Note: Descriptions are shown in the official language in which they were submitted.
_ 217277
- 1 - 19462
TITLE OF INVENTION
A METHOD FOR PREVENTING HEARTBURN
BACKGROUND OF THE INVENTION
Heartburn, or pyrosis, is a sensation of pain or burning
located substernally or high in the epigastrium with radiation into the
neck and occasionally to the arms, associated with regurgitation of acid-
peptic gastric juice into the esophagus. Occasional heartburn is common
in normal persons, but frequent and severe heartburn is generally a
1 o manifestation of esophageal dysfunction. Heartburn may result from
abnormal motor activity or distention of the esophagus reflux of acid or
bile into the esophagus, or direct esophageal mucosa irritation
(esophagitis).
Heartburn is most often associated with gastroesophageal
i s reflux. In this setting, heartburn typically occurs after a meal, with
stooping or bending, or when the patient is supine. It may be
accompanied by the spontaneous appearance in the mouth of fluid which
may be salty, sour, or bitter and green or yellow. Heartburn may arise
following the ingestion of certain foods (e.g. citrus fruit juices) or
2o dings (e.g. alcohol or aspirin).
Reflux esophagitis consists of esophageal mucosal damage
resulting from reflux of gastric or intestinal contents into the esophagus.
Esophagitis, an inflammation of the esophagus from regurgitation of
acid gastric contents, producing substernal pain, develops when the
25 mucosal defenses that normally counteract the effect of injurious agents
on the esophageal mucosa succumb to the onslaught of the refluxed acid
pepsin or bile. Mild esophagitis shows microscopic changes of mucosal
infiltration with granulocytes or eosinophils, hyperplasia of basal cells,
and elongation of dermal pegs. Erosive esophagitis shows
3 o endoscopically visible damage to the mucosa in the form of marked
redness, friability, bleeding, superficial linear ulcers, and exudates.
Famotidine (available from Merck & Co., Inc., Whitehouse
Station, NJ, under the name PEPCID~), an antagonist of the histamine
H2 receptor, is 3-{ { { 2-[(aminoiminomethyl)amino]-4-
2178277
- 2 - 19462
thiazolyl]methyl]thio]-N-(aminosulfonyl)propanimidamide, having the
structural formula:
~~ S02NH2
s H2Nv
/C=N~N/ CH2SCH2CH2
H2N S
NH2
The primary clinically important pharmacologic activity of famotidine
is inhibition of gastric secretion. Both acid concentration and volume of
gastric secretion are reduced by famotidine. Famotidine is used to treat
acid-related disorders such as gastric and duodenal ulcer,
1 s gastroesophageal reflux disease and Zollinger Ellison syndrome. Its
safety and efficacy have been well established in controlled clinical
studies. It is used by over 31 million patients worldwide.
Trials have shown famotidine to be beneficial in a dose
dependent manner in relief of symptoms associated with ulcerations and
2o gastritis.
Gitlin et al., Amer. Journal of Gastroenterolo~v ( 1985)
vol. 80 pp. 840 examines famotidine efficacy in the treatment of active
duodenal ulcers. The results suggest that duodenal ulcer healing rates
are famotidine dosage dependent. 20 mg twice daily, 40 mg twice daily
2 s and 40 mg at bedtime were administered over a four week period.
Healing rates of 67, 75, 70% , respectively, were seen.
Similarly, Miyoshi ~ al., Naika Hokan ( 1987) vol. 34 pp.
442-457 demonstrates that the efficacy of famotidine as a gastritis
therapy is dose-related. Miyoshi et al. evaluated dosage regimens of 5,
30 10, or 20 mg twice daily in the treatment of gastritis symptom relief.
Patients treated with 10 to 20 mg of famotidine had fewer erosions and
mucosal haemorrhages than those treated with 5 mg famotidine.
McCallum et al., Dig. Dis. Sci. (1985) vol. 30 pp. 1139-
1144 describes a study of healthy patients demonstrating that 5 mg of
famotidine produces has an effect on gastric acid secretion. Laskin et
CA 02178277 2000-06-23
-3-
al., J. Clin. Pharmacol. (1993) vol. 33 pp. 636-639 describes a study
demonstrating that single doses of 5 and 10 mg of famotidine produces
statistically significant decreases in intragastric acidity, beginning at 90-
100
minutes and persisting for approximately 9 hours.
