Note: Descriptions are shown in the official language in which they were submitted.
21731~1
ELECTROCHEMICAL PROCESS
The present invention relates to a method of producing
pulp comprising electrochemical treatment of green liquor to
oxidize sulfide therein.
S Production of pulp normally involves recovery of the
cooking chemicals. In alkaline cooking, particularly in the
sulfate process, black liquor from the cooking is
concentrated by evaporation, combusted in a recovery boiler
in which a great deal of the sulfur and the sodium are
recovered in the form of green liquor mainly consisting of
sodium carbonate and sodium sulfide. The green liquor is
transformed to white liquor by treatment with slaked lime
(causticizing) involving formation of sodium hydroxide and
precipitation of calcium carbonate. The white liquor is then
used for cooking the wood in the digester.
Green liquor can also be obtained by combustion of
bleaching effluents.
It has been disclosed by E.Venemark, "Some Ideas on
Polysulfide Pulping", Svensk Papperstidning No. 5, 15 March
1964, that the yield of wood from alkaline pulp cooking can
be improved if the white liquor contains polysulfides.
WO 95/00701 describes electrochemical treatment of white
liquor involving oxidation of sulfides to polysulfides as
well as production of alkali metal hydroxide.
WO 94/12720 discloses that sodium hydroxide can be
produced by electrolysis of green liquor obtained from
combustion of bleaching effluents. However, it is stated
that any sulfide present in the green liquor should be
oxidized with air before the electrolyses. Thus, the process
does neither involved electrochemical oxidation of sulfide
nor formation of polysulfides.
The present invention seeks to provide an improved method
of producing cellulose pulp involving electrochemical treatment
of cooking liquors to oxidize sulfide. It is another object to
provide an efficient process producing alkali metal hydroxide
from raw materials available at a pulp mill. The invention
also seeks to provide an improved method of obtaining
cooking liquor containing polysulfides. Still further, the
invention seeks to provide a method of obtaining sulfide
2173191
free liquors for use in a pulp mill.
The invention concerns a method of producing pulp
comprising a step of forming green liquor containing alkali
metal sulfide and alkali metal carbonate. The method further
comprises a step of electrochemically treating the green
liquor to oxidize at least a part of the sulfide therein,
preferably~by operating an electrochemical cell comprising at
least two compartments with the green liquor as anolyte.
Suitably, the electrochemical treatment also comprises
production of alkali metal hydroxide, preferably by operating
an electrochemical cell comprising at least two compartments
with water or aqueous alkali metal hydroxide as catholyte.
Normally, green liquor is obtained in processes for
alkaline cooking of cellulose pulp, for instance sulfate pulp
or kraft pulp. The green liquor to be treated can be obtained
by thermal treatment of cooking effluents, normally in a
recovery boiler in which concentrated black liquor is
combusted in reducing atmosphere. However, the green liquor
can also be obtained by thermal treatment of bleaching
effluents, which treatment may involve concentration and
combustion as described in the already mentioned WO 94/12720.
The main constituents of green liquor normally are carbonate,
sulfide and hydroxide of alkali metals, the concentrations of
which may be from 0 to saturation. Typically, green liquor
obtained from cooking effluents contains from about 0.2 to
about 3 moles/litre, preferably from about 0.5 to about 1.5
moles/litre, of alkali metal carbonate, from o to about 2
moles/litre, preferably from 0 to about 1 mole/litre of alkali
metal sulfide, from 0 to about 2 moles/litre, preferably from
0 to about 1 mole/litre of alkali metal hydroxide, and from 0
to about 0.3 moles/litre of alkali metal chloride. Green
liquor obtained from bleaching effluents generally have a
similar composition, but the sulfide content is normally
within the lower area of the specified range. The alkali metal
is normally sodium, potassium or a mixture thereof. In a
typical system, from about 90 to about 97~ of the alkali metal
ions are sodium, the remainder essentially being potassium.
However, there might also be systems essentially based on
potassium and containing very small amounts of sodium.
