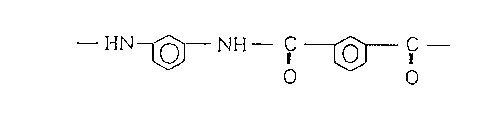Note: Descriptions are shown in the official language in which they were submitted.
~~.~~~~4
- 1 -
EASILY DYEABLE META-LINKA E-CONTAINING
AROMATIC POLYAMIDE FIBERS
TN-C425
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to meta-linkage-
containing aromatic polyamide fibers which are dyeable
with cationic dyes.
2. Description of the Related Art
Meta-linkage-containing aromatic polyamide
fibers have molecular skeletons consisting almost totally
of aromatic rings, and thus have excellent heat
resistance, flame retardance and flame proofness. As a
result, such fibers are suitable for use as industrial
materials for which heat resistance is required, and for
use in clothing and interior decoration for which flame
retardance and flame proofness are considered important;
they are rapidly attaining wider use especially in the
fields of clothing, bedding materials and interior
decoration which take advantage of their flame retardance
and flame proofness. These fields usually employ dyed
fibers, but while meta-linkage-containing aromatic
polyamide fibers have excellent physical characteristics,
their rigid polymer chains make them very difficult to
dye by conventional methods.
A number of improved methods have been proposed
in order to use meta-linkage-containing aromatic
polyamide fibers in these fields. For example, in
Japanese Unexamined Patent Publication No. 50-59522 there
have been proposed pigmented fibers obtained by
incorporating meta-linkage-containing aromatic polyamide
fibers with a specific pigment; because the fibers are
incorporated with the pigment during the production
process, however, there is a drawback of considerable
efficiency loss when colors are changed, which means that
the method cannot be adapted well to small-lot production
- 2 -
and more time is required to respond to client's orders.
As a means of improving the dyeing property, Japanese
Unexamined Patent Publication No. 55-21406 (U. S. Patent
No. 4,278,779) has proposed a method of adding a polymer
copolymerized with xylylenediamine; however, since a
third component is copolymerized with the polymer chain,
polymerization equipment and polymer stocking equipment
must be specialized for the particular polymer used,
which presents a problem of increased cost. In addition,
Japanese Examined Patent Publication No. 52-43930 (U. S.
Patent No. 3,695,992) proposes polyporous aromatic
polyamide fibers with an improved dyeing property, having
a pore size, void volume and density within specific
ranges; nevertheless, the dyeing property of these fibers
has not been sufficient, and associated drawbacks have
included difficulty in setting the dyeing conditions
because of the required pigments and organic dyeing aids
during the dyeing, as well as difficulty in disposal of
the waste liquors after use.
There has also been proposed in, for example,
Japanese Examined Patent Publication No. 44-11168 (U. S.
Patent No. 3,506,990), a meta-linkage-containing aromatic
polyamide prepared by copolymerization with a compound
having sulfonate groups introduced therein. This
polymer, however, has certain disadvantages in that
purification of the starting material is very difficult,
and it is impossible to obtain a stable polymer with the
necessary degree of polymerization and whiteness.
Furthermore, production of aromatic polyamides prepared
by copolymerization with components having sodium
sulfonate groups is disclosed in Japanese Unexamined
Patent Publication Nos. 48-96827 and 51-26320, and in
U.S. Patent Nos. 3,039,990, 3,142,662 and 3,409,596.
However, although aromatic polyamides are usually
prepared by reacting a dicarboxylic halide with a
diamine, the sodium sulfonate groups when present react
with the acid halide, making it impossible to obtain a
- 3 -
polymer with satisfactory physical properties.
SUMMARY OF THE INVENTION
In light of the aforementioned problems of the prior
art, it is an object of the present invention to provide,
by inexpensive and simple means, meta-linkage-containing
aromatic polyamide fibers with excellent dyeing
properties which may be used in the fields of bedding,
clothing and interior decorating.
