Note: Descriptions are shown in the official language in which they were submitted.
21 821 ~8
The invention relates to an agent for transdermal
administration, characterized in that it contains 14~,17a-
ethanoestra-1,3,5(10)-triene-3,17~-diol optionally in combination
with one or more gestagen(s).
14~,17~-Ethanoestra-1,3,5(10)-triene-3,17~-diol is a
substance of formula
OH
HO ~
A pharmacologically active compound with unusually strong
estrogenic action is generally known. (W0 88/01275)
It has now been found that 14~,17~-ethanoe~stra-1,3,5(10)-
triene-3,17~-diol optionally in combination with one or more
gestagen(s) can be used readily for the production of an agent
for the transdermal administration of the active ingredient or
active ingredient mixture.
Pharmaceutical agents to be administered transdermally have
the advantage, as is generally known, that they make possible a
more uniform release of the active ingredient over a longer
period than is generally possible in the case of other agents to
be administered -- for example, orally. These properties can
advantageously be used in a number of endocrine diseases. For
poorly water-soluble steroid hormones, such as, for example,
2 ~1 821 '~
. ,
estrogens, however, it is generally quite problematic to set up
transdermal systems that ensure a penetration of the active
ingredient through the skin that is sufficient for therapy.
It has now been found that it is possible, surprisingly
enough, with the aid of the agent according to the invention, to
achieve a therapeutically sufficient, very uniform penetration
speed of the steroid hormones through the skin, while this is
only conditionally possible in the case of the agents to be
administered transdermally that contain the known steroid
hormones. (EP-A 137278 and EP-A 275716)
Suitable gestagens for the agents according to the invention
are, for example, norethisterone, levonorgestrel, gestodene, 3-
keto-desogestrel or their esters. The combination preparations
according to the invention contain preferably 1 to 3 gestagens --
especially 1 to 2 gestagen(s) -- in addition to 14~,17~-
ethanoestra-1,3,5(10)-triene-3,17B-diol.
For the production of pharmaceutical preparations, the active
ingredient or the active ingredient mixture can be dissolved or
suspended in suitable volatile solvents and/or penetration-
enhancing agents. The solutions or suspensions obtained can be
mixed with the adjuvants that are commonly used, such as matrix
formers and bactericides, and optionally are decanted into
graduated containers after sterilization. In addition, it is
also possible, however, to further process these solutions or
suspensions with inclusion of emulsifiers and water into lotions
or ointments. Optionally with the addition of fuel gas, sprays
3 21 821 88
can also be produced, which can be decanted into ordinary
graduated containers.
Suitable volatile solvents are, for example, lower alcohols,
ketones or lower carboxylic acid esters, such as ethanol,
isopropanol, acetone or ethyl acetate, polar ethers, such as
tetrahydrofuran, lower hydrocarbons, such as cyclohexane or
gasoline or else halogenated hydrocarbons, such as
dichloromethane, trichloromethane, trichlorotrifluoroethane and
trichlorofluoromethane. It goes without saying that mixtures of
these solvents are also suitable.
Suitable penetration-enhancing agents are, for example,
monovalent or multivalent alcohols, such as ethanol, 1,2-
propanediol or benzyl alcohol, saturated and unsaturated fatty
alcohols with 8 to 18 carbon atoms, such as lauryl alcohol or
cetyl alcohol, hydrocarbons, such as mineral oil, saturated and
unsaturated fatty acids with 8 to 18 carbon atoms, such as
stearic acid or oleic acid, fatty acid esters with up to 24
carbon atoms or dicarboxylic acid diesters with up to 24 carbon
atoms.
Fatty acid esters that are suitable for the agents according
to the invention are, for example, those of acetic acid, caproic
acid, lauric acid, myristic acid, stearic acid and palmitic acid,
such as, for example, methyl esters, ethyl esters, propyl esters,
isopropyl esters, butyl esters, sec-butyl esters, isobutyl esters
or tert-butyl esters of these acids. Especially preferred esters
are those of myristic acid, such as their methyl esters and
especially their isopropyl esters. Suitable dicarboxylic acid
21~2188
~ .
diesters are, for example, diisopropyl adipate, diisobutyl
adipate and diisopropyl sebacate.
Other penetration-enhancing agents are phosphatidyl
derivatives, such as lecithin, terpenes, amides, ketones, urea
and its derivatives or ethers, such as, for example, diethylene
glycol monoethyl ether. It also goes without saying that
mixtures of these penetration-enhancing agents are also suitable
for the production of the agent according to the invention.
