Note: Descriptions are shown in the official language in which they were submitted.
r~ . ,~ . .
D-17147 217360~
lVlli~THOD A~l~ APPAR ~ FOR PROPORTIONING A~l)
G NON COMP~h~ R~ AI~T) COMPRl;~ Rl.F~ uIn~c:
Field of The ~nv~ntion
This invention pertains to mi~ing and proportioning a
compressible fluid and a non-compres~ible fluid. In a ~refe,.ed
embodiment of the invention the compressible fluid is a supercritical
fluid, the non-compressible fluid is a coating composition, and the
resultant mixture is applied to a substrate by spraying techniques.
Rack~ound Of The Inven~;on
Coating compositions are comple~ mixtures which often
include binders, pigments~ surfactants, flow-control agents, and organic
solvents. Organic solvents serve a variety of purposes related to
viscosity reduction, film foImation and adhesion. In spraying paints
and coatings, organic solvents reduce their viscosity. This viscosity
reduction is needed to enable atomization when the material is sprayed
and also to facilitate droplet coalescence on the surface, thus giving a
coherent, uniform film. Spray atomization requires a very low viscosity
to produce the fine droplets needed for high-quality coatings.
Despite the important role of volatile organic compounds
("VOC") play in the co~ting~s formulation, there has been a considerable
effort by the coating fo~nulators and applicators to reduce VOC
emissions for both economical and envirol-me~tal reasons.
A great deal of emph~cis has been placed on the
development of new coating technologies which will reduce the emission
of organic solvent vapors. A number of technologies have emerged as
having met most but not all, of the performance and application
requirements, and at the same time having met the emission
requirements and regulations. They are (a) powder, (b) waterborne,
dispersion, (c) waterborne, solution, (d) non-aqueous dispersion, and (e)
high solids coatings. Each of these tecnnologies has beer~ employed in
certain applications, and each has found a niche in a particular
~ -- )
D-17147 ~173~
- 2 -
industry. In a majority of cases, the coatings from these new
technologies are inferior to the old in one or more important application
or performance features.
U.S. Patent No. 4,923,720 discloses Dhethods and
apparatus for the production of the high solid co~tin~ formulation in
which substantial amounts of the liquid solvent component have been
removed and replaee~ w~th a w~-~xic an~ o~ çnt~lly comp~t;hle
supercritical fluid, such as supercritical carbon dio~de. Thig co~tine
composition is then sprayed onto a substrate at which time the
supercritical carbon dioa~ide vaporizes to assist spray ~tomi7~tion. In
order to produce a coating material solution or formulation with the
desired application characteristics, the relative proportion of the liquid
composition and supercritical carbon dioxide should be maintained at a
predetermined ratio or within a predetermined range. Howev~l, one
requirement of U.S. Patent No. 4,923,720 is to control the relative
proportion of liquid coating composition and supercritical fluid. The
liquid coating composition and supercritical fluid are each introduced
into the system by a separate pump. However, the volllme of the
supercritical carbon dio~ide is varied depending upon the system
pressure and temperature. This can result in deviation of the
supercritical carbon dioxide concelltlalion in the coating formulation,
resulting in inconsistent spray characteristics.
U.S. Patent No. 5,215,257 discloses an improved method
and apparatus for fo~ning and dispensing a coating material
formulation or solution con~inin~ a fluid coating composition and a
fluid diluent, such as a su~elc.;lical carbon dioxide. The control system
opens and closes the supply of supercritical carbon dio~cide and/or liquid
coating composition in accordance with variation of capacitance in the
formulation. The devices requires predetermined set point values to
control supercritical carbon dio~ide concentration in the co~t;n~
formulation. However, the correlation between the carbon dioxide
concentration in the coating formulation and the values obtained by
capacitance sensor can vary significantly depending upon system
D-17147 ~ ~173600
pressure, temperature and coating formulation. ~urthermore, with
respect to compositions having both liquid and gas components in a
multiple phase solution, it has been found that controlling carbon
dio~ide concentration is difficult. The signal fro~ tbe capacitance
~encing circuit produces a relativeiy widely fluctuating signal due to the
formation of bubbles. Another deficiency of the apparatus is that the
device requires the feed ~a~g ~ps~citance ~ormation of form~ tiQn
before carbon dioxide addition to calculate control set point values with
respect to carbon dioxide concentration.
Aforementioned U.S. Patent No. 4,923,720 discloses an
apparatus capable of pumping and proportioning a co~ting formlll~tion
and liquid carbon dioxide. In one embodiment, volumetric
proportioning of the coating formulation stream and the supercritical
carbon dio~ide stream is carried out by means of reciprocating pumps
which displace a volume of fluid from the pump during each one of its
pumping cycles. One reciprocating pump is used to pump the co~t;ng
formulation which is slaved to another reciprocating pump which is
used to pump the liquid carbon dio~ide. The piston rods for each pump
are attached to opposite ends of a shaft that pivots up and down on a
center fulcrum. The voll~me ratio is varied by sliding one pump along
the shaft, which changes the stroke length.
