Note: Descriptions are shown in the official language in which they were submitted.
''
~1~~~2~
"ALKYLATION WITH A FLUIDIZED SOLID ALKYLATION CATALYST"
FIELD
The invention specifically relates to the alkylation of hydrocarbons such
as aromatics or paraffins to produce useful chemicals and motor fuel using a
fluidized,
solid catalyst--for example, alkylation of isobutane to produce Cg
isoparaffins useful as
motor fuel blending components.
BACKGROUND
Large amounts of high octane gasoline are produced by the alkylation of
isobutane with butenes. Likewise, large amounts of valuable aromatic
hydrocarbons
including cumene, ethylbenzene and C,6 C2, linear alkylaromatics are produced
by the
alkylation of benzene with olefins of the appropriate carbon number. The
variety of
feed reactants and the passage of time has led to the development of a number
of
effective alkylation technologies which are employed in large scale commercial
facilities.
One of the most widely used processes for the production of motor fuel
2 0 is HF alkylation as described in US-A-4139573. One of the advantages of
the use of
liquid-phase HF as a catalyst is its resistance to deactivation, and the
relative ease with
which a slipstream may be removed from an onstream reaction zone for
"regeneration".
The HF itself is not chemically changed during use but various organic
reaction by-
products such as "acid soluble oils" (ASO) accumulate in the liquid-phase HF
and are
2 5 removed during this regeneration.
Regeneration is also necessary for all solid motor fuel alkylation catalysts
developed to date since they tend to suffer from a high deactivation rate.
Deactivation
of solid catalysts is due to different, possibly multiple, causes from those
encountered
with liquid HF as a catalyst and usually includes some accumulation of
3 0 hydrocarbonaceous deposits on the catalyst.
1
~~~~b2~
A common method of regenerating catalysts is by combustion of organic
deposits. This is often not desired for alkylation catalysts. US-A-3851004
describes an
alternative method for regenerating a solid bed alkylation catalyst comprising
a
hydrogenation component on a zeolitic support which comprises contacting the
catalyst
with a hydrogen-containing liquid-phase saturated hydrocarbon.
Any interruption in the operation of the reaction zone to regenerate or
replace catalyst is undesirable. Certain operating benefits are provided to
any process
by an ability to operate in a continuous manner, which makes it desirable to
find a
means to regenerate or replace the catalyst while the reaction zone is kept in
use. US-A-
4973780 describes a moving bed benzene alkylation process in which catalyst is
continuously or periodically replaced with regenerated catalyst to provide
countercurrent
catalyst-reactant flows. Cocurrent flow with catalyst added to the bottom of
the reactor
is also disclosed.
It has also been proposed to provide continuous operation by simulating
the movement of the catalyst through the reaction and regeneration zones. US-A-
4088291 and USA-4028430 describe the use of simulated countercurrent
operations to
perform a number of alkylation reactions including the production of motor
fuel. These
references provide separate reaction and catalyst reactivation zones, with an
external
regenerant stream being employed for the reactivation. In both references the
effluent
2 0 of the reaction zone is withdrawn from the alkylation zone immediately
upon its exit
from the reaction zone. These references also teach the use of a "pump around"
stream
to complete the simulation and provide a continuous liquid loop.
US-A-5157196 describes a moving bed paraffin alkylation process which
employs a plug flow in which the catalyst moves upward to a disengaging zone.
Used
2 5 catalyst from the disengaging zone is passed into a wash zone.
BRIEF SUMMARY
The invention is a fluidized process for the alkylation of hydrocarbons.
The invention provides a continuous reaction zone which is not interrupted for
the
2
_ ~~~~~2~ ,
periodic regeneration of catalyst. The invention also eliminates the need to
perform
motor fuel or aromatic alkylation reactions using volatile and hazardous
liquid phase
hydrofluoric acid. The invention is characterized by the use of a fluidized
riser-type
reaction zone with the upper end of the reaction zone discharging into a
separate zone
in which the reactants and products are separated from used catalyst and the
used
catalyst is then stripped and recirculated to the riser. A first portion of
the used catalyst
is mildly regenerated while a second portion is drawn off for full
regeneration in an
external fluidized regeneration zone.
