Note: Descriptions are shown in the official language in which they were submitted.
21 79476
MT~TMt~n FOR IMPROVING T~TT~ TRAN~MT~IVITY
OF AN ~T~T~lcTR~nE IN.~TAT,T,T~n TNTO T~T~ SO
RA('K(.Y~ NI~ OF T7rT~l INVENTION
1. ~ield of th~ inV~nt i r~n
The present irlvention relates to the
installation of electrodefi into a fioil deposit and, in
particular but not exclusively, to the i~ t of
1~ the electrical tranfimisfiivity of these electrodefi to
the 80il for purposes such as the electro-osmotic
drainage of soils, the grounding of metallic
equipmentfi or fitructures, the cathodic protection o~
buried metallic l-lem~nt.cl~ etc
In the present description and the
appended claims, the term "soil" is intended to
encompass not only soil but also any fine-grained
material in which electrodes are installed, such as
2~ clayey or silty natural deposits, dredged materials,
tailings, industrial wastefi, etc.
~ 21 79476
2. Brief description of th~ ~rior ;~rt: ~ ~
In various applications, electrodes are
installed into the ground to transmit an electric
5 potential to the soil. The performance of such
electrode~ is evaluated or measured as the ability of
the electrode to transmit (electrical transmi~sivity)
the electric potential to the soil without significant :
drop o~ 1088 of potential between the electrode and
10 the soil.
In mo3t of these applications, the
electrical transmi~slvity of the electrodes is of
primary importance and any electric resistance
15 building up at the immediate perlphery of the
electrodes decreases the performance~ of the system.
OB~ECTS OF TT~ rNVF NTION
An object of the present invention i9
therefore to improve the electrical ~r~n~ sivity of
an electrode to the soil in any installation where an
25 electric potential has to be transmitted from the ~oil
to the electrode including, amongst others, ~ystems
for electro-osmotically dewatering soil, systems for
2 1 79476
.
cathodically protecting buried metallic P-- tl~, and
~yste~s for grounding metallic er~uipments or
structure~ .
Another object of the ~ubject invention ls
to improve the electrical transmi~sivity of an
electrode to the ~oil by injecting an electrolyte in
the ~oil ~urrounding the electrode, in particular by
mean~ of the electro-o~otic flow generated by the
lo application of an electric potential to the electrode.
SUMM~RV OF T~T~ TNVFNTI
More specif ically, in acrnr~nr~ with the
pre~ent invention, there i8 provided a method for
improving the electrical tran~3missivity of an
electrode installed into the coil, in which an
electric potential i~ applied to the electrode Thi~
method comprise~ electro-o~motically inj ecting an
electrolyte into the ~oil in the region of the
electrode to create a low resistance zone around the
electrode and thereby improve the soil-electrode
25 contact and, there~ore, the tran~mi~ivity of the
electrode to the ~oil.
21 79476
The electrolyte can be Yelected f rom
different groups, for example a calcium chloride
solution and a calcium acetate solution.
Injecting an electrolyte into the 80il
preferably comprises electro-osmotically injecting an
electrolyte solution into the soil.
Applications of the method according to
the invention may comprise, ir. particular but not
exclusively, the ~ ~v~ ellt of the electrical
transmlssivity of an electrode installed into the soil
for grounding a metallic equipment or structure, for
electro-osmotically draining soil, for cathodically
protecting buried metallic elements, etc.
The present invention further relates to:
- a system comprislng at least one electrode installed
into the soil and means for applying an electric
potential to the electrode, ln -which the i ~ ,v~ t
comprises a low resistance zone formed around the
electrode by an electrolyte electro-osmotically
injected into the soil for improving the soil-
electrode contact and, thereby, the electrical
transmissivity of the electrode to the soil;
2 1 7~7~
- a system for grounding a metallic equipment or
~tructure compricing~ at least one electrode installed
into the soil and connected to the metallic equipment
or structure, and means for applying an electric
5 potential to the electrode, in which the improvenLent
comprises a low resistance zone formed around the
electrode by an electrolyte electro-osmotically
injected into the. soil for improving the soil-
electrode contact and, thereby, the electrical
10 transmissivity of the electrode to the soil;
- a system for electro-o~motically draining soil
comprising at least one anode, at least one cathode,
means f or applying a f irst higher electric potential
15 to the anode and means for applying a second lower
electric potential to the cathode, in which the
V. t comprises a low resistance zone formed
around the anode by an electrolyte electro-osmotically
injected into the 80il for improving the soil-anode
20 contact and, thereby, the electrical transmi~ivity of
the electrode to the 80il; and
- a system for cathodically protecting a buried
metallic element, compri~iing at leaot one electrode
25 installed into the soil and connected to the metallic
element, and means for applying an electric potential
to the electrode, wherein the improvement comprises a
2 1 794 76
low resistance zone formed around the electrode by an
electrolyte electro-osmotically injected into the soil
for improving the soil-electrode contact and, thereby,
the electrical transmissivity of the electrode to the
5 soil.
