Note: Descriptions are shown in the official language in which they were submitted.
W095/05890 ~ 6 ~ 5 ~ ~ PCT~S94/09432
.
MEMBRANES PREPARED ~ROM
CROSSLINKABLE SOLUBLE POLYMERS
- The present invention is related to composite
membranes useful in fluid separations and methods for
5 their preparation.
Semi-permeable composite membranes prepared
from various synthetic polymeric compositions are used
in various commercial and industrial applications for
the separation of various components found in liquids or
gases. Reverse osmosis and nanofiltration membranes are
typically relatively thin in order to provide a
desirable, i.e., relatively high, flux rate. Thus, it
is generally necessary that the reverse osmosis or
nanofiltration membrane be laminated onto a porous
support material. This support material will generally
possess cnaracteristics which make it desirable for such
a use. Such characteristics include a sufficient number
of pores large enough to permit water or other permeates
to pass through the support without adversely affecting
the flux rate or separation efficiency of the entire
composite membrane. Conversely, the pore size should
not be so large that the membrane tends to be forced
into the pores or rupture during use.
The present invention is directed to a
composite membrane which comprises a polymeric
discriminating layer affixed to a porous support layer.
3 The discriminating layer is formed by irradiating a
substantially aqueous polymer solution under conditions
which are sufficient to form a polymer film on the
~urface of the polymer solution at the interphase of the
3~ solution and a blanketing fluid which is immiscible with
the polymer solution. A significant feature of the
WO9S/05890 PCT~S94/09432
5 ~ --
present invention is that it is not necessary to dry the
polymer solution prior to irradiation t provided the bulk
concentration of the polymer in solution is at an
appropriate level to form the film upon irradiation.
The membranes of the present invention can be
made to exhibit a variety of molecular weight cut offs
(MWCOs) by altering the process for the preparation of
the membrane rather than by altering the polymer
backbone of the discriminating layer. The membranes
made by the process of the present invention preferably
have MWCOs ranging from 150 to 2000 daltons.
Further, the present invention largely avoids
the use of organic solvents. Additionally, the
membranes of the present invention are resistant to
degradation by chlorine and other oxidizers.
The discriminating layer of the membrane of the
current invention is prepared by irradiating a
substantially aqueous polymer solution under conditions
such that a thin film is formed on the surface of the
polymer solution at the interphase of the solution and a
blanketing fluid which is immiscible with the polymer
solution. Typically, the polymer sol~tion is open to
the air and the film is formed at the air/solution
interphase. However, the blanketing fluid may be an
atmosphere other than air or it may be a liquid which is
transparent to radiation and which does not interfere
with the formation of the discriminating layer.
When irradiated, the polymer solution from
which the discriminating layer is formed is
substantially aqueous. That is, it need not have been
subjected to a drying step and is not dry or in the form
W095/0589~ 5 ~ ~ ~ PCT~S94/09432
of a gel. The primary requirement is that the bulk
concentration of the polymer in solution is at an
appropriate level to form the film upon irradiation.
The appropriate level is from 0.01 to 30 weight percent
polymer. The ability to form the film in the absence of
drying is normally of particular advantage in an
industrial setting.
As noted above, the polymer solution is
substantially aqueous. By "aqueous", it is meant that
the solvent is typically water although water compatible
co-solvents may by used in conjunction with the water.
Water is generally preferred as a solvent due to cost
and availability and should constitute a minimum of
fifteen percent of the solution. At least fifty percent
water is preferred and at least seventy percent water is
more preferred. Compatible co-solvents include ethylene
glycol, lower alkanols and similar substances. The
primary restriction on co-solvents is that they do not
interfere with film formation.
Additionally, the polymer solution optionally
contains various additives including certain salts and
acids Specific examples of additives include NaCl,
H2S04, H3P04, CH3COOH, HN03, LiCl, MgCl2, NaS04, Na,
HP04, and HCl. Additionally, photosensitizers such as
the sodium salt of 2-naphthalenesulfonic acid may be
used. Depending on the desired properties of the
composite membrane, additives can be used to enhance
3 flux and/or selectivity.
The discriminating layer is formed at the
surface of the aqueous polymer solution and may then be
recovered and affixed to an appropriate support.
Alternatively, the aqueous polymer solution may be
WogS/05890 PCT~S94/09432
applied to a support and then irradiated to form the
discriminating layer ins~tu. It is a particular
advantage in an industrial setting that the
discriminating layer can be formed and affixed to the
porous support in a single process which may be
continuous.
In one preferred embodiment, the membrane of
the present invention is prepared in a process
comprising the following steps:
(1) contacting a support with at least one
polymer solution which is from 0.01 to 30
weight percent polymer in a substantially
aqueous solvent;
(2) irradiating the polymer solution under
conditions such that a discriminating layer
is formed at the blanketing fluid~solution
interphase; and
(3) affixing the discriminating layer to the
support.
