingerprints of BomHI+EcoRI double-digested
total DNA from UlmplO-derived hy~L-~ ycin B resistant trans-
formants, probed with a DIG labelled hpt probe. Lane 1: pAN7-1
cut with BomHI~EcoRI, lane 2: size-marker, phage Lambda DNA
double-digested with EcoRItHindIII, lanes 3-10: BamHI+EcoRI
double-digested DNAs from control non-transformed UlmplO and
transformants Bd25-1, Bb50-1, Bb50-2, Bb50-3, BblOO-l, BblOO-2,
respectively.
Fig.5A,B: D~ in~nt selection of a A.bisporus heterokaryon cont~;ning an
ade/hyg~ (from C25-1) and a Ul-derived nucleus (from protoclone
Ulp8). A: SDO-medium (no hy~L-c ycin B), with (I) or without
(II) A~Pn;ne. B: SD50-medium (50 ,ug.mL~l hy~L-~ ycin B), with (I)
or without (II) A~pnine. Strain Ulp8 shows clear growth on SDO-
medium, but unexpectedly thin, hardly visible growth in the
presence of ~n;ne. BII (double selective medium) shows
continued growth of mated colonies (duplicate inocula in the
middle), exclusively, whereas parental strains do not.
Fig. 6: PCR-analysis of Abade hygL-~ ycin-resistant transformants. Lanes
1, 10: phage Lambda digested with EcoRI and HindIII; lanes 2 -
5: template DNA isolated from non-transformed Abade control,
transformants C25-1, D10-1 and non-transformed Ul, exposed to
A. bisporus GPD2 promoter-specific primers (AbGPDl, sequence id
7, AbGPD2c, sequence id 9); lanes 6 - 9: template DNA from non-
transformed Abade control, transformants C25-1, D10-1 and pAN7-
1, respectively, exposed to hpt-specific primers (PR-HPTl,
~nc assing ATG at position 1:
5'-ATG.AAA.AAG.CCT.GAA.CTC.ACC.GCG.ACG.TCT-3',
sequence id 10 and PR-HPT2c [complement], encompassing TAG
around position 1050:
3'-GGG.TCG.TGA.GCA.GGC.TCC.CGT.TTC.CTT.ATC-5',
sequence id 11)
Fig. 7: Southern blot analysis of fruitbodies derived from UlmplO
primary transformants, hybridized to a DIG-labelled hpt-probe.
_
~ WO 95/02691 ~ 1 6 7 1 5 2 PCT~L94/00164
All DNAs were digested with BamHI plus EcoRI. Lane 1: pAN7-1;
lane 2: Ul; lanes 3 - 8: transformants UlmplO/Bb50-1 (var.
Miranda), UlmplO/Bb50-3 (var. Paula), UlmplO/BblOO-l (var.
Nicole), unstable transformant UlmplO/BblOO-2 (var. Nonna),
UlmplO/BblOO-3 (var. Nadine), UlmplO/BblOO-4 (var. Febeline),
respectively. kb, kilobase pairs.
Fig. 8A, B, C:
Southern blot analysis of total DNA from matings between
primary Abade transformants and Ul-derived protoclones,
hybridized to a DIG-labelled hpt-probe. All DNAs were digested
with BamHI plus EcoRI. 8A, 8B, 8C: generation 1, 2 and 3,
respectively. Lanes 1 - 18: D10-1, C25-1, Ulp6, Ulp6/D10-1,
Ulp6/C25-1, Ulp8, Ulp8/D10-1, Ulp8/C25-1, Ulpl2, Ulpl2/D10-1,
Ulpl2/C25-1, Ulpl5, Ulpl5/D10-1, Ulpl5/C25-1, Ulpl6, Ulpl6/D10-
1, Ulpl6/C25-1, Abade, respectively and lane 19: pAN7-1. kb,
kilobase pairs.
Fig. 9A, B, C:
Southern blot analysis of total DNA from an Abade-derived
hpt/bZe-cotransformant D20-1, hybridized to DIG-labelled
probes: A, hpt-probe; B, b~e-probe and C, mixed hpt/b~e-probe.