In order to achieve the above-mentioned object, the
present invention provides easily dyeable meta-linkage-
containing aromatic polyamide fibers consisting of a
composition which comprises a meta-linkage-containing
aromatic polyamide incorporating an alkylbenzenesulfonic
acid onium salt.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The meta-linkage-containing aromatic polyamide to be
used according to the present invention consists
substantially of aromatic rings constituting the main
skeleton, which have amide linkages at the meta-
positions. Particularly preferred among such meta-
linkage-containing aromatic polyamides is a poly-m-
phenylene isophthalamide consisting of repeating units
represented by the following chemical formula.
- HN~ NH- ~C O
0 0
The meta-linkage-containing aromatic polyamide may
also be a copolymer comprising less than 15 mole percent
of a third component. Monomers constituting the third
component may be aromatic diamine components such as, for
example, para-phenylenediamine, 3,4'-diaminodiphenyl
ether, 4,4'-diaminodiphenyl ether, para-xylylenediamine,
biphenylenediamine, 3,3'-dichlorobenzidine, 3,3'-
dimethylbenzidine, 3,4'-diaminodiphenylmethane, 4,4'-
diaminodiphenylmethane, 1,5-naphthalenediamine; and as
acid components, aromatic dicarboxylic acids such as, for
~~~q~44
- 4 -
example, terephthalic acid, naphthalene-2,6-dicarboxylic
acid, naphthalene-2,7-dicarboxylic acid, particularly
preferred of which is terephthalic acid. These aromatic
diamines and aromatic dicarboxylic acids may have a
portion of the hydrogen atoms on their aromatic rings
substituted with halogen atoms or alkyl groups such as
methyl.
This type of meta-linkage-containing aromatic
polyamide may be produced by a publicly known interfacial
polymerization or low-temperature solution polymerization
method. The degree of polymerization of the polymer, in
terms of the intrinsic viscosity (IV) of a 0.5 g/100 ml
solution in N-methyl-2-pyrrolidone at 30°C, is preferably
1.3 to 1.9 dl/g.
The alkylbenzenesulfonic acid onium salt combined
with the meta-linkage-containing aromatic polyamide may
be a compound such as tetrabutylphosphonium
hexylbenzenesulfonate, tributylbenzylphosphonium
hexylbenzenesulfonate, tetraphenylphosphonium
dodecylbenzenesulfonate, tributylphenylphosphonium
dodecylbenzenesulfonate, tetrabutylphosphonium
dodecylbenzenesulfonate, tributylbenzylammonium
dodecylbenzenesulfonate, etc. Among these,
tetrabutylphosphonium dodecylbenzenesulfonate and
tributylbenzylammonium dodecylbenzenesulfonate are
particularly preferred, because of their ready
availability, excellent thermal stability and high
solubility in dimethylacetoamide and N-methyl-2-
pyrrolidone, which are good solvents for the meta-
linkage-containing aromatic polyamide.
In order to obtain an adequate improving effect on
the dyeing property, the amount of the
alkylbenzenesulfonic acid onium salt to be combined with
the meta-linkage-containing aromatic polyamide is
preferably between 2.8 and 7.0 mole percent, and more
preferably between 3.5 and 7.0 mole percent with respect
to the repeating units of the meta-linkage-containing
21~~~4~
- 5 -
aromatic polyamide. At less than 2.8 mole percent, an
adequate improving effect on the dyeing property may not
be achieved, while at greater than 7.0 mole percent the
single filaments may be more prone to breakage during the
fiber production process.
The composition constituting the meta-linkage-
containing aromatic polyamide fibers of the present
invention, which contains the meta-linkage-containing
aromatic polyamide incorporating the onium
alkylbenzenesulfonate, preferably further contains a
halogen-containing alkyl phosphate or a halogen-
containing phenyl phosphate (hereunder referred to
collectively as "halogen-containing alkyl (phenyl)
phosphate"). The meta-linkage-containing aromatic
polyamide fibers made of such a composition have more
excellent dyeing properties.
