Note: Descriptions are shown in the official language in which they were submitted.
02451
2116561
_1 _
DESCRIPTION
BACKGROUND OF THE INVENTION
1. Field of the invention
This invention relates to novel 1,3-oxazin-4-one derivatives, herbicidal
compositions containing~same, and novel intermediates for preparing same.
2. Description of the Related Art
Certain types of 1,3-oxazin-4-one derivatives, such as 6-methyl-3-{1-methyl-1-
phenylethyi)-5-phenyl-2,3-dihydro-4H-1,3-oxazin-4-one, and their herbicidal
activities are
disclosed in, for example, WO 093/15064.
However, the compounds described in the above-mentioned international
publication differ from the compound of this invention since none of them have
an acid
amide substituent on the 3-position of the 1,3-oxazine ring. Further, the
herbicidal
activities and selective toxicities of the foregoing known compounds have been
unsatisfactory.
SUMMARY OF THE INVENTION
The inventors of the present invention have earnestly studied a variety of 1,3-
oxazin-4-one derivatives by synthesizing them and examining their
physiological
activities. As a result, the inventors found novel 1,3-oxazin-4-one
derivatives which have
remarkable selective herbicidal activity and exhibit excellent herbicidal
activity to
__ 2116561
-2-
various weeds at very small dosages without giving phytotoxicity to useful
crops. Thus
the present invention has been achieved. .
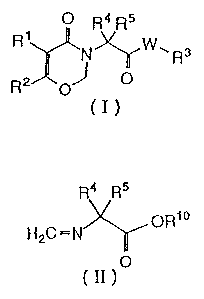
w According to the present invention, there are provided 9,3-oxazin-4-one
derivatives
represented by following general formula (1):
p Rd R5
Rt N~W~ Rs
R2 of o
c~~
in which R' represents a phenyl group which may be substituted; R2 represents
a
hydrogen atom or a lower alkyl, group; R3 represents a hydrogen atom, a lower
alkyl
group, an aralkyl group or a phenyl group which may be substituted; R4 and R5
each
independently represent a lower alkyl group, W represents an oxygen atom or a
group
represented by the formula -N(Rfi)- in which Rs represents a hydrogen atom, a
lower alkyl
group, a lower alkenyl group or a lower alkynyl group.
According to the present invention, there also provided herbicidal
compositions
containing the above derivatives and N-methylene amino acid ester derivatives
of
intermediates for preparing same, which are represented by following formula
(II):
Rd R5
ORIo
HzC ~ N
O
(II)
in which R4 and RS each independently represent a Power alkyl group;
R'° represents a
lower alkyl group or ari aralkyl group.
2176567
-3-
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The 1,3-oxazin-4-one derivatives and the intermediates for preparing same
according to the present invention represented by the general formulae (I) and
(ll),
respectively, are described in detail below.
Atoms and groups of the compounds represented in the general formulae (I) and
(I1) represented by R', R2, R3, R4, RS, Rs and R'° as defined above are
exemplified as
follows:
A phenyl group or a phenyl group which is substituted by a halogen atom, a
hydroxy group, a lower alkyl group, a lower alkoxy group, a phenoxy group, a
lower
alkylthio group, a lower alkylsulfonyl group, a lower haloalkyl group, a lower
haloalkoxy
group, an alkoxycarbonyl group, an alkoxycarbonylaikoxy group, an acyl group,
a cyano
group or a vitro group. Examples of these groups include a phenyl group, a
2-fluorophenyl group, a 3-chlorophenyl group, a 3,5-dichloro-~I-hydroxyphenyl
group, a 3-
toluyl group, a 2,5-xylyl group, a 3-anisyl group, a 3-phenoxyphenyl group, a
3-methyithiophenyl group, a 2-chloro-5-(methylsulfonyl)phenyl group, a
3-(trifluoromethyl)phenyl group, a 3,5-bis(difluoromethoxy)phenyl group, a
3-methoxycarbonylphenyl group, a 3-(1-methoxycarbonyl)ethoxyphenyl group, a -
3-nitrophenyl group, a 3-cyanophenyl group, a 3-acetylphenyl group, a 2-chloro-
5-
nitrophenyl group, a 3,5-dichlorophenyl group, a 2-fluoro-4-chforophenyl
group, a
2,5-dichlorophenyl group, a 3,5-dichloro-4-methylphenyl group, etc.
Halogen Atom
A fluorine atom, a chlorine atom, a bromine atom, or an iodine atom.
Lower Alkyl ~ro m
A lower alkyl group having one to six carbon atoms may be either straight
chained
or branched chained, such as a methyl group, an ethyl group, a n-propyl group,
an
isopropyl group, a n-butyl group, an isobutyl group, a sec-butyl group, a tent-
butyl group, a
n-pentyl group, a neopentyl group, a tert-pentyl group or a hexyl group, etc.
~1?b5~1
-4-
Lower Alkenvl t~rouo
A lower alkenyl group having two to five carbon atoms such as an allyl group,
a 2-
methyl-2-propenyl group, a 2-butenyl group, a 3-butenyl group, a 3-methyl-2-
butenyl
group, etc.
Lower Alkyd I ro m
A lower alkynyl group having two to five carbon atoms such as a 2-propynyl
group,
a 1-methyl-2-propynyl group, a 2-butynyl group, a 3-butynyl group, etc.
Lower Alkoxv C'
A lower alkoxy group whose alkyl moiety has the same meanings as defined
above,
such as a methoxy group, an ethoxy group, a propoxy group, an isopropoxy
group, a
butoxy group, a pentoxy group, etc.
ower A~[jsylthi r
A lower alkyithio group whose alkyl moiety has the same meanings as defined
above, such as a methylthio group, an ethylthio group, a propylthio group, an
isopropylthio group, a butylthio group, a pentylthio group, etc.
I ~w r AI ~rlsulfonvsro
A lower alkylsulfonyl group whose alkyl moiety has the same meanings as
defined
above, such as a methylsulfonyl group, an ethylsulfonyl group, a
propyisulfonyl group, an
isopropylsulfonyl group, a butylsulfonyl group, a pentylsulfonyl group, etc.
Lower Haioalkvl Groom
T
A lower haloalkyl group having one to four carbon atoms such as a bromomethyl
group, a difluoromethyl group, a dichloromethyl group, a trifluoromethyl
group, a 1-
chloroethyl group, an 2-iodoethyl group, a 3-chloropropyl group, a 2-methyl-2-
chloropropyl group, a 2,2,2-trifluoroethyl group, etc.
Lower Hatoalkoxv ~ros o
A lower haloalkoxy group whose haloalkyl moiety has the same meanings as
defined above, such as a trifluoromethoxy group, a difluoromethoxy group, a
chlorodifluoromethoxy group, a 2-chloroethoxy group, a 1,1,2,2-
tetrafluoroethoxy group, a
3-chloropropoxy group, etc.
1
2~?6551
An alkoxycarbonyl group having about two to eight carbon atoms such as a
methoxycarbonyl group, an ethoxycarbonyl group, an isopropoxycarbonyl group,
etc.
An aikoxycarbonylalkoxy group having about three to ten carbon atoms such as a
methoxycarbonylmethoxy group, a 1-(methoxycarbonyl)ethoxy group, a 1-
(ethoxycarbonyl)ethoxy group, a 1-methy!-3-(isopropoxycarbonyl)propyl group,
etc.
An acyl group such as an acetyl groin, a propionyl group, a butyryl group, an
isobutyryl group, etc.
An aralkyl group such as a benzyl group, a 1-phenylethyl group, a 2-
phenylethyl
group, a 1-methyl-1-phenylethyl group, a 1-methyl-2-phenylethyl group, a 1-
ethyl-2-
phenylethyl group, a 3-phenylpropyl group, etc.
Groups not specifically mentioned as examples of the above groups can be
selected by optional combinations based on the above atoms and groups or
according to
common sense in this field.
Among the compounds represented by general formula (I) described above;
preferred groups of the compound include those compounds of general formula
(I) in
which
R' is a phenyl group, a 2-fluorophenyl group, a 2-chlorophenyl group or a 2-
methylphenyl group;
RZ is a hydrogen atom, a methyl group or an ethyl group;
R3 is a phenyl group; a phenyl group substituted at the 3-posifion by one
substituent selected from the group consisting of a halogen atom, a lower
alkyl group, a
lower alkoxy group, a phenoxy group, a lower lialoalkyl group and a lower
haloalkoxy
group; or a phenyl group substituted at the 2- and 5-positions or 3- and 5-
positions by two
substituents selected from the group consisting of a halogen atom, a lower
alkyl group, a
~~ 165~~.
-s-
lower alkoxy group, a phenoxy group, a lower haloalkyl group or a lower
haloalkoxy group;
R4 and RS are each independently a methyl group or a ethyl group;
W is a group represented by the formula -N-(Rs)-, in which the preferred group
of
R6 is a hydrogen atom or a methyl group.
A more preferred compound of general formula (I) is a compound represented by
following general formula (1-'1 ):
~ H3C CH3 H Xr
~/ N~XZ
/ II
0 ~
H3c of X3
in which X', X2 and X3 each independently represent a hydrogen atom, a halogen
atom, a
lower alkyl group, a lower alkoxy group, a phenoxy group, a lower haloalkyl
group or a
lower haloalkoxy group.
Specific examples of the compound of genera! formula (I) above provided by the
present invention will be shown in Tables '1 to 5 hereinbelow. In the Tables,
abbreviations used have the following meanings.
Me: methyl group; Et: ethyl group;
Pr: n-propyl group;iPr: isopropyl
group;
Bu: butyl group; iBu: isobutyl group;
sBu: sec-butyl tBu: tent-butyl
group; group;
Hex: hexyl group; Ph: phenyl group;
Bn: benzyl group; 2-F-PH: 2 fluorophenyl; and
no substituent.
216551
-7-
Tabie 1
O H3C C H3 R6
_N N W
0 ~ /' Y
0
Compd. X Y RZ R6 Melting
No. Point ['Cj
1 - - Me H 134-137
2 ~ 2-F Me H i 29-131
-
3 - 3-F ~ Me H 131-132.5
4 - 4-F Me H 49-51
- 2-CI Me H 146-148.5
6 - 3-CI Me H 140.5-144
7 - 4-CI Me H 86-88
8 - 2-Br Me H
- 3-Br Me H 105.5-153
- 4-Sr Me~ H
11 - 2-I Me H
12 - 3-I Me H 154-156
13 - 4-1 Me H
14 - 2-F, 3-F Me H 155-161
~ 5 - 2-F, 4-F Me H 164-168.5
16 - 2-F, 5-F ~ Me H 115-120
17 - 2-F, 6-F Me H 176.5-178
18 - 3-F, 4-F Me H 129-130.5
19 - 3-F, 5-F Me H 177.5-178
. - 3-F, 4-F, 5-F Me H
21 . 2-F, 3-F, 4-F, 5-F, 6-F Me H 192-194
-
22 - 2-Cl, 3-CI Me H 138-142
23 - 2-Cl, 4-CI Me H 144-149
~~ ~b5b~
_$_
Table 1 (continued)
Compel. X Y . RZ R; Melting
No. Point ['Cj
24 - 2-Cl, 5-CI Me H 173-174
25 - 2-CI, 6-CI Me H
26 - 3-CI, 4-CI Me H 78-81
27 - 3-CI, 5-CI Me H 182-184.5
28 , 3-CI, 4-Ci, 5-C1 Me H 198.5-199.5
29 - 2-CI, 3-CI, 4-CI, 5-Ci, Me H
6-CI
30 - 2-F, 4-CI ~ ~ Me H 126-131
~
31 - 3-CI, 4-F Me H 168-169.5
32 - 3-Br, 5-Br Me H
33 - 3-CI, 5-F ~ Me H
34 - 2-Me Me H 169-171
35 - 3-Me Me H i 55-157
36 - 4-Me Me H 169-171
37 - 2-Me, 3-Me Me H
38 - 2-Me, 4-Me Me H
39 - 2-Me, ~-Me Me H 132-134
40 - 2-Me, 6-Me Me H 194-197
41 - 3-Me, 4-Me Me H
42 - 3-Me, 5-Me Me H 162-166
43 - 3-Me, 4-Me, 5-Me Me H
44 - 2-Me, 4-Me, 6-Me Me H
45 - 3-Me, 4-CI Me H
46 - 2-CI, 3-Me, 4-CI Me H 149-154
47 - 3-CI, 4-Me, 5-CI . Me H
48 - 2-Et ~ Me H
49 - 3-Et ~ Me H 118.5-120.5
50 - 4-Et Me H
51 - 2-Pr Me H
2) 76567
-9-
Table 1 (continued)
Compel. X Y - R~ Rs Metting
No. , Point (CJ
52 - 3-Pr Me H
63 - 4-Pr Me H
54 - 3-iPr ~ Me H '! 49-150
66 - 4-iPr Me H
_ 56 - 3-Bu Me H
57 - 4-Bu Me H
58 - 3-iBu ~ Me H
59 - 3-s8u Me H
'
60 - 4-tBu Me H
61 - 3-Hex Me H
62 - 4-Hex Me H
63 - 2-OMe Me H '! 62-164
64 - 3-OMe Me H 138.5-141
65 - 4-OMe Me H 154-i 55
66 - 2-OMe, 4-OMe Me H
67 - 2-OMe, 5-OMe Me H
68 - 3-OMe, 5-OMe Me H
69 - 3-.OMe, 4-OMe, 5-OMe Me H
70 - 3-CI, 4-OMe, 5-C! Me H
71 - 3-Br, 4-OMe, 5-Br Me H
72 - 3-CI, 4-OH, 5-CI Me H 170-172
73 - 3-Br, 4-OH, 5-Br Me H i 54-160.5
74 - 2-OEt Me H
75 - 3-OEt Me H
76 - 4-OEt - Me H
77 - 3-OPr Me H
7$ - 4-OPr Me H
79 - 3-OiPr Me H
CA 02176567 2004-03-03
-10-
Table1 (continued)
R' R6 Merong
Compel. X Y Point ('C]
No.
