Note: Descriptions are shown in the official language in which they were submitted.
r ~
~21 82699
1.3.2-1'~. ~~~ "'- ' l^Q~ide derivatives
The invention relates to the microbicidal use of 1,3,2-l:Pn7~ thi~7r~ I-oxides, to novel
1,3,2-i,...,...~ l-oxides and to processes for their preparation.
It is already known that certain 1,3,2~ ,..lr l-oxides have an ~IL;llly~,ul;C
5 action in l.l. -- .. , ~.. ll;. ~1 .. ,.. l.. ~ :~;.. ,.~ (see US-A 5 140 018).
F~~ llulc, structurally similar N-alkyl-benz[1,2];~..l1.;~.~1;l~-3-ones and their use in
the protection of materials are known (see e.g. DE-A 4 027 378, GB I 531 431,
EP 1~ 100). The activity and breadth of action of these ~'''''I''J'''''I` however, is not
always completely ~ .r.~ uly, especially at low ~ c Surprisingly, the
10 1,3,2-bPn~riithi~-lP l-oxides according to the invention have not only a greater
breadth of action but also a markedly higher activity.
It has been found that the 1,3,2-1....,l.li;ll.;~,..lr l-oxides - some of which are known
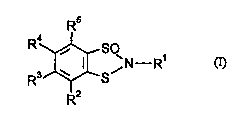
- of the general formula (I)
R4~ / N--R1 (I)
R3~--S
R2
15 in which
R' represents hydrogen, optionally substituted alkyl, alkoxy, alkenyl, alkinyl,
cycloalkyl, aryl, heteroaryl or aralkyl, or heteroarylalkyl, and
LeA3Q 141 -PCT
~A2 ~ 826gg
R2 R3 R4 and R5 each ;,.~ ly of one another represent hydrogen, halogen, alkyl,
h~ g~-n~l~lkyl, alkoxy, h~lo~n~ lkr~xy, alkylthio, hs~ ylLl~io,
dialkylamino, nitro or cyano
are suitable as ~ Ul,;uC;u.,~ for protecting industrial materials.
S A general definition of the 1,3,2-~...,.l;ll.;,.,~.l~. I-oxides which can be used in
accordance with the inYention is given by the formula (I). Preference is given to
,..."l,,...,..l~ of the formula (I) in which
R~ represents hydrogen, straight-chain and branched alkyl having I to 18 carbon
atoms, straight-chain or branched alkenyl having 2 to 18 carbon atoms or
lû straight-chain or branched alkinyl having 2 to 18 carbon atoms which is
optionally substituted one or more times by identical or different ,..1.~
consisting of halogen, alkoxy having I to 6 carbon atoms, hAlog " .~xy
having I to 6 carbon atoms and I to 9 identical or different halogen atoms,
alkylthio having I to 6 carbon atoms, l ' ~ " yllll;o having 1 to 6 carbon
atoms amd I to 9 identical or different halogen atoms, acyl having I to 6 carbonatoms, acyloxy having I to 6 carbon atoms, (alkoxy)-carbonyl having I to 6
carbon atoms, amino which is optionally substituted by identical or different
,.11.,l;l.. ~ consisting of alky~ or aryl, optionally substituted phenoxy or
pyridyloxy, nitro or cyano,
and represents cycloalkyl consisting of I to 4 ;~t~ uul~,~,t~d ring systems
having 3 to 18 carbon atoms which is optionally substituted one or more times
by identical or different ,"l,~l;ll,- ,~ consisting of halogen, alkyl having I to 4
carbon atoms, h/llogen~ lkyl having I to 4 carbon atoms and I to S identical or
different halogen atoms, alkoxy having I to 4 carbon atoms, I~ y
having I to 4 carbon atoms and I to 5 identical or different halogen atoms,
alkylthio having I to 4 carbon atoms, I~ enfls31kylthio having I to 4 carbon
atoms and I to S identical or different halogen atoms, nitro or cyano,
and represents aryl which is optionally substituted from one to five times by
halogen, alkyl having I to 10 carbon atoms, h~lf.g~nl-~lkyl having I to 8 carbonatoms and I to 8 identical or different halogen atoms, alkoxy having I to lû
Le A 3û 141 - 2 -
' ~ C~ 82~f~
carbon atoms, hqln~rnnqlkn~y having I to 8 carbon atoms and I to 8 identical
or different halogen atoms, alkylthio haYing I to 10 carbon atoms,
" y' ' - having I to 8 carbon atoms and I to 8 identical or different
halogen atoms, amino, Illullodlhyl~llillo having straight-chain or branched alkyl
radicals having I to 6 carbon atoms, dialkylamino having identical or differentjstraight-chain or branched alkyl radicals each having I to 6 carbon atoms,
cycloalkyl having I to 6 carbon atoms, methylenedioxy,
~linuululll~ l;u~y,cl.lvlunuululll~lllyl~ liv~y,d;~ lvlu~ ,v;u~y,
nitro or cyano,
and represents aralkyl in which the alkyl radical is straight or branched and
consists of I to 8 cOEbon atoms and aryl preferably represents phenyl which is
optionally substituted from one to four times by identical or different
t~ consistmg of halogen, alkyl having I to 6 carbon atoms,
hqlngt nnqlkyl having I to 6 carbon atoms and I to 6 identical or different
halogen atoms, alkoxy having I to 6 carbon atoms, 1 ~ lkn~ry having I to
6 carbon atoms and I to 6 identical or different halogen atoms, alkylthio havingI to 6 carbon atoms, l~ln~ 1 ylLIl;O having I to 6 carbon atoms and I to 6
identical or different halogen atoms, amino, monoalkylamino having straight-
chain and branched alkyl radicals having I to 6 carbon atoms, dialkylamino
having identical or different, straight-chain or branched alkyl radicals each
having I to 6 carbon atoms, cycloalkyl having I to 6 carbon atoms,
Ill~;L~ dioxy~dinuulul.l~ lylenedioxy~lllulunuululll~Lllyl~ diu~y~dichlor
methylenedioxy, nitro or cyano,
RZ, R3, R4 amd R5 each ;~ f ~lly of one another represent hydrogen, halogen, alkyl
having I to 6 carbon atoms, I I-,,f " yl having I to 6 carbon atoms and I to
6 identical or different halogen atoms, alkoxy having I to 6 carbon atoms,
halogenoalkoxy having I to 6 carbon atoms and I to 6 identical or different
halogen atoms, alkylthio having I to 6 carbon atoms, h,qln~nnqlkylthio having
I to 6 carbon atoms and I to 6 identical or different halogen atoms,
dialkylamino having identical or different, straight-chain or branched alkyl
radicals each having I to 6 carbon atoms, nitro or cyano.
Le A 3Q 1~1 - 3 -
C~21 826~
Particular preference is given to compounds of the formula (I) in which
R' represents hydrogen, straight-chain or branched alkyl having I to 14 carbonatoms, straight-chain or branched alkenyl having 2 to 14 carbon atoms or
straight-chain or branched alkinyl having 2 to 14 carbon atoms which is
optionally substituted from one to four times by identical or different
,"1,~1;l.,. .,1~ consisting of fluorine, chlorine, alkoxy having I to S carbon atoms,
~v --Iknxy having I to 5 carbon atoms and I to 5 fluorine and/or chlorine
atoms, alkylthio having I to 5 carbon atoms, h~ln~rnn~lkylthio having I to S
carbon atoms and I to 5 fluorine and/or chlorine atoms, acyl having I to 5
carbon atoms, acyloxy having I to 5 carbon atoms, alku,~y.,~bul.yl having I to
5 carbon atoms, amino which is optionally substituted by identical or different
~,.I,~lil.,~.,l~ consisting of alkyl haYing I to 4 carbon atoms and/or phenyl,
phenoxy or pyridyloxy, nitro or cyano,
and represents cycloalkyl consisting of I to 3 ring systems having 3 to 14
I S carbon atoms which is optionally substituted from one to four times by identical
or different ~ consisting of nuvl;u~/~,lllu~ , alkyl having I to 4
carbon atoms, alkoxy having I to 4 carbon atoms, alkylthio having I to 4
carbon atoms, nitro or cyano,
and represents aryl which is optionally substituted from one to four times by
fluorine, chlorine, alkyl having I to 8 carbon atoms, h~l~,, " yl having I to
6 carbon atoms and I to 6 fluorine and/or chlorine atoms, alkoxy having I to
8 carbon atoms, h~lngenn~lknYy having I to 6 carbon atoms and I to 6 fluorine
and/or chlorine atoms, alkylthio having I to 8 carbon atoms, ~ ylLII;o
having I to 6 carbon atoms amd I to 6 fluorine and/or chlorine atoms, amino,
monoalkylamino having alkyl radicals of I to 4 carbon atoms, di~lkyL Il;llo
having identical or different alkyl radicals each having I to 4 carbon atoms,
cycloalkyl having I to 6 carbon atoms, methylenedioxy,
dinuulu~ Lll~lenedioxy, chlorolluulul.l~Lllylenedioxy, dichloromethylenedioxy,
nitro or cyano,
and represents aralkyl in which the alkyl radical is straight or branched and
consists of I to 8 carbon atoms and aryl represents phenyl which is optionally
substituted from one to four times by fluorine, chlorine, alkyl having I to 4
Le A ~Q 141 . - 4 -
~A21 82~9~
. ~ carbon atoms, I ~'~enn~lkyl having I to 4 carbon atoms and I to 4 fluorine
and/or chlorine atoms, alkoxy having I to 4 carbon atoms, h~ enf~lkr~xy
haYing I to 4 carbon atoms and 1 to 4 fluorine and/or chlorine atoms, alkylthio
having I to 4 carbon atoms, I ' ~, Ikylthio having I to 4 carbon atoms and
I to 4 fluorine and/or chlorine atoms, amino, IllullOàlkyLull;llu having alkyl
radicals of I to 4 carbon atoms, d;dl~yla~ o having identical or different alkylradicals each having I to 4 carbon atoms, cycloalkyl having I to 6 carbon
atoms, m~ . y, din~ v~ llyL,.I~,l;u~y, chlulullllululll.,.llyl~,~cd;u~ y,
dichloromethylenedioxy, nitro or cyano,
lû R2, R3, R4 and R5 each ;".1~ ly of one another represent hydrogen, chlorine,
fluorine, alkyl having I to 4 carbon atoms, 1;~ yl having I to 4 carbon
atoms and I to 6 fluorine and/or chlorine atoms, alkoxy having I to 4 carbon
atoms, h~ ~n~ k~xy having I to 4 carbon atoms and I to 6 fluorine and/or
chlorine atoms, alkylthio having I to 4 carbon atoms, 'l~tl~ lkylthio having
I to 4 carbon atoms and I to 6 fluorine and/or chlorine atoms, d~alkylalllillo
having identical or different, straight-chain or branched alkyl radicals each
having I to 6 carbon atoms, nitro or cyano
The 1,3,2-~...,,..,1;11,:- ..1. 1-oxides of the formula (1) which can be used in accordance
with the invention are in some caser, known (cf US-A 5 14û û18) and cam be prepared
2û by analogous processes.
