Note: Descriptions are shown in the official language in which they were submitted.
~ ~ 21~93~4
Esters and amides of 9 ( Z )-retlnoic acid
The invention rRlates to esters and amides of 9 ( Z )-retinoic acid
for the treatment of carcinomas, to novel 9(Z)-retinoic acid
derivatives and to ph~rr~ utical compositions containing esters
and amides of 9(Z)-retinoic acid, and to the use thereof for the
treatment of carcinomas.
10 ~etinoic acids (vitamin A acids) are derivatives of vitamin A
( retinol ) covering a wide range of biological activities, such as
cellular differentiation and many others. Their biological activ-
lty is made possible by interaction with two families of nuclear
receptors (RA receptor and ~X receptor) (cf. Mol. Cell. Biol. 14
No. 4 (1994) 2323 - 30).
The use of all-(E)- or 13-(Z)-retinoic acids or their derivatives
in the therapy of acne has been known for a long time (cf.
US 3,729,568 and E~ 366 713). Use of 9(Z)-retinoic acid in the
20 treatment of cancer has been considered only since the identifi-
cation of specific receptor systems [cf. Cell 68 (1992) 397 -
406; Nature ~L (1993) 657 - 60; Nucl. Acid Research 21, No. 5
(1993) 1231 - 37; ~ifferentiation 54 (lg93) 123 - 29; J. Bio.
Chem. 26~ (1994) 16689 - 95, Blood ~2, No. 12 (1993) 35g2 - 99;
Blood ~, No. 10 (19g3) 22].
already known for the structural isomers all-E- and 13(Z)-
retinoic acid, in some cases severe side effects occur on use of
9(Z)-retinoic acid too, 80 that the extent of secondary reactions
30 disproportionately overcompensates for the effect achieved. In
addition, topical use of free g(Z)-ret$noic acid frequently leads
to severe derm~l irrltation and pain for the patient which
depends on the concentration and f requency of application . The
type and number of side effects reduce the possibility of therapy
with high doses of free 9 ( Z )-retlnoic acid and re3tri~ t the
number of areas of application.
We have now found that certain esters and amides of 9 ( Z ) -retinoic
acid can also be used for treating carcinomas but, at the same
gO time, show only few, if any, side effects. The present invention
therefore relates to derivatives of 9(2)-retinoic acid which can
easily be prepared and are of importance for the treafment of
carcinomas while simultaneously minim~7;ng the toxicological side
effects of free 9(Z)-vitamin A acid.
~ 2 2~7~3~
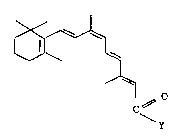
The invention therefore relates to compounds for use for treating
carcinomas, of the general formula I
X~
C~
~y (I),
where Y can be the following radicals:
--O--CRI--CO--R2,
where Rl
i5 H or Cl-C20-alkyl or Cl-C20-alkanoyl, preferably H or
20 Cl-C4-alkyl, and
R2 i8 Cl-C4-alkyl, -CH20H, 2 -CH2-o-co-R3
n ' Xn, Xn ~ Xn ~
and where R3 is H or Cl-C4-alkyl,
X is H, F, Cl, E~r, I, -OH, -oR3, -NH2, -N02, -CN, -NHR3, -NR3
30 -sH, sR3, -o-co-R3, -Co-R3, -CHO or -CO-ORI, 2
and n is an integer from O to S,
or else Y i8
~ 217~314
--O--CH2~ CH3 --O--CHz~ oCH3~
--O--CH2~ F; --~--CH2--CO-- NH~--CH3
CH3
-- N~ ~ COOH ; --NH ~ ;
COOH
--O~CE~2--CO ~ OCH3
OCE;3
`J~J~l ;
~
~o.~
-- O--CHR4 O--CHR3--O--CO--oR3
~ CHz--O--CO--R
--O--CRI--CH
O -- --CO --Rl
--N~ ; _ NR3 ~ ; --NR4 ~ xn r
o
4 17~
where Rl, R3, X and n have the abovementioned meanings, and R4 is
-Co-oR3, -Co-R3 or -Co-R3 ~~ Xn ~
where in the compounds which contain several R~, R2, R3 and/or R4
radicals, these can be ldentical or different, or Qlse
Y i8 one of the radicals
ORs ORs
Rs 1 Z~
ORs ORs
R300C ~ O- R300C /. ~ ,~NR3--
RsO ~ /, OR Rs \ ~ I~ ' ~ ORs
oR5 ORs
where R3 has the abovementioned meaning, and Rs is H or Cl--C4-
alkyl or C~--C4-alkanoyl.
