Note: Descriptions are shown in the official language in which they were submitted.
WO 96/04850 PCT/US94/09096
z~ soo25
ORAL COLLECTION FOR IMMUNOASSAY
The present invention relates to the field of immunological
testing. In particular, a system for analyzing immunoglobulins
and other substances derived from mucosal transudate is dis-
closed.
Because of the association between immunoglobulins of the
blood and saliva, as well as the occurrence of secretory IgA
peculiar to salival fluid, antigen-antibody tests have been
conducted on the saliva to assess the value of such tests as a
screening fool for diseases.
Collection of saliva from the salivary glands is complicated
by the low volumes secreted, the diverse anatomic dispersion of
the glands, and the relatively high viscosity of the fluid. Most
techniques for collection involve the use of capillary tubes,
suction into micropipettes, chewing on paraffin or aspiration
into syringes. These methods, however, are limited in that
viscosity of the saliva makes the recovery of bubble-free
material by these techniques difficult. Other methods of
collection have been suggested to eliminate or at least reduce
the quantity of bubbles in the sample. Among such methods
include collecting saliva in the mouth by direct absorption with
a sponge or flexible wad of osmotic membrane. After absorption,
the saliva can be separated from the absorptive material by
centrifugation or by compressing the absorptive material.
However, absorption is generally accomplished by using cotton,
nylon, or polyester as the absorptive material. These materials
can non-specifically bind proteins which can result in an
undesirably low recovery of immunoglobulins.
Testing of salivary specimens has not been extensively
developed. In addition to problems with collection, the samples
collected by the known methods typically contain about 0.01-0.1%
of the immunoglobulin found in blood serum. Because of the
highly reduced immunoglobulin content of saliva, it has been
necessary to use more accurate antigen-antibody assay methods in
screening patients for disease. Parry et al., "Rational
Programme for Screening Travellers for Antibodies to Hepatitis
A Virus", The Lancet, June 25, 1988, have discussed such methods
- 1 -
WO 96/04850 PCT/US94/09096
21 800 2 5
and have found that the more accurate IgG-capture radioimmunoas-
say (GACRIA) test is preferable to avoid false indications which
may occur in less sensitive methods. Of course, more sensitive
testing procedures usually require added time and expense to
achieve the test results. w
~Y..
The immune system of the mouth.not only interacts with the
general immune system of the body,. but also has its own central-
ized center for antigen-antibody response. Within the oral
cavity is found extraoral lymph nodes and intraoral lymphoid
aggregations. The extraoral lymph nodes are involved in the
drainage of the oral mucosa, gum and teeth. However, the
function of the intraoral lymphoid tissue is little understood.
The extraoral lymph nodes include a fine network of lymph
capillaries which are superficially located in the mouth, palate,
cheeks, lips, gingiva, and pulp of the teeth. The capillaries
join to larger lymph vessels which originate from a network deep
in the muscle of the tongue and other structures. An antigen can
gain entry into the oral lymphatic system directly through the
capillaries or be transported there by phagocytes. Once inside
the network, the antigen can induce an immune response.
Included in the intraoral lymphoid tissue are generally four
distinct tissue aggregations: (a) the tonsils, (b) scattered
submucosal lymphoid aggregations, (c) salivary gland lymphoid
tissue, and (d) gingival lymphoid tissue.
The tonsils (palatine and lingual) primarily produce B-cells
and T-cells which are generally contained within a cap of
lymphocytes and plasma cells. Antigen typically gains entry into
the tonsils through a distinct epithelial region wherein the
antigen can come into contact with the T- and B-cells to
stimulate an immune response. The predominant type of antibody
formed in the tonsils is found to be IgG followed, in order, by
IgA, IgM, IgD and IgE.
Scattered submucosal lymphoid cells have not been extensive-
ly studied. These cell masses are histologically similar to
tonsillar tissue.
Both the major salivary glands (parotid, submandibular and
sublingual) and the minor salivary glands have been found to
- 2 -
~ I ~ t _....
