Note: Descriptions are shown in the official language in which they were submitted.
2180034
BACRGROUND OF THE lNVh~. ~lON
The present invention relates to a process for producing
alkali metal hydroxides such as caustic soda (sodium
hydroxide) or potash (potassium hydroxide) by electrolysis of
alkali metal salts. More particularly, the present invention
relates to the production of sodium or potassium hydroxide by
electrolyzing non chloride salts such as sodium sulphate so
that the co-product of the process is not chlorine but other
valuable material such as potassium sulphate which can be used
in industrial chemical or fertilizer markets.
In the classical electrolytic process for producing
caustic soda, sodium chloride is used almost exclusively. In
some instances, there is sustained demand for caustic soda
while chlorine markets are steadily declining for a variety of
market and environmental factors, such as found with the pulp
and paper industry.
There is therefore a need to provide caustic soda
production without the usual chlorine production in a manner
that is economical and is sufficiently effective and efficient
that investment can be justified.
It is well known in the art that treating soda ash with
lime provides caustic soda and there are commercial plants in
the United States that do this. However, the economics are
such that these operations are only attractive to operate when
caustic soda prices are high. The primary reason for this
~AW or~lcEs
FINNEGAN,HENDERSON, cyclical operation is that soda ash based caustic soda plants
FARABOW, GARREl~
~ DWNER,L.L.P.
1300 I STQEET, N. W
'NAS~INGTON, DC 20005
202 408 '-000
~ 18003~
produce only one valuable product whereas chlor-alkali plants
produce two valuable products.
A number of processes have sought to produce caustic soda
by electrolysis of sodium sulphate such as those disclosed in
United States Patent Nos. 2,829,095; 3,135,673; 3,222,267;
3,398,069; 3,907,654; and 4,561,945, all incorporated herein
by reference. This art, in one manner or another, relates to
the production of caustic soda along with sulphuric acids of
varying strengths.
From an economic perspective, more dilute sulphuric acid
is less desirable. Attempts t-o produce sulphuric acid of
higher concentrations can result in significantly lower cell
efficiencies. These techno-economic difficulties have
resulted in very limited application of the art.
U.S. Patent No. 5,098,532 (incorporated herein by
reference) relates to the electrolysis of sodium sulphate to
produce caustic soda. This involves the use of a so-called
three compartment cell and the co-production of ammonium
sulphate. In this patent, back migration of protons from the
anolyte compartment, which causes reduced current efficiency,
is reduced by introducing ammonia to the cell compartment and
results in the production of ammonium sulphate.
While this approach reduces a techno-economic limitation
inherent in two or three compartment cells in the electrolysis
of sodium sulphate, the costs for utilization of ammonia may
L AW 0~1 C ES
FINNEGAN,HENDE~ON, well not be covered by the revenues due to ammonium sulphate. FARABOW, GARRETT
6 DWNER,L.L.P. This can then place the system at an economic disadvantage to
1~00 I STF~EET, N. W
W~ShlNGTON, DC 20005
Z02 - 40e - 4000 - 2-
- ~18003~
the production of caustic soda by conventional chlor-alkali
technology.
SUMMARY OF INVENTION
The features and advantages of the present invention
include providing a process for the production of an alkali
metal hydroxide such as sodium or potassium hydroxide which
will provide improved methods for utilizing non-chloride
alkali metal salts in electrolysis to produce caustic soda or
potash and other valuable products which do not contain
chlorine.
Additional objects and advantages of the invention will
be set forth in part in the description which follows, and in
part will be obvious from the description, or may be learned
by practice of the invention. The objects and advantages of
the invention will be realized and attained by means of the
elements and combinations particularly pointed out in the
appended claims.
To achieve the objects and in accordance with the purpose
of the invention, as embodied and broadly described herein,
the present invention relates to a process for producing an
alkali metal hydroxide, e.g., caustic soda or caustic potash,
by electrolysis using non-chloride salts such as sodium
sulphate or potassium sulphate. The sulphuric acid produced
in an anolyte compartment by electrolysis of the non-chloride
~w orr~crg
FINNEGAN, HENDE~ON, salt, such as sodium sulphate, is removed and brought into
FMABOW, G~RRE~
~3~NNERLL.P contact with a base ion exchange resin in the hydroxy form to
1300 I ST~EET, N. W.
W~S~I~NGTON, OC 20005
202 .~0~ 000
218003~
neutralize the sulphuric acid and thereby load the resin with
sulphate. The de-acidified or acid stripped anolyte liquor is
then returned to the anolyte compartment separately or with
additional non-chloride salt as needed. This enables the
anolyte compartment to be maintained at pH levels sufficiently
high enough to minimize back migration of protons and achieve
high current efficiencies. The alkali metal hydroxide is
produced as a catholyte, e.g., in a catholyte compartment.
