Note: Descriptions are shown in the official language in which they were submitted.
~TO 95/02580 PCT/EP94/02263
SUBSTITUTED 2-PHENYLPYRIDINES WITH HERBICIDAL ACTION
The present invention relates to novel substituted 2-phenylpyri-
dines of the formula I
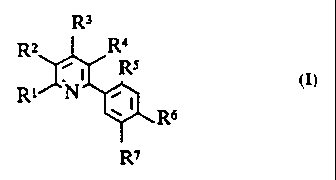
R3
R2 R4
.1 Rs
io R~ N . I
I R6
R~
in which the variables have the following meanings:
R1, R3, independently of one another,
hydrogen, halogen, Cl-C4-alkyl, Cl-C~-haloalkyl,
C1-C4-alkoxy-C1-C~-alkyl, C1-C~-alkoxy, C1-C~-alkoxy-C1-C4-alkoxy;
hydroxyl, C1-C4-haloalkoxy, (C1-C5-alkyl)carbonyloxy,
(C1-C5-Haloalkyl)carbonyloxy, SH, C1-C~-alkylthio,
C1-C4-alkylsulfinyl, C1-C~-alkylsulfonyl, C1-C4-haloalkylthio,
C1-C4-haloalkylsulfinyl, C1-C4-haloalkylsulfonyl, formyl, cyano,
hydroxycarbonyl, (C1-C~-alkoxy)carbonyl,
C1-C4-alkoxy-(C1-C4-alkoxy)carbonyl, (C1-C~-haloalkoxy)carbonyl,
(C1-C4-alkyl)carbonyl, (C1-C,~-haloalkyl)carbonyl,
C1-C4-alkoxy-(C1-C4-alkyl)carbonyl, CONH2, (C1-C4-alkyl)amino-
carbonyl,
di-(C1-C4-alkyl)aminocarbonyl, pyrrolidinylcarbonyl,
piperidinylcarbonyl, morpholinylcarbonyl, vitro, amino,
C1-C4-alkylamino, di-(C1-C4-alkyl)amino, pyrrolidinyl,
piperidinyl, morpholinyl, (C1-C4-alkyl)carbonylamino,
(C1-C4-haloalkyl)carbonylamino or C1-C,~-alkylsulfonylamino;
R2
halogen, cyano, vitro, C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, C1-C4-alkylthio, or .C1-C4-haloalkylthio
or together with R1 or with R3 a trimethylene or tetramethylene
chain;
R4
halogen, C1-C4-alkyl, C1-C4-haloalkyl, C1-C,~-alkoxy-C1-C4-alkyl,
C1-C4-alkoxy, C1-C~-alkoxy-C1-C4-alkoxy, hydroxyl,
C1-C4-haloalkoxy, (C1-C5-alkyl)carbonyloxy,
(C1-C5-haloalkyl)carbonyloxy, SH, C1-C~-alkylthio,
WO 95/02580 PCT/EP94/0226~
2
Ci-C4-alkylsulfinyl, Ci-C4-alkylsulfonyl, Ci-C4-haloalkylthio,
Ci-C~-haloalkylsulfinyl, Ci-C~-haloalkylsulfonyl, formyl, cyano,
hydroxycarbonyl, (Ci-C4-alkoxy)carbonyl,
Ci-C,~-alkoxy-(Ci-C4-alkoxy)carbonyl, (Ci-C4-haloalkoxy)carbonyl,
(Ci-C~-alkyl)carbonyl, (Ci-C,~-haloalkyl)carbonyl,
Ci-C4-alkoxy-(Ci-C4-alkyl)carbonyl, vitro, amino, C1-C4-alkylamino,
di-(Ci-C4-alkyl)amino, pyrrolidinyl, piperidinyl, morpholinyl,
(Ci-C4-alkyl)carbonylamino, (Ci-C4-haloalkyl)carbonylamino or
Ci-C4-alkylsulfonylamino;
R5
hydrogen or halogen;
Rs
halogen, cyano, vitro, hydroxyl, trifluoromethyl, Ci-C6-alkyl or
Ci-C4-alkoxy;
R~
chlorine, bromine, iodine, cyano, vitro, Ci-Ce-alkyl,
C2-Ce-alkenyl, C2-C8-alkynyl, Ci-C8-haloalkyl, C2-C8-haloalkenyl,
C2-C8-haloalkynyl, -(Ci-Ce-alkylene)-O-R8, -(C2-C8-alkenylene}-
O-R8, -(C2-C8-alkynylene)-O-Re, -(Ci-C8-alkylene)-S-R8,
-(CZ-Ce-alkenylene)-S-R8; -(C2-C8-alkynylene)-S-Re,
-(Ci-Ce-alkylene)-SO-R8, -(CZ-C8-alkenylene)-SO-R8,
-(C2-Ce-alkynylene)-SO-R8, -(Ci-C8-alkylene)-SOZ-R8,
-(C2-C8-alkenylene)-S02-R8; -(Cy-Ce-alkynylene)-SOZ-Re, -O-R8,
-S-R8, -SO-R8, -S02-R8, chlorosulfonyl, -S02-O-R8, -S02-N(R9,Rio),
-S02-NR9(CO-R12), -N(R9Rio), -NRii(CO-R12), -NRii(S02-R13)r
-N(S02_R13)(S02_Ri~), -N(S02-R13)~(CO-Ria), -NH-CO-O-R8, -O-CO-NH-R9.
-O-CO-R12, -NH-CO-NHR9, -O-CS-N(Ci-C,~-alkyl)2, -O-CS-NH2,
cyano-Ci-C,~-alkyl, -CO-O-R8, -CO-O-N=C(R26,R2~), -CO-
O-CH2-O-N=C(R3o,R3i)~ -CO-O-C(R28,R29)-CHZ-O-N=C(R3o,R31).
-CO-N(R9,Rio), -CS-N(R9,Rio), -CO-NH-S02-(Ci-C4-alkyl),
isoxazolidinylcarbonyl, formyl, -CO-R15,
hydroxycarbonyl-Ci-C6-alkyl, (Ci-C6-alkoxy)carbonyl-Ci-C6-alkyl,
_CR15=C(R1s)_CHO, -C(R15}aC(R1s)_CO_O_R8~
_C(R15)=C(Ris)_CO_N(R9~Rio)~ _C(R15)~C(R16)_CO-Ri~, -CH=N_O-R8.
-CH(XR18.YRig}, -CHy-CH(halogen)-CO-O-R8,
-CHZ-CH(halogen)-CO-N(R9,Rio), -CHy-CH(halogen)-CO-(Ci-C4-alkyl),
-CH2-CH(halogen)-CN, -C(Ci-C4-alkoxy)=N-O-R8,
-C(R15}=C(R16)-C(Ci-C4-alkoxy)~N-O-Re, -CH=CH-CH=CH-CO-O-R8,
-C(R5)= p -C(R15)=N-O-Re, -CO-OCH=N-OH, -CO-
O
OCH=N-O-(Ci-C4-alkyl), -CO-OC(Ci-C4-alkyl)=N-OH,
-CO-OC(Ci-C4-alkyl)=N-O-(Ci-C4-alkyl),
~'O 95/02580 ~ PCT/EP94102263
3
-CO-O-(Cl-C~-alkylene)-CH=N-OH,
-CO-O-(C1-C4-alkylene)-CH=N-O-(C1-C4-alkyl),
-CO-O-(C1-C,~-alkylene)-C(C1-C4-alkyl)~N-OH,
-CO-O-(C1-C,~-alkylene)-C(C1-C4-alkyl)=N-O-(C1-C~-alkyl),
-(C1-C8-alkylene)-O-CO-(C1-C~-alkyl), -CH=C$CHZ,
N N
-CH=C=CH-(Cl-C4-alkyl), -o-CH2 -CO~ -O-CH(C1-C4-alkyl) ~
O
N
_O_C ( C1_C,~-alkyl ) ~~
O '
R2o R2o
X R21 X R21
CH ~ y ~ CH ~ Y
f 23
R R2~
5_ or 6-membered heteroaryl with one to three hetero atoms
selected from a group~comprising one or two nitrogen atoms and
one oxygen or sulfur atom, it being possible for each
heteroaromatic ring atom which can be substituted to carry, if
desired, a radical selected from the group comprising vitro,
halogen, C1-C4-alkyl, CI-C4-alkoxy, C1-C4-alkylthio and
(C1-C~-alkoxy)carbonyl;
R$
hydrogen, C1-C8-alkyl, C1-Ce-haloalkyl, C4-C~-cycloalkyl,,which in
turn can carry one to three C1-C3-alkyl radicals, C3-C6-alkenyl,
C5-C~-cycloalkenyl, which in turn can carry one to three
C1-C3-alkyl radicals, C3-C6-haloalkenyl, cyano-C1-C8-alkyl,
C3-C6-alkynyl, C2-C8-alkoxyalkyl, 2-tetrahydrofuranyl-C1-CB-alkyl;
3-oxetanyl, 3-thietanyl, carboxyl-C1-C6-alkyl, (C1-C8-alkoxy)car-
bonyl-C1-C6-alkyl, (C1-C6-alkoxy)carbonyl-(C3-C~-Cycloalkyl),
C1-C4-alkoxy-(C1-C4-alkoxy)carbonyl-C1-C6-alkyl,cyclopropylmethyl,
(1-methylthiocyclopropyl)methyl, -CH(SH)-CO-OH,
-CH(SH)-CO-{C1-CB-alkoxy), -CH(C1-CB-alkylthio)-COON,
-CH(C1-C4-alkylthio)-CO-(C1-C8-alkoxy), -CH2-CO-N(R9)-Alo,
_CH(C1-C4-alkyl)-CO-N(R9)-Rlo, C(C1-C4-alkyl)2-CO-N(R9)-Rlo,
-CH2-CO-N(R9)-SOg-(C1-C~-alkyl),
-CH(CI-C4-alkyl)-CO-N(R9)-S02-(C1-C4-alkyl),
-C(C1-C4-alkyl)2-CO-N(R9)-S02-(C1-C,~-alkyl), -S-CO-NHa,
-S-CO-N(C1-C4-alkyl)-(C1-C~-alkyl),
_CHy-CO-O-(C1-C6-alkylene)-COOH,
-CH2-CO-O-(C1-C6-alkylene)-CO-(C1-C6-alkoxy),
-C(C1-C4-alkyl)2-CO-O-(C1-C6-alkylene)-COON,
WO 95/02580 ~ PCT/EP94I022E~
4
-C(Cl-Cq-alkyl)2-CO-O-(Cl-CQ-alkylene)-CO-(Cl-C6-alkoxy),
-CH(C1-Cq-alkyl)-CO-O-(C1-C6-alkylene)-COON,
-CH(C1-C4-alkyl)-CO-O-(C1-C6-alkylene)-CO-(C1-C6-alkoxy),
C3-C9-(a-alkylalkylidene)iminooxy-C1-C6-alkyl, phenyl,
phenyl-C1-C6-alkyl, phenyl-C3-C6-alkenyl, phenyl-C3-C6-alkynyl or s
phenoxy-C1-C6-alkyl, where the phenyl ring can in each case be
unsubstituted or carry one to three radicals selected from the
group comprising halogen, nitro, cyano, C1-Cq-alkyl, C1-C4-alkoxy,
C1-C4-alkylthio, C1-C4-haloalkyl and CZ-C6-alkenyl, 5- or
6-membered heteroaryl, heteroaryl-C1-C6-alkyl,
heteroaryl-C3-C6-alkenyl, heteroaryl-C3-C6-alkynyl or
heteroaryloxy-C1-C6-alkyl, where the heteroaryl radical in each
case contains one to three hetero atoms selected from a group
comprising one or two nitrogen atoms and one oxygen or sulfur
atom, and it being possible for each heteroaromatic ring atom
which can be substituted also to carry, if desired, a radical
selected from the group comprising hydroxyl, halogen, C1-Cq-alkyl,
C1-C4-alkoxy, C1-Cq-alkylthio and C1-Cq-haloalkyl;
R9 and R1° independently of one another,
hydrogen, C1-Ce-alkyl, C2-Ce-alkenyl, C3-Ce-alkynyl,
C1-C8-haloalkyl, C1-C4-alkoxy-C1-Cq-alkyl,
C1-C4-alkylthio-C1-C4-alkyl, cyano-C1-C$-alkyl,
carboxyl-C1-CQ-alkyl, (C1-CQ-alkoxy)carbonyl-C1-CQ-alkyl,
(C1-C6-alkoxy)carbonyl- (C3-C~-cycloalkyl),
C1-Cq-alkylsulfonyl-C1-C4-alkyl, C3-Ca-cycloalkyl, C1-C6-alkoxy,
(C3-C6-cycloalkoxy)carbonyl-C1-CQ-alkyl,
C1-CQ-alkoxy-(C1-Cq-alkoxy)carbonyl-C1-CQ-alkyl, phenyl,
phenyl-C1-Cq-alkyl, where the phenyl ring can in each case be
unsubstituted or carry one to three radicals selected from the
group comprising halogen, nitro, cyano, C1-C4-alkyl, C1-CQ-alkoxy,
C1-Cq-alkylthio, C1-C9-haloalkyl and C2-C6-alkenyl, 5- or
6-membered heteroaryl or heteroaryl-C1-C4-alkyl, where the
heteroaryl radical contains one to three hetero atoms selected
from a group comprising one or two nitrogen atoms and one oxygen
or sulfur atom, and it being possible for each heteroaromatic
ring atom which can be substituted also, if desired, to carry a
radical selected from the group comprising hydroxyl, halogen,
C1-C9-alkyl, C1-C4-alkoxy, C1-CQ-alkylthio and C1-C4-haloalkyl;
or
R9 and R1° together a tetramethylene, pentamethylene or
ethylerieoxyethylene chain, it being possible for each chain to
carry, if desired, a (C1-C6-alkoxy)carbonyl radical;
~O 95/02580 ~ ~ ~ PCTIEP94/02263
Rll
hydrogen, Ci-Cq-alkyl, C3-C6-alkenyl, C3-C6-alkynyl,
Ci-Cq-alkoxy-Ci-Cq-alkyl, sodium, potassium, calcium, magnesium,
ammonium or ammonium which is substituted by one to four
5 Ci-Cq-alkyl- or benzyl radicals and can, if desired, carry one to
three further Ci-C4-alkyl radicals:
R12
hydrogen, Ci-C6-alkyl, Ci-C6-haloalkyl, Ci-Cq-alkoxy-
Ci-Cq-alkyl, C3-C~-cycloalkyl, which can in turn carry one to
three radicals selected from the group comprising halogen,
Ci-Cq-alkyl, Ci-Cq-alkoxy and Ci-Cq-alkylthio, phenyl or
phenyl-Ci-C6-alkyl, where the phenyl ring can in each case be
unsubstituted or carry one to three radicals selected from the
group comprising halogen, vitro, Ci-Cq-alkyl, Ci-Cq-alkoxy,
Ci-Cq-alkylthio and Ci-Cq-haloalkyl;
R13 and Riq, independently of one another,
Ci-Cq-alkyl, phenyl or thienyl, where the phenyl or thienyl
radical can be unsubstituted or carry one to three radicals
selected from the group comprising halogen, vitro, Ci-Cq-alkyl,
Ci-Cq-alkoxy, Ci-Cq-alkylthio and Ci-Cq-haloalkyl;
RiS, R16 and Rl~, independently of one another,
hydrogen, halogen, Ci-Cq-alkyl, C2-Cq-alkenyl, Ci-Cq-haloalkyl,
Ci-Cq-alkoxy-Ci-Cq-alkyl or Ci-C4-alkylthio-Ci-Cq-alkyl:
Rie and Ri9, independently of one another,
C1-CB-alkyl, Ci-Cq-alkoxy-Ci-C4-alkyl or Ci-C8-haloalkyl;
R20 ~ R21 ~ R22 ~ R23 ~ R24 and R25, independently of one another,
hydrogen, cyano, Ci-C8-alkyl, Ci-Cq-alkoxy-Ci-Cq-alkyl,
halo-Ci-Cg-alkyl, Ci-C8-alkoxy, Ci-Cq-alkoxy-Ci-Cq-alkoxy, -CO-
O-R8, -CO-N(R9, Rio), -CO-Ris, -S-R8, -S02-Re, -O-CO-R12 or
C3-C~-cycloalkyl, Which can in turn carry from one to three
radicals selected from the group comprising halogen, Ci-Cq-alkyl,
Ci-Cq-alkoxy and Ci-Cq-alkylthio;
R26
Ci-C6-alkyl, Ci-C6-alkylthio, Ci-C6-alkoxycarbonyl or
Ci-C6-alkoxycarbonyl-Ci-CQ-alkyl;
R2~
Ci-C6-alkyl, trifluoromethyl, Ci-C6-alkoxy-Ci-Cq-alkyl,
C2-C~-alkoxycarbonyl-Ci-Cq-alkyl,
di-(Ci-C6-alkoxycarbonyl)-Ci-Cq-alkyl, C3-C6-cycloalkyl,
Ci-C6-alkoxy, Ci-C6-alkylthio, Ci-C6-alkanoyl,
CA 02167291 2004-04-15
6
C1-C6-alkoxycarbonyl, 2-furyl or phenyl which can be unsubstituted
or in turn carry one to three radicals selected from the group
comprising halogen, C1-CQ-alkyl and C1-CQ-alkoxy;
or
R26 and R2~ together with the carbon to which they are bonded a
cyclopentane or cyclohexane ring which can in turn, if desired,
carry one to three C1-Cq-alkyl radicals:
R28
hydrogen or C1-Cq-alkyl;
R2 9
hydrogen, C1-Cq-alkyl, phenyl or benzyl:
R3o
hydrogen or C1-C6-alkyl;
R31
C1-C6-alkyl, C3-C6-cycloalkyl or phenyl;
X and Y, independently of one another,
oxygen or sulfur:
and the N-oxides of I and the agriculturally utilizable salts of
I where these exist,
excepting those compounds I where R2 is C1-Cq-alkoxy and R1 and/or
R3 is carboxyl, its salt, ester or amide.
More particularly, the present invention is directed to
substituted 2-phenylpyridines of the formula I, wherein
Rl is hydrogen, methyl, methoxy, methylthio or
halogen;
R2 is halogen, Cl-Cq-haloalkyl with one to five
halogen atoms or Cl-C4-haloalkoxy with one to five
halogen atoms;
R4 is methyl, methoxy, methylthio or halogen;
CA 02167291 2004-04-15
6a
and R3 as well R5 to R31 and X and Y have the meanings
described above.
The invention furthermore relates to
- the use of the compounds I, their N-oxides and/or agricultur-
ally utilizable salts, as herbicides and for the desiccation
and/or defoliation of plants,
- herbicidal compositions and compositions for the desiccation
and/or defoliation of plants, which contain the compounds I,
their N-oxides and/or agriculturally utilizable salts, as
active substances,
- processes for the production of these herbicidal compositions
and compositions for the desiccation and/or defoliation of
plants,
~O 95/0258 PCT/EP94/02263
7
- methods for controlling unwanted plant growth and for the
desiccation and/or defoliation of plants using the compounds
I or compounds I' where I' corresponds to the formula I with-
out the disclaimer and R4 may additionally be aminocarbonyl,
(C1-C4-alkyl)aminocarbonyl, di-(C1-C4-alkyl)aminocarbonyl,
pyrrolidinylcarbonyl, piperidinylcarbonyl or morpholinylcar-
bonyl, as well as the N-oxides and the agriculturally utiliz-
e able salts of I and I', and
- processes for the preparation of the compounds I.
The invention additionally relates to the use of phenylpyridines
of the formula IV
R3
RZ R4
. ~ Rs
R N ~' IV
Rs
and of aromatic boronic acids or esters thereof of the formula
IIIa
8340
R5'
R33 ~ H ~ IIIa
Rs.
R~'
where
RS' is hydrogen, fluorine or chlorine;
R6' is halogen, hydroxyl or C1-C~-alkoxy;
R~' is hydrogen, C1-C4-alkyl or C1-C4-alkoxy;
R33 and R34 are, independently of one another, hydrogen or
C1-C4-alkyl or together are ethylene or propylene,
CA 02167291 2004-04-15
8
as intermediates for the preparation of the substituted
2-phenylpyridines I and
to novel aromatic boronic acids and esters thereof of the formula
IIIa'
R3q0
Rg.
Rss O~B i IIIa'
Halogen
lower alkyl
where
R5' is hydrogen, fluorine or chlorine;
halogen is a halogen atom;
lower alkyl is C1-Cq-alkyl and
R33 and R3q are, independently of one another, hydrogen or
C1-C9-alkyl or together are ethylene or propylene.
Some 2-phenylpyridines have previously been disclosed in the
following publications: EP-A 412 681: WO 94/05153; WO '~ /10118;
WO 92/22203; CA 114(11), 96724k: Izv. Timiryazevsk.S-Kh. Akad. 3,
155-160; Pestic. Sci. 2~(3), 175-179.
Highly fluorinated 2-phenylpyridines are provided as
intermediates for drugs and agrochemicals in T, Konakahara et
al., Nippon Kagaku Kaishi, (5), 466-71 {CA 113 (19): 171 837 j)
and der JP 12 11 586:
~3
F3C
F Id
, I
F3C-Fz N i I
Ra
l5
where Ra is hydrogen, dimethylamino, chlorine, methoxy or methyl.
~O 95/02580 PCT/EP94/02263
9
EP-A 167 491 discloses substituted thiobarbituric acids, eg. '
s F3C ~ ~ F3C ~ C1 H O O
O O I
N ~ ~ N NCH3 'N ~ I N N.CH3
H~~S H3C0 O N S
CH3 CH3
P. Boy et al. (Synlett 12, 923) disclose the preparation of
4-[(trifluoromethyl)pyridyl~phenols:
Rb
F3C
2s Rc
Rb ~ hydrogen or trifluoromethyl; R~ = hydrogen or tert-butyl.
N. Katagiri et al. (Chew. Pharm. Hull. ~ (9), 3354-72) describe
the preparation of substituted 2-phenylpyridines:
R
F3C ~ Rf
. I
Rd N
CH3
where Rd is hydrogen, chlorine or methoxy, Re is hydrogen, methyl,
ethyl or ethoxy and Rf is hydrogen or methyl or Re and Rf together
are (CHy)3 or (CH2)~.
WO 95/02580 r PCT/EP94/022~
Finally, DE-A 40 20 257 discloses 2,6-diarylpyridine derivatives
with herbicidal and defoliating properties:
R
5 ,
Rg Ri
20
where Rg and Rh are hydrogen, halogen, alkyl, alkoxy or haloalkyl,
Ri is hydrogen, halogen, cyano, alkyl, alkoxy or haloalkyl and Rk
and R1 are hydrogen or alkyl.
Those known compounds which in fact have a herbicidal,
defoliating, pesticidal or fungicidal action are not always
completely satisfactory.
It was an object of the present invention,to provide novel
compounds which have, in particular, herbicidal activity and
which can be used for the targeted control of unwanted plants
better than hitherto.