At least in clayey soils, a rather small
volume of in~ected electrolyte remains effective over
a very long period of time even under sustained
10 electric potential and electro-osmotic flow. In
clayey soils, when a solution, for exarnple a calcium
chloride solution is inj ected around a steel
electrode, a strong adherence rapidly develops between
the soil and the electrode ~due to physico-che~Lical
15 reactions, thus eliminating any 1088 of electric
potential at the soil-electrode interface. This soil-
electrode adherence appears to be permanent.
During the injection, some catio~ o~ the
20 electrolyte, for example calcium ions, which migrate
in the soil under the electric potential and electro-
osmotic ~low, appear to be ~ixed by the 80il in the
vicinity of the electrode i31 such a way that the soil
proximate the electrode remalns highly conductive a
25 long time after the injection has been made. Thus,
the zone of highly conductive soil ( low resistance
21 79476
zone) around the electrode enlarges the ef fective
diameter of the electrode.
Af ter inj ection of the electrolyte, the
5 electrode (8) can be used in any system in which an
electric potential has to be transmitted to the soil.
If the electrodes are used for the
electro-osmotic drainage of a f ine-grained soil
10 deposit, the potential losses at the anodes remain
practically zero during the duration of the treatment
and most of the voltage applied to the electrode is
effectively applied to the soil since the potential
losses at the cathode are rather small. For a given
15 voltage applied to the electrodes, the present
invention provides a much larger effective potential
gradient during the whole duration of the treatment
and thus produces an increased ràte of electro-osmotic
flow, a larger volume of drainage and a reduction in
20 the treatment time as well as in the energy cost.
If, under ~ome conditions of soil and
operations, the efficiency of an initial injection of
electrolyte decreases during the lif etime of the
25 electro~Les, additional electrolyte can be easily
injected using the same procedure.
2 1 79476
The objects, advantages and other features
of the present invention will become more apparent
upon reading of the following non restrictive
description o~ a preferred embodiment thereof, given
5 by way of example only with reference to the
accompanying drawings.
RRT~ n~ RrPTInN OF TTT~ nRAWTNr..
In the appended drawings:
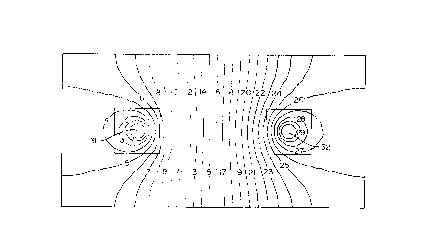
Figure 1 . is a theoretical electric
15 potential distribution in a horizontal plane between
two vertical electrodes of oppo~ite polarities driven
into the soil in a square electrode grid
Figure 2 is a graph showing potential
20 distr;hlltinn~, in time, between an anode and a cathode
in a field demonstration to illustrate the 1088 of
potential at the anode in previous art;
Figure 3 i~ a per pective, ~ichematic view
25 o~ a model test used in laboratory during the
d~velopment the present invention;
~ 21 79476
Figure 4 is a graE~h 3howing potential
distributions, in time, between the anodes and
cathodes of the laboratory model test of Figure 3, in
which the electrodes were simply pushed lnto the soil
5 without using the present invention; and
Figure 5 is a graph showing electric
potential distributions, in time, between the anodes
and cathodes of the laboratory model test of Figure 3,
10 in which the present invention was used.
Dl~T1~Trl~n DR.~'RTPTION OF TFTR pRR~RRR~n R~I~ODTMRl~T
Figure 1 presents the theoretical
potential distribution, in an horizontal plane,
between two generally elongated, vertical electrodes
31 and 32 of opposite polarities in a square electrode
20 grid. The diameter. of both electrodes 31 and 32 is
equal to 59~ of the spacing between these electrodes;
for example, the diameter of the electrodes 31 and 32
will be equal to 10 cm if the distance between these
two electrode~ is 2 . o m.