Applicants have now found that administration of famotidine,
prior to consumption by patients of heartburn-inducing food or beverage, to
patients who ordinarily experience heartburn episodes following consumption of
1o such meals, is an effective means for preventing or minimizing symptoms
associated with heartburn. Applicants have found that heartburn episodes can
be
prevented ill patients ordinarily susceptible to heartburn episodes, if
famotidine is
administered in doses of between 5 mg and 20 mg, prior to ingestion of
heartburn-inducing food and beverage. Applicants have also found that the risk
of
15 experiencing heartburn episodes can be reduced in patients ordinarily
susceptible
to heartburn episodes if such doses of famotidine are administered prior to
ingestion of heartburn-inducing food and beverage. Applicants have also found
that heartburn episodes in patients ordinarily susceptible to heartburn
episodes
can be relieved if such doses of famotidine are administered prior to
ingestion of
2o heartburn inducing food and beverage.
Applicants have also found that the effectiveness of such treatment
is not close dependent.
SUMMARY OF THE INVENTION
25 In accordance with the invention there is provided a composition
for preventing heartburn episodes in a patient susceptible to suffering
heartburn
episodes following ingestion of heartburn-inducing food or beverage, said
composition being for administration to the patient, prior to consumption by
the
patient of the food or beverage, and comprising an amount of famotidine
between
3o about 5 mg and 20 mg.
CA 02178277 2000-06-23
- 3a -
In accordance with another aspect of the invention there is
provided a composition for reducing the risk of heartburn episodes in a
patient
susceptible to suffering heartburn episodes following ingestion of heartburn-
inducing food or beverage, said composition being for administration to the
patient, prior to consumption by the patient of the food or beverage, said
composition comprising an amount of famotidine between about 5 mg and 20
mg.
to In accordance with still another aspect of the invention there is
provided a composition for relieving heartburn episodes in a patient
susceptible
to suffering heartburn episodes following ingestion of heartburn-inducing food
or
beverage, said composition being for administration to the patient, prior to
consumption by the patient of the food or beverage, and comprising an amount
of
15 famotidine between about S mg and 20 mg.
In still another aspect of the invention there is provided a
composition comprising an amount of famotidine between about 5 mg and 20 mg
for use in preventing heartburn episodes in a patient susceptible to suffering
heartburn episodes.
2o In yet another aspect of the invention there is provided a
composition comprising an amount of famotidine between about 5 mg and 20 mg
for use in reducing the risk of heartburn episodes in a patient susceptible to
suffering heartburn episodes.
In a still further aspect of the invention there is provided a
25 composition comprising an amount of famotidine between about 5 mg and 20 mg
for use in relieving heartburn episodes in a patient susceptible to suffering
heartburn episodes.
In another aspect of the invention there is provided a combination
comprising a composition of the invention, as defined hereinbefore, and
CA 02178277 2000-06-23
-3b-
directions for administration of the composition 30 minutes to 2 hours prior
to
consumption of heartburn-inducing food or beverage.
5 In a still further aspect of the invention there is provided a
composition comprising an amount of famotidine between about 5 mg and 20 mg
as an agent for administration prior to consumption of heartburn-inducing food
or
beverage, for reducing the risk of heartburn episodes following ingestion of
the
food or beverage.
to In still a further aspect of the invention there is provided a
composition comprising an amount of famotidine between about 5 mg and 20 mg
as an agent for administration prior to consumption of heartburn-inducing food
or
beverage, for reducing the risk of heartburn episodes following ingestion of
the
food or beverage.
15 In yet another aspect of the invention there is provided a
composition comprising an amount of famotidine between about 5 mg and 20 mg
as an agent for administration prior to consumption of heartburn-inducing food
or
beverage, for relieving heartburn episodes following ingestion of the food or
beverage.
2o The invention also provides a method for preventing heartburn
episodes in a patient susceptible to suffering heartburn episodes following
ingestion of heartburn-inducing food or beverage, comprising administering to
the patient, prior to consumption by the patient of the food or beverage, a
composition comprising an amount of famotidine between about 5 mg and 20
25 mg.
The invention also provides a method for reducing the risk of
heartburn episodes in a patient susceptible to suffering heartburn episodes
following ingestion of heartburn-inducing food or beverage, comprising
administering to the patient, prior to consumption by the
CA 02178277 2000-06-23
-4-
patient of the food or beverage, a composition comprising an amount of
famotidine between about 5 mg and 20 mg.
The invention still further provides a method for relieving
heartburn episodes in a patient susceptible to suffering heartburn episodes
following ingestion of heartburn-inducing food or beverage, comprising
administering to the patient, prior to consumption by the patient of the food
or
beverage, a composition comprising an amount of famotidine between about 5
1 o mg and 20 mg.