One broad embodiment of the invention may be characterized as a process
for the alkylation of a feed hydrocarbon which comprises the steps of passing
a first
catalyst stream, comprising fluidized regenerated catalyst, and a feed stream
comprising
the feed hydrocarbon and an alkylating agent into the bottom of a vertical
riser-reaction
zone maintained at reaction conditions and producing a reaction zone effluent
stream
comprising used catalyst, the feed hydrocarbon and a product hydrocarbon;
discharging
the reaction zone effluent stream into a separation zone in which used
catalyst is
separated from liquid phase hydrocarbons and thereby forming a liquid-phase
separation
zone effluent stream comprising the feed and product hydrocarbons, with the
thus
separated used catalyst descending downward within the separation zone;
transferring
an aliquot first portion of the used catalyst downward through a liquid phase
2 o regeneration zone wherein the first portion of the used catalyst is
contacted with feed
hydrocarbon containing dissolved hydrogen to form regenerated, washed
catalyst;
transferring a smaller second aliquot portion of the used catalyst or a
portion of the
regenerated, washed catalyst, together with feed hydrocarbon, from the
separation zone
into a high temperature regeneration zone wherein the catalyst is contacted
with vapor
2 5 phase hydrogen at vapor phase regeneration conditions and withdrawing high
temperature regenerated catalyst from the high temperature regeneration zone
as a
second catalyst stream; charging at least a portion of the second catalyst
stream to the
riser-reaction zone or to the liquid phase regeneration zone or to both of
these zones and
employing at least a portion of the regenerated, washed catalyst as the first
catalyst
3 o stream which is then charged to the riser-reaction zone; and, recovering
the product
3
I ~
hydrocarbon from the separation zone effluent stream.
BRIEF SUMMARY OF THE DRAWINGS
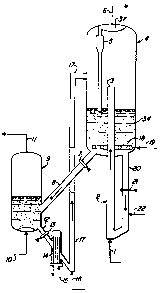
Figure 1 is a simplified diagram illustrating an embodiment of the
invention in which the riser-reactor 2 discharges into a large separation
vessel 4, which
retains a dense bed 34 of catalyst which is being subjected to mild liquid-
phase
regeneration.
Figure 2 illustrates a second embodiment of the invention in which the
riser reactor discharges into a hydrocyclone type separation zone 25 and the
majority of
the used catalyst is then subjected to mild regeneration in a segregated
washing section
l0 24 located below the hydrocyclone.
DETAILED DESCRIPTION
Hydrocarbon alkylation is widely used in the petroleum refining and
petrochemical industries to produce a variety of useful acyclic and cyclic
hydrocarbon
products which are consumed in motor fuel, plastics, detergent precursors, and
petrochemical feedstocks. Much of the installed base of alkylation capacity
uses liquid
phase hydrofluoric acid, generally referred to as HF, as the catalyst. The use
of HF in
these applications has a long record of highly dependable and safe operation.
However,
the potential damage from an unintentional release of any sizeable quantity of
HF and
the need to safely dispose of some byproducts produced in the process has led
to an
2 o increasing demand for alkylation process technology which does not employ
liquid phase
HF as the catalyst.
Numerous alkylation catalysts have been described in the open literature.
However, those that we have knowledge of all appear to suffer from
unacceptably high
deactivation rates when employed at commercially feasible conditions. While
some
2 5 catalysts have a sufficiently useful lifetime to allow the performance of
alkylation, the
rapid change in activity results in a change in product composition and also
requires the
periodic regeneration of the catalyst with the accompanying removal of the
reaction zone
4
~16~~2~
from operation. It is very desirable to provide a continuous process for
alkylation which
is not subjected to periodic reaction zone stoppages or variation in the
product stream
composition.
