Note: Descriptions are shown in the official language in which they were submitted.
L -k
X181553
SPECIFICATION
Pyrrolopyridazine derivatives
(Technological field]
The present invention concerns pyrrolopyridazine
derivatives or pharmaceutically acceptable salts thereof
which have an excellent gastric juice secretion inhibiting
activity, gastric mucosa protective activity and an
excellent antibacterial activity against H ii oba ,-
pylori; and an anti-ulcer agent comprising these
derivatives or salts thereof as the active ingredient.
(Background technology]
It is said that peptic ulcer occurs when the balance
between the attacking factors to gastric mucosa and
defensive factors for gastric mucosa is lost. Inhibition
of gastric juice secretion which is one of the attacking
factora.fs useful for prevention and therapy of gastric
ulcer. Hitherto, as drugs effective for inhibition of
gastric juice secretion, anticholinergic agents and
histamine H2 receptor antagonistic agents such as
cimetidine etc., have been widely employed in clinic.
However, when a histamine H2 receptor antagonistic agent
has been used for therapy for a long period,~~recurrence of
ulcer during interrupted administration of the drug is a
serfous problem. Though the recurrence of ulcers is
_' 2 2181553
considered to be due to decreased defensive factors at the
site of gastric mucosa, its relationship with Helicobacter
pylori has been recently indicated. Accordingly, an
excellent anti-ulcer agent is desired, which strongly
inhibits gastric juice secretion, i.e., an attacking
factor, protects gastric mucosa and has an excellent
antibacterial activity against Helicobact~pylori.
As pyrrolopyridazine derivatives having a gastric
juice secretion inhibiting activity and gastric mucosa
protective activity, a compound shown below, for example,
has been known (WO 91/17164, WO 92/06979, WO 93/08190
etc.). However, its effects are not sufficient, and it
has been desired to develop a compound having more potent
activity.
[Disclosure of Invention]
In order to solve the problem mentioned above, the
present inventors studied eagerly the synthesis of
pyrrolopyridazine derivatives and their pharmacological
activities for many years, aiming at the development of an
218155
excellent anti-ulcer agent which strongly inhibits gastric
juice secretion, i.e., an attacking factor, protects
gastric mucosa and has an excellent antibacterial activity
against Hel;cobacter gylori. Our study resulted in
finding that pyrrolopyridazine derivatives having specific
substituents have an excellent antibacterial activity
against ~3elicobacter by~or~ as well as a strong gastric
juice secretion inhibiting activity and gastric mucosa
protective activity; and in completion of the present
inventipn.
(Constitution of Invention)
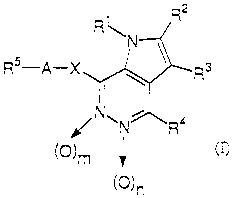
Pyrrolopyridazine derivatives of the present invention
have the general formula.
RS-A-X
(n
(O)n
In the formula above:
R1 represents a C2-C6 alkenyl group, a
halogeno-C2-C6-alkenyl group, a C6-C10
aryl-C2-C6-alkenyl group, a C2-C6 alkynyl group,
'1~~ 4 ~181~53
a C3-C~ cycloalkyl group, a (C3-C~ cycloalkyl)-
C1-C6-alkyl group, a (C5-C~ cycloalkenyl)-
C1-C6-alkyl group, or a halogeno-C1-C6-alkyl group;
R2 and R3 are the same or different, and each
represents a hydrogen atom, a C1-C6 alkyl group, or a
C6-C10 aryl group;
R4 represents a hydrogen atom, or a C1-C6 alkyl
group;
RS represents a C6-C10 aryl group, or a from 5-
to 10-membered heteroaryl group in which the hetero
atom.(s) are selected from the group.consisting of
nitrogen, oxygen and sulfur atoms;
A represents a C1-C3 alkylene group;
X represents an imino (NH) group, an oxygen atom, a
sulfur atom; or a methylene group;
m represents 0 or 1; and
n represents 0 or 1.
The C2-C6 alkeriyl group or the CZ-C6 alkenyl
' '~ 5 2181553
moiety of the halogeno-C2-C6-alkenyl group, and the _
C6-C10 aryl-C2-C6-alkenyl group included in the
definitions of R1 may be, for example: vinyl,
1-propenyl, 2-propenyl, isopropenyl, 1-butenyl, 2-butenyl,
3-butenyl, 1-methyl-1-propenyl, 1-methyl-2-propenyl,
2-methyl-1-propenyl, 2-methyl-2-propenyl, 1-pentenyl,
2-pentenyl, 3-pentenyl, 4-pentenyl, 1-methyl-1-butenyl,
2-methyl-2-butenyl, 3-methyl-2-butenyl, 1-hexenyl,
2-hexenyl, propan-1,2-dienyl, butan-L,2-dienyl,
pentan-1,2-dienyl or hexan-1,2-dienyl; preferably a
C2-CS alkenyl group (particularly, vinyl, 1-propenyl,
2-propenyl, isopropenyl, 1-butenyl, 2-butenyl, 3-butenyl,
2-methyl-2-propenyl, 2-pentenyl, 3-pentenyl,
3-methyl-2-butenyl or propan-1,2-dienyl group); and more-
preferably a C3-C4 alkenyl group (particularly,
1-propenyl, 2-propenyl, 1-butenyl, 2-butenyl or
2-methyl-2-propenyl~group).
The halogeno-C2-C6-alkenyl group included in the
definitions of R1 may be, for example:
2,2-difluorovinyl, 3-fluoro-2-propenyl,
2-chlaro-2-propenyl, 3-chloro-2-propenyl,
3-bromo-2-propenyl,
~
6 2 ~ 815 5 .~
3-iodo-2-propenyl, 3,3-difluoro-2-propenyl,
2,3-dichloro-2-propenyl, 3,3-dichloro-2-propenyl,
2,3-dibromo-2-propenyl, 3,3-dibromo-2-propenyl,
4,4,4-trifluoro-2-butenyl, 5-fluoro-2-pentenyl or
6-fluoro-2-hexenyl group; and preferably
3-chloro-2-propenyl, 3,3-dichloro-2-propenyl or
4,4,4-trifluoro-2-butenyl group.
The C6-C1~ aryl moiety of the (C6-ClQ aryl)-
C2-C6-alkenyl group included in the definitions of
Rl,and the C6-Clp aryl group included in the
definitions of R2, R3 and RS may be; for example: a
phenyl group or a naphthyl group; and preferably a phenyl
group. The group may have substituent(s) optionally on
the ring, and the substituents may be, for example: a
C1-C6 alkyl group mentioned later; a C1-C6 alkoxy
group such as methoxy, ethoxy, propoxy, isopropoxy,
butoxy, isobutoxy, pentoxy or hexyloxy; a halogen atom
such as fluoro, chloro, bromo or iodo; a
halogeno-C1-C6-alkyl group such as fluoromethyl,
chlorometliyl, difluoromethyl, trifluoromethyl,
2-fluoroethyl, 2-chloroethyl; 2-bromoethyl, 2-iodoethyl,
2,2,2-trifluoroethyl, 3-fluoropropyl, 4-fluorobutyl,
' X181.553
5-fluoropentyl or 6-fluorohexyl; or a
halogeno-Cl-C6-alkoxy group such as fluoromethoxy,
difluoromethoxy, trifluoromethoxy, 2-fluoroethoxy,
2-chloroethoxy, 2-bromoethoxy, 2-iodoethoxy,
2,2,2-trifluoroethoxy, 3-fluoropropoxy, 4-fluorobutoxy,
5-fluoropentoxy or 6-fluorohexyloxy; preferably a
CI-CQ alkyl group, a Cl-C~ alkoxy group, a halogen
atom group or a halogeno-C1-C4-alkyl group; and more
preferably methyl, methoxy, fluoro or chloro for the group
included in the definitions of Rl, R2 and R3 and
methyl, methoxy, fluoro, chloro trifluoromethyl or
difluoromethoxy (particularly flupro or chloro) for the
group included in the difinition of R5.
The (C6-C1~ aryl)-C2-C6 alkenyl group included
in the definitions of R1 may be, for example:
2-phenylethenyl, 3-phenyl-2-propenyl, 4-phenyl-3-butenyl,
5-phenyl-4-pentenyl, 6-phenyl-5-hexenyl,
3-methylphenyl-2-propenyl, 3-methoxyphenyl-2-propenyl,
3-fluorophenyl-2-propenyl, 3-chlorophenyl-2-propenyl or
3-naphthyl-2-propenyl; preferably 3-phenyl-2-propenyl,
4-phenyl-3.-butenyl, 5-phenyl-4-pentenyl,
2181553
, I
8
3-methylphenyl-2-propenyl, 3-methoxyphenyl-2-propenyl,
3-fluorophenyl-2-propenyl, 3-chlorophenyl-2-propenyl or
3-naphthyl-2-propenyl; and more preferably
3-phenyl-2-propenyl.
The C2-C6 alkynyl group included in the
definitions of R1 may be, for example; ethynyl,
2-propynyl, 2-butynyl, 2-pentynyl or 2-hexynyl; preferably
a C2-C4 alkynyl group; and more preferably 2-propynyl.
The C3-C~ cycloalkyl group or the C3-C~
cycloalkyl moiety of the (C3-C~
cycloalkyl)-C1-C6-alkyl group included in the
definitions of R1 may be, for example: cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl;
preferably cyclopropyl or cyclohexyl; and particularly
preferably cyclopropyl. The group may have optionally
substituent(s) on the ring; and the aubatituent may be,
for example: a CI-C6 alkyl group mentioned later;
preferably a Cl-C4 alkyl group; more preferably methyl
or ethyl; and particularly preferably methyl.
The (C3-C~ cycloalkyl)-C1-C6-alkyl group
' 9 2181553
included in the definitions of R1 may be, for example:
cyclopropylmethyl, methylcyclopropylmethyl,
cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl,
methylcyclohexylmethyl, cycloheptylmethyl,
2-cyclopropylethyl, 3-cyclopropylpropyl,
4-cyclopropylbutyl, 5-cyclopropylpentyl or
6-cyclopropylheptyl; preferably cyclopropylmethyl,
2-methylcyclopropylmethyl or cyclohexylmethyl; and
particularly preferably cyclopropylmethyl or
2-methylcyclopropylmethyl.
The (CS-C~ cycloalkenyl?-C1-C6-alkyl group
included in the definitions of R1 may be, for example:
cyclopentenylmethyl, cyclohexenylmethyl,
cycloheptenylmethyl, 2-cyclopentenylethyl,
3-cyclopentenylpropyl, 4-cyclopentenylbutyl,
5-cyclopentenylpentyl, 6-cyclopentenylhexyl,
2-cyclohexenylethyl, 3-cyclohexenylpropyl,
4-cyclohexenylbutyl, 5-cyclohexenylpentyl or
6-cyclohexenylhexyl; preferably cyclopenten-1-ylmethyl or
cyclohexen-1-ylmethyl; and more preferably
cyclopenten-1-ylmethyl.
The halogeno-C1-C6-alkyl group included in the
2181553
to
definitions of R1 may be, for example; fluoromethyl,
chloromethyl, difluoromethyl, trifluoromethyl,
2-fluoroethyl, 2-chloroethyl, 2-bromoethyl, 2-iodoethyl,
2,2-difluoroethyl, 2,2,2-trifluoroethyl, 3-fluoropropyl,
4-fluorobutyl, 5-fluoropentyl or 6-fluorohexyl; preferably
a halogeno-C1-C4-alkyl group; more preferably
difluoromethyl, 2-fluoroethyl, 2-chloroethyl,
2-bromoethyl, 2-iodoethyl, 2,2-difluoroethyl,
2,2,2-trifluoroethyl, 3-fluoropropyl or 4-fluorobutyl; and
particularly preferably 2,2,2-trifluoroethyl or ,
3-fluoropropyl.
The C1-C6 alkyl group included in the definitions
of R2, R3 and R4 may be, for example: methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, pentyl or hexyl;
preferably a C1-C4 alkyl group; more preferably methyl
or ethyl; and particularly preferably methyl.
The from 5- to 10-membered heteroaryl group having the
hetero atoms) selected from the group consisting of
nitrogen, oxygen and sulfur atoms included in the
definitions of RS may be, for example: pyrrolyl,
indolyl, furyl, benzofuryl, thienyl, benzothienyl,
oxazolyl, benzoxazolyl, isoxazolyl, benzisoxazolyl,
thiazolyl, benzothiazolyl,
a a
11 2181553
isothiazolyl, benzisothiazolyl, imidazolyl,
benzimidazolyl, pyrazolyl, benzopyrazolyl,
1,3,4-oxadiazolyl, 1,3,4-thiadiazolyl, pyridyl, quinolyl,
isoquinolyl, pyrimidinyl, pyrazinyl or pyridazinyl;
preferably furyl, thienyl, oxzzolyl, benzoxazolyl,
thiazolyl, benzothiazolyl, imidazolyl, benzimidazolyl,
1,3,4-oxadiazolyl, 1,3,4-thiadiazolyl, pyridyl, pyrazinyl
or pyridazinyl; and more preferably furyl, thienyl or
pyridyl. The heteroaryl group may have substituent(s) on
the ring and the substituent on the from 5- to 6-membered
hetero ring may be, for example: a C1-C6 alkyl group
or halogen atom mentioned above; particularly preferably
methyl, fluoro or chloro and the substituent on the phenyl
ring may be the same group as mentioned above for the aryl
group.
The C1-C3 alkylene group in the definition of A
may be, for example: methylene, ethylene, propylene or
trimethylene; and preferably methylene.
X may be preferably an oxygen.atom, a sulfur atom or a
methylene group; more preferably an oxygen atom or a
methylene group; and particularly preferably an oxygen
atom.
m may be preferably 0; and when m is 1, X may be
preperably a methylene group.
12 2181553
n may be preferably 0.
The compound having the general formula (I) mentioned
above can be converted, if necessary, to its
pharmaceutically acceptable salts. The salt may be,
preferably an acid addition salt, for example: a
hydrohalide such as hydrofluoride, hydrochloride,
hydrobromide or hydroiodide; a nitrate; a perchlorate; a
sulfate; a phosphate; a carbonate; a Cl-C~
alkylsulfonate such as methaneaulfonate,
trifluoromethanesulfonate or ethaneaulfonate; a C6-C10
arylaulfonate such as benzeneaulfonate or
p-toluenesulfonate; a carboxylate such as acetate,
propionate, butyrate, benzoate, fumarate, succinate,
citrate, tartarate, oxalate, malonate or maleate; or an
amino acid salt such as glutamate or asparate. In
addition, the scope of the present invention includes any
hydrate of Compound (I).
In Compound (I), there are optical isomers due to the
asymmetric carbon atoms) in the molecule, and/or
geometric .isomers due to the double bonds) in the
molecule in some cases. The scope of the present
invention covers all these stereoisomers and any mixtures
thereof.
~ 13 2181553
In the general formula (I), there may be mentioned as
a preferable compound:
(1) A compound in which R1 is a C2-CS alkenyl
group; a substituted C3-C4 alkenyl group with fluoro,
chloro or bromo; a C6 aryl-C3-CS-alkenyl group; a
C3-C4 alkynyl group; a cyclopropyl group; a C3-C6
cycloalkylmethyl group; or a halogeno-C1-C4-alkyl
group;
(2) A compound in which R1 is a C2-CS alkenyl
group; a C3-C4 alkenyl group substituted with fluoro
or chloro; a 3-(C6 aryl)-2-propenyl group; 2-propynyl
group; a cyclopropylmethyl group;
2-methylcyclopropylmethyl group; a cyclopeneten-1-ylmethyl
group; or a fluoro-C2-C3-alkyl group;
(3) A compound in which R1 is 1-propenyl, 2-propenyl,
1-butenyl, 2-butenyl, 2-methyl-2-propenyl,
propan-1,2-dienyl, 3-phenyl-2-propenyl, 2-propynyl,
cyclopropylmethyl, 2-methylcyclopropylmethyl,
cyclopenten-1_ylmethyl, 2,2,2-trifluoroethyl or
3-fluoropropyl;
14
(4) A compound in which R1 is 1-propenyl, 2-propenyl,
1-butenyl, 2-butenyl, 2-methyl-2-propenyl,
3-phenyl-2-propenyl, cyclopropylmethyl or
2-methylcyclopropylmethyl;
(5) A compound in which R2 and R3 are the same or
different, and each is a hydrogen atom, a C1-C4 alkyl
group or a C6 aryl group; '
(6) A compound in which R2 and R3 are the same or
different, and each is a hydrogen atom, a C1-C3 alkyl
group or a phenyl group;
(7) A compound in which R2 and R3 'are the same or
different, and each is a hydrogen atom or a C1-C2
alkyl group;
(8) A compound in which R2 and R3 are the same, and
each is a methyl group;
(9) A compound in which R4 is a hydrogen atom or a
C1-C4 alkyl group;
(10) A compound in which R4 is a hydrogen atom.or a
C1-C2 alkyl group;
(11) A compound in which R4 is a hydrogen, atom;
(12) A compound in which RS is a phenyl group
optionally substituted with Cl-C4 alkyl, C1-C4
alkoxy, halogen, halogeno-C1-C4-alkyl or
halogeno-C1-C4-alkoxy, a naphthyl group, a furyl
group, a thienyl group, an oxazolyl group, a benzoxazolyl
~5 2, 81553
group, a thiazolyl group, a benzothiazolyl group, an
imidazolyl group, a benzimidazolyl group, a
1,3,4-oxadiazolyl group, a 1,3,4-thiadiazolyl group, a
pyridyl group, a pyrazinyl group or a pyridazinyl group;
(13) A compound in which RS is a phenyl group
optionally substituted with methyl, methoxy, fluoro,
chloro, fluoromethyl, trifluoromethyl, fluoromethoxy or
difluoromethoxy, a furyl group, a thienyl group, an
oxazolyl group, a benzoxazolyl group, a thiazolyl group, a
benzothiazolyl group, an imidazolyl group, a .
benzimidazolyl group or a pyridyl group;
(14) A compound in which RS is a phenyl group
optionally substituted with methyl, methoxy, fluoro,
chloro, fluoromethyl, trifluoromethyl, fluoromethoxy or
difluoromethoxy, a furyl group, a thienyl group or a
pyridyl group;
(15) A compound in which RS is a phenyl group
optionally substituted with fluoro, chloro,
trifluoromethyl or difluoromethoxy;
(16) A compound in which RS is a phenyl group
optionally substituted with fluoro or chloro;
(17) A compound in which A is a methylene group;
(18) A compound in which X is an oxygen atom, sulfur atom
or methylene group;
(I9) A compound in which X is an oxygen atom or methylene
group;
(20) A compound in which X is an oxygen atom;
- 16 211553
(21) A compound in which m is 0; and
(22) A compound in which n is 0.
In addition, any optional combination of, from (1) to
(4), from (5) to (8), from (9) to (11), from (12) to (16),
(17), from (18) to (20), (21) and (22), may provide a more
preferable compound as mentioned below:
(23.) A compound in which:
R1 is an C2-CS alkenyl group; a C3-C4
alkenyl group substituted with fluoro, chloro or bromo; a
C6 aryl-C3-C5-alkenyl group; a C3-C4 alkynyl
group; a cyclopropyl group; a C3-C6.cycloalkylmethyl
group; or a halogeno-C1-C4-alkyl group;
R2 and R3 are the same or different, and each is a
hydrogen atom, a C1-C4 alkyl group, or a C6 aryl
group;
R4 is a hydrogen atom or a C1-C4 alkyl group;
. RS is a phenyl group optionally substituted with
C1-C4 alkyl, a C1-C4 alkoxy, halogen,
halogeno-C1-C4-alkyl or halogeno-C1-C4-alkoxy, a
naphthyl group, a furyl group, a thienyl group, an
oxazolyl group, a benzoxazolyl group, a thiazolyl group, a
benzothiazolyl group, an imidazolyl group, a
benzimidazolyl group, a 1,3,4-oxadiazolyl group, a
1,3,4-thiadiazolyl group, a pyridyl group, a pyrazinyl
group or a pyridazinyl group;
2181553
A is a methylene group;
X is an oxygen atom, sulfur atom or a methylene group;
and
when n is 1, m is 0;
(24) A compound in which:
R1 is an Cz-CS alkenyl group; a C3-C4
alkenyl group substituted with fluoro or chloro; a 3-(C6
aryl)-2-propenyl group; a 2-propynyl group; a cyclopropyl
group; a cyclopropylmethyl group;
2-methylcyclopropylmethyl group; a cyclopenten-1-ylmethyl
group; or a fluoro-CZ-C3-alkyl group;
R2 and R3 are the same or different, and each is a
hydrogen atom, an C1-C3 alkyl group, or a phenyl group;
R4 is a hydrogen atom or a C1-C2 alkyl group;
RS is a phenyl group optionally substituted with
methyl, methoxy, fluoro, chloro, fluoromethyl,
trifluoromethyl, fluoromethoxy or difluoromethoxy, a furyl
group, a thienyl group, an oxazolyl group, a benzoxazolyl
group, a thiazolyl group, a benzothiazolyl group, an
imidazolyl group, a benzimidazolyl group or a pyridyl
group;
A is a methylene group;
X is an oxygen atom, sulfur atom or a methylene group;
and
m is 0,~
2181553
18
(25) A compound in which:
R1 is a 1-propenyl, 2-propenyl, 1-butenyl,
2-butenyl, 2-methyl-2-propenyl, propan-1,2-dienyl,
3-phenyl-2-propenyl, 2-propynyl, cyclopropylmethyl,
2-methylcyclopropylmethyl, cyclopenten-1-ylmethyl,
2,2,2-trifluoroethyl br 3-fluoropropyl group;
R2 and R3 are the same or different, and each is a
hydrogen atom or a C1-C2 alkyl group;
R4 is a hydrogen atom or a C1-C2 alkyl group;
R is a phenyl group optionally substituted with
fluoro, chloro, trifluoromethyl or difluoromethoxy;
A is a methylene group;
X is an oxygen atom or a methylene group;
m is 0; and
n is 0;
(26) A compound in which:
R1 is a 1-propenyl, 2-propenyl, 1-butenyl,
2-butenyl, 2-methyl-2-propenyl, 3-phenyl-2-propenyl,
cyclopropylmethyl or 2-methylcyclopropylmethyl group;
R2 and R3 are the same, and each is methyl group;
R4 is a hydrogen atom;
R5 is a phenyl group optionally substituted with
fluoro or chloro;
A is a methylene group;
X is an oxygen atom;
m is 0; and
n is 0.
2181553
In Tables 1, 2 and 3 below, typical compounds of the
present invention are shown for example but these
compounds do not limit the scope of the present
invention. The compounds listed in Tables 1, 2 and 3 have
the structures of Compound (I=1), Compound (I-2) and
Compound (I-3), respectively.
RS-CH2-
(I-1)
R5-CHZ-
1
0
RS-CHz-
Q-3)
O
' ~ ~ 2p 2181553
[Table 1]
Example
Compd. No. R1 R2 R3 R4 RS X
1-1 CH=CH2 Me Me H Ph p
1-2 CH=CHCH3 Me Me H Ph p
1-3 CH2CH=CH2 Me Me H Ph p
1-4 C(CH3)=CH2 Me Me H Ph p
i-5 CH=CHCH2CH3 Me Me H Ph p
1-6 CH2CH=CHCH3 Me Me H Ph _ p
1-~ CH2CH2CH=CH2 Me Me H Ph p
1-8 C(CH3)=CHCH3 Me Me H Ph p
1-9 CH(CH3)CH=CH2 Me Me H Ph p
1-10 CH=C(CH3)CH3 Me Me H Ph p
1-11 CH2C(CH3)=CH2 Me Me H Ph p
1-12 CH=CHCH2CH2CH3 Me Me H Ph p
1-13 CH2CH=CHCH2CH3 Me Me H Ph p
1-14 CH2CH2CH=CHCH3 Me Me H Ph p
1-15 CH2CH2CH2CH=CH2 Me Me H Ph p
1-16 C(CH3)=CHCH2CH3 Me Me H Ph p
1-17 CH2C(CH3)=CHCH3 Me Me H Ph p
1-18 CH2CH=C(CH3)CH3 Me Me H Ph p
1-19 CH2CH=CHPh Me Me H Ph p
1-20 CH2CH2CH=CHPh Me Me H ph p
1-21 CH2CH2CH2CH=CHPh Me Me H Ph p
1-22 CH2CH=CH(2-FPh) Me Me H Ph 0
21 2181553
1-23 CH2CH=CH(3-FPh) Me Me H Ph p
1-24 CH2CH=CH(4-FPh) Me Me H Ph p
1-25 CH2CH=CH(2-ClPh) Me Me H Ph p
1-26 CH2CH=CH(3-ClPh) Me Me H Ph p
1-27 CH2CH=CH(4-ClPh) Me Me H Ph p
1-28 CH2CH=CH(2-MePh) Me Me H Ph p
1-29 CH2CH=CH(3-MeHh) Me Me H Ph 0
1-30 CH2CH=CH(4-MePh) Me Me H Ph 0
1-31 CH2CH=CH(2-OMePh) Me Me H ph p
1-32, CH2CH=CH(3-OMePh) Me Me H Ph p
1-33 CH2CH=CH(4-OMePh) Me Me H Ph p
1-34 CH2CH=CH(1-Naph) Me Me H Ph p
1-35 CH2CH=CH(2-Naph) Me Me H ph p
1-36 CH2CH=CF2 Me Me H Ph p
i-37 CH2CH=CHC1 Me Me H Ph p
1-38 CH2C(C1)=CH2 Me Me H Ph 0
1-39 CH2C(C1)=CHC1 Me Me H Ph p
1-40 CH2CH=CC12 Me Me H Ph p
1-41 CH2C(Br)=CHBr Me Me H Ph p
1-42 CH2CH=CHCF3 Me Me H Ph p
1-43 CH2CH=CBr2 Me Me H Ph p
1-44 C-CH Me Me H Ph p
1-45 CH2C=CH' Me Me H Ph p
1-46 CH2C_--CCH3 Me Me H Ph 0
1-47 CH2C=CCH2CH3 Me Me H Ph p
1-48 CH=C=CH2 Me Me H Ph 0
22 2 ~ 555
81
1-49 CH=C=CHCH3 Me Me H Ph p
1-50 CH=C=CHCH2CH3 Me Me H~ Ph p
1-51 CH2Pr Me Me H ph p
1-52 CH2Bu~ ~ Me Me H Ph p
1-53 CH2Pn~ Me Me H Ph p
1-54 CH2Hx~ Me Me H Ph p
1-55 CH2Hp~ Me Me H Ph p
I-56 CH2CH2Pr~ Me Me H Ph p
1-57 (CH2) Me Me H Ph
Pr~
3 p
1-58 (CH2) Me Me H ph
Pr
4 p
1-59 Pr Me Me H Ph p
1-60 Buy Me Me H Ph p
1-61 Pn~ Me Me H ph p
1-62 Hx~ Me Me H ph p
1-63 Hp~ Me Me H ph p
1-64 CH2(1-Pnte~) Me Me H Ph
p
1-65 . CH2(1-Hxe~) Me Me H Ph p
1-66 CH2(1-Hpte~) Me Me H Ph p
i-67 CHF2 Me Me H Ph p
1-68 CHZCH2F Me Me H Ph p
1-69 CH2CHF2 Me Me H Ph p
1-70 CH2CF3 Me Me H Ph p
1-71 (CH2)3F Me Me H Ph p
1-72 (CH2)4F Me Me H Ph p
1-73 CH2CH2C1 Me IHe H Ph p
1-74 CH2CH2Br Me Me H Ph p
23 2181553
1-75 CH2CH2I Me Me H ph p
1-76 CH=CH2 Me Me H '4-FPh
1-77 CH=CFiCH3 Me Me H 4-FPh 0
1-78 CH2CH=CH2 Me Me H 4-FPh 0
1-79 C(CH3)=CH2 Me Me H 4-FPh 0
1-80 CH=CHCH2CH3 Me Me H 4-FPh 0
1-81 CH2CH=CHCH3 Me Me H 4-FPh 0
1-82 CH2CH2CH=CH2 Me Me H 4-FPh 0
1-83 C(CH3)=CHCH3 Me Me H 4-FPh 0
1-84 CH(CH3)CH=CH2 Me Me H 4-FPh 0
1-85 CH=C(CH3)CH3 Me Me H . 4-FPh p
1-86 CH2C(CH3)=CH2 Me Me H 4-FPh 0
1-87 CH=CHCH2CH2CH3 Me Me H 4-FPh 0
1-88 CH2CH=CHCH2CH3 Me Me H 4-FPh 0
1-89 CH2CH2CH=CHCH3 Me Me H 4-FPh 0
1-90 CH2CH2CH2CH=CH2 Me Me H 4-FPh 0
1-91 C(CH3)=CHCH2CH3 Me Me H 4-FPh 0
1-92 CH2C(CH3)=CHCH3 Me Me H 4-FPh 0
1-93 CH2CH=C(CH3)CH3 Me Me H 4-FPh 0
1-94 CH2CH=CHPh Me Me H 4-FPh 0
1-95 CH2CH2CH=CHPh Me Me H 4-FPh 0
1-96 CH2CH2CH2CH=CHPh Me Me H 4-FPh p
1-97 CIi2CH=CH (2-FPh) Me Me H 4-FPh 0
1-98 CH2CH=CH(3-FPh) Me Me H 4-FPh p
1-99 CH2CH=CH(4-FPh) Me Me H 4-FPh 0
1-100 CH2CH=CH(2-ClPh) Me Me H 4-FPh p
24 2181 553
1-101 CH2CH=CH(3=ClPh) Me Me; H 4-FPh 0
1-102 CH2CH=CH(4-ClPh) Me Me H 4-FPh 0
1-103 CH2CH=CH(2-MePh) Me Me H 4-FPh 0
1-104 CH2CH=CH(3-MePh) Me Me H 4-FPh 0
1-105 CH2CH=CH(4-MePh) Me Me H 4-FPh 0
1-106 CH2CH=CH(2-OMePh) Me Me H 4-FPh p
1-107 CH2CH=CH(3-OMePh) Me Me H 4-FPh 0
1-108 CH2CH=CH(4-OMePh) Me Me H 4-FPh 0
1-109 CH2CH=CH(1-Naph) Me Me H 4-FPh 0
1-lI0 CH2CH=CH(2-Naph) Me Me H 4-FPh 0
1-111 CH2CH=CF2 Me' Me H 4-FPh 0
1-112 CH2CH=CHC1 Me Me H 4-FPh 0
1-113 CH2C(C1)=CH2 Me Me H 4-FPh 0
1-114 CH2C(C1)=CHC1 Me Me H 4-FPh 0
1-115 CH2CH=CC12 Me Me H 4-FPh 0
1-116 CH2C(Br)=CHBr Me Me H 4-FPh 0
1-117 CH2CHrCHCF3 Me Me H 4-FPh 0 -
I-118 CH2CH=CBr2 Me Me H 4-FPh 0
1-119 C=CH Me Me H 4-FPh 0
i-120 CH2C=CH Me Me H 4-FPh 0
1-121 CH2C=CCH3 Me Me H 4-FPh 0
i-122 CH2C=CCH2CH3 Me Me H 4-FPh 0
1-123 CH=C=CH2 Me Me H 4-FPh 0
1-i24 CH=C=CHCH3 Me Me H 4-FPh 0
1-125 CH=C=CHCH2CH3 Me Me H 4-FPh 0
1-126 CH2Pr~ Me Me H 4-FPh 0
21 8 1 ;~3
25
1-127 CH2Bu~ Me Me H 4-FPh 0
1-128 CH2Pn~ Me Me H 4-FPh p
1-129 CH2Hx~ Me Me H 4-FPh 0
1-130 CH2Hp~ Me Me H 4-FPh 0
1-131 CH2CH2Pr~ Me Me H 4-FPh 0
1-132 (CH2)3Pr~ Me Me H 4-FPh p
1-133 (CH2)4Pr~ Me Me H 4-FPh
1-134 Pry Me Me H 4-FPh 0
1-135 Hug Me Me H 4-FPh p
1-136 Pn~ Me Me H 4-FPh. 0'
1-137 Hx~ Me Me H 4-FPh p
1-138 Hp~ Me Me H 4-FPh 0
1-139 CH2(1-Pnte~) Me Me H 4-FPh 0
1-140 CH2(1-Hxe~) Me Me H 4-FPh 0
1-141 CH2(1-Hpte) Me Me H 4-FPh 0
1-142 CHF2 Me Me H 4-FPh 0
1-143 CH2CH2F ~ Me Me H 4-FPh 0
1-144 CH2CHF2 Me Me H 4-FPh 0 -
1-145 CH2CF3 Me Me H 4-FPh 0
1-146 . (CH2)3F Me Me H 4-FPh 0
1-147 (CH2)4F Me Me H 4-FPh 0
1-148 CH2CH2C1 Me Me H 4-FPh 0
1-149 CH2CH2Br . Me Me H 4-FPh 0
1-150 CH2CH2I Me Me H 4-FPh 0
1-151 CH=CH2 Me Me H 2,4-diFPh 0
1-152 CH=CHCH3 Me Me H 2,4-diFPh 0
26 2 ~ ~ ~ ~ 5 5
1-153 CH2CH=CH2 Me Me H 2,4-diFPh 0
1-154 C(CH3)=CH2 Me Me H 2,4-diFPh 0
1-155 CH=CHCH2CH3 Me Me H 2,4-diFPh 0
1-156 CH2CH=CHCH3 Me Me H 2,4-diFPh 0
1-157 CH2CH2CH=CH2 Me Me H 2,4-diFPh 0
1-158 C(CH3)=CHCH3 Me Me H 2,4-diFPh 0
1-159 CH(CH3)CH=CH2 Me Me H 2,4-diFPh 0
1-160 CH=C(CH3)CH3 Me Me H 2,4-diFPh 0
1-161 CH2C(CH3)=CH2 Me Me H 2,4-diFPh 0
1-162 CH=CHCH2CH2CH3 Me Me H 2,4-diFPh 0
1-163 CH2CH=CHCH2CH3 Me Me H 2,4-diFPh 0
'
1-164 CH2CH2CH=CHCH3 Me Me H 2,4-diFPh 0
1-165 CH2CH2CH2CH=CH2 Me Me H 2,4-diFPh H
1-166 C(CH3)=CHCH2CH3 Me Me H 2,4-diFPh 0
1-167 CH2C(CH3)=CHCH3 Me Me H 2,4-diFPh 0
1-168 CH2CH=C(CH3)CH3 Me Me H 2,4-diFPh 0
1-1.69 CH2CH=CHPh Me Me H 2,4-diFPh 0
1-170 CH2CH2CH=CHPh Me Me H 2,4-diFPh 0
1-171 CH2CH2CH2CH=CHPh Me Me H 2,4-diFPh 0
1-172 CH2CH=CH(2-FPh) Me Me H 2,4-diFPh 0
1-173 CH2CH=CH(3-FPh) Me Me H 2,4-diFPh 0
1-174 CH2CH=CH(4-FPh) Me Me H 2,4-diFPh 0
1-175 CH2CH=CH(2-ClPh) Me Me H 2,4-diFPh 0
1-176 CH2CH=CH(3-ClPh) Me Me H 2,4-diFPh 0
1-177 CH2CH=CH(4-ClPh) Me Me H 2,4-diFPh 0 _
1-178 CH2CH=CH(2-MePh) Me Me H 2,4-diFPh 0
27 2181553
1-179 CH2CH=CH(3-MePh) Me Me H 2,4-diFPh 0
1-180 CH2CH=CH(4-MePh) Me Me H' 2,4-diFPh 0
1-181 CH2CH=CH(2-OMePh) Me Me H 2,4-diFPh 0
1-I82 CH2CH=CH(3-OMePh) Me Me H 2,4-diFPh 0
1-183 CH2CH=CH(4-OMePh) Me Me H 2,4-diFPh 0
1-184 CH2CH=CH(1-Naph) Me Me H 2,4-diFPh 0
1-185 CH2CH=CH(2-Naph) Me Me H 2,4-diFPh 0
1-186 CH2CH=CF2 Me Me H 2,4-diFPh 0
1-187 CH2CH=CHC1 Me Me H 2,4-diFPh 0
1-188 CH2C(C1)=CH2 Me Me H 2,4-diFPh 0
1-189 CH2C(C1)=CHC1 Me Me H 2,4-diFPh 0
1-190 CH2CH=CC12 Me Me H 2,4-diFPh 0
1-191 CH2C(Br)=CHBr Me Me H 2,4-diFPh 0
1-192 CH2CH=CHCF3 Me Me H 2,4-diFPh 0
1-193 CH2CH=CBr2 Me Me H 2,4-diFPh 0
,
1-194 C_--CH Me Me H 2,4-diFPh 0
1-195 CH2C=CH Me Me H 2,4-diFPh 0
-
1-196 CH2C--__CCH3 Me Me H 2,4-diFPh 0
1-197 CH2C CCH2CH3 Me Me H 2,4-diFPh 0
1-198 CH=C=CH2 Me Me H 2,4-diFPh 0
1-199 CH=C=~CH3 Me Me H 2,4-diFPh 0
1-200 CH=C=CHCH2CH3 Me Me H 2,4-diFPh 0
1-201 CH2Pr~ Me Me H 2,4-diFPh 0
1-202 CH2Bu~ Me Me H 2,4-diFPh 0
1-203 CH2Pn~ Me Me H 2,4-diFPh 0
1-204 CH2Hx~ Me Me H 2,4-diFPh 0
2181553
28
1-205 CH2Hp~ Me Me H 2,4-diFPh 0
1-206 CH2CH2Pr~ Me Me H . 2,4-diFPh 0
1-207 (CH2)3Pr~ Me Me H 2,4-diFPh 0
1-208 (CH2)4Pr~ Me Me H 2,'4-diFPh 0
1-209 Pry Me Me H 2,4-diFPh 0
1-210 Buy Me Me H 2,4-diFPh 0
1-211 Pn Me Me H 2,4-diFPh 0
1-212 Hx~ Me Me H 2,4-diFPh 0
1-213 Hp~ Me Me H 2,4-diFPh 0
1-214 CH Me Me H 2
(1-Pnte) 4
i
2 , 0
-d
FPh
1-215 CH Me Me H 2
(1-Hxe~)
2 ,4-diFPh 0
1-216 CH2(1-Hpte~) Me Me H 2,4-diFPh 0
1-217 CHF2 Me Me H 2,4-diFPh 0
1-218 CH2CH2F Me Me H 2,4-diFPh 0
1-219 CH2CHF2 Me Me H 2,4-diFPh 0
1-220 CH2CF3 Me Me H 2,4-diFPh 0
1-221 (CH2)3F Me Me H 2,4-diFPh 0
1-222 (CH2)4F Me Me H 2,4-diFPh 0
1-223 CH2CH2C1 Me Me H 2,4-diFPh 0
1-224 CHaCH2Br Me Me H 2,4-diFPh 0
1-225 CH2CH2I Me Me H 2,4-diFPh 0
1-226 CH2CH~CH2 Me Me H 4-ClPh 0
1-227 CH2CH=CHCH3 Me Me H 4-ClPh 0
1-228 CH2C-_-CH Me Me H 4-ClPh 0
1-229 CH2Pr~ Me Me H 4-ClPh 0
1-230 CH2CH=CHPh Me Me H 4-ClPh 0
y 21 81553
29
1-231 CH2CH=CH2 Me Me H 2,4-diClPh 0
1-232 CH2CH=CHCH3 Me Me H 2,4-diClPh 0
1-233 CH2C--_CH Me Me H 2,4-diClPh 0
1-234 CH2Pr~ Me Me H 2,4-diClPh 0
1-235 CH2CH=CHPh Me Me H 2,4-diClPh 0
1-236 CH2CH=CH2 Me Me H 2-FPh 0
1-237 CH2CH=CHCH3 Me Me H 2-FPh
1-238 CH2C=CH Me Me H 2-FPh 0
1-239 CH2Pr Me Me H 2-FPh 0
1-240 CH2CH=CHPh Me Me H 2-FPh 0
1-241- CH2CH=CH2 Me' Me H 3-FPh 0
1-242 CH2CH=CHCH3 Me Me H 3-FPh 0
1-243 CH2C CH Me Me H 3-FPh 0
1-244 CH2Pr~ Me Me H 3-FPh 0
1-245 CH2CH=CHPh Me Me H 3-FPh 0
1-246 CH2CH=CH2 Me Me H 2-ClPh 0
1-247 CH2CH=CHCH3 Me Me H 2-ClPh 0
7:-248 CH2C=CH Me Me H 2-ClPh 0
.