Accordingly, we have found that this object is achieved by the
present substituted 2-phenylpyridines of the formula I and the
compounds I'. We have also found herbicidal compositions which
contain the compounds I and have a good herbicidal action. They
are tolerated or selective, preferably in graminaceous crops such
as wheat, corn and rice.
We have also found processes for the production of these
herbicidal compositions. We have additionally found novel
intermediates of the formula IIIa' for preparing the substituted
2-phenylpyridines I.
The compounds I and I' according to the invention are furthermore
suitable for the defoliation and desiccation of parts of plants
for, for example, cotton, potato, rape, sunflower, soybean or
broad beans.
The organic moieties specified above for the substituents R1 to
R34 or as substituents on (hetero)aromatic radicals represent,
like the meaning of halogen, collective terms for individual
lists of the individual group members. All the carbon chains, ie.
all alkyl, alkenyl, alkynyl, haloalkyl, haloalkenyl, haloalkynyl
s
~O 95/02580 .~ pCTlEP94/02263
I1
and haloalkoxy moieties and the a-alkylalkylidene moiety, can be
straight-chain or branched. Halogenated substituents preferably
have one to five identical or different halogen atoms.
Examples of specific meanings are:
- Halogen: fluorine, chlorine, bromine and iodine, preferably
fluorine and chlorine;
- C1-C4-Alkyl: methyl, ethyl, n-propyl, 1-methylethyl, n-butyl,
1-methylpropyl, 2-methylpropyl and 1,1-dimethylethyl;
C1-C6-Alkyl: C1-C4-alkyl as mentioned above, and n-pentyl,
1-methylbutyl, 2-methylbutyl, 3-methylbutyl,
2,2-dimethylpropyl, 1-ethylpropyl, n-hexyl,
1,1-dimethylpropyl, 1,2-dimethylpropyl, 1-methylpentyl,
2-methylpentyl, 3-methylpentyl, 4-methylpentyl,
1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl,
2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl,
1-ethylbutyl, 2-ethylbutyl, 1,1,2-trimethylpropyl,
1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl and
1-ethyl-2-methylpropyl;
- C1-C8-Alkyl: C1-C6-alkyl as mentioned above, and, inter alia,
n-heptyl, n-octyl;
- CZ-C4-Alkenyl: ethenyl, prop-1-en-1-yl, prop-2-en-1-yl,
1-methylethenyl, n-buten-1-yl, n-buten-2-yl, n-buten-3-yl,
1-methyl-prop-1-en-1-yl, 2-methyl-prop-1-en-1-yl,
1-methyl-prop-2-en-1-yl and 2-methyl-prop-2-en-1-yl;
- C3-C6-Alkenyl: prop-1-en-1-yl, prop-2-en-1-yl,
1-methylethenyl, n-buten-1-yl, n-buten-2-yl, n-buten-3-yl,
1-methyl-prop-1-en-1-yl, 2-methyl-prop-1-en-1-yl,
1-methyl-prop-2-en-1-yl, 2-methyl-prop-2-en-1-yl,
n-penten-1-yl, n-penten-2-yl, n-penten-3-yl, n-penten-4-yl,
1-methyl-but-1-en-1-yl, 2-methyl-but-1-en-1-yl,
3-methyl-but-1-en-1-yl, 1-methyl-but-2-en-1-yl,
2-methyl-but-2-en-1-yl, 3-methyl-but-2-en-1-yl,
1-methyl-but-3-en-1-yl, 2-methyl-but-3-en-1-yl,
3-methyl-but-3-en-1-yl, 1,1-dimethyl-prop-2-en-1-yl,
1,2-dimethyl-prop-1-en-1-yl, 1,2-dimethyl-prop-2-en-1-yl,
1-ethyl-prop-1-en-2-yl, 1-ethyl-prop-2-en-1-yl,
n-hex-1-en-1-yl, n-hex-2-en-1-yl, n-hex-3-en-1-yl,
n-hex-4-en-1-yl, n-hex-5-en-1-yl, 1-methyl-pent-1-en-1-yl,
2-methyl-pent-1-en-1-yl, 3-methyl-pent-1-en-1-yl,
4-methyl-pent-1-en-1-yl, 1-methyl-pent-2-en-1-yl,
WO 95/02580 ~ ~ PCT/EP94/022~
12
2-methyl-pent-2-en-1-yl, 3-methyl-pent-2-en-1-yl, 4-methyl-
pent-2-en-1-yl, 1-methyl-pent-3-en-1-yl,
2-methyl-pent-3-en-1-yl, 3-methyl-pent-3-en-1-yl,
4-methyl-pent-3-en-1-yl, 1-methyl-pent-4-en-1-yl,
2-methyl-pent-4-en-1-yl, 3-methyl-pent-4-en-1-yl,
4-methyl-pent-4-en-1-yl, 1,1-dimethyl-but-2-en-1-yl,
1,1-dimethyl-but-3-en-1-yl, 1,2-dimethyl-but-1-en-1-yl,
1,2-dimethyl-but-2-en-1-yl, 1,2-dimethyl-but-3-en-1-yl,
1,3-dimethyl-but-1-en-1-yl, 1,3-dimethyl-but-2-en-1-yl,
1,3-dimethyl-but-3-en-1-yl, 2,2-dimethyl-but-3-en-1-yl,
2,3-dimethyl-but-1-en-1-yl, 2,3-dimethyl-but-2-en-1-yl,
2,3-dimethyl-but-3-en-1-yl, 3,3-dimethyl-but-1-en-1-yl,
3,3-dimethyl-but-2-en-1-yl, 1-ethyl-but-1-en-1-yl,
1-ethyl-but-2-en-1-yl, 1-ethyl-but-3-en-1-yl,
2-ethyl-but-1-en-1-yl, 2-ethyl-but-2-en-1-yl,
2-ethyl-but-3-en-1-yl, 1,1,2-trimethylprop-2-en-1-yl,
1-ethyl-1-methyl-prop-2-en-1-yl,
1-ethyl-2-methyl-prop-1-en-1-yl and
1-ethyl-2-methyl-prop-2-en-1-yl, preferably ethenyl and
prop-2-en-1-yl;
C2-Ce-Alkenyls ethenyl, C3-C6-alkenyl as mentioned above and,
inter alia, n-hept-1-en-1-yl, n-kept-2-en-1-yl,
n-kept-3-en-1-yl, n-hept-4-en-1-yl, n-hept-5-en-1-yl,
n-kept-6-en-1-yl, n-oct-1-en-1-yl, n-oct-2-en-1-yl,
n-oct-3-en-1-yl, n-oct-4-en-1-yl, n-oct-5-en-1-yl,
n-oct-6-en-1-yl and n-oct-7-en-1-yl;
- C2-C6-Alkynyl: ethynyl and C3-C6-alkynyl such as
prop-1-yn-1-yl, prop-2-yn-3-yl, n-but-1-yn-1-yl,
n-but-1-yn-4-yl, n-but-2-yn-1-yl, n-pent-1-yn-1-yl,
n-pent-1-yn-3-yl, n-pent-1-yn-4-yl, n-pent-1-yn-5-yl,
n-pent-2-yn-1-yl, n-pent-2-yn-4-yl, n-pent-2-yn-5-yl,
3-methyl-but-1-yn-1-yl, 3-methyl-but-1-yn-3-yl,
3-methyl-but-1-yn-4-yl, n-hex-1-yn-1-yl, n-hex-1-yn-3-yl,
n-hex-1-yn-4-yl, n-hex-1-yn-5-yl, n-hex-1-yn-6-yl,
n-hex-2-yn-1-yl, n-hex-2-yn-4-yl, n-hex-2-yn-5-yl,
n-hex-2-yn-6-yl, n-hex-3-yn-1-yl, n-hex-3-yn-2-yl,
3-methyl-pent-1-yn-1-yl, 3-methyl-pent-1-yn-3-yl,
3-methyl-pent-1-yn-4-yl, 3-methyl-pent-1-yn-5-yl,
4-methyl-pent-1-yn-1-yl, 4-methyl-pent-2-yn-4-yl and
4-methyl-pent-2-yn-5-yl, preferably prop-2-yn-1-yl and
1-methyl-prop-2-yn-1-yl;
- Ca-C8-Alkynyl: ethynyl, C3-C6-Alkynyl as mentioned above and,
inter alia, n-hept-1-yn-1-yl, n-hept-2-yn-1-yl,
n-hept-3-yn-1-yl, n-kept-4-yn-1-yl, n-kept-5-yn-1-yl,
O 95/02580
PCT/EP94/02263
13
n-hept-6-yn-1-yl, n-oct-1-yn-1-yl, n-oct-2-yn-1-yl,
n-oct-3-yn-1-yl, n-oct-4-yn-1-yl, n-oct-5-yn-1-yl,
n-oct-6-yn-1-yl and n-oct-7-yn-1-yl;
- C3-C6-Haloalkenyls C3-C6-alkenyl as mentioned above with in
each case one to three hydrogen atoms being replaced by
fluorine, chlorine and/or bromine;
- CZ-C8-Haloalkenyl: C2-C8-alkenyl as mentioned above with in
each case one to three hydrogen atoms being replaced by
fluorine, chlorine and/or bromine;
- CZ-C8-Halolkynyls C2-Ce-alkynyl as mentioned above with in
each case one to three hydrogen atoms being replaced by
fluorine, chlorine and/or bromine;
- C3-C6-Cycloalkyls cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl, preferably cyclopropyl, cylopentyl and
cyclohexyl;
- C~-C~-Cycloalkyl: cyclobutyl, cyclopentyl, cyclohexyl and
cycloheptyl, preferably cylopentyl and cyclohexyl;
- C5-C~-Cycloalkenyl eg.s cyclopent-1-enyl, cyclopent-2-enyl,
cyclopent-3-enyl, cyclohex-1-enyl, cyclohex-2-enyl,
cyclohex-3-enyl, cyclohept-1-enyl, cyclohept-2-enyl,
cyclohept-3-enyl and cyclohept-4-enyl;
- (C3-C6-Cycloalkoxy)carbonyl: cyclopropoxycarbonyl,
cyclobutoxycarbonyl, cyclopentoxycarbonyl and
cyclohexoxycarbonyl, preferably cyclopropoxycarbonyl,
cyclopentoxycarbonyl and cyclohexoxycarbonyl;
- C1-C~-Haloalkyl: C1-C4-alkyl as mentioned above, which is
partially or completely substituted by fluorine, chlorine
and/or bromine eg. chloromethyl, dichloromethyl,
trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, chlorofluoromethyl, dichlorofluoromethyl,
chlorodifluoromethyl, 1-fluoroethyl, 2-fluoroethyl,
2,2-difluoroethyl, 2,2,2-trifluoroethyl,
2-chloro-2-fluoroethyl, 2-chloro-2,2-difluoroethyl,
2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl,
pentafluoroethyl and 3-chloropropyl, preferably
trifluoromethyl;
WO 95/02580 ~a PCTIEP94/022~
14
- C1-C6-Haloalkyls C1-C6-alkyl as mentioned above, which is
partially or completely substituted by fluorine, chlorine
and/or bromine;
- C1-CB-Haloalkyls C1-C8-alkyl as mentioned above, which is
partially or completely substituted by fluorine, chlorine
and/or bromine eg. the abovementioned C1-C4-haloalkyls;
- Cyano-C1-C8-alkyl: C1-Ca-alkyl as mentioned above, With in
10~ each case one hydrogen atom being replaced by the cyano
group, eg. cyanomethyl, 1-cyanoeth-1-yl, 2-cyanoeth-1-yl,
1-cyano-prop-1-yl, 2-cyano-prop-1-yl, 3-cyano-prop-1-yl,
1-cyano-prop-2-yl, 2-cyano-prop-2-yl, 1-cyano-but-1-yl,
2-cyano-but-1-yl, 3-cyano-but-1-yl, 4-cyano-but-1-yl,
1-cyano-but-2-yl, 2-cyano-but-2-yl, 1-cyano-but-3-yl,
2-cyano-but-3-yl, 1-cyano-2-methyl-prop-3-yl,
2-cyano-2-methyl-prop-3-yl, 3-cyano-2-methyl-prop-3-yl, and
2-cyanomethyl-prop-2-yl, preferably cyanomethyl and
1-cyano-1-methylethyl;
- Phenyl-C1-C4-alkyl: C1-C~-alkyl as mentioned above, with in
each case one hydrogen atom being replaced by the phenyl
group, eg. benzyl, 1-phenylethyl, 2-phenylethyl,
1-phenylprop-1-yl, 2-phenylprop-1-yl, 3-phenylprop-I-yl,
1-phenylbut-1-yl, 2-phenylbut-1-yl, 3-phenylbut-1-yl,
4-phenylbut-1-yl, 1-phenylbut-2-yl, 2-phenylbut-2-yl,
3-phenylbut-2-yl, 3-phenylbut-2-yl, 4-phenylbut-2-yl,
1-(phenylmethyl)-eth-1-yl,
1-(phenylmethyl)-1-(methyl)-eth-1-yl and
1-(phenylmethyl)-prop-1-yl, preferably benzyl;
- Phenyl-C1-C6-alkyls C1-C6-alkyl as mentioned above, with in
each case a hydrogen atom being replaced by the phenyl group,
eg. the abovementioned phenyl-C1-C~-alkyls;
- Phenyl-C3-C6-alkenyl: C3-C6-alkenyl as mentioned above, with
one hydrogen atom in each case being replaced by the phenyl
group;
- Phenyl-C3-C6-alkynyl: C3-C6-alkynyl as mentioned above, with
one hydrogen atom in each case being replaced by the phenyl
group;
- C1-C4-Alkoxy: methoxy, ethoxy, n-propoxy, 1-methylethoxy,
n-butoxy, 1-methylpropoxy, 2-methylpropoxy and
1,1-dimethylethoxy, preferably methoxy, ethoxy and
1-methylethoxy;
~'O 95/02580 ~~ PCTlEP94/02263
.
C1-C6-Alkoxy: C1-C~-alkoxy as mentioned above, and n-pentoxy,
1-methylbutoxy, 2-methylbutoxy, 3-methylbutoxy,
l,l-dimethylpropoxy, 1,2-dimethylpropoxy,
5 2,2-dimethylpropoxy, 1-ethylpropoxy, n-hexoxy,
1-methylpentoxy, 2-methylpentoxy, 3-methylpentoxy,
4-methylpentoxy, 1,1-dimethylbutoxy, 1,2-dimethylbutoxy,
1,3-dimethylbutoxy, 2,2-dimethylbutoxy, 2,3-dimethylbutoxy,
3,3-dimethylbutoxy, 1-ethylbutoxy, 2-ethylbutoxy,
10 1,1,2-trimethylpropoxy, 1,2,2-trimethylpropoxy,
1-ethyl-1-methylpropoxy and 1-ethyl-2-methylpropoxy;
- C1-Cg-Alkoxy: C1-C6-alkoxy as mentioned above, and, for
example, n-heptoxy and n-octoxy;
- C1-C4-Haloalkoxy: C1-C4-alkoxy as mentioned above, which is
partially or completely substituted by fluorine, chlorine
and/or bromine, eg. chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy,
trifluoromethoxy, chlorofluoromethoxy, dichlorofluoromethoxy;
chlorodifluoromethoxy, 1-fluoroethoxy, 2-fluoroethoxy,
2,2-difluoroethoxy, 2,2,2-trifluoroethoxy,
2-chloro-2-fluoroethoxy, 2-chloro-2,2-difluoroethoxy,
2,2-dichloro-2-fluoroethoxy, 2,2,2-trichloroethoxy and
pentafluoroethoxy, preferably C1-C2-haloalkoxy such as
trifluoromethoxy;
- C1-C4-Alkylthio: methylthio, ethylthio, n-propylthio,
1-methylethylthio, n-butylthio, 1-methyl-propylthio,
2-methylpropylthio and 1,1-dimethylethylthio, preferably
methylthio, ethylthio and methylethylthio;
- C1-C4-Haloalkylthio: chloromethylthio, dichloromethylthio,
trichloromethylthio, fluoromethylthio, difluoromethylthio,
trifluoromethylthio, chlorofluoromethylthio,
dichlorofluoromethylthio, chlorodifluoromethylthio,
1-fluoroethylthio, 2-fluoroethylthio, 2,2-difluoroethylthio,
2,2,2-trifluoroethylthio, 2-chloro-2-fluoroethylthio,
2-chloro-2,2-difluoroethylthio,
2,2-dichloro-2-fluoroethylthio, 2,2,2-trichloroethylthio and
pentafluoroethylthio, preferably C1-C2-haloalkylthio such as
trifluoromethylthio;
- C3-C6-Alkenyloxy: prop-1-en-1-yloxy, prop-2-en-1-yloxy,
1-methylethenyloxy, n-buten-1-yloxy, n-buten-2-yloxy,
n-buten-3-yloxy, 1-methyl-prop-1-en-1-yloxy,
2-methyl-prop-1-en-1-yloxy, 1-methyl-prop-2-en-1-yloxy,
WO 95/02580 ' ~ ~ ~ ~ . PCT/EP94/022~
16
2-methyl-prop-2-en-1-yloxy, n-penten-1-yloxy,
n-penten-2-yloxy, n-penten-3-yloxy, n-penten-4-yloxy,
1-methyl-but-1-en-1-yloxy, 2-methyl-but-1-en-1-yloxy,
3-methyl-but-1-en-1-yloxy, 1-methyl-but-2-en-1-yloxy,
2-methyl-but-2-en-1-yloxy, 3-methyl-but-2-en-1-yloxy,
1-methyl-but-3-en-1-yloxy, 2-methyl-but-3-en-1-yloxy,
3-methyl-but-3-en-1-yloxy, 1,1-dimethyl-prop-2-en-1-yloxy,
1,2-dimethyl-prop-1-en-1-yloxy,
1,2-dimethyl-prop-2-en-1-yloxy, 1-ethyl-prop-1-en-2-yloxy,
1-ethyl-prop-2-en-1-yloxy, n-hex-1-en-1-yloxy,
n-hex-2-en-1-yloxy, n-hex-3-en-1-yloxy, n-hex-4-en-1-yloxy,
n-hex-5-en-1-yloxy, 1-methyl-pent-1-en-1-yloxy,
2-methyl-pent-1-en-1-yloxy, 3-methyl-pent-1-en-1-yloxy,
4-methyl-pent-1-en-1-yloxy, 1-methyl-pent-2-en-1-yloxy,
2-methyl-pent-2-en-1-yloxy, 3-methyl-pent-2-en-1-yloxy,
4-methyl-pent-2-en-1-yloxy, 1-methyl-pent-3-en-1-yloxy,
2-methyl-pent-3-en-1-yloxy, 3-methyl-pent-3-en-1-yloxy,
4-methyl-pent-3-en-1-yloxy, 1-methyl-pent-4-en-1-yloxy,
2-methyl-pent-4-en-1-yloxy, 3-methyl-pent-4-en-1-yloxy,
4-methyl-pent-4-en-1-yloxy, 1,1-dimethyl-but-2-en-1-yloxy,
1,1-dimethyl-but-3-en-1-yloxy,l,2-dimethyl-but-1-en-1-yloxy,
1,2-dimethyl-but-2-en-1-yloxy,l,2-dimethyl-but-3-en-1-yloxy,
1,3-dimethyl-but-1-en-1-yloxy,l,3-dimethyl-but-2-en-1-yloxy,
1,3-d3.methyl-but-3-en-1-yloxy,2,2-dimethyl-but-3-en-1-yloxy,
2,3-dimethyl-but-1-en-1-yloxy,2,3-dimethyl-but-2-en-1-yloxy,
2,3-dimethyl-but-3-en-1-yloxy,3,3-dimethyl-but-1-en-1-yloxy,
3,3-dimethyl-but-2-en-1-yloxy, 1-ethyl-but-1-en-1-yloxy,
1-ethyl-but-2-en-1-yloxy, 1-ethyl-but-3-en-1-yloxy,
2-ethyl-but-1-en-1-yloxy, 2-ethyl-but-2-en-1-yloxy,
2-ethyl-but-3-en-1-yloxy, 1,1,2-trimethylprop-2-en-1-yloxy,
1-ethyl-1-methyl-prop-2-en-1-yloxy,
1-ethyl-2-methyl-prop-1-en-1-yloxy and
1-ethyl-2-methyl-prop-2-en-1-yloxy, preferably ethenyloxy and
prop-2-en-1-yloxy;
- Phenoxy-C1-C~-alkyls phenoxymethyl, 1-phenoxyethyl,
2-phenoxyethyl, 1-phenoxyprop-1-yl, 2-phenoxyprop-I-yl,
3-phenoxyprop-1-yl, 1-phenoxybut-1-yl, 2-phenoxybut-1-yl,
3-phenoxybut-1-yl, 4-phenoxybut-1-yl, 1-phenoxybut-2-yl,
2-phenoxybut-2-yl, 3-phenoxybut-2-yl, 4-phenoxybut-2-yl,
1-(phenoxymethyl)-eth-1-yl, 1-(phenoxymethyl)-1-(methyl)-
eth-1-yl and 1-(phenoxymethyl)-prop-1-yl, preferably
phenoxymethyl;
.~,~~r~,
~'O 95/02580 .~~ PCTIEP94/02263
17
- C1-C4-Alkylamino: methylamino, ethylamino, n-propylamino,
1-methylethylamino, n-butylamino, 1-methylpropylamino,
2-methylpropylamino and 1,1-dimethylethylamino, preferably
methylamino and ethylamino;
- Di-(C1-C4-alkyl)amino: N,N-dimethylamino, N,N-diethylamino,
N,N-dipropylamino, N,N-di-(1-methylethyl)amino,
N,N-dibutylamino, N,N-di-(1-methylpropyl)amino,
N,N-di-(2-methylpropyl)amino, N,N-di-(1,1-dimethyl-
ethyl)amino, N-ethyl-N-methylamino, N-methyl-N-propylamino,
N-methyl-N-(1-methylethyl)amino, N-butyl-N-methylamino,
N-methyl-N-(1-methylpropyl)amino, N-methyl-N-(2-methyl-
propyl)amino, N-(1,1-dimethylethyl)-N-methylamino,
N-ethyl-N-propylamino, N-ethyl-N-(1-methylethyl)amino,
N-butyl-N-ethylamino, N-ethyl-N-(1-methyl-propyl)amino,
N-ethyl-N-(2-methylpropyl)amino, N-ethyl-N-(l,l-dimethyl-
ethyl)amino, N-(1-methylethyl)-N-propylamino,
N-butyl-N-propylamino, N-(1-methylpropyl)-N-propylarnino,
N-(2-methylpropyl)-N-propylamino, N-(1,1-dimethylethyl)-N-
propylamino, N-butyl-N-(1-methylethyl)amino,
N-(1-methylethyl)-N-(1-methylpropyl)amino,
N-(1-methylethyl)-N-(2-methylpropyl)amino,
N-(1,1-dimethylethyl)-N-(1-methylethyl)amino,
N-butyl-N-(1-methylpropyl)amino, N-butyl-N-(2-methyl-
propyl)amino, N-butyl-N-(1,1-dimethylethyl)amino,
N-(1-methylpropyl)-N-(2-methylpropyl)amino,
N-(1,1-dimethylethyl)-N-(1-methylpropyl)amino and
N-(1,1-dimethylethyl)-N-(2-methylpropyl)amino, preferably
dimethylamino and diethylamino;
- C1-C,~-Alkylaminocarbonyl: methylaminocarbonyl,
ethylaminocarbonyl, n-propylaminocarbonyl,
1-methylethylaminocarbonyl, n-butylaminocarbonyl,
1-methylpropylaminocarbonyl, 2-methylpropylaminocarbonyl and
1,1-dimethylethylaminocarbonyl, preferably
methylaminocarbonyl and ethylaminocarbonyl;
- Di-(C1-C4-alkyl)aminocarbonyl: N,N-dimethylaminocarbonyl,
N,N-diethylaminocarbonyl, N,N-dipropylaminocarbonyl,
N,N-di-(1-methylethyl)aminocarbonyl,
N,N-dibutylaminocarbonyl, N,N-di-(1-methylpropyl)amino-
carbonyl, N,N-di-(2-methylpropyl)aminocarbonyl,
N,N-di-(1,1-dimethylethyl)aminocarbonyl,
N-ethyl-N-methylaminocarbonyl,
N-methyl-N-propylaminocarbonyl, N-methyl-N-(1-methyl-
ethyl)aminocarbonyl, N-butyl-N-methylaminocarbonyl, N-methyl-
N-(1-methylpropyl)aminocarbonyl, N-methyl-N-(2-methyl-
WO 95/02580 PCT/EP94/022
18
propyl)aminocarbonyl, N-(1,1-dimethylethyl)-N-methylamino-
carbonyl, N-ethyl-N-propylaminocarbonyl, N-ethyl-N-(1-methyl-
ethyl)aminocarbonyl, N-butyl-N-ethylaminocarbonyl, N-ethyl-
N-(1-methylpropyl)aminocarbonyl, N-ethyl-N-(2-methyl-
propyl)aminocarbonyl, N-ethyl-N-(1,1-dimethylethyl)amino-
carbonyl, N-(1-methylethyl)-N-propylami.nocarbonyl,
N-butyl-N-propylaminocarbonyl, N-(1-methylpropyl)-N-propyl-
aminocarbonyl, N-(2-methylpropyl)-N-propylaminocarbonyl,
N-(1,1-dimethylethyl)-N-propylami.nocarbonyl, N-butyl-
N-(1-methyl-ethyl)aminocarbonyl, N-(1-methylethyl)-
N-(1-methylpropyl)aminocarbonyl, N-(1-methylethyl)-
N-(2-methylpropyl)aminocarbonyl, N-(1,1-di-methylethyl)-
N-(1-methylethyl)aminocarbonyl, N-butyl-N-(1-methyl-
propyl)aminocarbonyl, N-butyl-N-(2-methylpropyl)amino-
carbonyl, N-butyl-N-(1,1-dimethylethyl)aminocarbonyl,
N-(1-methylpropyl)-N-(2-methylpropyl)aminocarbonyl,
N-(1,1-dimethylethyl)-N-(1-methylpropyl)aminocarbonyl and
N-(1,1-dimethylethyl)-N-(2-methylpropyl)aminocarbonyl,
preferably dimethylaminocarbonyl and diethylaminocarbonyl;
- C1-C4-Alkylsulfonyls methylsulfonyl, ethylsulfonyl,
n-propylsulfonyl, 1-methylethyl-sulfonyl, n-butylsulfonyl,
1-methylpropylsulfonyl, 2-methylpropylsulfonyl and
1,1-dimethylethylsulfonyl;
30
- C1-C~-Alkylsulfinyl: methylsulfinyl, ethylsulfinyl,
n-propylsulfinyl, 1-methylethyl-sulfinyl, n-butylsulfinyl,