However, liquid carbon dioxide is relatively CO~pl esfiible
at ambient temperature, the temperature at which it is typically stored
in a pressurized cont~iner. Such comprescihility may undesirably cause
fluctuations and osçill~tio~.c of the amount of carbon dioxide that is
present in the ~rlmiYed co~ting formulation that is to be sprayed. This
occurs due to the incompatible pumping characteristics of the relat*ely
non-compressible coating formulation and the relatively co~-essible
liquid carbon dioxide. With the coating formulation, pressure i5
immediately generated in the reciprocating pump as soon as its volume
is displaced. Inasmuch as the liquid carbon dioxide is subst~r~ti~lly
compressible, a larger volume is needed to be displaced in order to
generate the same pressure. Because mi~ing occurs when the fiow of
D-17147
2173~00
the coating formulation and of the liqu d carbon dioxide are at the same
pressure, the flow rate of carbon dio~ide lags behind the flow rate of the
coating formulation.
This oscillation is further accentuated if the driving force
operating the pump varies during the operating cycle, such as an air
motor rh5~nging direction dunng its cycle. Thus, if the driving force
declines, the pressure in the co~1;ng f~mtll~t;nn flow de~l~bes even
more rapidly, due to its non-co~ylessihility~ than the pressure in the
liquid carbon dio~ide flow.
Accordingly, the pressures generated in both flows may be
out of phase during the pllmping. U.S. Patent No. 4,621,927 discloses a
~L~LL~ e control apparatus controlling a flow rate of a second fluid to be
mL~ed with a first fluid so as to prepare a third fluid having a
predetermined concentration. A set point variable of the flow rate of the
second fluid is calculated in accordance with the flow rate of the third
fluid so as to improve controllability of the apparatus. However, the
invention in U.S. No. 4,621,927 cannot control the mixture of
compressible fluid(s) and non-compressible fluid(s) because the
thermodynamic properties of the fluids are influenced by variables such
as pressure, temperature, and concentration.
~umm~rv of The ~nv~n~on
By virtue of the present invention, the above deficiencies
have now been overcome. Methods and apparatus have been discovered
which are capable of accurately and continuously providing a
proportioned mi~ture comprised of a non-co~lessible fluid and a
compressible fluid.
In particular, the present invention measures the
volumetric flow of the non-compressible fluid stream before and after
the addition of compressible fluid to determine and to control the
amounts of co~)~ressible fluid. This invention ~imply and accurately
proportions the ~uids because it has been surprisingly discovered that
the density of the non-compressible fluid and compressible fluid mi~ture
D-17147 ~1736û0
does not vary significantly in many systems as long as the solubility
limit of the compressible fluid in the non-compressible fluid ~ix l,u}e is
not e~ceeded.
As used herein, the phrase "compres`sible fluid" is meant to
include a material whose density is affected by a change in pressure to
an extent of at least about 5%. As used herein, all fluids are understood
to be at one atmosphere pressure a~ Q~ ess otherw~ie aoted.
More specifically, the present invention in its broader
embodiment comprises an apparatus for continuously miYir~ a
6ubstantially compressible fluid and a subst~nt~ y non-co~ essible
fluid in a predetermined proportion which includes:
a) means for supplying substantially Co~ essible fluid;
b) means for supplying substantially non-compressible
fluid;
c) means for measuring the voll~metric flow rate of the
substantially non-compressible fluid;
d) means for generating a signal based upon the
volumetric flow rate of the substantially non-
compressible fluid;
e) means for forming a mLL~U~ e of the measured
substantially non-co~ressible fluid and subst~nti~lly
compressible fluid, 6uch that the density of the resulting
mixture behaves substantially like a non-co~ ressible
fluid;
f) means for measuring the volumetric flow rate of said
mixture;
g) means for generating a signal based upon the flow rate
of the substantially com~ressible fluid and
substantially non-coll-y, essible fluid ~ . e; and
h) means for controlling the flow rate of the subst~nti~lly
- compressible fluid in response to the sir~ls ~enerated
in (d) and (g).
- t
D-17147 ~ ~l73ç;ao
As used herein, the phrases "coating formulation" or
"coating composition" are understood to mean a typical, collvelltional
co~ting composition which does not have any supercritical fluid admiged
therewith. Also as used herein, the phrases "~ ed liquid ....~ . e" or
"admiged coating formulation" are meant to include an admisture of a
coating form~ tion with at least one supercritical fluid.
The present invention also comprises a method for formin~
a mixture of a substantially compressible fluid and a subst~nti~lly non-
compressible fluid in a predetermuned proportion which comyl;ses:
a) providing a non-compressible fluid;
b) measuring said non-compressible fluid's
volumetric flow rate;
c) providing a colllylessible fluid;
d) mimng the compressible fluid witb the
non-comyressible fluid such that the density of
the resulting migture behaves substantially as a
non-compressible fluid;
e) measuring the volumetric flow rate of the
migture; and
f) controlling the flow rate of the compressible fluid
based upon the volumetric flow rate of said
mixture.
As used herein "substantially as a non-co"~ essible fluid"
is understood to include a mixture whose density is unaffected by a
change in concentration of the components in the m~ re of less than
about 10~c, preferably of less than 5%, and most preferably of less than
2%.
