Note: Descriptions are shown in the official language in which they were submitted.
2 ! 7~037
'_
TITLE OF INVENTION
Targeting of Dosages of Medicine and Therapeutic Agents and Other
Glycosaminoglycans (GAGS).
5 FIELD OF THE INVENTION
This invention reiates to the targeting of medicines and therapeutic agents
to sites in the body of a mammal in need of treatment and, in one application,
finds use in the treatment of malignant tumours in humans.
BACKGROUND OF THE INVENTION
U.S. Application 07/675,908 owned by Hyal Pharmaceutical Corporation
and PCT Application PCT/CA90/00306, Publication No. WO91/04058 also owned
1 5 by Hyal Pharmaceutical Corporation teaches the use of dosages of at least 10 mg.
of forms of hyaluronic acid to transport effective amounts of medicines and/or
therapeutic agents to sites in need of treatment in the human body, to penetratethe tissue at the sites in need of treatment, including scar tissue, through allmembranes into the cells to be treated.
At page 25, line 17, the PCT Application teaches the additional benefit of
using at least about 200 mg. of forms of hyaluronic acid (for example, sodium
hyaluronate) in a dosage together with the medicine and/or therapeutic agent forreducing the side effects of the medicine and /or therapeutic agent when
25 administered (such as gastro-intestinal distress, neurological abnormalities,depression, etc. normally associated with the medicine and/or therapeutic agent)even at elevated amounts greater than the usual accepted dosage amounts of the
medicine and/or therapeutic agent when administered alone for example, an
NSAID (non-steroidal anti-inflammatory agent).
2 2~ 73037
.
The document continues at page 25, line 26:
"In addition, the responses that have been observed are
superior when the NSAID (for example, IndocidTM) is combined
with hyaluronic acid demonstrating clearly that the combination is
now "targeting" the pathological tissue even when administered by
the systemic intravenous route. Thus, it has been observed that
patients with neoplastic diseases when receiving in addition to
other chemicals (for example, ascorbic acid [Vitamin C], phloretin
and anti-cancer drugs), 50 - 200 mg NSAID - hyaluronic acid
(sodium hyaluronate) (for example indomethacin and hyaluronic
acid) experience dramatic relief of pain immediately. This is
followed within a short period of time by a resolution and
resorbtion of neoplastic lesions with an improvement of
pulmonaryj and liver function if there is tumor present in these
organs. Thus, the dead tumor material and the debris and tumor
toxins appear to be better eliminated by the body through the action
of the macrophages whose activity is enhanced by the addition of
the NSAID (or a steroidal anti-inflammatory drug) administered
with hyaluronic acid (or salt or other form thereof). Thus
Applicants believe that the addition of the NSAID for example with
hyaluronic acid (sodium hyaluronate) deblocks the macrophages by
preventing enzymatic production of prostaglandin synthetase
which blocks macrophage functioning. Thus the hyaluronic acid
(and salt and other forms) not only enhance the activity of the
NSAID but also reduce any side effects and toxicity that is associated
with the use of the prostaglandin synthesis inhibitors."
3 2 1 73Q37
-
U.S. Applications Serial No. 08/486,328 and 08/520,591 and PCT
Application PCT/CA95/00477, also owned by Hyal Pharmaceutical Corporation,
teach the modulation of cellular activity of tissue and cells expressing a high
affinity cell-surface receptor for hyaluronic acid by the use of forms of hyaluronic
5 acid. These cell surface receptors comprise adhesion molecule ICAM-1, adhesionmolecule CD44 and adhesion molecule HARLEC (Hyaluronic Acid [Hyaluronan]
Receptors Liver Endothelial Cells) and regulatory molecule RHAMM (Receptor
for HA Mediated Motility), for binding hyaluronan. HARLEC is expressed
(produced and put on the cell surface) in liver endothelial cells. The
10 administration of an effective amount of a form of hyaluronic acid to bind with
the cell-surface receptors modulates cellular activity of tissues and/or cells
expressing such high affinity cell-surface receptors for hyaluronic acid (for
example, an adhesion or regulatory molecule) in the human body.
l 5 As stated at page 19, line 30 of the Application, the binding capacity of the
liver has been found to be so great for hyaluronan that hyaluronan when
administered first goes to the liver and if not bound to the liver because the liver
has reached its binding capacity for hyaluronan, circulates in the system and
collects in for example, a tumour because of the tumour's receptors' ability to
20 bind with hyaluronic acid (hyaluronan) as a result of the tumour having excess
receptors for hyaluronic acid (more than normal tissue and cells).
Therefore, if hyaluronic acid is used as a vehicle for a medicine or
therapeutic agent to transport the medicine to a site in the body in need of
25 treatment, unless the combination is administered directly to the site in need of
treatment as by injection into a tumour, much of the combination ends up at the
liver with lesser amounts at the site in need of treatment, unless and until theliver has reached its binding capacity for hyaluronan.
4 21 73037
'~_
In an article entitled "Binding of hyaluronate and chondroitin sulphate to
liver endothelial cells" by Tovard C. Laurent, et al., Biochem J. (1986) 234, 653-658,
the authors discussed the fact that "Circulating sodium hyaluronate (HA) is
efficiently taken up and metabolized by the endothelial cells in the liver
5 sinusoids", and that Chondroitin sulphate (CSA) is also taken up and
metabolized by liver endothelial cells". The authors also state:
"The partial inhibition of HA binding by CSA (Smedared et al., 1984)
and the inhibition of CSA binding by HA (Smedared et al., 1985)
indicates that the two polysaccharides are recognized by the same
receptor. We have now confirmed this hypothesis by the use of
oligosaccharides of identical degree of polymerization prepared
from the two polymers."
I have now discovered that the liver endothelial cells carry at least two
different binding proteins for HA (hyaluronan) including a scavenger receptor
that binds to both chondroitin sulphate and hyaluronan, (and other
glycosaminoglycan (GAGS)) and, the majority of these different binding proteins
(the scavenger receptors on the liver) are inhibited from their take-up of GAGS
20 (Glycosaminoglycans) including chondroitin sulphate (CS) by their being
previously bound to, for example, chondroitin sulphate. I have also discovered
that the sites in need of treatment for example, metastatic tumours do not carrythe same scavenger receptors that bind to chondroitin sulphate. These sites carry
receptors that do bind to hyaluronan. Thus, by first administering for example,
2 5 chondroitin sulphate for take up by the scavenger receptors of the liver,
subsequently administered hyaluronan for example, will not be taken by the
liver.
It is therefore an object of this invention to provide a novel method of
21 73037
treatment of a disease or condition in a human which method permits
substantially more of the medicine and/or therapeutic agent to be brought to thesite in the body in need of treatment.
It is a further object of the invention to target sites in the body in need of
treatment so that any medicine or therapeutic agent reaches the site in need of
treatment rather than being taken up elsewhere in the body (for example! the
liver).
It is still a further object of the invention to provide a novel dosage kit or
combination of materials or chemicals which when used one after the other will
target the sites in the body in need of treatment for delivery of a medicine and/or
therapeutic agent to the site in need of treatment.
It is still a further object of the invention to reduce the amount of the
medicine and/or therapeutic agent that would otherwise be normally expected
to be used for treating a disease or condition. In this regard, if a transport agent is
used to transport the medicine and/or therapeutic agent to the site of the disease
and/or condition, it is a further object of the invention to use less of the usual
amount of the transport agent (for example, hyaluronan and a pharmaceutically
acceptable salt thereof) for transporting the medicine and/or therapeutic agent to
transport the medicine and/or therapeutic agent to the site of the treatment.
Thus, for example, less cytotoxic medicine (for example, methotrexate, cisplatinand the like) will be needed as an effective dosage amount of the medicine to
achieve successful treatment using less than the usual effective dosage amount
of the hyaluronan for example, as the transport agent.
Further and other objects of the invention will be realized by those skilled
in the art from the following Summary of Invention and Detailed Description of
6 21 73037
Examples thereof.