Lane 1: pAN7-1 digested with EcoRI plus BamHI (2.3 kb band
hardly visible); lanes 2 and 3: pUT720 digested with EcoRI and
EcoRI plus BamHI, respectively; lane 4: phage Lambda (digested
with EcoRI plus HindIII, not hybridizing); lanes 5, 6 and 7:
total DNA from D20-1 digested with NcoI, BamHI or HindIII,
respectively. Non-transformed Abade control did not hybridize
(not shown). kb: kilobase pairs.
3o
Fig. 10: Production of h( -l-Aryotic protoclones from primary
heterokaryotic transformants. Hy~ ycin-resistance was tested
of UlmplO/BblOO-l (var. Nicole)-derived protoclones grown on
DT80 agar medium contA;ning 50 ,ug.mL~l hy~L-~ ycin. A4: non-
35 transformed UlmplO control, B3: negative protoclone which has
lost the hy~ ycin-resistance (growth without hy~-~ ycin was
same as others, not shown).
wo gs/026gl 2 1 ~ 7 ~ ~ ~ PCT~L94/00164 ~
46
Fig. 11: PCR-analysis of donor DNA hpt- and lUC-markers after re-
extraction from electroporated Ul protoplasts. Lane 1: donor
DNA mixture cont~;ning plasmids pAN7-1 (hpt) and pT3T7-luc
(LUC); lane 2: re-extracted DNA after extensive washing plus
DNAse I-treatment of protoplasts; lane 3: re-extracted DNA
after extensive washing of protoplasts, only. DNA samples
serving as PCR-templates were exposed simultaneously to ~pt-
specific primers PR-HPTl (Pn~ ,~assing ATG at position 1:
sequence id 10) and PR-HPT2c (complement, Pn~ sing TAG
around position 1050: sequence id 11) and lUC-specific
primers PR-LUC-l (~n-- passing ATG at position 304:
sequence id 12) and PR-LUC-2c (complement, ~nc passing
position 1896: sequence id 13). Panel A, B: 15, 20 PCR-cycles,
respectively.
Fig. 12: Northern blot analysis of RNA extracted from fruitbodies
produced from UlmplO-derived primary transformants. Lane 1: RNA
from mycelium of control Abade transformant D10-2; lane 2: RNA
from non-transformed Ul-fruitbodies; lane 3: -; lane 4: RNA
from UlmplO/Bb50-1 fruitbodies and lane 5: same strain
harvested 4 days later. The arrow indicates the position of the
major transcript detected in A. niger (pAN7-1)-transformants,
correpon~ing to about 1450 nucleotides.
Fig. 13A, B:
Southern blot analysis of total DNA from pHAG3-1-derived Abade
transformants, using DIG-labelled AbGH3- (A) or hpt-probes (B).
Lane 1: mixture of pHAG3-1 digested with BgZII or BamHI; lane
2: mixture of non-digested and HindIII-digested phage Lambda
DNA (not hybri~i~;ng); lanes 3 - 6: Abade non-transformed
control DNA digested with CZaI, KpnI, B~ZII or EcoRI,
respectively; lanes 7 - 10: DNA from Abade transformant C10-
1.15/3 and lanes 11 - 14: DNA from Abade transformant C25-
1.15/3, also digested with CZaI, KpnI, BgZII or EcoRI,
respectively. kb: kilobase pairs.
Fig. 14: Southern blot analysis of total DNA from pHAG3-1-derived Abade
transformant C25-1.4/12 (showing homologous integration through
the AbGH3-sequence), mated to Ul-derived protoclones and
2167152
~ W O 95/02691 PCT~L94/00164
47
hybridized to a DIG-labelled AbGH3-probe. All A. bisporus
genomic DNAs from controls and mating products were digested
with CZaI. Lanes 1 and 2: pHAG3-1 digested with Bg~II or
HindIII, respectively; lane 3: phage Lambda DNA digested with
HfndIII plus EcoRI (not hybridizing); lane~ 4 and 5: non-
transformed Abade and Ul control DNAs; lane 6: DNA from Abade
transformant C25-1.4/12; lanes 7 - 11: DNA from Ul-protoclones
Ulp6, Ulp8, Ulpl2, Ulpl5 and Ulpl6, respectively; lanes 12 -
15: mating products between C25-1.4/12 and Ulp6, Ulp8, Ulpl2
and Ulpl5, respectively. kb: kilobase pairs.