In addition to these essential steps which may
be performed simultaneously or sequentially, the process
may comprise additional steps. In the preparation of
the membrane, it is desired that the support be "wet"
and that the discriminating layer be adhered to the
support sufficiently to prohibit unrestrained swelling
3 of the discriminating layer. Depending on the
particular support and discriminating layer used. this
may be accomplished by coating a support with a single
polymer solution that performs all the desired
functions. Alternatively, the support may be coated
with different solutions prior to irradiation. For
WO95/05890 ~ 5~ PCT~S94/09432
example, it may be desirable to treat a support with a
wetting solution; then with a polymer solution that
forms the basis for an affixing layer which, at a
minimum, functions to adhere the discriminating layer to
the support sufficiently to prohibit unrestrained
swelling of the discriminating layer; and then with a
solution from which the discriminating layer is formed.
Depending on the support and polymer solutions
used, the 'wetting' solution, the 'affixing' solution
and the discriminating layer solution may be the same
polymer solution and may be applied in a single layer or
in multiple layers. Alternatively, the solutions may
use the same polymer, but use a different solvent or a
different concentration. 41ternatively two or more
different polymer solutions may be used. In one
alternative embodiment, the process comprises the
following steps performed simultaneously or
sequentially:
(1) contacting a support with a wetting
solution;
(2) contacting the wet support with an affixing
layer forming polymer solution which is
from 0.01 to 10 weight percent polymer;
(3) contacting the wet support with a
di3criminating layer forming polymer
solution which is from 0.01 to 30 weight
percent polymer in a substantially aqueous
solvent;
(4) irradiating the discriminating layer
forming polymer solution under conditions
such that a discriminating layer is formed
W O 9S/OS890 PCTrUS94/09432
2~ 69~a
at the blanketing fluid/solution
interphase; and
(~) affixing the discriminating layer to the
support.
In another alternative embodiment, the process
comprises the following steps performed simultaneously
or sequentially:
(1) contacting a support with an affixing layer
forming polymer solution which is from 0.01
to 10 weight percent polymer;
(2) contacting the support with a
discriminating layer forming polymer
solution which is from 0.01 to 30 weight
percent polymer in a substantially aqueous
solvent;
(3) irradiating the discriminating layer
forming polymer solution under conditions
such that a discriminating layer is formed
at the blanketing fluid/solution
interphase; and
(4) affixing the discriminating layer to the
support.
In each embodiment, the process may be ccntinuous.
3 Polymer solutions and wetting solutions may be
applied to the support by techniques known to one
skilled in the art. Conventional techniques include
adsorption, dipping, casting,-spraying, wiping, rolling,
or filtration of the coating solution through the
substrate. Excess coating may be removed by draining or
W095/OS8YO ~ PCT~S94/09432
drawing a smooth instrument such as a blade or roller
across the surface. The temperature of the coating
solutions are selected so as to avoid conditions
detrimental to the resulting membrane. Other than as
discussed herein, operating parameters for applying the
polymer solutions are not critical so long as the
resulting membrane is not deleteriously affected. The
process may be conducted at temperatures ranging from
0 to 55C. Ambient temperatures, i.e., 10 to 45C are
generally convenient and therefore preferred.
The support is typically porous and does not
significantly impede the transport of fluids across the
membrane as compared to the discriminating layer. It is
used to provide mechanical strength to the membrane.
Examples of suitable supports include a microporous
polymer such as polysulfone, polyethersulfone,
polycarbonate, polyvinylidene chloride, Nylon,
polyetherether ketone, polybenzimidazole, cellulose
acetate or other cellulose esters.
The manner in which the discriminating layer is
affixed to the support is not critical to the present
invention so long as the resulting membrane has the
desired characteristics. The discriminating layer may
be affixed through chemical or physical means. Methods,
known to those skilled in the art, may be used such as
drying or acid catalyzed condensation. Activation of a
thermally sensitive crosslinking agent is a suitable
3 method. This may be accomplished in a separate step,
subsequent to the formation of the discriminating layer,
in which the membrane is subjected to elevated
- temperatures, in for example, an oven in a temperature
range from 50C to 200C, more preferably from 75C to
150C. In an alternative embodiment, the discriminating
WO 95/05890 PCT/US94/09432
layer maybe affixed to the support as a result of heat
that is incidental to the irradiation to ~orm the
di~criminating layer.
The polymer used in the discriminating layer
polymer solution must be capable of film formation upon
exposure to radiation. The polymer must also be surface
active in that it is necessary that the surface
concentration of the polymer in the discriminating layer
polymer solution be ~ufficient to form a surface film.
Multi-component polymers, such as those useful in the
present invention, usually consist of different
monomeric units each of which contributes a desired
characteristic to the resulting polymer and ultimately
to the finished membrane.
In order to impart the desired properties to
the membrane discriminating layer, polymeric reactants
may contain groups in the repeating unit in addition to
a moiety directly bearing or including a reactive
cationic or nucleophilic group, provided these groups do
not adversely affect the membrane or its formation. For
example, in cationic vinyl addition polymers, such
methacrylate derivatives as
3o
W095/05890 ~ PCT~S94/09432
c~3
~ CH2--~
ICI---t~2 to C3 alkylenet-~H
o
CH3
-tCH2- ~ and/or
f
c~3 O(C1 to Clg alkyl)
-tCH2--~t-
C=O
O-CH2CH2 ( 0-CH2C~O--~
(C6 to C22
alkyl or
alkenyl)
wherein m is an integer from l to 20, may be present to
advantage in membranes for reverse osmosis.