After the base ion exchange resin is loaded with sulphate ion,
the resin is given successive displacement washes to remove
anolyte entrained in the resin. The resin then is eluted with
alkali metal chloride, as with a hot concentrated alkali metal
chloride brine, such as potassium chloride and optionally
containing some alkali metal sulphate, such as potassium
sulphate. The resultant brine, now rich in alkali metal
sulphate, such as potassium sulphate, is cooled in a
crystallizer and treated with alkali metal chloride such as
potassium chloride, to yield alkali metal sulphate crystals,
such as potassium sulphate crystals, that are separated from
the brine and dried. The crystallizer liquor (primarily
alkali metal chloride) is heated and recycled to elute fresh
base ion exchange resin in the sulphate form. The base ion
exchange resin, now in the chloride form, is then subjected to
displacement washes to remove entrained alkali metal chloride
and then treated directly with an aqueous alkaline earth metal
LAW orrlcr~
FINNEGAN, HENDE~ON, hydroxide, such as aqueous calcium hydroxide, e.g., in a
FARABOW, GARREl-r
~D~NNE~,LLP slurry, to regenerate the base resin to the hydroxy form. The
1300 ~ STF~EET, N. W.
WASHINGTON, OC 20005
202-40~-4000
~180034
resin is subjected to a wash to remove entrained alkaline
earth metal hydroxide solids, such as calcium hydroxide, and
then a displacement wash to remove alkaline earth metal
chloride brine, such as calcium chloride. The displacement
wash may include use of sodium sulphate brine. The
regenerated resin is returned to absorption of sulphuric acid
from the anolyte.
The present invention also relates to a process wherein
the base resin in the hydroxy form is used to absorb sulphuric
acid from the anolyte as described above to produce alkali
metal hydroxide with subsequent treatment of the base resin by
ammonia, preferably in solution, to regenerate the base resin
to the hydroxy form and produce a solution of ammonium
sulphate. As in the preceding process, the base resin in the
hydroxy form is given a displacement wash to remove liquors
(primarily ammonium sulphate and ammonia solution) entrained
in the resin. The displacement wash may include the use of
solution of dilute caustic soda. The ammonium sulphate
solution can be brought up to higher concentrations by
additions of ammonia, e.g., in solution, and contacted with a
fresh bed of resin in the sulphate form. The solution can be
evaporated, the solids (e.g., ammonium sulfate) separated, and
dried. Ammonia can also be used to regenerate the resin from
the chloride form to the hydroxy form as well.
Additionally, the concentrated ammonium sulphate solution
L~W orrlcE~
FINNEGANHENDERsoN is passed over a potassium laden cation ion exchange resin to FMAsow, GARRETT
~D~ERLLP produce potassium sulphate brine preferably of high
1300 ~ 5TREET, N. W
W~S~II~IGT0~, DC ZOOO~S
20Z 40e-4000
" 2180034
concentration which can be processed in a manner similar to
the salt-out crystallizer cited above to yield potassium
sulphate. The resin now in the ammonium form is regenerated
in an alkali metal chloride, e.g., potassium chloride, which
then subsequently provides a resin in the potassium form and
forms ammonium chloride. The ammonium chloride brine can be
treated with an alkaline earth metal hydroxide such as calcium
hydroxide or magnesium hydroxide to regenerate the ammonia.
Strong or weak base resin in the chloride form can be
regenerated in two ways. In one approach, the resin is
regenerated with lime which will yield a resin in the hydroxy
form. The second approach is regeneration with ammonia. This
will form ammonium chloride solution and yield a resin in the
hydroxy form. The ammonium chloride solution can then be
treated with lime to produce ammonia and calcium chloride
brine. Lime based hydroxide ion for regeneration is likely to
be the most economic method to carry out the overall process.
Magnesia or partially calcined dolomite can also be employed
if warranted by economics. Other regenerants such as caustic
soda could be employed but normally this would not be economic
within the context of electrolytic production of caustic soda.
The process can be placed on recycle of sodium sulphate by use
of sodium rather than potassium chloride. The above process
conditions have application in two compartment cells because
the base resin in contact with the anolyte acts only to strip
~w o~r~c~s
FINNECAN,HENDE~ON, acidity (reduce acidity) and leaves sodium sulphate in
FARABOW, GARRErE
8 DUNNER, L. L.P.
1300 I STF?EET, N. W
WA5 1~1 ~JGT01~, 0 C 200 OS
20Z 40e - 4000 - 6-
- ~18003~
solution which is desirable for operation of lower cost two
compartment cells.
The present invention may also be applied to electrolysis
of sodium sulphate using three compartment cells wherein
sulphuric acid in the anolyte compartment is processed as
above with an ion exchange resin to yield potassium sulphate
and calcium chloride. These methods can be preferred when low
levels of sulphuric acid in the anolyte are desired for
reasons of minimal back migration of protons, high current
efficiencies, high quality caustic, and valuable co-products.
In an alternative approach, a cation resin may be used to
process acidic anolyte by passing the anolyte over a bed of
cation resin in the potassium form. In this manner, protons
from the acid liquor exchange with potassium ions, reducing
acidity. This results in the formation of a potassium
sulphate rich anolyte. This can be taken up to 120-180 g/l of
potassium sulphate at about 50~ to about 80~C. This liquor
can be bled from the system to an evaporative circuit with
recycle of water or the potassium sulphate brine can be cooled
and potassium chloride added to salt out the potassium
sulphate. The potassium sulfate can be separated out, dried,
and formulated into product by various means. The cation
resin in the hydrogen form can be treated with a base such as
aqueous ammonia, calcium hydroxide, magnesium hydroxide, or
partially calcined dolomite. The resin is then treated with
~AW or~lc~,
FINNEGAN,HENDERSON, potassium chloride to regenerate the resin. If ammonia is
FARABOW, GARRETT
~ ~UNNER,L.L.P. used with the anolyte to produce ammonium sulphate liquor,
1300 1 5TREET, N. W.