As halogen-containing alkyl (phenyl) phosphates
there may be mentioned compounds such as tris(~-
chloropropyl) phosphate, tris(2,3-dichloropropyl)
phosphate, tris(chloroethyl) phosphate,
phenyldichloropropyl phosphate and tris(dichlorophenyl)
phosphate. Although these compounds have been found to
have virtually no effect of improving dyeing properties
when they alone are combined with meta-linkage-containing
aromatic polyamides, they exhibit a specific dyeing
property-improving effect when used in tandem with an
alkylbenzenesulfonic acid onium salt.
The content of the halogen-containing alkyl (phenyl)
phosphate in the polymer is preferably 0.5 to 5.0 wto,
and more preferably 1.8 to 5.0 wtg based on the meta-
linkage-containing aromatic polyamide. At less than 0.5
wt~ the specific dyeing property-improving effect may not
be obtained, and at greater than 5 wt~ dye spots may
appear during the dyeing process, while the dyeing
property-improving effect may not be so greatly enhanced.
The above-mentioned meta-linkage-containing aromatic
polyamide composition constituting the meta-linkage
-- ~~~i ~4~
- 6 -
containing aromatic polyamide fibers of the present
invention preferably further contains an ultraviolet
absorber. Meta-linkage-containing aromatic polyamide
fibers made of such a composition not only have a more
excellent dyeing property, but also impart excellent
light fastness to the dyed product.
The ultraviolet absorber is preferably a
benzotriazole-based ultraviolet absorbing compound. This
is because meta-linkage-containing aromatic polyamides
have an ultraviolet absorbance range of from 340 to 360
nm, and most benzotriazole-based compounds have maximum
absorbance wavelengths within this range.
As is well-known, meta-linkage-containing aromatic
polyamides undergo considerable yellowing upon exposure
to light rays, as a result of their molecular structure
characteristics. Consequently, even if the dyeing
property is improved to provide finely dyed fibers, their
value as marketable dyed fibers is reduced by half when
the color shade is altered due to yellowing after dyeing.
Thus, by combining excellent light fastness with the
excellent dyeing property, their value is greatly
increased as meta-linkage-containing aromatic polyamide
fibers with an improved dyeing property.
Preferred examples of benzotriazole-based
ultraviolet absorbing compounds include 2-(5-methyl-2-
hydroxyphenyl)benzotriazole, 2-[2-hydroxy-3,5-bis(a,a'-
dimethylbenzyl)phenyl]-2H-benzotriazole, 2-(3,5-di-t-
butyl-2-hydroxyphenyl)benzotriazole, 2-(3-t-butyl-5-
methyl-2-hydroxyphenyl)-5-chlorobenzotriazole, 2-(3,5-di-
t-butyl-2-hydroxyphenyl-5-chlorobenzoazole and 2-(3,5-di-
t-amyl-2-hydroxyphenyl)benzotriazole. Among these, 2-[2-
hydroxy-3,5-bis(cc,a'-dimethylbenzyl)phenyl]-2H-
benzotriazole is particularly preferred because of its
heat resistance and high solubility in dimethylacetoamide
and N-methyl-2-pyrrolidone, which are good solvents for
the meta-linkage-containing aromatic polyamide.
The amount of the ultraviolet absorber to be added
is preferably 2.0 to 6.0 wt~, and particularly 3.0 to 5.0
wt$ based on the meta-linkage-containing aromatic
polyamide. If the amount is less than 2.0 wt~ the anti-
yellowing effect may not be exhibited, and even if it is
added at greater than 6.0 wt~ no further improvement in
the anti-yellowing effect may result, while the
workability of the fibers during the production process
may be reduced.
The mixture of the meta-linkage-containing aromatic
polyamide with the alkylbenzenesulfonic acid onium salt,
halogen-containing alkyl (phenyl) phosphate and
ultraviolet absorber may be accomplished by a method
wherein the meta-linkage-containing aromatic polyamide is
added to a solvent and mixed therewith to make a solution
to which solutions of the alkylbenzenesulfonic acid onium
salt, halogen-containing alkyl (phenyl) phosphate and
ultraviolet absorber each in appropriate solvents are
then added and mixed therewith, or an alternative method
in which the meta-linkage-containing aromatic polyamide,
alkylbenzenesulfonic acid onium salt, halogen-containing
alkyl (phenyl) phosphate and ultraviolet absorber are
combined into a mixture which is then dissolved in a
solvent. The dope obtained in this manner may then be
formed into fibers by a publicly known method.