80 - 4-OiPr Me H
g1 - 3-OBu Me H
g2 - 2-OiBu Me H
g3 - 3-OsBu Me H
g4 - 4-Ot8u ~ Me H
g5 - 3-OHex Me H
gg - 4-OHex Me H
g7 - 2-OPh Me H
Me H 140-142.5
gg - 3-OPh
gg - 4-OPh Me H
gp - 2-OPh, 5-OPh Me H
91 - 3-OPh, 5-OPh Me H
Me H 109.5-111
92 - 2-CF,
Me H 140.5-142.0
g3 3-CF,
Me H 74.5-78
g4 - 4-CF,
Me H 177.5-178.5
95 - 2-CF,, 5-CF,
Me H 172-175
gg - 3-CF,, 5-CF,
97 - 2-CHZCF, Me H
g8 - 3-CHZCF, Me H
99 - 4-CHZCF, Me H
100 - 2-CHzCF,, 5-CH~CF, Me H
101 - 3-CHZCF,, 5-CH~CF, Me H
Me H 134-140
102 - 2-OCHF,
Me H 132.5-134
103 - 3-OCHFZ
Me H 127-129
104 - 4-OCHFZ . ~
105 - 2-OCHF2, 5-OCHFz . Me H
106 - 3-OCHFz, 5-OCHFz Me H
107 - 3-Ct, 4-OCHF=, 5-CI Me H
276567
-11 -
Table 1 (continued)
Compd. X Y - RZ R6 Metting
No. Point ('Cj
108 - 3-Br, 4-OCHFZ, 5-Br Me H
109 - 2-OCF, ' Me H
110 - 3-OCF, Me H 146-150
111 - 4-OCF, Me H
112 - 2-OCF,, 5-OCF, Me H
i 13 - 3-OCF,, 5-OCF, ~ Me H
114 - 3-CI, 4-OCF,, 5-CI ~ Me H
' 115 - 3-Br, 4-OCF,, 5-Br Me H
116 - 2-OCHzCF, Me H
.
117 - 3-OCHZCF, Me H
118 - 4-OCHZCF, Me H
119 - 2-OCHzCF,, 5-OCH~CF, Me H
120 - 3-OCHZCF,, 5-OCH~CF, Me H
121 - 3-CI, 4-OCHZCF,, 5-CI Me H
122 - 2-CN Me H
123 - 3-CN Me H 70-72
124 - 4-CN Me H 84.5-87
125 - 2-CN, 5-CN Me H
126 - 3-CN, 5-CN Me H
127 - 2-NOZ Me H 172.5-174.5
128 - 3-NOZ Me H 165-168
129 - 4-N02 Me H 93.5-95
130 - 2-NOZ, 5-NOZ Me H
131 - 3-NO2, 5-NOZ Me H 110-115
132 - 2-NO~, 4-N02 - Me H
276561
-12-
Table 1 (continued)
Compel. X Y . R~ R6 MelSng
No. Point ['C]
133 - 2-CF,, 4-NO~ Me H
134 - 3-CF,, 4-NO_ Me H
135 - 2-COOMe Me H 182-184
13fi - 3-COOMe Me H 170.5-171
137 - 4-COOMe Me H 132.5-134
138 - 3-COOEt Me H 1 fi7.5-171.5
139 - 3-COOtBu ~ Me H
140 - 2-OCHZCOOMe Me H
141 - 3-OCHzCOOMe Me H 120-123.5
942 - 4-OCH~COOMe Me H
143 - 2-OCH(Me)COOMe , Me H
144 - 3-OCH(Me)COOMe Me H 131.5-i 34
145 - 4-OCH(Me)COOMe Me H
14fi - 2-SMe Me H 14fi-150
147 - 3-SMe Me H 135-137.5
148 - 4-SMe Me H 1 fig-170
149 - 2-SOZMe Me H 120-125-
150 - 3-SOzMe Me H
151 - 4-SOZMe Me~ H Measurement
Impossible
152 - 3-OH Me H 193-19fi
153 - 4-OH Me H
154 - 2-COMB Me H
155 - 3-COMB Me H 151.5-154
15fi - 4-COMB Me H
157 - 3-COEt . Me H
158 - - H H
159 - 3-CI H H
_ 2~7~561
-13-
Table 1 (continued)
Corr~pd. X Y . R' R6 Melting
No. . Point [Cj
160 - 3-Br H H
161 - 3-1 H H
162 - 3-F, 5-F : H H
163 - 2-Cl, 5-CI H H
164 - 3-Cl, 5-CI H H
165 - 3-Br, 5-Br H H .
166 - 3-Me ~ H H
167 - 2-Me, 5-Me H H
168 - 3-Me, 5-Me H H
169 - 3-Et H H
170 - 3-Pr H H
171 - 3-OMs H H
172 - 2-OMs, 5-OMs H H
173 - 3-OMe, 5-OMe H H
174 - 3-Cl, 4-OMe, 5-CI H H
175 - 3-OPh H H
176 - 3-CF3 H H
177 - 2-CF3, 5-CF, H H
178 - 3-CF,, 5-CF, H H
179 - 3-OCHF2 H H
180 - 2-OCHF2, 5-OCHFZ H H
181 - 3-OCHFz, 5-OCHFz H H
182 - 3-CN H H
183 - 3-NOZ H H
184 - 2-NO2, 5-NOz ~ H H
185 - 3-NOZ, 5-NOz H H
186 - - Et H
187 - 3-CI Et H 136-137
2'16561
-14-
Table 1 (continued)
Compel. X Y R~ R6 Metting
No. Point ['Cj
188 - 3-8r Et H
189 - 3-1 Et H
190 - 3-F, 5-F Et H
191 - 2-Ci, 5-Ci Et H
192 - 3-CI, 5-C) Et H
193 - 3-8r, 5-Br . Et H
194 - 3-Me ~ Et H
195 - 2-Me, 5-Me Et H
196 - 3-Me, 5-Me Et H
197 - 3-Et Et H
198 - 3-Pr Et H
199 - 3-OMe Et H
200 - 2-OMe, 5-OMs Et H
201 - 3-OMe, 5-OMe Et H
202 - 3-CI, 4-OMe, 5-Ci Et H
203 - 3-OPh Et H
204 - 3-CF, Et H 49-51 -
205 - 2-CF3, 5-CF, Et H
206 - 3-CF,, 5-CF, Et H
207 - 3-OCHFz Et H
208 - 2-OCHF~, 5-OCHFZ Et H
209 - 3-OCHFZ, 5-OCHFz Et H
210 _ _ Pr H
211 - 3-Ci Pr H 117.5-119
212 - 3-CF, - Pr H
.213 - 3-CF, . Bu H
214 2-F - Me H 139.5-141
215 2-F 3-CI Me H 129-134
._ ~ 2~Tb5b7
-15-
Table 1 (continued)
Compel. X Y . RZ R6 Metting
No. Point ['C)
21 fi 2-F 3-8r Me H
217 2-F 3-I Me H
218 2-F 3-F, 5-F Me H
219 2-F 2-CI, 5-Cl Me H
220 ~ 2-F 3-C1,~5-CI Me H 1fi8-169.5
221 2-F 3-Br, 5-Br Me H
222 2-F 3-Me ~ Me H
223 2-F 2-Me, 5-Me Me H
~
224 2-F 3-Me, 5-Me Me H
225 2-F 3-Et Me H
.
226 2-F 3-Pr Me H
227 2-F 3-OMe Me H
228 2-F 2-OMe, 5-OMe Me H
229 2-F 3-OMe, 5-OMe Me H
230 2-F 3-Cl, 4-OMe, 5-CI Me H
231 2-F 3-OPh Me H
232 2-F 3-CF, Me H -
233 2-F 2-CF3, 5-CF, Me H
234 2-F 3-CF,, 5-CF, Me H
235 ~ 2-F 3-OCHF2 Me H
236 2-F 2-OCHF2, 5-OCHFZ Me H
237 2-F 3-OCHF~, 5-OCHFz Me H
238 2-F 3-CN Me H
239 2-F 3-NO~ Me H
240 2-F 2-NOZ, 5-N02 ~ Me H
241 2-F 3-NO~, 5-NO~ Me H
242 2-CI - Me H
243 2-CI 3-CI Me H
- ~~ 16561
-16-
Table 1 (continued)
Compel. X Y . RZ Rs Matting
No. Point ['C)
244 2-Cl 3-Br Me H
245 2-Cl 3-1 Me H
246 2-CI &F, 5-F Me H
247 2-CI 2-CI, 6-CI Me H
248 2-CI 3-CI, 5-CI Me H
249 2-CI 3-Br, 5-Br Me H
250 2-C1 3-Me Me H
251 2-CI 2-Me, 5-Me Me H
252 2-CI 3-Me, 5-Me Me H
253 2-CI 3-Et Me H
254 2-Cl 3-Pr Me H
255 2-CI 3-OMe Me H
256 2-CI 2-OMe, 5-OMe Me H
257 2-Cl 3-OMe, 5-OMe Me H
258 2-CI 3-CI, 4-OMe, 5-CI Me H
259 2-CI 3-OPh Me H
260 2-C) 3-CF, Me H
261 2-CI 2-CF,, 5-CF, Me H
262 2-CI 3-CF,, 5-CF, Me H
263 2-Cl 3-OCHFZ Me H
264 2-CI 2-OCHF2, 5-OCHFz Me H
265 2-CI 3-OCHFZ, 5-OCHFz Me H
266 2-Cl 3-CN Me H
267 2-CI 3-NOZ Me H
268 2-CI 2-NOz, 5-NOZ . Me H
269 2-CI 3-NOz, 5-NO~ Me H
270 2-Me - Me H
2' 15~5~7
-17-
Table 1 (continued)
Compel. X Y _ R= R6 Melting
No. Point {'C]
271 2-Me 3-CI Me H
272 2-Me 3-Br Me H
273 2-Me 3-I Me H
274 2-Me 3-F, 5-F Me H
275 2-Me 2-CI, 5-CI ' Me H
276 2-Me 3-CI, ~-C) Me H
277 2-Me 3-Br, 5-Br ~ Me H
278 2-Me 3-Me Me H
279 2-Me 2-Me, 5-Me Me H
280 2-Me 3-Me, 5-Me Me H
281 2-Me 3-E# Me H
282 2-Me 3-Pr Me H
283 2-Me 3-OMe Me H
284 2-tvte2-OMe, 5-OMe Me H
285 2-Me 3-OMe, 5-OMe Me H
286 2-Me 3-CI, 4-OMe, 5-CI Me H
287 2-Me 3-OPh Me H
288 2-Me 3-CF, Me H
289 2-Me 2-CF,, 5-CF3 ~ Me H
290 2-Me 3-CF3, 5-CF, Me H
291 2-Me 3-OCHFz Me H
292 2-Me 2-OCHF2, 5-OCHFi Me H
293 2-Me 3-OCHFz, 5-OCHFz Me H
294 2-Me 3-CN Me H
295 2-Me 3-NOZ . Me H
298 2-Me 2-NOZ, 5-NOz . Me H
297 2-Me 3-N02, 5-NOZ Me H
298 - - Me Me 9 86-9 87
2~ ?65~~'
-,8 _
Table 'I (continued)
Compel. X Y _ RZ Rs Melting
No. Point (C)
299 . 3-CI Me Me
-
300 - 3-Br Me Me
301 - 2-CI, 5-Cl Me Me
~
302 - 3-CI, 5-CI Me Me 64-67
303 - 3-Me Me Me
304 - 2-Me, 5-Me ~ Me Me
305 - 3-Me, 5-Me ~ Me Me
306 - 3-Et Me Me
307 - 3-Pr Me Me
308 - 3-OMe Me Me
309 - 2-OMe, S-OMe Me Me
310 - 3-OMe, 5-OMe Me Me
311 - 3-OPh Me Me
312 - 3-CF3 Me Me
313 - 3-OCHF= Me Me
314 - 3-NOZ Me Me
315 - 2-NO2, 5-NOZ Me Me -
316 - 3-NOz, 5-NOz Me Me
317 - Me Et
318 - 3-CI Me Et
319 - 2-CI, 5-Cl Me Et
320 - 3-CI, 5-CI Me Et
321 - 3-Me Me Et
322 - 2-Me, 5-Me Me Et
323 - 3-Me, 5-Me ~ Me Et
2~~65f~
_19_
Table 1 (continued)
Compel. X Y R~ R6 Melting
No. Point ['C)
324 - 3-Et Me Et
325 - - Me CHZCH=CHz
326 - 3-CI Me CHZCH=CHI
327 - 2-CI, 5-CI Me CH~CH=CHZ
328 - 3-CI, 5-Cl . Me CHzCH=CHz
329 - 3-Me Me CHZCH=CHZ
330 - 3-CF3 ~ Me CHzCH=CH2
331 - 3-OCHFZ Me CHzCH=CHz
332 - - Me CHIC=CH
333 - 3-C! Me CHIC=CH
334 - 2-CI, 5-C! Me CH2CaCH
335 - 3-CI, 5-CI ~ Me - CHzCaCH
336 - 3-Me Me CHIC=-CH
337 - 3-CF3 Me CHzC=CH
338 - 3-OCHF2 Me CH2C=CH
339 - 2-F, 3-F, 4-F Me H 169.5-i 72.5
340 - 2-F, 3-F, 5-F Me H
341 - 2-F, 3-F, 6-F Me H 148.5-151
342 - 2-F, 4-F, 5-F Me H 137.5-139.5
343 - 2-F, 4-F, 6-F Me H 177-179
344 - 2-F, 3-F, 4-F, 5-F Me H 121.5-124.5
345 - 2-F, 3-F, 4-F, 6-F Me H 146-148
346 - 2-F, 3-F, 5-F, 6-F Me H 135.5-137
347 - 2-Cl, 3-CI, 5-CI Me H
348 - 2-CI, 4-Cl, 5-CI Me H
~
349 - 2-CI, 3-CI, 4-CI, 5-CI Me H 176-177
,
350 - 2-F, 5-CI Me H 14i-144.5
351 - 2-F, 5-Br Me H
~1T6561
-20-
Table 1 (continued)
Compel. X Y RZ R6 Melting
No. Point ['Cj
352 ~ 2-F, 5-1 Me H
-
353 - 2-F, 4-Br Me H 127-134
354 - 2-F, 3-F, 4-Br, 5-F, Me H 186-187
6-F
355 - 2-F, 3-Cl, 5-CI Me H
356 - 3-tBu, 5-t8u Me H 186.5-187.5
357 - 2-F, 5-Me ~ Me H
'
358 - 2-F, 5-Et ~ Me H
359 - 2-F, 5-iPr Me H
360 - 2-F, 5-tBu Me H
361 - 3-Br, 4-Me, 5-8r Me H 176.5-178.5
362 - 2-F, 3-CF3 Me H 126.5-128.5
363 - 2-F, 5-CF, ~ Me H 146.5-149
364 - 3-CF,, 4-F Me H 152-154
365 - 3-CF,, 4-CI Me H 153-155.5
366 - 2-F, 5-OH Me H
367 - 2-F, 5-OMe Me H
368 - 2-F, 5-OPh Me H
369 - 3-F, 5-OPh Me H 58-61
370 - 3-CI, 4-OH Me H 92-93.5
37i - 2-F, 4-CI, 5-Oil'r Me H 180-182.5
372 - 2-F, 5-OCHFz ~ Me H
373 - 3-F, 5-OCHFZ ~ Me H
374 - 2-F, 5-NOz Me H 148-150.5
375 - 3-F, 5-NOZ Me H
376 - 3-NO~, 4-F Me H 161.5-164
377 - 3-F, 5-CF, Me H
378 - 3-CI, 5-CF, Me H
379 - 3-F, 5-Me Me H
=2176567
-21 -
Table 1 {continued)
Compel. X Y RZ R6 Matting
No. Point [CJ
380 - 3-F, 5-OCHFZ Me H
381 2-F 2-F, 5-C) Me H
382 2-F 2-F, 5-Br Me H
383 2-F 2-F, 5-1 Me H
384 2-F 2-F, 5-CI Me H
.