When a vertical electrode of given
diameter is installed into th~ ground, a certain 1088
~ lo 2179476
or drop of electric potential develops at the
periphery of the electrode simply due to the
geometrical configuration of the soil-electrode
contact. In Figure 1, showing the theoretical
distribution of the equipotential lines in an
horizontal plane cutting the t~7o vertical electrodes
31 and 32 installed into a soil of uniform
re~istivity, the narrower spacings between the
e~[uipotential lines around the electrodes (see squares
in Figure 1) indicate a concentration of the poterltial
gradient around the~e electrodea. This potential
gradient is indicative of a drop or loss of electric
potential between the electrodes and the soil. In the
example of Figure 1, about 50~ of the applied
potential is lost, 25% at each electrode 31 and 32,
due only to the geometry of the electrode 31 and 32.
The 1OS-A of electric potential due to the
geometrical configuration varies with the diameter of
the electrodes 31 and 32. More bpecifically, increase
of the diameter of ~ the electrodes will reduce the loss
of potential due to the geometrical conf iguration of
the soil-electrode contact.
Other l?h~nA.--An~ taking place at the .,oil-
electrQde interface may also drastically increase the
loss of electric potential and reduce the
~ 21 79476
11
transmissivity of these electrodes, e~pecially at the
anode (positive electrode). In particular, drying and
shrinking of the ~oil at the soll-anode interface may
increase the 1088 of electric potential or reduce the
5 transmlssivity of the anode to a point that most of
the electric potential applied to the electrode is
lost at the soil-anode contact. The 1088 of electric
potential due to drying and shrinking of the soil
surrounding the anode, in addition to the 1088 due to
10 geometry i8 illustrated in Figure 2, using data from
a ca~e record which will be described hereinafter
The performance or transmissivity of the
electrodes is important in most applications where an
15 electric potential has to be applied to the soil.
Electrode for electro-o~motic drainage of ~oil~
The application of an electric potential
20 to a saturated or nearly saturated soil r~sults in the
migration o~ ions in the f luid of the soil pores . In
most soils, and in particular in fine-grained soils
like clays, soil particles are surrounded by
positively charged ions (cations) to canc~l the
25 negative charges existing at their surface. Under an
electric potential, these cations will move towards
the negative electrode (cathode), thus producing a
2 l 79476
12
,v~ -nt of the pore fluid toward this cathode. The
-- vl nt of the pore fluid i8 designated as an
electro-osmotic flow and depends on the level of
surface activity of the soil particles and not on the
pore sizes. For that reason, the electro-osmotic
permeability of a clayey soil is much larger than the
hydraulic permeability. For a glven soil, the
electro-osmotic flow is proportional to the electric
gradient (voltage/unit distance) effectively applied
to the soil.
The electro-osmotic permeability of f ine-
grained soils like clays is generally about 200 to 500
times larger than the hydraulic permeability of the
same soil. Thus, a fine-grained 8011 deposit can be
drained in a relatively short period of time by
applying an electrical gradient to the 80il without
the need of applying a load or a surcharge to the
deposit as is reguired for hydraulic drainage.
The electro-osmotic drainage or dewatering
of soil has been known for many years (see Casagrande,
L. (1952) ~'Electro-osmotic Stabilization of Soilsn,
soston Society of Civil Engineers ~ournal, 39~:1; pp.
51-83, also Harvard Soil Mechanics Series: 38, and
Bjerrum, L., Moum, J. and Eide, 0. (1967) "Application
of ElectrQ-osmotic to a Foundation Problem irL a
21 79476
13
Norwegian Quick Clay", Géotechnique, Vol. XVII, N~ 3,
pp. 214-235) . Some other prior art documents have
taught illl,~:L~.JV~ t~l to the electro-o~motic drainage of
80il depoE~its by promoting, in addition, a certain
bonding of the soil particles. For example, US patent
NQ 3,915,826 granted to Franceschini on October 28,
1975 counts for that purposa on the migration in the
soil of metallic ions produced by the oxidation of the
anode. US patent NQ 3,497,439 (O'Bannon) iEisued on
February 24, 1970 teaches the ~tabilization of some
clayey soils by electro-osmotically transporting into
the ~oil alkali metal or alkaline earth [netal ions.