WASI~INGTON, OC Z0005
202 408-'~000
- ~180034
this can be contacted with the cation resin in the potassium
form to produce potassium sulphate liquor which can be
processed as above to form solid potassium sulphate. The
cation resin now loaded with ammonium ion is treated with
potassium chloride to regenerate the resin to the potassium
form and the ammonium chloride is treated with calcium
hydroxide to release ammonia for recycling and to produce
calcium chloride. It is evident that ammonium sulphate and
potassium chloride can be reacted directly without the use of
ion exchange.
It is to be understood that both the foregoing general
description and the following detailed description are
exemplary and explanatory only and are not restrictive of the
invention, as claimed.
The accompanying drawing, which is incorporated in and
constitutes a part of this specification, illustrates one
embodiment of the invention and together with the description,
serve to explain the principles of the invention.
BRIEF DESCRIPTION OF THE DRAWING
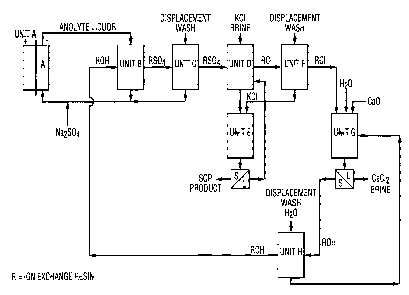
The Figure is a schematic diagram of an embodiment of the
process of the present invention.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The Figure is a schematic diagram of an embodiment of the
L~W orrlcr~
FINNEGAN, HENDER50N, present invention. Unit A is an electrolytic cell. Anolyte
FARAEOW, GARRE~
~ ~NNER,L.L.P. is withdrawn from the cell and passed through an anion ion
1300 ~ STF~EET, N. W
WAS~lNGTON~ ~C 20005
202-40~-~ooo --8--
- 2180034
exchange resin bed in the hydroxy form, Unit B, to reduce
acidity. The exhausted resin is given a displacement wash at
Unit C. The resin is then treated at Unit D with potash brine
to produce potassium sulphate for crystallization at Unit E.
The resin is given a displacement wash at Unit F and
regenerated to the hydroxy form with an alkaline earth metal
hydroxide, e.g., calcium hydroxide at Unit G which also forms
an alkaline earth metal chloride brine, e.g., calcium chloride
brine. The resin is given a displacement wash at Unit H
whereupon the resin is returned to reduction of acidity if the
anolyte.
The processes of the present invention provide improved
methods for utilizing non-chloride alkali metal salts, such as
sodium sulphate and potassium sulphate and the like, in
electrolysis to produce an alkali metal hydroxide such as
caustic soda and other valuable products, which do not contain
chlorine. For example, with sodium sulphate or potassium
sulphate as a feedstock to the electrolysis cell, a base resin
in the hydroxy form can be used to remove the required amount
of sulphuric acid from the anolyte liquor such that improved
cell current efficiencies are achieved. The anolyte is
preferably replenished with additional alkali metal sulphate
and returned to the anolyte compartment where additional
electrolysis occurs to form or produce alkali metal hydroxide
in the cathode compartment. Control of the acid level is
~AW orrlc~s
FINNEGAN, HENDE~ON, achieved through the volume of anolyte contacted with the
FAR~BOW, GARRE1T
~D~NNER,LLP hydroxy form of the base resin per unit time. The base resin,
~300 I STREET, N. W
WASNINGTON, DC 20005
202-40~3-4000
- ~180034
now in the sulphate form, can be treated directly with alkali
metal chloride (e.g., potassium chloride) to yield an alkali
metal sulphate (e.g., potassium sulphate), a valuable product.
The base resin, now in the chloride form, can then be directly
regenerated to the hydroxy form with an alkali earth metal
hydroxide (e.g., calcium hydroxide) to yield an alkali earth
metal chloride (e.g., calcium chloride). Alternatively, the
base resin in the chloride form can be treated with ammonia to
yield ammonium chloride. The ammonium chloride can then be
treated with a base such as calcium hydroxide and the like to
yield ammonia for recycle and calcium chloride. The
regeneration system produces base resin in the hydroxy form
for absorption of additional sulphuric acid.
U.S. Patent Nos. 4,504,458 and 4,707,34?, and European
Patent Application No. 86103871.9 (EP 0 199 104 A2), all
incorporated herein by reference, mention that sulphate may be
loaded on a base resin in the chloride form by means of
calcium, magnesium, and sodium sulphate brines, in solutions
of varying acidity. However, these processes have limitations
because sulphate only effectively displaces chloride ion on a
base resin within a dilute concentration range. Outside this
range, mixed brines are formed requiring expensive recovery
and separation methods to achieve effective operation.
Furthermore, it is not taught that under acid conditions,
acidity can build up in the production zone and steadily
L~W orr~c~
FINNECAN, HENDERSON, reduce productivity if not controlled.
F~RAsow, GARRErr
~ Dl.'N~ER, L. L. P.