The concentration at which the active ingredient or the
active ingredient mixture is optimally dissolved or suspended in
the solvent is usually 0.01 to 25% by weight for 14~,17-
ethanoestra-1,3,5(10)-triene-3,17B-diol. In the case of
gestagens, the concentration depends, of course, on the type of
active ingredient used and the individual dose desired; it must
be determined in individual cases by means of preliminary tests
that are familiar to one skilled in the art, such as, for
example, the determination of achievable blood-plasma
concentrations of the active ingredient, when agents that are
selected according to the invention are determined. In general,
active ingredient concentrations of 0.01 to 25% by weight of
estrogen on average according to the invention will also be
sufficient. The ratio by weight of 14~,17~-ethanoestra-
1,3,5(10)-triene-3,17B-diol to gestagen(s) is approximately 5:1
to 1:20 in the case of the combination preparations.
The therapeutically necessary transdermal daily dose for
14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol is a maximum of
250 ~g; thus an average percutaneous flow of 420 ng/cm2/hour is
2182188
,
necessary in the case of a TTS surface area of 25 cm2. In in
vitro studies with suitable formulations, it was shown that the
latter can be exceeded by about 10-fold with the agents according
to the invention.
Very uniform administration with a set dosage of the active
ingredient or active ingredient mixture can be achieved if the
active ingredient or the mixture is packed in a transdermal
therapeutic system (TTS). Suitable transdermal therapeutic
systems are those that are used conventionally for percutaneous
administration of active ingredients (Yie W. Chien: "Transder~al
Controlled Systemic Medications," Marcel Dekker, Inc., New York
and Basel, 1987, Dr. Richard Baker: "Analysis of Transdermal
Drug Delivery Patents 1934 to 1984" and "Analysis of Recent
Transdermal Delivery Patents, 1984-1986 and Enhancers" Membrane
Technology & Research 1030 Hamilton Court Menlo Park CA 94025
(415) 328-2228).
Thus, for example, a transdermal therapeutic system can be
used that consists of
a) an impermeable cover layer,
one to three matrix layer(s) that adhere to the cover layer
and that contain 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-
diol, optionally gestagen(s) and optionally penetration-
enhancing agents that are self-adhesive and permeable to
these components or are covered or surrounded by a skin
contact adhesive that optionally contains penetration-
enhancing agents, a removable protective layer, or
b) a cover provided with a contact adhesive that
6 2~ 8~1 a~
optionally contains penetration-enhancing agents,
one to three (in each case) matrix layer(s) that leave
uncovered a contact adhesive border and that are fastened by
means of a cover to the contact adhesive, and that contain
14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol, optionally
gestagen(s) and penetration-enhancing agents, and a
removable protective layer, or
c) an impermeable cover layer,
one to three pharmaceutical agent reservoir(s) that are
present on or in the cover layer and that contain 14~,17~-
ethanoestra-1,3,5(10)-triene-3,17B-diol, optionally
gestagen(s) and optionally penetration-enhancing agents,
one to three polymer layer(s) that are permeable to these
components, a permeable skin contact adhesive layer that
optionally contains penetration-enhancing agents, and a
removable protective layer.
A transdermal therapeutic system according to variant a)
represents a simple matrix system. It can be, for example,
round, oval or rectangular in shape and be produced as follows.
A solution or suspension of up to 25% by weight of active
ingredient or active ingredient mixture, 0-40% by weight of a
penetration-enhancing agent, 30-70% by weight of a medicinally
usual adhesive, supplemented with a suitable volatile solvent to
make 100% by weight, is painted on a flat, impermeable cover
layer. After drying, a second and optionally later even a third
layer that optionally contains an active ingredient, penetration-
enhancing agents and an adhesive can be applied to this layer and
2182188
dried. Then, the matrix system is provided with a removable
protective layer.
If a medicinally usual matrix former which, after the system
dries, does not adhere to the skin or does so inadequately is
used, the system can be covered or surrounded in addition with a
skin contact adhesive before the application of the removable
protective layer.
Suitable solvents and penetration-enhancing agents are, for
example, the already mentioned liquids of this type. As
medicinally usual adhesives, for example, polyacrylates,
silicones, polyurethanes, block polymers, styrene-butadiene
copolymers as well as natural or synthetic rubbers are suitable.
As additional matrix formers, cellulose ethers, polyvinyl
compounds or silicates are suitable. To increase adhesiveness,
the usual additives, such as, for example, adhesion-making resins
and oils, can be added to the obtained matrix.
As a protective layer, all films that are usually used in the
case of transdermal therapeutic systems are suitable. Such films
are, for example, siliconized or fluoropolymer-coated.
