Note: Descriptions are shown in the official language in which they were submitted.
z~ ~ ~t~o~
-1-
METHOD FOR THE PREPARATION OF UREA
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a method for
the preparation of urea from ammonia and carbon
dioxide.
2. Description of the Related Art
When ammonia and carbon dioxide are
introduced into a synthesis zone of a urea plant
at a suitable pressure (of for example 125-350
atm.) and a suitable temperature (of for example
170-250°C), ammonium carbamate is first formed
according to the exothermic reaction:
2 NH3 + COZ --> H2N-CO-ONH4
Then urea is formed from the obtained
ammonium carbamate through dehydration according
to the endothermic equilibrium reaction:
HZN-CO-ONH4 < ---> H2N-CO-NH2 + H20
The extent to which the conversion to urea
takes place is partly dependent on the temperature
and pressure employed, and the amount of excess
ammonia used. The reaction product obtained is a
solution that consists substantially of urea,
water, ammonium carbamate and unbound ammonia. The
ammonium carbamate and the ammonia are usually
removed from the solution and are in most cases
returned to the synthesis zone. The synthesis zone
may consist of separate zones for the formation of
ammonium carbamate and urea. These zones may,
however, also be combined in a single apparatus.
2z ~~oo~
-2-
A method that is frequently used for the
preparation of urea is described in European
Chemical News, Urea Supplement of 17 January 1969,
pp. 17-20. According to this method, the urea
synthesis solution that is formed in the synthesis
zone at a high pressure and temperature is
transferred to a stripping zone. There, the
synthesis solution is subjected to a stripping
treatment at a pressure that is substantially
identical to that in the synthesis zone by
contacting the solution with a countercurrent of
gaseous carbon dioxide while supplying heat, so
that the majority of the ammonium carbamate
present in the solution decomposes to ammonia and
carbon dioxide. These decomposition products are
stripped from the solution in gaseous form and are
evacuated together with a small amount of water
vapor and the carbon dioxide used for the
stripping. The gas mixture obtained in the
stripping treatment is transferred to a
condensation zone, in which the majority of the
mixture is condensed and absorbed into an aqueous
solution produced by the further treatment of the
urea-containing solution. Then both the aqueous
ammonium carbamate solution formed in this process
and the uncondensed gas mixture are passed from
the condensation zone to the synthesis zone for
the formation of urea. The heat required for the
conversion of ammonium carbamate into urea is here
obtained through further condensation of the gas
mixture, which releases the heat of condensation.
EP-B-155,735 describes a method for the
preparation of urea, and reports that a good
synthesis efficiency is obtained and the formation
of biuret and the hydrolysis of urea in the
stripping treatment remain within acceptable
limits. Moreover, the gas mixture obtained in the
~1 ~?Dad
-3-
stripping treatment reportedly is condensed at
such a temperature that a considerably smaller
heat-exchanging surface area suffices to discharge
the heat released, with low-pressure steam of for
example 3-5 bar being formed, or steam of a higher
pressure, for example of 5-10 bar, being obtained
or the heat released being used directly to heat
process streams.
According to EP-B-155,735, this is achieved
by causing urea to form in the condensation zone
as the gas mixture obtained in the stripping
operation is condensed. Because of the presence in
the condensation zone of relatively large amounts
of urea in water, which medium acts as a solvent
for the ammonium carbamate formed by the
condensation of the gas mixture obtained in the
stripping, the heat of condensation and of
solution becomes available at a higher temperature
level than when this medium is not used. When 30~
of the equilibrium amount of urea obtainable has
been formed, a heat effect is already clearly
noticeable. According to EP-B-155,735 the urea
formation is preferably continued to 50-80~ of the
equilibrium.
According to EP-B-155,735 the condensation
zone may be arranged either horizontally or
vertically. EP-B-155,735 further mentions that a
vertically arranged condensation zone affords the
possibility of combining the synthesis zone and
the vertical condensation zone in a single
apparatus.
JP-A-122,452/84 discloses that a horizontally
arranged reactor can be used for urea production.
However, JP-A-122,452/84 does not mention
combining condensation and synthesis zones.
Rather, it describes a method f or the preparation
of urea, in which a horizontal reactor, and a
.~:
2~?~a0~
-4-
separate stripper and condenser are used, the
gases from the stripper being condensed in the
condenser before the condensate is reintroduced
into the reactor.
U.S. Patent No. 3,024,280 describes a method
for the preparation of urea, in which a long
tubular winding reactor having a total length of
approximately 140 m is used. COZ is added to the
urea synthesis solution at various points along
the reaction tube while the solution is cooled
with the aid of cooling water that passes through
the tube jacket.