~173191
In a preferred embodiment the green liquor is treated in
an electrochemical cell comprising separate anode and cathode
compartments separated by a partially permeable barrier,
preferably a cation selective membrane. The preferred treat-
ment comprises the steps of introducing green liquor into theanode compartment, introducing water and preferably alkali
metal hydroxide into the cathode compartment, electrochemi-
cally oxidizing sulfide in the anode compartment, forming
hydroxide ions in the cathode compartment, and passing alkali
metal ions from the anode compartment to the cathode compart-
ment.
Preferably, the anode potential is so maintained that
the oxidation product substantially consists of polysulfides,
i.e. S22~, S32-, S42- and S52-, and so forth. The exact limits of
the anode potential depend on the magnitude of the over
voltage. Normally, however, the anode potential is suitably
maintained in a range of from about -0.6V, which is the
theoretical lower limit enabling the formation of polysulfide,
to the level at which oxygen begins to develop, normally at
about +0.6V. Preferably, the anode potential is maintained in
a range of from about -0.6V to about +0.5V, in particular in
a range of from about -0.2V to about +0.4V. By keeping the
anode potential somewhat higher, but preferably still below
the potential for oxygen formation, the oxidation of sulfide
essentially yields sulfate and/or thiosulfate. By keeping the
anode potential within the specified limits, it is also
possible to substantially avoid formation of chlorine.
The cathode potential is preferably so maintained that,
apart from hydroxide ions, mainly hydrogen gas forms, which in
practice normally takes place in a range of from about -o.9V
to about -1.2V. The hydrogen gas formed can be used as an
environment-friendly source of energy or as raw material in
other chemical processes. In another mode of operation, the
cathode potential is maintained in a range of from about +0.3V
to about -0.9V, while at the same time oxygen-containing gas,,
for example, air, is supplied to a cathode in the form of a
gas-diffusion electrode, resulting in the reduction of oxygen
and the formation of hydrogen peroxide and/or hydroxide ions.
In the presence of oxygen, it is also possible to have the
- 2173191
~_ 4
cell operate as a fuel cell, resulting in the generation of
electric power.
All figures indicating electrode potentials, anode as
well as cathode potentials, represent the potentials measured
against a reference electrode of Hg/HgO in 1 M NaOH at 25C.
The limits cannot be set at exact values, since the result at
a certain potential depends on the magnitude of the over
voltage in each particular case.
The green liquor introduced into the anode compartment
may have a degree of recycling from o to almost 100~. The
recycled green liquor, if any, may contain from about 0.2 mol
to about 1.5 mol of sulfide per litre, preferably from about
1 mol to about 1.5 mol of sulfide per litre. For instance, the
conversion of sulfides may be from about 0.5~ to 100~.
Preferably, the solution introduced into the cathode compart-
ment essentially consists of water and alkali metal hydroxide,
especially hydroxide of sodium, potassium or mixtures thereof.
The concentration of alkali metal hydroxide is not critical,
and may, for example, be from about 0.1 mol/l to about 15
mol/l, preferably from about 1 mol/l to about 10 mol/l. The
upper limit for what is regarded as suitable is generally
determined by the properties of the barrier separating the
anode and cathode compartments, since too much leakage of
hydroxide ions through the barrier reduces the current
efficiency.
In order to fully use the electrolytic cell, the process
is preferably carried out at a current density exceeding about
0.5 kA/m2, in particular exceeding about 2 kA/m2. At excessive
current densities, the anode is increasingly worn, and the
risk of undesirable by-products such as oxygen is increased.
If polysulfides are the desired main product, it is also
desired to avoid formation of sulfate and thiosulfate.
