Note: Descriptions are shown in the official language in which they were submitted.
~ W095/026~3 21 ~71 ~4 PCT~4/02059
"USE OF ~P:rn_,I DERIVATIVE8 A8 ANTI-META8TATIC AGENT8"
~he present invention relates to the use of cephem
derivatives as anti-metastatic agents.
~s known, malignancy of cancer is mainly due to
metastasis. Because therapy usually fails to destroy
multiple secondary tumor, their uncontrolled growth
:Leads to death of patients. Only very few patients die
from complications directly arising from primary tumor.
~ccordingly, there is a need in therapy of drugs able
to prevent and/or block the metastatic spread.
Several cephem derivatives were described as having
elastase inhibiting activity and can be used in the
treatment of inflammatory and degenerative diseases
caused by proteolytic enzymes in mammals including
humans.
]Now we have found that a selected class of compounds
~previously disclosed can prevent and/or block the
metastatic spread of tumors in mammals, including
t 20 humans.
Accordingly one object of the present invention is the
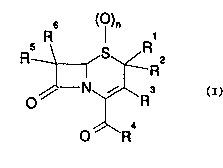
use of a compound of formula (I)
W095/02603 PCT~410205 ~
~ ~ 67 t 94
-- 2
6 ()n
5 R I R1
R \ ~S~ 2
/~--N,~R3
o
O //\R4
wherein n is zero, one or two;
Rl is hydrogen or an optionally substituted Cl-CI2 alkyl,
C2-CI2 alkenyl, C2-C~2 alkynyl, C6-C~0 aryl, C3-C8
cycloalkyl, Cs-C8 cycloalkenyl, or C7-C,4 aralkyl, C8-CI4
aralkenyl, C8-CI4 aralkynyl, (cycloalkyl)alkyl,
(cycloalkyl)alkenyl, heterocyclyl, (heterocyclyl)alkyl,
(heterocyclyl)alkenyl;
R2 represents an atom or group selected from the
following:
(1) halogen
(2) Rl as defined above
(3) an ether ORI wherein Rl is as defined above
(4) a thioether, sulphoxide or sulphone -S(O)nR
wherein n and Rl are as defined above
(5) acyloxy -OC(O)RI wherein Rl is as defined above;
(6) sulphonyloxy -OS(O)2RI wherein Rl is as defined
~ W095/02603 2 1 6 7 1 9 4 PCT~4/02059
above;
or Rl and R2 taken together form a methylene group of
formula =CHRI or =CH-CO2RI or =CH-CORI wherein Rl is as
defined above; or Rl and R2 taken together with the C-2
carbon atom of the cephem nucleus constitute a
carbocyclic or heterocyclyl group;
R3 represents one of the following:
(1) R2 as defined above
(2) an acyl group -C(O)RI, -C(O)ORI or -CO2H wherein R
as defined above
(3) on oxymethyl group -CH2-ORI wherein Rl is as
defined above
(4) a thiomethyl group or a derivative thereof of
formula -CH2S(O)nRI wherein n and Rl are as defined
lS above
(5) an acyloxymethyl group -CH2OC(O)RI wherein Rl is
as defined above or a -CH2o-R7 wherein R7 is a
mono, di- or tripeptide composed of D or L ~-
aminoacids chosen from Ala, Gly, Val, Leu, Ile,
Phe and with the terminal amino group either free
or protected as an amide -NHCORI or sulfonamide -
- NHSO2RI wherein Rl is as defined above
(6) an acylthiomethyl group -CH2SC(O) Rl wherein Rl is
as defined above
(7) a sulphonyloxymethyl group -CH2-OSO2R~wherein Rl is
as defined above
W095/02603 PCT~4/0205 ~
1 9 4
-- 4
(8) a group of formula -CH2-Z-NRIR8 wherein Z is a
bond, -o C(O)- or -OS(O)2-, Rl is as defined above
and R8, being the same or different, is as defined
above for Rl; or Rl and R8 taken together with the
nitrogen atom to which they are attached represent
a heterocyclic ring;
(9) ammoniomethyl -CH2N+RIR8R9 wherein Rl and R8 are as
defined above and R9, being the same or different,
is as defined for Rl; or Rl is alkyl and R8 and R9
together with the nitrogen atom to which they are
attached represent a heterocyclic ring;
R4 is either:
(1) a group Rl wherein Rl is as defined above
(2) a group ORI wherein Rl is as defined above
(3) a group SRl wherein Rl is as defined above
(4) a group NRIRs wherein Rl and R8 are as defined
above;
Rs is either Rl as defined above or halogen or C~-C6
alkoxy, Cl-C6 alkylthio or Cl-C6 acylamino;
R6 is a group selected from the following:
(1) R2 as defined above
(2) a group of formula -Z-N(RI)R8 wherein Z, Rl and R8
are as defined above
(3) a group of formula -NR8C(O)RI wherein Rl and R8 are
as defined above, or Rl and R8 taken together with
W095/02603 2 ! ~ PCT~4/02059
the aminocarbonyl group to which they are attached
constitute a heterocyclic ring
(4) an acylamino group -NHR7 wherein R7 is as defined
above
(5) an ammonio group -N~RIR8R9 wherein Rl R8 and R9 are
as defined above;
or R5 and R6 taken together with the C-7 carbon atom of
the cephem nucleus constitute a carbocyclic or
heterocyclic ring;
or Rs and R6 taken together constitute a methylene group
of formula =CHRI, =CH-CO-RI or =CH-SO2RI wherein Rl is as
defined above
or a pharmaceutically acceptable salt thereof, in the
preparation of a medicament for use in preventing
aild/or treating the metastatic spread of tumors.
