Note: Descriptions are shown in the official language in which they were submitted.
WO 95/05860 2170157 PCT/US94109489
-1-
Description
Improved Balloon Catheter
Technical Field
This invention generally relates to balloon catheters
and more particularly to the structure of and method of
manufacture of balloon catheters.
Backaround Art
Coronary balloon angioplasty involves the steps of
inserting a deflated balloon into a coronary artery,
advancing the balloon across a lesion until the balloon is
centered at the lesion and then inflating the balloon to
dilate and remove the stenosis. Significant efforts have
been directed toward constructing balloons with smaller
cross sections so that they can better cross a tight
lesion. However, experience with these smaller balloon
catheters has highlighted two desirable, but until now
antithetical, characteristics. First, the balloon should
exhibit very low coefficient of sliding friction to
facilitate initial positioning with minimal trauma.
Secondly the balloon should exhibit longitudinal or axial
stability during and after inflation. This stability is
needed to overcome any tendency for forces exerted by the
adjacent tissue to displace or shift the balloon
longitudinally in the vessel. Independent efforts have
been undertaken to address the issues of sliding friction
and of positional stability. However, no activities seem
to have been directed toward the development of a balloon
that incorporates both characteristics in a single device.
For example, Boston Scientific Corporation, the
assignee of this invention, manufactures a SliderT"' PTCA
Catheter having a lubricous, bonded coating covering the
= exterior of the balloon. This facilitates access to a
lesion and enhances the ability of the balloon to cross
the lesion.
Similarly the following patents disclose other
coatings adapted for use with balloon catheters:
4,810,543 (1989) Gould et al.
5,026,607 (1991) Kiezulas
WO 95/05860 Z170=157 PCT/US94/09489 ~
-2-
5,102,402 (1992) Dror et al.
United States Letters Patent No. 4,810,543 to Gould
et al. discloses articles having low friction surfaces and
processes for producing such articles. Specifically the
Gould et al. patent proposes treating a surface with a
mixture of concentrated sulfuric acid and a low molecular
weight polyhydroxy compound and removing any excess
treating mixture.
United States Letters Patent No. 5,026,607 to
Kiezulas discloses a method in which a protective
compound, such as urethane, is coupled with a slip
additive, such as siloxane and, optionally, a crosslinking
agent for a protective compound such as a polyfunctional
aziridine, coats the surface of medical apparatus. After
setting, the material provides a lubricous surface that is
tough and flexible and particularly adapted for use with
balloon catheters.
United States Letters Patent No. 5,102,420 to Dror et
al. discloses a balloon catheter with an exterior coating
of body effecting chemicals. In some embodiments a
balloon is inflated, dusted with microcapsules containing
a drug and then deflated prior to entry into the patient.
Alternately, cusps, folds and other corrugations are
formed when the balloon is deflated and capture
microcapsules containing the drug material. These
microcapsules are then presented when the balloon is
inflated.
Each of the Gould et al. and Kiezulas patents
discloses methods and procedures for making a device more
lubricous. However, none describes any method or
procedure for improving axial stability.
The following patents describe balloons that
incorporate stabilizing structures to enhance the
positioning, engagement and retention of a balloon at a
lesion:
4,447,227 (1984) Kotsanis
4,896,669 (1990) Bhate et al.
4,921,484 (1990) Hillstead
CA 02170157 2005-03-03
74810-11
3
4,927,412 (1990) Menasche
4,986,830 (1991) Owens et al.
5,002,531 (1991) Bonzel.
United States Letters Patent No. 4,447,227 to
Kotsanis discloses multipurpose medical devices. Each
device has a stabilizing structure for enhancing
positioning, engagement and retention of the balloon in a
desired lumen. The stabilizing structure is in the form of
an additional medical grade balloon or one or more vacuum
responsive members, such as active or passive microsuckers.
United States Letters Patent No. 4,896,669 to
Bhate et al. discloses a dilation catheter with an outer
tubular balloon portion. This balloon portion has
circumferential crimps at each of two end transitions and an
intermediate axially extended portion with longitudinal
crimps. The balloon portion expands readily to a
predetermined diameter while undergoing little change in
length. The transition portions are capable of longitudinal
extension in response to minor longitudinal contraction at
the two ends of the balloon portion thereby reducing axial
movement of the balloon relative to the catheter when the
balloon is inflated.
United States Letters Patent No. 4,921,484 to
Hillstead discloses a mesh balloon catheter device,
analogous to an expandable stent, in which the catheter has
a distal end with a tube of woven interlaced filaments
forming a tubular mesh. The proximal end of the mesh can be
moved toward the distal end of the mesh to expand the mesh
into surrounding tissue. This particular structure is
CA 02170157 2005-03-03
74810-11
3a
designed for location in a bladder where the mesh holds the
catheter in place while allowing an obstructed fluid flow.