Normally, a current density not exceeding about 20 kA/m2, in
particular not exceeding about 15 kA/m2, is preferred. The
formation of by-products is also reduced by keeping the
temperature of the anode compartment sufficiently high,
suitably in a range of from about 60C to boiling point, which
usually is about 110-120C. In practice, the upper temperature
limit depends on the material of the cell, especially when the
2173191
barrier is made of a polymer-based membrane, for which reason
the especially preferred temperature ranges from about 80C to
about 100C. For practical reasons, the temperature of the
cathode compartment is preferably substantially equal to that
of the anode compartment. Also, it has been found that the
amount of by-products is reduced if the anolyte flow is
sufficiently high. Preferably, the flow in the anode compart-
ment is turbulent, and suitably the average linear velocity is
higher than about 0.5 m/s. The catholyte flow is not critical
and is, in actual practice, conveniently determined by the
size of the lifting force of the generated gas. Otherwise,
pumps may be used.
It has been found that the precipitation of sulfur on
the anode can be avoided by the choice of a suitable material.
Without preference to any specific theory, it is assumed that
the oxidation of sulfide involves an intermediary stage in
which atomic sulfur is bound to the anode surface. If this
bond is too strong, the sulfur will not react further, and
some of the sulfur will remain on the anode surface and there
form a passivating surface layer. According to the invention,
use is advantageously made of an anode which is made of a
carrier material of high alkali resistance, such as titanium,
zirconium, hafnium, niobium and alloys thereof, or carbon,
nickel or nickel alloys, the carrier material being surface-
coated with one or more oxides of ruthenium, iridium, platinumand palladium. Electrodes made of suitable material and having
a suitable surface coating are commercially available, such as
the so-called DSA~ electrodes (dimensionally stable anode). It
has been found that DSA~ electrodes designed for oxygen or
chlorine-gas generation are suited for use in the invention,
such as those electrodes marketed under the designations ON
201, ON 120 and ON 101.
It is favourable if the anode has a large surface and
that the sulfide transport to the entire surface functions
well. Thus, the anode employed suitably is a three-dimensional
through-flow electrode, such as a three-dimensional mesh
electrode, balls of wire, layers of wire mats, particle beds
or metal foam. It is especially preferred to use a three-
dimensional mesh electrode composed of a plurality of layers
2173191
~_ 6
of expanded metal, for example interconnected by spot welding.
The cathode material is not critical, and use can be
made of such common alkali-resistant materials as steel,
stainless steel, nickel and ruthenium-coated nickel. The
cathode may consist of a flat plate, one or more layers of
mesh, or a three-dimensional through-flow electrode similar to
that used as anode. If oxygen-containing gas is to be blown
into the cathode compartment, use should be made of an oxygen-
reducing cathode, in which case a graphite-felt electrode is
convenient. Such electrodes are commercially available and
generally used, for example in fuel cells. The oxygen-reducing
cathode may be coated with a catalyst, such as platinum, in
order to increase the amount of hydroxide ions formed in
relation to the amount of hydrogen peroxide. By oxygen-
reduction, it is possible to produce an alkaline hydrogenperoxide solution which can be used as such for bleaching
cellulose pulp. Also, the presence of hydrogen peroxide in the
cathode compartment contributes to the resulting product being
perfectly sulfide-free, since any sulfides leaking in from the
anode compartment are at once oxidized by the peroxide to
sulfate.
Preferably, use is made of a two-compartment cell with
adjoining anode and cathode compartments, but cells having
three or more compartments may also be used, in which case the
green liquor may be introduced into the anode compartment as
well as into one or more compartments located between the
anode compartment and the cathode compartment. The barrier
separating the compartments of the cell, normally found
between the anode compartment and the cathode compartment,
should let alkali metal cations from the anode compartment
through to the cathode compartment, but should preferably to
the greatest possible extent prevent the passage of sulfides
and polysulfides and preferably that of other anions as well.