Photosensitivity in the polymer is preferably
obtained by the presence of onium groups on the polymer
backbone. Known onium groups include aryl cationic
moieties, which have been described as photoacid
generating initiators in the prior art. For example,
3o The Chemistry of the Sulfonium Group, edited by
C. J. M. Stirling and S. Patai, pp. 107-122,
John Wiley & Sons (1981), describes the photochemistry
of sulfonium compounds. Advances in Polymer Science,
62, pp. 1-48, Springer-Verlag Berlin, Heidleberg (1984),
describes the cationic polymerization using iodonium or
W095/OS890 b .` ~ PCT~S94/09432
sulfonium salt photoinitiators. It has been found that
in preferred embodiments, the polymer bearing a
plurality of photolabile onium groups will react at
ambient temperatures with even weakly reactive
nucleophile groups, such as amides, urea moieties or
5 sulfonic acid salts.
Preferred photolabile onium groups include
sulfonium, quaternary ammonium, phosphonium, pyridinium,
thiazolinium, imidazolinium or azetidinium groups.
Diazonium groups are not onium groups as the term is
used herein. Techniques and processes for making
compounds bearing the desired moieties are well known in
the prior art. U.S. Patents 2, 676,166; 2,891,025;
5 3,269,991; 3,329,560; 3,429,839; 3,544,499; 3,636,052;
3,723,386; 3,962,165; 4,002,586; 3,804,797; 4,337,185,
4,483,07~; 4,426.489; 4,444,977 and 4,477,640 are
incorporated herein by reference to illustrate
techniques for making such compounds. Especially
20 preferred as photolabile oniums are those containing a
sulfonium, quaternary ammonium or phosphonium group.
Preferably, the substituents on the photolabile onium
are each independently hydroxyalkyl, phenyl or alkyl
groups or are heterocyclic saturated moieties which
25 include the onium in the ring. Most preferably, the
photolabile onium group is bonded to the ~CH2~ moiety of
a benzyl group and is a dialkyl sulfonium, trialkyl
phosphonium or trialkyl ammonium moiety wherein each
alkyl has rom about 1 to about 16 carbon atoms or is a
sulfonium, alkyl phosphonium or alkyl ammonium where two
of the valences are part of a five- or six-member ring
including the onium.
The chromophore group is preferably an aromatic
group. The chromophore group may be joined to the onium
--10--
W095/OS891D 21 ~ PCT~S94109432
moiety by a linking group (chromophore-linking group-
onium) advantageously selected from methylene,
i.e.,(-CH2-), ethylidene (i.e.,
-CH- ) or -OCH2CH-CH2-
CH3 OH
Especially preferred as a chromophore is a phenyl group
which is pendant from a polymer backbone. Especially
preferred as a linking group is methylene or
OH
-OCH2CHCH2-
Preferably, the benzyl onium salt groups are
part of a a vinyl addition polymer. Such polymers can
readily be prepared by conventional vinyl addition
polymerization of vinyl benzyl chloride with other
compatible monomers followed by reaction of the benzyl
chloride with a suitable onium precursor. For example,
dialkyl sulfide will react with the benzyl chloride
group pendant from a vinyl addition polymer to form a
dialkyl sulfonium group. Tertiary amines or phosphines
will react with benzyl chloride in a similar manner.
Alternatively, a polystyrene or styrene copolymer can be
chloromethylated via conventional techniques to
introduce benzyl chloride groups. The benzyl chloride
groups can then be converted to onium groups as
described hereinbefore for the vinyl benzyl chloride
polymers.
The anion associated with the photolabile onium
group is advantageously selected so as to promote
WO9S/0S890 PCT~S94109432
5 ~ f~
reaction between the photolabile onium group and the
nucleophile group present, when exposed to radiation.
Any anion is operable so long as the reaction is not
deleteriously affected. Optionally, inner salts or
partial inner salts of onium compounds can be employed,
such as a polymer bearing both carboxylate and
photolabile onium groups. Some anions. such as
hydroxide, in some embodiments will also make a
sulfonium or certain other onium groups more susceptible
to the competing thermal reaction or degradation. The
counterion can be readily changed by contacting the
compound bearing the onium group with an appropriate ion
exchange resin in the conventional manner to effect
conversion to the desired anion.
Preferred polymers are those which provide
sufficient polymer concentration at the interphase with
the blanketing fluid to permit thin film ~ormation.
A preferred class of photoreactive systems is
represented by Formula I
~ I z_Qe pNue (I)
where illustrative embodiments of ArC, Z. Q~ and PNue
are presented in Table A. It should be noted that the
positive charge on Q~ and negative charge on PNae may
be one or greater with the proviso that in each instance
they are equal so that the overall charge is neutral.