Novel 1,3,2-L,. .,,,.,1;11.:-,.,!.- I-oxides are those of the formula (la)
R4~,~S` N--R1 (la)
R2
in which
LeA3û 141 ~~ ~
, _ ,
~A21 8~6qq
R' represents hydrogen, straight-chain or branched alkyl having 13 to 18 carbon
atoms, straight-chain or branched alkenyl or alkinyl having 17 to 18 carbon
atoms and represents straight-chain or branched alkyl having I to 18 carbon
atoms which is substituted one or more times by identical or different
S ~ consisting of halogen, alkoxy having I to 6 carbon atoms,
h~ genn~lk~xy having I to 6 carbon atoms and I to 9 identical or different
halogen atoms, alkylthio having I to 6 carbon atoms, h ~ ,,r - " yl~ll;o having
I to 6 carbon atoms and I to 9 identical or different halogen atoms, acyl havingI to 6 carbon atoms, amino which is optionally substituted by identical or
different b~ 1;` consisting of alkyl o} aryl, optionally substituted phenoxy
or pyridyloxy, nitro or cyano,
and represents straight-chairl or brarlched alkenyl or alkinyl of I to 18 carbonatoms which is substituted one or more times by halogen, alkoxy having I to 6
carbon atoms, ? ' ,, ~I-r~xy having I to 6 carbon atoms and I to 9 identical
or different halogen atoms, alkylthio having I to 6 carbon atoms,
h~ nn~lkylthio having I to 6 carbon atoms and I to 9 identical or different
halogen atoms, acyl having I to 6 carbon atoms, acyloxy having I to 6 carbon
atoms, (alkoxy)-carbonyl having I to 6 carbon atoms, amino which is optionally
substituted by identical or different ~..h~l;l.,..,~ consisting of alkyl or aryl,
optionally substituted phenoxy or pyridyloxy, nitro or cyano,
and represents ;cycloalkyl ~onsisting of I to 4 ;llt l~,o~ d rirlg systems
having 3 to 18 carbon atoms ~vhich is substituted one or more times by identical
ordifferent~l.l,~l;l.,~ .l~consistingofhalogen,alkylhavinglto4carbonatoms,
g~ yl having I to 4 carbon atoms and I to 5 identical or different
halogen atoms, alkoxy having I to 4 carbon atoms, h~ enn~lkl~xy having I to
4 carbon atoms and I to S identical or different halogen atoms, alkylthio havingI to 4 carbon atoms, h~log~l-nrls31kylthio having I to 4 carbon atoms and I to 5identical or different halogen atoms, nitro or cyano,
and represents aryl which is substituted from one to five times by alkyl having
5 to 10 carbon atoms, halogenoalkyl having 5 to 8 carbon atoms and I to 8
identical or different halogen atoms, alkoxy having S to 10 carbon atoms,
h~lngPno~lkoxy having I to 8 carbon atoms and I to 8 iderlùcal or different
halogen atoms, alkylthio having I to 10 carbon atoms, h- In~n~-~lkylthio having
LeA30 141 ~ -6-
C~2 1 ~P,2699
I to 8 carbon atoms and I to 8 identical or different halogen atoms, amino,
...u..ualkyLu~ o and straight-chain and branched alkyl radicals having I to 6
carbon atoms, cycloalkyl having I to 6 carbon atoms, ~ llylull~,d;vAy,
dinuv.v..l~Lllylenedioxy~ " Unuulu~ yl~ diù~y~ diulllulu~ LIIyl~ '' y,
S nitro or cyano,
and represents aralkyl in which the alkyl radical is straight or branched and
consists of 5 to 8 carbon atoms and aryl represents phenyl which is substituted
from one to four times by idcntical or different ~ consisting of
~G 'kYy having I to 6 carbon atoms and I to 6 identical or different
halogen atoms, alkylthio having I to 6 carbon atoms, hs~ lkyl~ o having
I to 6 carbon atoms and I to 6 identical or different halogen atoms, amino,
..u..oalkylc.~....;..u having straight-chain amd branched alkyl radicals having I to
6 carbon atoms, cycloalkyl having I to 6 carbon atoms, ~ .,I;u~y,
~I;nuululll~ iu~y~ Iulunuululll~Llljl~ l;u~y~ d;~l~lu~u~ l;u~y~
nitro or cyano,
R2, R3, R~ and R5 each ;"~ ly of one another represent hydrogen, halogen, alkyl
having I to 6 carbon atoms, hs~ Pnns31kyl having I to 6 carbon atoms and I to
6 identical or different halogen atoms, alkoxy having I to 6 carbon atoms,
hs~ Pn~ lkn~y having I to 6 carbon atoms and I to 6 identical or different
halogen atoms, alkylthio having I to 6 carbon atoms, h ~ lkyllll;o havirlg
I to 6 carbon atoms and I to 6 identical or different halogen atoms,
I' " yl~--u-o having identical or different, straight-chain or branched alkyl
radicals each having I to 6 carbon atoms, nitro or cyano.
The known and novel 1,3,2-bPn7. riithi l7olp I-oxides of the general formula (I)
in which
R', R', R3, R4, R5 have the meaning given above
are obtained if UOIll,UUUUldS of the general formula (Il)
Le A 30 141 - 7 -
~1 82699
Rs
R4~ ~ N--R' (Il)
R3~--S
R2
in which
R', R2, R3, R4 and R5 have the meaning given above
are reacted by analogous processes (see US-A 5 140 018) with oxidizing agents,
5 optionally in the presence of diluents.
The c~nnrol~n-lc with the general formula (Il) likewise show biological effects and are
suitable as microbicides for protecting industrial materials.
All customary oxidizing agents can be used for the oxidation; mention may be made
here by way of example of organic peroxides, for example m-~l~lu~uu~"b~l~uic acid
10 (mCPBA) or mul-u,u~.u~y~ l.Ll.dlic acid m~l~nr~ m salt ll~ all,ydla~ (MMPP) or organic
oxidizing agents, for example pyridinium CI.~ U~ (PCC), pyridine-SO3,
pyridinium dichromate (PDC) or t-butyl hypochloride, but also mild inorganic oxidizing
agents such as H~O2, sodium iodate, sodium perborate or oxone manganese dioxide.
The reaction Lt:lll,u.,la~ can be varied within a relatively large t~ a~ ; range.
The reaction is in general carried out at between -30C and +100C, preferably between
-10C and +60C. The reactions are preferably carried out in the presence of diluents.
Suitable diluents are water and organic solvents which are also attacked by the
oxidizing agents. These include, preferably, II,ydlU~ lVII~> such as toluene, xylene or
hexane, chlorinated llyd~uc~lJu~s such as chlorobenzene, chloroform, methylene
20 chloride, ethers such as methyl tert-butyl ether, or nitriles, such as a~ ~-tnnitrjl~
The known and novel ~h~ll7o~1ithi~701e5 of the general formula (Il)
LeA3û 141 ~ ~ ~
~1 826q~
in which
R', R2, R3, R4 and R5 have the meaning given above
are obtained if sulphenyl chlorides of the general formula (111)
R4`~SCI (111)
R2
5 in which
R2, R3, R4 and R5 have the mcaning given above
are reacted by analogous proccsses (see US-PA 5 140 180; C.H. Chen, J. Il~ u~,~.,l;c.
Chem., 16, 183 (1979)) with amines of the general formula (IV)
H2N-R' (IV)
10 in which
R' has the meaning given above,
if desired in the presence of æid-binding agents and if desired in the presence of
diluents.
In this process the reaction ~ Lulc:, can be varied within a relatively large
t~ Lulc range. The process is in general carried out at between -30C and +100C,
preferably between -10C and +50C. The reactions are optionally carried out in the
presence of acid-binding agents, in which case all customary acid-binding agents can
be used. These include, preferably, tertiary a~nines such as Llh,;ll~ lc and pyridine;
Le A 30 141 ~ 9 ~
~A21 ~269q
. alkali metal hydroxides, such as sodium hydroxide and potassium hydroxide, and alkali
metal carbonates and alkali metal halogen carbonates, such as potassium carbonate and
sodium hydrogen carbonate.
Suitable diluents, which are optionally used, are all inert organic solvents. These
S include, preferably, IIYVIVU~IJOIIS such as toluene, xylene or hexane; chlorinated
ll~dlucallJvll~ such as chlorobenzene amd ul~lvlurullll, ketones such as acetone; ethers
such as tetrahydrofuran, diethyl ether, methyl tert-butyl ether and dioxane; nitriles such
as a(`Pf~nitfilP
The known and novel sulphenyl chlorides of the general formula (III)
10 in which
R2, R3, R~ and R5 have the meaning given above
are obtained
if dithiols of the formula (V)
Rs
R4J~,SH
R3J$~SH (v)~
R2
l5 are reacted with chlorine gas in the presence of diluents.
Diluents employed are preferably chlorinated l~ydlu~ vll~, for exarnple carbon
tetrachloride, chloroforrn, methylene chloride or 1,2-dichlulu~ill~l~. rhe ~ UI~:~
can be varied within a large range; the process is in general carried out at between
-30C and +40C, preferably at ~ ,U~ UI~:~ below 20C.
I,e A 30 141 . - 10 -
~,A21 82699
In an alternative procedure, however, the sulphenyl chlorides can under identical
conditions also be obtained by ~ ";",,:;"~ the disulfide compounds with the general
formulae (VI) and (Vll) and their positional isomers (see e.g. E. Kuhle, The Chemistry
of the sulfenic acids, Georg Thieme Verlag, Stuttgart 1973).
R ~S--S ~R'~ R'~,,5--5 ~R
~1) ~11)
Another alternative for the preparation of the sulphenyl chlorides of the formula (111)
consists in the rl~ "~ of CU~ JUU~ of the general formula (Vlll)
R2
R3~S O--alky~ (Vlll)
R5
which can be prepared in accordance with literature ;..~L. u.,Liu..:, (J. Nakayama,
Synthesis 38 (1975)).