Fig. 15: Southern blot analysis of DNA from Abade transformants,
obtained after transformation with plasmids pAlH or pUlH
cont~in;ng A.bisporus GPD2-promoter sequences from Abade or Ul,
respectively and the modified hpt-gene. Genomic DNAs were
digested with EcoRV (odd numbered lanes) or with EcoRI plus
BamHI (even numbered lanes) and hybridized to a DIG-labelled
hpt-probe. Lanes 1 and 2: non-transformed Abade control; lanes
3 and 4: transformant E10-1.28/3; lanes 5 and 6: transformant
E20-1.28/3; lanes 7 and 8: transformant E20-2.28/3; lanes 9 and
10: transformant F10-2.28/3. kb: kilob~e pairs; 2.1 kb,
representing the EcoRI/BamHI-fragments from pAlH and pUlH
hybridizing to the hpt-probe.
Fig. 16A, B, C:
Southern blot analysis of total DNA from Abade co-transformant
D20-1.14/6 (simultaneously transformed with pAN7-1 and pUT720),
hybridized to DIG-labelled probes: hpt-probe (A), b~e-probe (B)
and mixed hpt-/~e-probes (C). Lane 1: phage Lambda digested
with EcoRI plus HindIII (not hybridizing); lanes 2, 3, and 4:
pAN7-1, digested with NcoI plus BamHI, EcoRI plus BamHI and
with BamHI, respectively; lanes 5, 6 and 7: pUT720, digested
- with NcoI plus BamHI, EcoRI plus BamHI and with BamHI,
respectively; lane 8: pABAGL-l digested with EcoRI plus NcoI
(not hybr~ ng); lane 9: -; lanes 10 - 14: non-transformed
Abade control, digested with NcoI plus BamHI, EcoRI plus BamHI,
HindIII, EcoRV or HindIII plus EcoRV (not hybridizing); lanes
15 - 19: D20-1.14/6, digested with NcoI plus BamHI, EcoRI plus
BamHI, HindIII, EcoRV or HindIII plus EcoRV; kb: kilobase
W O 95/02691 2 1 6 7 1 5 2 PCT~L94/00164 ~
48
pairs, sizes 6.0, 2.6 and 0.4 represent specific pUT720-
fragments.
Fig. 17A, B:
Effect of substrate composition on the efficiency of small
scale fruitbody formation. A: view from above, B: view from the
side. Grain kernels colonized with A. bisporus mycelium were
mixed with the substrates indicated. Jar 1: c ~-~ially
av~ hle ready to use compost; jar 2, 3 and 4: hemp (Cannabis
SRtiVa) core tissue, l ~ning after removal of bast tissue,
mixed with 0%, 1% and 10% (w/w) freeze-dried and finely ground
compost.
Fig. 18: Northern blot analysis of total RNA from Abade hy~L-~ ycin-
resistant strains, transformed with pHAG3-1 (A. n~du~ans GPD-
promoter and non-modified hpt-gene), pAlH or pUlH (ContAin~ng
A.bisporus Abade or Ul GPD2-promoter-sequences plus the
modified hpt-gene described above), hybridized to a 32P-dCTP-
labelled hpt-probe. Lane 1: Abade; lanes 2 and 3: pHAG3-1
transformant C25-1.4/12, RNA from lane 2 and 3 isolated after
10 generations and after 4 generations, respectively; lane 4 -
8: pAlH- transformants E10-1.28/3, E20-1.28/3, E20-2.28/3 and
pUlH-transformants F10-1.28/3, F10-2.28/3, respectively. Arrows
indicate the positions of major transcripts.
Approximate sizes: a, b, c and d, 1700 , 1450, 900, 400
nucleotides, respectively, as deduced from a co-electrophoresed
Gibco BRL 0.17 - 1.77 kb size marker.
