Note: Descriptions are shown in the official language in which they were submitted.
21 6q708
80311-7
MEDICATION INJECTOR WITH PROTECTED
CANNULA AND Y-8ITE LOC~OUT
Backqround of the Invention
This invention relates to injectors for administering
medication with a syringe and a hypodermic needle or
cannula.
Many different types of syringes and hypodermic
needles have been used for administering various types of
medications. U.S. Patent No. 3,378,008 to Ogle (1968)
discloses a hypodermic syringe and vial prefilled with an
injectable liquid, such as medication to be administered
to a patient. Although that type of syringe has been
successful, it has the disadvantage of presenting an
exposed needle, which can cause an accidental injury,
infection, or exposure to dangerous and toxic medication.
U.S. Patent No. Re 33,617 to Ogle, II (1991)
discloses injection apparatus which includes a protective
sheath disposed around the sharp end of a hypodermic
needle to prevent accidental sticking. This device has
been widely accepted by the industry. However, it does
not have any kind of a lockout feature to prevent
medication from being administered inadvertently through
an improper site, which might subject a patient to
medication at a rate in too high a concentration for the
safety of the patient. For example, some medication, such
as morphine sulfate, is sold as a fairly concentrated
medication, e.g., 50 mg of morphine sulfate per milliliter
21 6q708
_ 1 of solution, and must therefore be diluted, say, in an
intravenous bag or container of saline solution, or
mounted in a pump which limits delivery of the medication
to safe increments over a safe period of time.
Because of the dangerous nature of certain drugs or
medications, they have been sold in packages of relatively
low volume to minimize the possibility of overdosing.
However, this is inconvenient because it requires a large
number of individual containers and adds to the work of
medical personnel in administering the medication to
patients.
Summary of the Invention
This invention provides a medication injector with a
lS hypodermic needle surrounded by a protective sheath to
prevent accidental sticking. Moreover, a lockout device
on the sheath prevents the injector from being used to
administer medication in the syringe in a concentrated
form to a patient.
Briefly, this invention provides a medication
injector for use with an inlet port having an imperforate
elastomeric closure for a conduit connected to and
extended away from a container for liquid intended for
intravenous (i.v.) administration. Many such containers
have at least two conduits, one through which medication
can be injected into the solution in the container, and
the other for delivering liquid in the container to an
infusion catheter inserted in the vein of a patient. The
latter conduit often has a "Y-site", which carries a short
lateral tube to provide an injection site through which
additional or supplemental medication can be supplied
directly to the vein of a patient. Although many types of
medication can be safely applied through the injection
port of a Y-site, some drugs, such as a concentrated
solution of morphine sulfate, cannot. It is, therefore,
important that such medication be administered through a
procedure which prevents inadvertent overdosing. The
21 697Q8
1 injector of this invention prevents inadvertent
overdosing. The injector includes a boss which carries a
cannula or hypodermic needle with a scarf at one end
spaced from the boss. The cannula scarf is adapted to
s puncture the imperforate closure of an inlet port of an
i.v. container. An elongated and generally cylindrical
sheath is secured at one end to the boss, and extends away
from the boss to surround the cannula and extend beyond
the scarf end of the cannula at a first open end of the
sheath. The internal diameter of the first open end of
the sheath is sufficiently small to protect the user from
inadvertent puncture of the hands or fingers by the scarf.
An outwardly extending annular flange on the sheath, at
and around the first open end, is constructed and arranged
to engage a tubular portion adjacent an injection port of
a Y-site of an intravenous administration set and thereby
prevent the scarf end of the cannula from puncturing the
injection port of the Y-site. The end of the sheath
secured to the boss is adapted to make a fluidtight seal
with a source of medication to be introduced through the
cannula to the inlet port of the container.
The internal diameter of the first open end of the
sheath is sufficiently large to permit the sheath to slip
over the conduit which carries the imperforate closure to
form the inlet port for the container of liquid intended
for intravenous administration. The outside diameter of
the flange around the open end of the sheath is
sufficiently small to not interfere with the insertion of
the cannula through the imperforate closure for the inlet
port of the container. Preferably, the flange outside
diameter is greater than about 20 millimeters to insure
proper interference to prevent inadvertent connection of
the injector to an injection port of a Y-site.