DETAILED DESCRIPTION OF THE DRAWINGS
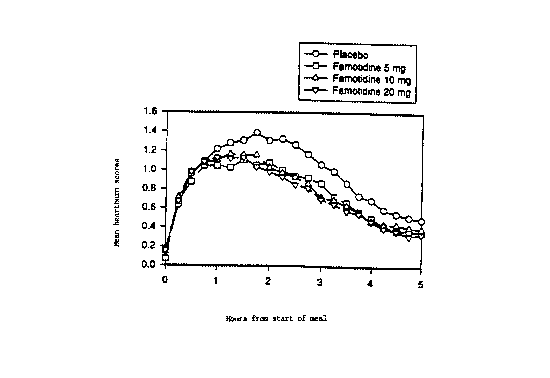
Figure 1 is a graph representing heartburn severity in patients in
response to administration to patients of famotidine 5 mg, famotidine 10 mg,
famotidine 20 mg, or placebo, and subsequent ingestion by the patients of
heartburn-inducing food or beverage.
Figure 2 is a graph showing mean area under the curve scores for
heartburn severity, acid sour stomach and overall discomfort.
Figure 3 shows global evaluations of effectiveness based on
2o subject ratings of famotidine administrations as excellent, good, fair,
poor or
ineffective.
DETAILED DESCRIPTION OF THE INVENTION
Compositions for use in the present invention contain famotidine
in an amount of between about 5 mg and 20 mg. In one embodiment of the
invention, the compositions include 5 mg famotidine. In another embodiment of
the invention, the compositions include 10 mg famotidine. In another
embodiment of the invention, the compositions include 20 mg famotidine.
The compositions may also contain pharmaceutically acceptable
3o carriers. Compositions may be formulated for oral administration in solid
or
liquid form, for example as effervescent or non-effervescent powders or
tablets,
capsules, suspensions or dispersions. Compositions may thus be formulated by
admixture with pharmaceutically acceptable vehicles additionally containing,
as
desired,
2178271
- 5 - 19462
pharmaceutically acceptable adjuvants including thickeners,
preservatives, and coloring and flavoring agents.
Powder formulations can be prepared by dry blending
ingredients under conditions of controlled temperature and humidity
using conventional equipment. Tablet formulations can be prepared by
combining the active components with tableting aids, fillers and
palatability aids in a conventional manner and tableting on a
conventional machine.
In the example shown below, famotidine tablets including 5
1 o mg famotidine and amounts of inactive ingredients hydroxypropyl
cellulose, hydroxypropyl methylcellulose, iron oxides, magnesium
stearate, microcrystalline cellulose, starch, talc, and titanium dioxide
sufficient to prepare an pharmaceutically acceptable tablet for delivery
of the active famotidine were prepared, as were tablets including 10 mg
1 s famotidine and 20 mg famotidine and appropriate amounts of such
inactive ingredients.
The term "preventing heartburn episodes" means
precluding symptoms, or reducing the severity of symptoms, associated
with heartburn in patients susceptible to heartburn following ingestion
20 of heartburn-inducing food or beverage.
The term "precluding symptoms" means making the
experience of symptoms impossible or largely ineffectual by removing
the conditions needed for them.
The term "reducing the severity of symptoms" means
25 substantially lowering the degree of pain associated with heartburn
symptoms that would ordinarily occur in patients susceptible to
heartburn following ingestion of heartburn-inducing food or beverage.
The term "reducing the risk of heartburn episodes" means
substantially lowering the tendency of patients susceptible to heartburn,
3 o following ingestion of heartburn-inducing food or beverage, to
experience symptoms associated with heartburn following ingestion of
heartburn-inducing food or beverage.
2118211
- 6 - 19462
The term "relieving heartburn episodes" means eliminating
or substantially eliminating symptoms, associated with heartburn
following ingestion of heartburn-inducing food or beverage.
The term "heartburn-inducing food or beverage" includes
s foods and beverages commonly associated with heartburn in patients
susceptible to food- or beverage-induced heartburn episodes, e.g.
tomatoes, chili, coffee, red wine, citrus juice, etc. For purposes of
describing the invention, the term "meal" is hereinafter to be
understood to mean heartburn-inducing food and/or beverage.
1 o The degree of heartburn pain associated with ingestion of
such foods varies among individuals and with food types. Thus, some
individuals may be more sensitive to certain heartburn-inducing foods
than are other individuals. The tendency for a given individual to
experience heartburn in response to ingestion of a particular food or
1 s beverage is predictable, however, and the individual is able to
determine, prior to ingestion, which food or beverage will induce
heartburn symptoms.