A further object the present invention is to provide a
compound of formula (I), as defined above, or a
pharmaceutically acceptable salt thereof, for u e in
preventing and/or treating the metastatic spread of
tumors.
The Cl-CI2 alkyl group is a straight or branched alkyl
group such as methyl, ethyl n-propyl isopropyl
n--butyl isobutyl sec-butyl tert-butyl n-pentyl
n--hexyl and so on.
The C2-CI2 alkenyl group is a straight or branched
a:Lkenyl group such as vinyl allyl, crotyl,
WOg5/02603 PCT~4/0205 ~
2 t ~
- 6 -
2-methyl-1-propenyl, 1-methyl-1-propenyl, butenyl,
pentenyl and so on.
The C2-CI2 alkynyl group is a straight or branched
alkynyl group such as ethynyl, propargyl, l-propynyl,
l-butynyl, 2-butynyl and so on.
The C6-C~0 aryl group is a monocyclic or bicyclic
aromatic
hydrocarbon group of 6 to lO carbon atoms, such as
phenyl and naphtyl.
The C3-C8 cycloalkyl group is a saturated carbocyclic
group of 3 to 6 carbon atoms, such as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl and so on.
The C5-C8 cycloalkenyl group is an unsaturated
carbocyclic group such as cyclopentenyl, cyclohexenyl
lS and so on.
The C~-CI4 aralkyl group is an alkyl group of 1 to 4
carbon atoms linked to a monocyclic or bicyclic
aromatic hydrocarbon group of 6 to lO carbon atoms.
Examples of aralkyl groups are benzyl, phenylethyl and
naphtylmethyl.
The C8-CI4 aralkenyl group is an alkenyl group of 2 to
4 carbon atoms linked to a monocyclic or bicyclic
aromatic hydrocarbon group of 6 to lO carbon atoms.
Examples of aralkenyl groups are styryl,
2-phenyl-1-propenyl, 3-phenyl-2-butenyl,
2-naphtylethenyl and so on.
The C8-CI4 aralkynyl group is an alkynyl group of 2 to
~ W095/02603 2 t 6 7 1 ~4 PCT~4/02059
~ carbon atoms linked to a monocyclic or bicyclic
aromatic hydrocarbon group of 6 to lO carbon atoms.
Examples of aralkynyl groups are 2-phenylethynyl,
2-naphtylethynyl and so on.
l'he (cycloalkyl)alkyl group is an alkyl group of 1 to
4 carbon atoms linked to a cycloalkyl group.
q'he (cycloalkyl)alkenyl group is an alkenyl group of 2
t:o 4 carbon atoms linked to a cycloalkyl ~-5Up or to an
aryl group.
I'he heterocyclyl group is a 3- to 6-membered
saturated or unsaturated heterocyclyl ring, containing
at least one heteroatom selected from O, S and N, which
is optionally fused to a second 5- or 6-membered ,
saturated or unsaturated heterocyclyl group or to a
cycloalkyl group or to an aryl group.
In particular, the heterocyclyl group may be for
example a tetrazole, thiadiazole, pyrrole, triazole,
imidazole, oxazole, thiophene, pyridine, pyrazine,
triazine, morpholine and the like.
The (heterocyclyl)alkyl group is an alkyl group of 1 to
4 carbon atoms linked to a heterocyclyl group.
The (heterocyclyl)alkenyl group is an alkenyl group of
2 to 4 carbon atoms linked to a heterocyclic group.
The term halogen (or halo) preferably encompasses
fluorine, chlorine or bromine.