SUMMARY OF INVENTION
I have now discovered that while chondroitin sulphate and hyaluronan
bind to liver cells and particularly, the scavenger receptors on the liver,
chondroitin sulphate does not bind with the receptors on for example, tumours
(for example, metastatic tumours) and particularly, the cell surface receptors for
hyaluronan comprising, the Regulatory molecule RHAMM (Receptor for HA
Mediated Motility), and adhesion molecules ICAM-l, HARLEC and CD44. This
has led me to develop my new methods of treatment of disease and conditions
including metastatic tumours. By "down regulating" the scavenger receptors on
the liver cells by binding them to administered chondroitin sulphate (or other
GAGS [Glycosaminoglycan] such as dextran sulphate, other than a form of
hyaluronan) and subsequently administering the form of hyaluronic acid
(hyaluronan) with the medicine and/or therapeutic agent, the subsequently
administered amounts of hyaluronan (which transport the medicine and/or
therapeutic agent) are picked up, not by the liver whose binding capacity has been
substantially fulfilled but, by other sites capable of binding with forms of
hyaluronan having excess unfilled hyaluronan receptors (such as on metastatic
tumours). At the same time, the hyaluronan transports any medicine (or
therapeutic agent) to treat the sites in need of treatment (for example, by an
effective amount of a cytotoxic agent to treat a tumour). As a result, I have, by
the pre-treatment with for example, chondroitin sulphate, found that less of theform of the transport agent for example, hyaluronan that is to be subsequently
administered is required than previously used. I have also found that less of the
medicine and/or therapeutic agent is required than was used previously to
provide a successful treatment of the disease and/or condition.
7 2 1 73037
The amount of the chondroitin sulphate that will "down regulate" the
liver cells is preferably in the order of at least about 3-5 mg. of chondroitin
sulphate per kilogram of body weight of the patient. However, preferably greateramounts (mg/kg) are administered to "turn off" the liver. Because the liver
processes the administered and "taken up" chondroitin sulphate rapidly, less
chondroitin sulphate is not as good as more, as after several hours the liver has
processed all the chondroitin sulphate. Thus, prolonged "blockage"/"down
regulating" or "immobilization" of the liver cells is preferred. Where
chondroitin sulphate (equivalent dose 1-2 grams/70 kg person) is administered,
the take-up of even small amounts of hyaluronan by the liver (0.5-1 mg/70
kg/person) can be inhibited for an extended time by the administration of the
chondroitin sulphate. Thus, the hyaluronan is available to transport the
medicine and/or therapeutic agent to the site in need of treatment (for example,methotrexate or cisplatin to a tumour or furosemide to a kidney or other use
proposed by the teachings of WO 91/04058 which is incorporated herein by
reference.
The amount thereafter required of the transport agent for example,
hyaluronan or a pharmaceutically acceptable salt thereof for example, sodium
hyaluronate having for example, a molecular weight less than 750,000 daltons
may be reduced substantially (for example, to an amount of substantially less
than 10 mg/70 kg person such as 0.1 mg/70 kg person) and the amount of
medicine and/or therapeutic agent likewise substantially reduced to a mere
fraction of what is normally used previously or proposed to be used previously.
It may be that with the liver shut down, only micrograms (,ug)/kg of the body
weight, of the transport agént for example, 20 ,ug/kg and micrograms (~g) of themedicine for example, depending on the medicine 10 ~g/kg of body weight may
be only required in the dosage.
8 21 73037
.
Other suitable compounds may be substituted for chondroitin sulphate
such as dextran sulphate including other GAGS. Some may be used in
substantially the same amounts as with chondroitin sulphate; others may be
used in higher or lower amounts. Other GAGS may include Dermatan sulphate,
5 Heparin sulphate, Heparin or their Proteoglycan forms, Keratan sulphate.
Keratan sulphate and the like, while not technically a
glycosaminoglycuronoglycan, will be considered to be included as a GAG herein.
Other scavenger receptor ligands may also be used such as acetylated low densitylipoproteins (LDL), acids such as poly-inosinic acid and the like.
Subsequent to administering the chondroitin sulphate (for example, after 3
to 4 minutes) (for example, by intravenous administration) the combination of
for example, hyaluronan (for example, the amounts, forms and molecular
weights taught in U.S. Application 07/675,908 and PCT Application WO91/04058
and Continuation-In-Part Applications Serial No. 08/468,328 filed June 6, 1995
and Serial No. 08/520,591 filed August 30, 1995 (the contents of all four of which
are incorporated herein by reference), together with the medicine and/or
therapeutic agent (whether excess amounts over and above the normally
administered amounts when at least 200 mg/70 kg person of the form of
20 hyaluronic acid is used or the normally used effective amounts of the medicine
or therapeutic agents are used or much lesser amounts (~g)/kg) is administered
by any suitable means. Because lesser amounts of medicine and/or therapeutic
agents and for example, hyaluronan may now be used, the amounts specified
above and in the documents which are incorporated herein by reference may be
25 substantially reduced. Because the amount of medicine and/or therapeutic agent
is reduced substantially, side effects are substantially reduced. Thus, even the 200
mg. amount per 70 kg. person of the form of hyaluronic acid will be reduced. In
fact, with the liver being "blocked", I have found that 5 ,ug/250 mg rat (20 ~1g/1
kg) of hyaluronan targets the site in need of treatment. I have also found that the
9 2 ~ 73037
'
same order of magnitude (~Ig/250 gm rat) medicine and/or therapeutic agent in
the dosage would be useful for treatment of the site in need of treatment. That is
because the liver has now been "blocked". Therefore, the teachings in WO
91/04058 with respect to dosage amounts may now be modified in light of the
5 above for use with this invention.
As stated in Application W091/04058, whose teachings are incorporated
herein by reference:
l O (i) at page 17, line 3 to page 18, line 16:
"Applicants have now discovered that combinations and
formulations (for example an injectable formulation) can be
provided for administration to a mammal for the treatment of a
disease or condition, which combinations or formulations employ
or incorporate as the case may be a therapeutically effective non-
toxic amount of a medicinal and/or therapeutic agent to treat the
disease or condition (for example a free radical scavenger (for
example ascorbic acid (Vitamin C)), Vitamin C (for the treatment of
mononucleosis), an anti-cancer agent, chemotherapeutic agent, anti-
viral agents for example a nonionic surfactant, e.g. nonoxynol-9
[nonylphenoxy polyethoxy ethanol] found in DelfenTM
contraceptive cream, and anionic surfactants (e.g. cetyl pyridinium
chloride) and cationic surfactants (e.g. benzalkonium chloride), non-
steroidal anti-inflammatory drugs ~NSAID) for example
indomethacin, naproxen and (+/-) tromethamine salt of ketorolac
(sold under the trademark ToradolTM) and steroidal anti-
inflammatory drugs, anti-fungal agent, detoxifying agents (for
example for administration rectally in an enema), analgesic,
lo 21 73037
,
bronchodilator, anti-bacterial agent, antibiotics, drugs for the
treatment of vascular ischemia (for example diabetes and Berger's
disease), anti-body monoclonal agent, minoxidil for topical
application for hair growth, diuretics (for example furosemide (sold
under the trademark LasixTM)), immunosuppressants (for example
cyclosporins), lymphokynes (such as interleukin - 2 and the like),
alpha-and-~-interferon and the like) administered with, or carried
in, an amount of hyaluronic acid and/or salts thereof (for example
the sodium salt) and / or homologues, analogues, derivatives,
complexes, esters, fragments, and/or sub units of hyaluronic acid
(preferably hyaluronic acid and salts thereof) sufficient to facilitate
the agent's penetration through the tissue (including scar tissue), at
the site to be treated through the cell membranes into the
indlvidual cells to be treated. When such combinations and
formulations are administered to patients suffering from the disease
or condition, the disease or condition is unexpectedly improved.
The formulation can be administered among other methods,
intravenously, intra arterially, intraperitoneally, intrapleurally,
transdermally, on the skin (topically), rectally, orally or by direct
injection (for example into a tumor, into an abscess or similar
disease focus) or put on a patch to be secured to the skin of the
patient. The hyaluronic acid and/or salts thereof and the agent can
be administered separately but are administered in sufficient
amounts and in an immediate time sequence or interval (preferably
concurrently and more preferably simultaneously), preferably at the
identical site (e.g. one given intravenously and the other "piggy
backed"), to treat the disease or condition."
