Note: Descriptions are shown in the official language in which they were submitted.
- 1-20520/A 21816 6 3
-1 -
Az-l dyes. pr~rPccPc for their ~ " and thPir uc~
The present invention relates to novel azo dyes, processes for their ,u, tl,Udl '' 1 and the use
of these dyes for dyeing and printing fibre materials, in particular textile fibre materials.
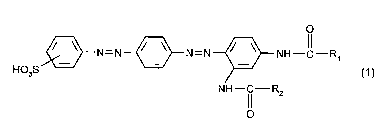
The present invention relates to azo dyes of the formula
Ho3S~3N=N~N=N~NH~ R
NH~ R2
o
in which
R1 and R2 il ,depel~d~ y of one another are unsl IhStitl ItPd or hydroxy-, C1-C4-alkoxy- or
halogen-substituted C~-C4-alkyl or C~-C~-alkoxy;
uns~h~tiilltpd or C1-C4-alkyl-, C,-C4-alkoxy- or halogen-substituted phenyl or phenoxy; or
a radical of the formula -N(R3)R4, in which R3 und R4 i, Id~,u~:l Id~:, Illy of one another are
hydrogen, C1-C4-alkyl, or C2-C~-alkanoyl which is urlcl Ih~titl ItPd or further substituted in the
alkyl part by hydroxyl or C1-C4-alkoxy,
with the proviso that R1 and R2 are not simultaneously methyl.
C1-C4Alkyl R1, R2, R3 and R4 i~ uelld~";ly of one another are, for example, methyl, ethyl,
propyl, isopropyl, butyl, sec-butyl, tert-butyl or isobutyl, in particular methyl or ethyl. The
C1-C4-alkyl radicals R1 and R2 can be further substituted by hydroxyl, halogen, for example
bromine or, preferably, chlonne, or in particular by C1-C4-alkoxy, for example ethoxy or,
preferably, methoxy. The methoxymethyl radical may be mentioned as an example of~o" ~,uu, Idil lg substituted radicals.
C1-C~Alkoxy R1 and R2 il l.k:pt:l Id~ of one another are, for example, methoxy, ethoxy,
propoxy, isopropoxy, butoxy or isobutoxy, in particular methoxy or, preferably, ethoxy. The
C1-C4-alkoxy radicals R1 and R2 can be further substituted by hydroxyl, halogen, for example
2181663
-- 2 -- =
bromine or, preferably, chlorine, or in particular by C1-C~-alkoxy, for example methoxy or
ethoxy. The co",::,,uol,-li"~ unslIhstitlItpd radicals are preferred.
C2-C4Alkanoyl R3 and R4 i, Ide:,u~l Idt:l ,;'J of one another are, for example, propionyl or, in
particular, acetyl. The C2-C4-alkanoyl radicals R3 and R4 can be further substituted by
hydroxyl or, in particular, C~-C4-alkoxy, for example methoxy or ethoxy. Preferably, these
radicals are further substituted by C~-C4-alkoxy, in particular by methoxy. M~l ,u,~yab~yl is
particularly preferred here.
Phenyl or phenoxy R~ and R2 are, in addition to the cu" t::~,UUI Idil ,9 url~ 1' -- ItPd radicals,
the radicals substituted by C,-C4-alkyl, such as methyl, ethyl, propyl, isopropyl, butyl, sec-
butyl, tert-butyl or isobutyl, C~-C4-alkoxy, for example methoxy, ethoxy, propoxy, isopropoxy,
butoxy or isobutoxy, or by halogen, for example fluorine, bromine or, in particular, chlorine.
The bUI I ~ UI ~ phenyl radicals are preferred here.
R3 is preferably hydrogen or C,-C4-alkyl, in particular hydro3en.