The Cl-C6 alkoxy group is a straight or branched
alkylthio group such as methoxy, ethoxy, n-propoxy,
W095/02603 2 ~ ~ 7 ~i ~ 4 PCT~4/020i9 ~
-- 8
isopropoxy, n-butoxy, isobutoxy, sec-butoxy,
tert-butoxy, n-pentoxy, n-hexyloxy and so on.
The C~-C6 alkylthio group is a straight or branched
alkoxy group such as methylthio, ethylthio,
n-propylthio,isopropylthio,n-butylthio,isobutylthio,
sec-butylthio, tert-butylthio, n-pentylthio,
n-hexylthio and so on.
The C~-C6 acylamino group is a straight or branched
acylamino group such as formamido, acetamido,
propionamido, pivalamido and so on.
The above said alkyl, alkenyl, alkynyl, cycloalkyl,
cycloalkenyl, aryl, aralkyl, aralkenyl, aralkynyl,
(cycloalkyl)alkyl, (cycloalkyl)alkenyl, heterocyclyl,
(heterocyclyl)alkyl, (heterocyclyl)alkenyl, alkoxy,
alkylthio, acylamino groups can be either unsubstituted
or substituted by one or more substituents selected
from the following ones:
- halo (i.e., fluoro, bromo, chloro or iodo);
- hydroxy or oxo;
- nitro;
- azido;
- mercapto (-SH);
- amino (i.e., -NH2, or -NHR' or -NR'R'') wherein R'
and R'', which are the same or different, are C~-CI2
straight or branched alkyl or phenyl or benzyl;
- formyl (i.e., -CHO);
W095/02603 2 1 6 7 ~ 9 ~ PCT~4/02059
- cyano;
- carboxy(alkyl) (i.e., (CH2)tCOOH or (CH2)tCOOR')
wherein R' is as defined above and t is O, 1, 2 or 3;
- sulpho (i.e., -SO3H);
- acyl (i.e., -C(O)R') wherein R' is as defined above
or trifluoroacetyl (i.e., -C(O)CF3);
- carbamoyl (i.e., -CONH2); N-methylcarbamoyl (i.e.,
-CONHCH3) or N-carboxymethylcarbamoyl (i.e.,
-CONHCH2COOH);
- carbamoyloxy (i.e., -OCONH2);
- acyloxy (i.e., -OC(O)R') wherein R' is as defined
above or formyloxy (i.e., -OC(O)H);
- alkoxycarbonyl or benzyloxycarbonyl (i.e., -C(O)OR')
wherein R' is as defined above;
- alkoxycarbonyloxy or benzyloxycarbonyloxy (i.e.,
-OC(O)OR') wherein R' is as defined above;
- alkoxy, phenoxy or benzyloxy (i.e., -OR') wherein R'
is as defined above;
- alkylthio, phenylthio or benzylthio (i.e., -SR')
wherein R' is as defined above;
- alkylsulphinyl, phenylsulphinyl or benzylsulphinyl
(i.e., -S(O)R') wherein R' is as defined above;
- - alkylsulphonyl, phenylsulphonyl or benzylsulphonyl
(i.e., -S(O)2R') wherein R' is as defined above;
- acylamino (i.e., -NHC(O) R' ' ' or -NHC(O) OR' ' ' ) wherein
R' ' ' is C,-C,2 straight or branched alkyl, phenyl,
benzyl, CH2CH2COOH or CH2CH2CH2COOH;
WO9~/02603 ~ .q~ PCT~4/0205 ~
-- 10 --
- sulphonamido (i.e., -NHSO2R') wherein R' is as
defined above;
- guanidino (i.e., -NHC(=NH)NH2);
- C~-C4 alkyl, C2-C4 alkenyl or alkynyl;
5 - C3-C6 cycloalkyl;
- phenyl
- substituted methyl selected from chloromethyl,
fluoromethyl, difluoromethyl, trifluoromethyl,
aminomethyl, N,N-dimethylaminomethyl, azidomethyl,
cyanomethyl, carboxymethyl, sulphomethyl,
carbamoylmethyl, carbamoyloxymethyl, hydroxymethyl, Cl-
C4 alkoxycarbonylmethyl, guanidinomethyl.