By measunng the volumetric flow rate of the non-
compressible fluid and ~oml)ressible fluid/non-compressed fluid mixture
and then controlling the flow rate of the compressible fluid pump, the
difficulties associated with handling a compressible fluid are
substantially elimin~ted. In a preferred embodiment of the invention
the density of the resulting fluid mixture is also measured to ensure
D-17147 - ~ ~ 7 3 6 0 0
that the fluid mi~tllre is behaving substantially as a non~ essible
fluid.
netailed nescr~Dtion Of The n~win~s
Figure 1 is a phase diagr~m for a supercritical carbon
dio2ide, polymer and solvent system.
Figure 2 is a graph of the density versus ~mMSitiDn of
ethanol/water and isopropyl alcohol/water systems.
Figure 3 is a graph of the density versus composition of a
dimethyl sulfoxide/acetone system.
Figure 4 is a graph of the density versus composition of an
acrylic polymer/methyl aryl ketone solution.
Figure 5 is a graph of the density versus composition of a
polymeric coating composition/carbon dioxide solution.
Figure 6 is a diagram of the apparatus suitable for
proportioning and spraying a compressible fluid and non-compressible
fluid.
Figure 7 is a diagram of the apparatus used to conduct the
experimental trials described herein.
Figures 8-11 are graphical representations of flow rate
versus time for the spray application of various coating mi~ es.
Figures 12 and 13 are graphs of the density versus
composition for two coating compositions in carbon dio2ide.
Figures 14 and 15 are graphs of the density versus
composition for two coating compositions in ethane.
netailed Descrintion Of The InvenRon
It is to be understood that while the following discussion
will primarily focus upon providing a proportionated ~mised liquid
mi~ture of a coating formulation and supercritical fluid, suc~ as carbon
dio~ide, which is suitable for being sprayed onto a substrate, ~he
present invention is in no way limited to this embodiment. As is readily
apparent from the foregoing discussion, the present invention
.
D-17147
3~0
encompasses the proportionation of any compressible and non-
compressible fluid to form a desired ~lu~ e for any intended
subsequent use.
The co~hne compositions employed~in this ~lvel~tion are
broadly defined to include paints, lacquers, adhesives and the like.
Such coating materials may also include those that are typically ltili7e.
in the agricultural field such as, but not limited to, fertilizers,
herbicides and insecticides.
The coating compositions employed in the present
invention typically co~l;ses a solids component cont~inine at least one
polymeric component, piem~nts~ melting agents, cross-linking agents,
ultraviolet light stabilizers. In addition to the solids component, a
solvent fraction is also employed, including active solvents, coupling
solvents and water. Other liquid components often found in coating
compositions may also be used such as curing agents, plasticizers,
surfactants and the like. The components of both the solvent fraction
and the liquid fraction of coating compositions are well known to those
with skill in the art. A more thorough ~liscllcsion of the components
found in coating compositions can be found in U.S. Patent No.
5,171,613.
Supercritical fluud phenomenon is well doc~lmente~, (6ee
pages F-62 - F-64 of the CRC Handbook of Ch~mistry and physics~ 67th
Edition, 1986-1987, pl~h1isher~ by the CRC Press, Inc., Boca Raton,
Florida). At high pressures above the critical point, the resulting
supercritical fluid, or "dense gas", will attain densities appro~-hir~g
those of a liquid and will assume some of the properties of a liquid.
These properties are dependent upon the fluid composition,
temperature, and pressure. As used herein, the "critical point" is the
transition point at which the liquid and gaseous states of a subst~nc~
merge into each other and represents the comhin~tion of the cFitical
temperature and critical pressure for a given substance. The critical
temperature", as used herein, is defined as the temperature above
which a gas cannot be liquefied by an increase in pressure. The "critical
D-17147 21731i00
pressure", as used herein, is defined as that pressure which is just
sufficient to cause the appearance of two phases at the "critical
temperature".
The compressihility of supercritical fluids is great just
above the critical temperature where small changes in pressure result
in large changes in the density of the supercritical fluid. The "liquid-
like" behavior of a supercritical fluid at higher pressures results in
gréatly enh~nced soll~hili7ing capabilities compared to those of the
"subcritical" compound, with higher diffusion coefflcients and an
extended useful temperature range comp~red to liquids. Compounds of
high molecular weight can often be dissolved in the supercritical fluid at
relatively low temperatures. An interesting phenomeno~ associated
with supercritical fluids is the OCCul 1 ellce of a "threshold pressure" for
solubility of a high molecular weight solute. As the pressure is
increased, the solubility of the solute will often increase by many orders
of magnitude with only a small pressure increase. The solvent
capabilities of the supercritical fluid, however, are not essential to the
broad aspects of the present invention.
Near-supercritical liquids also demonstrate solubility
characteristics and other pertinent properties ~imil~r to those of
supercritical fluids. The solute may be a liquid at the supercritical
temperatures, even though it is a solid at lower tem~ al~6. In
addition, it has been demonstrated that fluid "modifiers" can often alter
supercritical fluid properties significantly, even in relatively low
concentrations, greatly increasing 601ubility for 60me 601utes. These
variations are considered to be within the concept of a supercritical fluid
as used in the conte~t of this invention. Therefol e, as used herein, the
phrase "supercritical ~uid" denotes a compound above, at, or slightly
below the critical temperature and pressure (the critical point) of that
compound.