Of particular importance for use for treating carcinomas are com-
30 pounds of the general formula I where Y is the radical
--O--CRI--CO--~
Xn
where Rl is H,
X is F, -OH, -OCH3, -NHz, -CN, -NHCH3 or -N(CH3) 2, and
n is an integer from 1 to 5, preferably 1 to 3, in parti~ular
40 1 to 2,
and the compounds of the general ~ormula I
where Y is one of the radicals
21793I~
OR5
RsO~ ~ NR -- RsO :~ 7~ z NR
ORs
ORs
WherQ Rs i8 H or Cl-C4-alkanoyl such as CH3_CO-, C2Hs-CO-, C3H7-CO
10 or else HCO- and R3 18 H, Cl-C4-alkyl or Cl-C4-alkanoyl,
in particular
compounds of the general formula I where Y is one of the radicals
J ,~,~ ;
~J:~ ~
.~
O
-NH--~ ~ CO-OCH3 or
CH3CO-O ~~~ O-COCH3
O-COCH3
-NH--~ ~ CH2OH
CH3COO ~ ~-COCH3
OCOCH3
6 2179314
The 9 ( Z ) -retinoic acid derivatives according to the invention can
bQ used both locally, le. topically, for treating cancer of the
Rkin and orally, le. systemioally, for treating carcinomas or
precancerous states.
E~owever, under cQrtain conditions, local and systemic prophylaxis
of carcinomas is also possible.
The invention therefore also relates to pharmaceutical compos1-
10 tions containing compounds of the general formula I ln addltlon
to conventlonal pharmaceutlcal exclplents or diluents and
conventlonal pharmaceutical ancillary substances, and to the use
of ~ ullds of the general formula I in the form of pastes,
gels, ointments, oreams, lotlons, dustlng powders, solutions or
emulsions containing from 0 . 001 to 5~ of these compounds for the
local treatment of oarcinoman or precancerous states, and to the
use of a compound of the general formula I in the form of
tablQts, film-
coated tablets, sugar-coated tablQts, capsulQs, pills, powders,
20 granules, solutions or suspensions for the ~ystemic treatment of
carcinomas or precancerous states, and to the use of pharmaceuti-
cal composltions containing compounds of the general formula }
for the local or systemic prophylaxis of carcinomas.
The therapeutic compositions or formulations containing conven-
tional excipients or diluents and, wherQ appropriatQ, conven-
tion~l rh~rr-^eutical ancillary substances appropriate for thQ
requirQd modQ of adm$nistration with a dosage suitable for use
are produced in a conventional way, in partioular by mixing. ThQ
30 therapeutic compositions contain thQ . ~ul~ds to bo used accord-
ing to the invention in amounts of from 0.001 to 5~ by wQight,
prQferably from 0.001 to 1~ by weight, for local use, and
preferably ln a slngle dose of from 0.1 to 10 mg ~or systemic
use .
Tests relating to topical use are carried out by generally
customary oncology tQsting programs.
The supprQssion of uncontrolled cell growth is tested.
The preparation of compounds according to the invention of the
general formu a I is indicated by the following examples.
