Note: Descriptions are shown in the official language in which they were submitted.
WO 95/07750 PCT/SE94/00829
2~7~ 2
METHOD AND APPARATUS FOR ABSORBING HYDROGEN
SULPHIDE
.
This invention relates to a method and an apparatus for absorb-
ing hydrogen sulphide, more specifically a method and an apparatus
for selectively removing, by liquid absorption, hydrogen sulphide from
a gas containing both hydrogen sulphide and carbon dioxide.
It is well-known that hydrogen sulphide can be removed from
hydrogen-sulphide-cont~ining gases by being absorbed in an ~lk~line
aqueous solution (i.e. an aqueous solution having a pH > 7), such as
sodium hydroxide, or by using ethanolamine, such as monoethanol-
amine and diethanolamine. Absorption may, for instance, be used for
producing hydrogen sulphide in pure form, which optionally is further
processed to sulphur in a Claus process. If the gas contains carbon
dioxide in addition to hydrogen sulphide, the carbon dioxide will also
be absorbed in the ~lk~line solution. Carbon dioxide has approximate-
ly the sarne solubility in water as hydrogen sulphide, and the carbon
dioxide will therefore compete with the hydrogen sulphide for being
absorbed in the solution. Hydrogen sulphide and carbon dioxide are
absorbed in an ~lk~line aqueous solution of e.g. sodium hydroxide in
accordance with the formulae below:
H2S + OH- ~ HS-+ H20 (1)
H~S + 20H- ~ S2- + 2H20 (2)
C2 + OH- ~ HC03- (3)
C2 + 20H- ~ C032- + H20 (4)
25 The selectivity to hydrogen sulphide, i.e. the ratio of mole of absorbed
hydrogen sulphide to mole of absorbed (hydrogen sulphide + carbon
dioxide~, is directly proportional to the contents of hydrogen sulphide
and carbon dioxide in the gas. Thus, the competition on the part of
carbon dioxide is especially pronounced when the gas contains more
30 carbon dioxide than hydrogen sulphide, as is mostly the case. If a gas
contains, say, 1% by volume of hydrogen sulphide and 10% by volume
of carbon dioxide and efforts are made to absorb the hydrogen sul-
phide in a sodium hydroxide solution, the selectivity to hydrogen sul-
phide is but 10%, i.e. 90% of the gas absorbed consists of carbon
WO 9S/077S0 PCT/SE94/00829
~713~2
phide is but 10%, i.e. 90% of the gas absorbed consists of carbon
dioxide, which means that as much as 90% of the sodium hydroxide
is spent in absorbing carbon dioxide.
In an effort to remedy the above inconvenience in the absorption
of hydrogen sulphide from gases cont~ining both hydrogen sulphide
and carbon dioxide, methods for selective absorption of hydrogen
sulphide have been developed. For instance, efforts have been made to
selectively absorb hydrogen sulphide in solutions of strong oxidising
agents, such as potassium perm~ng~n~te, sodium dichromate or
ferric salts. In other selective methods, use is made of ~lk~line
solutions, such as sodium carbonate or potassium carbonate
solutions, the operational conditions during the absorption being
carefully adjusted. More detailed information about this prior-art
technique is found in an article by C. Oloman, F.E. Murray and
J.B. Risk entitled "The Selective Absorption of Hydrogen Sulphide
from Stack Gas", Pulp and Paper M~g~ine of C~n~ , 5 December
1969, p. 69ff, as well as an article by E. Bendall, R.C. Aiken and
F. Mandas entitled "Selective Absorption of H2S from Larger Quanti-
ties of CO2 by Absorption and Reaction in Fine Sprays", AICHE Jour-
nal (Vol. 29, No. 1), January 1983, p. 66ff.
By using a carbonate solution, such as a sodium carbonate
solution, instead of a hydroxide solution, such as a sodium hydroxide
solution, the selectivity for the absorption of hydrogen sulphide can be
augmented to about 30-50%. The reactions taking place during such
an absorption can generally be rendered as follows:
H2S + C032- ~ HS- + HCO3- (5)
C2 + C032- + H2O ~ 2HCO3- (6)
When the absorption solution is a carbonate solution, the
hydrogen sulphide is absorbed almost instantaneously, whereas the
carbon dioxide reacts only slowly with the carbonate ions to form
hydrogen carbonate ions. Owing to the high content of hydrogen car-
bonate generated when using a carbonate solution as absorption
medium, there is the additional advantage of a "counterpressure"
(equilibrium pressure) to the absorption of carbon dioxide, as appears
from the equilibrium formula (6) above.