Numerous prior art document3 also teach
the dewatering ~f ~lurries, ~ludges or ~uspension~ by
applying an electric potential by mean~ of electrode~.
Example~ are de~cribed in US patent NQ 4,376,022 (Porta
et al.) iscued on March 8, 1983; US patent NQ 4,382,341
(Bell et al . ) issued on May 10, 1983; US patent NQ
4,569,739 (Klinkowski) granted on February 11, 1986;
US pateIlt NQ 4,671,874 (Fremont et al.) i~ued June 9,
1987; US patent 4,755,305 (Fremont et al.) issued ~uly
5, 1988; US patent NQ 5,080,770 granted to= Culkin on
January 14, 1992; and US patent NQ 5,403,455 granted to
Candor on April 4, 1995.
~ 2 1 79476
14
However, the above discussed prior art
does not address the problem of 1088 of electric~
potential between the electrode and the soil.
The problem of loss of potential between
the electrode and the soil is particularly acute when
vertical electrodes are ins-talled in a soil deposit
for electro-osmotically draining this soil as
illustrated by the two iollowing examples
Figure 2 presents distributions of
electric potential between the anode and cathode for
a case record where electrodes were pushed into the
ground for the purpose of electro-osmotically draining
a soit clay deposit. In the graph o~ Figure 2, the X-
axis rep~e~ents the normalized distance between the
anode and cathode while the Y-axis represents the
normalized electric potential. Figure 2 has been
prepared using measurements made by Lo, K.Y, EIo, ~.S.
and Inculet, I.I (1991~ "Field Test of Electro-
osmotic Strengthening of Soft Sensitive Clay", Revue
~-~n~-l;Pnn~ de géotechnique, Vol. 28, N~ 1, pp 74-83,
at a test site, at Gloucester near the city of Ottawa,
in a clay deposit typical of Eastern ('~n~ n clays.
A8 indicated by Figure 2, the 1088 of el~ctric
potential at the anode increases ~[uickly with time.
After only 12 days of treatment, the voltage gradient
2 1 79476
effectively applied to the soil was only about 2096 of
the voltage gradlent applied to the electrode. A very
large partion, about 80~ of the applied electric
potential was lost between the anode and the soil.
The ph.~n~ ~n has also been studied in
laboratory using a physical model reproducing in-field
conditions. Two generally elongated vertical anodes
33 and 34 and two generally elongated vertical
lo cathodes 35 and 36 made of steel tube, 1.3 cm in
diameter were driven lnto a block 37 of undisturbed
Eastern r~n~i An clay, 25 cm in diameter and 12 to 14
cm high (see Figure 3). The block 37 of clay was
surrounded by an appropriate coating (not shown) to
15 prevent desiccation. ~he spacing between the two
anodes 33 and 3g was 6 cm, the spacing between the two
cathodes 35 and 36 w-às 6 cm, and the spacing between
the row of anodes 33 and 34 and the row of cathodes 35
and 3 6 was 12 cm .
The distribution of the electric potential
between the anodes 33 and 34 and the cathodes 35 and
35 for the laboratory test model is presented in
Figure 4. At the very beginning of the experiment,
25 the distributio~ of the electric potential was almost
linear, showing only a small 1088 of potential at the
electrode~ due to a rather large diameter of these
21 79476
16
electrodes when compared to the inter-row spaclng (11%
of the inter-row spacing). Also, due to the rather
large diameter of the electrodes 33-36 and the
relatively small distance between the electrodes of
5 the same polarity, i . e . the pair of anodes 33; 34 and
the pair of cathodes 35;36, the loss of electric
potential caused by the geometry of the electrodes was
rather small (about 209~), as shown in Figure 4 by the
distribution of the potential at the beginning of the
10 test (time = 0 hour). However, ~he loss of electric
potential increased very quickly due to the electro-
osmotic drying and shrinking of the soil at the soil-
electrode interface. As shown in Figure 4, the
potential loss at the anodes 33 and 34 was of the
order of 5096 after only 9 hours of electro-osmotic
drainage and reached 70~ after 21 hours of treatment.
At this stage, the gradient of electric potential
effectively applied to the soil had become too small
to cause- arly slgnificant electro-osmotic flow Qr
2 o drainage .