The carboxyl-protecting group may, for example, be a
lower alkyl group such as methyl, ethyl, propyl,
isopropyl or tert-butyl; a halogenated lower alkyl
group such as a 2,2,2-trichoroethyl or a
2,2,2-trifluoroethyl; a lower alkanoyloxyalkyl group
such as acetoxymethyl, propionyloxymethyl,
pivaloyloxymethyl, 1-acetoxyetyl, l-propionyloxyethyl;
a lower alkoxycarbonyloxyalkyl group such as
1 - ( m e t h o x y c a r b o n y l o x y ) e t h y l ,
1 - ( e t h o x y c a r b o n y l o x y ) e t h y l ,
1-(isopropoxycarbonyloxy)ethyl; a lower alkenyl group
such as 2-propenyl, 2-chloro-2-propenyl,
3-methoxycarbonyl-2-propenyl, 2-methyl-2-propenyl,
2-butenyl, cinnamyl; an aralkyl group such as benzyl,
p-methoxybenzyl, 3,4-dimethoxybenzyl, o-nitrobenzyl,
~ W095/02603 2 1 6 7 1 9 4 PCT~4/02059
-- 11 --
p-nitrobenzyl, benzhydryl, bis(p-methoxyphenyl)methyl;
a (5-substituted 2-oxo-1,3-dioxol-4-yl)methyl group
such as (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl; a lower
alkylsilyl group such as trimethylsilyl,
tert-butyldimethylsilyl, tert-butyldiphenylsilyl,
triphenylsilyl; or an indanyl group; a phtalidyl group;
a pyranyl group; a metoxymethyl or methylthiomethyl
group; a 2-methoxyethoxymethyl group. Particularly
preferred are a tert-butyl group, a p-nitrobenzyl
group, a p-methoxybenzyl group, a benzhydryl group, a
tert-butyldimethylsilyl, tert-butyldiphenylsilyl group
or a propenyl group.
The amino, hydroxy or mercapto protecting groups
possibly present may be those usually employed in the
chemistry of penicillins and cephalosporins for this
kind of functions. They may be, for instance,
optionally substituted, especially halo-substituted,
acyl groups, e.g. acetyl, monochloroacetyl,
dichloroacetyl, trifluoroacetyl, benzoyl or
p-bromophenacyl; triarylmethylgroups, e.g.
triphenylmethyl; silyl groups, in particular
trimethylsilyl, dimethyl-tert-butylsilyl,
diphenyl-tert-butylsilyl,; or also groups such as
tert-butoxycarbonyl, p-nitrobenzyloxycarbonyl,
2,2,2-trichloroethoxycarbonyl, benzyl and pyranyl.
Preferred protecting groups of the hydroxy function are
p-nitrobenzyloxycarbonyl; allyloxycarbonyl;
W095/02603 2 1 6 7 1 q 4 PCT~4/02059 ~
- 12 -
dimethyl-tert-butylsilyl; diphenyl-tert-butylsilyl;
trimethylsilyl; 2,2,2-trichloroethoxycarbonyl; benzyl;
dimethoxybenzyl; p-methoxybenzyloxycarbonyl;
p-bromophenacyl; triphenylmethyl, pyranyl,
methoxymethyl, benzhydryl, 2-methoxyethoxymethyl,
formyl, acetyl, tricloroacetyl.
As already said, the invention includes within its
scope
the salts of those compounds of formula (I) that have
salt-forming groups, especially the salts of the
compounds having a carboxylic group, a basic group
(e.g. an amino or guanidino group), or a ~uaternary
ammonium group. The salts are especially
physiologically tolerable salts, for example alkali
metal and alkaline earth metal salts (e.g. sodium,
potassium, lithium, calcium and magnesium salts),
ammonium salts and salts with an appropriate organic
amine or amino acid (e.g. arginine, procaine salts),
and the addition salts formed with suitable organic or
inorganic acids, for example hydrochloric acid,
sulphuric acid, carboxylic and sulphonic organic acids
(e.g. acetic, trifluoroacetic, p-toluensulphonic acid).
Some compounds of formula (I) which contain a
carboxylate and an ammonium group may exist as
zwitterions; such salts are also part of the present
invention.
W095/026~3 2 ~ PCT~4/02059
- 13 -
The present invention encompasses all the possible
stereoisomers as well as their racemic or optically
active mixtures.
~ urthermore, physiologically hydrolizable esters,
hydrates and solvates of compounds of formula (I) are
included within the scope of the present invention.
The physiologically hydrolizable esters of the
compounds (I) may include, for example,
methoxycarbonylmethyl, l-methoxycarbonyloxy-1-ethyl,
indanyl, phtalidyl, methoxymethyl, pivaloyloxymethyl,
ylycyloxymethyl, phenylglycyloxymethyl or 5-methyl-2-
oxo-1,3-dioxolan-4-yl esters, and other physiologically
hydrolizable esters which have been widely used in the
technical fields of penicilin and cephalosporin
antibiotics:morepreferably,methoxycarbonyloxymethyl,
1-methoxycarbonyloxy-1-ethyl, methoxymethyl or
pivaloyloxymethyl; and most preferably,
methoxycarbonyloxymethyl or methoxymethyl.
q'ypical solvates of the cephalosporin compounds of
formula(I) may include solvates with water miscible
solvents, e.g. methanol, ethanol, acetone or
acetonitrile or acetonitrile; and more preferably,
ethanol.