1300 ~ STREET, N. W
W~SIIINGTON, ~C 20005
Z02 40~-4000
-10-
'-- 2180034
In the present invention, a base resin in the chloride
form is first placed in the hydroxy form, by contact with an
alkaline earth metal hydroxide base such as calcium hydroxide
or aqueous forms thereof. The base resin in the hydroxy form
can then be readily and effectively loaded with sulphate ions
for the eventual production of potassium sulphate by
contacting the hydroxy form of the base resin with sulphuric
acid solutions, such as those found in anolytes of
electrolytic cells used for the production of caustic soda
from sodium sulphate.
According to a preferred process of the present
invention, anolyte typically containing about 10~ to about 40
sodium sulphate is electrolyzed at temperatures in excess of
50~C to produce caustic soda in the cathode compartment and
sulphuric acid in the anode compartment. The acidic anolyte
is contacted with the base resin in the hydroxy form to reduce
acid levels and thereby load the base resin in the sulphate
form. Before contact, steps must be taken to ensure that any
residual chlorine or hypochlorous acid is destroyed to prevent
damage to the resin. An agent suitable for this purpose is
sodium sulphite although those skilled in the art will
recognize that other agents can be used. The produced
chloride ion is absorbed by the base resin in the sulphate
form and is taken out of the system. Similar means may be
used to polish sodium sulphate feedstocks.
L~W orr-c~
FtNNEGAN,HENDE~ON, This contact may be carried out in several different
FARABOW, GARREl~'
~D~NNERLLP means including a stirred batch type reactor, a stirred
1300 I STFIEET, N. W.
WAS~INGTON, OC 20005
202-~0~-4000 - 11 -
!, _
180034
~co-current reactor, a conventional column type absorption
reactor or a moving bed of resin contacted with sprays of
anolyte. The type of contact process selected depends upon
the preferred steady state acid concentration of the anolyte
leaving the cell required for the effective and efficient
operation of the electrolytic cell, the required contact time,
and the volume per unit time of anolyte required to be acid
stripped by a given volume or equivalent of base resin.
In general, stirred contact reactors can be employed when
volumes of anolyte and resin are similar. Contact time is a
factor since neutralization of- acid with a base resin can be
significant in this mode. When anolytes need to be maintained
at less acidic conditions, due to operational requirements of
the cell, moving anolyte through a bed of resin in some manner
is preferred since this minimizes contact time needed between
resin and anolyte.
With a stirred batch reactor, acidic anolyte is added to
a base resin in the hydroxy form such that the pH of the
resin/anolyte slurry does not go below a pH of about 4 or 5.
The resin is now loaded in the sulphate form. At this point
the anolyte is drained and moved to an anolyte holding tank
for recycling and use in the cell. Additional purified sodium
sulphate is added as needed to the anolyte tank for proper
cell electrolysis conditions and this liquor is then returned
to the anolyte compartment in the cell.
LAW orrlcr~
FINNEC~, HENDERSON, The resin in the batch reactor is slurried to a
FMASOW, GARRETT
~ D~NNER,L.L.P. displacement washing device such as a pan filter, horizontal
1300 ~ 5'rREET, N. W.
WASIIINGTON, DC 20005
ZOZ- 408 - ~000 -12-
21 ~003~
vacuum filter or a fixed bed column wherein the resin is
washed, further processed as needed, and removed for further
processing, such as with a Himsley device noted in Perry's
Chemical Engineers' Handbook; the description of this device
is incorporated herein by reference.
With these devices, a displacement wash is carried out
wherein an amount of wash water, typically equal to 0.2 to 0.3
bed volumes of resin, is added to hydraulically displace or
force out anolyte entrained in the resin. This minimizes
carry over of sodium sulphate and residual acidity into the
alkali metal sulphate (e.g., potassium sulphate) production
circuit. In the case were there is significant residual
acidity in the resin, the displacement wash can first be
carried out with concentrated neutral sodium sulphate brines
and then followed with a water displacement wash.
A conventional fixed bed absorption column may be used to
reduce acidity in anolytes. This works quite effectively in
reducing acidity and produces anolyte effluent that is non
acidic until the capacity of the bed is exhausted. However,
a consideration with column absorption is that the column is
entrained with acidic anolyte when the resin bed acid
absorption capacity has been exhausted. As well, the bed
contains sodium sulphate in the entrained liquor.
Regarding entrained sodium sulphate, significant carry
over to the potassium sulphate production zone may not be
LAW OFI'IC~g
FINNEcANHENDER50N desirable under most circumstances. Carry over of sodium
FAR~sow, GARRErr
~ D~NER,L L.P. sulphate entrained in the base resin to the potassium sulphate
13O0 ~ 5~EET, N. W.
WASHlNGToN~ DC Z0005
Z02 - 40~- 4000 -13-
2180034
production zone is not in itself a problem. The processes of
the alkali metal sulphate (e.g., potassium sulphate)
production zone can accommodate sodium sulphate without
affecting the ion exchange production process provided that a
sodium bleed is provided for in an evaporator used for volume
control in the alkali metal sulphate (e.g., potassium
sulphate) production zone. However, carry over of large
amounts of sodium sulphate is not desirable since it is an
expensive feedstock for the electrolytic process. The sodium
sulphate is preferably removed by a displacement wash.