Preferably, the flange diameter is less than about 40
millimeters to avoid interference with adjacent conduits
when the injector is to be connected to the inlet port of
IV container. Preferably, the ratio of the outside
21 69708
1 diameter of the flange to the inside diameter of the
sheath is between about 2 to 1 and about 4 to 1.
In the preferred form of the invention, the injector
includes a guide with a second fitting shaped to make a
releasable fluidtight seal with the fitting connected to
the sheath portion. The second fitting has a passageway
extending through it to be connected with the passageway
extending through the fitting connected to the sheath. An
elongated second cannula has one end connected to the
passageway of the second fitting. The other end of the
second cannula is adapted to be connected to the source of
liquid medication with means for causing the liquid
medication to flow into the i.v. container and mix with
liquid stored in it. Preferably, a tubular guide is
secured to the second fitting to extend around the second
cannula. The guide is constructed and arranged to receive
a syringe barrel which holds the liquid medication so a
resilient stopper disposed at an open end of the barrel to
confine the liquid in the barrel can be penetrated by the
second cannula so that thereafter sliding the stopper
relative to the barrel causes the liquid in the barrel to
flow into the container and mix with the other liquid
stored in that container.
In the presently preferred form of the invention, one
of the fittings has a tapered socket, and the other has a
tapered nozzle, and the two fittings are adapted to be
held together by a threaded connection.
Preferably, the inside diameter of the protective
sheath around its open end is less than about 16 mm to
provide against accidental puncture or injury with the
scarf end of the cannula surrounded by the sheath. The
inside diameter sheath is less than the length of that
part of the cannula surrounded by the sheath to provide a
geometry which prevents accidental sticking of the user
with the scarf end of the cannula surrounded by the
sheath, and the length of the cannula projecting from the
boss is sufficient to penetrate the closure of the inlet
21 69708
1 port of the i.v. container when the injector sheath is
slipped over the inlet port.
2s
_5_
'~1 6q708
1 Brief Description of the Drawings
FIG. 1 is a perspective view, partly broken away,
showing an injector of this invention properly connected
to the inlet port of an intravenous container, and also
showing how the flange on the sheath prevents connecting
the injector to an injection port of a Y-site;
FIG. 2 is an enlarged exploded view, partly in cross
section, of the protective sheath disconnected from the
guide;
FIG. 3 is an enlarged fragmentary sectional view
showing the protective sheath connected to the guide;
FIG. 4 is an enlarged fragmentary sectional view
showing another embodiment of a protective sheath
connected to a guide; and
FIG. 5 is a sectional view of the protective sheath
and guide of FIG. 4 taken along line 5-5.
21 6'~708
_ 1 Description of the Preferred Embodiments
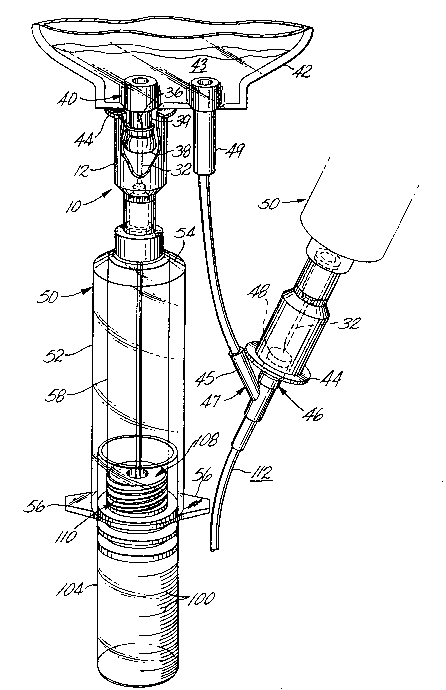
Referring to the drawings, an injector 10 includes an
elongated, hollow, cylindrical protective sheath 12 open
at a receiving (left as viewed in FIGS. 2 and 3) end 13.