Wo gs/07750 ~ 1 7 ~ ~ ~L 2 PCT/SE94/00829
A problem that arises when using a carbonate solution as
absorption medium is that only a fairly low hydrogen-sulphide con-
tent can be achieved in the solution, owing to the reduction of the
absorption capacity caused by the formation of hydrogen carbonate
5 ions. Thus, it is extremely difficult to attain hydrogen-sulphide
contents exceeding about 10 g/l. As a result, prior-art methods for
selectively absorbing hydrogen sulphide by means of an absorption
medium in the form of a carbonate solution have not met with much
success, despite the great demand for such a method in various fields
10 where hydrogen-sulphide-cont~inin~ and carbon-dioxide-con~ining
gases are generated. F`~mples of such fields of application are petro-
leum refinement, coal-gas production and, in particular, the combus-
tion of black liquor carried out in the sulphate pulp industry.
When recovering chemicals in the sulphate pulp industry in
15 accordance with the conventional Tomlinson process, the black liquor
is combusted in a soda recovery unit, resulting in the generation of
steam and the formation of a melt chiefly consisting of sodium car-
bonate and sodium sulphide. The melt is then dissolved in water and
causticised, so that the sodium carbonate is converted to sodium
20 hydroxide and white liquor is obtained, which may then again be used
for digesting wood. For many reasons, including the risk of an explo-
sion when a tube in the soda recovery unit bursts, efforts have in
recent years been made to develop new processes for the combustion
of black liquor, in which no melt is formed. Such processes can be
25 collectively referred to as "black-liquor evaporation", and one instance
thereof is the so-called SCA-Billerud process (E. Hornstedt and
J. Gomy, Paper Trade Journal 158 (1974): 16, pp 32-34). In this pro-
cess, the black liquor is pyrolysed in a reactor under such tempera-
ture conditions that dust, which chiefly consists of sodium carbonate
30 and carbon, and a combustible gas, which inter alia contains sulphur
compounds, are formed. Another instance of black-liquor evaporation
is given in Swedish PatentApplication 8605116-0, which concerns a
method for thermal decomposition of black liquor with concurrent
supply of oxy~e~l in an amount short of the stoichiometrically required
35 amount, at a pressure above 10 bar, and at such a temperature that
no melt is formed. The evaporation results in the formation of a solid
phase, which chiefly consists of sodium carbonate, and a gaseous
WO 95/077S0 . , PCT/SE9 1/00829
2~713~2
phase, which chiefly consists of hydrogen sulphide, carbon monoxide,
carbon dioxide, hydrogen, water vapour, and methane.
In order to be able to recover the chemicals used in black-liquor
evaporation as above and produce from these chemicals white liquor
5 to be used in the manufacture of pulp, it is necessary that the hydro-
gen sulphide can be removed from the generated gas. Since the gas
also contains carbon dioxide, the latter will compete with the hydro-
gen sulphide in the liquid absorption, and since the gas has a low
content of hydrogen sulphide (about 0.5-2%) and a carbon dioxide
content that is some 20 times higher (about 10-20%), conventional
liquid absorption results in lln.c~ti~f~ctory recovery of hydrogen sul-
phide.
There is thus a need to be able to separate, by liquid absorp-
tion, the hydrogen sulphide with a high degree of separation and a
15 high degree of selectivity from gases cont~ining both hydrogen sul-
phide and carbon dioxide.
According to Swedish Patent Application 9300533-8, which was
filed on 18 February 1993 but has not yet been published, it has been
found that absorption with a high degree of separation of hydrogen
20 sulphide and a high degree of selectivity for hydrogen sulphide can be
obtained by using, as a liquid absorption medium, a carbonate-
cont~ining ~lk~line solution and adjusting the pH of the solution
during the absorption of hydrogen sulphide by the addition of a
hydroxide, i.e. not by the addition of fresh carbonate. Thus, it is
25 possible to obtain a selectivity for hydrogen sulphide in the absorption
of 60-70%, a sulphide content in the absorption solution of about
30 g/l, and a degree of separation of hydrogen sulphide of about
90-99%.
To be more precise, this is attained by a method which is of the
30 type stated by way of introduction and in which the gas is contacted
in at least one step with a carbonate-cont~ining ~lk~line solution
whose pH is adjusted during the absorption by the addition of a
hydroxide.
The inventive method can be implemented with the aid of an
35 apparatus, which is characterised in that it comprises: a container
having a gas inlet and a gas outlet and cont~ining a packing arranged
in a number of successive steps; means for supplying a carbonate-
WO 95/07750 ~ :~L 71 3 4 2 PCT/SE94/00829
~ ~ i
containing solution to the last step, as seen in the feed direction of thegas, each step having means for supplying the carbonate-containing
solution through the step countercurrently to the gas and for recycl-
ing the solution over the step; conduits arranged between the steps
5 for supplying a partial flow of the solution from one step to a preced-
ing step, as seen in the feed direction of the gas; means for supplying
a hydroxide to the carbonate-cont~ining solution in at least one of the
steps; and an outlet conduit from the first step, as seen in the feed
direction of the gas, for discharging liquid cont~inin~ hydrogen sul-
1 0 phide.