The thiols of the forrnula (V) and the disulphide ~ of the formulae (Vl) and
(VII) are generally known and can be prepared in accordance with a variety of
literature instructions (see e.g. S. Hunig et. al., Liebigs Ann. Chem., 738, 192 (1979);
D.M. Giolando, Synthesis, 451 (1992);1. Degani et. al., Synthesis, 471 (1976); J.C.
Marten et. al., J. Am. Chem. Soc., ~L 658 (1989)).
The amines of the general forrnula (IV) are generally known and can in the majority of
cases be obtained commercially.
Le A 3~141 - 11 -
~.~2 1 8~6~
The active romrolm~c of the formulae (1), (la) and (Il) have a strong microbicidal
action and can be used in practice to combat unwanted Illil~l"'.l~,~..'l`'.'` The active
comrolln.1~ of t~he formulae (1), (Ia) and (Il) are suitable for protecting industrial
materials against infestation and dèstruction by unwanted llliUl~
S Industrial materials in the present context are to be umderstood as meaning non-living
materials which have been prepared for use in industry. Examples of industrial
materials which are to be protected from microbial alteration or destruction by active
~-,...l.."....l~ according to the invention cam be adhesives, sizes, paper and board,
textiles, leather, wood, coating ~ and plastics articles, cooling lubricants and10 other materials which can be infested with or destroyed by ~ s~ Within the
scope of the materials to be protected mention may also be made of parts of production
plants, for example ~,uol~ .. circuits, which may be adversely affected by the
..... llil.l;.~il-n of ll.;~,lUul~.. ,.,:.lllS. Industrial materials which may preferably be
mentioned within the scope of the present invention are adhesives, sizes, papers and
15 boards, leather, wood, coating ..~ , cooling lubricants and heat-transfer
liquids.
Examples of ..;~,.uc-~ capable of bringing about ~lP~r~ ti~m or alteration of the
industrial materials are bacteria, fungi, yeasts, algae and slime organisms. The active
r compound and/or ~ according to the invention preferably act against fungi,
20 in particular moulds, and against slime organisms and algae.
~Ih,~u-~ of the following genera may be mentioned by way of example:
Alternaria, such as Alternaria tenuis,
Aspergillus, such as Aspergillus niger,
('h~Pf~millnn such as ('~P-omil~m globosum,
25 Coniophora, such as Coniophora puetana,
Lentinus, such as Lentinus tigrinus,
Penicillium, such as Penicillium glaucum,
Polyporus, such as Polyporus versicolor,
AU-rU~ such as AU.ro~ pullulans,
30 Sclerophoma, such as S~,l.,lupllu~d pityophila,
Le A 30 141 - 12 -
~2 1 8~
Trichoderma, such as Tn~h~ rrn~ viride,
Fel~hrn~h; l, such as Escherichia coli,
r~r,..l.""~ c, such as P,- ~ ".,..~ aeruginosa,
Staphylococcus, such as Staphylococcus aureus.
S Depending on their particular physical and/or chemical properties, the active
UUIII~)U_~Id:~ of the formulae (1), (Ia) and (Il) can be converted to the customary
r~.,."..l~l,.",~, such as solutions, emulsions, ~ C;~ powders, foams, pastes,
granules, aerosols and very fine capsules in polymeric substances.
These fi rm~ til~nc and ~ are produced in a known manner, for example by
10 mixing the active c..~.,.l-u~ with extenders, that is, liquid solvents, liquefied gases
under pressure, and/or solid carriers, optionally with the use of surface-active agents,
that is, ~ ul~iryill~ agents and/or dispersing agents, and/or foam-forming agents. In the
case of the use of water as am extender, organic solvents can, for example, also be used
as auxiliary solvents. As liquid solvents, there are suitable in the main: aromatics, such
I 'i as xylene, toluene or alk~' , ' ' ' chlorinated aromatics or chlorinated aliphatic
h~ :llvcall,ul~s, such as chlulul,~ c~ IU~;LI~ or methylene chloride, aliphaticllydlu~allJul~s, such as ~ A~UIC or paraffins, for example mineral oil fractions,
alcohols, such as butanol or glycol as well as their ethers and esters, ketones, such as
acetone, methyl ethyl ketone,~ methyl iso~utyl ketone or ~y~ S, strongly polar
20 solvents, such as di~ ,Ll~ylru.~ or dimethyl 5~lrh~ P as well as water; by
liquefied gaseous exterlders or carriers are meant liquids which are gaseous at ambient
t lll,u~ ul~ and under ~ .l .. . ;c pressure, for example aerosol propellants, such as
ll~lo~ .L~J L~/d.uc~ul,ull~ as well as butane, propane, nitrogen and carbon dioxide; as
solid carriers there are suitable: for example ground natural minerals, such as kaolins,
25 clays, talc, chalk, quartz, attapulgite, montm/~rill~nif~ or ~ earth, and
ground synthetic minerals, such as highly disperse silica, alumina and silicates; as solid
carriers for granules there are suitable: for example crushed and rl ' natural
rocks such as calcite, marble, pumice, sepiolite and dolomite, as well as synthetic
granules of inorganic arld organic meals, and granules of organic material such as
30 sawdust, coconut shells, maize cobs and tobacco stalks; as ~IlluLryillg and/or foam-
forming agents there are suitable: for example non-ionic and anionic rm~ ifirrc such
Le A 30 141 - 13 -
~A21 ~9~
as polyoxyethylene fatty acid esters, ~ùlyu;~y~ ylene fatty alcohol ethers, for example
alkylaryl polyglycol ethers, alky' 'i ' alkyl sulphates, aryl 'l ' as well
as albumen hydrolysis products; as dispersing agents there are suitable: for example
lignin-sulphite waste liquors and methylcellulose.
5 Adhesives such as carboxy~ ;l.yl.,.,ll~llose and natural and synthetic polymers in the
form of powders, granules or latices, such as gum arabic, polyvinyl alcohol amd
polyvinyl acetate, as well as natural ~ I.rl;~ , such as cephalins and lecithins, and
synthetic rhncrhr,lirif~c, can be used irl the fr~rmlllzttir~nc Other additives can be
mineral and vegetable oils.
10 . It is possible to use color~mts such as inorganic pigments, for example iron oxide,
titanium oxide and Prussian Blue, and organic dyes, such as alizarin dyes, azo dyes amd
metal phthalocyanime dyes, and trace nutrients such as salts of iron, mztn~nf 5f, boron,
copper, cobalt, lllolybd.,.lulll and zinc.
The activity and the spectrum of action of the active rr/mrolln~C of the formulae (I),
15 (la) and (II) and/or of the ~.. rt.,~ l;.",~, precursors or, quite generally, frrmlll~tinnc
which can be prepared therefrom can be increased if, optionally, further ~l~illl;~,lub;~llly
active fr,nnrol.n~lc~ fungicides, 1.~ .;.lf~ herbicides, incfrt;riflfc or other active
are added in order to f nlarge the s~ectrum of action or to achieve particular
effects, for example additional protection against insects. rhese mixtures may possess
20 a broader spectrum of action than the compounds according to the invention.
In many cases synergistic effects are obtained if this is done; in other words, the
activity of the mixture is greater than the activity of the individual ~ t~
Examples are particularly favourable co-~...,.l,."...~ of the following uulllpuullda.
Triazoles such as:
25 amitrole, azocyclotin, BAS 480F, bitertanol, .l;r~..rc.,"~,..l~ r~llb~.~,ulla~vlc,
rr,.. lllr"~ f~ fenethanil, i~ ;"r~ lf flusilazole, flutriafol, ' to
isozofos, lllyclobll~fulil, ..,. t~..,.~,..!~ c~u~y~u~ .vle, l~t. I.~ 1, IJ~ ",lr
~-u~ c.~ --lr, (~)-cis-1-(4-~,l,lu,u,ullwlyl)-2-(lH-1,2,4-triazol-l-yl)-c,~ 1.~1.. 1.l.".~-l,
Le A 30 141 - 14 -
~21 8269S1
1~ , Ll;~ fvll~ trio~lim~nnl, 1~ riflllrni701~
mi~on~7Ole and their metal salts and acid adducts.
Imidazoles such as:
ima7alil, ~u.r~ , prochloraz, ~.;n...~ , 2~ tert-butyl)-1-(2-~ v,u,ul,.,~
3-(1,2,4-triazol-1-yl)-propan-2-ol, al;~vl~bu~allilides such as 2',6'-dibromo-
2-methyl-4-trifluoromethoxy-4'-trifluoromethyl-1 ,3-thiazole-5-carboxanilide,
I-imidazolyl-1-(4'-chlvluAuh_llu,~y)-3,3-dilll~,alyll,uL~l-2-one and their metal salts and
acid adducts.