1-methylpropylsulfinyl, 2-methylpropylsulfinyl and
1,1-dimethylethylsulfinyl;
- C1-C~-Alkylsulfonylamino: methylsulfonylamino,
ethylsulfonylamino, n-propylsulfonylamino,
1-methylethyl-sulfonylamino, n-butylsulfonylamino,
1-methylpropylsulfonylamino, 2-methylpropylsulfonylamino and
1,1-dimethylethylsulfonylamino;
- C1-C4-Haloalkylsulfonyl: C1-C4-alkylsulfonyl as mentioned
above, which is partially or completely substituted by
fluorine, chlorine and/or bromine, eg. chloromethylsulfonyl,
dichloromethylsulfonyl, trichloromethylsulfonyl,
fluoromethylsulfonyl, difluoromethylsulfonyl,
trifluoromethylsulfonyl, chlorofluoromethylsulfonyl,
dichlorofluoromethylsulfonyl, chlorodifluoromethylsulfonyl,
1-fluoroethylsulfonyl, 2-fluoroethylsulfonyl,
2,2-difluoroethylsulfonyl, 2,2,2-trifluoroethylsulfonyl,
2-chloro-2-fluoroethylsulfonyl, 2-chloro-
2,2-difluoroethylsulfonyl,
~O 95/02580 ~ ~ ~ PCT/EP94/02263
19
2,2-dichloro-2-fluoroethylsulfonyl,
2,2,2=trichloroethylsulfonyl and pentafluoroethylsulfonyl,
preferably trichloromethylsulfonyl and
trifluoromethylsulfonyl;
- C1-Cq-Haloalkylsulfinyl: C1-C4-alkylsulfinyl as mentioned
above, which is partially or completely substituted by
fluorine, chlorine and/or bromine, eg. chloromethylsulfinyl,
dichloromethylsulfinyl, trichloromethylsulfinyl,
fluoromethylsulfinyl, difluoromethylsulfinyl,
trifluoromethylsulfinyl, chlorofluoromethylsulfinyl,
dichlorofluoromethylsulfinyl, chlorodifluoromethylsulfinyl,
1-fluoroethylsulfinyl, 2-fluoroethylsulfinyl,
2,2-difluoroethylsulfinyl, 2,2,2-trifluoroethylsulfinyl,
2-chloro-2-fluoroethylsulfinyl, 2-chloro-
2,2-difluoroethylsulfinyl,
2,2-dichloro-2-fluoroethylsulfinyl,
2,2,2-trichloroethylsulfinyl and pentafluoroethylsulfinyl,
preferably trichloromethylsulfinyl and
trifluoromethylsulfinyl;
- C3-C9-(a-Alkylalkylidene)iminooxy eg.:
a-methylethylideneiminooxy and a-methylpropylideneiminooxy;
Suitable meanings for 5- or 6-membered heteroaryl and
heteroaryl-C1-C4-alkyl are the following heteroaromatics: 2-furyl,
3-furyl, 2-thienyl, 3-thienyl, 2-pyrrolyl, 3-pyrrolyl,
3-isoxazolyl, 4-isoxazolyl, 5-isoxazolyl, 3-isothiazolyl,
4-isothiazolyl, 5-isothiazolyl, 3-pyrazolyl, 4-pyrazolyl,
5-pyrazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, 2-thiazolyl,
4-thiazolyl, 5-thiazolyl, 2-imidazolyl, 4-imidazolyl,
1,2,4-oxadiazol-3-yl, 1,2,4-oxadiazol-5-yl, 1,2,4-
thiadiazol-3-yl, 1,2,4-thiadiazol-5-yl, 1,2,4-triazol-3-yl,
1,3,4-oxadiazol-2-yl, 1,3,4-thiadiazol-2-yl, 1,3,4-triazol-2-yl,
2-pyridinyl, 3-pyridinyl, 4-pyridinyl, 3-pyridazinyl,
4-pyridazinyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl,
2-pyrazinyl, 1,3,5-triazin-2-yl and 1,2,4-triazin-3-yl.
Particularly suitable agriculturally utilizable rations are those
which do not adversely affect the herbicidal action of the
compounds I, in particular the ions of the alkali metals,
preferably sodium and potassium, of the alkaline earth metals,
preferably calcium, magnesium and barium, and of the transition
metals, preferably zinc and iron, and the ammonium ion which can,
if desired, carry one to three C1-C4-alkyl, hydroxy-C1-Cq-alkyl
substituents and/or one phenyl or benzyl substituent, preferably
diisopropylammonium, tetramethylammonium, tetrabutylammonium,
WO 95/02580 PCT/EP94/0226~
20 ,
trimethylbenzylammonium and trimethyl-(2-hydroxyethyl)-ammonium,
tri-(C1-C4-alkyl)sulfonium, and sulfoxonium ions, preferably
tri- (C1-Cq-alkyl) sulfoxonium.
The ammonium ion and the abovementioned substituted ammonium ions
are very particularly preferred cations.
With a view to the use of the substituted 2-phenylpyridines I and
I' as herbicides or desiccant/defoliant compounds, the preferred
substituted 2-phenylpyridines I and I' are those in which the
substituents have the following meanings, in each case alone or
in combination:
R1 hydrogen, methyl, methoxy, methylthio or halogen, very
particularly preferably hydrogen;
R2 halogen, C1-Cq-haloalkyl with one to five halogen atoms or
C1-Cq-haloalkoxy With one to five halogen atoms, very
particularly preferably trifluoromethyl;
R3 hydrogen, methyl, methoxy, methylthio or halogen;
Rq methyl, methoxy, methylthio or halogen, very particularly
preferably halogen;
R5 hydrogen, fluorine or chlorine;
R6 chlorine and
R~ -0-R8, -S-R8, -SOZ-N (R9, Rl~) , -NR11 (S02-Rl~) , -CO-0-Re,
_CR15~C (R16) -CO-O-R8~ -CHaN-0-Re, -CH (XR18, YRl9) ,
-CHZ-CH ( Cl ) -CO-O-R8,
x R R21 x R~ R21
R~
. Y R2~22 ~ ~ Y R2~~tz3
The substituted 2-phenylpyridines of the formula I can be
obtained in a variety of ways, preferably by one of the following
processes:
~O 95/02580
PCT/EP94/02263
21
Reaction of a substituted 2-halopyridine II with an
organometallic compound III in the presence of a transition metal
catalyst in an inert solvent:
R3 R5
R2 R4
Me Cat.
+ ~I I
Ri ~ dial w Rs
R7
II III
In this case, Hal is chlorine or bromine, Me is Mg-Hal, Zn-Hal,
tri-(C1-C4-alkyl)tin, lithium, copper or B(OR32)(OR33), where R32
and R33 are, independently of one another, hydrogen or
C1-C4-alkyl, and Cat. is a transition metal catalyst, in
particular a palladium catalyst such as
tetrakis(triphenylphosphine)- palladium(O),
bis(1,4-diphenylphosphino)butanepalladium(II) chloride and
bis(triphenylphosphine)palladium(II) chloride, or a nickel
catalyst such as nickel(II) acetylacetonate,
bis(triphenylphosphine)nickel(II) chloride and
bis(1,3-diphenylphosphino)propanenickel(II) chloride.
Me is preferably B (OR32) (OR33) .
Reactions of this type are generally known, for example from the
following literature:
- Reactions with boronic acids (Me a B (OR32) (OR3) )
(1) W.J. Thompson and J. Gaudino, J. Org. Chem. ~Q (1984) 5237;
(2) S. Gronowitz and K. Lawitz, Chem. Scr. ~Q (1984) 5;
(3) S. Gronowitz et al., Chem. Scr. ,2~ (1986) 305;
(4) J. Stavenuiter et al., Heterocycles ~ (1987) 2711;
(5) V. Snieckus et al., Tetrahedron Letters ~$ (1987) 5093;
(6) V. Snieckus et al., Tetrahedron Letters ~ (1988) 2135;
(7) M.B. Mitchell et al., Tetrahedron Letters ~, (1991) 2273;
Tetrahedron g$ (1992) 8117;
(8) JP-A 93/301 870;
- Reactions with Grignard compounds (Me s Mg-Hal):
(9) L.N. Pridgen, J. Heterocyclic Chem., ,~,,~ (1975) 443;
(10)M. Kumada et al., Tetrahedron Letters, ~,1, (1980) 845, ibid ~
(1981) 5319;
WO 95/02580 PCTlEP94/022~
22
(11)A. Minato et al., J. Chem. Soc., Chem. Commun., (1984) 511;
- Reactions with organozinc compounds (Me = Zn-Hal):
(12) A.S. Bell et al., Synthesis, (1987) 843;
(13) A.S. Bell et al., Tetrahedron Letters, 2"~. (1988) 5013;
(14) J.W. Tilley and S. Zawoiski, J. Org. Chem. ~ (1988) 386,
see also Lit. (9);
- Reactions with organotin compounds
(Me = Sn(C1-Ce-alkyl)3}:
(15)T.R. Bailey et al., Tetrahedron Letters, ?~Z (1986) 4407;
(16) Y. Yamamoto et al., Synthesis, 1986, 564;
see also Lit. (6).
With a view to the preferred active substances I, the 2-halopyri-_
dines II are preferably reacted with an aromatic boronic acid of
the formula IIIa
8340
RS.
/ B IIIa
R33 O /
R6~
R~'
where
RS' is hydrogen, fluorine or chlorine;
R6' is hydroxyl, halogen or C1-C4-alkoxy;
R~' is hydrogen, C1-CQ-alkyl or C1-C4-alkoxy and
R33 and R34 are, independently of one another, hydrogen or
C1-CQ-alkyl or together are ethylene or propylene.
Among the boronic acids and esters thereof of the formula IIIa,
those of the formula IIIa'
~0 R3ap
R5,
/B / IIIa'
R33 O
Halogen
lower alkyl
~'O 95102580 PCT/EP94/02263
23
where
R5' is hydrogen, fluorine or chlorine;
halogen is a halogen atom;
lower alkyl is C1-C~-alkyl and
R33 and R3~ are, independently of one another, hydrogen or
C1-C4-alkyl or together are ethylene or propylene,
are novel.
The coupling of II + III may, where appropriate, be followed by
reactions on the phenyl ring to obtain further derivatives of the
compounds I.
The compounds I can be converted by conventional methods, eg. by
reaction with an organic peracid such as metachloroperbenzoic '
acid, into the N-oxides.
Substituted 2-phenylpyridines I where R1, R3 and/or R4 are an
alkali metal carboxylate radical can be obtained by treating com-
pounds I with R1, R3 and/or R~ = hydroxycarbonyl for example
- with sodium or potassium hydroxide in aqueous solution or an
organic solvent such as methanol, ethanol, acetone or toluene
or
- with sodium hydride in an organic solvent such as dimethyl-
formamide.
The salt formation normally takes place at a sufficient rate at
about 20~C.
The salt can be isolated, for example, by a precipitation with a
suitable inert solvent or by evaporating off the solvent.
Substituted 2-phenylpyridines I where R1, R3 and/or R4 is a car-
boxylate radical whose counterion is an agriculturally utilizable
cation not belonging to the group of alkali metals can normally
be prepared by metathesis of the corresponding alkali metal car-
boxylates.
Compounds I where R1, R3 and/or R~ is a carboxylate radical whose
counterion is, for example, a zinc, iron, calcium, magnesium or
barium ion can be prepared from the corresponding sodium
PCT/EP94/022~
WO 95/02580
24
carboxylates in a conventional way, as can compounds I where ~tl,
R3 and/or R4 is a carboxylate radical whose counterion is an ammo-
nium or phosphonium ion, using ammonia, phosphonium, sulfonium or
sulfoxonium hydroxides.
Unless otherwise indicated, all the reactions described above are
expediently carried out under atmospheric pressure or the autoge-
nous pressure of the particular reaction mixture.
The substituted 2-phenylpyridines I may result from the prepara-
tion as mixtures of isomers which, however, can if desired be
separated by the methods conventional for this purpose, such as
crystallization or chromatography, also on an optically active
adsorbate, into the pure isomers. Pure optically active isomers
can advantageously be prepared from corresponding optically
active starting materials.
The substituted 2-phenylpyridines I and I', their agriculturally
utilizable salts and N-oxides are suitable, both as mixtures of.
isomers and in the form of the pure isomers, as herbicides. They
are able to control weeds and obnoxious grasses very efficiently
in crops such as wheat, rice, corn, soybean and cotton with neg-
ligible damage to the crop plants. This effect occurs, in parti-
cular, with low application rates.
Depending on the particular application method, the compounds I
and I' or the herbicidal compositions containing them can also be
employed in a further number of crop plants to eliminate unwanted
plants. Examples of suitable crops are the followings
Allium cepa, Ananas comosus, Arachis hypogaea, Asparagus
officinalis, Beta vulgaris spp. altissima, Beta vulgaris spp.
rapa, Brassica napus var. napus, Brassica napus var.
napobrassica, Brassica rape var. silvestris, Camellia sinensis,
Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
sinensis, Coffea arabica (Coffea canephora, Coffea liberica),
Cucumis sativus, Cynodon dactylon, Daucus carota, Elaeis
guineensis, Fragaria vesca, Glycine max, Gossypium hirsutum,
(Gossypium arboreum, Gossypium herbaceum, Gossypium vitifolium),
Helianthus annuus, Hevea brasiliensis, Hordeum vulgare, Humulus
lupulus, Ipomoea batatas, Juglans regia, Lens culinaris, Linum
usitatissimum, Lycopersicon lycopersicum, Malus spp., Manihot
esculenta, Medicago sativa, Musa spp., Nicotiana tabacum (N.ru-
stica), Olea europaea, Oryza sativa , Phaseolus lunatus,
Phaseolus vulgaris, Picea abies, Pinus spp., Pisum sativum,
Prunus avium, Prunus persica, Pyrus communis, Ribes sylvestre,
Ricinus communis, Saccharum officinarum, Secale cereale, Solanum
~O 95/02580 ~~' ~ ~ ~ PCT/EP94/02263
tuberosum, Sorghum bicolor (s. vulgate), Theobroma cacao, Trifo-
lium pretense, Triticum aestivum, Triticum durum, Vicia faba,
Vitis vinifera and Zea ways.
5 In addition, the compounds I and I', their N-oxides and/or salts
can be employed in crops which have been made substantially
resistant, by breeding and/or genetic engineering methods, to the
effect of I.
10 Furthermore, the substituted 2-phenylpyridines I and I' are also
suitable for the desiccation and/or defoliation of plants. As
desiccants they are particularly suitable for drying out the
above-ground parts of crop plants such as potato, rape, sunflower
and soybean. This allows completely mechanized harvesting of
15 these important crop plants.
Also of economic interest is the facilitation of harvesting made
possible by the concentration in time of the abscission or
reduction in the strength of attachment to the tree in the case,
20 of citrus fruits, olives or other species and varieties of pomes-',
drupes and shell fruit. The same mechanism, ie. promotion of the
formation of separation tissue between fruit or leaf and stem
part of the plant, is also essential for easily controlled
defoliation of crop plants, especially cotton.
In addition, the shortening of the time interval in which the
individual cotton plants become mature results in an improved
quality of the fibers after harvest.
The active substances can be applied as such or in the form of
their formulations or the use forms prepared therefrom, eg. in
the form of directly sprayable solutions, powders, suspensions or
dispersions, emulsions, oily dispersions, pastes, dusting agents,
broadcasting agents, or granules, by spraying, atomizing,
dusting, scattering or watering. The application forms depend
entirely on the purposes for which they are used; they should
ensure in every case that distribution of the active substances
according to the invention is as fine as possible.
The formulations are produced in a conventional manner, eg. by
extending the active substance with solvents and/or carriers, if
desired using emulsifiers and dispersants, it also being possible
in the case of water as diluent to use other organic solvents as
auxiliary solvents.
Inert auxiliaries essentially suitable for this purpose are:
mineral oil fractions of moderate to high boiling points such as
WO 95/02580 PCT/EP94/022~
26
kerosene and diesel oil, also coal tar oils and minerals of '
vegetable~or animal origin, solvents such as aromatic compounds
(eg. toluene, xylene), chlorinated aromatic compounds (eg.
chlorobenzenes), paraffins (eg. petroleum fractions), alcohols
(eg. methanol, ethanol, butanol, cyclohexanol), ketones (eg.
cyclohexanone, isophorone), amines (eg. ethanolamine),
N,N-dimethylformamide, N-methylpyrrolidone and water; carriers
such as natural rock powders (eg. kaolins, aluminas, talc, chalk)
and synthetic rock powders (eg. highly disperse silica,
silicates); emulsifiers such as nonionic and anionic emulsifiers
(eg. polyoxyethylene fatty alcohol ethers, alkylsulfonates and
arylsulfonates) and dispersants such as lignin sulfite waste
liquors and methylcellulose.
Aqueous application forms can be prepared from emulsion
concentrates, dispersions, pastes, wettable powders or
water-dispensable granules by adding water. To prepare emulsions,
pastes or oil dispersions, the substances can be homogenized, as
such or dissolved in an oil or solvent, using wetting agents,
adhesion promoters, dispersants or emulsifiers, in water.
However, it is also possible to prepare concentrates which are
composed of active substances, wetting agent, adhesion promoter,
dispersant or emulsifier and, where appropriate, solvent or oil
and which are suitable for dilution with water.
Suitable surfactants are the alkali metal, alkaline earth metal
and ammonium salts of aromatic sulfonic acids, eg. lignin-,
phenol- of naphthalene- and dibutylnaphthalenesulfonic acid, and
of fatty acids, alkyl- and alkylarylsulfonates, alkyl sulfates,
lauryl ether sulfates and fatty alcohol sulfates, as well as
salts of sulfated hexa-, hepta- and octadecanols, and of fatty
alcohol glycol ether, products of the condensation of sulfonated
naphthalene and naphthalene derivatives with formaldehyde,
products of the condensation of the naphthalene or
naphthalenesulfonic acids with phenol and formaldehyde,
polyoxyethylene octylphenol ether, ethoxylated isooctyl-, octyl-
or nonylphenol, alkylphenol and tributylphenyl polyglycol ethers,
alkylaryl polyether alcohols, isotridecyl alcohol, fatty
alcohol/ethylene oxide condensates, ethoxylated castor oil,
polyoxyethylene alkyl ethers or polyoxypropylene, lauryl alcohol
polyglycol ether acetate, sorbitol esters, ligninsulfite waste
liquors or methylcellulose.
Powders and dusting agents and broadcasting agents can be
prepared by mixing or grinding the active substances together
with a solid carrier.
O 95/02580 PCT/EP94/02263
27
Granules, eg. coated, impregnated or homogeneous granules, can be
prepared kiy binding the active ingredients to solid carriers.
Solid carriers are mineral earths such as silicas, silica gels,
silicates, talc, kaolin, limestone, lime, chalk, bole, loess,
clay, dolomite, diatomaceous earth, calcium and magnesium
sulfates, magnesium oxide, ground plastics, fertilizers such as
ammonium sulfate, ammonium phosphate, ammonium nitrate, ureas and
vegetable products such as cereals flour, bark meal, wood meal
and nutshell meal, cellulose powders or other solid carriers.
The concentration of the active substances I and I' in the
formulations ready for use can vary within wide limits, for
example from 0.01 to 95% by weight. The active substances are
normally employed in a purity of from 90% to 100%, preferably 95%
to 100% (according to the NMR spectrum).