Preferred compounds of formula (I), according to the
invention, are the compounds of the formula (Ia)
WO 95/02603 ~ 9 ~ PCT/EP94/0205
-- 14 --
6 ()n
R \ j~ ~ R2
o// N R3
o "\R4
wherein n, Rl, R2, R3, R4, R5, and R6, are as defined
above, and the pharmaceutically acceptable salts
thereof. Examples of compounds according to the present
invention are the following:
1 ) t 6R, 7S ) -2 - ( 2, 2 -Dimethyl-propionyl ) -4- ( 6-hydroxy-2 -
methyl-5-oxo-2, 5-dihydro- [ l, 2, 4 ] triaz in-3 -ylsulf anyl ) -
7-methoxy-3-methyl-5, 5-dioxo-5-thia-1-aza-
bicyclo [ 4 . 2 . 0 ] oct-2-en-8-one
2 ) 2-Benzoyl-7-methoxy-4- ( 5-methyl- t l, 3, 4 ] t~ 701-2-
ylsulfanyl)-3-(5-methyl-tl,3,4]thiadiazol-2-
ylsulfanylmethyl) -5, 5-dioxo-5-thia-1-aza-
bicyclo [ 4 . 2 . O ] oct-2-en-8-one
3 ) 2- ( 2, 2 -Dimethyl-propionyl ) -7-methoxy-4 - ( l-methyl-lH-
tetrazol-5-ylsulfanyl) -3- ~l-methyl-lH-tetrazol-5-
ylsulf anylmethyl ) -5, 5-dioxo-5-thia-1-aza-
bicyclo [ 4 . 2 . 0 ] oct-2-en-8-one
4 ) 2-Benzoyl-4- ( 6-hydroxy-2-methyl-5-oxo-2, 5-dihydro-
[ l, 2, 4 ] triazin-3-ylsulfanyl) -7-methoxy-3-methyl-5, 5-
dioxo-5-thia-1-aza-bicyclo [ 4 . 2 . O ] oct-2-en-8-one
5 ) 2 -Benzoyl-7-methoxy-3 -methyl-4- ( 1-methyl-lH-
~ W095/0260.i 2 ~ 6 7 1 ~ 4 PCT~4/020~9
tetrazol-5-ylsulfanyl)-5,5-dioxo-5-thia-1-aza-
b:icyclot4.2.0]oct-2-en-8-one
6~2-(2,2-Dimethyl-propionyl)-7-methoxy-3-methyl-4-(5-
methyl-[1,3,4]thiadiazol-2-ylsulfanyl)-s,5-dioxo-5-
thia-1-aza-bicyclo[4.2.0]oct-2-en-8-one
7~ 2-(2,2-Dimethyl-propionyl)-7-methoxy-4-(5-methyl-
[1,3,4]thiadiazol-2-ylsulfanyl-3-(5-methyl-
[:L,3,4]thiadiazol-2-ylsulfanylmethyl)-5,5-dioxo-5-thia-
1-aza-bicyclo[4.2.0]oct-2-en-8-one
8~ 2-Benzoyl-7-methoxy-4-(1-methyl-lH-tetrazol-5-
ylsulfanyl) -3-(1-methyl-lH-tetrazol-5-
ylsulfanylmethyl)-5,5-dioxo-5-thia-1-aza-
bicyclo[4.2.0]oct-2-en-8-one
9 D 7-Allyl-2-benzoyl-4-(5-methyl-[1,3,4]thiadiazol-2-
ylsulfanyl)-3-(5-methyl-[1,3,4]thiadiazol-2-
ylsulfanylmethyl)-5,5-dioxo-5-thia-1-aza-
bicyclo[4.2.0]oct-2-en-8-one
lO) 7-Allyl-2-(2,2-dimethyl-propionyl)-4-(5-methyl-
[1,3,4]thiadiazol-2-ylsulfanyl)-3-(5-methyl-
[:L,3,4]thiadiazol-2-ylsulfanylmethyl)-5,5-dioxo-5-thia-
l--aza-bicyclo[4.2.0]oct-2-en-8-one
ll) 3-(6-Hydroxy-2-methyl-5-oxo-2,5-dihydro-
[1,2,4]triazin-3-ylsulfanylmethyl)-7-methoxy-5,5-dioxo-
2-(pyrrolidine-1-carbonyl)-5-thia-1-aza-
bicyclo[4.2.0]oct-2-en-8-one
12) 1-(3-Acetoxymethyl-7-methoxy-5,5,8-trioxo-5-thia-1-
aza-bicyclo[4.2.0]oct-2-enane-2-carbonyl)pyrrolidine-2-
W095l02603 PCT~ ~4/0205 ~
~6~1 941
- 16 -
carboxilic acid
13) 1-[3-Acetoxymethyl-5,5,8-trioxo-7-(2,2,2-trifluoro-
ace tylamino)-5-thia-1-aza-bicyclo[4.2.0]oct-2-enane-2-
