Note: Descriptions are shown in the official language in which they were submitted.
CA 02173862 2004-11-19
-1-
APPARATUS AND METHOD FOR TRANSFER OF A FLUID SAMPLE
FIELD OF THE INVENTION
The present invention relates generally to methods for fluid transfer, and in
particular to a method for transfering amplified nucleic acid from a reaction
chamber
to a detection chamber within a closed and sealed container.
BACKGROUND OF THE INVENTION
The amplification of nucleic acids is useful in a variety of applications. For
example, nucleic acid amplification methods have been used in clinical
diagnostics
and in typing and quantifying DNA and RNA for cloning and sequencing.
Devices for performing nucleic acid amplification reactions are known
generally as thermal cycling devices or thermal cyclers. One example of such a
device
is described in published PCT Application, WO 92J20778. The PCT application's
cycling device is useful in performing DNA amplification by techniques. The
device
described in WO 92J20778 includes a ring-shaped holder having a plurality of
wells
for accepting pipette tips containing samples. The samples are contained
within the
tips by heat sealing an open end of each tip. Means are provided for heating
and
cooling the ring, thereby allowing the device to cyclically heat and cool
samples in
the pipette tips. The means for cooling the ring includes a fan for drawing
cool air
over the ring, and cooling fins positioned radially inward from the ring to
assist in
directing cool air over the ring.
Methods of amplifying nucleic acid sequences are known in the art. For
example, the polymerase chain reaction ("PCR") method utilizes a pair of
oligonucleotide sequences called "primers" and thermal cycling techniques
wherein
one cycle of denaturation, annealing, and primer extension results in a
doubling of the
target nucleic acid of interest. PCR amplification is described further in
U.S.
2173ss2_
-2-
Patent No. 4,683,195 and U.S. Patent No. 4,683,202.
Another known method of amplifying nucleic acid sequences is the ligase
chain reaction ("LCR"). In LCR, two primary probes and two secondary probes
are
employed instead of the primers used in PCR. By repeated cycles of
hybridization and
ligation, amplification of the target is achieved. The ligated amplification
products are
functionally equivalent to either the target nucleic acid of interest or its
complement.
This technique was described in EP-A-320 308, and subsequently in EP-A-336-
731,
WO 89/09835, WO 89/12696, and Barany, Proc. Natl. Acad. Sci., 88:189-193
(1991).
Variations of LCR are described in EP-A439-182 and in WO 90/01069.
Other known methods of amplifying nucleic acids employ isothermal reactions.
Examples of such reactions include 3SR (Self sustained Sequence Replication)
E.Fahy,
D.Y.Kwoh & T.R.Gingeras, in PCR Methods and Applications 1:25 (1991); and SDA
(Strand
Displacement Amplification) G.T.Walker, M.C.Little, T.G.Nadeau & D.D.Shank, in
Proc.
Nat. Acad. Sci. U.S.A., 89:392 (1992).
Amplification of nucleic acids using such methods is usually performed in a
closed
reaction vessel such as a snap-top vial or a sealable pipette as disclosed in
WO 92/20778.
After the amplification reaction is completed, the reaction vessel is opened,
and the amplified
product is transferred to a detection apparatus where standard detection
methodologies are
used.
Typically, the amplified product is detected by denaturing the double stranded
amplification products and treating the denatured strands with one or more
hybridizing probes attached to a detectable label. The unhybridized labelled
probes
usually must be separated from the hybridized labelled probe, and this
requires an
extra separation step. In other detection methods, the amplification products
may be detected
by gels stained with ethidiurn bromide. Thus, 3zP tracings; enzyme immunoassay
[Keller et
al., 1, Clin. Microbiolo~y, 28:1411-6 (1990)]; fluorescence [Urdea et al.,
Nucleic Acids
Research,16:4937-56 (1988); Smith et al., Nucleic Acids Research, 13:2399-412
(1985)]; and
chemiluminescence assays and the like can be performed in a heterogenous
manner
[Bornstein and Voyta, Clin. Chem., 35:1856-57 (1989); Bornstein et al., Anal.
Biochem.,
180:95-98 (1989); Tizard et al., Proc. Natl. Acad. Sci., 78:4515-18 (1990)] or
homogenous
manner [Arnold et al., U.S. Patent No. 4,950,613; - Arnold et al., Clin Chem.,
A
WO 95/11437 PCT/US94/11016
21?~~~ 2 .
-3-
X5:1588-1589 (1989); Nelson and Kacian, Clinica Chimica Acts, 1,4:73-90
( 1990)].
These detection procedures, however, have serious disadvantages. Whcn
the reaction vessel containing a relatively high concentration of the
amplified
product is opened, a splash or aerosol is usually formed. Such a splash or
aerosol
can be a source of potential contamination, and contamination of negative, or
not-
yet amplified, nucleic acids may lead to erroneous results.
Similar problems concerning contamination may involve the work areas and
equipment used for sample preparation, reaction reagent preparation,
amplification,
and analysis of the reaction products. Such contamination may also occur
through
contact transfer (carryover), or by aerosol generation.
Furthermore, these previously described detection procedures are ti:r:e-
consuming and labor intensive. Probe hybridization techniques typically requue
denaturing the extension products, annealing the probe, and in some cases,
separating excess probe from the reaction mixture. Gel electrophoresis is also
disadvantageous because it is an impractical detection method if rapid results
are
desired.
US Patent 5,229,297 and corresponding EP 0 381 501 A2 (Kodak)
discloses a cuvette for carrying out amplification and detection of nucleic
acid
material in a closed environment to reduce the risk of contamination. The
cuvette is
a closed device having compartments that are interconnected by a series of
passageways. Some of the compartments are reaction compartments for amplifying
DNA strands, and some of the compartments are detection compartments having a
detection site for detecting amplified DNA. Storage compartments may also be
provided for holding reagents. Samples of nucleic acid materials, along with
reagents from the storage compartments, are loaded into the reaction
compartments
via the passageways. The passageways leading from the storage compartment are
provided with one-way check valves to prevent amplified products from back-
flowing into the storage compartment. The sample is amplified in the reaction
3 0 compartment, and the amplified products are transferred through the
interconnecting
passageways to detection sites in the detection compartment by applying
external
pressure to the flexible compartment walls to squeeze the amplified product
from
the reaction compartments through the passageways and into the detection
compartments. Alternatively, the cuvette may be provided with a piston
arrangement to pump reagents and/or amplified products from the reaction
compartments to the detection compartment.
WO 95111437 PCTlUS94/11016
-4-
Although the cuvette disclosed in EP 0 381 501 A2 (Kodak) provides a
closed reaction and detection environment, it has several significant
shortcomings.
For example, as illustrated in Figures 1 to 18 of the application, the
multiple
compartments, multiple passageways, check valves and pumping mechanisms
present a relatively complicated structure that requires some effort to
manufacture.
Also, the shape and configuration of the cuvette disclosed in EP 0 381 501 A2
do
not allow it to be readily inserted into conventional thermal cycling devices.
In
addition, the fluid transfer methods utilized by the cuvette call for a
mechanical
external pressure source, such as a roller device applied to flexible side
walls or the
displacement of small pistons. Conventional thermal cycling devices are not
readily
adapted to include such external pressure sources. Finally, the apparatus
described
in this reference is quite limited in terms of throughput of the disclosed
devices.
The system does not provide the desired flexibility for manufacturing.
French patent publication No. FR 2 672 301 (to 1.ar?ul) discloses a similar
hermetically closed test device for amplification of DNA. It also has multiple
compartments and passages through which sample and/or reagents are
transferred.
The motive forces for fluid transport are described as hydraulic, magnetic
displacement, passive capillarity, thermal gradient, peristaltic pump and
mechanically induced pressure differential (e.g. squeezing).
Methods for performing homogeneous amplification and detection have
been described in a limited manner. Higuchi et al., BiofTechnolo~"y, 10:413-
417
(1992) describe a method for performing PCR amplification and detection of
amplified nucleic acid in an unopened reaction vessel. Higuchi et al. teach
that
simultaneous amplification and detection is performed by adding ethidium
bromide
to the reaction vessel and the reaction reagents. The amplified nucleic acid
produced in the amplification reaction is then detected by increased
fluorescence
produced by ethidium bromide binding to ds-DNA. The authors report that the
fluorescence is measured by directing excitation through the walls of the
amplification reaction vessel before, after or during thermal cycling.
US Patent 5,210,015 also discloses a method of amplifying and detecting
target nucleic acid wherein detection of the target takes place during a PCR
amplification reaction. The reference teaches adding to the reaction mixture
labeled
oligonucleotide probes capable of annealing to the target, along with
unlabeled
oligonucleotide primer sequences. During amplification, labeled
oligonucleotide
fragments are released by the 5' to 3' nuclease activity of a polymerise in
the
217386 2
-5-
reaction mixture. The presence of target in the sample is thus detected by the
release
of labeled fragments from hybridized duplexes.
US Patent 5,585,242 entitled "Method and Device for Detection of Nucleic
Acid or Analyte Using Total Internal Reflectance" also discloses a reaction
vessel
wherein amplification and detection are accomplished in the same vessel.
Amplification products are captured on an optic element via specific binding
to
immobilized capture reagents. Combination of the amplification product with
the
capture reagent brings a fluorescent label within the penetration depth of an
evanescent wave set up in the optic element. A change in fluorescence results
from
the coupling of the fluorescent label and is detected.
In spite of these disclosures, neither closed reaction vessels nor homogeneous
assays have gained wide commercial use. Thus, there is a need for an
amplification
and detection system that avoids the shortcomings of the prior art, and also
provides
an efficient, reliable and sterile testing environment, in an easily
manufactured format.
SUMMARY OF THE INVENTION
In general, the present invention is directed to methods for transferring a
fluid
sample from a reaction area to a detection area. In the preferred method the
reaction
area is a thermocycling chamber for nucleic acid amplification analysis, but
it will be
understood that the invention has broad application to many other target
ligands,
assay configurations and/or type's bf chambers.
In one aspect, the invention relates to a method for transferring a fluid
sample
between a reaction chamber and a detection-chamber within a device, comprising
the
steps of a) providing a device having a reaction chamber and a detection
chamber
connected by means for fluid communication between the reaction and detection
chambers, and having a reaction sample disposed in said reaction chamber,
wherein a
propellant is also disposed in said reaction chamber such that the propellant
and
sample are intermixed or such that the sample is between the propellant and
the means
for fluid communication, and wherein further the propellant is inducible to
expand;
and b) inducing the propellant to expand to occupy a larger volume, thereby
forcing
the sample through the means for fluid communication into the detection
chamber.
,~,.:._
WO 95/11437 PCT/US94/11016
-6-
Preferably, the reaction chamber is an elongated or tubular construction
having at least one or two longitudinal segments and being closed at one end
and
having an opening into the detection chamber at the opposite end. The
propellant,
which may be any substance which can be induced to expand, ma~r.be the
reaction
sample itself or it may be a distinct substance lodged at or near the closed
end of the
reaction chamber. Ideally, the propellant may be induced to expand by a non-
mechanical stimulus, such as light or heat. Expansion of a propellant should
be
distinguished from mechanical pressure increases arising from non-expansion
events, such as hydraulic pressure or deformable septums.
Although not required by the invention, expansion of the propellant may
encompass a phase change, such as the vaporization of a liquid to a gas. In
such a
situation, it is convenient to localize the vaporization by using a nucleation
site in
the reaction chamber. Such a nucleation site may include inert particulate
matter,
such as boiling chips or glass or plastic microbeads, in the range of about
1.0 to
O.lmm in diameter, or a grooved, ridged or roughened surface inside the
reaction
chamber. Preferably, the nucleation site is localized at or near the bottom of
the
reaction sample to more efficiently force the sample from the reaction
chamber.
A preferred use of the method of the invention is for transferring a reaction
sample containing nucleic acid that has been amplified by a thermal cycling
process
2 o such as the ligase chain reaction or the polymerase chain reaction to a
detection
chamber without opening the sealed reaction/detection unit, thereby avoiding
or
significantly reducing the possibility of contamination of the work area by
amplified
nucleic. acid. Thus, a cycling reaction can be effected by applying
intermittent heat
to a first longitudinal segment and transfer can be effected by applying heat
to a
second longitudinal segment closer to the closed end. The means for applying
heat
to the two segments may be the same or different. Alternatively, in the case
of a
single longitudinal segment and a single means for applying heat, cycling can
be
effected by internlittently applying a first maximum amount of heat, and
transfer can
be effected by applying heat in excess of said first maximum to "superheat"
the
3 0 propellant.
BR_TEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates by block diagram the general components of the system
of the present invention;
Figure 2A to 2H illustrate several views of one variation of the reaction/
detection unit prior to assembly. Figure 2A, a partial cross-section taken
along line
WO 95/11437 PCT/US94/11016
2173 fi 2.
a-a in Figure 2C, shows the upper or detection chamber. Figure 2B shows the
lower or reaction chamber aligned for insertion into the detection unit.
Figure 2C is
a cross sectional view taken along lines c-c in Figure 2A. Figures 2D and 2E
are
cross sectional views taken along lines d-d and e-e, resepctively, in Figure
2C. It
can be seen that Figure 2D represents a front angle, while Figures 2A and 2E
represent side angles. Figures 2F, 2G and 2H show the reaction/detection unit
after
sealably engaging the reaction chamber to the detection chamber, and inserting
it
into the thenmal cycler holder. Figure 2F is a side cross sectional view like
2A,
while Figure 2G is a front cross sectional view and shows a variation in the
keying
means. Figure 2H is a cross section taken along line h-h in Figure 2F.
Figures 3A to 3D illustrate several embodiments and variations of a
reaction/detection unit in accordance with the invention. Figures 3A and 3B
illustrate a snap-fit embodiment of the reaction/detection unit after sealably
engaging
the reaction chamber to the detection chamber. Figures 3C and 3D show in cross-
section a variation of the reaction/detection unit, wherein the engaging means
and
detection configuration differ from those of Figures 3A and 3B.
Figures 4A to 4D illustrate enlarged views of the sealable engaging means
of the assembled reaction/detection unit. Figure 4A shows a standard friction
or
Luer fit in cross-section; Figure 4B shows a pawl or snap fit seal in cross-
section;
Figure 4C shows a different variation of a pawl or snap fit seal in schematic;
and
Figure 4D shows a screw thread type seal in cross-section.
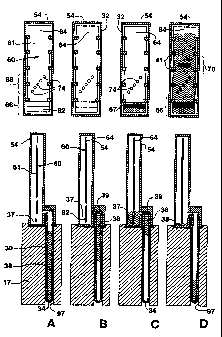
Figures SA to SD illustrate the transfer of an amplification reaction sample
from the reaction chamber to the detection chamber of the unit, according to
methods of the invention. Above each side view of the detection chamber is a
front
view of same.
Figure 6 illustrates a preferred embodiment of a two-tier heating element for
use in connection with ttye invention, each tier being configured as an
annular ring.
Figure 7 illustrates a partial cross-sectional view of a preferred thermal
cycler device of the invention.
Figures 8A to 8D illustrate altennative embodiments of preferred detection
systems of the invention. Figure 8A shows an embodiment with a motorized ring;
Figure 8B shows a stationary ring with motorized mirror and lamp; Figure 8C
depicts a reflectance detection arrangement; and Figure 8D depicts a
transmission
detection arrangement.
WO 95/11437 ,a~' PCTIUS94/11016
_g_
Figures 9A to 9K are flow charts illustrating a control program for
controlling the heating elements of a two-part thermal cycler according to the
invention.
Figure 10 illustrates a time and temperature profile for various aspects of
the
system of Figure 1.
Figures 11A to 11D are flow charts illustrating a computer program for
processing a video image according to the invention.
Figures 12A and 12B show enlarged read zone portions 68 of the strip
supports shown in Figures 2A and 3A, respectively.
Figures 13 and 14 are digitized photographic images of the results of six
reaction samples as described in Examples 6 and 12, respectively. In each
Figure,
the three samples on the left contained target DNA and a spot or band is
visible; the
three on the right did not.