By "carbonate-containing ~lk~line solution" is meant an
aqueous solution cont~ining carbonate ions (CO3 2-). Preferably, this
solution is an alkali metal carbonate solution, such as a solution of
sodium carbonate, potassium carbonate or lithium carbonate. Sodium
15 carbonate is especially preferred, being easily accessible as well as
fairly inexpensive. The carbonate concentration of the solution is not
critical, but conveniently is about 0.1-3 M with respect to carbonate,
preferably about 1-2.5 M, and most preferred about 1.7 M.
It is essential that the carbonate-cont~ining ~lk~line solution
20 has a pH of at least about 9. pH values below about 9 result in unsa-
tisfactory absorption of hydrogen sulphide, and there is even a risk
that hydrogen sulphide already absorbed be released from the solu-
tion. However, the pH of the solution should not be too high, since
this would have an adverse effect on the absorption of hydrogen sul-
25 phide, as compared with that of carbon dioxide. Thus, the pH of thesolution should preferably not exceed about 12 in order that the
absorption of carbon dioxide should not be too considerable. Prefer-
ably, the pH of the solution is in the range of about 10.0-11.0, and
most preferred in the range of about 10.2-10.8. If the pH of the solu-
30 tion is adjusted within this last narrow range, optimum separation ofhydrogen sulphide is obtained.
As appears from the equilibrium reactions (5) and (6), hydrogen
carbonate ions (HCO3-) are formed in the absorption of hydrogen sul-
phide and carbon dioxide. This means that the pH of the absorption
35 solution decreases as the absorption of hydrogen sulphide and carbon
dioxide proceeds. When the pH of the solution goes below about 9, the
absorption of hydrogen sulphide becomes unsatisfactory, as indicated
WO 95107750 ~ ~ PCT/SE94100829
in the foregoing, and there is instead a risk that hydrogen sulphide
already absorbed be released from the solution. If this is to be avoid-
ed, the solution has to be regenerated, i.e. its pH be increased to
above the lower permissible limit for a state of equilibrium between
gaseous H2S and sulphide content of the liquid at the temperature
and pH value at issue. However, the pH value must not be increased
to above about 12, in which case the absorption of carbon dioxide
would become predomin~nt. As a result of the increase of the pH of
the solution brought about by the addition of a hydroxide, such as an
alkali metal hydroxide, e.g. NaOH, the hydrogen carbonate ions form-
ed are reconverted to carbonate ions according to the following equi-
librium reaction:
OH- + HCO3- _ C032- + H2O (7)
Being thus regenerated, the carbonate solution can absorb
more hydrogen sulphide according to the reaction (5) above. Since
the pH of the solution is adjusted by the addition of a hydroxide and
maintained within the given range of about 9-12, preferably about
10.0-11.0, and most preferred about 10.2-10.8, the absorption of
carbon dioxide is kept on a comparatively low level.
As indicated in the foregoing, the carbonate-containing Alk~line
solution is regenerated by the addition of a hydroxide. Basically, use
can be made of any hydroxide that does not have an adverse effect on
the absorption of hydrogen sulphide and is capable of increasing the
pH of the solution from the given lower limit of about 9 to the desired
value, such as a value not exceeding about 12.0, preferably not
exceeding about 11.0, and most preferred not exceeding about 10.8.
According to the invention, use is preferably made of hydroxides of
alkali metals or AlkAline-earth metals, such as sodium hydroxide
(NaOH), potassium hydroxide (KOH), lithium hydroxide (LiOH), cal-
cium hydroxide (Ca(OH)2), and magnesium hydroxide (Mg(OH)2). For
reasons of availability and cost, sodium hydroxide is most preferred.
The temperature of the liquid used in the absorption is not par-
ticularly critical and may vary within a wide range, but preferably is
below about 80C, since there is a risk that the absorption of hydro-
gen sulphide decreases at temperatures of about 80C and above. It
WO 95/07750 ~ 1713 ~ 2 PCT/SE94/00829
.
is preferred that the temperature is in the range of from room tem-
perature, i.e. about 20C, to about 80C, more preferably about
40-70C, and most preferred about 60-70C.
In order to optimise the selectivity to hydrogen sulphide in the
5 liquid absorption, the absorption should be carried out in such a
mamler that the flow of gas and the flow of absorbing liquid are coun-
tercurrent, and the flow of gas should be turbulent and the flow of
liquid laminar. Moreover, the separation of hydrogen sulphide is pro-
moted if the volume of absorbing liquid is large compared with the
10 volume of gas from which hydrogen sulphide is absorbed. Such a high
ratio of liquid to gas is achieved by recycling the absorbing liquid that
is contacted with the hydrogen-sulphide-containing gas.