R4 is preferably hydrogen, C~-C4-alkyl, or C2-C4-alkanoyl which is urls~hetit~t~od or further
substituted in the alkyl part by C~-C~-alkoxy, in particular hydrogen, or C2-C4~1kanoyl which
is further substituted in the alkyl part by C1-Cralkoxy. R4 is particularly preferably hydrogen
or methoxyacetyl, in particular hydrogen.
R3 and R4 are especially preferably hydrogen.
R~ is preferably uns~hrfit~tPd or hydroxy-, C~-C4-alkoxy- or halogen-substituted C~-C4-alkyl
or C~-C4-alkoxy; or a radical of the fonmula -N(R3)R4, in which R3 and R4 are as defined and
preferred above. Preferably, in this case, R3 is hydrogen and R4 is hydrogen, or C2-C4-
alkanoyl which is further substituted in the alkyl part by C~-C4-alkoxy, in particular hydrogen.
R~ is preferably C~-C4-alkoxy; uns~ Ih~fifl Itf~d or C1-C4-alkoxy-substituted C~-C4-alkyl; or a
radical of the formula -N(R3)R4, in which R3 and R4 are as defined and preferred above.
Preferably, in this case, R3 is hydrogen and R4 is hydrogen, or C2-C4-alkanoyl which is
further substituted in the alkyl part by C1-C4-alkoxy, in particular hydrogen.
2181663
,~
-- 3 --
R~ is particularly preferably methyl; ethyl; methoxy; ethoxy; methoxy- or ethoxy-substituted
methyl; or amino.
R~ is especially preferably methyl; ethyl; ethoxy; methoxy-substituted methyl; or amino. Rl is
preferably methyl; ethyl; ethoxy; or methoxy-substituted methyl, in particular methyl.
The radical R2 is as preferred above for the radical R1, ethyl being especia~ly preferred.
Preferably, R~ and R2 il Iddpd~ ly of one another are uns' ' "' ~fPd or hydroxy-, C~-C4-
alkoxy- or halogen-substituted C1-C4-alkyl or C1-C4-alkoxy; or
a radical of the formula -N(R3)R4, in which R3 is hydro~en and R4 is hydrogen, or C2-C4-
alkanoyl which is further substituted in the alkyl part by C~-C4-alkoxy, in particular hydrogen.
Preferably, R1 and R2 il)dt:pelld~";'~ of one another are C~-C4-alkoxy; urlC~ Ihstitl It~od or
C,-,,-alkoxy-substituted C~-C4-alkyl; or amino.
R1 and R2 are particularly preferably i".l~,uel-d~, Illy of one another methyl; ethyl; methoxy;
ethoxy; methoxy- or ethoxy-substituted methyl; or amino.
R~ and R2 are especially preferably i, Id~,ut:l Id~ ly of one another methyl; ethyl; ethoxy;
methoxy-substituted methyl; or amino, in particular methyl; ethyl; ethoxy; or methoxy-
substituted methyl.
Azo dyes of the formula (1) which are of particular interest are those in which R~ is methyl
and R2 is ethyl.
The sulfo group in the azo dyes of the formula (1) is preferably bonded in the para-position
relative to the azo group.
The present invention furthermore relates to a process for the ,UI t:pdl dliUI I of azo dyes of the
formula (1), which comprises diazotizin~ an amine of the formula
2181663
--4 -
HO3S~N=N~NH2 (2)
coupling the ~ product to a coupling component of the fommula
~NH2
(3)
NH~ R2
o
and reacting the resulting reaction product with a compound which introduces the radical of
the formula
--C--R, (4)
in which
R~ and R2 are as defined underfommula (1).
The ~ of the compound of the formula (2) is can ied out in a manner known per
se, for example with a nitrite, for example with an alkali metal nitrite, such as sodium nitrite,
in a mineral acid medium, forexample in a h~lu~.l,l.,,i~,acid medium, attemperaturesfrom,
for example, -5 to 40'C, and preferably at 0 to 10C.