With respect to acidity, the pH of the residual anolyte
entrained in the resin can be quite acidic and in the range of
a pH of about 0.6 when the acid content of the anolyte exiting
the cell is in the range of 50 g/l of sulphuric acid. It can
be difficult to wash this acidity out with water alone,
particularly when using a weak base resin.
Acidity entrained with the resin can be transferred into
the salt out crystallizer or crystallizer from the resin when
the resin is treated with an alkali metal chloride (e.g.,
potassium chloride). A displacement wash with concentrated
sodium sulphate brine can be used to reduce acid transport to
the potassium sulphate crystallizer.
Acid entrainment by the resin in fixed bed columns is
more severe with the use of a weak base resin rather than a
strong base resin due to absorption or complexing of sulphuric
~AW orrlcr~
FINNECAN, HENDE~ON, acid with the weak base resin. With the weak base resin, and
F~sow, G~RRE~
~D~NNE~LLP depending upon the extent of water washing, as much as 20~ to
1300 I ST~EET, N. W
V~lASlllNGTON~ DC 2000~i
20Z - 4O~- ~000
-14-
~oo~
40% of the sulphate ion in the crystallizer can be due to
entrained acidity.
If acidity builds up in the salt out crystallizer, this
would gradually reduce the efficiency of the alkali metal
sulphate (e.g., potassium sulphate) production step since
undesirable and highly soluble potassium bisulphate and/or HCl
can be produced. Knudsen, U.S. Patent No. 4,504,458
(incorporated herein by reference), does not teach this nor
does this patent teach that acidic double salts of potassium
sulphate can be formed. Therefore, the process of Knudsen
would be unsuitable for this type of application.
Control of acidity levels in the alkali metal sulphate
(e.g., potassium sulphate) production zone can be carried out
by including a buffering system connected to the salt out
crystallizer whereby clarified liquor from the crystallizer is
passed through a reactor containing a cation exchange resin in
the potassium form. The cation resin absorbs acidity and
releases potassium into solution to form potassium sulphate.
This avoids the need to add expensive bases like potassium
hydroxide.
The cation resin in the hydrogen form can then be
converted to a calcium form with a hydrated lime slurry and
the resin can then be regenerated with potash to yield the
starting resin. In this manner, acid values originating from
the anolyte brine can be managed, particularly if upsets in
~w orrlc~s
FIN~EGAN,HENDERSON, the system occur. Base resin in the hydroxy form could also FARA~OW, GARRE~
~D~NER,LLP be used to adjust the acidity of the crystallizer circuit.
1300 I STREET, N. W
WASIIINGTON, OC Z0005
20Z - 40~ - ~000 -15-
2i 80U3~ ;
A preferred base resin desirably absorbs the full acid
equivalency above pH 4. The exchange of hydroxy groups on
strong base resin for sulphate is generally near completion
above pH 6 and this resin is preferred when used in
conjunction with aqueous ammonia. With a weak base resin, an
acceptable operating endpoint is usually lower. With weak
base resin such as Rohm and Haas IR-93, endpoints are in the
range of pH 4-5 leading to suitable working capacities.
When a high ratio of anolyte to resin, such as 10 to 1,
20 to 1, or higher, is used to maintain low levels of acidity
in the anolyte compartment, the natural flow rate, in terms of
USGPM, through batch reactors or fixed bed columns becomes
difficult.
With fixed bed columns, pressurization is needed and even
then, the geometry requires such a high ratio of diameter to
height that it is less than desirable for the plug flow
conditions needed for the remainder of the process wherein
potassium sulphate is produced. While removing the resin to a
more appropriate ion exchange device for further processing
can be done, there is a more convenient option.
An alternative approach to stripping acid from anolyte is
to employ a high surface area liquid/solid contacting device
such as a horizontal belt filter or pan filter. For
continuous operation, a horizontal belt filter equipped with
multiple spray bars may be used whereby base resin in the
~w Ol'~lC~g
FINNEC~,HENDERSON, hydroxy form is slurried on to the belt and the resin cake is FARABOW, GARRETT
~ ~NNER,L.L.P. sprayed or eluted with anolyte to load the resin in the
1300 I STQEET, N. W
WAs~llNGToN~ l~c ZOOOS
ZOZ 403-4000
-16-
218~039
sulphate form. The anolyte may be passed over the bed one or
more times in a counter current or co-current mode to ensure
effective acid stripping and thereafter washed. For high
anolyte to resin volumes, resin bed depths may be on the
shallow side for best stripping efficiencies when the belt is
operated in a single pass mode for high anolyte to resin
volumes.
In a multiple pass or recycle mode for resin employed to
achieve more suitable anolyte to resin volumes, one or more
belt filters may be run in a continuous batch mode wherein
resin is passed over the belt several times in a thicker layer
from at least about 2 inches, more preferably from about 2 to
about 18 inches under successive anolyte sprays, preferably in
the countercurrent mode, such that the pH of stripped anolyte
rises to an appropriate level of a pH 4-6 or more. When the
acid stripping capacity of the bed is exhausted, it is sent to
the potassium sulphate production zone and fresh base resin in
the hydroxy form is placed on the horizontal belt device.
Operations in this manner increase the contact time
between the resin and anolyte, which is desirable, and yield
increased resin bed depths which is known to be desirable by
practitioners of the arts in conducting effective ion exchange
processes such as stripping.
