Note: Descriptions are shown in the official language in which they were submitted.
21 7q82~
wo 9S/188~
PHOSPHONIC ACID DERIVATIVES CONTAINING A TRIAZOLE RING AS HERBICIDES
The p}esent invenion relates to novel triazoles which have a herbicidal action and are
plant-growth-inhibiting, to processes for their ~ lC~i~.lll, to ~;ollll.o~;~iOIlS containing
them as active 5--hCt ~ 5, and to their use for controlling weeds, especially selectively in
crops, or for inhibiting plant growth.
Triazole ~ which have a herbicidal action are generally known. For example,
WO 93/1561(~ and US-A-5,248,655 describe herbicidally active triazole ~
It has been found that ~ ~ ....l..,,....l~ of the formula (I) have a herbicidal and
plant-growth-inhibiting action. They are therefore suitable as active substances in weed
killers and in ~ for inhibiting plam-growth.
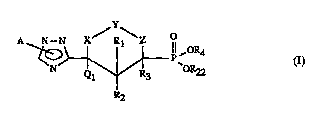
The triazoles according to the invention are those of the formula I
N--N X/P\Z O
N~\~ Ii,o~4
r~2
in which
A is hydrogen, Cl-C4-alkyl, C2-C4-alkenyl, ~ yllllcthyl, benzyl, a
group ~CH2-N~CH2)1, S02N(CH3)2, CH(CI-C4-alkoxy)2 or
2-(trimethylsilyl)ethoxymethyl;
t is 4 or 5;
Rl is hydrogen, Cl-C6-alkyl, OR19 or NR20R2l;
R2 is hydrogen, Cl-C6-alkyl, ORI9 or NR20R2l, or Rl and R2 together form an oxo group;
R3 is hydrogen, Cl-C6-alkyl, Cl-C6-alkoxy or represents together with R2 a chemical
bond;
R4 is hydrogen, Cl-C4-alkyl1 Cl-C4-alkyl substituted by halogen, cyano, COOH,
Cl-C4-alkoxycarbonyl, amino, di(C~-C4)-alkylamino, Cl-C4-alkylamino, phenyl, benzyl or
: 2 1 7~822
wo 9~/18811 r~
- 2 -
an aL~ali metal, aLIcaline earth metal, ~ ., mono-, bis- or
tris-CI-C6-~yl- ,-,",--"-~ - triaL~yl, ~ t~iaL~cylsuifoxonium, ~ ,";",., or
amidinium cation;
X is oxygen, S(O)m, NR5 or CR6R7;
Y is oxygen, S(O)p, NRs or CR8Rg;
Z is oxygen, S(O)q, NR5 or CRIoRll;
m, p and q are each ~ y of the others 0, 1 or 2;
R5 is hydrogen, Cl-C6-aL~cyl which is ~ i or substituted by halogen,
Cl-C4-aLlcoxy, COOH, Cl-C4-aL~u~y~,~l,ullyl, ' ' phenyl or by phenyl which
itself is substituted by Cl-C4-aLl~yl, halogen, Cl-C4-haloaLIcyl, Cl-C4-aL~oxy, cyano, nitro,
Cl-C4-alkylthio, Cl-C4-a3kylsulfinyl, Cl-C4 aL~ ulrul,~l or Cl-C4-alku,.y~.cul,.,llyl, or R5
is C3-C6-aLlcenyl which is ~ -t ~ or substituted by Cl-C4-al~u~y-~ul,ullyl, halogen,
., . .~- 1 .~l; 1. .:~ .-i phenyl or by phenyl which itself is substituted by Cl-C4-aL~cyl, halogen,
Cl-C4-haloalkyl, Cl-C4-aL~coxy, cyano, nitro, Cl-C4-a~cylthio, Cl-C4-aLkylsulfinyl,
Cl-C4-aL~l ,lru.,~l or Cl-C4-aL~u,-y~i,ul,yl, C3-C6-alkynyl which is ~ i or
substituted by ~ i phenyl or by phenyl which itseif is substituted by Cl-C4-alkyl,
halogen, Cl-C4-haloalkyl, Cl-C4-aL~coxy, cyano, nitro, Cl-C4-aLlcylthio,
Cl-C4-aLkylsulfinyl, Cl-C4-aL~cylsulfonyl or Cl-C4-aL~u.~y1~l,ullyl, Cl-C6-aLI;ui.y.all,ul-yl,
.1 phenyl or phenyl which itself is substituted by Cl-C4-alkyl, halogen,
Cl-C4-haloaLkyl, Cl-C4-aLlcoxy, cyano, nitro, Cl-C4-aL~ylthio, Cl-C4-aLlcylsulfinyl,
Cl-C4-aL~yl~ulrul.yl or Cl-C4-alkuAy~l,ullyl;
R6, R7, R8, Rg, Rlo and Rll are each ;~ L" 'f~ y of the others hydrogen, Cl-C6-aL~yl
which is ~ i or substituted by hydroxy, amino, Cl-C4-aL~coxy, Cl-C4-aLlcylamino
or phenyl, which itself is ""~ i or substituted by C1-C4-alkyl, halogen,
Cl-C4-haloalkyl, Cl-C4-aL~coxy, cyano, nitro, Cl-C4-aL~ylthio, Cl-C4-aL~cylsulfinyl,
Cl-C4-aL~cylsulfonyl or Cl-C4-al~u~y~l,ullyl, phenyl, benzyl which are 1l--~ ,l or
substituted by Cl-C4-aL~cyl, halogen, Cl-C4-haloaLlcyl, Cl-C4-alkoxy, cyano, nitro,
Cl-C4-alkylthio, Cl-C4alkylsulfinyl, Cl-C4 aLkylsulfonyl or C1-C4-alhu,-y.albullyl, or R6
and R7 or R8 and R9 or R1o and R1 1 together form an oxo group;
Ql is ORI2 or NR13RI4;
O O
R12 is hydrogen, a group 1¦ or a group ¦¦ ;
-C-Q-RI5 -c-Rl6
Rl3 is hydrogen, C1-C6-aL~cyl, Cl-C6-aL~oxy, hydroxy, C1-C6-aL~yl.a.~ol.yl,
C1-C6-aL~u,~y.~ul,ull~l, berlzoyl which is ""~ i or substituted by C1-C4-aL~cyl,
wo ss/lssll ~ t~ 2 1 7 q 8 2 2 1 1~ s ~
- 3 -
halogen, Cl-C4-halo,aL~cyl, Cl-C4-aL~coxy, cyano, nitro, Cl-C4-aL~ylthio,
Cl-C4-aL~y~ull~llyl, Cl-C4-aL~yl~llru,.~l or Cl-C4-aL~u,~y.,~u~u,lyl, a group
-c!N-Rl7Rl8 ' Cl-C6-alkyl which is sUbstitUted by halogen, Cl-c4 aL~cOxy
C1-C4-aL~u,.y~,~ubullyl, phenyl or by phenyl which itself is substituted by Cl-C4-aLkyl,
halogen, Cl-C4-haloaL~cyl, Cl-C4-aLtcoxy, cyanû~ nitro, Cl-C4-aL~ylthio,
Cl-C4-aLlcylsulfinyl, Cl-C4-aLlcylsulfonyl or Cl-C4-al~u,~y~.~l,ul.yl,
Rl4 is hydrogen or Cl-C6-aL'cyl;
Rl5 is Cl-C6-aL~yl, C3-C6-allcenyl, C3-C6-aL~cynyl; or Cl-C6-alkyl, C3-C6-aLkenyl,
C3-C6-alkynyl substituted by halogen or Cl-C4-aL~coxy; or phenyl, benzyl; or phenyl,
benzyl substituted by Cl-C4-aL~cyl, halogen, halomethyl, Cl-C4-aL~coxy, cyano, nitro,
Cl-C4-all~u~y~,~l,ullyl, Cl-C4-aL~cylthio, Cl-C4-aL~yl~ulrLlyl or Cl-C4-aL~yl~ulrullyl,
Rl6 is Cl-C6-alkyl, C2-C6-aL~enyl, C2-C6-aL~ynyl; or Cl-C6-aL~cyl, C2-C6-aL~enyl,
C2-C6-aLkynyl substituted by halogen or Cl-C4-alkoxy; or phenyl, benzyl; or phenyl,
benzyl substituted by Cl-C4-aLkyl, halogen, Cl-C4-haloaL~cyl, Cl-C4-aL~coxy, cyano, nitro,
Cl-C4-aL~uAy~ l,v,lyl, Cl-C4-aL~yltbio, Cl-C4-aLI.yl~ulrL.yl or Cl-C4-aL~cylsulfonyl; or is
Cl-C6-aLIcoxyaLkyl, Cl-C6-aLI~yl~ul,ullylu,.y~Llcyl, Cl-C6-aL~u,.y1~ul,ul,ylaL~cyl or
C3-C6-cycloaL~yl;
Rl7 is hydrogen or Cl-C6-aL~cyl;
Rls is hydrogen or Cl-C6-aL'cyl;
O O
Rl is hydrogen, a group ¦¦ or a group 1¦ ;
9 -C-Q-R23 -C-R24
Q is oxygen or sulfur;
R20 is hydrogen, Cl-C6-aLkyl, Cl-C6-aL~coxy, hydroxy, Cl-C6-aL'~yl~albu,lyl,
Cl-C6-aL~u,.y.,~ul,ullyl, benzoyl which is " ~ ;1 or substituted by Cl-C4-aL'cyl,
halogen, Cl-C4-haloaLkyl, Cl-C4-a~coxy, cyano, nitro, Cl-C4-alkylthio,
Cl-C4-aL~cylsulf~nyl, Cl-C4-alkylsulfonyl or Cl-C4-aL~w~y~,~ul,u.,yl, a group
-c!N-R2sR26 ~ Cl C6 a~yl which is sUbStituted by halogen, Cl-C4-aL~cOxy
Cl-C4-alku~yl,~ubull~l, phenyl or by phenyl which itself is substituted by Cl-C4-alkyl,
halogen, Cl-C4-haloaLIcyl, Cl-C4-aL~coxy, cyano, nitro, Cl-C4-aL~ylthio,
Cl-C4-aLlcylsulfinyl, Cl-C4-alkylsulfonyl or Cl-C4-a~uAy-,~ul,u.,yl,
~ :, 2l798
W095/18811 22 P ~ c~
-- 4 --
R2l is hydrogen or Cl-C6-aLI~yl;
R22 is hydrogen, Cl-C4-aLIcyl, Cl-C4-alkyl substituted by halogen, cyano, COOH,
Cl-C4-aL~u~yL~bullyl7 amino, di(CI-C4)-aL~ylamino, Cl-C4-aJ~ylamino, phenyl, benzyl or
an aLlcali metal, aL~aline earth metal, m~nillm mono-, bis- or tris-aLI~l~................