1-249 CH2Pr~ Me Me H 2-ClPh 0
1-250 CH2CI3=CHPh Me Me H 2-ClPh 0
1-251 CH2CH=CH2 Me Me H 3-ClPh 0
1-252 CH2CH=CHCH3 Me Me H 3-ClPh p
1-253 CH2CH=CH2 Me Me H 4-MePh 0
1-254 CH2CH=CHCH3 Me Me H 2-MePh 0
1-255 CH2C--_CH Me Me H 3-MePh 0
1-256 CH2CH=CH2 Me Me H 2-OMePh 0
2181553
1-257 CH2CH=CHCH3 Me Me H 3-OMePh 0
1-258 CH2C=CH Me Me H 4-OMePh 0
1-259 CH2CH=CH2 Me Me H 4-CF3Ph 0
1-260 CH2CH=CHCH3 Me Me H 4-CF3Ph 0
1-261 CH2CH=CH2 Me Me H 4-OCHF2Ph 0
1-262 CH2CH=CHCH3 Me Me H 4-OCHF2Ph 0
1-263 CH2CH=CH2 Me Et H Ph 0
1-264 CH2CH=CH2 Me Pr H Ph 0
1-265 CH2CH=CH2 Me Pr1 H Ph 0- -
-
1-266 CH2CH=CH2 Me Bu H Ph 0
1-267 CH2CH=CH2 Me Bu1 H Ph 0
1-268 CH2CH=CH2 Me Bus H Ph 0
1-269 CH2CH=CH2 Me But H Ph 0
1-270 CH2CH=CH2 Me Ph H Ph 0
1-271 CH2CH=CH2 Me 2-FPh H Ph 0
1-272 CH2CH=CH2 Me 3-FPh H Ph 0
1-273 CH2CH=CH2 Me 4-FPh H Ph 0
1-274 CH2CFI=CH2 Me 2,4-diFPh H Ph 0
1-275 CH2CH=CH2 Me 4-ClPh H Ph 0
1-276 CH2CH=CH2 Me 4-MePh H Ph 0
1-277 CH2CH=CH2 Me ~-OMePh H Ph 0
1-278 CH2CH=CH2 Me Et H 4-FPh 0
1-279 CH2CH=CH2 Me Pr H 4-FPh 0
1-280 CH2CH=CH2 Me Prl H 4-FPh 0
1-281 CH2CH=CH2 Me Bu H 4-FPh 0
1-282 CH2CH=CH2 Me Bul H 4-FPh 0
31 2181553
1-283 CH2CH=CH2 Me Bus H 4-FPh 0
1-284 CH2CH=CH2 Me But H 4-FPh 0
1-285 CH2CH=CH2 Me Ph H 4-FPh 0
1-286 CH2CH=CH2 Me 2-FPh H 4-FPh 0
1-287 CH2CH=CH2 Me 3-FPh H 4-FPh 0
1-288 CH2CH=CH2 Me 4-FPh H 4-FPh 0
1-289 CH2CH=CH2 Me 2,4-diFPh H 4-FPh 0
1-290 CH2CH=CH2 Me 4-ClPh H 4-FPh 0
1-291 CH2CH=CH2 Me 4-MePh H 4-FPh 0
1-292 CH2CH=CH2 Me 4-OMePh H 4-FPh 0
1-293 CH2CH=CH2 Me Et H 2,4- 0
d iFPh
1-294 CH2CH=CH2 Me Pr H 2,4-diFPh 0 ._
1-295 CH2CH=CH2 Me Pri H 2,4-diFPh 0
1-296 CH2CH=CH2 Me Bu H 2,4-diFPh 0
1-297 CH2CH=CH2 Me Bu1 H 2,4-diFPh 0
1-298 CH2CH=CH2 Me Bus H 2,4-diFPh 0
I-299 CH2CH=CH2 Me But H 2,4-diFPh 0
i-300 CH2CH=CH2 Me Ph H 2,4-diFPh 0
1-301 CH2CH=CH2 Me 2-FPh H 2,4-diFPh 0
1-302 CH2CH=CH2 Me 3-FPh H 2,4-diFPh 0
1-303 CH2CH=CH2 Me 4-FPh H 2,4-diFPh 0
1-304 CH2CH=CH2 Me 2,4-diFPh H 2,4-diFPh 0
1-305 CH2CH=CH2 Me 4-ClPh H 2,4-diFPh 0
1-306 CH2CH=CH2 Me 4-MePh H 2,4-diFPh 0
I-307 CH2CH=CH2 Me' 4-OMePh H 2,4-diFPh 0
1-308 CH2CH=CHCH3 Me Et H Ph 0
32 2181553
1-309 CH2CH=CHCH3 Me Pr H ph p
1-310 CH2CH=CHCH3 Me Prl H Ph p
1-311 CH2CH=CHCH3 Me . Bu H Ph 0
1-312 CH2CH=CHCH3 Me Bui H Ph p
1-313 CH2CH=CHCH3 Me Bu9 H Ph 0
1-314 CH2CH=CHCH3 Me But H Ph p
1-315 CH2CH=CHCH3 Me Ph H Ph p
1-316 CH2CH=CHCH3 Me 2-FPh H Ph p
1-317 CH2CH=CHCH3 Me 3-FPh H Ph p
1-318 CH2CH=CHCH3 Me 4-FPh H Ph p
1-319 CH2CH=CHCH3 Me 2,4-diFPh H Ph p
1-320 CH2CH=CHCH3 Me , 4-ClPh H ph p
1-321 CH2CH=CHCH3 Me 4-Meph H Ph p
1-322 CH2CH=CHCH3 Me 4-OMePh H Ph p
1-323 CH2CH=CHCH3 Me Et H 4-FPh 0
1-324 CH2CH=CHCH3 Me Pr H 4-FPh p
1-325 . CH2CH=CHCH3 Me Pri H 4-FPh 0
1-326 CH2CH=CHCH3 Me Bu H 4-FPh 0
1-327 CH2CH=CHCH3 Me Bul H 4-FPh 0
1-328 CH2CH=CHCH3 Me Bus H 4-FPh 0
1-329 CH2CH=CHCH3 Me But H 4-FPh 0
1-330 CH2CH=CHCH3 Me Ph H 4-FPh 0
1-331 CH2CH=CHCH3 Me 2-FPh H 4-FPh 0
1-332 CH2CH=CHCH3 Me 3-FPh H 4-FPh p
1-333 CH2CH=CHCH3 Me 4-FPh H 4-FPh. 0
1-334 CH2CH=CHCH3 Me 2,4-diFPh H 4-FPh p
21$1 553
33 _
1-335 CH2CH=CHCH3 Me 4-ClPh H 4-FPh 0
I-336 CH2CH=CHCH3 Me 4-MePh H 4-FPh p
1-337 CH2CH=CHCH3 Me 4-OMePh H 4-FPh 0
1-338 CH2CH=CHCH3 Me Et H 2,'4-diFPh 0
1-339 CH2CH=CHCH3 Me Pr H 2,4-diFPh 0
1-340 CH2CH=CHCH3' Me Pri H 2,4-diFPh 0
1-341 CH2CH=CHCH3 Me Bu H 2,4-diFPh 0
1-342 CH2CH=CHCH3 Me Bu1 H 2,4-diFPh 0
1-343 CH2CH=CHCH3 Me Bus H 2,4-diFPh 0
1-344 CH2CH=CHCH3 Me But H 2,4-diFPh 0
1-345 CH2CH=CHCH3 Me Ph H. 2,4-diFPh 0
1-346 CH2CH=CHCH3 Me 2'-FPh H 2,4-diFPh 0
1-347 CH2CH=CHCH3 Me 3-FPh H 2,4-diFPh 0
1-348 CH2CH=CHCH3 Me 4-FPh H 2,4-diFPh 0
1-349 CH2CH=CHCH3 Me 2,4-diFPh H 2,4-diFPh 0
1-350 CH2CH=CHCH3 Me 4-ClPh H 2,4-diFPh 0
1-351 CH2CH=CHCH3 Me 4-MePh H 2,4-diFPh 0
1-352 CH2CH=CHCH3 Me 4-OMePh H 2,4-diFPh 0
1,-353 CH2CH=CH2 Et Me H Ph p
1-354 CH2CH~Cg2 Pr Me H Ph p
1-355 CH2CH=CH2 Pr1 Me H Ph p
1-356 CH2CH=CH2 Bu Me H Ph p
1-357 CH2CH=CH2 Bul Me H Ph p
1-358 CH2CH=CH2 Bus Me H Ph p
1-359 CH2CH=CH2 But Me H Ph p
1-360 CH2CH=CH2 Ph Me H Ph p
21 81553
34
1-361 CH2CH=CH2 2-FPh Me H ph 0
1-362 CH2CH=CH2 3-FPh Me H Ph
1-363 CH2CH=CH2 4-FPh Me H Ph 0
1-364 CH2CH=CH2 2,4-diFPh Me H Ph p
1-365 CH2CH=CH2 4-ClPh Me H Ph
1-366 CH2CH=CH2 4-MePh Me H Ph p
1-367 CH2CH=CH2 4-OMePh Me H Ph ~ p
1-368 CH2CH=CH2 Et Me H 4-FPh 0
1-369 CH2CH=CH2 Pr Me H 4-FPh p
1-370 CH2CH=CH2 Prl Me H 4-FPh 0
1-371 CH2CH=CH2 Bu Me H 4-FPh 0
1-372 CH2CH=CH2 Bui Me H 4-FPh 0
1-373 CH2CH=CH2 Bus Me H 4-FPh 0
1-374 CH2CH=CH2 But Me H 4-FPh 0
1-375 CH2CH=CH2 Ph Me H 4-FPh 0
1-376 CH2CH=CH2 2-FPh Me H 4-FPh 0
1-377 CH2CH=CH2 3-FPh Me H 4-FPh 0
1-378 CH2CH=CH2 4-FPh Me H 4-FPh 0
1-379 CH2CH=CH2 2,4-diFPh Me H 4-FPh p
1-380 CH2CH=CH2 4-ClPh Me H 4-FPh p
1-381 CH2CH=CH2 4-MePh Me H 4-FPh 0
1-382 CH2CH=CH2 4-OMePh Me H 4-FPh 0
1-383 CH2CH=CH2 Et Me H 2,4-diFPh 0
1-384 CH2CH=CH2 Pr Me H 2,4-diFPh 0
1-385 CH2CH=CH2 Prl Me H 2,4-diFPh 0
1-386 CH2CH=CH2 Bu Me H 2,4-diFPh 0
2181553
1-387 CH2CH=CH2 Bu1 Me H 2,4-diFPh 0
1-388 CH2CH=CH2 Bus Me H 2,4-diFPh 0
1-389 CH2CH=CH2 But Me H 2,4-diFPh 0
1-390 CH2CH=CH2 Ph Me H 2,4-diFPh 0
1-391 CH2CH=CH2 2-FPh Me H 2,4-diFPh 0
1-392 CH2CH=CH2 3-FPh Me H 2,4-diFPh 0
1-393 CH2CH=CH2 4-FPh Me H 2,4-diFPh 0
1-394 CH2CH=CH2 2,4-diFPh Me H 2,4-diFPh 0
1-395 CH2CH=CH2 4-ClPh Me H 2,4-diFPh 0
1-396 CH2CH=CH2 4-MePh Me H 2,4-diFPh 0
1-397 CH2CH=CH2 4-OMePh Me H 2,4-diFPh 0
1-398 CH2CH=CHCH3 Et Me H Ph p
1-399 CH2CH=CHCH3 Pr Me H Ph p
1-400 CH2CH=CHCH3 Prl Me H Ph p
1-401 CH2CH=CHCH3 Bu Me H Ph p
1-402 CH2CH=CHCH3 Bui Me H Ph p
1-403 CH2CH=CHCH3 Bus Me H Ph p
1-404 CH2CH=CHCH3 But Me H Ph p
1-405 CH2CH=CHCH3 Ph Me H Ph p
1-406 CH2CH=CHCH3 2-FPh Me H Ph p
1-407 CHaCH=CHCH3 3-FPh Me H Ph p
1-408 CH2CH=CHCH3 4-FPh Me H Ph p
1-409 CH2CH=CHCH3 2,4-diFPh Me H Ph p
1-410 CH2CH=CHCH3 4-ClPh Me H Ph p
1-411 CH2CH=CHCH3 4-MePh Me H Ph p
1-412 CH2CH=CHCH3 4-OMePh Me H Ph p
2181553
36
1-413 CH2CH=CHCH3 Et Me H 4-FPh 0
1-414 CH2CH=CHCH3 Pr Me H 4-FPh 0
1-415 CH2CH=CHCH3 Pr1 Me H 4-FPh 0
1-416 CH2CH=CHCH3 Bu Me H 4-FPh p
1-417 CH2CH=CHCH3 Bul Me H 4-FPh 0
1-418 CH2CH=CHCH3 Bu8 Me H 4-FPh 0
1-419 CH2CH=CHCH3 But Me H 4-FPh 0
1-420 CH2CH=CHCH3 Ph Me H 4-FPh p
1-421 CH2CH=CHCH3 2-FPh Me H 4-FPh 0
1-422 CH2CH=CHCH3 3-FPh Me H 4-FPh 0
1-423 CH2CH=CHCH3 4-FPh Me H 4-FPh p
1-424 CH2CH=CHCH3 2,4-diFPh Me H 4-FPh p
1-425 CH2CH=CHCH3 4-ClPh Me H 4-FPh 0
1-426 CH2CH=CHCH3 4-MePh Me H 4-FPh 0
i-427 CH2CH=CHCH3 4 -OMePh Me H 4-FPh 0
1-428 CH2CH=CHCH3 Et Me H 2,4-diFPh 0
1-429 CH2CH=CHCH3 Pr Me H 2,4-diFPh 0
1-430 CH2CH=CHCH3 Prl Me H 2,4-diFPh 0
I-431 CH2CH=CHCH3 Bu Me H 2,4-diFPh 0
1-432 CH2CH=CHCH3 Bui Me H 2,4-diFPh 0
1-433 CH2CH=CHCH3 Bue Me H 2,4-diFPh 0
1-434 CH2CH=CHCH3 But Me H 2,4-diFPh 0
1-435 CH2CH=CHCH3 Ph Me H 2,4-diFPh 0
I-436 CH2CH=CHCH3 2-FPh Me H 2,4-diFPh 0
1-437 CH2CH=CHCH3 3-FPh Me H 2,4-diFPh 0
1-438 CH2CH=CHCH3 4-FPh Me H 2,4-diFPh 0
2181553
37
1-439 CH2CH=CHCH3 2,4-diFPh Me H 2,4-diFPh 0
1-440 CH2CH=CHCH3 4-ClPh Me H 2,4-diFPh 0
1-441 CH2CH=CHCH3 4-MePh Me H 2,4-diFPh 0
1-442 CH2CH=CHCH3 4-OMePh Me H 2,4-diFPh 0
1-443 CH2CH=CH2 Me Me H Ph NH
1-444 CH2Pre Me Me H Ph
1-445 CH2CH=CH2 Me Me H 4-FPh S
1-446 CH2CH=CH2 Me Me H 4-FPh NH
1-447 CH2CH=CH2 Me Me H 2,4-diFPh S
1-448 CH2CH=CH2 Me Me H 2,4-diFPh NH
1-449 CH2CH=CH2 Me Me H 4-ClPh S
1-450 CH2CH=CH2 Me Me H 4-ClPh NH
1-451 CH2CH=CH2 Me Me H 2,4-diClPh S
1-452 CH2CH=CH2 Me Me H 2,4-diClPh NH
1-453 CH2CH=CH2 Me Me Me Ph 0
1-454 CH2CH=CH2 Me Me Me 4-FPh 0
1-455 CH2CH=CH2 Me Me Me 2,4-diFPh 0
.
1-456 CH2CH=CH2 Me Me Me 4-ClPh 0
1-457 CH2CH=CH2 Me Me Me 2,4-diClPh 0
1-458 CH2CH=CHCH3 Me Me H Ph S
1-459 CH2CH=CHCH3 Me Me H Ph NH
1-46'0 CH2CH=CHCH3 Me Me H 4-FPh S
1-461 CH2CH=CHCH3 Me Me H 4-FPh NH
1-462 CH2CH=CHCH3 Me Me H 2,4-diFPh S
1-463 CH2CH=CHCH3 Me Me H 2,4-diFPh NH
1-464 CH2CH=CHCH3 Me Me H 4-ClPh S
38 2781 553
1-465 CH2CH=CHCH3 Me Me H 4-ClPh NH
1-466 CH2CH=CHCH3 Me Me H ~ 2,4-diClPh S
1-467 CH2CH=CHCH3 Me Me H 2,4-diClPh NH
1-468 CH2CH=CHCH3 Me Me Me Ph 0
1-469 CH2CH=CHCH3 Me Me Me 4-FPh 0
1-470 CH2CH=CHCH3 Me Me Me 2,4-diFPh 0
1-471 CH2CH=CHCH3 Me Me Me 4-ClPh 0
1-472 CH2CH=CHCH3 Me Me Me 2,4-diClPh 0
1-473 CH2CH=CH2 Me Me H 2-Thi 0
1-474 CH2CH=CH2 Me Me H 3-Thi 0
1-475 CH2CH=CH2 Me Me H. 2-Fur 0
1-476 CH2CH=CH2 Me Me H 2-Thiaz 0
1-477 CH2CH=CH2 Me Me H 2-Pyr 0
1-478 CH2CH=CH2 ~ Me Me H 3-Pyr 0
1-479 CH2CH=CH2 Me Me H 4-Pyr 0
1-480 CH2CH=CH2 Me Me H 2-Oxaz 0
1-481 CH2CH=CH2 Me Me H 2-Imidz 0
1-482 CH2CH=CH2 Me Me H 2-Bezoxaz 0
1-483 CH2CH=CH2 Me Me H 2-Bezthiaz 0
1-484 CH2CH=CH2 Me Me H 2-Bezimidz 0
1-485 CH2CH=CH2 Me Me H 3-Pyridz 0
I-486 CH2CH=CH2 Me Me H 2-Pyraz 0
1-487 CH2CH=CH2 Me Me H 2-(1,3,4-TDA) 0
1-488 CH2CH=CHCH3 Me Me H 2-Thi 0
1-489 CH2CH=CHCH3 Me Me H 3-Thi 0
1-490 CH2CH=CHCH3 Me Me H 2-Fur 0
y 21 81553
39
1-491 CH2CH=CHCH3 Me Me H 2-Thiaz 0
1-492 CH2CH=CHCH3 Me Me H 2-Pyr 0
1-493 CH2CH=CHCH3 Me Me H 3-Pyr 0
1-494 CH2CH=CHCH3 Me Me H 4-Pyr 0
-
1-495 CH2CH=CHCH3 Me Me H 2-Oxaz 0
1-496 CH2CH=CHCH3 Me Me H 2-Imidz 0
1-497 CH2CH=CHCH3 Me Me H 2-Bezoxaz 0
1-49B CH2CH=CHCH3 Me Me H 2-Bezthiaz 0
1-499 CH2CH=CHCH3 Me Me H 2-Bezimidz 0
1-500 CH2CH=CHCH3 Me Me H 3-Pyridz 0
1-501 CH2CH=CHCH3 Me Me H 2-Pyraz 0
1-502 CH2CH=CHCH3 Me Me H 2-(1,3,4-TDA) 0
1-503 CH2CH=CHCH3 Me Me H 2,6-diFPh 0
1-504 CH2CH=CHCH3 Me Me H 3,5-diFPh 0
1-505 CHZCH=CHCH3 Me Me H 2-C1-6-FPh 0
1-506 CH=CHCH3 Me Me H Ph I~IH
1-507 CH2C$=CH2 Me Me H Ph S
1-508 CH2CH=CHCH2CH2CH3 Me Me H, Ph 0
1-509 CH2CH=CHCH2CH2CH3 Me Me H 4-FPh 0
1-510 CH2CH=CHCH2CH2CH3 Me Me H 2,4-diFPh 0
1-511 CH2CH=CH2 H Me H Ph 0
1-512 CH2CH=CHCH3 H Me H Ph 0
1-513 CH2CH=CH2 H Me H 4-FPh 0
1-514 CH2CH=CHCH3 H Me H 4-FPh 0
1-515 CH2CH=CH2 H Me H 2,4-diFPh 0
1-516 CH2CH=CHCH3 H Me H 2,4-diFPh 0
' 21 $1 553
40
1-517 CH2CH=CH2 Me H H Ph 0
1-518 CH2CH=CHCH3 Me H H Ph 0
1-519 CH2CH=CH2 Me H H 4-FPh 0
1-520 CH2CH=CHCH3 Me H H 4-FPh 0
1=521 CH2CH=CH2 Me H H 2,4-diFPh 0
1-522 CH2CH=CHCH3 Me H H 2,4-diFPh 0
.
1-523 CH2CH=CH2 Me Me H 4-C1-2-FPh 0
1-524 CH2CH=CHCH3 Me Me H 4-Cl-2-FPh 0
1-525 CH2CH=CH2 Me Me H 2,6-diCIPh 0
1-526 CH2CH=CHCH3 Me Me H 2,6-diClPh 0
1-527 CH2CH=CH2 Me Me H 2,5-diClPh 0
1-528 CH2CH=CHCH3 Me Me H 2,5-diClPh 0
1-529 CH2CH=CH2 Me Me H 2,4,6-triFPh 0
1-530 CH2CH=CHCH3 Me Me H 2,4,6-triFPh 0
1-531 CH2CH=CH2 Me Me H 2,4,6-triClPh 0
1-532 CH2CH=CHCH3 Me Me H 2,4,6-triClPh 0
1-533 CH2CH=CH2 Me Me H 2,6-diFPh 0
1-534 CH2CH=CH2 Me Me H 3,5-diFPh 0
1-535 CH2CH=CH2 Me Me H 2-C1-6-FPh 0
1-536 ' CH2CH=CH2 Me Me H 2,5-diFPh 0
1-537 CH2CH=CHCH3 Me Me H 2,5-diFPh 0
1-538 CH2(2-MePr) Me Me H Ph 0
1-539 CH2(2-MePr~) Me Me H 4-FPh 0
1-540 CH2(2-MePr) Me Me H 2,4-diFPh 0
1-541 CH=CH2 Me Me H Ph S
1-542 CH=CHCH3 Me Me H Ph S
' 41 2181553
1-543 CH2C(CH3)=CH2 Me Me H Ph S
1-544 CH2CH=CHCH2CH3 Me Me H Ph S
1-545 CH2CH=C(CH3)CH3 Me Me H Ph S
1-546 CH2CH=CHPh Me Me H Ph S
1-547 CH2CH=CH(4-FPh) Me Me H Ph S
1-548 CH2CH=CF2 Me Me H Ph S
1-549 CH2CH=CHC1 Me Me H Ph S
1-550 CH2C(C1)=CH2 Me Me H Ph S
1-551 CH2CH=CC12 Me Me H Ph S
1-552 CH2C=CH Me Me H Ph S
1-553 CH=C=CH2 Me Me H Ph S
1-554 CH2Pr~ Me Me H Ph S
1-555 Pry Me Me H Ph S
1-556 Hx~ Me Me H Ph S
1-557 CH2CH2F Me Me H Ph S
1-'558 CH2CHF2 Me Me H Ph S
1-559 CH2CF3 Me Me H Ph S -
1-560 (CH2)3F Me Me H Ph S
1-561 CH=CH2 Me Me H 4-FPh S
1-562 CH=CHCH3 Me Me H 4-FPh S
1-563 C(CH3)=CH2 Me Me H 4-FPh S
1-564 CH=CHCH2CH3 Me Me H 4-FPh S
1-565 CH2C(CH3)=CH2 Me Me H 4-FPh S
1-566 CH2CH=CHPh Me Me H 4-FPh S
1-567 CH2CH=CH(4-FPh) Me Me H 4-FPh S
i-568 CH2CH=CF2 Me Me H 4-FPh S
42 21 81 553
1-569 CH2CH=CHC1 Me Me H 4-FPh S
1-570 CH2CH=CCI2 Me Me Ii 4-FPh S
1-571 CH2C=CH Me Me H 4-FPh S
1-572 CH2Pr~ ' Me Me H 4-FPh S
1-573 CH2Hx~ Me Me H 4-FPh S
1-574 Pry Me Me H 4-FPh S
1-575 Hx~ Me Me H 4-FPh S
1-576 (CH2)3F Me Me H 4-FPh S
1-577 C(CH3)=CH2 Me Me H 2,4-diFPh S
1-578 CH=CHCH2CH3 Me Me H 2,4-diFPh S
1-579 CH2CH=CHPh Me Me H 2,4-diFPh S
1-580 CH2CH=CF2 Me Me H 2,4-diFPh S
1-581 CH2CH=CHC1 Me Me H 2,4-diFPh S
1-582 CH2C=CH Me Me H 2,4-diFPh S
1-583 CH=C=CH2 Me Me H 2,4-diFPh S
1-584 CH2Pr~ Me Me H 2,4-diFPh S
1-585 . CH2Hx~ Me Me H 2,4-diFPh S
1-586 Pry Me Me H 2,4-diFPh S
1-587 CH2Pr~ Me Me H 4-ClPh S
1-588 CH2Pr~ Me Me H 2,4-diClPh S
1-589 CH2CH=CH2 Me Me H 2-FPh S
1-.590 CH2CH=CHCH3 Me Me H 2-FPh S
1-591 CH2Pr~ Me Me H 2-FPh S
1-592 CH2CH=CH2 Me Me H 3-FPh S
1-593 CH2CH=CHCH3 Me Me H 3-FPh S
1-594 CH2Pr~ Me Me H 3-FPh S
2181553
43
1-595 CH2CH=CH2 Me Me H 2-ClPh S
1-596 CH2CH=CHCH3 Me Me H ~ 2-ClPh S
1-597 CH2Pr~ Me ~ Me H 2-ClPh S
1-598 CH2CH=CH2 Me Me H 3-ClPh S
1-599 CH2CH=CHCH3 Me Me H 3-ClPh S
1-600 CH2CH=CH2 Me Me H 4-CF3Ph S
1-601 CH2CH=CHCH3 Me Me H 4-CF3Ph S
1-602 CH2CH=CH2 Me Et H Ph S
1-603 CH2CH=CH2 Me Et H 4-FPh S
1-604 CH2CH=CH2 Me Et H 2,4-diFPh S
1-605 CH2CH=CHCH3 Me Et H Ph S
1-606 CH2CH=CHCH3 Me Et H 4-FPh S
1-607 CH2CH=CHCH3 Me Et H 2,4-diFPh S
1-608 CH2CH=CH2 Et Me H Ph S
1-609 CH2CH=CH2 Et Me H 4-FPh S
1-610 CH2CH=CH2 Et Me H 2,4-diFPh S
1-611 CH2CH=CHCH3 Et Me H Ph S
1-612 CH2CH=CHCH3 Et Me H 4-FPh S
1-613 CH2CH=CHCH3 Et Me H 2,4-diFPh S
1-614 CH2CH=CH2 Me Me H 2-Thi S
1-615 CH2CH=CH2 Me Me H 2-Fur S
1-616 CH2CH=CH2 Me Me H 3-Pyr 5-
1-617 CH2CH=CHCH3 Me Me H 2-Thi S
1-618 CH2CH=CHCH3 Me Me H 2-Fur S
1-619 CH2CH=CHCH3 Me . Me H 2-Pyr S
1-620 CH2CH=CHCH3 Me Me H 3-Pyr S
' 44 21 81553
1-621 CH2CH=CHCH3 Me Me H 2,6-diFPh S
1-622 CH2CH=CHCH3 Me Me H 3,5-diFPh S
1-623 CH2CH=CHCH3 Me Me H 2-Cl-6-FPh S
1-624 CH2CH=CH2 Me Me H 4-C1-2-FPh S
1-625 CH2CH=CHCH3 Me Me H 4-C1-2-FPh S
'
1-626 CH2CH=CHCH3 Me Me H 2,6-diClPh S
1-627 CH2(2-MePr~) Me Me H 4-FPh S
1-628 CH2(2-MePr) Me Me H 2,4-diFPh S
1-629 CH=CH2 Me Me H Ph CH2
1-630 CH=CHCH3 Me Me H Ph CH2
'
1-631. CH2CH=CH2 Me . Me H Ph CH2
1-632 CH2CH=CHCH3 Me Me H Ph CH2
1-633 CH2C(CH3)=CH2 Me Me H Ph CH2
1-634 CH2CH=CHCH2CH3 Me Me H Ph CH2
1-635 CH2CH=C(CH3)CH3 Me Me H Ph CH2
-
1-636 CH2CH=CHPh Me Me H Ph CH2
1-637 CH2CH=CH(4-FPh) Me Me H Ph CH2
1-638 CH2CH=CF2 Me Me H, Ph CH2
1-639 CH2CH=CHC1 Me Me H Ph CH2
1-640 CH2C(C1)=CH2 Me Me H Ph CH2
1-641 CH2CH=CC12 Me Me H Ph CH2
1-642 CH2C--_CH Me Me H Ph CH2
1-643 CH=C=CH2 Me Me H Ph CH2
1-644 CH2Pr~ Me Me H Ph CH2
-
1-645 Pry Me Me H Ph CH2
1-646 Hx Me Me H Ph CH2
-
' 45 21815 53
1-647 CH2CH2F Me Me H Ph CH2
1-648 CH2CHF2 Me Me H Ph CH2
1-649 CH2CF3 Me Me H Ph CH2
1-650 (CH2)3F Me Me H Ph CH2
1-65i CH=CH2 Me Me H 4-FPh CH2
1-652 CH=CHCH3 Me Me H 4-FPh CH2
1-653 CH2CH=CH2 Me Me H 4-FPh CH2
1-654 C(CH3)=CH2 Me Me H 4-FPh CH2
1-655 CH=CHCH2CH3 Me Me H 4-FPh CH2
1-656 CH2CH=CHCH3 Me Me H 4-FPh_ CH2
1-657 CH2C(CH3)=CH2 Me Me H 4-FPh CH2
1-658 CH2CH=CHPh Me Me H 4-FPh CH2
1-659 CH2CH=CH(4-FPh) Me Me H 4-FPh CH2
1-660 CH2CH=CF2 Me Me H 4-FPh CH2
1-661 CH2CH=CHC1 Me Me H 4-FPh CH2
1-662 CH2CH=CC12 Me Me H 4-FPh CH2
1-663 CH2C=CH ' Me Me H 4-FPh CH2
1-664 CH2Pr~ Me Me H 4-FPh CH2
1-665 CH2Hx~ Me Me H 4-FPh CH2
1-666 , Pr Me Me H 4-FPh CH2
1-667 Hx~ Me Me H 4-FPh CH2
1-668 (CH2)3F Me Me H 4-FPh CH2
1-669 CH2CH=CH2 Me Me H 2,4-diFPh CH2
1-670 C(CH3)=CH2 Me Me H 2,4-diFPh CH2
1-671 CH=CHCH2CH3 Me Me H 2,4-diFPh CH2
1-672 CH2CH=CHCH3 Me Me H 2,4-diFPh CH2
~
46 2181553
1-673 CH2CH=CHPh Me Me H 2,4-diFPh CH2
1-674 CH2CH=CF2 Me Me H 2,4-diFPh CH2
1-675 CH2CH=CHC1 Me Me H 2,4-diFPh CH2
-
I-676 CH2C--_CH Me Me H 2,4-diFPh CHZ-
1-677 CH=C=CH2 Me Me H 2,4-diFPh CH2
1-678 CH2Pr~ Me Me H 2,4-diFPh CH2
1-679 CH2Hx~ Me Me H 2,4-diFPh CH2
1-680 Pry Me Me H 2,4-diFPh CH2
1-681 CH2CH=CH2 Me Me H 4-ClPh CH2
1-682 CH2CH=CHCH3 Me Me H 4-ClPh CH2
1-683 CH2Pr~ Me Me H 4-ClPh CH2
1-684 CH2CH=CH2 Me Me H 2,4-diClPh CH2
1-685 CH2CH=CHCH3 Me Me H 2,4-diClPh CH2
1-686 CH2Pr~ Me Me H 2,4-diClPh CH2
1-687 CH2CH=CH2 Me Me H 2-FPh CH2
1-688 CH2CH=CHCH3 Me Me H 2-FPh CH2
1-689 CH2Pr~ Me Me H 2-FPh CH2
1-690 CH2CH=CH2 Me Me H 3-FPh CH2
1-691 CH2CH=CHCH3 Me Me H 3-FPh CH2
1-692 CH2Pr Me Me H 3-FPh CH2
-
1-693 CH2CH=CH2 Me Me H 2-ClPh CH2
1-694 CH2CH=CHCH3 Me Me H 2-ClPh CH2
1-695 CH2Pr~ Me Me H 2-ClPh CH2
-
1-696 CH2CH=CH2 Me Me H 3-ClPh CH2
1-697 CH2CH=CHCH3 Me Me H 3-ClPh CH2
1-698 CH2CH=CH2 Me Me H 4-CF3Ph CH2
2181553
47
1-699 CH2CH=CHCH3 Me Me H 4-CF3Ph CH2.
1-700 CH2CH=CH2 Me Et Ii Ph CH2
1-701 CH2CH=CH2 M~ Et H 4-FPh CH2
1-702 CH2CH=CH2 Me Et H 2,4-diFPh CH2
1-703 CH2CH=CHCH3 Me Et H Ph CH2-
1-704 CH2CH=CHCH3 Me Et H 4-FPh CH2
1-705 CH2CH=CHCH3 Me Et H 2,4-diFPh CH2-
1-706 CH2CH=CH2 Et Me H Ph CH2
1-707 CH2CH=CH2 Et Me H 4-FPh CH2
1-708 CH2CH=CH2 Et Me H 2,4-diFPh CH2
1-709 CH2CH=CHCH3 Et Me H Ph CH2
1-710 CH2CH=CHCH3 Et Me H 4-FPh CH2
1-711 CH2CH=CHCH3 Et Me H 2,4-diFPh CH2
1-712 CH2CH=CH2 Me Me H 2-Thi CH2
1-713 CH2CH=CH2 Me Me H 2-Fur CH2
1-714 CH2CH=CH2 Me Me H 3-Pyr CH2
1-715. CH2CH=CHCH3 Me Me H 2-Thi CH2
1-716 CH2CH=CHCH3 Me Me H 2-Fur CH2
1-717 CH2CH=CHCH3 Me Me H 2-Pyr CH2
1-718 CH2CH=CHCH3 Me Me H 3-Pyr CH2
1-719 CH2CH=CHCH3 Me Me H 2,6-diFPh CH2
1-720 CH2CH=CHCH3 Me Me H 3,5-diFPh CH2
1-721 CH2CH=CHCH3 Me Me H 2-C1-6-FPh CH2
1-722 CH2CH=CH2 Me Me H 4-C1-2-FPh CH2
1-723 CH2CH=CHCH3 Me Me H 4-Cl-2-FPh CH2
1-724 CH2CH=CHCH3 Me Me H 2,6-diClPh CH2
4a 2181553
1-725 CH2(2-MePr~) Me Me H 4-FPh CH2
1-726 CH2(2-MePr~) Me Me H 2,4-diFPh CH2
1-727 CH2(2-MePr~) Me Me H Ph S
1-728 CH2(2-MePr~) Me Me H Ph CH2
I-729 CH2CH=CHCH3 Me Pn H Ph 0
1-730 CH2CH=CHCH3 Me Pa H 4-FPh 0
1-731 CH2CH=CHCH3 Me Pn H 2,4-diFPh 0
1-732 CH2CH=CHCH3 Me Pn H 4-FPh CH2
1-733 CH2CH=CHCH3 Me Pn H 2,4-diFPh CH2
1-734 CH=CHCH3 H Me H 4-FPh 0
1-735 CH2Pr~ H Me H 4-FPh 0
1-736 CH2Pr~ H Me H 2,4-diFPh 0
1-737 CH2(2-MePr) H Me H 4-FPh 0
1-738 CH2(2-MePr~) H Me H 2,4-diFPh 0 -
49 2181553
[Table 2]
Example
Compd. No. R1 R2 R3 R4 RS X -
2-1 CH=CH2 Me Me H Ph 0
2-2 CH=CHCH3 Me Me H Ph 0
2-3 CH2CH=CH2 Me Me H Ph 0
2-4 CH2CH=CHCH3 Me Me H Ph 0
2-S CH2C(CH3)=CH2 Me Me H Ph 0-
2-6 CH2CH=CHCH2CH3 Me Me H Ph. 0
2-7 CH2CH=C(CH3)CH3 Me' Me H Ph 0.