Methyl(0-2-[2-[6-(2-~,y~ulu,u}.c.lv~y)pyrimidin-4-yloxy]phenyl]3-methoxyacrylate,
methyl(O-2-[2-[6-(2-i' ` `~ )~~' y)pyrimidin-4-yloxy]phenyl]-3-1llr;alw~ya~,ly'
methyl(O-2-[2-[6-(2-nuululul..,llux~y)pyrimidin-4-yloxy]phenyl]-3-metllv~-y.l~,ly'
methyla3-2-[2-[6-(2,6-,linuulu,uh llv,~y)u,y ` `'` 1 yloxy]phenyl]-3-...~,;llu,.y~ly' ',
methyl(O-2-[2-[3-(pyrimidin-2-yloxy),ull~,l.v,.y]~ l.yl]-3-lll~l,ùAy~ly~ , ' yle~)-2-
[2-[3-(5-1l~alyll~yl;lll;~ -2-yloxy)-phenoxy]phenyl]-3-methu~y~ly', -~hyl(o-2-[2
[3-(phenyl-~ul,ullullylo~y)phenoxy]phenyl]-3-l,l~,llv~yu~ly~, methyl(O-2-[2-[3-(4-
Il;LIuul~llv~y)phenoxy]phenyl]-3-methu~y~ yl..~, methyla~)-2-[2-~ll.l~u?.y~ull~llyl]-3
methv~y~..,ly' , methyl(~)-2-[2-(3~s-~ lyl~ ~vyl)pyrrol-l-yl]-3-lllr;~llv~y~l~lylate~
methyla~)-2-[2-(3-1ll~,illu,.y,ull.llv~y)phenyl]-~3-lll~,Lllu,~yul,lyl~., methyl(O-2-t2-(2-
phenylethen-l-yl)-phenyl]-3-methoxyacrylate, methyl(E)-2-[2-(3,5-dichlorû-
phenoxy)pyridin-3-yl]-3-methoxyacrylate,methyl(O-2-(2-(3-(1,1,2,2-lr;LI~lluulu~alu~y)-
phenoxy)phenyl)-3-methoxyacrylate, methyl(O-2-(2-[3-(alpha-llydlo~yl,~,ll~l)-
phenoxy]phenyl)-3-methu~y~.~,lyl..~., methyl(O-2-(2-(4-~ ,llù~,y,u~lhl;ll-2-yloxy)-
phenyl)-3-methv~y,l.lyl~L~, methyl(O-2-[2-(3-n-propylvl~y,ull~,llu~y)phenyl]3-methoxy-
acrylate, methyl(o-2-[2-(3-isopropylu~y~ull~llu~y)phenyl]-3-lll~illu~y~l ly . ' Yla~)-
2-[2-[3-(2-nuoluull~,llu~y)pehnoxy]phenyl]-3-methoxyacrylate, methyl(E)-2-[2-(3-
ethoxy-phenoxy)phenyl]-3-1ll.illu~y~.~,ly' , methyl(O-2-[2-(4-tert~-l,uLyll yl;dil~-2-
yloxy)-phenyl]-3-methoxyacrylate, methyl(E)-2-[2-[3-(3-
cyO luull~lv~y)phenoxy]phenyl]-3-~ illu~y~ly' , ' yl~-2-[2-(3-1llc:al~1u,~ 1;AI-2-
yloxymethyl)phenyl]-3-methoxyacrylate, methyl(O-2-[2-[6-(2-1ll.alyl,ul~;llu~y)pyrimi-
30 din-4-yloxy]phenyl]-3-methw~y~lyl.. ., methyl(O-2-[2-(5-bromopyridin-2-yloxy-methyl)phenyl]-3-methoxyacrylate, methyl(O-2-[2-(3-(3-iodopyridin-2-yloxy)phenoxy)-
Le A 30 141 - 15 -
~A21 82~9~
phenyl]-3-m~Lilu~yd~ly' , methyl-(O-2-[2-[6-(2-chloropyridin-3-yloxy)pyrimidin ~-
yloxy]phenyl]-3-lll~lllu~ya~ly~ (omethyl-2-[2-(5~6~ yl~uylr~ -2-ylmethyl-
..~;,.".""". :l~yl)phenyl]-3-methoxy-ærylate~ (E)-methyl-2-{2-[6-(6-1ll~,Lilyl~ idi.r2-
yloxy)pyrimidin-4-yloxy]phenyl}-3-methoxyacrylate, (E),(E)methyl-2-{2-(3-
S methu~yl,i~ l)methyll.~;.I,;.,~.,.. :1,~I]phenyl}-3-methu,.~r.~,ly' ' '~E)methyl-2-{2-(6-(2-
y)-pyrimidin-4-yloxy]phenyl}3-methoxyærylate~ (O,(Omethyl-2- {2-[6-
phenylpyrimidin-4-yl)-methyloximino-methyl]phenyl} -3-methoxyacrylate,
(O,(Omethyl-2-{2-[(4~ ' ' ulJll~ yl)-methylox-illli~ l]phenyl}-3-metllu~d~lyl~
(Omethyl-2-{2-[6-(2-n-p.ul,yl,ul,~,..u,,y)-l ,3,5-triazin-4-yloxy]phenyl}-3-methoxy-
10 ærylate,(O,@)methyl-2-{2-[(3- u,ul~ yl)lll~,lilyll -~yl]phenyl}-3-methoxy-
ærylate;
Succinate d~h,.' ,, inhibitors such as:
fenfuram, furcarbanil, uy, ' '' 1, furmecyclox, Seedvax, I~,t~ulruvr~"u~ ,r~bulid,
u~y~,rllJu~;ll, shirlan, mebenil (mepronil), benodanil, flutolanil (Moncut); ~ l.Al..,æ
I 'i derivatives such as terbinafine, naRifine, butenafine, 3-chloro-7-(2-aza-2,7,7-trimethyl-
oct-3-en-5-ine);
.,1l.1.~..~.";.~. ~ such as dirhl~fl~ , tolylfluanid, folpet, fluorfolpet; captan, captofol;
, such as l,r~lJ~II~;III, benomyl, ~rlll;u~ r,.l.. .i.1 ,..ir, ~hjf-rh~
methyl, 11~;,.~.. 1_~.. ~1.' or salts thereof;
morpholine derivatives, such as tridemorph, r~ ulJ;lllul~ falimorph, .1;.. :1.. ,.,.. ,1.1. ?
dodemorph, aldimorph, fenpropidin and their ~uyl~ullJl~ullh, acid salts, for example
p-l,~ll.. ,i. ~.,II,I.~.,:r acid and p-dod~,~,yl,ul~ l-sulphonic acid;
d;~ crll, , cufraneb, ferbam, mancopper, mancozeb, maneb, metam, metiram,
thiram zeneb, ziram;
25 b~.,~..ll~;,.,..les such as 2-l.,~ . -,...le;
~h~n7Anni~ such as 2,6-dichioro-N-(4-Llinuvlull-cthyibenzyl)-benzamide;
boron compounds, such as boric acid, boric esters, borax;
fu~lal~i~,lly~ie and formaidehyde donor cv",,l,uu"~i~, such as benzyl alcohol mono-
(poly)-ll I .; f. .l l l .Al oxazolidines, hexa-hydro-S-triazines, N-methylol. l,lu,u~ : . "i~
30 ~ r~ A~1~h~ie, nitropyrin, oxolinic acid, tecloftalam;
tris-N-(cyclohexyl ll ;,., ~ .; l l l, ..l ;oyy) ~lllminillm N-(cyclo-hexyldiazeniumdioxy)-
tributyltin and K salts, bis-N-(cyclohexyl.i;~, ..;I,.~,l;..~y)-copper;
Le A 30 141 -16 -
~.2 ~ ~2~
. N~ LllyliauLlli~ulin-3-one, 5-chloro-N-I~ yl,~ ,.lin-3-one, 4,5-dichloro-
N-o~lyl;soLll;~vlin-3-one~N-octyl-icrlthi:l7~\lin-3-one~4~s-trimethylene-isothiazolinone
4,5-L~ .LI~ i, N-methylohl.lu~ ";~1.
aldehydes such as cinnamaldehyde, formaldehyde, glutaraldehyde,
5 ,I~-bIUIIIU~ ` ',yde;
ll~io~ ,;" such as Llliu~,~allaLulll~,llyllllio~ lr, methylene l,;~Ll.;~
etc;
quaternary ~n~ nrnillm ~ 1 ull~ C~ such as ben-y~ L~rlLc;Llr~d
chloride, ben7yldimethyldod~,.,yl- , .I-- 1~ chloride, didecyl(~ llr~
10 chloride;
iodine derivatives, such as .I;;o~ .yl p-tolyl sulphone, 3-iodo-2-propinyl alcohol,
4~ 1 ' u~ l 3-iodulJlu~ yl fûrmal, 3-bromo-2,3-diiodo-2-propenyH~
2,3,3-triiodoallyl alcohol, 3-bromo-2,3-diiodo-2-propenyl alcohol, 3-iodo-2-propinyl
n-butylcarbamate, 3-iodo-2-propinyl n-hexylcarbamate, 3-iodo-2-propinyl
15 ~.loll.,.~yl~ l,rl.... ~" 3-iodo-2-propinyl phenylcarbamate;
phenol derivatives, such as ~ 1, tetracblorophenol, 3-methyl-4~ lo.u~l.,l.ol,
3,5-dimethyl-4-chlorophenol, phenoxyethanol, dichlorphen, o-pl.~,~.yluh~..ol,
m~ l.yl,ul.~,.lol, p-pl-.,l.yl~ ol, 2-ben7yl-4-,,l.lu.u~ ,l.ol and their alkali metal and
alkaline earth metal salts;
20 ~ lub;cid~ with an activated halogen group, such as CIIIUI~J~ ,; lf, bronopol,
bronidox, tectamers such as 2-bromo-2-nitr~-1,3-propanediol, 2-bromo-4'-hydroxy-"",~, 2,2-dibromo-3-nitrile-~.u~ ";~lP, 1,2-dibromo-2,4-d;~"~a.lol,~,~.."
!3-bromo-~-1.;llu~lyl~.lc,
pyridines, such as l-hydroxy-2-pyril1inrthir~np (and its Na, Fe, Mn and Zn salts),
tetrachloro-4-l.l.,llly'~ ~1' yl~y~;~;lle, ~yl;, ~ I;Ill, dipyrithion,
I -hydroxy-4-methyl-6-(2,4,4-trimethylpentyl)-2(1 H)-pyridine;
metal soaps, such as tin, copper, zinc ~ , octoate, 2-t:~llylll~ lo..-~" oleate,phosphate, benzoate;
metal salts, such as copper l~y~hu~y~,~bl , sodium dichromate, potassium
30 d;~l..u ~, potassium chromate, copper sulphate, copper chloride, copper borate, _inc
fluorosilicate, copper fluorosilicate;
oxides, such as tributyltin oxide, Cu2O, CuO, ZnO;
dialkyldithio.,r~ ~, such as Na salts and Zn salts of dialkyldithio~,~l,~ul...t~
Le A 30 14~ - 17 -
.