Fig l9A, B, C:
Restriction maps of plasmids pHAG3-1 (A), pAnHB (B) and pAnHL
(C). Abbreviations: Bm, BamHI; Bg, BgIII; E, EcoRI; ERV, EcoRV;
H, HindIII; K, KpnI; Nc, NcoI; Nh, NheI; No, NotI; X, XbaI;
b~e, phleomycin-resistance gene; hpt, hygromycin-
phosphotransferase gene; LUC, Photinus pyralis (firefly)
luciferase gene; ~, start codons; P~, TTRPC. A.niduZans GPD-
promoter- and TRPC-terminator-sequences, respectively; ssa,
excretion signal sequence of the Trichoderma reesei
ce~lobiohydrolase I-gene. Essential bZe-, hpt- and lUC-coding
2167152
W O 95/02691 PCTn~L94/00164
49
sequences in grey. Numbers refer to approximate positions (bp).
Individual drawings and DNA segments not to the same scale.
WO 95102691 2 1 6 7 1 5 2 PCT~Lg4/00164 ~
REFERENCES
Alic M, Kornegay JR, Pribnow D, Gold MH ( 1989) Appl Env Microbiol 55:
406-411
Banks GR, Kanuga N, Ealing J, Spanos A, Holden DW ( 1992) Curr Genet 22:
483-489
Rinninger DM, Skrzynia C, Pukkila PJ, Casselton LA (1987) The EMBO J 6:
835-840
Castle AJ, Horgen PA, Anderson JB ( 1988) Appl Environm Microbiol 54:
1643-1648
Ch~llen MP, Rao BG, Elliot TJ (1991) In:
Genetics and breeding of Agaricus. Procee~ing.~ of the
First International S- inAr on Mushroom Science. LJLD van Griensven
(ed.). Pudoc, Wageningen, The Netherlands. Pp 135-139
Chater K. Saps, hydrophobins and aerial growth. Curr Biol 1 (1991)
318-320
Dijkstra FY (1976) PhD thesis, University of Delft, The Netherlands
Faber KN, Swaving GJ, Faber F, Ab G, Harder W, Veenhuis M, Haima P.
(1992) J Gen Microbiol 138: 2405-2416
Fincham JRS ( 1989) Microbiol Rev 53: 148-170
Fritsche G Maint~nAnce (1991), rejuvenation and i ,ro~ t of Hors~ U1
In: Genetics and breeding of Agaricus. Proceedings of the First
International S nAr on Mushroom Science, LJLD van Griensven (ed)
Puaoc~ Wageningen, The Netherlands. Pp 145-160
Gausing K (1987) Planta 171: 241-246
Gritz L, Davies J (1983) Gene 25: 557-580
Harmsen MC, van Griensven LJLD, Wessels JGH ( 1989) J Gen Virol 70:
1613-1616
Harmsen MC, Tolner B, Kram A, Go SJ, de Haan A, Wessels JGH (1991) Curr
Genet 20: 137-144
Hiatt A, (1990) Nature 344: 469-470
Horgen PA, Jin T, Anderson JB (1991) In:
Genetics and breeding of Agaricus. Proceedings of the First
International S inAr on Mushroom Science. LJLD van Griensven (ed.).