Those skilled in the art will recognize from the present
invention that use of a horizontal belt filter to carry out
~w orrlca3
FINNECAN, HENDE~ON, ion exchange in the foregoing manner can be extended beyond an
F~RABOW, GARRETT
1300 ~ ST~ET, N W. acid stripping operation to production, washing and
WA5~INGTON, DC 20005
z02-.Oe-4ooo -17-
- 2180Q~4
regeneration steps as used in other types of devices listed in
the Encylodpedia of Chemical Technology or Perrys' Chemical
Engineers' Handbook.
Use of two horizontal belt filters, one for production
and one for regeneration, with appropriate sprays for
production liquor, regeneration liquors, and wash sprays,
offers an alternative approach that separates out individual
unit operations in ion exchange rather than combining them in
a single device. This separation allows processing
flexibility and close control that permits optimization of
individual steps in a simple, open and continuous manner.
The shallower than normal bed depth can be compensated
for by multiple passes of the resin over the belt as needed
along with recycle of liquors. This could be termed a moving
bed, horizontal moving bed, or a thin layer bed.
In a preferred method of the present invention, once the
anolyte has been stripped by contact with base resin in the
hydroxy form, the sulphate loaded base resin, which has had a
displacement wash with sodium sulphate brine and then water to
remove entrained anolyte, passes into the potassium sulphate
production zone. The sulphate loaded resin and brine of
potassium chloride and potassium sulphate, saturated with
respect to each other at about 25~C to minimize potassium
sulphate content, are contacted at about 25~C to about 95~C
but preferably at about 50~C to about 75~C in an ion exchange
~AW OrFlC~
FINNECAN, HENDE~ON, reactor such as a fixed bed column or a moving bed device.
FAR~BOW, GARRErr
~ DUNNER,L.L.P.
1300 I STREET, N. W.
WA51-IINGTON, DC 20005
202-40~--1000
-18-
- 21800~4
The volume of anolyte to resin is preferably in the range
of two to one. In one arrangement of the process, the exit
anolyte, rich in potassium sulphate, is passed through a flash
cooler and then on to one or more crystallizer tanks wherein
solid potassium chloride is added to saturate the brine at or
near room temperature. Potassium sulphate, also known as
sulfate of potash (SOP), is precipitated out during the
addition of potassium chloride to the process. SOP is
separated from the liquor, sent to the dryer, and then to a
granulation circuit for formulation into granular product.
High grade SOP product can be made by repulping
centrifuged potassium sulphate crystals in potassium sulphate
brine, centrifuging, then drying the product. Alternatively,
a potassium sulphate wash on the centrifuge may be employed.
The chloride ion content of the product that has been treated
in this manner is 0.05%.
The salt out crystallizer mother liquor which is rich in
potassium chloride is made ready for the production step by
heat exchanging the brine at about 50~C to about 70~C prior to
contact with the resin. The design contains a provision for
evaporative capacity to control circuit volume. The
evaporative load can range between 1-3 tons of water per ton
of product although lower evaporative loads can be achieved by
using a displacement wash of potassium chloride amounting to
about 0.2 to 0.3 bed volumes of base resin in the sulphate
L~W orrlcrg
FINNEC~N,HENDE~ON, form to displace wash water entrained in the resin as it
FARABOW, GARRErP
1300 I ST~EÉT', N. W enters the potassium sulphate production zone. In an
W~SHINGTON, DC 20005
202 40~-4000
-19-
- 21~0034
alternate process configuration, a conventional crystallizer
can be used to recover potassium sulphate from the production
liquor.
When the base resin in the sulphate form is treated with
hot liquor containing primarily potassium chloride, the resin
is converted to the chloride form. A displacement wash is
used to remove potassium liquors entrained in the resin. This
is sent to the production brine circuit.
The base resin in the chloride form can be converted to
the hydroxy form by slurrying the resin into a stirred reactor
at about 50~ solids and adding typically 5-7~ excess hydrated
lime to the slurry in a controlled manner so that the rate of
addition does not significantly exceed the rate of
consumption. Partially or fully calcined dolomite may be used
in place of lime. U.S. Patent No. 4,708,804 (incorporated
herein by reference) does not teach that hydrated lime can be
used in this manner nor does it teach that chloride ion, which
is difficult to displace from base resin, can be removed in
this manner and the resin placed in the hydroxy form.
After treatment with a hydrated lime slurry, the resin
and the residual hydrated lime slurry are separated. The base
resin in the hydroxy form is thoroughly washed in a
conventional manner to clean the resin surfaces and by a
displacement method to ensure no calcium carryover. The
displacement wash can include the use of 0.2 to 0.3 bed
LAW orrlc~s
FINNECAN, HENDERSON, volumes of hot brine rich in sodium sulphate. Resin is then
FARA80W, GARRE~
6 DUNNER, L. L P.
1300 ~ STREET, N. W
WASH~NGTON, OC 2000~5
Z02 40e-'-000
-20-
- 2:18003~
returned to duty in the absorption of sulphuric acid from the
anolyte liquor.
The hydrated lime slurry is sent to a settling tank and
some material is sent for recycle and some is discharged. The
settling circuit contains a bleed for calcium chloride brine.