DETAILED DESCRIPTION OF SOME EMBODIMENTS OF THE INVENTION
OUTLINE OF DETAILED
DISCLOSURE:
1. System Overview
2. Reaction/Detection Units
a. Reaction Chambers
b. Detection Chambers
c. Detection Supports
d. Sealing Mechanisms
3 . Thenmal Cycling and Transfer Device
a. Cycler Devices
b. Transfer Methods
4. Detection Systems
5. Computer/Circuit Controls
6. Heat Control
a. Hardware
b. Software
7. Video Processing
8. Methods for Amplifying and Detecting
Nucleic Acids
9. Kits of the Invention
~ 10. Examples
11. Sequence Listing
1. System Overview .
Figure 1 is a generalized schematic diagram of an amplification and detection
apparatus configured in accordance with the invention. The apparatus 10
includes a
thermal cycling device 16, including first and second heating element tiers 17
and 18
and associated thermosensors 122, 123, a fan motor 19 and a detection system
22, each
of which will be described in more detail below. The apparatus 10 also
includes a
WO 95/11437 PCTIUS94/11016
217~~fi 2
-9-
computer controller 26 coupled to the thermal cycling device 16. In general,
the
thermal cycling device 16, under control of the computer 26 which sends
independent
signals to each of heater tier 1 (17) and heater tier 2 (18), is capable of
independent:-.
delivering prescribed tempesature(s) to localized segments of reaction
containers housed
inside the thermal cycler device 16, in order to amplify and/or transfer
target nucleic
acid present in the reaction samples. Details of the computer control of the
device 16
are described in later sections.
The apparatus l0 also includes a plurality of reaction/detection units <~ (see
Figures 2-3). The units 20 have a two-part, sealable construction that
includes a
reaction chamber 30 and a detection chamber 32, as shown in Figures 2A to 2H
and 3A
to 3D. The reaction chamber 30 houses the reaction sample for carrying out the
desired
amplification reactions. The detection chamber 32 is provided with means for
generating a detectable indication of the results of the amplification
reaction. Specific
aspects and variations of these reaction/detection units 20 are described in
detail later in
this disclosure.
The amplification reaction methods begin by ins~:~ting a reaction sample 38
into
the reaction chamber 30, along with desired amplification reagents. The
detection
chamber 32 is then mated with the reaction chamber 30 to form the sealed unit
20 which
is then placed into the heating tiers 17, 18 of the thermal cycling device 16
as best
shown in Fig 2F and SA-SD. After the reaction and detection chambers 30, 32
are
mated, the unit 20 remains sealed, thus providing a closed environment for
carrying out
both amplification and detection.
The computer 26 controls the temperature settings and the timing of any
temperature cycles, depending on the type of amplification reaction that is
being
performed. For amplification reactions such as PCR or LCR, the computer 26 is
programmed to take the heating tiers through one or more cycles of a
high/denaturing
temperature, followed by a low/annealing temperature. Where two tiers are
provided,
the computer 26 is capable of controlling the temperature of the upper heating
tier 17
independently of the lower heating tier 18, although they may also follow
identical
protocols.
At the end of the amplification reaction and without opening the sealed
reaction/detection unit, the reaction sample is transferred from the reaction
chamber 30
to the detection chamber 32 of the sealed unit 20. The reaction sample is
preferably
transferred by expanding a propellant in the reaction chamber 30 to force the
sample
and reagents into the detection chamber.
WO 95111437 ~ ,~ ~ ~ - PCT/US94111016
-10-
The detection chamber 32 includes detection means for generating a detectable
indication of the results of the amplification reaction. Generally, the
detection means
includes a support 60 having one or more capture sites 74 for immobilizing and
accumulating amplified target nucleic acid present in the reaction sample 38.
The
immobilized amplified target nucleic acid is associated with a detectable
indicator at the
capture sites 74, and this indicator is detected and analyzed by the detection
system 22
and the computer 26.
The various components of the apparatus 10 will now each be described in
greater detail, including multiple variations on the general overview set
forth above.
2. Reaction/Detection Unit
a. Reaction Chambers
Reaction/detection units 20 of the present invention are shown in Figures 2A
to
2E, 3A to 3D and in other figures as well. Each unit 20 includes a reaction
chamber 30
and a detection chamber 32. The unit 20 may be disposable.
The nucleic acid amplification reaction takes place in the reaction chamber
30.
The reaction chamber 30 is made of a material such as glass or plastic that
can
withstand the temperatures necessary for denaturation of nucleic acids,
typically 80-
110 °C. The bottom end 34 of elongated reaction chamber 30 is closed,
and the top end
36 is open to accept a reaction sample 38 and, if desired, amplification
reaction
reagents. Such reaction reagents may be added to the reaction chamber 30 by
the user,
but they are preferably included during manufacture and enclosed by a
removable or
rupturable seal (not shown), in which case only the test sample is added by
the user.
Test sample can be inserted in the reaction chamber 30 by any known means. For
example, it can be placed in a syringe (not shown) and inserted into the-
reaction
chamber 30 by removing the seal or puncturing it with a hollow-bore syringe
tip.
Thus, reaction sample 38 in the chamber 30 includes both the test sample and
amplification reagents. It may additionally include a propellant 40 and one or
more
components of the detection system.
3 o The size of the chamber 30 should be selected so as to barely contain the
relatively small quantities of reaction sample 38. Preferably, the chamber 30
is
dimensioned to hold a reaction sample of about 10 N,L to about 200 N.L. Even
more
preferably, the chamber 30 holds about 50 ~.L to about 120 EtL. The reaction
chamber
should also be of suitable dimensions so that surface tension in the reaction
chamber
30 is reduced and bubbling of the reaction sample during heating is avoided.
Further,
the reaction chamber 30 should have a high surface area to volume ratio to
enhance the
WO 95/11437 PCTlUS94/11016
217362
-ll-
rate of heat transfer to the reaction sample. Preferably, the reaction chamber
30 is an
elongated tubular shape having a longitudinal axis. In one preferred
cmbodiment, the
reaction chamber 30 is a microsyringe tube or capillary tube sealed at the
bottom end.
It has bean found that smooth interior-walled reaction chambers perform poorly
compared to chambers that have irregular surfaces in the interior,
particularly at the
closed or bottom end 34. For example, open microsyringe or capillary tubes
that are
heated to seal one end perform well, the heating apparently introducing
irregularities in
the interior surface; while a closed-end capillary tube (e.g. from Varivest,
Grass Valley,
CA: see example 4) performed less well unless it too was melted first. It is
hypothesized that the irregular surface provides a nucleation site for
vaporization to
begin at or near the bottom of the sample. However, applicants do not intend
to be
limited to or bound by any particular theory or mechanism of operation.
Mechanically grinding or roughening of the interior of the tubes will also
improve performance as will grooves or ridges in the interior. Performance may
also
be improved by the addition of small boiling chips or sticks, or microparticle
beads to
~'~~'~ottom of the reaction tube. For example, beads of polystryene, glass,
ceramic,
ess steel or other suitable inert material ranging in size from about 1.0 to
0.1 mm
diameter are useful as nucleation sites. Particle size is not thought to be
critical,
provided the particles fit within the reaction chamber. Such particles should
be inert to
the reaction reagents and should be more dense than the reaction sample.
b . Detection Chambers
The separation of amplified target nucleic acid from the reaction sample takes
place in the detection chamber 32, as shown in Figures 2 and 3. The detection
chamber
32 is made of a transparent material, such as plastic or glass, and has an
open end 48
and a closed end 54. Reaction sample 38 flows into the detection chamber 32
via the
open end 48, where it encounters a detection support 60 (described in detail
below).
In a preferred embodiment (Fig. 2) the detection chamber includes a reservoir
37 for holding sample fluid delivered from the reaction chamber. This may be
3 0 accomplished, for example, by directing the sample fluid into open end 48
and through
a flow path having an orifice 39 above the level of the floor of the detection
chamber
32, so that fluid enters from the side of the chamber. Alternatively, a
standpipe inlet
can create a reservoir. The reservoir 37 maintains a supply of reaction sample
fluid
available to the detection support means 60, even in the face of cooling and
receding of
the fluid sample within the reaction chamber 30 (Compare Figures SC and SD, in
which
fluid in the reservoir is absorbed by the strip 61 rather than receding back
down the
PCT/US94/11016
WO 95/11437
-12-
reaction tube). For elongated detection chambers having reservoirs and a side
entry
orifice 39, it may also be helpful to mold angled fins 43 to bestow additional
strength
on the entire detection chamber.
In another preferred feature, -the cross sectional shape (Figure 2C) of the
detection chamber is polygonal or asymmetric such that it may be seated in a
matching
groove in the heating tier in only one possible orientation. This is best
shown in
Figures 2F and 2H, which depicts a trapezoidal shaped seat. For transmission
detection configurations (see infra) it is preferable that the front and rear
faces of the
chamber remain substantially parallel. A trapezoid is the simplest polygon
that does
this while still dictating a fixed orientation. However, other polygonal or
asymmetric
shapes may be envisaged. For reflectance detection configurations (see infra),
the front
and rear faces need not be parallel and other polygons are suitable. If a
rounded seat
configuration is employed it may possess a cam or a flat side to dictate a
single
orientation. The seat need not have the same configuration as the optical
face(s).
The detection chamber 32 (and/or the reaction chamber 30) may include tab
members 58 (shown in Figs 2G and 7) which support the chamber within the
thermal
cycling device 16 and which provide for easy handling. The tab member 58 may
also
include means for engaging a key groove 91 (shown in Figures 2G and 7) located
in
the heating tier 17. This alternative to the polygon shape also ensures a
prescribed
orientation for the detection chamber 32 with respect to the heating tier, and
also with
respect to the detection system 22 provided the detection system is fixed with
regard to
the heating tier.
Figures 3A - 3D show alternative embodiments to the preferred embodiment of
Figure 2. These embodiments have similar components and features and these
have
been given the same reference numeral as in the embodiment of Figure 2. The
embodients of Figure 3 do not, however, include the reservoir feature.
The unit 20 can also be provided with a bar code (not shown) which is
preferably located on the detection chamber 32. A bar code reader (not shown)
provided on the thermal cycling device 16 for reading the bar code can then
communicate the encoded information to the computer 26. The bar code can
identify
the particular unit 20 and can provide other pertinent information about the
sample and
the reaction to be performed. Some of this information may include the patient
identity
and/or the configuration of the capture sites 74 as described later in this
disclosure in
connection with the video processing program implemented by the computer 26.
c. Detection supports
WO 95/11437 PCTIUS94/11016
~1~3~6 2
-13-
The detection chamber 32 also includes detection support means 60 for
accepting the reaction sample, separating the amplified target DNA and
generating a
visible indication of the results of the amplification reaction. Typically the
detection
support means includes a solid support on which signal indicative of the
presence of
target can be accumulated, as is well known in heterogeneous assays.
Such solid supports include, for example, plastics, glass, natural and
synthetic
polymers and derivatives thereof, including cellulose esters, microporous
nylon,
polyvinylidine difkuoride, paper and microporous membranes. Supports may be
shaped, for example, as fibers, beads, slides, cylindrical rods or strips. In
a preferred
embodiment, the detection support means 60 is a microporous strip 61 shown in
Figures 2, 3 and 5 capable of supporting capillary migration. More preferably,
the
porous support is nitrocellulose, such as nitrocellulose having pore size of
about 2 ~tm
to about 20 ~,m, usually S or 10 ~xn. Preferably, the porous support is inert,
or
rendered inert through the use of blocking agents and/or transport
facilitating agents
(see, e.g. U.S. Patent 5,120,643) and does not generally react physically or
chemically
with any of the reagents or target nucleic acid in the reaction sample. The
use of
transport facilitating agents is known in the art, and is further discussed in
Example 3.
Porous and microporous supports exhibit wicking by capillarity and
chromatographic
properties; however, non-chromatographic supports and non-porous supports are
contemplated by the invention as well.
The detection support means 60 can be any suitable shape, including a round or
disc shape, or rectangular shape. The size or dimensions of the detection
means 60
should be selected to provide sufficient resolution of the visible indicator
produced ~by
amplified target nucleic acid immobilized on the detection means 60. The
detection
means 60 is preferably small and/or thin in order to shorten the time needed
for
detection of immobilized target nucleic acid and to min: .ze material usage.
Those
skilled in the art will be able to optimize dimensions of the detection means
60 in
relation to the volume of the reaction sample 38, the amount of amplified
target, and the
size of the reaction chamber 30 and the detection chamber 32. The detection
chamber
32 may be configured to house the detection means 60.
Typically, different support materials 60 will accept and transport the
reaction
sample 38 at varying rates depending, for instance, on pore size and thickness
of the
support. The support should be selected so that it does not transport the
reaction
sample 38 past specific binding pair members or capture molecules, described
further
below, at a rate that exceeds the time rewired for binding amplified target
nucleic acid.
WO 95/11437 ~ °°~~ ~ '~ PCT/US94/11016
-14-
The preferred support 60 is a strip 61 that includes a first end 62 at which
reaction sample transport begins, a second end 64 at which reaction sample
transport
ends, and one or more regions 66, 68, 70 containing the mechanisms for
allowing
amplified target nucleic acid to be isolated in the detection chamber 32.
As shown in Figs 2D and SD, the strip 61 comprises at least two regions,
wherein a first region 66 at or near the first end 62 of the strip 61
functions in labeling
amplified target nucleic acid present in the reaction sample, and a second
region 68
functions in separating the labeled amplified target nucleic acid from the
reaction sample
by immobilizing the amplified target on the strip 61. The second region 68 may
include
one or more zones, with each zone including at least one capture site 74 for
immobilizing target nucleic acid and providing a visible indication when the
target
nucleic acid has been immobilized on the capture site. Capture sites 74 may be
arranged as continuous bands, as in Figures 2D and 3C; as discontinuous bands,
as in
Figure 2G; or as individual spots, as in Figures 3A and SA-SD. The
significance of
multiple capture sites and replicate sites within a capture area is discussed
infra.
It will be realized that the labelling function need not occurr on the strip
itself,
but may occur at any point between the reaction sample and the capture sites,
including
within the reaction sample. For example, a conjugate pad may be attached to
the
bottom end of a detection support medium. Such a pad might also be placed in
the
open end 36 of the reaction chamber, in the open end 48 of the detection
chamber, or in
the orifice 39 or the reservoir 37 of the embodiment shown in Figure 2. If the
conjugate pad is not attached to the strip it appears preferable to at least
have it contact
the strip.
The strip 61 may include a third region 70 which functions as a control zone
or
reference standard for the detection system 22. Preferably, all such regions
66, 68, 70
are spatially distinct areas of the support 61. The functions of the regions
66, 68, 70
are described in further detail below in connection with the methods for
detection of
amplified target nucleic acid(s).
The support 61 may, if necessary, be affixed to an inert substrate preferably
made of a transparent material such as glass, plastic or nylon which is
sufficiently rigid
to provide structural support. In the embodiment depicted in Figures 2 and 5,
the
detection chamber is equipped with pins or fingers 41 which hold the strip
rigidly in
position. Such pins or fingers 41 can be molded into the chamber housing
during
manufacture. The support and substrate are preferably in a fixed location or
angle
within the detection chamber 32 so that detection of amplified target nucleic
acid
immobilized on the support 61, as described further below in connection with
the
WO 95/11437 PCT/US94/11016
21736 ~
-ls-
methods of the invention, can take place at a predetermined location or angle
with
respect to the detection system 22.
d. Sealing Mechanisms
Detection chamber 32 is designed to sealingly mate with the reaction chamber
30 to prevent the escape of any amplified nucleic acid once the amplification
reaction is
performed. For this reason, reaction/ detection unit 20 includes engagement
means for
sealably engaging the chambers 30, 32 together. The engagement means may be
accomplished by any of several known means. The engagement means should form a
secure seal so that the chambers 30, 32 do not leak potentially contaminating
fluids; in
other words, they should not become unsealed or disconnected under conditions
of
increased temperature or pressure, or under normal handling and/or disposal.
Figures 4A to 4D illustrate several mechanisms for sealably engaging or mating
the two chambers 30, 32 of the unit 20. Perhaps the simplest mechanism is the
standard Luer or friction fit. This is illustrated in enlarged detail in
Figure 4.~ as well
as in Figure 2 and others. The open top end 36 of the reaction chamber 30
includes an
angled facing 44 around its outside perimeter, and the open end 48 of the
detection
chamber 32 includes an angled facing s0 around its inside perimeter. The angle
of the
bevel on the two faces 44, 50 is matched so that a tight friction fit is
achieved when the
two chambers are pressed together as shown in Figures 2E, 2F, 3C, 3D and 4A.
Althc~ngh not shown, variations on this sealing mechanism include the Luer
lock
syste:a and a bayonet locking system.