A "patient susceptible to suffering heartburn episodes
following ingestion of heartburn-inducing food or beverage" means any
2o patient who ordinarily experiences symptoms of heartburn caused by
ingestion of heartburn-inducing food or beverage
The famotidine compositions are administered prior to
consumption by the patient of the heartburn-inducing food or beverage,
at a time such that the anti-secretory activity of famotidine will be
2 s engaged prior to stimulation, by heartburn-inducing food or beverage,
of the acid-secreting parietal cells. Such administration can be within
six hours prior to ingestion of the heartburn-inducing food or beverage.
In one embodiment, administration is between 30 minutes and 2 hours
prior to meal consumption, e.g. 1 hour prior to meal consumption.
EXAMPLE
Adult male and female subjects having a history of
heartburn, acid indigestion, or sour/upset stomach of at least 2 months'
-- 2178277
- 7 - 19462
duration, with a minimum of three episodes per week, were studied.
Subjects had to be able to identify specific foods or beverages that
produced their symptoms and had to have used antacids in the past to
relieve their discomfort. If not post-menopausal, female subjects had to
s be practicing an approved form of contraception. A history of serious
gastrointestinal conditions, such as duodenal or gastric ulcer, atrophic
gastritis within the previous 6 months, esophageal strictures, irritable
colon, inflammatory bowel disease, biliary tract disease, or
diverticulosis excluded subjects from the trial. Other exclusion criteria
i o included the presence of a clinically significant laboratory abnormality,
pregnancy or lactation (among females), recent use of various
prescription drugs, particularly H2 antagonists, omeprazole or
sucralfate, and a history of substance abuse.
Following an initial screening assessment, eligible subjects
i s were given a validation meal consisting of commercially available chili
and burgundy wine. Only subjects who reported at least moderate
symptoms of upper gastrointestinal discomfort within 90 minutes after
the start of the meal qualified for treatment with placebo, famotidine 5
mg, famotidine 10 mg, or famotidine 20 mg. Subjects were randomly
2o assigned to one of four treatment sequences.
Subjects were given four test meals (identical in
composition to the validation meal), with the interval between meals and
trial medications approximately 7 days. Trial medication, in tablet
form, was administered 1 h before each test meal. Rescue medication
2s consisting of an antacid with a neutralizing capacity of 56 mEq/two
tablet (Maalox~ TC) was available throughout the trial for subjects who
insisted on additional relief.
Subjects rated the severity of their heartburn,
acid/sour/upset stomach, and overall upper gastrointestinal discomfort
3o prior to the validation and four test meals, and at 15 minute intervals
thereafter for 5 hours. Symptom severity was rated on a six-point scale
as none (0), slight ( 1 ), mild (2), moderate (3), severe (4), or very
severe (5). Subjects provided a global evaluation of the study treatment
at the end of the 5 hour assessment period or immediately prior to
v- 2178277
- 8 - 19462
administration of rescue medication. Treatments were rated on a five-
point scale as ineffective (0), poor (1), fair (2), good (3), or excellent
(4). Efficacy was additionally assessed by examining the use of rescue
medication and the time to rescue medication in each treatment group.
The tolerability of the four study treatments was
determined by recording all adverse experiences reported by subjects
during the trial.
Approximately equal numbers of males (58) and females
63) were studied, ranging in age from 20 to 61 years. Subjects had
z o been experiencing meal-provoked gastrointestinal symptoms for
approximately 7 years, having an average of 5.6 episodes per week.
Ninety-seven subjects (80%) indicated that the validation meal produced
symptoms similar to those provoked by a typical meal, and the severity
of symptoms following the validation meal was rated as about the same
i s or worse by 83% of subjects.
Global evaluations of study medication were significantly
more favorable following all three doses of famotidine than following
placebo for all four meals combined (p < 0.001 ). More than half of all
subjects receiving famotidine (54-63%) rated the drug as either
20 "excellent" or "good" versus only 38% of subjects receiving placebo.
The global evaluations of all treatments for the four test meals
combined are shown in Fig. 3.
A peak heartburn rating was recorded for each participant.
Each individuals' peak heartburn rating was ranked in a manner
2 s consistent with the symptom severity evaluation described above.
Significantly milder peak heartburn ratings were evident following
treatment with all three doses of famotidine compared to placebo (p <
0.001 for famotidine 5 mg and famotidine 20 mg versus placebo; p =
0.004 for famotidine 10 mg versus placebo). Approximately three-
3o quarters of famotidine subjects (74-76%) rated their peak heartburn
severity as "mild", "slight", or "none". In contract, only 57% of
subjects gave similar ratings following placebo treatment.