It is an objective of this invention to provide an alkylation process which
does not employ liquid phase HF as the catalyst. It is a further objective of
the subject
invention to provide an alkylation process which utilizes a fluidized solid
catalyst. It
is a specific objective of the invention to provide a solid catalyst
alkylation process for
the production of motor fuel blending hydrocarbons. A further objective of the
subject
process is to provide a continuous process which delivers a uniform quality
and quantity
of product and which employs a fluidized solid alkylation catalyst.
The subject process achieves these objectives by the use of unique
fluidized catalyst flow schemes in which a riser-type reactor delivers the
product
hydrocarbons and used catalyst to a liquid phase separation zone from which
catalyst is
removed for division between one of two regeneration zones.
The hydrocarbon feedstock to the subject process may be essentially any
hydrocarbon which is retained as an easily flowable liquid phase material at
the
conditions employed in the reaction and mild regeneration zones and which may
be
alkylated via solid catalyst at the conditions maintained in the riser
reaction zone. The
feed hydrocarbon may therefore be an aromatic hydrocarbon such as benzene or
toluene.
2 0 This feed hydrocarbon or substrate is often reacted with an alkylating
agent comprising
an acyclic light olefin such as ethylene, propylene or butylene to produce
such chemicals
as ethylbenzene and cumene. A large amount of benzene is alkylated with higher
carbon
number olefins having from about 10 to about 15 carbon atoms per molecule to
produce
linear alkyl benzenes which are then sulfonated to produce detergents. For
motor fuel
2 5 production the preferred feed hydrocarbons are light paraffinic
hydrocarbons such as the
butanes. An especially preferred paraffinic feed hydrocarbon is isobutane.
The entering feed hydrocarbon is typically alkylated with a linear olefin
having from 2 to 15 carbon atoms per molecule. The feed hydrocarbon may also
be
reacted with an alkylating agent chosen from a variety of compounds other than
olefins
3 o including monohydric alcohols. Examples of the alcohols which may be
employed as
S
the alkylating agent include ethanol and methanol. For instance, methanol is
widely
described in the literature as being useful in the paraselective methylation
of benzene
and toluene.
The operation of the subject invention may be best discerned by reference
to the Drawings. In both figures, isobutane is the feed hydrocarbon and it is
reacted
with C4 olefins to produce C8 hydrocarbons which may be recovered by normal
product
recovery methods such as fractional distillation. Although there are
differences between
the two embodiments, the same numbering system has been employed on each one
to
the extent that the same or analogous equipment is employed.
1 o As used herein the term "substantially free" means a molar concentration
less than 1.5 mole percent. The term "rich" is intended to indicate a
concentration of
the specified compound or class of compounds greater than 50 mole percent.
Referring to Figure 1, a liquid phase feed stream comprising an admixture
of isobutane and C4 olefins enters the process through line 1 at the bottom of
the riser-
reactor 2. The injection of this liquid results in the upward flow of the
contents of the
riser-reactor 2 including solid catalyst which travels downward through the
transfer line
at a rate controlled by the slide valve 21. Liquid phase isobutane flows into
the
transfer line 20 through line 22 at a rate sufficient to cause a continuous
minor net
upward liquid flow through the conduit 20. This upward liquid flow is intended
to strip
2 0 hydrogen from the catalyst and surrounding liquid to prevent the entrance
of hydrogen
into the riser and to wash heavy hydrocarbon contaminants from the surface of
the
catalyst. Conduit 20 will therefore deliver a stream of hydrogen-free freshly
regenerated, washed catalyst to the bottom of the riser reactor. This catalyst
is admixed
with the entering reactant feed stream and catalyzes the reaction of olefins
with the
2 5 entering C4 isobutane or recirculating isobutane to form C8 product
hydrocarbons. The
reaction products, the residual isobutane and the now used catalyst exit from
the top 3
of the riser reactor 2 and enter into a large volume cylindrical separation
chamber 4.