11 2 1 73037
(ii) at page 25, line 18 to page 26, line 14:
"Thus and according to another aspect of the invention when
an NSAID for example indomethacin (dissolved in n-methyl
glucamine) or other NSAID is administered with greater than
200mg hyaluronic acid for 1 - 2 mg/kg body weight of the NSAID (in
one instance indomethacin and NMG), no major toxic side effects
occur such as gastro-intestinal distress, neurological abnormalities,
depression, etc., even at elevated amounts of indomethacin (if
l O necessary). If the amount of hyaluronic acid is decreased below thatamount, the usual side effects may begin to reoccur. In addition, the
responses that have been observed are superior when the NSAID
(for example IndocidTM) is combined with hyaluronic acid
demonstrating clearly that the combination is now "targeting" to
the pathological tissue even when administered by the systemic
intravenous route. Thus, it has been observed that patients with
neoplastic diseases when receiving in addition to other chemicals
(for example ascorbic acid [Vitamin C], phloretin and anti-cancer
drugs), 50 - 200 mg NSAID - hyaluronic acid (sodium hyaluronate)
(for example indomethacin and hyaluronic acid) experience
dramatic relief of pain immediately. This is followed within a short
period of time by a resolution and resorbtion of neoplastic lesions
with an improvement of pulmonary, and liver function if there is
tumor present in these organs. Thus the dead tumor material and
the debris and tumor toxins appear to be better eliminated by the
body through the action of the macrophages whose activity is
enhanced by the addition of the NSAID (or a steroidal anti-
inflammatory drug) administered with hyaluronic acid (or salt or
other form thereof). Thus Applicants believe that the addition of
12 2~ 73037
-
the NSAID for example with hyaluronic acid (sodium hyaluronate)
deblocks the macrophages by preventing enzymatic production of
prostaglandin synthetase which blocks macrophage functioning.
Thus the hyaluronic acid (and salt and other forms) not only
enhance the activity of the NSAID but also reduce any side effects
and toxicity that is associated with the use of the prostaglandin
synthesis inhibitors.
Examples of agents suitable for use as chemotherapeutic
l O agents are novantrone (Mitoxantrone), Methotrexate, 5-FU ~5-
Fluouracil), carboplatinum, methyl CCNU administered orally and
Mitomycin C."
(iii) at page 26, lines 32 to 37:
"The hyaluronic acid and salts thereof may be utilized at
varying doses - 10 to 1000 mg/70 kg person with the optimal doses
tending to range between 50 and 350 mg/70 kg individual. As there
is no toxicity, the hyaluronic acid can obviously be administered in a
20 dose excess (for example 3000 mg/70 kg individual) without any
adverse effects."
(iv) at page 29, line 27 to page 33, line 31:
"One form of hyaluronic acid and/or salts thereof (for
example sodium salt) and homologues, analogues, derivatives,
complexes, esters, fragments, and sub units of hyaluronic acid,
preferably hyaluronic acid and salts and thereof suitable for use with
Applicant's invention is a fraction supplied by Sterivet Laboratories
13 2~ 73037
Limited. One such fraction is a 15 ml vial of Sodium hyaluronate
20mg/ml (300mg/vial - Lot 2F3). The sodium hyaluronate fraction
is a 2% solution with a mean average molecular weight of about
225,000. The fraction also contains water q.s. which is triple distilled
and sterile in accordance with the U.S.P. for injection formulations.
The vials of hyaluronic acid and/or salts thereof may be carried in a
Type 1 borosilicate glass vial closed by a butyl stopper which does
not react with the contents of the vial."
1 0 The fraction of hyaluronic acid and/or salts thereof (for
example sodium salt) and homologues, analogues, derivatives,
complexes, esters, fragments, and sub units of hyaluronic acid,
preferably hyaluronic acid and salts thereof may comprise
hyaluronic acid and/or salts thereof having the following
1 5 characteristics:
a purified, substantially pyrogen-free fraction of hyaluronic
acid obtained from a natural source having at least one characteristic
selected from the group consisting of the following:
i) a molecular weight within the range of 150,000-
225,000;
ii) less than about 1.25% sulphated mucopoly-
saccharides on a total weight basis;
iii) less than about 0.6% protein on a total weight basis;
iv) less than about 150 ppm iron on a total weight basis;
v) less than about 15 ppm lead on a total weight basis;
vi) less than 0.0025% glucosamine;
vii) less than 0.025% glucuronic acid;
viii) less than 0.025% N-acetylglucosamine;
ix) less than 0.0025% amino acids;
14 2 1 73037
x) a UV extinction coefficient at 257 nm of less than
about 0.275;
xi) a UV extinction coefficient at 280 nm of less than
about 0.25; and
xii) a pH within the range of 7.3-7.9. Preferably the
hyaluronic acid is mixed with water and the fraction of
hyaluronic acid fraction has a mean average molecular
weight within the range of 150,000-225,000. More preferably
the fraction of hyaluronic acid comprises at least one
1 0 characteristic selected from the group consisting of the
following characteristics:
i) less than about 1% sulphated
mucopolysaccharides on a total weight basis;
ii) less than about 0.4% protein on a total weight basis;
1 5 iii) less than about 100 ppm iron on a total weight
basis;
iv) less than about 10 ppm lead on a total weight
basis;
v) less than 0.00166% glucosamine;
vi) less than 0.0166% glucuronic acid;
vii) less than 0.0166% N-acetylglucosamine;
viii) less than 0.00166% amino acids;
x) a UV extinction coefficient at 257 nm of less
than about 0.23;
xi) a UV extinction coefficient at 280 nm of less
than 0.19; and
xii) a pH within the range of 7.5-7.7
Other forms of hyaluronic acid and/or its salts, and
homologues, derivatives, complexes, esters, fragments and sub
15 2173~37
units of hyaluronic acid may be chosen from other suppliers, for
example those described in the prior art documents previously
referred to. In addition Applicants have successfully employed
sodium hyaluronate produced and supplied by LifeCoreTM
Biomedical, Inc. having the following specifications
Characteristics Specification
Appearance White to cream
colored particles
Odor No perceptible odor
Viscosity Average < 750,000 Daltons
Molecular Weight
UV/Vis Scan, 190-820nm Matches reference scan.
OD, 260nm < 0.25 OD units
Hyaluronidase Sensitivity Positive response
IR Scan Matches reference
pH, 10mg/g solution 6.2- 7.8
Water 8% maximum
Protein < 0.3 mcg/mg NaHy
16 2 1 73037
-
Acetate < 10.0 mcg/mg NaHy
Heavy Metals, maximum ppm
As Cd Cr Co Cu Fe Pb Hg N i
2.0 5.0 5.0 10.0 10.0 25.0 10.0 10.0 5.0
Microbial Bioburden None observed
Endotoxin < 0.07EU/mg NaHy
Biological Safety Testing Passes Rabbit Ocular
Toxicity Test
The following references teach hyaluronic acid, sources
1 5 thereof and processes of the manufacture and recovery thereof.
United States Patent 4,141,973 teaches hyaluronic acid
fractions (including sodium salts) having:
'(a) an average molecular weight greater than about 750,000,
preferably greater than about 1,200,000 - that is, a limiting
viscosity number greater than about 1400 cm3/g., and preferably
greater than about 2000 cm3/g.;
(b) a protein content of less than 0.5% by weight;
(c) ultraviolet light absorbance of a 1% solution of sodium
hyaluronate of less than 3.0 at 257 nanometers wavelength and
less than 2.0 at 280 nanometers wavelength;
(d) a kinematic viscosity of a 1% solution of sodium
hyaluronate in physiological buffer greater than about 1000
centistokes, preferably greater than 10,000 centistokes;
17 2 1 73037
(e) a molar optical rotation of a 0.1 - 0.2% sodium
hyaluronate solution in physiological buffer of less than -11 X
103 degree - cm2/mole (of disaccharide) measured at 220
nanometers;
(f) no significant cellular infiltration of the vitreous and
anterior chamber, no flare in the aqueous humor, no haze or
flare in the vitreous and no pathological changes to the cornea,
lens, iris, retina, and choroid of the owl monkey eye when one
milliliter of a 1% solution of sodium hyaluronate dissolved in
1 O physiological buffer is implanted in the vitreous replacing
approximately one-half the existing liquid vitreous, said HUA
being
(g) sterile and pyrogen free and
(h) non-antigenic.'
Canadian Letters Patent 1,205,031 (which refers to United
States Patent 4,141,973 as prior art) refers to hyaluronic acid fractions
having average molecular weights of from 50,000 to 100,000; 250,000
to 350,000; and 500,000 to 730,000 and discusses processes of their
manufacture.
Where high molecular weight hyaluronic acid (or salts or
other forms thereof) is used, it must be diluted to permit
administration and ensure no intramuscular coagulation."