WO 96104850 PCT/US94/09096
21 800 2 5
contain lymphocytes and plasma cells. Most of the plasma cells
secrete IgA and some IgG or IgM. The IgA synthesized in the
salivary glands has a dimeric structure. This type of IgA is
referred to as secretory IgA (sIgA) and is the major immunoglobu-
lin component in saliva.
Both T-cells and B-cells are found in the gingival lymphoid
tissue. In subjects having clinically normal gingival tissue,
T-cells predominate. During an infectionary period, such as
during the development of gingivitis, B-cells have been found to
predominate.
Plasma cells are also found in the gingival lymphoid tissue.
Clusters of these cells are generally located near the blood_
vessels and predominantly produce IgG. To a lesser extent, IgA
and IgM are also manufactured. More importantly, Brandtzaeg et
al. in, Human Saliva: Clinical Chemistry and Microbioloay edited
by Jorma 0. Tenovuo, have shown that the immunoglobulins from the
secretions from the gingival tissue area are directly related to
the immunoglobulins found in the blood.
In order to eliminate or greatly reduce the problems
inherent in antigen-antibody analysis of salival fluid, we
previously proposed a method for collecting immunoglobulins from
the oral cavity with the aid of a hypertonic solution as a rinse.
See EP 0 418 739 A1. We also previously proposed the use of a
collecting pad, which has been treated with a hypertonic
solution, in the oral cavity to absorb oral immunoglobulin for
immunological testing. See WO 91/13355.
We have now found that an untreated pad can be used to
collect a sufficient quantity of oral immunoglobulin for
immunological testing. The use of the pad results in a yield of
immunoglobulins greater than would be expected and can incorpo-
rate basic antigen-antibody testing techniques as a screening
tool for diseases. We have also found that the present invention
can be used to collect substances other than immunoglobulins for
testing. In fact, the invention has been successfully used to
collect substances having molecular weights ranging from about
176 (cotinine) to about 950,000 (IgM). There is no limit to the
size of the molecule which can be collected using the present
- 3 -
21 800 2 5
invention. If the molecule can pass through the walls of the
capillaries and other oral tissue, it can be collected using
the present invention. The pad is contacted with the oral
mucosa without chewing.
More specifically, the present invention provides a
method of immunological testing which comprises:
(a) obtaining a mucosal transudate sample to be tested
by
(i) inserting a substantially salt-free absorbent
pad into the oral cavity,
(ii) contacting the pad with the oral mucosa
without masticating said pad,
(iii) removing the pad from the oral cavity;
(b) subjecting the sample to at least one immunological
test known to reveal the presence or absence of at least one
immunologically active analyte, and
(c) analyzing the test results for the presence or
absence of the analyte.
Fig. 1 is a longitudinal section of one embodiment
of a container for storing the pad;
Fig. 2 is a longitudinal section of the embodiment
of Fig. 1 with the pad and holder shown;
Fig. 3 is an elevational view of another embodiment
of a container useful in the present invention with the cap
removed;
Fig. 4 is a top plan view of the container of Fig.
3;
Fig. 5 is a longitudinal cross-sectional view of
- 4 -
24271-19 (S)
21 800 2 5
the container of Fig. 4 taken along the line 5--5 of Fig. 4;
Fig. 6 is an elevational view of a cap for use with
the container shown in Fig. 3;
Fig. 7 is a top plan view of the cap of Fig. 6; and
Fig. 8 is a cross-sectional view of the cap taken
along the line 8--8 of Fig. 7.
The present invention is concerned with collecting
oral immunoglobulins for immunological testing and other
substances for testing. A pad is used to collect a specimen
having a high concentration of immunoglobulins or the other
substances. High levels of immunoglobulins from the oral
cavity are considered to be concentrations in excess of 50 ~g
total Ig per ml. The specimen can be subjected to a basic
testing technique which can be used as a tool for screening a
patient for diseases or for the presence of certain foreign
substances.