385 2-F 2-F, 3-CI, 5-CI Me H
386 . 2-F 2-F, 5-Me ~ Me H
387 2-F 2-F, 5-Et Me H
388 2-F 2-F, 5-iPr Me H
389 2-F 2-F, 5 t8u Me H
390 2-F 2-F, 3-CF, Me H
391 2-F 2-F, 5-CF, Me H
392 2-F 3-F, 5-CF, Me H
393 2-F 3-CF3, 4-CI Me H
394 2-F 2-F, 5-OH Me H
395 2-F 2-F, 5-OMe Me H
396 2-F 2-F, 5-OPh Me H
397 2-F 3-F, 5-OPh Me H
398 2-F 2-F, 5-OCHFZ Me H
399 2-F 3-F, 5-OCHF2 Me H
- = 2~ 76567
- 22 -
Table 2
0 Rd Rs H
Ph N~ N
I ~ o I
H3C O
Compd. R' Rs Y Melting
No. Point [C]
400 Me Et
401 Me Et 3-CI
402 ~Me Et 3-Br
403 Me Et 3-!
404 Me Et 3-F, 5-F
405 Me Et 2-CI, 5-CI
406 Me Et 3-CI, 5-Ci
407 Me Et 3-Br, 5-Sr
.408 Me Et 3-Me
409 Me Et 2-Me, 5-Me.
410 Me Et 3-Me, 5-Me
411 Me Et 3-Et
412 Me Et 3-Pr
413 Me Et 3-OMe
414 Me Et 2-OMe, 5-OMe .
415 Me Et 3-OMe, 5-OMe
416 Me Et 3-CI, 4-OMe, 5-Cl
417 Me Et 3-OPh
.418 Me Et 3-CF,
419 Me Et 2-CF,, 5-CF,
420 Me Et 3-CF,, 5-CF,
421 Me Et 3-OCHF2
422 Me Et 2-OCHFZ, 5-OCHF~
217b5b1
- 23 -
Table 2 (continued)
Compel. R' RS . Y Melting
No. Point ['C]
423 Me Et 3-OCHFZ, 5-OCHF=
424 Me Et 3-CN
425 Me Et 3-NOz
426 Me' Et 2-NOZ, 5-NOz
427 Me Et 3-NO~, 5-NOZ
428 Me iPr
429 Me iPr 3-Ci
430 Me iPr 2-CI, 5-Cl
431 Me iPr 3-Ci, 5-CI
432 Me iPr 3-Me
433 Me iPr 2-Me, 5-Me
434 Me iPr 3-Me, 5-Me
.435 Me iBu
436 Me iBu 3-Cl
437 Me iBu 2-CI, 5-CI
438 Me iBu 3-CI, 5-CI
439 Me iBu 3-Me
440 Me i8u 2-Me, 5-Me
441 Me iBu 3-Ms, 5-Me
442 Et Et
443 Et Et 3-CI
444 Et Et 2-CI, 5-CI
445 Et Et 3-CI, 5-CI
446 Et Et 3-Me
447 Et Et 2-Me, 5-Me
448 Et Et 3-Me, 5-Me
_ 2176567
- 24 -
Table 3
0 R4 Rs
Ph N 0
J o t,
N3C O
Compel. R' Rs Y Melting
No. Point [C]
500 Me Me
501 Me Me 3-CI
502 Me Me 3-Br
503 Me Me 3-I
504 Me Me 3-F, 5-F
505 Me Me 2-CI, 5-C!
506 Me Me 3-Cl, 5-CI Oily
507 Me Me 3-Br, 5-Sr
508 Me Me 3-Me
509 Me Me 2-Me, 5-Me
510 Me Me 3-Me, 5-Me
511 Me Me 3-Et
512 Me Me 3-Pr -
513 Me Me 3-OMe
514 Me Me 2-OMe, 5-OMe
515 Me Me 3-OMe, 5-OMe
576 Me Me 3-CI, 4-OMe, 5-CI
517 Me Me 3-OPh
518 Me Me 3-CF,
519 Me Me 2-CF,, 5-CF,
520 Me Me 3-CF,, 5-CF,
521 Me Me 3-OCHF~
522 Me Me 2-OCHFz, 5-OCHFZ
523 Me Me 3-OCHF2, 5-OCHFz
21165b1
-25-
Table 3 {continued)
Compel. R' R5 . Y Melting
No. ~ Point [Cj
524 Me Me 3-CN
525 Me Me 3-NOZ
526 Me Me 2-NOz, S-NO~
527 Me Me 3-NOZ, 5-NOz
528 Me Et
529 Me Et 3-CI
'
530 Me Et 2-CI, 5-CI
531 Me Et 3-CI, 5-CI
532 Me Et 3-Me
533 Me Et 2-Me, 5-Me
534 . Me Et 3-Me, 5-Me
535 Me Et 3-Et
536 Me Et 3-OMe
537 Me Et 3-OPh
538 Me Et 3-CF3
539 Me Et 3-OCHF,
540 Me Et 3-NO~
541 Me iPr
542 Me iPr 3-CI
543 Me iPr 2-CI, 5-CI
544 Me iPr 3-CI, 5-Cl
545 Me iPr 3-Me
546 Me iPr 2-Me, 5-Me
547 Me iPr 3-Me, 5-Me
548 Me iBu
549 Me iBu 3-CI
550 Me iBu 2-CI, 5-CI
551 Me iBu 3-CI, 5-CI
-2fi-
Table 3 (continued)
Compel. R' Rs . Y Melting
No. Point ('Cj
552 Me iBu 3-Me
553 Me i8u 2-Me, 5-Me
554 Me i8u 3-Me, 5-Me
555 Et Et
556 Et Et 3-CI
557 t Et 2-CI, 5-Cl
558 Et Et 3-CI, 5-CI
559 Et Et 3-Me
560 Et Et 2-Me, 5-Me
561 Et Et 3-Me, 5-Me
- . = 2~765~1
-27-
Table 4
0 .Ra R5
Ph N W. R3
0
H3C 0
Compel. R' Rs W R' Melting
No. Point jCJ
600 Me Me NH H 185.5-189
601 Me Me NH Me
602 Me Me NH Et
603 Me Me NH Pr
604 Me Me NH if'r 129-131.5
605 Me Me NH Bu
606 Me Me NH ii3u 118.5-121
607 Me Me NH sBu
608 Me Me NH tBu 145.5-147
609 Me Me NH Hex
610 Me Me NH 8n
611 Me Me NH CH(Me)Ph
612 Me Me NH C(Me)zPh 200-201.5
613 Me Me NH CHZCH2Ph 130-132
614 Me Me NH CHZCHZCHzPh
615 Me Et NH H
616 Me Et NH Me
617 Me Et NH Et
618 Me Et NH Bn
619 Me Et NH CH(Me)Ph
620 Me Et NH C(Me)2Ph
621 Me Et NH CHZCHzPh
622 Me iBu NH~ Bn
623 Me Me NMe H
-28-
Table 4 (continued)
Compel. R' Rs W R' Meit;ng
No. Point [~Cj
624 Me Me NMe Me 112-117
625 Me Me~ NMe Et
626 Me Me NMe Pr
627 Me Me NMe iPr
628 Me Me NMe Su
629 Me Me NMe Bn
630 Me Me NMe CH(Me)Ph
631 Me Me NMe C(Me)~Ph
632 Me Me NMe CHZCH~Ph
633 Me Me NMe CHZCHZCHZPh
634 Me Me O H 202-205
635 Me Me O Me , 67-69
636 Me Me O Et Oiiy
637 Me Me O Pr
638 Me Me O ii'r Oiiy
839 Me Me O Bu
649 Me Me O iBu
642 Me Me O sBu
643 Me Me O t8u
644 Me Me O Hex
645 Me Me O Bn OiiY
646 Me Me O CH(Me)Ph
647 Me Me O C(Me)ZPh
648 Me . Me O CHZCHzPh
649 Me Me O CHzCHzCH2Ph
650 Me Et O H 184-187
_ 216561
- 29 -
Table 4 (continued)
Compd. R' Rs W R' Melting
No. Point ['CJ
651 Me Et O Me ~i~Y
652 Me Et O Et
653 Me Et O Pr
654 Me Et O iPr
655 Me Et O Bu
656 Me Et O i8u
657 Me Et O Bn
658 Me Et O CH{Me)Ph
659 Me Et O C(Me)ZPh
660 Me Et O CHZCHzPh
661 Me iPr O H
682 Me iPr O Me
663 Me iPr O Et
664 Me ii'r O Pr
665 Me iPr O iPr
666 Me iPr O Bu
667 Me iPr O iBu
668 Me iPr O Bn
669 Me ii'r O CH(Me)Ph
670 Me iPr O C(Me)zPh
671 Me iBu O H
672 Me iBu O Me
673 Me iBu O Et
674 Me iBu O iPr
875 Me iSu O iBu
676 Me ii3u . O Bn
_. 21 ?6~E7
-30-
Table 5
O H3C C H3
~' Rs
~J o
R2 O
Compd. R' RZ W R' Melting
No. Point ['Cj
680 2-F-Ph Me O H 182-183.5
681 2-F-Ph Me O Me
682 2-F-Ph Me O Et '
683 2-F-Ph Me O Bn Oily
684 Ph Et O H 152.5-153.5
685 . Ph Et O Me
686 Ph Et O Et
687 Ph Et O 8n 99-100
688 Ph Pr O H 120-122
~
689 Ph Pr O Et
690 Ph Pr O Bn 53-56
The compound according to the present invention may be manufactured using any
methods known in the art. For example, the compound represented by general
formula
(I) may be manufactured using the following methods.
O Ra Rs
R1 O . . RQ Rs Rt ORja
O H2C~ ORio N
+ N~~ ~ O
~.CH3 O R
R O C H3 ( I I ) ( I-2 )
( III )
~1?6567
-31 -
in which R', R2, R4 and RS are as defined in general formula {I) and
R'° is as defined in
general formula (11).
The compound of formula (I-2) can be obtained by reacting a compound of
formula
{il) with a compound of formula (III) in the presence or absence of an
adequate solvent.
The reaction temperature can be arbitrarily determined so far as it ranges
from
90°C to '160°C or to the boiling point of the solvent.
The solvent, if used,. is not particularly limited so tar as it is inert with
starting
materials under the conditions of Method A, but in view of the reaction
temperature, it is
preferably a solvent having a higher boiling point, such as toluene, xylene or
mesitylene.
Although the reaction time varies depending upon the setting conditions, the
reaction can usually be completed in 1 to 240 minutes.
Although the quantitative ratio of the compounds of formulae (Il) and (ill) is
not
particularly limited, the. compound of formula {lll) is usually 0.5 to 2
moles, preferably 0.9
to 1.1 moles, per 1 mole of the compound of formula (Il).
The products of formula.(I-2) can be isolated and purified from the reaction
mixture
using a known method, such as extraction, recrystallization or chromatography.
The N-methylene amino acid ester derivative represented by general formufia
(11)
shown below and used in the above reaction as a starting material is a
novel.compound,
which is also included in the scope of the present invention.
R~ Rs
ORj°
H2C=N
~II~
__ 2 ~ 165b1
-32-
in which R° and RS each independently represent a lower alkyl group,
and R'° represents
a lower alkyl group or an aralkyl group.
Among the compounds represented by general formula (11) described above,
preferred groups of the compound include those compounds of general formula
(il) in
which R° and RS are each independently a methyl group or an ethyl
group; and R'° is a
methyl group, an ethyl group or a benzyl group.
Specific examples of.the compound of general formula (I1) above provided by
the
present invention are shown in Table 6 hereinbelow. in Table 6, the
abbreviations used
are the same as in the foregoing Tables.
__ - 2~ 7G~6~
-33-
Table 6
R~
OR1°
H2C=N
O
Compel. No. R' Rs R'
2-i Me Me Me
2-2 Me Me Et
2-3 Me Me ' Pr
2-4 Me Me iPr
2-5 Me Me Bu
2-6 Me Me iBu
2-7 Me Me si3u
2-8 Me Me. tBu
2-9 Me Me Hex
2-10 Me Me Bn
2-1.1 Me Me CH(Me)Ph
2-12 Me Me C(Me)ZPh
2-13 Me Me CH~CHZPh
2-14 Me Me CHzCHZCH2Ph
2-i 5 Me Et Me
2-16 Me Et Et
2-17 Me Et Pr
2-i 8 Me Et iPr
2-19 Me Et Bu
2-20 Me Et ~ iBu
2-21 Me Et ~ sBu
2-22 Me Et tBu
2-23 Me Et Hex
2~1b561
Table 6 (continued)
Compd. No. R' Rs R'
2 24 Me Et Bn
2-25 Me Et CH(Me)Ph
2-26 Me Et C(Me)~Ph
2-27 Me Et CHZCHZPh
2-28 Me Et CHZCHzCHZPh
2-29 Me ~ iPr Me
2-30 Me iPr Et
2-31 Me iPr ~ iPr
2-32 Me ii'r Bu
2-33 Me iPr tBu
2-34 Me iPr 8n
2-35 Me iPr CH(Me)Ph
2-36 Me iPr C(Me)zPh
2-37 Me ii'r CH~CHzPh
2-38 Me iBu Me
2-39 Me i8u Et
2-40 Me iBu iPr
2-41 Me iSu Bu
2-42 Me iBu tBu
2-43 Me i8u Bn
2-44 Me iBu CH(Me)Ph
2-45 Me iBu C(Me),Ph
2-46 N1e iBu CH~CHzPh
2-47 Et Et Me
2-48 Et Et Et -
2-49 Et Et Bn
= 2' 1656'
-35-
The compound of formula (II) may be manufactured using any method known in
the art. For example, it may be manufactured using the following method.
R~ R5 R4 Rs
Formalin
HzN ~Ri° H =N oRio
1 z
tII~)o (II)o
in which R4 and RS are as defined in general formula (!) and R'° is as
defined in general
formula (11).
The compound of formula (II) can be obtained by reacting one of amino acid
esters of formula (il') with formalin in the presence or absence of an
adequate solvent.
The reaction temperature can be arbitrarily determined so tar as it ranges
from
about 0°C to 140°C.
The solvent, if used, is not particularly limited so far as it is inert with
the
materials under the conditions of this method, and preferred examples of the
solvent
include hydrocarbons such as toluene or xylene; ethers such as diethyl ether,
diisopropyl ether or tetrahydrofuran.