carbonyl]-pyrrolidine-2-carboxilic acid
14) 1-(7-Benzoylamino-3-methyl-5,5,8-trioxo-5-thia-1-
aza-bicyclo[4.2.0]oct-2-enane-2-carbonyl)-pyrrolidine-
2-carboxylic acid
15) 3-Methyl-5,5,8-trioxo-5-thia-1-aza-
bicyclo[4.2.0]oct-2-ene-2-carboxylic acid 4-carboxy-
benzyl ester
16) 2-Benzoyl-7-ethylsulfanyl-4-(5-methyl-
[1,3,4]thiadiazol-2-ylsulfanyl)-3-(5-methyl-
tl,3,4]thiadiazol-2-ylsulfanylmethyl)-5,5-dioxo-5-thia-
l-aza-bicyclot4.2.0]oct-2-en-8-one
17) 2-Benzoyl-7-ethylsulfanyl-3-methyl-4-(5-methyl-
tl,3,4]thiadiazol-2-ylsulfanyl)-5,5-dioxo-5-thia-1-aza-
bicyclo[4.2.0]oct-2-en-8-one
18)3-(1-Methyl-lH-tetrazol-5-ylsulfanylmethyl)-5,5,8-
trioxo-7-(2,2,2-trifluoro-acetylamino)-5-thia-1-aza-
bicyclo[4.2.0]oct-2-ene-2-carboxylic acid 4-carboxy-
benzyl ester
19) 2-Acetylamino-3-[7-methoxy-3-(1-methyl-lH-tetrazol-
5-ylsulfanylmethyl)-5,5,8-trioxo-5-thia-1-aza-
bicyclo[4.2.0]oct-2-enane-2-carbonylsulfanyl]-propionic
acid
20) 2-Acetylamino-3-[7-allyl-3-(1-methyl-lH-tetrazol-5-
ylsulfanylmethyl)-5,5,8-trioxo-5-thia-1-aza-
~ wo 95~026cl3 2 1 ~ 71 ~4 PCT~4/02059
- 17 -
bicyclo[4.2.0]oct-2-enane-2-carbonylsulfanyl]-propionic
~ acid
and the pharmaceutically acceptable salts thereof.
Cephems of formula (I) defined under the present
invention are known compounds or can be prepared from
known compounds by known methodologies.
For example, suitable methods for the preparation of
the claimed compounds can be found in the following
bibliografic references, listed according to the site
o~ functionalization of the cephem nucleus:
2--substituted cephems: Noveau Journal de Chimie 1, 85
(1977); Synthetic Communations 15, 681 (1985); Chem.
Pharm. Bull. 31, 1482 (1983); Bull. Chem. Soc. Jpn. 56,
2~85 (1983); Tetrahedon Letters 21, 1293, (1980); J.
Org. Chem. 44, 811 (1979); Tetrahedron Letters 4751
(1978); J. Am. Chem. Soc. 100, 1886 (1978); J. Chem.
Soc. Perkin I 2298 (1977); Tetrahedron Letters 3611
(~977); J. Chem. Soc. Chem. Comm. 671 (1973);
Tetrahedron Letters 3717 (1972); US 3.660.395; Eur. J.
Med. Chem. 24, 599 (1989); J. Med. Chem. 14, 420
(1971); J. Med. Chem. 14, 426 (1971); Heterocycles 29,
1107 (1989); J. Med. Chem. 27, 1225 (1984).
3--substituted cephems: Heterocycles 24, 1653 (1986); J.
Chem. Soc. Perkin I 1361 (1991); SynLett 389 (1990);
SynLett 391 (1990); J. Org. Chem. 55, 5833 (1990);
Tetrahedron Letters 31, 3389 (1983); Tetrahedron 41,
2()25 (1985); Chem. Pharm. Bull. 33, 5534 (1985); J.
W095/02603 2 ~ ~ 7 ~ q 4 PCT~4/0205 ~
- 18 -
Chem. Soc. Perkin I 2281 (1983); J. Org. Chem. 53, 983
(1988); Gazz. Chim. II. 115, 169 (1985); Tetrahedron
39, 461 (1983); J: Antibiotics 39, 380 (1986); J. Am.