A second sealing mechanism is illustrated in detail in Figure 4B. This is a
snap-
fit or pawl variation of the standard Luer fit. The top end 36 includes the
beveled face
44 and an annular shoulder or pawl 46 around its outer periphery. The
detection
chamber 32 includes the beveled face s0 and an annular pawl or shoulder s2.
Again,
the bevel angle is matched tc~ produce a tight seal, and the annular shoulders
46, s2 lock
with one another to prevent the two portions from becoming separated. Another
variation of a snap fit seal is illustrated in Figure 4C. Although shaped
somewhat
differently, the elements are all similar and have been given identical
reference
numerals. A snap-fit is achie~~°ed by engaging the ends such that
shoulder s2 moves
over facing 44 and into engagement with shoulder 46. .~
In a final sealing mechanism, illustrated in Figure 4D, the open end 36 of the
reaction chamber 30 is fitted with male screw threads 47. The inside of the
open end
48 of the detection chamber 32 is similarly fitted v: .rh matching female
screw threads
49. By twisting the n"action°chamber into the detection chamber, a
sealed
reaction/detection unit is obtained. Many other equivalent seal variations are
possible
WO 95/11437 PCT/US94I11016
-16-
and within the scope of the invention: Ideally, the seal mechanisms are
virtually
irreversible under normal handling conditions.
Reaction/detection units 20 according to the invention may be used with either
one or two tier thermal cycling devices, as described below.
3. Thermal CXcling and Transfer Device
a. Cycler Devices
Figures 6 and 7 illustrate the details of a preferred embodiment of the
thermal
cycling and transfer device 16 shown schematically in Figure 1. It should be
understood, however, that both one-tier and mufti-tier heating/transfer units
are suitable
for use with the devices and methods of the invention. Thus, the cycler 16
includes at
least one heating tier 17, and optionally two heating tiers 17 and 18 for
delivering the
desired temperatures) to the reaction chamber 30 under control of the computer
26. In
one embodiment the heating tiers constitute an annular upper heating ring 90
that is
spatially separated from an annular lower heating ring 92. The airspace
between the
heating rings 90, 92 acts as an insulator, although other insulating materials
may be
employed. The heating tiers may have a variety of other shapes such as linear,
planar
or wedge (not shown). One or more cooling fins 93 are placed on the rings 90,
92,
typically spaced radially inward to assist in reducing the temperature of the
rings 90, 92
during cooling periods. A fan 94 is positioned below the cooling fins 93 to
further
assist in reducing the temperature of the rings 90, 92 during cooling periods.
The heating rings 90, 92 are made from a heat conducting material such as
aluminum, copper or gold. Heat may be delivered to the rings 90, 92 via
conventional
resistive heat strips 95, 96 attached to the rings, preferably along a
perimeter surface of
the rings 90, 92 as shown in Figure 6, or by other known means such as a
manifold or
by conductance. In mufti-tier systems, the computer 26 can independently
control the
temperature of each heating ring 90, 92 by supplying power independently to
the each
of the heat strips 95, 96. It can also track the two tiers together as if one.
As shown in Figure 7, the unit 20 is placed inside one of several apertures or
wells 97 in the heating rings 90, 92 such that a first longitudinal segment 33
of the
reaction chamber 30 is exposed to the upper ring 90, and a second longitudinal
segment
of the reaction chamber 30 is exposed to the lower ring 92. As shown in
Figures 6
and 7, the wells 97 are each made from an aperture 98 in the upper ring 90 in
registration with an aperture 99 in the lower ring 92. The upper ring
apertures 98
35 extend completely through the upper ring 90. The lower ring apertures 99
may extend
wholly through the lower ring 92, as shown in Figures 2G and 7, provided there
is
WO 95/11437 PCT/US94/11016
21~3~62.~
-17-
some means for supporting the reaction/detection unit 20 in the well 97 such
as the tab
member 58 described earlier. Alternatively, apcrrures 99 may extend only
partially
through the lower ring 92 to allow the closed bottom end 34 of the reaction
chamber 30
to rest in the lower ring 92.
, The computer 26 (see Fig 1) controls the upper heating ring 90, the optional
and
lower heating ring 92 and the fan 94 to direct preselected temperatures) to
the reaction
sample 38 in the reaction chamber 30. The heating and cooling cycles of the
thermal
cycling device 16 and their control by the computer 26 are described in more
detail
below in the disclosure relating to Computer/Circuit Controls. When the
amplification
reaction is complete, the computer 26 directs the heating element to deliver
heat to the
propellant 40 at or above its threshold expansion temperature. When the
threshold
temperature a reached, the propellant 40 expands, thereby forcing the reaction
sample
38 upward into the detection chamber 32. In one embodiment the propellant is
expanded by heating the lower ring 92 in excess of the upper ring 90.
b . Transfer Methods
Figures SA-SD illustra~e the reaction sample 38 as it is transferred from the
reaction chamber 30 to the detection chamber 32 in a one tier apparatus. The
unit 20 is
placed inside aperture 97 in the heating element 16. In an alternate two tier
system, the
reaction chamber 30 is placed in the apertures such that a first longitudinal
segment 33
(Figures 2B and 3A) of the reaction chamber 30 is exposed to the upper ring
90, and a
second longitudinal segment 35 (Figures 2A and 3A) of the reaction chamber 30
is
exposed to the lower ring 92.
In Figure SA, the amplification reaction has been completed, and the heating
element 16 is being raised to the threshold temperature of the propellant 40.
In two tier
systems the upper ring 90 may initially be held to a temperature below the
threshold
temperature to reduce the potential for evaporating the reaction sample 38
after the
amplification reaction is complete. It is preferred that the propellant
threshold
temperature be above the highest amplification reaction temperatures) so that
the
3 o propellant 40 does not expand during the amplification reaction.
As used in the present invention, "propellant" refers to any substance that
expands in response to a stimulus, preferably a non-mechanical stimulus. For
instance,
the propellant 40 may be a gas (such as air), a liquid, or a solid compound.
In the case
of liquid and solid propellants, they are generally vaporizable to cause
expansion. The
stimulus for expanding the propellant 40 may be, for example, heat, light, or
a '
combination thereof, but preferably is heat in the present invention. The
reaction
WO 95/11437 PCT/CIS94/11016
n~f:
i
-18-
sample 38 itself may serve as propellant 40. Mechanical pressures, such as
hydraulics
or septum deformation do not result in expansion of a propellant.
In Figure 5B, the heating element 16 has heated the propellant 40 to its
threshold temperature, and the propellant 40 has expanded to push the reaction
sample 38 upward toward the detection chamber 32. In two tier systems at this
point,
the upper heating ring 90 may be brought to the threshold temperature to
assist in
expanding the propellant 40 as it moves up through the first longitudinal
segment 33.
As will be described later in connection with Fig 10, the computer 26 is
provided with a
programmable time delay to allow the upper heating ring 90 to be superheated
to the
threshold temperature after the lower heating ring 92.
The heating element 16 (or both upper and lower heating rings 90, 92) continue
to deliver the threshold temperature to expand the propellant 40, as shown in
Figure SB
and SC, until the reaction sample 38 has been transferred completely into the
detection
chamber 32, preferably into reservoir 37 thereof via side opening 39.
In Fig SC> the first region 66 of the detection strip 61 is beginning to
become
wetted. This region (or a prior portion of the sample path, see above)
preferably
contains a label (e.g. zone 67) which becomes associated with the amplified
target
nucleic acid passing through this region. One method for accomplishing this
association is by means of a hapten bound to the nucleic acid and a colloidal
particle
conjugated with anti-hapten antibody. Colloidal gold or selenium are suitable
labels, as
is colored latex particles. Haptens and haptenation is lmown in the art,
especially bi-
haptenation methods in connection with LCR and PCR amplifications of nucleic
acid.
For example, see EP-A 357 011 and EP-A-439 182. As the haptenated nucleic acid
passes through zone 67, label conjugate is solubilized and mobilized by the
reaction
solution and it binds with the haptens on the nucleic acid. As an alternative,
one may-
attach a detectable label directly to the probe,/primer provided it does not
interfere with
hybridization or any required enzymatic activity, such as extension and
ligation.
As the solution migrates up the strip 61, it encounters the capture sites 74
in
region 68, and optionally the control sites in region 70. At the capture sites
74, a
3 0 second antibody against a second hapten is immobilized against transport.
All nucleic
acid bound to this hapten becomes immobilized at these sites. If the
immobilized
nucleic acid was amplified and thereby contains the first hapten as well, then
conjugate
will accumulate at the capture site and become detectable (Fig SD). Each
capture site 74
may contain immobilized antibody against a different hapten, thus enabling
multiplex
amplification and detection by the methods of the invention. Alternatively,
multiple
WO 95/11437 PCT/US94/11016
217~~62 .
-19-
capture sites 74 may contain antibodyagainst the same hapten, thus enabling an
averaging of the signal among each of the sites.
It should also be understood that the transfer by thermal expansion aspects of
this invention are not limited to nucleic acid assays or to thermal cyclers.
The transfer
aspect is useful any time it is desired to move a reaction sample from a
reaction location
to a detection location. It is especially useful in situations where it is
desirable (e.g. for
contamination reasons) to make the transfer within a sealed or closed
container.
However, it may be used in non-amplified and non-nucleic acid assays, such as
immunoassays, provided the reagents can tolerate the levels of heat necessary
to effect
the transfer.
4. Detection Systems
The results of the amplification reaction are detected and analyzed by the
detection system 22 and the computer controller 26. The detectable label is
preferably a
visible label, but other detectable labels, such as W,1R or fluorescent
labels, are also
possible. The preferred detection system 22 generates a video image of the
support 60
and includes a video camera 100 and a light source 104 (both shown in Figures
7 and
8A to 8D) for illuminating the support 60. An image of the support 60 is
pro~~ided to
the camera 100, either directly or by reflection, and the camera 100 generates
a video
image which is fed to the computer 26. For simplicity, visible labels will be
discussed
2 0 further.
A ~ ariety of configurations are suitable for the detection system 22; some
are
depicted in Figs 8A to 8D. In general, the detection system 22 should include
a light
source 104 for illuminating the detection means 60 and a camera 100 for
creating video
images of the detection means 60. The camera lens may be pointed directly at
the
detection means 60, or a mirror may be provided for reflecting an image of the
detection
means 60 to the camera lens.
As shown in Fig 8B, the detection system 22 includes a camera 100, a camera
lens 102, a light source 104, a mirror 106 and a motor 108 (preferably a
stepper motor)
coupled to the mirror 106. The light source 104 is positioned such that the
camera lens
102 measures the colorimetric signals reflected from the support 61. The
camera 100
and the mirror 106 are positioned axially with respect to the heating rings
90, 92, and
the mirror 106 is positioned at an angle such that it reflects an image of the
porous
support 61 to the camera lens 102. The camera 100 is stationary, and the
mirror 106 is
rotated by the motor 108 under computer control to successively present an
image of
the strip 61 of each detection chamber 32to the camera lens 102. The camera
100
generates a video image of the strip 61 of each detection chamber 32 and
passes this
CA 02173862 2004-11-19
WO 95/11437 PGT/US94/11016
-20-
image to the computer 26 for analysis. The software for analyzing this image
is
described later in the Video Processing section.
Figure 8A illustrates another configuration of the detection system 22. This
detection system includes a camera 100, a camera lens 102, a light source 104,
a mirror
106, and a motor 109 coupled to the heating rings 90, 92. The light source 104
is
positioned such that the camera lens 102 measures the colorimetric signals
reflected
from the support 61. The camera 100 and the mirror 106 are positioned axially
with
respect to the heating rings 90, 92, and the mirror 106 is positioned at an
angle chosen
so that it reflects an image of the support 61 to the camera lens 102. The
camera 100
and the mirror 106 are stationary, and the heating rings 90, 92 are rotated by
the motor
109 under computer control to successively move each detection means into view
to
present an image of the strip 61 of each detection chamber 32 to the mirror
106 which
reflects the image to the camera lens 102. The camera 100 generates a video
image of
the support 61 of each detection chamber 32 and passes this image to the
computer 26
for analysis.
In an alternative embodiment, the camera lens 100 can be pointed directly at
the
support 61, thus eliminating the need for the mirror 106. In another
alternative, the
light source may be inside the ring while the camera is outside the ring, or
vice versa
These alternatives utilize transmission detection, discussed below in
connection with
Figure 8D.
In Figure 8C, a reflectance fluorescence detection system is provided with a
camera 100, a camera lens 102, a light source 104, an excitation filter 110
and an
emission filter 112. The light source 104 and the camera 100 are positioned
such that
the camera lens 102 receives the fluorescent signals emitted from the support
61 in the
detection chamber 32. The excitation filter 110 is positioned between the
light source
104 and the support 61, and the emission filter 112 is positioned between the
support
61 and the camera lens 102.
In Figure 8D, another fluorescence detection system is provided with a camera
100, a camera lens 102, a light source 104, an excitation filter 110 and an
emission
3 0 filter 112. The light source 104 and the camera 100 are positioned such
that the support
61 is between the light source 104 and the camera 100. Thus, the camera lens
102
nxeives the fluorescent signals transmitted through the support 61. The
excitation filter
110 is-positioned between the light source 104 and the support 61, and the
emission
filter 112 is positioned between the support 61 and the camera lens 102.
CA 02173862 2004-11-19
WO 95/11437 PGT/US94/11016
-21-
Circuitry suitable for transmission detection is generally known,
although a particular circuit is described in co-owned v . s . Patent
5,387,790, entitled Light Intensity Detection and
Measurement Circuit for Measuring the Duration of the
Discharge Cycle of a Capacitor Network, issued
February 7, 1995.
It is contemplated that detection systems could utilize either the
transmission or
reflectance methods shown in Figures 8C and 8D; and either method for
presenting
successive detection means 60 to the camera. In particular, the detection
systems could
incorporate the rotating mirror and motor shown in Figure 8B, or the rotating
heating
rings 90, 92 and motor shown in Figure 8A (with or without the mirror).
5. Computer/Circuit Controls
~ 5 As shown in Figure 1, the computer controller 26 may be implemented as an
IBM AT-compatible personal computer having a monitor 113, keyboard 114 and
data
storage means. The computer 26 includes an image frame grabber card 116, a 16-
bit
analog/digital I/O card 118 and a custom printed circuit board (PCB) 120. A
suitable
frame grabber card 116 is the Coreco'~' OC-300 which is available from Coreco
(Montreal, Canada). A suitable analog/digital I/O card 118 is that available
from Data
Translation Company.
The diagram of Figure 1 illustrates a simplified representation of the
circuitry
contained in the frame grabber card 116, I/O card 118 and the PCB 120. The
frame
grabber card 116 accepts video signals from the camera 100 for processing and
analysis. The 1/O card 118 and the PCB 120 combine to control the heating and
cooling cycles by controlling the heating strips 95, 96 and the fan 19. The
PCB 120
contains conventional circuitry which is used to deliver the appropriate power
to the
heating strips 95, 96 and the fan 19, and also to monitor the actual
temperature of the
heating strips 95, 96. A pair of thetmistors 122,123 are coupled to the
heating rings
3 0 90, 92 to sense the temperature of the rings 90, 92. The thermistors 122,
123 generate
an output signal representing the temperature of the rings ~90, 92, and this
signal is fed
back to the PCB 120.
The computer 26 includes software programs that control the temperature of the
heating rings 90, 92 by controlling the hating strips 95, 96 and the fan 19.
The
3 5 computer 26 also includes software programs for grabbing and analyzing the
video
signal input at the frame grabber card 116. Figures 9A to 9K illustrate a flow
chart of a
WO 95111437 PCT/US94/11016
-22-
suitable heat control program 200. Figures 1 lA to 11D illustrate a flow chart
of a
suitable video processing program 600. The heat control program 200 and the
video
processing program 600 may be implemented using commercially available
prograrntt>utg languages such as BASIC or C.
6. Heat Control
a. Hardware
In general, the heat control program 200 provides instructions to the PCB 120
via the I/O card 118. For example, the heat control program 200, which
communicates
with digital signals, sets a desired "set" temperature for the upper and lower
heating
rings 90, 92. The I/O card 118 converts the digital computer signals into
analog signals
at the D/A converters 126, 128. One D/A converter is provided for each heating
strip
and thus, when two heating blocks are employed, the temperature of each may be
controlled separately. The analog output from D/A converter 126 is coupled to
the
upper heating tier 17 via comparator 130 and solid state relay 132, and the
analog
output from D/A converter 128 is coupled to the lower heating tier 18 via
comparator 134 and solid state relay 136.