Furthermore, the contact between the sulphur-contAining gas
and the absorbing liquid (the carbonate-contAining solution) may
15 involve one or more steps, preferably two or three steps, and most
preferred three steps. Such multistep contact has the advantage of
shortening the length of each individual step, such that the pH of the
carbonate-contAining solution does not have time to fall below about 9
in the individual step, while at the same time the sulphide content
20 can be kept low in the uppermost step. Preferably, each step has such
an extent or length that the pH of the solution at the end of the step
has fallen to about 10.0-10.2, the liquid being then drawn offto be
regenerated by means of a hydroxide and then recycled to the step at
issue.
According to the invention, it has been found that the removal
of hydrogen sulphide in a method of the above type can be rendered
more efficient, while drastically reducing the chemical consumption,
if the method is divided into two stages, namely a first stage, in which
the main part of the hydrogen sulphide is removed in accordance with
30 the method described above, and a second stage, in which the
remAinin~ hydrogen sulphide is essentially removed by combustion to
sulphur dioxide, which is absorbed in an AlkAline solution in a wet
- cleaner (scrubber). The reason for this is as follows.
As hydrogen sulphide is absorbed in the different steps of the
35 first stage, the hydrogen-sulphide content of the gas decreases, which
results in a reduction of the previously-defined selectivity to hydrogen
sulphide. When the selectivity is reduced, the consumption of alkali
WO 95/07750 2~ 4 ~ PCT/SE94/00829
'~
increases dramatically in accordance with the reactions (5), (6) and (7)
indicated above. As appears from these reactions, 1 mole of OH- is
spent per mole of hydrogen sulphide absorbed, and 2 mole of OH- is
spent per mole of carbon dioxide absorbed. When the selectivity to
hydrogen sulphide is reduced and the absorption of carbon dioxide
increases, as is the case in the last absorption step or steps of the
first stage, the consumption of alkali (OH-) increases considerably.
This means that it takes a comparatively larger amount of alkali to
absorb the "last" hydrogen sulphide of the gas than to absorb the
"first" hydrogen sulphide of the gas. By refraining from carrying the
absorption of hydrogen sulphide to the full in the first stage, but
purposely leaving a certain amount of hydrogen sulphide in the gas
discharged from the first stage, one avoids that the selectivity to
hydrogen sulphide is dramatically reduced and, consequently, that
the consumption of alkali increases dramatically in the first stage.
The hydrogen-sulphide absorption in the first stage is so carried out
that the main part of the hydrogen sulphide, i.e. at least 50%, is
removed. Conveniently, about 60-97%, preferably about 80-95%, and
most preferred about 90%, of the hydrogen sulphide is removed in the
first stage. This results in a low consumption of alkali in the first
stage, which involves good process economy.
Before the gas from the first stage can be let out into the sur-
rounding atmosphere, the rem~ining hydrogen sulphide must, how-
ever, be removed. According to the invention, this takes place by
combusting the hydrogen-sulphide-cont~ining gas from the first
stage, such that the hydrogen sulphide is oxidised to sulphur dioxide,
whereupon the sulphur dioxide formed is absorbed in an aqueous
solution, whose pH is adjusted by the addition of an ~lk~line solution
or an ~lk~line substance, in a wet cleaner (scrubber).
The present invention thus provides a method for selectively
removing, by liquid adsorption, hydrogen sulphide from a gas con-
t~ining both hydrogen sulphide and carbon dioxide, characterised in
that the gas, in a first stage for removing the main part of the hydro-
gen-sulphide content of the gas, is contacted in at least one step with
a carbonate-containing ~lk~line solution whose pH is adjusted during
the absorption by the addition of a hydroxide, and that the gas is then
in a second stage combusted in order to convert the rem~ining hydro-
W095/07750 ~ ~ 713 4 ~ PCT/SE94/00829
gen sulphide to sulphur dioxide, which is absorbed in an alkali-con-
taining solution.
The invention further provides an apparatus for selectively
removing, by liquid absorption, hydrogen sulphide from a gas con-
5 taining both hydrogen sulphide and carbon dioxide, characterisedin that it comprises
a) a container having a gas inlet and a gas outlet and containing
a packing arranged in a number of successive steps; means for sup-
plying a carbonate-contAining solution to the last step, as seen in the
10 feed direction of the gas, each step having means for supplying the
carbonate-cont~ining solution through the step countercurrently to
the gas and for recycling the solution over the step; conduits arranged
between the steps for supplying a partial flow of the solution from one
step to a preceding step, as seen in the feed direction of the gas;
15 means for supplying a hydroxide to the carbonate-cont~ining solution
in at least one of the steps; and an outlet conduit from the first step,
as seen in the feed direction of the gas, for discharging liquid con-
taining hydrogen sulphide,
b) a combustion device having an inlet for hydrogen-sulphide-
20 contAining gas from the gas outlet of the container; combustionmeans for combusting the gas supplied and converting the hydrogen
sulphide therein to sulphur dioxide; and an outlet for discharging
sulphur-dioxide-cont~ining gas from the combustion device, and
c) a wet cleaner adapted to absorb the sulphur dioxide formed
25 upon combustion, and comprising a container which has an inlet for
sulphur-dioxide-containing gas from the combustion device; an outlet
for cleaned gas; means for supplying, circ~]lAting and finely dividing
an aqueous solution, whose pH is adjusted by the addition of Alk~line
solution or ~lk~line substance, so as to contact it with the sulphur-
30 dioxide-contAining gas supplied; and means for discharging the solu-
tion that has absorbed sulphur dioxide.