Pudoc, Wageningen, The Netherlands. Pp 62-72
Kay E, Vilgalys R (1992) Mycologia 84: 173-182
Khush RS, Morgan L, Becker E, Wach M (1991) In:
~ W O 9~/02691 2 1 6 7 1 5 2 PCT~L94/00164
51
Proceedings of the First International .S ;n~r on Mushroom Science,
Mushroom experimental Station, Horst, The Netherlands. LJLD van
Griensven (ed.) Wageningen, Pudoc III. Pp 73-80
Lerch K, (1982) J. Biol. Chem. 257: 6414-6419
Liou CM, Yanai K, Horiuchi H, Takagi M
(1992) Biosci Biotech Biochem 56: 1503-1504
Maniatis T, Fritsch EF, Sambrook J
(1982) Molecular cloning: A laboratory ~n~lAl. Cold Spring Harbor
Laboratory, Cold Spring Harbor, NY
Mooibroek H, Sietsma JH, Wessels JGH
(1987) Abstracts l9th Lunteren Conference on Molecular Genetics, p52
Mooibroek H, Kuipers AGJ, Sietsma JH, Punt PJ, Wessels JGH
(1990) Mol Gen Genet 222: 41-48
Munoz-Rivas A, Specht CA, Drummond BJ, Froeliger E, Novotny CP, Ullrich
RC (1986) Mol Gen Genet 205: 103-106
Murashige T, Skoog F (1962) Physiol Plant 15: 473-497
Nishiyama Y, Nakayama S, Okada Y, Min K-S, Onosaka S, Tanaka K
(1990) Chem Pharm Bull 38: 2112-2117
Peng M, Singh NK, Lemke PA (1992) Curr Genet 22: 53-59
Pl~ckthun A, (1990) Nature 347: 497-498
Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CAMJJ
(1987) Gene 56: 117-124
Raeder U, Broda P (1985) Microbiology 1: 17-20
Raguz S, Yague E (1991) In:
Science and cultivation of edible fungi, Maher ed.) Balkema
Rotterdam, ISBN 9054100214, pp 43-50
R~nd~ll TA, Reddy CA (1992) Curr Genet 21: 255-260
Raper CA, Raper JR, Miller RE (1972) Mycologia 64: 1008-1117
Raper CA (1988) SchizophyZZum commune, a model for genetic studies of the
Basidiomycetes. In: GS Sidhu (ed.): Genetics of plant pathogenic
fungi. ~cAd ;c Press, Tondon~ p511-522
Royer JC, Horgen PA (1991). In:
Genetics and breeding of Agaricus. Procee~ingc of the First
International S in~r on Mushroom Science. LJLD van Griensven (ed.).
Pudoc, Wageningen, The Netherlands. Pp 135-139
SonnPnl~erg AS, Wessels JG, van Griensven LJ
(1988) Curr Microbiol 17: 285-291
.
W 0 95tO2691 ~ l 6 7 1 5 2 PCTn~L94/00164 ~
52
Stringer MA, Dean RA, Sewall TC, Timberlake WE. Rodlet-less, a new
AspergiZlus developmental mutant induced by direct gene
inactivation. Genes Dev. 5 (1991) 1161-1171
Tsai H-F, Siegel MR, Schardl CL (1992) Curr Genet 22: 399-406
Wang J, Holden DW, Leong SA (1988) Proc Natl Acad Sci USA 85: 865-869
Wessels JGH, de Vries OMH, Asgeirsdottir SA, Springer J. The thn mutation
of SchizophyZZum commune, which suppresses formation of aerial
hyphae, affects expression of the Sc3 hydrophobin gene. J. Gen.
Microbiol 137 (199la) 2439-2445
Wessels JGH, de Vries OMH, Asgeirsdottir SA, Schuren FHJ. HydL-ophobin
genes involved in formation of aerial hyphae and fruit bodies in
SchizophyZZum. The Plant Cell 3 (199lb) 793-799
Wood DA, Claydon N, Burton KS, Matcham SE, Allan M, Perry C, Thurston CF,
Raguz S, Yague E (1991) In: Science and cultivation of edible fungi,
Maher (ed.) Balkema Rotterdam, ISBN 9054100214, pp 43-50.
~wo 95,026gl 5 3 2 1 6 7 1 5 2 PCT/NL94/00164
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT:
(A) NAME: ATO-DLO Instituut voor Agrotechnologisch
Onderzoek
(B) STREET: Haagsteeg 6
(C) CITY: Wageningen
(D) STATE: Gelderland
(E) COUNTRY: The Netherlands
(F) POSTAL CODE (ZIP): 6708 PM
(A) NAME: CNC Cooperatieve Nederlandse
Champignnnkwekersver~n;g;ng B.A.