The coupling to the coupling component of the formula (3) is carried out in a manner known
per se at acidic, neutral or weakly alkaline pH, for example at a pH from 3 to 7, and at
temperatures from, for example, -5 to 30C, preferably 0 to 25C.
Compounds of the formula
218~663
-- 5 --
o
Il (5),
Hal--C--R~
in which
Hal is halogen, such as chlorine, bromine or iodine, in particular chlorine,
can be used, for example, for introduction of the radical of the fonmula (4). Examples of
compounds of the formula (5) are acetyl chloride, propionyl chloride, methoxyacetyl chloride
and ethyl 1,l llu",ru", Idlt:. Further examples of compounds which introduce the radical of the
formula (4) are acetic anhydride and propionic anhydride.
The introduction of the radical of the abo\,t:",~"liu"ed formula (4) can be carried out, for
example, in dipolar aprotic solvents, for example dimethylf~" ",d",id~ or dimethyl sulfoxide, or
in water or, preferably, in pyridine, at a temperature from, for example, 1 û to 80CC, in
particular 10 to 50C
In the process according to the invention for the ,IJI~:,Udl " ~ of the azo dyes of the formula
(1), the substituents of the compounds of the forrnulae (2), (3), (4) and (5) are as preferred
above.
The compounds of the fonmulae (2), (3) and (5) are known or can be prepared analogously
to known compounds.
The azo dyes of the formula (1 ) are either in the form of their free acid or, preferably, in the
form of salts thereof.
Salts are, for example, the alkali metal or ammonium salts or the salts of an organic amine.
Examples are the sodium, lithium, potassium or ammonium salts or the salt of mono-, di- or
Il i~ll Idl lOIdl l lil l'3.
2181663
- 6 -
The azo dyes of the fonmula (1) according to the invention are suitable, by methods known
per se, for dyeing and printing, in particular of fibre materials containing nitrogen or
containing hydroxyl groups, for example textile fibre materials of cellulose, silk and, in
particular, wool and synthetic polyamides. Dyeing or printing of natural or synthetic
polyamide fibre materials is preferred. The azo dyes of the formula (1) according to the
invention can be used in the generally customary form, if d,U,UI U,UI idlt~ prepared b~rul ~l ~dl 11.1,
for dyeing or printin3. Level dyeings with good all-round properties, in particular good
fastness to nubbing, wet processing, wet nubbing and light, are obtained. Furthermore, the
dyes according to the invention are readily water-soluble and can easily be combined with
other dyes. The abov~",t:l " led textile material can be in the most diverse fomms of
processing, for example as fibres, yarns, woven fabric or knitted fabric.
In the following Examples, parts are parts by weight. The temperatures are degrees Celsius.
Parts by weight bear the same relation to parts by volume as the gram to the cubic
centimetre.
Example 1: 22.2 parts of 4-d,,,i,,od~uL,t,,,~,,e4'-sulfonic acid are dissolved in 400 parts of
water at a temperature of 75C, and 2û parts of a 4 normal sodium nitrite solution are added.
The solution is added dnopwise to 400 parts of ice and 20 parts of cù, Ict:"L, d~tll.l hydrochloric
acid at a temperature of 0 to 5C in the course of 20 minutes. The brown suspension thus
obtained is stirred for one hour and the pH is then brought to a value of 4 with a sodium
carbonate solution (10%). The diazo suspension is then added dropwise to a coupling
solution which comprises 150 parts of 3-d,,,i,,u,ul~e,,jlurea ll~luulllulid~: in 400 parts of
water and 63.5 parts of 1 normal sodium hydroxide solution at a temperature of 0 to 5C in
the course of 60 minutes. During the coupling, the pH is kept at a value of 5. The mixture is
allowed to react completely at a temperature of 0 to 5C for 2 hours and then at room
temperature overnight. The brown suspension is filtered and the residue is washed with
water. The moist residue is then stirred with 1000 parts of wakr and the pH is brought to a
value of 8 with 67 parts of 1 normal sodium hydroxide solution. 70 parts of sodium chloride
are added at a temperature of 60C and the fine suspension is filtered at a temperature of
50C. Afier drying, a compound which, in the form of the free acid, uOIlt:~,uulnl:, to the
fonmula
21816~3
-- 7 --
Ho3S~3N=N~3N=N~NH2 , =
(101)
NH--C--NHz
is obtained.