The flush stream of line 22 may be passed into the conduit above the slide
valve 21.
The low liquid velocities present within the separation vessel 4 allow the
3 0 solid particulate catalyst to settle downward and form a dense fluid bed
34 located in
6
'~
a lower portion of the vessel. This bed is preferably maintained in a dense
fluidized
state by the passage of a liquid phase stream comprising isobutane and
dissolved
hydrogen into the vessel 4 through line 19. A distribution grid 18 is employed
near the
bottom of vessel 4 to achieve a uniform distribution of the entering liquid-
phase
isobutane containing dissolved hydrogen throughout the dense fluid bed. The
catalyst
retained within the fluid bed is therefore subjected to a mild regeneration
procedure by
contact with hydrogen saturated isobutane. This added isobutane of line 19
together
with that from line 22 and the olefin-free liquid phase material exiting from
the riser
reactor 2 will gradually travel upward through the vessel 4 and enter into a
cyclone type
("hydroclone") liquid-solids separator 5. The cyclone 5 effects the further
separation of
any entrained catalyst or catalyst fragments from the liquid phase
hydrocarbons before
the liquid is discharged into a plenum 37 at the top of the vessel 4. The
plenum may
be utilized to facilitate the installation of two or more separate cyclones
into the
separation vessel 4. The thus collected liquid phase hydrocarbons are removed
from the
process through line 6 as a product stream and transferred to the appropriate
product
recovery facilities not shown on the drawing.
The major portion of the used catalyst retained in the dense bed is
withdrawn as a continuous stream through line 20 at the rate set by the slide
valve 21.
This first catalyst stream flows downward countercurrent to some of the
hydrogen-free
2 0 isobutane charged to the process via line 22. The remainder of this
isobutane flows
from line 22 into the riser 2. The purpose of this procedure is to prevent the
entrance
of hydrogen into the riser where it could saturate olefins added by line 1. If
the catalyst
employed in the process does not promote the hydrogenation of the olefins,
then this
washing procedure may be eliminated. Slide valve 21 will need to always be
slightly
2 5 open, or other means provided, to allow the flow of liquid upward through
line 20 and
ensure hydrogen does not enter the reactor 2. The hydrogen-free isobutane of
line 21
can alternatively be passed into line 20 at a point above the slide valve 21.
A second and smaller stream of the catalyst present in the dense fluid bed
at the bottom of the separation vessel 4 is withdrawn through line 8 at a flow
rate
3 0 controlled by the slide valve 7. This smaller stream comprises both solid
catalyst and
7
~.~6~~~~
liquid-phase hydrocarbons and is passed into an external regeneration vessel
9, with the
catalyst being retained in the regeneration vessel 9 for some average time set
by the
transfer rate in line 8. It is currently preferred that the second catalyst
stream has a
uniform flow rate but a variable rate could be used to facilitate batch
regeneration.
While in the regeneration vessel the catalyst is agitated and fluidized by the
addition of
a high temperature vapor phase stream comprising hydrogen and isobutane
through line
10. This stream has been heated by means not shown to a sufficient temperature
to
cause the vaporization of at least a major portion of the liquid phase
hydrocarbons which
enter the regeneration vessel through line 8 in the second catalyst stream.
There is
thereby formed a vapor phase regeneration zone effluent stream comprising
hydrogen,
isobutane and any other hydrocarbons which enter the regeneration zone through
line 8
or result from the regeneration process. This higher temperature hydrogen-rich
stripping
is a much more intense regeneration procedure and is preferably performed at
conditions
yielding a catalyst residence time of at least 30 minutes within the
regeneration vessel
9.