~ Ys~mples of compounds which are known to have utility as
supercritical fluids are listed in aforementioned U.S. Patent No.
4,723,920.
D-17147 ~ ~1~3600
- 10-
Due to the low cost, enviro~mental acceptability, non-
flAmm~qhility and low critical tempel at~l~e of carbon dioxide,
supercritical carbon dio~ide fluid is preferably used with the coating
formulations. For many of the same reasons, nitrous o~ide (N20) is a
desirable supercritical fluid for a~mirtt~re with the coating
formulations. However, any ofthe supercritical fluids and the mi~tvres
eof are to be-considered as being applicable for use with the coating
formulations.
The solvency of supercritical carbon dioxide is
substantially simil7~r to that of a lower aliph~t;c hydrocarbon and, as a
result, one can consider supercritical carbon dioxide as a replAcemPnt
for the hydrocarbon solvent of a conventional coating formulation. In
addition to the enviromnental benefit of replacing hydrocarbon solvents
with supercritical carbon dioxide, there is a safety benefit also, because
carbon dioxide is non-flAmmAhle.
Due to the solvency of the supercritical fluid with the
coating formulations, a single phase liquid mixture is formed which is
capable of being sprayed by airless spray techniques.
Coating formulations are commonly applied to a substrate
by pAssing the coating formulation under pressure through an orifice
into air in order to for_ a liquid spray, which imp~ s the substrate and
forms a liquid co~ting. In the coatings industry, three types of orifice
sprays are commonly used; namely, air spray, airless spray, and air-
assisted airless spray.
Air spray, airless spray, and air-assisted airless spray can
also be used with the liquid coatin~ forml~lat;on heated or with the air
heated or with both heated. Heating reduces the viscosity of the liquid
coating formulation and aids ato_ization. The present invention can
also be applied by electrostatic applications as described in U.S. Patent
No. 5,106,650.
- In essentially every process in which a miYtllre is preparedfor a particular purpose, the constituents of that mi~ture usually need
to be present in particular, accurately proportionated amounts in order
D-17147 ~173~iO0
for the mixture to be effective for its intended use. In the
aforementioned related patent, the underlying objective is to reduce the
amount of organic solvent present in a coating form~ tion by the use of
supercritical fluid. Understandably, with this objective in mind, it is
generally desirable to utilize as much supercritical fluid as possible
while still ret~ining the ability to effectively spray the liquid ~ e of
coating fc~ulations and supercritical fluid and also obtain a ~eRi
coating on the substrate. Accordingly, here too, it is particularly
preferred that there be prescribed, proportionated amounts of
supercritical fluid and of coating formulation present in the liquid
coating mixture to be sprayed.
Generally, the preferred upper limit of supercritical fluid
addition is that which is capable of being miscible with the coating
formulation. This practical upper limit is generally recogni7~b1e when
the a~mi~zture cont~inin~ coating formulation and supercritical fluid
breaks down from one phase into two fluid phases.
To better understand this phenomenon, reference is made
to the phase diagram in Figure 1 wherein the supercritical fluid is
supercritical carbon dio~ide fluid. In Figure 1, the vertices of the
triangular diagram represent the pure components of an ~miYe~
coating formulation which for the purpose of this discussion cont~inc no
water. Vertex A is an organic solvent, vertex B i8 carbon dioxide, and
vertex C represents a polymeric material. The curved line BFC
represents the phase boundary between one phase and two ph~ces. The
point D represents a possible composition of a coating formulation in
which supercritical carbon dio~de has not been added. The point E
represents a possible composition of an ad_ixed coating formulation,
after admi~ture with supercritical carbon dioxide.
Thus, afler ~tomi7~tion, a majority ofthe carbon dio~ide
vaporizes, leaving subst~nt,;~11y the composition of the original co~ting
formulation. Upon contacting the substrate, the rem~ining li4uid
e of the poly_er and solvent(s) component(s) will flow, i.e.,
coalesce, to produce a unifonn, smooth film on the substrate. The film
D-17147 217360~
forming pathway is illustrated in Figure 1 by the line seEments EED
(at~mi7~tion and decG~ ression) and DC (coalescence and film
formation).
However, the ~mount of supercritica~ fluid, such as
6upercritical carbon dio~ide, that can be rnLYed with a coating
formulation is generally a fllnctio~ of the miscibility of the 6upercritical
fluid with the coating formulation as can be-st b~gllr~i7e~ by ~ g
to Figure 1.
As can be seen from the phase diagram, particularly as
shown by arrow 10, as more and more supercritical carbon dioYide is
added to the coating formulation, the composition of the ~miYe~ liquid
coating mixture approaches the two-phase boundary represented by line
BFC. If enough supercritical carbon dioside is added, the two-phase
region is reached and the composition correspondingly breaks down into
two fluid phases. Sometimes, it may be desirable to admis an amount
of supercritical fluid (in this case, supercritical carbon dio~ide) which is
even beyond the two phase boundary. Generally, however, it is not
preferable to go much beyond this two phase boundary for optimum
spraying performance and/or coating formation.
In addition to avoiding the two-phase state of the
supercritical fluid and the coating formulation, proper proportionation
is also desirable to provide o~ ,m spraying conditions, 6uch a6,
formation of desired s~lmiyei3 viscosity, formation of desired particle
size, formation of desired S~l ayed fan shape, and the like.