An example of a typical fiber-forming method
involves addition of the alkylbenzenesulfonic acid onium
salt, halogen-containing alkyl (phenyl) phosphate and
benzotriazole-based ultraviolet absorber to an N-methyl-
2-pyrrolidone solution containing a poly-m-phenylene
isophthalamide polymer, to prepare a dope. The dope is
extruded from a nozzle into an aqueous inorganic solution
whose main component is calcium chloride, stretched after
coagulation and washing with water, and then further
stretched on a hot plate at 300-325°C and crystallized,
and finally subjected to oiling to complete the fibers.
For staple fibers, they are crimped, cut and then spun to
obtain easily dyeable spun fibers.
CA 02169344 1999-10-OS
- g
The easily dyeable meta-linkage-containing aromatic
polyamide fibers of the present invention obtained in the
manner described above have an excellent dyeing property
and light fastness without any loss in the excellent heat
resistance, flame retardance and flame proofness of the
original meta-linkage-containing aromatic polyamide
fibers, and thus may be effectively applied for clothing,
bedding and interior decorations which require coloring.
In particular, the addition of the halogen-containing
alkyl (phenyl) phosphate provides further improvement in
the flame retardance while drastically improving the
dyeing property and lowering the dyeing cost compared to
addition of the alkylbenzenesulfonic acid onium salt
alone, and addition of the ultraviolet absorber,
particularly a benzotriazole-based ultraviolet absorbing
compound, drastically improves the light fastness;
allowing the fibers to be applied in the fields of
clothing, bedding and interior decorations in the same
manner as existing common fibers.
The present invention is explained below by way of
the examples. The measured values in the examples and
comparative examples were obtained by the following
methods.
1. Dyeing property
Crimped fibers were collectively cut to a
length of 50 mm and dyed at 30°C for.90 minutes using a
dyeing solution comprising 8~ o.w.f: *Estrol Navy Blue N-
RL (product of Sumitomo Chemical Co., Ltd.), 0.3 g/1
acetic acid and 25 g/1 sodium nitrate to a fiber/dyeing
solution ratio (liquor ratio) of 1:40, after which a
solution comprising 1 g/1 hydrosulfite, 1 g/~ *Amiladin D
(product of Dai-ichi Kogyo Seiyaku Co., Ltd.) and l g/1
sodium hydroxide was used at a liquor ratio of 1:40 for
reductive washing at 80°C for 30 minutes, followed by
water washing and drying: A 1.3g portion of the fibers
was stuffed into a cell having a diameter of 31 mm and a
depth of 13 mm, and a'*CM-2002 Minolta spectrophotometric
*Trade-mark
--
- g _
colorimeter was used for colorimetry with a 10° field of
view, a D76 light source and regular reflection
elimination, using the value L* as an index of the dyeing
property.
2. Intrinsic viscosity (IV)
The polymer was dissolved in N-methyl-2-
pyrrolidone (NMP) to a concentration of 0.5 g/100 ml, and
an Ostwald's viscometer was used for measurement at 30°C.
3. Fineness
The fineness of the fibers was measured
according to JIS-L-1015.
4. Tensile strength
The tensile strength of the fibers was measured
according to JIS-L-1074, with a 20 mm long test sample,
an initial load of 1/20 g/de and an elongation rate of
2 0 mm/min .
5. Alkylbenzenesulfonic acid onium salt content of
f fibers
As a standard sulfur sample, 0.1 cc of an
aqueous (NH~)ZSO,, solution of known concentration is
dropped onto test filtration paper, and a calibration
curve is prepared based on fluorescent X-ray quantitative
analysis after vacuum drying. About 50 mg of the fibers
and about 20 mg of calcium chloride are dissolved in 5.0
cc of NMP by heating at 110°C for 1 hour, and after
dropping a standard amount (0.1 cc) on the test
filtration paper, it is vacuum dried. The sample is then
quantitatively analyzed by fluorescent X-ray analysis,
the sulfur concentration of the fibers is calculated
based on the previously prepared calibration curve, and
this is converted into the content on the assumption that
all of the sulfur is derived from the
alkylbenzenesulfonic acid onium salt.