United States Letters Patent No. 4,927,412 to
Menasche discloses a catheter adapted for use in a coronary
sinus where the sinus walls are slippery, extensible and
tapered in a distal direction. Prior
WO 95/05860 PCT/US94/09489
~~ ~~15,7,
catheters normally were subject to axial displacement
while being inflated. In accordance with this patent a
balloon has a truncated conical surface with outwardly
facing, spaced apart, parallel concentric lands for
frictionally engaging the coronary sinus. This structure
is stated to provide a high retentive force for
stabilizing the catheter and preventing its ejection from
the coronary sinus.
United States Letters Patent No. 4,986,830 to Owens
et al. discloses a valvuloplasty catheter with a balloon
that remains positionally stable during inflation.
Stability is achieved by providing first and second
inflation ports of differing sizes so that the expanding
member inflates to create a dog-bone effect that allows
the balloon to surround and stabilize the expander member
relative to the valve being treated.
United States Letters Patent No. 5,002,531 to Bonzel
discloses an inflatable balloon with a hose-like outer
skin to which is connected at axially oriented edges and
an inner skin also having a hose-like shape. The outer
skin acts as a holding membrane. In this particular
structure, the inner skin is elastic and undergoes a
considerable reduction in diameter when the balloon is
deflated. This eases passage of the catheter as it is
advances through or retracts from an artery.
Each of the foregoing references therefore proposes
some structure for improving axial stability during
inflation. Although the Bonzel patent recognizes a need
for easing passage through a lesion, neither it nor any
other of these references describe any method or procedure
for making a balloon more lubricous. Consequently the
prior art defined by these references can be characterized
as providing either reduced friction when a balloon is
deflated or increased friction when a balloon is expanded,
but not both.
Disclosure of Invention
Therefore it is an object of this invention to
provide an improved balloon that facilitates placement at
CA 02170157 2005-03-07
74810-11
a lesion and yet retains its position at the lesion during
inflation.
Still another object of this invention is to
provide an improved balloon catheter that exhibits different
5 frictional characteristics in its inflated and noninflated
states.
Yet another object of this invention is to provide
a balloon catheter that has a low coefficient of sliding
friction in a deflated state and a higher coefficient of
friction in an inflated state.
According to one aspect the invention provides a
medical balloon, said balloon being expansible from a
compact state to an expanded state in a patient's vessel,
said balloon having a first external surface portion having
a first coefficient of friction and a second external
surface portion having a second coefficient of friction
different from the first coefficient of friction, said
balloon, in its compact state, being folded to expose
essentially only said first external surface portion and, in
its expanded state, exposing both said first and second
external surface portions.
According to another aspect the invention provides
a medical balloon, said balloon being expansible from a
compact state to an expanded state in a patient's vessel,
said balloon having a plurality of first external surface
areas having a first coefficient of friction and a plurality
of second external surface areas having a second coefficient
of friction different from the first coefficient of
friction, said balloon, in its compact state, being folded
to expose essentially only said plurality of first external
surface areas and, in its expanded state, exposing both said
CA 02170157 2005-03-03
74810-11
5a
plurality of first external surface areas and said plurality
of second external surface areas.
Brief Description of the Drawings
The appended claims particularly point out and
distinctly claim the subject matter of this invention. The
various objects, advantages and novel features of this
invention will be more fully apparent from a reading of the
following detailed description in conjunction with the
accompanying drawings in which like reference numerals refer
to like parts, and in which:
FIG. 1 is a front plan view of a portion of a
balloon catheter constructed in accordance with this
invention in an uninflated or compact state;
FIG. 2 is a cross-section taken along lines 2-2 in
FIG. 1;
FIG. 3 is a front plan view of the balloon
catheter in FIG. 1 in an inflated or expanded state;
FIG. 4 is a cross-section taken generally along
lines 4-4 in FIG. 3;
FIG. 5 is an enlarged cross-section taken along
lines 5-5 in FIG. 4;
CA 02170157 2005-03-03
-6-
FIG. 6 is an enlarged cross-section taken along lines
6-6 in FIG. 4;
FIG. 7 depicts an alternate embodiment of the
structure shown in FIG. 6;
FIG. 8 shows still another alternative embodiment of
the structure shown in FIG. 6;
FIG. 9 depicts the application of this invention to
an alternative form of a balloon catheter and is a front
plan view of the balloon catheter in an uninflated or
compact state;
FIG. 10 is a section taken along lines 10-10 in FIG.
9;
FIG. 11 is a front plan view of the balloon catheter
in FIG. 9 in an inflated or expanded state; and
FIG. 12 is a section taken along lines 12-12 in FIG.
11.