Af~7 ~ 82~
tetram~LllylL~iu,~, disulphide, potassium N-methyl-diLl.ivcal' ,
nitriles such as 2,4,5,6-tetrachloroisophthalodinitrile, disodium cyano-
quinolines, such as 8-l~y~u~yu"l~lolille amd Cu salts thereof;
S IllL~VO-,lllUliC acid, S-hydroxy-2(5H)-furanone;
4,5-dichloro.l;~ ,"l;,.r.lf, 4,5-~ f)l;l~ f- 4~5-LI~ lyll~lf~l;lh~
4,5 -dichloro-(3H)- 1 ,2-dithiol-3 -one, 3 ,5 -dimethyl ~ I y dl u- 1,3 ,5-thiadiazine 2-thione,
N-(2-p-~,lllulu~t.,l~vylc;Lllyl) ! ' ehloride, potassium N-llydlu,yll.cthyl-
N'-methyl-d;Lllio~
10 2-oxo-2-(4-hydroxy-phenyl)~c~,~llyJlu,;illlic acid ehloride,
phenyl 2-ehloro-eyano-vinyl sulphone,
phenyl 1,2-dichloro-2-cyano-vinyl sulphone;
Ag, Zn or Cu-corltaining zeolites alone or enclosed in polymeric active
Very partieular preferenee is given to mixtures with
sl~rrml7rtlf, ltl.. ,.. ,.,.,.. lf ~"y,ulu~,ullo.~.ulf, diehlobutra7ol, .~ ,,lr,
t~.t~ C, ~.~f ~ .llr~ lUl~ ;f ~ `, tf l~ lf, methyl
(E)-mf~thrtyiminl~[f~-(o-tolyloxy)-o-tolyl)]~ yl (E)-2-{2-[6-(2-L,yal-u~tll~.llu~-y)-
pyrimidin-4-yl-oxy]phenyl}-3-l,l.Lllu,y~l~, nl~,llr~llUfdlll, earboxin, ~nrirl~tnil,
4-(2,2-difluoro-1,3-lt..1,...1;..,...1-4-yl)-lH-pyrrol-3-,. ~ ' ''-, butenafine, imazalil,
20 N-methyl-isuLh;~ùlill-3-one,S-ehloro-N-~ l.yI;~ ,.1;- ,-3-one,N-UI~LYI;~ULI.:,. ,..,lin-
3-one, 1, ,~:~..11,:-.,l;ll.. ~, N-(2-llyllu,y,ulu,uyl)-amino-methanol, berlzyl aleohol
(hemi)-formal,j' '' ' yde,omadine,dimethyl~ and/or3-iodo-2-propinyl
n-LttlLylLal~ LLt:~.
FUILII~IIIIUI~ highly effeetive mixtures are also prepared with the following aetive
25 ~,u~ uo~
F ''
aeypetaes, 2-~min~tbl~fStnf, cullu.u,uylru~, anilæine, benalaxyl, bupirimate,
ehinomethionat, ehloroneb, rhln7n'' , eymoxanil, dæomet"l~ lf ~ diehloram,
diethofenearb, .1;.". 1l.;, ;,,.~.l, dioeab, difhianon, dodine, drazoxolon, f rliff nrh~e
30 ethirimol, etridia_ole, fenarimol, fenitropan, fentin aeetate, fentin hydroxide, ferimzone,
Le A 30 141 - 18 -
CA 2 ~ 82~
fluazinam, fluromide, flllcl~lfArni~f flutriafol, fosetyl, fthalide, furalaxyl, guazatine,
hymexazol, iprobenfos, iprodione, isoprothiolane, metalaxyl, m~ lr(l. ~.1" nitrothal-
isopropyl, nuarimol7 of urace, oxadiyl, p~.n. ~, pencycuron, l.l...~.l;l,l~., pimaricin,
piperalin, UlUUylllivu~ )lu~uàlllocculJ7 propineb, ~ylf~ uullvs, pyrifenox, pyroquilon,
5 AIllintA,A.. nf tar oils, tecnazene, thicyofen, Lll;Ul' -methyl, tolclofos-methyl,
triazoxide, l. ;. I,IA. ..;flf ~ Ll;.,,~,l~lC, triforine, vinclozolin.
,. . ~
phosphoric esters, such as azinphos-ethyl, azinphos-methyl, a-1(4-clllv.vl' yl)-4-O-ethyl, S-propyl)~lloa,ul.u.ylûxy-pyrazole, ~,LIvl~J~lirus, co~nnA~hne~ demeton,
lû demeton-S-methyl, diazinon, dichlorvos, Villl '- ~ ', ethoate, eLIlo~lu~Jl.oa, etrimfos,
'' U~ll;UIl~ fenthion, Il~ L~ parathion, parathion-methyl, phosalone, phoxim,
pirimiphos-ethyl, pirimiphos-methyl, profenofos, prothiofos, sulfprofos, triazophos and
Lli-,lllvl,ullvl~
such as aldicarb, b~,lldiO~,all" a-2-(1-~ Lll~ ,lv~yl)-phenyl
15 methylcarbamate, bu~u~,albu~.illl, bu~uA~,allJu~dul, carbaryl, carbofuran, ~,albvaulrall,
Clo~lllu~ isoprocarb, methomyl, oxamyl, pirimicarb, promecarb, propoxur and
thiodicarb;
or~An~Aici~ n ~ v....~l~ preferably dimethyl(phenyl)silyl-methyl 3-l' ~b~l~yl
ethers, such as dimethyl-(4-elllu~y~ lyl)-silylmethyl 3-~ luAyb~l~yl ether, or
20 . (di~ Lll~lyll~llyl)-silyl-methyl 2-phenoxy-6-~y,;v~ Lllyl ethers, such as, for example,
dimethyl-(9-ethoxy-phenyl)-silylmethyl 2-phenoxy-6-pyridylmethyl ether, or [(phenyl)-
3-(3-~ luA~,ull~,.lyl)-propyl](dimethyl)-silanes, for example (4-t LIIUA~
[3-(4-fluoro-3-p}l~ vAyul.~llyl-propyl]dimethyl-silane, silafluofen;
uyl~iLIuuids, such as allethrin, Allll.A ,l. :1.,;,. b;ulcalll~Llllill, byfenthrin, cy~,lv,ulvLluill~
25 cyfluthrin,.~ cyhalothrin, Cy~ lifI IA111~ alpha-cyano-3-phenyl-
2-methylbenzyl 2,2-dimethyl-3-(2-chloro-2-trifluoro-methylvinyl)cyclopropane-
u~ulJvAylaLe, Lll~ulv~Ja~luill, fenfluthrin, fenvalerate, llu~,yl' , flumethrin, fluvalinate,
rPrm~-Afh~in resmethrin and trAl~mf~thrin
nitroimines and luLIulll~ lenes, such as 1-[(6-chloro-3-pyridinyl)-methyl]-4,5-dihydro-
30 N-nitro-lH-imidazol-2-amine(illliddclu~lid),N-[(6-chloro-3-pyridyl)methyl-]N2-cyano-
N'-methylacetamide (NI-25);
abamectin, AC 303, 630, acephate, fArinAthrin, alanycarb, aldoxycarb, aldrin, amitraz,
Le A 3Q 141 -19 -
-
C~21 826qq
~7~mPthirhnc Bacillus 11"..;"~;. .,~ic phosmet, ~ .";~ phosphine, prallethrin,
propaphos, ~ _."~ s, prothoate, pyraclofos, pyrethrins, pyridaben, pyri~l~fPnthion
uAyf~ll, qllin:~lrhrs, RH-7988, rotenone, sodium fluoride, sodium
II~AaLlUUII " , sulfotep, sulphuryl fluoride, tar oils, ~,nu~.l~ulull~ tefluthrin,
5 temephos, terbufos, Lellaulllul~ ullos~ l;ll, 0-2-tert-butyl-pyrimidin-5-yl-
o-isopropyl-pho ,lulluluil thiocyclam, thiofanox, thiometon, ~ J~ h. ;"
~linuulll~ull, Ll;lll~Llla~alb~ l,;"." Verticillium lacanii, XMC, xylylcarb,
b~,l rulauallJ, bensultap, bifenthrin, hi~l~llPfhrin MERhio^'lcthrin (S)~ ,lu~ t~,..yl
isomer, bromophos, bromophos-ethyl, buprofe_in, cadusafos, calciurn ~01~
10 ~al~lulll....,Il,i.,.. cartap,, ' ' , chlordane, chlulf..lv;l.,uho~, ,1.1.,. ni._,...ul"
~ " , ' ,uhlulu,u;~;ll,chlorpyrifos,cyanophos,beta-cyfluthrin,âlpha--:y~ Llu;
~;y~ cylul.la~ " da_omet, DDT, demeton-S-Il",;llyl:,ulul,oll, d;af~lLll;ulull~dialifos, d;~ulu~llùs, dinul,...~l,ull, dinoseb, .l~ rus~ diaxacarb, disulfoton,DNOC, ~nnrPnfhrin Pn~r~elllfs~n EPN, ~f..l~ I , eLll;~f~ alb~ ethion, etofenprox,
15 fenobucarb, fenoxycarb, F~ . .I r. ,1~ ,. " fipronil, rl~ uAulull~ flufenprox, llur~lluAuluii,
fonofos, fonr~Pt~nptp~ fr,nnrlthir~n, ~ennpthih~rl, rL.ath;U~IJ, heptachlor, Il~,Aalluululull,
hydramethylnon, hydrogen cyanide, hydroprene, IPSP, isa_ofos, isofenphos,
;~u,uluLlliOlall~, isoxathion, ir.~fPnrhr,s kadethrin, lindane, malathion, mecarbam,
mPrhnefolrn, mercurous, chloride, metam, M~ iulll, anisopliae, I,.~II "- ;l`,~i,20 Ill~Lllalllidu~llo~, ,.. :1.,.1~11.:~,.., methiocarb, methoprene, III~LIIUAY~IIIUI~ methyl
isothiocyanate, mPthol~ rh, mevinphos, IIIUIIO~,IULU~I105, naled, Neodiprion sertifer NPV,
nicotine, omethoate, uAyd~ tull-methyl, u~ La~ ulu~ llùl~ petroleum oils, phenothrin,
rhPntl ' phorate;