A stream of fully regenerated catalyst is removed from the regeneration
zone via line 12 at a rate controlled by slide valve 13. This rate is
preferably
approximately equal to the rate at which catalyst is fed into the regeneration
zone but
may fluctuate over short periods. The highly regenerated catalyst first flows
through a
2 o catalyst cooler 14 which receives low temperature isobutane through line 1
S and is then
passed into the bottom of a second riser 17. The catalyst is preferably cooled
to a
temperature below 38°C. A stream of liquid-phase isobutane from line 16
then fluidizes
the highly regenerated catalyst and causes it to flow upward via line 17 into
the
separation vessel 4 where it is commingled with the catalyst which has been
subjected
2 5 to the mild regeneration. It has been noted that highly regenerated
catalyst tends to
produce lower octane number product and the use of a blend of fresh and mildly
regenerated used catalyst should reduce this tendency. As shown on the
drawing, the
catalyst from the transfer riser 17 preferably enters the separation vessel 4
in the upper
half of the vessel 4 to avoid the pressure head of the catalyst retained in
the vessel 4.
3 0 Alternatively, the transfer riser 17 may direct the highly regenerated
catalyst into the
8
. ~.
.
dense bed of catalyst present in the lower portion of the separation vessel.
This may be
done to increase the degree of fluidization at the bottom of the separation
vessel.
Alternatively, a portion or all of the highly regenerated catalyst could also
be fed
directly into the riser 2. The higher volume of mildly regenerated catalyst
and its
greater rate of circulation overwhelms the addition rate from line 17.
The circulation of the catalyst through the high temperature regeneration
zone requires the catalyst to be heated and cooled. The utility requirements
of the
process also require that the heat of reaction of the alkylation reaction be
removed.
These activities can be integrated with the operation of the products recovery
section of
the process. For instance, the heat available in the vapor phase stream
discharged via
line 11 from the high temperature regeneration vessel 9 can be used to aid in
reboiling
a fractionation column. Heat can also be supplied to the product recovery
section from
the cooler 14 used to cool catalyst being returned to the riser-reactor.
Alternatively, the
heated coolant may be passed into the regeneration zone 9.
Figure 2 illustrates a different embodiment of the subject process. Like
the embodiment of Figure l, the feed hydrocarbon and feed olefin reactants
enter the
bottom of a riser reactor 2 through line 1. Regenerated catalyst flowing
downward
through the transfer conduit 20 at a rate controlled by the slide valve 21 is
admixed with
the entering feed hydrocarbons from line 1 and fluidized upward through the
riser 2.
2 0 At the upper terminal end of the riser 2, the olefin-free reactant-
catalyst admixture is
directed horizontally into a hydrocyclone 25 which functions as the separation
zone.
The hydrocyclone is the sole solids-liquid separation device employed in this
embodiment. The residual feed hydrocarbon which has not been converted in the
riser-
reactor and the product hydrocarbons exit from the upper end of the
hydrocyclone
2 5 through line 6 for transfer to the product recovery zone. The used
catalyst separated in
this manner from the reactants passes downward through the lower portion of
the
hydrocyclone. A first small portion of this used catalyst is diverted from the
bottom of
the hydrocyclone through conduit 8 at a rate controlled by slide valve 7. This
small
portion of the used catalyst is passed directly into the high intensity
external regeneration
3 0 vessel 9. In comparison, the embodiment of Figure 1 diverts catalyst which
has been
9
,,
subjected to mild regeneration.
Regeneration vessel 9 is operated in a manner similar to the external
regeneration
zone of the embodiment of Figure 1. The catalyst is preferably confined within
this
regeneration zone for an average residence time of at least 30 minutes while
being
contacted with a heated stream of hydrogen and isobutane fed to the bottom of
the
regeneration zone 9 through line 10. This hot hydrogen-hydrocarbon stripping
removes
liquid phase hydrocarbons and deposits from the catalyst and produces a vapor
phase
regeneration zone effluent stream removed from the regeneration zone 9 through
line 11.
This regeneration zone effluent stream is preferably cooled sufficiently to
condense
l0 substantially all of the hydrocarbons contained within this stream and then
subjected to
vapor-liquid separation. The recovered liquids are passed into the products
recovery
zone and the hydrogen may be recycled to the bottom of the regeneration zone.