Although the reaction time varies depending upon the conditions, the reaction
can usually be completed in 1 hour to 1 day.
Although the quantitative ratio of the compounds of formula (II') and formalin
is
not particularly limited, formalin is usually 1 to 5 moles, preferably 1.1 to
2 moles, per 1
mole of the compound of formula (II').
The products of formula (11) can be isolated and purified from the reaction
mixture
using a known method, such as extraction, distillation, recrystallization or
chromatography.
~~165~1
-36-
The amino acid esters of formula {il') used in the above reaction as a
starting
material can be obtained by known methods or methods similar thereto.
The compound of formula (II) often makes an equilibrium condition with trimers
thereof around room temperature, and thus said compound may be present as a
mixture
of the compound itself, that is monomers and trimers thereof. In addition, the
whole
compound may be present in the form of a trimer depending upon the condition.
However, the monomer name of said compound will be used no matter what form it
takes so as to avoid unnessecary complication.
The compound of formula (111) is another starting material for synthesizing
the
compound of forri~ula (I-2) and can be obtained by several methods, for
example by the
method described in Chem. Pharm. Bull., ~.1.{6), 1896-7901 (1983) or methods
similar
(hereto.
O R~ Rs . O
R~ R5
R ~ ORi° H20 R1 OH
R2 O~ O Alkali ~ ~ O
( I_2 ) R O
in which R', R2, R4 and RS are as defined in general formula (I) and
R'° is as defined in
general formula (II).
The compound of formula (I-3) can be obtained by hydrolyzing the compound of
formula (I-2) with an alkali.
Examples of an alkali include a solution of sodium hydroxide or potassium
hydroxide.
-
-37-
The solvent, if used in addition to water, is not particularly limited so far
as it is
inert under the conditions of Method B, and preferred examples of the solvent
include
alcohols such as methanol.or ethanol; ethers such as tetrahydrofuran or
dioxane.
The reaction temperature is preferably about from room temperature to
80°C.
The products of formula (I-3) can be isolated and purified from the reaction
mixture using a known method, such as extraction, recrystallization or
chromatography.
p R~ Rs
OBn ~ H2
~N N OH
O O Metal catalyst 2 J O
R2 R O
(I-4) (I-3 ~
in which R', R2, R4 and RS are as defined in general formula (I) and the
abbreviation of
Bn means a benzyl group. -
The compound of formula (I-3) can be obtained by hydrogenating the compound
of formula (i-4) in the presence of a metal catalyst.
Most of the metal catalysts commonly used as a catalyst for promoting
hydrogenation, such as palladium-carbon, rhodium-carbon or platinum black, can
be
used as a metal catalyst for this method.
The solvent used is not particularly limited so far as it is inert under the
condition
of Method C, and preferred examples of the solvent include alcohols such as
methanol
or ethanol; acetic acid esters such as ethyl acetate; and acetic acid.
This reaction may be completed under the following condifions; hydrogen
_._
-38-
atmosphere; normal pressure; room temperature; reaction time of from 1 hour to
1 day,
and it also may be promoted by applying heat and/or pressure.
The amount of the catalyst added can be arbitrarily determined according to
the
reaction rate.
The products of formula {)-3) can be isolated and purified from the reaction
mixture using a known method, such as extraction, recrystallization or
chromatography.
O Rd Rs O R4 Rs
N OH Ph3p R3(IV) H R~ N
J O CCy J o
O Base R2 O -
~I_3 ) CI)
in which R', R2, R3, R4, RS and W are as defined in general formula (I).
The compound of formula (I) can be obtained by reacting the compound of
formula (I-3) with carbon tetrachloride and triphenylphosphine followed .by
treatment with
the compound of formula (IV) in the presence of base.
The reaction temperature is preferably from room temperature to about
140°C or
to the boiling point of the solvent for the first process above, and
0°C to about 60°C for
the second process.
The solvent used is not particularly limited so far as it is inert under the
conditions
of Method D, and preferred examples of the solvent include halogenated
hydrocarbon
solvents such as carbon tetrachloride, chloroform or methylene chloride;
hydrocarbon
solvents such as toluene, xylene or mesitylene; and ether solvents such as
diethyl ether,
tetrahydrofuran or dimethoxyethane.
-- 2176567
-39-
Examples of a base include tertiary amines such as triethyl amine,
diisopropylethylamine or pyridine; inorganic bases such as sodium hydroxide or
sodium
carbonate. If required, the base can also be applied as a water solution or as
a salt
formed with the compound.of formula (IV). Furthermore, when the compound (IV)
is an
amine, an excess of said compound of formula (IV) can also be used as a base.
The products of formula (I) can be isolated and purified from the reaction
mixture
using a known method, such as extraction, recrystallization or chromafography.
R4 R5 . n ~ O R4 Rs
Ri N~OH N.~N~N~.N R3-~'H R1 YV
I O (IV) N
R2 OJ O R2 IOJ O
(I_3) (I)
in which R', R2, R4, RS and W are as defined in general formula (i).
The compound of formula (I) can be obtained by reacting the compound of
formula (1-3) with carbonyldiimidazoie followed by treatment with the compound
of
formula (IV) or the salt thereof.
The reaction temperature is preferably from 0°C to about 60°C
for the first
process above, and from room temperature to about 100°C or to the
boiling point of the
solvent for the second process.
The reaction time is usually from 0.5 to 24 hours.
The solvent used is not particularly limited so far as it is inert under the
conditions
of Method E, and the preferred examples of the solvent include halogenated
hydrocarbon solvents such as carbon tetrachloride, chloroform or methylene
chloride;
hydrocarbon solvents such as toluene, xylene or mesitylene; ether solvents
such as
~~T6551
-.ao -
diethyl ether, tetrahydrofuran or dimethoxyethane; ketone solvents such as
acetone or
methyl ethyl ketone; and polar aprotic solvents such as acetonitrile, N,N-
dimethylformamide or N, N-dimethylacetoamide.
The products of formula (I) can be isolated and purified from the reaction
mixture
using a known method, such as extraction, recrystallization or chromatography.
R~ R$
Ri N OH R~~_z R O R4 R5 0
I (v) N''~ ~Rz,
R2 of ~ R2 ~ J o
( ~-~ , Hase O
C x-$
in which R', R2, R4 and RS are as defined in formula (I); R"~ is a primary or
secondary
lower alkyl group or an aralkyl group; Z is a halogen atom, a p-
toluensulfonyloxy group,
a methyisulfonyloxy group, a trifluoromethylsuifonyloxy group or a group which
can be a
good leaving group by the nucleophilic reaction as represented by formula
OS020R".
The compound of formula (1-5) can be obtained by reacting the compound of
formula (I-3) with the compound of formula (V) in the presence of base.
The reaction temperature is preferably from room temperature to about
140°C or
to the boiling point of the solvent.
The solvent used is not particularly limited so far as it is inert under the
conditions
of Method F, and preferred examples of the solvent include polar aprofic
solvents such
as N,N-dimefhylformamide, dimethylsulphoxide, acetonitrile or acetone; ether
solvents
such as tetrahydrofuran or dioxane; alcohols such as methanol or ethanol; and
a mixfure
2116561
-41 -
of water and the above-mentioned solvents.
Examples of base include inorganic carbonates such as potassium carbonate,
potassium bicarbonate, sodium carbonate or sodium bicarbonate; inorganic bases
such
as sodium hydroxide or potassium hydroxide; sodium methoxide; and sodium
hydride.
The base can also be applied as a salt formed with the compound of formula (1-
3), if
required.
The product represented by general formula (I-5) can be isolated and purified
from the reaction mixture using a known method, such as extraction,
recrystallization or
chromatography.
O R4 R5 O R4.Rs
R1 ~~~ \ Oxidizing agent R~
w
J o ~ , l
O J 2 OJ O
~SRtz \ ~2
(~S, (I-7 ) SOZR
in which R', R2, R3, R4, RS and W are as defined in general formula (I), and
R'2 is a lower
alkyl group.
The compound represented by general formula (1-7) can be obtained by oxidizing
the compound of formula (I-6) with an adequate oxidizing agent.
Examples of the oxidizing agent include hydrogen peroxide, m-chloroperbenzoic
acid, sodium metaperiodate, peracetic acid and potassium permaganate.
The reaction temperature is preferably from 0°C to about 140°C
or to the boiling
point of the solvent.
The solvent used is not particularly limited so far as it is inert under the
conditions
of Method G, and preferred examples of the solvent include halogenated
hydrocarbon
2t1G561
- 42 -
solvents such as 1,2-dichloroethane, carbon tetrachloride, chloroform or
methylene
chloride; methanol; acetic acid; water; and the mixture thereof.
The product represented by general formula (1-7) can be isolated and purified
from the reaction mixture using a known method, such as extraction,
recrystallization or
chromatography.
p R4 Rs . O R4 Rs Rt3
R~s_z R1
N N~R3 (YZ) N~~~~Rs
J o R ~ J o
R O Base 2 O
( z-8 ) ( z_9)
in which R', R2, R3, R4 and RS are as in formula (!); R'3 is a primary or
secondary lower
alkyl group, a lower aralkenyl group or a lower alkynyl group; Z is as defined
in. general
formula (V).
The compound of formula (I-9) can be obtained by reacting the compound of
formula (1-8) with the compound of formula (Vl) in the presence of base.
The reaction temperature is preferably from room temperature to about
140°C or
to the boiling point of the solvent.
The solvent used is not particularly limited so far as it is inert under the
conditions
of Method H, and preferred examples of the solvent include polar aprotic
solvents such
as N,N-dimethylformamide, dimethylsulphoxide, acetonitrile or acetone; ether
solvents
such as tetrahydrofuran or dioxane; alcohols such as methanol or ethanol; and
a mixture
of water and the above-mentioned solvents.
Examples of base include inorganic carbonates such as potassium carbonate,
CA 02176567 2005-03-14
-43-
potassium bicarbonate, sodium carbonate or sodium bicarbonate; inorganic bases
such
as sodium hydroxide or potassium hydroxide; sodium methoxide; and sodium
hydride.
The products of formula (1-9) can be isolated and purified from the reaction
mixture using a known method, such as extraction, recrystallization or
chromatography.
The compound of general formula (() according to the present invention has
strong herbicidal activities against many kinds of weeds and very weak
phytotoxicities to
useful crops. - . .
When the compound represented by general formula (I) is used as a herbicide,
it
is mixed with an agriculturally and horticulturally acceptable carrier,
diluent or additive
and adjuvant by a known method while being formed into a formulation which is
usually
employed as agricultural chemicals, for example, wettable powder; granule,
wafer-
dispersible granule, emulsion concentrate or suspension concentrate. The
compound
may be mixed or used together with other agricultural chemicals, for example,
fungicides, insecticides, miticides, herbicides, plant growth regulators,
fertilizers and soil
conditioners.
!n particular, the mixed use with other herbicides can lead not only fo
reductions
in doses, reductions in manpower, but also to the broadening of the herbicidal
spectrum
attributable to cooperative activities and further improved effects
attributable to
synergistic activities by the both agents.
The following can, for example, be mentioned as specific examples of other
herbicides usable in a state such that they are mixed with the compounds of
the present
invention represenfed by general formula (() (the term in parentheses denote
common
names unless otherwise defined).
TM
Methyl 3,4-dichlorophenylcarbamate (Swep), isopropyl 3-chlorophenylcarbamate
TM TM
(Chloroproham), S-(4-chlorobenzyi)-diethylthiocarbamate (Benthiocarb), S-ethyl
N,N-
hexamethylenethiocarbamate (Molinate), S-(1-methyl-1-phenylethyi)-piperidine-1-
carbothioate (Dimepiperafe), S-benzyl N-ethyl-N-(1,2-dimethylpropyl)
thiolcarbamate
CA 02176567 2005-03-14
-44-
TM
(Esprocarb), 3-(methoxycarbanyl)aminophenyl N-(3-mefhytphenyi) carbamate
TM TM
(Phenmedipham), ethyl 3-phenyicarbamoyloxyphenyicarbamate (Desmedipham), etc.
urea h _rbi ides
:~"
1-(a,a-Dimethyibenzyl)-3-(4-methylphenyi)urea (Dymron),.3-{3,4-dichiorophenyl)-
1,1-dimethylurea (Diurov), 'i,1-dimethyl-3-(a,a,a-trifluoro-m-tolyl}urea
(Fluometuron), 3-
TM
(4-(4-chioroptienoxy)phenyl]-1,1-dimethyiurea (Chloroxuron), 3-(3,4-
dichlorophenyl)-1-
methoxy-1-methylurea (Linuran), 3-(4-chlorophenyl)-1-methoxy-1_methylurea
TM - TM
(Monolinuron), 3-(4-bromo-3-chlorophenyl)-1-methoxy-1-methylurea
(Chlorbromuron), 1-
TM
(a,a-dimethylbenzyl)-3-(2-chlorobenzyl)urea (Code number JC-940), etc.
)-laloacetarnide herb' itc des.
2-chloro-2',6'-diethyl-N-methoxymethytacetaniiide (Alachlor), N-butoxymethyl-2-
TM
chloro-2',6'-diethylacetanilide (Butachlor), 2-chloro-2',6'-diethyl -N-(2-
propoxyethyl)
TM TM
acetanilide (Pretilachlor), 2-chloro-N-isopropyiacetanifide (Propachior), etc.
Amide~.r fides
TM
3',4'-Dichioropropionanilide (Propanil), 2-bromo-N-(1,1-dimethylbenzyl)-3,3-
dimetiiyibutanamide (Bromobutide), 2-benzothiazol-2-yloxy-N-methylacetanilide
TM TM
(Metenacet), N,N-dimethyldipf~enylacetamide (Diphenamid), etc.
Dinitro~e~~l he_rbiside~
TM TM
4,6-dinitro-o-cresol (DNOC), 2-tent-butyl-4,6-dinitrophenol (Dinoterb), 2-sec-
butyl-
4,6-dinitrophenol (Dinoseb), N,N-diefhyl-2;6-dinitro-4-trifluoromethyl-m-
phenylenediamine (Dinitramine), a,a,a-trifluoro-2,6-dinitro-N,N-dipropyl-p-
toluidine
TM TM
(Trififuralin), 4-methyl-sulfonyl-2,6-dinitro-N,N-dipropylanifine (Nitralin),
N-(1-ethylpropyl)-
TM
2,6-dinitro-3,4-xylidine (Pendimethafin), etc.
p~lP,~lpXy herbs:idgs
2,4-Dichforophenoxyacetic acrd (2,4-D), 2,4,6-trichforophenoxyacetic acid
(2,4,6-
TM TM
T), 4-chloro-o-tolyloxyacetic acid (MCPA), S-ethyl-(4-chloro-2-methylphenoxy)-
TM TM
ethanethioate (MCPA thioethyl), 4-(4-chforo-o-tolyfoxy) butyric acid (MCPB), 4-
(2,4-
TM
dichlorophenoxy) butyric acid (2,4-DB}, 2-(4-chloro-o-tolyloxy) propionic acid
TM TM
(Mecoprop), 2-(2,4-dichlorophenoxy) propionic acid (Dichiorprop), {RS)-2-(4-
(2,4-
CA 02176567 2005-03-14
- 45 -
TM
dichlorophenoxy)phenoxyJ propionic acid (Diclofop) and its esters, (RS)-2-[4-
(~-
trifluoromethyl-2-pyridyloxy)phenoxyJ propionic acid.(F(uazifop) and its
esters, 2-(2,4-
dichloro-3-methylphenoxy) propionanilide (Clomeprop), S-ehtyl 4-chloro-2-
TM TM
methylphenoxy-thioacetate (Phenothiol), 2-(2-naphthoxy) propionanilide
(Naproanilide),
etc.