trial~y~ .. ;, ., ", triaL~ . ,1 r~. ~ ,, .. ,, " I ~ . ~ or amidinium cation;
R23 is Cl-C6-aUcyl, C3-C6-aL~cenyl, C3-C6-aLlcynyl; or C~-C6-aL~cyl, C3-C6-alkenyl,
C3-C6-aL~cynyl substituted by halogen or Cl-C4-alkoxy; or phenyl, benzyl; or phenyl,
benzyl substituted by Cl-C4-aL~cyl. halogen, halomethyl, Cl-C4-alkoxy, cyano, nitro,
Cl-C4-alkuAyL~ulJull~l, Cl-C4-aLlcylthio, Cl-C4-aLIcylsulfinyl or C~-C4 aL~cylsulfonyl;
R24 is Cl-C6-alkyl, C2-C6-aL~enyl, C2-C6-alkynyl; or Cl-C6-aLI~yl, C2-C6-aLkenyi,
C2-C6-aLIcynyl substituted by halogen or Cl-C4-alkoxy; or phenyl, benzyl; or phenyl,
benzyl substituted by Cl-C4-alkyl, halogen, Cl-C4-haloaL~yl, C~-C4-aL~coxy, cyano, nitro,
Cl-C4-aL~u,.yL~ul,u.l~l, Cl-C4-aL~cylthio, Cl-C4-alkylsulfinyl or Cl-C4-alkyl~ulru.lyl, or is
Cl-C6-aL~coxyaLIcyl, Cl-C6-~l~ubullylu~-ydLkyl, Cl-C6-aL~u,.yL~ubLl.vldL~cyl or
C3-C6-cycloaLlcyl;
R2s is hydrogen or C~-C6-aL~yl; and
R26 is hydrogen or CI-C6-aLI~yl, with the proviso that a) at least two of the ,~ X,
Y and Z are carbon atoms, b) that Rl or R2 is ORI9 or NR20R2, if cimlllt~n~o~lcly X, Y and
Z are carbon atoms and QI is ORI2 and c) that Rl and R2 are not ~ y ORl9,
NR2LlR2, or both of them.
In the above ~ finiri~mc, halogen is to be understood as being fluorine, chlorine, bromine
and iodine, preferably fluorine, chlorine and bromine.
ALlcyl is, for example, methyl, ethyl, isopropyl, n-propyl, n-butyl, isobutyl, sec-butyl,
tert-butyl and the various isomeric pentyl or hexyl radicals
HaloaLIcyl is, for example, nuululll.,.llyl, LlilluLlul..~Lll,vl, l. ;n~ .;l,yl, chloromethyl,
dichloromethyl, trichloromethyl, 2,2,2-~1illuulu~,.llyl, 2-fluoroethyl, 2-chloroethyl and
2,2,2-LIiLIllVlU~,LIl.~l.
ALicoxy is, for example, methoxy, ethoxy, propoxy, i~V~JIUl~U~y, n-butoxy, isobutoxy,
sec-butoxy and tert-butoxy; preferably methoxy.
ALkenyl is to be l~n~ rct~d as being straight-chain or branched aL~cenyl, for example
vinyl, allyl, methallyl, I-~ ,L;lylvillyl, but-2-en-1-yl, 3-pentenyl, 2-hexenyl ûr 3-heptenyl.
i 2 ~ 7~822
WO 95/18811
AL~cenyl radicals having a chain length of 2 or 3 carbon atoms are preferred.
The aLkynyl radicals occurring in the definitions of the, ~ may be
straight-chained or branched, for example ethynyl, propargyl, 3-butynyl,
l-~u~ yl~JIu~)~u~yl~ 2-pentynyl or 2-hexynyl. Ethynyl and propargyl are preferred.
Al~u~.y~, ubul~l is, for example~ uAy~,~ul)u~ l, GLIIu~y~,rubullyl, n-~lu~w~y~ ubul~yl,
i~U~lU~U~y~ ub~ and n-buLu~.y~,~ubu.,yl, preferably Ill~llluAyc ubullyl and ethoxy-
carbonyl.
If the ~ .u~ of the formula I conkain an ~yllllll~u;c carbon atom, this results in the
fact that the c ~..,; .u, .~ can occur in optically isomeric forms. If there is an aliphatic C=C
double bond, geometric isomerism can also occur. The formula I therefore also embraces
all ~ . rA ) ~ which are possible and which are in the form of
or their mixtures.
ThetermorganicAmmr~n~ AcationisintendedtoincludeA~..,....",~.,cationsprepared
from low molecular weight amines, that is to say those having a molecular weight below
about 300. Examples of such amines include aL~cylamines, aL~,Ilyla.lli.._s, and
Alk ~ .r 5 containing not more than two amino groups, such as Illl~illyllulli
ethylamine, n-~lul!yllull;l.~" iso-~,lu~ . the four isomeric ~uL~Ir~lllul~,S,
n-arnylamine, iso-amylamine, Il~".y' , Il~,lJL~lau~ c, ocly' llullylaull;llc,
lalllul~" penta~l~,~ylaulli~ ,~dd~yl~l~i~lC, I~ La~l~yl ullilll" O~ La~ ull;lll"methyl-ethylamine, methyl-;~u~lu~yl_lllillc, methyl-ll~ lal..;lll" methyl-nollylaullillG,
methyl-l,~,llLa~ylalllill~" methyl-o~ Ladl,.~' ~, ethyl-butylamine, ethyl l.~,~JLyl_llillG,
ethyl-octylamine, hexyl }I~lJLyldlllil~c, hexyl-octylamine, dilll~ ylalllillc, diGLl.yl~..il.e,
di-n-l!lu~yla l.ill~,, di-iso-~lu~,~laulliulc, di-n-butylamine, di-n-amylamine,
di-iso-amylamine, dill~"~ylalllille, dill~,"Lylalllille, ~lio~ Lyl,ullillc, ~ nl - . l l; l ~.,
n ~ . . i, . iso Aulv~ . i, - N,N--~ llyl. .1 1 .A 1~ N--~ y¦~ r,1 _ "
N-buyl. I11Allnl_ l~ , allylamine, n-butenyl-2-arnine, n-pentenyl-2-arnine,
2,3~imethylbutenyl-2-amine, di-butenyl-2-amine, n-hexenyl-2-amine, propylPn~iAminP,
~iPIl~^^nlSlminP tri-iso-~lul~ylaullill~" tri-n-butylamine, tri-iso-butylamine.