Examples of such formulations are:
I. 20 parts by weight of compound No. I.068 are dissolved in a,
mixture composed of 80 parts by weight of alkylated benzene;
10 parts by weight of the adduct of 8 to 10 mol of ethylene
oxide and 1 mol of oleic acid N-monoethanolamide, 5 parts by
weight of calcium dodecylbenzenesulfonate and 5 parts by
weight of the adduct of 40 mol of ethylene oxide and 1 mol of
castor oil. Fine dispersion of the solution in 100,000 parts
by weight of water results in an aqueous dispersion which
contains 0.02% by weight of the active ingredient.
II. 20 parts by weight of compound No. I.106 are dissolved in a
mixture composed of 40 parts by weight of cyclohexanone,
30 parts by weight of isobutanol, 20 parts by weight of the
adduct of 7 mol of ethylene oxide and 1 mol of isooctylphenol
and 10 parts by weight of the adduct of 40 mol of ethylene
oxide and 1 mol of castor oil. Fine dispersion of the solu-
tion in 100,000 parts by weight of water results in an
aqueous dispersion which contains 0.02% by weight of the
active ingredient.
III.20 parts by weight of active ingredient No. I.163 are dis-
solved in a mixture composed of 25 parts by weight of cyclo-
hexanone, 65 parts by weight of a mineral oil fraction of
boiling point 210 to 280~C and 10 parts by weight of the
adduct of 40 mol of ethylene oxide and 1 mol of castor oil.
Fine dispersion of the solution in 100,000 parts by weight of
water results in an aqueous dispersion which contains 0.02%
by weight of the active ingredient.
WO 95/02580 PCT/EP94/022~
28
IV. 20 parts by weight of active ingredient No. I.188 are tho- '
roughly mixed with 3 parts by weight of sodium diisobutyl-
naphthalene-a-sulfonate, 17 parts by weight of the sodium
salt of a lignosulfonic acid from a sulfite waste liquor and
60 parts by weight of powdered silica gel and ground in a
hammer mill. Fine dispersion of the mixture in 20,000 parts
by weight of water results in a spray liquor which contains
0.1% by weight of the active ingredient.
V. 3 parts by weight of active ingredient No. I.512 are mixed
with 97 parts by weight of finely divided kaolin to result in
a dusting agent which contains 3% by weight of the active
ingredient.
VI. 20 parts by weight of active ingredient No. I.901 are inti-
mately mixed with 2 parts by weight of calcium dodecylbenze-
nesulfonate, 8 parts by weight of fatty alcohol polyglycol
ether, 2 parts by weight of sodium salt of a phenol/urea/
formaldehyde condensate and 68 parts by weight of a paraf-
finic mineral oil to result in a stable oily dispersion.
The active ingredients or the herbicidal and growth-regulating
agents can be applied by a pre-emergence or post-emergence
method. Normally, the plants are sprayed or dusted with the
active ingredients, or the seeds of the test plants are treated
with the active ingredients. If the active ingredients are less
well tolerated by certain crops, the application techniques can
be such that the herbicidal agents are sprayed with the aid of
spraying equipment so as to avoid as far as possible the leaves
of the sensitive crops, while the active ingredients reach the
leaves of unwanted plants growing underneath them or the uncov-
ered surface of the soil (post-directed, lay-by).
The application rates of the active ingredient may vary depending
on the aim of the control, the season and the stage of growth.
When used as herbicides or defoliants, the application rate is
preferably from 0.001 to 3.0, in particular 0.01 to 1.0, kg/ha
active substance.
To widen the sprectrum of action and to achieve synergistic
effects, the substituted 2-phenylpyridines I and I' can be mixed
and applied together with numerous representatives of other
groups of herbicidal or growth-regulating active ingredients.
Examples of suitable components of the mixture are diazines,
4H-3,1-benzoxazine derivatives, benzothiadiazinones, 2,6-dini-
troanilines, N-phenylcarbamates, thiolcarbamates, halo carboxylic
acids, triazines, amides, ureas, diphenyl ethers, triazinones,
~O 95/02580 ~ PCTlEP94/02263
29
uracils, benzofuran derivatives, 1,3-cyclohexanedione derivatives
which have, for example, a carboxyl or carbimino group in pos~.-
tion 2, quinolinecarboxylic acid derivatives, imidazolinones,
sulfonamides, sulfonylureas, aryloxy- and heteroaryloxyphenoxy-
- 5 propionic acids and their salts, esters and amides, and others.
It may additionally be beneficial to apply the compounds I or I',
alone or in combination with other herbicides, also mixed with
other crop protection agents, together with, for example, agents
for controlling pests or phytopathogenic fungi or bacteria. Also
of interest is the miscibility with mineral salt solutions which
are used to eliminate deficiencies of nutrients and trace ele-
ments. It is also possible to use non-phytotoxic oils and oil
concentrates.
Preparation examples
Example 1: 3-Chloro-2-(4-chloro-3-methoxyphenyl)-5-
trifluoromethylpyridine (Table 1, Example I.001)
The preparation took place as shown in the following scheme:
H2N i 1.) NaN02, HC1 I i 1.) Mg, Ether
~ ~ 2.) NaI ~ ( 2.) B(OCH3)3
~ C1 ~ C1 3 ~ ) aq. H2S04
OCH3 OCH3
(OH)2$ F3C / / C1
\ ~ F'2C , / C1
N Cl
Cl N
OCH3 ~Pd(P(C6H5)s)~~ Cat.
C1
OCH3
1st reaction step: 2-Chloro-5-iodoanisole
123.4 g (0.7835 mol) of 4-chloro-3-methoxyaniline were added to
190 ml concentrated hydrochloric acid in 760 ml of water. This
suspension was vigorously stirred at 60°C for one hour and then
cooled to 0°C, and a solution of 59.5 g (0.862 mol) of sodium
nitrite in 170 ml of water was added dropwise at below 5°C. The
resulting mixture was then stirred at this temperature for
20 minutes and subsequently a solution of 129.2 g (0.862 mol) of
PCT/EP94/022~
WO 95/02580
sodium iodide in 220 ml of water was added dropwise. After the
reaction mixture had warmed to about 20~C it was stirred at
40-50~C for one hour and subsequently decolorized with a little
dilute sodium bisulfite solution. The solution obtained after
5 removal of the solids was extracted three times with 200 ml of
ether each time. The combined ether phases were dried over sodium
sulfate and concentrated. Yield: 190.6 g (96%) of a dark oily
residue which, according to the 1H-NMR spectrum, had a purity of
about 95%. The crude product can be purified by distillation at
10 100-120~C under 0.1 mbar to afford colorless crystals of melting
point 38~C. However, this purification is unnecessary for further
reactions.
1H-NMR (270 MHz, in CDC13): b [ppm] = 3.87(s,3H), 7.06(d,lH),
15 7.20(d,lH), 7.22(dd,lH).
2nd reaction steps 4-Chloro-3-methoxybenzeneboronic acid
In a flame-dried flask, 1.93 g (79.2 mmol) of magnesium turning
20 were etched with a small crystal of iodine, and 50 ml of anhyd-
rous ether were added. Then, under a nitrogen atmosphere, a solu-
tion of 20.0 g (79.2 mmol) 2-chloro-5-iodoanisole in 50 ml of
anhydrous ether were added dropwise in such a way that the ether
was kept boiling by the heat of reaction. After the addition was
25 complete, the mixture was refluxed for 1 1/2 hours and then fil-
tered through glass wool, with exclusion of moisture, into a
dropping funnel.
This Grignard solution and, synchronously but separately, 8.24 g
30 (79.2 mmol) of trimethyl borate were added dropwise to 50 ml of
anhydrous ether in a flame-dried flask under a nitrogen atmo-
sphere at -60 to -70~C. The resulting suspension was they stirred
at the stated temperature for one hour and, after it had warmed
to about 20~C, acidified to pH 3 with 5% strength sulfuric acid.
After separation of the phases, the aqueous phase was extracted
three times with ether. The combined organic phases were dried
over sodium sulfate and then concentrated. The residue was
extracted by boiling three times with 100 ml of water each time.
The aqueous phases were combined and cooled, when 4.6 g (32%) of
colorless crystals separated out and were removed and dried under .
reduced pressure at 20-25~C.
1H-NMR (270 MHz, in d6-DMSO): b [ppm] = 3.90(s,3H), 7.39(s,2H),
7.56(s,lH), 8.2(s,br.,2H).
~O 95/02580
PCT/EP94/02263
31
3rd reaction step: 3-Chloro-2-(4-chloro-3-methoxyphenyl)-
5-trifluoromethylpyridine
38.8 g (0.180 mol) of 2,3-dichloro-5-trifluoromethylpyridine,
33.5 g (0.180 mol) of 4-chloro-3-methoxybenzeneboronic acid,
0.7 g (0.61 mmol) of tetrakis(triphenylphosphine)palladium(O) and
45.3 g (0.539 mol) of sodium bicarbonate in a mixture of 550 ml
of dimethoxyethane and 550 ml of water were refluxed for four
hours. The mixture was then acidified to pH 4-5 with dilute
hydrochloric acid, the dimethoxyethane was removed by
distillation, and the remaining aqueous phase was extracted with
methylene chloride. The combined methylene chloride phases were
washed with water, dried over sodium sulfate and evaporated. The
residue was stirred with a little cold n-hexane, filtered off
with suction and dried. Yield: 44.2 g (76%) of colorless crystals
of melting point 72~C.
1H-NMR (270 MHz, in CDC13)s b [ppm~ = 3.96(s,3H), 7.30-7.38(m,2H),
7.48(d,lH), 8.05(s,lH), 8.84(s,lH).
Example 2: 3-Chloro-2-(4-chloro-2-fluoro-5-methoxy-
phenyl)-5-trifluoromethylpyridine (Table 4,
Example I.501)
In a preparation similar to that described above for 3-chlo-
ro-2-(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine, reac-
tion of 8.9 g (41 mmol) of 2,3-dichloro-5-trifluoromethylpyridine
and 7.9 g (41 mmol) of 4-chloro-2-fluoro-5-methoxybenzeneboronic
acid and subsequent purification of the crude product by chroma-
tography on silica gel (methylene chloride as eluent) resulted in
2.6 g of colorless crystals.
Yield: 19%; melting point: 105-106~C.
Example 3: 3-Chloro-2-(4-chloro-3-hydroxyphenyl)-5-trifluorome-
thylpyridine (Table 1, Example I.021)
20.0 g (62.1 mmol) of 3-chloro-2-(4-chloro-3-methoxyphe-
nyl)-5-trifluoromethylpyridine in 110 ml of 47% strength aqueous
hydrobromic acid were refluxed for five hours. The reaction mix-
ture was then diluted, while cooling in ice, with about 400 ml of
water and extracted three times with 150 ml of methylene chloride
each time. The combined organic phases were dried over sodium
sulfate and evaporated to give a residue of 17.9 g (94%) of col-
orless crystals of melting point 105-107~C.
1H-NMR (270 MHz, in CDC13): 8 [ppm~ = 6.0(s,br.,lH), 7.28(dd,lH),
7.40(d,lH), 7.43(d,lH), 8.05(s,lH), 8.83(s,lH).
PCT/EP94/022~
WO 95/02580
32
Example 4: 3-Chloro-2-(4-chloro-2-fluoro-5-hydroxy-
phenyl)-5-trifluoromethylpyridine (Table 4,
Example I.521)
A preparation similar to that described above for 3-chlo-
ro-2-(4-chloro-3-hydroxyphenyl)-5-trifluoromethylpyridine re-°
sulted in 1.4 g of colorless crystals from 1.6 g (4.7 mmol) of
3-chloro-2-(4-chloro-2-fluoro-5-methoxyphenyl)-5-trifluoromethyl-
pyridine.
Yield: 91%; melting point: 111-112°C.
Example 5: 3-Chloro-2-(4-chloro-3-propargyloxyphenyl)-
5-trifluoromethylpyridi,ne (Table 1, Example 1.012)
1.73 g (14.6 mmol) of propargyl bromide were added dropwise to a
mixture of 3.00 g (9.74 mmol) of 3-chloro-2-(4-chloro-3-hydroxy-
phenyl)-5-trifluoromethylpyridine, 4.0 g (29 mmol) of potassium
carbonate and 100 ml of anhydrous dimethylformamide. The mixture
was stirred at 20-25°C for about 15 hours and then poured into
400 ml of water. The solution was kept cold for a few hours, an8
the resulting crystals were then separated off, washed with water
and dried under reduced pressure. Yield: 3.1 g (92%) of colorless
crystals of melting point 102-103°C.
1H-NMR (250 MHz, in CDC13): b [ppm~ = 2.57(t,lH), 4.86(d,2H),
7.38(dd,lH), 7.48-7.54(m,2H), 8.05(s,lH), 8.85(s,lH).
35
45
O 95/OZ58~
PCT/EP94/02263
33
Example 6: 3-Chloro-2-(4-chloro-2-fluoro-5-propargyloxy-
phenyl)-5-trifluoromethylpyridine (Table 4,
Example 512)
In a preparation similar to that described above for
3-chloro-2-(4-chloro-3-propargyloxyphenyl)-5-trifluoromethylpyri-
dine, reaction of 1.4 g (4.5 mmol) of 3-chloro-2-(4-chlo-
ro-2-fluoro-5-hydroxyphenyl)-5-trifluoromethylpyridine and 0.6 g
(5 mmol) of propargyl bromide resulted in 1.1 g of colorless
crystals. Yield: 67%; melting point: 97-98~C.
Example 7: 3-Chloro-2-(4-chloro-3-isopropoxyphenyl)-5-trifluoro-
methylpyridine (Table 1, Example 1.004)
0.23 g (7.8 mmol) of an 80% suspension of sodium hydride in min-
eral oil was washed with anhydrous pentane to remove the mineral
oil and then suspended in 50 ml of anhydrous dimethylformamide.-A
solution of 2.0 g (6.5 mmol) of 3-chloro-2-(4-chloro-3-hydroxy-
phenyl)-5-trifluoromethylpyridine in 50 ml of anhydrous dimethyl-
formamide was added dropwise to this suspension at O~C. After the
addition is complete, the mixture was stirred for 15 minutes and
then 1.3 g (7.8 mmol) of isopropyl iodide were slowly added drop-
wise. The mixture was then stirred at 20-25~C for about 15 h and
subsequently poured into 400 ml of water and extracted three
times with methyl tert-butyl ether. The combined organic phases
were washed with water, dried over sodium sulfate and evaporated
under reduced pressure. Yield: 1.9 g (83%) of a colorless oil.
1H-NMR (250 MHz, in CDC13): b [ppm] = 1.41(d,6H), 4.63(h,~lH),
7.30(dd,lH), 7.35(d,lH), 7.49(d,lH), 8.08(s,lH), 8.85(d,lH).
Example 8: 3-Chloro-2-[4-chloro-3-(methoxycarbonylmethoxy)-
phenyl]-5-trifluoromethylpyridine (Table 1,
Example I.014)
A preparation similar to that described above for 3-chlo-
ro-2-(4-chloro-3-propargyloxyphenyl)-5-trifluoromethylpyridine
resulted in 2.2 g (89%) of colorless crystals of melting point
109-110~C from 2.0 g (6.5 mmol) of 3-chloro-2-(4-chloro-3-hydroxy-
phenyl)-5-trifluoromethylpyridine, 1.5 g (9.7 mmol) of methyl
2-bromoacetate, 1.8 g (13 mmol) of potassium carbonate and a to-
tal of 100 ml of dimethylformamide.
1H-NMR (250 MHz, in CDC13): 8 [ppm] = 3.82(s,3H), 4.80(s,2H),
7.29(d,lH), 7.40(dd,lH), 7.52(d,lH), 8.05 (s,lH), 8.84(s,lH).
WO 95/02580 ~ PCTIEP94/0226~
34
Example 9: 3-Chloro-2-(4-chloro-3-(1-ethoxycarbonylethoxy)-
phenyl]-5-trifluoromethylpyridine (Table 1,
Example I.017)
A preparation similar to that described above for 3-chloro-
2-(4-chloro-3-propargyloxyphenyl)-5-trifluoromethylpyridine
resulted in 2.4 g (90%) of a colorless oil from 2.0 g (6.5 mmol)
of 3-chloro-2-(4-chloro-3-hydroxyphenyl)-5-trifluoromethylpyri-
dine, 1.8 g (9.7 mmol) of ethyl 2-bromopropionate, 1.8 g
(13 mmol) of potassium carbonate and a total of 100 ml of dime-
thylformamide.
1H-NMR (270 MHz, in CDC13): & [ppm] = 1.28(t,3H), 1.73(d,3H),
4.21(q,2H), 4.80(q,lH), 7.31(d,lH), 7.39 (dd,lH), 7.50(d,lH),
8.10(s,lH), 8.84(s,lH).
Example 10: 3-Chloro-2-[4-chloro-3-(cyanomethoxy)phenyl]-
5-trifluoromethylpyridine ,(Table 1, Example I.019)-
A preparation similar to that described above for 3-chlo-
ro-2-(4-chloro-4-3-propargyloxyphenyl)-5-trifluoromethylpyridine
resulted in 2.0 g (89%) of colorless crystals of melting point
85-86~C from 2.0 g (6.5 mmol) of 3-chloro-2-(4-chloro-3-hydroxy-
phenyl)-5-trifluoromethylpyridine, 1.2 g (9.7 mmol) of bromo-
acetonitrile, 1.8 g (13 mmol) of potassium carbonate and a total
of 100 ml of dimethylformamide.
1H-NMR (250 MHz, in CDC13): 8 [ppm] = 4.93(s,2H), 7.50-7.55(m,3H),
8.08(s,lH), 8.87(s,lH).~
Example 11: 3-Chloro-2-[4-chloro-3-(1-cyanoethoxy)phenyl]-5-tri-
fluoromethylpyridine (Table 1, Example I.02J)
A preparation similar to that described above for 3-chloro-
2-(4-chloro-3-propargyloxyphenyl)-5-trifluoromethylpyridine
resulted in 2.1 g (90%) of colorless crystals of melting point
75-76~C from 2.0 g (6.5 mmol) of 3-chloro-2-(4-chloro-3-hydroxy-
phenyl)-5-trifluoromethylpyridine, 1.3 g (9.7 mmol) of
(+)-2-bromopropionitrile, 1.8 g (13 mmol) of potassium carbonate
and a total of 100 ml of dimethylformamide.
1H-NMR (270 MHz, in CDC13): b [ppm] = 1.88(d,3H), 4.98(q,lH),
7.55(s,2H), 7.61(s,lH), 8.07(s,lH), 8.85(s,lH).
~VO 95/02580 ~ PCTlEP94/02263
Example 12: 3-Chloro-2-(4-chloro-3-nitrophenyl)-5-trifluoro-
methylpyridine (Table 1, Example I.064)
5 The preparation took place as shown in the following scheme:
F3C \ ~ Cl + (OH)2H \ I {pd[p(C6H5)3]4~ (Cat.)
N C1 C1
NaHC03
F3C / Cl X03 F3C / C1
~N \ I H2S0~ ~N
~Cl ~Cl
N02
1st reaction step: 3-Chloro-2-(4-chlorophenyl)-5-trifluoromethyl-
pyridine
A preparation similar to that described above for 3-chloro-2-
(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine resulted in
11.1 g (72%) of colorless crystals of melting point 78-79~C from
11.5 g (53.1 mmol) of 2,3-dichloro-5-trifluoromethylpyridine,
8.3 g (53.1 mmol) of 2,3-dichloro-5-trifluoromethylpyridine,
8.3 g (53.1 mmol) of 4-chlorobenzeneboronic acid, 120 mg
(0.10 mmol) of tetrakis(triphenylphosphine)palladium(0) and
13.4 g (159 mmol) of sodium bicarbonate.
1H-NMR (250 MHz, in CDC13): b [ppm] ~ 7.49(d,2H), 7.75(d,2H),
8.05(s,lH), 8.84(s,lH).
2nd reaction steps 3-Chloro-2-(4-chloro-3-nitrophenyl)-5-tri-
fluoromethylpyridine
3.6 g (57 mmol) of concentrated nitric acid were added dropwise
to a mixture of 11.1 g (38.0 mmol) of 3-chloro-2-(4-chloro-phe-
nyl)-5-trifluoromethylpyridine in 50 ml of concentrated sulfuric
acid while stirring and cooling in ice at 0-S~C. The mixture was
stirred at this temperature for two hours and then poured into
500 ml of ice-water. The product was extracted three times with
150 ml of ethyl acetate each time. The combined organic phases
were washed twice with a little water, dried over sodium sulfate
and finally concentrated under reduced pressure. The residue was
purified by chromatography on silica gel (mobile phase: cyclohex-
ane/ethyl acetate 95:5). Yield: 11.8 g (90%) of colorless crys-
tals of melting point 68-69~C.
WO 95/02580 PCTIEP94/022f~
36
1H-NMR (250 MHz, in CDC13): S [ppm] = 7.70(d,lH), 8.02(dd,lH),
8.12(d,lH), 8.40(s,lH), 8.88(s,lH).
Example 13: 2-(3-Amino-4-chlorophenyl)-3-chloro-5-trifluoro-
methylpyridine (Table 1, Example 1.065)
A preparation similar to that described above for 3-chloro-2-
(3-amino-4-hydroxyphenyl)-5-trifluoromethylpyridine resulted in '
12.8 g (91%) of colorless crystals of melting point 88-90~C from
15.4 g (45.7 mmol) of 3-chloro-2-(4-chloro-3-nitrophenyl)-5-tri-
fluoromethylpyridine (prepared as in Example 12), 7.7 g (137
mmol) of iron powder, 80 ml of methanol and 40 ml of glacial
acetic acid.
1H-NMR (250 MHz, in CDC13): 8 [ppm] = 4.18(s,br.,2H),
7.00-7.13(m,2H), 7.35(d,lH), 8.03(s,lH), 8.80(s,lH).
Example 14: 3-Chloro-2-[4-chloro-3-bis(methylsulfonyl)amino-
phenyl]-5-trifluoromethylpyridine (Table 1,
Example I.067)
2.4 g (21.0 mmol) of methanesulfonyl chloride were slowly added
dropwise to a mixture of 3.0 g (9.77 mmol) of 2-(3-amino-4-
chlorophenyl)-3-chloro-5-trifluoromethylpyridine, 2.2 g
(22.4 mmol) of triethylamine and 50 ml of anhydrous methylene
chloride at 0-S~C. The mixture was stirred at 20-25~C for about
15 hours and then washed ~:wice with water, dried over sodium sul-
fate and finally concentrated. The residue was stirred with
ether, filtered off with suction and dried under reduced pres-
sure. Yield: 3.6 g (87%) of colorless crystals of melting point
230-231~C.
1H-NMR (270 MHz, in d6-DMSO)s 8 [ppm] = 3.62(s,6H), 7.84(d,lH),
7.92(dd,lH), 8.09(d,lH), 8.68(s,lH), 9.12(s,lH).