WO 95/05890 ~ 5 ~ ~ PCT/US94/09432
(D ~ ~
o~ o 0 ~ o
V o
3 ~ ~ / ~ v/ \''
o ~ o I o
V O O N ~U~//
V ~ (D 2 ~ ~
~ C~ \ / N N ~$ ) ~ <~=>
1~ N
~ PCT~S94/09432
In Table A, each moiety at each occurrence is
independently selected from the group consisting of
R' = a polymer or copolymer backbone optionally
inertly substituted or bearing a plurality of Q~ and/or
pNue;
R1 and R2 are each independently hydrogen
C1-C1g alkyl, or -CH2(CH2)uOH, preferably CH3 or
tertiary-butyl, where u = 1 to 12;
RF is a fluorinated alkyl.
RF may be an alkyl which is not fully
fluorinated, but no more than one atom of hydrogen or
chlori~e should be present in place of fluorine for each
carbon atom.
RF is preferably ~CF2)VCF3, where v is an
integer from 1 to 12, more preferably from 6 to 12, or
RF is preferably -(CH2tx~CF2)F, where x is an integer 1
or 2 and y is an integer from 1 to 12, more preferably
from 6 to 12; and
R = C1 to C1g alkyl, phenyl or a polymer or
copolymer which is optionally inertly substituted or
bears a plurality of Q~ and/or PNue.
The photoreactive moiety, ArC-Z-Q~, may be used
as a low molecular weight species, for example
3o
QfflCH2~ CH2Q
-14-
W095/05890 ~ 5 ~ ~ PCT~S94109432
OH OH
Q-CH2--C-CH2-~3+~ OCH2-C-CH2-Q
The photoreactive moiety is preferably attached
to a polymer, either as a pendant group or as an end
group. For example, a class of polymers can be
represented by Formula II:
A-(B)m(c)n(D)o- E (II)
- - P
A and E are each terminal groups resulting from a vinyl
polymerization, and B, C and D are internal covalently
bonded groups which can be arranged in any sequence.
The subscripts m, n and o represent molar ratios and
m+n~o = 1.00 where m is in the range from about 0.03 to
about 1.00, n is in the range from O to 0.97 and o is in
the range from O to 0.96. The subscript p is the
average degree of polymerization, preferably from abou~
2 to 1,000, more preferably from about 100 to about
1 000 .
In Formula II, B is a photoreactive moiety
which has the formula
Rq-Y-ArC-Z-Qffl
wherein Rq is a group which includes a carbon-carbon
- single bond formed during vinyl addition polymerization
of the polymer and Y is a chemical bond or a
noninterfering, bivalent moiety. ArC is a chromophore,
W095/05890 - PCT~S94/09432
~
Z is a linking group and Q~ a photolabile onium as
defined hereinbefore. Preferably, Rq is the residue of
an ethylenically unsaturated monomer, more preferably
~CH2-CH~ or
CH3
~CH2-C3
1 0
and Y is a chemical bond (in which case B is
Rq-ArC-Z-Q~) or a noninterfering connecting group. such
as
-C ~o -C ~ ~
O-~CH2~V \NH~CH2~V O~CH2cH2o~u -C-O-
~CH2CH-O~u
-C-O~CH2CH0~u ; ~CH2CH20~U and CH3
CH3
wherein u is independently at each occurrence an integer
from 1 to 20 and v is an integer from 1 to 12, but
preferably 1. Illustrative examples of B include
3o
-16-
W095/05890 ~ 5 5 PCT~S94/09432
.
H H
5-CH2-C -CH2-C
- _ 1-
CH2Q~ 0CH2-C-cH2Q
CH3
-CH2-C -
C=0
CH2 ~ CH2-
In Formula II, "C" is a group derived from anethylenically unsaturated monomer which has the formula:
RP-Y-PNue
where RP is a residue of an ethylenically unsaturated
monomer, PNue (as defined hereinbefore), and Y' is a
chemical bond, in which case "C" is RP-PNue, or Y' is a
noninterfering group, such as
-C(0)-(CH2)u or -C(o)o(CH2tU
wherein u is an integer from 1 to 20. Illustrative of
"C" are
W09S/05890 `~ PCT~S94/09432
~1~9~
C,H3 o
~CH2-C~ff-Oe ~CH2-CH~
[~CH2COO~
In Formula II D has the ~ormula
I
_Rh_G
where Rh is an organic group and the residue from a
polymerized ethylenically unsaturated monomer, more
preferably
~CH2-CH3 or ~CH2-C(CH3)3
and G is an organic noninterfering group, such as
R 0
~ -CO(CH2)uOH, -cocH2cH2RF
O O
3 _c-0-R or -C(OCH2CH2 ~ OR"
wherein R is as previously defined for Table A, RF is as
previously defined for Table A, R" i-~ a C1 to C18 alkyl
-18-
wo gs/osggo 2 1 6 ~ 1 5 S rcTlusg4l~\9~3~
or aralkyl, and u is an integer from 1 to 20 and v is an
integer from 1 to 40.
A and E in Formula II are each independently
end groups consistent with vinyl addition
polymerization. Illustrative end groups are CH3(CH2)S-,
H-, CH3 , (CH3)3CO-, Cl- and -OH.
In another embodiment of the subject invention,
the first and second compounds can be prepared in situ
from polymerizable moieties bearing at least one
nucleophilic or onium group. For example, vinylbenzyl
chloride, hydroxyethylmethacrylate and methacrylic acid
can be copolymerized using a free radical initiator.