Also hydroxide ions should preferably be prevented by the
barrier, even if some may be permitted to pass. Preferably,
use is made of a cation-selective membrane permeable to alkali
metal cations but essentially impermeable to sulfides and
polysulfides. If the cell has more than two compartments,
different combinations of anion-selective and cation-selective
2173191
membranes may be used for separating the different compart-
ments of the cell. Furthermore, one or more porous diaphragm
may be used as barriers, optionally in combination with one or
more ion-selective membranes. Suitable membranes may, for
instance, be made of perfluorinated, sulfonated or teflon-
based polymers, or ceramics. Also polystyrene-based membranes
or diaphragm of polymers or ceramics may be used. There are
several commercially available membranes suitable for use,
such as Nafion~.
A plurality of electrolytic cells can be arranged in
bipolar as well as monopolar fashion.
The electrochemically treated green liquor can be
transferred to a causticizing step in which slaked lime
(calcium hydroxide) is added, converting the carbonate to
hydroxide. If the green liquor contains polysulfides, these
will be present in the resulting white liquor which then can
be used in the cooking. If the green liquor is substantially
free from sulfide and polysulfides, the liquor obtained from
the causticizing can be used in the bleachery. The electro-
chemically treated green liquor can also be further treatedelectrochemically to remove carbonate in the form of carbon
dioxide and simultaneously produce alkali metal hydroxide.
In a preferred mode of operating an alkaline pulp
process, green liquor obtained from cooking effluents is so
treated that its content of polysulfides is increased before
it is supplied to the causticizing process. Then, the whole
amount of green liquor can be treated at a low degree of
conversion, for example ranging from about 0.5~ to about 1~,
based on the sulfide present in the green liquor, or a part
flow can be treated at a higher degree of conversion, for
example ranging from about 10~ to 100~, preferably ranging
from about 60~ to about 95%, based on the sulfide present in
the green liquor. The catholyte is conveniently recycled in a
special circuit, a steady state being maintained by recovering
a part flow as a product, which, for example, can be used in
the cooking process or the bleaching process or be completely
- removed from the system of the pulp mill.
In another mode of operation, a partial flow of the
green liquor is so treated that a large amount of the sulfides
2173191
are converted to polysulfides, preferably about 70-100~,
whereupon the polysulfides are converted to sulfur or some
solid sulfur compound, for example by cooling crystallisation,
and are removed from the system. This mode of operation is
suited for use in mills where excessive amounts of sulfur
compounds are supplied to the process along with the raw
material. The catholyte may be treated as in the mode of
operation described above.
In yet another mode of operation, a partial flow of the
green liquor is so treated that the sulfides are oxidized to
sulfate and/or thiosulfate. If so, the treated anolyte may be
used as a substantially sulfide free liquor in the pulp mill,
for example as a pH regulator in the bleachery. The substan-
tially sulfide free green liquor may also be causticized with
slaked lime to convert the carbonate to hydroxide and then be
used in an oxygen delignification step or in alkaline bleach-
ing steps. In another embodiment, the electrochemically
treated and preferably substantially sulfide free green liquor
is further treated electrochemically to remove carbonate in
the form of carbon dioxide and simultaneously produce alkali
metal hydroxide. Such treatment is preferably performed by
mixing preferably substantially sulfide free green liquor with
an acid aqueous anolyte, preferably containing sulfate,
resulting in formation of carbon dioxide and an acid anolyte
containing less carbonate then the original green liquor,
removing carbon dioxide from the acid anolyte, introducing the
acid anolyte into the anode compartment of an electrochemical
cell, introducing water and preferably alkali metal hydroxide
in the cathode compartment of said cell, electrochemically
forming protons in the anolyte, electrochemically forming
hydroxide ions in the catholyte, passing alkali metal ions
from the anolyte to the catholyte through a separator,
preferably a cation selective membrane, and mixing part of the
acid enriched anolyte with new green liquor. Normally, also
oxygen and/or chlorine forms in the anolyte, thus enabling
removal of chloride from the green liquor.