(v) and, at page 33, line 37 to page 35, line 30:
"Thus Applicant has combined hyaluronic acid (and sodium
hyaluronate and/or other forms) with medicinal and/or therapeutic
18 21 73037
agents for the treatment of conditions and diseases with totally
unexpected results:
For Example
Condition/Disease Chemicals & Drugs
1. Cancer, increasing activity free radical scavenger,
of macrophages superoxide dismutase,
ascorbic acid(Vitamin C)
anti-cancer drugs, NSAID,
Chemotherapeutic Agents,
detoxifying Agents (e.g.
cholestyramine)
lA. Reduction of swelling
in brain of Dimethyl Sulfoxide
(DMSO)person suffering brain trauma
2. Hair growth minoxidil - combination -
grow more hair when applied
topically
3. Herpes, canker sore, nonionic surfactants, e.g.,
shingles nonoxynol-9 and
anionic, (e.g. cetyl
pyridinium chloride) and
cationic (e.g.
benzalkonium chloride),
2 5 surfactants
4. Renal failure, cardiac diuretics - furosemide
insufficiency, hypertension,
edema
5. Infection, acne, antibiotics, antibacterials,
mononucleosis antimicrobials, etc.,
ascorbic
acid and hyaluronic acid
6. Transplants cyclosporins
7. Inflammation, elimination of non-steroidal anti-inflamma-
tumor break down material tories, NSAID e.g.
(toxins and debris),
diclofenac,
decreasing side effects, indomethacin, piroxicam,
19 2~73037
' -
relief of pain (e.g. ibuprofen, tromethamine salt
back pain) of Ketorolac, naproxen,
8. Detoxification enema, detoxifying agent,
peritoneal dialysis
9. Bronchodilation bronchodilators, e.g. beclo-
methasone diproprionate
(sodium cromoglycate although
not specifically a broncho-
dialator), theophylline
10. Vascular ischemia treat limbs in respect of
diabetes, Berger's disease, etc. with
suitable medicine e.g. Trental
11. HIV (AIDS) DMSO, Vitamin C, NSAID (e.g.
indomethacin, naproxen,
ketorolac tromethamine),
interferon, VibramycinTM,
(doxcycline), tetracycline
12. Diabetes insulin
13. Post-menopause estrogens replacement
14. Prevention of topical antimetabolites (e.g. infection
sulfonamides)
15. Reduction of swelling DMSO
16. Hypertension, cardiac Calcium channel blockers e.g.
insufficiency- Nifedipine ~-
Blockers e.g. atenolol, propranolol
17. Prostaglandin acetylsalicylic acid
Synthesis inhibition
18. Enhance oxygenation of perfusate
tissue by perfusion fluid
bathing the tissue (for transplantation
purposes"
The above description and proposals will apply herein only modified to
reflect the benefits of this invention. Thus, the administration of the form of
hyaluronic acid and, medicine and/or therapeutic agent described above and
identified in the Applications incorporated herein by reference may now be
preceded by an effective amount (for example, exceeding in the order of about 3 -
2 ~ 73037
5 mg/kg of body weight) of chondroitin sulphate (for example, 200 - 400 mg/70 kgperson). As a result, less medicine and/or therapeutic agent for example, a
cytotoxic agent, and transport agent for example, hyaluronan, than would be
normally or usually used for the treatment, may now be required because the
5 hyaluronan together with the medicine and/or therapeutic agent now goes to thesite in need of treatment (tumour, for example) and is not taken up by the liverwhich has now been "down regulated". Thus, the liver would not be as damaged
by the for example, cytotoxic agent as in the past.
l O The chondroitin sulphate preferably may have a molecular weight
exceeding 20,000 daltons for example, in the order of about between 20,000 and
40,000 daltons although there is a benefit irrespective of the molecular weight of
chondroitin sulphate administered. Preferably, higher molecular weight
chondroitin sulphate is used so long as it is in a dosage form that can be
15 administered effectively (for example, in sufficient sterile water for intravenous
purposes). The dextran sulphate or other agents (such as other
glycosaminoglycans) may have a molecular weight for example, in the range
between about 20,000 and 500,000 daltons or higher provided the dosages can be
effectively administered.
Thus, according to one aspect of the invention, a method of treating a
disease or condition in a human treatable by a medicine and/or therapeutic agentwhich may be transported by an agent (for example, a form of hyaluronic acid
such as sodium hyaluronate) to the site in need of treatment in the body and
25 which agent may also transport the medicine and/or therapeutic agent to the
liver (by for example, the transport agent binding to receptors on the liver) isprovided comprising:
(a) administering an effective amount of a first agent such as
21 21 73037
-
chondroitin sulphate which does not bind to receptors at the
site in need of treatment but which binds with receptors of
the liver thereby "down regulating" the liver;
and,
(b) thereafter (for example, 3 to 4 minutes after the
administration under sub-paragraph (a) of this paragraph)
administering an effective non-toxic amount of a medicine
and/or therapeutic agent and an effective amount of a second
agent which is a transport agent [for the medicine and/or
l O therapeutic agent] and is a different agent from the first agent
(for example, a form of hyaluronic acid) and which second
agent binds to the site in need of treatment and would be
capable of binding to the sites of the liver if the liver had not
been "down regulated" so that the liver's binding capacity for
the second agent has been substantially reduced (preferably
substantially eliminated or blocked) by the up-take by the
liver of the first agent (for example, chondroitin sulphate
Molecular Weight 20,000) administered under sub-paragraph
(a) by for example, binding with the scavenger receptors of the
2 O liver .
Because the liver has been "down regulated", the amounts of medicine
and/or therapeutic agent that may be effective to treat the site in need of
treatment is substantially reduced. As well, the amount of the for example, form25 of hyaluronic acid (transport agent) is substantially reduced so that the effective
amount is substantially less than 10 mg/70 kg person (for example, 20 ,ug/kg of
body weight of the patient being treated).
According to another aspect of the invention, a method of protecting the
22 2 1 73037
'
liver from taking up medicines and/or therapeutic agents toxic to the liver whenadministering the medicine and/or therapeutic agent to a site in need of
treatment, is provided comprising:
(a) administering an effective amount of a first agent such as
chondroitin sulphate which does not bind to receptors at the
site in need of treatment but which binds with receptors of
the liver thereby "down regulating" the liver;
and,
l O (b) thereafter (for example, 3 to 4 minutes after the
administration under sub-paragraph (a) of this paragraph)
administering an effective non-toxic amount of a medicine
and/or therapeutic agent and an effective amount of a second
agent which is a transport agent and is a different agent from
the first agent, (for example, a form of hyaluronic acid) and
which second agent binds to the site in need of treatment and
would be capable of binding to the sites of the liver if the liver
had not been "down regulated" so that its binding capacity for
the second agent has been substantially reduced (preferably
substantially eliminated or blocked) by the up-take by the
liver of the first agent (for example, chondroitin sulphate,
Molecular Weight 20,000) administered under sub-paragraph
(a) by for example, binding with the scavenger receptors of the
liver.
The amount of the first agent administered under (a) for example, in the
order of at least about 3 - 5mg/kg of body weight (for example, 200 - 400 mg/70 kg
person) having preferably a molecular weight in the range of 20,000 to 40,000
daltons, may be administered by any suitable manner such as systemically for
23 21 73037
'
example, orally, intravenously, subcutaneously or by direct injection proximate,adjacent, or into, the liver (by direct administration into the hepatic artery).Thereafter, (for example, after 3 to 4 minutes) the amount of the second agent for
transport in sub-paragraph (b) for example, sodium hyaluronate having a
molecular weight less than 750,000 daltons is administered in an effec~lve
amount now found to be substantially less than 10mg/70kg person discussed in
WO 91/04058 together with the medicine and/or therapeutic agent. The at least
200 mg/70kg person of for example, the sodium hyaluronate provided in
Application WO91/04058 to reduce the side effects of the medicine and/or
1 0 therapeutic agent may now be substantially reduced because the amount of themedicine and/or therapeutic agent that is now effective is substantially less than
previously provided. Thus, less of for example, the form of hyaluronic acid may
now be administered in a dosage together with a lesser amount of what is now
an effective amount of medicine and/or therapeutic agent to reduce the side
effects of the medicine and/or therapeutic agent. Thus, the amounts of the
medicine and/or therapeutic agent and hyaluronan transport agent may now be
(,ug) microgram amounts per kilogram of body weight to be effective. .
The first agent may be chondroitin sulphate (preferably) or other suitable
agent (such as dextran sulphate or other GAGS [Glycosaminoglycans] and/or
their proteoglycan forms which are not a form of hyaluronic acid). Other
scavenger receptor ligands which are effective may also be used as the first agent.
The second agent is preferably a form of hyaluronic acid such as hyaluronan or
sodium hyaluronate.