In general, after the polymers bearing onium
groups or nucleophilic groups are prepared, it is
desirable to separate the oligomers so that only higher
molecular weight polymers are used as the first and
second compounds. Oligomers can be conveniently
separated by use of conventional dialysis techniques or
ultrafiltration membranes.
The polymers bearing onium and/or nucleophilic
(or anionic) groups can optionally be derived from
unsaturated moieties bearing other compatible groups.
In some instances it may be desirable to use such
compatible monomers in order to enhance certain
properties of the resulting compounds, such as their
3 hydrophobic or hydrophilic properties, their
film-forming properties or glass transition temperature.
For example, nonylphenoxy polyoxyethylene (10)
methacrylate (9N10MA) or other surface active monomers
can be used to render the polymer more wetting. Other
compatible monomers include a C1 to C12 alkyl
-19-
W095/05890 i PCT~S94/09432
methacrylate or hydroxyethyl methacrylate. Preferably,
the first and second compounds display a good
combination of properties. For example, it is desirable
that said compound is soluble or dispersible in aqueous
media. At the same time the compound should be
sufficiently wettable such that it can readily be
deposited on the substrate on which the compounds are to
be reacted.
In a preferred embodiment, the polymer used to
form the discriminating layer is a terpolymer of
hydroxyethylmethacrylate, vinylbenzyldimethyl sulfonium
chloride and methacrylic acid. Other preferred polymers
include viny~benzyltrimethyla onium/methyacrylic acid
and vinylbenzyldimethylsulfonium/methyacrylic acid.
As discussed above, it may be desired to use a
separate solution to "wet" the support to facilitate
interaction between the support and other coating
solutions. Those skilled in the art will recognize that
various solutions are suitable for this. Examples of
useful solutions include dilute alcohol solution and
various polymer solutions. It should be noted that
solutions should be avoided which may interfere with
formation of the film. As discussed herein, the polymer
solution useful in the formation of the discriminating
layer may be used.
The affixing layer serves to affix the
discriminating layer to the support. The discriminating
layer is preferably physically affixed to the support.
By affixed is meant that the discriminating layer is
stabilized, i.e., unrestrained swelling of the layer is
prevented and delamination of the discriminating layer
is substantially prevented. To accomplish this, the
-20-
W095/05890 PCT~S94/09432
affixing layer polymer solution is preferably capable of
insolubilization. Insolubilization may be accomplished,
for example, by reaction with the solvent or via
~ubsequent crosslinking and forming covalent bonds with
residual reactive groups in the discriminating layer.
It should be noted that in the alternative, or in
conjunction with the affixing layer polymer solution,
any unreacted portion of the discriminating layer
polymer solution also serves to affix the discriminating
layer to the support. By unreacted in this context, it
is meant any portion of the discriminating layer polymer
~olution which does not form the discriminating layer at
the air/blanketing layer interphase upon irradiation.
Various polymers are suitable for use in the
affixing layer polymer solution, so long as they serve
this purpose and do not detrimentally affect the
finished membrane. The polymers may be multi-component
polymers. Multi-component polymers usually consist of
different monomeric units each of which contributes a
desired characteristic to the resulting polymer and
ultimately to the finished membrane. For example,
monomers may be used which contribute nucleophilic
groups for reacting with or crosslinking through a
cationic group, enhance the hydrophobic or hydrophilic
properties of the membrane, exert a special afinity for
the species which is to be separated using the finished
membrane or adjust the mechanical properties of the
3 resulting membrane.
The polymer in the affixing polymer solution
may be the same as or different from the polymer in the
- discriminating layer forming polymer solution and can be
selected from those discussed herein in connection with
the discriminating layer polymer solution. Other
~ '}~i~ 21-
34,061~--F
.
suitable polymers are well known to those skilled in the
art and include those discussed in, for example, U. S.
Patent 4,~39,203 to Davis et al. issued June 13, 1989,
relevant portions thereof hereby being incorporated by
reference.
It is preferred that the discriminating layer
is formed by exposure to ultraviolet (UV) radiation,
although other types of radiation may be used.
Generally, the radiation exposure should be at a
combination of time and wavelength sufficient to form
the desired film. Generally, a typical dose of UV
radiation is from 0.01 to 20 joules/cm2 although any
dosage which result in the preparation of the membranes
of this invention is acceptable.
If the dosage is too low, the film formed is
too thin and thus lacks the necessary mechanical
strength to be useful. If the dosage is too high, it
2~ results in a thick, brittle film and results in a
membrane having undesirably low flux.
The discriminating layer of the membranes of
this invention is generally very thin. The
discriminating layer is typically about 50 to 1000
nanometers (500 to 10,000 Angstroms).
~ he MWCOs of the composite membranes of the
present invention are altered by modifying the
3 conditions under which the membranes are produced. For
example, membrane characteristics are influenced by
adjusting the concentrations of polymer solutions used
in the production of the membranes; modifying the
radiation dosage; varying the identity and concentration
of additives used, and varying the affixing conditions.
AMENDlED SHE~
.