(B) STREET: Driekronenstraat 6
(C) CITY: Milsbeek
(E) COUNTRY: The Netherlands
(F) POSTAL CODE (ZIP): 6596 MA
(ii) TITLE OF INVENTION: Production and application of transgenic
mushroom mycelium and fruitbodies
(iii) NUMBER OF SEQUENCES: 13
(iv) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.25 (EPO)
(2) INFORMATION FOR SEQ ID NO: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
2167152
W 0 95/02691 5 4 PCTt~ 94tO0164
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: Nco-HPT5
- 10 (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 1:
GACATCACCA TGGCTGAACT C 21
(2) INFORMATION FOR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: AnTRPC3C
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 2:
CCGCTCGAGT GGAGATGTGG A 21
(2) INFORMATION FOR SEQ ID NO: 3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 20 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
~wo 95,026gl 5 5 ~ 1 6 7 1 5 2 PCT~L94/00164
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: HPT-E2
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 3:
AGTTCAGCGA GAGCCTGACC 20
(2) INFORMATION FOR SEQ ID NO: 4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 20 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
( ii ) MOLECULE l'YPE: cDNA
(iii) ANTI-SENSE: NO
(vii) IMMEDIATE SOURCE:
(B) CLONE: HPT-NTC
3o
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 4:
CATAGCCTCC GCGACCGGCT 20
(2) INFORMATION FOR SEQ ID NO: 5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 35 base pairs
(B) TYPE: nucleic acid
W O 95/02691 6 2 1 6 7 1 5 2 PCT~Lg4/00164 ~
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(iii) ANTI-SENSE: NO
(vii) IMMEDIATE SOURCE:
(B) CLONE: EN-LUC-l
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 5:
GGGAATTCCA TGCCATGGAA GACGCCAAAA ACATA 35
(2) INFORMATION FOR SEQ ID NO: 6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 20 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(iii) ANTI-SENSE: NO
(vii) IMMEDIATE SOURCE:
(B) CLONE: T7
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 6:
TAATACGACT CACTATAGGG 20
(2) INFORMATION FOR SEQ ID NO: 7:
~ W O 95/02691 5 7 2 1 6 7 1 5 2 PCT~L94/00164
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 3O base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(iii) ANTI-SENSE: NO
(vii) IMMEDIATE SOURCE:
(B) CLONE: AbGPDl
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 7:
GG M TTCGTT GTCATCACCG CTCCTGGGAG 3O
(2) INE~ORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 26 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(iii) ANTI-SENSE: NO
(vii) IMMEDIATE SOURCE:
(B) CLONE: AbGPD3
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 8:
G M GAAGAAT TCAGAGGTCC GC M GT 26
W O 95/02691 5 8 2 ~ ~ 7 ~ ~ 2PCT~Lg4l00l64 ~
(2) INFORMATION FOR SEQ ID NO: 9:
(i) SE W ENCE CHARACTERISTICS:
(A) LENGTH: 24 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(iii) ANTI-SENSE: NO
(vii) IMMEDIATE SOURCE:
(B) CLONE: AbGPD2c
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 9:
GCTTATCGCC AlG~ lC TCTC 24
(2) INFORMATION FOR SEQ ID NO: lO:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 30 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
3o
(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: PR-HPTl
(xi) SEQUENCE DESCRIPTION: SEQ ID NO lO:
~ wo gs/0269~ 5 9 2 1 6 7 1 5 2 PCT/NLg4/00164
ATG M AAAGC CTGAACTCAC CGCGACGTCT 30
(2) IMFORMATION FOR SEQ ID NO: 11:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: PR-HPT2c
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 11:
GGGTCGTGAG CAGGCTCCCG TTTCCTTATC 30
(2) INFORMATION FOR SEQ ID NO: 12:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 30 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: PR-LUC-l
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 12:
ATGGAAGACG CCAAAAACAT AAAGAAAGGC 30
W O 95/02691 6 ~ ~ ~ 6 7 1 5 ~ PCT~Lg4/00l64 ~
(2) INFORMATION FOR SEQ ID NO: 13:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 30 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
10(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: PR-LUC-2c
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 13:
CACCGGGGCC GACTTAACCT TAGCTATAAC 30