Therefore, according to another aspect of the invention, I have provided a
dosage kit for maximizing the amount of medicine and/or therapeutic agent to
be delivered to a site in the body in need of treatment and/or for protecting the
liver from taking up medicine and/or therapeutic agent (particularly cytotoxic
24 21 73037
agents) when medicines and/or therapeutic agents must be delivered to treat
sites other than the liver, comprising:
(a) an effective dosage amount of a first agent such as
chondroitin sulphate which does not bind to receptors at the
site in need of treatment but which binds with receptors of
the liver to thereby "down regulate" the liver;
and, separately from the dosage amount of (a), and,
(b) an effective dosage amount comprising an effective non-toxic
dosage amount of a medicine and/or therapeutic agent and
an effective amount of a second agent which is a transport
agent and is a different agent from the first agent (for
example, a form of hyaluronic acid) and which second agent
will bind to the site in need of treatment and would be
capable of binding to the sites of the liver if the liver is not
down regulated so that the liver's binding capacity for the
second agent has been substantially reduced (preferably
eliminated or blocked) by the up-take by the liver of the first
agent (for example, chondroitin sulphate Molecular Weight
20,000) when administered after for example, at least about 3-4
minutes after administration of the dosage amount of the
first agent under sub-paragraph (a) by for example, binding
with the scavenger receptors of the liver.
The dosage amounts for sub-paragraph (bj may be microgram
(,ug) per kilogram of body weight for example, 20 ~g/kg.
Further, according to another aspect of the invention a method is
provided, comprising:
25 21 73~3~
(a) administering an effective amount of a first agent which does
not bind to receptors at the site in need of treatment but
which binds with receptors of the liver thereby "down
regulating" the liver;
and,
(b) thereafter administering an effective amount of a medicine
and/or therapeutic agent (for example, an NSAID [non-
steroidal anti-inflammatory agent] or cytotoxic agent (for
example, methotrexate and cisplatin and combinations
thereof) and an effective amount of a second agent which is a
transport agent and is a different agent from the first agent
and which second agent is a transport agent which binds to
the site in need of treatment and transports to the interstitial
fluid, lymph and lymph nodes, and would be capable of
binding to the sites of the liver if the liver had not been
"down regulated" so that its binding capacity for the second
agent has been substantially reduced by the up-take by the
liver of the first agent administered under sub-paragraph (a)
by binding with the scavenger receptors of the liver.
The first agent may be chondroitin sulphate and the second agent may be a
form of hyaluronic acid. The amounts of each may be as previously discussed.
For example, the amount of chondroitin sulphate may exceed at least about 3-
25 5mg/kg and the effective amount of the form of hyaluronic acid may exceed 0.1mg/70kg person and may have a molecular weight less than 750,000 daltons.
The invention will now be illustrated by reference to the following Figures
and examples and discussion with respect thereto:
26 2 1 73037
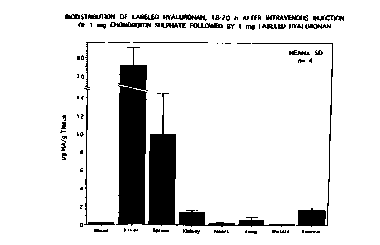
Figure 1 illustrates the Biodistribution of Labeled Hyaluronan, 18-
20h after Intravenous Injection of 1 mg Chondroitin Sulphate Followed by 1 mg
Labeled Hyaluronan;
Figure 2 illustrates the Uptake of 1 mg Labeled Hyaluronan (HA)
With or Without Preinjection of 1 mg Chondroitin Sulphate (CS);
Figure 2b illustrates the Uptake of 1 mg Labeled Hyaluronan;
Figure 3 is made up of two drawings, the top drawing comparing
MCPM/rat v. Time (min.), and the lower drawing comparing the MCPM/organ
lO when 1 mg of chondroitin sulphate was administered followed by l25I-HA (l25I- Hyaluronan).
Figure 4 illustrates the inhibition of labeled hyaluronan (HA)
binding to NGW cells at 37~C. (The chondroitin sulphate does not interfere
whereas the labeled hyaluronan does.)
Figure 5 shows in vivo images of the reduced liver uptake of labeled
hyaluronan (HA) after pre-treatment of rats by chondroitin sulphate (CS).
Figure 6 illustrates the targeting of tumours by trace amount of
labeled HA (hyaluronan) after administration of chondroitin sulphate (CS) in an
effective amount of 200 - 400 mg/70 kg person of CS.
A summary of the data I have developed on tumour targeting using
radiolabelled hyaluronan (HA) administered after chondroitin sulphate (CS)
pretreatment is set out in the Figures and is discussed below. I used female
Wistar FU rats (weight in the order of 250 gm per rat on average) inoculated in
one hind leg with a rat colon carcinoma called NGW to develop the data. When
the tumours appeared, the rats received an intravenous injection of 1 mg
chondroitin sulphate (200 - 400 mg/70 kg person) followed 30 seconds later by 1
mg HA (hyaluronan) of low specific radioactivity. 18 to 20 hours later the
animals were sacrificed and the radioactivity in the organs measured (see Fig. 1).
27 21 73037
I have with the same model used 1 mg HA (hyaluronan) of similar specific
activity alone and found a tumour to non-tumour ratio of 7.79+5.00.
Using chondroitin sulphate (CS) pretreatment the ratio is increased to
16.23+2.48. The increase is mainly due to a lower uptake in the non-tumour
tissue (muscle of the healthy leg) but there is also a 27% increase in total amount
bound (see Fig. 2).
The individual values show that 4 out of 5 "controls" have a tùmour to
non-tumour ratio of about 4 (the relatively high ratio of 7.79 is due to one single
experiment with a high ratio). In earlier published studies with labeled HA
alone, I have seen tumour to non-tumour ratios of about 4 using similar, but notidentical tumour systems. See "Accessible hyaluronan receptors identical to
ICAM-1 in mouse mast cells", Stefan Gustafson, et al., Glycoconjugate Journal
(1995) 12:350-355. Therefore, I have now developed a real improvement using
chondroitin sulphate (CS) to the delivery of medicine and/or therapeutic agents.- The administration of chondroitin sulphate (CS) effectively blocks liver uptake
of labeled HA (hyaluronan) at 10-15 minutes by about 80% (see Fig. 3) (without
chondroitin sulphate (CS) the liver would have absorbed 95% of the radioactivityat this time).
Invitro data-also developed by me establishes the NGW tumour cells
have HA (hyaluronan) take-up receptors that are not inhibited
(blocked/immobilized for a period of time from taking up the second agent (for
example, hyaluronan)) by chondroitin sulphate (CS).
Figure 4 illustrates three determinations which clearly show that the
uptake of radiolabelled hyaluronan is not interfered with by the chondroitin
28 21 73037
sulphate but is interfered with by the unlabeled hyaluronan.
Composite Figure 5 shows in vivo images of the reduced liver uptake of
labeled HA after CS pre-treatment of rats. This figure shows that also uptake of5 trace amounts of HA (equivalent to 0.5-1 mg/70 kg person) can be effectively
inhibited for an extended time by CS (equivalent dose 1-2 gm/70 kg person).
Thus, one may use low amounts of not only active drug (medicine and/or
therapeutic agent) (~g/kg) but also of HA (~g/kg of body weight of the patient).Thus, low amounts of HA (much less than 10 mg/70 kg person) can be used as
1 O the transport agent. Thus, I have developed a treatment whereby a low dose of
HA and low dose of active drug (medicine and/or therapeutic agent) that really
targets the site of treatment (for example diseased tissues) which is best has been
provided and which also reduces side effects.
l 5 I have also performed experiments on tumour rats using 1 mg CS (200 -
400 mg /70 kg person) followed by a trace dose of labeled HA (equivalent to 0.5-1
mg/70kg person) and seen good targeting to the tumour (see Figure 6). The
experimental conditions are identical to those previously described for NGW
tumour rats, except for the low dose of HA. The relatively high radioactivity in20 the control muscle is most likely due to circulating degradation products at this
time point (about 20 h after injection). Even so, the tumour to non-tumour ratiois 8.8 (p<0.001, n=4), showing that the targeting works also at lower HA doses.
Some preliminary size exclusion chromatographic studies were conducted
25 on the Mw (molecular weight) distribution of radiolabeled HA (hyaluronan) in
serum and urine of rats receiving a pretreatment of 5mg CS and then 5 ,ug 125I-
HA (hyaluronan) with a molecular weight of about 400 kDa (400,000 daltons).