Further distinctive features of the invention appear from the
following description and are stated in the appended cl~3im~.
As indicated in the foregoing, the carbonate-containing ~lkAline
35 solution used in the first stage for hydrogen-sulphide absorption is an
aqueous solution contAining carbonate ions, such as a solution of
alkali metal carbonate, preferably sodium carbonate. When the inven-
WO 95/07750 2 ~ i3 4 2 PCT/SE94/00829
10tive method is used for removing hydrogen sulphide in the sulphate
pulp industry, for instance when combusting black liquor, one has
found that green liquor may be used as the carbonate-containing
~lk~line solution according to the invention, and this constitutes a
5 particular aspect of the invention. Green liquor is an aqueous solution
that usually contains about 1.3-1.4 mole of Na2CO3 per litre, about
0.4-0.6 mole of Na2S per litre, and about 0.3-0.5 mole of NaOH per
litre. According to the invention, it has also been found that white
liquor may likewise be used in the first stage as the hydroxide for
10 regenerating the carbonate-containing ~lk~line solution, when the
invention is employed in the sulphate pulp industry for removing
hydrogen sulphide. White liquor is an aqueous solution that con-
tains NaOH, Na2S and Na2CO3, usually in the proportions of about
2.3-2.6 mole of NaOH per litre, about 0.4-0.6 mole of Na2S per litre,
and about 0.25-0.4 mole of Na2CO3 per litre. Also the use of white
liquor as the hydroxide for regenerating the carbonate-cont~ining
~lk~line solution constitutes a particular aspect of the invention.
Instead of white liquor, use can be made of weak liquor or
oxidised white liquor for regenerating the carbonate-cont~ining
20 ~lk~line solution. By weak liquor is meant an aqueous solution
that contains NaOH, Na2S and Na2CO3, usually in the proportions
of about 0.3-0.4 mole of NaOH per litre, about 0.05-0.08 mole of Na2S
per litre, and about 0.05-0.07 mole of Na2CO3 per litre.
Since green liquor, white liquor as well as weak liquor contain
25 both carbonate and hydroxide, they may be used either separately or
together, both as the carbonate-containing solution and for regenerat-
ing the carbonate-cont~ining ~lk~line solution.
In the second stage, the combustion of the hydrogen-sulphide-
cont~ining gas takes place in known m~nner in a combustion device
30 of k~own type, such as a lime sludge reburning kiln or a bark burning
boiler when the invention is used in the sulphate pulp industry. If the
heat content of the hydrogen-sulphide-containing gas is too low, an
auxiliary fuel, such as oil or natural gas, may be used in the com-
bustion to produce s~ti~f~ctory oxi-l~tion of the hydrogen sulphide to
35 sulphur dioxide. When the hydrogen-sulphide-containing gas is
burnt, the hydrogen sulphide is, at normal combustion temperatures,
~ wo 95/07750 21 7~ 3 ~ 2 PCT/SE94/00829
completely oxidised to sulphur dioxide, and the gas thus contains
only negligible amounts of hydrogen sulphide after the combustion.
After the combustion of the hydrogen-sulphide-containing gas
in the second stage, the sulphur dioxide formed should be removed,
5 which according to the invention takes place by it being absorbed in
an alkali-containing solution in a wet cleaner (scrubber). To selectively
remove, by absorption in an alkali-containing solution in a scrubber,
sulphur dioxide from a gas which in addition contains carbon dioxide,
is a well-known technique, which need not be described in more detail
10 here. Conveniently, use is made of a scrubber where the absorbing
alkali-cont~ining solution is recycled to a high extent. The pH of the
alkali-containing solution in the SO2 scrubber is preferably in the
range of about 6-8, and most preferred in the range of about 7-7.5. At
these fairly low pH values, the absorption of carbon dioxide is neglig-
15 ible, and the selectivity to the sulphur-dioxide separation consequent-
ly is 100%. During the absorption, the sulphur dioxide undergoes one
of the following reactions:
S2 + OH- ~ HSO3- (8)
S2 + S082- + H2O ~ 2HSO3-
HSO3- + OH- ~ H2O + S032- (10)
As appears from the reaction formulae (8)-(10), 2 mole of OH- is
spent per moie of SO2 absorbed. Since no carbon dioxide is absorbed,
the absorption of sulphur dioxide can be carried out to almost 100%
of the sulphur dioxide present without there being any noticeable
increase in the alkali consumption. This considerably improves pro-
cess economy, as compared with the case when all the sulphur is
absorbed as hydrogen sulphide in the first stage, which results in
poor selectivity.