tri-sec.-buLylaullille, tli-n-amylamine, i ' ' ylaull;llc, Llh.illyl~ull;-.e, Ll;~lulJylàlllille,
heterocyclic amines as, for example, pyridine, chinoline, iso-chinoline, ~ P,
piperidine, pyrrolidine, indoline" I.i"". I;.li." . and azepine; primary arylamines as, for
example, aniline, methoxyaniline, ethoxyaniline, o,m,p-toluidine, I,~l~,lyl~ ...1;A,.,i"r
woss/1ssll 2 ~ 79822
- 6 -
benzidine, naphthylamine and o,m,p-chloroaniline; in particular ethyl-, propyl-, diethyl-
oder ~ preferably iso-l,lu~yl~ullil.e and f~
Tetra-substituted ~ m cations are also included, for example
tVi~ villJ~ tetra~v~liy' ~ yl~
vvll~L~ lly' tetra-e~ and ' ~ - " ,.,; ~ ., cations.
TriaLkylsulfonium cations include those, for exa~nple, in which each of the three aL~cyl
groups, which are not necessary all the same, may contain from I to 6 carbon atoms.
Trialkyl~ r.,~ cations likewise include those in which each of Ihe three alkyl
grorlps, which may be the same or different, may contain from I to 6 carbon atoms.
Fl ~ r~ cations include, for example, cations in which the ~ u ~ U~ US atom bears
four ~ lf 'Il~, each of which may be an alkyl group of one to ten carbon atoms or a
phenyl group, forexample, the t~t~ullv~ k ~ LV~ VU~YI~ and
tUll~lJllvllylllht~ - I cations.
Amidinium cations include, for example, straight chain amidinium cations of formula
R27-C(NH2)=NH2+, wherein R27 is an aL~cyl radical of, for example, from I to 10 carbon
atoms, and cyclic amidinium cations such as 1,5-D;~lv;uy~,10[5,4.0]undec-5-ene ~DBU).
Alkali metal cations include lithium, sodium and potassium; and aLlcaline earth metal
caions include l l~Af,. l. ~ 1'' ll, calcium, strontium and barium.
Preferred ~ lU~ of the formula I are those, in which A is hydrogen.
m~ n~i~ of the formula I which are of particular interest are those in which R3 is
hydrogen or Cl-C6-aL~cyl.
Further preferred ~ of the formula I are those in which R6, R7, R8, R9, Rlo and
Rll are hydrogen.
Preferred ~ ,v --~ of the formula I which must be ~ i are those in which Ql is
hydroxy or amino.
- . ~ 1 2 1 79822
wo 95/1881~
-- 7 -
Further preferred l,Ulll~JUUlld~ of the formula I are those in which Rl, R2, R3, R4 and R22
are hydtogen.
r~u (ll~llllu~ of the formula l which are of parlicular interest are those in
which X is CR6R7, Y is CR8Rg and Z is NR5. Further preferred ~ I"J~ from amongstthose of the formula I are those in which Ql is hydroxy amd A, R4, R6, R7, R8, Rg and R22
are hydrogen.
A preferred individual compound from the scope of formula I is
2-Oxa-3-hydroxy-3-(1,2,4-triazol-3-yl)-~-,lull~,A~u-~ U~ acid.
Preferred ~.UIII~JUUII~ of the formula I which are of particular interest are those, in which
the ~ ` Ql and--P--OR4 are in trans-position.
R22
A further subject of the present invention is a process for the 1"~ of . ..".1...",.~1~ of
the formula I which comprises a) for the ~ of a compound of the formula I, in
which Ql is hydroxy, reacting a compound of the formula 11
A ~N--N
~N)>--Li
wherein A has the meanings given for formula I except for hydrogen, with a compound of
the formula III
X/ R\Z
11 ,OR4
O /~ OR22 (~)'
~;2
in which Rl, R2, R3, X, Y and Z have the meanings given for formula I and A, R4 and R22
have the meanings given for formula I except for hydrogen, to a compound of the formula
2 1 79~22
WO95/18811 r._~,s,'~[[ l
A N--N X R Z
N~ P`OR (Ia)
R2
in which Rl, R2, R3, X, Y and Z have the meanings given for for~nula I and A, R4 and R22
have the meanings given for formula I except for hydrogen, tind optionally cleaving
protecting groups A andlor R4 and R22 and further converting them into a salt;
b) for the In~ iUII of a compound of the formula Ib
A N--N X R Z
N~ P`OR (Ib)
r~2
O O
in which Rl2 is a group 1¦ or a group ¦¦ and A, Rl, R2, R3, R4, Rl5,
-C-Q-R15 -c-Rl6
R~6, R22, X, Y and Z have the meanings given for formula r, reacting a compound of the
formula IV
A N--N X R Z
N~ R22 ( )
R2
withacompoundoftheformu~av: X CfQR5 (V),VI: X2C!Rl6 ( )
(Rl6CO)20 (VII), wherein Xl and X2 are halogen and Rl5 and Rl6 have the meaningsgiven for forrnula 1,
wo 95/18811 ? 1 7 9 8 2 ~ L.5,
c) for the ~ of a compound of the formula I7 in which Ql is NH2, converting a
compound of the formula Ia
~ ~ P ~OR (Ia)
in which Rl, R2, R3, X, Y and Z have the meamings given for formula I and A, R4~and R22
have the meanings given for formula I except for hydrogen, in the presence of NaN3 and
CF3COOH to the ~ r~ azide amd ~ y reducing the azide,
d) for the ~ iUII of a compound of for nula I, in which Ql is NRI3RI4 and A, if bound
to the S-carbon atom of the triazole ring, is as defined for formula I or A, if bound to one
of the 3 nitrogen atoms of the triazole ring, is hydrogen, reacting a compound of the
formula Ia
A N--N X/R\z O
N> ¦ 11 'oORR42 (Ia),
r~2
in which Rl, R2, R3, X, Y and Z have the meanings given for formula I and R4 and R22
have the meanings given for formula I except for hydrogen, with a compound of formula
XVI
Q0-Zo (XVI),
wherein Z0 is a leaving group, for example halogen, preferably chlorine, and Q0 is for
2l 79822
o95/18811 r~1,~ .c
- 10-
H3C \ /CH3
xample S(O)CI-, C(O)C~-, PCh-, C(O)CI-C(O)- or ~C~C~CH3 to form a
CH3
compound of formula xvn
A N--N X/R\Z
N~ ll`OOR242 (XVII),
1~2
wherein A, Rl, R2, R3, R4, R22, X, Y, Z and Z~ are as defined aboYe, which is converted
by reaction with a compound of formula XVII:[
HNRI3RI4 (xvm),
wherein Rl3 and Rl4 are as defined under formula I, and optionally cleaving the groups R4
and R22, for example with trimethylsilyl bromide and a solvent, for exatnple
.1.. 1,1. .111.. .11. ~ ~, followed by treatment with an alcohol and propylene oxide, and furthe}
converting them into a salt,
e) for the ~lc~ iu11 of a compound of the formula I, in which Ql is NHRI3 and Rl3 is
hydrogen, Cl-C6-aL~cyl, Cl-C6-aL~coxy, hydroxy, Cl-C6-aLkylcarbonyl,
Cl-C6-aL~u~yLa.l,ull~l, benzoyl which is - - ~ l or substituted by Cl-C4-aLkyl,
halogen, Cl-C4-haloaL'cyl, Cl-C4-aLcoxy, cyano, nitro, Cl-C4-aL~cylthio,
Cl-C4-aLkylsulfinyl, Cl-C4-aL~cylsulfonyl or Cl-C4-alku,~y.,~l,u11yl, a group
1 , c c -aL~c I which is substituted by halogen, Cl-C4-aL~coxy,
-C-N-R17R18 1 6 Y
Cl-C4-aL~uayL~lJu1lyl, phenyl or by phenyl which itself is substituted by Cl-C4-alkyl,
halogen, Cl-C4-haloaLkyl, Cl-C4-aL~coxy, cyano, nitro, Cl-C4-aL~ylthio,
Cl-C4-aLlcylsulfinyl, Cl-C4~-aLlcylsulfonyl or Cl-C4-aL~u~-yLall u11yl,
or Rl3 is a protecting group, for example arylthio, especially phenylthio or
4-chlorophenyl~io, reacting a compound of the formula XI
ii,; 21 79~22
WO 95/18811 P~,llll., . I
A
N--N
N M
wherein A has the meanings given for formula I except for hydrogen and M is Sodium,
, Lithium, Cerium or Copper, with a compound of the formula
x~ ~z o
3 ~N~ P--OR4 ~Xll)
R3 R22
R2
in which Rl, R2, R3, Rl3, X, Y and Z have the meanings given for formula I and R4 and
R22 haYe the meanings given for formula I except for hydrogen in the presence of an inert
solvent, or
f) for the preparation of a compound of the formula I, in which A is hydrogen and Ql is
NR~3RI4, wherein Rl3 and Rl4 have the meamings given for formula I except for hydrogen
or Rl3 and Rl4 are protecting groups, for example arylthio, especially phenylthio or
4-chlulu~ io, reacting a compound of the formula XIII
~ \z o
X R22
wherein R3, R4, R22, X, Y and Z have the meanings given for formula I, in the presence ûf
NaCN and HNRI3Rl4 to a compound of the formula XIV
~ ."- i~ 2~ 2~
W095/18811 r~l,.. ,,5,
- 12-
y
x~ \z o
NC~ ~ R4 ~XIV)
R13R14N R3 R22
wherein R3, R4, R22, X, Y and Z have the meanings given fol formula I and Rl3 and Rl4
have the meanings given above and converting said compound in the presence of sodium
methylaoe and formyl hydrazine to the compound of the formula I.