Catalyst which has been subjected to the high temperature stripping is
withdrawn from vessel 9 through line 12 at a rate controlled by the slide
valve 13. This
hot catalyst is admixed with liquid phase isobutane from line 32 and then
passed into
the heat exchanger 14. Isobutane coolant supplied by line 15 is used to cool
the catalyst
to less than about 38° C and the catalyst then flows into the stripping
section 26.
Catalyst cooling may be used to heat and/or vaporize the isobutane.
Vaporization has
some advantages.
2 0 The majority of the catalyst collected in the bottom of the hydrocyclone
passes downward through a liquid-filled wash section 24 which functions as the
mild
regeneration zone in this embodiment. The descending catalyst preferably
passes
through a series of funnel-shaped baffles 23 intended to admix and stir the
catalyst and
promote uniform contacting of the descending catalyst with a rising stream of
hydrogen
2 5 saturated isobutane injected into the bottom of the wash section through
line 29. The
countercurrent contacting within the wash zone 24 imparts a mild regeneration
to the
used catalyst descending from the hydrocyclone and washes off some of the
deactivating
deposits. The hydrogen saturated isobutane rises into the hydrocyclone and is
removed
with the product stream of line 6.
3 0 The regenerated, washed catalyst descending through the wash section
I ~ /
~.~~~~2~
enters the stripping section 26 where it is contacted with an additional
quantity of
upward flowing isobutane from line 31. The catalyst descends through the
stripping
zone 26 countercurrent to the rising hydrogen-free isobutane. A second series
of
inclined conical or funnel-like baffles 30 is provided in the stripping zone
at various
locations to ensure admixing of the rising isobutane with the descending
catalyst and a
thorough removal of hydrogen and deactivating deposits from the catalyst.
Although the
baffles 30 are shown only above the junction with the transfer conduit 12 they
may be
located below this point also. At the midpoint of the stripping section, the
descending
catalyst stream from the wash section is joined and admixed with the stream of
highly
l0 regenerated catalyst removed from the external regeneration zone 9.
Additional cooling
is provided to the bottom of the stripping section 26 by means of indirect
heat exchanger
35 located at the bottom of the stripping section which receives coolant
through lines
36. This cooling results in the catalyst being brought to the desired reaction
zone inlet
temperature before passage into the riser 2. The upward flow of hydrogen-free
isobutane from line 31 is relied upon to flush hydrogen from the catalyst
stream of line
12.
The steps of the subject invention include the regeneration of catalyst
located in one regeneration zone by contact with a liquid-phase hydrocarbon,
which is
preferably the feed hydrocarbon such as isobutane. Hydrogen is preferably
dissolved in
2 0 this liquid-phase stream up to the point of the stream being saturated
with hydrogen.
The average residence of catalyst particles in the liquid-phase hydrocarbon
regeneration
zone is preferably from about 0.5 to 15 minutes. The liquid-phase or "mild"
regeneration is performed in a vessel or conduit in relatively open
communication with
the reaction zone. The temperature and pressure conditions employed in this
2 5 regeneration zone will therefore be very similar to those in the reaction
zone. T h a
invention also includes a second regeneration operation in which catalyst is
contacted
with a vapor-phase gas stream at an elevated temperature in the range of 80 to
500°C
and more preferably from 100 to 250°C. The zone in which this "hydrogen
stripping"
or severe regeneration step is performed is operated in a manner which
provides a longer
3 0 average residence time for the catalyst particles than the liquid-phase
regeneration step.
11
~~68~i2
The average residence time of a catalyst particle should be at least 30
minutes and can
reach 12 to 24 hours. This regeneration step is performed using a vapor-phase
hydrogen
rich gas stream. The presence of some isobutane in this gas stream may be
desirable
to increase the heat capacity of the gas and therefore increase catalyst heat
up rates. The
longer residence time required for this regeneration step allows the high
temperature gas
charged to the regeneration zone to vaporize liquid which flows into the
severe
regeneration zone.