As shown in Fig. l, mean heartburn severity tended to be
equivalent in the placebo and famotidine dosage groups for the first
2178271
- 9 - 19462
hour, but for the remainder of time, it was greater in the placebo group
than in the famotidine groups. Mean AUC scores (area under the
curve) for heartburn across the 5 hour evaluation interval were
significantly lower than in the famotidine 5-, 10-, and 20-mg dosage
s groups than in the placebo group (Fig. 2).
Figure 2 also shows that mean AUC scores across the 5
hour assessment period for acid/sour stomach and overall discomfort
were significantly smaller for each of the famotidine dosages than for
placebo (p < 0.025 for acid/sour stomach; p < 0.008 for overall
i o discomfort). In addition, peak rating of both acid/sour stomach and
overall discomfort were also milder with famotidine prophylaxis than
with placebo. Peak acid/sour stomach was rated as "mild", "slight", or
"none" by 73%, 69%, and 6$% of subjects following treatment with 5,
10, and 20 mg famotidine, respectively, and by 54% of subjects
1 s following placebo treatment. Similar percentages of subjects in the
three famotidine dosage groups (77%, 66%, 74%) rated their peak
overall discomfort as "mild" or less compared to only 54% of those
treated with placebo. The comparison with placebo was statistically
significant for all three dosages of famotidine for acid/sour stomach (p
20 < 0.034) and was statistically significant for the 5- and 20-mg dosages
for overall discomfort (p < 0.001).
A rescue antacid was used by only 17-18% of subjects in
the three famotidine dosage groups compared to 37% of those treated
with placebo (p < 0.001 ). The differences between the famotidine and
2s placebo groups were most evident 90 minutes following test meal
ingestion.
With one exception, the differences between the three
famotidine dosage groups were not statistically significant for any of the
efficacy parameters (p >_ 0.09), nor was there evidence of a carryover
3 o effect of previous treatment (p > 0.09). The significant difference
between the dosage groups was for overall discomfort where peak ,
ratings following the 5- and 20-mg dosages were milder than those after
mg (p < 0.019).
- 2178277
- 10 - 19462
A total of 61 subjects reported an adverse experience
during the trial, with the incidence being approximately equal during
each of the four treatment periods. No subject had a serious adverse
experience during this trial, nor did any subject discontinue the study
s prematurely for safety reasons.
The results of the study show that administration of
famotidine 1 h before a food and beverage challenge was significantly
more effective than placebo in preventing provoked upper
gastrointestinal symptoms. Peak ratings of heartburn and acid/sour
1 o stomach were significantly milder following administration of single
oral doses of famotidine 5, 10, and 20 mg compared to placebo, and
approximately three-quarters of subjects rated these symptoms as
"none" to "mild" following prophylactic treatment with famotidine
compared to slightly more than half following placebo. In addition,
i s overall discomfort was rated as "mild" or less by a larger percentage of
subjects following famotidine doses of 5, 10, and 20 mg (77%, 66%,
and 74% respectively) than following placebo (54%). This difference
was only statistically significant, however, for the 5- and 2-mg dosages.
Global evaluations performed at the end of each test period also
2o significantly favored famotidine over placebo, with 54-63% of subjects
rating famotidine 5, 10, and 20 mg as "good" or "excellent" compared
to only 38% of subjects for placebo. Consistent with these finding,
rescue antacids were used by a significantly smaller percentage of
subjects following famotidine treatment compared to placebo (17-18%
2s versus 37%).
With the exception of peak overall discomfort ratings,
there were no significant differences among the three famotidine
dosages in this trial for any of the efficacy parameters evaluated,
indicating that a dose as low as 5 mg was as effective as higher doses of
3 0 10 and 20 mg.
Famotidine was well tolerated in this trial, with the type
and frequency of reported adverse experiences similar to those observed
with placebo. There was no distinction in the tolerability profile among
the three famotidine dosages, confirming the notable absence of any
_ 2178277
- 11 - 19462
dose-related change in the incidence of side effects reported in other
investigational trials.
In summary, single oral doses of 5, 10, and 20 mg
famotidine were significantly more effective than placebo in preventing
food- beverage-induced heartburn and related upper gastrointestinal
symptoms when given 1 hour before a provocative meal challenge. The
tolerability of three dosages of famotidine was comparable to that of
placebo.
to
20
30