Preferably, all components of the injector, except the
cannulas, are made of a suitable moldable plastic, such as
a polycarbonate, which is of radiation grade to permit
sterilization by conventional irradiation. A bore 14
extends through the sheath from the receiving end to a
transfer end 15, which is integrally formed with an
inwardly extending, transverse, annular wall 16 (FIGS. 2
and 3), which has a central bore 18 around which is formed
integrally with wall 16 a collar 17 which makes a snug fit
around a hollow, cylindrical boss 20 coaxially disposed in
the transfer end of the sheath. For the embodiment of
FIG. 3, the collar and boss may be sealed together with an
adhesive (not shown), or by spin-welding.
A relatively small diameter bore 22 in the boss
adjacent end wall 16 steps up to a larger diameter bore
24, which tapers outwardly to the right (as viewed in FIG.
3) to form a first fitting in the shape of a Luer-Lock
female socket 30 connector. The Luer-Lock may be of the
type shown in U.S. Patent No. 4,737,144 to Choksi (1988).
A first or short cannula 32 is sealed at its right
(as viewed in FIGS. 2 and 3) end in the small bore 22 of
the Luer-Lock female fitting. The left end of the first
cannula includes a scarf or tapered section to present a
sharp point 36 extending toward the receiving (left) end
of the injector. The scarf 36 is adapted to penetrate a
resilient, imperforate closure 38 covering a conduit 39 to
form an inlet port 40 of a container 42, such as an i.v.
bag, which holds an i.v. solution 43.
An outwardly extending annular flange 44 is formed
integrally with the receiving end 13 of the protective
sheath. The outside diameter of the flange is
sufficiently large to contact a delivery conduit 45 (FIG.
1) and thus prevent the injector sheath from being slipped
1 69708
1 far enough over an injection port 46 of a Y-site 47 to
cause the cannula scarf to pierce a closure 48 for the
port. However, the outside diameter of the flange is not
so large as to contact an outlet conduit 49 of the i.v.
bag and prevent the injector sheath from being slipped
onto the injection port so the cannula can properly
penetrate the closure 38 for the inlet port to permit
medication to be injected into the bag as shown on the
left side of FIG. 1, and described in more detail below.
A tubular injector guide 50 includes an elongated
cylindrical outer sleeve 52 formed integrally at a
discharge end (left as viewed in FIGS. 2 and 3) with the
outer periphery of an annular discharge end wall 54, which
slopes inwardly to the left (as viewed in FIGS. 2 and 3).
A pair of diametrically opposed and outwardly
extending ears 56 (FIG. 1) are formed integrally with the
inlet end (right as viewed in FIGS. 2 and 3) of the
injector outer sleeve 52 to lie in a plane perpendicular
to the longitudinal axis of the injector and guide. A
cylindrical inner sleeve 58 spaced from and disposed
coaxially within the outer sleeve is formed integrally at
its discharge end (left) with the interior surface of
annular wall 54. The inlet end (right) of the inner
sleeve is substantially co-extensive with the inlet end of
the outer sleeve and has internal threads 60, as described
below.
The discharge end of the injector includes an
external annular boss 62 formed integrally with the
exterior of the annular wall 54 around a central bore 64
extending through the end wall and boss.
A second fitting in the shape of an externally
tapered Luer-Lock nozzle 66 connector is formed integrally
on the left (as viewed in FIGS. 2 and 3) end of the boss
62, and makes a snug fit in a matching tapered socket 30
of the Luer-Lock female first fitting. External threads
70 around the right (as viewed in FIG. 3) end of the Luer-
Lock socket 30 engage internal threads 72 in a cylindrical
21 69708
_ 1 skirt 74 formed integrally with the boss 62 and disposed
to surround part of the Luer-Lock nozzle. When the
threads on the two fittings are screwed together, they
releasably lock and seal the nozzle in the socket, as
shown in FIG. 3.
An external annular shield 81 is formed integrally
with the exterior of the annular wall 16 at the right (as
viewed in FIG. 3) end of the injector. The shield 81
surrounds and is spaced from collar 17. The interior
surface 82 of the shield tapers outwardly toward the right
and makes a snug fit around a matching external surface of
the skirt 74, which surrounds the nozzle of the Luer-Lock.