All of the catalyst passing from the separation zone to the bottom of the
riser is preferably subject to one of the two forms of regeneration. A much
smaller
1 o quantity of catalyst flows through the hydrogen stripping regeneration
zone compared
to the flow through the liquid-phase regeneration. The flow through the high
temperature regeneration zone will be only about 0.2 to about 20 weight
percent, and
preferably from about 0.4 to about 5 weight percent of the total catalyst flow
through
the riser.
The catalyst flow into the bottom of the riser is preferably as close to a
continuous steady state flow as the equipment and catalyst system allow. The
flow can,
however, be in the form of numerous small quantities of catalyst transferred
in rapid
sequence.
The Drawing and above description are presented in terms of controlling
2 0 catalyst flow through the use of slide valves. Alternative means can be
used for this
purpose including, for example, other types of valves, lockhoppers, fluid flow
control
(reverse flow of liquid), screw conveyors, etc. One particular alternative is
the use of
an "L valve", which would reduce the amount of isobutane flush needed in the
process.
The embodiment shown in the Drawing may also be varied by the use of
2 5 other types of heat exchangers and by the use of other coolants. While the
use of
isobutane as coolant and integration with the product fractionation zone is
preferred,
other coolants including water, air or other hydrocarbons can be employed. A
further
variation encompasses the use of countercurrent fluid flow to simultaneously
cool newly
regenerated catalyst and to flush hydrogen from the catalyst and liquid
surrounding the
3 o catalyst.
12
I
The subject process can be performed using any solid, that is,
heterogeneous, catalyst which is stable and has the required activity and
selectivity for
the desired reaction at the conditions needed to maintain liquid-phase
reactants in the
reaction zone. A large number of catalysts have been proposed for the
production of
motor fuel by alkylation including various zeolites and superacid catalysts.
For instance,
US-A-4384161 describes the use of a large pore zeolite and a Lewis acid. The
zeolites
referred to include ZSM-4, ZSM-3, the faujasites including zeolite Y and
mordenite.
The Lewis acids mentioned in this reference include boron trifluoride and
aluminum
chloride. The alkylation of isoparaffins using a somewhat similar catalyst
system
comprising a large pore crystalline molecular sieve such as a pillared
silicate or an
aluminophosphate or silicoaluminophosphate together with a gaseous Lewis acid
is
disclosed in US-A-4935577. The use of these Lewis acids is not preferred in
the subject
process as they provide their own waste handling and safety problems. They
also will
probably require provisions for the circulation of the Lewis acid, which
complicates the
process. US-A-5157200 describes an isoparaffin alkylation process using a
catalyst
comprising a crystalline transition alumina, preferably eta or gamma alumina,
which has
been treated with a Lewis acid under anhydrous conditions. Previously referred
to US-
A-5157196 describes an isoparaffin alkylation process using a slurried solid
catalyst,
with the preferred catalyst being an acid washed silica which has been treated
with
2 o antimony pentafluoride. Both of these last two references describe a
number of prior
art solid alkylation catalysts.
A preferred alkylation solid catalyst comprises a refractory inorganic
oxide impregnated with a monovalent cation, especially an alkali metal cation
or an
alkaline earth metal cation, and whose bound surface hydroxyl groups have been
at least
2 5 partially reacted with a Friedel-Crafts metal halide. Analogs of these
catalysts without
the metal cations are described in US-A-2999074 and US-A-3318820 which
describe
preparation techniques which can be applied to the preferred catalysts. The
preferred
refractory oxide is alumina having a surface area greater than 50 m2/g, but
the use of
other oxides including titanic, zirconia, silica, boric and aluminum phosphate
is
3 o contemplated. The preferred catalyst also contains a metal component
active for olefin
13
hydrogenation deposited on the inorganic oxide prior to reaction of the bound
surface
hydroxyl groups with the metal halides. This metal may be chosen from the
group
consisting of nickel, platinum, palladium, and ruthenium with the first three
of these
metals being preferred. The catalyst contains one or more monovalent metal or
alkaline
earth metal cations selected from the group consisting of lithium, sodium,
potassium,
cesium, silver, copper, beryllium, magnesium, calcium and barium. Subsequent
to the
deposition of these metals and the controlled calcination of the composite,
the composite
is reacted with a Friedel-Crafts metal halide. The metal may be aluminum,
zirconium,
tin, tantalum, gallium, antimony or boron. Suitable halides are the fluorides,
chlorides
1 o and bromides.