2 1 ~ 5
34,061E-F
The properties of the composite membranes of
this invention wiil vary with their MWCOs. Generally,
membranes having 2 lower MWC0 will have pure water
fluxes lower than those of higher MWC0. For example, a
membrane having a MWC0 of 60 daltons or less generally
should have a pure water flux of greater than .0407
liters per square centimeter of membrane per day at 1.7
MegaPascals (mPa) (10 gallons per square foot of
membrane ?er day (gfd) at 250 psi). Membranes with a
MWC0 of about 200 daltons generally should have a pure
water flux of greater than .102 liters per square
centimeter per day at 1.7 mPa (25 gfd at 250 psi).
Membrane devices of the spiral, tubular hollow
fiber or plate and frame configuration can be fabricated
from the membranes prepared as described herein. These
devices are assembled in accordance with conventional
techniques once the membrane is prepared.
The following examples are provided to
illustrate the invention and should not be considered as
limiting its scope.
Figures 1-8 show results obtained when
membranes prepared by the process of this invention are
tested.
Exam~le 1 - PreDaration of Membrane
A polymerization initiator, 2,2'-azobis-
3 isobutyronitrile was used to initiate free radical
polymerization of 2-hydroxyethylmethacrylate,
vinylbenzyl chloride and methacrylic acid in
tetrahydro~uran. After polymerization was complete,
approximately 1.2 equivalents of dimethyl sulfide
perequivalent of vinylbenzyl chloride was added to the
-23-
~IOED SHEET
.
34,061E-r
polymer solution. The solution w2S then heated to
promote the conversion of the benzyl chloride moiety to
benzylsulfonium chloride. Water was added the reaction
mixture as necessary to keep the polymer soluble. The
tetrahydrofuran and residual dimethyl sulfide were
removed from the polymer solution under reduced
pressure. The aqueous solution was then dialyzed
against deionized water using dialysis tubing. The
polymer solution was stored at 4C until used.
A polysulfone ultrafiltration membrane was made
by casting a 1~ weight percent solution of polysulfone
in dimethyl formamide on a glass plate with .0127
centimeters (0.005 inch) doctor blade and quickly
immersing the plate in a water bath at room temperature.
This membrane was used as the support in the composite
membrane synthesis.
An aqueous solution containing 0.1 weight
percent of the polymer made as described above and 3
weight percent of H2S04 was placed in a dish. A 5.08
centimeter (2 inch) in diameter disk of the polysulfone
support was submerged under the solution which was
2~ maintained at room temperature. The solution was
irradiated with a 450 W Ace-Hanovia 7825-34 UV lamp.
The total dose of radiation delivered between 280 nm and
390 nm wavelength was 4 joules/cm2. The irradiation
caused the formation of a thin polymer film at the
air~Qolution interface. The film was loosened from the
edge of the dish. The volume of the solution below the
film was increased four times by the addition of water
through a syringe. The submerged support was drawn up
through the film to laminate the film on to the support
which was then placed for one hour in an oven pre-heated
to 90C. Next, a 3.81 centimeter (1.~ inch) in diameter
-2~-
AI~ENI~.D SHEEl
216g~
34,061E-r
disk was cut ou, of the support. This dlsk was placed
in a solution of 1:1 weigh~:weight isopropanol and water
to rewet the polysulfone support. The disk was then
immersed in deionized water for a few minutes and then
assembled into a reverse osmosis test cell and tested
sequentially with the following feed solutions: 2000
ppm NaCl in deionized water at 1.7 mPa (250 psi); 2000
ppm MgS04 in deionized water at 1.7 mPa (2~0 psi); 6
percent corn syrup in deionized water containing 50 ppm
Thimerosal antimicrobial. Feed was circulated at a rate
of 100 cm3/min.
After steady state had been reached, permeate
was collected. Flux of the permeate was determined by
weighing the amount of permeate collected in a given
time. The solute concentrations of the feeds and
permeates were evaluated either by measuring the
conductance of the solutions associated with the NaCl-
or MgS04-containing feeds, or by high performance liquid
chromatography (HPLC) of aliquots of the solutions
associated with the corn syrup-containing feed. For the
solutions associated with the NaCl or MgS04 feeds,
conductance measurements were converted to absolute
concentrations through calibrations. HPLC was conducted
with an acetonitrile/water eluant with detection based
on the index of refraction. The column separated the
glucose oligomers present in the corn syrup. Oligomers
containing from 1 to 13 glucose units were detectable.
3 The amount of each oligomer present in the permeate
solution was determined relative to that of feed
solution by comparing the peak heights of the
corresponding chromatograms. The percent solute
3~ rejection, SR, of the solute was calculated as for the
NaCl and MgS04-containing feeds as:
~NDED SHE~T
~ 2 ~ 5
34 ~ 06 1 r _1.
[solute]permeate
SR=100 x 1 -
[solute]feed
and for the glucose oligomer with i glucose units, dpi,
as
SR-100 x(1-relative concentration of dPi in the
permeate)
The data on flux and solute rejection for the
membrane are shown in Table I. The units of flux,
liters per square centimeters per day, are liters/cm2-
day (gfd, are gallons/ft2-day). The limits of detection
of the HPLC technique were such that when the rejection
of a glucose oligomer was 98.5 percent, that glucose
oligomer was not detectable in the permeate
.