Accol ~i..gly, in order to spray liquid co~ing formulations
cont~ining supercritical fluid as a diluent on a continuous, semi-
continuous, and/or an intennittent or peAodic on-demand basis, it is
necess~ to prepare such liquid co~t;n~ fonnulations in response to
such 6l 1 ayh.g by accurately miYing a proportioned amount of the
coating formulation with the 6upercritical ~uid. Ho~ve~e~, the
compressihility of supercritical fluids is much greater than that of
liquids. Consequently, a small change in pressure results in large
changes in the density of the 6upercritical fluid.
D-17147 ~173600
- 13-
The non-coml..essible ~uid in the present invention is
typically in the liquid state. The liquid state is characterized by the
strong interaction of the molecules, which di~ uishes liquids from
gases, and the state of disorder of the molectlkt-`motion, which
distinguishes liquids from solids. The behavior of liquids are generally
well understood and their properties tend not to vary ~i~tificstntly over
dis~rete ranges.
However, no known liquid solutions are esactly ideal.
Solutions of high]y ~imilstr components may only show slight deviations,
whereas greater deviation are observed in almost all other 601utions,
where the components differ in size, mass and chemical nature. It has
been observed that polymers do not easily blend to form true solutions.
As a result, polymers separate into distinct phases when brought
together if there are appreciable di~el ellces in the molecules. One of
the easiest ways to characterize the differences in behavior of liquid
mi~ture is to measure the density of the ~ e.
Figure 2 is a plot of liquid density versus composition of
water and ethanol and water and iso-propyl alcohol at atmospheAc
pressure at 20C. With the addition of ethanol or isol.ro~yl alcohol to
the mi~ture, the density of the mixture gradually decreases to the
density of the undiluted plcohol. Figure 3 demonstrates a ~jmils~r result
with a plot of the liquid density of dimethyl sulfoxide and ~cet4~e at
atmospheric temperature and pressure.
Some polymers in liquid solvents also behave ~imil~ly.
Referring to Figure 4, an acrylic polymer (AT954, Rohm & Haas Co.)
and n-methyl aryl ketone (MAK) were mi~ced at atmospheric pressure
and 25C. With increasing MAK levels, the density of the ~ e
decreased gradually to the density of pure MAK
Surprisingly, it has been discovered that in contrast to the above
~l~es wherein the density of the ~ e compositions uniformly
decreases, ~l~ es of polymeric compositions, solvents and
compressible fluids undergo a period wherein the density is relatively
constant. This relatively constant density mi~ture rem~inc until a two
D-17147 ~173600
- 14 -
phase mi~ture is created at which point the density of the mi~ture
changes rapidly.
Referring to Figure 6, a plot of ,.lule density ofthe components
listed in Table 1 below, in carbon dioxide is presehted .
T~T F l
COMPONF.~TS W~IGHT PF~CF~T
Alkyd, Reichhold 6256-03 21.6%
Nitrocellulose, 6.0%
Plasticizer 2.4%
Urea, Bettle 80 resin 10.0%
Solvents 60.0%
(mi~ture of MAK, i-propyl alcohol, n-butanol, and
ethyletho~y propionate (EEP))
With the addition of carbon dioxide (up to 30 weight
percent) to the composition in Tab!e 1, the mixture density decreased
less than 1.2 percent. With the addition of more than 40 percent carbon
dio~ide, mixture densities decreased siFnifis~ntly and two distinct
ph~ses were created, 8 carbon dioxide rich phase and a polymer rich
phase.
Without wishing to be bound by any theory, we believe
that the arrangement of the polymer and solvent molecules change with
the continuing addition of carbon dio~nde to a polymer-solvent ~ e
such that the coating forrmll~t;on maintains a constant ratio between
the total mass and the total volume (the density of the system). Such
effect would be a result of an çnh~ncetl interaction between the solvent
and the polymer due to the presence of carbon dio~ide. The large free
volume contributed to the system by the carbon dioxide would l,el~t a
better solvent and polymer interaction, resulting in a smaller density
reduction than expected.
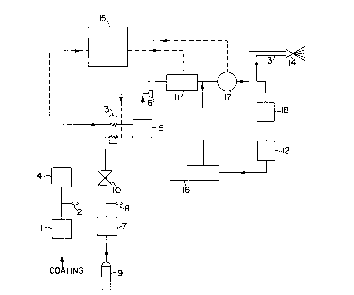
Referring now to Figure 6, apparatus is depicted which by
taking advantage of the relative constant density, is capable of
~ : } ~
D-17147 ~173600
- 16-
pllmping, pressurizing, proportioning, heAting, and miYing a co~ting
composition with carbon diogide to form an a-~mixed liquid ~ ure
through only volumet;ric measurem~nts The co~ c~mposit;on and
supercritical carbon dioYide is provided at the precisely desired
proportions ready for being sprayed. The a~& allls depicted herein is
able to simply and e!egantly p~ ,Ol ,ion the liquid miYture by m~king
use of-~e constant density pherlomen~ described herein. As notes~
above, while this ~i~cllcsion is focused on carbon dioxide it is not limited
to this material and the present invention may include any
compressible fluid.