6. Alkyl (phenyl) phosphate content of fibers
In the same manner as above, a calibration
curve is prepared by fluorescent X-ray analysis of a
~~~~44
- 10 -
standard phosphorus sample of known concentration, the
phosphorus concentration of the fibers is calculated, and
this is converted into a content on the assumption that
all of the phosphorus is derived from the alkyl (phenyl)
phosphate.
7. Benzotriazole-based ultraviolet absorbing
compound content of fibers
About 20 mg of a sample dried at 105°C for 60
minutes is measured into a test tube (120 mm x 10 mm~,
pyrex), 4 ml of concentrated hydrochloric acid is added
and the tube is sealed. The sealed tube is heated at
130°C for about 6 hours for hydrolysis. After completion
of hydrolysis, the sealed tube is cooled to room
temperature and opened. The total contents of the test
tube are then transferred to a 50 ml separatory funnel, 5
ml of chloroform is added, and the mixture is adequately
shaken and allowed to stand, after which the chloroform
phase is removed. This extraction procedure with
chloroform is repeated 3 times, all of the chloroform
phases are combined and concentrated to 2-3 ml, and the
solution is then analyzed by liquid chromatography (LC).
Separately, a test sample is prepared for a calibration
curve, which is obtained by the same procedure as
described above. The calibration curve is used to
quantify the benzotriazole-based compound in the sample.
8. Measurement of light fastness
About 2g of sample (crimped and cut to lengths
of 38-76 mm) is taken and dispersed with a hand card.
The dispersed sample is affixed to a mount to a width of
18-22 mm and a thickness of 2-3 mm, as shown in Fig. 1.
The mount is then set onto a metallic flask. The
metallic flask is set into a fade tester (Model CF-20N,
product of Shimazu Laboratories), and irradiated for a
prescribed time with an arc current of 15-17 A and an
internal temperature of 42-45°C. The difference in the
degree of discoloration of the "irradiated" and "non-
irradiated" sections of the irradiated sample is judged
- 11 -
visually against the difference in discoloration of a
simultaneously irradiated blue scale (JIS L0841, product
of Japan Standards Association).
Example 1
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 110g of NMP and
further mixed with 3.6g of phosphonium
dodecylbenzenesulfonate, and the solution was subjected
to vacuum degassing to make a spinning dope.
The dope was heated to 85°C and then used for wet
spinning into a spinning bath from a spinning nozzle with
200 holes, each with a diameter of 0.07 mm. The
composition of the spinning bath was 40 wt% calcium
chloride, 5 wt~ NMP and 55 wt~ water, and the temperature
of the spinning bath was 85°C. The filaments were given
a course of about 100 cm through the spinning bath, and
were drawn at a rate of 6.2 m/min. The filaments were
then washed with water, stretched to a draw ratio of 2.4
in 95°C hot water, and dried using a roll at 200°C, after
which they were stretched to a draw ratio of 1.75 on a
320°C hot plate to obtain stretched filaments with
400 de/200 filaments. The total draw ratio was 4.2.
Example 2
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 110g of NMP and
further mixed with a solution of 3.6g of
tributylbenzylammonium dodecylbenzenesulfonate in 2g NMP,
and the solution was subjected to vacuum degassing to
make a spinning dope. This dope was used for spinning
and stretching in the same manner as in Example 1, to
obtain stretched filaments with 400 de/200 filaments.
Example 3
A 5.7g portion of tetrabutylphosphonium
dodecylbenzenesulfonate was dissolved in 110g of NMP, and
then 30g of poly-m-phenylene isophthalamide with an IV of
1.35 dl/g was dissolved therein and the solution was
subjected to vacuum degassing to make a spinning dope.