Representative molecules which have been
successfully collected by the use of the present invention
are:
Analyte Molecular Weight
Cotinine 176
Glucose 180
Theophylline 180
Cocaine 303
Beta2-microglobulin 11,818
Hepatitis B surface antigens 24,000
Beta-human chorionic gonadotropin 37,900
- 4a -
24271-19(S)
WO 96/04850 ' PCT/US94l09096
21 800 2 5
IgG -- human antibody 150,000
Total IgG (antigen not specified)
HIV-1
Hepatitis A
Hepatitis B
Rubeola (measles)
Syphilis non-treponemal antigen
IgA -- human antibody 160,000
Total IgA (antigen not specified)
IgM -- human antibody 950,000
Total IgM (antigen not specified)
Hepatitis A
Hepatitis B
In order to minimize degradation in a collected specimen,
container in which the pad is stored after use the hypertonic
solution of the present invention can include a preservative.
Such a preservative can act to inhibit proteolytic enzymatic
activity which can be responsible for the destruction of antibody
molecules. Compounds contemplated as a preservative include
anti-bacterial agents, anti-fungal agents, bacteriostatic agents,
fungistatic agents, and enzyme inhibitors. In a preferred
embodiment benzoic acid, sorbic acid or the salts thereof are
used as anti-fungal agents. As bacteriostatic agents, salts in
high concentration and compounds capable of maintaining the
hypertonic solution at low pH are contemplated. Such salts
include thimerosal (or merthiolate), phenyl mercuric acetate,
phenyl mercuric nitrate and sodium azide. Other preferred
preservatives include preservatives which are typically used in
medicines and mouthwashes. Examples include ethyl alcohol and
chlorhexidine gluconate. Another class of preferred anti-
microbial agents are detergents which can be used as topical
germicides or in mouthwashes. An example is benzalkonium
chloride. It is preferred to use these preservatives in a range
of about 0.01% to about 0.2% by weight.
In the present invention, a pad is used to absorb mucosal
secretions from the oral cavity. The pad is made of an absorbent
material which can be effectively placed into the oral cavity.
A plastic or carbohydrate material such as cellulose can be used
as the absorbent material, but a thick, absorbent cotton paper
is preferred. An example of a thick, absorbent cotton paper is
product n300 manufactured by Schleicher and Schuell in Keene, New
WO 96/04850 PCT/US94/09096
2~ 800 2 5 '
Hampshire. The pad is preferably not in the form of a foam or
sponge, although foam or ,spvonge could be used.
Most materials from which the pad is made can non-specifi-
cally bind protein. Thus, some immunoglobulins can undesirably
bind to the pad and it is desired to block proteins from binding
to the pad by using a blocking agent. Non-specific binding is
not normally a problem in the collection of blood samples since
blood contains its own blocking agent (i.e., human serum
albumin).
To reduce non-specific binding in the collection of oral
specimens, a blocking agent can be added to the hypertonic
solution to be incorporated into the pad. A blocking agent is
generally a soluble protein which is used to prevent non-specific
binding of another protein to a solid surface. Compounds which
can be added as blocking agents include albumin and gelatin, but
any water soluble, non-toxic protein can be used as a blocking
agent as long as the protein does not adversely affect antibody
molecules. It is preferred to use bovine gelatin. In general,
blocking agents can be made into a solution at a concentration
of between about 0.01% and 0.2% by weight. The solution is then
incorporated into the pad by soaking or spraying followed by
drying.
To collect a substance from the oral cavity, the pad can be
placed into the mouth with the aid of a holder. The pad and
holder are shown in the drawing. The pad holder 1 can be a
hollow, plastic stick having a groove 2 at one end. The pad 3
is inserted into the groove and the holder can be manipulated to
place the pad into the oral cavity, preferably between the lower
gums and cheek. Placement of the pad between the lower cheek and
gums facilitates absorption of secretions originating from
gingival lymphoid tissue as well as secretions from submucosal
lymphoid tissue and salivary gland lymphoid tissue. It is
preferable that the specimen be collected by rubbing the pad back
and forth between the gums and cheek for about ten seconds and
then holding the pad in position for about two minutes. The pad
should not be chewed since chewing stimulates salivation which
is undesirable.