The output from one relay 132 is coupled to the upper heating strip 95 which
is
coupled the upper heating ring 90. The output from another relay 136 is
coupled to the
lower heating strip 96 which is coupled to the lower heating ring 92. The
relays 132,
136 enable power to the heating strips 95, 96 which in turn deliver heat to
the heating
rings 90, 92. Thermistors 122, 123 are coupled to the heating rings 90, 92 for
sensing
the temperature of the heating rings 90, 92 and developing electric signals
corresponding to the sensed temperature. The signals from thermistor 122 are
coupled
through an operational amplifier 138 to comparator 130, and the signals from
the other
thetmistor 123 are coupled through an operational amplifier 140 to comparator
134.
The outputs from the operational amplifiers 138, 140 are also fed to A/D
converters
142, 144 on the 1/O card 118 to provide the computer 26 and the heat control
software
with digital signals representing the current temperatures of the upper
heating ring 90
and the lower heating ring 92.
The computer 26 generates a digital signal represeriting the desired or "set"
temperature for each tier. These are accepted by the PCB 120 at the D/A
converters
126, 128 and converted to analog signals to control the heating strips 95, 96
in order to
achieve these set temperatures. Comparators 130, 134 continuously compare the
voltages an its two input lines. For comparator 130, the input voltages
correspond to
the upper heating ring 90 temperature (from thermistor 122) and the set
temperature
WO 95/11437 PCT/US94/11016
2'~~3~6 2
-23-
received from the D/A converter 126. For comparator 134, the input voltages
correspond to the lower heating ring 92 temperature (from thermistor 123) and
the set
tempcrature received from the D/A converter 128. When the sensed temperature
of
either t the heating rings 90, 92 is less than its set temperature, the
corresponding
comparator, 130 or 134, continues to output the set temperature to the heating
strips 95,
96 via the relays 132, 136. When the sensed temperatures of the heating nines
90_ 92
exceed the set temperatures, the comparators 130, 134 cut off the output to
the heating
strips 95, 96. The program may then direct the PCB via solid state relay 137
to turn on
the fan motor 19, and conversely, to tum it off when the cooling period is
complete;
i.e. when the low set temperature is reached.
b . Software
The flow chart illustrated in Figures 9A to 9K uses conventional block symbols
to represent the major functions performed by the heat control program. The
heat
control program 200 has four major sections or routines. The first section is
the
"Initialize" section 202, shown in Figure 9A, which gets the computer hardware
ready
to receive data by defining software variables and fixed hardware parameters
in a
conventional manner. The initialize section 202 is executed once when the
computer 26
is powered up. The second section is the "Edit" section 204, shown in Figures
9B to
9D, which allows the operator to set and/or alter the different parameter
choices that
2 0 define the particular denature protocol, if any, and Cycle/Superheat
protocol, if any.
The third section is the "Denature" section 206, shown in Figures 9E to 9G,
which
instructs the PCB 120 to take the heating rings 90, 92 to the temperature
chosen for the
denature protocol. The fourth section is the "Cycle/Superheat" section 208,
shown in
Figures 9H to 9K, which instructs the PCB 120 to take the heating rings 90, 92
to the
temperatures chosen for the cycling protocols and the superheat, or threshold,
protocol.
As described earlier in this disclosure, the superheat protocol expands the
propellant 40
in the reaction chamber 30 to thereby transfer the reaction sample 38 from the
reaction
chamber 30 to the detection chamber 32. The program 200 preferably repeats the
high
and low temperature cycling for a predetermined number of cycles X and then
moves to
3 0 the superheating cycle
As shown in Figure 9A, the Initialize section 202 starts the program 200 at
bloc': 210 and then initializes the software constants and variables at block
212. Block
212 performs such conventional steps as allocating and defining memory
locations on
the computer hardware and defining program variables. These steps are
necessary in
order to allow a computer program to communicate efficiently with the computer
hardware. At blocks 214, 216 and 218, the program 200 allows the operator to
either
WO 95/11437 PCT/US94/11016
-24-
specify a desired protocol file (stored in computer memory or data storage) or
to accept
a set of default protocol values. The protocol file contains values for a set
of
parameters that define the characteristics of a particular cycling/superheat
protocol. In
either event, the protocol parameters may be altered by the operator in the
Edit section
204 described below. For the disclosed embodiment of the heat control program
200,
the following parameters are included in the protocol file, and exemplary
values are
given in the far right column. In the disclosed program 200 the Shutoff
Temperature
(which is used only at the end of the operation to turn the fan off) is not an
editable
parameter, but is preset.
Param. Name Description Example Value
TEMP.DEN= Denature Temperature 95C
TIh~IE.DEN= Denature Time 120 sec
TEMPLO= Low Cycle Temperature 60C
TIIvvIELO= Low Cycle Time 60 sec
TEMPHI= High Cycle Temperature 80C
TIMEHI= High Cycle Time 60 sec
TIIVVIELEAD= Lead Time For Superheat 15 sec
TIMESUPER= Overall Superheat Time 30 sec
TEMPSUPER2= Upper Block Superheat Temperature 95C
TEMPSUPER= Lower Block Superheat Temperature 110C
CYCLEMAX= Total Number Of Cycles 8
TRACK= Tracking (on/off) off
SHUTOFF= Shutoff Temperature At End Of Reaction
50C
TIIVViEIMAGE= Image Delay Time 120 sec
The parameters will
be described with
reference to Figure
10, which is a
plot of
temperature vs.
time for the heating
rings) (and consequently
the reaction chamber
30)
as they are taken denature protocol, a cycling protocol
through a and a superheat
protocol. Figure
10 assumes there
are two heating
tiers, but that
either they parallel
one
another or only
one is in use until
the superheat cycle.
As shown, the heating
rings) start at a particular temperature at Time To. This temperature may be
any value
at or below the holding temperature from the end of the last amplification
reaction. For
the illustrated example, the heating rings) are about room temperature at To.
After To,
the heat control program 200 instructs the PCB 120 to bring the heating rings)
to a first
"set" temperature, in this case the "Denature Temperature", the value of which
is
selected for denaturing nucleic acid in the sample and/or any probe or primer
reagents.
The Denature Temperature typically ranges from about 80-100°C; the
exemplary value
is 95°C. As the set temperature cannot be attained instantaneously, the
temperature
gradually rises or "ramps" up to the set temperature during the period from To
to Tt.
Via feedback thermistor(s) the program 200 senses when the heating rings) have
WO 95/11437 PCT/US94/11016
21~'3~6 2
-25-
reached the selected set temperature and holds this temperature for the
predetermined
period from Tl to T2 (the "Denature Time's in order to denature the sample DNA
and
any reagent probes or primers.
At the conclusion of the Denature Timc (T~ the program resets the set
temperature to the "Low Cycling Temperature" and the heating rings) "ramp"
down to
this new set temperature during the period from T2 to T3 , which is maintained
for the
"Low Cycling Time". Preferably the ramp down times (e.g. T2 to T3 and T6 to
T~) are
minimized by turning on the fan 19 to help cool the heating ring(s). The
values for
these parameters are selected to provide the temperature and time for
reannealing
primers or probes to the suspected target or amplicons made from target.
Annealing
temperatures depend on probe length and the content of guanosine and cytosine
residues, as is known in the art, and are typically set several degrees below
the
predicted Tm for the probes or primers. For typical probe and primer lengths,
Low
Cycling Temperatures can range from about 45-70 °C; the exemplary value
being set at
60 °C. This period is shown in Figure 10 from T3 to T4.
Next, the program resets the set temperature and ramps up to the "High Cycling
Temperature" which is held for the "High Cycling Time" as shown in Figure 10
from
T4 to TS and TS to T6. Values for the High Cycling Temperature and High
Cycling
Time are selected to again denature the probes or primers from the target or
a.mplicons.
Generally the High Cycling Temperature is slightly lower than the sample
Denature
Temperature, but it must be greater than the Tm of the amplicons. Values
ranging from
about 70-95°C are common; the exemplary value is 80°C.
After the High Cycle Time r~as expired, the program resets the set temperature
to the "Low Cycling Temperature", the heating rings) "ramp" down to T7 and the
process repeats. Each cycle consists of a high and a low temperature, as shown
in
Figure 10. "Total Number of Cycles" is the parameter whose value controls the
number of cycles. The number of cycles will vary greatly depending on the
assay
being performed. For both PCR and LCR, it is not uncommon to have between 10
and
70 cycles, generally between 25 and 50.
After the Total Number of Cycles has been achieved, the program moves into
the Superheat aspect to transfer the reaction sample 38 from the reaction
chamber 30 to
the detection chamber 32 as described above in connection with Figures SA -SE.
In
two tier systems, this is generally accomplished by superheating the lower
tier first and
the upper tier second for reasons described above. Optionally, the lower tier
is also
superheated to a higher temperature than the upper tier as shown in Figure 10.
The
WO 95/11437 5 PCTIUS94/11016
-26-
Lower Block Superheat Temperature and the Upper Block Superheat Temperature
are
the parameters that hold the values for these superheat stages. As mentioned
earlier,
these values are selected to expand a propellant, thereby forcing the reaction
sample into
the detection chamber. This temperature is generally as high or higher than
the
denature temperature, but it need not be since the propellant can be shielded
from the
denaturing temperatures by placing it low in the reaction chamber (i.e. within
the lower
tier) and not tracking the two tiers. For simplicity, an aqueous reaction
sample may
serve as propellant and the superheat temperatures will generally range from
about 90-
120°C.
In two tier systems, the "Lead Time For Superheat" is an optional time period
during which the lower heating ring 92 is brought to its superheat temperature
before
the upper heating ring 90 is brought to its superheat temperature. The Lead
Time For
Superheat is shown in Figure 10 from TS to T"_ An exemplary value is given
above as
seconds. Depending on the value for Lead Time and the slope of the superheat
15 ramp-up, the Lead Time (TS to T,~ may be greater than, equal to or less
than the ramp
time (TS to Tp); in other words, the relative positions of T" and Tp may be
reversed
from that depicted.
The "Overall Superheat Time" holds the time value for the superheat stage,
commencing when the upper tier (or the single tier if only one is used)
reaches its set
temperature (e.g. the Upper Block Superheat Temperature). This time is shown
in
Figure 10 from Te to Tr and needs only be sufficiently long to uansfer an
adequate
volume of the reaction sample to the detection chamber. This of course is
dependent on
the sample volume and the detection means, but is easily determinable by
simple
experiment. An exemplary value is 30 seconds. It should be noted, however,
that all
exemplary times and time ranges are subject to the specific embodiments
utilized herein
and that the use of other ranges is easily within the ability of those stalled
in the art
The "Tracking" parameter determines in the case of a two tier heating element
whether both the upper and the lower heating rings 90, 92 participate in the
denature
protocol and the cycling protocols. If the Tracking parameter is on, both
heating
rings 90, 92 participate in the denature protocol and the cycling protocols.
If the
Tracking parameter is off, only one of the heating rings 90, 92 participates
in the
denature protocol and the cycling protocols.
The "Shutoff Temperature At The End Of The Reaction" is the set temperature
at which the program 200 turns off the fan motor that cools the heating rings
90, 92 at
the end of the testing protocol, represented in Figure 10 by Th.
WO 95/11437 PCT/US94/11016
2~73~fi 2
-27-
The "Image Delay Time" merely signals the computer to wait a specified tune
before beginning the detection procedures. This time should be sufficient to
pemut the
signal in the detection chamber to fully develop, arI may range from about 1-
10
minutes or more, depending on the ty p~e of signal and detection means
employed.
It will be appreciated that one may select an amplification protocol that
calls for
a high cycle temperature before the first low cycle temperature. In this case,
the period
from T2 to T3 is simply expanded to include a plateau at the high cycling
temperature
for a time determined by the selected protocol before continuing its ramp down
to the
low temperature.
Figure 10 also shows the Program States for the Denature and Cycle/Superheat
routines. These are described below in connection with the software.
Returning again to Figure 9A, after the protbcol file is selected (blocks 214,
216
and 218), the program 200 then places a help text and the current protocol
parameters
on the monitor 113 screen at blocks 220 and 222. Block 220 provides help
information
to assist operators in deciding what steps to take to continue the program
200. The
screen headings at block 222 also provide prompts regarding keystroke entries
to obtain
a desired result.
The program 200 initializes a thermistor look-up table at block 224. Although
the resistance of the thermistors 122, 123 varies with temperature, these
temperature
2 0 changes are not linear. Thus, a look-up table is provided so that the
program 200 does
not have to recalculate the temperature every time a reading is delivered from
either of
the thermistors 122, 123. The I/O card 118 is initialized at block 226. This
sets the
various values that will be used on the 1J0 card 118 suchwas the gain settings
on the pre-
amp stages or the use of unipolar (0 volts to 10 volts) or bipolar (-5 volts
to +5 volts)
signal ranges. At block 228, the protocol parameters are initialized and the
1!O card 118
is prepared to convert temperatures to digital. Block 230 moves the program
200 to the
Edit section 204.
The Edit section 204 of the program 200 is shown in Figures 9B, 9C and 9D.
In general, the Edit section 204 allows the operator to change some or all of
the
protocol parameters chosen at blocks 216 and 218 of the Ir'valize section 202.
The
Edit section 204 clears the keyboard 114 at block 236, wiii~;n is equivalent
to setting
Key=0, and displays the current protocol parameters at block 238. The program
200
provides a continuous display of the current temperature of the heating rings
90, 92.
This is accomplished at blocks 240 and 242 by reading the analog inputs from
the
upper and lower heating rings 90, 92, converting these inputs into temperature
values at
the themiistor look-up table, and displaying the temperature on the monitor
113. In
WO 95/11437 PCTIUS94/11016
-28-
block 244, the program 200 also displays on the monitor 113 the parameter edit
command instructions which provide prompts to the operator for editing the
protocol
parameters.
The Edit section 204 then looks for a keyboard input at block 246 until one is
received. The operator may now edit protocol parameters by hitting any of the
keys
shown in blocks 250, 256, 260, 264, 270, 280, 284, 288, 294 and 298. The "U"
key,
shown at block 250, takes the program 200 to block 251 which allows the
operator to
reset the high cycling temperature and the time duration of the high cycling
temperature.
Similarly, the "L" key, shown at block 256, takes the program 200 to block 258
which
allows the operator to reset the low cycling temperature and the time duration
of the low
cycling temperature. The "C" key, shown at block 260, takes the program 200 to
block
262 which allows the operator to set the maximum number of cycles. The "W"
key,
shown at block 264, takes the program 200 to blocks 266 and 268 which allow
the
operator to save the edited parameter protocols in a file in the computer's
memory. The
"F" key, shown at block 270, takes the program 200 to block 272 which allows
the
operator to turn on the fan 94 and thereby bring down the temperature of the
heating
rings 90, 92, if desired. The "D" key, shown at block 280, takes the program
200 to
block 282 which allows the operator to edit the denature temperature and the
time
duration of the denature protocol. The "H" key, shown at block 284, takes the
program 200 to block 286 which allows the operator to edit the superheat
parameters.
The superheat parameters include the superheat temperature for the lower
heating ring,
the lag-time for superheating the upper heating ring, the superheat
temperature of the
upper heating ring, and the overall time period for the superheating. The "T"
key,
shown at block 288, takes the program 200 to block 290 which allows the
operator to
edit the tracking parameter. After the program 200 polls the T key at block
288, the
timers are set at block 292 in anticipation of starting the Denature section
206. The "E"
key, shown at block 294, takes the program 200 to block 296 which exits the
program
200. The "S" key, shown at block 298, sets the "state," "cycle number",
"RTime" and
"key" all to 0 (block 300), and moves the program 200 to the Denature section
206
from block 304. If the S key is not pressed, the program 200 returns to the
beginning
of the Edit section 204.
The Denature section 206 (Figures 9E, 9F and 9G) begins at block 310 and
displays the current protocol parameters at block 312. Block 314 clears the
keyboard
inputs, and block 316 examines the value that was entered for the denature
temperature
(TEMP.DEI~. If the denature temperature has been set to 0, the program 200
skips the
denature protocol and sets the "cyclenum" flag to 1 and the state flag to 0
(block 318)
WO 95/11437 PCT/US94/11016
~'~~36 2
-29-
before moving into the Cycle/Superheat routine via block 320. By entering the
Cycle/Superheat section 208 via block 420, the program starts the sample out
at the
High Cycling Temperature by setting SETTEMP equal to TEMPHI at block 422 and
by
entering the Cycle/Superheat routine 208 with the state flag at 0.