3o
A~ENt~ED SHE~
6 !9 r3 5 5
34,061_-F
.
TABLI~I
Membrane Performa~ce
5Membrane PerformanceMembrane Performance
Flux. 200 DDm .3374 liters/cm2-day
NaCl@ 1.7 mPa (250 psi)(82.8 gfd)
NaCl rejection 32.4%
10MgS04 rejection 79.5S
dpi rejection 60.7%
dp2 rejection 91.1,~
dp3 rejection >98.5%
15dp4 rejection 298.5%
dps rejection ~ 98.5%
dp6 rejection ~ 98.5%
dp7 rejection ~98.5Z
20dp8 rejection 298.5%
dpg rejection 298.5%
dp~o rejection ~ 98.5
Exam~le 2
In this example, the procedure outlined in
Example 1 W2S followed with the exception that
commercially available polysulfone ultrafilters
available from FilmTec Corporation were used as the
3 support and the area of the support was 250 cm2. The
polymer concentration was 0.55 weight percent and the
radiation dose was 2.5 J/cm3. In each experiments
~1-7), two to four samples are cut from each membrane.
35 The results are shown in Table II.
A~E~ED S~E~
.
21695~
~ ...
34 ~ 06 lF--~
.
TABLE II
Variation of Membrane Performance for Samples
Made Under Identical Conditions
NaCl Flux
Experiment SampleRe jectionlitersJcm2-day
(S) (g~d)
a 18.05 .212
(52.0)
b 20.84 .120
(49.0)
2 a 45.58 .119
(29.3)
b 37.59 .198
(48.7)
c î 9.19 .225
(55.1)
d 11.14 .203
(49.7)
3 a 28.21 .216
(53.0)
b 58.03 .141
(34 5)
4 a 20.24 .275
(67.5)
b 14.97 .22~ ~
(55.1)
a 21.62 .254
(62.4)
b 24.25 .204
( ~0 . 1 )
6 a 19.21 .207
(50.8)
b 17.50 .226
(55.4)
7 a 22.19 .218
(53.5)
b 18.89 .194
(47.6)
--28--
~Eil tDED SHE~
~ 2~ ~i3~ ~
34,061~-F
.
General Procedure for Membrane Pre~aration
The general method, which will be the same for
alL of the membranes synthesized in the following
examples unless otherwise specified, was as follows:
Approximately 12 cm diameter circles were cut
from a sheet of machine-made polysulfone. These circles
were stored in 1:1 isopropanol alcohol (IPA) IPA:H20
until used. Prior to use, a circle was removed from the
storage solution and immersed in approximately 11 of
deionized (dI) dI H20. A~ter about 10 minutes, the
dI H20 was placed with fresh dI ~2-
The circles were then mounted on a specialholder. The holder consisted of a 12 cm diameter disk
of perforated stainless steel. Three posts were welded
approximately equally spaced a few millimeters from the
edges of the disk. A stainless steel ring a~out 0.; cm
wide with three holes to accommodate the posts was used
to hold the polysulfone flat. After the polysulfone was
- mounted-on the holder, it was i~me~sed in d-I E2~ until -
it was used.
A solution containing 0.1 weight percent of
polymer and 3.0 weight percent H2S04 was prepared. The
polysulfone mounted in the holder was removed from the
dI E20- The holder and the polysulfone were blotted dry
with a paper towel. The holder was then quickly placed
in a 13.8 cm x 1.5 cm petri dish. 60 cc of the polymer
soLution was added. 60 cc was sufficient to immerse the
polysulfone to a depth of a few millimeters.
The petri dish containing the polymer solution
and immersed polysulfone, at ambient temperature unless
otherwise noted, was inserted into an UV irradiation
-29-
A~ENUEDSHE~
~ 9~5
34.06 îE-F
chamber and irradiated, until 2.00 Joules/cm2
accumuLated on the radiometer detector.
The irradiation was then terminated, and the
dish removed. The film was loosened from the sides o~
the dish with a scalpel blade. The polysulfone holder
was then lifted up through the interface at a shallow
angle. When the holder was clear of the solution, it
was positioned to be nearly vertical. The bottom side
of the holder was then blotted on paper towel. The
holder -~as then placed in an oven set at 100C for
1 hour. During the last 5 minutes, a vacuum was drawn
in the oven.
1~ The sample was removed from the oven and cooled. The polysulfone circle was removed from the
holder. As many as three 3.0 cm diameter circles were
punched from the polysulfone circle and assembled into
test cells. The cells filled with a solution of
1~.5 percent IPA in dI ~2- After about 15 minutes, the
cells were flushed for 25 minutes with a stream of dI
water. The cells were successiveLy pLaced on lines
circulating one of four different feeds: 2000 ppm NaCl;
2~ 300 ppm CaC12; 2000 ppm MgS04; or 10 percent glucose
containing ~0 ppm Thimerosal antimicrobial. The feed
pressure was in all cases .90 mPa (130 psi).