The alkali in the alkali-containing solution conveniently is a
hydroxide or a carbonate of an alkali metal or an ~lk~line-earth metal,
such as sodium hydroxide, potassium hydroxide, calcium carbonate,
calcium hydroxide or magnesium hydroxide. Sodium hydroxide is pre-
ferred, especially if the sulphur is to be recycled directly in the pro-
WO 95/077~iO PCT/SE94/00829
~7 ~
cess, e.g. when the method is employed in the sulphate pulp industry.
If so, use is conveniently made of a sodium-hydroxide-based spray
scrubber, for instance of the type used by ABB Flakt Industri AB and
designated the MoDo scrubber. The scrubber may then comprise one
5 or more heat-recovery steps, in which cold water is sprayed towards
the hot gas, so that hot water having a temperature of about 45-65C
is obtained. After the absorption of sulphur dioxide, the solution leav-
ing the scrubber contains sodium sulphite (Na2SO3), sodium hydro-
gen sulphite (NaHSO3) and sodium sulphate (Na2SO4). In the sul-
10 phate pulp industry, this solution can be mixed with other combust-
ible process liquids, e.g. the black liquor in a pulp mill. A combustion
under reducing conditions results in sulphur in the form of hydrogen
sulphide, and a combustion under oxidising conditions results in sul-
phur in the form sulphur dioxide.
In the pulp industry, the method according to the invention is
conveniently so designed that its different stages are integrated in the
existing equipment. This means that the combustion of the hydrogen-
sulphide-containing gas takes place in an existing bark burning boiler
or lime sludge reburning kiln. This reduces the need for other fuels in
these process steps. The sulphur-dioxide-cont~ining flue gases from
the bark burning boiler or the lime sludge reburning kiln can then be
conducted to an existing SO2 scrubber. In this case, the only new
equipment needed is a scrubber for the separation of hydrogen sul-
phide.
Pulp mills may be equipped with separate SO2 scrubbers for
flue gases from the bark burning boiler and the lime sludge reburning
kiln and from the soda recovery boiler, respectively. When the hydro-
gen-sulphide-cont~ining gas is combusted elsewhere, for example in a
separate boiler, the sulphur-dioxide-cont~ining flue gases can be con-
ducted to the existing SO2 scrubber for the flue gases from the soda
recovery boiler.
It is evident from the foregoing that the present invention
enables good process economy, a closed chemical system in which
all the sulphur is recycled, as well as very low emi~sions to the sur-
rounding atmosphere.
For clarifying purposes, the invention will now be described in
more detail with reference to the accompanying drawings, in which
WO 95/07750 PCT/SE94/00829
~17134~
Fi~ 1 shows a preferred apparatus according to the invention
for implementing the first stage of the method according to the inven-
tion; and
Fig. 2 is a schematic flow chart illustrating the method accord-
ing to the invention.
The apparatus shown in Fig. 1 comprises a tower or container 1
having an inlet 2 for a gas 3 which contains hydrogen sulphide as well
as carbon dioxide. At the opposite end of the apparatus, there is pro-
vided an outlet 4 for gas 5 from which hydrogen sulphide has been
removed by liquid absorption. The contact between the hydrogen-sul-
phide-containing gas and the carbonate-cont~ining solution involves
three steps 6, 7 and 8. Each step contains a p~king 9, as hinted at in
step 6 in the Figure. In order to optimise the selectivity to hydrogen
sulphide in the absorption, the packing 9 has such a shape as to
generate a l~min~r flow of liquid through the steps 6, 7 and 8. It has
been found that a p~cking in the form of corrugated plates is espe
cially suitable for this purpose. The packing may, for instance, be
made of plastic or metal.
The contact between the hydrogen-sulphide-absorbing, carbo-
nate-containing solution and the hydrogen-sulphide-containing gas
is carried out in countercurrent fashion. To this end, each step has
means for supplying the carbonate-cont~ining solution through the
step countercurrently to the gas, as well as for recycling the solution
over the step. As shown in Fig. 1, these means are made up of pumps
10, 11 and 12 which, via conduits 13, 14 and 15, feed the carbonate-
cont~ining ~lk~line solution to the respective steps 6, 7 and 8, as well
as conduits 16, 17 and 18 which conduct the solution from the
respective steps to collecting vessels 19, 20 and 21. From these col-
lecting vessels, the solution is recycled over the steps through con-
duits 22, 23 and 24, which are connected to the pumps 10, 11 and
12, respectively. Fresh carbonate solution, preferably sodium carbo-
nate solution, is fed to the last step 6, as seen in the feed direction
of the gas, through a conduit 25 from a supply (not shown) of sodium
carbonate solution.
Instead of supplying fresh carbonate solution to the last step,
the carbonate solution can be generated in the last step by supplying
sodiurn hydroxide solution to this step and allowing the hydroxide
WO 95/07750 PCT/SE94/00829 ~
2~7:~3~
14
solution to absorb carbon dioxide from the gas, such that a carbo-
nate-cont~ining solution is obtained according to the reactions (3)-(4)
above.