- 12 -
This dope was used for spinning and stretching in the
same manner as in Example 1, to obtain stretched
filaments with 400 de/200 filaments.
Example 4
A 2.7g portion of tetrabutylphosphonium
dodecylbenzenesulfonate was dissolved in 110g of NMP, and
then 30g of poly-m-phenylene isophthalamide with an IV of
1.35 dl/g was dissolved therein and the solution was
subjected to vacuum degassing to make a spinning dope.
This dope was used for spinning and stretching in the
same manner as in Example 1, to obtain stretched
filaments with 400 de/200 filaments.
Example 5
A 6.Og portion of tetrabutylphosphonium
dodecylbenzenesulfonate was dissolved in 110g of NMP, and
then 30g of poly-m-phenylene isophthalamide with an IV of
1.35 dl/g was dissolved therein and the solution was
subjected to vacuum degassing to make a spinning dope.
This dope was used for stretching in the same manner as
in Example 1, to obtain stretched filaments with
400 de/200 filaments.
The measurement results of the physical properties
of the fibers obtained in Examples 1 to 5 are given in
Table 1 below. All of the fibers in these examples were
found to have satisfactory dyeing properties, high
strength, and satisfactory spinning properties.
Table 1
Opium salt Strength Ductility Dyeing
content of property
fibers (g/de) ($) I,* value
Example 4.4 5.0 44 22.0
1
Example 4.0 4.9 45 22.5
2
Example 7.0 4.6 42 19.5
3
Example 3.3 5.0 43 25.0
4
Example 7.4 4.0 39 19.0
5
Opium salt: alkylsulfonic acid opium salt
~
.-
- 13 -
Content: mole percent with respect to polymer
Example 6
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 1108 of NMP, and
then 3.6g of phosphonium dodecylbenzenesulfonate and 1.5g
of tris(j3-chloropropyl) phosphate were mixed therewith
and the solution was subjected to vacuum degassing to
make a spinning dope.
The dope was heated to 85°C and then used for wet
spinning into a spinning bath from a spinning nozzle with
200 holes each with a diameter of 0.07 mm. The
composition of the spinning bath was 40 wt~ calcium
chloride, 5 wt~ NMP and 55 wt~ water, and the temperature
of the spinning bath was 85°C. The filaments were given
a course of about 100 cm through the spinning bath, and
were drawn at a rate of 6.2 m/min. The filaments were
then washed with water, stretched to a draw ratio of 2.4
in 95°C hot water, and dried using a roll at 200°C, after
which they were stretched to a draw ratio of 1.75 on a
320°C hot plate to obtain stretched filaments with
400 de/200 filaments. The total draw ratio was 4.2.
Example 7
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 1108 of NMP, and
then a solution of 3.6g of tributylbenzylammonium
dodecylbenzenesulfonate and 1.5g of tris(~3-chloropropyl)
phosphate in 2g of NMP was mixed therewith and the
solution was subjected to vacuum degassing to make a
spinning dope. This dope was used for spinning and
stretching in the same manner as in Example 6, to obtain
stretched filaments with 400 de/200 filaments.
Example 8
A 3.6g portion of tetrabutylphosphonium
dodecylbenzenesulfonate and l.Sg of tris(dichlorophenyl)
phosphate were dissolved in 1108 of NMP, and then 30g of
poly-m-phenylene isophthalamide with an IV of 1.35 dl/g
was dissolved therein and the solution was subjected to
- 14 -
vacuum degassing to make a spinning dope. This dope was
used for spinning and stretching in the same manner as in
Example 6, to obtain stretched filaments with 400 de/200
filaments.
Example 9
A 2.258 portion of tetrabutylphosphonium
dodecylbenzenesulfonate and 2.4g of tris(J3-chloropropyl)
phosphate were dissolved in 1108 of NMP, and then 30g of
poly-m-phenylene isophthalamide with an IV of 1.35 dl/g
was dissolved therein and the solution was subjected to
vacuum degassing to make a spinning dope. This dope was
used for spinning and stretching in the same manner as in
Example 6, to obtain stretched filaments with 400 de/200
filaments.