~, .. . . ,
25 Fentin acetate, metaldehyde, methiocarb. nirl~lc~nni~lP thiodicarb, trimethacarb.
Algicides:
Copper sulphate, di~lllulul~ l, endothal, fentin acetate,
~1, .1.:. ;~1. _
acetochlor, ~riflllr,rf~n aclonifen, acrolein, alachlor, alloxydim, ametryn, ~mi~ln~clllfilron,
30 amitrole, s~mmrnillnn sulfamate, anilofos, asulam atra_ine, a~i~JllutlyllC, bena_olin,
Le A 3Q 141 - 20 -
~ h2 1 826~9
b~nfl~ in lu~llru~ , b~l~ulrulull, bensulfide, benta_one, ben_ofencap,
b~ ;a~ulvll, bifenox, bilanafos, borax, dichlorprop, dichlorprop-P, diclofop, diethatyl,
dircllv~uuull, ~lif! n7nqll~t ~l;n~ " dimefuron, .~ ;rr~ imf th:~hlnr
,1;",. ~ .". ;~yll~ flim~thirirl, dilll~;.llyLI.D;ll;c acid, flinitrr~in~, dinoseb, dinoseb, dinoseb
5 acetate, dinoseb, bromacil, bromobutide, bromnf-rmYim~ bromoxynil, butachlorj
butamifos, fuenachlor, butralin, butylate, <,alb~L~Il;dc, CGA 184927, rhlnrm~thnxyfen,
chloramben, ~ ' ' I)IUIIIUIUII~ chlorbutam, chlorfurenol, ~.hl.~ IIIUI;IIIUIUII,chlornitrofen, ~,lllulua~,~,Lic acid, ~ llUlU~ iU~ chlorotoluron, I,IIIUIU~UIUII,
chlorprepham, ~ hlv~ulru~u~, chlorthal, ~,lllulLh.al.l;d, cinmethylin, u;l~urul,ulul~,
10 clethodim, ~-lnm~7nn~, clomeprop, clopyralid, cyanamide, cyanazine, dinoseb acetate,
dinoterb, ~ .} ,- ,.;~, di,ululu~,LIyll, diquat, dithiopyr, diduron, DNOC, PPX-A 788,
DPX-E96361, DSMA, eglina7ine, endothal, EPTC, esprocarb, rll, '11..."1;"
~;LLdill~uuull~ etdlurull~ , fenoxaprop, fenoxaprop-P, fenuron, flamprop, flamprop-M,
fl~a~ulrulul~, fluazifop, flua_ifop-P, n.,.l-l...,l;." flumeturon, llu~lu~ ,uf~
15 lluUlulllLlul~.l, r.~ lu~ " flurenol, fluridone, llul. ' ' ' , Lluulu~yu~l, cycloate,
~;yulu~ydilll, 2,4-D, daimuron, dalapon, dazomet, 2,4-DB, ~ -, desmetryn,
dicamba, ~linhlnrbrnil~ isuAuluLulul., isouron, isoxaben, i~u~ yl;ruAu, lactofen, lenacil,
linuron, LS830556, MCPA, MCPA-thioethyl, MCPB, mecoprop, mecoprop-P,
mefenacet, m~fllli~ , metam, III~.Lalll;Llull~ --u
20 metha7ole, metllul~lu,uLIyll~,, methyldymron, Ill..llyli:~uLlliu.,~ UblUIII~AIU
fomosafen, fosamine, rulylù-iyf~ IIIf- ~-, glyphosate, haloxyfop, hrY~7innn~
., imazapyr, ima_aquin, illl~iLlla~r, ioxynil, isopropalin, ~JIuuy~llidc,
prosulfocab, ~ uly , l~yl~cul~ulrulull, I,~la u~-yr~ yl;l~uL;~alb, pyridate,
quinclorac, quinmerac, .~ lo, ~ , quizalofop, quzizalofop-P, S-23121, sethoxydim,
25 sifuron, simazine, simetryn, SMY 1500, sodium chlorate, sulfometuron, tar oils, TCA,
m~tol ~(~hln~ r, metoxuron, metribzin, ~ ,L ,ulrulu-l, molinate, monalide, monolinuron,
MSMA, naproamilide, napropamide, naptalam, nebuton"I;cù.ulrulull, nipyraclofen,
.,.., n,..~,,..~ orbencarb, oaryzalin, oxadiazon, U:.ynuulrtll, paraquat, pebulate,
F )-1;~ - pc~llLh,lllulu~ .llul, pentaochlor, petroleum oils, 1.11..Il,l..1;1.1.....
30 picloram, piperophos, ~I~LilaClllul, I~l;lll;.ulrulull, prodiamine, ~,u~;l;ll~;lle, propmeton,
prometryn, propachlor, tebutam, Lc;buLll;ulun~ terbacil, terbumeton, t~lbu~llyla~;ll~,
terbutryn, Lll;~dnuulull, thif~nc~llfilron, Lll;Ob~ all" thiocarbazil, tioclorim,
tralkoxydim, tri-allate, triasulfuron, tribenzuron, triclopyr, tridiphane, trietazine,
Le A 30 141 - 21-
~A2 ~ 82699
. ~ trifluralin, IBI-C4874 vemolate, propanil, ~lo,u~u,u;~arup, propazine, propham.
The weight ratios of the active rnmrC!Iln~ in these active-compound c~,..,l: ' ;., ~ can
be varied within relatively large ranges.
The active-compound ~ 15 preferably receive the active compound in a
proportion of from 0.1 to 99.9%, in palticular from I to 75%, ~I;.,ul~ly preferably
from 5 to 50%, the remainder to 100% being made up by one or more of the
alJu~...~-- ;---.--1 CO~
The Ill;-,lviliCi~kLl ~....1..~;1;....~ or ~-- used to protect the industrial materials
contain the active compound and/or the active-compoumd ~ , "~1;..l. in a
c~nr~ntr~tinn of from 0.01 and 95% by weight, in particular from 0.1 to 60% by
weight.
The cnn~ntrPtinnC in which the active compounds andlor active-compound
~...,,I,;,,,.I;n..c which are to be used are employed depends on the nature and the
incidence of the Ill;CIùul~ , to be combated and on the ~omrc\~itinn of the material
15 to be protected. The optimum quantity for use can be determined by series of tests. The
~nn~rntr,qtil~nc for use are in general in the range from 0.001 to 5% by weight,preferably from 0.05 to 1.0% by weight, based on the material to be protected.
The active .. I .. u.. 1c and .~ ;.. ,.c according to the invention make it possible in
an ad~ a~ uu~ manner to replace the l~ lub;~,idal ~nmrn~itinn~ which have been
20 available to date by more effective ~nmrncitinnc They exhibit good stability and,
alvall~d~uu~ly, have a broad spectrum of action.
The examples which follow serve to illustrate the invention. The invention is not
limited to the examples.
LeA~0 141 -22-
CA21 82699
PreparatiQn h'
ExamPle I
~ ~N--C H
1.35 g (4.2 mmol) of N-(n-dodecyl)-b .. ,n.l,~ are dissolved in 20 ml ofchloroform, the solution is cooled to -20C, and a total of 1.26 g (4 mmol) of 56%
strength m-~LIulup~.b~.l~uic acid are added in po}tions. The mixture is ~ r~ ly
stirred at 0C for 2 h, washed with saturated NaHCO3 solution and saturated NaCIsolution, dried over Na2SO~ and ~
The residue is purified by Llu~ y on silica ~el (toluene:ethyl acetate = 1:1).
Yield: 0.7 g (~ 50% of theory) of a pale yellow oil
'H-NMR (CDCI}) o = 0.9 (3H, t); 1.1-1.7 (23H, m), 3.5 (2H, t); 7.2-7.9 (4H,
m).
In analogy to this example and in accordance with the general instructions aboYe, the
CU1~ U~UII~ of the formula (I) which are listed in Table I below are also prepared.
Le.A30 141 -23-
- ~A2182699
Table I
Rs
R4~ / N--R1 (1)
R
R2
Ex. R' R2 R3 R~ R5 Physic,21 const2al3ts
No.
S I -C,rH2s H H H H see Preparation Example
2 -CtH,, H H H H 'H-NMR (CDCI3)
~ = 0.85 (3H,t), 1.2-~.5
(tOH, m), 1.85 (2H,tq), 3.50
(2H,t), 7.30 (IH,m), 7.48
(2H,m), 7.85 (IH,d).
3 -H H H H H
4 -CH3 H H H H 'H-NMR (CDCI3)
o = 3.25 (3H,s); 7.3
(IH,m); 7.5 (2H,m); 7.83
(IH,d).
5 ~C2Hs H H H H 'H-NMR (CDCI3)
o = 1.43 (3H,t); 3.57
(2H,q); 7.26 (IH,m); 7.49
(2H,m); 7.81 (IH,d).
0 6 -C3H, H H H H 'H-NMR (CDCI3)
~3 = 1.0 (3H,t); 1.82
(2H,m); 3.47 (2H,t); 7.29
(IH,m); 7.4 (2H,m); 7.83
( I H,d).
7 -CH(CH3)2 H H H H 'H-NMR (CDCI3)
o = 1.38 (3H,d); 1.44
(3H,d); 4.12 (IH,m); 7.3
(IH,m); 7.46 (2H,m); 7.8
(IH,d).
8 -C~H9 H H H H 'H-NMR (CDCI3)
~ = 0.95 (3H,t); 1.45
(2H,m); 1.75 (2H,m); 3.50
~2H,t); 7.30 (IH,m); 7,45
(2H,m); 7.80 (IH,d).
9 -CH2CH(CH3)2 H H H H 'H-NMR (CDCI3)
o = 1.0 (6H,m); 2,1
(IH,m); 3.27 (2H,d); 7.26
(IH,m); 7.45 (2H,m); 7.82
( I H,d).
Le A ;~0 141 - ~4 ~
CA2182699
Table I - Continued
Ex. R' R3 R3 R4 Rs Physical consta73ts
No.
10 ~CH H H H H 'H-NMR (CDCI3)
--CH a (3H2xd);.'.26/1.43
C2Hs (3H,2xt); 1.5-1.9
(2H,m); 3.9 (IH,m);
7.3 (IH,m); 7.48
(2H,m); 7.82 (IH,d).
(Diastereomer mixture)
S 11 -CH2CH=CH2 H H H H 'H-NMR (CDCI3)
~ = 4.1-(2H,d); 5.3-5.5
(2H,m); 6.0 (IH,m);
7.30 (IH,m); 7.45
(2H,m~; 7.80 (IH,d),
12 -CH2C_CH H H H H 'H-NMR (CDCI3)
o = 2.37 (2H,m); 4.29
(2H,AB system); 7.3
(IH,m); 7.45 (2H,m);
7.8 (IH,d).
13 -C,H" H H H H 'H-NMR (CDCI3)
o = 0.9 (3H,t); 1.3
(4H,m); 1.78 (2H,m);
3.49 (2H,t); 7.3
(IH,m); 7.45 (2H,m);
7.80 (IH,d).
14 -CsH,3 H H H H 'H-NMR (CDCI3)
o = 0.9 (3H,t); 1.3
(6H,m); 3.50 (2H,t);
7.32 (IH,m); 7.45
(2H,m); 7.80 (IH,d).
-C,H H H H H 'H-NMR (CDCI2)
'5 o = 0.9 (3H,t); 1-35
(8H,m); 3.50 (2H,t);
7.30 (lH,m); 7.45
(2H,m); 7.80 (IH,d).
10 16 -CgC19 H H H H
17 -CloH2, H H H H
18 -CllH23 H H H H
19 -Cl3H3, H H H H
-CH2CH20CH3 H H H H 'H-NMI~ (CDCI3)
o = 3.25 (3H,s); 3.5-
3.8 (4H,m); 7.27
(IH,m); 7.48 (2H,m);
7.80 (IH,d).