Process a) is cartied out in a manner or analogously as given by A.R. Katritzky,Tetrahedron 46 (1990), page 641 ff. The protecting group A as L~ yLII~,Lllyl, benzyl, a
group CH2-N3CH~)t SO2N(CH3)2, CH(CI-C4-alkoxy~2 or
2-(LI Ul~,llylsilyl)~lluAylll~,.llyl can be cleaved by:
A) I~y&uO_,loly ,;~, acidic hy&rolysis or reductive conditions for the L
group,
B) I~ydlu~ Olysis with palladium on active charcoal or by reductive cleavage by so&um
in liquid ammonia for the benzyl group,
C) cleavage with NaBH4 for Lhe group CH2-N~ CH~)t
D) cleavage with so&um hydroxide, sodium cyanide, tetra-n-buLy~ "",,;,~, fluoride,
H2SO4 or LiAlH4 for the dimethylsul~l.~.luyl group,
E) acid hydrolysis for the &aL .".y,l.~,Lllyl group,
F) acid hy&-olysis or cleavage with teLra-n-l,u~yl.u ~ fluoride for the
2-(trimethylsilyl)ethoxymethyl group.
Said reactions are carried out in a manner or analogously as given by US-A-5,248,655,
D.K. Anderson, Heoerocyclic Chemistry 23 (1986), pages 1257ff; A R. Katritzky,
Tetrahedron 46 (1990), page 641 ff.; A.J. Carpenter, Tetrahedron 1986, 42. 2351; P.J~
Dudfield, Synlett. 1990, 277; S. Ohta, Chem. Pharm. Bull. 1993, 41, 1226 and W. Holzer,
Heterocycles 1992, 34, 303.
Processes b), c), e) and f) are carried out in a manner or ~lal~;uu~ly as given in Chem.
Rev~ 1953, ~, pages 237 - 416 (b); D. R~ nn, Organic Synthesis 1981, 60, 104 (c);
, 7~ 22
wo gS/18811 ~3 r~
- 13 -
E. Ciganek, J. Org. Chem. 1992, 57, 4521; F.A. Davis, J. Org. Chem. 1977, 42., 398 and B.
Lipschut~, Tetrahedron 1986, 27., 4241 (d) and S.L. Crooks, J. Med. Chem. 1986, 29. 1988
and T. Murakami, IL,h,lucy~,h,~ 1981, 15, 301 (e). In process vatiant b), the meaning
chlorine is preferred for the ~ Xl and X2-
Process d) is carried out in a manner anal.ogously as given in J. Org. Chem. 39, 940(1974).
~mro~ ic of formula n are well known and for instdnce are prepared by reacting a1,2j4-triazole i~ an organic solvent, especially tetral,yd.uru~ or d.~ h l~,., in the
presence of n-butyllithium at i , between -50 to -90C The preparation of
of the formula m is described, for example, in US-A-5,248,655.
t~i mrolln~fc of the formula III, in which R2 is hydrogen, can also be prepared by
1) reacting a compound of the formula vm
o
,~ R 1
Z R3
in which Rl, R3, X, Y and Z have the meanings given for formula I, with a compound of
the formula IX
HOP(OR4)0R22 (IX)
in which R4 and R2~ have the meanings given for formula I except for hydrogen, in an
inert solvent, p}eferably dichlulu,.,~.i'..d.~c, in the presence of
N,O-bis(t-imethylsilyl)acetamide and trimethy~silyl 1.;n -.. l. 1l . ~llfonate at
~IlI,OCl~UiC~ of from -78C to 40C, preferdbly -20C to 20C, or
2) reacting a compound of the formula VIII
; 1 ~`` 21 ~2~
W095118811 r_l~lL,5~c- 1
- 14-
o
XJ~ R~ (VIII)
Z R3
in which R1, R3, X, Y and Z have the meanings given for formula 1, with a compound of
tbe formula X
(CH3)2R2sSiOP(OR4)0R7z (X)
in which R4 and R2z have the meanings given for formula I except for hydrogen and Rzs is
methyl or tert.-buyl, in an inert solvent, preferably dichloromethane, in the presence of a
compound of the formula XV
(CH3)zRzsSiOSOzCF3 (XV)
in which R2s is methyl or tert.-butyl, or
3) reacting a compound of the formula VIII
o
X J~R1 (vm
`Z R3
in which R1, R3, X, Y and Z have the meanings given for formula 1, preferably Z is NR5
and Rs is ben~yl, with a compound of the formula XVI
P(OR4)(0R22)OR22 ax)
in which R4 and R2z have the meanings given for formula I except for hydrogen,
preferably R4 and R2z are i-propyl, in an inert solvent, preferably dichlulu~ l"".e, in the
presence of a base and of a si~ylating agent, preferably trimethylsilyl
llinuu~ ,l. ,.,1' ortert.-butyldimethylsilylLinuw,..,-~ r, ~lfonate at
t~ al~ucS of from -7gC to 40~C and cleavage of the resulting silyl ether with acleaving agent, preferably tetrabut~l~ .. ,.". ".;. " ., n". ., ;",- in an inert solvent, preferab~y
~LIallyd~u~ula~le, in the presence of a base.
/ ~ ,; 2 1 79~22
wo 95/18811 ~ ~ - r~ [ ~ ~
- 15-
Process variants 1), 2) and 3) have been developed specifically for the preparation of the
active ingredients of the formula I. The present invention therefore likewise relates to
them. Con~rollnri~ of the formula VIII are known and a}e described, for example, in
Danishefsky, S.; Webb II, RRJ. Org. Chem. 1984, 49, 1955, Guerry, P.; Neier, R.;Synthesis, 1984l 485; Kozikowski, A.P.; Park, P.; J. Org. Chem., 1990, S5, 4688; Ziegler,
FE.; Bennet, G.B.; J. Am. Chem. Soc., 1973, 95, 7458; Chen, L.; Wang, E.; Lin, L.; Wu,
S.; II~,L~ULYLICS, 1984, 22, 2769; Terasawa, T.; Okada, T.; J. Org. Chem., 1977, 42, 1163;
Batten, R.J.; Coyle, J.D.; Taylor, RJ.K.; Synthesis, 1980, 910.
The c~ of the formula I are employed in unaltered form, as obtainable by the
synthesis, or preferably together with the auxiliaries cu., ~ ic,.,dlly used in r. ,, ~ " "1~
technology, and they are therefore processed in a known manner to give, for example,
. ""l~iri~ . , directly sprayable or dilutable solutions, dilute emulsions,
wettable powders, soluble powders, dusts, granules, and also ~nr~re~ tjt~nc for example
in polymeric substances. The application methods, such as spraying, atomising, dusting,
scattering or pouring, as well as the type of c~ are selected to suit the intended
aims and the prevailing L~
The f~ ione, i.e. the ~ c~Liulls or c~ n ~ -nc ~ ; the
active substance of the formula I and, if desired, one or more solid or liquid additives, are
prepared in a known manner, for example by intimately mixing and/or grinding the active
substances with extenders, for example with solvents, solid carriers and, if desired,
surface-active . ~ " l~ (surfactants).