SUMMARY OF THE INVENTION
It is an object of the present invention is
to provide an improved method for the preparation
of ur ea .
It is a further object of the present
invention to provide a method for the preparation
of urea entails lower investment costs.
To accomplish these and other objects, an
embodiment of the present invention provides a
method for the preparation of urea, the urea being
formed along with ammonium carbamate from a
reaction between carbon dioxide and ammonia,
preferably at a pressure of about 125 bar to about
350 bar in a urea synthesis solution in a urea
reactor. The urea reactor preferably contains a
horizontally arranged condensation zone which
includes a heat exchanger. According to this
method, ammonia (NH3) and carbon dioxide (C02 are
supplied to the reactor and are largely absorbed
into a urea synthesis solution. A substantial
portion of heat is generated by condensation.
This heat is discharged by, for example, means of
the heat exchanger. The residence time of the
urea synthesis solution in the reactor is selected
21?ldQ3
.~.
-5-
so that at least about 85~ of the theoretically
obtainable amount of urea is prepared, whereupon
the urea synthesis solution can be processed into
a urea solution or solid urea.
The method according to the present invention
is advantageous in that, among other things, the
method can be carried out in a plant with
substantially lower investment costs, because of
the integration of a heat exchanger/condenser in a
single reactor. Accordingly, fewer apparatuses
and pipelines - which must be resistant to high
pressure in a highly corrosive environment - are
required. Because the present invention can
suitably be (and preferably is) practiced with a
condenser/heat exchanger part having a horizontal
orientation, a smaller (shorter) plant and plant
installations are required. This offers further
investment benefits and also promotes safety.
Preferably the entire reactor is designed as
a horizontally arranged reactor.
In addition, in accordance with the present
invention the supply of the gas phase (C02 and NH3)
can then be distributed across the reactor so that
a higher degree of conversion can be achieved, if
desired. It is also possible to obtain a
particular degree of conversion at a lower
pressure. In this manner, the operation of a
stripper in the process is simplified. Another
advantage of a completely horizontally arranged
reactor is that it can be started up in a simple
manner, because the entire plant can also be
operated when the reactor is only partly filled.
These and other objects, features, and
advantages of the present invention will become
apparent from the following detailed description
when taken in conjunction with the accompanying
217~03
-6-
drawings which illustrate, by way of example, the
principles of the present invention.
BRIEF DESCRIPTION OF THE DRAWING
The accompanying drawings illustrate the
present invention. In such drawings:
FIG. 1 is a schematic view of a high-pressure
section of a urea plant suitable for practicing an
embodiment of the process of the present
invention.
FIG. 2 is a cross-sectional view (along X-X
in FIG. 1) of a reactor suitable for urea plant of
FIG. 1 and, in particular, the condensation zone
of the reactor.
FIG. 3 is a cross-sectional view (along Y-Y
in FIG. 1) of the reactor and heat exchanger and,
in particular, the condensation zone of the
reactor.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In accordance with an embodiment of the
present invention, a method is provided for
preparing urea from ammonium carbamate, which is
formulated from carbon dioxide and ammonia. This
method comprises the steps of supply NH3 and COZ to
a synthesis zone of a reactor operated at a
pressure of about 125 bar to about 350 bar. The
reactor includes a synthesis zone, and a
horizontally arranged condensation zone with a
heat exchanger disposed therein. Ammonia and
carbon dioxide are supplied to the reactor so that
the ammonia and the carbon dioxide undergo
condensation and are substantially absorbed into a
urea synthesis solution, which is maintained in
the reactor for a residence time selected so that
at least about 85~ of a theoretically obtainable
amount of urea is prepared. A substantial portion
CA 02177003 2003-03-27
22772-1264
-7-
of heat formed from the condensation can thereby
be discharged to a fluid passing through the heat
exchanger.
In accordance with a further embodiment, at
least a portion of the carbamate is removed from
the reactor and decomposed in a stripping zone to
form carbamate decomposition products. These
products are then passed to the reactor for
condensation in the condensation zone, with a
substantial portion of heat formed from the
condensation being discharged to the fluid passing
through the heat exchanger.
The theoretically obtainable amount of urea
is determined by the thermodynamic position of the
equilibrium and is dependent on for example the
NH3/C02 ratio, the H20/C02 ratio and the
temperature and can be calculated with the aid of
the models as described in for example Bull. of
the Chem. Soc. of Japan 1972, Vol. 45, pp.
1339-1345, and J. Applied Chem. of the USSR
(1981), Vol. 54, pp. 1898-1901,
Preferably the residence time of the urea
synthesis solution is selected so that at least
about 90~ of the theoretically obtainable amount
of urea is prepared, and more preferably more than
about 95~.