7 217931~
Example 1
2- [ 9 ( Z ) -Retinoyloxy ] -4 ' -methylacetophenone
50 ml of dimethylformamide were introduced into a flask and then
0.5 g of potassium carbonate and 1.12 g of 2-chloro-4'-mQthyl-
acetophenone plus 2 g of 9 ( Z ) -retinoic acid were added with
exclusion of light. The mixture was stirred under nitrogen at
10 room temperature (RT) for 24 hours (h). 100 ml of water were then
~dded to the reaction mixture, and stirring was continued for
2 h. The re~ulting crystals were filtered off with suction and
dried under nitrogen at 30 C for 12 h. 2.4 g (~vLLe~vl~ding to a
yiQld of 84& of theory) of the abovementioned product were
obtained. The product was purified by recrystallization ~rom
isopropanol. 'rhe melting point of the recrystallized product was
126 C. The NMR spectra agree with the proposed structure.
Example 2
2--[ 9 ( Z ) -Retinoyloxy l--4 ~ -methoxyace~nphc-~n~
A mixture of 35 ml of dimethylformamide, 0.5 g of potassium
carbonate, 1. 23 g of 2-chloro-4 '-methoxyacetophenone and 2 g of
9 ( Z )-retinoic acid was stirred under N2 at RT with exclusion of
light for 24 h. 100 ml of water were then added to the reaction
mixture at 30 C, and stirring was continued for 2 h. The resulting
crystals were filtered off with suction and dried under nitrogen
for 12 h. 2.5 g (corresponding to 88.1~ of theory) of the above-
30 mentioned product were obtained. The melting point afterrecrystallization from isopropanol was 124-126 C.
Example 3
2-[ 9 ( Z )-Retinoyloxy]-4 '-fluoroacetophenone
A mixture of 30 ml of dimethylformamide, 0.2 g of pot~ssium
carbonate, 0.54 g of 2-chloro-4'-fluoroacetophenone and 1 g of
9 ( Z ) -retinoic acid was atirred under N2 at RT with exclusion of
40 light for 24 h. 100 ml of water were then added, and stirring was
continued ~or 2 h. Removal of the crystals and drying under
nitrogen for 12 hours resulted in 1.1 g (84.69~ of theory) of the
abovementioned product. The melting point after purification by
recrystallization from methanol was 99 C.
8 2179314
ExamplQ 4
2--[9(Z)-Retinoyloxy]--3'--fluoroacetophenone
A mixture of 50 ml of dimethylformamide, 0.75 g of potassium
carbonate, 1. 8 g of 2-chloro-3 '-fluoroacetophenone and 3 g of
9 ( Z )-retinoic acid was stirred under nitrogen at RT with
exclusion of light for 24 h. 100 ml of water were then added to
the reaction mixture, and stirring was continued for 2 h. The
10 resulting crystals were filtered off with suction and recrystal-
lized from isopropanol. 3.3 g (85% of theory) of the
abovementioned product were obtained with a melting point of
76-79 C.
Example 5
2--[ 9 ( Z ) -Retinoyloxy ] -N- ( 2, 6-dimethylphenyl ) acetamide
A mixture of 35 ml of dimethylformamide, 0 . 5 g of potassium
20 carbonate, 1.3 g of 2-chloro-N-(2,6-dimethylphenyl)acQtamide and
2 g of 9 ( Z )-retinoic acid was stirred under nitrogen at RT with
exclu~ion of light for 24 h. 100 ml of water were then added to
the reaction mixture, and the mixture was thQn stirred for 2 h.
The resulting crystals were dried under nitrogen for 12 h.
Recrystalli2ation from isopropanol resulted in 2.4 g (91% of
theory) of the abovementioned product with a solidification point
of 147-148 C.
Example 6
N- ( p-Carboxyphenyl ) -9 ( Z ) -retinoamide
A mixture of 10 g of 9 ( Z )-retinoic acid and 2 . 9 g of pyridine in
650 ml of diethyl ether was cooled to 0 C. Then, at 0 C, 3.5 g of
thionyl chloride were added dropwise, and the mixture was stirred
at 0 C for 2 h. Precipitated pyridinium hydrochloridQ was removed.
The filtrate waD then cooled to -25 C and a solution of 4.1 g of
p-aminobenzoic acid in 150 ml of diethyl ether and 2.9 g of
pyridine was added. The mixture was stirred at RT for 2 h. For
40 workup, the reaction mixture was qxtracted with ice-cold 1 N i~Cl
and saturated NaCl solution. The aqueous phase was separated of f
~nd the solvent was removed under reduced pressure to result in
11.7 g (93r of theory) of the abovementioned product which, after
recrystallization from isopropanol/ethanol, had a solidification
point of 187 C.