Example 15: 3-Chloro-2-(4-chloro-3-methylsulfonylaminophenyl)-
5-trifluoromethylpyridine (Table 1, Example I.066)
A solution of 3.6 g (7.78 mmol) of 3-chloro-2-[4-chloro-3-
bis-(methylsulfonyl)aminophenyl]-5-trifluoromethylpyridine (pre-
pared as in Example 13) and 100 mg of sodium methoxide in 100 ml
of methanol were stirred at 20-25~C for three hours. Then most of
the methanol was distilled off under reduced pressure. The resi-
due was taken up in dilute hydrochloric acid, after which the
product was extracted three times with ethyl acetate. The com-
bined organic phases were dried over sodium sulfate and concen-
trated. The oily residue was treated with ether/petroleum ether
~'O 95/02580 ~ ~ ~~~~ PCT/EP94/02263
37
to afford 1.6 g (53%) of colorless crystals of melting point
133-134~C.-
1H-NMR (250 MHz, in ds-DMSO)s b [ppm~ = 3.06(s,3H), 7.55(dd,lH),
7.68(d,lH), 7.84(d,lH), 8.62(s,lH), 9.08(s,lH), 9.7(s,br.,lH).
Example 16: 3-Chloro-2-[4-chloro-3-(2-chloro-2-methoxycarbonyl-
ethyl)phenyl]-5-trifluoromethylpyridine (Table 1,
Example I.163)
11.2 g (130 mmol) of methyl acrylate and 2.2 g (16.3 mmol) of
copper(II) chloride were added to a solution of 2.0 g (19.5 mmol)
of tert-butyl nitrite in 100 ml of anhydrous acetonitrile at O~C.
Subsequently, while stirring at O~C, a solution of 4.0 g
(13.0 mmol) of 2-(3-amino-4-chlorophenyl)-3-chloro-5-trifluoro-
methylpyridine in 100 ml of anhydrous acetonitrile was slowly
added dropwise. After the addition was complete, the mixture was
stirred at 20-25~C for 5 h, then filtered, concentrated and chro-
matographed on silica gel with cyclohexane/ethyl acetate (98:2)
as mobile phase. Yield: 3.2 g (59%) of a colorless oil.
1H-NMR (270 MHz, in CDC13): b [ppm~ ~ 3.35(dd,lH), 3.58(dd,lH),
3.78(s,3H), 4.66(t,lH), 7.50(d,lH), 7.65(dd,lH), 7.72(d,lH),
8.05(s,lH), 8.84(s,lH).
Example 17: 3-Chloro-2-(4-chloro-3-methylphenyl)-5-trifluorome-
thylpyridine (Table 1, Example I.76)
The preparation took place as shown in the following scheme:
HyN 1.) NaNOy I 1.) Mg
HC1 ~ ~ I 2.) B(OCH3)3
( C1 2.) NaI ~ C1 3.) H2S04, Hy0
CH3 CH3
F3C / I Cl F3C / Cl
(OH)yB Cl
N Cl
N
bpd[p(C6H5)3~4~ \
\ ' C1
Cl CH3
WO 95/02580 PCT/EP94/022c'~
38
1st reaction step: 4-Chloro-3-methyliodobenzene
A preparation similar to that described above for 2-chloro- 5-io-
doanisole resulted in 206.0 g (93%) of a colorless liquid of
boiling point 57-58~C/0.6 mbar from 125.0 g (0.883 mol) of
4-chloro-3-methylaniline, 62.0 g (0.899 mol) of NaN02 and 135.0 g
(0.900 mol) of NaI.
1H-NMR (200 MHz, in CDC13): b [ppm] = 2.32(s,3H), 7.15(d,lH),
7.44(dd,lH), 7.59(d,lH).
2nd reaction step: 4-Chloro-3-methylbenzeneboronic acid
A preparation similar to that described above for 4-chloro-
3-methoxybenzeneboronic acid resulted in 35.2 g (58%) of color-
less crystals of melting point 255-258~C, which can be reacted
without further purification, from 90.0 g (0.356 mol) of
4-chloro-3-methyliodobenzene, 8.7 g (0.358 mol) of magnesium
turnings and 37.0 g (0.356 mol) of trimethyl borate.
1H-NMR (400 MHz, in ds-DMSO): b [ppmJ = 2.38(s,3H), 7.37(d,lH),
7.7o(dd,lH), 7.82(d,lH).
3rd reaction step: 3-Chloro-2-(4-chloro-3-methylphenyl)-5-
trifluoromethylpyridine
A preparation similar to that described above for 3-chloro-
2-(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine resulted
in 120.0 g (99%) of colorless crystals of melting point 40-42~C
from 93.4 g (0.432 mol) of 2,3-dichloro-5-trifluoromethylpyri-
dine, 67.0 g (0.393 mol) of 4-chloro-3-methylbenzeneboronic acid
and 0.4 g (0.35 mmol) of tetrakis(triphenylphosphine)palla-
dium(0).
1H-NMR (200 MHz, in CDC13): b [ppmJ = 2.45(s,3H), 7.45(d,lH),
7.55(dd,lH), 7.64(d,lH), 8.04(d,lH), 8.84(d,lH).
Example 18: 2-(3-Bromomethyl-4-chlorophenyl)-3-chloro-5-tri-
fluoromethylpyridine (Table 1, Example I.080)
A solution of 9.6 g (31.4 mmol) of 3-chloro-2-(4-chloro-3-methyl-
phenyl)-5-trifluoromethylpyridine (prepared as in Example 16) and
5.6 g (31.5 mmol) of N-bromosuccinimide in 150 ml of tetrachloro-
methane was irradiated with a 150 W high-pressure Hg lamp for one
hour. For the workup, the precipitated succinimide was removed,
and the filtrate was concentrated under reduced pressure. The
~O 95/02580
PCT/EP94/02263
39
residue was taken up in cyclohexane. The solids were removed and
discarded, and the cyclohexane solution was concentrated again.
The crude product was purified by chromatography on silica gel
(mobile phase: n-pentane/methyl tert-butyl ether 20:1). Yield:
6.2 g (51%) of colorless crystals of melting point 71-72~C.
1H-NMR (250 MHz, in CDC13): & [ppm] = 4.66(s,2H), 7.50(d,lH),
7.71(dd,lH), 7.91(d,lH), 8.08(s,lH), 8.85(s,lH).
Example 19: 3-Chloro-2-(4-chloro-3-methoxymethylphe-
nyl]-5-trifluoromethylpyridine (Table 1,
Example I.081)
A solution of 1.05 g (19.4 mmol) of sodium methoxide in 5 ml of
methanol was added to a solution of 5.0 g (12.9 mmol) of
2-(3-bromomethyl-4-chlorophenyl)-3-chloro-5-trifluoromethylpyri-
dine (prepared as in Example 17) in 100'ml of methanol. After
stirring at room temperature for 96 hours, most of the methanol
was removed by distillation under reduced pressure. The residue
was taken up in water. Esterification with dilute hydrochloric
acid was followed by extraction three times with 50 ml of n-hex-
ane each time. The combined hexane phases were dried over sodium
sulfate and then concentrated. The oily residue was induced to
crystallize by trituration with cyclohexane. Yield: 3.5 g (81%)
of colorless crystals of melting point 52-54~C.
Example 20: 3-Chloro-2-(4-chloro-3-chlorosulfonylphenyl)-5-tri-
fluoromethylpyridine (Table 1, Example I.050)
40.0 g (0.137 mol) of 3-chloro-2-(4-chlorophenyl)-5-trifluoro-
methylpyridine (see above for preparation) were added in portions
to 75 ml of chlorosulfonic acid while stirring and cooling in
ice. After the addition was complete, the mixture was stirred at
130~C for four hours. The cooled mixture was cautiously poured
into ice-water which was then extracted three times with methyl-
ene chloride. The combined organic phases were dried over sodium
sulfate and concentrated. Yield: 45.0 g (84%) of a dark oil.
Example 21: 2-(3-Aminosulfonyl-4-chlorophenyl)-3-chloro-5-tri-
fluoromethylpyridine (Table 1, Example I.051)
4 ml of concentrated aqueous ammonia solution were added all at
once to a solution of 4.0 g (10.2 mmol) of 3-chloro-2-(4-chloro-
3-chlorosulfonylphenyl)-5-trifluoromethylpyridine (prepared as in
Example 19) in 50 ml of tetrahydrofuran. After stirring at 20-25~C
for one hour, most of the tetrahydrofuran was removed by dis-
tillation under reduced pressure. The residue was kept in the
WO 95/02580 s PCT/EP94/0226~
cold for a few hours, after which the crystals which had sepa=
rated out~were removed and stirred in diisopropyl ether. Yield:
3.2 g (84%) of colorless crystals of melting point 176~C.
5 Example 22: 3-Chloro-2-(4-chloro-3-methylaminosulfonylphenyl)-
5-trifluoromethylpyridine (Table 1, Example I.052)
A preparation similar to that described above for 2-(3-arnino-
sulfonyl-4-chlorophenyl)-3-chloro-5-trifluoromethylpyridine
10 resulted in 3.6 g (91%) of a colorless oil from 4.0 g (10.2 mmol)
of 3-chloro-2-(4-chloro-3-chlorosulfonylphenyl)-5-trifluoro-
methylpyridine and 4 ml of 40% strength aqueous methylamine solu-
tion.
15 Example 23: 3-Chloro-2-(4-chloro-3-dimethylaminosulfonylphenyl)-
5-trifluoromethylpyridine (Table 1, Example I.053)
A preparation similar to that described above for 2-(3-amino-
sulfonyl-4-chlorophenyl)-3-chloro-5-trifluoromethylpyridine
20 resulted in 3.6 g (88%) of a colorless oil from 4.0 g (10.2 mmol-)
of 3-chloro-2-(4-chloro-3-chlorosulfonylphenyl)-5-trifluoro-
methylpyridine and 4 ml of 40% strength aqueous dimethylamine
solution.
25 Example 24: 2-(4-Chloro-3-methoxyphenyl)-5-trifluoromethylpyrid-
ine (Table 6, Example I.902)
A preparation similar to that described above for 3-chloro-2-
(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine resulted in
30 3.5 g (76%) of colorless crystals of melting point 74~C from 2.7 g
(16.1 mmol) of 2-chloro-5-trifluoromethylpyridine and 3.0 g
(16.1 mmol) of 4-chloro-3-methoxybenzeneboronic acid.
Example 25s 3-Chloro-2-(4-chloro-3-methoxyphenyl)pyridine
35 (Table 6, Example I.903)
A preparation similar to that described above for 3-chloro-2-
(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine resulted in
2.6 g (63%) of colorless crystals of melting point 116~C from
40 2.4 g (16.2 mmol) of 2,3-dichloropyridine and 3.0 g (16.1 mmol)
of 4-chloro-3-methoxybenzeneboronic acid.
Example 26: 2-(4-Chloro-3-methoxyphenyl)-3,6-dichloro-5-tri-
fluoromethylpyridine (Table 6, Example 1.905)
~O 95/02580 ~ ~ ~ PCT/EP94/02263
41
A preparation similar to that described above for 3-chloro-2- '
(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine resulted in
2.24 g (39%) of colorless crystals of melting point 88-90~C from
4.03 g (16.1 mmol) of 2,3,6-trichloro-5-trifluoromethylpyridine
and 3.0 g (16.1 mmol) of 4-chloro-3-methoxybenzeneboronic acid.
1H-NMR (250 MFiz, in CDC13: b [ppm~ ~ 3.97(s, 3H), 7.35-7.40(m,2H),
7.48(d, 1H), 8.10(s,lH)
Example 27: 3-Chloro-2-(4-chloro-3-dibromomethylphenyl)-5-tri-
fluoromethylpyridine (Table 1, Example I.079)
A solution of 75.0 g (0.245 mol) of 3-chloro-2-(4-chloro-3-me-
thylphenyl)-5-trifluoromethylpyridine (prepared as in Example 16)
and 109.0 g (0,613 mol) of N-bromosuccinimide in 2 1 of tetra-
chloromethane was irradiated under reflux with a 150 watt Hg
immersion lamp for three hours. The mixture was cooled and then
the succinimide which was formed and unreacted N-bromosuccinimide
were removed. The solvent was removed by distillation under '
reduced pressure, after which the oily residue was induced to
crystallize by trituration with hexane. Yield: 105.0 g (92%) of
colorless crystals of melting point 75-77~C.
Example 28: 3-Chloro-2-(4-chloro-3-formylphenyl)-5-trifluoro-
methylpyridine (Table l, Example I.113)
6.99 g (15.1 mmol) of 3-chloro-2-(4-chloro-3-dibromomethylphe-
nyl)-5-trifluoromethylpyridine in 100 ml of 96% strength sulfuric
acid were stirred at 100~C for one hour during which a vigorous
stream of nitrogen was passed through the reaction mixture. The
mixture was cooled and then poured into ice-water. The solid
product was separated off, washed with water and dried under
reduced pressure. Yield: 4.2 g (87%) of colorless crystals of
melting point 94~C.
Example 29
2-(4-Chloro-3-methoxyphenyl)-5-trifluoromethyl-3-methylthiopyri-
dine (Table 6, Example I.901)
3.0 g (9.3 mmol) of 3-chloro-2-(4-chloro-3-methoxyphenyl)-5-tri-
fluoromethylpyridine and 0.7 g (10 mmol) of sodium thiomethoxide
in a mixture of 50 ml of methanol and 20 ml of dimethylformamide
were stirred at 80~C for 7 h and then at 23~C for 72 h. The mix-
ture was then poured into 500 ml of ice-water, which was then ex-
tracted three times with 150 ml of tert-butyl methyl ether each
time. The combined organic phases were washed twice with 100 ml
of water each time, dried over sodium sulfate and concentrated.
WO 95/02580 ~ ~ ~ ~ ~ ~ PCT/EP94/0226~
42
Yield: 2.9 g (94~) of colorless crystals; melting point:
100-103~C.
Example 30
2-(4-Chloro-3-methoxyphenyl)-5-trifluoromethyl-3-methoxypyridine
(Table 6, Example 1.904)
33.5 g of a 30~ strength methanolic solution of sodium methoxide
were added to a solution of 3.0 g (9.3 mmol) of 3-chloro-2-
(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine in 100 ml of
methanol. The mixture was refluxed for 20 h and then poured into
about 500 ml of ice-water. The product was extracted from the
aqueous phase with 3 x 100 ml of methylene chloride. The combined
organic phases were dried over sodium sulfate and concentrated.
The oily residue was induced to crystallize by trituration with
n-hexane. Yield: 1.3 g of colorless crystals; melting point:
62-63~C (purity about 85~).
Example 31
3-Chloro-2-(4-chloro-3-methoxyphenyl)-6-ethoxy-5-trifluoromethyl-
pyridine (Table 6, Example I.925)
6.5 g (18 mmol) of 3,6-dichloro-2-(4-chloro-3-methoxyphenyl)-
5-trifluoromethylpyridine and 6.1 g (109 mmol) of potassium hy-
droxide in 100 ml of ethanol were stirred at 23~C for 41 h. The
solvent was then removed by distillation and the residue was tak-
en up in 100 ml of dilute hydrochloric acid. Extraction was car-
ried out three times with 100 ml of methylene chloride each time.
The combined extracts were dried over sodium sulfate and then
concentrated. Yield: 6.3 g (85~) of a colorless oil (purity about
90~) .
1H-NMR (270 MHz, in CDC13): 8 [ppm] = 1,44 (t, 3H), 3.97 (s,3H),
4.52 (q,2H), 7.38 (dd,lH), 7.40 (d, 1H), 7.47 (d, 1H), 7.94 (s,
1H).
Example 32
3-Chloro-2-(4-chloro-3-methoxyphenyl)-5-trifluoromethylpyridine
N-oxide (Table 5, Example I.802)
A solution of 4.5 g (14 mmol) of 3-chloro-2-(4-chloro-3-methoxy-
phenyl)-5-trifluoromethylpyridine and 9.7 g (31 mmol) of
3-chloroperbenzoic acid in 80 ml of methylene chloride was
stirred at 23~C for 4 days and then at 40~C for 16 h. The mixture
Was then extracted with 100 ml of 10~ strength aqueous sodium bi-
sulfate solution, with 100 ml of a 10~ strength aqueous sodium
bicarbonate solution and three times with 80 ml of water each
~O 95/02580 ~ PCT/EP94/02263
43
time. The organic phase was concentrated, and the residue was
chromatographed on silica gel with cyclohexane/ethyl acetate
(5s1). Yield: 3.6 g (76%) of colorless crystals; melting point:
156-157°C.
Example 33
3-Chloro-2-(4-chloro-3-mercaptophenyl)-5-trifluoromethylpyridine
The preparation took place as shown in the following scheme:
FgC / Cl 1, NaH, DMF C1
~ FgC
~N / I 2. C1-CS N(CHg)2
\N
cl ~
OH 2 h C1
230~C O-CS-N(CH3)2
F3C / C1 1, NaOH
g3C Cl
2. hydro-
N
chloric ~N
C1 acid
SH Cl
S-CO-N(CH3)2
1st reaction step:
3-Chloro-2-(4-chloro-3-dimethylaminothiocarbonyloxyphenyl)-5-tri-
fluoromethylpyridine
A solution of 65.0 g of 3-chloro-2-(4-chloro-3-hydroxyphe-
nyl)-5-trifluoromethylpyridine in 200 ml of dimethylformamide,
and then 31.3 g of dimethylthiocarbamoyl chloride, were added
dropwise to a suspension of 6.7 g of 80% sodium hydride in 300 ml
of anhydrous dimethylformamide. The solution was stirred at 80°C
for one hour and then poured into 2.5 1 of 1% by weight sodium
hydroxide solution. After extraction three times with 250 ml of
tert-butyl methyl ether each time the combined organic phases
were washed twice with 150 ml of water each time and then dried
over sodium sulfate and concentrated until crystallization
started. After removal of the crystals, the mother liquor was
concentrated further until more of the product started to crys-
tallize out.
Total yield: 61.3 g (74%) of colorless crystals;
melting point: 101-103°C.
WO 95/02580 . PCT/EP94/0226~
44
2nd reaction step:
3-Chloro-2-(4-chloro-3-dimethylaminocarbonylthiophenyl)-5-tri-
fluoromethylpyridine
61.3 g of 3-chloro-2-(4-chloro-3-dimethylaminothiocarbonyloxyphe-
nyl)-5-trifluoromethylpyridine in 100 ml of sulfolane were heated
at 230°C for 2 h. The mixture was cooled and then poured into
400 ml of water, which was then extracted three times With 100 ml
of ethyl acetate each time. The combined organic phases were
washed twice with 100 ml of water each time and then dried over
sodium sulfate and concentrated. The residue was purified by
chromatography on silica gel (mobile phases cyclohexane/ethyl
acetate = 6a1). Yield: 41.2 g (67%) of colorless crystals; melt-
ing points 85-86°C.
3rd reaction steps
3-Chloro-2-(4-chloro-3-mercaptophenyl)-5-trifluoromethylpyridine
A solution of 41.2 g of 3-chloro-2-(4-chloro-3-dimethylaminocar-
bonylphenyl)-5-trifluoromethylpyridine and 20.9 g of sodium
hydroxide in 300 ml of methanol was stirred at 23°C for 16 h. The
methanol was removed by distillation and then the residue was
taken up in 400 ml of water. The solution was extracted with
ether and, after solidification With dilute hydrochloric acid,
three times more with 100 ml of ether each time. The three last
ether phases were combined, dried over sodium sulfate and concen-
trated. Chromatography on silica gel with cyclohexane/ethyl acet-
ate as mobile phase afforded 28.7 g (85%) of a colorless oil.
1H-NMR (270 MFiz, in CDC13): $ [ppm] = 4.00 (s,lH), 7.43-7.54 (m,
2H), 7.77 (s,lH), 8.04 (s,lH), 8.83 (s,lH).
Example 34
3-Chloro-2-[4-chloro-3-(2-propynylthio)-phenyl]-5-trifluoro-
methylpyridine (Table 1, Example I.036)
2.0 g of 3-chloro-2-(4-chloro-3-mercaptophenyl)-5-trifluoro-
methylpyridine (prepared as in Example 33) and 2.1 g of potassium
carbonate were introduced into 50 ml of anhydrous dimethylform-
amide at 23°C. After a dropwise addition of 0.73 g of propargyl
bromide, the mixture was stirred for 16 h and then poured into
300 ml of water. After 30 minutes, the crystals which had formed
were removed, washed with water and dried.
Yield: 1.8 g (81%) of colorless crystals; melting point: 91-92°C.
~O 95/02580 ~' ~~ PCT/EP94/02263
Example 35
3-Chloro-2-(4-chloro-3-methylthiophenyl)-5-trifluoromethylpyri-
dine (Table 1, Example I.025)
5 A preparation similar to that described above for 3-chloro-2-
[4-chloro-3-(2-propynylthio)phenyl]-5-trifluoromethylpyridine
resulted in 1.2 g (50%) of colorless crystals of melting point
96-97~C from 2.0 g of 3-chloro-2-(4-chloro-3-mercaptophenyl)-5-
trifluoromethylpyridine and 0.9 g of methyl iodide.
Example 36
3-Chloro-2-[4-chloro-3-(1-ethoxycarbonylethylthio)phenyl]-5-tri-
fluoromethylpyridine (Table l, Example I.042)
In a preparation similar to that described above for 3-chloro-
2-[4-chloro-3-(2-propynylthio)phenyl]-5-trifluoromethylpyridine,
reaction of 2.0 g of 3-chloro-2-(4-chloro-3-mercaptophenyl)-5-
trifluoromethylpyridine with 1.11 g of ethyl 2-bromopropionate
and extraction of the product with tert-butyl methyl ether af-
2~ forded 2.4 g (92%) of a colorless oil.
1H-NMR (270 MHz, in CDC13) 8 [ppm] = 1.14 (t,3H), 1.58 (d,3H),
4.00 (q,lH), 4.12 (q,2H), 7.54 (d,lH), 7.66 (dd,lH), 7.97 (d,lH),
8.07 (s,lH), 8.85 (s,lH).
Example 37
4-[2-Chloro-5-(3-chloro-5-trifluoromethyl-2-pyridinyl)phenyl-
aminosulfonyl]-3,5-dimethylisoxazole (Table 1, Example I.176)
A solution of 2.5 g of 2-(3-amino-4-chlorophenyl)-3-chloro-5-tri-
fluoromethylpyridine and 2.3 g of 3,5-dimethylisoxazole-4-sulfo-
nyl chloride in a mixture of 100 ml of toluene and 100 m1 of
pyridine was refluxed for 16 hours. The mixture was concentrated
and the residue was taken up in 50 ml of ethyl acetate. The solu-
tion was washed with 50 ml each of 10% strength hydrochloric acid
and 10% strength aqueous sodium bicarbonate solution and was
dried over sodium sulfate and concentrated. Chromatography on
silica gel With cyclohexane/ethyl acetate (6:1) resulted in 1.9 g
of colorless crystals; melting point: 161-162~C.