In order to adjust (increase) the pH of the absorption solution,
5 a hydroxide, preferably a sodium hydroxide solution, may be supplied
to the collecting vessels 19, 20 and 21 through conduits 26, 27 and
28, respectively, conducting alkali from a supply (not shown), which
preferably is common to all the conduits. The supply of sodium
hydroxide solution for adjusting the pH of the absorption solution is
10 regulated on the basis of the measured pH values of the solutions in
the collecting vessels 19, 20 and 21 (not shown).
As appears from Fig. 1, the different steps are further inter-
connected by conduits 29 and 30 for feeding a partial flow of the
absorption solution from one step to the preceding step, i.e. from
15 the step 6 to the step 7 as well as from the step 7 to the step 8.
Finally, an outlet conduit 31 is arranged for discharging hydro-
gen-sulphide-cont~ining liquid from the collecting vessel 21 and the
step 8.
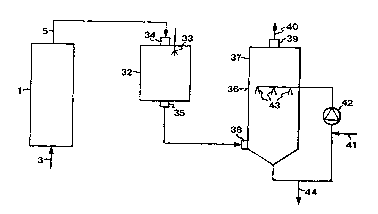
Fig. 2 is a schematic flow chart illustrating the method accord-
20 ing to the invention. As indicated in the flow chart, a hydrogen-sul-
phide-cont~ining and carbon-dioxide-containing gas 3 is, in a first
stage, treated in an apparatus which includes a container 1 for the
absorption of hydrogen sulphide, as described in the foregoing with
reference to Fig. 1. According to the invention, the absorption of
25 hydrogen sulphide is not, in the first stage, carried out to 100%, i.e.
total absorption of hydrogen sulphide in the first stage, but only so far
that at least the main part of the hydrogen sulphide, i.e. at least 50%,
preferably about 60-97%, more preferably about 80-95% and most
preferred about 90%, of the hydrogen sulphide is absorbed in the first
30 stage. Preferably, a total of at least about 98% of the hydrogen-sul-
phide content of the gas is absorbed by the first and the second stage.
The thus-cleaned gas 5, which still contains hydrogen sulphide
and carbon dioxide, is then fed from the first stage to the second
stage, in which the gas is first combusted in order to convert hydro-
35 gen sulphide to sulphur dioxide, and then wet-cleaned in a scrubber
in view of the absorption of the sulphur dioxide formed.
~ WO 95/07750 PCTISE94/00829
I ~17~3~2
As indicated in Fig. 2, the combustion takes place in a combus-
tion device 32, which has combustion means 33, e.g. a burner, for
combusting the gas 5 supplied through an inlet 34 and oxidising the
hydrogen sulphide to sulphur dioxide. As mentioned in the foregoing,
the combustion device 32 may consist of existing equipment, such as
a bark burning boiler or a lime sludge reburning kiln. The sulphur-
dioxide-containing flue gas is discharged through an outlet 35 and
conducted to a wet cleaner 36, which comprises a container 37 having
an inlet 38 for the sulphur-dioxide-containing gas, an outlet 39 for
the cleaned gas 40, and means 41 for supplying an alkali-cont~ining
solution. As mentioned above, the alkali-containing solution contains
a hydroxide of an alkali metal or ~lk~line-earth metal, preferably
sodium hydroxide. The solution is circulated by means of a pump 42
to nozzles 43, where it is finely divided and is countercurrently
brought into contact with the sulphur-dioxide-cont~ining gas, such
that the sulphur dioxide is absorbed by the solution. In order to opti-
mise the absorption of sulphur dioxide, without any competing
absorption of carbon dioxide, the pH of the solution is adjusted to
about 6-8, preferably 7-7.5. The solution that has absorbed sulphur
dio~ide is discharged from the second stage at 44.
The invention will now be further elucidated with the aid of a
non-restricting Example.
F~x~rnple
A test was performed for the selective removal of hydrogen sul-
phide from a gas generated in black-liquor evaporation. Use was made
of an apparatus of the type described above and shown in Figs 1 and
2.
In the first stage of the method, the absorption of hydrogen sul-
phide took place at atmospheric pressure, and the feed gas had a
temperature of about 60C and contained 1.13 mole % of hydrogen
- sulphide and 16.9 mole % of carbon dioxide. The gas was saturated
with water vapour at the temperature at issue, which corresponded to
about 18.7 mole % of water. The feed gas flow was 38280 Nm3/h,
giving the gas a velocity of about 3.1 m/ s in the absorption tower. The
absorption tower had a height of 6.25 m, and the two first steps each
had a height of 1.5 m, whereas the last step, as seen in the feed
direction of the gas, had a height of 1 m. Each step was provided with
WO 95/07750 2 ~ 71`~ 4 ~ PCT/SE94/00829
16
a packing of the type Mellapack 500 from Sulzer. The diameter of the
tower was 2.3 m.