This brine can be concentrated up for sales by conventional
evaporation as outlined in the Encyclopedia of Chemical
Technology (lst Ed.). Alternatively, initial concentration
steps can be achieved by cation exchange using sodium chloride
or reverse osmosis.
In an alternative method of regeneration, ammonia is used
as the working base and lime is used to regenerate ammonia.
In this method, base resin in the chloride form leaving the
production zone for regeneration is contacted with aqueous
ammonia. This can be done using a fixed bed column that is
capable of resin removal such as the Himsley device or a
stirred reactor can be used wherein ammonia gas is sparged
into a higher density solids/liquids slurry of resin and
water.
In contacting resin with ammonia solution in a column,
there is a better opportunity to achieve higher ammonium
chloride solution concentrations than with a stirred reactor.
With a fixed bed, a displacement production volume of aqueous
ammonia in an amount equal to 0.2 to 0.3 bed volumes of resin
can be introduced and taken out of the column by displacement
~AW O~IC'S
FINNEGAN,HENDERSON, washes of water. In either case, ammonium chloride liquor is
FARABOW, GARRErr
6 DU~N~R,L.L.P.
~300 I ST~EET, N. W
WAS~INGTON, DC Z0005
202 40~ 4000
-21-
~1 8003~
produced and the base resin is transformed from the chloride
form to the hydroxy form.
Care may need to be taken to remove residual ammonia
values from the resin before it is returned to acid absorption
from anolyte so ammonia values do not end up in the cell or
lost to other parts of the circuit. This can be accomplished
by a displacement wash of water, followed by one of sodium
sulphate and/or dilute caustic soda. The resultant ammonium
chloride brines can be treated with hydrated lime to release
ammonia for recycle and generate calcium chloride brines for
concentration to saleable products.
The individual method chosen for regeneration of base
resin in the chloride form to the hydroxy form depends upon
several factors. Weak base resin in the chloride form
regenerates better than strong base resin and hydrated lime or
ammonia may be used to regenerate weak base resin. Ammonia
solutions can be used to generate higher initial
concentrations of calcium chloride than direct use of lime and
this is beneficial in economically concentrating calcium
chloride brines to saleable products.
Lime is not very effective in regenerating strong base
resin from the chloride form to the hydroxy form; however the
higher levels of hydroxide with ammonia solution over hydrated
lime enable strong base resin to be regenerated with ammonia
solution, preferably in a column type operation where a
~AW O~IC~S
FINNE~AN,HENDE~ON, displacement volume of aqueous ammonia equal to about 0.2 to
FAR~OW, GARRE~
~ DUNNER,L.L.P. O . 3 bed volumes is used to remove chloride ion from the resin.
1300 I STREET, N. W.
WA5~INGTON, DC Z0005
202 - '~0_- ~000 --22--
~ 2'1gO03~ ~
Strong base resin in the hydroxy form can be usefully employed
to absorb sulphuric acid from anolyte since strong base resin
generally is neutralized by acid at higher pHs than weak base
resin.
EXAMPLES
A number of experiments were carried out whereby
solutions of 50 g/l of sulphuric acid at 50-70~C, with and
without sodium sulphate, and simulating anolyte liquor, were
contacted with base ion exchange resin in the hydroxy form in
a fixed bed column to strip the sulphuric acid from the
solution and load sulphate ion on the base resin. Elution of
the resin was stopped when the pH of the effluent liquor
declined from basic values and reached a pH of 4-6. The base
resin was then treated with a hot potash brine at about
50-70~C which had been prepared from a solution saturated with
respect to potassium chloride and potassium sulphate at room
temperature. The eluant was cooled to crystallize out
potassium sulphate and further potassium sulphate was
precipitated by the addition of potassium chloride to saturate
the brine with respect to each potassium salt. The amount of
potash added varied with dilution due to water entrained in
the resin, wash water and pH of the production brine. This
liquor was then used as a production brine for another trial.
The resin in the chloride form was then regenerated to the
L~W OrFlC~9
FINNECAN,HENDE~ON, hydroxy form by making up a 50~ slurry of resin with water.
FARAsow, CARRE~
~ DUNNER,L.L.P. Calcium hydroxide was then added in portions, with stirring,
1300 I STQEET, N. W.
W~SH~NGl'ON, DC 20005
202 408-4000
-23-
' ~ 2180034
' until the slurry became permanently cloudy. The results are
contained in Table 1 using about 0.65 L of IR-93 weak base
resin.
TABLE 1
Volume (L) Pota~sium
of Anolyte Sulphate
50 g/l Thermal Calcium
Sulphuric Step Hydroxide
Acid (q) (~)
A. 1.3 34 30
B. 1.05 39 30
C. 1.10 45 26
D. 1.1 44 26
E. 1.1 45 24
With Run E, a full material balance was taken. The
amount of potassium sulphate recovered through salt out as
potassium chloride was added to the cooled production brine
amounted to 10.8 g for a total yield of 55.6 g of potassium
sulphate. A density gauge was used to indicate the saturation
point of potassium chloride addition. The amount of potassium
chloride added was 57 g.
On average about 0.55 moles of acid was absorbed and
entrained in the resin producing about 0.32 moles of potassium
sulphate through the addition of 0.77 moles of potassium
chloride while about 0.37 moles of calcium hydroxide was
needed to regenerate the resin. The results are consistent
indicating that the regeneration step works effectively and
LAwOr~,c~5 consistent yields of potassium sulphate are obtained.