As demonstrated by these two examples, the
loss of potential at the anodes is caused by a
combination of geometrical loss as illustrated in
25 Figure 1, of passivation of the anode contact by
oxidation, and of drying of the soil immediately
surrounding the anode. Accordingly, the loss of
21 ~9476
17
potential at the electrode-soil contact is one of the
most important problem met in electro-osmotic drainage
used to improve the properties of sof t soils .
5 Electrode~ for cathodic protection o~ buried
RtrUctureEi or elementR
The corrosion of underground metallic
elements such as structures, pipeA, reinforcing bars
10 in cDncrete, etc. is accelerated when the electric
potential of the buried metallic element is higher
than the electric potential of the surrounding soil
since the metallic element then acts as an anode. It
is well known in the art that corrosion of buried
15 metallic P1A~Ant.q can be prevented by cathodic
protection. A small electric potential is applied to
the surrounding soil by means of electrodes installed
in the sDil and connected as an anode while the buried
metallic elements are r~AnnAAtA~1 as a cathode.
Typically, catho~ic protection requires a
current of 1 milliampere (mA) per square foot ~0 . 093
m2) of metal to be protected. Depending on the
electrode transmissivity, the soil conductivity and
25 the distance between the installed anodes and metallic
~lAm~n~A to be protected, a fairly high voltage could
be required to supply the neces~qary current thereby
2 1 794 76
18
causing a rise of temperature possibly followed by a
failure of the electrodes (see Burkhart, W.H. (1980)
"Temperature Rise in Underground Impres3ed Current
Anodes", Corrosion, Vol. 36, No 4, pp. 161-167) . This
5 problem is generally overcome by installing many
anodes resulting in a fairly costly protection syseem.
The drop or 1088 of electric potential
between the installed ano~es and the ~u~ ~ ~)ullding soil
10 is a major problem in a system for cathodically
protecting buried metallic elementH. In fact, the
loss of electric potential is caused by an electric
resistance surrounding~ the anode which must be
compensated by supplying additional power to the
system. Indeed, US patent NQ 4,388,168 (Burkhart)
issued on June 14, 1983 reports that in cathodic
protection systems, most of the resistance between an
anode and the soil is ~~n~ ntrated in a shell of soil
immediately surrounding the anode.
The problem of electrode performance or
transmissivity in cathodic protection systems has been
addressed, for example in prior art US patents NQ
4,018,715 (Tatum) issued on April 19, 1977, and
4,710,644 granted to Baach on December 1, 1937 mainly
by providing a low resistance backfill around the
anodes .
21 79476
19
In addition to instaIling the anode in a
hole of large dlameter, typically 12" (30 cm),
backfilled with low resistance material, such as coke,
around the anode, US patent NQ 4,388,168 teaches the
5 installation above each anode of a ~ ntil;n~r in~ iin~
a saturated solution of salt and mixing arrangements
where fresh water is mixed with the saturated solution
of salt and is continuously seeped into the soil above
the anode. ~ccording to this patent, the c~ntinll~)us
10 seeping of a saline solution from above the anode
should create a large cone having a low resistivity
around the electrode, thus increasing the performance
or the transmissivity of the anode.
In summary, performance of electrodes for
cathodic protection of buried metallic elements can be
improved according to the prior art by fairly costly
installation cor~sisting of large diameter drill hole
backf illed with low resistance material and in the
case of US patent NQ 4,388,168, by adding a seeping
system which, periodicaIly, should need maintenance
and refill.