Blood was collected at 2-4, 10-12, 22-24 and 70 minutes after the completion of the
administration. Urine was collected from the bladder at 70 minutes when the
i 29 2 1 73037
animals were killed and organs collected. The results show that the Mw
(molecular weight) of hyaluronan goes down (reduces in the body) by time and at
70 minutes, most of the hyaluronan that is left in the circulation has a molecular
weight less than 39 kDa (39,000 daltons). At this time, about 10% of the injected
5 radioactivity was found in the urine and had a mean Mw (molecular weight) of
about 20 kDa (20,000 daltons). The radioactivity in the liver was only 6-7% while
kidneys contained about 9%, I believe, due to material being filtered out into
urine. Blood contained around 35% and as other organs contained only
negligible amounts, about 40% has been filtered out into peripheral tissues. ThelO chondroitin sulphate (CS) blocking is therefore an ideal way of getting some
hyaluronan out into the tissues. This is an additional factor in the increased
binding of intravenously administered hyaluronan (HA) to tumour tissue that I
found using chondroitin sulphate pretreatment. This provides a further method
of delivering a form of hyaluronan (HA) together with a medicine and/or
15 therapeutic agent into interstitial fluid, lymph and lymph nodes for the
treatment of disease for example, cancer and metastases. This treatment may alsobe used to prevent metastases.
21 73037
_ 30
Hyaluro~ thYaluron~c acid; ~IA) is a high mC~ Ar weie~t poly~a~ ;~ concicti~to of
~pç~in~ uDit~ of ~ ."~ ;r acid a~ld N uctyl~ o~ . It i~ found in high con~ - rlions
in ~onn~cr~ tlssue~ such ~ skin and ca~il~, in the vitreou~ body of the eye ~nd in synovi~l
d (1) The pGl ~ c~ With sevcral prote~n~ in the extracellular matrix and
also wi~ so~ ccll-sur~ace HA-bi~ding p~oteins ~2).
T~e seram lervel of HA is nonnally very low (10-50~g/1), but elevatet i~ certain disease state~
such ~ .1.~ ;d ~thritis, live~ ~ho5i~, ~d v~iou~ ms~ (3). Circula~ng
hy~uro~ con~e~ from ~o pc.;~hc~l tissues ~vhere ~ost i5 as~ated with ~ or binding
h~At some e7~sts in f~eely ~obili7~d compartmcntS. The ~l~;a~ ~h~r~de e~ters thc
ge~cral ci~ula~ion via ~c Iymph (4) aftcr 8~90 % is ~Gn. ~ A in lymph nodcS before, ~ -hinc
the bloodstream (S). The ~Iw in ~er~ is in the orter of 1.5x~0~ whilo tho Mw of HA in
lymph i~ about ~xlO6 t6). The major ~te for eliminadon o~ HA from the ~ dst~n~, undler
normal ~ r~ It~ S~ i5 via rtc2~r m~A;atPA ~d~ytosis by the li~er (1,7). T~e t~2 of
h~ ..uu~ nio~ered l~y~i~u to e l ...._.,~..l anima~s ~t m~ is ~Il ~o order of a few
minuteJ, and already Rer 15-20 n~in ~e dcgMda~on p.u-lwl~ 9tl~t to appel~r in the c~ ti~
(7- 9). The UPta]U i4 Via COa~et Pi~ ~nd COated Ve~iC1e~ h liver ~ colls a.EC), Whi1e
K11PffOr Ce11~ and l~cp~ s 8rO eQQ~11Y nega~i~e fOr ~Iptake both in ViYo ~t ~ vitro (9-
11~.
The HA ta1~en UP bY LEC iS ~pOr~d tO 1~G~b~ 3 Wh6X it iS d~g~ tO ~C e;~ A
that ~]t - -t-'~Y ~re l~roke~ tOWn tO C~rbOn tiOXide, Urea ant WatOr i~ the ~F ~ 2).
Inhibiti~ dia wi~ EC ~n culture sho~v tbat t~e r~ce~ reCO~ Other liga~ds be~ide~
HA, ~uch ~ c~uJ~iti~ sulphate (CS), dcx~ s~h~e(DxS) and desulphated CS (13-lS).
Wo ha~e n~w ~v~ r4 inhibition studios w~th poly~ oT~ 3 that m~t bmd to t.he HA
~ t ~ on LEC in vivo,s~t c~ in the present paper show that CS and l~S. but not hepar~n,
inhibit ~he cb~rs~ce of HA by the liver. The HA that ~ema~ in tho circul~o~ i~ brolcen down
to ~ agment~ by what ~ t~ be a speci~lc ~d ~ahrablo ",rr.~
31 . 2173037
.
collrP~ on~ of HA i~hibit this d~ ti~n ~hi9 ~ 5m could explain why circulati~g
H~ has a much lower Mw th~n the HA C~ ~e general circula~o~ via the Iymph.
S~ inn of thig r~rrh~ s~ could also explain ~vhy e~ ~ei~ high cixulating levels of HA
ca~ gi~e r~se to i~ ,l pla9ma v~cosi~ from hi~h Mw HA.
32 2 1 73037
-
Materials and methods
Po~ss~char~ 'rhe HA uset ~or 1~bçllin~ d upt~ d tw~G~ smdies wa~ s~pplied by
COI~ n~l ~EIPC), Toro~to, Canada. The ~ c~ wcight tistnbution
of thc HA wa~ ~).h -~ cd by ChrOl~tGgr~p~ on a c~librated colum~ of Sephacryl'HR w~h
po~ositiw notcd as 400, 1000 and 2000 (F -~ia. Uppsala, Swcdcn) in 0.25M NaC1,0.05% chl~ ol (16). The HA content ~n each fraction wa~ l.,ol~lur~d by ~-t~ I ";"gt;on of
~e absorbance at 214 ~m. R~ was mea6u~ed by gamma-c~ntin~ on a P~t au~
g~nma ~ - counter.
Cho~d~vl~n s -lrh-'~ A from ~ovino trachea ~v~ from Sigma C~ C~ 'l'pel'ly, St Loui~,
U.S.~. (product number 8529). This batch csl~tai~)~A 1.9 ng HA/ 1lg CS ~ ~ ned by a
specific radioassay for HA (HA-50, Pham~ , Upssala, Sweden).
ncxtr~l sulphate with a Mw of appro~ ty 500 OOODa ~vas from ~ Biotech,
Upps-l~, Sweden ~C~te No. 17~0340-01).
~Iep~ ~om inte~tinal mucosa and purified by ~epea~ed p~ P~;on ~ith cet~lpy~diniu~
c~ (17) wa~ a kind gift from ~ r.~ w T ;n ~ 9J I U~ of UppRala, Sweden.
T.~ f ~ CS ~ncl hepan~: The HA, CS a~d hepa~n was l~h~lle~l with DL-ty~osine
(Sigma c~ ~ 1 compa~y St Louis, U.S.A) as ~ io~lily ~esori~ (18), after CNB~-
acti~a~ion of the polysacc~aride. Bnc~y, lS mg HA. CS or hep~ wa~ at~d at pH 11 by
8 mg CNBr for S ~in. 'rhe activated poly~acch~rido was separated ~om the
a sm~ll colum~ OI Sc~hAAI~- G2S ~ 10, ~ia, Upp85~ Swedc~ ilibratet wit~ 0.2
M borato buffe~ pH 8Ø The activated poi~ h~rl~Ç was ~ncuba1ed ovor n~ght with 1 nlg
tyros~e ~) (siema cl~r~ company, St Louis, U.S.~). Tke T bound to H~ (T-HA), CS
' 2 1 73037
(T-CS) or hep~n (T-Hep) was sepa~ated from ~ kuu~d T on a Pl~ 10 column cqu~librated
with phosphate buffered saline (p~ 7,5) (PBS), CQntPining NaCI (8 gl), KCl (0,~ g/l),
KH2PO4 (0,~ d Na2HPO4 (1,15 g/l).
A part o~ the T-HA, T-CS or T-Hep wa~ loaJIut~d tt~ 25I by placi~ O~g of T~ elled
E,ol~ - - c~ t~F..,~ r with O.S mCi l25I i~ a 9mal1 glass hlbc co~re~d with a film o~ lO~g
1,3,4,6-~r~ehloro-3a,6a-tiphenylPl~ou~l (Sigma ch~rnicsl company, St Loui~, U.S.~.).
U~ c~po~ w~s ~e~o~,cd oll a PD 10 colun~ cq~ hr~d with PBS and the ioAin~t~l
T-HA (l~5I-T-HA), T-CS (l25I~T-cs) or heparln (l25I-T-Hep~ stored at S C. ~he SpCCiflC
radioac~vity wa~ u~ually lSOO-5000 dpmlng.
Tne l2~I-T-HA kept a high ~ ul~r wcight-profile upo~ gcl filtration c~v~tu~aphy with a
mca,~ Mw of arount 0.5~c106 Da, and wa~ found to be cleared from thc e~ t;~n w~th tbe
kinctics and orga~ Ai~ ution repolb~d forbio~yhlhct~cally labeled HA o~ Mw. The l2SI-
ls~ .A T-~IA w~ ~l~o talce~ up by isol~d rat liver P~l~ioth~ alls both in v~o a~d in ~ ro,
;n~ ~ the l9l.e~ not ;.-h - f~ -~ci with the birlting ,ho speci~ic cell-surface .
found on ~e cells (1, 2, ~11).