As a cover layer, for example, 10 to 100 ~m thick films made
of polyethylene or polyester can be used selectively pig~ented or
metallized in this system. The pharmaceutical agent layer that
is applied thereupon preferably has a thickness of 20 to 500 ~m.
The dispensing of active ingredients preferably is done over a
surface area of 5 to 100 cm2.
In the case of multilayer matrix systems, the 14~,17~-
ethanoestra-1,3,5(10)-triene-3,17B-diol and optionally the
2182188
penetration enhancers can be introduced into, for example, the
matrix that is applied to the impermeable cover layer, while the
layer or layers that are present below contains the estrogens and
optionally also penetration enhancers. In contrast, it is also
possible, however, to place several active ingredient-containing
matrices beside one another in such a transdermal system.
According to variant b, a transdermal therapeutic matrix
system can also be, for example, round, oval or rectangular and
can be produced as follows.
A cover is coated with a skin contact adhesive. Then, one to
three punched-out areas of a matrix layer that is provided with
an impermeable cover and that contains 14~,17~-ethanoestra-
1,3,5(10)-triene-3,17~-diol, optionally gestagen(s) and
penetration-enhancing agents, are attached to the latter via TTS
so that the cover has a sufficient edge to attach to the skin
and, in the case of multiple areas, also sufficient interspaces,
and provides it with a removable protective layer. The materials
used in this matrix system can be the same as those of variant a.
A transdermal, therapeutic reservoir system according to
variant c can also be, for example, round, oval or rectangular
and can be represented as follows;
An impermeable film is worked by heat and/or suction in such
a way that one to three blisters holding 0.1 to 3 ml result. The
latter are filled with an active ingredient-containing solution
or suspension that contains 1-50% by weight of active ingredient
or active ingredient mixture with a penetration-enhancing agent.
2l8218~
The active ingredient-containing solution or suspension can also
be thickened with up to 10% by weight of matrix former.
As a cover for the reservoir to the skin, a welded or glued
permeable polymer layer, to which a permeable skin contact
adhesive layer and a removable protective layer are attached, is
used.
In this system, the above-mentioned penetration-enhancing
agents can be used. As a permeable polymer layer, for example, a
20 to 200 ~m thick film made of cellulose esters, cellulose
ethers, silicones or polyolefin compounds is used. By altering
this polymer layer, the rate of diffusion of the active
ingredient or active ingredient mixture can be varied within wide
limits.
For an adhesive and protective layer, the same materials that
are described in the transdermal therapeutic system according to
variant a are suitable.
In the case of the production of transdermal therapeutic
systems with two or three active ingredient-containing matrix
layers or pharmaceutical agent reservoirs that are arranged
beside one another, it is often suitable to introduce 14~,17~-
ethanoestra-1,3,5(10)-triene-3,17B-diol into one and gestagen or
gestagens into the other. In such cases, the active ingredient-
containing matrix systems or pharmaceutical agent reservoirs can
contain not only differing active ingredients, but also differing
penetration-enhancing agents.
In the case of the matrix systems according to variants a or
b, care must be taken to leave sufficient distance between the
21 821 88
areas to prevent diffusion of active ingredients into the
respective other areas. In the case of the reservoir systems
according to variant c, it is possible to provide the individual
reservoirs with differing permeable polymer layers to adapt the
diffusion flow of the individual active ingredients to the
respective needs.
Other features of the transdermal systems according to the
invention can be explained based on the attached drawings, which
are not true-to-scale.
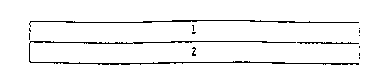
Fig. 1 shows a cross section through a simple round matrix
system according to variant a without the removable protective
layer. It consists of impermeable cover layer 1 and
pharmaceutical agent-containing matrix layer 2.
Fig. 2 shows a cross section through a matrix system
according to variant b without the removable protective layer.
Fig. 3 shows the top view of this system. The system consists of
cover 3, which is provided with a contact adhesive layer 4. Two
pharmaceutical agent-containing matrix layers 6 and 8 are
fastened to this contact adhesive layer by means of impermeable
covers 5 and 7.
Fig. 4 shows a cross section through a round, one-chamber
reservoir system according to variant c, without the removable
protective layer. It consists of impermeable cover layer 9,
pharmaceutical agent reservoir 10, permeable polymer layer 11 and
skin contact adhesive 12.
Fig. 5 shows a cross section through a round, two-chamber
reservoir system according to variant c without the removable
11 21 821 88
protective layer. It consists of impermeable cover layer 13, two
semi-circular pharmaceutical agent reservoirs 14 and 15,
permeable polymer layer 16 and skin contact adhesive layer 17.