Electrodes for grounding eg~l~ t.~ or structure~
Many structures or equipments require
grounding either for~ safety or to improve their
~ 21 79476
performance by dissipating in the ground aceidental
overpotential or residual pot,~nt;~l~. Aecordingly,
the transmissivity of grounding electrodes is of
primary importance and any electric resistanee
5 building up at the immediate periphery of such
eleetrodes decreases the performance. The solutions
whieh are presently available to eompensate for the
increased resistance or 1088 of potential; 1; ~tely
at the periphery of grounding eleetrodes is to
10 inerease the number of grounding electrodes and to
enlarge the diameter of the electrodes or to provide
a low resistance backfill around the electrodes,
De~cription of the pref erred ~ t of the pre~3ent
15 inve~tion
To overcome the above discussed drawbacks
of the prior art, the present invention proposes to
in] eet an electrolyte solution in the soil around the
20 electrode using the electro-osmotic flow generated by
the application of an electric ~C (Direct Current)
potential to the soil. In m~st of the cases, an
electro-osmotic flow develops in the soil from the
anode to the cathode; therefore, the electrolyte
~5 solution will be injectecl in the soil around the
anode. When an electrical potential is transmitted to
the soil by means of such an anode to which a higher
~ ' 2~ 79476
21
electric potential is applied, and another electrode
connected as a cathode and to which a lower electric
potential i8 applied, the pore fluid of the soil is
electro-osmotically displaced from the anode (s) to the
5 cathode (8) and the cations of the anodic solution
(calcium ions for example) moves from the anode (s) to
the cathode (s) . In the vicinity of the anode, the
displaced pore fluid of the soil is replaced by the
electrolyte solution. -
As indicated in the fore~oing description,in the present description and the appended claims,
the term "soil" is in~Pn-qPri to ~n~ not only soil
but also any fine-grained material in which electrodes
15 are installed, such a~3 clayey or silty natural
deposits, dredged materials, tailings, industrial
wastes, etc
Without limiting the scope of the
20 invention, a solution of calcium chloride (lN:one
normality) has been found very effective for clayey or
silty soils. After the injection of a rather small
volume of a calcium chloride solution, a strong
adherence develops between the soil and the anode,
25 made for ~example of steel, and the soil immediately
surrounding the electrode becomes highly conductive in
the same manner as when the diameter o~: the electrode
21 79476
22
is enlarged many times. Therefore, the ~ubject ~-
invention enables a substantial reduction of the
geometrical 1088 of electric potential without having
to install large and costly electrodes.
Referring to Figure 5, the soil
immediately surrounding the anode remains, after
injection of the electrolyte solution, highly
conductive even after a long period of electro-osmotic
treatment, for example electro-o~motic drainage. It
should be noted that this zone of highly conductive
soil (low resistance zone) extends all around the
anode and not only in the portion facing the cathode.
A~ illustrated in Figure 1, equipotential lines in the
vicinity of the electrodes are closed around the
electrodes and have a more or le~s circular shape.
This means that the potentlal gradients extent
radially from the anodes in all directions and provide
electro-osmotic injection in all directions and not
only towards the cathodes. The effectivene~E~ of the
subj ect invertion can ~e readily appreciated by
comparing Figure 4, involving no inj ection of
electrolyte solution in the soil around the anode, and
Figure 5 where a calcium chloride solution has been
injected. In Figure 4, the 1089 of electric potential
at the anode is already important af ter only 9 hour~
of treatment and after 21 hours, most of the apFlied
~ 2 ~ 79476
23
potential was lost at the soll-anode contact. During
the test of Figure 4, the anodes became quickly loose
in the clay specimen and could be freely extracted
from the soil, indicating a very poor soil-electrode
5 contact.
The model test of Figure 5 was crn~ rt~
with exactly the same conditions as the model test of
Figure 4 with the e~cèption that a small quantity of
10 a calcium chloride solution (lN) was injected in the
soil around the anode at the beginning of the
treatment, using the electro-osmotic flow during the
first 10 hours of the treatment. As a consequence of
this injection, no potential loss was observed at the
15 anode during all the subser~uent stages of the test as
evidence by the graph of Figure 5. Even at the e~d of
the treatment, after 140 hours, there was no potential
loss at the anode and most of the voltage gradient
applied to the electrodes was effectively applied to
20 the soil. At the end of the treatment reported on
Figure 5, the soil was still adhering solidly to the
anodes and the electrodes could not be removed f reely
from the clay specimen thus confirming the good soil-
electrode contact.
Typically, the electrolyte solution is
inj ected in clayey soil deposits under an electric
~ 21 79476
24
potential of about 20 volt per meter. The volume of
electrolyte solution to be inj ected depends, somewhat,
on the porosity of the soil. The clayey soil which
was used in the model test to develop the invention
5 had a natural water content of 80~ and thus a very
high porosity. In the laboratory model te~t (~ee
Figure 3 ), very good anode tran~mis~ivity was obtained
after the injection of 20 to 40 ml of a calcium
chloride solution ( lN) in the soil arolmd each anode
10 33 and 34. Based on the laboratory results, an
in~ection of about 35 liters of a calcium chloride
solution (lN) per meter of electrode length will
provide a high in-field electrode transmissfvity even
for soils having a high porosity. The duration of the
15 injection will vary from one soil to the other but
will be of the order of 4 or 5= days for most soils
when a potential gradient of 20 volts per meter is
supplied .