In particular, carbon dioxide is supplied as a liquid from
any suitable source (9), such as a t~nk or cylinder. Preferably, the
liquid carbon diogide is supplied on a continuous basis. The carbon
diogide is then fed to carbon dioxide feed pump (7) through an optional
0-3000 psi pressure indicator (8). The carbon dioxide is sent to a control
valve (10) then heated to about 30-80C in the prehe~ter (3) and then
sent to mixer (5). Refernng now to the coating composition, the co~1;n~
is supplied by a pllmp (1) through an optional pressure gauge (2),
through a flow meter (4) to the preheater (3). The coating composition
is then sent to the miYing unit (5) to form the a~mixed liquid ~LI~e.
The flow rate of the coating composition and carbon dio~ide
are then measured by the 6econd flow meter (11). An optional
thermocouple (6) is preferably provided. An optional density meter (17)
is preferably provided to monitor the density of the ~miyer7 coating
formulation. In a most preferred embo~ime~t a density meter is
employed to ensure that the flow rate of carbon dioYide does not becoTne
so large as to create a significant change in the density of the ~miye~
coating formulation. A sight gauge (18) is ~.efelsbly employed for
phase analysis. The prlmiYetl co~ting formulation miYture can then be
adjusted to desired final temperature by an optional heater (not shown)
and provided through a conduit (13) to the spray gun (14). Tbe mixture
of coating and carbon dioYide also r~n be recirculated through the
heater (12) and recirculation pump (16) to m~int~in constant spray
D-17147 il73600
- 16-
temperature if desired. A multi-ch~nnel flow ratio co~ u~er (15)
receives the cign~l~ of the flow rates from both the flow meters and is
used to output Eignal to control the flow rate of tbe carbon dio~cide via
control valve (10).
The specific equipment items employed in Figure 1 are
listed in Table 2 below.
TART ~ ~.
ITF~l~I n~c~rpTIoN
Coatings feed pump, Graco Model 205-630
2 Pressure in~ tQr, range from 0 to 3000 psi
3 Nordson H-400 series paint beater
4 Precision gear meter, ZHM-01, AW. Co.
Sparger and static KenicsTM mixer
6 Thermocouple, k-type
7 Carbon dioxide feed pump, Haskel Model No.
DSF-25 witb 51050 Spool
8 Pressure indicator, range from 0 to 3000 psi
9 Carbon dioxide
Cylinder
Jordan control valve, Model 708, 0.002cv,
linear trim
11 Precision gear meter, ZHM-01, AW. Co.
12 Nordson paint heater
13 High pressure ~pray hose
14 High pressure spray gun
Multi-ch~nne1 flow ratio computer, EMO-1005
16 Ross MF-24-11-10-AAAA Recirculation pltmp
17 Micromotion Model No. D40HSS
Density meter
18 JergensonTM sight gauge
-_ ~ Q
D-17147 2173600
The type of volumetric flow meter used in the present
invention is not critical. Any suitable volumetric flow meter such as
gear meters, turbines and rotameters and the like may be used of which
gears meters are preferred.
Whereas, the exact scope of the present invention is set
forth in the appended ~ imc~ the following specific eY~mples illustrate
oertain aspects o~e present invention and more particularly, point o~t =
methods of evaluating the s~me. However, the examples are set forth
for illustration only and are not to be construed as limitations on the
present invention as set forth in the appended claims. All parts and
percentages are by weight unless otherwise` specified.
T~XAMPT,T~ 1
Apparatus suitable for studying the controll~hility of
compressible fluid, specifical~y carbon dio~ide, was constructed and is
depicted in Figure 7. The unit was comprised of feed pumps for coating
formulation (101) and carbon dioxide (107), two ~ow gear meters (104)
and (111), a control valve (110), heaters (103), a micro-processor based
flow controller (115) and a homogeneous mi~ing unit for the two fluids
(105). The co~ting material was fed from a cor~iner, and pressurized
to 1500-2200 psig at room temperature by an air-driven liquid co~ting
pump. The coating material was preheated to 30-40C through a heater
(103). The flow rstes of co~qting materisl were measured by a pre~i~iQn
gear meter (104). Liquid carbon dioxide was fed from a cylinder, and
pressurized to 1500-2200 psi at room temperature by an air-driven
carbon dioside liquid pump (107). Then carbon dio~ide was preheated
to 30-40C through a heater (103). These two ~uids were mixed
through a ~nising unit (105), which was comprised of a ~a,~el, and two
Kenies~M mixers.
The flow rates of the ~l~ e of coating material and
carbon dio~ide were measured by a precision gear meter (11~,), and
heated in heater (112) to 45-60C before spray application. The
D-17147 - ~173600
- 18-
mi~ture of coating and carbon dioxide were re-circ~ te-l through the
spray gun (114) to m~int~in constant spray tempelstula.
A multi-ch~nnel flow ratio co~u~er (115) ~ac~ived ~i~n~l~
of the flow rates from both gear meters, displayed the totaled flow rates,
and was used to manipulate the position of a carbon dionde control
valve (110) to control a required carbon dioxide cQncentration in the
coating mixture. ~or the data analysis, the flow rate of carbon dioxide
was also monitored with a mass flow meter (109), and the data from
gear meters (a) and (b) were interfaced to a computerized data
acquisition system (116).