The conversion of carbamate into urea and
water in the reactor can be effected by ensuring
that the residence time of the reaction mixture in
the reactor 3s sufficiently long. The residence
time will generally be more than about 10 min..
and preferably more than about 20 min. On the
other hand, the residence time also will generally
be shorter than about 2 hours, and preferably
shorter than about 1 hour. At a higher reactor
temperature and pressure, a short residence time
~1??~0.3
_8_
will often suffice to obtain a high degree of
conversion.
The pressure in the reactor is preferably
between about 130 bar and about 210 bar, and the
temperature is preferably between about 170°C and
about 200°C.
The reactor is usually designed to generally
have the shape of a wide pipe with a diameter of
between about 1 m and about 5 m, preferably
between about 2 m and about 4 m. The length of the
reactor is generally between about 5 m and about
40 m, preferably between about 10 m and about
25 m.
The reactor is generally provided with means
that ensure that the liquid flows through the
reactor substantially in plug flow. To this end,
the reactor is provided with, for example, a
structured packing (in one or more places) or with
partitions, which divide the reactor into
compartments. The compartments are somewhat
similar in appearance to "continuously stirred
tank reactors" (CSTRs) arranged in series.
Although reference is made herein to CSTRs and
compartments, these terms are included in the
interest of brevity and are not intended to
restrict the invention thereto.
The number of compartments or CSTRs in the
reactor arranged in series is generally greater
than 2 and preferably greater than 5. In general
the number of compartments (CSTRs) will be smaller
than 40, preferably smaller than 20.
The compartments are preferably defined by
substantially vertical partitions. The partitions
preferably have a surface area that is at least
about 50~ of the area of the vertical
cross-sectional area of the horizontally placed
reactor, and more preferably at least about 85~ of
21 ??00~
-9-
the vertical cross-sectional area of the reactor.
Preferably the area of the partitions is at most
about 98~ of the vertical cross-sectional area of
the horizontally placed reactor.
Preferably turbulence in the reactor
compartments is provided by introducing a gas via
a distributing device, for example via a pipe
having holes therein, the pipe being disposed at
or near the bottom of the reactor. Ammonia, carbon
dioxide and/or inert gas can suitable be used as
the introduced gas.
The liquid ammonia preferably is introduced
into the reactor in the portion thereof that also
contains the both the condensation zone and the
heat exchanger. The C02 and any gaseous ammonia
present preferably are fed into all the
compartments of the reactor, but a larger
proportion, preferably more than about 60~, is
preferably fed to the portion of the reactor that
contains both the condensation zone and the heat
exchanger. Inert gas can be fed into all or any
combination of the compartments. The inert gas
will often be present in recycle streams, together
with COZ and gaseous ammonia.
The gas phase that is fed to the reactor can
be divided optimally between the series-arranged
CSTRs by suitable dimensioning of the distributing
device. This makes it possible, for example, to
minimize the amount of non-condensable gases in
the off-gas of the last CSTRs. In this manner, the
vapor pressure of the condensable components is
maximized in that part of the reactor where the
thermodynamically determined equilibrium is
approached. This means that, at a given total
pressure (i.e., the sum of the vapor pressure of
the condensable components plus the pressure of
the non-condensable components), the temperature
21 ??0 0~
.... _
-10-
in this part of the reaction zone is maximized, as
a result of which a higher degree of conversion is
obtained. Alternatively, this arrangement is also
suitable for minimizing the total pressure at a
given temperature, so that for example a better
performance of the stripping operation can be
realized.
The heat or energy that is discharged in the
heat exchanger of the reactor is generally more
than about 125 kWh per ton of urea produced. In
general, the energy will be less than about 800
kWh per ton of urea produced.
The heat released in the reactor can then be
transferred to and discharged by water or other
fluid that is passed through tubes of the heat
exchanger and is in the process converted into
low-pressure steam of preferably about 3 bar to
about 10 bar, and more preferably about 4 bar to
about 7 bar. The heat can also be discharged by
passing a process stream that is to be heated
through it, f or example a urea solution that is to
be evaporated at about 2 bar to about 8 bar or a
urea solution that is to be expanded at about
15 bar to about 40 bar. The heat exchanger is
preferably disposed in the condenser portion of
the reactor. The condenser portion preferably
accounts for about 10~ to about 70~ of the total
length of the reactor, and more preferably about
30~ to about 50~ of the total length.