Optionally, if an even tighter fit is desired, a pair of
inwardly extending and longitudinally spaced annular
protrusions 84, which are semicircular in the cross-
sectional view shown in FIG. 3, bear against the external
surface of the skirt 74 to provide a tight seal against
contamination when the Luer-Lock fitting is in the
connected position shown in FIG. 3. However, these
protrusions are optional, and a sufficient seal can be
attained without them.
A second or long cannula 86, disposed coaxially
within the injector guide 50, is sealed at its left (as
viewed in FIGS. 2 and 3) in a central bore 88 extending
through the nozzle and collinear with the central bore 22
in the boss in the end wall 16 of the injector. The right
end of the second cannula includes a scarf 90 (FIG. 2),
which terminates just short of the right end of the inner
sleeve 58.
When the injector and injector guide are assembled,
as shown in FIG. 3, the cannulas and the Luer-Lock
fittings form a passageway through which a liquid (not
shown) can pass to mix with i.v. solution in the i.v. bag,
when the injector is slipped over the inlet port of the
i.v. bag, as shown on the left side of FIG. 1.
Referring to FIG. 2, an injector cap 91 having a
centrally located cylindrical and inwardly extending plug
21 6~708
_ 1 92 with external grooves 93 can be snapped into the left
(as viewed in FIGS. 2 and 3) end of the protective sheath
and thus seal it against contamination. An outwardly
extending handle 94 in the shape of a web is formed
integrally across the diameter of the cap 91 to facilitate
installing and removing the cap from the protective
sheath. An inwardly extending annular projection 95,
which is semicircular in cross section as viewed in FIG.
3, around the interior of the left end of the protective
sheath engages the grooves 93 of cap 91 to act as a detent
and thus releasably secure the cap in the protective
sheath. A similar cap (not shown) is adapted to be fitted
into the right (as viewed in FIGS. 2 and 3) end of the
injector guide to seal it against contamination.
In another preferred embodiment, as shown in FIGS. 4
and 5, an injector 210 includes a sheath 212 in which a
collar 217 for the sheath is joined by a friction fit to
a boss 220, which holds the cannula 232. As shown in FIG.
5, the boss has four radially and longitudinally extending
fins 221 spaced equidistant around the cannula 232. The
collar includes eight inwardly opening, longitudinal slots
223 spaced equidistant around its central bore 218. Each
slot lies between adjacent inwardly and longitudinally
extending ridges 224, which are semicircular in cross
section (as viewed in FIG. 5) to present inwardly facing
convex surfaces 226 to facilitate fitting the boss in the
collar. In assembling the injector, the boss is pressed
into the collar so that the four fins mesh with four of
the eight slots of the collar. By including eight slots
with the adjacent convex surfaces, the slots are
symmetrically disposed relative to the fins so assembly is
simplified because the orientation of the parts to one
another is not critical.
In the embodiment of FIGS. 4 and 5, a tight fit
between the collar and boss is promoted by proper
selection of materials of construction. Preferably, the
sheath, including the collar, is made of a material such
--10--
21 69708
1 as polycarbonate while the boss is made of a softer
material such as polypropylene. Preferably, the fins
extend out slightly further than the inner diameter of the
collar, as measured from the bottom of one groove to the
bottom of a diametrically opposed groove. Thus, the
bottoms of the grooves lie in a cylindrical plane which
has a diameter slightly less than that of a cylindrical
plane defined by the outermost portions of the fins. Upon
insertion of the boss into the collar, the softer fins
deform plastically to create a tight friction fit with the
collar. The travel of the boss into the collar is limited
by the right (as viewed in FIG. 4) ends of the ridges 224
on the collar abutting the left end of the boss. The fit
of the boss and collar is further improved by including a
sharp edge 228 at the juncture of the annular wall 216 and
the bore 218 of the collar 217 so the softer and deformed
fins cold-flow outwardly as they pass the sharp edge so
the inner end of each fin forms a respective hook 230 over
the sharp edge to lock the boss firmly to the collar.
This is best illustrated in FIG. 4.
With the injector and injector guide assembled as
shown in FIG. 3 or FIG. 4, and the protective caps in
their respective positions, the assembled apparatus is
packaged and sterilized (say, by conventional irradiation)
until ready for use as shown in the operating position of
FIG. 1.