FY~rnples 2 to 4: The compounds of the formulae
H03S~N=N ~N=N~NH2
(1 02),
NH--C--CH3
Ho3S~N=N~N=N~3NH2
(103) and
NH--ICl--CH2OCH3
HO3S~N=N~N=N~NH2
(1 04)
NH--C--C H
can be obtained in a manner analogous to that described in Example 1.
F-r~ple 5: 6.6 parts of the compound of the formula (104) are stirred in 150 parts of
pyridine, and 5.9 parts of acetyl chloride are added dropwise in the course of 10 minutes, the
218166~
--8 -
temperature risin3 to 45~C. The reaction mixture is subsequently stirred for one hour and 50
parts of methanol are then added. The orange-coloured suspension is pouned onto 750
parts of a uu~ ldlt:d aqueous sodium chloride solution and filtered and the residue is
washed with a 5% aqueous sodium chloride solution and with methanol. After drying, a dye
which, in the form of the free acid, c~" t::~pu"~ to the compound of the formula
HO3S~N=N~N=N~NH--C--CHy
NH--C--C2Hs
o
is obtained. The dye of the fommula (105) dyes wool and polyamide in orange colour shades.
EY~mples ~ to 14: The procedure in Example 5 is repeated, but usin3 an equimolar amount
of oneofthecompoundsofthefommulae(101), (102)and(103),whered,~,u,uu,idl~,insteadof 6.6 parts of the compound of the formula (104), and an equimolar amount of propionyl
chloride or ~cll~uxyau~:lyl chloride, where ~,U,UIU,UIid~l~, instead of 5.9 par~s of acetyl chloride.
The dyes, shown in the fomm of the free acid, of the formulae
o
HO3S--~N=N~N=N~NH--C--CH3
(1 06),
NH--ICl--NH2
o
HO3S ~3 N = N ~3 N = N ~
(1 07),
NH--C--CH20CH3
o
~ 218166~
g
HO3S ~ N = N ~ N = N ~
(1 08),
o
HO3S ~3 N = N ~ N = N ~
(109),
NH--ll--NH2
HO3S ~ N = N ~3 N = N ~
(1 1 O),
NH--C--CH2OCH3
Ho3S~3N=N~N=N~NH--~--CH20CH3
(111),
o
Ho,S ~N=N~3N=N~NH--C--CH20CH3
(112),
NH--C--CH3
o
` 218166~
-10-
HO3S~N=N~N=N~NH--C-CH2OCH3
NH--C--CH2OCH3
o
HO3S~N=N~N=N~NH C--C2Hs
NH--C--CH3
are obtained. The dyes of the formulae (106) to (114) dye wool and polyamide in orange
colour shades
FY~rnple 15: The procedure described in Example 5 is repeated, but using an equimolar
amount of the compound of the formula (10) instead of 6.6 parts of the compound of the
formula (104), and an equimolar amount of ",~ ac~Lyl chloride instead of 5.9 parts of
acetyl chloride. A dye which, in the form of the free acid, w" t:~,uu, Id~ to the compound of
the formula
HO3S~N=N~N=N~NH--C--CH2OCH3
NH--ICl--NH-CO-CH2OCH3
is obtained. The dye of the formula (115) dyes wool and polyamide in orange colour shades
F~ rnpel 16: 4.4 parts of the compound of the formula (102) are stirred in 50 parts of
py~dine at a temperature of 80C. 6.5 parts of ethyl ~,llolUrUlllld~ are then added dropwise
218166~
-11 -
at a temperature of 25C in the course of 10 minutes, during which the temperature rises to
42C, The mixture is subsequently stirred at this temperature for one hour and 75 parts of
methanol and 25 parts of a 25% aqueous sodium chloride solution are then added to the
reaction mixture at room temperature. The suspension is filtered and the residue is washed
with a 10% aqueous sodium chloride solution and with methanol. The moist residue is then
recrystallized from a mixture of alcohol and water in the ratio 1:1. After drying, 2.8 parts of a
dye which, in the fonm of the free acid, cu,,~:~po~ to the compound of th~ formula
o
Ho3S~N=N~3N=N~NH--C--OC2H5
~/ (1 16)
NH--C--CH3
o
are obtained. The dye of the formula (116) dyes wool and polyamide in orange colour
shades .