The presence of a highly active metal hydrogenation component on the
catalyst will promote hydrogenation of the feed olefin if both the olefin and
hydrogen
simultaneously contact the catalyst. This potential waste of the olefin and
hydrogen can
be avoided by careful design and operation of the process to avoid having both
the
olefin and hydrogen in simultaneous contact with the catalyst. This can be
done by
flushing the hydrogen or olefin from the catalyst before inserting it into a
zone
containing the other compound as described above.
Silicalites have been described as useful alkylation catalysts for the
production of monoalkylbenzenes in US-A-4489214 and as useful in methylating
toluene
2 o to produce paraxylene in US-A-4444989. The use of ZSM-5 zeolites in
aromatic
alkylation is described in US-A-3751506. ZSM-5 zeolites that have been treated
with
one or more compounds or elements to improve their selectivity for para-
selective
alkylation of aromatic hydrocarbons are described in US-A-4420418. The use of
zeolite
L, zeolite Omega and zeolite beta as alkylation catalysts for the selective
alkylation of
2 5 benzene is described in US-A-4301316. The use of a number of natural and
synthetic
zeolites including clinoptilolite and zeolite Y as alkylation catalysts is
described in US-
A-3251897.
The catalyst may be in the form of any suitable shape and size which
results in a solid catalyst which flows readily in both dry and wet states and
which is
3 o readily fluidized at the moderate liquid flow rates employed in the riser.
The catalyst
14
,.
~1~~~2
can therefore be present as small irregular particles or as uniformly shaped
particles.
It is preferred that the catalyst is present as "microspheres" having an
average diameter
less than 0.16 cm and more preferably less than 0.08 cm.
Suitable operating conditions for the reaction zone include a temperature
of about -50 to 100°C, preferably 20 to 50°C, and a pressure as
required to maintain the
hydrocarbons present as a liquid. A moderate pressure in the general range of
about 120
to about 3500 kPa is preferred with 2000-3000 kPa being highly preferred. The
weight
hourly space velocity for the olefin may range from about 0.1 to 5.0 hr''. The
riser
reaction zone is preferably designed and operated in a manner intended to
promote plug
flow (no backmixing) of the reactants, products and catalyst within the riser.
However,
the liquid must flow upward faster than the catalyst in order to transport it.
It is generally preferred that the reaction zone is operated with an excess
of the feed hydrocarbon compared to the alkylating agent. That is, it is
preferred to
operate with a ratio of the feed paraffinic or aromatic hydrocarbon to a feed
olefin at
the reactor entrance greater than 1:1, and preferably from 2:1 to 5:1 or
higher as
measured by the flow rates into the reaction zone. It is highly preferred to
operate with
an abundance of isoparaffin compared to alkylating agent in a motor fuel
alkylation
process. Specifically, it is preferred that the molar ratio of isoparaffin to
olefin being
charged to the reaction zone is greater than 2:1 and more preferably greater
than 3:1.
2 0 Ratios from 10:1 to about 100:1 or higher can be employed for motor fuel
alkylation.
The known technique of feeding the olefin at a number of points along the flow
path
of the feed hydrocarbon may be employed to maintain a higher average paraffin
to
olefin ratio.
Provisions may be made for removing used catalyst from the reaction
2 5 zone and to replace the used catalyst with fresh catalyst. Conventional
valued
lockhopper systems may be used for this purpose.