2-8 CH2CH=CHPh Me Me H Ph 0
2-9 CH2CH=CH(4-FPh) Me Me H Ph 0
2-10 CH2CH=CF2 Me Me H Ph 0
2-11 CH2CH=CHC1 Me Me H Ph 0-
2-12 CH2C(C1)=CH2 Me Me H Ph 0
2-13 CH2CHtCCI2 Me Me H Ph 0
2-14 CH2C=CH Me Me H Ph 0
2-15 CH=C=CH2 Me Me H Ph 0
2-16 CH2Pro Me Me H Ph 0
2-17 Pro Me Me H Ph 0 _
2-I8 Hxo Me Me H Ph 0
2-19 CH2CH2F Me Me H Ph 0
2-20 CH2CHF2 Me Me H Ph 0
2-21 CH2CF3 Me Me H Ph 0
2-22 (CH2)3F Me Me. H Ph 0
so 21 81553
2-23 CH=CH2 Me Me H 4-FPh 0
2-24 CH=CHCH3 Me Me H 4-FPh 0
2-25 CH2CH=CH2 Me Me H 4-FPh 0
2-26 C(CH3)=CH2 Me Me H 4-FPh 0
2-27 CH=CHCH2CH3 Me. Me H 4-FPh 0
2-28 CH2CH=CHCH3 Me Me H 4-FPh 0
2-29 CH2C(CH3)=CH2 Me Me H 4-FPh 0
2-30 CH2CH=CHPh Me Me H 4-FPh 0-
2-31 CH2CH=CH(4-FPh) Me Me H 4-FPh 0
2-32 CH2CH=CF2 Me Me H 4-FPh. 0
2-33 CH2CH=CHC1 Me Me H 4-FPh 0
2-34 CH2CH=CC12 Me Me H 4-FPh 0
2-35 CH2C=CH Me Me H 4-FPh 0
2-36 CH2Pr~ Me Me H 4-FPh 0
2-37 CH2Hx~ Me Me H 4-FPh 0
2-38 Pry Me Me H 4-FPh 0
2-39 Hx ' Me Me H 4-FPh 0
2-40 (CH2)3F Me Me H 4-FPh 0
2-41 CH2CH=CH2 Me Me H 2,4-diFPh 0
2-42 ~ C(CH3)=CH2 Me Me H 2,4-diFPh 0
2-43 CH=CHCH2CH3 Me Me H 2,4-diFPh 0
2-44 CH2CH=CHCH3 Me , H 2,4-diFPh 0
Me
2-45 CH2C~i=CHPh Me Me H 2,4-diFPh 0
2-46 CH2CH=CF2 Me Me H 2,4-diFPh 0
2-47 CH2CH=CHC1 Me Me H 2,4-diFPh 0
2-48 CH2C=CH Me Me H 2,4-diFPh 0
2181553
51
2-49 CH=C=CH2 Me. Me H 2,4-diFPh 0 -
2-50 CH2Pr~ Me Me H 2,4-diFPh 0
2-51 CH2Hx~ Me Me H 2,4-diFPh 0
-
2-52 Pry Me Me H 2,4-diFPh 0
2-53 CH2CH=CH2 Me Me H 4-ClPh 0
2-54 CH2CH=CHCH3 Me Me H 4-ClPh 0
2-55 CH2Pr~ Me Me H 4-ClPh 0
2-56 CH2CH=CH2 Me Me H 2,4-diClPh 0
2-57 CH2CH=CHCH3 Me Me H 2,4-diClPh 0
2-58 CH2Pr~ Me Me H 2,4-diClPh 0
2-59 CH2CH=CH2 Me Me H 2-FPh D
2-60 CH2CH=CHCH3 Me Me H 2-FPh 0
2-61 CH2Pr~ Me Me H 2-FPh 0
2-62 CH2CH=CH2 Me Me H 3-FPh 0
2-63 CH2CH=CHCH3 Me Me H 3-FPh 0
2-64 CH2Pr~ Me Me H 3-FPh 0
2-65 CH2CH=CH2 Me Me H 2-ClPh 0
2-66 CH2CH=CHCH3 Me Me H 2-ClPh 0
2-67 CH2Pr~ Me Me H 2-CIPh 0
2-68 CH2CH=CH2 Me Me H 3-ClPh 0
2-69 CH2CH=CHCH3 Me Me H 3-ClPh 0
2-70 CH2CH=CH2 Me Me H 4-CF3Ph 0
2-71 CH2CH=CHCH3 Me Me H 4-CF3Ph 0
2-72 CH2CH=CH2- Me Et H Ph 0
2-73 CH2CH=CH2- Me Et H 4-FPh 0
2-74 CH2CH=CH2 Me Et H 2,4-diFPh 0
2181553
52
2-75 CH2CH=CHCH3 Me Et H Ph 0
2-76 CH2CH=CHCH3 Me Et H 4-FPh 0
2-77 CH2CH=CHCH3 Me Et H 2,4-diFPh 0
2-78 CH2CH=CH2 Et Me H Ph 0-
2-79 CH2CH=CH2 Et Me H 4-FPh 0
2-80 CH2CH=CH2 Et Me H 2,4-diFPh 0
2-81 CH2CH=CHCH3 Et Me H Ph 0
2-82 CH2CH=CHCH3 Et Me H 4-FPh 0
2-83 CH2CH=CHCH3 Et Me H 2,4-diFPh 0
2-84 CH2CH=CH2 Me Me H 2-Thi 0
2-85 CH2CH=CH2 Me Me H 2-Fur D
2-86 CH2CH=CH2 Me Me H 3-Pyr 0
2-87 CH2CH=CHCH3 Me Me H 2-Thi 0
2-88 CH2CH=CHCH3 Me Me H 2-Fur 0
2-89 CH2CH=CHCH3 Me Me H 2-Pyr 0
2-90 CH2CH=CHCH3 Me Me H 3-Pyr 0
2-91 CH2CH=CHCH3 Me Me H 2,6-diFPh 0
2-92 CH2CH=CHCH3 Me Me H 3,5-diFPh 0
2-93 CH2CH=CHCH3 Me Me H 2-C1-6-FPh 0
2-94 CH2CH=CH2 Me Me H 4-C1-2-FPh 0
2-95 CH2CH=CHCH3 Me Me H 4-C1-2-FPh 0
2-96 CH2CH=CHCH3 Me Me H 2,6-diClPh 0
2-97 CH2(2-MePr~) Me Me H 4-FPh 0
2-98 CH2(2-MePr~) Me Me H 2,4-diFPh 0
2-99 CH2(2-MePr~) Me Me H Ph 0
2-100 CH=CH2 Me Me H Ph S
53 zTSls ~3
2-101 CH=CHCH3 Me Me H Ph S
2-102 CH2CH=CH2 Me Me H Ph S
2-103 CH2CH=CHCH3 Me Me H Ph S
2-104 CH2C(CH3)=CH2 Me Me H Ph S
2-145 CH2CH=CHCH2CH3 Me Me H Ph S
2-106 CH2CH=C(CH3)CH3 Me Me H Ph S
2-107 CH2CH=CHPh Me Me H Ph S
2-108 CH2CH=CC12 Me Me H Ph S
2-109 CH2C=CH Me Me H Ph S
2-110 CH=C--CH2 Me Me H Ph S
2-111 CH2Pr~ Me Me H Ph S
2-112 - Pry Me Me H Ph S
2-113 Hx Me Me H Ph S
2-114 CH2CH2F Me Me H Ph S
2-115 CH2CHF2 Me Me H Ph S
2-116 CH2CF3 Me Me H Ph S
2-117 (CH2)3F Me Me H Ph S
2-118 CH=CHCH3 Me Me H 4-FPh S
2-119 CH2CH=CH2 Me Me H 4-FPh S -
2-120 CH2CH=CHCH3 Me Me H 4-FPh S
2-121 CH2C(CH3)=CH2 Me Me H 4-FPh S
2-122 CH2CH=CHPh Me Me H 4-FPh S
2-123 CH2Pr~ Me Me H 4-FPh S
2-124 CH2Hx~ Me Me H 4-FPh S
2-125 CH2CH=CH2 Me Me H 2,4-diFPh S
2-126 CH2CH=CHCH3 Me Me H 2,4-diFPh S
54 2181553
2-127 CH2CH=CH2 Me Me H 4-ClPh S
2-128 CH2CH=CHCH3 Me Me H 4-ClPh S
2-129 CH2CH=CH2 Me Me H 2,4-diClPh S
2-130 CH2CH=CHCH3 Me Me H 2,4-diClPh S
2-131 CH2CH=CH2 Me Me H 2-FPh S
2-132 CH2CH=CH2 Me Me H 3-FPh S
2-133 CH2CH=CH2 Me Me H 4-CF3Ph S
2-134 CH2CH=CH2 Me Et H Ph S
2-135 CH2CH=CHCH3 Et Me H Ph S
2-136 CH2CH=CHCH3 Et Me H 4-FPh S
2-137 CH2CH=CHCH3 Me Me H 2-Pyr S
2-138 CH2CH=CHCH3 Me Me H 2-C1-6-FPh S
2-139 CH2CH=CH2 Me Me H 4-Cl-2-FPh S
2-140 CH2CH=CHCH3 Me Me H 4-C1-2-FPh S
2-141 CH2CH=CHCH3 Me Me H 2,6-diClPh S
2-142 CH2(2-MePr~) Me Me H 4-FPh S
2-143 CH2(2-MePr~) Me Me H 2,4-diFPh S
2-144 CH2(2-MePr~) Me Me H Ph S
2-145 CH=CH2 Me Me H Ph CH2
2-146 CH=CHCH3 Me Me H Ph CH2
2-147 CH2CH=CH2 Me Me H Ph CH2
2-148 CH2CH=CHCH3 Me Me H Ph CH2
2-149 CH2C(CH3)=CH2 Me Me H Ph CH2
2-15-0 CH2CH=CHCH2CH3 Me Me H Ph CH2
2-151 CH2CH=C(CH3)CH3 Me Me H Ph CH2
2-152 CH2CH=CHPh Me Me H Ph CH2
2181553
2-153 CH2CH=CC12 Me Me H Ph CH2
2-IS4 CH2C=CH Me Me H Ph CH2
2-155 CH=C=CH2 Me Me H Ph CH2
2-156 CH2Pr Me Me H Ph CH2
2-157 Pr Me Me H Ph CH2
2-158 Hx Me Me H Ph CH2
2-159 CH2CH2F Me Me H Ph CH2
2-160 CH2CHF2 Me Me H Ph CH2
2-161 CH2CF3 Me Me H Ph CH2
2-162 (CH2)3F Me Me H Ph CH2
2-163 CH=CHCH3 Me Me H 4-FPh CH2
2-164 CH2CH=CH2 Me Me H 4-FPh CH2
2-165 CH2CH=CHCH3 Me Me H 4-FPh CH2
2-166 CH2C(CH3)=CH2 Me Me H 4-FPh CH2
2-167 CH2CH=CHPh Me Me H 4-FPh CH2
2-168 CH2Pr~ Me Me H 4-FPh CH2
2-169 CH2Hx~ Me Me H 4-FPh CH2
2-170 CH2CH=CH2 Me Me H 2,4-diFPh CH2
2-171 CH2CH=CHCH3 Me Me H 2,4-diFPh CH2
2-172 CH2CH=CH2 Me Me H 4-ClPh CH2
2-173 CH2CH=CHCH3 Me Me H 4-ClPh CH2
2-174 CH2CH=CH2 Me Me H 2,4-diClPh CH2
2-175 CH2CH=CHCH3 Me Me H 2,4-diClPh CH2
2-176 CH2CH=CH2 Me Me H 2-FPh CH2 -
2-177 CH2CH=CH2 Me Me H 3-FPh CH2
2-178 CH2CH=CH2 Me Me H 4-CF3Ph CH2
i'
56 X1 8 1553
2-179 CH2CH=CH2 Me Et H Ph CH2-
2-180 CH2CH=CHCH3 Et Me H Ph CH2
2-181 CH2CH=CHCH3 Et Me H 4-FPh CH2
2-182 CH2CH=CHCH3 Me Me H 2-Pyr CH2
2-183 CH2CH=CHCH3 Me Me H 2-C1-6-FPh CH2
2-184 CH2CH=CH2 Me Me H 4-C1-2-FPh CH2
2-185 CH2CH=CHCH3 Me Me H 4-C1-2-FPh CH2
2-186 CH2CH=CHCH3 Me Me H 2,6-diClPh CH2
2-187 CH2(2-MePr~) Me Me H 4-FPh CH2
2-188 CH2(2-MePr~) Me Me H 2,4-diFPh CH2
2-189 CH2(2-MePr~) Me Me H Ph CH2
2-190 CH2CH=CHCH3 Me Pn H 4-FPh 0
1-191 CH2CH=CHCH3 Me Pn H 2,4-diFPh 0
2-192 CH2CH=CHCH3 Me Pn H Ph CH2
2-193 CH2CH=CHCH3 Me Pn H 4-FPh CH2
2-194 CH2CH=CHCH3 Me Pn H 2,4-diFPh CH2
57 2? 8155
[Table 3]
Example
Compd. No. R1 R2 R3 R4 RS X
3-1 CH2CH=CH2 Me Me H Ph 0
3-2 CH2CH=CHCH3 Me Me H Ph 0 -
3-3 CH2C(CH3)=CH2 Me Me H Ph 0
3-4 CH2C_--CH Me Me H Ph 0
3-5 CH2Pr~ Me Me H Ph 0
3-6 CH=CH2 Me Me H 4-FPh 0
3-7 CH=CHCH3 Me Me H 4-FPh 0
3-8 CH2CH=CH2 Me Me H 4-FPh 0
3-9 CH2CH=CHCH3 Me Me H 4-FPh 0
3-10 CH2C-CH Me Me H 4-FPh 0
3-11 CH2Pr Me Me H 4-FPh 0
3-12 CH2Hx Me Me H 4-FPh 0
3-13 CH2CH=CA2 Me Me H 2,4-diFPh 0
3-14 CH2CH=CHCH3 Me Me H 2,4-diFPh 0
3-15 CH2C CH Me Me H 2,4-diFPh 0
3-16 CH2Pro Me Me H 2,4-diFPh 0
3-17 CH2CH=CH2 Me Me H 4-ClPh 0
3-18 CH2CH=CHCH3 Me Me H 4-ClPh 0
3-19 CH2Pro Me Me H 4-CIPh 0 -
3-20 CH2CH=CH2 Me Me H 2,4-diClPh 0
3-21 CH2CH=CHCH3 Me Me H 2,4-diClPh 0
3-22 CH2Pro Me Me H 2,4-diClPh 0
58
2181553
3-23 CH2CH=CHCH3 Me Me H 2,6-diFPh 0
3-24 CH2CH=CHCH3 Me Me H 3,5-diFPh 0
3-25 CH2CH=CHCH3 Me Me H 2-C1-6-FPh 0
3-26 CH2(2-MePr~) Me Me H 4-FPh 0
3-27 CH2(2-MePr~) Me Me H 2,4-diFPh 0
3-28 CH2(2-MePr~) Me Me H Ph 0
3-29 CH2CH=CH2 Me Me H Ph S
3-30 CH2CH=CHCH3 Me Me H Ph S
3-31 CH2C(CH3)=CH2 Me Me H Ph S
3-32 CH2C_--CH Me Me H Ph S
3-33 CH2Pr~ Me Me H Ph S
3-34 CH=CH2 Me Me H 4-FPh S
3-35 CH=CHCH3 Me Me H 4-FPh S
3-36 CH2CH=CH2 Me Me H 4-FPh S
3-37 CH2CH=CHCH3 Me Me H 4-FPh S
3-38 CH2C=CH Me Me H 4-FPh S
3-39 CH2Pr~ Me Me H 4-FPh S
3-40 CH2Hx~ Me Me H 4-FPh S
3-41 CH2CH=CH2 Me Me H 2,4-diFPh S
3-42 CH2CH=CHCH3 Me Me H 2,4-diFPh S
3-43 CH2C=CH Me Me H 2,4-diFPh S
3-44 CH2Pr~ Me Me H 2,4-diFPh S
3-45 CH2CH=CH2 Me Me H 4-CIPh S
3-46 CH2CH=CHCH3 Me Me H 4-ClPh S
3-47 CH2Pr Me Me H 4-ClPh S
3-48 CH2CH=CH2 Me Me H 2,4-diClPh S
59 2181553
3-49 CH2CH=CHCH3 Me Me H 2,4-diClPh S
3-50 CH2Pr~ Me Me H - 2,4-diClPh S
3-51 CH2CH=CHCH3 Me Me H 2,6-diFPh S
3-52 CH2CH=CHCH3 Me Me H 3,5-diFPh S
3-53 CH2CH=CHCH3 Me Me H 2-Cl-6-FPh S
3-54 CH2(2-MePr) Me Me H 4-FPh S -
3-55 CH2(2-MePr) Me Me H 2,4-diFPh S
3-56 CH2(2-MePr~) Me Me H Ph S
3-57 CH=CH2 Me Me H Ph CH2
3-58 CH=CHCH3 Me Me H Ph CH2
3-59 CH2CH=CH2 Me Me H Ph CH2
3-60 CH2CH=CHCH3 Me Me H Ph CH2
3-61 CH2C(CH3)=CH2 Me Me H Ph CH2
3-62 CH2CH=CHCH2CH3 Me Me H Ph CH2
3-63 CH2CH=C(CH3)CH3 - Me Me H Ph CH2
3-64 CH2CH=CHPh Me Me H Ph CH2
3-65 CH2CH=CC12 Me Me H Ph - CH2 -
3-66 CH2C=CH Me Me H Ph CH2
3-67 CH=C=CH2 Me Me H Ph CH2
3-68 CH2Pr~ Me Me H Ph CH2
3-69 Pry Me Me H Ph CH2
3-70 Hx~ Me Me H Ph CH2
3-71 CH2CH2F Me Me H Ph CH2
3-72 CH2CHF2 Me Me H Ph CH2
3-73 CH2CF3 Me Me H Ph CH2
3-74 (CH2)3F Me Me H Ph CH2
.,
60 211553
3-75 CH=CHCH3 Me Me H 4-FPh CH2
3-76 CH2CH=CH2 Me Me H 4-FPh CH2
3-77 CH2CH=CHCH3 Me Me H 4-FPh CH2
3-78 CH2C(CH3)=CH2 Me Me H 4-FPh CH2
3-79 CH2CH=CHPh Me Me H 4-FPh CH2
3-80 CH2Pr~ Me Me H 4-FPh CH2
3-81 CH2Hx~ Me Me H 4-FPh CH2
3-82-- CH2CH=CH2 Me Me H 2,4-diFPh CH2
3-83 CH2CH=CHCH3 Me Me H 2,4-diFPh CH2
3-84 CH2CH=CH2 Me Me H 4-ClPh CH2
3-85 CH2CH=CHCH3 Me Me H 4-ClPh CH2
3-86 CH2CH=CH2 Me Me H 2,4-diClPh CH2
3-87 CH2CH=CHCH3 Me Me H 2,4-diClPh CH2
3-88 CH2CH=CH2 Me Me H 2-FPh CH2
3-89 CH2CH=CH2 Me Me H 3-FPh CH2
3-90 CH2CH=CH2 Me Me H 4-CF3Ph CH2
3-91 CH2CH=CH2 Me Et H Ph . CH2
.
3-92 CH2CH=CHCH3 Et Me H Ph CH2
3-93 CH2CH=CHCH3 Et Me H 4-FPh CH2
3-94 CH2CH=CHCH3 Me Me H 2-Pyr CH2
3-95 CH2CH=CHCH3 Me Me H 2-C1-6-FPh CH2
3-96 CH2CH=CH2 Me Me H 4-C1-2-FPh CH2
3-97 CH2CH=CHCH3 Me Me H 4-C1-2-FPh CH2
3-98 CH2CH=CHCH3 Me Me H 2,6-diClPh CH2
3-99 CH2(2-MePr) Me Me H 4-FPh CH2
3-100 CH2(2-MePr~) Me Me H 2,4-diFPh CH2
3-101 CH2{2-MePr~) Me Me H Ph CH2
2181553
In these Tables above, the group names are abbreviated
as follows.
Benzimidz: Benzimidazolyl group
Benzoxaz: Benzoxazolyl group
Benzothiaz: Benzothiazolyl group
Bu: Butyl group
Buc: Cyclobutyl group
Bul: Isobutyl group
Bus: s-Butyl group
But: t-Butyl group
Et: Ethyl group
Fur: Furyl group
Hxc: Cyclohexyl group
Hxec: Cyclohexenyl group
Hptec: Cycloheptenyl group
Hpc: Cycloheptyl group
Imidz: Imidazolyl group
Me: Methyl group
Naph: Naphthyl group
Oxaz: Oxazolyl group
Pntec: Cyclopentenyl group
Ph: Phenyl group
Pn: Pentyl group
Pnc: Cyclopentyl group
' ~~ 2181553
62
Pr: Propyl group
Prc: Cyclopentyl group
Prl: Isopropyl group
Pyr: Pyridyl group
Pyraz: Pyrazinyl group
Pyridz: Pyridazinyl group
TDA: Thiadiazolyl group
Thi: Thienyl group
Thiaz: Thiazolyl group
Among the compounds listed above:
preferable compounds are as follows: Compounds Nos.
1-1, 1-2, 1-3, 1-6, 1_-11, 1-13, 1-18, 1-19, I-24, 1-36,
1-37, 1-38, 1-39, 1-40, 1-42, 1-45, 1-48, 1-51, 1-59,
1-62, 1-68, 1-69, 1-70, 1-71, 1-76, 1-77, 1-78, 1-79,
1-80, 1-81, 1-86, 1-94, 1-99, 1-111, 1-112, 1-115, 1-12Q,
1-126, 1-129, 1-I34, 1-137, 1-146, 1-153, 1-I54, 1-I55,
1-156, 1-169, 1-186, I-187, 1-195, 1-198, 1-201, 1-204,
1-209, 1-226, 1-227, 1-229, 1-231, 1-232, 1-234, 1-236,
1-237, 1-239, 1-241, 1-242, 1-244, 1-246, 1-247, 1-249,
1-251, 1-252, 1-259, 1-260, 1-263, 1-278, 1-293, 1-308,
1-323, 1-338, 1-353, 1-368, 1-383, 1-398, 1-413, 1-428,
1-445, 1-447, 1-449, 1-451, 1-458, 1-460, 1-461, 1-462, -
1-464, 1-466,
2181553
63
1-473, 1-475, 1-478, 1-488, I-490, 1-492, 1-493, 1-503,
1-504, 1-505, 1-506, 1-507, 1-523, 1-524, 1-526, 1-539,
1-540, I-619, 1-623, 1-63I, 1-632, I-644, 1-653, 1-656,
1-664, 1-669, 1-672, 1-678, 1-725, 1-726, 1-728, 1-729,
1-734, 2-3, 2-4, 2-5, 2-6, 2-8, 2-13, 2-14, 2-16, 2-17,
2-21, 2-24, 2-25, 2-28, 2-36, 2-38, 2-41, 2-44, 2-50,
2-52, 2-53, 2-54, 2-55, 2-56, 2-57, 2-58, 2-59, 2-60,
2-61, 2-65, 2-66, 2-67, 2-76, 2-93, 2-94, 2-95, 2-96,
2-97, 2-98, 2-1I9, 2-120, 2-123, 2-126, 2-127, 2-128,
2-130, 2-I38, 2-139, 2-140, 2-142, 2-143, 2-147, 2-148,
2-156, 2-164, 2-165, 2-168, 2-170, 2-171, 2-173, 2-I75,
2-183, 2-184, 2-185, 2-186, 2-187, 2-188, 2-189, 3-59,
3-60, 3-.68, 3-76, 3-77, 3-80, 3-82, 3-83, 3-99, 3-100 and
3-101;
more preferable compounds are as follows: Compounds
Nos. 1-1, 1-3, 1-6, 1-11, I-13, 1-18, 1-19; I-39, 1-40, -
1-42, 1-45, 1-48, 1-51, 1-59, 1-62, 1-68, 1-69, 1-70,
1-71, 1-77, 1-78, 1-81, 1-86, 1-94, 1-126, 1-129, 1-I53,
1-156, 1-226, 1-231, 1-232, 1-236, 1-241, 1-259, 1-263,
1-323, 1-413, 1-445, 1-447, 1-449, 1-451, 1-458, 1-460,
1-462, 1-464, 1-466, 1-492, 1-505, 1-507, 1-523, 1-524,
1-526, 1-539, 1-540, 1-619, 1-623, 1-631, 1-632, 1-644, -
1-653, 1-656, 1-664, 1-669, 1-672, 1-678, 1-725, 1-726,
1-728, 1-729, 2-3, 2-4, 2-5, 2-6, 2-8, 2-13, 2-14, 2-16,
2-17, 2-21, 2-24, 2-25, 2-28, 2-36, 2-41, 2-44, 2-50,
2-53, 2-54, 2-56, 2-57, 2-59, 2-76, 2-93, 2-94, 2-95,
2-96, 2-97,
281553
64
2-98, 2-119, 2-120, 2-126, 2-I27, 2-128, 2-I30, 2-138,
2-139, 2-I40, 2-I42, 2-143, 2-148, 2-I64, 2-165, 2-168,
2-171, 2-187, 3-60, 3-76, 3-77, 3-83, 3-99, 3-I00 and
3-101;
much more preferable compounds are as follows:
Compounds Nos. I-3, 1-6, 1-11,-1-13, 1-19, 1-39, 1-40,
I-42, 1-45, 1-51, 1-59, 1-70, 1-77, 1-78, 1-81, 1-126,
I-153, 1-156, 1-226, 1-231, 1-232, 1-236, 1-323, 1-445,
I-447, 1-449, I-451, 1-460, 1-462, 1-464, 1-466, I-505,
1-523, I-524, 1-526, 1-539, 1-540, 1-619, 1-623, 1-631, -
1-632, 1-644, 1-653, 1-656, 1-664, 1-669, 1-672, 1-678,
1-725, 1-726, 1-728, 2-4, 2-5, 2-16, 2-24, 2-25, 2-28,
2-36, 2-41, 2-44, 2-50, 2-53, 2-56, 2-76, 2-93, 2-95,
2-97, 2-98, 2-119, 2-120, 2-148, 2-164, 2-165, 2-168,
2-171, 2-187, 3-60, 3-77 and 3-83; and -
particularly preferable componds are as follows; -
Compound No. 1-6: 1-(2-BUtenyl)-7-benzyloxy-2,3-
dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-11: 7-Benzyloxy-2,3-dimethyl-1-(2-
methyl-2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-45: 7-Benzyloxy-2,3-dimethyl-1-(2-
propynyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-51: 7-Benzyloxy-1-cyclopropylmethyl-2,3-
dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-77: 7-(4-Fluorobenzyloxy)-2,3-
dimethyl-1-(1-propenyl)pyrrolo[2,3-d]pyridazine;
218155.3
Compound No. 1-78: 7-(4-Fluorobenzyloxy)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-81: 1-(2-Butenyl)-7-(4-
fluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-126: 1-Cyclopropylmethyl-7-(4-
fluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-153: 7-(2,4-Difluorobenzyloxy)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-156: 1-(2-Butenyl)-7-(2,4-
difluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-226: 7-(4-Chlorobenzyloxy)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-231: 7-(2,4-Dichlorobenzyloxy)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-232: 1-(2-Butenyl)-7-(2,4-
dichlorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-236: 7-(2-Fluorobenzyloxy)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-323: 1-(2-Butenyl)-3-ethyl-7-(4-
fluorobenzyloxy)-2-methylpyrrolo[2,3-d]pyridazine;
Compound No. 1-445: 7-(4-Fluorobenzylthio)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-460: 1-(2-Butenyl)-7-(4-
fluorobenzylthio)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-462: 1-(2-BUtenyl)-7-(2,4-
difluorobenzylthio)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
2181553
66
Compound No. 1-505: 1-(2-BUtenyl)-7-(2-chloro-6-
fluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-524: 1-(2-BUtenyl)-7-(4-chloro-2-
fluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-539: 7-(4-Fluorobenzyloxy)-2,3-
dimethyl-1-(2-methylcyclopropylmethyl)pyrrolo[2,3-
d]pyridazine;
Compound No. 1-540: 7-(2,4-Difluorobenzyloxy)-2,3-
dimethyl-1-(2-methylcyclopropylmethyl)pyrrolo[2,3-
d]pyridazine;
Compound No. 1-631: 2,3-Dimethyl-7-phenethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-632: 1-(2-Butenyl)-2,3-dimethyl-7-
phenethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-653: 7-(4-Fluorophenethyl)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-656: 1-(2-Butenyl)-7-(4-
fluorophenethyl)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-664: 1-Cyclopropylmethyl-7-(4-
fluorophenethyl)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-669: 7-(2,4-Difluorophenethyl)-2,3-
dimethyl-1-(2-propenyl)pyrrolo[2,3-d]pyridazine;
Compound No. 1-672: 1-(2-Butenyl)-7-(2,4-
difluorophenethyl)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
Compound No. 1-678: 1-Cyclopropylmethyl-7-(2,4-
difluorophenethyl)-2,3-dimethylpyrrolo[2,3-d]pyridazine;
2181555
67
Compound No. 1-725: 7-(4-Fluorophenethyl)-2,3-
dimethyl-1-(2-methylcyclopropylmethyl)pyrrolo[2,3-
d]pyridazine;
Compound No. 1-726: 7--(2,4-Difluorophenethyl)-2,3-
dimethyl-1-(2-methylcyclopropylmethyl)pyrrolo[2,3-
d]pyridazine;
Compound No. 1-728: 2,3-Dimethyl-1-(2-
methylcyclopropylmethyl)-7-phenethylpyrrolo[2,3-d]-
pyridazine;
Compound No. 2-28: 1-(2-Butenyl)-7-(4-
fluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine-5-
oxide;
Compound No. 2-44: 1-(2-Butenyl)-7-(2,4-
difluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine-5-
oxide;
Compound No. 2-171: I-(2-Butenyl)-7-(2,4-
difluorophenethyl)-2,3-dimethylpyrrolo[2,3-d]pyridazine-5-
oxide; and
Compound No. 3-83: 1-(2-Butenyl)-7-(2,4-
difluorophenethyl)-2,3-dimethylpyrrolo[2,3-d]pyridazine-6-
oxide .
The pyrrolopyridazine derivatives in the present
invention can easily be prepared by the methods summarized
in the following reaction scheme:
68 ~i8~~~3
Method A
Step A1
Y + Rs-A-Xa H
g Step A2 RS-A-Xa
R -A-X R
, (Ia) t0)
m 1 (Ib)
(O)~~
Method B
Rs-A- ~ Step B?
R -Z
M (v)
Rs-A-
(Ia)
2?815'3
69
Method C
COR4
STEP C1
---~ RS-A-C
R2/ 'N'
R' Y (~
Rs-A (Ic)
STEP C2
In the above formulae,
R1, R2, R3, R4, RS and A are as defined above;
Xa represents an imino group, an oxygen or sulfur atom;
Y represents a halogen atom (preferably chlorine, bromine
or iodine);
Z represents a halogen atom (preferably chlorine, bromine
or iodine); a C1-C4alkanesulfonyloxy group optionally
substituted with halogen atoms) (such as
methanesulfonyloxy, ethanesulfonyloxy, propanesulfonyloxy,
butanesulfonyloxy, trifluoromethanesulfonyloxy or
trichloromethanesulfonyloxy); a C6-Cl~azylsulfonyloxy
group (such as benzenesulfonyloxy or
p-toluenesulfonyloxy); or a halogeno-acetoxy group (such
as trifluoroacetoxy or trichloroacetoxy);
m' is o or 1; and
~O)n yu~
2181553
n' is 0 or 1, provided that both of m' and n' are not
concurrently 0.
Method A involves the preparation of the compounds of
formulae (Ia) and (Ib), that is, a formula (I) wherein X
represents an imino group, an oxygen or a sulfur atom.
Step A1 is a step to prepare a compound of formula
(Ia), that is, a formula (I) wherein X represents an imino
group, an oxygen or a sulfur-atom and n is 0, by reacting
a compound of general formula (II) with a compound of
general formula (III) in a solvent or without solvent in
the presence or absence of a base.
The base used in this step may be, for example, an
alkali metal hydride such as lithium hydride, sodium
hydride or potassium hydride; alkali metal amides such as
lithium amide, sodium amide or potassium amide; alkali
metal carbonates such as sodium carbonate, potassium
carbonate or lithium carbonate; alkali metal alkoxides
such as sodium methoxide, sodium ethoxide, potassium
tert-butoxide or lithium ethoxide; or organic amines such
as triethylamine, tributylamine, diisopropylethylamine, -
N-methylmorpholine, pyridine, picoline,
4-(N,N-dimethylamino)pyridine, 2,6-di(tert-butyl)-4-
methylpyridine, quinoline, N,N-dimethylaniline,
N,N-diethylaniline, 1,5-diazabicyclo[4.3.0]non-5-ene
(DBN), 1,4-diazabicyclo[2.2.2]octane (DABCO) or
1,8-diazabicyclo[5.4.0]undec-7-ene (DBU); preferably an
2181.553
71
alkali metal hydride (particularly sodium hydride) or
alkali metal alkoxide (particularly potassium
tert-butoxide). The reaction in this reaction proceeds in -
the absence of a base. In order to carry out efficiently
the reaction, it may be conducted in the presence of
quaternary ammonium salts such as benzyltriethylammonium
chloride or tetrabutylammonium chloride, crown ethers such
as 18-crown-6 or dibenzo-18-crown-6 etc.
There is no particular limitation upon the nature of _
the solvent used in this step, provided that it has no
adverse effect upon the reaction. Examples of suitable
solvents include: for example, aliphatic hydrocarbons such
as hexane, heptane, ligroin or petroleum ether; aromatic -
hydrocarbons such as benzene, toluene or xylene;
halogenated hydrocarbons such as dichloromethane,
chloroform, carbon tetrachloride, dichloroethane,
chlorobenzene or dichlorobenzene; ethers such as diethyl
ether, diisopropyl ether, tetrahydrofuran, dioxane,
dimethoxyethane or diethylene glycol dimethyl ether;
ketones such as acetone, methyl ethyl ketone, methyl
isobutyl ketone, isophorone or-cyclohexanone; nitriles
such as acetonitrile or isobutyronitrile; amides such ae
formamide, dimethylformamide, dimethylacetamide,
N-methyl-2-pyrrolidone or hexamethylphosphoric triamide;
sulfoxides such as dimethylaulfoxide or sulfolane; and
mixtures of two or more of these organic solvents; and
'2 2181553
preferably ethers (particularly tetrahydrofuran or
dioxane).
The compound of formula (Ia) may also be prepared by
reacting a compound of formula (III) wherein Xa is an
oxygen or sulfur atom, with an alkali metal (preferably
sodium) in the presence of a solvent (preferably ethers)
to give the corresponding alkolate or thiolate and
subsequently by reacting the product with a compound of
formula (II).
The reaction temperature is usually from 0° to 250°C
(preferably from room temperature to 200°C). The time
required for the reaction varies depending upon the
reaction temperature and other factors, but it is from one
minute to 50 hours (preferably from 5 minutes to 30 hours).
Where a compound of formula (II) wherein Rl is an
alkenyl or alkynyl group, is used as a reactant, a
compound of formula (Ia) produced may be converted to an
isomer by isomerization.
After completion of the reaction, the desired compound
of formula (Ia) in this reaction may be recovered from the
reaction mixture by conventional means. An example of one
such technique comprises: filtering conveniently off
insoluble material, if any; and distilling off the solvent
211553
73
under reduced pressure; or-after distilling off the
solvent under reduced pressure, adding water to the
residue; extracting with a Water-immiscible organic
solvent such as ethyl acetate; drying the extract over
anhydrous magnesium sulfate or the like; and finally
distilling off the solvent. The product, if necessary, -
may be purified by conventional means such as
recryatallization, column chromatography and the like.
Step A2 is a step to prepare a compound of formula
(Ib), that is, a compound of formula (I) wherein X
represents an imino group, an oxygen or sulfur atom; m is
m'; and n is n' (m' and n' are as defined above), by
reacting a compound of formula (Ia) with an oxidizing
agent in the presence of an inert solvent.
Examples of oxidizing agents used include: for-
example, peroxy acids such as peracetic acid, perbenzoic
acid or m-chloroperoxybenzoic acid; hydrogen peroxide; or
alkali metal salts of peroxyhalogenous acid such as sodium
meta-perchlorate, sodium meta-periodate or potassium
meta-periodate; preferably peroxy acids or hydrogen
peroxide; and particularly preferably
m-chloroperoxybenzoic acid.
There is no particular limitation upon the nature of
the solvents used in this step, provided that it has no
adverse effect upon the reaction and may dissolve the
starting material to some extent. Examples of suitable
r
~1$15~3
solvents include: for example, hydrocarbons such as
hexane, benzene, toluene or xylene; halogenated
hydrocarbons such as dichloromethane, chloroform, carbon
tetrachloride, dichloroethane, chlorobenzene or -
dichlorobenzene; alcohola such as methanol, ethanol,
propanol or butanol; esters such as ethyl acetate, propyl
acetate, butyl acetate or ethyl propionate; carboxylic
acids such as acetic acid or propionic acid; water; and
mixtures of two or more of these solvents; and preferably
halogenated hydrocarbons (particularly dichloromethane or
chloroform) or carboxylic acids (particularly acetic acid).
The reaction temperature is usually from -20° to 150°C
(preferably from 0°C to 100°C). The time required for the
reaction varies depending upon the reaction temperature
and other factors but it is from 10 minutes to 5 hours
(preferably from 20 minutes to 2 hours).
After completion of the reaction, the desired compound
of formula (Ib) in this reaction may be recovered from the
reaction mixture by conventional means. An example of one
such technique comprises: filtering conveniently off
insoluble material, if any; and distilling off the solvent
under reduced pressure; or after distilling off the
solvent under reduced pressure, adding water to the
residue; extracting with a water-immiscible organic
?181553
solvent such as ethyl acetate; drying the extract over
anhydrous magnesium sulfate or the like; and finally
distilling off the solvent. The product, if necessary,
may be purified by conventional means such as
recrystallization, column chromatography and the like.
Method B is an alternative method to prepare a
compound of formula (Ia).
Step B1 is a step to prepare a compound of formula
(Ia) by reacting a compound of general formula (IV) with a
compound of general formula (V) in an inert solvent or
without solvent in the presence or absence of a base. The
reaction may be carried out in a similar manner to that of
Step A1 in Method A.
Method C is a method to prepare the compounds of
formulae (Ic) and (Id) that is, a compound of formula (I)
wherein X represents a methylene group.
Step C1 is a step to prepare a compound of formula
(Ic) by reacting a compound of formula (VI) with hydrazine
or its hydrate in an inert solvent.
There is no particular limitation upon the nature of
the inert solvent used in this step, provided that it has
no adverse effect upon the reaction and may dissolve the
starting material to some extent. Examples of suitable
solvents include: for example, ethers such as diethyl
ether, diisopropyl ether, tetrahydrofuran, dioxane,
dimethoxyethane or diethylene glycol dimethyl ether;
21$1553
76
alcohols such as methanol, ethanol, propanol or butanol;
carboxylic acids such as acetic acid or propionic acid;
amides such as formamide, dimethylformamide,
dimethylacetamide, N-methyl-2-pyrrolidone or
hexamethylphosphoric triamide; amines such as
triethylamine or pyridine; and water; and preferably
alcohola (particularly ethanol) or carboxylic acids
(particularly acetic acid).
The reaction temperature is usually from -50° to 150°C __
(preferably from -10° to 100°C). The time required for
the reaction varies depending upon the reaction
temperature and other factors, but it is from 10 minutes
to 12 hours (preferably from 30 minutes to 5 hours).
After completion of the reaction, the desired compound
of formula (IC) in this reaction may be recovered from the
reaction mixture by conventional means. An -example of one
such technique comprises: filtering conveniently off
insoluble material, if any; and distilling off the solvent
under reduced pressure; or after distilling off the
solvent under reduced pressure, adding water to the
residue; extracting with a water-immiscible organic
solvent such as ethyl acetate; drying the extract over
anhydrous magnesium sulfate or the like; and finally
distilling off the solvent. The product, if necessary,
1
2181553
may be purified by conventional means such as
recrystallization, column chromatography and the like.
Step C2 is a step to prepare a compound of formula
(Id), that is, a compound of formula (I) wherein X
represents a methylene group; m is m'; and n is n' (m' and
n' are as defined above), by reacting a compound of
formula (Ic) with an oxidizing agent in an inert solvent
and the step may be carried out in a similar manner to
that of step A2.
The starting materials of formulae (II), (IV) and
(VI), may easily be prepared by methods summarized in the
following reaction scheme:
Method D
R3 Rs
STEP D1 R3 CHO
STEP D2
2~ 2 ~ s Rz~N COzRs
R 0 R Y R NHCHZC02R
R~ (X)
2
R, R
STEP D3 R ( ~ COR STEP D4 \N ~ Rs
~ RZ N COzRs O
RW~ HNwN~ Ra (XIn
STEP D5
Y
2181553
Method E
3
Ra CHO R
Step E1
S tep E2
R2 y R~NHCH2COzRs R2 ~ CO2Rs
(~ (IXa) R~ (Xa)
R3
Step E3 R~/CORd Step E4
R2 N CO2Rs ~ 2~ ~ s -a
H R N CO2R
(Xb)
H (XIa)
Step ES
Y
(XIIa) , :a)
Ste~ Rs-A-X2
RS-A-Xa H
Method F
O
Step F1 II
----~. CH3CC=CHOM
O HCO2Rs CH3
(VIIa) ~ (XIV)
Step F2
CH3CCHZCO2Rs -. CH3CCC02Rs
(X~ NOH
C H3
Step F3
CH3 N CO2Rs
H (Xc)
79
2?81553
Method G
Method H
4
R3 I I COR Step G1 R3 I I COR4
R2~N~COzRs R -Z (~ Rz~N COzRs
I
H (XIa) ~ R' (XI)
R3 R3.
Step H1
z~ I O I ~ ~CH2-A-Rs
R H Y-C-CH2 A-RS R H O (XIX)
(
Steg H2
' -A-Rs Step H3
R' Z M
KX)
A-Rs
Method I
Method J
Step I1
R NHz ~- YCHZCOZRs ~ R' NHCHZCOzRs
cxxn (xxm
g Step ,71
R -Y ..~. H2NCHzC02R ~ R NHCHZCOzR
(XXIIn (XXM (IXa)
as 218155
In the above formulae, Rl, R2, R3, R4, R5,
A, Xa, Y and Z are as defined above;
R6 represents a C1-C6 alkyl group;
R~ represents an amino-protecting group, and preferably
a tert-butoxycarbonyl group, a C6-arylmethyl group such
as a benzyl, p-methoxybenzyl or p-bromobenzyl or a
C6-arylmethoxycarbonyl group such as benzyloxycarbonyl,
p-methoxybenzyloxycarbonyl or a p-bromobenzyloxycarbonyl;
and
M represents an alkali metal such as lithium, sodium or
potassium (preferably sodium).
Method D is a method to prepare a compound of formula
(II) .
Step D1 is a step to prepare a compound of general
formula (VIII) by reacting a compound of general formula
(vII) with a Vilsmeier reagent such as phosphorus
oxychloride-dimethylformamide, phosphorus
oxybromide-dimethylformamide or oxalyl
chloride-dimethylformamide in an inert solvent (for
example, halogenated hydrocarbons such as dichloromethane,
chloroform, carbon tetrachloride or 1,2-dichloroethane; or
amides such as a dimethylformamide) at from -10° to 150°C-
(preferably from 0°C to 100°C) for from 15 minutes to 12
hours (preferably from 30 minutes to 5 hours).
Step D2 is a step to prepare a compound of general
formula (X) by reacting a compound of formula (VIII) with
2181553
a compound of general formula (IX) in an inert solvent
(for example, aromatic hydrocarbons such as benzene or
toluene; halogenated hydrocarbons such as dichloromethane
or chloroform; ethers such as diethyl ether, diisopropyl
ether, tetrahydrofuran or dioxane; alcohols such as
methanol, ethanol or propanol; amides such as
dimethylformamide or dimethylacetamide; or amines such as
triethylamine or pyridine) in the presence of a base (for _ _ .
example, organic amines such as triethylamine or pyridine)
at a temperature of from -10° to 150°C (preferably from
0°
to 50°C) for from 30 minutes to 24 hours (preferably 1 to -
hours).
Step D3 is a step to prepare a compound of general
formula (XI). A Compound of formula (XI) wherein R4 is
a hydrogen atom, may be prepared by reacting a compound of
formula (X) with a Vilsmeier reagent in a similar manner -
to Step D1. A compound of formula (XI) wherein R4
represents a C1-C6 alkyl group may be prepared by
reacting a compound of formula (X) with an acid anhydride-
or acid halide having a formula:
(R4aC0)20 or R4aCOY
(wherein Y is as defined above, and R4a represents a
C1-C6 alkyl group) in an inert solvent (for example,
aromatic hydrocarbons such as benzene, toluene or
2181553
82
nitrobenzene; halogenated hydrocarbons such as
dichloromethane, chloroform, carbon tetrachloride 9r
1,2-dichloroethane; or carbon disulfide) in the presence
of a Lewis acid (for example, aluminium chloride, stannic -
chloride or zinc chloride) at from -10° to 150°C
(preferably from 0° to 100°C) for from 10 minutes to 12
hours (preferably 30 minutes to 5 hours).
Step D4 is a step to prepare a compound of general
formula (XII) by reacting a compound of formula (XI) with
hydrazine or its hydrate in an inert solvent in a similar
manner to Step C1 in Method C described above.
Step D5 is a step to prepare a compound of formula
(II) by reacting a compound of formula (XII) with a
halogenating agent (for example, phosphorus oxychloride,
phosphorus oxybromide, oxalyl chloride, thionyl chloride,
phosphorus pentachloride or phosphorus pentabromide) in an
inert solvent (for example, halogenated hydrocarbons such
as dichloromethane or chloroform; ethers such as diethyl
ether, tetrahydrofuran or dioxane; amides such as
dimethylformamide or dimethylacetamide; or aulfoxides such
as dimethylaulfoxide) or without solvent at from 10° to
150°C (preferably from 50° to 120°C) for from 30 minutes
to 12 hours (preferably from 1 to 5 hours).
' 2181553
83
Method E is a method to prepare a compound of formula
(IV) .
Step E1 is a step to prepare a compound of general
formula (Xa) by reacting a compound of general formula
(VIII) with a compound of general formula (IXa) in a
similar manner to Step D2 in Method D described before. -
Step E2 is a step to prepare-a compound of general
formula (Xb) by removing the amino-protecting group of a
compound of formula (Xa).