Comparative Example 1
A 3.6g portion of tris((3-chloropropyl) phosphate was
dissolved in 1108 of NMP, and then 30g of poly-m-
phenylene isophthalamide with an IV of 1.35 dl/g was
dissolved therein and the solution was subjected to
vacuum degassing to make a spinning dope. This dope was
used for spinning and stretching in the same manner as in
Example 6, to obtain stretched filaments with 400 de/200
filaments.
Example 10
A 2.25g portion of tetrabutylphosphonium
dodecylbenzenesulfonate and 0.458 of tris(~3-chloropropyl)
phosphate were dissolved in 1108 of NMP, and then 30g of
poly-m-phenylene isophthalamide with an IV of 1.35 dl/g
was dissolved therein and the solution was subjected to
vacuum degassing to make a spinning dope. This dope was
used for spinning and stretching in the same manner as in
Example 6, to obtain stretched filaments with 400 de/200
filaments.
Example 11
A 3.6g portion of tetrabutylphosphonium
dodecylbenzenesulfonate and 0.9g of tris(~i-chloropropyl)
phosphate were dissolved in 1108 of NMP, and then 30g of
i~
poly-m-phenylene isophthalamide with an IV of 1.35 dl/g
was dissolved therein and the solution was subjected to
vacuum degassing to make a spinning dope. This dope was
used for spinning and stretching in the same manner as in
Example 6, to obtain stretched filaments with 400 de/200
filaments.
The measurement results of the physical properties
of the fibers obtained in Examples 6 to 11 and
Comparative Example 1 are given in Table 2 below. All of
the fibers in the examples were found to have
satisfactory dyeing properties, high strength, and
satisfactory spinning properties. However, the fibers of
Comparative Example 1 had a very poor dyeing property,
and thus it was found that the tris(~i-chloropropyl)
phosphate by itself had no effect of improving the dyeing
property.
Table 2
Onium salt Phosphate Strength DuctilityDyeing
content content (g/de) property
of of (~) L* value
fibers fibers
Ex. 6 4.4 3.0 4.9 44 19.0
Ex. 7 4.0 3.0 4.9 44 19.5
Ex. 8 4.4 3.0 5.0 44 19.0
Ex. 9 2.8 4.8 4.8 39 21.5
Comp. Ex. 0 7.2 4.0 36 34.0
1
Ex. 10 2.8 0.9 5.0 45 26.5
Ex. 11 2.8 6.0 4.4 31 21.5
Onium salt: alkylsulfonic acid onium salt
Onium salt content: mole percent with respect to polymer
Phosphate: halogen-containing alkyl (phenyl) phosphate
Phosphate content: weight percent based on polymer
Example 12
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 1108 of NMP, and
then 3.6g of tributylbenzylammonium
dodecylbenzenesulfonate, 1.5g of tris(/3-chloropropyl)
- 16 -
phosphate and 0.6g of 2-[2-hydroxy-3,5-bis(a,a'-
dimethylbenzyl)phenyl]-2H-benzotriazole were mixed
therewith and the solution was subjected to vacuum
degassing to make a spinning dope. The dope was heated
to 85°C and then used for wet spinning into a spinning
bath from a spinning nozzle with 200 holes each with a
diameter of 0.07 mm. The composition of the spinning
bath was 40 wt~ calcium chloride, 5 wt~ NMP and 55 wt~
water, and the temperature of the spinning bath was 85°C.
The filaments were given a course of about 100 cm through
the spinning bath, and were drawn at a rate of 6.2 m/min.
The filaments were then washed with water, stretched to a
draw ratio of 2.4 in 95°C hot water, and dried using a
roll at 200°C, after which they were stretched to a draw
ratio of 1.75 on a 320°C hot plate to obtain stretched
filaments with 400 de/200 filaments. The stretched
filaments were crimped with a crimper, and cut to a
length of 51 mm with a cutter. The filaments were dyed
with Estrol Navy Blue N-2RL (product of Sumitomo Chemical
Co., Ltd.) by the method described above, and the dyed
filaments were measured for light fastness with a fade
tester (Model CF20N, Shimazu Laboratories) by the method
described above.