The present invention enables of alkali on the basis of
raw material available in pulp mills, without the formation of
any undesirable by-products and without any need for
217319:L
~ g
pretreatment of the green liquor with oxygen or air. Thanks to
the comparatively low anode potential required for sulfide
oxidation, the alkali production is very energy effective. If
the alkali metal hydroxide produced is not utilised in the
closed part of the pulp process, it is also possible to reduce
the risk of increasing potassium concentration, thus avoiding
the problems that may arise in the soda recovery unit at
excessive potassium contents. The yield of wood in the
manufacture of pulp can be augmented by increasing the
polysulfide content of the white liquor obtained by causti-
cizing the treated green liquor. It is also possible to obtain
substantially sulfide free liquors for use in the bleachery.
Thus, through the invention green liquor obtained from cooking
effluents can be treated to be useful in the bleachery and
vice versa, depending on the material balance for the indi-
vidual pulp mills. Since electrochemical treatment of green
liquor normally also involves removal of alkali metal cations
and some water, the load on the causticizing plant will be
lower than if the corresponding treatment is performed on the
white liquor. Another advantage of treating the green liquor
is that calcium added in the causticizing decreases the
lifetime of cell membranes.
The invention will be described in more detail below
with reference to the accompanying drawings, in which
Fig. 1 is a schematic view of an electrolytic cell;
Fig. 2 is a view of a three-dimensional mesh electrode;
and
Fig. 3 is a schematic flow chart illustrating how the
invention can be applied to the manufacture of cellulose pulp.
The invention is not restricted to the embodiments
shown, but is defined by the scope of the appended claims.
The electrolytic cell 1 illustrated in Fig. 1, comprises
an anode compartment 2 provided with a three-dimensional
through-flow electrode serving as an anode 4. A cathode
compartment 3 provided with a preferably three-dimensional
cathode 5 is separated from the anode compartment 2 by means
of a cation-selective membrane 6. The anode 4 and the cathode
5 are connected to a direct-current source (not shown). The
anode compartment 2 has an inlet 7 and an outlet 8 for the
2173191
anolyte. The cathode compartment 3 has an inlet 9 and an
outlet 10 for the catholyte and gaseous products, extending to
a gas separator 13 which has an outlet 12 for gas and an
outlet 11 for liquid. When the cell 1 is in operation, green
liquor is introduced into the anode compartment 2 through the
inlet 7. Thus, sulfides are oxidized to polysulfides, and
alkali metal cations are transported through the membrane 6
into the cathode compartment 3. Polysulfide-concentrated green
liquor is discharged through the outlet 8. An aqueous solution
of alkali metal hydroxide is introduced into the cathode
compartment 3 through the inlet 9, and water is decomposed
into hydrogen gas and hydroxide ions. The hydrogen gas is,
along with an aqueous solution concentrated with respect to
alkali metal hydroxide, discharged through the outlet 10. In
the gas separator 13, the hydrogen gas 12 is separated from
the alkali metal hydroxide 11.
Figs 2a and 2b illustrate a three-dimensional mesh
electrode from above and from the front, respectively. The
illustrated electrode is composed of four nettings of expanded
metal 40 which, by spot welding, are connected to a current
supply in the form of metal strips 41.
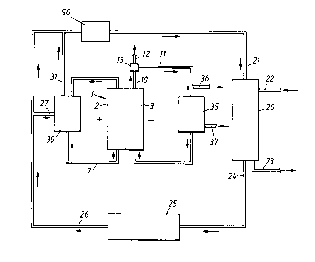
Fig. 3 illustrates how an electrolytic cell 1 of the
type shown in Fig. 1 may be used for alkaline production of
cellulose pulp, such as sulfate pulp. For reasons of clarity,
only one cell 1 is shown, but it is obvious to those skilled
in the art that any number of cells, for example from two to
several hundreds, can be interconnected in parallel or in
series. A preferred mode of operation will now be described.