When the amino-protecting group is a
tert-butoxycarbonyl group, it may be removed by treating
with an acid (for example, inorganic acids such as
hydrogen chloride, hydrochloric acid, sulfuric acid or
nitric acid; or organic acids such as acetic acid,
trifluoroacetic acid,-methanesulfonic acid or
p-toluenesulfonic acid) in an inert solvent (for example,
halogenated hydrocarbons such as dichloromethane,
chloroform or carbon tetrachloride; or ethers such as
ether, tetrahydrofuran or dioxane) at a temperature of
from -10° to 100°C (preferably from -5° to 50°C)
for from
minutes to 48 hours (preferably from 30 minutes to 10 -
hours) .
When the amino-protecting group is a C6 arylmethyl
or C6 arylmethoxycarbonyl group, it may be removed by
reacting with hydrogen of from 1 to-10 atmospheric
pressures in the presence of a catalyst (for example,
palladium on charcoal, palladium black, platinum oxide,
platinum black or the like, preferably palladium on
$4 2181553
charcoal) in an inert solvent (for example, alcohols such
as methanol, ethanol or isopropanol; ethers such as ether,
tetrahydrofuran or dioxane; or mixtures of two or more of
these solvents) from 0° to 100°C (preferably from 20° to
70°C) for from 5 minutes to 48 hours (preferably from 1 to
24 hours).
Step E3 is a step to prepare a compound of general
formula (XIa) by acylating a compound of formula (Xb) in a
similar manner to Step D3 in Method D described before.
Step E4 is a step to prepare a compound of general
formula (XIIa) by reacting a compound of formula (XIa)
with hydrazine or its hydrate in a similar manner to Step
C1 in Method C described before.
Step ES is a step to prepare a compound of general
formula (IIa) by reacting a compound of formula (XIIa)
with a halogenating agent in a similar mannerto Step DS
in Method D described before.
Step E6 is a step to prepare a compound of general -
formula (IV) by reacting a compound of formula (IIa) with
a compound of formula (III) in a similar manner to Step A1
in Method A described before.
Method F is a method to prepare a compound of formula
(Xc) which is an intermediate in Method E, that is, a
compound of formula (Xb) wherein each of R2 and R3 is
a methyl group.
Step F1 is a step to prepare a compound of general
2181~~3
formula (XIV) by reacting a compound of general formula
(VIIa) with a compound of general formula (XIII) in an
inert solvent (for example, hydrocarbons such as hexane,
benzene or toluene; ethers such as diethyl ether,
diisopropyl ether, tetrahydrofuran, dioxane,
dimethoxyethane or diethylene glycol dimethyl ether; or
amides such as dimethylformamide or dimethylacetamide) in
the presence of a base (for example, alkali metals such as
lithium, sodium or potassium; alkali metal hydrides such
as lithium hydride, sodium hydride or potassium hydride;
alkali metal amides such as lithium amide, sodium amide or
potassium amide; or alkali metal alkoxides such as lithium
ethoxide, sodium methoxide, sodium ethoxide or potassium
tent-butoxide) at from -10° to 100°C (preferably from 0°
to 50°C) for from 30 minutes to 48 hours (preferably from
2 to 20 hours).
Step F2 is a step to prepare a compound of general
formula (XVI) by reacting a compound of general formula
(XV) with an alkali metal nitrite (for example, lithium
nitrite, sodium nitrite, potassium nitrite or the like) in
an inert solvent (for example, ethers such as diethyl
ether, tetrahydrofuran, dioxane or dimethoxyethane;
carboxylic acids such as acetic acid or propionic acid;
amides such as dimethylformamide or dimethylacetamide;
water; or mixtures of two or more of these solvents) at
from -20° to 50°C (preferably from 0° to 20°C) for
from 15
' ~ 2181553
86
minutes to 48 hours (preferably from 30 minutes to 20
hours).
Step F3 is a step to prepare a compound of formula
(Xc) by reacting a compound of formula (XVI) with a
compound of formula (XIV) in an inert solvent (for
example, ethers such as diethyl ether, tetrahydrofuran,
dioxane or dimethoxyethane; carboxylic acids such as
acetic acid or propionic acid; amides such as
dimethylformamide or dimethylacetamide; water; or mixtures
of two or more of these solvents) in the presence of a
reducing agent (for example, zinc, tin, iron or the like)
at from 20° to 150°c (preferably from 50° to
100°c) for
from 30 minutes to 10 hours (preferably from 1 to 5 hours).
Method G is an alternative method to prepare a
compound of formula (XI) which is an intermediate in
Method D.
Step G1 is a step to prepare a compound of formula
(XI) by reacting a compound of general formula (XIa) with
a compound of formula (V) in a similar manner to Step Bl
in Method B described before.
Method H is a method to prepare a compound of formula
(VI) .
Step H1 is a step to prepare a compound of general -
formula (XIX) by reacting a compound of general formula
(XVII) with a Grignard reagent having a general formula:
! 87 2I81553
R6-Mg-Y
(wherein R6 to Y are as defined above) in an inert
solvent (for example, ethers such as diethyl ether,
diisopropyl ether, tetrahydrofuran, dioxane or
dimethoxyethane) at from -10° to 100°C (preferably from
0°
to 70°C) for from 10 minutes to 6 hours (preferably from
20 minutes to 2 hours) to give a magnesium compound and
subsequently by reacting the magnesium compound with a
compound of general formula (XVIII) at from -100° to 50°C
(preferably from -78° to 0°C) for from 10 minutes to 6
hours (preferably from 30 minutes to 3 hours).
Step H2 is a step to prepare a compound of general
formula (XX) by reacting a compound of formula (XIX) with
a compound of formula (V) in a similar manner to Step B1
in Method B described before.
Step H3 is a step to prepare a compound of general
formula (VI). A compound of formula (VI) wherein R4
represents a hydrogen atom may be prepared by reacting a
compound of formula (XX) with a Vilsmeier reagent in a
similar manner to Step D1 in Method described before. A
compound of formula (VI) wherein R4 represents a
C1-C6 alkyl group may be prepared by reacting a
compound of formula (XX) with a compound having formula:
' X181553
88
(R4aC0)20 or R4aC0Y
(wherein R4a and Y are as defined above) in a similar -
manner to Step D3 in Method D described before and
subsequently by reacting the product with a Vilsmeier
reagent in a similar manner as.Step D1 of Method D
described before.
Method I is a method to prepare a compound of formula
(IX) which is a starting compound in Method D.
Step I1 is a step to prepare a compound of formula
(IX) by reacting a compound of general formula (XXI) with
a compound of general formula (XXII) in an inert solvent
(for example, hydrocarbons such as hexane, benzene or
toluene; halogenated hydrocarbons such as dichloromethane
or chloroform; ethers such as diethyl ether, diisopropyl
ether, tetrahydrofuran, dioxane or dimethoxyethane;
ketones such as acetone or methyl ethyl ketone; amides
such as dimethylformamide or dimethylacetamide; or -
sulfoxides such as dimethylsulfoxide) in the presence or
absence of a base (for example, alkali metal carbonates
such as lithium carbonate, sodium carbonate or potassium
carbonate; or organic amines such as triethylamine,
tributylamine, diisopropylethylamine, N-methylmorpholine,
pyridine, picoline or 4-(N,N-dimethylamino)pyridine) at
from -10° to 150°C (preferably from 0°C to 100°C)
for from
30 minutes to 48 hours (preferably from 1 to 20 hours).
2181553
89
Method J is a method to prepare a compound of general
formula (IXa) which is a starting compound in Method E.
Step J1 is a step to prepare a compound of formula
(IXa) by reacting a compound of general formula (XXIII)
with a compound of general formula (XXIV) in a similar
manner to Step I1 in Method I described before.
A compound of formula (Ia), (Ib), (IC), (Id), (II),
(VI), (X), (XI) or (XII), wherein R1 represents a
halogeno-alkyl group, if desired, may be
dehydrohalogenated by treating with a base (for example,
organic amines such as DBN, DBU, DABCO or the like) in an
inert solvent (for example, ethers such as diethyl ether,
tetrahydrofuran or dioxane) at from 0° to 150°C
(preferably from 50° to 100°C) for from 30 minutes to 20
hours (preferably from 1 to 10 hours) to give an alkenyl
derivative.
After completion of the reaction, each of the desired
compounds in the above reactions may be recovered from the
reaction mixture by conventional means. For example, one
such techniqv.e comprises: filtering conveniently off -_
insoluble material, if any; and distilling off the solvent
under reduced pressure; or after distilling off the
solvent under reduced pressure, adding water to the
residue; extracting with a water-immiacible organic
21~i~53
solvent such as ethyl acetate; drying the extract over-
anhydrous magnesium sulfate etc.; and finally distilling
off the solvent. The product, if necessary, may be
purified by conventional means, such as recrystallization,
column chromatography or the like.
(Effect of Invention)
The pyrrolopyridazine derivatives of the present
invention have an excellent gastric secretion inhibiting
acitivty, gastric mucosa protective activity and
antibacterial activity against Helicobacter ~rlori.
Therefore, the derivatives are useful as a preventive and
therapeutic agent for ulcerous diseases such as peptic
ulcer, acute or chronic gastric ulcer, duodenal ulcer,
gastritis, reflux esophagitis, gastro-esophageal
parareflexia, dyspepsia, gastric hyperacidity,
Zollinger-Ellison syndrome etc., as a preventive agent for
postoperative ulcerous diseases or as an antibacterial
agent against Helicobacter p~rlori.
[Possible usefulness in industry]
As mentioned above, the pyrrolopyridazine derivatives
(I) of the present invention have an excellent gastric
secretion inhibiting activity and so on, and the
derivatives are useful as a preventive or therapeutic
agent for ulcerous diseases. The mode of administration
of the pyrrolopyridazine derivatives (I) to use as a
2181553
91
preventive or therapeutic agent for ulcerous diseases, may
be oral administration by use of, for example, tablets,
capsules, granules, powders, syrups etc.; or parenteral
administration by use of, for example, injections. These
drug preparations can be prepared according to
conventional means by use of additives including: vehicles
such as lactose, mannite, corn starch, crystalline
cellulose etc.; binders such as cellulose derivatives, gum
arabic, gelatin etc.; diaintegrators such as calcium
carboxymethylcelluloae etc.; lubricants such as talc,
magnesium stearate etc.; stabilizers; corrigents; solvents
for injection such as water, ethanol, glycerin etc. The
dosage may be variable depending on the symptom, age of
patients etc., but a dosage from 1 mg to 1000 mg
(preferably from 10 mg to 500 mg) for an adult may be
administered once or divided into several doses a day.
',
92 2181553
M&C FOLIO: 71849/FP-9501 WANGDOC: 20791
[The best embodiment in order to perform the invention]
The following Examples, Referential Examples and
Teat Examples illustrate the invention in more detail.
However such examples are not to be construed as being
limitative of the scope of the invention.
Example 1
1- (2-Butenyl) -7- (4-fluorQbenzvlo~r) -2 3-dimethyl~vrxolo f2 __
3-dlpvridazine
0.85 g (0.0076 mole) of potassium tert-butoxide was
added to a solution of 0.48 g (0.0038 mole) of
4-fluorobenzyl alcohol and 0.08 g (0.0003 mole) of
18-crown-6 in 30 ml of tetrahydrofuran and the mixture
was stirred at room temperature for 10 minutes. 0.45 g
(0.0019 mole) of 1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine was then added to the
mixture and stirred at room temperature for 8 hours.
After completion of the reaction, the reaction mixture
was poured into ice-water and the aqueous mixture was
extracted with dichloromethane. The extract was dried
over anhydrous sodium sulfate and the solvent was
distilled off under reduced pressure. The residue was
purified by column chromatography through silica gel
using a 4:1 mixture of toluene and ethyl acetate as an
eluent. An oily material thus obtained was crystallized
in hexane to give 0.39 g of 1-(2-butenyl)-7-
21$1553
93
(4-fluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine
(cis/trans=13/87) as a pale brown powder.
m.p.: 93-103°C.
Mass spectrum (CI, m/z): 326 (M+ + 1).
NMR spectrum (CDC13, 5 ppm):
1.50-1.67 (m, 3H), 2.26 (s, 3H); 2.31 (s, 3H),
4.79-4.89 (m, I.74H), 4.98-5.04 (m, 0.26H),
5.10-5.58 (m, 2H), 5.67 (s, 2H), 7.00-7.12 (m,
2H), 7.41-7.54 (m, 2H), 8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C17H20FN30: C, 70.17; H, 6.07; N,
12.91,
Found: C, 70.20; H, 6.18; N, 12.84.
Example 2 -
7-Benzvlo_~-2,3-dimethyl-1-(3-methyl-2-hmrPnp lnvrrolof2 3
-dlpvridazine
The title compound was prepared as a White powder in
6.2% yeild in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(3-methyl-2-
butenyl)pyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 104-105°C.
Mass spectrum (CI, m/z): 322 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.60 (s, 3H), 1.63 (s, 3H), 2.26 (s, 3H), 2.33
(s, 3H), 4.98 (d;J=6 Hz, 2H), 5.11 (t;J=6 Hz,
' 2181553
94
1H), 5.73 (s, 2H), 7.30-7.42 (m, 3H), 7.50-7.55
(m, 2H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C20H23N3W C, 74.74;- H, 7.21; N,
13.07,
Found: C, 74.74; H, 7.28; N, 12.99.
Example 3
7-BenzvzQx<r-2.3-dimethyl-1- (2-
pro~enyl)pyrrolof2.3-d]~vridazine
The title compound was prepared as a white powder in
63.2% yield in a similar procedure to that described in _
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 115-116°C.
Mass spectrum (CI, m/z): 294 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.27-(s, 3H), 2.30 (s, 3H), 4.61 (d;J=16 Ha, IH),
4.92-5.00 (m, 2H), 5.08 (d;J=10 Hz, 1H), 5.69 (s,
2H), 5.83-5.97 (m, 1H), 7.30-7.53 (m, 5H), 8.99
(s, 1H) .
Elementary analysis (%):
Calc'd for C18H19N30: C, 73.70; H, 6.53; N,
14.32,
Found: C, 73.69; H, 6.59; N, 14.14.
95 2181553
Example 4
7-Benzyloxv-1-cyrclo~9 lm r yi-2 3-
dimethylgyrrolof2 3-dlpyridazine _
The title compound was prepared as a white powder in
69.2% yield in a similar procedure to that described in
Example 1 by using 7-chloro-1-cyclopropylmethyl-2,3-
dimethylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 123-124°C.
Mass spectrum (CI, m/z): 308 (M+ + 1).
NMR spectrum (CDC13, Sppm):
0.2i-0.45 (m, 4H), 1.06-1.21 (m, 1H), 2.28 (s,
3H), 2.39 (s, 3H), 4.24 (d;J=8 Hz, 2H), 5.70 (s,
2H), 7.29-7.56 (m, 5H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C19H21N3W C, 74.24; H, 6.89; N,
13.67,
Found; C, 74.42; H, 6.90; N, 13.66.
Example 5
7-Benzvla
5311I1CL~l~~y~lyrsOiOIG 3-niy~~rinazme
The title compound (cis/trans=21/79) was prepared as
pale brown crystals in 78.6 % yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine [cis/trans (18/82)] and
benzyl alcohol.
96 2181553
m.p.: 81-84°C.
Mass spectrum (CI, m/z): 308 (M+ + 1).
NMR spectrum (CDC13, Sppm):
1.50-1.66 (m, 3H), 2.25 (s, 3H), 2.31 (s, 3H),
4.79-4.91 (m, 1.58H), 4.97-5.07 (m, 0.42H),
5.10-5.61 (m, 2H), 5.71 (s, 2H), 7.27-7.56 (m,
SH), 8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C19H21N3o: C, 74.24; H, 6.89; N,
13.67,
Found: C, 74.14; H, 6.97; N, 13.57.
Example 6
7-Benzp_OXV_2 3-dimr~thlrl _~ _ (3~henyl 2 _.
x~ropen5~~yrroiof2 3-dlgvr~d~zjne
The title compound (traps) was prepared as pale brown
crystals in 85.4 % yield in a similar procedure to that
described in Example 1 by using
7-chloro-2,3-dimethyl-1-(3-phenyl-2-
propenyl)pyrrolo[2,3-d]pyridazine (traps) and benzyl
alcohol.
m.p.: 132-134°C. -
Mass spectrum (CI, m/z): 370 (M+ + 1).
NMR spectrum (CDC13, sppm):
2.28 (s, 3H), 2.37 (s, 3H), 5.10 (d;J=5 Hz, 2H),
5.71 (s, 2H), 6.07 (d;J=16 Hz, 1H), 6.22 (dt;J=16
Hz, 5 Hz, 1H), 7.10-7.55 (m, lOH), 9.00 (s, 1H).
2181553
Elementary analysis (%):
Calc'd for C24H23N3~~ C, 78.02; H, 6.27; N,
11.37,
Found: C, 78.09; H, 6.28; N, 11.32.
Example 7
7- (4-Fluorobea~zv~ oar) _2 3-dimArr,p -i - (2-
proy~enyl) nvrro~ o (2 3-dl 8vr; rya ~i nP
The title compound was prepared as a white powder in
22.1% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and 4-fluorobenzyl
alcohol.
m.p.: 125-126°C.
Mass spectrum (CI, m/z): 312 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
2.27 (s, 3H), 2.30 (s, 3H), 4.62 (d;J=14 Hz, 1H),
4.90-4.97 (m, 2H), 5.07 (d;J=10 Hz, 1H), 5.65 (s,
2H), 5.81-5.96 (m, 1H), 7.01-7.1i (m, 2H),
7.43-7.42 (m, 2H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C18H18FN30: C, 69.43; H, 5.83; N,
13.50,
Found: C, 69.23; H, 5.94; N, 13.45.
Example 8
7-l3-Fluorobenzyioxv)-2 3-dime hyl-~-(2-
propenylLgvrro~o(2 3-dl~vridazine
98
2181555
The title compound was prepared as white cottony
crystals in 71.4% yield in a similar procedure to that
described in Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and 3-fluorobenzyl
alcohol.
m.p.: 85-86°C.
Mass spectrum (CI, m/z): 312 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
2.28 (s, 3H), 2.31 (s, 3H), 4.63 (d;J=14 Hz, 1H),
4.94-5.02 (m, 2H), 5.11 (d;J=10 Hz, 7.H), 5.70 (s,
2H), 5.86-6.01 (m, 1H), 6.98-7.07 (m, 1H),
7.16-7.40 (m, 3H), 9.00 (s, iH).
Elementary analysis. (%):
Calc'd for C18H18FN30: C, 69.44; H, 5.83; N,
13.50,
Found: C, 69.34; H, 5.85; N, 13.40.
Example 9
7-l2 4-Difiuorobenzyloxv)-2 3-dimethyl-i-(2-
propei~p ~ p5rrrol o f2 ~-dl gyrs daze ne
The title compound was prepared as a white powder in
26.6% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and 2,4-difluorobenzyl
alcohol.
m.p.: 125-126°C.
Mass spectrum (CI, m/z): 330 (M+ + 1).
21 R 1553
NMR spectrum (CDCI3, bppm):
2.25 (s, 3H), 2.30 (s, 3H), 4.62 (d;J=14 Hz, 1H),
4.90 (d;J=5 Hz, 2H), 5.05 (d;J=10 Hz, 1H), 5.71 -
(s, 2H), 5.81-5.91 (m, 1H), 6.80-6.90 (m, 2H),
7.51-7.57 (m, 1H), 8.98 (s, 1H).
Elementary analysis (%):
Calc'd for C18H17F2N30: C, 65.64; H, 5.20; N,
12.76,
Found: C, 65.64; H, 5.21; N, 12.74.
Example 10
7- (2-Fluo obenzy7ynx~r) -2 3-dimethvl-1- (2-
pro enyl)gyrrolof2 3-dlpyridazine
The title compound was prepared as a white powder in
74.8% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and 2-fluorobenzyl
alcohol.
m.p.: 83-84°C.
Mass spectrum (CI, m/z): 312 (M+ + 1).
NMR spectrum (CDCI3, bppm):
2.25 (s, 3H), 2.30 (s, 3H), 4.63 (d;J=14 Hz, 1H),
4.89-4.95 (m, 2H), 5.04 (d;J=10 Hz, 1H), 5.77 (s,
2H), 5.81-5.95 (m, 1H), 7.04-7.19 (m, 2H),
7.27-7.38 (m, 1H), 7.51-7.59 (m, 1H), 8.99 (s,
1H) .
1 218153
1~~
Elementary analysis (%):
Calc'd for C18H18FN30: C, 69.44; H, 5.83; N,
13.50,
Found: C, 69.42; H, 5.87; N, 13.45.
Example 11
7-Benzylo~r-2.3-dimethvl-1-(2-
pentenyl)pyrrolof2.3-dlpyridazine
The title compound (trans) was prepared as a white
powder in 75.9% yield in a similar procedure to that
described in Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
pentenyl)pyrrolo(2,3-d]pyridazine (trans) and benzyl
alcohol.
m.p.: 92-93°C.
Mass spectrum (CI, m/z): 322 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.95 (t;J=12 Hz, 3H), 1.98-2.12 (m, 2H), 2.26 (s,
3H), 2.31 (s, 3H), 4.92-5.08 (m, 2H), 5.23-5.34
(m, 1H), 5.39-5.52 (m, 1H), 5.71 (s, 2H),
7.28-7.55 (m, SH), 8.96 (s, 1H).
Elementary analysis (%):
Calc'd for C20H23N3~' C' 74.74; H, 7.21; N,
13..07,
Found: C, 74.86; H, 7.31; N, 13.02.
Example 12
7-(4-Chlorobenz~yloxv)-2,3-dimethyl-I-f2-
propenyl)gyrrolof2 3-dlovridazine
101 2181553
The title compound was prepared as white crystals in
50.7% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and 4-chlorobenzyl
alcohol_
m.p.: 98-99°C.
Mass spectrum (CI, m/z): 328 (M+ + 1), 330 (M+ +
3).
NMR spectrum (CDC13, bppm):
2.26 (s, 3H), 2.31 (s, 3H), 4.62 (d;J=14 Hz, 1H),
4.89-4.97 (m, 2H), 5.09 (d;J=10 Hz, 1H), 5.66 (s,
2H), 5.82-5.97 (m, 1H), 7.35 (d;J=8 Hz, 2H), 7.43
(d;J=8 Hz, 2H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C18H18C1N30: C, 65.95; H, 5.53; N,
12.82,
Found: C, 65.95; H, 5.56; N, 12.78.
Example 13
7-BenzyloxY-2 3-dimethvl-1-vinylpyrrolo~2 3-dlgyridazine
The title compound was prepared as a white powder in
59.7% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-
vinylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 85-86°C.
Mass spectrum (CI, m/z): 280 (M+ + 1).
.~ .
2181553
102
NMR spectrum (CDC13, bppm):
2.28 (s, 3H), 2.43 (s, 3H), 5. I8 (d;J=8 Hz, 1H),
5.22 (d;J=17 Hz, 1H), 5.72 (s, 2H), 7.29-7.57 (m,
6H), 9.00 (s, 1H).
Elementary analysis (%):
Calc'd for C17H17N30: C, 73.10; H, 6.13; N,
15.04,
Found: C, 73.04; H, 6.30; N, 14.71.
Example 14
7-Benzvloxv-2.3-dimethvl-~.-(2-methvl-
The title compound was prepared as white crystals in
89.3% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-methyl-2-
propenyl)pyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 106-107°C.
Mass spectrum (CI, m/z): 308 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.60 (s, 3H), 2.26 (s, 6H), 4.01 (s, 1H), 4.76
(s, 1H), 4.81 (s, 2H), 5.66 (s, 2H), 7.30-7.51 -
(m, 5H), 9.00 (s, 1H).
Elementary analysis(%):
Calc'd for C19H21N30: C, 74.24; H, 6.89; N,
13.67,
Found: C, 74.18; H, 6.92; N, 13.67.
S 103 2181553
Example 15
7-Benzyloxv-2.3-dimethyl-1-(2 2 2-
~rifluoroethy~)pvrrolof2 3-dlpyr;r~a~;nA
The title compound was prepared as a whitepowder in
65.2% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2,2,2-
trifluoroethyl)pyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 83-84°C.
Mass spectrum (CI, m/z): 336 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
2.27 (s, 3H), 2.37 (s, 3H), 4.93 (q, J=9 Hz, 2H),
5.70 (s, 2H), 7.30-7.58 (m, 5H), 9.01 (s, 1H).
Elementary analysis (%):
Calc'd for C17H16F3N30: C, 60.89; H, 4.81; N, -
12.53,
Found: C, 60.96; H, 4.77; N, 12.45.
Example 16
7-Benzvloxv-1-cvcl
The title compound was prepared as a white powder in
78.6% yield in a similar procedure to that described in
Example 1 by using 7-chloro-1-cyclopropyl-2,3-
dimethylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 121-122°C.
Mass spectrum (CI, m/z): 294 (M+ + 1).
2181553
104
NMR spectrum (CDC13, bppm):
0.87-1.10 (m, 4H), 2.22 (s, 3H), 2.43 (s, 3H),
3.18-3.28 (m, 1H), 5.69 (s; 2H), 7.30-7.59 (m,
5H), 8.95 (s, 1H).
Elementary analysis (%):
Calc'd for C18H19N3~~ C, 73.69; H, 6.53; N,
14.33,
Found: C, 73.78; H, 6.56; N, 14.37.
Example 17
7-(2,4-Dichlorobenzyloxy)-2.3-dimethyl-1-(2-
pro~yl)~yrrolof2,3-dl~yridazine
The title compound was prepared as white crystals in
76.5% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and 2,4-dichlorobenzyl
alcohol.
m.p.: 98-99°C.
Mass spectrum (CI, m/z): 361 (M+), 363 (M+ + 2).
NMR spectrum (CDC13, 5ppm):
2.26 (s, 3H), 2.30 (s, 3H), 4.63 (d, J=16 Hz,
1H), 4.91-4.98 (m, 2H), 5.05-5.09 (d;J=11 Hz,
1H), 5.77 (s, 2H), 5.83-5.98 (m, 1H), 7.20-7.29
(m, 1H), 7.42-7.56 (m, 2H), 9. OD (s, 1H).
Elementary analysis (%):
Calc'd for C18H17C12N30: C, 59.68; H, 4.73; N,
11.6D,
Found: C, 59.71; H, 4.79; N, 11.52.
.
2181553
105
Example 18
7-Benzyloxy-1-(2-fluoroethyl)-2.3-
dimethyl_pvrrolof2 3-dlpyridazine
The title compound was prepared as white crystals in
76.2% yield in a similar procedure to that described in
Example 1 by using 7-chloro-1-(2-fluoroethyl)-2,3-
dimethylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: l06-107°C.
Mass spectrum (CI, m/z): 300 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.27 (s, 3H), 2.35 (s, 3H), 4.51 (s, 2H), 4.64
(dt;J=21 Hz, 4 Hz, 2H), 5.69 (s, 2H), 7.29-7.51
(m, SH), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C17H18FN3~: C, 68.21; H, 6.06; N,
14.04,
Found: C, 68.05; H, 6.09; N, 14.03.
Example 19
7-Benzyl~r-1-(3-fluoropro~yl)-2,3-
dimethvlgyrrolof2,3-dlpvridazine
The title compound was prepared as white crystals in -
71.2% yield in a similar procedure to that described in
Example 1 by using 7-chloro-1-(3-fluoropropyl)-2,3-
dimethylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
2181553
106
m.p.: 100-l0I°C.
Mass spectrum (CI, m/z): 314 (M+ + 1).
NMR spectrum (CDC13, sppm):
1.95-2.15 (m, 2H), 2.25 (s, 3H), 2.35 (s, 3H),
4.23 (dt;J=48 Hz, 6 Hz, 2H), 4.40 (t;J=8 Hz, 2H),
5.69 (s, 2H), 7.31-7.53 (m, 5H), 8.98 (s, 1H).
Elementary analysis (%):
Calc'd for C18H20FN30: C, 68.99; H, 6.43; N,
13.41,
Found: C, 69.05; H, 6.52; N, 13.20.
Example 20
7-Benzylox~r-1-(2 2-dif~~oroethv~)-2 3-
;m hy~pyrrplpf2.3-dlpyridazine
The title compound was prepared as a white powder in
71.6% yield in a similar procedure to that described in
Example 1 by using 7-chloro-1-(2,2-difluoroethyl)-2,3-
dimethylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 128-131°C.
Mass spectrum (CI, m/z): 318 (M+ + 1).
NMR spectrum (CDC13, sppm):
2.26 (s, 3H), 2.35 (s, 3H), 4.62 (tt;J=14 Hz, 5
Hz, 2H), 5.72 (s, 2H), 5.96 (tt;J=56 Hz, 5 Hz,
1H), 7.31-7.53 (m, 5H), 9.00 (s, 1H).
Elementary analysis (%):
l07 2 P ~ 1553
Calc'd for C17H17F2N30: C, 64.34; H, 5.40; N,
13.24,
Found: C, 64.35; H, 5.33; N, 13.11.
Example 21
1-(2-Butenyl)-7-(2 4-dichlorobenzy~oxvl-2 ~-
dimeth5~8yrrolol2,3-dlgyridazine
The title compound (cis/trans=25/75) was prepared as
white crystals in 72.4% yield in a similar procedure to
that described in Example.l by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trana=21/79) and
2,4-dichlorobenzyl alcohol.
m.p.: 133-134°C.
Mass spectrum (CI, m/z): 376 (M+ + 1), 378 (M+ +
3), 380 (M+ + 5).
NMR spectrum (CDC13, Sppm):
1.56-1.67 (m, 3H), 2.26 (s, 3H), 2.32 (s, 3H),
4,.82-4.89 (m, 1.5H), 5.00-5.05 (m, 0.5H),
5.16-5.25 (m, 0.75H), 5.30-5.39 (m, 0.25H),
5.47-5.60 (m, iH), 5.80 (s, 2H), 7.20-7.27 (m,
1H), 7.43 (s, IH), 7.50-7.56 (m, 1H), 8.97 (s,
1H) .
Elementary analysis (%):
Calc'd for C19H19C12N30: C, 60.65; H, 5.09; N,
11.17,
Found: C, 60.76; H, 5.10; N, 11.14.
2181553
Example 22
1-(2-Butenyl)-7-(2.4-difluorob n~yloxy)-2 3-
dimethylpyrrolo[2.3-dlpyridazine
The title compound (cis/trans=18/82) was prepared as a
pale yellow powder in 43.1% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=21/79) and
2,4-difluorobenzyl alcohol.
m.p.: 93-95°C.
Mass spectrum (CI, m/z): 344 (M+ + 1).
NM& spectrum (CDC13, 5ppm):
1.54-1.65 (m, 3H), 2.24 (s, 3H), 2.31 (s, 3H),
4.80-4.85 (m, 1.64H), 4.97-5.01 (m, 0.36H),
5.15-5.34 (m, 1H), 5.42-5.57 (m, 1H), 5.72 (s,
2H), 6.81-6.90 (m, 2H), 7.51-7.60 (m, 1H), 8.97
(s, 1H) .
Elementary analysis (%):
Calc'd for C19H19F2N3~~ C, 66.46; H, 5.58; N,
12.24,
Found: C, 66.56; H, 5.56; N, 12.15.
Example 23
7-Benzyl~r-1-~rclohexvl -2:3-
dimethgl_pyrrolo(2,3-dl,gyridazine
The title compound was prepared as a white powder in
92.8% yield in a similar procedure to that described in
io9 2181553
Example i by using 7-chloro-1-cyclohexyl-2,3-
dimethylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 174-177°C.
Mass spectrum (CI, m/z): 336 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.10-1.44 (m, 2H), 1.48-2.08 (m, 4H), 1.76 (s,
3H), 2.13-2.59 (m, 4H), 2.27 (s, 3H), 3.91-4.19
(m, 1H), 5.70 (s, 2H), 7.29-7.66 (m, SH), 8.95
(s, 1H) .
Elementary analysis (%):
Calc'd for C21H25N3W C, 75.19; H, 7.51; N,
12.53,
Found: C, 75.17; H, 7.63; N, 12.50.
Example 24
7-(4-Fluorobenzyl~)-2 3-dim~thvl-~-(3-x~henvl-2-
The title compound (traps) was prepared as pale yellow
crystals in 47.7% yield in a similar procedure to that
described in Example 1 by using
7-chloro-2,3-dimethyl-1-(3-phenyl-2-
propenyl)pyrrolo[2,3-d]pyridazine (traps) and
4-fluorobenzyl alcohol.
m.p.: 124-126°C.
Mass spectrum (CI, m/z): 388 (M+ + 1).
NMR sepctrum (CDC13, bppm):
2.29 (s, 3H), 2.38 (s, 3H), 5.09 (d;J=5 Hz, 2H),
a
ll0 2181553
5.68 (s, 2H), 6.03 (d;J=17 Hz, 1H), 6.20 (dt;J=I7
Hz, 5 Hz, 1H), 6.91-7.51 (m, 9H), 9.00 (s, 1H).
Elementary analysis (%):
Calc'd for C24H22FN3~~ C, 74.40; H, 5.72; N,
10.85,
Found: C, 74.65; H, 5.75; N, 10.75.
Example 25
2 3-Dimethyl-1- f2-x~rox?envl) -7- (4-
trifluoromethvibenzyloxvlpyrrolof2 3-dlgyr~~az~nP~
The title compound was prepared as pale yellow
crystals in 52.5% yield in a similar procedure to that
described in Example.l by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and -
4-trifluoromethylbenzyl alcohol.
m.p.: 95-96°C.
Mass spectrum (CI, m/z): 362 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
2.27 (s, 3H), 2.32 (s, 3H), 4.63 (d;J=I6 Hz, 1H),
4.96 (d;J=4 Hz, 2H), 5.10 (d;J=10 Hz, 1H), 5.75
(s, 2H), 5.87-6:00 (m, 1H), 7.46-7.78 (m, 4H),
9.00 (s, 1H).
Elementary analysis (~):
Calc'd for C19HI8F3N30: C, 63.15; H, 5.02; N,
11.63,
Found: C, 63.23; H, 5.02; N, 11.66.
V
111 ~~ g~ 553
Example 26
7- l4-Fluorobenz,3rlox<r) -2 3-dimefihyi -i - (2-methyl-2-
propenyl~~vrrolof2.3-dlpyridazine
The title compound was prepared as a grayish white
powder in 38.7% yield in a similar procedure to that
described in Example 1 by using
7-chloro-2,3-dimethyl-1-(2-methyl-2-
propenyl)pyrrolo[2,3-d]pyridazine and 4-fluorobenzyl
alcohol.
m.p.: 118-120°C.
Mass spectrum (CI, m/z): 326 (M+ f 1).
NMR spectrum (CDC13, Sppm):
1.62 (s, 3H), 2.27 (s,, 6H), 4.02 (s, 1H), 4.76
(s, 1H), 4.80 (s, 2H), 5.61 (s, 2H), 7.01-7.11
(m, 2H), 7.41-7.50 (m, 2H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C19H20FN30: C, 70.13; H, 6.20; N,
12.91,
Found: C, 70.29; H, 6.28; N, 12.68.
Example 27
7-BenzyloxSr-3-eth~cl-2-methvl-1 - t2- _
propenyl)myrrolo(2,3-dlgyridazine
The title compound was prepared as a white powder in
97.0% yield in a similar procedure to that described in
Example-1 by using 7-chloro-3-ethyl-2-methyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and benzyl alcohol.
112 2181553
m.p.: 82-83°C.
Mass spectrum (CI, m/z): 308 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.22 (t;J=8 Hz, 3H), 2.32 (s, 3H), 2.73 (q;J=8
Hz, 2H), 4.61 (d;J=18 Hz, IH), 4.91-4.99 (m, 2H),
5.08 (d;J=10 Hz, 1H), 5.70 (s, 2H), 5.83-6.00 (m,
1H), 7.27-7:53 (m, 5H), 9.03 (s, 1H).
Elementary analysis (%):
Calc'd for C19H21N3W C, 74.24; H, 6.89; N,
13.67,
Found: C, 74.33; H, 6.99; N, 13.61.
Example 28
1-(2-Butenyl)-2 3-dimethvl-7-(2-
thieny m hyloxylpyrrolo~2 3-dlnvridazine
The title compound (cis/trans=20/80) was prepared as a
grayish white powder in 20.6% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=20/80) and
2-thiophenemethanol.
m.p.: 72-75°C.
Mass spectrum (CI, m/z): 314 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.54-1.70 (m, 3H), 2.25 (s, 3H), 2.31 (s, 3H),
4.82-4.89 (m, 1.6H), 4.99-5.04 (m, 0.4H),
5.17-5.38 (m, 1H), 5.43-5.60 (m, 1H), 5.86 (s,
113
2181553
2H), 6.96-7.04 (m, 1H), 7.15-7.21 (m, 1H)',
7.29-7.35 (m, 1H), 8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C17H19N30S: C, 65.15; H, 6.11; N,
13.41,
Found: C, 65.13; H, 6.12; N, 13.38.
Example 29
1-(2-Hutenyl)-7-(4-flmnrnhan~yl_nxy 2 3
d' m h~F~Y''ro~ o f -.~BS~r; d
The title compound (cis/trans=98/2) was prepared as
pale brown crystals in 59.3% yield in a similar procedure
to that described in. Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=94/6) and
4-fluorobenzyl alcohol.
m.p.: 108-112°C.
Mass spectrum (CI, m/z): 326 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
1.58-1.68 (m, 3H), 2.26 (s, 3H), 2.31 (s, 3H),
4.83-4.89 (m, 0.04H), 4.97-5.04 (m, 1.96H),
5.29-5.60 (m, 2H), 5.68 (s, 2H), 7.00-7.52 (m,
4H), 8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C19H20FN30: C, 70.14; H, 6.20; N,
12.91,
Found: C, 69.95; H, 6.22; N, 12.90.
114
Example 30
7-Benzyl~-1-f2-ch~oro-2-~t~op~nyl)-2 3-
dimethy~bvrrolof2 3-d]~vradazsne
The title compound was prepared as a white powder in
10.9% yield in a similar procedure to that described in
Example 1 by using 7-chloro-1-(2-chloro-2-propenyl)-2,3-
dimethylpyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 88-90°C.
Mass spectrum (CI, m/z): 328 (M+ + 1), 330 (M+ +
3) .
NMR spectrum (CDC13, bppm):
2.27 (s, 3H), 2.32 (s, 3H), 4.48 (s, 1H), 5.05
(s, 2H), 5.23 (s, 1H),. 5.69 (s, 2H), 7.30-7.54
(m, SH), 9.00 (s, 1H).
Elementary analysis (%):
Calc'd for C18H18C1N30: C, 65.95; H, 5.54; 12.82,
Found: C, 66.00; H, 5.51; N, 12.74.
Example 31
1-l2-Buteny~)-7-(4-difluoromefihnxv n~yloxr -2 3-
dimethyly~yrrolol2,3-dl8yridazine _
The title compound (cis/trans=21/79) was prepared as a
white powder in 37.8% yield in a similar procedure to that
described in Example 1 by using 1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=20/80) and
4-difluoromethoxybenzyl alcohol.
115 2181553
m.p.: 109-110°C.
Masa spectrum (CI, m/z): 374 (M+ + 1).
NMR spectrum (CDC13, Sppm):
1.56-1.68 {m, 3H), 2.25 (s, 3H), 2.33 (s, 3H),
4.84-4.89 (m, 1.58H), 5.00-5.04 (m, 0.42H),
5.14-5.25 (m, 0.79H), 5.30-5.38 (m, 0.21H),
5.45-5.60 (m, 1H), 5.69 (s, 2H), 6.52 {t;J=51 Hz,
1H), 7.13 (d;J=8 Hz, 2H), 7.51 (d;J=8 Hz, 2H),
8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C20H21F2N3C2' C~ 64.43; H, 5.67;
N, 11.25,
Found: C, 64.28; H, 5.57; N, 11.32.
Example 32
1-(2-Butenyl)-2.3-dimethvl-7-(3-
b2yridy~methvl~)gyrro~.of2 3-dlgyridazine _
The title compound (cis/trans=22/78) was prepared as a
pale yellow powder in 45.9% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=20/80) and
3-pyridinemethanol.
m.p.: 80-8i°C.
Mass spectrum (CI, m/z): 309 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
2181553
116
1.57-1.66 (m, 3H), 2.26 (s, 3H), 2.32 (s; 3H),
4.85-4.90 (m, 1.56H), 5.00-5.04 (m, 0.44H),
5.14-5.23 (m, 0.78H), 5.31-5.39 (m, 0.22H),
5.47-5.60 (m, 1H), 5.74 (s, 2H), 7.29-7.34 (m,
1H), 7.82-7.88 (m, 1H), 8.57-8.62 (m, 1H), 8.78
(s, 1H), 8.98 (s, 1H).
Elementary analysis (%):
Calc'd for C18H20N4W C, 70.11; H, 6.54; N, -
18.17,
Found: C, 69.83; H, 6.51; N, 18.08.
Example 33
1-(2-BUtenvl)-2-ethyl-7-(4-fluoroben~ylo.~y~
methy~~yrro~of2 3-dlpyridazine
The title compound (traps) was prepared as a white
powder in 41.7% yield in a similar procedure to that
described in Example 1 by using
1-(2-butenyl)-7-chloro-2-ethyl-3-
methylpyrrolo[2,3-d]pyridazine (cis/trans=4/96) and
4-fluorobenzyl alcohol.
m.p.: 74-76°C.