The following are possible as solvents: aromatic IIYLIIUL~IJOI~S~ in particular the fractions
C8 to Cl2, such as mixtures of alkylbenzenes, for example xylene mixtures or alkylated
s aliphatic and cyrl~lirh~ti~ IIYLIIUL~UbU~I~ such as paraffins, LYLIO~ IG or
IGI~}IYL.~ 1-'.n- alcohols, such as ethanol, propanol or butanol; glycols as well as
their ethers and esters, such as propylene glycol or dipropylene glycol ether, ketones such
as LY~ . , isophorone or diacetone alcohol, strongly polar solvents such as
N-methyl-2-pyrrolidone, dimethyl sulfoxide or water; vegetable oils as well as their esters,
such as rapeseed oil, castor oil or soybean oil; and if ~ lu~ ~ also silicone oils.
Suitable surface-active ~ are non-ionic, cationic andlor anionic surfactants
having good emulsifying, dispersing and wetting properties, depending on the nature of
W095/18811 2 i 1 9822 r ~
- 16 -
the active substance of the formula I to be fnrml-lqt~-fi Surfactants are also to be
understood as meaning mixtures of 5~-~fqr-qntc
Anionic surfactants which are suitable can be either so-called water-soluble soaps or
waoer-soluble synthetic surfæe-active ~
Suitable soaps which may be mentioned are the aLIcali metal salts, aLkaline earth metal
salts or substituoed or ~ salts of higher fatty acids (Cl0-C2~), such
as the Na salts or K salts of oleic or stearic acid, or of natural mixtures of fatty acids which
can be obtained, for example, from coconut oil or tallow oil. Mention must also be made
of the fatty acid ~
However, so-called synthetic surfactants are used more fref~uently, in particular fatty
alcohol sulfonates, fatty alcohol sulfaoes, sulfonated bf .,,;."i~ . derivatives or
alkylaryl~--lf
The fatty alcohol sulfonates or fatty alcohol sulfates are generally in the form of alkali
metal salts, aL~caline earth metal salts or substituoed or, ~ ~n~m~njllrn salts, and
have an alkyl radical having B to æ c atoms, alkyl also including the aLkyl moiety of acyl
radicals, for example the Na or Ca salt of lib..;ll~ull`ollic acid, of the d df ~,yl~l;îu.ic ester
or of a fatty alcohol sulfate mixture prepared from natural fatty acids. This group also
includes the salts of the sulfuric esters and sulfonic acids of fatty alcohoUethylene oxide
adducts. The sulfonated l,~ ,i,.,irlA, i~ derivatives preferably contain 2 suifonyl groups
and one fatty acid radical having 8 to 22 C atoms. Exarnples of alkylarylsulfonates are the
Na, Ca or ~ n~ f salts of dod~ fnnir acid. of
diL ulyl ~ nPcl~lfnnir acid or of a ~ f ~. .lfnnir acid/formaldehyde
",.,,i~ product.
Other suitable l ~ are the ~ rhncrhAt~c~ such as the salts of the
phosphoric ester of a p-no~ U(4-14)-ethylene oxide adduct, or rh~crhnliritic
Suitable non-ionic surfactants are mainly polyglycol ether derivatives of aiiphatic or
~,y~ lnA~ alcohols7 of saturated or llncqtllrqt~d fatty acids and of al~yl~ ols~ which
can contain 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic)
llyd~ lJull radical and 6 to 18 carbon atoms in the alkyl radical of the alkylphenols.
Other non-ionic surfactants which are suitable are the wate}-soluble polyethylene oxide
. 21 79822
wo ss/lss~ r
- 17 -
adducts with poly~lul!yl~ glycol, cl~y~ r~y~lu~/yl~,.lc glycol and
- aLkyl~.oly~,lul.yl~ , glyeol which have 1 to 10 earbon atoms in the alkyl ehain and whieh
contain 20 to 250 ethylene glyeol ethe} groups and 10 to 100 propylene glyeol ether
groups. The abu.. ~ ,u ....l~ eustomarily eontain 1 to 5 ethylene glyeol units
per propylene glyeol unit.
Examples of non-ionie surfactants whieh may be mentioned are
llol~ Jt,~ ,ol~ l-u~y.,illc.~olc, castor oil polyglycol ethers, ~July~JIulJyh,.le/~ul~ I.yl~,llt
oxide adducts, tril,u~yll,h_..u/~y~ol.~ l.u~ u.ol, pol~ , glycol and
~yl~ lu~y~ul~
Other suitable substanees are fatty aeid esters of polyu..y~,~l.yl. l, ,, l.~ " sueh as
polyu,~ yl~ ulb;~ trioleate.
The cationic surfaetants are mainly quatemary ~n~r,ni.lm salts, which contain at least one
alkyl radical having 8 to 22 C atoms as N-~ and which have lower l; ~
or free alkyl, benzyl or lower hydroxyaL~yl radicals as further ~ ~..l;I,,. ..l~ The salts are
preferably in the form of halides, Ill~ y' r or ~ ulL.~,i., for example
~t~yl~ cillyl~ chlorideorbenzyldi(2-~''~ u~ ,lhyl~ll. ..l-l...bromide.
The surfaetants eustomary in r. 1 .. ;. . teehnology are described, inter alia, in the
following ~",l,l;~l;,.,~c
"MrC-l~rh~rn's Detergents and Prn..lcifil~rc Annual", Mc Publishing Corp., Glen Roek,
New Jersey, 1988;
M. and J. Ash. "Euuy~.lu~Alhl of Surfactants", Vol. I-III, Chemical Publishing Co., New
York, 1980-1981.
~r. Helmut Stache, ''Tensid-Tc~ h- ..1l...~ Il [Surfaetant Guide]", Carl Hanser Verlag,
Munieh, Vienna, 1981;
As a rule, the pestieidal ~IC~U~I~iUII~ eontain 0.1 to 99 %, in partieular 0.1 to 95 %, of the
aetive substanee of the for;nula I, I to 99 % of a solid ûr liquid additive and 0 to 25 %, in
partieular 0.1 to 25 %, of a surfactant.
While .... ,..., 1 "~ r ~ are more preferred as c ... ,1, . ,...: ~l goods, the user
generally uses dilute cr mrrcihc nc
WO 9511881 1 ~ 2 1 7 9 8 2 2 P~ 1 11 s
~ 18-
The ~ can also comprise further additives such as stabilisers, for example
epoxidised or llnPp-l~iAic~i vegetable oils (epoxidised coconut oil, rapeseed oil or soybean
oil), defoamers, for example silicone oil, ~ >~ v.~ viscosity regulators, binders,
tackifiers, as well as fer~lisers or other active substances for achieving specific effects.
In particular, preferred r.., ."..~ have the following ~c~ c-~ " (% = per cent by
weight)
Fm~lleifi5~b~p ~ S. - ~ '
Acive ingredient: 1 to 20 %, preferably 5 to 10 %
Surface-active agent: 5 to 30 %, preferably 10 to 20 %
Liquid carrier: 15 to 94 %, preferably 70 to 85 %
Dusts: - -
Active ingredient: 0.1 to 10 %, preferably 0.1 to I %
Solid carrier: 99.9 to 90 %, preferably 99.9 to 99 %
~llerlpne;~n ~'Y!~ ~C
Active ingredient: 5 to 75 %, preferably lO to 50 %
Water: 94 to 24 %, preferably 88 to 30 %
Surface-actiYe agent: 1 to 40 %, preferably 2 to 30 %
Wensble l~owders~
Active ingredient: 0.5 to 90 %, preferably 1 to 80 %
Surface-active agent: 0.5 to 20 %, preferably I to 15 %
Solid carrier: 5 to 95 %, preferably 15 to 90 %
Granules: ~
Active ingredient: 0.5 to 30 %, preferably 3 to 15 %
Solid carrier: 99.5 to 70 %, preferably 97 to 85 %
As a rule, the active substances of the formula I are sucessfully employed at application
rates from 0.001 to 10 kglha, in particular 0.005 to 2 kg/ha. The dosage rate which is
required for the desired action can be APtPrminPA by tests. It depends on the nature of the
action, the development stage of the crop plant and the weed, as well as on the application
~location, time, method~ and, due to these parameters, can vary within wide limits.