The l2~ r-CS a~d W-T-Hep was, by ~el filtr~tion c~u-.,-~g. aph~r on Sepha~.lyl S-1000 and
S-300 calibratcd with HA s~ ~P~, found to have thc same mcan Mv~ as the u~ belcd CS
v~ 30 000 Da) ant hcpann tapprs~im~ b 20 000 Da) a~d showed similar s~ze
Cell~: A s~ngle cell s~ epc ~ n was p~epared from ~4e liver of Spra~ue Dawley rat~, weighing
200-300~, by cQlls~l~ perfusion for 10 minute~ at 3~ ~C. Liver en~th~liRl cell~, Kupffe~
ceL~ ~md p~;l~l cell~ we~ pu~fied by PG~COII J c~ r~.O~O~ ~d s~.cc~ P.dh~"r~
de~ et by Pertoft nd S~cdD~d (19), giYi~g ~ u~ ~- 9~ 95% purc coll~ ~10, 19).
~ A10~r c~ woro ~s;n~s;nPd u~dor ~t~r1 c~ g CQ~ A;~
21 73037
34
SupplGm~t~ with L-glu~ (2 mM), gentanuci~ (50 ~u~Jml) a~d, in the case of
p~ cl~y~al cclls, 10% (vlv) fetal calf scnum. Livcr cn~i~t~e~iPl ~ells wcre cultured entirely
without se~um, All cells wcrc cultivatcd over n~g~t beforc the ~ta~t of the e
Uphke .~t~ G with cells in ~ e~ T-hyaluronan, and in CO.,,l,G~;I tn~ e,~
la~ d L~o}~sr--hsru~ were added to cold RP~ i ~e~ c~ h ..;1~, L_gl..l,..";"F. (2 mM)
a~d g~ "~ (S0 ,ugl~l), a~d given to cultures of 100 00~200 000 livcr, ~-A~ cells ~
fil"u~ec~in-coated di~hes with a ~S~m~t~r of 16 rnm, l'he culture~ were kept u~der ~d&rd
cltlh~ tg e ~~n~ s ia 3~ m~ t
After the te~~ tiQn of the ;n~ubation~, the m~A;um was re~o~ d analyzet for
radioacti~ity, lhcr~l~r it was i~ some e~l c- :",r~rit~ subje¢ted to g61 cromGt~ hy 0~ a 24 rnl
Sop~yl 300 column to separate ~ from ~ F~ ,~h~ ~c cells washed
three ~mes in p~sph~ bufr~.~d saline (pH 7,5) ~PBS), er-~t~ ~""~ NaC 1 ~8 g~l), KCl (~,2
gll), KH2PO4 ~0,2 ~) and Na2HPO4 (1,15 gl), anal~zed for mdioacti~ , or hu~o~cluzed
and L~rtion~çd a~ de~cribed earl~er (18). U~sp6clflc bhdin~ was co.~led for by
m~JdlJ~G~ of radio~cti~ity ~socia~ed to dishes ~vithout cell~, which gene~ally Yva~ ju~t above
b~ ul,d levcl~ .
Tn ~ivo 9h~ a~ Sprague l~awley ra~, wcighing 200-300 g, were P~-e~th~t;7~ wit~
pentobsrbi~l (45 mg/ l~g bod~ wcight). rney ~ce.~cd an i~jc~t;o in tho tail Yein of S,ug
T h~l~oll~ ar l2~I-T-Hep (8-1S~ 6 cpm), in 0.8-1.0 ml 0.1S M NaC1, 10 mM
NaH2PO4, pH 7.4. In some s~udies ~e ~ r~ce;ic~ 1-Smg ~nlr~led polysa~ s~ s 30
s~d~ prior to s~e labelled po1ysac~h~ 100d samples were ~ h~ ly CC~ t~ i~tO~II the
di~tal p~t of tho tail dur~ng the c~rc~ t;nn per~od. ~ 60me c~ses senum w~ subjected to size
e~cludon chroma~l~ on a Sephacryl S 300 and thc radioactivi~r of i~c duate ~l),~cd.
21 73037
'
After 10min-22h the rat was killed. I.i~rer, lungs, kid~eys, heart, spleen and in some inS!~ 5
ur~e were assayod for radioactivity. The dah were p;o.essed using a hrat~-n~osh S~300,
Maci~to5~ or ~-~tosh 7200 cu ~ pple co~l ..te. I~c, Cupcrtino, CA, U.S.A.).The graph~ werc corlstructed using the Cricl~et Graph~ y.v~la~ (vergio~ 1.3, Crick~t
6~t . ~, Malvern, PA, U.S.A.) ant CauYas (version 3Ø2., Deneba Sy~tems Irlc, ~ami, ~71,
U.S.A.). St~t;o~irsl ana~ysis was pe F~- ",1~ u9ing Sta~ tYersion 1.1, Crickct Software,
Malver4, PA, U.S.A.).
~;ntl Ai~..c The rats wore an~ and injected as dc~r~ cd above. In ~ua~lc
studios the ~.jc,c~o~ were made with tho rat~ placed o~ a ~uji ph~pho;~ er sc~cen with a
high :J:~UtiQ~ ~r~ collimator ~.,t~ rat and screerl. ~he scree~ w~ ~ ,~9~ ~or 10 m~n
a~d the imagc de~dop~d and analyzed on a Puji 2000 ~ko~Fh~ gcr. So~ ~ma~e analysi~
~tudies woro p~. r~ d u~ing the NIH Imap ~. n~
2~ 73037
36
Results
A tracer dosc of i"h~ O~ y 2~mi~ictPred 125I-T-~ was by phosphoim~ shown to be
rapstly cbaret by the rat liver (Pigls A ;m~ll amount of radiou~i~, att~ibutable to small
""""".t. of ~ r lost from thc labeled pol~ ec~ could be visu~i7c.d in thc urinary
bladder (Fl~ lS.Dudng 22 ~, a~s,~;...~t~l~ 20% of the ~d;cectivi~y di~ ~d from the liver
~md could bc foulld ~ the url~c (bedding in thc ca~).
Whcrl CS, at a dose of about 20 mg/kg body weight, was ~ 1 prior to ~ T-HA the
rapit c1~e ..nce ws~ lost and thc major part of ra' ~~ vity could be vi~u~1i7~d sc~ ed over
t~e entire anim~l for hours (Fig lS. The r~dia~ctiv~ty ~r CS bloc~in~ wa~ m~y found in th~
blood with some up~e ~ liver, splecn and kidrley (Pi~ 2S ~nd wa~ fouut to rapitly dr- ~
in Mw (~i~ 3~. So~ labclled polysacchandes with ~w of about 10000~0000 Da were fou~d
nne ~Fig 2'and 3~. Only minute ~Vu~l~ of labelled HA coult be fou~t ~n thc ~Irine when
er uptalce was bloekcd by ~nl~bel~d HA (1 mglkg b.w.) (~ ). The rapid d~n~ In Mwof c;~ ati~ T-HYA seen afte~ Cs bl~~ g was not secn with HA b';r~no alld rnore
r~dio~nqr ~hycd within ~e Fneral circula~o~ afte~ HA bl~ç~ ing d~ a~.ee CS 1~1nr~jo~ (Flg
2 ~d ~ig 4). However, trace ~ n~ of radio~ctivi~r could bo fount in tbe ~ne af~cr 70 m~,
t~is ~ato~ ad the same M~l;v as the radioactivity fourld in urine s*er Cs bl~c~ (~gs 2, 3
ant 4).
DxS wa~ found to e:f~ ely bloc~ liver uptake at a dose of 200 mg/k~ b. w., and result in
inc~oaud outflo~v of labeled HA from the general circulation a~ ~een by low Uver uptal~e and
low .uco.~ jec~ed HA (Fig S). Wheu a dosc of 1 mg/kg bw. wa~ tested it was ~ouIld that
the li~ror uptako wa~ inhihi~ by 30~0~o (Fig ~S
Hepanu at a tosc of 20 mg/kg b.w. did ~ot ~ffect the clea~ance of 125I-T-HYA (Fig 2), ~or
could HA at a dos~ of 20 mg/kg bw. inhiblt ~hc et ~ - 0~ t2~I-T-Hep at a traca dose (fig 6~.
CS could parda~ly iAhibit tbe liYer uptake o~ T-Hep (Pig 6S.