Best Mode for Carrying out the Invention
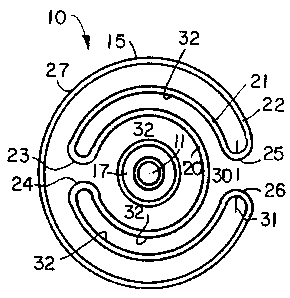
In the embodiment of FIGS. 1 through 4, a
catheter 10 slides over a guidewire 11 and includes
tubular portions 12 and 13 at a distal end 14 of the
catheter 10. A balloon 15 lies longitudinally between and
attaches to the tubular portions 12 and 13. Ports 16,
shown in phantom in FIG. 3, allow fluid to be admitted to
the area of the balloon 15 for expansion. The fluid is
supplied either through a lumen 17, in FIG. 1 that carries
the guidewire 11 or through an auxiliary lumen (not
shown), all is well known in the art.
FIGS. 1 and 2 depict the disposition of thin balloon
material about the catheter 10 in a compact position. For
clarity, FIG. 2 depicts the material out of scale in
spaced adjacent layers. In an actual balloon the layers
would be tightly packed. The balloon 15 is formed in
three concentric layers including an inside layer 20, and
intermediate layer 21 and an outer layer 22. The
intermediate layer 21 folds back over the inside layer 22
such that the folds 23 and 24 are circumferentially
adjacent on the back side of the balloon 15. The
WO 95/05860 PCT/US94/09489
2170157
-7-
intermediate layer 21 and outer layer 22 produce adjacent
folds 25 and 26 as shown in FIGS. 1 and 2.
In accordance with this invention, a first exterior
surface 27 of the outer layer 22 between points marked by
the intersection of the axes 30 and 31 with the folds 25
and 26, respectively, is treated to have a first
coefficient of sliding that facilitates transferring the
balloon 15 across a lesion. The second or remaining
exterior surface 32 has a greater coefficient of sliding
friction. As will be apparent, the second surface 32 also
has a greater surface area than the first surface area 27.
When the balloon 15 expands to the configuration
shown in FIGS. 3 and 4, all of the exterior surfaces 27
and 32 are exposed. However, the second surface 32 with
its greater coefficient of friction and greater area
dominates, so it increases the__overall coefficient of
friction for the expanded balloon 15. Thus the
coefficient of friction for the entire balloon 15 in its
expanded form is greater than the coefficient in the
collapsed or compact form. Consequently, the balloon 15
exhibits different coefficients of friction in its
compacted and expanded forms. If the exterior surface 27
is treated to reduce its coefficient of friction, the
balloon 15 has a low coefficient of sliding friction in
its compact form that facilitates its placement at a
lesion. As the balloon 15 expands, its overall
coefficient of friction increases as the surface 32 is
exposed, so the balloon 15 retains its position during and
after inflation.
There are several methods and structures for
producing surfaces of different coefficients of friction.
FIGS. 5 and 6, for example, disclose portions of the
balloon catheter in FIG. 4 corresponding to the first
surface 27 in FIG. 5 and the second surface in FIG. 6 in
which the balloon 15 has a cellular or tubular honeycomb
core 33. In accordance with one method, the balloon,
during manufacture, is expanded in the form shown in FIGS.
3 and 4 and coated with diverse coatings over portions
WO 95/05860 PCT/US94/09489 4D
2170151
-8-
coextensive to the surfaces 27 and 32. The first surface
27 would be coated with a material that optimizes
lubricousness while the surface 32 would be coated with a
material that has a higher coefficient of friction. After
the coating cures, the balloon is collapsed and folded
into the form shown in FIGS. 1 and 2. Coatings for the
first surface 27 include those composed of hydrogel,
silicone and hydrophilic oil materials. The second
surface 32 could remain uncoated or be formed of a tacky
coating, such as a polyurethane coating or even be coated
with the same material as the first surface 27 that is
roughened after application.
FIG. 7 shows another embodiment of a balloon 15 in
which the surface 32 is textured by forming
circumferentially extending, axially spaced ribs 34 at the
exterior surface 32. The ribs 34 can press gently into
and anchor with surrounding tissue as the balloon 15
expands. This effectively provides an overall coefficient
of friction that is greater than the coefficient of
friction of a smooth surface 27.
FIG. 8 depicts another embodiment in which the
surface 32 is treated with an array of molded pockets 35
bounded by circumferentially and longitudinally extending
ribs 36 and 37. When expanded this waffle-like surface
gently contacts adjacent tissue and anchors the balloon 15
in place. In either of the embodiments of FIGS. 7 or 8,
the material forming the surfaces 32 and 27 may be the
same. The ribs 34 in FIG. 7 and the ribs 36 and 37 in
FIG. 8 would be coextensive only with the surface 32. In
accordance with one manufacturing process, a slippery
coating, such as a hydrogel material, would be applied to
the entire surface of the balloon. Then a material
etching process, such as laser etching, would form the
ribs 34 or ribs 36 and 37 by removing the intermediate
portions of the coating.