However, using the example value above, the Denature temperature is set to a
value greater than zero (95°C), so the program 200 initializes the
Denature temperature
and Denature time at block 322 which includes several subroutines for getting
the
uacking information, setting the Denature temperature and turning the fan 94
off.
"Setting" a temperature or a time involves creating a variable such as
SETTEMP,
SETTEMPO or SETTEMP1 for temperature, and RTIME for time, and assigning a
value to said variable the value being selected from one of the parameters
described
above: namely, TEMP.DEN, TEMPLO, TEMPHI, TEMPSUPER and
TEMPSUPER2 for temperature variables and TIME.DEN, TIMELO, TIMEHI,
TIMELEAD and TIMESUPER for the time variable. Thus, at block 322, the
SETTEMP variable assumes the value stored in the protocol for the Denature
Temperature.
Blocks 311, 324 and 326 show that Denature section 206 continuously polls the
keyboard 114 for parameter edit inputs from the operator. If a keyboard input
is
received, the program 200 moves to the Edit section 204, and the operator can
then edit
any of the current protocol parameters. The program 200 updates the
temperature
display at blocks 328 and 330.
At block 332 the program 200 branches to poll either temperature or time
depending on the value of the program state flag, the key flag and the RTime.
Since
RTime (as well as other variables) was set to 0 at block 300, the program
polls
temperature on this first pass through the loop and moves on to block 336.
Here, the
program 200 examines the TRACK variable to determine if both blocks of a two
tier
system should be cycled in parallel or not. If TRACK=on, block 338 sends the
program to block 356 which examines both blocks. If TRACK=off, block 340
causes
the program to examine only one block-the upper block in this example. For the
remainder of this description, is will be assumed that TRACK=off, but one
skilled in
the art will readily recognize the mirror-like nature of certain sections of
the flow
diagrams. Of course, in a single heating element system, the TRACK variable is
unnecessary and only one block is examined. The following description assumes
a two
block system wherein the upper block only is used for denaturing and cycling,
it being
understood that this is just one embodiment.
WO 95/11437 PCT/US94111016
-30-
In the Denature section 206, the program state flag can have four values from
0
to 3. In general, when the program state flag is 0 (see block 344), the
program 200 has
signaled the PCB 120 to take the heating rings to the denature temperature,
and the
program 200 (at block 332) polls the A/D converters 142, 144 on the I/O card
118 to
determine when the upper heating ring has reached the denature temperature
(see block
346). If the upper heating ring has not yet reached the denature temperature,
the
program 200 moves through blocks 350, 372 and 382, and returns to the main
denature
loop near the beginning at block 311. From there, the program returns to block
346
and again inquires as to whether the upper heating ring has reached the
denature
temperature (95°C).
The program 200 continues this loop until the upper heating ring 90 has
reached
the denature temperature. The answer at block 346 is now yes, and the program
200
sets the key flag to 1 at block 348. When the heating rings 90, 92 reach the
denature
temperature, the key flag is set to 1 at block 348, and the program state flag
is
incremented to 1 at block 374. In addition, the variable RTime is set to
assume the
value of parameter TIZviE.DEN (Denature Time) at block 378, the timer is
started at
block 380 and the program returns to the main denature loop (blocks 382 and
311).
Because RTime now holds a value (120 seconds in the example), the program
branches at block 332 to the "Timecheck" subroutine at block 396 and inquires
if
RTime has timed out. RTime "times out" when the period set for the particular
activity
(in this case, the 120 sec. Denature Time) expires. If the answer to this
inquiry is no,
the program loops back through the beginning of the Denature section 206 and
returns
via blocks 332 and 334 to the timeout inquiry at block 398. If the answer to
the timeout
inquiry is yes, then the program 200 increments the program state flag (to 2
now) at
block 400 and resets Key and RTime to 0 at block 402. The program 200 then
resets
the SETTEMP variable to equal the parameter value TEMPLO (block 406) and turns
on
the fan (block 408) to ramp the heating block 90 down to the Low Cycling
Temperature.
Upon return to the Main Denature Loop (block 311) with the program state flag
at 2 and RTime reset to 0, the program 200 branches through blocks 336, 340,
342 and
344 to block 350, and again polls the upper heating block 90 at block 352 to
determine
if it has reached the SETTEMP (now the Low Cycling temperature). If the upper
heating block 90 has not yet reached its set temperature (60°C in the
example), the
program 200 loops back to block 352 through blocks 372, 382, 311, 332, 336,
340,
342, 344 and 350. When the Low Cycling SETTEMP is reached, the program
increments the Key to 1 and the state flag to 3 (blocks 348 and 374) and turns
the fan
WO 95/11437 PCTIUS94/11016
~'~~~~6 2
-31-
off (block 390). Then it resets Key to 1 and the state flag to 2 before moving
into the
main loop of the Cycle/Superheat section 208 (blocks 392 and 394). It should
be
appreciated that when entering the Cycle,/Superheat routine 208 after the
denaturing
routine, the Cycle/Superheat routine begins at the Low Cycling Temperature,
whereas
when Denaturingis skipped the program enters the Cycle/Superheat routine at
the High
Cycling Temperature (see blocks 318, 320, 420 and 422 as described above).
In the example the Cycle/Superheat section 208 (Figures 9H to 9K) begins at
block 421, the SETTEMP having already been initialized. As with the Denature
section
206, the Cycle/Superheat section 208 also continuously polls the keyboard 114
for
parameter edits inputs, and returns the program 200 to the Edit section 204
whenever it
receives the appropriate input from the keyboard 114. The current temperature
of each
of the heating blocks 90, 92 is fed to the 1/O card 118 and displayed at
blocks 428 and
430.
At block 432, the program 200 asks whether it should check time or
temperature depending on the value of RTime. The RTime is 0 here (having been
reset
last at block 402), so the program branches to block 436 to check the
temperature of the
heating blocks. Tracking is off, so the inquiry at block 436 leads to the
state inquiry at
block 438 and then to the state inquiry at block 462. In the Cycle/Superheat
section
208, the program state flag can have eleven values from 0 to 10, but was set
to 2
leaving the Denature Section (block 392), thus the program asks at block 464
whether
the upper heating block has reached the Low Cycling temperature of
60°C. Since this
temperature was reached at the end of the Denature section 206, (and even if
it had not
been, block 392 reset Key =1 ) and thus Key = 1 at this point. The program 200
then
flows through blocks 476 478, 484 and 490 to the inquiry at block 496, which
is "yes"
at this point, causing the program to move into a "Change State" subroutine.
It can be observed generally that in this program 200 when the program state
is
zero or an even number the heating blocks) is ramping up or down to a new set
temperature and the program branches to poll the A!D converter(s) 142, 144 on
the I/O
card 118 for temperature information fed from the thermistor(s) 122, 123.
Conversely,
when the program state is an odd number the set temperature has been reached
so the
program branches to poll the timer so that it can determine if the heater
blocks) have
held the set temperature for the appropriate time period. This can be seen in
Figure 10
also.
In the Change State subroutine at block 510, the program state flag is
incremented (to 3) at block 512. Block 514 is answered no and block 518 is
answered
yes, causing the program 200 to reset RTime to assume the value of TllvIELO
(the Low
WO 95/11437 ~ PCT/US94/11016
-32-
Cycling Time of 60 seconds in our example) at block 520. The program also
turns the
fan off at block 522 and starts the timer at block 536 before moving back to
the
beginning of the Cycle/Superheat section 208 at block 421.
The program 200 moves through the beginning of the Cycle/Superheat section
to block 432. Because the RTime now holds a value (60 sec), the program
branches
from block 432 to the Checktime subroutine beginning at block 550. If the
RTime has
not expired, the program returns to the main loop until the 60 seconds in the
RTime has
timed out. When the RTime has timed out, the answer to the inquiry at block
552 is
yes, and thus the program 200 increments the state flag to 4 at block 555 and
resets
RTime and Key to 0 before moving on to block 562 via block 556.
When the program reaches state 4 and block 562, the cyclenum flag is
incremented at block 564 (to 1 in our example since the Cycle/Superheat
routine 208
was entered via blocks 392 and 394, where cyclenum was set = 0). The program
then
queries the "cyclenum" flag. If the cyclenum flag has not exceeded the maximum
number of cycles, stored as protocol parameter CYCLEMAX, the program 200
resets
the program state flag to 0 and sets the variable SETTEMP to the value of the
High
Cycle Temperature parameter and turns the heating elements) on for beginning
the next
cycle (blocks 568 and 586) and then returns to the main loop at block 421. For
the
illustrated example, CYCLEMAX is 8 and TEMPHI is 80°C. Thus, the
program
returns to block 421 with SETTEMP=80.
This time through the main loop, the program moves through blocks 424, 428
and 430 to the RTime test at block 432. Since RTime was reset to 0 at block
555, the
program branches to block 436 to check the temperature of the heating
block(s). With
Tracking off, the inquiry at block 436 leads to the state inquiry at block
438, where the
answer is now yes. This sends the program to block 440 to determine if the
heating
blocks) has reached the new set temperature. If not, the program moves through
blocks 444, 460, 462, 476, 478, 484, 490 and 496 to return to the main loop
and
continue its polling of the heater block temperature. When the heating blocks)
reach
the set temperature the answer at block 440 increments the key flag to 1 at
block 442.
Continuing through blocks 444, 460, 462, 476, 478, 484 and 490 to block 496,
the
program branches over to the "Change State" subroutine .because Key = 1.
In the Change State subroutine at block S 10, the program state flag is
incremented (to 1) at block 512 and block 514 is answered yes, causing the
program
200 to reset RTime to assume the value of TnVIEHI (the High Cycling Time of 60
seconds in our wxample) at block 516. The program also starts the timer at
block 536
before moving back to the beginning of the Cycle/Superheat section 208 at
block 421.
WO 95/11437 PC1YUS94/11016
21~3~~ 2
-33-
The program 200 moves through the beginning of the Cycle/Superheat section 208
to
block 432. Because the RTime now holds a value (60 sec), the program branches
from
block 432 to the Checktime subroutine beginning at block 550. If the RTime has
not
expired, the program returns to the main loop (block 554) until the 60 seconds
in the
RTime has timed out. When the RTime has timed out, the answer to the inquiry
at
block 552 becomes yes, and thus the program 200 increments the state flag to 2
at
block 555 and resets RTime and Key to 0 before moving on to block 556.
At block 556 the answer is yes causing the program to reset the variable
SETTEMP to the value of the Low Cycle Temperature parameter ('TEMPLO) at block
558 and at block 560 turns on the fan for cooling the heating blocks) before
returning
to the main loop at block 421.
Once again in the main loop, the program reached block 432 and decides to poll
the temperature (block 464) since RTime is 0. This continues until the desired
(TEMPLO) temperature is reached, upon which key set to 1 at block 466. This
sends the program back to the "Change State" subroutine (block 510) where the
state
flag is incremented (to 3) and RTime is reset to TITvvIELO for holding the
heating
blocks) at TEMPLO for the desired time period. This causes the program to
branch at
block 432 to the Checktime subroutine (block 550) to poll the timer. As
before, when
RTime times out, the state flag is incremented at block 555 (to 4), RTime and
Key are
reset to 0 and the cyclenum flag is again evaluated. The program 200 continues
to
execute cycles as described above using program states 0, 1, 2 and 3 until
CYCLEMAX is reached (e.g. until the cyclenum flag is incremented to 9 at block
564).
When the cyclenum flag exceeds the maximum number of cycles (block-566),
the program 200 examines the value of TEMPSUPER at block 570. If it is 0, the
superheat portion is skipped by setting the program state flag to 8 at block
574. In the
illustrated example, the value of TEMPSUPER is 110°C, which starts the
lower ring
superheat process by setting the variable SETT'EMPl equal to 110'C at block
572
before returning the main loop at 421. SETTEMP1 is a variable that holds a
value for
the set temperature of the lower block only, whereas SETTEMP was applied to
the
3 0 upper block or to both blocks if Tracking was on.
In the main loop, the program once again polls temperature at block 432 since
RTime is 0, and skips through inquiries at 438 and 462 to reach the inquiry at
478,
which is answered yes. The program assumes here that if Tracking was off, the
lower
heating block is at a lower temperature than the upper block and state 4 is
maintained
until the lower block comes up to the temperature of the upper block. When the
inquiry
at block 480 is yes, the key flag is set to 1 which causes a state change via
blocks 496,
WO 95/11437 ~ PCT/U594/11016
~, "..
-34-
500 and S 10. This increments the state flag (to 5) and loads the TnVIELEAD
value into
the variable RTime at block 526 and restarts the timer at block 536 before
returning to
the main loop. The TIMELEAD value is the time period by which the superheat of
the
lower heating block 92 leads the superheat of the upper heating block 90. This
is
represented by the exemplary 15 seconds and in Figure 10 by the time period
between
TS and T".
The main loop now branches at block 432 to the Checktime subroutine and
determines when RTime (=TIMELEAD) times out, whereupon the program 200
increments the state flag (to 6). With state flag = 6 the program branches at
block 576
to load the value of TEMPSUPER2 into the variable SETTEMPO at block 578 and to
enable superheating of the upper block. SETTEMPO is a variable that holds a
value for
the set temperature of the upper block only, as distinct from the lower block
or both
blocks (as when Tracking is on). Returning to the main loop, the program
branches to
poll temperatures at block 432 and reaches block 484 and 486 to examine
whether the
upper block has reached its set temperature (TEMPSUPER2). When it has, the key
flag
is changed to 1 at block 488 to move the program 200 to the Change State
subroutine at
block 510. This again increments the program state (to 7) which via block 528
causes
the variable RTime to assume the value of TnVIESUPER at block 530 and to
restart the
timer at block 536. In the example TIMESUPER was 30 seconds and represents the
2 0 period of time during which the upper block is maintained at the superheat
temperature.
In the main loop, block 432 branches to the Checktime subroutine and
determines when
the RTime (=TIMESUPER) is allowed to time out. When it does, the program 200
increments the state flag (to 8), resets the key flag and RTime and moves to
block 580
where the program turns off the temperature outputs to the upper and lower
heating
rings at block 582. In preparation for cool down, the program at block 584
turns the
fan on and resets the SETTEMP variables for both heating blocks to the value
of
SHUTOFF. This value, 50°C in the example, is selected so that the fan
will not run
constantly trying to cool the heating blocks below ambient temperature.
Upon return to the main loop with the program state at 8 and RTime reset to 0,
the program branches at block 432 to poll temperatures. At block 490 the
answer is
yes so at block 492 the program polls the temperature of~fhe upper block to
determine if
it has cooled to the set temperature of 50°C. When it has, the key flag
is set to 1 at
block 494, causing a state change via blocks 496, 500, 510 and 512 to state 9.
At
block 532 the program branches to turn the fan off (block 534) and to load the
value of
TIMEIMAGE into the variable RTime (block 535) before starting the timer (block
536)
and returning to the main loop. As mentioned, the TIMEIMAGE parameter is
selected
WO 95/11437 PtVT/US94/11016
217362_
-35-
to allow the unit to compete its development of signal before starting the
detection
process. In the main loop, block 432 branches to the Check Time subroutine
and,
upon timeout; increments the state flag (to 10) causing the program via blocks
581 and
583 to begin the detection procedures, described below in connection with
Figures 11A
to 11D.
7. Video Processing
The detection system 22, described in an earlier section, utilizes a video
processing program such as the Detection Program 600 illustrated in Figures
11A-11D.
When the computer control program reaches a program state of 10, control is
transferred over to the detection program 600. In general, the detection
program uses
digital video analysis techniques to analyze the video image of the detection
means 60
(e.g. strip 61) generated by the camera 100 of the detection system 22.
Preferably, the
video processing program uses the digital data acquired from replicate capture
sites to
improve the accuracy and reliability of the overall amplification reaction as
described
below. First, however, it is important to define terms used in the
description. Each
detection means 60 includes at least a read zone 68 as shown in Figures 2A, 2G
and
SA-SD. The read zones 68 of the devices of Figures 2A and 5 are shown in
enlarged
view in Figures 12A and 12B. The detection means 60 preferably also includes a
reference bar and/or a control zone 70.