Le A ~Q 141 - 25 -
CA2 ~ 826~9
Table I - Contimled
Ex. R' R3 R3 R~ Rs Physical constants
No.
21 H H H H 'H-NMR (CDCI3)
1.5-2.2 (8H.m);
4,07 (IH,m); 7.30
(~H,m); 7.48 (2H,m);
7.80 (IH,d).
22 H H H H 'H-NMR (CDCI3)
--CH~ ~ = 4.63 (2H.AB
2 \~/ system); 7.2-7.4
(8H,m); 7.80 (IH,d).
23 H H H H 'H-NMR (CDCI3)
t3 s 0.9-1.1 (4H,m);
2.93 (IH,m); 7.3
(IH,m); 7.48 (2H,m);
7.80 (IH,d).
24 H H H H 'H-NMR (CDCI3)
~ o = 7.1-7.6 (8H,m);
\~/ 7.88 (IH,d).
5 25 CH H H H H 'H-NMR (CDCI3)
3 O s 1.85 (3H,t);
4.90/4 .90 ( I H,2xq);
~3 ( i H,d).
26 C H H H H H 'H-NMR (CDCI3)
/ 2 s o = 0.8-1.0 (6H,m);
--CH2--CH\ 1.2-1.6 (8H,m); 1.79
C4H9 (IH,m); 3.39 (2H,m);
7.3 (IH,m); 7.48
(2H,m); 7.82 (IH,d).
27 -C,sH H H H H 'H-NMR (CDCI3)
33 o = 0.87 (3H,t); 1.2
(26H,m); 181 (2H,m);
3.50 (2H,t); 7.3
(IH,m); 7.48 (2H,m);
7.82 (IH,d).
28 -Cl3H37 H H H H 'H-NMR (CDCI~)
o = 0.87 (3H,t); 1.2
(28H,m); 1.80 (2H,m);
3,48 (2H,t); 7.3
(IH,m); 7.48 (2H,m);
7.80 (IH,d).
eA~Q 141 -~6-
2 1 ~6~
Table I ~ Continued
Ex. R' R2 R' R~ Rs Physical constants
No.
29 H H H H 'H-NMR (CDCI3)
~ o = 2.6-2.9 (4H,m)
(CH2)4 ~ 2.67 (2H,t): 3.50
(2H,t); 7.1-7.5 (8H,m);
7.82 (lH~d)
-C,~H29 H H H H 'H-NMR (CDCI3)
~3 = 0.87 (3H.t): 1.2
(24H,m): 1.81 (2H,m):
3.49 (2H,t) 7.30
. (IH,m); 7.50 (2H,m)
7.82 (IH,d).
31 ~CH ) OC H H H H H 'H-NMR (CDCI3)
' 3 ' 9 o = 0.92 (3H,t): 1.35
(2H,m); 1.55 (2H,m):
2.07 (2H,m) 3.44
(2H,t): 3.49 (2H,t);
3.65 (2H,t); 7.31
(IH,m): 7.50 (2H,m);
7,81 (IH,d).
32 H H H H 'H-NMR (CDCI3)
~ = 4.6 (2H,AB
CH2 ~O~ system); 6.35 (IH,d);
6.46 (IH,d) 7.25-7.55
(4H,m) 7.83 (IH,d).
5 33 -C3C,7 H H F H 'H-NMR (CDCI3)
~ = 0.8 (3H,t) 1.2
( l OH,m) 0.8-2.0
(2H,m): 3.5/3.9
(2H,2xt) 7.0-8.0
(3H,m).
34 H H H H 'H-NMR (CDCI3)
--(CH2)2o~3 (2H,m), 6.9-7.5
(8H,m) 7.84 ( I H,d).
H H H H 'H-NMR (CDCI3)
r\ ~3 = 1.0-2.2 (lOH,m):
\ 3.7 (I H,m) 7.3
(I H,m) 7.48 (2H,m);
7.84 (IH,d).
36 -C(CH,)~ H H H H 'H-NMR (CDCI3)
~ = 1.61 (9H,s): 7.26
(IH,m) 7.48 (2H,m):
7.80 (IH,d).
Le A 30 141 - 27 -
C~21 82~99
Table I - Continued
lex. R' R2 R' R' Rs Physical constmts
No.
37 ~CH3 H H H H oil
--CH
C3H7
38 CH CH OCH H H H H 'H-NMR (CDCI3)
_~ 2 2 3 o 3 1.1-1.2 (6H,m);
1.48 (2H,d); 1.7-2.0
CH3 (2H,m); 3.3-3.7
(4H,m); 4.2-4.4
. ~lH,m); 7.3 (IH,m);
7.48 (2H,m); 7.81
( I H,d).
39 H H H H 'H-NMR (CDCI,)
~OEt o = 1.23 (3H,t); 1.3-
/ 2.3 (8H,m); 3.2
(IH,m); 3.5 (2H,q); 3.7
(IH,m); 7.3 (IH~m);
7.45 (2H,m); 7.81
(IH,d),
40 -cycloC,H,3 H H H H 'H-NMR (CDCI;)
5 5 1.3-2.1 (14H,m);
4.05 (IH,m); 7.35
(IH,m); 7.45 (2H,m);
7.85 (IH,d).
541 OC H H H H H 'H-NMR (CDCI3)
2 5 0 = 1.3 (6H,m); 3.4-
--CHzCH\ 3.8 (6H,m); 4.74
OC H (IH,m); 7.26 (IH,m);
2 5 7.48 (2H,m); 7.84
(IH,m).
42 -CH3 H Cl H Cl 'H-NMR (CDCI3)
o=326(3H,s);7.45
( I H,s); 7.71 ( I H,s).
43 -CH2CH=CH2 H Cl H Cl 'H-NMR (CDCI3)
o = 4.1 (2H,m); 5.5
(2H,m); 5.98 (IH,m);
7.2517.3417.46 7.71
(2H,4xs).
44 -C,H" H Cl H Cl 'H-NMR (CDCI3)
5 = 0.88 (3H,t); 1.3
(9H,m); 1.90 (2H,m);
3.84 (2H,t); 7.6-7.9
(2H,4xs).
Le A 3Q 141 - 28 -
-
C~2~ 82699
Table I - Continued
Ex. R' R3 R3 R~ Rs Physical const~u3ts
No.
45 -(CH,CH2O)3OC2H5 H H H H 'H-NMR (CDCI3)
~ = 1.21 (3H.t); 3.45-
3.9 (14H,m); 7.35
(IH~m); 7.48 (2H,m);
7,82 (IH,d).
46 -CH CH OH H H H H 'H-NMR (CDCI3)
2 li = 2.95 (IH,br), 3.6-
4,0 (4H,m); 7.35
(IH,m); 7.52 (2H,m);
7.85 (IH,d).
47 -CH2CO2Et H H H H 'H-NMR (CDCI3)
o = 1.19 (t,3H); 4.16
(q,2H); 4.26 (d,2H);
7.32-7.51 (m,3H); 7.85
(m,lH).
48 -CH(Ph)CO 'Bu H H H H 'H-NMR (CDCI3)
o = 1.38 arld 1.43
(s,9H); 216 (s,lH);
7.26-7.52 (m,8H); 7.75
(m, I H).
5 49 -CH(CH3)COICH3 H H H H 'H-NMR (CDCI3)
~ = 1.65 (3H); 3.49
and 3.69 (3H); 4.49-
4.71 (IH); 7.27-7A9
(3H); 7.83 (IH).
50 -CH(CH2CH(CH,)2)CO2CH3 H H H H 'H-NMR (CDCI3)
~ = 3.45 and 3.68
(3H); 4,52 (IH); 7.30-
7.48 (3H); 7.86 (IH).
51 CH(i-Pr)CO,Et H H H H 'H-NMR (CDCI3)
o = 0.87-1.26 (9H);
2.43 (IH); 3.88 (IH);
4.03-4.20 (2H); 7.21-
7.34 (m,lH); 7.47
(IH); 7.82 (IH).
52 -CH(CH2Ph)CO2Et H H H H MS: m/z 348
Le A ~Q 141 - ~9 ~
CA2 1 826~
s
[~S ~ N C,2H25
2.11 g (10 mmol) are dissolved in ether and the solution is added dropwise to a
mix~ure, cooled at -20C, of 1.85 g (10 mol) of ~I~KI~ylal~ and 2.42 g (24 mmol)5 of L~;~ a~yl~ . The mixture is stirred at room t~ lu-t; for S h and filtered and the
filtrate is .~ ff1 on a rotary evaporator. Purification b~ ch~m~lt~-~hy on silica
gel (toluene).
Yleld: 2.5 g (~ 74% of theory)
'H-NMR (CDC13) o= 0.85 (3E~ t), 1.1-1.3 (21H, m), 1.5
(2H, m), 2.85 (2H, t), 7.1-7.5 (4H,
m).
In analogy to this example and in accordance with the general ;, ~ above, the
~Illlql~lllllll~ of the formula (II) which are listed in Table 2 below are also prepared
- T ~ A 3Q 141 ~ 30 - - = ~
.
C~21 82~9
Rs
R4~S~ N--R1 (II)
R3~ S
R2
E~ R' R2 R3 R4 R5 Phy~3ical cons~nts
No.
5 1 -C,2-H2s see P~ ation E~ample
2 -C~HI7 'H-NMR (CDCI3)
o = 0.85 (3H,t), 1.1-1.4
(10~ m), I.SS (2H,m),
290 (2H,t), 7.15 (2~m),
7,35 (2H,m)
3 -H H H H H
4 -CH3 H H H H 'H-NMR (CDCI3), ~ = 2.9
(3H,s); 7.15 (2H,m); 7.35
(2H,m).
-C2H3 H H H H 'H-NMR (CDCI,), o =
1.05 (3H,t); 2.95 (2H,q);
7,20 (2H,m); 735 (2H,m).
10 6 -C3H7 H H H H 'H-NM~ (CDCI3), o =
0.90 (3H,t); I.SS (2~m);
2.85 (2H,fl; 7.15 (2H,m);
7.35 (2H,m).
7 -CH(CH3)l H H H H 'H-NMR(CDCI3), o=
1.05 (6H,d); 3.0 (IH,dq);
7.10 (2H,m); 7.30 (2H,m).