Preferably the condensation zone and heat
exchanger of the reactor are designed as a
so-called submerged condenser, in which a portion
of the gaseous mixture to be condensed, the
ammonia, and dilute carbamate solution are fed
into the shell side of a shell-and-tube heat
exchanger The heat of solution and condensation
released is transferred to and discharged by a
CA 02177003 2003-03-27
22772-1264
-11-
medium flowing through the tubes, for example
water. which is converted into low-pressure steam.
The method according to the invention can be
used in a so-called conventional urea process, but
is preferably used in a stripping process as
described in ECN Brea Supplement of 17 January
1969, pp. 17-20, in Hydrocarbon Processing of July
1975. pp. 102-104, or in Nitrogen. May-June 1990,
pp. 22-29~ In this preferred embodiment of the
method according to the invention the urea
synthesis solution formed in the reactor is fed to
a stripper, where carbamate is decomposed.
whereupon the gases obtained are returned to the
reactor.
The decomposition of the ammonium carbamate
present in the urea synthesis solution is
generally effected by supplying heat. If only heat
is supplied, this process is also known as thermal
stripping. However, the decomposition preferably
is effected by stripping with the aid of a
stripping gas in countercurrent to the urea
synthesis solution, with addition of heat.
Ammonia. carbon dioxide, and an inert gas. alone
or in any combination, can be used as the
stripping gas.
The stripping operation can be carried out at
a pressure that is the same as the synthesis
pressure of at a slightly higher or lower
pressure. Preferably substantially the same
pressures are used in the reactor and in the
stripping zone, since this makes it possible to
return the gases formed in the stripping zone to
the reactor in a non-complex manner.
In another embodiment of the present
invention the gases from the stripper are used to
convert water into steam or to heat a process
21~~003
-12-
liquid in a first heat exchanger disposed outside
the reactor, after which the partially condensed
gases are sent to the reactor according to the
invention. In this manner, at most about 70~ and
preferably at most about 50~ of the energy to be
discharged from condensation is discharged in this
first heat exchanger; correspondingly, at least
about 30~, and preferably more than about 50~, of
the heat of condensation is discharged in the heat
exchanger of the reactor. This embodiment is
advantageous when, for example, a process stream
needs to be heated (for example a urea solution to
be concentrated) and the generation of steam as a
by-product is desirable.
A particularly advantageous embodiment of the
present invention involves preparing the urea in a
reactor with a condensation zone and a heat
exchanger, in which the gases from the stripper
are returned directly to the condensation zone. In
this case all of the energy to be discharged is
discharged in and transferred to the heat
exchanger of the reactor. This is particularly
preferable because of the simplicity, and hence
inexpensive nature of the design.
Preferably carbon dioxide (C02) is used as
the stripping gas so that the stripping gas is
hence used as the C02-containing gas that is fed
to all the reactor's compartments. Besides C02,
this stripping gas contains NH3 and inert gas. In
addition to all the extra liquid ammonia to be
supplied, preferably more than about 60~ of this
stripping gas, in particular more than about 70~a,
is fed to the condenser zone of the reactor.
The present invention is illustrated by the
following figures and the examples, without being
limited thereto.
CA 02177003 2003-03-27
22772-1264
-13-
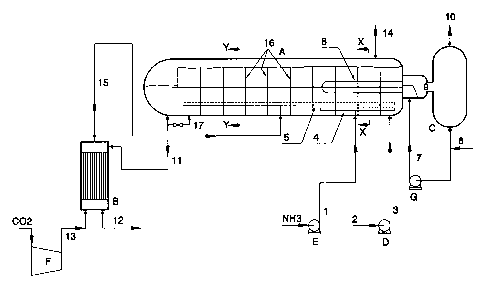
As shown in FIG. 1, A represents a reactor
with a condensation zone and a heat exchanger, B a
stripping zone, C a steam reservoir, and D, E, F
and G pumps or compressors.
Pump E supplies compressed liquid ammonia to
the reactor's condensation zone via conduit (1)
and via a pipe (4) provided with openings. A
carbamate solution that has been obtained
elsewhere in the process, in particular by washing
off-gases with an aqueous solution obtained in the
evaporation of the urea solution, is introduced
into the reactor (A) via conduits (2) and (3) by
means of pump D. A gaseous mixture containing
ammonia and carbon dioxide is introduced into the
liquid via a pipe (5) provided with openings. This
gaseous mixture - supplied via pipe (15) - is
obtained by subjecting the urea synthesis solution
formed in the reactor (A) to a stripping treatment
in the stripping zone (H), with the addition of
heat and in countercurrent contact to a stripping
gas, for example carbon dioxide, supplied via pipe
(13). The pressures in the reactor (A) and
the-stripping zone (B) are the same in the
embodiment shown, for example about 140 bar. The
pressures in these zones may, however, differ from
one another. The dimensions of the reactor (A) are
selected so that the residence time of the
reaction mixture in this reactor is sufficiently
long to ensure that at least about 85~ of the
theoretically possible amount of urea is formed in
the reactor. The reactor (A) is provided with
partitions (16) (or (23) in FIGS. 2 and 3) which
divide the reactor into compartments. The last
partition (i.e., located at the tar left of the
reactor depicted in FIG. 1) has an aperture at the
upper portion thereof only to enable the level of
the condensate in the last compartment to be
zI77003
-14-
controlled. At startup the penultimate and last
compartments must then be (temporarily) connected
to one another via a bypass pipeline (17), so that
a urea synthesis solution can already be
discharged when the reactor is half full.