- 6 -
I I ~ T __.... __
WO 96/04850 PCT/US94/09096
21 800 2 5
After the specimen has been collected, the pad is stored in
a container until immunological testing can be performed. One
type of container is shown in Figs. 1 and 2. It is desired that
the container have a centrifuge tube as an outer portion of the
container, and that an inner portion of the container have an
inner tube which mounts into the centrifuge tube. The pad is to
be placed into the inner tube, and the contents therein are
secured by a tube cap.
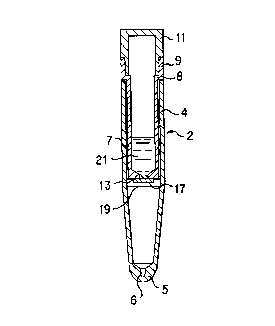
Reference is had to Fig. 1 wherein there is shown an
assembly which has been modified for use with the instant
invention. The container 2 comprises a centrifuge tube 4 having
a tapered lower end or base 5 with a downwardly tapering recess
6 in which solid matter accumulates upon centrifugation; an upper
tube or container 7 having a radially outwardly projecting
annular flange s and a cylindrical upper portion 9 at its upper
end; and a plug or stopper 11. The cylindrical portion 9 and
stopper 11 are of the same size and shape as the upper part of
centrifuge tube 9 so they are flush with the outer surface of the
centrifuge tube and the assembly presents a uniform appearance,
although this feature is not important to the practice of the
instant invention. In the floor 13 at the bottom of container
7 is a bore 17 to allow liquid to flow from container 7 to
centrifuge tube 4 when the complete assembly is centrifuged. The
container 7 is made of any suitable material such as polyethyl-
ene, glass, etc. Similarly, the stopper 11 is made of any
suitable material such as polyethylene as is well-known in the
art.
There is a removable plug 19 in bore 17. The plug could be
made of any suitable material such as wax, a plastic, etc. A
suitable quantity of a preservative solution 21 is placed in the
container 7.
Once the pad is placed in the inner tube, a preservative
solution 21 is added. Such a preservative solution can act to
inhibit enzymatic activity which can be responsible for the
destruction of antibody molecules or can function as an anti-
microbial agent.
WO 96/04850 PCT/US94/09096
2'! 800 2 5
Compounds contemplated for use in the inner tube as a
preservative include anti-bacterial agents, anti-fungal agents,
bacteriostatic agents, fungistatic agents~and enzyme inhibitors.
As an antibacterial agent, it is preferred to use chlorhexidine
gluconate or thimerosal.
The preservative solution to be used in the inner tube can
contain one or a combination of the preservatives. In general,
the preservatives are included in a concentration which limits
microbial contamination and does not adversely effect the
immunoglobulins absorbed into the pad.
The preservative solution to be used in the inner tube can
also contain a detergent which improves removal of antibody from
the pad during centrifugation. Tween 20 (polyoxyethylene
sorbitan monooleate) is a preferred detergent since it can also
prevent non-specific binding of antibody to a solid surface. It
is preferred to use a combination comprising about 0.01%-0.2%
chlorhexidine gluconate and 0.20-0.7% Tween 20. A combination
comprising about 0.1% chlorhexidine gluconate and 0.5o Tween 20
is most preferred.
After the preservative solution is added to the inner tube,
the tube cap is inserted into the container to seal in the
contents. The pad can be stored in this manner for several days
until immunological testing can be initiated.
In this embodiment, the pad 3 on holder 1 is used as already
described. After the pad 3 is removed from the user's mouth,
stopper 11 is removed from container 7 and the pad is placed
within the container 7. The holder 1 is broken off at a point
outside the mouth of container 7 so it will project upwardly from
the container. Then the stopper 11 is replaced. Since stopper
11 is hollow, it will securely seal the container 7 with the
broken end of holder 1 extending into it. Holder 1 is preferably
scored at a suitable location to provide for easy breaking. When
the pad 3 is inserted in container 7, it will absorb at least a
part of preservative solution 21.