Masa spectrum (CI, m/z): 340 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.20 (t;J=8 Hz, 3H), 1.59 (d;J=7 Hz, 3H), 2.28
(s, 3H), 2.75 (q;J=8 Hz, 2H), 4.81-4.90 (m, 2H),
5.08-5.26 (m, 1H), 5.42-5.57 (m, 1H), 5.66 (s,
2H), 7.07 (t;J=9 Hz, 2H), 7.49 (dd;J=7, 9 Hz,
2H), 8.98 (s, 1H).
2181553
117
Elementary analysis (%):
Calc'd for C20H22FN30: C, 70.77; H, 6.53; N,
12.38,
Found: C, 70.78; H, 6.44; N, 12.34.
Example 34
7-(4-Fiuoroben25r~th~o)-2 3-dimethyi 1 (2
~ropenyl)pyrrolof2.3-dlpvr;rta~;r,P
The title compound was prepared as a pale yellow
powder in 61.6% yield in a similar procedure to that
described in Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo[2,3-d]pyridazine and
4-fluorophenylmethanethiol.
m.p.: 106-109°C.
Mass spectrum (CI, m/z): 328 (M+ t 1).
NMR spectrum (CDC13, bppm):
2.27 (s, 3H), 2.31 (s, 3H), 4.48 (d;J=16 Hz, 1H),
4.72 (s, 2H), 5.00-5.10 (m, 2H), 5.12 (d;J=10 Hz,
1H), 5.88-6.02 (m, 1H), 6.90-7.00 (m, 2H),
7.37-7.47 (m, 2H), 9.07 (s, 1H).
Elementary analysis (%):
Calc'd for C18H18FN30S: C, 66.04; H, 5.54; N,
12.83,
Found: C, 66.45; H, 5.54; N, 12.58.
Example 35
1 ~yclogronvlmethyl-7- (4-fluoroben~p nr~.~ -2 3-
d; m ~yy~ bvrro~ o (2 3-dl pyre daze ne
118 2181553
The title compound was prepared as a white powder in
74.1% yield in a similar procedure to that described in
Example 1 by using 7-_chloro-1-cyclopropylmethyl-2,3-
dimethylpyrrolo[2,3-d]pyridazine and 4-fluorobenzyl
alcohol.
m.p.: 137-138°C.
Mass spectrum (CI, m/z): 326 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
0.21-0.27 (m, 2H), 0.38-0.45 (m, 2H), 1.05-1.20
(m, 1H), 2.28 (s, 3H), 2.39 (s, 3H), 4.22 (d, J=8
Hz, 2H), 5.66 (s, 2H), 7.05-7.12 (m, 2H),
7.48-7.53 (m, 2H), 8.99 (s, 1H).
Elemetary analysis (%):
Calc'd for C19H20FN30: C, 70.13; H, 6.20; N,
12.91,
Found: C, 70.22; H, 6.24; N, 12.89.
Example 36
1-i2-BUtenyl)-7-furfu~rloxy-23-
dim h5rlpyrrolof2.3-dlpyridazine
The title compound (cis/trans=15/85) was prepared as a
flesh-colored powder in 12.2% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=20/80) and
furfuryl alcohol.
m.p.: 84-85°C.
Mass spectrum (CI, m/z): 298 (M+ + 1).
119 2181553 -
NMR spectrum (CDC13, 5ppm):
1.58-1.65 (m, 3H), 2.25 (s, 3H), 2.32 (s, 3H),
4.52-4.$4 (m, 1.64H), 4.98-5.00 (m, 0.36H),
5.28-5.35 (m, 1H), 5.44-5.49 (m, 1H), 5.66'(s,
2H), 6.38-6.39 (m, 1H), 6.51 (s, 1H), 7.44 (s,
1H), 8.95 (s, 1H).
Elementary analysis (%):
Calc'd for C17H19N302: C, 68.67; H, 6.44; N,
14.13,
Found: C, 68.45; H, 6.52; N, 14.14.
Example 37
~vclohexl~lmet~yl-7- (4-fhoro~~yl~xy) -2 3-
d~m hp gyrrolo[2.3-dlzwridaz~ne
The title compound was prepared as a white powder in
45.6% yield in a similar procedure to that described in
Example 1 by using 7-chloro-1-cyclohexylmethyl-2,3-
dimethylpyrrolo[2,3-d]pyridazine and 4-fluorobenzyl
alcohol.
m.p.: 108-109°C.
Mase spectrum (CI, m/z): 368 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.77-0.90 (m, 2H), 0.93-1.09 (m, 3H), 1.24-1.32
(m, 2H), 1.56-1.67 (m, 4H), 2.25 (s, 3H), 2.32
(s, 3H), 4.01 (d, J=7 Hz, 2H), 5.63 (s, 2H), 7.08
(t, J=6 Hz, 2H), 7.50 (dd, J=6 Hz, 3 Hz, 2H),
8.96 (s, 1H).
2i8i553
~a
120
Elementary analysis (%):
Calc'd for C22H26~3C~ C, 71.90; H, 7.09; N,
11.44,
Found: C, 71.71; H, 7.05; N, 11.19.
Example 38
1-(2-Butenyl)-7-(2 6-difluoroben~y~~~1-2 3-
;m h5ri pvrYo~ o f2 3-d1 ywridazine
The title compound (cis/trans=22/78) was prepared as a
white powder in 58.3% yield in a similar procedur8 to that
described in Example 1 by using 1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=24/76) and
2,6-difluorobenzyl alcohol.
m.p.: 85-94°C.
Mass spectrum (CI, m/z): 344 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.46-1.60 (m, 3H), 2.26 (s, 3H), 2.31 (s, 3H),
4.65-4.79 (m, 1.56H), 4.86-4.94 (m, 0.44H),
5.09-5.51 (m, 2H), 5.78 (s, 2H), 6.87-7.02 (m,
2H), 7.27-7.42 (m, 1H), 8.98 (s, H).
Elementary analysis. (%):
Calc'd for C19H19F2N30: C, 66.46; H, 5.58; N,
12.24,
Found: C, 66.13; H, 5.45; N, 12.25.
Example 39
1-(2-Butenyl)-7-(3,5-difluorobenzylO~y)-2 3-
dimethKl_pyrrolo(2.3-dlcvridazine
2181553
121
The title compound (cis/trans=29/71) was prepared as a
white powder in 41.6% yield in a similar procedure to that
described in Example 1 by using 1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=24/76) and
3,5-difluorobenzyl alcohol.
m.p.: 78-84°C.
Mass spectrum (CI, m/z): 344 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
1.51-1.76 (m, 3H), 2.27 (s, 3H), 2.34 (s, 3H),
4.83-4.96 (m, 1.42H), 5.01-5.10 (m, 0.58H),
5.11-5.75 (m, 2H), 5.70 (s, 2H), 6.67-6.82 (m,
1H), 6.93-7.10 (m, 2H), 8.98 (s, H).
Elementary analysis.(%):
Calc'd for C19H19F2N30: C, 66.46; H, 5.58; N,
12.24,
Found: C, 66.28; H, 5.58; N, 12.20.
Example 40
1-(2-BUtenvl)-7-(2-chloro-6-fluorobenzvloxv)-2.3-
The title compound (cis/trans=21/79) was prepared as a
pale brown powder in 57.6% yield in a similar procedure to
that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=24/76) and
2-chloro-6-fluorobenzyl alcohol.
X1$1,553
122
m.p.: 103-i12°C.
Mass spectrum (CI, m/z): 360 (M+ + 1), 362 (M+ +
3).
NMR spectrum (CDC13, 5ppm):
1.41-1.58 (m, 3H), 2.25 (s, 3H), 2.30 (s, 3H),
4.66-4.77 (m, 1.58H), 4.84-4.92 (m, 0.42H),
5.03-5.51 (m, 2H), 4.83 (s, 2H), 6.99-7.12 (m,
1H), 7.21-7.38 (m, 2H), 8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C19H19C1FN30: C, 63.42; H, 5.32; N,
11.68,
Found: C, 63.52; H, 5.34; N, 11.60.
Example 41
7-Benzyloxv-1-(3,3-dichloro-~robenyl)-2,3-
dimethylgyrrolof2,3-dlpyridazine
0.13 g (0.0011 mole) of potassium tert-butoxide was
added to a suspension of 0.29 g (D.0011 mole) of
7-benzyloxy-2,3-dimethylpyrrolo[2,3-d]pyridazine and 0.03
g (0.0001 mole) of 18-crown-6 in 8 ml of tetrahydrofuran
and the resulting mixture was stirred at room temperature
for 44 minutes. 0.22 g (0.0011 mole) of
3,3-dichloro-2-propenyl bromide was then added to the
mixture and stirred at room temperature for 5 minutes. The
reaction mixture was poured into ice-water and the aqueous
mixture was extracted with dichloromethane. The extract
was dried over anhydrous sodium sulfate and the solvent
2181553
123
was distilled off under reduced pressure. The residue was
purified by column chromatography through silica gel using
a 20:1 mixture of chloroform and methanol as an eluent to . _.
give 0.090 g of
7-benzyloxy-1-(3,3-dichloro-2-propenyl)-2,3-
dimethylpyrrolo[2,3-d]pyridazine as a yellow powder.
m.p.: 149-151°C.
Mass spectrum (CI, m/z): 362 (M+ + i), 364 (M+ +
3), 366 (M+ + 5).
NMR spectrum (CDC13, 5ppm):
2.26 (s, 3H), 2.35 (s, 3H), 5.06 (d, J=5 Hz, 2H),
5.72 (s, 2H), 5.90 (t, J=5 Hz, 1H), 7.29-7.58 (m,
5H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C18H17C12N30: C, 59.68; H, 4.73; N,
11.60,
Found: C, 60.06; H, 4.99; N, 11.32.
Example 42
7-Benzvlnx
vropvny~~pyrroiQf2 '~-dlgyridazinP
The title compound was prepared as a pale yellow
powder in 27.4% yield in a similar procedure to that
described in Example 41 by using
7-benzyloxy-2,3-dimethylpyrrolo[2,3-d]pyridazine and
3-bromo-1-propyne.
m.p.: 116-117°C.
Mass spectrum (CI, m/z): 292 (M+ + 1).
124 ~ ~ ~ ~ '""'S _
NMR spectrum (CDCl3, bppm):
2.26 (s, 3H), 2.30-2.35 (m, 1H), 2.44 (s, 3H),
5.18 (d, J=2 Hz, 2H), 5.74 (s, 2H), 7.30-7.44 (m,
3H), 7.53-7.6i (m, 2H), 9.00 (s, 1H).
Elementary analysis (%):
Calc'd for C18H17N30: C, 74.20; H, 5.88; N,
14.42,
Found: C, 73.88; H, 5.85; N, 14.36.
Example 43
~- w-wloro-L-b.-O~ym -7- (4- ~ mnrnhenv~~1 p~7)? ~ 3
dim hKl_~yrrolof2 3-dlgyr'~a~snP
The title compound (cis/trans~l/1) was prepared as a
pale yellow powder in 31.4% yield in a similar procedure
to that described in Example 41 by using
7-(4-fluorobenzyloxy)-2,3-dimethylpyrrolo[2,3-d]pyridazine
and 1,3-dichloropropene (a mixture of cis and trans
isomers).
m.p.: 110-115°C.
Mass spectrum (CI, m/z): 346 (M+ + 1), 348 (M+ +
3) .
NMR spectrum (CDC13, bppm):
2.25 (s, 1.5H), 2.26 (a, 1.5H), 2.33 (s, 1.SH),
2.34 (s, 1.SH), 4.89-4.91 (m, 1H), 5.15-5.17 (m,
1H), 5.64-6.14 (m, 4H), 7.04-7.12 (m, 2H),
7.47-7.50 (m, 2H), 8.98 (m, 1H).
Elementary analysis (%):
,.
125 2 ~ 81553
Calc~d for C18H17C1FN30'1/4H20: C, 61.72; H,
5.04; N, 11.99,
Found: C, 61.83; H, 4.86; N, 12.04.
Example 44
7-(4-Fi~oroben~yloxy)_2 3 dsmerhp i (i .
t~ropenyl)gyrro~of2 3-dlgvr~~a~snA
1.62 g (0.014 mole) of potassium tert-butoxide was
added to a solution of 1.02 g (0.0081 mole) of
4-fluorobenzyl alcohol and 0.12 g (0.00045 mole) of
18-crown-6 in 10 ml of tetrahydrofuran and the resulting
mixture was stirred at room temperature for 25 minutes. A
solution of 0.60 g (Ø0027 mole) of
7-chloro-1-(2-propenyl)-2,3-
dimethylpyrrolo(2,3-d]pyridazine in 5 ml of
tetrahydrofuran was added dropwise to the mixture and
stirred at room temperature for 10 hours. After
completion of the reaction, the reaction mixture was
poured into ice-water and the aqueous mixture was
extracted with dichloromethane. The extract was dried
over anhydrous sodium sulfate and the solvent was
distilled off under reduced pressure. The residue was
purified by column chromatography through silica gel using
a 2:3 mixture of ethyl acetate and hexane as an eluent to
give 0.33 g of 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-(1-
propenyl)pyrrolo[2,3-d]pyridazine (trans) as a pale yellow
powder.
m.p.: 114-115°C.
126 21 ° 1555
Mass spectrum (CI, m/z): 312 (M+, + 1).
NMR spectrum (CDC13, 6ppm):
1.43 (d, J=8 Hz, 3H), 2.25 (s, 3H), 2.29 (s, 3H),
5.62 (s, 2H), 5.85-5.95 (m, 1H), 6.65-6.71 (m,
1H), 7.02-7.10 (m, 2H), 7.43-7.50 (m, 2H), 9.00
(s, 1H) .
Elementary analysis (%):
Calc'd for C18H18FN30: C, 69.44; H, 5.83; N,
13.50,
Found: C, 69.79; H, 5.91; N, 13.51.
Example 45
7-Ben~W o~r~,_2, 3-dimethyl -~ - (p.~QBane-i ~_
diengl_Zgvrrnlnf7 3-d~~«yir7a~ina
The title compound was prepared as pale brown crystals
in 37.6% yield in a similar procedure to that described in
Example 44 by using 7-chloro-2,3-dimethyl-1-(2-
propynyl)pyrrolo[2,3-d]pyridazine and benzyl alcohol.
m.p.: 77-79°C.
Mass spectrum (CI, m/z): 292 (M+ + 1).
NMR spectrum (CDC13, Sppm):
2.27 (s, 3H), 2.41 (s, 3H), 5.37 (s, 1H), 5.40
(s, 1H), 5.73 (e, 2H), 7.28-7.68 (m, 6H), 8.98
(s, 1H) .
Elementary analysis (%):
Calc'd for C18H17N30: C, 74.21; H, 5.89; N,
14.42,
Found: C, 74.29; H, 5.86; N, 14.31.
127 ~ ~ ~ ~ 55~
Example 46
7-Ben~y~amino-2 3-dimethyl-~-ri-
prpnenY~~yrrpl p ~2 't -dl b7yri (ia ~i na
The title compound (tram) was prepared as a beige -
powder in 31.5% yield in a similar procedure to that
described in Example 44 by using
7-chloro-2,3-dimethyl-1-(2-propenyl)pyrrolo(2,3-d]pyridazine
and benzylamine.
m.p.: 130-131°C.
Mass spectrum (CI, m/z): 292 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.44-1.62 (m, 3H), 2.2,0 (s, 3H), 2.25 (s, 3H),
4.88 (d;J=5 Hz, 2H), 5.11-5.22 (m, 1H), 6.03-6.14
(m, 1H), 6.71-6.78 (m, 1H), 7.20-7.42 (m, 5H),
8.83 (s, 1H).
Elementary analysis (%):
Calc'd for C18H20N4' C, 73.04; H, 6.95; N, 18.93,
Found: C, 73.53; H, 6.99; N, 18.83.
Example 47
1-(2-Butenyl)-7-(4-fluoroben~~iaminnl-2 3-
dim hyipyrrolof2,3-dlxwridazine
A solution of 0.35 g (0.0015 mole) of
1-(2-butenyl)-7-choro-2,3-dimethylpyrrolo[2,3-d]pyridazine
dissolved in 3.5 ml of 4-fluorobenzylamine was heated at
128
~~~~J~~S
180°C for 2.5 hours. After completion of the reaction,
the reaction mixture was allowed to cool to room
temperature and then poured into ice-water. The aqueous
mixture was extracted with dichloromethane. The extract
was dried over anhydrous sodium sulfate and the solvent
was distilled off under reduced pressure. The residue was
purified by column chromatography through silica gel using
a 30:1 mixture of chloroform and methanol as an eluent to
give 0.22 g of 1-(2-butenyl)-7-(4-fluorobenzylamino)-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=1/4) as a
flesh-colored powder.
m.p.: 135-138°C.
Mass spectrum (CI, m/z): 325 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.38-1.41 (m, 0.6H), 1.55-1.59 (m, 2.4H), 2.25
(s, 3H), 2.30 (s, 3H), 4.70-4.89 (m, 5H),
5.13-5.46 (m, 1H), 5.51-5.65 (m, 1H), 7.00-7.08 -
(m, 2H), 7.33-7.42 (m, 2H), 8.85 (s, 1H).
Elementary analysis (%):
C3lc'd for C19H21FN4: C, 70.35; H, 6.53; N,
17.27,
Found: C, 70.08; H, 6.62; N, 17.08.
Example 48
1-(2-Butenyl)-7-(4-chloro-2- imnrnha~~r~~nr -2 3-
d; m hylgyrrolo f 2 3-dl pyr; r9a ~;.,P
The title compound (cis/trans=24:76) was prepared as
C
129 Z ~ ~ 155
a white powder in 63.8% yield in a similar procedure to
that described in Example 1 by using using
1-(2-butenyl)-7-chloro-2,3-dimethylpyrrolo[2,3-d]pyridazine
(cis/trans=24/76) and 4-chloro-2-fluorobenzyl alcohol.
m.p.: 106-109°C.
Mass spectrum (CI, m/z): 360 (M+ + i), 362 (M+
+ 3).
NMR spectrum (CDC13, bppm):
1.59 (d;J=6 Hz, 2.28H), 1.65 (d;J=6 Hz, 0.72H),
2.24 (s, 3H), 2.30 (s, 3H), 4.82 (d;J=6 Hz,
1.52H), 4.99 (d;J=6 Hz, 0.48 Hz), 5.12-5.60 (m,
2H), 5.73 (s, 2H), 7.12 (d;J=9 Hz, 2H), 7.51
(t;J=9 Hz, 1H), 8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C19H19C1FN30: C, 63.42; H, 5.32; N,
11.68,
Found: C, 63.41; H, 5.17; N, 11.54.
Example 49
1-(2-Buten~rl)-7-(2 6-dichlorobenzyloxyl-2 3-
dime hyi~yrroiof2 3-dlRyridazine
The title compound (cis/trans=21:79) was prepared as a
pale yellow powder in 83.5% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-dimethylpyrrolo(2,3-d]pyridazine
(cis/trans=24/76) and 2,6-dichlorobenzyl alcohol.
130 2181553
m.p.: 133-140°C.
Mass spectrum (CI, m/z): 376 (M+ + 1), 378 (M+ +
3), 380 (M+ + 5).
NMR spectrum (CDC13, bppm):
1.34-1.60 (m, 3H), 2.24 (s, 3H), 2.30 (s, 3H),
4.71 (d;J=6 Hz, 1.58H), 4.89 (d;J=6 Hz, 0.42 Hz),
5.02-5.50 (m, 2H), 5.94 (s, 2H), 7.19-7.47 (m,
3H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C19H19C12N30: C, 60.65; H, 5.09; N,
11.17,
Found: C, 60.53; H, 5.03; N, 11.17.
Example 50
1-(2-~utenyl)-7-(4_FlLnrnhan~rlthinl-7 '7-
d~m hylgyrrolof2 3-dlg3rridazinP
The title compound (cis/trans=20:80) was prepared.as a
pale yellow powder in 64.9% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=23/77) and
4-fluorophenylmethanethiol.
m.p.: 110-115°C.
Mass spectrum (CI, m/z): 342 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.58-1.65 (m, 2.4H), 1.73-1.79 (m, 0.6H), 2.25
(s, 3H), 2.31 (s, 3H), 4.74 (s, 2H), 4.93-5.00
t
a
131 2~g~555
(m, 1.6 Hz), 5.07-5.33 (m, 1.4H), 5.46-5.61 (m,
1H), 6.91-7.02 (m, 2H), 7.39-7.48 (m, 2H), 9.06
(s, 1H) .
Elementary analysis (%):
Calc'd for C19H20FN3S: C, 66.84; H, 5.90; N,
12.31,
Found: C, 66.92; H, 5.90; N, 12.23.
Example S1
1-l2-Butenyl)-7-12,4-difluorobenzylthio)-2 3-
d~m hyl8yrrolof2,3-dlgyridazine
The title compound (cis/trana=16:84) was prepared as
pale brown crystals in 39.0% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=22/78) and
2,4-difluorophenylmethanethiol.
m.p.: 122-127°C.
Mass spectrum (CI, m/z): 360 (M+ + 1).
NMR spectrum (CDCI3, bppm):
1.48-1.68 (m, 2.52H), 1.71-1.80 (m, 0.48H), 2.24
(s, 3H), 2.31 (s, 3H), 4.77 (s, 2H), 4.88-5.68
(m, 4H), 6.65-6.84 (m, 2H), 7.47-7.63 (m, 1H),
9.06 (s, 1H) .
Elementary analysis (%):
Calc'd for C19H19F2N3S: C, 63.49; H, 5.33; N,
11.69,
Found: C, 63.67; H,- 5.32; N, 11.66.
t
132 218153
Example 52
1- (2-B~yl ) -7- (2-Cl'll nrp-6-Flnnrnhan~ylthinl 7 '1
dim hylgyrrolo(2 3-dlp~rri~a,a;aP
The title compound (cis/trans=18:82) was prepared as
pale brown crystals in 70.1% yield in a similar procedure
to that described in Example 1 by using
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo(2,3-d]pyridazine (cis/trans=(22/78) and
2-chloro-6-fluorophenylmethanethiol.
m.p.: 95-117°C.
Masa spectrum (CI, m/z): 376 (M+ + 1).
NMR spectrum {CDC13, 6ppm):.
1.51-1.66 {m, 2.46 H), 1.67-1.77 (m, 0.54H), 2.27
(s, 3H), 2.32 (s, 3H), 4.81-5.62 (m, 6H),
6.93-7.31 (m, 3H), 9.09 (s, 1H).
Elementary analysis (%):
Calc'd for C19H19C1FN3S: C, 60.71; H, 5.09; N,
11.18,
Found: C, 60.79; H, 5.13; N, 11.11.
Example 53
1- (2-Butenyt ) -7- (2 4-d; nhl nrnhan l rr,; n~ ~ z
ZY
dimethvlovrrolo f 2 . 3 -r71 nvr; ria ~; "A
The title compound (cis/trans=16:84) was prepared as
pale brown crystals in 63.2% yield in a similar procedure
to that described in Example 1 by using
x.
2181553
133
1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=22/78) and
2,4-dichlorophenylmethanethiol.
m.p.: 8I-85°C.
Mase spectrum (CI, m/z): 392 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.47-1.81 (m, 3H), 2.25 (s, 3H), 2.31 (s, 3H),
4.85 (s, 2H), 4.91-5.02 (m, 1.68H), 5.03-5.13 (m,
0.32H), 5.13-5.64 (m, 2H), 7.07-7.68 (m, 3H),
9.05 (s, 1H).
Elementary analysis (%):
Calc'd for C19H19C12N3S: C, 58.16; H, 4.88; N,
10.71,
Found: C, 58.01; H, 4.87; N, 10.69.
Example 54
i- m-rsucenym -z j-cl~meryl5u _~_ (2
bvrj dylmeth~ ~ ' o) p.Yrroi o f dl gvr; Q
The title.compound (cis/trana=20/80) was prepared as a
yellow oil in 64.8% yield in a similar procedure to that
described in Example 1 by using 1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=22/78) and
2-pyridylmethanethiol.
Mass spectrum (CI, m/z): 325 (M+ + 1).
NMR spectrum (CDC13, Sppm):
1.50 (d;J=8 Hz, 2.4H), 1.77 (d;J=S Hz, 0.6H),
2.26 (s, 3H), 2.31 (s, 3H), 4.92 (s, 2H),
134 2 ~ g 1555
4.96-5.04 (m, 1.6H), 5.10-5.38 (m, 1.4H),
5.48-5.63 (m, 1H), 7.09-7.17 (m, 1H), 7.56-7.62
(m, 2H), 8.54-8.60 (m, 1H), 9.04 (s, IH).
Elementary analysis (%):
Calc'd for ClBHZON4S'3/4H20: C, 63.97; H,
6.41; N, 16.58,
Found: C, 64.16; H, 6.14; N, 16.23.
Example 55
7-(4-Chlorobenzylthio)-2,3-dimethvl-1-(2-
proHenyl)pyrrolof2,3-dlpyridazirae
The title compound was prepared as a yellow solid in
70.6% yield in a similar procedure to that described in
Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
propenyl)pyrrolo(2,3-d]pyridazine and
4-chlorophenylmethanethiol.
m.p.: 107-108°C.
Mass spectrum (CI, m/z): 344 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.26 (s, 3H), 2.30 (s, 3H), 4.58 (d;J=14 Hz, 1H),
4.70 (s, 2H), 5.00-5.13 (m, 3H), 5.87-6.01 (m,
1H), 7.19-7.27 (m, 2H), 7.35-7.42 (m, 2H), 9.07
(s, 1H).
Elementary analysis (%):
Calc'd for C18H18C1N3S: C, 62.87; H, 5.28; N,
12.22,
Found: 62.90; H, 5.44; N, 12.00.
7
S 2181553
135
Example 56
1-(2-Butenyl)-3-ethvl-7-(4-fi~mrnhP"~ylo,~r)_2_
methylgyrrolof2,3-dlgyridazinP __
The title compound (cis/trans=22/78) was prepared as a
white powder in 63.1% yield in a similar procedure to that
described in Example 1 by using
1-(2-butenyl)-7-chloro-3-ethyl-2-
methylpyrrolo[2,3-d]pyridazine (cis/trana=26/74) and
4-fluorobenzyl alcohol.
m.p.: 78-83°C.
Mass spectrum (CI, m/z): 340 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.22 (t;J=8 Hz, 3H), 1.56-1.68 (m, 3H), 2.32 (s,
3H), 2.71 (q;J=8 Hz, 2H), 4.81-4.89 (m, 1.56H),
5.02 (d;J=8 Hz, 0.44H), 5.13-5.29 (m, 1H),
5.42-5.58 (m, 1H), 5.69 (s, 2H), 7.01-7.12 (m,
2H), 7.42-7.55 (m, 2H), 9.01 (s, 1H).
Elementary analysis (%):
Calc'd for C20H22FN30: C, 70.77; H, 6.53; N,
12.38,
Found: C, 70.75; H,.6.56; N, 12.40.
Example.57
7-(4-F~uorobenzv~~)-2 3-dimethyl-1-(2-
m lm t~yrrQlof2.3-dlovridazine
The title compound (traps) was prepared as a white
powder in 91.6% yield in a similar procedure to that
described in Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
methylcyclopropylmethyl)pyrrolo[2,3-d]pyridazine and
Y
2181553
136
4-fluorobenzyl alcohol.
m.p.: 121-122°C.
Mass spectrum (CI, m/z): 340 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
0.15 (dt;J=8 Hz, 4 Hz, 1H), 0.39 (dt;J=8 Hz, 4
Hz, 1H), 0.58-0.67 (m, 1H), 0.76-0.85 (m, 1H),
0.90 (d;J=7 Hz, 3H), 2.27 (s, 3H), 2.37 (s, 3H),
4.14 (dd;J=15 Hz, 7 Hz, 1H), 4.28 (dd;J=15 Hz, 7
Hz, 1H), 5.64 (d;J=16 Hz, IH), 5.68 (d;J=16 Hz,
1H), 7.06-7.13 (m, 2H), 7.48-7.53 (m, 2H), 8.99
(s, 1H) .
Elementary analysis (%):
Calc'd for C20H22FN30: C, 70.77; H, 6.53; N,
12.38,
Found: C, 70.77; H, 6.57; N, 12.37.
Example 58
7-(2,4-Difluorobenzvlox<r)-2 3-dimethyl-1-(2-
methy~y~~bvlmethvl)pyrrolof2 3-dlpyridazine
The title compound (trans) was prepared as a white
powder in 63.8% yield in a similar procedure to that
described in Example 1 by using 7-chloro-2,3-dimethyl-1-(2-
methylcyclopropylmethyl)pyrrolo[2,3-d]pyridazine and
2,4-difluorobenzyl alcohol.
m.p.: 101-102°C.
Mass spectrum (CI, m/z): 358 (M+ + 1).
2181553
137
NMR spectrum (CDC13, bppm):
0.10-0.17 (m, 1H), 0.35-0.41 (m, 1H), -0.59-0.63
(m, 1H), 0.78-0.86 (m, 1H), 0.89 (d;J=7 Hz, 3H),
2.26 (s, 3H), 2.36 (s, 3H), 4.10 (dd;J=15 Hz, 7
Hz, 1H), 4.26 (dd;J=15 Hz, 7 Hz, 1H), 5.67-5.77
(m, 2H), 6.84-6.92 (m, 2H), 7.54-7.62 (m, 1H),
8.97 (s, 1H).
Elementary analysis (%):
Calc'd for C20H21F3N3W C, 67.20; H, 5.92; N,
11.76,
Found: C, 67.28; H, 5.91; N, 11.74.
Example 59
~ (2-BUtenyl) -7- (4-fl ~or~hPn~yl~) 2 m t yi '~ __,
den vlpyrrolof2,3-r~lgyridazine
The title compound (cis/trans=14/86) was prepared as a'
white powder in 49.8% yield in a similar procedure to that
described in Example 1 by using
1-(2-butenyl)-7-chloro-2-methyl-3-
pentylpyrrolo[2,3-d]pyridazine (cis/trans=20/80) and
4-fluorobenzyl alcohol.
m.p.: 65-69°C.
Mass spectrum (CI, m/z): 382 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.89 (t;J=8 Hz, 3H), 1.21-1.40 (m, 9H), 2.33 (s,
3H), 2.68 (t;J=8 Hz, 2H), 4.82-4.89 (m, 1.72H),
5.02 (d;J=8 Hz, 0.28H), 5.07-5.24 (m, 1H),
2181553
138
5.43-5.58 (m, 1H), 5.66 (s, 2H), 7.02-7.12 (m,
2H), 7.45-7.52 (m, 2H), 8.99 (s, 1H).
Elementary analysis (%):
Calc'd for C23H28FN30: C, 72.41; H, 7.40; N,
11.02,
Found: C, 72.44; H, 7.29; N, 11.03.
Example 60
7-Benzvloxv-2 3-dimethyl-1-(4 4 4 r~fi"ors
butenv~)gyrrolof2 3-dlpyridaz ne
The title compound was prepared as pale yellow
crystals in 20.7 yield in a similar procedure to that
described in Example 41by using
7-benzyloxy-2,3-dimethylpyrrolo[2,3-d]pyridazine and
4,4,4-trifluoro-2-butenyl methanesulfonate.
m.p.: 138-140°C.
Mass spectrum (CI, m/z): 362 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.28 (s, 3H), 2.29 (s, 3H), 4.98-5.11 (m, 3H),
5.66 (s, 2H), 6.37-6.48 (m, 1H), 7.32-7.50 (m,
5H), 9.01 (s, 1H). -
Elementary analysis (~):
Calc'd for C19H18F3N30: C, 63.15; H, 5.02; N,
11.63,
Found: C, 63.21; H, 5.06; N, 11.59.
Example 61
A
' 2181553
139
7-BenzvloxV-1-(2 3-dichloro-2-gropenyl)-2 3-
dimethvlgyrrolof2.3-dlgvridazine __
The title compound was prepared as ocherous powdery
crystals in S.0% yield in a similar procedure to that
described in Example 41 by using
7-benzyloxy-2,3-dimethylpyrrolo[2,3-d]pyridazine and
1,2,3-trichloro-1-propene.
Mass spectrum (CI, m/z): 362 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
2.32 (s, 3H), 2.43 (s, 3H), 5.15 (s, 2H), 5.66
(s, 2H), 5.80 (s, 1H), 7.31-7.52 (m, 5H), 9.18
(s, 1H) .
Example 62
1-(2-BUtenyl)-7-(4-fluorophenethyl)-2 3-
dimethy~gyrrolof2.3-dlgyridazine
0.10 g (0.0020 mole) of hydrazine hydrate was added to
a solution of 0.39 g (0.00113 mole) o~ I-(2-
butenyl)-2-[1-chloro-3-(4-fluorophenyl)-I-propenyl]-3-
formyl-4,5-dimethylpyrrole in 7 ml of ethanol and the
resulting mixture was stirred at 75°C for an hour. After
completion of the reaction, the reaction mixture was
concentrated under reduced pressure and the concentrate
was diluted with ice-water. The aqueous mixture was
extracted twice with 30 ml of ethyl acetate. The combined
extracts were washed with a saturated aqueous solution of
sodium chloride and dried over anhydrous sodium sulfate.
The solvent was distilled off under reduced pressure and
r 2181553
140
the residue.was purified by column chromatography through
silica gel using a 50:1 mixture of chloroform and methanol
as an eluent to give 0.23 g of the title compound
(cis/trans=22/78) as white powdery crystals.
m.p.: 108-113°C.
Mass spectrum (CI, m/z): 324 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.60-1.68 (m, 3H), 2.30 (s, 3H), 2.35 (s, 3H),
3.18-3.26 (m, 2H), 3.49-3.57 (m, 2H), 4.75-4.80
(m, 1.56H), 4.85-5.05 (m, 1H), 5.20-5.30~(m,
0.44H), 5.50-5.67 (m, 1H), 6.94-7.02 (m, 2H),
7.18-7.24 (m, 2H), 9.20 (s, 1H).
Elementary analysis-(%):
Calc'd for C20H22FN3~ C, 74.28; H, 6.85; N,
12.99,
Found: C, 74.41; H, 6.99; N, 12.90.
Example 63
7-(4-Fluoronhenethyl)-2 3-dimet~rl-1-(2-
methy~yeloproy~~lmethyl)gyrrolof2 3-dlovridazine
The title compound Was prepared as pale yellow powdery
crystals in 72.7% yield in a similar procedure to that
described in Example 62 by using
7-[1-chloro-3-(4-fluorophenyl)-1-propenyl]-3-
formyl-4,5-dimethyl-1-(2-methylcyclopropylmethyl)pyrrole. -
m.p.: 112-114°C.
Mass spectrum (CI, m/z): 338 (M+ + 1).
2181553
141
NMR spectrum (CDC13, bppm):
0.27-0.41 (m, 2H), 0.57-0.79 (m, 2H), 0.97 (d;J=6
Hz, 3H), 2.29 (s, 3H), 2.39 (s, 3H), 3.19-3.25
(m, 2H), 3.57-3.63 (m, 2H), 4.20 (d;J=6 Hz, 2H),
6.95-7.02 (m, 2H), 7.20-7.27 (m, 2H), 9.18 (s,
1H) .
Elementary analysis (%):
Calc'd for C21H24FN3~ C, 74.75; H, 7.17; N,
12.45,
Found: C, 74.63; H, 7.27; N, 12.42.
Example 64
1-t,~vclox~ropylmethp _7_~4_fluoronhenArhyl)-2 3-
~~m hy~gyrroio[2 3-dlwrridazinP
The title compound was prepared as pale yellow powdery
crystals in 53.4% yield in a similar procedure to that
described in Example 62 by using
2-[1-chloro-3-(4-fluorophenyl)-1-propenyl-1-
cyclopropylmethyl]-3-formyl-4,5-dimethylpyrrole.
m.p.: 172-173°C.
Mass spectrum (CI, m/z): 324 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
0.20-0.26 (m, 2H), 0.52-0.59 (m, 2H), 1.02-1.10
(m, 1H), 2.29 (s, 3H), 2.39 (s, 3H), 3.19-3.25
(m, 2H), 3.58-3.64 (m, 2H), 4.20 (d;J=6 Hz, 2H),
6.95-7.01 (m, 2H), 7.19-7.25 (m, 2H), 9.18 (s,-
1H) .
'~' 2181553
142
Elementary analysis (%):
Calc'd for C20H22~3' C~ 74.28; H, 6.86; N,
12.99,
Found: C, 74.19; H, 6.88; N, 12.90.
Example 65
7- (4-Fi uorogh_eneth5rl ) -2 3-dimethvl-~ - (2-
~ro~yl)gyrroloC2,3-dl~yridazine
The title compound was prepared as pale yellow powdery
crystals in 55.8% yield in a similar procedure to that
described in Example 62 by using
2-[1-chloro-3-(4-fluorophenyl)-1-propenyl]-3-
formyl-4,5-dimethyl-1-(2-propenyl)pyrrole.
m.p.: 123-124°C.
Mass spectrum (CI, m/z): 310 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.30 (s, 3H), 2.34 (s, 3H), 3.19-3.25 (m, 2H),
3.45-3.51 (m, 2H), 4.46 (d;J=17 Hz, 1H),
4.81-4.84 (m, 2H), 5.16 (d;J=10 Hz, 1H),
5.91-6.04 (m, 1H), 6.94-7.01 (m, 2H), 7.18-7.23
(m, 2H), 9.20 (s, IH).
Elementary analysis (%):
Calc'd for C19H20FN3: C, 73.76; H, 6.52; N,
13.58,
Found: C, 73.72; H, 6.61; N, 13.45.
Example 66
1-(2-butegyl)-2.3-dimethvl-7-
phenethylg,~rrroloC2 3-dlgyridaz~ne
S
2181553
143
The title compound (cis/trans=14/86) was prepared as
an ocherous powder in 53.2% yield in a similar procedure
to that described in.Example 62 by using
1-(2-butenyl)-2-(1-chloro-3-phenyl-1-propenyl)-3-
formyl-4,5-dimethylpyrrole (cis/trans=23/77).
m.p.: 98-106°C.
Mass spectrum (CI, m/z): 306 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.58-1.65 (m, 3H), 2.29 (s, 3H), 2.34 (s, 3H),-
3.19-3.25 (m, 2H), 3.50-3.57 (m, 2H), 4.76-4.79
(m, 1.72H), 4.84-4.89 (m, 0.28H), 4.94-5.02 (m,
1H), 5.56 (br.d, 1H), 7.20-7.35 (m, 5H), 9.20 (s,
1H).
Elementary analysis (%):
Calc'd for C20H23N3' C' 78.65; H, 7.59; N, 13.76,
Found: C, 78.78; H, 7.61; N, 13.76.
Example 67
2.3-D~.methvl-7-
The title compound was prepared as yellow crystals in
56.3% yield in a similar procedure to that described in
Example 62 by using
2-(1-chloro-3-phenyl-1-propenyl)-3-formyl-4,5-
dimethyl-1-(2-propenyl)pyrrole.
m.p.: 96-98°C.
Mass spectrum (CI, m/z): 292 (M+ + 1).
218155
144
NMR spectrum (CDC13, 5ppm):
2.,30 (s, 3H), 2.34 (s, 3H), 3.20-3.26 (m, 2H),
3.48-3.54 (m, 2H), 4.45 (d;J=17 Hz, 1H),
4.82-4.85 (m, 2H), 5.16 (dd;J=10 Hz, 2 Hz, 1H),
5.98 (ddt; J=17 Hz, 10 Hz, 4 Hz, 1H), 7.16-7.39 -'
(m, 5H), 9.21 (s, 1H).
Elementary analysis (%):
Calc'd for C19H21N3: C, 78.31; H, 7.26; N, 14.42,
Found: C, 78.28; H, 7.42; N, 14.20.
Example 68
'~_D;metY~yl-1-(2-merhy~~yclo _ropYlmPr yip 7
phenethylgyrrolo f 2 -c)l~rrida~~ ne
The title compound was prepared as a creamy powder in
50.0% -yield in a similar procedure to.that described in
Example 62 by using
2-(1-chloro-3-phenyl-1-propenyl)-3-formyl-4,5
dimethyl-1-(2-methylcyclopropylmethyl)pyrrole.
m.p.: 102-103°C.
Mass spectrum (CI, m/z): 320 (M+ + 1).
NMR spectrum (CDC13, sppm):
0.26-0.41 (m, 2H), 0.57-0.67 (m, 1H), 0.73-0.80
(m, 1H), 0.97 (d;J=6 Hz, 3H), 2.28 (s, 3H), 2.38
(s, 3H), 3.20-3.26 (m, 2H), 3.60-3.66 (m, 2H),
4.22 (d;J=6 Hz, 2H), 7.14-7.38 (m, SH), 9.18 (s,
1H) .
' 2181553
1
145
Elementary analysis (%);
Calc'd for C21H25N3- C, 78.95; H, 7.89; N, 13.16,
Found: C, 78.95; H, 7.93; N, 13.11.
Example 69
~Ycio ''o~ylmethyl-2-'~-~;me~-hy~-7
~henethxl_Ryrrolo(2.3-dlpyridazine
The title compound was prepared as a creamy powder in
69.9% yield in a similar procedure to that described in
Example 62 by using 2-(1-chloro-3-phenyl-1-propenyl)-1-
cyclopropylmethyl-3-formyl-4,5-dimethylpyrrole.
m.p.: 133-135°C.