In addition to transdermal therapeutic systems, also other
galenical preparations are suitable for transdermal
administration of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-
diol.
An emulsion gel for transdermal administration consists of,
for example, the active ingredient or active ingredient mixture,
penetration-enhancing agents, emulsifiers (whereby ambiphilic
representatives of penetration-enhancing agents can be used as
emulsifiers) and optionally matrix formers. A typical
formulation consists of 0.1-25% by weight of active ingredient or
active ingredient mixture, 0-10% by weight of emulsifier, 0-5% by
weight of matrix former, 0 to 50% by weight of penetration-
enhancing agents and water to make 100% by weight. The agent is
emulsified in the conventional way and mixed, if necessary, with
the conventional antioxidants, preservatives, etc.
One-phase gels are obtained, for example, by detaching or
suspending the active ingredient or the active ingredient mixture
in solvents such as water, lower alcohols or their mixtures,
optionally with the addition of penetration-enhancing agents and
thickening with matrix formers.
Typical formulations for such gels contain 0.01-25~ by weight
of active ingredient or active ingredient mixture, 1-20% by
weight of matrix former, 0 to 40% by weight of penetration-
12 ~l 821 ~
enhancing agents supplemented with the solvent to make 100% byweight.
Also, these gels can optionally contain antioxidants,
preservatives, etc.
A typical spray formulation is, for example, the following:
1-25% by weight of active ingredient or active ingredient
mixture, 0-20% by weight of matrix former, 0-60% by weight of
penetration-enhancing agents supplemented with solvents and
optionally propellants to make 100%. If pressurized-gas packings
are used, the propellant can be omitted.
The agents that contain 14~,17~-ethanoestra-1,3,5(10)-triene-
3,17B-diol for transdermal administration according to the
invention can be used for treating the same diseases as the
previously known agents, for example, ones to be administered
orally that contain highly effective estrogens. Moreover, the
optionally gestagen-containing preparations according to the
invention can also be used for contraception. The agents
according to the invention have special advantages in treating
diseases that require long-term treatment with relatively high
dosages of active ingredients. Here, the frequency of
administration can be significantly reduced and a significantly
more uniform blood plasma level can be achieved. It is also
advantageous that no gastrointestinal side-effects are to be
expected and the first liver passage is avoided.
These advantages make the gestagen-free monotherapeutic
agents of this invention appear especially suitable. For
example, for hormone replacement therapy in the case of
13
2182188
menopausal symptoms, atrophic vaginitis, craurosis vulvae,
treatment of estrogen-dependent tumors for osteoporosis
prophylaxis or treatment, or for treating other diseases, in
whose therapy an estrogen substitution is indicated.
The transdermal use of gestagens in sequential or continuous
combination with 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol
offers special advantages, for example, for treating menopausal
symptoms, for the prevention of osteoporosis, for cycle
regulation and for cycle stabilization, for transdermal
contraception and for treating other diseases, in which a
combined estrogen/gestagen substitution is indicated.
The embodiments below are used for a more detailed
explanation of the invention. The following commercial products
are used in them:
- Polyester film of 0.074 mm thickness (Skotchpak~R) 1009 of the
3M manufacturer; polypropylene film (celgard(Q) 2500) of the
Celanese manufacturer, Linerfolie Skotchpak [liner-foil
Scotchpak] (R~ 1022 and 1360 of the 3M manufacturer; Transferkleber
[transfer adhesive] 9871 of the 3M manufacturer, polyacrylate
adhesive of Sichello ~R) J 6610-21 type of the Henkel KG
manufacturer, polyacrylate adhesive of the Gelva(Q) 788 type of
the Monsanto manufacturer, silicone adhesive of X-7-2960 type of
the Dow Corning manufacturer and hydroxypropyl cellulose of the
Klucel(Q) HXF type of the Hercules manufacturer.
14
- 21 821 88
Example 1
In 62.4 g of a 50% solution of silicone adhesive in gasoline,
0.8 g of 14~,17a-ethanoestra-1,3,5(10)-triene-3,17~-diol
8.0 g of 1,2-propanediol
are introduced in succession while being stirred. After the
batch is degassed, the mixture is introduced onto polyester film
by means of a coating device, so that after the volatile solvent
is removed, a uniform film of 40 g/m2 of solid deposit results.
Then, it is laminated with a fluoropolymer-coated polyester
liner. The laminate thus obtained is divided into round
individual patches with a surface area of 10 cm2 by means of a
punching device and packaged in aluminum foil. Fig. 1 shows a
cross section through this patch without a polyester liner.