FINNECAN, HENDERSON,
FAR~OW,GA~ Entrained acid, as measured by titration with KOH solution,
~i DIJ'NNE~, L, L. P.
WA5~1NGTON, DC Z0005 can be significant amounting to about 40~ of total acid
Z02- 40~3-4000
-24-
- 2~8~34
absorbed when this is carried out in a fixed bed column.
Entrained acid can be partly washed out or neutralized in the
crystallizer mother liquor or this can be minimized by a
displacement wash with sodium sulphate solution. With sodium
sulphate added to the acidic anolyte to simulate a cell exit
solution, the initial sodium level was 12,200 ppm while it was
12,400 ppm after being passed over the weak base resin. This
indicates little or no interference due to sodium sulphate
with the acid stripping process. In a measurement to examine
potential calcium carryover from the lime regeneration step
and thereafter into the cell anode compartment, the initial
calcium level for the anolyte was 25 ppm while the effluent
level was 22 ppm, indicating little change as anolyte passed
over the resin.
In another type of contact between anolyte and resin
whereby anolyte is added to base resin in the hydroxy form
with agitation, the addition of 0.5 equivalents of acid to 0.6
equivalents of resin dropped the pH of a resin/water slurry
from about 10.8 to 4.6 using IR-93 resin and the like. Ion
exchange resins are also described in Encyclopedia of Polymer
Science and Eng'r., Vol. 8, pp. 341-393 (1985), which is
incorporated herein by reference. Selection of a base resin
that exhausts most of its acid absorption capacity by pHs
above 4-5 is desirable. Resin could be regenerated with
ammonia to a pH of about 9.0 before a type buffering set in.
LAW OrCICf5 It will be recognized that these examples are indicative
FARABOW, GARRETr
~ 3WNER,L.L.P. of how base ion exchange resin can be usefully employed to
1300 I STI~cEr, N. W
W~SHINGTON, OC 20005
20Z - ~-0~ 000
-25-
- ~1 80034
remove sulphuric acid from the anode compartments of two and
three compartment cells used in the production of caustic soda
from sodium sulphate.
In view of the present invention, it will be evident to
those skilled in the art that while acid stripping from
anolyte is operationally simpler with higher acid levels in
the anolyte leaving the cell, the nature of cell membranes,
with two compartment cells in particular, is such that lower
levels of acidity may be preferred for high current
efficiencies. The process and equipment specified will allow
sufficient flexibility for overall economic operation.
In another manner of acid removal, cation resin may be
usefully employed with three compartment cells wherein a
potassium laden cation resin is contacted with anolyte. The
sulphuric acid is effectively converted to potassium sulphate
which can be built up to as high as 150-180 g/l, at
temperatures up to 95~C, and removed from the anolyte system
and processed to recover potassium sulphate by evaporation.
Use of strong acid resin typically yields a more acidic pH for
anolyte liquor than weak acid resin.
The choice of cation ion resin depends upon the most
desired operating and pH conditions for the anolyte
compartment. Weak acid cation resin is preferred for cells
requiring less acidic anolyte liquor for efficient
functioning. A two staged absorption may be employed whereby
~AW or~l c ~
FINNEG~,HENDE~ON, anolyte first contacts strong acid resin in the potassium form FAR~sow, G~RRErr
~ ER,L.L.P. and then weak acid cation resin in the potassium form.
1300 I ST~EET, N. W.
W~S~INGTON, DC Z0005
202 40~1-4000 --26--
21800~4
Contact of this resin with a base such as calcium hydroxide
neutralizes the acidity of the resin and loads the resin with
calcium. The resin can then be more effectively treated with
potassium chloride to regenerate the potassium form of the
resin.
U.S. Patent No. 3,096,153 (incorporated herein by
reference) uses cation ion exchange to produce potassium
sulphate from sulphuric acid; however, this method uses
- potassium chloride directly to regenerate the resin while the
present invention uses first a base and then potassium
chloride, which is more efficient in terms of minimizing
losses of costly potassium chloride. Potassium ion is not
very effective in displacing hydrogen ions from cation resin.
Processes have been presented whereby two or three
compartment cells may be employed to electrolyze non chloride
alkali metal salts such as sodium sulphate or potassium
sulphate. The choice of cell type depends upon the
comparative capital and operating cost of each and
consideration of factors related to cell operations such as
membranes, appropriate electrolyte concentrations, and
suitable circulation rates for anolyte.
Generally speaking, two compartment cells are less costly
in terms of capital and operating expense, and are therefore
preferred. However, with two compartment cells, it is more
difficult to select membranes and efficient cell operating
FINNEGAN,HENDERSON, conditions suitable for the higher acid anolyte conditions
FARABOW, GARRETT
~ D~ E~, L. L. P.
~300 ~ STi~EET, N. W
WASI~NGTON, DC 20005
202- ~01~ 000
-27-
2180034
which are more desirable for balanced volume contact between
anolyte liquor and resin.
L~W o~rlcr~
FINNEGAN, HENDERSON, .
FARAEOW, GARRErT
li D UNN ER, L. L. P .
1300 I STREET, N. W.
WAS~INGTON, OC 20005
20Z - ~0~ - "000
-28-