Mass spectrum (CI, m/z): 306 (M+ + 1).
NMR spectrum (CDC13, Sppm):
0.20-0.26 (m, 2H), 0.51-0.58 (m, 2H), 1.02-1.12
(m, 1H), 2.29 (s, 3H), 2.39 (s, 3H), 3.19-3.25
(m, 2H), 3.60-3.67 (m, 2H), 4.22 (d;J=6 Hz, 2H),
7.16-7.36 (m, SH), 9.19 (s, 1H).
Elementary analysis (%):
Calc'd for C20H23N3: C, 78.65; H, 7.59; N, 13.76,
Found: C, 78.42; H, 7.62; N, 13.66.
Example 70
3.- (2-Butenyl) -7- (2 4-d; fi mnr hanc~th 1 1
QF' Y 2 3 _
d; m hyi pyr,-of o -d1 py,-; daz; ne
2181553
146
The title compound (cis/trans=20/80) was prepared as
pale ocherous powdery crystals in 59.2% yield in a similar
procedure to that described in Example 62 by using
1-(2-butenyl)-2-[1-chloro-3-(2,4-difluorophenyl)-1-
propenyl]-3-formyl-4,5-dimethylpyrrole.
m.p.: 114-118°C.
Mass spectrum (CI, m/z): 342 (M+ + 1).
NMR spectrum (CDC13, Sppm):
1.58-1.71 (m, 3H), 2.30 (s, 3H), 2.35 (s, 3H),
3.17-3.26 (m, 2H), 3.45-3.55 (m, 2H), 4.80-5.03
(m, 2.8H), 5.19-5.28 (m, 0.2H), 5.51-5.66 (m,
1H), 6.77-6.84 (m, 2H), 7.22-7.32 (m, 1H), 9.19
(s, 1H) .
Elementary analysis (%):
Calc'd for C20H21F2N3' C~ 70.36; H, 6.20; N,
12.31,
Found: C, 70.52; H, 6.23; N, 12.27.
Example 71
7-(2 4-Difluorobhenethvl)-2 3-dimethyl-1-(2-
methy~yclogrogylmet ~l ) pyrroi o f 2 3-dl ~,yridazine
The title compound was prepared as pale yellow powdery
crystals in 64.0% yield in a similar procedure to that
described in Example 62 by using
2-[1-chloro-3-(2,4-difluorophenyl)-1-propenyl]-3-
formyl-4,5-dimethyl-1-(2-methylcyclopropylmethyl)pyrrole.
m.p.: 105-106°C.
~1~1~53
147
Mass spectrum (CI, m/z): 356 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.26-0.42 (m, 2H), 0.59-0.80 (m, 2H), 0.97 (d;J=6
Hz, 3H), 2.29 (s, 3H), 3.40 (s, 3H), 3.17-3.24
(m, 2H), 3.55-3.61 (m, 2H), 4.28 (d;J=6 Hz, 2H),
6.78-6.85 (m, 2H), 7.23-7.32 (m, 1H), 9.18 (s,
1H) .
Elementary analysis (%):
Calc'd for C21H23F2N3' C' 70.97; H, 6.52; N,
11.82,
Found: C, 71.11; H, 6.54; N, 11.86.
Example 72
Ice- velopropvlmethyl-7-(2 4-di i»~ro methyl)-2 3-
dimethylgyxrOlof2-'~-dlgyr;rla~;na
The title compound was prepared as pale flesh-colored
powdery crystals in 69.0% yield in a similar procedure to
that described in Example 62 by using
2-[1-chloro-3-(2,4-difluorophenyl)-1-propenyl]-1-
cyclopropylmethyl-3-formyl-4,5-dimethylpyrrole.
m.p.: 159-160°C.
Mass spectrum (CI, m/z): 342 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.22-0.28 (m, 2H), 0.52-0.59 (m, 2H), 1.01-I.13
(m, 1H), 2.29 (s, 3H), 2.41 (s, 3H), 3.17-3.23 -
(m, 2H), 3.56-3.62 (m, 2H), 4.28 (d;J=6 Hz, 2H),
6.78-6.85 (m, 2H), 7.23-7.32 (m, 1H), 9.18 (s,
1H) .
2181553
148
Elementary analysis (%):
Calc'd for C20H21F2N31~SH20: C, 69.63; H,
6.25; N, 12.18,
Found: C, 69.71; H, 6.22; N, 12.12.
Example 73
7-(2 4-Diflunro8 nP yl)-2 3-dimethyl-1-(2-
propeny~pyrrolo f 2 3 -dlg~rri ria ~i nP
The title compound was prepared as pale yellow powdery
crystals in 66.2% yield in a similar procedure to that
described in Example 62 by using
2-(1-chloro-3-(2,4-difluorophenyl)-1-propenyl]-3-
formyl-4,5-dimethyl-1-(2-propenyl)pyrrole.
m.p.: 118-119°C.
Mass spectrum (CI, m/z): 328 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.30 (s, 3H), 2.35 (s, 3H), 3.17-3.23 (m, 2H),
3.43-3.49 (m, 2H), 4.43 (d;J=17 Hz, 1H),
4.90-4.93 (m, 2H), 5.14 (d;J=10 Hz, 1H),
5.94-6.05 (m, 1H), 6.76-6.84 (m, 2H), 7.22-7.31
(m, 1H), 9.20 (s, 1H).
Elementary analysis (%):
Calc'd for C19H19F2N3: C, 69.71; H, 5.85; N,
12.84,
Found: C, 69.67; H, 5.90; N, 12.81.
Example 74
1-(2-Buteny~)-7-(4-fluorob nzyiyn~y -2 3-
dim hy~ gyrr010 (2 3-dl ~vri r7avi na ~rt3rnrh~ nri ria ___--
2181553
149
A solution of 0.36 g (0.01 mole) of hydrogen chloride
in 3.0 ml of ethanol was added dropwise with ice-cooling -
to a solution of 2.00 g (0.00615 mole) of
1-(2-butenyl)-7-(4-fluorobenzyloxy)-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trans=1/99) in 160
ml of dry diethyl ether and the resulting mixture was
stirred at the same temperature for 20 minutes. The
reaction mixture was concentrated at room temperature and
the residue was washed with a mixture of 10 m1 of ethanol
and 120 ml of dry diethyl ether. The solid mass thus
obtained was,then collected by filtration to give 1.88 g
of the title compound (traps) as a white powder. -
m.p.: 203-220°C.-
Mass spectrum (CI, m/z): 325 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.62 (d, J=9 Hz, 3H), 2.32 (s, 3H), 2.46 (s, 3H),
5.00 (d, J=5 Hz, 2H), 5.18-5.72 (m, 4H),
6.99-7.20 (m, 2H), 7.36-7.60 (m, 2H), 9.29 (s,
1H), 17.18 (s, 1H).
Elementary analysis (%):
Calc'd for C19H20FN30'HC1: C, 63.07; H, 5.85;
N, 11.61,
Found: C, 63.09; H, 5.91; N, 11.61.
Example 75
1-(2-Butenyl)-7-(4-fl~ornhAn~ylo
dimethvlp5rrrolo(2 '~-dlovr~daz~n -5-oxid
A solution of 0.72 g (0.0029 mole) of
218153
150
m-chloroperoxybenzoic acid (purity: 70~) in 20 ml of=
dichloromethane was added to a solution of 0.72 g (0.0029
mole) of 1-(2-butenyl)-7-(4-fluorobenzyloxy)-2,3-
dimethylpyrrolo[2,3-d]pyridazine in 70 ml of
dichloromethane at room temperature and the resulting
mixture was stirred at the same temperature for 30
minutes. After completion of the reaction, the reaction
mixture was washed three times with 40 ml each of a
saturated aqueous solution of sodium hydrogencarbonate. -
The dichloromethane layer was dried over anhydrous sodium
sulfate and the solvent was distilled off under reduced -
pressure. The residue was purified by column
chromatography through silica gel using a 100:1 to 100:4
mixture of chloroform and methanol as an eluent to give
crystals, which were washed with a mixture of ether and
hexane to give 0.63 g of the title compound
(cis/trana=27/73) as a pale yellow powder.
m.p.: 138-148°C.
Mass spectrum (CI, m/z): 342 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.42-1.68 (3H, m), 2.13 (3H, s), 2.30 (3H, s),
4.69-4.83 (1.46H, m), 4.88-4.97 (0.54H, m),
5.11-5.66 (4H, m), 6.98-7.13 (2H, m), 7.39-7.52
(2H, m), 8.27 (1H, s).
Elementary analysis (~):
Calc'd for C19H20FN3~2' C, 66.85;'H, 5.91; N,
12.31,
Found: C, 66.78; H, 5.88; N, 12.29.
2181553
151
Example 76
1-(2-Butenyl)-7-(2.4-difluorobenzyloxyl-2 3- .
dim hy~y~yrrolo(2,3-dlpyridazine-5-oxide
The title compound (cis/trans=7/93) was prepare-d as
pale yellow powdery crystals in 87.0% yield in a similar
procedure to that described in Example 75 by using
1-(2-butenyl)-7-(2,4-difluorobenzyloxy)-2,3-
dimethylpyrrolo[2,3-d]pyridazine.
m.p.: 166-168°C.
Mass spectrum (CI, m/z): 360 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.58-1.61 (m, 3H), 2.14 (s, 3H), 2.29 (s, 3H),
4.72-4.75 (m, 1.86H), 4.88-4.91 (m, 0.14H),
5.15-5.31 (m, 1H), 5.38-5.50 (m, 1H), 5.59 (s,
2H), 6.83-6.94 (m, 2H), 7.49-7.58 (m, 1H), 8.23
(s, 1H) .
Elementary analysis (%):
Calc'd for C19H19F2N302: C, 63.50; H, 5.33; -
N, 11.69,
Found: C, 63.49; H, 5.28; N, 11.69.
Example 77
1-(2-Butenyl)-7-(2.4-difluorophenethyl)-2.3-
dim h~y~yrrolof2.3-dlpyridazine-5-oxide and
1-(2-butenyl)-7-(2,4-difluoronheneth3rl)-2.3-
dimethylpvrrolof2.3-dlpyridazine-6-oxide
152 2181553
A solution of 0.35 g (0.00142 mole) of
m-chloroperoxybenzoic acid (purity: 70%) in 5 ml of
dichloromethane was added dropwise to a solution of 0.47 g
(0.00138 mole) of
i-(2-butenyl)-7-(2,4-difluorophenethyl)-2,3-
dimethylpyrrolo[2,3-d]pyridazine in 10 ml of
dichloromethane at room temperature over a period of 30
minutes and the resulting mixture was stirred at the same
temperature for an hour. After completion of the
reaction, the reaction mixture was washed three times with
30 ml each of a saturated aqueous solution of sodium
hydrogencarbonate. The dichloromethane layer was washed
with a saturated aqueous solution of sodium chloride and
dried over anhydrous sodium sulfate, and the solvent was
distilled off under reduced pressure. The residue was
purrfied by column chromatography through silica gel using
a 50:1 mixture of chloroform and methanol as an eluent.
The purified product was triturated with a mixture of
ether and hexane to give 0.058 g of the 5-oxide of the
title compound (cis/trans=25/75) and 0.290 g of the
6-oxide of the title compound (cis/trans=25/75) as a pale
yellow powder, respectively.
5--Ox~ O mA
m.p.: 104-112°C.
Mass spectrum (CI, m/z): 358 (M+ + 1).
NMR spectrum (CDC13, bppm):
r 153 2181555
1.62-1.70 (m, 3H), 2.25 (s, 3H), 2.30 (s, 3H),
3.08-3.18 (m, 2H), 3.41-3.51 (m, 2H),, 4.63-4.67
(m, 1.5H), 4.74-4.78 (m, O.5H), 4.97-5.07 (m,
0.75H), 5.07-5.13 (m, 0.25H), 5.50-5.66 (m, 1H),
6.75-6.83 (m, 2H), 7.28-7.37 (m, 1H), 8.52 (s,
1H) .
Elementary analysis (%):
Calc'd for C20H21F2N3~~1/SH20: C, 66.54;
H, 5.97; N, 11.64,
Found: C, 66.64; H, 5.88; N, 11.55.
6-OX7.de COm~,1 mri
m.p.: 135-141°C.
Mase spectrum (CI, m/z): 358 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.62-1.69 (m, 3H), 2.19 (s, 3H), 2.33 (s, 3H),
3.12-3.32 (m, 4H), 4.76-4.78 (m, 1.SH), 4.86-4.87
(m, O.5H), 4.96-5.09 (m, 0.75H), 5.19-5.25 (m,
0.25H), 5.50-5.67 (m, 1H), 6.76-6.84 (m, 2H),
7.20-7.29 (m, 1H), 8.40 (s, 1H).
Elementary analysis (%):
Calc'd for C20H21F2N3~~ C, 67.21; H, 5.92; N,
11.76,
Found: C, 66.98; H, 5.99; N, 11.62.
Referential Example 1
Ethvl i - (2-bu Pny i l -5-et~v~ a mArr,yyr,-rni A ~ a r ,tyl
(1) 3-Chin,-o-2-methy~-a pentenai (~ msh ~rP of cis nd
traps)
154 2 ~ ~ 1553
27 ml (0.30 mole) of phosphorus axychlor_de were addad
dropwise to 29.2 g (0.40 mole) of dimethylformamide with
ice-cooling over a period of 30 minutes and the resulting -
mixture was stirred at the same temperature for 30 minutes _
and then at room temperature for-40 minutes. 21 m1 (0.21
mole) of 3-pentanone were added thereto over a period of
20 minutes to keep the reaction temperature below 40°C by
ice-cooling, and the mixture was stirred with ice-cooling -
for 10 minutes and then at room temperature for 2 hours. - -
The reaction mixture was poured into about 300 ml of
ice-water by portions and the diluted mixture was
neutralized to pH 7-8 with sodium hydrogencarbonate. The
mixture was extracted with diethyl ether (once with 200 m1
and three times with 100 ml). The combined extracts were
washed with a saturated aqueous solution of sodium
chloride and dried over anhydrous sodium sulfate. The
solvent Was distilled off using a rotary evaporator in a
bath kept below 30°C. The residue was purified by
distilling at 58-61°C/15 mmHg to give 14.8 g of
3-chloro-2-methyl-2-pentenal as a colorless transparent
oil.
Masa spectrum (CI, m/z): 133 (M+ + 1), 135 (M+ +
3) .
NMR spectrum (CDC13, bppm):
1.23 (t;J=8 Hz, 3/4H), 1.30 (t;J=8 Hz, 9/4H),
1.84 (s, 3/4H), 1.91 (s, 9/4H), 2.65 (q;J=8 Hz,
2/4H), 2.94 (s, 6/4H), 10.02 (s, 3/4H), 10.21 (s,
1/4H).
2181553
155
(2) Ehyl 1-(2-butenyl)-5-ethyl-4-merh~~~nvrrole-2-
carboxvlate
3.74 g (0.024 mole) of ethyl N-(2-butenyl)glycinate
were added to a solution of 5.0 g (0.038 mole) of
3-chloro-2-methyl-2-pentenal in 10 ml of ethanol with
stirring, and subsequently 6 ml (0.043 mole) of
triethylamine were added thereto and stirred at room
temperature for 7 hours. After the precipitates deposited
were filtered off, 5.35 g (0.048 mole) of potassium
tert-butoxide were added to the filtrate by portions and
the mixture was stirred for 30 minutes. The reaction
mixture was poured into about 150 ml of a saturated
aqueous solution of ammonium chloride and extracted with
ethyl acetate (once with 100 ml and twice with 50 ml).
The combined extracts were washed with a saturated aqueous -
solution of sodium chloride and dried over anhydrous
sodium sulfate, and the solvent was distilled off. The
residue was purified by column chromatography through
silica gel using a 3:97 mixture of ethyl acetate and
hexane as an eluent to give 1.53 g of ethyl
1-(2-butenyl)-5-ethyl-4-
methylpyrrole-2-carboxylate (cis/trans=76/24) as an orange
oil.
Mass spectrum (CI, m/z): 236 (M+ + 1).
NMR spectrum (CDC13, bppm):
2181555
156
1.10 (t;J=8 Hz, 3H), 1.32 (t;J=7 Hz, 3H),,
1.61-1.65 (m, 2.28H), 1.74-1.78 (m, 0.72H), 2.03 -
(s, 3H), 2.55 (q;J=8 Hz, 2H), 4.20 (q;J=7 Hz,
2H), 4.84-4.90 (m, 1.52H), 4.97-5.03 (m, 0.48H),
5.30-5.38 (m, iH), 5.52-5.61 (m, 1H), 6.79 (s,
IH) .
Referential Example 2
~hvl 1-cvclopro~vl-4 5-d~mAth~~lgyr,-r,iA ~ c rboxvla
to
The title compound was prepared as a yellow oil in
41.6% yeild in a similar procedure to that described In
Referential Example 1 by using 2-butanone and ethyl
N-cyclopropylglycinate.
Mass spectrum (CI, m/z): 208 (M+ +1).
NMR spectrum (CDC13, 5ppm):
0.79-0.87 (m, 2H), 1.08-1.16 (m, 2H), 1.35 (t;J=8
Hz, 3H), 1.98 (s, 3H), 2.26 (s, 3H), 3.12-3.24
(m, 1H), 4.24 (q;J=8 Hz, 2H), 6.71 (s, 1H).
Referential Example 3
Ethv1 T-cvciohexv~-4 5- ~mAth i~vrrole 2 carboxvlatv
The title compound was prepared as a yellow oil in
18.9% yeild in a similar procedure to that described in
Referential Example 1 by using 2-butanone and ethyl
N-cyclohexylglycinate.
Mass spectrum (CI, m/z): 250 (M+ + 1).
157
2181553
NMR spectrum (CDC13, nppm):
1.32 (t;J=8 Hz, 3H), 1.63-1.94 (m, 6H), 1.98 (s,
3H), 2.03-2.24 (m, 4H), 2.30 (s, 3H), 3.39-3.70
(m, 1H), 4.21 (q;J=8 Hz, 2H), 6.89 (s, 1H).
Referential Example 4
Ethvl 4-ethvl-5-methvi-~-(2-p~0'g~,rlyl)pvrrole-2-carboxv~ar -
The title compound was prepared as a yellow oil in
25.6% yeild in a similar procedure to that described in
Referential Example 1 by using 2-pentanone and ethyl
N-(2-propenyl)glycinate, there was obtained the desired
compound as a yellow oil in 25.6%. yield.
Mass spectrum (CI, m/z): 222 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.17 (t;J=8 Hz, 3H), 1.34 (t;J=8 Hz, 3H), 2.16
(s, 3H), 2.42 (q;J=8 Hz, 2H), 4.23 (q;J=8 Hz,
2H), 4.68-4.8I (m, 1H), 4.91-5.00 (m, 2H),
5.04-5.12 (m, 1H), 5.87-6.02 (m, 1H), 6.84 (s,
1H).
Referential Example 5
Ethyl ?-(2-butenvl)-5-ethyl-3-formvl-4-methvlnvrrole-2-
1.5 ml (0.0069 mole) of phosphorus oxychloride was
added to a solution of 1.38 g (0.0059 mole) of ethyl
1-(2-butenyl-5-ethyl-4-methylpyrrole-2-carboxylate
(trans/cis=76/24) in 3 ml of dimethylformamide and the
resulting mixture was stirred in an oil bath kept at
2181553
158
100°C for 2 hours. The reaction mixture cooled, was goured
into about 50 ml of ice-water by driblets and neutralized
to pH 7-8 with a saturated aqueous solution of sodium
hydrogencarbonate. The aqueous mixture was then extracted
with 50 ml each of ethyl acetate for four times. The
combined extracts were washed with a saturated aqueous
solution of sodium chloride and dried over anhydrous
sodium sulfate, and the solvent was distilled off. The
residue was purified by column chromatography through
silica gel using a 1:10 mixture of ethyl acetate and
hexane as an eluent to give 1.33 g of ethyl
I-(2-butenyl)-5-ethyl-3-forrnyl-4-methylpyrrole-2-
carboxylate (trans/cis = 77/23) as a yellow-orange oil.
Mass spectrum (CI, m/z): 264 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.11 (t;J=8 Hz, 3H), 1.39 (t;J=7 Hz, 3H),
1.65-1.69 {m, 2.31H), 1.74-1.79 (m, 0.69H), 2.25
(s, 3H), 2.60 (q;J=8 Hz, 2H), 4.38 (q;J=7 Hz,
2H), 4.84-4,91 (m, 1.54H), 4.98-5.02 (m, 0.46H),
5.32-5.43 (m, 1H), 5.52-5.56 (m, 1H), 10.47 (s,
1H).
Referential Example 6
E~yi i-cyclop~ogyl-3-formyl-4 5-dimethyl_ovrrole 2 -
carboxvlate
The title compound was prepared as a yellow oil in
159 2 i 81553
37.7% yeild in a similar procedure to that described in
Referential Example 5 by using ethyl 1-cyclopropyl-4,5-
dimethylpyrrole-2-carboxylate.
Mass spectrum (CI, m/z): 236 (M+ + 1).
NMR spectrum (CDC13, 6ppm) : -
0.75-0.83 (m, 2H), 1.11-1.20 (m, 2H), 1.40 (t;J=7 -
Hz, 3H), 2.24 (s, 3H), 2.29 (s, 3H), 3.22-3.33
(m, 1H), 4.41 (q;J=7 Hz, 2H), 10.32 (s, IH).
Referential Example 7
F~hvl 1-cyclohexv~-3-formyl-4 5-dimethy~5~yrrole 2
The title compound was prepared as a yellow oiI in
47.1% yeild in a similar procedure to that described in
Referential Example 5 by using ethyl 1-cyclohexyl-4,5-
dimethylpyrrole-2-carboxylate.
Mass spectrum (CI, m/z): 278 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.17-1.51 (m, 4H), 1.40 (t;J=8 Hz, 3H), 1.69-2.15
(m, 6H), 2.22 (s, 3H), 2.31 (s, 3H), 4.38 (q;J=8
Hz, 2H), 4.61-4.82 (m, 1H), 10.29 (s, 1H).
Referential Example 8
Ethvl 4-ethyl-3-formv~ s morr,yi i m p,-nnPny~~,rrole ~ .,-
The title compound was prepared as a yellow-orange oil
in
160 2181553 -
42.8% yeild in a.similar procedure to that described in
Referential Example E by using ethyl 4-ethyl-5-methyl-1-(2-
propenyl)pyrrole-2-carboxylate.
Mass spectrum (CI, m/z): 250 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.10 (t;J=7 Hz, 3H), 1.37 (t;J=7 Hz, 3H), 2.18
(s, 3H), 2.74 (q;J=7 Hz, 2H), 4.37 (q;J=7 Hz,
2H), 4.79 (d;J=17 Hz, 1H), 4.92-4.98 (m, 2H),
5.15 (d;J=11 Hz, 1H), 5.88-6.04 (m, 1H), 10.50
(s, 1H) .
Referential Example 9
Methvi i-(2-butenvl>-3-formvl-4 5-dimethyl_pvrrole 2 -
carbox~ lr ate
2.21 g (0.0199 mole) of potassium tert-butoxide were
added to a solution of 3.60 g (0.0199 mole) of methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and 0.36 g
(0.0014 mole) of 18-crown-6 in 220 ml of tetrahydrofuran
and the resulting mixture was stirred at room temperature
for 45 minutes. 3.60 g (0.0398 mole) of 1-chloro-2-butene
(a mixture of cis and trans isomers) was added to the
mixture and heated under reflex for 7 hours. 1.80 g
(0.0199 mole) of 1-chloro-2-butene were then added thereto -
and heated under reflex for 22 hours. The reaction
mixture was allowed to cool to room temperature,
subsequently ice-water was added thereto and the mixture
~
2181553
161
was extracted with ethyl acetate. The extract was, washed
with water and dried over anhydrous sodium sulfate, and -
the solvent was distilled off. The residue was purified
by column chromatography through silica gel using a 95:5
mixture of toluene and ethyl acetate as an eluent to give
4.50 g (0.0192 mole) of methyl 1-(2-butenyl)-3-
formyl-4,5-dimethylpyrrole-2-carboxylate (cis/trans =
24/76) as a yellow oil.
Mass spectrum (CI, m/z): 236 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
1.64-1.70 (m, 2.3H), 1.74-1.80 (m, 0.7H), 2.18
(s, 3H), 2.27 (s, 3H), 3.90 (s, 3H), 4.82-4.91
(m, 1.SH), 4.97-5.03 (m, O.SH), 5.27-5.70 (m,
2H), 10.45 (s, 1H).
Referential Example 10
Methyl '~-formv~-4 5-d~merhp -,-(3-merhri
butenvl)pyrro~e-2-carboxviaro
The title compound was prepared as a pale
yellow-orange solid in 85.4% yeild in a similar procedure
to that described in Referential Example 9 by using methyl
3-forntyl-4,5-dimethylpyrrole-2-carboxylate and
1-bromo-3-methyl-2-butene.
Mass spectrum (CI, m/z): 250 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.:'1 (s, 3H), 1.77 (s, 3H), 2.16 (s, 3H), 2.25
(s, 3H), 3.90 (s, 3H), 4.92 (d;J=6 Hz, 2H), 5.10
(t;J=6 Hz, 1H), 10.44 (s, 1H).
162 2181553
Referential Example 11
Methvl 3-formyl-4.5-dimethyl-1-(2-proggr~y~y~xrrole-2-
carbox~ 1r ate
The title compound was prepared as a mellow solid in
95..1% yeild in a similar procedure to that described in
Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
3-bromo-1-propene.
Mass spectrum (CI, m/z): 222 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.15 (s, 3H), 2.26 (s, 3H), 3.88 (s, 3H), 4.78
(d;J=16 Hz, 1H), 4.97 (d;J=5 Hz, 2H), 5.15
(d;J=10 Hz, 1H), 5.89-6.02 (m, 1H), 10.47 (s, 1H).
Referential Example 12
Met$y~ i-cyclo~rpy~ylmethyi-3-formvl-4 5-dimethyl8yrrole-2-
carboxvlate
The title compound was prepared as a pale yellow-white
solid in 95.1% yeild in a similar procedure to that
described in Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and cyclopropyl
bromide.
Mass spectrum (CI, m/z): 236 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
0.32-0.60 (m, 4H), 1.08-1.28 (m, 1H), 2.21 (s,
3H), 2.25 (s, 3H), 3.90 (s, 3H), 4.16 (d;J=8 Hz,
2H), 10.43 (s, 1H).
2181553
163
Referential Example 13
Methyl 3-formyl-4 5-dimethyl-1-(3-ghenvl-
propenyl)pyrrole-2-carboxylarP
The title compound (traps) was prepared as a pale
brown oil in 93.9% yeild in a similar procedure to that
described in Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-
carboxylate and 3-chloro-1-phenyl-1-propene (traps).
Mass spectrum (CI, m/z): 298 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.22 (s, 3H), 2.27 (s, 3H), 3.90 (s, 3H), 5.12
(d;J=4Hz, 2H), 6.12-6.34 (m, 2H), 7.17-7.43 (m, -
5H), 10.48 (s, 1H).
Referential Example 14
Methyl 3-fom!yl -4.5-dimethyl-1- (2-oentea2yl)pyrrole-2- _
carboxvlate
The title compound (traps) was prepared as a
yellow-orange oil in 85.1% yeild in a similar pro-cedure to
that described in Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
1-bromo-2-pentene (traps).
Mass spectrum (CI, m/z): 250 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.05 (t;J=8 Hz, 3H), 2.09-2.26 (m, 8H),-3.90 (s,
2181553
164
3H), 5.00 (d;J=5 Hz, 2H), 5.21-5.34 (m, 1H,),
5.46-5.60 (m, 1H), 10.43 (s, 1H).
Referential Example 15
Methyl 1-(2-bromo rt,yl)-3-formvi-4 5 dimethylpyrrole 2
carboxvlate
The title compound was prepared as ocherous crystals
in 9.4% yeild in a similar procedure to that described in
Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
1,2-dibromoethane.
Mass spectrum (CI, m/z): 288 (M+ + 1), 290 (M+ +
3) .
NMR spectrum (CDC13, bppm):
2.27 (s, 6H), 3.60 (t;J=7 Hz, 2H), 3.92 (s, 3H),
4.64 (t;J=7 Hz, 2H), 10.48 (s, 1H).
Referential Example 16
Methvl 3-form3rl.-4.5-dimethyl-1-(2-methvl-2-
oro~envl)pyrro~e-2-carboxvlatP
The title compound was prepared as a pale yellow oil
in 86.2% yeild in a similar procedure to that described in
Referential Example 9 by using but using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
3-chloro-2-methyl-1-propene.
Mass spectrum (CI, m/z): 236 (M+ + 1).
NMR spectrum (CDC13, bppm):
2181 X53 _.
165
1.78 (s, 3H), 2.12 (s, 3H), 2.27 (s, 3H), 3.88
(s, 3H), 4.13 (s, 1H), 4.81 (s, 1H), 4.86 (s,
2H), 10.48 (s, 1H).
Referential Example 17
Methyl 3-fop -4 5-dimethvl-1-(2 2 2-
trifluoroethy~)pyrrole-2-carboxvlate ,
The title compound was prepared as pale brown crystals
in 5.5% yeild in a similar procedure to that described in
Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-
carboxylate and 1,1,1-trifluoro-2-iodoethane.
Masa spectrum (CI, m/z): 264 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.22 (s, 3H), 2.27 (s, 3H), 3.93 (s, 3H),
4.91-5.30 (m, 2H), 10.47 (s, 1H).
Referential Example 18
I~thy1 1-(2-fluoroethyl)-3-formyl-4 5-dimethylg~r-rrole-2-
carbaxvlate
The title compound was prepared as pale brown crystals
in 77.4% yeild in a similar procedure to that described in
Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
1-bromo-2-fluoroethane..
Mass spectrum (CI, m/z): 228 (M+ + 1).
166 2 0 81555
NMR spectrum (CDC13, bppm):
2.23 (s, 3H), 2.27 (s, 3H), 3.90 (s, 3H),
4.46-4.86 (m, 4H), 10.49 (s, 1H).
Referential Example 19
Methyl 1-(2,2-difluoroethyl)-3-formyl-4 S- -
dimethylpyrrole-2-carboxulate
The title compound was prepared as flesh-colored
crystals in 90.4% yeild in a similar procedure to that
described in Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
2-bromo-1,1-difluoroethane.
Mass spectrum (CI, m/z): 246 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.24 (s, 3H), 2.26 (s, 3H), 3.93 (s, 3H), 4.66
(dt;J=4 Hz, 14 Hz, 2H), 6.11 (tt;J=4 Hz, 54 Hz,
1H), 10.49 (s, 1H).
Referential Example 20
Methyl 1-(2-butenyl)-3-formyl-4 5-dimethgl_~2yrrole-2- -
~arbox~rlate
The title compound (cis/trana=93/7) was prepared as a -
pale brown oil in 33.4% yeild in a similar procedure to
that described in Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-
carboxylate and 2-butenyl methanesulfonate
(cis/trans=96/4).
167 2181553
Mass spectrum (CI, m/z): 196 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.64-1.70 (m, 0.2H), 1.71-1.82 (m, 2.8H), 2.17
(s, 3H), 2.25 (s, 3H), 3.90 (s, 3H), 4.85-4.91
(m, 0.1H), 4.96-5.06 (m, 1.9H), 5.27-5.70 (m, -
2H), 10.44 (s, 1H).
Referential Example 21
Methvl 1-(2-chloro-2-~r penyl)-3-formyl-4 5-
dimethv pvrrole-2-carbo~rs~late _ -
The title compound was prepared as pale yellow
crystals in 77.5% yeild in a similar procedure to that
described in Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
2,3-dichloro-1-propene.
Mass spectrum (CI, m/z): 256 (M+ + 1), 258 (M+ +
3) .
NMR spectrum (CDC13, bppm):
2.19 (s, 3H), 2.27 (s, 3H), 3.90 (s, 3H), 4.70
(s, 1H), 5.10 (s, 2H), 5.29 (s, 1H), 10.49 (s,
1H) .
Referential Example 22
Methyl 3-formvl-4,5-dimethyl-1-(4 4 4-trif~~~nrn-~-
b~ -nyl)pvrrole-2-carhnxv~la P
The title compound (trans) was prepared as a pale
yellow oil in 60.0% yeild in a similar procedure to that
described
168 2181553
in Referential Example 9 by using methyl , -
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
4,4,4-trifluoro-2-butenyl methanesulfonate (trans).
Masa spectrum (CI, m/z): 290 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
2.15 (s, 3H), 2.27 (s, 3H), 3.90 (s, 3H),
5.09-5.13 (m, 2H), 5.22-5.31 (m; 1H), 6.49-6.57
(m, IH), 10.48 (s, 1H).
Referential Example 23
Meth~rl 1- (3 3-difluoro-2-~p~y1) -3-formvl-4 5-
dim hp pyrrole-2-carbo~rlate
The title compound was prepared as a pale yellow oil
in 70.4% yeild in a similar procedure to that described in
Referential Example 9 by using methyl
3-forinyl-4,5-dimethylpyrrole-2-carboxylate and
3-bromo-3,3-difluoro-1-propene.
Masa spectrum (CI, m/z): 258 (M+ + 1).
NMR spectrum (CDC13, Sppm):
2.20 (s, 3H), 2.25 (s, 3H), 3.91 (s, 3H),
4.50-4.67 (m, IH), 4.91 (d;J=7 Hz, 2H), 10.46 (s,
1H).
Referential Example 24
Methyl 1-(3-fluor9pro_pyl)-3-formyl-4 5-dimethylHyrrole-2-
carboxvlate
169 2181555
The title compound was prepared as pale yellow .
crystals in 90.8% yeild in a similar procedure to that
described in Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
1-bromo-3-fluoropropane.
Mass spectrum (CI, m/z): 242 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.01-2.30 (m, 2H), 2.22 (s, 3H), 2.26 (s, 3H),
3.90 (s, 3H), 4.33-4.60 (m, 4H), 10.46 (s, 1H).
Referential Example 25
Mewl 3-formyl-4.5-dimethvl-1-(2-progyn_vl)gvrrole-2-
carboxylate
The title compound was prepared as white crystals in
89.3% yeild in a similar procedure to that described in
Referential Example 9 by using methyl
3-formyl-4,5-dimethylpyrrole-2-carboxylate and
3-bromo-1-propyne.
Mass spectrum (CI, m/z): 220 (M+ + 1).
NMR spectrum (CDC13, Sppm):
2.27 (s, 3H), 2.29 (s, 3H), 2.31 (s, 1H), 3.93
(s, 3H), 5.18 (s, 2H), 10.48 (s, 1H).
Referential Example 26
I~ethSri 1- (3 3-dichloro-2-proTJ2~y~ 1 -3-formvl-4 5- __
dimethyipyrrole-2-carboxzrlate
The title compound was prepared as grayish-white
crystals in 87.1% yeild in a similar procedure to that
170 2181553
described in Referential Example 9 by using methyl
3-forrnyl-4,5-dimethylpyrrole-2-carboxylate and
1,1,3-trichloro-1-propene.
Mass spectrum (CI, m/z): 290 (M+ + 1), 292 (M+ +
3), 294 (M+ + 5).
NMR spectrum (CDC13, sppm):
2.20 (s, 3H), 2.25 (s, 3H), 3.91 (s, 3H), 5.05
(d;J=6 Hz, 2H), 5.99 (t;J=6 Hz, 1H), 10.46 (s,
1H) .
Referential Example 27
Methyl 1-cyclohex~lr met~rl-3-formyl-4 5 di~y~pyrrole 2
carboxylate
The title compound was prepared as an orange oil in
79.6% yeild in a similar procedure to that described in
Referential Example 9 by using methyl
3-forrnyl-4,5-dimethylpyrrole-2-carboxylate and
cyclohexylmethyl bromide.
Mass spectrum (CI, m/z): 278 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.83-1.33 (m, 5H), 1.48-1.80(m, 6H), 2.17 (s,
3H), 2.26 (s, 3H), 3.89 (s, 3H), 4.17 (d;J=7 Hz,
2H), 10.42 (s, IH).
Referential Example 28
171 2 i 81555
1-(2->3utenvl)- 3-d'methvi-6 7-
dihydrogyrrolo f2 3-dl g~rri r~a~i np 7 on
1.10 g (0.0220 mole) of hydrazine hydrate was added to
a solution of 4.50 g (0.0191 mole) of methyl
1-butenyl-3-formyl-4,5-dimethylpyrrole-2-carboxylate
(cis/trans=24/76) in 47 ml of acetic acid and the
resulting mixture was stirred at 100°C for 2 hours. The
reaction mixture cooled to room temperature was poured
into ice-water. Precipitated crystals were collected by
filtration and washed with water. --The crystals thus
obtained were dissolved in 300 ml of dichloromethane and
the solution was dried over anhydrous sodium sulfate. -
Distilling off the solvent gave 3.53 g (0.0163 mole) of
1-(2-butenyl)-2,3-dimethyl-6,7-dihydropyrrolo[2,3-d]-
pyridazine-7-one (cis/trans=21/79) as pale brown crystals.
Mass spectrum (CI, m/z): 218 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.59-1.69 (m, 2.4H), 1.78-1.85 (m, 0.6H), 2.20
(s, 3H), 2.29 (s, 3H), 5.06-5.16 (m, 1.6H),
5.24-5.31 (m, 0.4H), 5.34-5.68 (m, 2H), 8.07 (s,
1H), 10.29 (s, 1H).
Referential Example 29
1-Cvclo~~yl-2.3-dim hp -6 7-
dihvdroy~yrrolo f 2 , 3 - dl~vrj rya z i n - 7 - one
The title compound was prepared as a pale creamy
powder in 86.0% yeild in a similar procedure to that
described in Referential Example 28 by using ethyl
172 2181553
1-cyclopropyl-3-formyl-4,5-dimethylpyrrole-2-carboxxlate.
Mass spectrum (CI, m/z): 204 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.05-1.13 (m, 2H), 1.23-1.31 (m, 2H), 2.19 (s,
3H), 2.40 (s, 3H), 3.27-3.37 (m, 1H), 8.00 (s,
1H), 9.88 (brs, 1H).
Referential Example 30
l~yclohee~rl-2.3-dimethyl-6 7-
dil~rdropyrrolo(2 3-dlpyridazin-7-one
The title compound was prepared as a pale creamy
powder in 95.3% yeild in a similar procedure to that
described in Referential Example 2B by using ethyl
1-cyclohexyl-3-formyl-4,5-dimethylpyrrole-2-carboxylate
Mass spectrum (CI, m/z): 246 (M+ + 1).
NMR spectrum (CDC13, bppm):
I.15-1.60 (m, 4H), 1.60-2.00 (m, 6H), 2.17 (s,
3H), 2.38 (s, 3H), 4.00-4.4I (m, 1H), 8.03 (s,
1H), 10.06 (brs, 1H).
Referential Example 3I
3-Et~2v~-2-methyl-1-(2-~rop2ny~~-6 7-
dihydroy~yrrolo(2 3-dl~yridazsn-7-one _
The title compound was prepared as a white powder in
86.3% yield in a similar procedure to that described in
Referential Example 28 by using ethyl 4-ethyl-3-formyl-
5-methyl-1-(2-propenyl)pyrrole-2-carboxylate '
173
'~ 21$1553
Mass spectrum (CI, m/z): 218 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.20 (t;J=8 Hz, 3H), 2.29 {s, 3H), 2.65 (q;J=8
Hz, 2H), 4.79 (d;J=18 Hz, 1H), 5.I5 (d;J=9 Hz,
1H), 5.17-5_22 (m, 2H), 5.93-6.08 (m, 1H), 8.11
(s, 1H), 10.17 (brs, 1H).
Referential Example 32
1-(2-Butenyl)-2-ethyl-3-methyl-6 7-
dih~rdrogyrrolo~2 3-dlgyridazin-7-one
The title compound (cis/trans=15/85) was prepared as a
white powder in 72.7% yeild in a similar procedure to that
described in Referential Example 28 by using ethyl
I-(2-butenyl)-5-ethyl-4-methylpyrrole-2-carboxylate
(cis/trans=23/77).
Mass spectrum (CI, m/z): 232 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.20 {t;J=8 Hz, 3H), 1.66 (d;J=7= Hz, 2.55H), 1.82
(d;J=7 Hz, 0.45H), 2.21 (s, 3H), 2.73 (q;J=8 Hz,
2H), 5.13 (d;J=7 Hz, 1.7H), 5.28 (d;J=7 Hz,
0.3H), 5.35-5.52 (m, 1H), 5.57-5.70 (m, 1H), 8.07
(s, 1H), 10.35 (brs, 1H).
174 2181553
Referential Example 33
2 3-Dimethy~-i-(3-methyl-2-butenyll-6 7-
dihydroovrr~~o(2 3-dlpyridazin-7-one
The title compound was prepared as beige crystals in-
90.3% yeild in a similar procedure to that described in
Referential Example 28 by using methyl 3-formyl-
4,5-dimethyl-1-(3-methyl-2-butenyl)pyrrole-2-carboxylate
Mass spectrum (CI, m/z): 232 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
1.70 (s, 3H), 1.82 (s, 3H), 2.20 (s, 3H), 2.29
(s, 3H), 5.20 (s, 3H), 8.08 (s, 1H), 10.20 (brs,
1H) .