4~
Example 38
3-Chloro-2-[4-chloro-3-(4-chlorophenylsulfonylamino)phe-
nyl]-5-trifluoromethylpyridine (Table l, Example I.167)
In a similar way to Example 37, reaction of 2.5 g of 2-(3-
amino-4-chlorophenyl)-3-chloro-5-trifluoromethylpyridine and
1.9 g of 4-chlorobenzenesulfonyl chloride, and purification of
WO 95/02580 ~ ~ PCT/EP94/0226~
46
the crude product by recrystallization from ether, resulted in
1.2 g of colorless crystals. Yield: 31%; melting point: 156-I57~C.
Example 39
Ethyl (~)-2-[2-Chlorv-5-(3-chloro-5-trifluoromethyl-2-pyri-
dinyl)phenylsulfonylamino]propionate (Table 1, Example I.179)
4.0 g of 3-chloro-2-(4-chloro-3-chlorosulfonylphenyl)-5-tri-
fluoromethylpyridine, 2.4 g of D,L-alanine ethyl ester hydro-
chloride and 5.2 g of triethylamine in 50 ml of anhydrous tetra-
hydrofuran were stirred at 23~C for 18 hours, after which the mix-
ture was concentrated. The residue was taken up in 100 ml of
methylene chloride. The solution was extracted twice with 30 ml
of water each time, dried over sodium sulfate and concentrated.
Chromatography of the residue on silica gel with cyclohexane/
ethyl acetate (4:1) resulted in 2.8 g of a colorless oil. Yield:
58%;
1H-NMR (270 MHz, in CDC13): b [ppm] = 1.15 (t,3H), 1.45 (d,3H),
3.98-4.14 (m,3H), 5.84 (d,lH), 7.68 (d,lH), 7.95 (dd,lH), 8.10
(s,lH), 8.53 (d,lH), 8.88 (s,lH).
Example 40
(S)-3-Chloro-2-[4-chloro-3-(2-methoxycarbonyl-1-pyrrolidinyl)phe-
nyl]-5-trifluoromethylpyridine (Table 1, Example I.181)
3.0 g of 3-chloro-2-(4-chloro-3-chlorosulfonylphenyl)-5-tri-
fluoromethylpyridine, 1.9 g of L-proline methyl ester hydrochlo-
ride and 3.9 g of triethylamine in 100 ml of anhydrous tetrahy-
drofuran were stirred at 23~C for 18 hours, after which the mix-
ture was concentrated. The residue was taken up in 100 ml of me-
thylene chloride. The solution was extracted twice with 30 ml of
water each time, dried over sodium sulfate and concentrated.
Chromatography of the residue on silica gel with cyclohexane/
ethyl acetate (4:1) resulted in 2.9 g of a colorless oil. Yield:
78%;
1H-NMR (270 MHz, in CDC13)s b [ppm] = 1.86-2.37 (m,4H), 3.48-3.79
(m,2H), 3.63 (s,3H), 4.65 (dd,lH), 7.66 (d,lH), 7.93 (dd,lH),
8.10 (s,lH), 8.57 (d,lH), 8.87 (s,lH).
Example 41
3-Chloro-2-[4-chloro-3-(2,5-dichloro-3-thienylaminosulfonyl)phe-
nyl]-5-trifluoromethylpyridine (Table 1, Example I.182)
~'O 95/02580 ~ PCTlEP94102263
47
3.0 g of 3-chloro-2-(4-chloro-3-chlorosulfonylphenyl)-5-trif-
fluoromethylpyridine and 1.7 g of 3-amino-2,5-dichlorothiophene
hydrochloride in a mixture of 100 ml of toluene and 100 ml of
pyridine were refluxed for 6 hours. The residue after concentra-
tion was dissolved in 100 ml of ethyl acetate. The solution was
extracted with 50 ml of dilute hydrochloric acid and 50 ml of
water, dried over sodium sulfate and concentrated. Chromatography
on silica gel with methylene chloride resulted in 0.6 g of color-
less crystals. Yield: 15%, melting point: 106-108~C.
Example 42
3-Chloro-2-(4-chloro-3-methoxymethylphenyl)-5-trifluoromethyl-
pyridine (Table 1, Example I.081)
7.0 g of a 30% by weight solution of sodium methoxide in methanol
were added to a solution of 5.0 g of 2-(3-bromomethyl-4-chloro-
phenyl)-3-chloro-5-trifluoromethylpyridine in 100 ml of anhydrous
methanol. The mixture was refluxed for 8 hours and then ,concen-
trated. The residue was taken up in 100 ml of 10% strength hydrp-
chloric acid, after which three extractions with 50 ml of ether'
each time were carried out. The ether phases were dried over
sodium sulfate and concentrated. The oily residue was induced to
crystallize with a little cold cyclohexane. Yield: 2.9 g (67%) of
colorless crystals; melting points 52-54~C.
Example 43
3-Chloro-2-(4-chloro-3-ethylthiomethylphenyl)-5-trifluoromethyl-
pyridine and 2-(4-chloro-3-ethylthiomethylphenyl)-3-ethyl-
thio-5-trifluoromethylpyridine (Table 1, Example I.097 and
Table 2, Example I.304)
A solution of 4.0 g of 2-(3-bromomethyl-4-chlorophenyl)-3-chloro-
5-trifluoromethylpyridine and 1.3 g of sodium thioethoxide in
80 ml of anhydrous dimethylformamide was stirred at 23~C for
18 hours and then refluxed for 4 hours. The cooled reaction mix-
ture was then poured into 400 ml of ice-water, which was then
extracted three times with 100 ml of tert-butyl methyl ether each
time. The combined organic phases were extracted with 100 ml of
water, dried over sodium sulfate and concentrated. The residue
was chromatographed on silica gel with n-heptane/tert-butyl
methyl ether (20:1).
Yield fraction 1: 1.0 g (26%) of a colorless oil (first compound
mentioned);
WO 95/02580 PCT/EP94/022~
48
1H-NMR (200 MHz, in CDC13): 8 [ppm] = 1.28 (t,3H), 2.55 (q,2H),
3.92 (s,2H), 7.50 (d,lH), 7.64 (dd,lH), 7.83 (d,lH), 8.06 (s,lH),
8.85 (s,lH).
Fraction 2s 1.5 g (37%) of colorless crystals; melting point:
62-63°C (second compound mentioned).
Example 44
3-Chloro-2-(4-chloro-3-hydroximinomethylphenyl)-5-trifluoro-
methylpyridine (Table 1, Example 1.129)
22.6 g of 3-chloro-2-(4-chloro-3-formylphenyl)-5-trifluoromethyl-
pyridine, 6.5 g of sodium bicarbonate and 5.4 g of hydroxylamine
hydrochloride in 100 ml of tetrahydrofuran were stirred at 23°C
for 24 hours. The tetrahydrofuran was then removed, after which
the residue was taken up in 100 ml of methylene chloride. The
solution was washed twice with 100 ml of water. The solid formed
during this was removed, washed with water and dried. ConcentraL
tion of the organic phase resulted in a residue which was puri-
fied by chromatography on silica gel With cyclohexane/ethyl ace=
tate (7:3). Yield: 26.1 g (82%) of colorless crystals; melting
point: 173-174°C
Example 45
3-Chloro-2-(4-chloro-3-ethoximinomethylphenyl)-5-trifluoromethyl-
pyridine (Table 1, Example 1.131)
4.0 g of 3-chloro-2-(4-chloro-3-formylphenyl)-5-trifluoromethyl-
pyridine and 4.4 g of a 45% strength aqueous ethylhydroxylamine
solution in 100 ml of tetrahydrofuran were refluxed for 3 hours
and then stirred at 23°C for 16 hours. The residue obtained after
evaporation was purified by chromatography on silica gel (mobile
phase: n-heptane/tert-butyl methyl ether $ 10:1). Yields 4.1 g
(91%) of an oil which slowly crystallized; melting point: 58-59°C.
Example 46
3-Chloro-2-(4-chloro-3-methoxycarbonylmethoximinomethylphe-
nyl)-5-trifluoromethylpyridine (Table 1, Example I.136)
4.0 g of 3-chloro-2-(4-chloro-3-hydroximinomethylphenyl)-5-tri-
fluoromethylpyridine, 1.8 g of potassium carbonate and 2.0 g of
methyl bromoacetate in 90 ml of dimethylformamide were stirred at
80°C for 8 hours and then at 23°C for 55 hours. The mixture was
then poured into 800 ml of ice-water, followed by extraction four
times with 150 ml of tert-butyl methyl ether each time. The com-
bined organic phases were washed with 150 ml of water, dried over
sodium sulfate and concentrated. The residue was purified by
~O 95/02580 PCT/EP94102263
49
chromatography on silica gel with cyclohexane/ethyl acetate
(10:1). Yield: 3.9 g (81%) of colorless crystals; melting point:
49~C.
Example 47
3-Chloro-2-[4-chloro-3-(1-ethoxycarbonylethoximinomethyl)phe-
nyl]-5-trifluoromethylpyridine (Table 1, Example I.140)
4.0 g of 3-chloro-2-(4-chloro-3-hydroximinomethylphenyl)-5-tri-
fluoromethylpyridine, 5.1 g of potassium carbonate and 6.7 g of
ethyl 2-bromopropionate in 80 ml of dimethylformamide were
stirred at 100~C for 12 hours. The mixture was cooled and then
poured into 800 ml of water. The mixture was then extracted four
times with 150 ml of tert-butyl methyl ether each time. The com-
bined organic phases were washed with 150 ml of water, dried over
sodium sulfate and concentrated. The residue was purified by
chromatography on silica gel with cyclohexane/ethyl acetate
(10:1). Yield: 4.0 g (77%) of a colorless oil.
1H-NMR (270 MHz, in CDC13): b [ppm] = 1.29 (t,3H), 1.56 (d,3H),
4.23 (q,2H), 4.85 (q,lH), 7.50 (d,lH), 7.72 (dd,lH), 8.05 (s,lH),
8.27 (d,lH), 8.65 (s,lH), 8.84 (s,lH).
Example 48
3-Chloro-2-(4-chloro-3-dimethoxymethylphenyl)-5-trifluoromethyl-
pyridine (Table 1, Example I.114)
50 ml of trimethyl orthoformate were added to 40.0 g of montmo-
rillonite R-10 in 250 ml of anhydrous dichloromethane, after
which a solution of 20.0 g of 3-chloro-2-(4-chloro-3-formylphe-
nyl)-5-trifluoromethylpyridine in 50 ml of dichloromethane was
added dropwise while stirring and cooling in ice. After stirring
at 23~C for 20 hours, the clay was removed and thoroughly washed
with dichloromethane. The dichloromethane phase was concentrated.
The residue was purified by chromatography on silica gel with
cyclohexane/ethyl acetate (10:1). Yield: 22.1 g (96%) of a color-
less oil.
1H-NMR (270 MHz, in CDC13): 8 [ppm] = 3.42 (s,3H), 5.70 (s,lH),
7.52 (d,lH), 7.72 (dd,lH), 8.06 (s,lH), 8.10 (d,lH), 8.87 (s,lH).
Example 49
3-Chloro-2-[4-chloro-3-(4-methyl-1,3-dioxolan-2-yl)phenyl]-5-tri-
fluoromethylpyridine (Table 1, Example I.118)
WO 95!02580 PCT/EP94/022~
2.0 g of 1,2-propanediol and 100 mg of p-toluenesulfonic acid
were added to 3.2 g of 3-chloro-2-(4-chloro-3-dimethoxymethylphe-
nyl)-5-trifluoromethylpyridine in 100 ml of anhydrous toluene.
The mixture was then refluxed for two hours and stirred at 23~C
5 for 16 hours. Extraction was carried out first with 50 ml of a
10% by weight sodium bicarbonate solution and then three times
with 80 ml of water each time, after which the organic solution
was dried over sodium sulfate and concentrated. The residue was
purified by chromatography on silica gel with cyclohexane/ethyl
10 acetate (lOsl). Yield: 3.1 g (94%) of a colorless oil (1:1 mix-
ture of diastereomers);
1H-NMR (270 MHz, in CDC13): S [ppm] = 1.39(d,3H), 1.41(d,3H),
3.58-3.68(m,2H), 4.12-4.49(m,4H), 6.23(s,lH), 6.37(s,lH),
15 7.50(d,2H), 7.74(dd,2H), 8.06(s,2H), 8.14(d,2H), 8.86(s,2H).
Example 50
3-Chloro-2-[4-chloro-3-(4-vinyl-1,3-dioxolan-2-yl)phenyl]-5-tri-
fluoromethylpyridine (Table 1, Example I.119)
3.2 g of 3-chloro-2-(4-chloro-3-dimethoxymethy!phenyl)-5-trifluo-
romethylpyridine, 2.3 g of 1-butene-3,4-diol and 100 mg of p-to-
luenesulfonic acid in 100 ml of anhydrous toluene were refluxed
for 12 hours. The solution was cooled and then washed with 50 ml
of 10% by weight aqueous sodium bicarbonate solution and three
times with 50 ml of water each time, dried over sodium sulfate
and concentrated. The crude product was purified by chromatog-
raphy on silica gel (mobile phase: cyclohexane/ethyl acetate =
10:1). Yield: 3.2 g (95%) of a colorless oil (1:1 mixture of
diastereomers);
IR (RHr): v [cm-1] = 1602, 1324, 1217, 1194, 1162, 1137, 1100,
1083, 1047, 988.
Example 51
3-Chloro-2-[4-chloro-3-(4,5-bis(methoxycarbonyl)-1,3-dioxolan-2-
yl)-phenyl]-5-trifluoromethylpyridine (Table 1, Example I.123)
3.0 g of 3-chloro-2-(4-chloro-3-dimethoxymethylphenyl)-5-tri
fluoromethyl- pyridine, 5.4 g of dimethyl L(+)-tartrate and 100
rng of p-toluenesulfonic acid in 180 ml of anhydrous toluene were
refluxed for 10 hours. The solution was cooled and then washed
with 50 ml of 10% by weight sodium bicarbonate solution and three
times with 50 ml of water each time, dried over sodium sulfate
and concentrated. Chromatography on silica gel resulted in 3.0 g
(77%) of colorless crystals; melting points 48-53~C.
~O 95102580 PCTlEP94/02263
51
Example 52
3-Chloro-2-[4-chloro-3-(2-chloro-2-methoxycarbonylvinyl)phenyl]-5
-trifluoromethylpyridine (Table 1, Example I.142)
13.7 g of methyl propiolate and 2.7 g of copper(II) chloride were
added to a solution of 2.5 g of tert-butyl nitrite in 100 ml of
anhydrous acetonitrile at O~C. Subsequently, while stirring at
O~C, a solution of 5.0 g of 2-(3-amino-4-chlorophenyl)-3-
chloro-5-trifluoromethylpyridine in 100 ml of anhydrous aceto-
nitrile was added dropwise. After the addition was complete, the
mixture was stirred at 20-25~C for 5 hours and then filtered. The
crude product obtained after concentration of the filtrate was
chromatographed on silica gel with cyclohexane/ethyl acetate
(100:1). Yield: 1.3 g (19%) of colorless crystals (1:1 mixture of
E and Z isomers); melting point: 71-73~C.
Example 53
(E)-3-Chloro-2-[4-chloro-3-(2-ethoxycarbonylvinyl)phenyl]-5-tri-
fluoromethylpyridine (Table 1, Example 1.146)
A solution of 1.35 g of sodium ethoxide in 20 ml of anhydrous
ethanol was added dropwise to 4.0 g of 3-chloro-2-(4-
chloro-3-formylphenyl)-5-trifluoromethylpyridine and 2.9 g of
triethyl phosphonatoacetate in 70 ml of anhydrous toluene. After
stirring at about 20~C for 44 hours, the solvent was removed. The
residue was taken up in 100 ml of 10% strength hydrochloric acid
and extracted three times with 100 ml of ethyl acetate each time.
The combined organic phases were washed with 100 ml of water,
dried over sodium sulfate and concentrated. Chromatography on
silica gel with cyclohexane/ethyl acetate (10:1) resulted in
3.4 g (71%) of colorless crystals; melting point: 118-120~C.
Example 54
3-Chloro-2-[4-chloro-3-(2-ethoxycarbonyl-2-methylvinyl)-
phenyl]-5-trifluoromethylpyridine (Table 1, Example I.149)
A solution of 1.75 g of sodium ethoxide in 30 ml of anhydrous
ethanol was added dropwise to 8.0 g of 3-chloro-2-(4-
chloro-3-formylphenyl)-5-trifluoromethylpyridine and 6.0 g of
triethyl 2-phosphonatopropionate in 80 ml of anhydrous toluene.
The mixture was stirred at about 20~C for 2 hours and then concen-
trated. The residue was taken up in 150 ml of ethyl acetate. The
solution was washed with 100 ml of 5% strength hydrochloric acid
and then three times with 100 ml of water each time, dried over
sodium sulfate and concentrated. The crude product was chromato
graphed with cyclohexane/ethyl acetate (10:1) on silica gel to
WO 95102580 ~ ~ ,~ PCT/EP94/022~'
52
afford 9.2 g (91%) of colorless crystals (85:15 mixture of E and
Z isomersj; melting point: 88-91~C.
Example 55
3-Chloro-2-(4-chloro-3-hydroxycarbonylphenyl)-5-trifluoromethyl-
pyridine (Table 1, Example I.102)
38.5 g of sodium perborate tetrahydrate were added in portions
over the course of 30 minutes to a solution of 16.0 g of
3-chloro-2-(4-chloro-3-formylphenyl)-5-trifluoromethylpyridine in
100 ml of glacial acetic acid at 100~C, followed by stirring at
100~C for 1.5 hours. The mixture was cooled and then poured into
400 ml of water. The product was extracted from the aqueous phase
with 3 x 100 ml of tert-butyl methyl ether. The combined organic
phases were dried over sodium sulfate and then concentrated. The
residue was triturated with n-hexane. The material insoluble in
hexane was removed and extracted by boiling twice with ether, the
pyridine N-oxide byproduct remaining undissolved. The combined '
ether phases were dried over sodium sulfate and concentrated to:
result in 13.7 g (81%) of colorless crystals; melting point:
149-151~C.
Example 56
3-Chloro-2-(4-chloro-3-methoxycarbonylphenyl)-5-trifluoromethyl-
pyridine (Table 1, Example I.103)
4.8 g of 3-chloro-2-(4-chloro-3-formylphenyl)-5-trifluoromethyl-
pyridine, 8.4 g of N-iodosuccinimide, 5.2 g of potassium car-
bonate and 120 ml of methanol were stirred at 23~C for 20 hours.
Most of the methanol was removed by distillation and then a solu-
tion of 7 g of Na2S203 pentahydrate in 200 ml of water was added,
after which the mixture was extracted three times with 100 ml of
tert-butyl methyl ether each time. The combined organic phases
were washed With 50 ml of water, dried over sodium sulfate and
concentrated. Yield: 4.9 g (93%) of a colorless oil;
1H-NMR (270 MHz, in CDC13): b [ppm] = 3.95(s,3H), 7.59(d,lH),
7.88(dd,lH), 8.08(s,lH), 8.32(d,lH), 8.87(s,lH).
Example 57
3-Chloro-2-(4-chloro-3-isopropoxycarbonylphenyl)-5-trifluoro-
methylpyridine (Table 1, Example I.106)
A small piece of sodium was added to a solution of 4.0 g of
3-chloro-2-(4-chloro-3-methoxycarbonylphenyl)-5-trifluoromethyl-
pyridine in 20 ml of anhydrous isopropanol, followed by stirring
~'O 95/02580 ~ C~ ~ PCT/EP94I02263
53
at O~C for 20 hours. The solvent was then removed. The residue was
taken up is 50 ml of water. The aqueous phase was extracted three
times with 50 ml of tent-butyl methyl ether each time. The com-
bined organic phases were dried over sodium sulfate and concen-
trated. Yield: 2.5 g (58%) of a colorless oil;
1H-NMR (270 MHz, in CDC13): b [ppm]: 1.41(d,6H), 5.32(h,lH),
7.58(d,lH), 7.86(dd,lH), 8.09(s,lH), 8.24(d,lH), 8.87(d,lH).
Example 58
3-Chloro-2-(4-chloro-3-chloroformylphenyl)-5-trifluoromethylpyri-
dine
23 g of 3-chloro-2-(4-chloro-3-hydroxycarbonylphenyl)-5-trifluo-
romethylpyridine in 23 ml of thionyl chloride were refluxed for
5 hours. The excess thionyl chloride was then removed by dis-
tillation. The remaining dark brown oil was used without~further
purification for the subsequent reaction.
Example 59
2-(3-Carbamoyl-4-chlorophenyl)-3-chloro-5-trifluoromethylpyridine
(Table 1, Example I.185)
A solution of 4.0 g of 3-chloro-2-(4-chloro-3-chloroformyl-
phenyl)-5-trifluoromethylpyridine in 10 ml of methylene chloride
was added dropwise to 100 ml of a 25% by weight aqueous ammonia
solution cooled to O~C. After 3 hours, the crystals which had
formed were separated off, stirred with n-hexane, again separated
off, washed with n-hexane and finally dried. Yield: 2.8 g (75%)
of colorless crystals; melting point: 167-168~C.
Example 60
3-Chloro-2-(4-chloro-3-dimethylaminocarbonylphenyl)-5-trifluoro-
methylpyridine
A solution of 3.5 g of 3-chloro-2-(4-chloro-3-chloroformyl-
phenyl)-5-trifluoromethylpyridine in 10 ml of methylene chloride
was added dropwise to 75 ml of a 40% by weight aqueous di-
methylamine solution cooled to O~C. The mixture was stirred at 0
to 5~C for 4 hours and then diluted with 225 ml of water, after
which it was extracted three times with 100 ml of tert-butyl
methyl ether each time. The combined organic phases were washed
twice with 100 ml of water each time, dried over sodium sulfate
and concentrated. The residue was chromatographed on silica gel
WO 95/02580 PCT/EP94/0226~
54
with cyclohexane/ethyl acetate (7:3). Yield: 2.2 g (61%) of
colorless~crystals; melting point: 90-91'C.
Example 61
3-Chloro-2-(4-chloro-3-ethoxyaminocarbonylphenyl)-5-trifluoro-
methylpyridine (Table 1, Example I.187)
A solution of 4.0 g of 3-chloro-2-(4-chloro-3-chloroformyl-
phenyl)-5-trifluoromethylpyridine in 10 ml of methylene chloride
was added dropwise to 80 ml of a 45% by weight aqueous ethyl-
hydroxylamine solution at 0 to 5'C. The mixture was stirred for
2 hours and diluted to 300 ml with water, after which it was
extracted three times with 100 ml of tart-butyl methyl ether each
time. The combined organic phases were washed twice with 100 ml
of water each time, dried over sodium sulfate and concentrated.
The residue was chromatographed on silica gel with cyclohexane/
ethyl acetate (7:3). Yield: 3.2 g (76%) of colorless crystals;
melting point: 162-163'C.