As mentioned above, each read zone 68 preferably includes multiple capture
sites 74 for the purpose of multiplexing the assay. Multiplexing refers to
performing an
assay for more than one analyte at the same time; for example, testing for
both
Chlamydial organisms and gonococcal organisms, or testing for genetic
mutations at
multiple sites in a gene or even in multiple genes. Multiplexing can also
refer to the
simultaneous assay of one analyte along with a positive and/or
negative.control reagent.
These multiple capture sites 74 are depicted as continuous bands or lines in
Figures 2A
and 12A, and as a diagonal array of "spots" in Figure 5 and 12B. They were
also
described earlier as discontinuous bands or line as seen in Figure 2G
These multiple capture sites 74 must be distinguished from what will be
described below as replicate sites 72 or replicate zones. Preferably the area
of each
distinct capture site 74 is large enough to support several "reading windows"
which are
referred to herein as replicate sites or replicate zones. These are depicted
in Figure 12A
as the boxed areas on the top capture site 74, and as multiple scan lines on
the spot 74
in Figure 12B. In Figure 2G, the discontinuous bands create natural replicate
zones,
while with continuous bands the replicate zones are created arbitrarily (see
boxes 72 in
Figure 12A) by the reading software. It should be understood that each
replicate site or
WO 95/11437 PCT/US94/11016
-36-
zone of a capture site 74 contains additional data for the same analyte, as if
"replicate"
assays were being performed for that analyte. Having a plurality of replicate
sites
permits discarding of statistical "outliers" and increases the confidence
level that the
image of the capture site is correctly and faithfully evaluated.
Turning now to the video processing features of the invention, the computer 26
and the video processing program detect the presence of amplified target
nucleic acid
immobilized on the support 61. In general, the camera 100 detects an image of
the
support 61, usually in accord with one of the configurations illustrated in
Figure 8.
The camera 100 then outputs a video signal to the frame grabber card 116 of
the
computer 26. The frame grabber card 116 digitizes a video frame and stores the
digital
values in RAM 124. Thus, the digital values are accessible to the computer 26
and may
be manipulated by the video processing program 600. The computer 26 uses an 8-
bit
gray scale having a resolution of 512 x 484 pixels. A numerical value is
assigned to
each pixel such that a zero (0) represents a black image, and two hundred and
fifty-five
(255) represents a white image. The values between 0 and 255 each represent a
particular shade of gray. The digitized representation of the video signal may
be shown
on the computer monitor 113 for viewing by an operator.
The video processing program 600 is illustrated by the flow chart shown in
Figures 11 A to 11 D. The flow chart uses conventional symbols to represent
the major
functions performed by the video processing program 600. The video processing
program 600 has two major sections or loops. The first section is the "Read"
section
which begins in block 606, and the second section is the "Assay" section which
begins
in block 634 and is a subroutine of the Read section 606. The Read section is
executed
once for each reaction/detection unit 20, and the Assay section is executed
once for each
capture site 74 imaged from the detection means 60 of each unit 20.
The program 600 starts in block 602 and initializes a position counter in
block
604. The position counter keeps track of the number of reaction and detection
units in a
particular batch. For the disclosed dual annular ring embodiments, the heating
rings
90, 92 include forty wells 97 for holding reaction/detection units 20. Block
608
advances the motor 108 or 109 to the next sample read position. In detection
systems
22 using a mirror 106 for reflecting an image of the detection means 60 to the
camera
lens 102, the motor 108 would rotate the mirror as well in order to present
successive
images of each detection means 60 to the camera 100.
The reaction/detection units 20 preferably are provided with a bar code (not
shown) which identifies the reaction sample 38 and the unit 20, and contains
information about the assay to be performed for this reaction/detection unit.
The bar
_. WO 95/11437 PCT/US94/11016
2'~7~~62
-37-
code preferably also provides the computer 26 with information about the
configuration
of the detection means 60, such as information about the presence, location of
and
geometry (e.g. bands or spots) of control zones 70, capture sites 74, and
replicate
zones 72. Preferably, there are a limited number of such configurations and
configuration information is stored in the computer's memory, to be retrieved
by the
computer upon receipt of a bar code signal that is associated with a
particular
configuration. Alternatively, if only one configuration is used, a single
reference bar
can provide a frame of reference for image analysis.
The cycler 16 and/or the computer 26 are then provided with a code reader (not
shown) for reading the bar code. The program 600 reads the bar code
information in
block 610 and determines in block 612 whether the bar code was read
successfully. If
the read was unsuccessful, the program 600 indicates in block 614 that no bar
code was
read for this unit. In systems where bar code information is needed to locate
the
position and number of capture sites, the computer will not know how to
process the
particular unit 20 if the barcode is not successfully read and no result can
be reported so
the program 600 moves to block 6l 6 which sends the program 600 to the sample
end
routine at block 678. If the read was successful, the program 600 moves to
block 618
in which the zone configuration information is processed in preparation for
obtaining
and examining the digitized image.
Once the video image is fed from the camera 100 to the frame grabber card 116,
the image is digitized at block 620 and scanned for the control zone 70 at
block 622.
The control zone is typically a prescribed zone that is ordinarily positive
for any
reaction sample. The control zone generally serves two functions. First, it
indicates to
the operator that the amplification reaction and transfer of the sample to the
detection
chamber proceeded properly. Second, it provides a reference point for
determining the
location of the capture sites as defined by the bar coded configuration
inforniation. In
block 624, the program inquires whether the control zone was found. If the
answer to
the inquiry at block 624 is no, the program indicates an error code for the
current
sample and proceeds to block 616 which sends the program 600 to the sample end
routine at block 678. If the answer to the inquiry at ;.. ~ock 624 is yes, the
program 600
proceeds to block 628 which sends the program 600 to the Assay Read routine at
block
630.
The Assay Read routine moves to block 632 and, using the zone configuration
information provided by the unit bar code or by other input, selects the first
analyte
zone for processing. Each analyze zone is divided into a plurality of scan-
lines having a
plurality of pixels in each scan-line. Each pixel was assigned a grayscale
numerical
WO 95/11437 ~ ; PCT/US94/11016
-38-
value during the digitizing procedure in block 620. In block 636, the program
600
examines the scan-lines in the current analyte zone and calculates the pixel
mean,
standard deviation (SD) and range values for each scan-line in the current
analyte zone.
The program 600 then moves to block 638 and asks whether any of the scan-
s lines in the current analyte zone are statistically different from other
scan-lines in the
current analyte zone. If the answer to the inquiry in block 638 is no, the
program
moves to block 640 and reports a negative result for the current analyte zone.
The
program 600 then moves from block 640 to block 642 which sends the program 600
to
the next zone routine at block 670. If the answer to the inquiry in block 638
is yes, the
program has detected a positive result for the current analyte zone and moves
to
block 644.
It will be appreciated that for a scan-line to be statistically different from
the
others it must contain a signal area whereas the other scan lines do not.
Thus, it can be
seen that the configuration of capture sites 74 and replicate sites 72 must
leave some
space between the sites. This is depicted in Figures 12A and 12B by the spaces
75. In
the band configuration, the bands are placed sufficiently far apart that some
scan-lines
will examine the space between bands. In the spot configuration, adjacent
spots should
be separated by a vertical space 75 if horizontal scan-lines are employed. If
this space
75 is not present and all capture sites 74 yielded positive signals, then all
scan lines
would contain signal and none would be statistically different.
Block 644 begins a background normalization procedure, where the program
classifies each scan-line of the current analyte zone as containing some
signal or only
background. Using scan-lines classified as background only, the program 600
then
establishes a background gradient for the current analyte zone in block 646,
and uses
25~ this gradient to account for variance in lighting and position. Background
gradients
may be established in a variety of known ways such as by derivative and
row/column
analysis as is known in the art. In block 648, the program performs background
adjustments or nor<nalizations on the signal scan-lines using the background
gradient
information. Background normalization is traditionally used to establish a
signal
baseline and improve data interpretation, and may also be accomplished in a
variety of
known ways such as by subtraction or horizontal/vertical mean subtraction. The
program then moves from block 648 to block 650 which transfers the program 600
to
block 652.
The image processing subroutine begins at block 652. In block 654, the
. 35 program uses contour enhancement to identify the perimeter of signal area
77 in a
successful replicate site 72. Contour enhancement is a known digital image
processing
w0 95/11437 PCTlUS94/11016
21~'3~~ 2
-39-
technique for feature extraction and is applied here to determine the contours
or
boundaries of the signal area for each replicate site. In block 656, the
program
calculates the mean, standard deviation and range values for all pixels within
the
perimeter of each replicate site signal area. The analysis is now focused on
the signal
areas of the replicate sites.
In block 658, the program identifies any anomalous results by asking whether
any of the signal area statistics in one replicate site are significantly
different from the
signal area statistics from other replicate sites 72. If the answer to this
inquiry is no, all
of the replicate sites 72 are judged to be the same, and the program 600 then
calculates
at block 660 the mean pixel value of the signal areas within all the replicate
sites and
stores this value as a result for the current analyte zone. From block 660,
the program
moves to block 662 which transfers the program to the next zone routine at
block 670.
If the answer to the inquiry in block 658 is yes, the program 600 moves to
block 664 which removes at~errant results which are referred to as statistical
"outliers"
or "fliers". Aberrant results can be defined statistically in a number of
ways, including
results falling too far from the mean, "too far" being defined in terms of the
number of
standard deviations, or in terms of the statistical significance within preset
confidence
limits. In block 666, the program determines whether there are enough
acceptable sites
remaining after discarding the aberrant or anomalous sites to obtain a
reliable test result.
Any of several criteria may be used to make the determination set forth in
block 666.
For example, the program may require a fixed percentage (e.g. at least 50%) of
the
identified replicate sites to be acceptable. If the number of acceptable
replicate sites
exceeds the established minimum, the program proceeds to block 660 to
calculate the
mean pixel value of the signal areas within the acceptable replicate sites and
stores this
value as a result for the current analyte zone. If the number of acceptable
capture sites
does not exceed the established minimum, the program proceeds to block 668
which
sets the indeterminate result flag for the current analyte zone. In other
words, the
program could not find sufficient reliable data in the scanned image to reach
a firm
conclusion regarding the assay. The program then moves from block 668 to block
662
3 o which takes the program to the next zone subroutine at block 670.
The program then moves to block 672 and asks whether the current zone is the
last zone. If the answer to the inquiry in block 672 is no, the program
selects the next
analyte zone in block 674 and then moves to block 676 which returns the
program to
the assay loop at block 634. If the answer to the inquiry in block 672 is yes,
the
detection for the current reaction/detection unit 20 is complete, and the
program moves
into the sample end subroutine which begins at block 678.
WO 95/11437 ~ ~~ V PCT/US94111016
-40-
~~s;,~n block 680, the program 600 stores all of the sample results and then
displays
andloi:prints all sample results in block 682. Alternatively, the program can
be
configured to store -all the data and print it at the end of a run. The
position counter is
then incremented in block 684, and the program asks in block 686 whether the
last
position has been completed. If the answer to the inquiry in block b$6 is no,
the
program moves to block 690 which returns the program to the read~loop at block
606.
If the answer to the inquiry in block 686 is yes, the program ends at block
688.
- n~; It should be understood that use of the video imaging aspects of this
invention
are ~,Qt limited to the preferred two tier cycling element and, in fact, are
not limited to
nucleic acid analysis at al. Rather, the video imaging aspects may be utilized
on any
form of assay, including for example immunoassa~;where a signal can be
generated
such that it can be distinguished from the background using a camera means,
and
preferably some form of electromagnetic illumination.
8. Methods For Amplif~g And DetectinE Nucleic Acids -
In accordance with another aspect of the invention, there are provided methods
for performing nucleic acid amplification and detection. As described in the
Background of the Invention, various methods for amplifying nucleic acids are
known
in the art Amplification reactions contemplated by the present invention
include, but
are not limited to, PCR, LCR, 3SR, and SDA. In the present invention, the
amplification reaction sample generally comprises target nucleic acid, at
least one
enzymatic agent that induces amplification, and a buffer. Enzymatic agents
contemplated by the invention include, but are not limited to, ligases and
polymerises,
and combinations thereof. The reaction sample may also include primers or
probes,
which are described further below. Preferably, primers or probes are added in
molar
excess of the amount of target nucleic acid in the reaction sample.
It will be readily apparent to those persons skilled in the art that certain
additional reagents may be employed, depending on the type of
amplification~reaction.
For instance, for PCR amplification reactions, the reaction sample will
generally also
include nucleotide triphosphates, dATP, dCTP, dGTP, and dTTP. LCR reaction
samples usually include NAD. The amounts of all such reagents in the reaction
sample
may be determined empirically by those persons skilled in the art. Examples of
reaction
samples for particular amplification reactions are described further in
Examples 4, 9,
and 11 of this disclosure.
The nucleic acid of ir..terest to be amplified, referred to as the target
nucleic acid,
may comprise deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), and may be
WO 95/11437 PC1'/US94/11016
21~~~6 ~ ._
-41-
natural or synthetic analogues, fragments, and/or derivatives thereof. The
target nucleic
acid is preferably a naturally-occurring viral nucleic acid or DNA of
prokaryotic or
eukaryotic origin.
The teams "primer" and "probe" as used in the present application are intended
to refer generally to an oligonucleotide which is capable of sufficiently
hybridizing with
the target nucleic acid. The term "primer" is typically used in connection
with PCR,
and the term "probe" is typically used in connection with LCR. The term
"primer/probe" will be used in the present application where general
discussions can
apply to both primer and probe sequences.
In the methods of the invention, the primer/probe is preferably selected to be
complementary to various portions of the target nucleic acid. The length of
the
primer/probe will depend on various factors, including but not limited to,
amplification
reaction temperature, source of the primer/probe, complexity of the target
nucleic acid,
and the type of amplification reaction. Preferably, each primer/probe is
sufficiently
long to have a desired specificity and avoid hybridization with random
sequences that
may be present in the reaction sample. More preferably, each primer/probe
comprises
about 15 to about 100 bases, and even more preferably, about 15 to about 40
bases.
The primer/probe may be chemically synthesized using methods known in the
art. Preferably, the primer/probe is synthesized using nucleotide
phosphoramidite
chemistry techniques known in the art and/or instruments commercially
available from
Applied Biosystems, Inc. (Foster City, CA), DuPont (Wilmington, DE) or
Milligen
(Bedford, MA).
Pimer/probes may be directly linked to detectable label which does not
interfere
with hybridization. Alternatively, a specific binding pair member is attached
to at least
one primer/probe employed in the amplification reaction. Preferably, a
specific binding
pair member is attached to each primer in a primer pair, or to at least two
probes in a set
of probes employed in the amplification reaction. More preferably, the
specific binding
pair members thus attached to the primers in the primer pair or to the at
least two probes
in the set of probes are two different speck binding pair members. As
described
3 0 further below, a first specific binding pair member attached to a primer
pair or probe set
can be used to couple amplified target with a reporter molecule conjugated to
a
detectable label. The second specific binding pair member can then be used to
bind the
labeled amplified target to a capture molecule immobilized on the support 61.
Preferably, the two specific binding pair members do not cross react with each
other
and do not cross react with the labeled reporter molecules or the capture
molecules
immobilized on the support 61.
WO 95/11437 ~~~ °~ PCT/US94/11016
-42-
Typically, the specific binding pair member comprises an antigen, hapten,
chemical compound, or polynucleotide capable of being bound by another
molecule
such as an antibody or complementary polynucleotide sequence. The specific
binding
pair member may also be a magnetic particle. Specific binding pair members
contemplated by the present invention include, but are not limited to, biotin,
T3,
oligonucleotides, polynucleotides, and drug compounds such as theophylline,
digoxin,
and salicylate. Such specific binding pair members are known in the art and
are
commercially available.
Methods of attaching or linking specific binding pair members to the
primer/probe are also known in the art. For example, the specific binding pair
member
may be attached to the primer/probe through covalent bonding or standard ~3-
cyanoethyl-phosphoramidite chemistry techniques. Enzo Biochemical (New York)
and
Clontech (Palo Alto, CA) have also described and commercialized primer/probe
labeling techniques. The methods employed will vary depending, for instance,
on the
type of specific binding pair member and the position of the binding pair
member on the
primer/probe sequence. The binding pair member should, however, be attached by
thermostable means to survive any temperature cycling employed in the
amplification
reaction.