Example 13
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 1108 of NMP, and
then 3.6g of tributylbenzylammonium
dodecylbenzenesulfonate, 1.5g of tris(/3-chloropropyl)
phosphate and 1.2g of 2-[2-hydroxy-3,5-bis(a,a'-
dimethylbenzyl)phenyl]-2H-benzotriazole were mixed
therewith and the solution was subjected to vacuum
degassing to make a spinning dope. This dope was used
for spinning and stretching in the same manner as in
Example 12, to obtain stretched filaments with 400 de/200
filaments. The obtained stretched filaments were
crimped, cut, dyed and measured for light fastness by the
same method as in Example 12.
~~~'~3~
Example 14
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 110g of NMP, and
then 3.6g of tributylbenzylammonium
dodecylbenzenesulfonate, 1.5g of tris(~3-chloropropyl)
phosphate and 1.8g of 2-[2-hydroxy-3,5-bis(a,a'-
dimethylbenzyl)phenyl]-2H-benzotriazole were mixed
therewith and the solution was subjected to vacuum
degassing to make a spinning dope. This dope was used
for spinning and stretching in the same manner as in
Example 12, to obtain stretched filaments with 400 de/200
filaments. The obtained stretched filaments were
crimped, cut, dyed and measured for light fastness by the
same method as in Example 12.
Example 15
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in NMP, and then
3.9g of tributylbenzylammonium dodecylbenzenesulfonate
and 1.5g of tris(j3-chloropropyl) phosphate were mixed
therewith and the solution was subjected to vacuum
degassing to make a spinning dope. This dope was used
for spinning and stretching in the same manner as in
Example 12, to obtain stretched filaments with 400 de/200
filaments. The obtained stretched filaments were
crimped, cut, dyed and measured for light fastness by the
same method as in Example 12.
Example 16
A 30g portion of poly-m-phenylene isophthalamide
with an IV of 1.35 dl/g was dissolved in 110g of NMP, and
then 3.6g of tributylbenzylammonium
dodecylbenzenesulfonate, 1.5g of tris(j3-chloropropyl)
phosphate and 2.4g of 2-[2-hydroxy-3,5-bis(a,a'-
dimethylbenzyl)phenyl]-2H-benzotriazole were mixed
therewith and the solution was subjected to vacuum
degassing to make a spinning dope. This dope was used
for spinning and stretching in the same manner as in
Example 8, to obtain stretched filaments with 400 de/200
.r ~~e~~~~
- 18 -
filaments.
There was considerable breakage of single filaments
during the spinning and stretching process, and a great
deal of fuming on the 320°C hot plate.
The obtained stretched filaments were crimped, cut,
dyed and measured for light fastness by the same method
as in Example 12.
The measurement results for Examples 12 to 16 are
given in Table 3. All of the fibers in the examples had
satisfactory filament quality, dyeing properties and
light fastness, except that the fibers of Example 15 did
not have an improved light fastness.
Table 3
Onium Phosphate UltravioletStrengthDuctilityLight
salt content absorber fastness
content of content (g/de) (X)
of fibers of (degree)
fibers fibers
Ex. 4.7 5.0 2.0 S.0 44 3
12
Ex. 4.7 5.0 4.0 4.9 43 4
13
Ex. 4.7 S.0 6.0 4.9 42 4-S
14
Ex. 5.1 5.0 0.0 5.1 46 1-2
15
Ex. 4.7 5.0 8.0 4.4 38 4-5
16
Onium salt: tributylbenzylammonium
dodecylbenzenesulfonate
Onium salt content: mole percent with respect to polymer
Phosphate: tris((i-chloropropyl) phosphate
Phosphate content: weight percent with respect to
polymer
Ultraviolet absorber: 2-[2-hydroxy-3,5-bis(a,oc'-
dimethylbenzyl)phenyl]-2H-
benzotriazole
Ultraviolet absorber content: weight percent based on
polymer