Example 62
Ethyl (2S)-2-[2-chloro-5-(3-chloro-5-trifluoromethyl-2-pyri-
dinyl)- benzoylamino]-3-methylbutanoate (Table 1, Example I.188)
A solution of 3.55 g of 2-chloro-2-(4-chloro-3-chloroformyl-
phenyl)-5-trifluoromethylpyridine in 10 ml of methylene chloride
was added dropwise to a mixture of 3.63 g of L-valine ethyl ester
hydrochloride, 7.9 g of pyridine and 30 ml of methylene chloride
at 23°C. After stirring for 60 hours, 160 ml of methylene chloride
were added. The mixture was washed three times with 150 ml of
water each time, dried over sodium sulfate and then concentrated.
The crude product was purified by chromatography on silica gel
with cyclohexane/ethyl acetate (10:1). Yield: 2.2 g (48%) of
colorless crystals; melting point: 90-92'C.
Example 63
The preparation took place as shown in the following scheme:
45
~O 95/02580 '~ ~ PCT/EP94/02263
F3C / I 1 (HO)~g ~ ~ F F3C / I C1
\N
N~C1 Pd [P (CsHs) s] 4 ~ I
w
5
F
F3C C1
I
HN03
10 -~ N ~ I
H2SOQ
F
N02
3-Chloro-2-(4-fluoro-3-nitrophenyl)-5-trifluoromethylpyridine
(Table 3, Example I.409)
In a preparation similar to that described above for
3-chloro-2-(4-chloro-3-nitrophenyl)-5-trifluoromethylpyridine,
nitration of 66.6 g of 3-chloro-2-(4-fluorophenyl)-5-trifluorome-
thylpyridine with 22.8 g of 100 strength nitric acid resulted in
62.6 g (81~) of a colorless oil which slowly crystallized.
1H-NMR (250 MHz, in CDC13): 8 [ppm] = 7.47(t,lH), 8.09-8.19(m,2H),
8 . 60 (dd,1H) , 8 . 89 (s,1H) .
Precursor: 3-chloro-3-(4-fluorophenyl)-5-trifluoromethylpyridine
In a preparation similar to that described above for
3-chloro-2-(4-chlorophenyl)-5-trifluoromethylpyridine, use of
55.0 g of 2,3-dichloro-5-trifluoromethylpyridine, 35.6 g of
4-fluorobenzeneboronic acid, 1.0 g of tetrakis(triphenylphos-
phine)palladium, 64.2 g of sodium bicarbonate, 300 ml of dime-
thoxyethane and 500 ml of water resulted in 65.0 g (93~) of
colorless crystals; melting point: 41-42°C.
Example 64
3-Chloro-2-(4-cyano-3-ethylsulfonylami.nophenyl)-5-trifluoro-
~0 methylpyridine (Table 3, Example 1.404)
The preparation took place as shown in the following scheme:
WO 95/02580 PCT/EP94/0226.~
56
F3C / Cl KCN F3C , Cl
~N / I CHON(CH3)2 \N
\ \
NOy F CN
N02
Fe F3C / C1 2 CZHS-S02C1
CH3COOH ~N I / N(C2H5)3~ CH2C12
CN
NH2
F3C , Cl F3C , Cl
N ~ ~ NaOCyHS N
\ \
CN CaHsOH CN
/ N\ / N\
C2H5-02S S02C2H5 H S02C2H5
1st reaction step:
3-Chloro-2-(4-cyano-3-nitrophenyl)-5-trifluoromethylpyridine
(Table 3, Example I.401)
5.0 g of 3-chloro-2-(4-fluoro-3-nitrophenyl)-5-trifluoromethyl-
pyridine and 1.5 g of potassium cyanide in 50 ml of dimethyl-
formamide were heated at 50~C for 4 hours and then stirred at 23~C
for 20 hours. The mixture was then poured into 200 ml of water.
The aqueous phase was extracted three times with 100 ml of tert-
butyl methyl ether each time. The combined organic phases were
washed twice with 50 ml of water each time, dried over sodium
sulfate and concentrated. The residue was purified by chroma-
tography on silica gel (mobile phase: cyclohexane/ethyl acetate =
9:1). Yield: 2.7 g (53%) of a yellow oil which slowly crystal-
lized.
1H-NMR (400 MHz, in CDC13): 8 [ppm] = 8.10(d,lH), (8.20 (s,lH),
8.36(dd,lH), 8.82(d,lH), 8.94(s,lH).
~'O 95/02580 ~"~ ~ ~~ ~ PCT/EP94/02263
57
2nd reaction step:
2-(3-Amino-4-cyanophenyl)-3-chloro-5-trifluoromethylpyridine
(Table 3, Example I.402)
In a preparation similar to that described above for 3-chlo-
ro-2-(3-amino-4-hydroxyphenyl)-5-trifluoromethylpyridine, use of
21.1 g of 3-chloro-2-(4-cyano-3-nitrophenyl)-5-trifluoromethylpy-
ridine, 10.8 g of iron powder, 116 ml of methanol and 58 ml of
glacial acetic acid resulted, after final trituration in a little
ether, in 18.7 g (98%) of a dark oil.
1H-NMR (270 MHz, in d6-DMSO): b [ppm] = 6.30(s,br.,2H),
6.85(s,br.,lH), 7.15(s,br.,lH), 7.55(s,br.,lH), 8.60 s,br.,lH),
9.05(s,br.,lH).
3rd reaction step:
3-Chloro-2-[4-cyano-3-bis(ethylsulfonyl)aminophenyl]-5-tri- '
fluoromethylpyridine (Table 3, Example I.403)
In a preparation similar to that described above for
3-chloro-2-[4-chloro-3-bis(methylsulfonyl)aminophenyl]-5-tri-
fluoromethylpyridine, use of 4.0 g of 2-(3-amino-4-cyano-
phenyl)-3-chloro-5-trifluoromethylpyridine, 1.7 g of ethane-
sulfonyl chloride, 1.5 g of triethylamine and 100 ml of dichloro-
methane resulted in 4.2 g (65%) of colorless crystals.
1H-NMR (270 MHz, in CDC13): b [ppm] = 1.53(t,6H), 3.7-3.82(m,4H),
7.91(s,lH), 8.02(d,lH), 8.07(d,lH), 8.12(s,lH), 8.90(s,lH).
4th reaction step:
3-Chloro-2-(4-cyano-3-ethylsulfonylaminophenyl)-5-trifluoro-
methylpyridine (Table 3, Example I.404)
In a preparation similar to that described above for 3-chlo
ro-2-(4-chloro-3-methylsulfonylaminophenyl)-5-trifluoromethyl
pyridine, use of 4.2 g of 3-chloro-2-[4-cyano-3-bis(ethyl-
sulfonyl)aminophenyl]-5-trifluoromethylpyridine, about 100 mg of
sodium ethoxide and 100 ml of ethanol resulted in 2.0 g (59%) of
colorless crystals;
1H-NMR (270 MHz, in CDC13): b [ppm] = 1.44(t,3H), 3.24(9,2H),
7.45(s,lH), 7.63(d,lH), 7.72(d,lH), 8.10 (s,2H), 8.90(s,lH).
Tables 1 to 6 which follow list further compounds I which have
been or can be prepared by one of the processes described.
WO 95/02580 ~ PCT/EP94/022~'
58
Table 1
F3C ~ C1
Ia (Rl=R3=RS=H
~ CI R2=CF3; R4, R6=Cl)
R~
No. R~ M.p. / IR (cm-1] ~ iH-NMR
(ppm]
L001 -OCH3 72 ~C
L002 -O-CH2-CH3
L003 -O-CH2CHZCH3
L004 -O-CH(CH3h 1.41(d,6H), 4.63(h,lH), 7.30(dd,lH),
7.35(d,lH), 7.49(d,lH), 8.08(s,lH),
8.85(d,lH)
L005 -O-CH2-CHZ-CZHS
L006 -O-CH(CH3)-C2Hg
L007 -O-CHZ-CH(CH3n
L008 -O-CHZ-CHZ-CHZ-C2H5
L009 -O-CH2-CH=GH2
LO10
H ~ / C1
C=
-OCHy / ' H
LOll
H~ / H
C=
-OCHy / ~ Cl
L012 -O-CH2-C-C-H 102 - 103 ~C
L013 -O-CH(CH3)C=C-H 96-98C
L014 -O-CH2-CO-OCH3 109 - 110 ~C
LO15 -O-CHZ-CO-OCZHS 62 - 63 oC
L016 -O-CH(CH3)-CO-OCH3 102 -103 ~C
L017 -O-CH(CH3)-CO-OC2H5 1.28(t,3H), 1.73(d,3H), 4.21(q,2H),
4.80(q,lH), 7.31(d,lH), 7.39(dd,iH),
7.50(d,lH), 8.10(s,iH), 8.84(s,iH).
L018 -O-cyclopentyl
L019 -O-CH2-C-N 85 - 86 C
L020 -O-CH(CH3)-C-N 75 - 76 oC
L021 -O-H 105 -107 C
L022 -O-CH2-CO-O-(CH2)a-CH3
~'O 95/02580 PCT/EP94/02263
59
No. R~ M.p. / IR (cm-1) / 1H-NMR
(ppm)
L023 -O-CH(CH3)-CO-O-(CH2)4-CH3
L024 -O-CH2-phenyl _
L025 -S-CH3 96-97C
L026 -S-CZHS
L027 -S-CH2-C2H5 -
L028 -S-CH(CH3~ 1.39 (d,6H), 3.57 (h,lH),
7.45-7,58
(m,2H), 7.78 (d,iH), 8.05
(s,lH9, 8.86
(s,iH)
L029 -S-CHZ-CH2-C2H5
L030 -S-CH(CH3)-CZHS
L031 -S-CH2-CH(CH3h
L032 -S-CH2-GHZ-CH2-C2H5
L033 -S-CH2-CH=CH2 3.65 (d,2H), 5.15 (d,lH),
5.26 (d,lH),
5.84-6.02 (m,lH), 7.44-7.59
(m,2H), 7.72
(s,iH), 8.06 (s,iH), 8.86
(s,lH)
L034
H ~
C1
/
C=
~ \
-SCH2
H
L035
H~ H
~
C=
/ \
Cl
-SCHZ
L036 -S-CH2-C-C-H 91-92C
L037 -S-CH(CH3)-C=C-H
L038 -S-CH2-CO-OH 144-145C
L039 -S-CH2-CO-OCH3 88-89C
L040 -S-CH2-CO-OC2H5 73C
L041 -S-CH(CH3)-CO-OCH3 1.59 (d,3H), 3.68 (s,3H),
3.99 (q,lH), 7.55
(d,lH), 7.67 (dd,lH), 7.97
(d,lH), 8.07
(s,lH), 8.86 (s,lH)
L042 -S-CH(CH3)-CO-OC2H5 1.14 (t,3H), 1.58 (d,3H),
4.00 (q,lH), 4.12
(q,2H), 7.54 (d,lH), 7.66
(dd,IH), 7.97
(d,lH), 8.07 (s,lH), 8.85
(s,lH)
L043 -S-cyclopentyl
L044 -S-CH2-C-N 97-98C
L045 -S-CH(CH3)-C-N 1.72 (d,6H), 4.09 (q,lH),
7.62 (d,lI~, 7.78
(dd,lH), 8.07 (d,lH), 8.13
(s,lH), 8.85
(s,lH)
PCTIEP94/022~
WO 95/02580
No. R M.p. / IR [cm'1] ! 1H-NMR
[PPmJ
L046 -S-CH2-CO-O-(CH2)4-CH3 1.86 (t,3H), 1.18-1.42 (m,4H),
1.58
(p,2H), 3.76 (s,2H), 4.11
(t,2H), 7.51
(d,lH), 7.60 (dd,lH), 8.83
(d,lH), 8.06
(s,lH), 8.85 (s,lH)
L047 -S-CH(CH3)-CO-O-(CH2)4-CH3
L048 -S-CH2-phenyl
L049 -S-CH2-(4-Cl-phenyl)
LO50 -SOZ-Cl 7.79 (d,lH), 8.10(d,iH),
8.13(dd,lH), 8.65
(d,lH), 8.88(d,lH).
LO51 -S02-NH2 176 ~C
L052 -SOZ-NH-CH3 5.15 (q,lH), 7.68(d,iH),
7.98 (dd,lH),
8.10 (d,lH), 8.57(s,lH),
8.89 (s,lH).
L053 -S02-N(CH3)2 2.95 (s,6H), 7.68(d,lH),
7.94(dd,lH, 8.10
(d,iH), 8.53(d,lH), 8.88(d,lH).
L054 -S02-NH-CZHS 1.14(t,3H), 3.04(q,2H), 5.02(t,lH),
7.68(d,lH), 7.95(d,lH), 8.10(d,lH),
8.59(d,lH), 8.89(d,lH).
LO55 -SOZ-NH-CH(CH3~y 104-108C
L056 -S02-N(CH3)-C2H5
L057 -S02-N(C2Hgh 77 ~C; 1.18 (t,6H), 3.42
(q,4H), 7.65
(d,lH), 7.90 (dd,lH), 8.10
(d,lH), 8.57
(d,lH), 8.87 (d,lH)
L058 -SOZ-(pyrrolidin-1-yl) 104 - 105 C
L059 -SOZ-(piperidin-1-yl) 87-88C
L060 -S02-(morpholin-4-yl) 114 -115 C
L061 -S02-NH-phenyl
L062 -S02-N(CH3)-phenyl
L063 -S02-NH-CH2-phenyl
L064 -N02 68 - 69 ~C
L065 -NH2 88 - 90 0C
L066 -NH-SOZ-CH3 133 -134 ~C
L067 -N(SOZ-CH3)Z 230 - 231 ~C
L068 -NH-SOZ-C2Hg 100 -102 ~C
L069 -N(S02-C2Hg) 204 - 205 ~C
L070 -NH-SOZ-CHZ-CZHS
L071 -NH-CHO
L072 -NH-CO-CH3
L073 -NH-CO-CZHS
L074 -N(CO-CH3)-S02-CH3
L075 -N(CO-CH3)-S02-C2H5
L076 -CH3 40 - 42 oC
~'O 95/02580 , 5, ~ PCT/EP94/02263
61
No. R M.p. l IR [cm-1) / iH-NMR
[ppm)
L077
\ / CH3
H/C C \ H
L078
\ /H
C= C
H / \CH3
L079 -CHBr2 75-77C
L080 -CH2-Br 71- 72 oC
L081 -CHZ-O-CH3 52 - 54 oC
L082 -CHZ-O-CzHS ~ oC
L083 -CHZ-O-(CHZ)2-CH3 0.97(t,3H), 1.68(se,2H), 3.56(t,2H),
4.68(s,2H), 7.46(d,lH), 7.64(dd,lH),
7.95(d,lH), 8.05(d,IH), 8.85(d,lH)
L084 -CH2-O-CH(CH3)2 89 oC
L085 -CHZ-O-(CH2~-CH3
L086 -CHZ-O-CH(CH3)-CZHS
L087 -CH2-O-CHZ-CH(CH3n
L088 -CHZ-O-GHZ-CH=CH2 4.15(d,2H), 4.70(s,2H), 5.19-5.44(m,2H),
5.88-6.09(ml,H), 7.50(d,lH),
7.68(dd,iH), 7.98(d,lH), 8.08(d,lH),
8.86(d,lH)
L089 -CH2-O-CHz-C-C-H 49 oC
L090 -CH2-O-CH2-CO-OCH3
L091 -CH2-O-CH2-CO-OC2H5
L092 -CHZ-OCH(CH3)-C-C-H 86-88C
L093 -CH2-O-CH(CH3)-CO-OCH3
L094 -CH2-O-CH(CH3)-CO-OC2H5
L095 -CH2-O-cyclopentyl
L096 -CH2-SCH3 62 - 64 C
L097 -CH2-SCZHS 1.28 (t,3H), 2.55 (q,2H),
3.92 (s,2H), 7.50
(d,lH), 7.64 (dd,lH), 7.83
(d,iH), 8.06
(s,lH), 8.85 (s,lH)
L098 -CHZ-S-(CHZ)2-CH3 0.98 (t,3H), L62 (se,2H),
2.50 (t,2H), 3.88
(s,2H), 7.44 (d,lH), 7.63
(dd,lH), 7.82
(d,lH), 8.04 (s,iH), 8.84
(s,iH)
L099 -CH2-S-CHZ-CO-OCH3
L100 -CH2-S-CHZ-CO-OC2H5
L101 -CH2-N(CH3)Z
L102 -CO-OH 149-151C
WO 95/02580 t ~ PCTIEP94/0226~
62
No. R~ M.p. l IR [cm'1J / iH-NMR
[ppm]
L103 -CO-OCH3 3.95 (s,3H), 7.59 (d,lH),
7.88 (dd,lH),
8.08 (s,iH), 8.32 (d,lH),
8.87 (s,iH)
L104 -CO-OC2H5 1.42 (t,3H), 4.44 (q,2H),
7.57 (d,iH), 7.88
(dd,lH), 8.08 (s,lH), 8.30
(d,lH), 8.87
(s,lH)
L105 -CO-O-(CHZ)2-CH3 1.07 (t,3H), 1.83 (se,2H),
4.34 (t,2H), 7.59
(d,lH), 7.88 (dd,lH), 8.09
(s,iH), 8.32
(d,lH), 8.88 (s,lH)
L106 -CO-OCH(CH3)2 1.41 (d,6H), 5.32 (h,lH),
7.58 (d,lH), 7.86
(dd,lH), 8.09 (s,iH), 8.24
(d,lH), 8.87
(d,lH)
L107 -CO-O-(CH2)3-CH3
L108 -CO-OCH(CH3~C2H5
L109 -CO-OCHZ-CH(CHg~
L110 -CO-O-(CH2)4-CH3
L111 -CO-OCHZ-CHZ-OCH3
L112 -CO-OCH2-CH2-OCZHS
L113 -CHO 94 ~C
L114 -CH(OCH3h 3.42 (s,3H), 5.70 (s,lH),
7.52 (d,lH), 7.72
(dd,lH), 8.06 (s,iH), 8.10
(d,lH), 8.87
(s,lH)
L115 -CH(OCZHsh 1.27 (t,6H), 3.53-3.80 (m,4H),
5.80 (s,lH),
7.49 (d,iH), 7.70 (dd,lH),
8.04 (s,iH),
8.13 (d,lH), 8.86 (s,lH)
L116 -CH(OCH2-CZHS)2
L 117 1,3-Dioxolan-2-yl
L118 4-Methyl-1,3-dioxolan-2-yl1.39 (d,3H), 1.41 (d,3H),
3.58-3.68
(m,2H), 4.12-4.49 (m,4H),
6.23 (s,lH),
6.37 (s,iH), 7.50 (d,2H),
7.74 (dd,2H),
8.06 (s,2H), 8.14 (d,2H),
8.86 (s,2H)
(ca. 1:1 mixture of diastereomers)
L119 4-V'myl-1,3-dioxolan-2-yl 1602, 1324, 1217, 1194, 1162,
1137, 1100,
1083, 1047, 988
(ca. 1:1 mixture of diastereomers)
L120 4,5-Dimethyl-1,3-dioxolan-2-yl1602, 1380, 1324, 1194, 1162,
1138,1101,
1084, 1041, 909
(ca. 1.5:1.5:1 mixture of
diastereomers)
L121 4-Ethyl-1,3-dioxolan-2-yl 1602,1382, 1324, 1217,1194,
1162, 1138,
1100, 1082, 1046
(ca. 1.5:1 mixture of diastereomers)
L122
O
- CH
~S
CA 02167291 2005-02-07
63
No. R ~ M.p. / IR [cm-1] / 1H-NMR
[ppm]
L123 4,5-Bis(methoacy- 4853C
carbonyl)-1,3-dioaolan-2-yl
.