Chem. Soc. 108, 1685 (1986); J. Chem. Soc. Chem. Comm.
1012 (1974); Chem. Pharm. Bull. 28, 2116 (1980); Gazz.
Chim. IC 110, 519 (1980); Phil. Trans. R. Soc. Lond. B
289, 173 (1980); Chem. Pharm. Bull. 28, 62 (1980); J.
Antibiotics 37, 1441 (1984); Tetrahedon Letters 29,
6043 (1988); Tetrahedron Letters 29, 5739 (1988);
~eterocycles 1799 (1986); J. Org. Chem. 54, 5828
(1989); J. Antibiotics 42, 159 (1989); Heterocycles 28,
657 (1989); SynLett 888 (1991); J. Antibiotics 43, 533
(1990), Eur. J. Med. Chem. 27, 875 (1992).
4-substituted cePhems: Tetrahedron Letters 52, 5219
(1978); Tetrahedron Letters 33, 2915 (1977); J. Org.
Chem. 51, 4723 (1986); Synthesis 52 (1986); J. Org.
Chem. 35, 2429 (1970); J. Org. Chem. 35, 2430 (1970);
US 4992-541-A; EP 0124001-A2; EP 0267723-A2; US
4.547.371; J. Med. Chem. 33, 2522 (1990); Tetrahedron
Letters 32, 6207 (1991); Eur. J. Med. Chem. 27, 875
(1992), J. Med. Chem. 20, 173 (1977); J. Med. Chem. 15,
1172 (1972); US 5.077.286; PCT WO 89/10926.
7-substituted ce~hem: J. Org. Chem. 43, 3788 (1978); J.
Org. Chem. 42, 2960 (1977); J. org. Chem. 42, 3972
(1977); Tetrahedron Letters 1303 (1976); J. Med. Chem.
25, 457 (1982); Tetrahedron Letters 16, 1441 (1979); J.
Chem Soc. Chem. Comm. 276 (1988); J. Chem. Soc. Perkin
~ wo 95,02603 2 1 ~ 7 l ~ PCT~4/02059
-- 19 --
I 635 (1987); J. Org. Chem. 54, 3907 (1989); J.
Antibiotics 52, 159 (1989); Tetrahedron Letters 30,
2375 (1989); Tetrahedron Letters 30, 2379 (1989)
Thetrahedron Letters 375 (1972); Tetrahedron Letters
lg, 1637 (1979).
As stated above, the compounds of the invention have
been found to be active as anti-metastatic agents.
Accordingly, they can be used in mammals, including
humans, for preventing and/or treating the metastatic
spread of tumors.
The antimetastatic activity of the compounds was proved
experimentally in vivo against the highly metastatic
B16F10 murine melanoma. B16F10 tumor cells were
maintained in vitro by serial soil. For experimental
purpose, tumor cells were pretreated in vitro with
1000~ for 6 hrs, whereas control were ;ncllh~ted with
medium. Cells were then harvested and injected
intravenously into C57/Bl6 mice at the concentration of
105 cells/mouse. Animals were treated intraperitoneally
with the compound for 6 days at the dose of 200 mg/kg.
AEter 22 days mice were sacrificed and the number of
lung metastatic foci were counted.
- Data reported in table 1 show that a representative
compound of the invention, namely (6R,7S)-2-(2,2-
dimethyl-propionyl)-4-(6-hydroxy-2-methyl-5-oxo-2,5-
dihydro-[1,2,4]triazin-3-ylsulfanyl)-7-methoxy-3-
methyl-5,5-dioxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-en-8-
W095/02603 ~ t 9 ~ PCT~4/0205
- 20 -
one (internal code FCE26238) is clearly active as
antimetastatic agent. An evident reduction of the
metastasis number was observed after in vitro
pretreatment and after in vivo treatment. No evidence
of toxicity was observed.
Tabl~ 1
Group Treatment with FCE26238 median
number of
metastasis
in vitro in vivo
(range)
Control - - 20 (7-72)
- 200 mg/kg x6 4 (2-24)
lOOOy x 6 hrs - 0 (0-0)
lOOOy x 6 hrs 200 mg/kg x6 0 (0-0)
The compounds of the invention can be administered by
the usual routes, for example, parenterally, e.g. by
intravenous injection or infusion, intramuscularly,
subcutaneously, topically or orally, intravenous
injection or infusion being the preferred. The dosage
depends on the age, weight and condition of the patient
and on the administration route.
A suitable dosage for the compounds of the invention,
e.g. FCE26238 for administration to adult humans may
~ WO9~/02603 2 1 6 7 1 9 ~ PCT~4/02059
- 21 -
range from about 0.5 to about 300 mg per dose 1-4 times
a day.