Referential Example 34
2 3-D~methvi-i-(2-~x;envl)-6 7-
dihvdro~yrrolof2 3-dlpyrida~~n-7-onp
The title compound was prepared as a grayish-white
solid in 99.5% yeild in a similar procedure to that
described in Referential Example 28 by using methyl
3-formyl-4,5-dimethyl-1-(2-propenyl)pyrrole-2-carboxylate
Mass spectrum (CI, m/z): 204 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
2.20 (s, 3H), 2.28 (s, 3H), 4.81 (d;J=16 Hz, 1H),
5.15 (d;J=10 Hz, 1H), 5.21 (d;J=6 Hz, 2H),
5.91-6.08 (m, 1H), 8.07 (s, 1H), 10.09 (brs, 1H)
r
175 2181553
Referential Example 35
~ycloprogylmeth~rl-2 3-dimet.h -6 7-
dihydropyrrolof2 3-dl~yridazin-7-one
The title compound was prepared as a white powder in
98.4% yeild in a similar procedure to that described in
Referential Example 28 by using methyl
1-cyclopropylmethyl-3-fozmyl-4,5-dimethylpyrrole-2-
carboxylate.
Mass spectrum (CI, m/z): 218 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.46-0.55 (m, 4H), 1.14-1.29 (m, 1H), 2.20 (s,
3H), 2.36 (s, 3H), 4.43 (d;J=8 Hz, 2H), 8.08 (s,
1H), 10.05 (brs, 1H).
Referential Example 36
2 3-Dimethyl-1- (3-girl-2-~ggnyl) -6 7-
dihydropyrrolof2.3-dl~yridazin-7-one
The title compound (trans) was prepared as pale brown
crystals in 89.9% yeild in a similar procedure to that
described in Referential Example 28 by using methyl
3-formyl-4,5-dimethyl-1-(3-phenyl-2-propenyl)pyrrole-2-
carboxylate (trans).
Mass spectrum (CI, m/z): 280 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.21 (s, 3H), 2.33 (s, 3H), 5.33-5.40 (m, 2H),
6.32 (s, 2H), 7.16-7.35 (m, SH), 8.07 (s, 1H),
9.73 (s, 1H).
176
M&C FOLIO: 71849/FP-9501 ? ~ ~ ~ ~~~: 20811
Referential Example 37
2 3-D~methyi-~-(2- en nyl)-6 7-
dihydropyrrolo~2 ~-dlpvridazin-7-one
The title compound (traps) was prepared as pale brown
crystals in 89.3% yield in a similar procedure to that
described in Referential Example 28 by using methyl
3-formyl-4,5-dimethyl-1-(2-pentenyl)pyrrole-2-carboxylate
(traps).
Mass spectrum (CI, m/z): 232 (M+ + 1).
NMR spectrum (CDC13, Sppm):
1.04 (t;J=8 Hz, 3H), 2.15-2.30 (m, 8H), 5.22-5.60
(m, 4H), 8.06 (s, 1H), 10.23 (s, 1H).
Referential Example 38
2,3-Dimethvl-1-vinyl-6 7-dihydrogyrrnlr,r2.~-dyyridazin-7-
ong
The title compound was prepared as a pale yellow foam
in 92.6% yield in a similar procedure to that described in
Referential Example 28 by using methyl
3-formyl-4,5-dimethyl-1-vinylpyrrole-2-carboxylate.
Mass spectrum (CI, m/z): 190 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.22 (s, 3H), 2.43 (s, 3H), 5.23 (d;J=9 Hz, 1H),
5.32 (d;J=18 Hz, 1H), 7.84 (dd;J=18 Hz, 9 Hz,
1H), 8.08 (s, 1H), 9.92 (brs, 1H).
177 281555
Referential Example 39
2 ~-Dimethy~-1-(2-methyl-2-pjrgpg~yl)-6 7-
dihvdropyrrolof2 3-dlpyridazin 7 one
The title compound was prepared as a flesh-colored
powder in 90.2% yield in a similar procedure to that
described_in Referential Example 28 by using methyl
3-formyl-4,5-dimethyl-1-(2-methyl-2-propenyl)pyrrole-2-
carboxylate.
Masa spectrum (CI, m/z): 218 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
1.72 (s, 3H), 2.21 (s, 3H), 2.25 (s, 3H), 4.30
(s, 1H), 4.82 (s, IH), 5.12 (s, 2H), 8.09 (s,
1H), 10.30 (brs, 1H).
Referential Example 40
2 3-D~metkzvi -i - (2 2 -tr'7.fluornathY~ ~ ~ ''
dihydrogyrro~o 2 '~-dlpyr~dazin-7 one
The title compound was prepared as pale brown crystals
in 70.0% yield in a similar procedure to that described in
Referential Example 28 by using methyl 3-formyl-4,5-
dimethyl-1-(2,2,2-trifluoroethyl)pyrrole-2-carboxylate. -
Mass spectrum (CI, m/z): 246 (M+ + 1).
NMR spectrum (CDC13 + DMSO-D6, bppm):
2.22 (s, 3H), 2.35 (s, 3H), 5.29 (q;J=9 Hz, 2H),
8.08 (s, IH), 11.27 (s, 1H).
178
218155.
Referential Example 41 ,
1-(2-Fluoroerj~yl)-2,3-dimethyl-6 7-
dihydropyrrolof2 3-dlgyridazin-7 one
The title compound was prepared as white crystals in
100% yield in a similar procedure to that described in
Referential Example 28 by using methyl 1-(2-fluoroethyl)-3-
formyl-4,5-dimethylpyrrole-2-carboxylate
Mass spectrum (CI, m/z): 210 (M+ + 1).
NMR spectrum (CDC13 + DMSO-d6, bppm):
2.21 (s, 3H), 2.32 (s, 3H), 4.62-4.89 (m, 4H),
8.04 (s, 1H).
Referential Example 42
1-(2 2-Difluoroethy~)-2 3-dimethyl-6 7-
dihydroovrrolof2 3-dl~yridazin-7-one
The title compound was prepared as a grayish-white
powder in 91.0% yield in a similar procedure to that
described in Referential Example 28 by using methyl
1-(2,2-difluoroethyl)-3-formyl-4,5-dimethylpyrrole-2-
carboxylate.
Masa spectrum (CI, m/z): 228 (M+ + 1).
NMR spectrum (CDC13 + DMSO-d6, 5ppm):
2.21 (s, 3H), 2.35 (s, 3H), 4.85 (dt;J=4 Hz, 14
Hz, 2H), 6.20 (tt;J=4 Hz, 54 Hz, 1H), 8.07 (s,
1H), 12.10 (brs, 1H).
179 218155x'
Referential Example 43
1-(2-Butenyl)-2 3-dimethvl-6 7-
d;hydrobvrrolof2 3-dly~yridazin-7-one
The title compound (cis/trans=97/3)-was prepared as
pale brown crystals in 74.6% yield in a similar procedure
to that described in Referential Example 28 by using
methyl 1-(2-butenyl)-3-formyl-4,5-dimethylpyrrole-
2-carboxylate (cis/trans=93/7).
NMR apecCrum (CDC13, bppm):
i.62-1.68 (m, 0.09H), 1.75-1.85 (m, 2.9IH), 2.20
(s, 3H), 2.29 (s, 3H), 5.08-5.14 (m, 0.06H),
5.21-5.31 (m, 1.94H), 5.34-5.70 (m, 2H), 8.05 (s,
1H), 9.89 (s, 1H).
Referential Example 44 _
1-(2-Chioro-2-grogg~yyi)-2 3-dimethvl-6 7-
dihvdropyrrolo~2,3-dTnvr;~a~;n-7-~nA
The title compound was prepared as pale yellow
crystals in 100% yield in a similar procedure to that
described in Referential Example 28 by using methyl
1-(2-chloro-2-propenyl)-3-formyl-4,5-dimethylpyrrole-2-
carboxylate (trans).
Mass spectrum (CI, m/z): 238 (M+ + 1), 240 (M+ +
3) .
NMR spectrum (CDC13, bppm):
2.21 (s, 3H), 2.30 (e, 3H), 4.90 (s, 1H), 5.32
(s, 1H), 5.35 (s, 2H), 8.09 (s, 1H), 10.09 (brs,
1H).
2181553
180
Referential Example 45
2 3-Dimethp -i-(4 4 4-trifluoro-2-hmrP yl)-6 7-
dihydro~yrrolof2,3-dlpyridazin-7-one
The title compound (trans) was prepared as a pale
grayish-white powder in 40.0% yield in a similar procedure
to that described in Referential Example 28 by using
methyl 3-formyl-4,5-dimethyl-1-
(4,4,4-trifluoro--2-butenyl)pyrrole-2-carboxylate (trans).
Mass spectrum (CI, m/z): 272 (M+ + 1).
NMR spectrum (CDC13 + DMSO-D6, bppm):
2.22 (s, 3H), 2.29 (s, 3H), 5.25-5.40 (m, 3H),
6.55-6.63 (m, 1H), 8.08 (s, 1H), 11.90 (brs, 1H).
Referential Example 46
1-(3 3-Dif~uoro-2~nro~yl)-2 3-dime~rl-6 7-
dihydrop~rrrolof2 3-dlpyridazin-7-one
The title compound was prepared as a pale yellow
powder in 83.0% yield in a similar procedure to that
described in Referential Example 28 by using methyl
1-(3,3-difluoro-2-propenyl)-3-formyl-4,5-dimethylpyrrole-
2-carboxylate
Masa spectrum (CI, m/z): 240 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.20 (s, 3H), 2.31 (s, 3H), 4.59-4.72 (m, 1H),
5.17 (d;J=8 Hz, 2H), 8.07 (s, 1H), 10.20 (brs,
1H) .
181 2181553
Referential Example 47
~(3-FluoroHropvl)-2 3-dim hy1-6 7-
dihydro~yrrolot2 3-dlpyridazin-7-one
The title compound was prepared as white crystals in
93.0 yield in a similar procedure to that described in
Referential. Example 28 by using methyl 1-(3-fluoropropyl)-
3-formyl-4,5-dimethylpyrrole-2-carboxylate -
Mass spectrum (CI, m/z): 224 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
2.05-2.37 (m, 2H), 2.20 (s, 3H), 2.33 (s, 3H),
4.47 (dt;J=48 Hz, 6 Hz, 2H), 4.60 (t;J=8 Hz, 2H),
8.07 (s, 1H), 10.20 (brs, 1H).
Referential Example 48
2 3-Dimethyl-1-(2~py~yl)-6 7-
dlhVdrO'DyrrOl n ,f 2 '~ -dl pV~"i ria ~ i n _ 7- One
The title compound was prepared as white crystals in
100% yield in a similar procedure to that described in
Referential Example 28 by using methyl
3-formyl-4,5-dimethyl-1-(2-propynyl)pyrrole-2-carboxylate. -
Mass spectrum (CI, m/z): 202 (M+ + 1).
NMR spectrum (CDC13 + DMSO-d6, bppm):
2.21 (s, 3H), 2.42 (s, 3H), 2.46 (s, 1H), 5.50
(s, 2H), 8.05 (s, 1H), 11.49 (s, 1H).
2181553
182
Referential Example 49
1- (3 3-DS chl oro-2-~o-~gxlv~ ) -4 5-dimethyl-6 7-
dihydro~yrrolo~2.3-dlgyridazin-7-one
The title compound was prepared as a grayish-white
powder in 96.5% yield in a similar procedure to that
described in Referential Example 28 by using methyl
1-(3,3-dichloro-2-propenyl)-3-formyl-4,5-dimethylpyrrole-2-
carboxylate.
Mass spectrum (CI, m/z): 272 (M+ + 1), 274 (M+ +
3), 276 (M+ + 5).
NMR spectrum (CDC13, 5ppm):
2.20 (s, 3H), 2.32 (s, 3H), 5.33 (d;J=6 Hz, 2H),
6.09 (t;J=6 Hz, 1H), 8.10 (s, 1H), 10.63 (brs,
1H) .
Referential Example 50
1-Cyclohexvlmethy7-~,3-dimethvl-6 7- _
di~dropyrrolof2 3-dlpyridazin-7-one
The title compound was prepared as a white powder in
85.2% yield in a similar procedure to that described--in
Referential Example 28 by using methyl 1-cyclohexylmethyl-
3-formyl-4,5-dimethylpyrrole-2-carboxylate.
Mass spectrum (CI, m/z): 260 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
1.00-1.29 (m, 5H), 1.47-1.88 (m, 6H), 2.20 (s,
183 21~155j
3H), 2.31 (s, 3H), 4.33 (d;J=7 Hz, 2H), 8.08 (s,
1H), 9.82 (brs, 1H).
Referential Example 51
1-(2-Butenyl)-7-chloro-2 3-dimethvlbvrrolo(2 3-dlgy,-i a~iw A
39 ml (0.43 mole) of phosphorus oxychloride were added
to 3.53 g (0.0163 mole) of I-(2-butenyl)-2,3-
dimethyl-6,7-dihydropyrrolo(2,3-d]pyridazin-7-one
(cis/trans=24/76) and the mixture was stirred at 97°C for
2.5 hours. The reaction mixture was allowed to cool to
room temperature and added dropwise to water with
ice-cooling. The mixture was neutralized with a 40~
aqueous solution of sodium hydroxide and extracted with
dichloromethane. The extract was washed with water and
dried over anhydrous sodium sulfate, and the solvent was
distilled off. The residue was purified by column
chromatography through silica gel using a 1.1 mixture of
toluene and ethyl acetate as an eluent to give 3.59 g
(0.0152 mole) of 1-(2-butenyl)-7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine (cis/trana=21/79) as pale
brown crystals.
Mass spectrum (CI, m/z): 236 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.62-1.69 (2.4H, m), 1.79-1.85 (0.6H, m), 2.29
(3H, s), 2.39 (3H, s), 5.02-5:71 (4H, m), 9.17
(1H, s) .
184 ~ ~ g ~ ~ ~ 5
Referential Example 52
7-Chl Yo-2 ~-d~methyl-1-(3-met12y1-2-
buteny~lpyrrolof2 3-dlgyridazine
The title compound was prepared as a pink powder in
67.2% yield in a similar procedure to that described in
Referential Example 51 by using 2,3-dimethyl-1-(3-methyl-2-
butenyl)-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Masa spectrum (CI, m/z): 250 (M+ + 1), 252 (M+ +
3) .
NMR spectrum (CDC13, bppm):
1.72 (s, 3H), 1.82 (s, 3H), 2.30 (s, 3H), 2.40
(s, 3H), 5.05-5.19 (m, 3H), 9.19 (s, 1H).
Referential Example 53
7-Ch~oro-2 3-di ethyl-1-(2-
pro~~yl)pvrrolo(2 3-dlgyrida~
The title compound was prepared as a pale yellow
powder in 94.0% yield in a similar procedure to that
dESCribed in Referential Example 51 by using
2,3-dimethyl-1-
(2-propenyl)-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 222 (M+ + 1), 224'(M+ +
3) .
NMR spectrum (CDCl3, 5ppm):
2.30 (s, 3H), 2.39 (s, 3H), 4.63 (d;J=16 Hz, 1H),
5.06-5.21 (m, 3H), 5.93-6.08 (m, iH), 9.17 (s,
1H).
185
2181555
Referential Example 54
7-Chloro-~-cy~lQ~R:QRYImerh~
dylpyrrolof2-'~-dlpyr;dazinP
The title compound was prepared as a pale yellow
powder in 79_7% yield in a similar procedure to that
described in Referential Example 51 by using
1-cyclopropylmethyl-2,3-
dimethyl-6,7-dihydropyrrolol2,3-d]pyridazin-7-one
Mass spectrum (CI, m/z): 236 (M+ + 1), 238 (M+ +
3) .
NMR spectrum (CDC13, bppm):
0.38-0.60 (m, 4H), 1.16-1.22 (m, 1H), 2.30 (s,
3H), 2.44 (s, 3H), 4.44 (d;J=8 Hz, 2H), 9.I6 (s,
1H) .
Referential Example 55
7-Chloro-2 _ ~-~7i math~r~ _
p n nyl)Rvrro~of. -dlpvr~daz~ne
The title compound (trans) was prepared as pale brown
crystals in 89.7% yield in a similar procedure to that
described in Referential Example 51 by using
2,3-dimethyl-1-(2-pentenyl)-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one (trans).
Mass spectrum (CI, m/z): 250 (M+ + 1), 252 (M+ +
3) .
NMR spectrum (CDCl3, bppm):
1.09 (t;J=8 Hz, 3H), 2.12-2.3I (m, 5H), 2.40 (s,
3H), 5.19 (d;J=7 Hz, 2H), 5.24-5.62 (m, 2H),- 9.16
(s, 1H) .
186 2181553
Referential Example 56
7-Chloro-2 3-dimethyl-1-f3-bhen 1~-7~- _..
prop~n_y1)gvrro~of2 3-dlgyridazine
The title compound (trans) was prepared as pale brown
crystals in 82.2% yield in a similar procedure to that
described in Referential Example 51 by using
2,3-dimethyl-1-(3-phenyl-2-propenyl)-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one (trans).
Mass spectrum (CI, m/z): 298 (M+ + 1), 300 (M+ +
3) .
NMR spectrum (CDC13, bppm):
2.33 (s, 3H), 2.46 (s, 3H), 5.25-5.34 (m, 2H),
6.13 (d;J=17 Hz, 1H), 6.32 (dd;J=17 Hz, 5 Hz,
1H), 7.18-7.40 (m, SH), 9.21 (s, 1H).
Referential Example 57
7-Chloro_2,3_d;me hyi-~-v;n~gyrro~of2 z-dlpyridazinP
The title compound was prepared as a white powder in
65.1% yield in a similar procedure to that described in
Referential Example 51 by using 2,3-dimethyl-1-vinyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 208 (M+ + 1), 210 (M+ +
3) .
NMR spectrum (CDC13, 6ppm):
2.31 (s, 3H), 2.42 (s, 3H), 5.37 (d;J=17 Hz, 1H),
5.62 (d;J=9 Hz, 1H), 7.34 (dd;J=17 Hz, 9 Hz, 1H),
9.20 (s, 1H).
187 2181553
Referential Example 58
7-Ch~oro-. -d~methyl-1-(2-methyl-2_
g~g~yl) gyrro~ o f2 '~-dl gyridazine _
The title compound was prepared as a white powder-in _
91.2% yield in a similar procedure to that described in
Referential Example 51 by using 2,3-dimethyl-1-(2-methyl-2-
propenyl)-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 236 (M+ + 1), 238 (M+ +
3).
NMR spectrum (CDC13, 5ppm):
1.81 (s, 3H), 2.30 (s, 3H), 2.35 (s, 3H), 3.95
(s, 1H), 4.85 (s, 1H), 4.99 (s, 2H), 9.18 (s, 1H).
Referential Example 59
7-Chloro-2,3-di ethyl-1-(2 2 2-
trifiLOroethy?]~vrrW oft 3-dlgyridazine
The title compound was prepared as a white powderin
89.1% yield in a similar procedure to that described in
Referential Example 51 by using 2,3-dimethyl-1-(2,2,2-
trifluoroethyl)-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 264 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
2.32 (a, 3H), 2.47 (s, 3H), 5.19 (q;J=9 Hz, 2H),
9.22 (s, 1H).
Referential Example 60
7-Chloro-1-cvclonro~y~-2 3- smethyl~vrrolof2.3-dl~vridazir~e
188 2181553
The title compound was prepared as a pale creamy
powder in 95.5% yield in-a similar procedure to that
described in Referential Example 51 by using
1-cyclopropyl-2,3-dimethyl-
6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Masa spectrum (CI, m/z): 222 (M+ + 1), 224 (M+ +
3).
NMR spectrum (CDC13, 5ppm):
1.05-1.12 (m, 2H), 1.31-1.39 (m, 2H), 2.23 (s,
3H), 2.52 (s, 3H), 3.36-3.45 (m, 1H), 9.10 (s,
1H) .
Referential Example 61
7-Chloro-1-(~-fluoroethyl)-2.3-
dim hyigyrrolof2 3-dl~yridazine
The title compound was prepared as a pale brown powder
in 56.2% yield in a similar procedure to that described in
Referential Example 51 by using
1-(2-fluoroethyl)-2,3-dimethyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 228 (M+ + 1), 230 (M+ +
3).
NMR spectrum (CDC13, Sppm):
2.31 (s, 3H), 2.46 (s, 3H), 4.67-4.82 (m, 2H),
4.88 (s, 2H), 9.19 (s, 1H).
Referential Example 62
7-Chloro-~-!2 2-difluoroethyl)-2 3-
dimeth,~lpyrrolof2.3-dlpyridazine
i
189 2$1553
The title compound was prepared as pale beige c,xystals
in 75.0% yield in a similar procedure to that described in
Referential Example 51 by using 1-(2,2-trifluoroethyl)-2,3-
dimethyl-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 246 (M+ + 1), 248 (M+ +
3).
NMR spectrum (CDC13, bppm):
2.30 (s, 3H), 2.45 (s, 3H), 4.87 (dt;J=14 Hz, 4
Hz,-2H), 6.14 (tt;J=54 Hz, 4 Hz, 1H), 9.20 (s,
IH) .
Referential Example 63
7-Chloro-?-cvcTOhexvl-2 3-dimethyl_gvrrolof2 ~-~1y r;r;a~ine
The title compound was prepared as a creamy powder in
84.8% yield in a similar procedure to that described in
Referential Example 51 by using 1-cyclohexyl-2,3-
dimethyl-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 264 (M+ + 1), 266 (M+ +
3).
NMR spectrum (CDC13, bppm):
1.17-1.64 (m, 4H), 1.89-2.11 (m, 6H), 2.27 (s,
3H), 2.59 (s, 3H), 5.58-5.76 (m, 1H), 9.11 (s,
1H) .
Referential Example 64
7-Chloro-3-ethyl-2-methyl-1-(2-
propenyl)bvrrolof2 3-dlpyridazine
~
190 2181553
The title compound was prepared as a pale brown powder
in 68.8% yield in a similar procedure to that described in
Referential Example 51 by using
3-ethyl-2-methyl-1-(2-propenyl)-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 236 (M+ + 1), 238 (M+ +
3).
NMR spectrum (CDC13, 5ppm):
1.24 (t;J=8 Hz, 3H), 2.40 (s, 3H), 2.76 (q;J=8
Hz, 2H), 4.63 (d;J=17 Hz, 1H), 5.07-5.20 (m, 3H),
5.94-6.09 (m, 1H), 9.21 (s, IH).
Referential Example 65
1-(2-Hutenyl)-7-chloro-2 3-dimethyl_gvrrolof2 3-dlpyridaz'ne ,
The title compound (cis/trans=98/2) was prepared as a
pale yellow oil in 84.9% yield in a similar procedure to
that described in Referential Example 51 by using
1-(2-butenyl)-2,3-dimethyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one (cis/trans=97/3).
Masa spectrum (CI, m/z): 236 (M+ + 1), 238 (M+ +
3) .
NMR spectrum (CDC13, 5ppm):
1.62-1.69 (m, 0.2H), 1.78-1.87 (m, 2.8H), 2.29 -
(s, 3H), 2.39 (s, 3H), 5.01-5.08 (m, O.1H),
5.15-5.23 (m, 1.9H), 5.30-5.73 -(m, 2H), 9.14 (s,
1H).
Referential Example 66
191
7-Chloro-~-(2- hloro-2-pryll-2 3- _
dim lovrrolof2 3-d]~vridazinP
The title compound was prepared as grayish-white
crystals in 94.4% yield in a similar procedure to that
described in Referential Example 51 by using
1-(2-chloro-2-propenyl)-2,3-
dimethyl-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 256 (M+ + 1), 258 (M+ +
3), 260 (M+ + 5).
NMR spectrum (CDCI3, bppm):
2.31 (s, 3H), 2.40 (e, 3H), 4.50-4.55 (m, 1H),
5.18-5.26 (m, 2H), 5.30-5.37 (m, IH), 9.20 (s,
1H) .
Referential Example 67
1-(2-But nyl)-7-ch oro-2-erhyi-'~-
meth~pyrrolof2.3-dlx~yrjdaz~ne
The title compound (cis/trana=4/96) was prepared as a
pale reddish-white powder in 63.4% yield in a similar
procedure to that described in Referential Example 51 by
using 1-(2-butenyl)-2-ethyl-3-methyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one (cis/trans=15/85).
Mass spectrum (CI, m/z): 250 (M+ + 1), 252 (M+ +
3) .
NMR spectrum (CDC13, Sppm):
1.23 (t;J=8 Hz, 3H), 1.58-1.67 (m, 2.88H),
2.77-2.84 (m, 0.12H), 2.30 (s, 3H), 2.82 (q;J=8
Hz, 2H), 5.00-5.09 (m, 2H), 5.16-5.30 (m, 1H),
192 2181553
5.53-5.69 (m, IH), 9.14 (s, 1H).
Referential Example 68
7-Ch~oY'o-2 3-d~ ethyl-~-(4 4 4-try >»nrn-2-
butenv» p~rrroi o f2 3-dl gyridazine
The title compound (trana) was prepared as a pale
yellow solid in 78.0% yield in a similar procedure to that
described in Referential Example 51 by using
2,3-dimethyl-1-(4,4,4-trifluoro-2-
butenyl)-6,7-dihydropyrrolo(2,3-d]pyridazin-7-one (trans).
Mass spectrum (CI, m/z): 290 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.23 (s, 3H), 2.39 (s, 3H), 5..08-5.17 (m, 1H),
5.24-5.30 (m, 2H), 6.55-6.63 (m, 1H), 9.22 (s,
1H) .
Referential Example 69
7-Chloro-1-(3.3-difluoro-2-prnyl)-2 3-
dime hy~pyrrolo)2 3-dl~yr~da~~nP
The title compound was prepared as a white powder in
79.1% yield in a similar procedure to that described in
Referential Example 51 by using
1-(3,3-difluoro-2-propenyl)-2,3-
dimethyl-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one
Mass spectrum (CI, m/z): 258 (M+ + 1), 260 (M+ +
3).
NMR spectrum (CDC13, 6ppm):
2.30 (s, 3H), 2.43 (s, 3H), 4.50-4.61 (m, 1H),
193- 21 81 553
5.11-5.18 (m, 2H), 9.18 (s, IH).
Referential Example 70
7-Chloro-1-13-fluor progwll-2 3- __
dimethylgyrrolof2 3-dlRyridazine ._
The title compound was prepared as white crystals in
86.9% yield in a similar procedure to that described in
Referential Example 51 by using
1-(3-fluoropropyl)-2,3-dimethyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 242 (M+ + 1), 244 (M+ +
3) .
NMR spectrum (CDC13, bppm):
2.08-2.36 (m, 2H), 2.30 (s, 3H), 2.44 (s, 3H),
4.49 (dt;J=54 Hz, 6 Hz, 2H), 4.64 (t;J=8 Hz, 2H),
9.17 (s, 1H).
Referential Example 71
7-Chloro-2.'3-c3lmathvl -
ro~yr~yl ~vrroi o f 2 3 -dl ~~rridazine
The title compound was prepared as a white powder in -
67.2% yield in a similar procedure to that described in
Referential Example 51 by using
2,3-dimethyl-1-(2-propynyl)-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 220 (M+ + 1), 222 (M+ +
3).
NMR spectrum (CDC13, bppm):
194 211555
2.30 (s, 3H), 2.39 (s, 1H), 2.50 (s, 3H), 5.31
(s, 2H), 9.19 (s, 1H).
Referential Example 72
7-Chloro-~-(3.~-d~ch~oro-2-~openYi)-2 3-
dimethyly~yrrolof 3-dlpyridazine
The title compound was prepared as an ocherous powder
in 89.7% yield in a similar procedure to that described in
Referential Example 51 by using
1-(3,3-dichloro-2-propenyl)-2,3-
dimethyl-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 290 (M+ + 1), 292 (M+ +
3), 294 (M+ + 5).
NMR spectrum (CDC13, bppm):
2.30 (s, 3H), 2.42 (s, 3H), 5.27 (d;J=6 Hz, 2H),
5.96 (t;J=6 Hz, 1H), 9.17 (s, 1H).
Referential Example 73
7-Chl oro-i -~ycloh~rlm fi yl-2 -'~-
dim rlbvrrolo~2 3-dl~yridazine
The title compound was prepared as a white powder in
95.5% yield in a similar procedure to that described in
Referential Example S1 by using
1-cyclohexylmethyl-2,3-dimethyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 278 (M+ + 1), 280 (M+ +
3).
NMR spectrum (CDC13, bppm):
'~ 195 2181553
0.95-1.30 (m, SH), 1.45-1.90 (m, 6H), 2.28, (s,
3H), 2.40 (s, 3H), 4.29 (d;uT=8 Hz, 2H), 9.I4 (s,
1H).
Referential Example 74
Methyi 4 5-dimethylgyrrole-2-carboxv~arA
(1) 2-Methyl-3-oxobutanal'sodium salt
A mixed solution of 67.6 g (0.93 mole) of 2-butanone
and 71.7 g (0.93 mole) of ethyl formate was added to a
mixture of 20.5 g (0.891 mole) of sodium and 720 ml of dry
diethyl ether with stirring under ice-cooling over 2 hours
and the resulting mixture was stirred at the same
temperature for 6.5 hours. Precipitated solids were
collected by filtration and washed with diethyl ether to
give 104 g of 2-methyl-3-oxobutanal'sodium salt as an
ocherous solid.
(2) Methy~ 4 5-dimethy~DVrrole 2 carboxv~arP
A solution of 40.7 g (0.59 mole) of sodium nitrite in
68 ml of -water was added dropwise to a solution of 61.5 g
(0.53 mole) of methyl acetoacetate in 208 ml of acetic
acid over a period of 3 hours with ice-cooling, and the
resulting mixture was stirred at the same temperature for
3 hours and allowed to stand at room temperature
overnight. A solution of 104 g (0.852 mole) of
2-methyl-3-oxobutanal'sodium salt prepared in the above
(1) in 200 ml of water was added to the reaction mixture,
and subsequently 90 g (1.38 moles) of zinc powder was
added thereto at 60-64°C over a period of 2 hours and the
196 ~ ~ ~ ~ 5 ~ j
mixture was heated under reflux for 30 minutes. The hot
reaction mixture thus obtained was poured into 1 kg of
ice-water, and the ocherous solids precipitated were
collected by filtration and washed with water. The solids
were dissolved in 800 ml of ethyl acetate and the
insoluble materials originated from zinc was filtered
off. The filtrate was dried over anhydrous sodium sulfate -
and the solvent was distilled off. The concentrate thus
obtained was allowed to stand at room temperature -
overnight, and the precipitated crystals were collected by
filtration and washed twice with 25 ml each of a 2:1
mixture of hexane and diethyl ether to give 15.0 g (0.0981
mole) of methyl 4,5-dimethylpyrrole-2-carboxylate as
ocheroua powdery crystals.
Mass spectrum (CI, m/z): 154 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.00 (s, 3H), 2.21 (s, 3H), 3.81 (s, 3H), 6.68
(s, 1H), 9.20 (br.s, 1H).
Referential Example 75
Methv~ a-formp -4 5-dimerhy~pvrrole 2 carboxvlate
15.0 g (0.0979 mole) of phosphorus oxychloride were
dropwise added to a solution of 13.7 g (0.0898 mole) of
methyl 4,5-dimethylpyrrole-2-carboxylate in 13 ml (0.17
mole) of dimethylformamide over a period of 1.3 hours with
ice-cooling, and the resulting mixture was stirred at room
temperature for 0.5 hour and then at 90-100°C for 0.5 _-
197 2181553
hour. The hot reaction mixture thus obtained was poured
into ice-water and dissolved. The solution was adjusted
to pH 5-6 with a 10~ aqueous solution of sodium hydroxide. -
The solids precipitated were collected by filtration and
then dissolved in 600 m1 of ethyl acetate. The filtrate-
was extracted twice with 200 ml each of-ethyl acetate.
The combined extracts were washed with water and dried
over anhydrous sodium sulfate, and the solvent was
distilled off. The residue was washed with a mixture of
hexane and ethyl acetate and collected by filtration to
give 10.6 g (0.0583 mole) of methyl 3-formyl-4,5-
dimethylpyrrole-2-clirboxylate as a brown powder.
Mass spectrum (CI, m/z); 182 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.24 (s, 3H), 2.26 (s, 3H), 3.92 (s, 3H), 9.29
(br.s, 1H), 10.54 (s, 1H).
Referential Example 76
MethSr~ 3-acetyl-4 5-dimethy_lyvrrole-2-carh~x~ la P _
5.0 ml of acetic anhydride were added to a solution of
1.50 g (0.0098 mole) of methyl
4,5-dimethylpyrrole-2-carboxylate in 15 ml of
dichloromethane at room temperature and subsequently a
mixture of 1.5 ml (0.013 mole) of tin tetrachloride and 4
ml of dichloromethane was dropwise added thereto over a -
198
period of 10 minutes. The mixture was stirred for 30 -
minutes, poured into ice-water, neutralized to pH 7-8 with
an aqueous solution of sodium hydrogencarbonate and
extracted with dichloromethane. The extract was dried
over-anhydrous sodium sulfate and the solvent was
distilled cff under reduced pressure. The residue was
purified by column chromatography through silica gel using
a 1:2 mixture of ethyl acetate and hexane as an eluent to -
give 1.61 g of methyl
3-acetyl-4,5-dimethylpyrrole-2-carboxylate as a
grayish-white solid.
Mass spectrum (CI, m/z): 196 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.02 (s, 3H), 2.20 (s, 3H), 2.57 (s, 3H), 3.85
(s, 3H), 8.95 (brs, 1H).
Referential Example 77
-D?methW -6 7-d~h5rdro~yrrolof2 3-dlgyridazin 7 one
1.50 g (0.030 mole) of hydrazine monohydrate were
dropwised added slowly to a solution of 4.00 g (0.022
mole) of methyl 3-formyl-4,5-dimethylpyrrole-2-carboxylate -
in 80 ml of acetic acid at room temperature and the
resulting mixture was stirred at 110°C for 2 hours. After
completion of the reaction, thereaction mixture was
poured into ice-water. The resulting precipitates were
collected by filtration and washed well with water. The
199 2181553
precipitates were dissolved in dichloromethane and the
solution was dried over anhydrous sodium sulfate. The
solvent was distilled off under reduced-pressure to give
3.40 g of 2,3-dimethyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one as a grayish-white
powder.
Mass spectrum (CI, m/z): 164 (M+ + I).
NMR spectrum (CDC13 + DMSO-d6, 5ppm):
2.18 (s, 3H), 2.3I (s, 3H), 8.03 (s, 1H), 12.04
(brs, 2H).
Referential Example 78
7-C~ioro-2 3-dimethylgyrrolo[2 3-dl~vridazinP
45 ml of phosphorus oxychloride were added to 3.35 g
(0.021 mole) of
2,3-dimethyl-6,7-dihydropyrrolo[2,3-d]pyridazin-7-one and
the mixture was heated under reflux for 2 hours. After
completion of the reaction, the reaction mixture was
slowly poured into ice-water and the aqueous mixture was
neutralized with an aqueous solution of sodium hydroxide_
The precipitated yellow solids were collected by
filtration and washed well with water. The solids were -
disaolved in dichloromethane and dried over anhydrous
sodium sulfate, and the solvent was distilled off under
reduced pressure. The residue was purified by column
chromatography through silica gel using a 15:1 mixture of
200 218 ~ 553
chloroform and methanol as an eluent to give 2.33 g, of
7-chloro-2,3-dimethylpyrrolo[2,3-d]pyridazine as a pale
yellow powder:
Mass spectrum (CI, m/z): 182 (M+ + 1), 184 (M+ +
3) .
NMR spectrum (CDC13 + DMSO-d6, bppm):
2.29 (s, 3H), 2.46 (s, 3H), 9.14 (s, 1H), 11.70
(brs, 1H).
Referential Example 79
7-Benzylox<r-2 3-dimethylgyrrolo[2 3-dlpyr;~a~;nA
1.35 g (0.0074 mole) of 7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine was added to a solution,
obtained by adding 0.26 g (0.011 mole) of sodium to 25 ml
of benzyl alcohol at room temperature, and the resulting
mixture was heated at 115°C and, in the course of the
heating, 10 ml of benzyl alcohol were additionally added
thereto. The heating was continued for 30 hours with
stirring. After completion of the reaction, the reaction
mixture was poured into ice-water and extracted with
dichloromethane. The extract was dried over anhydrous
sodium sulfate, the solvent was removed under reduced
pressure and benzyl alcohol was distilled off under
reduced pressure. The residue was purified by column
chromatography through silica gel using a 20:1 mixture of
chloroform and methanol as an eluent to give 1.15 g of
7-benzyloxy-2,3-dimethylpyrrolo[2,3-d]pyridazineas a pale
yellow powder.
201 2181555
Mass spectrum (CI, m/z): 254 (M+ + 1).
NMR spectrum (CDCI3 + DMSO-d6, bppm):
2.23 (s, 3H), 2.38 (s, 3H), 5.69 (s, 2H),
7.30-7.60 (m, 5H), 8.99 (s, 1H), 10.65 (brs, 1H).
Referential Example 80
7-(4-Fluorobenzyl~)-2 3-dimethylpvrro~nf2 3-dlgyridazine
The title compound was prepared as pale yellow
crystals in 29.8% yield in a similar procedure to that
described in Referential Example 79 by using 7-chloro-2,3-
dimethylpyrrolo[2,3-d]pyridazine and 4-fluorobenzyl
alcohol.
Mass spectrum (CI, m/z): 272 (M+ + 1).
NMR spectrum (CDC13 + DMSO-d6, bppm):
2.23 (s, 3H), 2.39 (s, 3H), 5.66 (s, 2H),
7.06-7.12 (m, 2H), 7.52-7.61 (m, 2H), 8.92 (s,
1H), 11.40 (brs, 1H).
Referential, Example 81
M~hy1 3-formyl-4 5-dimethyl-1-vinyl_ovrrole-2-carboxvlate
A solution of 0.15 g (0.00052 mole) of methyl 1-(2-
bromoethyl)-3-formyl-4,5-dimethylpyrrole-2-carboxylate and -
0.08 g (0.00052 mole) of 1,8-diazabicyclo[5.4.0]undec-7-
ene dissolved in 2 ml of tetrahydrofuran was heated under
reflux for 6 hours. The reaction mixture was allowed to _
cool at room temperature and purified by column
chromatography through silica gel using a 9:1 mixture of -
202 2181553
toluene and ethyl acetate as an eluent to give 0.080 g
(74% yield) of methyl 3-formyl-4,5-dimethyl-1-
vinylpyrrole-2-carboxylate as pale yellow crystals.
Masa spectrum (CI, m/z): 208 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.20 (s, 3H), 2.27 (s, 3H), 3.90 (s, 3H), 5.20
(d;J=17 Hz, 1H), 5.44 (d;J=9 Hz, 1H), 7.12
dd;J=17 Hz, 9 Hz, 1H), 10.49 (s, 1H).
Referential Example 82
Ethyl 1-(2-butenyl)-4-ethyl-5-methyigyrrol2-2-carboxvlate
The title compound (cis/trans=25/75) was prepared as a
pale yellow oil in 30.1% yield in a similar procedure to
that described in Referential Example 1 by using 2-pentane
and ethyl N-(2-butenyl)glycinate.
Mass spectrum (CI, m/z): 236 (M+ + 1).
NMR spectrum (CDC13, -6ppm):
1.13 (t;J=7 Hz, 3H), 1.32 (t;J=7 Hz, 3H), 1.62
(d;J=7 Hz, 2.25H), 1.75 (d;J=7 Hz, 0.75H), 2.16
(s, 3H), 2.40 (q;J=7 Hz, 2H), 4.24 (q;J=7 Hz,
2H), 4.87 (d;J=7 Hz, 1.5H), 5.00 (d;J=7 Hz,
0.5H), 5.25-5.41 (m, 1H), 5.49-5.66 (m, 1H), 6.83
(s, 1H) .
203 218 T 553
Referential Example 83
Ethyl 1-(2-b~rAnyl)-5-methyl-4 genryl~yrroiP ~ rarh
oacvlate
The title compound (cis/trans=21/79) was prepared as a
red oil in 26.1% yield in a similar procedure to that
described in Referential Example 1 by using 2-octanone and
ethyl N-(2-butenyl)glycinate.
Mass spectrum (CI, m/z): 278 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
0.90 (t;J=7 Hz, 3H), 1.18-1.44 (m, 7H), 1.44-1.60
(m, 2H), 1.65 (d;J=8 Hz, 2.37H), 1.73 (d;J=8 Hz,
0.63H), 2.15 (s, 3H), 2.37 (t;J=7 Hz, 2H), 4.22
(q;J=7 Hz, 2H), 4.84-4.89 (m, 1.58H), 5.01 (d;J=8
Hz, 0.42H), 5.23-5.42 (m, 1H), 5.48-5.63 (m, IH),
6.79 (s, 1H).
Referential Example 84
Ethyl I-(2-bLtenv~)-4-methy~~yrrole- - arn~n,larP
The title compound (cis/trans=30/70) was prepared as a
pale yellow oil in 44.1% yield in a similar procedure to
that described in Referential Example 1 by using
propionaldehyde and ethyl N-(2-butenyl)glycinate.
Mass spectrum (CI, m/z): 208 (M+ + 1).