2 ~ 79822
W095~18811 ;, ~ ~ t' r~ s~
- 19-
Controlled ~ c-- of ~tjYe sPbst~ce
The dissolved active substance is applied to mineral granule carriers or polymerised
grapules (Preal~u-,.,ald~ ~) and allowed to dry. If desired, a coatiPg can be applied
(coated granules), which permits slow release of the active substance over a certain period
The followiPg examples are intended to illustrate the invention in greater detail.
A~ uaLiull of ~ of formula I
Preparation of Compound No. l.00l
MeO O O
(CH20)n ~3 HOP(OEt)2 ~
Me3SiO~ ZnCI2 cat. TMSOTf, p(o)(oEt)2
CH2CI2 2
Nq 1) n-suLi, THF ~N~>~ 1) TMSBr, CH2CI2
<~N,N-Tr 2) 2 N-N OH 2) MeOH
-- ~ ' 3) propyleneoxide
1~ ~ P(O)(OH)2 (Tr is T ilJl,.,.,yl-~-~,l-yl, TMSBr is trimethyl-
N NH OH ~ u~
MethodA: HOP(OEt)2 + ~=N CH2CI2, û C
o ~ ~PO(OEt)2
Method B: ~ + Me3SiOP(OEt)2 cat.Me3SiOSO2CF3 2
CH2CI2, û'C
Method A:
To a solution of diethyl phosphite (9.1 ml, 70.6 mmol), N,O-bis(trimethylsilyl)acetamide
t~ 2~ 22
wo 95/18811 'i r~-"
-20 -
(18.9 ml, 77.2 mmol) in 60 ml of dichlulu~ l.dlle was added trimethylsilyl
Llinuu~ r - - r- (1.45 ml, 6.4 mmol) at room ~ d~uu~ and stirred for 25 min.
The resulting mixture was cooled to 0C and 2,3-dihydro4H-pyran-4-on 1 (6.3 g,
64.2 mmol) in 8 ml of dichlùlu~ . was added and stirred for 1.5 h. The reaction was
quenched with 30 ml of water and extracted several times with dichl~Jlu~ ,Lll~ ,. The
combined organic layers were dried over MgSO4, . . ~ and purified by silica gel
y (hf-Y,~ ,rf n~ = 3:2) to give 4.0 g (26 % yield) of pure portion of the
desired compound 2 (as a colorless oil).
(I) Contains ca. 30 % of trans-4-methoxy-3-buten-2-on.
HNMR (400 MHz, CDCL3)~ 4.47-4.41 (m,1), 4.26-4.I7 (m,4), 4.02-3.96 (m,l), 3.75 (dt,
1,J=11.8,3.0),2.82-257(m,3),2.43-2.37(m,1),1.36(t,6,J=7.1).
Method B:
To a rnixture of enon 1 (0.80 g, 8.15 mmol) and diethyl tlimethylsilyl phosphite (1.89 g,
8.97 mmol) in 10 ml of dichlulul.l~ , was added trimethylsilyl
~linuul. .~ r~ (18 111, 0.08 mmol) at OC under nitrogen. After stirring for l hat 0C, water (~ ml) was added and the mixture was stirred for 1 h at room t~ dLUl~.
The organic layer was separated and the aqueous layer was extracted with
di~ lulul~ lldl~. The combined orgamic layers were dried over MgSO4, cG...,~,.IL~ and
purified by silica gel ~,IIluI~ld~U~;ld~Jlly (4 % ethanol in ethyl acetate) to give 600 mg (31 %
yield) of the desired compound 2.
(1) Contains ca. 30 % of 4-methoxy-3-buten-2-on. Danishefsky, S.; Webb II, R.RJ. Org.
Chem. 1984, 49,1955.
Ph3C, 1) nBuLi, THF, -78 C N Ph3C` ~ PO(OEt)2
N~ ~N 2) ~ ~ Po(oEf)2 N N
o PO(OEt)2 4A 4B
To a solu~ion of l-Lli~ yl~ lyl-1,2,4-triazole (4.3 g, 13.8 mmol) in 100 ml of TH~ was
added n-BuLi (1.63 M in hexane, lû.2 ml, 16.5 mmol~ in 10 min at -78C under nitrogen.
WO95/18811 .~ 2 ~ 7q822 r~
-21 -
Themixturewasstirredforlhat-78C,saturated,""",.~ ,.,chloridewasaddedandthe
mixture was allowed to warm to room t,~ UU~. Most of organic solvent was removedumder reduced pressure and the residue was extracted with ethyl acetate (3 x 50 ml). The
combimed organic layers were dried over MgSO4,, - ,- - ~ ri and purified by silica gel
~ y (1~ = 3:2 to 2:3) to give 4A (2.5 g, 33 % yield) and 4B (1.5
g, 20 % yield).
4A
mp: 174-189C
lHNMR (400 MHz, CDCI3)s 7.89 (S,l), 7.35-7.12 (m,15), 4.16-4.08 (m,4), 3.78-3.69(m,2), 3.44-3.38 (m, l), 2.45 (dt,l,J = 13.1, 9.4), 2.26 (dt,l,J = 13.5, 5.0), 1.36-1.33 (m,l),
1.32 (t,3,J =7.1), 1.31 (t,3,J = 7.1), 1.00 (s,1), 0.85-0.81 (m,1).
B
mp: 145-151C
1HNMR (400 MH~, CDCI3)~ 7.86 (S,l), 7.30-7.08 (m,15), 4.43 (dt,l,J = 5.9, 12.7), 4.12
(quintet,2,J = 7.4), 4.03 (quintet,2,J = 7.3), 3.86 (t,2,J = 5.4), 3.57 (S,l), 2.38-2.26 (m,l),
1 82-1.76 (m,l), 1.45-1.38 (m,l), 1.31 (t,3,J = 7.15), 1.30 (t,3,J = 7.17), 1.22-1.16 (m,l).
CPh3 H
N 1) Me3SiBr, CH2CI2
N ~ ~--P(Et)2 2~ MeOH N ~_ PO(OH)2
OH 3) propyleneoxide OH
~A
4A
To a solution of 4A (1.8 g, 3.29 mmol) in 36 ml of di~l~lvlu~ e was added
trimethylsilyl bromide (2.2 ml, 16.4 mmol) and the mixture was stirred overnight at room
t~ Lul~. After stirring with 20 ml of methanol for 2 h, propylene oxide (2 ml) and
then ether was added until the ~.~ r was completed. The ~)lci~,i~.iLdt~,~ were
collected on a glass filter, washed with ether, and dried in vacuo to give 800 mg (98 9'o
yield) of 5A.
4B was ~IuL~Lt;d in the same way as 4A to give ~Udl-tiLdLiV~ yield of 5B.
5A
mp: 169-175C (decomp.)
;~
WO95/18811 ~ ~ ~ ~79822
-22-
IHNMR (400 MHz, D2O~ o 8.92 ~s,l), 4.04-3.98 (m,2), 3.90 (apparent t,l,J = 11.3),
2.31-2.13 (m,3), 1.92 (broadd,1, J=1.4).
mp: 155-1~3C
IHNMR (400 ~qHz, D20) ~ 9.02 (s,1), 4.094.05 (m,l), 3.61 (apparent t,1,J = 12.0), 3.50
(apparent t,1,J = 12.0), 2.~6 (broad d,1,J = læ4), 2.4~ (broad d,1,J = 14.0), 2.0S-1.g9
(m,2)-
~clJal aLiOIl of Compound I~o. 1.017 and 1.019:
N pO3H2 NH po3H2
1.017 1.019
N Li /M~Br2 N ~ H
~ Ph3C THF, -78'C <~ HCI In C 2C 2
O~OEt 2) worku with 01 N HCI CPh3 then Et3N
P (A) 69 % yield
N ~ NaBH4, THF/EtOH N ~ MesCVEt3N/CH2Cl2
</N-lN 93%yield N~N (C) 2)(EtO)2PONa,Nal,THF
Ph3C PhoC 33 % yield
N~ PO(OEt)2 --/ Me <~ ~po(oEt)2
Ph3C 45 YO yield Ph3C (E)
N
13 TMSBr ~ N
2) propylene oxide N J~ P3H2
quant OH OH
1.017
2 1 ~982
O 95/188~1 2
-23 -
DMSO,CICOCOcl, CH2C12
<N 1~PO(OEt)2 30 /O yi3eld N ~?PO(OEt)2
Ph3C (E) Ph3C
1 ) TMSBr. CH2C12 H
2) propylene oxide N ~ po3H2
OHO
1.019
~NH~N HO~ PO3H2
N ~ PO3H2 N OH
1) NaBH4, EtOH/THF OH OH ~N N
2) TMSBr, CH2CI2 1.017 H
3) propylene oxide H OH
4) separation N HO
N OH N \~ P3H2
N ~ PO3H2 IN
OH ~H
Prep2rat;0n of ('rlmrolln~ No. 1.010 and 1.002:
NH
`N po(oH)2 ~N ~ PO(OH)2
NH2 NH2
I 010 1.00~
i
9~2
WO 95118811 1 ~I/Ib75.