The specific items listed in Figure 7 are as follows:
TART F. 3
ITEM n~SCR~PTION
101 Coatings feed pump, Graco Model 206-530
102 Pressure indicator, range from 0 to 3000 psi
103 Nordson H-400 series paint heater
104 Precision gear meter, ZHM-01, AW. Co.
105 Sparger and static KenicsTM mixer
106 Thermocouple, k-type
107 Carbon dioxide feed pump, ~kel
108 Pressure indicator, range from 0 to 3000 psi
109 Mass flow meter, Micro Motion meter Model No. D6
110 Jordan con-trol valve, Model 708, 0.002cv, linear
trim
111 Precision gear meter, ZHM-01, AW. Co.
112 Nordson heater H-400
113 High pressure spray hose
114 High pressure spray gan
115 Multi-channel flow ratio computer, EMO-1005
116 Computerized data acquisition system Cole Palmer,
L-08338-20
D-17147 ~173600
Figure 8 is a plot of co~tingS flow rate versus time (120
seconds) for continuous sl,~ay~lg of an A~mi~ed coating form~ t;on
from a spray apparatus depicted in Figure 7. The coAt;n g form~ t;oI~
was a mi~ture of acrylic and mel~mine polymers~and organic solvents.
Point #1 in tbe Figure 8 is the coatings flow rate measured by a
precision gear meter (104). Point #2 in the Figure 8 is tbe flow rate of
the admi~ed coatings formula~ion flow rate measured by a precicion
gear meter (111). From the disclosure of tbis invention, ca~l,oll dioxide
flow rate is the difference between tbe re~rlin~ of gear meter (111) and
the readings of gear meter (104).
~XAl~
Figure 9 illustrates carbon dioxide flow rates from a spray
unit using the coating formulation described in Table 1 in an appa atus
~imil~r to Figure 7 determined by two methods; 1) calc~llAti~ the
differences in flow rate between the two flow gear meters from Figure 7,
and 2) actual carbon dioxide flow rates measured by the mas6 flow
meter (109). The differences in the graphs is believed to be c~-lce~ by
response time delays and the effect of data averaging in the mas6 flow
meter, because it requires 0.2-0.6 second time delays for the flow
calculations to be conducted However, overall flows for 120 6ec~nrlR
were 89.0 cubic centimeters (cc) from this invention, and B9.~ gram6
from mass flow meter, indicating that the:
1. Density of the mixture of coAtings and carbon dioxide is
close to 1.0 grams/cc, which is almost the s~me as the density of co~tin~
material alone; and
2. The combir~tion of two volumetric flow meters can be
used to measure and accurately control carbon dio~nde con~entrations.
A~ .~ 3
Fi~re 10 shows three plots of flow rates; l)co~tin~
composition,; 2) mi~ture of coating composition and carbon dio~cide; and
3)carbon dio~ide for a 90 second continuous spray interval using the
D-17147 ~173~00 ~-
- 20 -
coating formulation in ~Y~mple 1. Apparatus simil~r to that disclosed
in Figure 7, without a recirculation loop was used. Line #1 in Figure 10
indicates co~tings flow rates measured by a precision gear meter. Line
#2 in Figure 10 was the coatings and carbon dio~de mi~cture flow rates
measured by a precision gear meter. Line #3 in Figure 10 carbon
dio~ide flow rates calculated from the differences between the re~iing~
of the gear meter and the reP~ings of the flow meters. Overall these
plots follow the same trends, and the totalized flow rates of coatings and
~lul e of coatings and carbon dio~ide for 90 seconds were 219.~ cc and
310.9 cc, respectively. Therefore, carbon dioxide flows for 90 seconds
were 91.4 cc from the method of the present invention, and 92.0 grams
as measured by the mass flow meter, indicating that the comhin~tion of
the two volumetric flow meters can be used to accurately measure and
control carbon dioxide flowrates.
~Al~ ~ 4
Figure 11 shows two flow rates: 1) coatings; and ~miYe~
coating formulations intermittently sprayed for 800 seCo~lc from a
spray unit described in Figure 7 without a recirculation loop. Point #1
in the Figure 11 indicates coatings flow rates measured by a precision
gear meter 104. Point #2 in the Figure 11 inrli~tes the flow rate of
coating and carbon dio2ide flow mixture measured by a pre~i~ion gear
meter 111. Overall, these plots followed the same trends, and the
totalized flow rates of coatings and ~lmi~ed coating formulation for 800
~econds were 219~ cc and 3109 cc, respectively. Therefore, carbon
dio~ide flows for 800 seconds were 914 cc as measured by the method of
the present invention, and 920 grams from mass flow meter. The
Example once again demonstrates that the comhin~tion of two -
volumetric flow gear meters can be used to measure and control carbon
dioxide concentrations accurately.
D-17147 2 i 73600
- 21 -
~XA~ ~ 5
In this e~ample, the total flowrate of carbon dioxide
obtained from a carbon dioxide mass flow meter and two volumetric
gear meters were compared at the different process conditions.
Apparatus depicted in Figure 7 was employed to make the comparisons.