Calcium chloride is a low cost but
effective electrolyte for the purpose of the present
invention in particular when an electrode made of
cteel is used, :iince the Cl- anions react with the
ferrous ions instead of forming chloride gas.
However, when anbdes made of inert material are used
for example to avoid oxidation over the year in a
cathodic protection system, injeotion of calcium
21 79476
chloride in the soil around such anode will produce
toxic chlorine gas. In such a case, other :_
electrolytes such as calcium acetate can be selected
for injection into the soil.
In the model test of Figure 5, the water
content of the soil in the area of the anode has been
reduced by electro-osmotic drainage from a value of
8096 initially to a value of about 6096 after 140 h of
10 treatment. ~Iowever, even after this significant
drying of the soil, the small quantity of electrolyte
electro-osmotically injected before the treatment is
still as effective as it was at the beginning of the
treatment (Figure 5) and the transmissivity of the
15 anode (electrode raised to the higher electric
potential, the cathode l~eing the electrode r-;n~A;ni~d
to the lower electric potential) ic still excellent at
the end of the treatment. Under less severe
conditions o~ electro-osmotic drainage or drying as in
20 electrode system for catalytic protection or for
grounding equipments or structures, the high electrode
transmissivity should be permanent or last for a very
long period of time, at least in clayey soils.
When used for the in-s~itu electrokinetic
treatment of soil or other fine-grained material, the
present invention can, for a given voltage supplied to
21 79476
26
the electrodes, increase the voltage gradient
e~fectively applied to the soil by a factor of 2 to 4
thus eignif icantly reducing the duration of the
treatment and the energy cost. In many cases in which
5 treatment by electro-osmotic drainage is carried out,
the present invention, i . e . the electro-osmotic
in~ ection of an electrolyte in the soil around the
electrode appears to be the only economical method for
.n~int~;n;n~ an effective voltage gradient in the soil
10 for a period of time sufficient for conducting a
significant drainage of the soil.
In a system ~or cathodic protection of
buried metallic P-~ -nt.q, the electrodes, preferably
15 made of perforated pipes, are sirnply driven into the
soil without requiring drilling of a large diameter
hole and backfilling the hole with low resistance
material. The electrodes are then temporarily
connected into pairs of anodes and cathodes for the
20 duration of the injection of the electrQlyte. The
current recti~ier which is normally part of the
cathodic protection system is used to apply a DC
(direct current) potential between the anodes and
cathodes in order to in~ect the electrolyte solution.
25 After the necessary volume has been in~ected, the
electrodes are connected as anodes in the cathodic
protection sy~qtem.
27 21 79476
In some ca~ec, for example, when the
anodes have to be in~talled at a short di~tance from
the buried metallic element~, the electric potential
of operation of the cathodic protection ~ystem may be
5 ~ufficient to electro-o~motically inject the
electrolyte ~olution. In ~uch cases, the application
of the pre~ent invention would ~imply con~i~t of
supplying the electrolyte ~olution in~ide the
perforated electrode.
The in~tallation of electrodes for
grounding e~uipments or ~tnlctures would follow the
~ame procedure a~ in the case of a cathodic protection
sy~tem. For that purpose, at least two electrode~3 are
15 implanted in the 80il to form a pair of anode and
cathode. A DC potential ~ource il3 needed ~or the
inj ection of the electrolyte ~olution . Contrary to
the electrode3 of a cathodic protection system,
grounding electrode~ are not submitted to a
20 ~ignificant ~uEitained potential and the effect of the
initial injection ~hould normally ~e permanent, at
least in clayey ~ioil~.
If, under some condition~ of ~oil and
25 operation~i, the l-f f i ~ y of an initial inj ection of
electrolyte decrease~ during the l; f,ot; -- of the
2 1 794 76
28
electrode~, additional electrolyte can be eaE3ily
inj ected u~ing the ~ame procedure .
Although the precent invention has been
5 described hereinabove with reference = to a preferred
embodiment thereof, thi~ embodiment can be modified at
will, within the ~cope of the appended claim~~, without
departing ~rom the ~3pirit and nature o~ the 3ubject
invention .