The pad 3 is stored in container 7 until testing can be
initiated. At the laboratory, the container 7, with the stopper
11 securely in place, is inverted, the seal 19 of wax or other
_ g _
1 C , t .
21 800 2 9
suitable substance is removed, and the container 7 is placed
in a centrifuge tube 4. The complete assembly 2 is then
centrifuged whereby all the liquid, including preservative
solution, saliva, etc., is drawn down through bore 17 into the
centrifuge tube 4. Testing is then performed using known
techniques.
To simplify the collection and analysis of an oral
specimen using the pad collection system, a kit can be
provided. The kit can include a combination of the pad and
implements used to collect and prepare the oral specimen for
analysis. One preferred embodiment of the kit includes the
treated pad 3 and pad holder 1; the container 7 having the
stopper 11 and the storage preservative 21. Optionally, a
centrifuge tube 4 could be included.
Another container for storing collected substances
for subsequent testing according to the present invention
comprises an open upper end adapted to be sealed with a
removable stopper and a lower end having an opening
communicating the interior of the container with the outside,
the opening being selectively sealed by a frangible nipple
during storage of the substances and unsealed for removal of
the collected substances for subsequent testing. For both
comfort and safety of the user, the distal end of the
frangible nipple is enlarged, preferably in the form of a
ball. The open upper end is preferably sealed with a screw-on
"plug seal" cap. In addition, the floor of the container,
which slopes towards the opening in the center thereof, has a
plurality of upstanding webs to prevent the pad described in
9 24271-19(S)
. . ",, ..w~..._~___. . _.___.~.._.__... _ _._ _.~..-.. ..~_ __..._ .__ r_ ._
.._ _. .,.. .
_w ~1 800 2 5
U.S. Patent No. 5,103,836 from resting on the bottom of the
container and blocking the opening at the bottom.
The container is dimensioned to permit its insertion
into a standard 15 ml, conical centrifuge tube. This allows
centrifugation after the nipple is broken off, thereby
transferring the vial contents into the centrifuge tube.
The container is intended to hold a volume of
preservative liquid (from about 0.5 ml. to about 2.0 ml.).
When the pad is placed in the container, the preservative is
absorbed into the pad. Since the container with the
preservative will be stored for up to a year without
significant loss of liquid volume, the
9a
WO 96/04850 PCT/US94/09096
21 800 2 5
water transmission rate must be low. The container is fabricated
of any suitable plastic such as polyethylene, polycarbonate,
polystyrene, PET (polyethylene terephthalate), polypropylene, EPC
(ethylene propylene copolymer), and the,like. Polycarbonate is
the least desirable due to excessive °"breathing," that is,
allowing moisture to escape. The preferred material is poly-
propylene.
Referring to Figs. 3 and 4, the container of this embodi-
ment, generally designated by the numeral 10, comprises an
elongated body portion 12. Body portion 12 is generally
cylindrical although, it is preferably slightly tapered from the
upper end to the floor 15. The upper end 16 is open and is
provided with a screw thread 18 to accommodate the cap.
A nipple 20 extends downwardly from the floor 15. The
floor 15 preferably has a slight slope at an angle a from the
outside to the center. Angle a is preferably about 20°. At the
center of the inside of the floor 15 is a depression 22,
preferably "V" shaped and preferably forming an angle of about
88.50°. The depression 22 causes the base end of nipple 20 to
be weakened, thereby allowing the same to break off when
sufficient pressure is applied. A bulb 29 is formed at the
distal end of nipple 20 to make it easier and safer to break off
the frangible nipple 20. In order to prevent the pad which
will be inserted in the container 10 from resting on the floor
15 at the hold created by breaking off nipple 20, a plurality of
upstanding webs 26 are provided on floor 15. Preferably, there
are four webs 26.
Turning now to Figs. 5~, 6, and 7, there is shown a cap,
generally designated by the numeral 28 for sealing the open upper
end 16 of container 10. Cap 28 is of the type commonly known as
a "plug seal" cap. Cap 28 has internal screw threads 30 to
cooperate with threads 18 on the container. Annular depression
32 creates an annular space 39 bounded by annular depression 32
and the inside of outer wall 36. The wall of the container thus
fits into annular space 34 and creates a perfect seal. The
outside surface of cap 28 is knurled as shown at 38 to facilitate
removal and replacement of the cap.