37 2 ~ 730~7
The b~ndlng of ~ RYA to LEC i~ culeuro wa9 effec~vely inhibitod by H~, CS ~nd DxS
~Fig 7S
Whon the b;o~istributiQn of inh~h~OUS l2sI-T CS was gtudied it was fou~t that the livcr
uptalco wa~ lowor than for ~ T-HYA, a~ wa~ thc total ~~co~ o~ ratioacti~vi~,'while the
un~nary CA~ 3 was high (E~i3 85. The liver uptako could ¢~tiiitul~ be inhibited by ll~laboled
CS ~nd H~ s 1~; ~8 in ~.~a30d u~:y cl~ e (~ig 8)
38 21 73037
_
Dlscus~ion
. .
That the receptor mediatct cndoc~a;s of HA by LEC is ~ot specific for HA has earlier been
show~ by c~ w~th isolated L~C in culture (13~15). I~ such studies the .cic~pt~ ~ also
~ e ligand~ such ~ CS and r)xS. The p~esent i~ves~gatio~ wa~ p~ F~ d in order to see
if ~omo llc~ti~ ch~rgcd polys~cch~tcs ~ n~ ~nGe the ~o.~,. of cir~u!~ HA in
v~vo.We ha~re u~ed a l~be~ tech~ique that does ~ot ~lter the Mw nor i~ with the cdl
binding ~.opo.l cs of pol~;a: :h~ and resulh in a teriYativc wit~ y-radiation of hi~h
spcciflc activity ~18, 20-23). Such a poly6acch~rido is ~d~ ant~ many .~s~ eg. it i~
ea~y to detect in low amollntg an.t the d;~lri~ ~I n~ can be ._co~dot in thc li~o ~nimal u~ng
S~:r~ h;r o~ p~sFh~ g t~. h" ~ s~ We choose the rat for th~o 6t~tio~ a~ many
L~,ove, and uptako studias havo earlier bee~ ~c.~....ct wi~ ~8 spocies and nosmal blood
bvob ~t atimated t~uo~_. r~tu ar~ dmilar to tho ones found in m~n (1, 3, 9,10, 24).
Our studio~ ~how that CS and, l:)xS, but not heparin, iohibit the ck~ rc of HA from the
bloo~3~hoam via i~h;bi~i9n of tho ~Jt~.r m~ ted c~toc~,to~is by tho liver (Pig 1 and 5~.
ee~u~ h bo ck~red by m~l~io~s not affected by ~IA, while CS seem~ ~o par~ally
reduce tb,e liver upt~e of labeled hoparin (l~lg C~. but the inhi~itil~n lS only pa~al and not as
e~ tho blocking o~ labeled H~. As ~ result of CS or l~xS bln~ of li~e~ HA~t~c HA that ~in~ o c;~l~'ion is rapidly broken down to sm~ller fralr~ "
by w~ 9Ce~U to be a ~ nd 4a~blc ~ .; .., u high CQ~ ations of KA inhibit~
~i~ dcgr~dadon (E~l~ 3 and 4). l'he h ~-~ tion re~ in low recovery of i~ected to~ t
the low Mw H~ et out illtO the ti~su6~ ant ~ia the kldneys out into the un~e ~ 3
and 4). ~Ve ha~rc to~c thc ~izc d~h~ n of cifc"l~ti~ mst~i~l b~ s~e ~rl~iQn
chrom~togr~phy of sen~ ar pla~ o~ a col~ o$ Se~ha~yl S 300. Thi~ ~d will not ~eparate
HA abo~o 50 l~Da ve~y well ~o it i~ po~siblo thst thc b~wn of tho infectet ,..~ with a
M~v of about 400 kOa L~ brokon down by Jhear force~ to a Mw above 50 lcDa whe~ t~n~ PA
~ is ~ive~ to reduce livcr upt~ko. However, the brealcdown to ~m~116r rr.~ t~ re~
the ~p~co of m~d chmmatq6.4~d~ on, OCCllr8 e~rly af~ je~
39 2 1 73037
only in the case of CS bloc~ing ant not to any great extent when u~ heled HA ~ usc~ a~
blselr~ agellt (Fi~ 3 and 45
l~iat CS and HA arc recogr i7rd by the same ~e~r ~ in tb~ er i~ o show~ by thc fact t~
not o~ly llnl~hel~ CS but also ~IA c~ ibit the Ihrer uptake of 12sI-T-CS (Fig 8~. That thc
liver upta~e of CS is not as hi~h as t~at of H~ probably ~ on the ~ast that thc CS used
only ha~ a Mw of ~round 30 ~a compared to about 400 kDa for thc HA, ~nd some is Lh~ .fo~
xapidly .~,~o.~ from thc c~ulation by filtration so that only ~ ~action of t~t injectod remains
long e~ough ~n ~e general ''Uc~ tir~n tO be taken up by ehe li~er~
D~S seem~ also to bind to tl~e samc r~p~ol.~ as Cs ~nd HA but wi h lo~ver af~nity as a higher
dose i6 nceded eo i~hibit li~er uptake of 125I-T~HA win~ D~S than using CS ~r HA (Pig 5~
Our ~esult!~ indicatc that the lul~o~ of tbe nan~rally OC~ pol~acc~arida ~A ~d CS are
par~ally an cffoct of li~rer uptake of tbe circ~lln~;~ pol~sacch~;~c3 vi~ a co ~ L~C6pti;~ on
LE!C. ~t is t~(~refi~ ~ possible, t}lat high level~ of c~rculating HA i~ ~o~ co~ c~ bo
secon~ary to i~crcascd outflow of CS into tho ge~eral circulation from thc tissuos. and vicc
ven& Th~ pre6cnt rosul~ also arguo, tuo to the p,~s- -~e of a moro SpWiflC tegradadve
r~ccha~ism of HA ul the circulation, that if thc IEC arc blockct by CS, thc unnary excretioD of
HA should be gro~sly i~cr~asot in relation to the effect on sen~ levels, while ~ er~C~ in
serum leYcls of HA d~e to i~ ~d outflow of HA into the circulation or dec~ clearance
by the li~cr, would result ~n only ...odcrAt~ ir~."c~se~ in urinary cxcretion. Such a lack of
comla~ b ~ or m lev~ls and unn~y excte~on hao bee~ d~ ~hfd earlier ~n a study of"i.1 ~ritis (RA), p~ biliary ci~-h~ (PBC) a~d Wcmer~ sy~dromc (25). All
throe disoasos cau~o ~ n --e~ serum le~rels of HA. IIo~ .r, thc urinary exore~ion ~n RA al~d
PBC ~ only sliehtly il.c.~g~cdl w}~ .as a tcnfold inrrea~~ in excretio~ is sccn in Werners
s~ , de8pi~ tho f~cl ~at ~e serum~ }c~el i~ c~e wa8 lower th~n for PBC a~d RA.
The prcsoDco of a sa~ble ~ tivc ~chani~m fot HA h the ci~rulo~iQn could aJ~o expbi~ why
21 73037
circ~8 HA h~ a much lower Mw ~an the ~ entering the gcneral c~rclllSTtiorT via the lymph (6,
26). Sizo ,~j.t ~it;~ of dle poly".~,. by ~ 9 ~ir~ T~rT~g ha~ been ~lggrs~d a~ a .. IT-.. ;5m by
Fra~e~ (26). Howc~cr, thc Mw a~d co~c~ntr~on of the ~ Rbell~d EIA used to block l~ve~ uptake in
the proaent study ~ it u~likoly that ~e ~ w~ i .rl.,. .n.~d to wch an e~cte~t that brcak~own
b~l eh~Tng wou~d be h;l~it~d, F~aser ha~ ~tatcd that degradation by ~orum hy~luronid,ase does not
occur under physhol~~ Tl COT~T~ that f~ee radical acti~ity in serum would be too low to causo the
81&e 1~ h~ ~;on and t~at thc degradation of high Mw HA i9 not inhihit~d by high dose3 of low Mw
HA. 'rhe presc~t study also argue3 ~gainst free radical attack as the degr~tion occurs ~n the
presenco of a high dose of CS ~at p~o~a~l), would ~avenge any acdve free r~ e site of
degraclati~ could bc via a hya~ ;Ao~D~ fixed on a cell surface ~nd with a Mw~rp~do~t bi~ding
of HA . rc~ for act~ity. FU ~ ttudic8aro needed to characterize ~is ~ el~ ;io~ that might
Erove an ~u~u.~l p~t of HL~ tl~l;r-..
As many changes can be made to the embodi t~ ~ithout departing from
the scope of the invention, it is inten~e~ that all material cont~in~
herein be interpreted as illustrative of the invention and not in a limiting
sense.