After the liner-foil is removed, the patch adheres to the skin.
The determination of content yields a unifor~ active
ingredient distribution of 0.08 mg/cm2 on average.
Example 2
In 170 g of a 50~ solution of polyacrylester adhesive in
acetone/gasoline,
5.0 g of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol
and
10.0 g of isopropyl myristate
are dissolved in succession while being stirred. After the batch
is degassed, the solution is introduced onto polyester film by
means of a coating device, so that after the volatile solvent is
removed, a uniform film of 100 g/mZ of solid deposit results.
;~182188
.
Then, it is laminated with a siliconized active ingredient-free
liner-foil. The laminate thus obtained is divided into
individual patches with a surface area of 10 cm2 by means of a
punching device and packaged in aluminum foil. After the liner-
foil is removed, the patch adheres to the skin.
The content of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-
diol is 0.5 mg/cm2 on average.
Example 3
In 112 g of a 50~ solution of polyacrylester adhesive in
acetone/gasoline,
3.5 g of 3-keto-desogestrel
3.5 g of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol and
7.0 g of 1,2-propanediol with 10% 1-dodecanol
are dissolved or suspended in succession while being stirred.
After the batch is degassed, the mixture is introduced onto
polyester film by means of a coating device, so that after the
volatile solvent is removed, a uniform film of 70 g/m2 of solid
deposit results. Then, it is laminated with a siliconized active
ingredient-free liner-foil. The laminate thus obtained is
divided into individual patches wi~h a surface area of 10 cm2 by
means of a punching device and packaged in aluminum foil. After
the liner-foil is removed, the patch adheres to the skin.
In a like manner, the content of 3-keto-desogestrel and
14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol is 0.35 mg/cm2
each.
. 16 21 821 ~8
Example 4
Analogously to Example 1, two different segment-like matrix
systems are produced that have the design depicted in Fig. 2 and
3. Matrix system I consists of matrix 8, provided with a
polyester film 7, of the following composition
1.0 mg of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol
5.0 mg of isopropyl myristate and
44 mg of acrylate adhesive
and has a surface area of 5 cm2.
Matrix system II consists of matrix layer 6, provided with a
polyester film S, of the following composition
2.0 mg of gestodene
lO.O mg of isopropyl myristate and
88 mg of acrylate adhesive
and has a surface area of lO cm2.
Both matrix systems are stuck onto a cover film that is
coated with a skin contact adhesive, as Fig. 3 shows. After
lamination and punching out, patches of the type shown in Fig. 2
and 3 result.
Example 5
A polyester film of a 7.4 cm diameter is worked by suction
and heat, so that a round blister with a surface area of lO cm2
results. The latter is filled with l ml of a suspension of
2.5 mg of norethisterone acetate and
2.5 mg of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol
- 2182188
in 1,2-propanediol, which contains 10% lauric acid. A
polypropylene or cellulose acetate butyrate film is welded on the
edge. Depending on the pressure per unit of time, the sealing
temperature is between 70C and 100C. Skin-adhesive film is
transferred to the permeable polymer layer. The patch is
provided with a liner and packaged in aluminum foil.
Fig. 4 shows a cross section through a patch of this type
without a liner.
Example 6
Analogously to Example 5, a polyester film is worked so that
two semicircular blisters with a surface area of 7.5 cm2 each
that are separated from one another by a ridge result.
Reservoir I is filled with 0.75 ml of a suspension of
1.5 mg of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol
in 1,2-propanediol
and reservoir II is filled with 0.75 ml of such a one of
3.0 mg of levonorgestrel
in 1,2-propanediol.
The further completion of the patch is carried out as described
in Example 5. Fig. 5 shows a cross section through such a patch
without a liner.
Example 7
In 76.78 g of ethanol (96~ by vol.) or isopropanol,
0.2 g of 14~,17~-ethanoestra-1,3,5(10)-triene-3,17B-diol
0.02 g of gestodene
218218~
10.0 g of 1,2-propanediol and
10.0 g of isopropyl myristate
are dissolved in succession. Then, 3 g of hydroxypropyl
cellulose is added to the solution, and air is removed from it.
After 2 hours of steeping time, the gel is filled in aluminum
tubes with three-fold inner protective varnishing.
The determination of content yields a homogeneous active
ingredient distribution in gel at values of 95% at 105% of the
setpoint value.
Example 8
20.00 g of 14~,17~-ethanoestra-1,3,5tlO)-triene-3,17B-diol is
dissolved in 1000 g of isopropyl myristate, sterilized by
filtration and decanted in 5 ml medicine bottles under aseptic
conditions.