Ca, rboxil~r,~~3cid herbi~id
2,2-Dichloropropionic acid (Dalapone), trichloroacetic acid (TCA), 2,3,6-
~ TM
trichlorobenzoic acid (2,3,6-TBA), 3,6-dichloro-o-_ anisic acid (Dicamba), 3-
amino-2,5-
dichlorobenzoic acid (Chloramben), etc.
0-Ethyl O-(2-vitro-5-methylphenyl)-N-sec-butyl-phosphoramidethioate
TM
(Butamifos), 0,0-diisopropyl S-(2-benzenesulfonylaminoehtyl)phosphorodithioate
(SAP), S-(2-methyipiperidin-1-yl) carbonylmethy) O,O-dipropyl-
phosphorodithioate
TM
(Piperophos), etc.
F3 nzonifrll~bicidQ~
TM
2,6-Dichlorobenzonitrite (Dichlobenil), 3,5-dibromo-4-hydroxybenzonitrile
TM TM
(Bromoxynil), 4-hydroxy-3,5-diiodobenzonitrile (laxynil), etc.
Dinhenylether herbicide
TM
2,4-Dichlorophenyl 4-nitrophenyl ether (Nitrofen), 2,4,6-trichlorophenyl 4'-
TM
nitrophenyl ether (Chlornitrofen), 2,4-dichlorophenyl 3-methoxy-4-vitro-phenyl
ether
,.M TM
(Ghlomethoxyfen), ri~ethyl 5-(2,4-dichlorophenoxy)-2-nitrobenzoate (Bifenox),
4-
~,
nitrophenyl a,a,a-triffuoro-2-vitro-p-Poly! ether (Fluoroditen), 2-chloro-4-
trifiiuoromethyiphenyl 3-ethoxy-4-nitrophenyt ether (Oxyfluorfen); 5-(2-chloro-
a,a,a-
trifluoro-p-tolyloxy)-2-nitrobenzoic acid (Acifluorfen), etc. ,
-Cia~'~rse~rbi id ~
4-Amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one (Metamitron), 4-amino-6-tert-
butyl-3-methylthio-1,2,4-triazin-5(4H)-one (Metribuzin), 2-chloro-4,6-bis-
(ethylamino)-
TM
1,3,5-triazine (Simazine)., 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-
triazine .
TM TM
(Atrazin), 2,4-bis(ethylamino)-6-methylfhio-1,3,5-triazine (Simetryn), 2,4-
CA 02176567 2005-03-14
- 46 -
TM
bis(isopropylamino)-6-methylthio-1,3,5-triazine (Prometryn), 2-(1,2-
TM
dimethylpropylamino)-4-ethylamino-6-methy(thio-1,3,5-triazine (Dimethametryn),
etc.
Sulfon~rlurea H~ i id _~
2-Chloro-N-(4-methoxy-6-methyl-1,3,5-triazin-2-yi) aminocarbonytj
TM
benzenesulfonamide (Chlorosuifuron), methyl 2-{(((4,6-dimethoxypyrimidin-2-yl)
TM
aminocarbonyl) aminosulfonylj methyl}benzoate (Bensulfuron meth yl), ethyl 2-
(((4-
chforo-6-methoxypyrimidin-2-yl) aminocarbonylJaminosulfonyl}benzoate
(Chlorimuron
TM
ethyl), etc.
D~7inQ~bic:ido~
TM
4-(2,4-Dichlorobenzoyl)-1,3-dimethylpyrazol-5-yl-p-toluenesulfonate
(Pyrazolate), ,
TM
1,3-dimethy!-4-(2,4-dichlorobenzoyl)-5-phenacyloxypyrazole (Pyrazoxyfen), 1,3-
dimethy!-4-(2,4-dichloro-3-methyl-benzoyt)-5-(4-methylphenacyloxy) pyrazole
(Benzofenap), etc. .
Other herbicides
TM
3,6-Dichloropyridine-2=carboxylic acid (Clopyralid), 4-amino-3,5,6-
TM
trichloropyridine-2-carboxylic acid (Picloram), 5-amino-4-chloro-2-phenyl-
pyridazin-
3(2H)-one (Chloridazon), 3-cyclohexyf-1,5,6,7-tetrahydrocyclo
pentenopyrimidine-
TM TM
2,4(3H)-dione (LenaciJ), 5-bromo-3-sec-butyl-6-methy)uracil (Bromacil), 3-tent-
butyl-5-
chloro-6-methyuracile (Terbacil), 3-isopropyl-(1H)-2,1,3-benzofhiadiazin-
~!(3H)-one-2,2-
TM TM
dioxide (Bentazone), N-1-naphthyiphthalamic acid (Naptalam), etc.
As the agriculturally and horficutturally acceptable carriers or diluents used
in the
formulation of the compounds of this invention alone or in mixing with other
herbicides,
solid or liquid carriers usually used in agriculture may be used.
Examples of such solid carriers or diluents include clays represented by
kaolinites, montmorillonites, illites, palygorskites, etc., more specifically
pyrophyllite,
attapulgite, sepiolite, kaolinite, bentonite, vermiculite, mica, talc, etc.;
and other
inorganic substances such as gypsum, calcium carbonate, dolomite, diatomaceus
earth,
magnesium line, phosphorus lime, zeoiite, silicic anhydride, synthetic calcium
silicate,
etc.; organic substances of vegetable origin such as soybean flour, tobacco
flour, walnut
2~765b7
flour, wheat flour, wood flour, starch, crystalline cellulose, etc.; synthetic
or natural
polymers such as coumarone resin, petroleum resin, alkyd resin, polyvinyl
chloride,
polyalkylene glycol, ketone resin, ester gum, copal gum, dammar gum, etc.;
waxes such
as carnauba wax, beeswax, etc.; or urea and the like.
Examples of suitable liquid carriers or diluents include paraffin or naphthene
hydrocarbons such as kerosene, mineral oil, spindle oil, white oil, etc.;
aromatic
hydrocarbons such as xylene, ethylbenzene, cumene, methylnaphthalene, etc.;
chlorinated hydrocarbons such-as trichloroethylene, monochlorobenzene, o-
chlorotoluene, etc.; ethers such as dioxane, tetrahydrofuran, etc.; ketones
such as
acetone, methyl ethyl ketone, diisobutyl ketone, cyclohexanone, acetophenone,
isophorone; esters such as ethyl acetate, amyl acetate, ethylene glycol
acetate,
diethylene glycol acetate, dibuty) maleate, diethyl succinate, etc.; alcohols
such as
methanol, n-hexanol, ethylene glycol, diethylene glycol, cyclohexanol, benzyl
alcohol,.
etc.; ether alcohols such as ethylene glycol ethyl ether, diethyiene glycol
butyl ether,
etc.; polar solvents such ass dimethylformamide, dimethyl suifoxide, etc., or
water.
In addition, surfactants and other auxiliary agents may be used for various
purposes such as emulsification, dispersion, humidification, spreading,
dilation, .
combination destruction control, stabilization of active ingredients,
improvement of
flowability, prevention of corrosion, prevention of freezing, etc., of the
compounds of the
invention.
As the surfactant, although one of nonionic, anionic, cationic and amphoteric
surfactants may be used, nonionic and (or) anionic sun'actants are usually
used.
Examples of suitable nonionic surfactants include addition polymerization
products of
ethylene oxide with higher alcohols such as lauryl alcohol, stearyl alcohol,
oleyl alcohol,
etc.; addition polymerization products of ethylene oxide with aikylnaphthols
such as
butylnaphthol, octylnaphthol, etc.; addition polymerization products of
ethylene oxide
with higher fatty acids such as palmitic acid, stearic acid, oleic acid, etc.;
higher fatty
acid esters of polyhydric alcohols such as sorbitan, and addition
polymerization
products of ethylene oxide therewith; etc.
211561
_~8_
As suitable anionic surfactants, there can be cited, for example, alkyl
sulfate salts
such as sodium laurylsulfate, amine salts of sulfuric acid ester of oleyl
alcohol, etc., alkyl
sulfonate salts such as sodium dioctyl sulfosuccinate, sodium 2-
ethylhexylsulfonate,
etc., arylsulfonate salts such as sodium isopropyl naphthalenesulfonate,
sodium
methylene bisnaphthalenesulfonate, sodium lignosulfonate, sodium dodecyl
benzenesulfonate, etc., and the like.
Further, for the purpose of improving the properties of formulations,
enhancement
of effects, etc., the herbicides of this invention may be used in combination
with
polymers and other auxiliary agents such as casein, gelatin, albumin, glue,
sodium
alginate, carboxymethylcellulose, methylcellulose, hydroxyethylcellulose,
polyvinyl
alcohol, etc.
The above-described carriers or diluents and various auxiliary agents are used
singly or in combination with others depending on the purpose taking into
consideration
forms of formulation, conditions of application, etc.
The contents of active ingredients in the various formulations of this
invention
thus prepared may vary widely depending on forms of formulation, and suitable
content
is within the range of usually 0.1 to 99 % by weight, and preferably 1 to 80 %
by.weight,
which is most suitable.
Wettable powder contain active ingredient compounds in amounts of usually 25
to 90 %, and the remainder solid carriers or diluents and dispersion wetting
agents. If
necessary, colloid protection agents, defoaming agents, etc. may be added
thereto.
Granule contain, for example, active ingredient compounds in amounts of
usually
1 to 35 %, and the remainder may be solid carriers or diluents and
surfactants. The
active ingredient compounds may be mixed with solid carriers or diluents
uniformly, or
fixed to or adsorbed on the surfaces of solid carriers or diluents uniformly.
It is
preferred that the diameter of the granules be within the range of about 0.2
to 1.5 mm.
Emulsion concentrate contain, for example, active ingredient compounds of
usually 5 to 30 %, and in addition about 5 to 20 % by weight of emulsifiers,
the
remainder being liquid carriers or diluents. If necessary, spreading agents
and
~~~~5E1
-49-
anticorrosive agents may be added thereto.
Suspension concentrate contain, for example, active ingredient compounds in
amounts of usually 5 to 50 %, and in addition 3 to 10 % by weight of
dispersion wetting
agents, with the remainder. being water. If necessary, protective colloid
agents,
preservatives, defoaming agents, etc. may be added thereto.
The compounds of this invention may be used as herbicides as they are or in
any
forms of formulation described above.
The herbicides of this invention may be applied in effective amounts to
various
places to be protected, for example, farm-lands such as paddy fields and
upland, or
non-crop lands, prior to germination of weeds or to weeds of various stages
from after
germination to growth period. The dose is generally, as amount of active
ingredients,
on the order of 0.7 to 10,000 glha, preferably 7 to 5,000 g/ha. The dose may
be varied
properly depending on the kind of objective weeds, their growth stages, places
of
application, weather, etc.
The compound of formula (1) and the herbicide provided by this invention have
strong herbicidal activities against many kinds of weeds and very weak
phytotoxicities to
useful crops, as will be apparent from the test examples described later on.
For example, the compound of this invention exhibits excellent herbicidal
effects
at very low doses over a wide range of time from germination to and including
the
growth period of annual weeds such as Echinochloa crus-galfr, Cyperus
ditformis,
Monochoria vaginalis, Rotala indica, Lindernia procumbens, Dopafrium junceum,
Eleocharis acicularis, and Alisma canaliculatum, and perennial weeds such as
Scirpus
juncoides, and Cyperus serofinus, while simultaneously being very safe towards
paddy
field rice plants. Another feature of the compound of the present invention is
that when
applied to soil or stem and leaves, it exhibits high herbicidal activities on
various weeds
which also cause problems in uplands to include perennial and annual
Cyperaceous
weeds such as Cyperus rotundus, Cyperus esculontus, Cyperus brevifolius,
Cyperus
microiria, and Cyperus iria and Echinochloa crus-galli, Drigitaria
sanguinalis, Setaria
viridis, Poa annua, Sorghum halepense, Avena sativa, and Alopecurus
myosuroides as
-50-
well as broad-leaved weeds such as Polygonum Japathifolium, Amaranthus
viridis, and
Chenopodium album, while simultaneously being very safe toward soybeans,
cotton,
sugar beets, maize, upland rice plants, wheat, etc.
Further, the compound according to the present invention can be used not only
in
paddy fields, and uplands, but also in orchards, mulberry fields, lawns, and
non-crop
lands.
in addition, when the compound according to the present invention is used in
combination with other known agricultural chemicals having herbicidal
activities, they
exhibit complete herbicidal effects on weeds which are difficult to control
with each of
the compounds applied alone, and effectively control various weeds by
synergistic
herbicidal effects at doses at which a single compound is not effective. They
are also
very safe towards paddy field rice plants, soybeans, cotton, sugar beets,
maize, upland
rice plants, wheat, etc., so that they can provide herbicides which are very
useful in
agriculture.
EXAMPLES
Next, production of the compound of formula (!) and the intermediate compound
of formula (II) will be described in more detail in the following examples.
Example 1
To methyl 2-amino-2-methylbutyrate (2.62 g) was dropped at room temperature
37 % formalin (2.27 ~g), and the mixture was stirred for 4 h. Then the
reaction mixture
was dissolved in ether and washed with water. The organic phase was dried over
magnesium sulfate, filtered and evaporated to get the captioned compound (2.80
g).
Example 2
-51 -
To a mixture of 2,2,6-trimethyl-5-phenyl-4H-1,3-dioxin-4-one (2.18 g) and
methyl
2-(N-methylenamino)-2-methylbutyrate (1.5 g) was added 20 ml of xylene, and
the
mixture was refluxed for 1 h. The solvent inias evaporated and the residue was
pur~ied
by chromatography on a silica gel column to get the captioned compound (2.4
g).
Example 3
To a solution of methyl 2-methyl-2-(6-methyl-5-phenyl-2,3-dihydro-4-oxo-4H-1,3-
oxazin-3-yl)-butyrate (1.91 g) in 20 ml of ethanol was added at room
temperature 30 ml
of aq. NaOH (0.3N). After stirring for 24 h, ethanol was evaporated, and the
mixture
was acidified by hydrochloric acid. The precipitate was filtered off and dried
to get fhe
captioned compound (1.36 g).