-24-
~NHpotoc2H~ NII potoc2H5)2
OH NH2
7a
NH
~ pOtOC2H5)2
N--l
~ 'h
NH
7b
NH I ) Mc35i)3r, CHC12
< N 2) MeOH ~NH~N
Potoc2H5)2~ 3) propylene oxide N ~ PotOH~2
NH2 NH2
7a 1.010
1.635 g (13,74 mmol) Thionylchlorid was added to a suspension of 1.062 g (3.50 mmol) of
compound no. _ in I ml toluene. The solution becomes 1~ v~ ~ and was stirred for10 mmutes at room L~ dlule. 20 ml diGLl~yL,ill~,- was added under stirring. A sticky
p}ecipitate came out which was separdted from the solvent by fll'f ~nr~rifm and the residue
was washed with ether and driGd with the vacuum pump.
This product was dissolved in ~ ml dichlv.v---.,~l.dll~ and added slowly to a solution of
ammonia in Lc~ull~Lvrula--e with stirring at 0C. A white precipitate came out which was
separated by ~ ~ ~VI dLiU-- of the solvent and the residue was dissolved in 1 vO ml methanol,
stirred with 3 g potassium cdrbonate~ dissolved in 20 ml toluene and again
evaporated. Column chromatography on silica gel yields 218 mg of compound~ 156 mg
of compound 7b. 61 mg of a mixture of CVIII~)OUII~::I 7a and ~2, and as a by-product 582 mg
of the olefin 8
., i
~ ~; 2~ 79822
W095118811 r~.,~ s~
-25 -
~ OCZH5
N~ OC2H5 (8).
IH-NMR (400 MHz, CDCI3) of eompound 7a: 7.98 ppm ~s, lH), 4.12,ppm (m, 4H), 2.43ppm (m, lH), 2.06 ppm (m, 4H), 1.78 ppm (m, 2H), 1.48 ppm (m, 2H), 1.32 ppm (t, 6H).
D~l~,t~liull of the compound 7a was executed with trimethylsilyl bromide in
di~ lulu~ctharle followed by treatment with methanol and propylene oxide as deseribed
above, to yield tile desired produet 1.010. IH-NMR (400 ~Iz, CD30D): 8.62 ppm (s,
lH), 2.38 ppm (m, lH), 2.12 ppm (m, 5H), 1.97 ppm (m, lH), 1.73 ppm (m, lH), 1.53
ppm (m, lH).
Compound no. 1.002 ean be obtained analogously, starting from the e~ .". ~,~ ,...l;"~
eompound 6b
NH
(6b~
IH-NMR (400 MHz, CD30D) of eompound no. 1.002: 8.46 ppm (s, lH), 4.08 ppm (dxd,
lH), 3.81 ppm (t, 2H), 2.48 ppm (m, 2H), 2.28 ppm (d, lH), 1.94 ppm (d, lH).
B. Preparation of a eompound of the formula UIa:
cdt.TMSOTf/HOP(OEt)2 1N-HCI
BSA b~ (rIa)
OEt BSA: N,O-i~ ld~ PO(OEt)2
To a solution of di~.llyl~llu~ll;t~, (4.2 ml, 32.5 mmol) in 50 ml of diehlululll~,Lllallc was
added N,O-bistrimethylsilyla~,la lli~iG (8.0 ml, 32.6 mmol) and trimethylsilyl trifiate (0,28
ml, 1.45 mmol) at room t~ Lul~ and stirred for 30 min. After the mixture was cooled
to 0C, 3-ethoxy-2-eyclohexen-1-one (4.10 g 29.2 mmol) in ~ ml o~dichlu.u,~ l,all~ was
WO 95/18811 9 8 2 2 r ~ "~,s ~
-26-
added and stirred at 0C for 20 min and then al room t~ ly~ uu~i for 15 min. To the
resulting solution was added 50 ml of lN hydrochloric acid and stirred at room
tcllly~la~ulc for 2o mitL~ The mixture was extracted with Birh~ "., and the organic
phase was dried over MgSO4 and ' under reduced pressure. The residue was
purified by silica geH.ll--~ .,.u-l~yl acetate = 1:3 to ethyl acetate) to give
compound ma ~6.55 g, 90% yield, containing ca. 10% of ~iGLllyl~llu~ ;t~,) as a colorless
oil.
IHNMR (400 MHz, CDCl3~ ~ 657 (dt, 1,J = 21.1, 1.8), 4.20-4.10 (m, 4), 2.55-2.45 (m, 4),
2.13-2.06 (m, 2), 1.36 (t, `6, J = 7.1).
C. Preparation of a compound of the formula mb:
O OSitBuMe O
iPrO)3P, CH2C12 ~ 2 ~ (IIlb)
~N 2. tBuMe2SiOTf, -78 C ~N PO~ojp~)2 THF, O C ~N po~oiPr)2
Ph I ~ Ph -- Ph 3
~C~ a~;UII of 2
To a mixture of Lliiaul)lu~yl phosphite (2.91 ml, 11.8 mmol) and 1-benzyl-1,6-
dibydro-3(2H)-pyridinone I (2.02 g, 10.7 mmol) in 24 ml of dichlo~ -lc was addedtert-butyldimethylsilyl ,.inu.,l. ." .I.~ ,~r ,,.lr~" ~t. (2.71 ml, 11.8 mmol) in 7 min at -78C.
After stirring for 1 h at -78C, 20 ml of saturated NaHCO3 was~added. The mixture was
extracted with 200 ml of ether. The organic layer was washed with 2 x 15 ml of brine,
dried over MgSO4, and . - - ' to give 6.74 g of a crude product. rul irl~aLioll by
silicagel-,lll.-",~l--~---l,l,y(di~lllu~ l, =4:1)gave2.75g(55%yield)of_asa
colorless oil.
IHNMR (400 MHz, CDCI3) ~ 7.35-7.25 (m, 5), 4.9I (d, ï, J = 9,0). 4,70-4,63 (m, 2), 3.60
(m, 2), 2.97-2.80 (m, 4), 2.48 (dt, 1, J = 7.8, 11.1), 1.34-1.24 (m, 12), 0.89 (s, 9), 0.14
(s, 6).
2 1 7 9 8 22
WO~5118811 1
-27 -
Preparation of 3
To a solution of 2 (2.75 g, 5.88 mmol) in 15 ml of THF was added L~,LIablu~
fluoride (7,06 mmol, 1.0 M in THF) in 7 min at 0C. After stirring for 10 min, 3 ml of
water and 15 ml of saturated NaHCO3 was added. The mixture was extracted 150 ml of
ether. The organic layer was dried over MgSO4 arld ~ A to give 3.1 g of a crude
product. The crude product was purified by silica gel ~ l . y (dichloro-
f ~ ,l acetate = 3:1:1) to give 950 mg (46% yield) of 3 as an orange oil.
IHNMR (400 MHz, CDCl3) ~ 7.34-7.26 (m, 5), 4.72-4.65 (m, 2), 3.63 (d, 1, J = 13.0), 3.58
(d, 1, J = 13.0), 3.23 (d, 1, J = 15.0), 3.14 (bt, 1, J = 6.2), 2.82 (d, 1, J = 15.0), 2.62-2.43
(m, 4) 1.31-1.25 (m, 12).
Table L
~'f~m rol-nA~ of formula Ic:
N-N R1
N ~ P3H2 (Ic)
Cpd. Ql Rl R2 Rg Rg Z phys. data
No. m.p.C
. .