- 10 -
WO 96/04850 PCT/US94/09096
21 80025
The container 10 is used in the same manner as the contain-
ers described above with respect to Figs. 1 and 2. The following
examples show the effectiveness of the pad of the present
invention.
EXAMPLE 1
Comparison of IgG in Saliva versus Mucosal Transudate
The purpose of this study was to compare the levels of IgG in
saliva versus mucosal transudate.
Oral samples were collected from ten subj ects . The subj ects
collected saliva by chewing on the "absorbent body" sold as part
of the Sarstedt SalivetteT" kit with a cotton plug as an absor-
bent body until the cotton plug became saturated with saliva.
Approximately 4 hours later, the subject then collected mucosal
transudate by collecting oral fluid using the treated pad
described in WO 91/13355. After the saliva or transudate was
collected, the liquid was removed from the cotton plug and
treated pad by centrifugation. The oral specimens were analyzed
for the presence of IgG antibody using an enzyme immunoassay.
Table 1 shows the levels of IgG collected by the cotton plug
versus the treated pad.
Table 1
Subject Number Saliva IgG Transudate IgG
( /cg/ml ) ( ~cg/ml )
1 1.6 38.3
2 3.2 G0.3
3 0.7 21.0
4 1.8 48.5
2.4 135
4.9 86.7
7 1.1 72.3
g 1.3 31.1
g 3.0 63.1
4.1 37.5
Mean IgG 2.4 59.4
- 11 -
WO 96/04850 ~ PCT/US94/09096
~1; 800 2 5
Saliva samples collected by chewing on an "absorbent body"
of the Sarstedt Salivette'=" kit contain approximately 25 times
less IgG antibody than samples collected by the treated pad of
WO 91/13355.
EXAMPLE 2-
Tests comparing the treate~i'vpad of WO 91/13355 and the
untreated pad according to the instant invention were run.
A total of four pads were used for oral specimen collection
from each voluntary participant, two of the pads being treated
and two untreated. Each collection (two like pads collected
simultaneously) lasted two minutes. The participant rinsed
his/her mouth with tap water after the initial collection and
waited at least one-half hour before the second collection.
Participants in the study were divided into two groups. Half of
the participants initially collected an oral sample using twa
untreated pads, followed later by two treated pads. The other
participants collected with two treated pads first, and two
untreated pads later.
After collection the participant inserted the pad into an
empty vial which has been labeled with the specimen number and
the type of pad used for collection (treated, or untreated). The
stick was snapped-off and the vial capped tightly. The specimen
remained at 4°C before and during centrifugation. Once centri-
fuged, the oral specimens collected by the two like pads from
each participant were pooled. Specimens were stored at 4°C.
Each specimen pool was assayed using Epitope, Inc. ~s EIA for
Total IgG to quantitate the IgG present in each specimen.
- 12 -
I ~ ~ 1
WO 96f04850 ' PCT/I1S94/09096
21 804 2 5
Table 2
Subj ect Number IgG ( ~,g/ml )
Untreated Pads Treated Pads
1* 6.7 9.1
2* 4.9 7.8
3 4.6 11.2
4 12.4 23.9
5* 10.2 26.4
6* 9.2 43.9
7 5.3 45.4
* untreaLea paas colleczea =irsL.
Specimens collected with the treated pads contained higher
levels of immunoglobulin G (IgG) than those collected with the
untreated pads, as analyzed by an in-house IgG-specific enzyme
immunoassay (EIA).
From the analysis of the matter collected by the pads of WO
91/13355 and the matter collected by the Sarstedt "absorbent
body", it is concluded that the pad collected the IgG from
mucosal transudate and not saliva, and that the difference is
significant.
From the analysis of the matter collected by the treated
pads of WO 91/13355 and the untreated pads of the instant
invention, it is concluded that while the untreated pads
collected less IgG than the treated pads, the untreated pads
still collected significantly more IgG than would be expected to
be collected from saliva (5.3 ~,g/ml vs. 2.4 ~cg/ml).
- 13 -