Example 4
To benzyl 2-amino-2-methylbutyrate (12.96 g) was dropped at room temperature
37 % formalin (7.62 g), and the mixture was stirred for 4 h. The reaction
mixture was
dissolved in ether and washed with water. The organic phase was dried over
magnesium sulfate, filtered and evaporated to get the captioned compound (13.7
g).
Example 5
To a mixture of 2,2,6-trimethyl-5-phenyl-4H-1,3-dioxin-4-one (13.97 g) and
benzyl
2-(N-methylenamino)-2-methylpropionate (13.8 g) was added 130 ml of xylene,
and the
mixture was refluxed for 2 h. The solvent was evaporated and the residue was
puri~ied
by chromatography on a silica gel column to get the captioned compound (21.1
g).
~~~b~b~
-52-
Example 6
To a solution of benzyl 2-methyl-2-{6-methyl-5-phenyl-2,3-dihydro-4-oxo-4H-1,3-
oxazin-3-yi)-propionate (21.1 g) in 100 ml of ethanol was added 1 g of 5% Pd
on carbon
and hydrogenated at room temperature under normal pressure. After ethanol was
evaporated, saturated aq. sodium bicarbonate was added to the mixture and the
catalyst
was filtered off. The filtrate was acidified by hydrochloric acid. The
precipitate was
fltered off and dried to get the captioned compound ('! 0.8 g).
Example 7
To a mixture of 2-methyl-2-(6-methyl-5-phenyl-2,3-dihydro-4-oxo-4H-1,3-oxazin-
3-yl)-propionic acid (0.83 g) and potassium carbonate {0.45 g) in 4 ml of
dimethylformamide (DMF) was added ethyl iodide (0.56 g), and the mixture was
stirred
at 80 °C for 5 h. The reaction mixture was poured into water and
extracted with ethyl
acetate. The organic layer was washed with brine, dried over magnesium sulfate
and
evaporated. The residue was purified by chromatography on a silica gel column
~o get
the captioned compound (0.87 g).
Example 8
To a suspension of 2-methyl-2-(6-methyl-5-phenyl-2,3-dihydro-4-oxo-4H-1,3-
oxazin-3-yl)-propionic acid (1.1 g) in 14 ml of CC14-CH2C12 (1:1 ) was added
triphenylphosphin (1.38 g), then the mixture was refluxed for 40 min. The
reaction
mixture was ice-cooled and 3,5-dichlorophenol {0.65 g) and triethylamine (0.4
g) were
slowly added, then it was stirred at room temperature for 1 h. After
evaporation of the
CA 02176567 2004-03-03
-53-
solvent, the residue was dissolved in ethyl acetate. The insolubles were
filtered off and
the filtrate was evaporated. The residue was purified by chromatography on a
silica gel
column to get the captioned compound (0.7 g).
Example 9
To a suspension of 2-methyl-2-(6-methyl-5-phenyl-2,3-dihydro-4-oxo-4H-1,3-
oxazin-3-yl)-propionic acid (0.83 g) in 10.4 m! of CC14 CHZCIZ (1:1) was added
triphenylphosphin (1.04 g), then the mixture was refluxed for 40 min. The
reaction
mixture was ice-cooled and aniline (0.28 g) and triethylamine (0.3 g) were
slowly added,
then it was stirred at room temperature for 1 h. After evaporation of the
solvent, the
residue was dissolved in ethyl acetate. The insolubles were filtered off and
the filtrate
was evaporated. The residue was purified by chromatography on a silica gel
column to
get the captioned compound (0.58 g).
Example 10
' _ _' __ _-- __ _ _' r _ _
The captioned compound (1.05 g) was prepared in the same manner as
described in Example 9 except 3,5-dichloroaniline was used as a starting
material.
Example 11
pre ap ratiop~of N13-(trifluoromethyl~phenylj-2-methyl-2-(6-methyl-5-phenyl-2
3-dihydro-4-oxo
-4H-1 3-oxazin-3- 1 ro anamide Com . No.93
The captioned compound (0.72 g) was prepared in the same manner as
described in Example 9 except 3-(trifluoromethyl)aniline was used as a
starting material.
Example 12
-
-4
To a solution of 2-methyl-2-(6-methyl-5-phenyl-2,3-dihydro-4-oxo-4H-1,3-oxazin-
3-yl)-propionic acid {0.83 g) in 6 ml of tetrahydrofuran (THF) was added
carbonyldiimidazole (0.59 g) . After stirring at room temperature for 30 min.,
isopropylamine (0.23 g) was added and the reaction mixture was stirred at
60°C for 5 h.
Then it was poured into water and extracted with ethyl acetate. The organic
layer was
washed with brine, dried over magnesium sulfate and evaporated. The residue
was
purified by chromatography on a silica gel column to get the captioned
compound (0.38
g)~
Example 13
A solution of N-{3,5-dichlorophenyl)-2-methyl-2-(6-methyl-5-phenyl-2,3-dihydro-
4-
oxo-4H-'f,3-oxazin-3-yl)-propanamide (0.6 g) in 2 ml of DMF was cooled in an
ice bath
and 60% sodium hydride in oii (0.06 g) was added. The mixture was stirred at
room
temperature for 30 min. and subsequently methyl iodide (0.31 g) was added and
the
mixture was stirred at room temperature for 5 h. Then the reaction mixture was
poured
into water and extracted with ethyl acetate. The organic layer was washed with
brine,
dried over magnesium sulfate and evaporated. The residue was purified by
chromatography on a silica gel column to get the captioned compound (0.51 g).
Example 74
A solution of N-(4-methylthiophenyl)-2-methyl-2-(6-methyl-5-phenyl-2,3-dihydro-
4-oxo-4H-1,3-oxazin-3-yl)-propanamide (0.6 g) in 12 ml of 1,2-dichloroethane
was
cooled in an ice bath and 70% methachloroperbenzoic acid (0:8 g) was added.
After
-55-
stirring at room temperature for_24 h, the reaction mixture was washed with
saturated
aq. sodium bicarbonate, dried over magnesium sulfate and evaporated. The
residue
was purified by chromatography on a silica gel column to get the captioned
compound
(0.5 g). '
Example 15
dihyrdro-4-oxo-4H-1 '~-oxa in-'~-yL~opanamide'( omndNo~,~.~1
The captioned compound (0.93 g) ) was prepared in the same manner as
described in Example 9, except 2 fluoro-5-{trifluoromethyl)aniline was used as
a starting
material.
The physical properties of the various compounds prepared by the similar
methods as described in the Examples are shown in Tables 1 to 6 previously
mentioned,
and'H-NMR data of those compounds are shown in Tables 7 and 8 below.
~~~~55~
-56-
Table 7
Compd. 'H-NMR (300MHz) a (ppm) Solvent CDCI, TMS=Oppm
No.
1 1.72 (s, 6H),1.95 (s, 3H), 5.30 (s, 2H), 7.07 (t,1H),
7.23-7.38 (m, 7H), 7.49 (d, 2H),
8.39 {brs,1 H) .
2 1.69 (s, 6H),1.96 (s, 3H), 5.32 (s, 2H), 6.95-7.3 (m,
8H), 8.i3 (brs,1H),
8.25-8.34 {m,1 H) .
3 1.70 {s, 6H), 1.95 (s, 3H), 5.30 (s, 2H), 6.70-6.80 (m,
1 H), 7.06-7.52 (m, 8H),
8.57 {brs, 1 H) .
4 1.71 (s, 6H),1.95 (s, 3H), 5.30 (s, 2H), 6.91-7.46 (m,
9H), 8.41 (brs, 1 H) .
1.68 (s, 6H),1.96 (s, 3H), 5.34 {s, 2H), 7.00 (ddd,1 H),
7.20-7.37 (m, 7H), 8.38 {dd,
i H), 8.42 (brs,1 H) '
6 7.70 (s, 6H),1.95 (s, 3H), 5.30 (s, 2H), 7.01-7.07 (m,
1H), 7.16-7.41 (m, 7H),
7.65 (t,1 H), 8.51 (brs, i H) .
7 1.70 (s, 6H), 1.95 (s, 3H), 5.30 (s, 2H), 7.20-7.48 (m,
9H), 8.50 (brs, 1 H)
9 1.70 (s, 6H),1.95 (s, 3H), 5.30 (s, 2H), 7.10-7.42 {m,
8H), 7.78 (m,1 H), 8.50 {brs, i H)
12 i.69 (s, 6H),1.95 (s, 3H), 529 (s, 2H), 6.99 (t,1H), 7.22-7.47
{m, 7H), 7.94 {t,1H),
8.44 (brs, i H)
i 4 1.68 (s, 6H),1.96 (s, 3H), 5.32 (s, 2H), 6.855 (m,1 H),
7.02 (m, 1 H),
7.22-7.37 (m, 5H), 8.05 (m,1 H), 8.19 (brs,1 H)
1.68 (s, 6H),1.96 (s, 3H), 5.32 (s, 2H), 6.77-6.92 (m,
2H), 7.20-7.38 (m, 5H),
8.05 (brs,1 H), 8.15-8.26 (m,1 H)
16 1.67 (s, 61-x, i.96 (s, 3H), 5.32 (s, 2H), 6.64-6.73 {m,1
H), 6.94-7.04 (m, 1 H),
7.2i-7.37 {m, 5H), 8.12-8.24 (m, 2H) . _
i7 1.7i (s, 6H),1.94 (s, 3H), 5.29 (s, 2H), 6.9i (m, 2H),
7.15 (m, iH), 7.26-7.39 (m, 5H),
7.93 (brs,1 H)
18 i.69 (s, 6H),1.95 (s; 3H), 5.30 (s, 2H), 6.98-7.10 (m,
2H), 7.22-7.39 (m, 5H),
7.54-7.63 (m, 1 H), 8.54 (brs, 1 H)
19 i.68 (s, 6H), i.95 (s, 3H), 5.29 (s, 2H), 6.50 (tt, iH),
7.11 {m, 2H), 7.22-7.40 (m, 5H),
8.67 (brs,1 H)
21 1.69 (s, 6H),1.95 (s, 3H), 5.29 (s, 2H), 7.22-7.40 (m,
5H), 8.16 (brs, 1 H)
22 1.67 {s, 6H), 1.97 (s, 3H), 5.34 (s, 2H), 7.16-7.37 (m,
7H), 8.34 (dd, 1 H), 8.51 (brs,
1 H)
23 1.66 (s, 6H), 1.96 (s, 3H), 5.33 (s, 2H), 7.20-7.38 (m,
7H), 8.35 (d, 1 H), 8.37 (brs, 1 H)
24 1.67 (s, 6H),1.96 (s, 3H), 5.33 (s, 2H), 6.98 (dd,1 H),
7.21-7.37 (m, 6H), 8.4i (brs,
i H), 8.51 (d, 1 H)
26 1.69 (s, 6H), 1.95 (s, 3H), 5.30 (s, 2H), 7.21-7.39 (m,
7H), 7.75-7.78 (m, 1 H),
8.62 (brs, i H)
~~ ~~56~'
-s~-
Table 7 (continued)
Compd. 'H-NMR (300MHz) b (ppm) Solvent CDCi, TMS=Oppm
No.
27 1.69 (s, 6H),1.96 (s, 3H), 5.29 (s, 2H), 7.05 (t, i H),
7.22-7.40 (m, 5H), 7.48 (d,1 H),
8.66 (brs,1 H)
28 1.67 (s, 6H),1.95 (s, 3H), 5.29 (s, 2H), 7.22-7.40 (m,
5H), 7.62 (s, 2H), 8.72 (brs,1 H)
30 1.67 (s, 6H), 1.96 (s, 3H), 5.31 (s, 2H), 7.05-7.12 (m,
2H), 720-7.37 (m, 5H),
8.11 (brs,1 H), 8.21-8.28 (m, 1 H)
31 1.69 (s, 6H),1.95 (s, 3H), 5.30 (s, 2H), 7.03 (t, 1 H),
7.21-7.40 (m, 6H), 7.69 (dd, 1 H),
8.51 (brs,1 H)
34 1.71 (s, 6H),1.95 {s, 3H), 2.22 (s, 3H), 5.31 {s, 2H),
7.03 (t,1H), 7.11-7.39 (m, 7H),
7.84 (d,1 H), 8.00 (brs,1 H) .
35 1.70 (s, 6H), 1.94 (s, 3H), 2.31 (s, 3H), 5.29 (s, 2H),
6.89 (d,1 H), 7.13-7.42 (m, 8H),
8.32 (brs, 1 H)
36 1.67 (s, 6H), 1.92 (s, 3H), 2.27(s, 3H), 5.27 (s, 2H),
7.07(d, 2t~, 7.21-7.39 (m, 7H),
8.25 {brs,1 H)
39 1.72 (s, 6H),1.95 (s, 3H), 5.32 (s, 2H), 6.85 (d,1H),
7.02 (d,1H), 7.23-7.38 (m, 5H),
7.71 (brs,1 H), 7.94 {brs,1 H)
40 1.76 (s, 6H),1.94 (s, 3H), 2.23 (s, 6H), 5.33 (s, 2H),
7.01-7.10 (m, 3H),
7.24-7.39 (m, 5H), 7.74 (brs, 1 H) .
42 1.70 (s, 6H),1.94 (s, 3H), 2.26 (s, 6H), 5.30 (s, 2H),
6.72 (brs,1 H), 7.14(brs, 2H),
7.23-7.38 (m, 5H), 8.27 (brs, 1 H)
46 ~ 1.67 (s, 6H), 1.96 (s, 3H), 2.46 (s, 3H), 5.33 (s, 2H),
7.21-7.38 (m, 6H),
8.30-8.37(m, i H), 8.21 (d,1 H), 8.45(brs,1 H)
49 1.21 (t, 3H), 1.71 (s, 6H),1.94 (s, 3H), 2.61 (q, 2H),
5.30 (s, 2H), 6.92 (d,1 H),
7.16-7.43 (m, 8H), 8.34 (brs, 1 H)
54 1.22 (d, 6H),1.72 (s, 6H),1.95 (s, 3H), 2.87 (sep,1 H),
5.30 (s, 2H), 6.95 (m,1 H),
7.17-7.38 (m, 7H), 7.42 (t,1 H), 8.34 (brs,1 H)
63 1.69 (s, 6H), 1.96 {s, 3H), 3.81 (s, 3H), 5.33 (s, 2H),
6.80-7.04(m, 3H),
721-7.36(m, 5H), 8.34(dd,1 H), 8.48(brs,1 H)
64 1.69 (s, 6H), 1.94 (s, 3H), 3.77 (s, 3H), 5.28 (s, 2H),
6.62 (dd, 1H), 6.92 (dd, 1H),
7.16 (t,1 H), 7.21-7.37 (m, 6H), 8.37 (brs,1 H)
65 1.70 (s, 6H), 1.94 (s, 3H), 3.77 (s, 3H), 5.29 (s, 2H),
6.82 (d, 2H), 7.22-7.44 (m, 7H),
8.23 (brs, 1 H)
72 1.66 (s, 6H), 1.94 (s, 3H), 5.29 (s, 2H), 7.22-7.40 {m,
5H), 7.44 (s, 2H), 8.38 (brs, 1 H)
73 1.68 (s, 6H), 1.95 (s, 3H), 5.29 (s, 2H), 7.22-7.40 (m,
5H), 7.66 (s, 2H), 8.41 {brs, 1 H)
88 1.68 (s, 6H), 1.94 (s, 3H), 5.28 (s, 2H), 6.72 (dd,1 H),
6.99 (dd, 2H), 7.07 (t, 1 H),
7.20-7.40(m, 1 OH), 8.37(brs, 1 H)
-58-
Table 7 (continued)
Compd. 'H-NMR (300MHz) b (ppm) Solvent CDCI, TMS=Oppm
No.