ExampLe 3
Various membranes were prepared using a
hydroxyethylmethacrylate/vinylbenzyl
dimethylsulfoniom/methacrylic acid polymer and the
General Procedure set forth above with the only
variations being that no vacuum was pulled on the oven
at the end of the curing period and the concentration of
-30-
~$~N~EDS~E~
~ 1 6 ~
34,061E-r
H2S04 used was varied as follows:
Acid Concentration pH
neat 3.64
10 ppm 3.51
0.01% 2.78
0.1% 1.73
1.0% 0-94
3.0Z 0.54
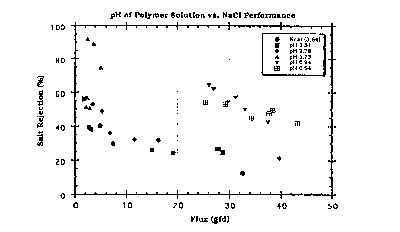
The results obtained when the membranes are tested using
feed solutions containing NaCl, CaC12, MgS04 and Glucose
are shown in Figures 1-4.
Exam~le 4
The procedure in Example 3 was followed with
the exception that the membranes were prepared by adding
two weight percent of each of the following salts to the
polymer solutio~: NaCl, LiCl, MgÇl:2,MgS04, Na2S04,
Na2~P04. The results are shown in Figures 5-8.
Exam~le 5
Various mem~ranes were prepared using a
hydroxyethylmethacrylate/vinylbenzyl
dimethylsulfoniom/methacrylic ac~d polymer and the
General Procedure set forth above with the only the
temperature at which irradiation occurred. The
temperatures used were 0C, 33C and 55C.
-31-
A~E~DEDSff~
. .
WO 95/05890 PCT/US94/09432
5 5 5
Exam~le 6
In this example, the polymer used was
poly(vinylbenzyltrimethylammonium
bicarbonate/methacrylic acid in the preparation of a
membrane using the General Procedure set forth above.
Example 7
Various membranes were prepared using a
0 hydroxyethylmethacrylate/vinylbenzyl
dimethylsulfoniom/methacrylic acid polymer and the
General Procedure set forth above with the only
variation being the oven temperature at which the
1~ membranes were cured after irradiation. The
temperatures used were 70C, 100 and 120C.
Example 8
Various membranes were prepared using the
General Procedure outlined above using the following
polymers:
0.5 weight percent of a
vinylbenzyltrimethylammonium/methyacrylic acid
(1:1)
0.5 weight percent of a
vinylbenzyldimethylsulfonium/methyacrylic acid
(1:1)
0.1 weight percent of
hydroxyethylmethacrylate/vinylbenzyldimethylsul
fonium/methyacrylic acid (45:22.5:32.5)5
~ 216g5~
34,061~-F
hydroxyethylmethacrylate/vinylbenzyldimethylsul
fonium/methyacrylic acid (5:47.~:47.5)
l.0 weight percent of a
vinylbenzyltrimethylammoniumlmethyacrylic acid
(l:l) which received 4 J/cm2 of radiation
Example 9
The procedure set forth in Example 3 was
followed using the additives and varying the ultraviolet
radiation dose as shown in Table I below. In the last
three entries in Table I, "NSA'~ refers to the sodium
salt of 2-naphthalenesulfonic acid, a photosensitizer
1~ which was added to the polymers solution at O.Ol weight
percent. Three samples were cut from each membrane and
then tested as described in the General Procedure with
the exception that no test was run using glucose. Flux
is reported in units of liters per square centimeter of
membrane per day (liters/cm2-day) (gallons per s~uare
foot of membrane per day (gfd)).
3o
-33-
A~ENDEDS~E~
34 , 06 1 E-F
¢ ~ C ¢ C ¢
o Z Z Z Z Z Z
V~
Il .
r 1~ _ r~ r-l N ~ r-l N NO ~1 eD r-l ¢ ¢ ¢ ~ I~C ¢
~ O O _ O N
O ~ _ ~ ~ ~ ~ _ ~ '' ~ Z Z Z Z Z Z
I:t
r~
U ~ C N r~ O N ~D t~l 1115 O ~r O
N ~ r-i N ~ ~ r-t r~l r-l t'q r I _I
r
r-~ ~,o V~ . . _ _ _ _ , , _ _ _ _ _ _ . _
U ~ ~
.r l
_l
_~ r ~ ~ r~ ~ ¢ ¢
~ r-i N ~ IC~ L I ~ . . .
¢r 1~: ~ z z z z z z z
.
~I
r
~,?~ 1_''0'-~ 0 ~ _o_ owe ~c ¢ ¢ ,~ ¢
z t
~n
_ ~ ~ ~ O O O O O O
O O O O ~ ~ ~ X X X
r~ 0 0 0 0 0 0 ~ ~ X X X
C~' _, ,_, "~, _,_, ,_, ".,_, _ ,_, _, ,_, x x x Q
¢ ¢ ¢ ~
.~ ~ ~ ~ o
~ ~ ~ ~ o o o Z~ _I ~ ~ O O O u~
~ _ -- ~ o ~ ~ -- s 3 -- -- _
o~- e~ oP O O O ~ ~ J ~ ~ ~ ~
~ I O O O C
O O o _ -- _ ~ .~ ) o o o ~ '1 z
--34--
~IEM3ED SHEEr
.