9 3 I ~
Example 7
2- [ 9 ( Z ) -Retinoyloxy ]--3 ', 4 ' -dimethoxyacetophenone
A mixture of 50 ml of dimethylformamide, 0.75 g of potassium
carbonate, 2 . 2 g of 2-chloro-3 ', 4 ' -dimethoxyacetophenone and 3 g
of 9 ( Z ) -retinoic acid was stirred under nitrogen at RT with
excluslon of light for 24 h. For workup, 100 ml of water were
added to the reaction mixture, which was then stirred at RT for
10 2 h. The precipitated crystals were filtered off with suction,
recrystallized from isopropanol and dried under nitrogen. This
resulted in 3.6 g (969~ of theory) of crystallized product of
melting point 130-C.
Example 8
N- [ 1--( D-Glucopyranosyluronosyl ) ] -9 ( Z ) -retinoamide
A mixture of 10 g of 9(Z)-retinoic acid and 2.9 g of pyridine in
20 500 ml of diethyl ether was cooled to 0 C under N2 with exclusion
of light. Then, at 0 C, 3.5 g of thionyl chloride were added
dropwise, and the mixture was stirred at 0 C for 2 h. Precipitated
pyridinium hydrochlorida was removed. The filtrate was then
cooled to --25 C and a solution of 10 g of methyl (2,3,4-tri-o-
acetyl-f~--D--glucopyranosyluronosyl)amine in 150 ml of diethyl
ether and 4 . 2 g of potassium carbonate were added. The mixture
was stirred at -25 C fo 2 h and warmed to 0 C over the course of
1 h. Then 2 g of potassium carbonate and 100 ml of methanol were
added to the reaction mixture. To remove the protective groups, a
30 20~ strength solution of ROE~ in 150 ml of methanol was then
added. The reaction mixture was heated at 60 C for 0.5 h and then
cooled to RT. After extraction with heptane/ethyl acetatQ, the
aqueous phases were extracted several times with methylene
chloride. The methylene chloride phases were then concentrated
under reduced pressure. The residue was fractionated into a 1:1
mixture of the two anomers by chromatography on silica gel. This
1:1 mixture of isomers of the abovementioned product was obtained
in amounts of 8.2 g (corre~ponding to 609~ of theory) with a
solidification point of 56-58 C.
Example 9
Tocopheryl 9 ( Z ) -retinoate
6 g of 9(Z)-retinoic acid were dissolved in 150 ml of diisopropyl
ether, and the solution was cooled to 5 C. Then 4 . 6 g of tri-
f luoroacetic anhydride were added dropwise, and the reaction
lo 2~9~1d
mixture waa warmed to RT . A solut1on of 9 . 6 g of D, L-tocopherol
in 10 ml of diisopropyl ether was then added dropwise, and the
mixture was stirred at RT for 1 h. For workup, 20 ml of agueous
ammonia were added to the mixture at 20 - 25 C. After phase
Eieparation, the organic phase was wa3hed with water, dried and
then distllled to remove the solvent. 12 g (corresponding to 84
of theory) of the abovementioned product were obtained in the
form of an orange-yellow oil whose NMR data agree with the
proposed structure.
Example 1 0
Ergocalciferyl 9 ( Z )-retinoate
A mixture of 10 g of 9(Z)-retinoic acid, 2.9 g of pyridine and
500 ml of diethyl ether was cooled under N2 protective gas with
exclusion of light to 0 C and, at 0 C, 3.5 g of thionyl chloride
were added dropwise, and the reaction mixture was stirred at 0 C
for 2 h. Precipitated pyridinium hydrochloride wa~ removed. The
20 filtrate was cooled to -2S C and a solution o~ 13.2 g of ergo-
calciferol in lS0 ml of diethyl ether and 4.2 g of potassium
carbonate were added. The mixture was stirred at -25 C for 2 h and
then warmed to 0 C over the course of 1 h. 'rhen lO~t strength
aqueous KHC03 solution was added to the reaction mixture, followed
by wash$ng with water. Removal of the solvent by distillation
resulted in lS g of the abovementioned product in the form of a
dark orange oil. The NMR spectra are consistent with the propo3ed
~ tructure .