To conduct the amplification reaction, the reaction sample 38 is placed in the
reaction chamber 30. Because the quantity of reaction sample is typically
small, it may
be preferable to place the sample 38 in the reaction chamber 30 using a
microsyringe
pipette (not shown), or to briefly centrifuge the chamber to force the sample
38 to the
bottom of the chamber. The reaction chamber 30 and detection -chamber 32 are
then
engaged to form a sealed unit 20, and the unit 20 is placed in a thermal
cycling device
16, preferably, a thermal cycling device 16 as shown in Figures 6-8 and
described
herein. The reaction sample 38 is then exposed to temperature conditions
sufficient to
amplify target nucleic acid present in the reaction sample. For some
amplification
reactions, such as PCR and LCR, the reaction sample will be exposed to thermal
cycling. Other amplification reactions, however, such as SDA and 3SR, may
employ
isothermal conditions. Under thermal cycling conditions, the reaction samples
are
typically exposed to a range of temperatures for set periods of time. For LCR,
there is
usually temperature cycling at two different temperatures. For example, as
described in
Example 5, the reaction sample is cycled at 85°C and 55°C. Those
skilled in the art can
determine empirically, without undue experimentation, suitable temperatures,
cycling
times, and the number of cycles needed to complete the amplification reaction.
Under
CA 02173862 2004-11-19
WO 95/11437 PCTNS94111016
-43-
appropriate temperature conditions, and is the presence of target nucleic acid
in the
reaction sample, the primers or probes will hybridize to the target nucleic
acid as the
amplification reaction proceeds.
When the amplification reaction is completed, the nacxion sample is
transferred
from the reaction chamber 30 to the detection chamber 32 so that the reaction
sample 38
comes into contact with the support 61 (Figures SA to SE). During transfer of
the
reaction sample 38 to the detection chamber 32, the unit 20 remains sealed.
The
transfer of sample may occur by various means such as by creation of a vapor
phase or
expansion of fluid or propellant caused by increased temperature.
t 0 Preferably, transfer of the reaction sample 38 to the detection chamber 32
occurs by expansion of a propellant 40 at the bottom end of the reaction
chamber 30.
In the preferred embodiment, the expansion of the propellant 40 is caused by
the
computer 26 raising the temperature of the lower heating element 18 (or only
heating
element 17) above the propellant's threshold temperature. More particularly,
the
computer 26 directs the heating element 17 or 18 to deliver heat to the second
longitudinal segment 35 of the reaction chamber 30 so that the propellant 40
is exposed
to a temperature above the propellant's threshold temperature. Typically, the
element is
super-heated to a temperature above 95'C, usually at or above 100 'C. The heat
thus
delivered to the reaction chamber 30 causes the propellant to expand, thereby
transferring the reaction sample upward toward the detraction chamber 32. The
temperature needed to expand the propeDant 40 will depend on the nature and
co=.: vposition of the propellant 40. It is preferred that the propellant 40
has a threshold
temperature above the amplification.reaction tempeTatuce(s) so that the
propellant 40
does not expand during the course of the amplification reaction.
In a preferred embodiment, one region 66 of the support 61 comprises multiple
conjugate molecules capable of binding to a first specific binding pair member
attached
to the amplified target in the reaction sample. The conjugate molecules are
deposited on
the support 61 using methods known to persons skilled in the art. For example,
the
conjugate molecules can be deposited on the support 61 by spotting and drying.
Preferably, the conjugate molecules are dried on the support 61 in the
presence of meta-
soluble proteins, such as casein, to aid in the transport and resolubilization
of the
conjugate molecules. The conjugate molecules can also bo deposited on the
support by
methods described in U.S. Patent No. 3,120,643.
The conjugate molecules in the region 66 are not immobilized on the support
but rather
are capable of resolubilizing in the presence of sa~r~ple andlor aqueous
solvent
and move along the support by capillary movement. Examples of conjugate
WO 95!11437 PCT/US94/11016
.~~.,
components capable of binding to the specific binding pair members described
above
include, but are not limited to, antibiotin antibodies, anti-theophylline
antibodies,
avidin, carbohydrates, lectins, complementary oligonucleotide or
polynucleotide
sequences, streptavidin, and protein A.
The conjugate molecules thus deposited on the support are conjugated to a
label.
The term "label" as used in the present application refers to a molecule which
can be
used to produce a detectable signal. The signal should be able to be detected
visually,
optically or upon excitation by an external light source. Suitable labels are
known in
the art and include latex, colored latex particles, and colloidal metals such
as gold or
selenium. Alternatively, the label may be a fluorescent molecule such as
fluorescein,
rhodamine, acridine orange, and Texas red. Additional labels which may be
employed
in the invention are described in U.S. Patent No. 4,166,105; U.S. Patent No.
4,452,886; U.S. Patent No. 4,954,452; and U.S. Patent No. 5,120,643. Such
labels
may be conjugated or linked to the reporter molecules according to methods
generally
known in the art. [See, e.~, U.S. Patent No. 5,120,643; U.S. Patent No.
4,313,734].
As the reaction sample contacts a first region 66 of the support 61 modified
as
described above, the amplified target nucleic acid coupled to specific binding
pair
members binds to the labeled reporter molecules. Also, the reporter molecules
on the
support are resolubilized and are mobilized with the amplified target nucleic
acid in the
reaction sample. As has been mentioned, the conjugate need not be present on
the strip
and is not needed at all if a detectable label is directly linked to the
primer/probe.
By capillary movement, the reaction sample, along with the labeled amplified
target, is transported to a second region 68 of the support 61. The second
region 68 of
the support 61 preferably includes a plurality of capture molecules (capture
sites 74)
capable of binding to a second specific binding pair member attached to the
amplified
target nucleic acid. Where the second specific binding pair member attached to
the
amplified target is a magnetic particle, the capture molecules) should be
selected so as
to be able to capture and immobilize the amplified target by magnetic
attraction. All
such capture molecules are immobilized on the support 61. Methods of
immobilizing
the capture molecules on the support 61 are known in the art and include
adsorption,
absorption, and covalent binding, as well as those methods described in U.S.
Patent
No. 5,120,643. The amount of capture molecules immobilized on the support 61
will
vary, depending, for instance, on the binding affinity for the specific
binding pair
member. Preferably, the concentration of capture molecules immobilized on the
support 61 is in molar excess of the amplified target.
WO 95/11437 PCTlUS94111016
2~~3fi 2 -
-45-
Preferably, the plurality of capture molecules are immobilized on the support
61
at predetermined locations or zones (capture sites 74) on the support 61. The
capture
molecules can be immobilized in any desired geometric form or configuration,
such as a
diagonal, vertical, or horizontal configuration, or in the form of circles or
bars. It is
more preferable to spatially separate any such circles or bars so that the
results of the
amplification reaction can be suitably detected and resolved.
As the reaction sample and labeled am-~.3ified target contacts the second
region
68 of the support 61, labeled amplified target nucleic acid in the reaction
sample 38 will
bind to the immobilized capture molecules (capture sites 74) on the support 61
and will
become immobilized at that location. Sample components not bearing the capture
hapten will be cleared from the second region 68 to any additional zones
and/or to the
second end 64 of the support 61 by capillary movement of the reaction sample
38.
Further, the support 61 may also comprise a third region referred to herein as
a
"control" zone 70. The control zone 70 is modified so as to provide a control
or
reference standard in the detection method. Preferably, the control zone 70
includes
some reagent that will capture a detectable label at a predetermined location
on the
support 61. The support 61 can, of course, comprise additional regions or
zones for
conducting further analysis. Alternatively, or additionally, the support 61
may
comprise a reference spot or zone including a detectable dye which, while not
reactive
with reagents, provides a detectable signal that serves as a frame of
reference for
automated imaging by the camera.
The labeled amplified target nucleic acid immobilized on the support 61
produces a visible indicator, and this visible indicator is detected and
analyzed by the
detection system 22 and computer 26. The visible indicator thus produced is an
indication of the presence or amount of amplified target nucleic acid in the
reaction
sample 38. If no amplified target nucleic acid is present in the reaction
sample 38, no
labeled amplified target will bind to the immobilized capture molecules and no
visible
indicator will be measured. The density or intensity of the indicator on the
support 61
can be read optically by any means. As described herein for one embodiment,
the
3 0 signal is reflected onto a video camera lens 102 by a reflecting mirror
106. As the
mirror 106 rotates, each of the supports 61 in each of the detection chambers
32 can be
read.
In addition to the preferred embodiments described above, the invention
contemplates alternative methods for labeling and immobilizing target nucleic
acid. For
instance, the primer/probe may be coupled to a detectable label during
manufacture.
Alternatively, the primer/probe may be coupled a ~g manufacture with a
specific
WO 95111437 PCTIUS94/11016
,.L~ ~ _;;~ ~2
-46-
binding pair member that allows it to bind to a detectable label that is
conjugated to a
complementary specific binding pair member. The binding of the complementary
specific binding pair members can take place either during or after the
amplification
reaction. Thus, it is contemplated that amplified target nucleic acid in the
reaction
sample can be coupled to a detectable label prior to being transferred to the
detection
chamber 32.
In a further embodiment, labeled amplified target nucleic acid is detected in
the
detection chamber 32 by means of microparticle agglutination. In this
embodiment, a
pair of primers or a set of probes is coupled during manufacture with the same
specific
binding pair member. Microparticles conjugated to complementary specific
binding
pair members are then included as part of the detection means 60. As the
reaction
sample 38 is transferred to the detection chamber 38 and comes into contact
with the
detection means 60, amplified target present in the reaction sample 38 binds
to the
coated microparticles. By virtue of the bivalency of the amplified target, the
microparticles agglutinate. Unamplified probes or primers may bind only one
microparticle, and will not be able to initiate agglutination. The
agglutination can then
be detected and analyzed by the detection system 22 as described above.
9. Kits of the Invention
2 0 The invention also provides kits for amplifying and detecting nucleic
acids. The
kits comprise multiple disposable reaction chambers 30, multiple disposable
detection
chambers 32, and engagement means for sealably securing each reaction chamber
30 to
a detection chamber 32. Each of the disposable detection chambers 32 include a
support 61 modified for immobilizing amplified target nucleic acid. The kit
also
comprises one or more containers holding in a suitable buffer reagents for
perforniing
amplification reactions. For PCR, such reagents include DNA polymerase, dATP,
dCTP, dTTP, dGTP and at least two primers specific for a predetermined target
nucleic
acid. For LCR, such reagents include DNA ligase, NAD, and at least four probes
specific for a predetermined nucleic acid. Suitable containers for the
reagents include
bottles, vials and test tubes. In a preferred embodiment, the disposable
reaction
chambers 32 in the kit are pre-packaged with selected reagents and closed with
a
puncturable seal.
CA 02173862 2004-11-19
WO 95111437 Pt;.'T/US94/11016
-47-
10. E~cam~le~
Example 1: Construction of Thermal Cvclers
A.. A dual-ring thermal cycla was constructed from two aluminum rings
having the following dimensions: 105 mm outer diameter, 95 mm inns diameter,
and
13 mm height. The gap betw~n the rings was 2 mm. Each ring contained 40
aligned
wells for holding reaction/detection units 20, each well having a diameter of
approximately 2.3 mm. The rings were equipped with radial cooling fins on the
internal surface as shown in Figure 7. Self adhesive heating strips (Mince
Products,
Minneapolis, Ml~ was attached to the outer circumference of the upper and
lower
rings. The heating strips thus attached were capable of delivering about 300
watts of
power to each ring. The temperature of the rings was controlled by electronics
and the
software as described above. A Charge Coupled Device (CC~) camera and movable
mirror were installed along the center axis of the rings above the cooling
fan.
B. A thermal cycler was constructed from a single annular ring of aluminum
with dimensions: outer diameter 105 mm, inner diameter 94 mm, and height 36
mm.
The ring contained 36 wells for reaction tubes, each well being 3.5 mm
diameter. The
ring was equipped with radial cooling fins on the internal surface. A self
adhesive
heating strip (Mince Products, Minneapolis, MIA was attached to the outer
circumference. The temperature of the ring was controlled by control
electronics and the
software as described above. A CCD camera was installed external to the ring
and a
light source was installed in the center.
C. A dual-tier them~al cycler was constructed from two rectangular aluminum
blocks having the following dimensions: 84 mm x 25 mm x 6 mm. Each block
contained 12 wells for holding reaction/detection units 20, each well having a
diameter
of approximately 0.31 cm. The blocks were equipped with cooling fins on one
surface. Self adhesive heating strips (Mince Products, Minneapolis, MIA were
attached to the other surface. The temperature of the blocks was controlled by
electronics and software as described above.
Ex~mole 2: Prrnaration of Antibody Reaeenis
A. Antiserum: Antiserum to biotin, adamantane, quinoline, dibenzofuran,
thiophene-carbazole, and aQidine were raised in rabbits against each napten
conjugated
to BSA. Details of preparing antibodies to adamantsne, quinoline,
dibenzofuran,
thiophene-carbazole, and acridine are known in the art .
Monoclonal antibody to fluorescein was raised in mouse
WO 95/1143? PCT/US94/11016
PN6i EI
-48-
using standard techniques. Antiserum against dansyl was a mouse monoclonal
obtained
from the University of Pennsylvania (S-T. Fan and F. Karush, Molecular
Immunoloev, 21, 1023-1029 (1984). The antisera were purified by passage
through
protein A Sepharose~ or protein G Sepharose~ (Phartnacia, Piscataway, Nn and
diluted in 0.1 M TRIS pH 7.8, 0.9% NaCI, 0.1% BSA, 1% sucrose, 1% isopropanol,
and a trace of phenol red.
B. Conjugates: Colloidal selenium was prepared following the procedure of D.
A. Yost, et al (U.S. Patent 4,954,452 (1990)). The colloid was diluted in
water to
achieve an optical density of 16 at 545 nm. To 1 mL of this suspension was
added 1 ~,L
of anti-biotin at 1 mg/mL and 60 p.L of BSA at 100 mg/mL. This suspension was
mixed on a vortex mixer for 1 minute. A 0.5 mL portion of this mixture was
diluted
with 0.5 mL of 40 mM TRIS pH 7.8, 4% casein, and allowed to soak into a 10 x
1.25
cm glass fiber-based pad (Lypore 9254, Lydall Inc., Rochester, NY). The pad
was
lyophilized and cut into 6 x 6 mm sections.
Anti-biotin antiserum was also conjugated to polystyrene uniformly-dyed blue
latex particles (Bangs Laboratories, Carmel, III. The latex particles (380 nm
diameter)
were diluted 1:25 in water to give 1 mL at 0.4% solids, and 10 N.L, of anti-
biotin at 1
mg/mL was added. The suspension was mixed on a vortex mixer for 45 seconds,
and
5 ~,L of 5% casein in 0.1 M TRIS (pH 7.8) was added. A 0.5 mL portion of this
mixture was diluted with 0.5 mL of 40 mM TRIS (pH 7.8), 4% casein, and allowed
to
soak into a 10 x 1.25 cm pad (Lypore 254T"', Lydall, Inc., Rochester, NY). The
pad
was lyophilized.
C. Solid supports: Anti-dansyl antibody (1 mg/mL) was applied to
nitrocellulose sheets (S ~,m pore size, precast onto Mylar~, Schleicher and
Schuell,
Keen, NH) using a motor-driven microsyringe. In addition, anti-adamantine,
anti-
acridine, anti-quinoline, anti-dibenzofuran, anti-thiophenecarbazole, and anti-
fluorescein antibodies at 0.5-1 mg/mL were applied to different nitrocellulose
sheets (S
~m pore size, Schleicher and Schuell, Keen, NH) by reagent jetting as
described in
U.S. Patent 4,877,745 (Abbott) to form a multiplex capture support.
Example 3: Preparation of Detection Chambers .
A. Tubular: Tubular detection chambers were constructed of plexiglass tubes of
approximately 3 mm internal diameter. The top ends of the detection chambers
were
closed, and the bottom ends were tapped to fit threaded microtube reaction
chambers
described in Example 4A below.
WO 95/11437 PCT/US94I11016
...
-49-
The Lydall antibiotin conjugate pad of Example 2B was affixed to the bottom
of the antidansyl nitrocellulose supports 61 (Example 2C) with adhesive tape.
The
nitrocellulose-Lydall pad support was then sliced into 3 x 50 mm strips, which
were
inserted, with the Lydall pad portion downward, into detection chambers made
of
plexiglass tubes of approximately 3mm internal diameter.