92 1.65 (s, 6H),1.96 (s, 3H), 5.3i (s, 2H), 7.11-7.36 (m,
6H), 7.48-7.60 (m, 2H),
823 (brs,1 H), 8.37 (d, i H)
93 1.72 (s, 6H),1.96 (s, 3H), 5.31 (s, 2H), 7.21-7.42 (m,
7H), 7.65 (d,1 H), 7.85 (brs,1 H),
8.64 (brs,1 H) .
94 1.7i (s, 6H),1.96 (s, 3H), 5.31 (s,~2H), 7.21-7.39 (m,
5H), 7.53, 7.62 (ABq, 4H),
8.73 (brs,1 H) .
95 1.65 (s, 6H),1.97 (s, 3H), 5.32 (s, 2H), 722-7.36 (m,
5H), 7.41 (brd, 1 H),
7.69 (brd, 9 H), 8.41 (brs,1 H), 8.86 (hrs, i H) '
96 i.68 (s, 6H), 1.96 (s, 3H), 5.32 (s, 21-~, 72i-7.37 (m,
5H), 7.49 (brs, 1 H),
7.96 (brs, i H), 8.92 (brs,1 H)
102 1.67 (s, 6H),1.95 (s, 3H), 5.32 (s, 2H), 6.40 (t,1H),
6.99-7.i0 (m, 2H),
7.16-7.36 (m, 6H), 8.40(dd,1 H), 8.45(brs,1 H)
103 1.70 (s, 6H),1.95 (s, 3H), 5.30 (s, 2H), 6.50 (t,1 H),
6.80-6.83 (m, 1 H),
7.19-7.39 (m, 7H), 7.50(brs,1H), 8.53(brs,1H)
104 1.70 (s, 6H),1.95 (s, 3H), 5.30 (s, 2H), 6.43 (t,1 H),
7.04,7.48 (ABq, 4H),
7.22-7.39 (m, 5H), 8.46 (brs,1 H)
110 1.67 (s, 6H),1.94 (s, 3H), 5.29 (s, 2H), 6.90 {m,1 H),
7.20-7.37 (m, 7H),
7.57 (brs,1 H), 8.57 (brs,1 H)
'123 1.65 (s, 6H),1.94 (s, 3H), 5.31 (s, 2H), 721-7.36 (m,
7H), 7.57-7.65 (m,1 H),
7.s7 (brs,1 H), 8.64 (brs,1 H)
124 1.71 (s, 6H), 1.96 (s, 3H), 5.30 (s, 2H), 7.20-7.39 {m,
5H), 7.56, 7.63 (ABq, 4H),
8.90 (brs,1 H)
127 1.67 (s, 6H), 1.99 (s, 3H), 5.39 (s, 2H), 7.12 (ddd,1
H), 7.7 8-7.34 (m, 5H), 7.61 (t, 1 H),
8.19(dd,1 H), 8.81 (dd, 1 H), 71.01 (brs,1 H)
128 1.70 (s, 6H), 1.96 (s, 3H), 5.32 (s, 2H), 7.21-7.45 (m,
6H), 7.85-7.92 (m, 2H),
8.33-8.37 (m,1 H), 8.74 (brs,1 H)
129 1.71 (s, 6H),1.96 (s, 3H), 5.32 (s, 2H), 7.20-7.40 {m,
5H), 7.67, 8.15 (ABq, 4H),
9.06 (firs, 7 H)
131 1.69-(s, 6H), 1.98 (s, 3H), 5.35 (s, 2H), 7.22-7.38 (m,
5H), 8.64 (t,1 H), 8.67 (d, 2H),
9.25 (brs, 1 H)
135 1.88 (s, 6H), 1.96 (s, 3H), 3.84 (s, 3H), 5.42 (s, 2H),
7.02 (ddd,1 H), 7.18-7.35 (m,
5H), 7.49 (ddd,1 H), 7.98 (dd,1 H), 8.71 (dd, 1 H),11.52
(brs,1 H)
137 1.85 (s, 6H), 1.92 (s, 3H), 3.85 {s, 3H), 5.28 (s, 2H),
7.20-7.35 (m, 5H),
7.57, 7.92 {ABq, 4H), 8.65 (brs,1 H)
138 1.37 (t, 3H), 1.69 (s, 6H), 1.95 (s, 3H), 4.35 (q, 2H),
5.31 (s, 2H), 7.20-7.38 (m, 6H),
7.74 (d,1 H), 7.88 (dd, 1 H), 7.99 (brs,1 H), 8.39 (brs,
1 H)
_ ~~~65~7
-59-
Table 7 (continued)
Compd. 'H-NMR (300MHz) a (ppm) Solvent CDCI, TMS=Oppm
No. _
141 1.69 (s, 6H),1.95 (s, 3H), 3.77 (s, 3H), 4.62 (s, 2H),
529 (s, 2H), 6.65 (dd,1 H), 7.01 (d,1 H), 7.14-7.38(m,
7H), 8.41 (brs, 1 H)
144 1.58 (d, 3H),1.68 (s, 6H),1.94 (s, 3H), 3.71 (s, 3H),
4.77(q,1 H), 5.28 (s, 2H), 6.60
(dd, i H), 7.04 (d,1 H), 7.12-7.38 (m, 7H), 8.39 (brs,1
H)
146 1.69 (s; 6H),1.96 (s, 3H), 2.26 (s, 3H), 5.36 (s, 2H),
7.Oi (t,1 H), 7.20-7.35 (m,SH),
7.45 (dd,1 H), 8.38 (dd,1 H), 9.08 '(brs,1 H)
147 1.71 (s, 6H),1.95 (s, 3H), 2.47 (s, 3H), 5.30 (s, 2H),
6.94-6.99 {m, 1 H), 7.16-7.39 (m,
7H), 7.5s (brs,1 H),
8.3s (brs,1 H)
148 1.69 (s, 6H),1.94 (s, 3H), 2.44 (s, 3H), 529 {s, 2H),
7.18-7.48 (m, 9H), 8.38 (brs, i H)
149 1.67 (s, 6H),1.96 (s, 3H), 2.59 (s, 3H), 5.43 (s, 2H),
7.16(t, i H), 7.25-7.38 (m, 5H),
7.56 (t;1 H), 7.85 (dd,1 H), 8.30 (d, 1 H), 9.99 (d,1
H)
151 1.67 (s, 6H),1.95 (s, 3H), 2.99 (s, 3H), 5:3i (s, 2H);
7.20-7.40 (m, 5H), 7.67, 7.78
{ABq, 4H), 8.79 (brs,1 H)
152 1.65 (s, 6H),1.94 (s, 3H), 5.32 (s, 2H), 6.5-6.6 (m, 1
H), 6.7-6.8 (m,1 H),
7.1-7.4 (m, 7H)
155 1.70 (s, 6H),1.96 (s, 3H), 2.59 (s, 3H), 5.32 (s, 2H),
721-7.42 (m, 6H), 7.66 (dd, 1 H),
7.79-7.86 (m,1 H), 8.01-8.04 (m,1 H), 8.42 (brs,1 H)
187 1.10 (t, 3H),1.70 (s, 6H), 2.23 (q, 2H), 5.30 (s, 2H),
7.0-7.7 (m, 9H), 8.5i {brs,1 H)
204 1.12 (t, 3H),1.73 (s, 6H), 2.22 (q, 2H), 5.33 (s, 2H),
7.2-7.9 (m, 9H), 8.64 (brs,1 H)
211 0.88 (t, 3H),1.4-1.65 (m, 2H),1.71 (s, 6H), 2.20 (t, 2H),
5.31 (s, 2H), 7.0-7.7 (m, 9H),
8.52 (brs, i H) -
214 1.71 (s, 6H),1.93 (s, 3H), 5.33 (s, 2H), 7.04-7.17 (m,
3H), 7.25-7.35 (m, 4H),
7.49 (d, 2H), 8.37 (brs,1 H)
215 1.69 (s, 6H), 1.94 (s, 3H), 5.32 (s, 2H), 7.01-7.24 (m,
4H), 7.24-7.35 (m, 3H),
7.64 (t,1 H), 8.48 (brs,1 H)
220 1.67 (s, 6H), 1.94 (s, 3H), 5.32 (s, 2H), 7.04 (t, 1H),
7.04-7.18 (m, 2H),
7.26-7.31 (m, 2H), 7.47 (d, 2H), 8.60 (brs,1 H)
298 1.59 (s, 6H), 1.74 (s, 3H), 3.19 (s, 3H), 4.20 (brs, 2H),
7.14-7.20 (m, 2H),
7.26-7.42 (m, 8H)
302 1.57 (s, 6H),1.82 (s, 3H), 3.21 (s, 3H), 4.66 (brs, 2H),
7.15 (d, 2H), 7.26-7.40 (m, 6H)
339 1.66 (s, 6H), 1.96 (s, 3H), 5.31 (s, 2H), 7.22-7.37 (m,
5H), 7.93 (m, 1 H), 8.90 (brs,1 H)
341 1.72 (s, 6H), 1.96 (s, 3H), 5.30 (s, 2H), 6.86 (m, 1 H),
7.14 (m, 1 H), 7.25-7.40 (m, SH),
8.09 (brs, 1 H)
342 1.66 (s, 6H), 1.95 (s, 3H), 5.31 (s, 2H), 6.93 (m, 1 H),
7.21-7.38 (m, 5H),
8.1'1 (brs,1 H), 8.26 (m, 1 H)
_ 2~ ~~561
-60-
Table 7 (continued)
Compd. 'H-NMR (300MHz) a (ppm) Solvent CDCI, TMS=Oppm
No.
343 1.72 (s, 6H), 1.95 (s, 3H), 529 (s, 2H), 6.71 (m, 2H),
7.23-7.40 (m, 5H), 7.93 (brs, 1 H)
344 1.65 (s, 6H),1.96 (s, 3H), 5.30 (s, 2H), 7.18-7.40 (m,
5H), 8.01-8.14 (m, 1 H),
8.19 (bts,1 H)
345 1.70 (s, 6H),1.95 (s, 3H), 529 (s, 2H), 6.76-6.87 (m,1
H), 724-7.40 (m, 5H),
8.03 (brs,1 H)
346 1.70 (s, 6H), 1.95 (s, 3H), 5.29 (s, 2H), 6.94 (m, 1
H), 7.25-7.40 (m, 5H), 8.19 (bts,1 H)
348 1.66 (s, 6H),1.95 (s, 3H), 5.32 (s, 2W), 7.21-7.36 (m,
5H), 7.43 (s,1 H), 8.38 (brs,1 H),
8.63 (s,1 H)
349 1.64 (s, 6H),1.96 (s, 3H), 5.31 (s, 2H), 7.20-7.36 (m,
5H), 8.49 (brs,1H), 8.62 (s,1H)
350 1.67 (s, 6H), 1.96 (s, 3H), 5.32 (s, 2H), 6.95-7.00 (m,
2H), 7.22-7.37 (m, SH),
8.17 (brs,1 H), 8.39-8.43 (m,1 H)
353 1.67 (s, 6H),1.96 (s, 3H), 5.31 (s, 2H), 7.20-7.37 (m,
8H), 8.13 (brs,1 H), 8.21 (t, 1 H)
354 1.70 (s, 6H), i.96 (s, 3H), 529 (s, 2H), 7.24-7.40 (m,
5H), 825 (brs,1H)
336 1.30 (s,18H),1.72 (s, 6H),1.95 (s, 3H), 5.31 (s, 2H),
7.15 (m,1H), 7.23-7.38 (m, 7H),
826 (brs,1 H)
357 1.68 (s, 6H),1.95 (s, 3H), 2.29 (s, 3H), 5.32 (s, 2H),
6.79 (m,1 H), s.88-s.96 (m,1 H),
7.22-7.38 (m, 5H), 8.06 {brs, 1 H), 8.13 (dd,1 H)
361 1.68 (s, 6H), 1.95 (s, 3H), 2.49 (s, 3H), 5.29 (s, 2H),
7.23-7.39 (m, 5H), 7.73 (s, 2H),
8.46 (brs,1 H)
362 1.67 (s, 6H),1.97 (s, 3H), 5.32 (s, 2H), 7.17-7.38 (m,
7H), 8.22 (brs,1 H), 8.52 (t,1 H)
363 1.69 (s, 6H), 1.96 (s, 3H), 5.33 (s, 2H), 7.15 (t, 1H),
7.21-7.38 (m, 6H), 8.3i (brd, 1H),
8.71 (dd,1 H)
364 1.70 (s, 6H), 1.95 (s, 3H), 5.31 (s, 2H), 7.10 (t,1 H),
722-7.40 (m, 5H), 7.65 (m, 1 H),
7.79 (dd,1 H), 8.fi1 (brs,1 H)
365 1.69 (s, 6H), 1.95 (s, 3H), 5.31 (s, 2H), 7.20-7.40 (m,
6H), 7.64 (dd, 1H), 7.86 (d, 1H),
8.68 (brs, 1 H)
369 1.66 (s, 6H), i .93 (s, 3H), 5.28 (s, 2H), fi.4-6.6 (m,
1 H), 6.84 (brs,1 H), 6.9-7.4 (m,
11 H), 8.42 {brs, 1 H)
370 1.68~(s, 6H), 1.94 (s, 3H), 5.30 (s, 2H), 6.84 (s,1 H),
7.09 (dd,1 H), 7.2-7.4 (m, 5H),
7.63 (d,1 H), 8.23 (brs, i H)
371 1.35 (d, 6H),1.67 (s, 6H),1.96 (s, 3H), 4.53 (sep, 1
H), 5.32 (s, 2H), 7.09 (d,1 H),
7.2-7.4 (m, 5H), 8.10 (d,1 H), 8.10 (brs, 1 H)
376 1.65 (s, 6H),1.94 (s, 3H), 5.32 (s, 2H), 7.10 (dd, 1
H), 7.21-7.36 (m, 5H), 7.73 (m, 1 H),
8.7 5 (dd,1 H), 8.72 {brs,1 H)
~~7~5G1
-61 -
Table 7 (continued)
Compd. 'H-NMR (300MHz) a (ppm) Solvent CDCI, TMS=Oppm
No.