The pharmaceutical compositions of the invention may
contain a compound of formula (I) or a pharmaceutically
acceptable salt thereof, as the active substance, in
association with one or more pharmaceutically
acceptable excipients and/or carriers.
The pharmaceutical compositions of the invention are
usually prepared following conventional methods and are
administered in a pharmaceutically suitable form. For
instance, solutions for intravenous injection or
infusion may contain as carrier, for example, sterile
water or, preferably, they may be in the form of
sterile aqueous isotonic saline solutions.
Suspensions or solutios for intramuscular injections
may contain, together with the active compound, a
pharmaceutically acceptable carrier, e.g. sterile
water, olive oil, ethyl oleate, glycols, e.g. propylene
glycol, and, if desired, a suitable amount of lidocaine
hydrochloride.
In the form for topical application, e.g. creams,
lotions or pastes for use in dermatological treatment,
the active ingredient may be mixed with conventional
oleoginous or emulsifying excipients.
The solid oral forms, e.g. tablets and capsules, may
contain, together with the active compound, diluents,
e.g. lactose, dextrose, saccharose, cellulose, corn
W095/02603 2 1 6 7 1 9 ~ PCT~4/0205 ~
starch and potato starch; lubricants, e.g. silica,
talc, stearic acid, magnesium or calcium stearate,
and/or polyethylene glycols; binding agents, e.g.
starches, arabic gums, gelatin, methylcellulose,
carboxymethyl cellulose, polyvinylpyrrolidone;
disaggregating agents, e.g. a starch, alginic acid,
alginates, sodium starch glycolate; effervescing
mixtures; dyestuffs; sweeteners; wetting agents, for
instance, lecithin, polysorbates, laurylsulphates; and,
in general, non-toxic and pharmacologically inactive
substances used in pharmaceutical formulations. Said
pharmaceutical preparations may be manufactured in a
known manner, for example by means of mixing,
granulating, tabletting, sugar-coating, or film-coating
processes.
An object of the invention is also to provide a method
of treatment of the above mentioned pathological
conditions comprising both separate and substantially
contemporaneous administration of a composition
containing a compound of formula (I), or a
pharmaceutically acceptable salt thereof, and a
pharmaceutical composition contA i n; ng a different
pharmaceutically active agent, typically an antitumor
agent.
Antitumor agents that can be formulated with a compound
of the invention or, alternatively, can be administered
in a combined method of treatment are e.g. doxorubicin,
W09~/02603 ~l 6 7 1 ~4 PCT~P94/02059
- 23 -
daunomycin, epirubicin, idarubicin, etoposide,fluorouracil, paclitaxel, melphalan, cyclophosphamide,
bleomycin, vinblastin and mitomycin or a mixture of two
o.r more thereof.
The compounds of the invention can therefore be used in
a treatment to ameliorate a cancer.
E~AMPLE A
Tablets:
Per 10,000
Inqredients Per Tablet Tablets
1. .~ctive ingredient 40.0 mg 400 g
~pd of Form I
2. ~orn Starch20.0 mg 200 g
3. Alginic acid20.0 mg 200 g
4. Sodium alginate20.0 mg 200 g
5. Magnesium
Stearate 1.3 mq 13 g
101.3 mg 1013 g
Procedure for tablets:
Step 1. Blend ingredients No. 1, No. 2, No. 3 and No.
4 in a suitable mixer/blender .
Step 2. Add sufficient water portionwise to the blend
from Step 1 with careful mixing after each
W095/02603 ~ t ~ ~ ~i q ~ PCT~4/0205 ~
- 24 -
addition. Such additions of water and mixing
until the mass is of a consistency to permit
its conversion to wet granules.
Step 3. The wet mass is converted to granules by
passing it through an oscillating granulator
using a number 8 mesh (2.38) screen.
Step 4. The wet granules are dried in an oven at 60C
until dried.
Step 5. The dried granules are lubricated with
ingredient no. 5.
Step 6. The lubricated granules are compressed on a
suitable tablet press.
Exam~le B
Intramuscular injection:
Inqredients Per ml Per liter
1. Active ingredient 10.0 mg 10 g
Cpd of Form I
2. Isotonic buffer q.s. q.s.
solution pH 4Ø
Procedure:
Step 1. Dissolve the active ingredient in the buffer
solution.
W095102603 2 1 6 7 1 9 4 PCT~P94/02059
- 25 -
Step 2. Aseptically filter the solution from step 1.
Step 3. The sterile solution is aseptically filled
into sterile ampoules
Step 4. The ampoules are sealed under aseptic
conditions
7,f~ f r~