FY~mriles 17 to 19: The procedure described in Example 16 is repeated, but using an
equimolar amount of one of the compounds of the fommulae (101), (103) and (104) instead of
4 4 parts of the compound of the formula (102). The dyes, shown in the form of the fnee
acid, of the formulae
o
HO3S ~ N = N ~3 N = N ~ NH C OC2Hs
o
2181663
. ~
-- 12 --
HO3S ~ N = N ~ N = N ~ N H--C--OC2Hs
NH--C--C2Hs
o
HO3S~N=N ~N=N ~NH--C--OC2Hs
NH--C--CH20CH3
o
are obtained. The dyes of the formulae (117), (118) and (119) dye wool and polyamide in
orange colour shades.
~Y~mple 20: 4.4 parts of the compound of the formula (102) are stirred in 70 parts of water
at room temperature, and 3 parts of triethylamine and 10 parts of acetone are added. 5.7
parts of methyl ~ lul ~,ru", ~d~e: are added dropwise to the orange solution in the course of 10
minutes, during which the temperature rises to 32C. The pH is kept at a value of 7 here by
addition of an aqueous, 1 normal sodium hydroxide solution. After addition of 2û parts of
acetone, the mixture is stirred for 30 minutes and 50 parts of methanol are then added. The
mixture is then stirred for 20 minutes and the product is filtered off and washed successively
with 40 parts of a 5% sodium chloride solution, 50 parts of ethanol and 20 parts of methanol.
After drying, 4 parts of a dye which, in the form of the free acid, co, ,~,u, ~d~ to the formula
Ho3S~3N=N~3N=N~3NH--C--OCH3
NH--C--CH3
` ~ 218166~
-13-
are obtained. The dye of the formula (120) dyes wool and polyamide in orange colour
shades.
EY~rnples 21 ~ 2~ The procedure described in Example 20 is repeated, but using an
equimolar amount of one of the compounds of the formulae (101) and (104) instead of 4.4
parts of the compound of the formula (102). The dyes, shown in the form of the free acid, of
the formulae
HO3S~N=N~N=N ~NH--C--OCH3
NH--ICl--NH2
o
HO3S~N=N~N=N~NH--C--OCH3
NH--C--CzHs
o
are obtained. The dyes of the formulae (121) and (122) dye wool and polyamide in orange
colour shades.
Dyeing ~Y~ - 1 part of a levelling assistant (based on the cu, Id~l ISd~iUI~ product of a
higher aliphatic amine and ethylene oxide) is added to 2000 parts of d~",i, IC~ d water at
room temperature. The bath is then brought to a pH of 6 with acetic acid. 0.5 part of the
dye of the formula (105) according to Example 5 is then added. 100 parts of polyamide 6,6
fibre material (Helanca tricot) are introduced into the resulting dye solution and the dyebath
is heated to a temperàture of about 96C in the course of 45 minutes. The temperature is
maintained for 45 to 60 minutes, the bath is then cooled to a temperature of 70C and the
dyed goods are removed and rinsed with water and then dried. A fabric dyed in an orange
colour shade is obtained.
_ . _ _ _ _ _ . .. ... .. . ..