506 1.66 {s, 6H),1.94 (s, 3H), 5.3i (s, 2H), 7.07 (d, 2H),
7.20 (t,1 H), 7.23-7.41 (m, 5H)
600 1.59 (s, 6Hj, i.92 (s, 3H), 5.26 (s, 2H), 7.06 (brs,
2H), 7.2-7.4 (m, 4H), 7.62 (brs,1H)
604 1.13 (d, 6H),1.61 (brs, 6H), 1.92 (s, 3H), 4.03 (sep,
1 H), 5.24 (s, 2H), 5.87 (d, 1 H),
720-7.38 (m, 5H) ,
606 0.89 (d, 6H),1.61 (s, 6H);1.7-1.9 (m,1 H), 1.92 (s, 3H),
3.06 (t, 2H), 5.25 (s, 2H),
628 (brs,1 H), 7.2-7.4 (m, 4H) -
608 1.33 (s, 9H),1.57 (s, 6H),1.91 (s,.3H), 523 (s; 2H),
5.91 (brs,1H), 7.22-7.38 (m, 5H)
611 1.48 {d, 3H),1.61 (s, 3H), 1.62 (s, 3H),1.90 {s, 3H),
5.09 (dq, 1 H),
5.19, 523 (ABq, 2H), 6.38 (d,1 H), 7.19-7.39 (m,1 OH)
612 1.60 (s, 6H),1.68 (s, 6H),1.91 (s, 3H), 521 (s, 2H),
6.46 (brs, 1 H),
7.18-7.42 (m,1 OH)
613 1.54 {s, 6H),1.91 (s, 3H), 2.80 (t, 2H), 3.50 (q, 2H),
5.19 (s, 2H), 6.18 (brt, 1 H),
7.16-7.38 (m, 10H)
624 1.59 (s, 6H),1.92 (s, 3H), 3.04 (brs, 6H), 5.20 (s, 2H),
7.2-7.4 (m, 5H)
634 1.59 (s, 6H),1.92 (s, 3H), 5.27 (s, 2H), 7.22-7.38 (rn,
5H)
635 1.55 (s, 6H), '1.92 (s, 3H), 5.27 (s, 2H), 7.20-7.37
(m, 5H)
636 122 (t, 3H), 1.55 (s, 6H),1.91 (s, 3H), 4.15 (q, 2H),
5.27 (s, 2H), 721-7.38 (m, 5H)
638 1.20 (d, 6H),1.59 (s, 6H),1.91 (s, 3H), 4.9-5.1 {m,1
H), 5.25 (s, 2H), 7.2-7.4 (m, 5H)
645 1.58 (s, 6H), 1.90 (s, 3H), 5.11 {s, 2H), 5.25 (s, 2H),
7.20-7.38 (m, 10H)
650 0.93 {t, 3H),1.53 (s, 3H),1.77-1.91 (m, 1 H), 1.93 (s,
3H), 2.21-2.36 (m,1 H),
522, 5.25 (ABq, 2H), 722-7.39 (m, 5H)
651 0.91 (t, 3H), 1.48 (s, 3H),1.75-1.89 (m,1 H),1.92 (s,
3H), 2.12-2.27 (m,1 H),
3.69 (s, 3H), 522, 5.25 (ABq, 2H), 7.23-7.38 (m, 5H)
683 1.57 (s, 6H), 1.88 (d, 3H), 5.11 (s, 2H), 5.28 (s, 2H),
7.02-7.16 (m, 2H),
7.24-7.33 (m, 2H), 7.30 (s, 5H)
687 1.07 (t, 3H),1.58 (s, 6H), 2.15 (q, 2H), 5.12 (s, 2H),
5.25 (s, 2H), 7.2-7.5 (m, 10H)
690 0.84 (t, 3H), 1.5-1.8 (m, 8H), 2.15 (t, 2H), 5.11 (s,
2H), 5.24 (s, 2H), 7.1-7.4 (m, 1 OH)
2176561
-62-
Table 8 D c
H~C~CH3 -~-- A
H C CH~
HZC.N~ORtt '~ /3 H~'N~H""-' B
O ' H3C~N~N~CH~
ttR02C CH~ H C_ c02Rt t
Compel. 'H-NMR (300MHz) a (ppm) Solvent CDCI, TMS=Oppm
No.
2-10 1.34 (s, 6H), 3.67 (s, 2H), 5.06 (s, 2H), 725-7.39 (m,
SH) (trimer)
2-15 0.80-0.92 (m, 3H),1.28-1.42 (m, 3H);1.68-1.90 (m, 2H),
3.60-3.76 (m, 3H+B), 7.42,
7.48 (ABq, D)
Next, several embodiments of formulations using the compound of this invention
will be shown. In the following formulations, all "parts" are by weight.
Formulation Example 1 (Emulsion Concentrate)
Compound No. 1 20 parts
Xylene 63 parts
Calcium dodecylbenzenesulfonate 7 parts
Polyoxyefhylenestyryl phenyl ether 5 parts
Dimethylformamide , 5 parts
The above materials were uniformly mixed and dissolved, to obtain 100 parts of
emulsion concentrate.
Formulation Example 2 (Wettable Powder)
Compound No. 9 20 parts
Kaolinite . 70 parts
Calcium lignosulfonate 7 parts
Condensate of alkylnaphthalenesulfonic acid 3 pads
The above materials were mixed and crushed using a jet mill, to obtain 100
parts
of wettable powder.
~~~~561
- 63 -
Formulation Example 3 (Suspension Concentrate)
Compound No. 1 . 20 parts
Sodium di(2-ethylhexy!)sulfosuccinate 2 parts
Polyoxyethylene nonylphenylether ~ 2 parts
Defoaming agent 0.5 parts
.
Propylene glycol 5 parts
~
Xanthan gum ~ _ 0.01 parts
Water ~ 70.49 parts
The above materials were crushed and uniformly mixed by using a wet-type ball
mill, to obtain 100 parts of suspension concentrate.
Formulation Example 4 (Granule)
Compound lVo.1 1 parts
Sodium di(2-ethylhexyl)suifosuccinate 2 parts
Bentonite 30 parts
Talc 67 parts
The above materials were sufficiently mixed, kneaded with an addition of a
suitable amount of water and granulated by a granulator to obtain 100 parts of
a
granule.
The herbicidal effects of the compound of this invention will be explained
below
according to the test examples.
Test Example 1 (Paddy field soil application)
Paddy field soil was filled in 500 cm2 wagner pots, suitable amounts of water
and
chemical fertilizers were added thereto, and kneaded to convert it to a paddy
field state.
A stock of paddy field rice plant (variety; Koshihikari) comprising a pair of
two
seedlings that had been grown in advance in a greenhouse to a stage of two
leaves,
were transplanted in each pot in a population of one stock per pot. Further,
in each
pot, there were sown predetermined amounts of seeds of Echinochloa crus-galli,
Monochoria vaginalis, Lindernia procumbens and Scirpus juncoides,
respectively, and
water was filled to a depth of 3cm. ~ .
On the next day, wettable powders were prepared using the compounds shown in
Table 9 below according to Formulation Example 2, and they were diluted with a
suitable amount of water.so that they contained active ingredients in an
amount of 50
g/ha. They were applied by dropping with a pipette.
After 29 days from the application with the chemicals, herbicidal effects on
each
weed and phytotoxicity on paddy field rice plants were evaluated according to
the
following criteria. The results obtained are shown in Table 9 below.
Evaluation criteria (1'i ranks)
Score Herbicidal effects: Phytotoxicrty to crop:
Ratio of trifled weeds Ratio of injured plants compared
compared to the
t0 the COntfOf (%) COntfOf (%~
0 0
1 Above 0 to 10
2 Above 10 to 20 .
3 Above 20 to 30 -
4 Above 30 to 40
Above 40 to ~0 Same as the left column
6 Above SO to fi0
7 Above 60 to 70
8 Above 70 to 80
9 Above 80 to 90
Above 90 to 100
(withered)
~~~6561
-65-
Table 9
Compd. Active - Herbicidal Phytotobcity
No. ingredienteffects
dose Weed A Weed B Weed Weed 0 Padd field
g alma . . . C rice pl nt
-- -
1 50 10 10 . 10 10 0
3 50 10 10 10 10 0
6 50 ~ 10 - 10 10 10 0
22 50 . 10 ~10 10 10 0
24 50 10 10. 10 ~ 10 0
26 50 10 10' 10 9 0
27 50 10 10 10 10 0
30 50 10 10 10 10 0
35 50 10 10 10 10 0
39 50 10 10 i0 10 0
42 50 10 10 10 10 0
49 50 10 10 10 10 0
64 50 10 10 10 10 0
93 50 10 10 10 10 0
103 50 10 10 10 10 0
123 50 10 10 10 10 0
128 50 10 10 10 9 0
138 . 50 10 10 10 10 0
363 50 10 10 10 10 0
in the Tables 9-12, abbreviations of weeds are as follows.
Weed A: Echinochloa crus-galli
Weed B: Monochoria vaginalis
Weed C: Lindernia procumbens
Weed D: Scripus Juncoides
Weed E: Digitalia sanguinalis
Weed F: Setaria viridis
_ ~~~~561
- ss -
Weed G: Abufilon fheophrasti
Weed H: Xanthium strumarium
Weed I: Polygonum lapathifolium
Weed J: Datura stramonium
Test Example 2 (Paddy field foliar application)
Paddy field soil was filled-in 500 cm2 wagner pots, suitable amounts of water
and
chemical fertilizers were added thereto, and kneaded to convert it to a paddy
field state.
A stock of paddy field rice plant (variety; Koshihikari) comprising a pair of
two seedlings
that had been grown in advance in a greenhouse to a stage of two leaves, were
transplanted in each pot in a population of one stock per pot. Further, in
each pot,
there were sown predetermined amounts of seeds of Echinochloa crus~alli,
Monochoria
vaginalis, Lindernia procumbens and Scirpus juncoides, respectively, and wafer
was
filled to a depth of 3cm.
After having grown the plants in a greenhouse until Echinochloa crus~alli
reached
a stage of 1.5 leaves, wettable powders were prepared using the compounds
shown in
Table 10 below according to Formulation Example 2, and they were diluted with
a
suitable amount of water so that they contained active ingredients in an
amount of 100
glha. They were applied by dropping with a pipette.
After 21 days from the application with the chemicals, herbicidal effects on
each
weed and phytotoxicity on paddy field rice plants were evaluated according to
the
criteria shown in Test Example 1 above. The results obtained are shown in
Table 10
below.
~'l T 6561
-s7-
Table 10
comps. Active Herbicidal Pnytotobcity
No. ingredienteffects
g a'Uha Weed Weed Weed C Weed Paddy field
A B D rice plant
1 100 10 ~10 10 10 0
3 .100 ~10 ~ 10 10 . 10 0
~
6 100 10 ~ 10 10 10 0
22 100 10 10 10 9 0
24 100 10 10 10 10 0
26 100 10 10 ' 10 8 0
27 100 10 10 10 9 0
30 100 10 10 10 9 0
35 100 10 10 10 10 0
39 100 10 10 10 9 0
42 100 10 10 10 10 0
49 100 10 10 10 10 0
64 100 10 10 10 10 0
93 100 10 10 10 10 0
103 100 10 10 10 10 0
123 100 10 10 10 10 0
128 100 10 10 10 8 0
138 100 10 10 10 10 0
363 100 10 10 10 10 0
Test Example 3 (Upland soil application)
Upland soil was filled in 900 cm2 plastic pots, in which there were sown
predetermined amount of seeds of Echinochloa crus-galli, Drigitaria
sanguinalis, Setaria
viridis, Abutilon theophrasti, Xanthium strumarium, Polygonum lapathifolium
and Datura
stramonium, respectively, and soil was placed thereon to a thickness of 1 cm.
On the day after sowing, wettable powders were prepared using the compounds
_ ~~~6561
-s8-
shown in Table 11 below according to Formulation Example 2, and they were
diluted
with a suitable amount of water so that they contained active ingredients in
an amount of
9 kglha. They were sprayed uniformly over the surface of soil.
After 21 days from the application with the chemicals, herbicidal effects on
each
weed were evaluated according to the criteria shown in Test Example 1 above.
The
results obtained are shown in Table 11 beloinr.
Table 11
c"'y Aaire ~ Herbicidal
No. ingredienteffects
' dose
Weed Weed Weed Weed Weed Weed Weed
A E F G H 1 J
1 1000 10 10 10 10 8 9 8
3 1000 10 10 10 10 8 9 7
6 100D 10 ~ 10 10 10 9 10 9
24 1000 10 10 i0 10 9 10 ' 9
35 1000 10 10 10 10 9 10 9
42 1000 10 10 10 10 9 10 9
49 1000 10 10 10 10 8 9 8
93 1000 10 10 10 9 7 8 8
363 1000 10 10 10 10 9 10 10-
Test Example 4 (Upland foliar application)
Upland soil was filled in 900 cm2 plastic pots, in which there were sown
predetermined amount of seeds of Echinochloa crus-galli, Drigifaria
sanguinalis, Setaria
viridis, Abutilon fheophrasti, Xanthium strumarium, Polygonum lapathifolium
and Datura
stramonium, respectively, and soil was placed thereon to a thickness of 1 cm.
After having grown the plants in a greenhouse until each plant reached a stage
of
from 2 to 4 leaves, wettable powders were prepared using the compounds shown
in
Table 12 below according to Formulation Example 2, and they were diluted with
a
~~~6561
-69-
suitable amount of water so that they contained active ingredients in an
amount of 1
kg/ha. They were sprayed uniformly over the surface of leaves.
After 21 days from the application with, the chemicals, herbicidal effects on
each
weed were evaluated according to the criteria shown in Test Example 1 above.
The
results obtained are shown in Table 12 below.
Table 12
c~"~' ~"'e . ~ .
ISO. ltlgfEdefll Herbicidal
dose Weed Weed effects Weed Weed
g ailha A E I J
Weed
F
Weed
G
Weed
H
1 1000 10 10 ~ 10 10 9_ 9 9
3 1000 10 10 90 10 9 10 8
6 1000 10 10 10 10 9 10 10
24 1000 10 10 10 10 9 10 10
35 1000 10 10 10 10 9 10 10
42 1000 10 10 10 10 9 10 10
49 1000 10 10 10 10 9 10 9
93 1000 10 10 10 10 8 10 9
363 1000 10 10 10 ~ 10 I 9 ~ 10 , 8