NMR apecrtrum (CDC13, 5ppm):
1.32 (t;J=7 Hz, 3H), 1.68 (d;J=8 Hz, 2.1H), 1.74
(d;J=8 Hz, 0.9H), 2.06 (s, 3H), 4.26 (q;J=7 Hz, -
204 2 i 81553
2H), 4.79 (d;J=6 Hz, 1.4H), 4.93 (d;J=6 Hz,
0.6H), 5.49-5.68 (m, 2H), 6.62 (s, 1H), 6.77 (s, -
1H).
Referential Example 85
8~v1 1-(2-buten~i~-4-ethp -a-fn,-myl-5-methVipyrrole 2
carboxvlate
The title compound (cis/trans=27/73) was prepared as
an orange oil in 26.5% yield in a similar procedure to
that described in Referential Example 5 by using ethyl
1-(2-butenyl)-4-ethyl-5-methylpyrrole-2-carboxylate -
(cia/trans=25/75).
Mass spectrum (CI, m/z): 264 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
I.08 (t;J=8 Hz, 3H), 1.38 (t;J=8 Hz, 3H), 1.67
(d;J=6 Hz, 2.19H), 1.75 (d;J=6 Hz, 0.81H), 2.19
(s, 3H), 2.72 (q;J=8 Hz, 2H), 4.37 (q;J=8 Hz,
2H), 4.87 (d;J=6 Hz, 1.46H), 4.99 (d;J=6 Hz,
0.54H), 5.30-5.49 (m, 1H), 5.52-5.68 (m, 1H),
10.48 (s, 1H) .
Referential Example 86
r~etnv! 3-iormyl-4 5-dimethvi-1-(2-
methvlcvclopropylmethyl)pvrrole-2-Garb- xvla P
The title compound was prepared as yellow crystals in -
92.0% yield in a similar procedure to that described in
Referential Example 9 by using methyl 3-formyl-
2a5 21815.53
4,5-dimethylpyrrole-2-carboxylate and
1-bromoethyl-2-methylcyclopropane.
Macs spectrum (CI, m/z): 250 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.22-0.31 (m, 1H), 0.46-0.53 (m, IH), 0.70-0.92
(m, 2H), 1.00 (d;J=6 Hz, 3H), 2.21 (s, 3H), 2.28
(s, 3H), 3.90 (s, 3H), 4.25 (d;J=6 Hz, 2H), 10.43
(s, 1H) .
Referential Example 87
Ethyl 1- (2-buteny~ ) -3-formwl-5-met~rl-4~oenty~pyrrole 2 -
carbox< lr ate
The title compound (cis/trans=22/78) was prepared as
an orange oil in 41.4% yield in a similar procedure to
that described in Referential Example 5 by using ethyl
1-(2-butenyl)-5-methyl-4-pentylpyrrole-2-carboxylate
(cis/trans=21/79).
Mass spectrum (CI, m/z): 306 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
0.88 (t;J=7 Hz, 3H), 1.21-1.53 (m, 9H), 1.68
(d;J=8 Hz, 2.34H), 1.79 (d;J=8"Hz, 0.66H), 2.20 -
(s, 3H), 2.70 (t;J=7 Hz, 2H), 4.36 (q;J=7 Hz,
2H), 4.85 (d;J=6 Hz, 1.56H), 4.99 (d;J=7 Hz,
0.44H), 5.30-5.47 (m, 1H), 5.49-5.66 (m, 1H),
10.47 (s, 1H).
2181555
206
Referential Example 88
1-l2-Butenyll-3-ethyl-2-mPrhYl-6,7
dihydrog~rrroiof2 3-dl~rridazin 7 on
The title compound (cis/trans=23/77) was prepared as a
pale yellow powder in 99.0% yield in a similar procedure
to that described in Referential Example 28 by us-ing ethyl
1-(2-butenyl)-4-ethyl-3-formyl-5-methylpyrrole-2-carboxylate
(cis/trans=25/75).
Masa spectrum (CI, m/z): 232 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
1.20 (t;J=8 Hz, 3H), 1.67 (d;J=8 Hz, 2.31H), 1.80
(d;J=8 Hz, 0.69H), 2.30 (s, 3H), 2.65 (q;J=8 Hz,
2H), 5.13 (d;J=7 Hz, 1.54H), 5.27 (d;J=7 Hz,
0.46H), 5.36-5.53 (m, 1H), 5.55-5.69 (m, 1H),
8.14 (s, 1H), 10.20 (br.s, 1H).
Referential Example 89
2 3-Dimethyi-i-(2-meth~~yclox~ro~Ylmet 1~ 6 7
dihvdro~yrroiof2 3-dlgyr~daz~n-7-one
The title compound was prepared as white flaky
crystals in 88.7% yield in a similar procedure to that
described in Referential Example 28 by using ethyl
3-formyl-4,5-dimethyl-1-(2-
methylcyclopropylmethyl)pyrrole-2-carboxylate.
Mass spectrum (CI, m/z): 232 (M+ + 1).
207 2181553
NMR spectrum (CDC13, bppm):
0.19-0.26 (m, 1H), 0.61-0.70 (m, 1H), 0.84-1.02
(m, 5H), 2.23 (s, 3H), 2.38 (s, 3H), 4.44 (d;J=6
Hz, 2H), 8.08 (s, 1H), 10.13 (br.s, 1H).
Referential Example 90
1-( -B, nvl)-2-m y1-3-3-y~ent5~-~ ~
dlhydrOb7VrrOlOf2-3-dl~yririavin-7-nna
The title compound (cis/trans=20/80) was prepared as a -
brownish-white powder in 80.1% yield in a similar
procedure to that described in Referential Example 28 by
using ethyl
1-(2-butenyl)-3-formyl-5-methyl-4-pentylpyrrole-2-
carboxylate (cis/trans=22/78).
Mass spectrum (CI, m/z): 274 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.90 (t;J=7 Hz, 3H), 1.21-1.42 (m, 4H), 1.48-1.63
(m, 2H), 1.69 (d;J=7 Hz, 2.4H), 1.80 (d;J=8 Hz,
0.6H), 2.28 (s, 3H), 2.61 (t;J=7 Hz, 2H),
5.07-5.15 (m, 1.6H), 5.28 (d;J=8 Hz, 0.4H),
5.34-5.52 (m, 1H), 5.54-5.69 (m, 1H), 8.06 (s,
1H), 9.82 (br.s, 1H).
Referential Example 91
1-(2-Butenyl)-7-chloro-~-Arhyl-2-
methy~y~.~rrolQf2 '~-dl~yrid~zsne
The title compound (cis/trans=26/74) was prepared as
ocherous crystals in 80.8% yield in a similar procedure to
208 218 ~ 553
that described in Referential Example 51 by using
1-(2-butenyl)-3-ethyl-2-methyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one (cis/trans=23/77).
Mass spectrum (CI, m/z): 250 {M+ + 1), 252 (M+ +
3) .
NMR spectrum (CDC13, bppm):
1.23 (t;J=8 Hz, 3H), 1.66 (d;J=8 Hz, 2.22H), 1.8I
(d;J=8 Hz, 0.78H), 2.40 (s, 3H), 2.76 (q;J=8 Hz,
2H), 5.03-5.10 (m, 1.48H), 5.20 (d;J=8 Hz,
0.52H), 5.24-5.41 (m, 1H), 5.55-5.69 (m, 1H),
9.20 (s, 1H).
Referential Example 92
7-Chloro-2 3-dimethyl-1-(2-
m~t.hylc c o rooylmethyl)pyrrolof2 3-dlpvridazine
The title compound was prepared as white crystals in
98.3% yield in a similar procedure to that described in
Referential Example 51 by using 2,3-dimethyl-i-(2-
methylcyclopropylmethyl)-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one.
Mass spectrum (CI, m/z): 350 {M+ + 1), 352 (M+ +
3).
NMR spectrum (CDC13, bppm):
0.26-0.34 (m, 1H), 0.50-0.59 (m, 1H), 0.76-1.03
(m, SH), 2.30 (s, 3H), 2.46 (s, 3H), 4.46 (d;J=7
Hz, 2H), 9.04 (s, 1H).
209 2181555
Referential Example 93
1-(2-BLtenY~)-7-ChlOrO-2-mP hyl-3
n ylgyrrolof2 ~-dlgyrid
The title compound (cis/trans=20/80) was prepared as
an orange oil in 88.5% yield in a similar procedure to
that described in Referential Example 51 by using
1-(2-butenyl)-2-methyl-3-pentyl-6,7-
dihydropyrrolo[2,3-d]pyridazin-7-one (cis/trans (20/80).
Mass spectrum (CI, m/z): 292 (M+ + 1), 294 (M+ +
3).
NMR spectrum (CDC13, 6ppm):
0.88 (t;J=8 Hz, 3H), 1.23-1.40 (m, 4H), 1.55-1.69
(m, 4.4H), 1.81 (d;J=7 Hz, 0.6H), 2.39 (s, 3H),
2.72 (t;J=8 Hz, 2H), 5.05-5.08 (m, 1.6H),
5.19-5.32 (m, 1.4H), 4.56-5.64 (m, 1H), 9.18 (s,
1H) .
Referential Example 94
~-i.s-t4-r'~»~ropheny~. ropionyll-2 3 dimethylgyrrole
A solution of 2.50 g (0.263 mole) of
2,3-dimethylpyrrole in 10 m1 of dry tetrahydrofuran was
added dropwise to a solution of ethylmagnesium bromide in
17 ml of dry tetrahydrofuran, which was prepared from 0.83
g (0.0341 mole) of magnesium chips and 4.02 g (0.0361
mole) of ethyl bromide, at room temperature over a period
of 10 minutes. Thereafter, the refiuxing mixture was
1 2181553
210
allowed to cool to room temperature over a periad of 30
minutes to give 4,5-dimethyl-2-pyrrolemagneaium bromide.
A tetrahydrofuran solution of
4,5-dimethyl-2-pyrrolemagneaium bromide prepared above was
dropwise added to a solution of
3-(4-fluorbphenyl)propionyl chloride in 25 ml of dry
tetrahydrofuran, which was prepared from 9.50 g (0.0565
mole) of 3-(4-fluorophenyl)propionic acid and 6.0 ml of -
thionyl chloride, at -78°C over a period of about 35
minutes under a stream of nitrogen, and the reaction
mixture was allowed to rise to room temperature over a
period of about 2 hours. 15 ml of a saturated aqueous
solution of ammonium chloride and 50 ml of water were
added to the mixture, and the aqueous layer was separated
and extracted with ether. The combined ether extract (250
ml) was washed twice with 100 ml each of an about 10%
aqueous solution of sodium hydroxide and subsequently with
50 ml of a saturated aqueous solution of sodium chloride,
and dried over anhydrous sodium sulfate. The solvent was
distilled off under reduced pressure, and the residue was
purified by column chromatography through silica gel using
a 1:10 mixture of ethyl acetate and hexane as an eluent to -
give 3.42 g of the title compound as a pale brown solid.
Mass spectrum (CI, m/z): 264 (M+ + 1).
NMR spectrum (CDC13, Sppm):
2.01 (s, 3H), 2.22 (s, 3H), 2.98 (br.s, 4H), 6.66
(d;J=2 Hz, 1H), 6.92-6.99 (m, 2H), 7.15-7.20 (m,
211 2i8i555
2H), 9.31 (br.s, 1H).
Referential Example 95
5-(3-Phen~proy~ionvl)-2 3-dimethylpyrrole
The title compound was prepared as a pale violet solid
in 51.4% yield in a similar procedure to that described in
Referential Example 94 by using 3-phenylpropionic acid.
Mass spectrum (CI, m/z): 228 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.00 (s, 3H), 2.22 (s, 3H), 3.00 (br.s, 4H), 6.66
(d;J=2 Hz, 1H), 7.17-7.32 (m, 5H), 9.41 (br.s,
1H).
Referential Example 96
~-ij-ci,4-Dmluorog~y~pr~ionvi~-2 3-dimethylgyrrole
The title compound was prepared as a brownish-yellow
solid in 48.0% yield in a similar procedure to that
described in Referential Example 94 by using
3-(2,4-difluorophenyl)propionic acid.
Mass spectrum (CI, m/z): 264 (M+ + 1).
NMR spectrum (CDC13, bppm):
2_00 (s, 3H), 2.22 (s, 3H), 2.94-3.05 (m, 4H),
6.66 (d;J=3 Hz, 1H), 6.67-6.81 (m, 2H), 7.14-7.22
(m, 1H), 9.44 (br.s, 1H).
'~ 212 2181553
Referential Example 97
1-(2-ButeriVl)-5-f3-(4-f mnrnnhanyl)hroginnvll 2 3
dimethyly~yrro
0.82 g (0.00731 mole) of potassium tert-butoxide was
added to a solution of 1.39 g (0.00567 mole) of 5-[3-(4-
fluorophenyl)propionyl]-2,3-dimethylpyrrole and 0.19 g
(0.00074 mole) of i8-crown-6 in 40 ml of tetrahydrofuran
and the resulting mixture was stirred at room temperature
for 20 minutes. 1.80 g tO.OlI5 mole) of 1-bromo-2-butene
(a mixture of cis and trans isomers) were added to the
mixture and stirred at room temperature for 4 hours.
After completion of the reaction, the reaction mixture was
poured into ice-water and the aqueous mixture was
extracted twice with 80 ml each of ethyl acetate. The
extract was waked with a saturated aqueous solution of
sodium chloride and dried over anh_.-drous sodium sulfate.
The solvent was distilled off under reduced pressure and
the residue was purified by column chromatography through
silica gel using a 1:10 mixture of ethyl acetate and
hexane as an eluent to give 0.90 g of the title compound
(cis/trans=22/78) as a pale yellow oil.
Mass spectrum (CI, m/z): 300 (M+ + 1).
NMR spectrum (CDC13, sppm):
1.58-1.65 (m, 2.34H), 1.71-1.77 (m, 0.66H), 2.01
(s, 3H), 2.14 (s, 3H), 2.90-3.04 (m, 4H),
4.89-4.94 (m, 1.56H), 5.03-5.08 (m, 0.44H),
213 -
5.28-5.41 (m, 1H), 5.49-5.60 (m, 1H), 6.76 (s,
1H), 6.92-7.00 (m, 2H), 7.13-7.21 (m, 2H).
Referential Example 98
S-[3-l4-5'luorophenyl)orogionyll-2 3-d'me~-hyl-1-(2
me_thy3 c,~groRylmethyl ) pyrrole -
The title compound was prepared as a pale yellow oil -
in 74.2 yield in a similar procedure to that described in
Referential Example 97 by using
5-[3-(4-fluorophenyl)propionyl]-2,3-
dimethylpyrrole and 2-methylcyclopropylmethyl bromide.
Mass spectrum (CI, m/z): 314 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.14-0.21 (m, 1H), 0.41-0.48 (m, 1H), 0.67-0.90
(m, 2H), 0.97 (d;J=6 Hz, 3H), 2.01 (s, 3H), 2.17
(s, 3H), 2.91-3.07 (m, 4H), 4.25-4.28 (m, 2H),
6.77 (s, 1H), 6.91-6.98 (m, 2H), 7.16-7.22 (m,
2H).
Referential Example 99
1-~vcloorogy~emethyl-5-!3-!4-fluorophen;rl)aro~yll 2 3
dim yiy~yr_ro~e
The title compound was prepared as a pale yellow oil
in 64.8% yield in a similar procedure to that described in
Referential Example 97 by using
5-[3-(4-fluorophenyl)propionyl]-2,3-
dimethylpyrrole and cyclopropylmethyl bromide.
214 ~~~~553
Mass spectrum (CI, m/z): 300 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
0.29-0.36 (m, 2H), D.39-0.49 (m, 2H), 1.07-1.21
(m, iH), 2.02 (s, 3H), 2.18 (s, 3H), 2.93-3.06
(m, 4H), 4.27 (d;J=7 Hz, 2H), 6.78 (s, 1H),
6.91-6.99 (m, 2H), 7.14-7.22 (m, 2H).
Referential Example 100
5-f3-(4-Fluorophenyl)x~rop~yl)-2 3-dsmPrhyl 1 (2
pro~renyl)pvrrole
The title compound was prepared as a pale yellow oil
in 60.3% yield in a similar procedure to that described in
Referential Example 97 by using
5-[3-(4-fluorphenyl)propionyl]-2,3-
dimethylpyrrole and 3-bromo-1-propene.
Mass spectrum (CI, m/z): 286 (M+ + 1).
NMR spectrum (CDC13, sppm):
2.01 (s, 3H), 2.13 (s, 3H), 2.91-3.05 (m, 4H),
4.72 (d;J=17 Hz, 1H), 4.99-5.02 (m, 2H), 5.06
(d;J=11 Hz, 1H), 5.86-5.98 (m, 1H), 6.77 (s, 1H),
6.90-6.99 (m, 2H), 7.13-7.21 (m, 2H).
Referential Example 10i
1- t2-Butenyl) -2 3-d~mPr~ri -5- (3- henylr proyl)pyrrole
The title compound (cis/trans=23/77) was prepared as a
yellow oil in 75.7% yield in a similar procedure to that
described in Referential Example 97 by using
~
2181553
215
2,3-dimethyl-5-(3-phenylpropionyl)pyrrole and
1-bromo-2-butene.
Mass spectrum (CI, m/z): 282 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.64 (d;J=8 Hz, 2.31H), 1.77 (d;J=8 Hz, 0.69H),
2.01 (s, 3H), 2.17 (s, 3H), 3.02 (br.s, 4H),
4.89-5.96 (m, 1.54H), 5.08 (d;J=7 Hz, 0.46H),
5.28-5.42 (m, 1H), 5.49-5.61 (m, 1H), 6.77 (s,
1H), 7.15-7.32 (m, 5H).
Referential Example 102
2 3-DSmethvl-5- (3~henylTJ~'O ionyl) -1- (2-gr~nenyl)ovrrole
The title compound was prepared as a yellow oil in
86.6% yield in a similar procedure to that described in
Referential Example 97 by using 2,3-dimethyl-5-(3-
phenylpropionyl)pyrrole-and 3-bromomethyl-1-propene.
Masa spectrum (CI, m/z): 268 (M+ + 1).
NMR spectrum (CDC13, 6ppm):
2.02 (s, 3H), 2.13 (s, 3H), 2.94-3.10 (m, 4A),
4.70-4.77 (m, 1H), 4.99-5.09 (m, 3H), 5.94
(ddt;J=17 Hz, 11 Hz, 7 Hz, 1H), 6.79 (s, 1H),
7.12-7.33 (m, 5H).
Referential Example I03
2 3-Dimethyl-1-(2-methvl~ycionrogylmethyl)-5-(3-
ghenylpropionyl)pvrro~e
216 2 ~ ~ ~ ~ 53
The title compound was prepared as a yellow ail in
99.1% yield in a similar procedure to that descrihed in
Referential Example 97 by using 2,3-dimethyl-5-(3-
phenylpropionyl)pyrrole and
1-bromomethyl-2-methylcyclopropane.
Mass spectrum (CI, m/z); 296 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.14-0.21 (m, 1H), 0.42-0.49 (m, 1H), 0.68-0.93
(m, 2H), 0.97 (d;J=6 Hz, 3H), 2.01 (s, 3H), 2.17
(s, 3H), 3.05 (br.s, 4H), 4.25-4.34 (m, 2H), 6.78
(s, 1H), 7.12-7.33 (m, 5H).
Referential Example 104
1-CvcloprQB ly me hyl-2 3-dimethy~-5-(3-
phenylgropionyl~vrrole
The title compound was prepared as an orange oil in
93.0% yield in a similar procedure to that described in
Referential Example 97 by using 2,3-dimethyl-5-(3-
phenylpropionyl)pyrrole and bromomethylcyclopropane.
Mass spectrum (CI, m/z): 282 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.30-0.49 (m, 4H), 1.10-1.25 (m, 1H-), 2.02 (s,
3H), 2.17 (s, 3H), 3.03 (br.s, 4H), 4.28 (d;J=7
Hz, 2H), 6.80 (s, 1H), 7.14-7.31 (m, 5H).
217 2~~~~53
Referential Example 105
I-(2-Butenvl)-5-[3-(2 4-diflunry~~nrnoionvll-2 3-
dimethylpyrrole
The title compound (cis/trans=23/77) was prepared as a -
yellow oil in 55.4% yield in a similar procedure to that
described in Referential Example 97 by using
5-[3-(2,4-difluorophenyl)propionyl]-2,3-
dimethylpyrrole and 1-bromo-2-butene.
Mass spectrum (CI, m/z): 318 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.62-1.64 (m, 2.31H), 1.74-1.77 (m, 0.69H), 2.00
(s, 3H), 2.14 (s, 3H), 2.99 (br.s, 4H), 4.89-4.92
(m, 1.54H), 5.04-5.07 (m, 0.46H), 5.28-5.40 (m,
1H), 5.51-5.60 (m, 1H), 6.73-6.80 (m, 3H),
7.14-7.22 (m, 1H).
Referential Example 106
5- f3- (2,4-I)ifluoT"oy~heny~grnninnyl] -2 3-dimethvl-1- (2-
methy~yclo~~ylmethyl)gyrrole
The title compound was prepared as a yellow oil in
80.2% yield in a similar procedure to that described in
Referential Example 97 by using 5-[3-(2,4-
difluorophenyl)propionyl]-2,3-dimethylpyrrole and
2-methylcyclopropylmethyl bromide.
Mass spectrum (CI, m/z): 332 (M+ + 1).
2I8155~ _
218
NMR spectrum (CDC13, bppm): ,
0.14-0.20 (m, IH), 0.41-0.48 (m, 1H), 1.67-1.92
(m, 2H), 0.97 (d;J=6 Hz, 3H), 2.01 (s, 3H), 2.17
(s, 3H), 3.00 (br.s, 4H), 4.20-4.32 (m, 2H),
6.73-6.80 (m, 3H), 7.15-7.23 (m, 1H).
Referential Example 107
1-Cvclog~ogylmethyl-5- [3- (2 4-
difluorophenyl)p~Qgio~yll-2 3-dimethylgyrrole
The title compound was prepared as a yellow solid in
85.1% yield in a similar procedure to that described in
Referential Example 97 by using
5-[3-(2,4-difluorophenyl)propionyl]-2,3-dimethylpyrrole
and cyclopropylmethyl bromide.
Mass spectrum (CI, m/z): 318 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.30-0_50 (m, 4H), 1.06-1.12 (m, IH), 2.02 (s,
3H), 2.18 (s, 3H), 3.00 (br.s, 4H), 4.27 (d;J=7
Hz, 2H), 6.72-6.81 (m, 3H), 7.14-7.22 (m, 1H).
Referential Example 108
grogenyl)gvrrole
The title compound was prepared as a pale yellow oil
in 81.7% yield in a similar procedure to that described in
Referential Example 97 by using
5-[3-(2,4-difluorophenyl)propionyl]-2,3-dimethylpyrrole
2181553
219
and 3-bromo-1-propene.
Mass spectrum (CI, m/z): 304 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
2.01 (s, 3H), 2.13 (s, 3H), 2.g9 (br.s, 4H), 4.72
(d;J=17 Hz, 1H), 4.98-5.01 (m, 2H), 5.06 (d;J=10
Hz, 1H), 5.86-6.00 (m, 1H), 6.73-6.80 (m, 3H),
7.13-7.2I (m, 1H).
Referential Example 109
1-(2-Buten~tl)-2-[1-ch~~r~-3-(4-fluoronhenyl) 1 ~r p~yll 3
formvl-4 5-dimet)3,yi~yrrole
0.38 ml (0.00408 mole) of phosphorus oxychloride was
added to 0.29 g (0.00397 mole) of dry dimethylformamide
and the mixture was stirred at room temperature for 30
minutes. A solution of 0.89g (0.00297 mole) of
1-(2-butenyl)-5-[3-(4-fluorophenyl)propionyl]-2,3-
dimethylpyrrole in 4 ml of dichloromethane was added
dropwise to the mixture and stirred at room temperature
for 30 minutes. The reaction mixture was poured into
ice-water and neutralized with an aqueous solution of _
sodium hydroxide. The aqueous mixture was extracted with
50 ml each of dichloromethane for three times. The
extract was washed-with 30 ml of a saturated aqueous
solution of sodium hydrogencarbonate and 30 ml of a
saturated aqueous solution of sodium chloride in turn
21$1553
220
and dried over anhydrous sodium sulfate. The solvent was
distilled off under reduced pressure and the residue was
purified by column chromatography through silica gel using
a 1:12 to 1:9 mixture of ethyl acetate-and hexane as an
eluent to give 0.4I g of the title compound
(cis/trans=22/78) as a yellow oil.
Mass spectrum (CI, m/z): 346 (M+ + 1).
NMR spectrum (CDC13, bppm):
1.55-1.74 (m, 3H), 2.10 (s, 3H), 2.23 (s, 3H),
3.72 (d;J=7 Hz, 2H), 4.39-4.43 (m, 1.56H),
4.51-4.55 (m, 0.44H), 5.27-5.66 (m, 2H), 6.08
(t;J=7 Hz, 1H), 6.97-7.04 (m, 2H), 7.16-7.24 (m,
2H), 9.77 (s, 1H).
Referential Example 110
2- [i _Ch~ 0,-0-3- (4-fl ~oroph~Yi ~ 1~R~Y11 3 form~r~ 4 5
dime hvl-1 - (2-methyl ~~~roy:~rlm~thyl) gyrrole _
The title compound was prepared as a pale brown oil in
71.2% yield in a similar procedure to that described in
Refereetial Example 109 by using
5-[3-(4-fluorophenyl)propionyl]-2,3-
dimethyl-1-(2-methylcyclopropylmethyl)pyrrole.
Mass spectrum (CI, m/z): 360 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.22-0.28 (m, 1H), 0.34-0.41 (m, 1H), 0.59-0.78
(m, 2H), 0.94 (d;J=6 Hz, 3H), 2.17 (s, 3H), 2.24
221 2' 8 ? 5 ~~
(s, 3H), 3.68-3.78 (m, 4H), 6.12 (t;J=7 Hz, 1H),
6.98-7.04 (m, 2H), 7.19-7.26 (m, 2H), 9.76 (s,
1H) .
Referential Example 111
2-fl-Chloro-3-(4-fluoroyl)-1-prog~mrll-1-
~yclo~gvlmethvi-3-formyl-4 5-dimethyl~yrrole
The title compound was prepared as a pale yellow oil -
in 72.3% yield in a similar procedure to that described in
Referential Example 109 by using
1-cyclopropylmethyl-5-[3-(4-
fluorophenyl)propionyl]-2,3-dimethylpyrrole.
Mass spectrum (CI, m/z): 346 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
0.19-0.30 (m, 2H), 0.48-0.57 (m, 2H), 0.98-1.11
(m, 1H), 2.18 (s, 3H), 2.24 (s, 3H), 3.68 (d;J=7
Hz, 2H), 3:77 (d;J=7 Hz, 2H), 6.13 (t, J=7 Hz, -
IH), 6.97-7.04 (m, 2H), 7.19-7.25 (m, 2H), 9.77
(s, 1H) .
Referential Example 112
2-fl-Ghloro-3-(4-fluorophenyl)-1-~tro~~~yll-3-formvl-4 5-
dimethyl-i-(2-~ropenyl)pyrr~~P
The title compound was prepared as a yellow oil in
70.8% yield in a similar procedure to that described in
Referential Example 109 by using
5-[3-(4-fluorophenyl)propionyl]-2,3-
dimethyl-1-(2-propenyl)pyrrole.
222 2181 X53
Mass spectrum (CI, m/z): 332 (M+ + 1).
NMR spectrum (CDC13, sppm):
2.09 (s, 3H), 2.24 (s, 3H), 3.72 (d;J=7 Hz, 2H),
4.46-4.51 (m, 2H), 4.83 (d;J=17 Hz, 1H), 5.14
(d;J=10 Hz, 1H), 5.78-5.92 (m, 1H), 6.09 (t;J=7
Hz, 1H), 6.96-7.04 (m, 2H), 7.15-7.34 (m, 2H),
9.78 (s, 1H).
Referential Example 113
1-(2-Butenvl)-2-(1-chloro-3 yl-1-prop~rY~1-3-
formvl-4 S-dimethy~Byrrole
The title compound (cis/trana=23/77) was prepared as -
an orangish-yellow oil in 61.6% yield in a similar
procedure to,that described in Referential Example 109 by
using i-(2-butenyl)-2,3-dimethyl-5-(3-
phenylpropionyl)pyrrole (cis/trans=23/77).
Mass spectrum (CI, m/z): 328 (M+ + 1), 330 (M+ +
3).
NMR spectrum (CDC13, bppm):
1.61-1.73 (m, 3H), 2.10 (s, 3H), 2.23 (s, 3H),
3.76 (d;J=7 Hz, 2H), 4.40-4.43 (m, 1.54H),
4.50-4.55 (m, 0.46H), 5.30-5.45 (m, 2H), 5.12
(t;J=7 Hz, 1H), 7.22-7.35 (m, 5H). 9.79 (s, 1H).
Referential Example 114
2-(1-Chloro-3-phenyl-1-pr envl)-3-formvl-4 5-dimethyl-1-
(2-pro~enYl)pvrrole
223
The title compound was prepared as a red-brown oil in
73.6% yield in a similar procedure to that described in
Referential Example 109-by using 2,3-dimethyl-5-(3-
phenylpropionyl)-1-(2-propenyl)pyrrole.
Mass spectrum (CI, m/z): 314 (M++1), 316 (M+ + 3).
NMR spectrum (CDC13, 5ppm):
2.09 (s, 3H), 2.24 (s, 3H), 3.75 (d;J=7 Hz, 2H), -
4.47-4.52 (m, 2H), 4.83 (d;J=17 Hz, 1H), 5.14-
(d;J=9 Hz, 1H), 5.85 (ddt;J=17 Hz, 9 Hz, 7 Hz,
1H), 6.16 (t;J=7 Hz, 1H), 7.14-7.41 (m, 5H), 9.80
(s, iH) .
Referential Example 115
2-(i-Chloro-3-~,henvl-l ~rog~yl)-3-formvl-4 5 dimethyl i
(2-methvlcycloprQxwlmethvl)ovrrole
The title compound was prepared as a yellow-orange oil
in 65.9% yield in a similar procedure to that described in
Referential Example 109 by using 2,3-dimethyl-1-(2-
methylcyclopropylmethyl)-5-(3-phenylpropionyl)pyrrole.
Masa spectrum (CI, m/z): 342 (M+ + 1), 344 (M+ +
3).
NMR spectrum (CDC13, bppm):
0.21-0.28 (m, 1H), 0.34-0.40 (m, 1H), 0.62-0.73
(m, 2H), 0.93 (d;J=6 Hz, 3H), 2.16 (s, 3H), 2.24 -
(s, 3H), 3.68-3.84 (m, 4H), 6.15 (t;J=7 Hz, 1H),
7.15-7.40 (m, 5H), 9.78 (s, 1H).
224 21 ~ 1555
Referential Example 116
2-f1-Chloro-3-phenyl-1-grox~~nyll-1-cyelopropylmethyl-3-
formyl-4,5-r3amethKl_pyrrole
The title compound was prepared as a yellow-orange oil __
in 75.5% yield in a similar procedure to that described in -
Referential Example I09 by using
1-cyclopropylmethyl-2,3-dimethyl-5-
(3-phenylpropionyl)pyrrole.
Mass spectrum (CI, m/z): 328 (M+ + 1), 330 (M+ +
3) .
NMR spectrum (CDCl3, 6ppm):
0.21-0.27 (m, 2H), 0.47-0.54 (m, 2H), 0.99-1.08
(m, 1H), 2.18 (s, 3H), 2.24 (s, 3H), 2.70-2.84
(m, 4H), 6.17 (t;J=7 Hz, 1H), 7.16-7.40 (m, 5H),
9.78 (s, 1H).
Referential Example 117
1-l2-BUtenyl)-2-f1-chloro-3-(2 4-di ~»~royl)-1-
pro~~yl)-3-formvl-4 5-dimethylpvrrole
The title compound (cis/trans=23/77) was prepared as a
pale yellow oil in 85.7% yield in a similar procedure to
that described in Referential Example 109 by using
1-(2-butenyl)-5-[3-(2,4-
difluorophenyl)propionyl]-2,3-dimethylpyrrole.
Mass spectrum (CI, m/z): 364 (M+ + 1).
NMR spectrum (CDC13, 5ppm):
1.59-1.71 (m, 3H), 2.10 (s, 3H), 2.22 (s, 3H),
3.72 (d;J=7 Hz, 2H), 4.39-4.41 (m, 1.54H),
225 2181553
4.51-4.53 (m, 0.46H), 5.27-5.67 (m, 2H), 6.05
(t;J=7 Hz, 1H), 6.77-6.87 (m, 2H), 7.18-7.27 (m,
1H), 9.75 (s, 1H).
Referential Example 118
2-fl-Ch~oro-3-(2 4-d~f~~nrnghenvl)-7.-orop~n_y11 3
formvi -4 s-d~merhyl-1- (2-merh~rlcycloprpg~ ~r m thv~PYrrole . . .,
The title compound was prepared as a pale brown oil in
70.8% yield in a similar procedure to that described-in -
Referential Example 109 by using
5-[3-(2,4-difluorophenyl)propionyl]-2,3-dimethyl-1-(2-
methylcyclopropylmethyl)pyrrole.
Mass spectrum (CI, m/z): 378 (M+ + I).
NMR spectrum (CDC13, 5ppm):
0.21-0.28 (m, 1H), 0.33-0.40 (m, 1H), 1.56-1.80
(m, 2H), 0.93 (d;J=6 Hz, 3H), 2.16 (s, 3H), 2.24
(s, 3H), 3.70-3.77 (m, 4H), 6.09 (t;J=7 Hz, 1H),
6.78-6.88 (m, 2H), 7.20-7.30 (m, 1H), 9.75 (s,
1H) .
Referential Example 119
~m-~.W 1~1i7-~- 4G 4-Q~ r W nrnnnanvl
a -1-8~b~~Yl.~
cvclonropvlmethyi-3-formvl-4 5- ~mernyi~yrrole
The title compound was prepared as a pale brown oil in
67.8% yield in a similar procedure to that described in _
Referential Example 109 by using 1-cyclopropylmethyl-
5-[3-(2,4-difluorophenyl)propionyl]-2,3-dimethylpyrrole.
i
226
Mass spectrum (CI, m/z): 364 (M+ + 1).
NMR spectrum (CDC13, bppm):
0.20-0.26 (m, 2H), 0.47-0.54 (m, 2H), 0.98-1.11
(m, 1H), 2.18 (s, 3H), 2.24 (s, 3H), 3.70-3.79
(m, 4H), 6.10 (t;J=7 Hz, 1H), 6.78-6.88 (m, 2H),
7.21-7.29 (m, 1H), 9.75 (s, 1H).
Referential Example 120
2-fl-ChTOro-3-(2 4-d~fl"nrng n.~ll-~-pr~Yll 3
formyl-4 5-d~methv~-1-L2-x~ropenvl)nvrrole
The title compound was prepared as a pale
brownish-yellow oil in 80.2% yield in a similar procedure -
to that described in Referential Example 109 by using
5-[3-(2,4-difluorophenyl)propionyl]-2,3-dimethyl-1-(2-
propenyl)pyrrole.
Mass spectrum (CI, m/z): 350 (M+ + 1).
NMR spectrum (CDC13, bppm):
2.09 (s, 3H), 2.23 (s, 3H), 3.72 (d;J=7 Hz, 2H),
4.46-4.49 (m, 2H), 4.81 (d;J=17 Hz, 1H), 5.14
(d;J=10 Hz, 1H), 5.77-5.91 (m, 1H), 6.06 (t;J=7
Hz, 1H), 6.77-6.86 (m, 2H), 7.18-7.23 (m, 1H),
9.76 (s, 1H) .
' ~ 218153
227
Test Example 1
PrOtOn,~Otaaaimm-a~eriQcln2 tr~~hOSphata8e- . ...
(H+.K+-ATPase) activation tent
According to the method reported by Sachs et al. [J.
Biol. Chem., 25~, 7690 (1976)], a microsomal fraction -
obtained from homogenized fresh porcine gastric mucosa by
density gradient ultracentrifugation was used as the '
proton. potassium - adenosine triphosphatase preparation,
~1 of a solution of a test compound in
dimethylsulfoxide were added to 0.75 ml of 70 mM tris-HC1
buffer solution (magnesium chloride S mM, potassium
chloride 20 mM and pH = 6.85) containing from 30 to 80
~g/ml (in terms of protein concentration) of the enzyme
preparation and the mixture was incubated for 45 minutes
at 37°C with shaking at 200 times per minute. The enzyme
reaction was initiated by adding 0.25 ml of 8 mM disodium
adenosine triphosphate solution. After 20 minutes of the
reaction time, the reaction was stopped by adding 1 ml of
a 10~ trichloroacetic acid - active carbon (100 mg)
solution. The reaction solution was centrifuged (4°C,
3000 rpm, 15 minutes) and the inorganic phosphate in the
supernatant prepared from adenosine triphosphate by
hydrolysis was measured by colorimetry, according to the
method of Yoda and Hokin [Biochem. Biophys. Res. Commun.,
~, 880 (1970)]. In a similar manner, the amount of
228 2181553
inorganic phosphate was measured in a reaction solution in
the absence of potassium chloride. From the difference
between the phosphate amounts in-the presence and absence
of potassium chloride, the proton'potassium - adenosine
triphosphatase activity was calculated. Based on the
activity value in the control and the values in the test
compound at the various concentrations tested, the
inhibiting rates (%) and then the 50% inhibiting
concentrations (IC50) to proton.potasaium - adenosine
triphosphatase activity were obtained. The compounds of
Examples 1, 4, 7, 9, 17, 21, 22, 35, 44, 48, 49, 57, 58,
74, 75 and 76 show an excellent activity.
Test Example 2
Gastrsc ac~d aecrer;~n act;vitv test u~; lnrir
~1~L.RY
licr r-; r ;n rats (Shay ra m rnr,~~
Pyloric ligation was conducted based on the method of
Shay et al_ [Gastroenterology, 5_, 43 (1945)]. Male rats
of SD strain were fasted for 24 hours, their abdomens were
sectioned during anesthesia under ether. The duodenal and
pyloric regions were exposed and the latter was ligated.
A solution of a test compound (i0 mg/ml) prepared by use
of 1.5% parts of dimethylacetamide, 68.5% parts of
polyethyleneglycol (PEG-400) and 30% parts of
physiological saline solution, was injected into the
2181555
229
duodenal region for the dose to be 20 mg/kg by use of a
1-ml syringe and an injection needle (26G). After -
injection of.the test compound, the abdominal region was
autured_ The animals were allowed to a stand for 4 hours
without feeding food and water and then sacrified with
carbon dioxide gas. The stomach was excised and gastric -
juice was gathered. The gastric juice sample was
centrifuged.(4°C, 2500 rpm, 15 minutes). The amount of
the supernatant was measured to be as the gastric
secretion. The acidity of the gastric juice was
determined by the amount (ml) of 0.01 N sodium hydroxide
solution required for titration of 0.1 ml of the
supernatant to pH 7.0 by use of an auto-titrating
apparatus. The value of gastric acid secretion was
calculated from the value of gastric secretion and the
acidity of gastric juice. The inhibiting rate was obtained
from the values of gastric acid secretion of the control
group and of the administered group. The compounds of
Examples 1, 3, 5, 7, 9, 11, 12, 14, 16, 17, 22, 26, 32,
33, 35, 37, 40, 41, 42, 44, 48, 57, 58, 62 and 68 show an
excellent activity.
Test Example 3
~lt3baCte~"j a~ aCti V~ r~ aQaillBt H ~ ~ nnhanrar R~,lpri
The antibacterial activity of the compounds of the
present invention were evaluated by measuring the minimum
2181555
230
inhibitory concentration values (MIC) of the present
compounds against H >; obac avlors strains 9470, 9472
and 9474.
These strains of H 1i obac r ~;rlori were incubated
for 4 days on a plate medium. The medium was prepared by
dissolving brain heart infusion agar (Product of DIFCO) in
a defined amount of distilled water, by sterilization in
an autoclave, injecting horse blood (Product of Nihon
Seibutsu Zairyo Co.) into each plate for the medium to
contain 7% of the blood and by solidifying.
Under slightly aerobic conditions, Helicobacter gvlor; -
cultivated at 37°C for 4 days was suspended in a
physiological saline solution for its inocular size to be
about 108 CFUJml. The suspension was diluted 100 times,
and about 10 ~1 of the diluted suspension was inoculated
on a medium for MIC determination.
The medium for MIC determination used had the same
compounds as those for preculture. The medium for MIC
determination was prepared by mixing one part of 2-fold
diluted solutions of the compound dissolved in
dimethylaulfoxide with sterilized distilled water and 99
parts of the medium and by solidifying on a dish.
231 2_181553
In a similar way to the preculture, Hel'nn a r
pylori was cultivated at 37°C for 3 days under slightly
aerobic conditions. After completion of the culture, the
bacterial growth at every inoculated site was observed
with the naked eye. The minimum concentration which gave
no bacterial growth was regarded as MIC of the compound of
the present invention. The compounds of Examples 1, 2, 3,
4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19,
20, 21; 22, 23, 24, 25, 27, 29, 34, 44, 45, 48, 49, 50,
51, 52, 56, 57, 58, 75 and 76 show an excellent
antibacterial activity.