L 124
o
- CH
~S
L125 1,3-Dithiolan-2-yl 3.3-3.5 (m,4H), 6.12 (s,iH),
7.47 (d,lH),
7.62 (dd,1H), 8.04 (s,1H),
8.29 (d, i H),
8.85 (s,lH)
L 126 4-Methyl-1,3-dithiolan-2-yl1601,1451, 1377,1324,1162,
1137, 1099,
1084, 1045, 766
(ca 60:40 mixture of diastereomers)
L127
0
~O
L128 90-91~C
S_
~S
L129 -CH=N-OH I73 - 174 C
L 130 -CH=N-OCH3 65 - 67 C
L131 -CH=N-OCzHs 58 - 59 ~C
L132 -CH=N-OCHz-CZHS
L133 -CH=N-OCH(CHg}~
L134 -CH=N-OCHZ-CHz-CZHS
L135 -CH=N-OCHZ-CO-OH
L136 -CH=N-OCHz-CO-OCH3 49C
L137 -CH=N-OCHZ-CO-OC2Hg 49C
LI38 -CH=N-OCH(CHg)-CO-OH
L139 -CH=N-OCH(CH3~C0-OCH3 1.56 (d,3H), 3.77 (s,3H),
4.88 (q,iH), 7.50
(d,iH), 7.72 (dd,lH), 8.06
(s,iH), 8.27
(d,lH), 8.66 (s,lH), 8.87
(s,iH)
L140 -CH=N-OCH{CH3)-CO-OC2H5 1.29 (t,3H), 1.56 (d,3H),
4.23 {q,2H), 4.85
(q,lH), 7.50 (d,lH), 7.72
(dd,lH), 8.05
(s,iH), 8.27 (d,lH), 8.65
(s,lH), 8.84
(s,lH)
~
I.141 -CH=C(CI)-COOH
L142 -CH=C(Cl)-CO-OCH3 71 - 73 C
(cis/trans ca. 1:1)
WO 95/02580 PCT/EP94/022t~
64
No. R~ M.p. ! IR [cm-1] / iH-NMR
[PPS]
L143
\ / Cl
C = C \
OC
H
~ CO
y
S
H
-
L144
\ H
C=C~
~ COOH
H
L 145 97 - 98 C
\ H
C = C ~
OCH
~ CO
H
-
3
L146 118-120C
-
\ H
C = C ~
H
OC
~ CO
5
-
2
H
L147
\ / CH3
C = C \
~ COOH
H
L148 -CH=C(CH3)-CO-OCH3 103C
(cis/trans ca. 5:95)
L 149 -CH=C(CH3)-CO-OC2Hg 88-91C
(cis/trans ca. 15:85)
L150
\ / Cl
C=C\
~ CO
NH
H
-
2
L 151
\ / Cl
C = C
\
H ~ CO-NHCH
L152
\ / C1
C = C \
CH
)
~ CO-N
g
y
H
(
2.~ ~ ~'~~~ ~
O 95/02580 PCT/EP94/02263
No. R M.p. / IR [cm-1) / 1H-NMR
[PPS)
L153
\ /H
C= C
/ \CO
NH
-
H
y
L154
\ /H
C= C
H ~ \CO
NHCH
-
3
L 155
\ /H
C=C
H ~ \CO
N
CH
-
(
g ) y
L156
\ / CH3
C= C
H ~ \CO
-NH2
L157
\ / CHg
C= C
H ~ \
CO-NHCHg
L158
\ / CH3
C= C
H ~ \
CO-N ( CH3 ) 2
L159
\ / Cl
C= C
H ~ \C
O-CH3
L 160
\ /H
C= C
H ~ \CO
CH
-
3
L161
\ / CH3
C=C
H ~ \CO
-CH3
WO 95/02580 ~ ~ PCT/EP94/022~
66
No. R~ M.p. / IR (cm'1) / 1H-NMR (ppm)
L 162 -CH2-CH(Cl)-CO-CH3
L163 -CHZ-CH(Cl)-CO-OCH3 1749, 1602, 1324, 1217, 1199, 1163, 1138,
11(D0, 1085, 1049
L164 -CH2-CH(Cl)-CO-OC2H5
L165 ~ CF3 133 - 134 °C
- ~~ ~ \
I I
0
L166 Cl 158 - 159 °C
II
- rt- s
II
0
ci
L167 ~ 156 -157 °C
II ~\
- rr- s ~ c1
II
0
L168 ~ 191-193°C
(I- ~~ \- No
-r1-s V
II
0
L 169 0 114 - 115 °C
_ II I
N- II s ci
0
L 170
II i
- rr- s s
II
0
L171 ~ II
- I~ S
s
L172 -N[S02-(5-chlorthien-2-yl))2 167-168°C
L173 -NH-SOZ-(2,5-dichlorthien-3-yl) 132-133°C
L174 -NH-SOZ-(4,5-dichlorthien-2-yl) 154-155°C
L175 -NH-S02-(4,5-dibromthien-2-yl) 157-158°C
CA 02167291 2005-02-07
67
No. R M.p. / IR (cm-1] / 1H-NMR
[ppm]
L176 -NH-S02-(3,5-dimethylisozazol-4-yl)161-162C
L177 -NH-S02-thien-2-yl 144-145C
L178 -NH-S02-tluen-3-yl
L179 -S02-NH-CH(CH3)-CO-OCZHS 1.15 (t,3H), 1.45 (d,3H),
3.98-4,14
(aus D,IrAlanin) (m,3H), 5.84 (d,lH), 7.68
(d,lH), 7.95
(dd,lH), 8.10 (s,lH), 8.53
(d,lH), 8.88
(s,l~
L180 -S02-N(CH2-CZHS)2 0.85 (t,6H), 1.58 (se,4H),
3.32 (t,4H), 7.64
(d,lH), 7.90 (dd,lH), 8.10
(s,lH), 8.57
(d,lH), 8.87 (s,lH)
L181 1.86-2.37 (m,4H), 3.48-3.79
(m,2H), 3.63
(s,3H), 4.65 (dd,lH), 7.66
(d,lH), 7.93
(dd,lH), 8.10 (s,lH), 8.57
(d,l.H), 8.87
(s,1H)
H3COOC
L182 -S02-NH-(2,4-dichlorthien-3-yl)106-108C
LI83 -CH2-SCH(CHjn 1.30 (d,6H), 2.92 (h,lH),
3.94 (s,2H), 7.50
(d,lH), 7.62 (dd,lH), 7.89
(d,lH), 8.07
(s,iH), 8.88 (s,iH)
L184 -SCH2-CO-O(cyclopentyl) 71-72C
L185 -CO-N~iZ 167-168C
L186 -CO-NH-CH3 163-164C
L187 -CO-NH-OC2H5 162-163C
L188 -CO-NH-CH(CH(CH3~]- 90-92C
COOC2H5
L189 -CO-NH-CH(CH3)-CO-OCH3 120-124C
L 190 -NH-CO-OCH3 120-122C
L191 -NH-CO-CO-OCH3 95-97C
L192 -Cl 1601, 1449, 1321, 1164,1137,
1098, 1082,
1034, 766, 723
L193 -CH2-O-N=C(CHg~ 85-86C
L194 -S02-CH3 116-117C
WO 95/02580 ~, . PCT/EP94/0226:~
68
Table 2
R2 R4
I I~R1~R3~R5=H;
N i I R . Cl)
R~
No. R2 R4 R~ p~ ~~
H
1
L301 CF3 -S-CH(CH3)2 CH2-S-CH-(CH3yl 1.28 (d,6H), 1.32
(d,6H), 2.94 (h,lH),
3.35 (h,lH), 3.92
(s,2H), 7.43-7.50
,
(m,2H), 7.75 (d,lH),
7.90 (s,lH), 8.72
(s,lHj
L302 CF3 -S-CH2-C2H3 CH2-S-CHZ-C2H5
L303 CF3 -S-CH3 CH2-S_CH3 106_108C
L304 CF3 -S-C2H3 CH2-S-CZHS 1.29 (t,3H), 1.32
(t,3H),
2.55 (q,2H), 2.90
(q,2H), 3.90 (s,2H),
7.45-7.58 (m,2H),
7.73
(s,lH), 7.81 (s,lH),
8.70 (s, l H);
62-63C
L305 CF3 -O-CH2-CH=CH2 CHZ-O-CH2-CH=CH2 2
X
4.67 (m,4H),
4.6
-
5.18-5.50 (m,4H),
5.88-6.14 (m,2H),
7.40-?.49 (m,2H),
7.88
(dd,lH), 8.18
(s,lH),
8.57 (s,lH)
L306 CF3 -O-CH(CH3)-C=C-H -CH20-CH(CH3)-C=CH66-67C
L307 CF3 -S-CH3 -O-CH2-C_--._C-H 103-104C
L308 Cl Cl -O-CH2-CN 136-137C
L309 CI Cl -O-CHZ-CH=CHZ 64-66C
L310 CI Cl -O-CH2-C-C-H 133-134C
L311 Cl Q -O-CH(CH3)-C-C-H 146-147C
L312 CI Cl -O-CH(CH3~ 1.41 (d,6H), 4.64
(h,lH), 7.25 (dd,1H),
7.32 (d,lH), 7.45
(d,lH), 7.82 (d,lH),
8.53 (d,lH)
~O 95/02580 ~ ~~ PCT/EP94/02263
69
No. R2 R4 R m
H ~R p
J
L313CI CI -O-CH(CH3)-CO-OCH31.72 (d,3H), 3.77
(s,3H), 4.87 (q,lH),
7.26 (d,1H), 7.34
(dd,lH), 7.49
(d,lH),
7.84 (d,iH), 8.54
(d,lH)
L314CI Cl -O-CH(CH3)-CO-OCZHS1.26 (t,3H), 1.72
(d,3H), 4.22 (q,2H),
4.83 (q,iH), 7.26
(d,lH), 7.34 (d,lH),
7.48 (dd,iH),
7.82
(d,lH), 8.54 (d,lH)
L315CI Cl -O-CHZ-CO-OCH3 140-141C
L316CI Cl -O-CHZ-CO-OC2H5 67-68C
L317CI CI -O-CH3 122-123C
Table 3
F3C Cl
I~R1~R3~R5=H
N ~ I R -
R6
R~
No. R6 R~ M.p./IR (cm-1]/
iH-NMR [PPmJ
L401 CN -N02 8.10 (d,lH), 8.20 (s,iH),
8.36 (dd,iH),
8.82 (d,lH), 8.94 (s,lH)
L402 CN -NH2 6.30 (s,br.,2H), 6.85 (s,br.,lH),
7.15
(s,br.iH), 7.55 (s,br.lH),
8.60 (s,br.,lH),
9.05 (s,br.,iH).
(Solvent: DMSO-d6)
L403 CN -N(S02-CZHS)2 1.53 (t,6H), 3.7-3.82 (m,4H),
7.91 (s,lH),
8.02 (d,iH), 8.07 (d,lH),
8.12 (s,lH),
8.90 (s,iH)
L404 CN -NH-S02-C2H5 1.44 (t,3H), 3.24 (q,2H),
7.45 (s,lH),
7.63 (d,lH), 7.72 (d,lH),
8.10 (s,2H),
8.90 (s,lH)
L405 CN -N(S02-CH3)2 3.58 (s,6H), 7.91 (s,iH),
7.96 (d,lH),
8.09 (s,lH), 8.12 (s,iH),
8.90 (s,lH)
L406 CN -NH-S02-CH3 3.20 (s,3H), 7.32 (s,lH),
7.68 (dd,lH),
7.78 (d,lH), 8.12 (s,2H),
8.90 (s,lH)
WO 95/02580 ~, ~. PCT/EP94/0226~
No. R6 R~ M.p./IR [cm-ij/
- 1H-NMR [PPmj
1,407 CN -CH2-CH(CI)-CO-OC2H5 1.29 (t,3H), 3.49 (dd,lH),
3.72 (dd,lH),
4.27 (q,2H), 4.64 (t,iH),
7.77-7.88
(m,3H), 8.10 (s,lH), 8.89
(s,lH)
L408 F CH3 2.35 (s,3H), 7.10 (t,lH),
7.55-7.63
(m,2H), 8.02 (s,lH), 8.81
(s,iH)
L409 F NOZ 7.47 (t,lH), 8.09-8,19 (m,2H),
8.60
(dd,lH), 8.89 (s,lH)
Table 4
g3C ~ C1
N Ia (Rl=R3=H~ R2=~3~ R4=R6=Cl)
R~
No. R M.p. / IR [cm-lj
~ -
iH-~ [PPmj
L501 -OCH3 105-106C
L502 -O-CH2-CH3
L503 -O-CH2~2~3
L504 -O-CH(CH3h
Ia505 -O-CHZ-CH2-CZHS
Ia506 -O-CH(CH3)-CZHS
L507 -O-CHz-CH(CH3~
L508 -O-CHZ-CHZ-CH2-C2H5
L509 -O-CH2-CH=CH2
L510
H ~ / C1
C= ~
-OCH2 / H
L511
H~ / H
C=
~ C1
-OCHy
L512 -O-CH2-C-C-H 97-98C
L513 -O-CH(CH3)C-C-H
L514 -O-CH2-CO-OCH3
L515 -O-CHZ-CO-OCZHS
L516 -O-CH(CH3)-CO-OCH3
~'O 95/02580 PCT/EP94/02263
71
No. R~ M.p. / IR [cm'1)
/
iH-NMR [PPm)
L517 -O-CH(CH3)-CO-OCZHS
L518 -O-cyclopentyl
L519 -O-CH2-C-N
L520 -O-CH(CH3)-C-N
L521 -OH 111-112C
L522 -O-CH2-CO-O-(CH2)4-CH3
L523 -O-CH(CH3)-CO-O-(CH2)4-CH3
L524 -O-CHZ-phenyl
L525 _S_~3
L526 -S-CZHS
L527 -S-CH2-C2H5
L528 -S-CH(CH3h
-
L529 _S-CH2-CH2-C2H5
L530 -S-CH(CH3)-C2H5
L531 -S-CHZ-CH(CH3)2
L532 -S-CH2-CH2-CHZ-C2H5
L533 -S-CHZ-CH=CH2
L534
H \ / C1
C=
-SCHZ ~ \ H
L535
H\ / H
C=
-SCH2 / \ C1
L536 -S-CH2-C-C-H
L537 -S-CH(CH3)-CC-H
L538 -S-CHZ-CO-OCH3
L539 -S-CH2-CO-OC2H5
L540 -S-CH(CH3)-CO-OCH3
L541 -S-CH(CH3)-CO-OC2H5
L542 -S-cyclopentyl
L543 -S-CHZ-C-N
I L544 -S-CH(CH3)-C-N
L545 -S-CHZ-CO-O-(CHZ)4-CH3
L546 -S-CH(CH3)-CO-O-(CH2)4-CH3
L547 -S-CHZ-phenyl
WO 95/02580 ~ PCT/EP94/0226~
72
No. R~ M.p. / IR [cm'1]
/
iH-NMR [PFm]
L548 -S-CHZ-((4-Cl-phenyl)
L549 -SOZ-Cl
L550 -S02-~2
L551 -S02-NH-CH3
L552 -S02-N(CH3~
L553 -S02-NH-CZHS
L554 -SOZ-N(CH3)-C2Hs
L555 -S02-N(CZHs~
L556 -S02-(pyrrolidin-1-yl)
L557 -S02-(morpholin-4-yl)
L558 -SOz-NH-phenyl
L559 -SOZ-N(CH3~PhenY1
L560 -SOZ-NH-CH2-phenyl
L561 -N02 '
L562 -NH2
L563 -NH-SOZ-CH3
L564 -N(S02-CH3h
L565 -NH-SOZ-C2H5
L566 -N(SO~-CZHS)
L567 -NH-S02-CHZ-CZHS
L568 -NH-CHO
L569 -NH-CO-CH3
L570 -NH-CO-CZHS
L571 -N(CO-CH3)-S02-CH3
L572 -N(CO-CH3)-SOZ-C2H5
L573 -~3
L574
\ CH3
C = C ~
H~ H
L575
\ H '
C = C ~
H ~ CHg ,
L576 -CH2-Br
L577 -CH2-O-CH3
L578 -CHZ-O-C2H5
L579 -CH2-O-(CHZ)2-CH3
~O 95/02580 ~ ~ PCT/EP94/02263
73
No. R~ M.p. / IR (cm-1] /
iH-NMR [PPm]
L580 -CH2-O-CH(CH3)2
L581 -CH2-O-(CH2)3-CH3
L582 -CHZ-O-CH(CH3)-C2H5
L583 -CH2-O-CHZ-CH(CH3)2
L584 -CH2-O-CHZ-CH=CHZ
L585 -CHZ-O-CH2-C-C-H
L586 -CHZ-O-CHZ-CO-OCH3
L587 -CHZ-O-CHZ-CO-OC2H5
L588 -CHZ-O-CH(CH3)-CO-OCHg
L589 -CHZ-O-CH(CH3)-CO-OCZHS
L590 -CHZ-O-cyclopentyl
L591 -CHZ-S-CH3
L592 -CHZ-S-C2H5
L593 -CH2-S-(CH2n-CH3
L594 -CHZ-S-CHZ-CO-OCH3
L595 -CHZ-S-CH2-CO-OC2H5
L596 -CHZ-N(CH3n
L597 -CO-OH w
L598 -CO-OCH3
L599 -CO-OC2H5
L600 -CO-O-(CHZ~y-CH3
L601 -CO-OCH(CH3)2
L602 -CO-O-(CH2~-CH3
L603 -CO-OCH(CH3)-CZHS
L604 -CO-OCH2-CH(CH3)2
L605 -CO-O-(CH2)4-CH3
L606 -CO-OCH2-CH2-OCH3
L607 -CO-OCHZ-CH2-OC2H5
L608 -CHO
L609 -CH(OCH3)Z
L610 -CH(OC2H5)2
L611 -CH(OCH2-C2H5~
L612 1,3-Dioxolan-2-yl
L613 4-Methyl-1,3-dioxolan-2-yl
L614 4-Vinyl-1,3-dioxolan-2-yl
L615 3,4-Dimethyl-1,3-dioxolan-2-yl
WO 95/02580 ~ ~ PCTlEP94/0226~
74
No. R~ M.p. / IR [cm-1) /
1H-NMR [PPm)
L616
O
- CH
~S
L617
O
- CH
~S
L618 4-Methyl-1,3-dithiolaa-2-yl
L619 1,3-Dioxan-2-yl
L620 1,3-Dithian-2-yl
L621 -CH=N-OH
L622 -CH=N-OCH3 I
L623 -CH=N-OCZHS
L624 -CH=N-OCHZ-C2H5
L625 -CH=N-OCH(CH3h
L626 -CH=N-OCHZ-CHZ-C2H5
L627 -CH=N-OCHZ-CO-OH
L628 -CH=N-OCH2-CO-OCH3
L629 -CH=N-OCHZ-CO-OC2H5
L630 -CH=N-OCH(CH3)-CO-OH
L631 -CH=N-OCH(CH3)-CO-OCH3
L632 -CH=N-OCH(CHg)-CO-OC2Hg
L633
Cl
C= C
H ~ ~cooH
L634
C1
C=C~
H ~ CO-OCH3
L635
C1
C=C~
H ~ CO-OC2H5
~VO 95/02580
PCT/EP94102263
No. R~ M.p. / IR [cm'1]
/
lIi-NMR [ppm]
L636
\ /H
C=C
H ~ \C
OOH
L637
\ /H
C= C
H ~ \CO
-OCH3
L638
\ /H
C=C
H~ \C
O-OCyHS
L639
\ / CH3
C= C
H ~ \
COOH
L640
\ / CH3
C= C
H ~ \
CO-OCHg
L641
\ / CH3
C= C
H ~ \C
O-OC2H5
L642
\ j Cl
C= C
H ~ \
CO-NHZ
L643
\ / Cl
C= C
H ~ \
CO-NHCH3
WO 95/02580 PCT/EP94/0226~
76
No. R~ /
p~
NMR ( pm]
H
L644 -
\ / C1
C = C \
~ CO
N
CH
H
-
(
3)2
L645
\ /H
C = C \
~ CO-NH
H
2
L646
\ / H
C = C \
~ CO-NHCH
3
H
L647
\ H
C=C~
~ CO
N
CH
H
-
(
3)2
L648
\ / CH3
C = C \
~ CO
NH
H
-
2
L649
\ / CH3
C=C\
NHCH
~ CO
-
3
H
L650
\ / CH3
C= C \
CH
)
~ CO
N
3
H
-
(
L651
\ / C1
C = C \
~ CO
CH
H
-
3
~'VO 95/02580 ~~ ~~ ~ PCT/EP94/02263
77
No. R~ M.p. / ~ (wi] /
1H-NMR [PPm]
L652
\ /H
C= C
H ~ \CO
-CH3
L653
\ / CH3
C= C
H ~ \C
O-CH3
L654 -CHZ-CH(Cl)-CO-CH3
L655 -CHZ-CH(Cl)-CO-OCH3
L656 -CH2-CH(Cl)-CO-OC2H5
Table 5
R2 R4
I (R1~ R3~ R5 = H~ R6 ~ Cl
O N
O Cl
R~
No. R4 R~ R2 M.p. \ IR [cm-1] \
1H-NMR [ppm]
L801 H -OCH3 CF3 133-134C
L802 Cl -OCH3 CF3 156-157C
L803 Cl -O-CH2-CN Cl 167-168C
L804 Cl -O-CH(CH3)-CO-OC2H5 Cl 85-87C
L805 Cl -O-CH2-CO-OC2H5 Cl 128-129C
WO 95!02580 ~ , PCTlEP94/0226~
78
Table 6
R3
R2 R4
I I (RS=H; R6=C1; R~=OCH3)
R N I
1
OCH3
No. Rl R2 R3 R4 M.p. / IR [cm-1)/
iH-NMR [PPmJ
L901 H CF3 H SCH3 100-103C
L902 H CFg H H 74 C;
4.02(s,3H), 7.49(s,2H),
7.75(s,lH), 7.83(d,lH),
7.99(d,lH), 8.94(s,lH)
L903 H H H CI 120-121 ~C;
3.97(s,3H), 7.20-7.35(m,3H),
7.45(d,lH), 7.82(dd,iH),
8.59(d,lH)
L904 I-~ CF3 H OCH3 62-63C
L905 CI CF3 H Cl 88-90 ~C;
3.97(s,3H),7.35-7.40(m,2H),
7.48(d,lH), 8.10(s,lH)
L906 H H H H
L907 H C1 H H
L908 H H H CF3
L909 Cl CN H H
L910 Cl CI Cl Cl
L911 Cl NOZ H H
L912 H H CF3 H 83-84 C
L913 Cl H CF3 H
L914 CF3 H H H 80-81 C
L915 H Cl H CF3
L916 Cl CF3 H H
L917 H NOZ H Cl
L918 OCH3 H H H
L919 Cl Cl SCH3 Cl
L920 H H Cl NOZ
L921 H H OC2H5 NOZ
L922 H NOZ H H
~VO 95102580 PCT/EP94102263
79
No. R1 R2 R3 R4 M.p. / IR (cm'lj/
iH-NMR [PPmj
L923 H H H NOZ
L924 Cl Cl CF3 Cl
L925 OC2H5 CF3 H Cl 1.44 (t,3H), 3.97 (s,3H),
4.52
(q,2H), 7.38 (dd,lH),
7.40
(d,lH), 7.47 (d,lH),
7.94 (s,lH)
WO 95/02580 ~~ PCTlEP94/0226~
Examples of the herbicidal activity
The herbicidal action of the substituted 2-phenylpyridines I and
5 I' was shown in glasshouse tests:
Plants were grown in plastic flowerpots containing loamy sand ,
with about 3.0~ humus as substrate. The seeds of the test plants
were sown separately according to species.
For pre-emergence treatment, the active ingredients suspended or
emulsified in water were applied immediately after sowing using
finely distributing nozzles. The pots were watered lightly in
order to promote germination and growth and subsequently covered
with transparent plastic domes until the plants had started to
grow. This covering results in uniform germination of the test
plants unless this has been impaired by the active ingredients. ,
The application rate for pre-emergence treatment was 0.0313 kg/ha
active substance.
For post-emergence treatment, the test plants were grown to a
height of 3 to 15 cm, depending on the species, and only then
treated with the active ingredients suspended or emulsified in
water. For this purpose, the test plants were either sown direct-
ly and grown in the same vessels or they were germinated
separately and transplanted into the test vessels a few days
before the treatment. The application rate for post-emergence
treatment was 0.25, 0.125, 0.0625, 0.0313 or 0.0156 kg/ha active
substance.
35
The plants were kept at 10-25~C or 20-35~C depending on the
species. The tests lasted 2 to 4 weeks during which the plants
are tended and their reaction to the individual treatments was
evaluated.
Evaluation was on a scale from 0 to 100, where 100 means no
emergence of the plants or complete destruction of at least the
above-ground parts and 0 means no damage or normal growth.
The plants used in the glasshouse tests comprised the following
species:
CA 02167291 2004-04-15
ai
Botanical name Common name
Abutilon theophrasti velvet leaf
Amaranthus retroflexus redroot pigweed
Chenopodium album lambsquarters
(goose foot)
Ipomoea subspecies momingglory
Setaria faberii faber's foxtail:
giant foxtail
Slanum nigrum black nightshade
Stellaria media common chickweed
Zxa mays com (maize)
At an application rate of 0.25 and 0.125 kg/ha, unwanted plants
can be controlled very efficiently by the post-emergence method
with compounds Nos. 1.163, I.068, I.106, I.188 and I.512.
Setaria faberii in corn is controlled very efficiently by the
pre-emergence method with compounds Nos. I.106 and I.512.
Examples of the growth-regulating activity
The test plants were young, 4-leaved (without seed leaves) cotton
plants of the variety Stoneville 825 which had been grown under
glasshouse conditions (rel. humidity 50-70~; day/night tempera-
ture 27/20°C).
The young cotton plants underwent foliage application until drip-
ping wet with aqueous formulations of the stated active ingredi-
ents (with the addi~ion of 0.15 by weight of the fatty alcohol
alkoxylate Plurafac LF 700, based on the spray liquor). The
amount of water applied was equivalent to 1000 1/ha. After
13 days, the number of leaves lost and the degree of defoliation
in ~ were determined. No leaf loss occurred in the untreated con-
trol plants.
ao
dg * Trademark