A digester 20 is supplied with white liquor 21, as well as
wood and other chemicals 22 required, such as alkali metal
hydroxide. Cooking and washing (not shown) result in pulp 23
and black liquor 24 undergoing different treatment stages 25
to obtain green liquor, which stages are well known among
persons skilled in the art of pulp manufacture and normally
include evaporation, addition of make-up chemicals, such as
sodium sulfate, and combustion in reducing atmosphere. The
treatment of the black liquor yields green liquor 26 which
normally contains from 0 to about 0.4 mol/l of sulfides, from
about 0.7 to about 1.2 mol/l of carbonate ions, from 0 to
2173191
11
about 0.6 moles/litre of hydroxide ions, and from about 0.7 to
about 2.5 mol/l of alkali metal cations, of which from about
90~ to about 97% normally is sodium, the remainder essentially
being potassium. Some of the green liquor 27, for example from
5 about 1~ to about 30~, is conducted to a tank 30 holding
polysulfide-containing green liquor. Green liquor whose
polysulfide-content increases in electrolysis, for example in
such a manner that from about 65~ to about 95~ of the sulfide
is converted to polysulfide, circulates between the tank 30
and -the anode compartment 2 of the electrolytic cell 1.
Polysulfide-rich green liquor 31 is drawn off from the tank 30
to be mixed with the main flow 2 6, such that the aimed-at
polysulfide content is achieved, for example from about 0.5~
by weight to about 1. 5~ by weight, whereupon the resulting
mixture is supplied to a causticizer 50 in which slaked lime
is added and white liquor 21 forms. The white liquor 21 is
transferred to the digester 20 for cooking. An alkali metal
hydroxide solution, for instance containing from about 2 mol
to about 15 mol of alkali metal hydroxide per litre, circu-
20 lates between the cathode compartment 3 of the cell 1 and atank 35 via the gas separator 13. Some of the alkali metal
hydroxide solution 11 from the gas separator 13 is drawn off
as a product 36 and may, for example, be used in the manufac-
ture of pulp, or in completely different processes. Water 37
25 is supplied to the tank 35, thereby to maintain the volume and
the concentration essentially constant.
In a particular mode of operation, sulfur may be
expelled from the system by carrying the sulfide oxidation to
high contents of polysulfides in the tank 30, preferably to a
30 conversion exceeding 70~, based on the sulfide in the green
liquor. Green liquor from the tank 30 may then be so treated
that sulfur is precipitated, for example through cooling
crystallisation. This can be brought about by circulating
polysulfide-rich green liquor between the tank 30 and a
crystallizer (not shown) from which precipitated sulfur is
removed, the mother liquor being recycled to the tank 30.
In another mode of operation, the sulfides in the green
liquor are to a great extent oxidized to sulfate and/or
thiosulfate, which can be performed by filling the tank 30
2173191
12
with green liquor, which then is circulated through the anode
compartment 2, no green liquor leaving the circulation system
until essentially the entire amount of sulfides has been
converted to sulfate or thiosulfate. The resulting sulfate-
rich, substantially sulfide-free green liquor may then be used
in the pulp mill, particularly in the bleachery, optionally
after causticizing or further electrochemical treatment to
obtain alkali metal hydroxide.
The invention will now be further described through the
following example.
EXAMPLE: The experiment was performed in a micro-flow
cell consisting of an anode compartment and the cathode
compartment separated by a Nafion~324 cation selective
membrane. The anode was a DSA~ON201 mesh electrode and the
cathode was a flat stainless steel electrode. The surface area
of the membrane and the projected area of the electrodes was
cm2. Synthetic green liquor consisting of an aqueous
solution of 1.4 M sodium carbonate and 1.275 M sodium sulfide
was used as the anolyte and 2.633 M sodium hydroxide was used
as the catholyte. 125 ml anolyte and 125 ml catholyte were
placed in separate vessels and were then circulating through
the cell with a flow of 160 ml/min. The voltage over the cell
was about 1.3 V and the temperature was about 90C. After 64
minutes the sodium hydroxide concentration in the catholyte
was 2.8 M and the sulfide concentration in the anolyte was 1.0
M. The current efficiency for the hydroxide production was
60~.