Fresh absorption solution, which consisted of 8.8 m3/h of 2 M
sodium carbonate solution having a temperature of about 60C, was
supplied to the last step in the tower along with recycled absorption
solution, such that a total of about 50 m3/h of absorption solution
was supplied to the last step in the tower. The pH of the absorption
solution supplied was about 11.0, which was reduced to about 10.2
during the passage of the solution through the step owing to the
10 absorption of hydrogen sulphide. After passing through the step, the
solution was supplied to a 1.5 m3 collecting vessel, where the solution
was regenerated by the addition of a 2.5 M sodium hydroxide solution
having a temperature of about 60C, such that the pH of the solution
was again increased to about 11Ø Then, the regenerated solution
15 was recycled by means of a pump to the last step in the absorption
tower for renewed absorption of hydrogen sulphide.
About 11 m3/h of the absorption solution was drawn off from
the collecting vessel to the collecting vessel of the intermediate step,
whence about 50 m3/h of the absorption solution having a pH of
20 about 11.0 was pumped, as in the previous step, to the intermediate
step, whence the absorption solution was drawn off at a pH of about
10.2 to be recycled to the collecting vessel. In the collecting vessel, the
solution was regenerated by the addition of a 2.5 M sodium hydroxide
solution having a temperature of about 60C, as in the previous step.
From the collecting vessel of the intermediate step, about
13.5 m3/h of the absorption solution was drawn off to the collecting
vessel of the first (lowermost) step, whence 50 m3/h of the absorption
solution having a pH of about 11.0 was pumped to the first step, as
seen in the feed direction of the gas. After passing this step and there
30 absorbing hydrogen sulphide, the solution, now having a pH of about
10.2, was drawn off to be recycled to the collecting vessel. In the col-
lecting vessel, the solution was regenerated as in the previous steps
by the addition of a 2.5 M sodium hydroxide solution having a tempe-
rature of about 60C, such that the pH of the regenerated solution
35 was about 11Ø All in all, about 8.6 m3/h of the 2.5 M sodium
hydroxide solution was added to the collecting vessels of the three
steps.
~ WO 95/07750 PCT/SE94/00829
2~7~2
From the collecting vessel of the first (lowermost) step, about
17.4 m3/h of the solution having a HS- concentration of 1 mole/l was
drawn off. The gas leaving the absorption tower contained 0.113 mole
% of hydrogen sulphide and 16.4 mole % of carbon dioxide. In the
5 first stage of the method, the degree of separation of hydrogen sul-
phide was about 90%, and the selectivity to hydrogen sulphide in the
separation was about 0.67%.
Then came the second stage of the method. Thus, the gas leav-
ing the absorption tower in the first stage was combusted in a fur-
nace to which was added 31700 Nm3/h of air, which resulted in
66000 Nm3/ h of flue gas cont~ining 650 ppm of SO2 (1930 mole/h).
This flue gas was conducted to an SO2 scrubber of conventional type
desclibed in the foregoing. In the scrubber, 1.54 m3/h of the 2.5 M
sodium hydroxide solution was spent, and 2.0 m3/h of fresh water
was in addition supplied to the scrubber in order to cool the incoming
flue gas to saturation temperature. The gas leaving the scrubber had
an So2 concentration of 12 ppm, which corresponded to 35 mole/h
(1.12 kg/h of sulphur). The supplied absorption solution was kept at
a constant pH of 7.2. The absorption solution was recycled in th
scrubber at a total flow rate of 170 m3/h. From the scrubber was
drawn off 1.8 m3/h of the solution, which had a total sulphur con-
centration (S032- + HSO3- + S042-) of 1.05 mole/l. The total degree
of separation of sulphur, i.e. the degree of separation of hydrogen
sulphide in the first stage plus the degree of separation of sulphur
dioxide in the second stage, thus was 99.8%. In the first stage,
860 kg/h of sodium hydroxide was spent, and in the second stage,
152 kg/h of sodium hydroxide was spent, i.e. a total of 1012 kg/h of
NaOH was spent in order to obtain a degree of separation of sulphur
of 99.8%.
For comparative purposes, the method was repeated, but this
time only with the first stage, i.e. the combustion of hydrogen sul-
phide to sulphur dioxide and the absorption of the formed sulphur
dioxide in a wet scrubber were not performed. Instead, the separation
of hydrogen sulphide in the first stage was carried out to such an
extent that 98% of the hydrogen sulphide was separated. The selec-
tivity to hydrogen sulphide in a 90-98% separation of hydrogen sul-
phide is 0.15. The total selectivity for the whole stage then is about
WO 95/07750 21 7 ~ 3 4 2 PCT/SE94/00829
' . . ,
18
NaOH consumption more than 70% higher than in the inventive
method, despite the fact that the total degree of separation of sulphur
was lower.