FIGS. 9 through 12 depict a balloon catheter assembly
50 with an expandable balloon 51 that extends to a distal
end over a guidewire 52. Spaced tubular portions 53 and
WO 95/05860 PCTIUS94/09489
2,i70M
-9-
54 of the catheter 50 support the balloon 51. The
catheter is generally similar to that shown in respect to
FIGS. 1 through 4.
In this particular embodiment, however, the balloon
51 is compacted by pleating. More specifically, when the
balloon 51 deflates, it forms into pleats, eight pleats in
this example, about a central tube 56 interconnecting the
tubular portions 53 and 54 and a centrally disposed marker
57. The pleats 60 through 67 shown in FIGS. 9 and 10 are
laminated structures with a base film 68 and a plurality
of coatings. Specifically the pleat 60 includes a central
coating 60A that is at the outer surface of the compacted
balloon 51 and that is coextensive longitudinally with the
balloon 51. Likewise the pleats 61 through 67 have
corresponding central, exteriorly exposed, longitudinal
sections 61A through 67A. Each of these surface sections
60A through 67A has a low coefficient of friction. These
are essentially the only surface sections that are exposed
when the balloon 51 is in a compact form.
When the balloon 51 expands about the central tube 56
as shown in FIGS. 11 and 12, the pleats 60 through 67 open
into a generally circular configuration, depending of
course on the tissue into which the balloon 51 expands.
At pleat 60, this exposes areas 60B and 60C on either side
of the central area 60A. Similarly, areas 61B through 67B
and 61C through 67C are exposed on opposite sides of the
central areas 61A through 67A respectively. Each of the
areas 60B through 67B and 60C through 67C has a higher
coefficient of friction than the surfaces of coatings 60A
through 67A respectively. Moreover, the total area of the
areas 60B through 67B and 60C through 67C exceeds the
total area of the areas 60A through 67A.
In FIG. 12, radial lines, such as radial line 70,
depict the boundary between areas such as areas 61B and
62C. In actual practice the areas 61B and 62C would be
formed as a continuous coating. The radial line 70 and
other similar radial lines are shown for purposes of
description only.
WO 95/05860 Z1 PCT/US94/09489 ~
'~~.~5~
-10-
As will be apparent, surface treatment as shown and
described with FIGS. 7 and 8 can be used in the surfaces
60B through 67B to achieve spaced areas of a greater
coefficient of friction. Moreover, the embodiment shown
in FIGS. 9 through 12, like the embodiment shown in FIGS.
1 through 4, provides a balloon catheter that has
different coefficients of friction in its compact and
expanded forms. Thus, like the embodiment of FIGS. 1
through 4, the embodiment in FIGS. 9 through 12
facilitates its placement at a lesion. Further, this
embodiment also retains its position during inflation
because its overall coefficient of friction increases
during inflation. This embodiment differs from that shown
in FIGS. 1 through 4 because in FIGS. 9 through 12 the
balloon has plural surfaces of differing coefficients of
friction rather than one area of each coefficient of
friction.
In another specific embodiment, an uncoated balloon
15 as shown in FIG. 1 is folded into the form shown in
FIG. 2, albeit more compactly form. A coating then is
applied to the balloon 15 in a conventional manner as
described, for example, in United States Letters Patent
No. 5,091,205 issued February 25, 1992 describing
hydrophilic lubricous coatings. The entire exterior
surface 27 of the balloon 15 is coated when the balloon 15
is compacted as shown in FIG. 2. When the balloon 15
expands to the form shown in FIG. 3, the coating remains
limited to the surface 27. The surface 32 remains
uncoated and provides a surface of greater friction.
In each of the specifically disclosed embodiments and
in other evident variations a balloon catheter in its
compact or deflated form produces a balloon configuration
with a low coefficient of friction during the transfer of
the balloon to and across a lesion. When the balloon
expands, it produces at least one section having a surface
with a higher coefficient of friction that dominates and
increases the overall coefficient of friction for the
balloon because this surface is greater than the surface
WO 95/05860 PCT/US94/09489
~ ~ tr~~ ~ ~
-11-
exposed when the balloon is uninflated. This stabilizes
the balloon in a lesion and minimizes the chances for its
unwanted longitudinal displacement.
This invention has been disclosed in terms of certain
embodiments. It will be apparent that many modifications,
particularly in the form of different coatings and surface
treatments can be made to the disclosed apparatus without
departing from the invention. Therefore, it is the intent
of the appended claims to cover all such variations and
modifications as come within the true spirit and scope of
this invention.