T~A~,
CONrlITION~ l2 E
Conditions of
at mi~ing,
Temperature (C) 33 36 40 45 50
Pressure(psi) 1600 1600 1600 1600 1600
CO2, measured from
mass meter (grams) 101 115 105 96 86
C2 measured(cc) 105 121 112 120 130
from flow meters
Relative error (~c) 3.5 5.2 6.7 25 51
Relative error is defined as (CO2 from this invention-CO2 from mass
meter)/CO2 from mass meter.
As expected with increasing temperatures, the density of
the admixed coating formulation changes. The c~nging density of the
e results in a larger l,e~ ce~-tage error when relying on volumetric
measurements.
~XAl~IP~,~ 6
The total amount of carbon dio~ide mixed with a coating
formulation and sprayed from apparatus depicted in Figure 7 was
measured. The amount of carbon dio~ide used was measured using a
mass flow meter and two voIumetric gear meters. The coating
formulation consisted of 69 weight percent AT-954 Acrylic, available
D-17147 ~3600
- 22 -
from Rohm & Haas, and 31 weight percent MAK. The pressure and
temp~ at~ll e at the coatings and carbon dioxide mi~ing unit were
maintained at 1600 psi and 36C, respectively.
T~TA~ .
coNrlITIoN~
CO2, concentration in
the formulation 15% 27.6% 39%
CO2, measured from
mass meter (grams) 31 50 71
CO2, measured from
volumetric meter (cc) 30.5 60.0 74.9
solution appearance clear clear haze
Relative error (~) 1.6% 0.05'o 5.5%
Relative error is defined as
(C2 from this invention-CO2 from mass meter)/CO2 from mass meter
A clear solution appearance is indicative of a single phase
solution. A hazy appearance indicates that the solution is in two
distinct phases. This ~mple demonstrates the highly accurate
proportionation of the fluids when the single phase solution is
maintained. When a two phase solution is created, the density of the
60lution typically bègins to change rapidly and the accuracy of the
proportionation apparatus is liminished.
l;~Al~p~,~ 7
The apparatus of ~mple 1 was used to 6pray the
formulation of Table 1 with carbon dio~ide at 1500 psi and 50C.
At 1500 psi and 50C, densities of coating formulation a~d
supercritical carbon dio~ide were 0.9652g/cc and 0.3978 g/cc,
respectively. With the addition of carbon dio~ide into the form~ ;on
(a) up to 30 percent, the mixture densities decreased les6 than 1.2
percent. However, with the addition of more than 40 percent~ arbon
dio~de, mixture densities decreased significantly, and the co~ting
D-17147 ~ 3 ~ 0 0
- 23 -
formulation displayed two distinct ph~es; a carbon dioxide rich phase
and a polymer rich phase.
~ XAl~qP~J~ 8
Figure 13 is a graph o~ miYture density-of the coating
formulation listed below with carbon diogide at 1500 psi and 50C as
measured by the spray unit depicted in Figure 6. At 1500 psi and
50C, the densities of the coating formulation and supercritical carbon
dioxide were measured as 0.9700 g/cc and 0.3978 g/cc, respectively.
Adding carbon diogide into the admi~ed coating formulation up to
levels appro~çhing 40 percent, the a~rni~ed coating fonnulation
mixture density decreased less than 1.5 percent. However, with the
addition of more than 45 percent carbon dio~ide into the ~rlmiye~
coating formulation, the miYture density significantly decreased, and
the mi~ture separated into distinctive two phases.
Components Weieht ~er~nt
Alkyd, 6255-03 20.6%
Nitrocellulose, 5.7%
Plasticizer 9.5%
Water 4.8%
Solvents 57.1%
(miYture of MAK, i-propyl alcohol, n-bllt~nol, EEP)
~XAl~p~ ~ 9
Figure 14 is a plot of mi~ture density of co~tin~
formulation from ~Y~mple 8 with supercritical ethane at 1500 psi and
50C measured from a spray unit in Figure 5. At 1500 psi and 50C,
the densities of the coating formulation and supercritical ethane were
measured as 0.9652 g/cc and 0.203 g/cc, respectively. With the addition
D-17147 217 360n
- 24 -
of ethane into the formulation at levels up to about 25 percent, the
mixture was a fiingle clear phase. However, the mi~ture density
decreased more than 19 percent.
~Al~P~,~ 10
Figure 15 is a plot of ~l.u e density of coating
formulation from F~y~mple 7 with supercritical ethane at 1500 psi and
50C measured from the fipray unit depicted in Figure 6. At 1500 psi
and 50C, the densities of 100 percent of coating formulation and
supercritical ethane were measured as 0.9652 g/cc and 0.203 g/cc,
respectively. With the addition of ethane into the formulation (a) up to
17 percent, the mi~ture showed a fiingle clear phase. However, the
mi~ture densities decreased more than 11.7 percent.
Figures 12-15 clearly demonstrate that the une~pected
constant density properties of the admi~ed coating formulations
especially when using supercritical carbon dioxide as a viscosity
reducing agent. However, when a two phase solution is created, tbe
density of the mixture can vary significantly witb increasing
COlll~l essed fluid levels. The ethane mixtures did not exhibit a
fiubst~nti~lly constant density region when admi~ed, therefore the
present invention would not be fiuitable for accurately proportionately
these mi~tures.