To use the injector/injector guide assembly, it is
removed from the sterile container, and the cap (not
shown) is removed from the right (as viewed in FIGS. 2 and
3) end of the injector guide 50. A cylindrical syringe
barrel 104, which may be of conventional construction such
- as that shown in U.S. Patent No. 3,378,008, includes the
usual calibration marks 106 indicating the amount of
liquid medication (not shown) in the barrel. A slidable
stopper 108 is disposed in the outlet end (upper end as
viewed in FIG. 1) of the barrel to store the liquid in the
barrel under sterile conditions until ready for use. The
--11--
21 6~708
1 stopper includes a reduced-diameter portion 110, which is
externally threaded, and which extends toward the upper
end of the syringe barrel. A syringe cap (not shown)
usually makes a snug fit within the outlet of the syringe
barrel and fits over the reduced portion 110 of the
stopper. In this condition, the cap, stopper, syringe
barrel, and the liquid within are sterilized in a sealed
package (not shown) until ready for use, as explained
below.
lo To use the apparatus of this invention, the cap is
removed from the calibrated syringe barrel. The stoppered
end of the syringe barrel is then inserted into the
annular space between the inner and outer sleeves of the
injector guide until the external threads on the reduced
portion 110 of the stopper 108 in the syringe barrel first
engage the internal threads 60 in the inner sleeve of the
injector. This contact is made gently, and then, without
pushing, the syringe barrel is rotated about three turns
to engage the threads until a slight resistance is felt.
An additional half turn of the syringe barrel causes the
scarf end of the long cannula to pass through the syringe
barrel stopper so that the liquid medication within the
barrel is now in communication through the two cannulas
with the liquid in the i.v. bag.
With the syringe barrel assembled as described, the
injector protective sheath is slipped up over the inlet
port to the position shown in FIG. 1 so that the scarf end
of the short cannula 32 penetrates the closure 38 to
provide communication with the interior of the i.v. bag.
To this end, and as shown in FIGS. 3 and 4, the distance
from the scarf end of the cannula to the open end of the
sheath is less than that of the internal diameter of the
sheath around the scarf end of the cannula. As previously
stated, the outside diameter of the flange 44 is
sufficiently small so as not to contact the adjacent
outlet conduit 49. Thus, the injector can be properly
positioned over the inlet port 40, as shown in FIG. 1.
2 i 69708
_ 1 Conversely, as shown on the right side of FIG. l, the
outside diameter of the flange 44 is sufficiently large to
prevent the protective sheath from being slipped far
enough onto the injection port 46 of the Y-site 47 to
permit cannula 32 to penetrate the closure 48 of the
injection port.
With the apparatus assembled as shown in FIG. 1, the
syringe barrel is then pushed into the injector guide,
using the ears 56 on the injector to facilitate operation
with one hand, if desired. The stopper is held in a fixed
position in the threaded end of the inner sleeve of the
injector guide so that the closed end of the syringe
barrel slides toward the stopper and forces liquid out of
the barrel, through the cannulas, and into the container
42, where it mixes with the i.v. solution there. After
the required amount of medication is injected into the
i.v. bag, the injector is moved away from the i.v. bag,
causing the short cannula to be withdrawn from the inlet
port.
Thus, with the apparatus assembled as shown in
FIG. 1, the medication in the syringe barrel 104 can be
injected only into the inlet port of the i.v. bag, where
the medication is safely mixed and diluted with i.v.
solution before being administered to a patient (not
shown) through the outlet conduit 49, Y-site 47, and a
delivery tube 112, which is connected to a catheter (not
shown) inserted in a vein of the patient.
If the medication in the syringe barrel is to be
administered in some manner other than that shown in FIG.
1, say, by a pump, or directly into an injection port, or
into a vein of a patient, the injector can be removed from
the injector guide by simply unscrewing the Luer-Lock
connection. Thereafter, the male nozzle of the Luer-Lock
can be connected to a "needleless" connection, and the
injector guide and syringe barrel mounted in a pump (not
shown), which is driven in a controlled manner to deliver
medication at the required rate. If desired, the nozzle
-13-
2 1 6 `~ -1 0 8
1 end of the Luer-Lock fitting can be connected to a
conventional hypodermic needle for administration of the
medication.