1.001 OH H H H H O 169-175 (decomp.)
1.002 NH2 H H H H O solid; IH-NMR
1.003 O,C,CH3 H H H H O
1.004 OH H H CH3 H O 175-185
1.005 OH H H Ph H O
1.006 OH H H CH2OH H O
1.007 OH H H CH2NH2 H O 223
1.008 OH H H H H S solid
1.009 OH H H H H SO2 248-260
1.010 NH2 H H H H CH2 solid; IH-NMR
. ; 2 ~ 7~22
wo95118811 r~1,~.S~ t
-~8 -
1.011 NH2 H H H H S 246-260
1.012 OH H H H H NH 197
1.013 OH H. ~~ H H H NCH3 258-260
1.014 OH H H H H NCH2Ph
1.015 OH H H H H NCH2CO2CH3
1.016 NH2 H H H H NH ~ 270
1.017 OH H OH H H CH2 ~ 147-153 (decomp.)
1.018 OH H H =O O
1.019 OH =O H H CH2 138-148 (decomp.)
1.020 OH NH2 H H H CH2
1.021 OH H NH2 H H CH2
1.022 NH2 H H H H NCH2COOH
1.023 NH2 H H H H NCH3
1.024 OH H H C3H~(n)H O 138
,CH2~
N-N I 2
1.025 ~ ~ P3H2 2l7-æ6
OH
Table 2
mro~nt1c of the folTnul;~ Id:
N-N ~Rl,
~Y~ P3H2 (Id)
Qt
Cpd.No. Ql X Y Rlo Rll phys.data
2.001 OH O CHz H H solid
2.002 OH CH2 O =O
2.003 OH CH2 NH H H solid
2.004 OH CH2 S H H 180C (decomp.)
2.005 ~H CH2 O H H solid
2.006 NH2 CH2 0 H H solid
wo9s/l881l ~' ~ 2 ~ 79822 .~
-29-
2.007 NH2 CH2 NH H H 265C (decomp.)
2.008 OH CH2 N-CH3 H H 200C (decomp.)
2.009 NH2 CH2 N-CH3 H H
2.010 OH NH CH2 H H
2.011 OH N-CH3 CH2 H H
2.012 OH CH2 NCH2PHH H 200C (decomp.)
2.013 OH CH2 SO H H solid
2.014 OH CH2 SO2 H H solid
COOC2H5
N--N
2.015 ~ ~ po3H2 150C
OH
Table 3
~omrolmflc of the fo~mula Ie
R R8
)2 (Ie)
~, ,N
NH
Cpd. Ql Rl R2 R8 Rg Z phys. data
No. ` m.p. C
3.001 OH H H H H O 155-163
3.002 OH H H H H NCH3 213-223
3.003 OH H H CH3 H O 160-165
3.004 OH H H H H NH solid
3.005 OH H H H H S 145
3.006 NH2 H H H H NH 250
3.007 NH2 H H H H S 243-266
WOgS/18811 ~ ~ ;79~22 r l,~. r -, ~
- 30 -
Table 4 . . ..
Cnmro~lr~c of formula If
Rlo
Ql~k PO(OH)
~, N
NH
Cpd. Ql X Y Rlo Rll phys. data
No.
4.001 OH CEI~ O E~ H solid
4.002 NH2 OE~2 S H H solid
4.003 OH CE~, S H H solid
5.001 N-N~PO(OH)2 solid
NH2
NH-N
6.001 ~ ,~Po(oc2Hs)2 liquid
NH2
\,~PO(OC H
7.001 2 5)2 = I;quid
N--
N
W095/18811 ` ~-t; ' 21 79822 p~"",,5,~ 1
- 31 -
Formulation examples for acive inFeciients of thç formula I
(% = per cent by wçi~ht)
l. Wettable powder a) b) c)
Acive ingredient according to
Tables 1-2 20 % 50 % 0.5 %
Sodiumligninsulfonate 5 % 5 % 5
Sodium lauryl sulfate 3 % - _ %
SOdium ~ ul~u~y~ r 6 % 6%
Octylphenol pol~ yl~ , glycol
ether (7-8 mol of ethylene oxide) - 2 % 2
Highly disperse silicic acid 5 % 27 % 27 %
Kaolin 67 % - %
Sodium chloride - - 59.5 %
The acive ingredient is mixed thoroughly with the additives and the mixture is ground
thoroughly in a suitable mill. Wettable powders which can be diluted with water to give
of any desired ~ are obtained.
2.Emulsion ~ a) b)
active ingredient according to
Tables 1-2 10 % 1%
Calcium dodc~yll - ~ 3 % 3 %
Octylphenol IJol~,Ll,yl~ , glycol
ether (4-5 mol of ethylene oxide) 3 % 3 %
Castor oil pvly~LllyL,Il~, glycol
ether (36 mol of ethylene oxide) 4 % 4
Cyl ~ , .""", 30 10 %
Xylenemixture 50 % 79 %
Emulsions of any desired nt)n~ n can be prepared from such :OIl~ L ,~ by dilution
with water.
W0 9S/18811 " ' ~ 9 ~ 2 ;~ r~" ~ c~c- ~ --
-32-
3. Dusts a) b)
Active ingredient àccording to
Tables 1-2 0.1% 1%
Talc 99.9 % -
Kdolin - 99 %
Ready-to-use dusts are obtainèd by intimate mixing of the carriers
v~ith the active ingredient.
4. Extruded~rdnules - a) b)
Active ingredient according to
Tables 1-2 10 % 1%
Sodium li~ ullul.~t~, 2 % 2 %
C~bu~ yL~llulose 1% 1%
Kaolin 87 % 96 %
The active ingredient is mixed with the additives and the mixture is ground and moistened
with water. This mixture is extruded and the extrudate is then dlied in a stream of air.
5. Coated ~ranules
Active ingredient according to
Tables 1-2 3 %
~ol~ L,.-~, glycol (molecular
weight 200) 3 %
Kaolin 94 %
The finely ground active ing~dient is applied uniformly to the kaolin, which has been moistened
with ~ol~ lylu~ glycol, in a mixer. Dust-free coated grdnules are obtained in this manner.
6. S~ n~ . ."~ .. a) b)
active ingredient according to
Tables 1-2 5 % 40 %
Ethylene glycol 10 % 10 %
No~lyl~ ol polyethylene glycol
ether (15 mol of ethylene oxide) 1 % 6 %
Sodiumli~;ll;l.~.ll~u,.dL~ 5 % 10 %
WO95/18811 . ~,~;~, ~t,V~ 21 79822 r~l,~,s,~ Jtl
- 33 -
C~ubu~y~ ,d-yl~-,llulose 1% 1%
37% aqueous formaldehyde solution 0.2 % 0.2 %
Silicone oil in the form of a
75% aqueous emulsion 0.8 % 0.8 %
Water 77 % 32 %
The finely ground active ingredient is intimately mixed with the additives. A suspension
. is thus obtained, from which ~ r~;- -- c of any desired ~ " can beprepared by dilution with water.
7. Salt solution
Active ingredient according to
Tables 1-2 5 %
I~ul~u~ e 1%
Octylphenol poly~ , glycol
ether (78 mol of ethylene oxide) 3 %
water 91 %
The c~ .u~ of the formula I are employed in unchanged form or, preferably, as
r.. l.r.~ together with the auxiliaries customary in fnrm~ hrn technology, and are
therefore processed in a known manner for example to emulsion r- ~~ ~~ ' ~, directly
sprayable or dilutable solutions, dilute emulsions, wettable powders, soluble powders,
dusts, granules and also capsules in, for example, polymeric c-lhct~-nrPs The methods of
use, such as spraying, misting, dusting, scattering or pouring, like the nature of the
c are chosen according to the desired effets and the given ~ . 5
Biolotical Examples
Example B 1: Post-emer~ence her~hicidal action (contact herbicide)
Monocotyledonous and dicotyledonous test species are raised in plastic pots containing
standard soil. At the 4-6 leaf-stage, the plants are sprayed with an aqueous suspension of
the test--- -- ,l--J~ at rates of 2 kg ailha ~5001 water/ha) and transferred to the
gr~ -c~ for futther cultivation under optimal conditions. The evaluation takes place
after 18 days, whereby the raùng scale of 1-9 is employed (1 = 100 % to 9 = no herbicidal
effect). Ratings of 1 to 4 (especially 1 to 3) indicate good to excellent herbicidal effect. In
this test, the co~ uu.ld~ according to tables I and 2 show good herbicidal activity. An
Wo 95118811 ; S .~ i, ` ' ~ i 2 ~ ~ 9 8 2 2 . ~1,~. ' .
-34-
example for the herbicidal activity of compound 1.001 is given in Table B 1:
Table B1: POSt e.~ ,e herbicidal action:
ComPound: Avena Setaria Sinapis Stellaria
1 . 001 2 = 2 1 2