The heat that is released in the reactor (A)
is discharged with the aid of water, which is
supplied via conduit (6), which is passed through
the heat exchanger (8) installed in the reactor
(A) by means of pump G via conduit (7). The water
is converted into low-pressure steam in that
process. The steam formed is sent to steam
reservoir C via conduit (9) and is discharged
therefrom via conduit (10) to a low-pressure
steam-consuming installation (not shown) which can
be, for example, the recycling and/or evaporation
section. Instead of discharging the heat as a
steam formation, it is also possible to pass a
process stream that needs to be heated, for
example an aqueous urea solution that is to be
concentrated, such as stream (12), through the
cooling elements.
The inert gases which contain ammonia and C02
are discharged from the reactor (A) via outlet
(14). NH3 and COZ are removed from these gases in a
known manner. The urea synthesis solution passes
from the reactor (A) via conduit (11) to the
stripping zone (B). The stripped urea synthesis
solution is discharged via stream (12) and can be
further processed to an aqueous urea solution and
concentrated in a known manner, whereupon the
concentrated solution is optionally converted into
solid urea.
In FIGS. 2 and 3, which represent
cross-sections of the reactor A, (21) represents
the reactor wall. For practical reasons there can
be an opening (22) defined between the partitions
CA 02177003 2003-03-27
22772-1264
-15-
(23) which divide the reactor into compartments
and the wall (21). The gas from the stripper is
introduced into the reactor A through the
distributing device (24). For practical reasons
.there are manholes (25) and (25') in each
partition. When the reactor is in operation, the
manholes may be open or closed, depending on the
desired throughput of the urea synthesis solution.
The manholes in the partitions near the heat
exchanger (29) are preferably closed, while each
of the remaining partitions preferably has only
one manhole open, with the open manholes being
alternated a staggered fashion so that a zigzag
liquid stream is obtained. The top side of a
partition (23) defines an opening (26) as a gas
discharge area. The partitions may be provided
with vertical baffle partitions (27), which are
however not essential. In Figure 2 ammonia is
introduced into the reactor by means of the
distributing device (28) at the level of the
condenser/heat exchanger and the heat exchanger is
represented as (29).
A method for the preparation of urea is
disclosed in NL 1,000,416.
The following non-limiting examples serve to
explain embodiments of the present invention in
more detail.
Example
A plant with a high-pressure synthesis unit
as shown in Fig. 1 was used to produce 70 tons of
urea per hour. The pressure was 149 bar; under
these conditions the degree of conversion in the
reactor was 95~ relative to the theoretical
equilibrium. In the heat exchanger 78.7 tons of
steam of 4.5 bar (148°C) was produced per hour
°
~ 21?7003
-16-
from 78.7 tons of condensate of 175°C; this
corresponds to some 600 kWh per ton of urea.
The streams through the pipelines were as
follows.
Amounts
in
tons
/ hour
Compo- 1 2 11 12 13 14 15
vent
Urea 75.2 70.0
NH3 39.7 20.8 62.9 8.7 12.1 57.1
1 0 C02 17.i 35.8 11.7 5i.3 5.4 79.2
H20 9.7 36.5 30.2 0.6 4.8
NZ 1.3 1.3 1.3
OZ 0.2 0.2 0.2
TOTAL 39.7 47.6 210.3 120.5 52.9 19.7 142.7
Temp. 20 186 173 120 176 187
(~C)
The stripped urea synthesis solution obtained
from pipeline (12) was further processed into
solid urea in a manner known to those skilled in
the art.
Although the present invention has been
described in detail with reference to its
presently preferred embodiments, it will be
understood by those of ordinary skill in the art
that various modifications and improvements to the
present invention are believed to be apparent to
one skilled in the art. Accordingly, no
limitation upon the invention is intended, except
as set forth in the appended claims.