B. Rectangular Chamber with Reservoir: Strip holders of the design shown in
figure 2A-2E were molded of polycarbonate. Into the base, in the orifice
leading from
the reaction tube to the reservoir, was placed a 6 x 6 mm section of the
selenium
antibiotin conjugate pad of example ZB. A multiplex capture support strip with
immobilized antibody (example 2C), was placed in the strip holder. The lid was
welded
to the base of the strip holder by ultrasound such that the strip was held in
place by the
pins.
Example 4: Reaction Chamber Preparation
A. P.C.~~. Microsyringe Tips, were purchased from Tri-Continent Scientific,
Inc., Grass Valley, CA and the open tips (bottoms) were sealed closed with
heat.
These reaction chambers were made of polypropylene, had a volume of 100 p.L
and an
internal diameter of 1.8 mm. The tops were threaded as shown in Figure 13.
B. Custom reaction chambers were ordered from Varivest, Inc. Grass Valley,
CA. These chambers were constructed of polypropylene capillary tubes to have a
volume of 100 EtL, 3.5 mm OD, 2 mm ID and a length of 3.5 cm. Curiously, in
tests
where the reaction sample alone served as propellant, these tubes performed
very
poorly unless the already sealed bottoms were first melted, presumably
introducing
surface irregularities at or near the lower, closed end.
Example 5: Reaction Sample Preparation. J3.11
Oligonucleotide probes were synthesized by phosphoramidite chemistry on an
ABI DNA synthesizer and were haptenated with either biotin ~or da~syl haptens
as
indicated. The sequences (SEQ ID NOS 1, 2, 3 and 4 shown below) were used to
amplify a portion of human chromosome 7 coding for the J3.11 polymorphism
which
is loosely linked to cystic fibrosis. (I. Bartels, et al., Am~J. Human
Genetics. x$:280-7
(1986). They align on the target (SO-base synthetic target: SEQ ID NO. 5) as
shown
below:
WO 95/11437 ~ ~ ~ ''PCT/US94111016
-50-
SEO ID NO. SEQUENCE and ALIGNMENT
1. 5'-biotin-GTGTCAGGACCAGCATTCC-3'
2. GTAAAGGGGAGCAATAAGGT-3'
5. 5'-ATATTGTTGTGTCAGGACCAGCATTCCGGGAAAGGGGAGCAATAAGGTCA-3'
5'. (3'-TATAACAACACAGTCCTGGTGCTAAGGCCCTTTCCCCTCGTTATTCCAGT-5')
3. 3'-biotin-CACAGTCCTGGTCGTAAG
4. CCATTTCCCCTCGTTATTCCA-dansyl-5'
To perform "double-gap" LCR as described in Backman, et al. European Patent
Application 439 182, reaction sample mixtures contained the following reagent
concentrations in a total volume of 100 p,L: 50 mM EPPS, titrated with KOH to
achieve pH 7.8; 20 mM K+; 30 mM MgCl2; 10 ~tM NAD, 1.7 ~tM dGTP, 9000 units
DNA ligase from Thermos thermophilus; 1 unit DNA polymerise from Thermos
aquaticus; 1 ~,g herring sperm carrier DNA; 4 x 1012 copies (6.7 nmole) of
each
oligonucleotide probe (SEQ ID NOS. 1, 2, 3 and 4); and 10~ copies target DNA
(SEQ
ID NO. 5).
The reaction samples were pipetted into reaction chambers of example 4A. The
reaction chambers were then centrifuged briefly to force the reaction sample
to the
bottom of the chamber. The reaction chambers were screw-threaded to the
detection
chambers described in Example 3A to form sealed reaction/detection units 20.
Example 6: Amplifying DNA and Transferring Reaction Sample
From Reaction Chamber To Detection Chamber
The sealed reaction/detection units of Example 5 were inserted into a split
ring thermal cycler. (See Example lA). The upper and lower rings were subject
to
the following protocol of temperature in order to effect the LCR reaction: 40
cycles
of 82°C for 5 seconds and 55°C for 60 seconds. Each cycle took
approximately 2
minutes to complete, for a total LCR time of about 80 minutes.
Following completion of the temperature cycling, the lower ring was heated
3o to 110°C, and the upper ring was heated to 100'C. These temperatures
were held
for 25 seconds. By thermal expansion and vaporization of the reaction sample
in
the reaction chamber, the sample was transferred from the reaction chamber to
the
detection chamber, where the reaction sample contacted the first end of the
support
61 containing the labeled anti-biotin conjugate. The labeled anti-biotin was
re-solubilized, and the reaction sample proceeded by chromatography up the
nitrocellulose support 61. In reaction samples containing amplified target
DNA, the
amplification product was bound at the anti-dansyl capture sites on the
support 61
WO 95/11437 PCT/US94/11016
zl~~~s 2
-51-
and visible color development was observed. The results of six reaction
samples
are shown in Figure 13. The three samples on the left contained target DNA and
dark spots are visible on the detection strip (see arrow). The three samples
on the
right contained no target DNA and no spots are visible.
Example 7: Detection Image
The detection chambers of Example 6 were scanned to a TIFF file with a
flatbed scanner (ScanJet C, Hewlett-Packard, Palo Alto, CA) using grayscale
settings of brightness 140 and contrast 150. The TIFF file was imported into
Image'' (available from the National Institutes of Health, Research Services
Branch, NIH), and the images of the developed bands analyzed for pixel
density.
The results are tabulated in Table 1 below, where maximum density and minimum
density refer to the gray level of the image in the immediate vicinity of the
band.
TABLE 1
strip 1 strip 2 strip 3 strip 4 strip 5 strip 6
(pos) (pos) (pos) (peg) (peg) (peg)
maz density 183 203 210 164 159 183
min density 143 147 186 135 129 159
difference 40 56 34 ~ 29 30 24
Example 8: Video Processing
A photographic image of the color reaction product described in Example 6
was taken by the CCD camera. The presence or absence and amount of color
reaction in the specified regions of the support 61 was determined by analysis
of
gray scale data files generated from the image, using software described
earlier in
this disclosure.
Example 9: Alcohol Prod
The reaction sample of Example 5 is prepared in a microsyringe-barrel
reaction vessel, except that 2 ~.I. of 1-propanol is placed at the bottom end
of the
reaction chamber, and the reaction sample is placed in the chamber so that the
sample and the 1-propanol are separated by about 2.5 p.L air. The reaction
chamber
is then sealably fitted with the detection chamber 32 to form a sealed
reaction/detection unit 20 as in Example 5. DNA amplification, and the post-
heating protocol of Example 6 are executed, except that the upper and lower
ring are
both heated to 100°C. The vaporization of the 1-propanol forces the
reaction
WO 95111437 ~ PCT/US94111016
-52-
sample upwards so as to contact the support 61 in the detection chamber. The
color
reaction product on the support strips 61 can then be analyzed by the imaging
detection system described in Example 7 or 8.
Example 10' Nucleation of Propellant Expansion
The reaction sample of Example 5 was prepared except that several glass
microbeads (average diameter 0.2 mm) (Homogenizing beads, Virtis Corporation,
Gardiner, NY) were added. The steps described in Examples 6 and 7 were then
performed. The glass beads act as nuclei for initiation and localization of
boiling at
the bottom end of the reaction chamber, and the vapor thus generated serves to
transfer the reaction sample into the detection chamber. The color reaction
product
on the support strips 61 were then analyzed by the imaging detection system
and
procedure described in Example 7.
Example 11: Reaction Sample Preparation, (3~globin
Oligonucleotide probes (SEQ. ID NOS. 6, 7, 8, and 9) which hybridize
with the human (3-globin gene (SEQ. ID NO. 10) were synthesized by
phosphoramidite chemistry on an ABI DNA synthesizer and were haptenated with
biotin or adamantane as shown.
SEO ID NO. ~ROUENCE and ALTGNMENT
6. 5'-adam-GGGCAAGGTGAACGTGGA
7. GAAGTTGGTGGTGAGGCC-biotin-3'
10. 5'-CCTGTGGGGCAAGGTGAACGTGGATGAAGTTGGTGGTGAGGCCCTGG-3'
10'. (3'-GACACCCCGTTCCACTTGCACCTACTTCAACCACCACTCCGGGACCC-5')
8. 3'-CCCGTTCCACTTGCACC
9. ACTTCAACCACCACTCCGG-biotin-5'
To perform the so-called "double-gap" LCR method described by Backman, et
al European Patent Application 0 439 182 (1991) reaction sample mixtures
contained
the following final concentrations in a total volume of 100 ~tL: 50 mM EPPS pH
7.8,
KCl titrated with KOH to achieve pH 7.8 and 20mM K+, .30 mM MgCl2, 101tM
NAD, 1.7 pM dGTP, 9000 units DNA ligase (from Thermos thermophilus), 1 unit
DNA polymerase (from Thermos aquaticus), and 1 x 1012 copies (1.7 pmole) of
each
oligonucleotide (SEQ ID NOS. 6, 7, 8 and 9). Targets were 250 ng human
placental
DNA (about 105 copies), which contain SEQ ID NO. 10, or water.
WO 95/11437 PCT/US94/11016
~17~~62_
-53-
Reaction mixtures were pipetted into 100 ~1. rcaction chambers according to
example 4B, the bottoms of which had been melted and cooled. The reaction
chambers
were centifuged briefly to force the reaction mixture to the bottom of the
tube. The
tubes were capped with the detection units of Example 3B to form sealed
reaction/detection units.
Example 12- Amp~g DNA and Transferring Reaction Same
From Reaction Chamber To Detection Chamber
The combined reaction/detection units of example 11 were inserted into the
thermal cycler of example 1B and subjected to the following sequence of
temperature
in order to effect the LCR reaction: 35 cycles of 88°C for 10 seconds
and 53°C for 60
seconds. Each cycle took approximately 2 minutes to complete, for a total LCR
time of
about 80 minutes. Following the completion of the amplification cycles, the
ring was
heated to 104°C. This temperatures was held for 25 seconds. By virtue
of thermal
expansion and vaporization of the reaction mixture, the liquid sample was
ejected from
each reaction element to the affixed detection element, where the amplified
sample
entered the dried pad containing anti-biotin conjugate. The labeled antibody
in the pad
was solubilized, and the mixture proceeded by chromatography up the
nitrocellulose
strip. When the appropriate DNA sequence was present in the test sample, the
resultant amplification product was retained at the anti-adamantine capture
site and
visible color development was seen. No color was seen at any other antibody
locus.
The reaction units are shown in figure 14.
Example 13: Video Processi~
The reaction/detection units of examples 11 and 12 are imaged and processed
according to the procedures of Examples 7 and 8.
Example 14: Multi lex SuRports
Support strips 61 were prepared as in Example 3B with a plurality of antibody
3 0 binding sites, each antibody specific for a different hapten. The strips
also contain
biotin-labeled egg albumin at a specific location on the support. The biotin
labeled
protein serves as a control or reference standard.
WO 95111437
-54-
PCTNS94111016
Example 15 ~ Multiplex Detection
Oligonucleotide probes are synthesized as described in Example 5 or 11, and:
~ The four probes of example 11 hybridize with the human ~i-
globin gene. Two of the probes contain terminal biotin moieties,
allowing them to bind with anti-biotin-latex conjugate and one contains
terminal adamantane, allowing them to bind with anti-adamantane at a
specific binding zone on the support strip. This serves as a positive
control.
~ Four other probes hybridize with a sequence unknown in
nature. Two of the probes contain terminal biotin moieties, allowing
them to bind with anti-biotin-latex conjugate, and two of them contain
terminal dibenzofuran, allowing them to bind with anti-dibenzofuran at
a specific binding zone on the support strip. This serves as a negative
control.
~ Four other probes hybridize with the portion of human
chromosome 7 coding for the ~F5o8 mutation of cystic fibrosis. Two
of the probes contain terminal biotin moieties, allowing them to bind
with anti-biotin-latex conjugate, and two of them contain terminal
fluorescein, allowing them to bind with anti-fluorescein at a specific
2 0 binding zone on the support strip.
~ Four other probes hybridize with the portion of human
chromosome 7 coding for the GSSiD mutation of cystic fibrosis. Two
of the probes contain terminal biotin moieties, allowing them to bind
with anti-biotin-latex conjugate, and two of them contain temunal
thiophene-carbazole, allowing them to bind with anti-thiophene-
carbazole at a specific binding zone on the support strip.
~ Four other probes hybridize with the portion of human
chromosome 7 coding for the GSa2X mutation of cystic fibrosis. Two
of the probes contain terminal biotin moieties, allowing them to bind
with anti-biotin-latex conjugate, and two of them contain terminal
quinoline, allowing them to bind anti-quinoline at a specific binding
zone on the support strip.
~ Four other probes hybridize with the portion of human
chromosome 7 coding for the W12g2X mutation of cystic fibrosis.
Two of the probes contain terminal biotin moieties, allowing them to
WO 95/11437 PCT'/US94111016
21~3~6 2
-ss-
bind with anti-biotin-latex conjugate, and two of them contain terminal
dansyl, allowing them to bind with anti-dansyl at a specific binding
zone on the support strip.
The DNA sequences surrounding each of these mutations can be found in the
literature. LCR amplification is then performed using conditions of examples s-
6 and
11-12, the strips are developed, and the spots are visualized as described in
Examples 7-8.
Example 16: Multiplex Video Processing
Support strips 61 are prepared as in Example 11, except that each antibody (or
biotin-labeled protein) appears at three or more specific locations on the
strip. A
plurality of specific capture sites 74 or binding areas allows the video
processing
program 600 to average the signal from similar spots, thus increasing the
confidence
of the assignment of a particular result. In addition spurious signal may be
rejected if
similar spots do not exhibit color.
While the above-described embodiments of the invention are preferred, those
skilled in this art will recognize modifications of structure, arrangement,
composition
and the like which do not depart from the true scope of the invention. The
invention
for which protection is sought is defined by the appended claims.
WO 95/11437 PCT/US94/11016
-56-
(1) GENERAL INFORMATION:
SEQUENCE LISTING
(i) APPLICANT: PETER ZAUN
STANLEY R. BOUMA
JULIAN GORDON
JOHN J. KOTLARIK
NATALIE A. SOLOMON
(ii) TITLE OF INVENTION: APPARATUS AND METHOD FOR TRANSFER OF
A FLUID SAMPLE
(iii) NUMBER OF SEQUENCES: 10
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Abbott Laboratories
(B) STREET: One Abbott Park Road
(C) CITY: Abbott Park
(D) STATE: Illinois
(E) COUNTRY: USA
(F) ZIP: 60064-3500
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy diskette
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: Wordperfect
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Thomas D. Brainard
(B) REGISTRATION NUMBER: 32,459
(C) REFERENCE/DOCKET NUMBER: 5359.US.01
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 708-937-4884
(B) TELEFAX: 708-938-2623
(2) INFORMATION FOR SEQ ID NO: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
100 (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 1:
WO 95/11437 PCTlUS94l11016
217~~62
-s~-
GTGTCAGGAC CAGCATTCC 1g
(3) INFORMATION FOR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 20
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 2:
GTAAAGGGGA GCAATAAGGT 20
(4) INFORMATION FOR SEQ ID N0: 3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 18
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 3:
GAATGCTGGT CCTGACAC 1g
(5) INFORMATION FOR SEQ ID NO: 4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 4:
ACCTTATTGC TCCCCTTTAC C 21
( 6 ) I'~' T2MF.TION FOR SEQ ID NO : 5
(i; SEQUENCE CHARACTERISTICS:
(A) LENGTH: 50
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double stranded
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: genomic DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 5:
ATATTGTTGT GTCAGGACCA GCATTCCGGG AAAGGGGAGC AATAAGGTCA 50
(7) INFORMATION FOR SEQ ID NO: 6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 18
(B) TYPE: nucleic acid
WO 95111437 PCT/US94/11016
-58-
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 6:
GGGCAAGGTG AACGTGGA 18
(8) INFORMATION FOR SEQ ID N0: 7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 18
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 7:
GAAGTTGGTG GTGAGGCC 18
(9) INFORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 17
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
(xi) SEQUENCE DESCRIPTION: SEQ ID N0: 8:
CCACGTTCAC CTTGCCC 17
(10) INFORMATION FOR SEQ ID N0: 9:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: Other nucleic acid (synthetic DNA)
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 9:
GGCCTCACCA CCAACTTCA 19
(11) INFORMATION FOR SEQ ID NO: 10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 47
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double stranded
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: genomic DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 10:
CCTGTGGGGC AAGGTGAACG TGGATGAAGT TGGTGGTGAG GCCCTGG 47