8 -C4H7 H H H H 'H-NMR (CDC13~
0.85 (3H,t); 1.30 (2H,m);
1.55 (2H~m); 2.90 (2H,t);
7.15 (2H,m); 7.35 (2H,m).
g -CH2CH(CH3)2 H H H H 'H-NMR(CDCI3), o=
0.90 (6H,d); 1.90 (IH,m);
2.70 (2H,d); 7.15 (2H,m);
7.35 (2H,m).
,CH H H H H 'H-NMR(CDCI3), o =
--CH 2 0.85 (3H,t), 1.0 (3H,d);
~C H 1.35 (IH,m), 1.60 (IH,m);
2 5 æ75 (l~m); 7.10 (2H,m);
7.35 (2H m).
reA30 141 -31-
~A 2 ~ 82699
T1~ TT-I (continued)
Ex. Rl Rl R3 R~ R' Physical co~5tants
No.
I l -CH CH =CH H H H H 'H-NMR (CDCI,),
2 2 ~i = 3.50 (2H,d);
5.1-5.3 (2H,m); 5.
(IH,m); 7.18
(2H,m); 7.35
(2H,m).
12 -CH C--CH H H H H 'H-NMR (CDC~3),
2 ~; = 2.30 (IH,t);
3.65 (2H,d); 7.15
(2H,m); 7.30
(2H,m) .
13 -CsHIl H H H H 'H-NMR (CDCI3),
= 0.85 (3H,t);
1.2 (4H,m); 1.5
(2H,m); 2.85
(2H,t); 7.15
(2H,m); 7.30
(2H,m).
-C,H H H H H 'H-NMR (CDCI3
14 13 ~ = 0.85 (3H,t);
1.2 (6H,m); 1.50
(2H,m); 2.90
(2H,t); 7.15
(2H,m); 7.35
(2H,m).
15 -C H H H H 'H-NMR (CDCII
12 25 0 = 0.90 (3H,t);
1.1-1.7 (20H.m);
2.88 (2H,t); 7.15
(2H,m); 7.35
(2H,m).
16 H H H H 'H-NMR (CDCI
o = 0.6 0.8
(4H,m); 2.90
(IH,m); 7.20
(2H,m); 7.38
(2H,m).
17 H H H H 'H-NMR (CDCI
~\ ~ = 7.0-7.5
\= / (9H,m)
r~A30 141 -32-
C~2~ ~2699
18 -C H H H H H 'H-NMR (CDCI3),
7 15 ~; = 0,90 (3H,t);
1.2 (.3H,m); 1.50
(2H,m); 2.85
(2H,t); 7.15
(2H,m); 7.35
(2H ,m) .
19 H H H H 'H-NMR (CDCI3),
--CH2~ 7, 1 7,4 (9H,m).
H H H H 'H-NMR (CDCI3),
8= 1.4-1.8
\ J (8H,m); 3.30
(IH,m): 7.15
(2H,m); 7.35
(2H,m).
21 -(CH2),OCH3 H H H H 'H-NMR (CDCI,),
~ = 3.0 (2H,t);
3.30 (3H,s); 3.5
(2H,t); 7.2 (2H,m);
7.45 (2H,m).
S æ CH H H H H 'H-NMR (CDCI,),
_~ 3 ~; = 1.45 (3H,d);
3.90 (IH,q); 7.10
Ph (2H,m); 7.2-7.4
(7H.m).
23 C H H H H H 'H-NMR (CDCI3),
_~ 4 9 S = 0.7-0.9
(6H,m) 1.1-1.7
C2H5 (8H,m); 2.90
(2H,d); 7.15
(2H,m); 7.35
(2H,m).
24 -Cl6H33 H H H H 'H-NMR (CDCI3),
= 0.90 (3H,t);
1.2 (26H.m); 1.5
(2H,m); 2.85
(2H,t); 7.20
(2H,m); 7.30
(2H,m).
-C H H H H H 'H-NMR (CDCI3),
15 37 ~j = o.gO (3H,t);
0.2 (28H,m); 2.85
(2H,t); 7.15
(2H,m); 7.35
(2H,m).
C~21 82699
26 A H H H H 'H-NMR (CDCI3),
--(CH2)~ ) 2.5 (2H,t); i.9
(2H,t); 7.1-7.4
(9H,m).
27~ H H H H 'H-NMR (CDCI,),
--(CH2)3 N 0 2.40 (6H), 2.95
(2H,t); 3.70
(4H,m); 7.15
(2H,m); 7.35
(2H,m).
28-Cl4H29 H H H H 'H-NMR (CDCI3),
~i = 0.85 (3H,t);
1.25 (22H,m); 1.5
(2H,m); 2.90
(2H,t); 7.15
(2H,m); 7.35
(2H,m),
29-(CH2)rOc~H2 H H H H 'H-NMR (CDCI,),
= 0.8 (3H,t); 1.2
(2H,m); 1.5
(2H,m); 1.8
(2H,m); 3.0 (2H,t);
3.4 (4H,m); 7.1
(2H,m); 7.35
(2H,m).
530 --CHz CH2 H H H H 'H-NMR (CDC13),
\~ 3.4 (2H,s); 4.85
CH (IH,s); 4.90
3 (IH,s); 7.15
(2H,m); 7.35
(2H,m).
31 ~ H H H H m.p. = 158C
~, SO2
32 -C H H H F H 'H-NMR (CDCI3),
s ,, ~ = 0.8 (3H,t); 1.2
(lOH,m); 1.5
(2H,m); 2.9 (2H,t);
6.85 (IH,m); 7.05
(IH,m); 7.20
(IH,m).
33 H H H H 'H-NMR (CDCI3),
/~ S = 3.95 (2H,s);
--CH2 ~ 6.35 (2H,m); 7.2
O (2H,m); 7.3
(3H,m).
rPA30 141 ~34~
.. . .
~A2~ 826q9
34 -CH3 H H F H 'H-NMR (CDCI3).
S = 2.8 (3H.s);
6.9 (IH,dt): 7.1
(IH.dd); 7.25
(IH,m).
H H H H 'H-NMR (CDCI3).
(CH2~20~3 ~3 _ 3;2 (2H,t); 4.0
(9H.m).
36 _~ H H H S = 1.0-2.0
/ (lOH,m); 2.50
(IH,m); 7.2
(2H.m); 7.35
(2H.m).
37 -CH2CHlOH H H H H 'H-NMR (CDCI3).
~3 = 2.0 (IH.br);
3.05 (2H.t); 3.70
(2H.t); 7.15
(2H.br); 7.35
(2H.br).
5 38 --CH2CI H--CH3 H H H ~; = 1.05 (3H.d);
OH 2.50 (IH.br); 2.70
(IH,m); 3.05
(IH,m); 3,g5
(IH,m); 7.15
(2H,m); 7.35
(2H,m) .
39 -C(CH3)~ H H H H 'H-NMR (CDCI3),
~ = 1.05 (9H,s);
7.05 (2H,m); 7.15
(2H.m).
40 C H H H H H 'H-NMR (CDCII),
7 ~i = 0.80 (3H,t);
1.0 (3H,d); 1.20
CH3 (2H.m); 1.50
(2H,m); 2.80
(IH,m).
41 N H H H H 'H-NMR (CDCI3),
--(CH )--N ~ ~i = 2.05 (2H,m);
2 3 ~ N 2.85 (2H,t); 4.30
(2H.t): 7.15
(2H,m); 7.35
(2H,m); 7.95
(IH,s); 8.10
(IH,s).
Le A 30 141 - 3~ -
~,A21 82699
. ~
42 CH2CH(C2Hs)(CH3) H H H H 'H-NMR (CDCI3),
~,~ ~=0.7-0.8
C H (9H,m); 7.15
2 5 (2H,m); 7.35
(2H,m).
43 CH CH OC H H H H H 'H-NMR (CDCI3),
_~ 2 2 2 5 ~i = 0.9 (3H,d);
CH 1.05 (3H,t); 1.5
3 (H,m); 1.9 (IH,m);
3.05 (IH,m); 3.45
(4H,m): 7.15
(2H,m); 7.35
(2H,m) .
44 H H H H 'H-NMR (CDCI~),
~OC H ~i = 1.05 (3H,t);
2 5 ~ ~ 1.1-2.5 (9H,m);
3.2-3.5 (3H,m)
7.15 (2H,m); 7.30
(2H,m) .
H H H H 'H-NMR (CDC12),
--CH2{ ~ (IIH,m); 2 75
(2H,d): 7.20
(2H,m) 2.35
(2H,m).
5 46 -CH2CH(OC2H~)2 H H H H 'H-NMR (CDC12).
~ = 1.05 (6H,t)
3.5-3.8 (6H,m); 4.5
(2H,t); 7.15
(2H,m); 7.35
(2H,m).
47 ,CH2CN H H H H 'H-NMR (CDCI3),
--CH ~ = 0.95 (3H,t);
I.sO (2H,m); 2.50
C2Hs (IH,m); 2.40
(2H,d); 7.1
(2H,m): 7.3
(2H,m).
48 -cycloCqC~5 H H H H 'H-NMR (CDCI,),
~ = 1.2-2.0
(14H,m): 2.80
(IH,m); 7.15
(2H,m); 7.30
(2H,m).
A ~0 141 - 36 -
~A21 82699
. ~
49 -(CH,CH2O)2-C~H9 H H H H 'H-NMR (CDCI,),
~ = 0.85 (3H,t);
1.30 (2H,m); 1.50
(2H,m); 3.15
(2H,t); 3.40
(ZH,m~; 3.5-3,7
(6H,m); 7.15
(2H,m); 7.35
(6H,m).
50 -(CH CH O) CH H H H H 'H-NMR (CDCI3),
2 2 2 3 ~ = 3.30 (3H,s);
3.3-3.9 (8H,m);
7.15 (2H,m); 7.35
(2H,m).
51 -(CH CH2O) CH3 H H H H 'H-NMR (CDCI3),
2 3 ~ ~ -3.15 (3H,t);
3,38 (3H,s); 3.5-
3,7 (lOH,m) 7.1
(2H,m); 7.3
(2H,m).
52 -(CH2CH2O)3C2H, H H H H 'H-NMR (CDCI3),
3.12 (2H,t); 3.5-3.8
(12H,m); 7.1
(2H,m); 7.35
(2H,m).
S 53 -CH(Ph)CO2'Bu H H H H m.p.: 132-133C
54 -CH(CH Ph)CO Et H H H H 'H-NMR (CDCI3),
2 2 ~ = 1.01 (3H);
2.98-3.27 (2H);
3.73 (IH); 3.90
(2H); 7.09-7.36
(9H).
55 -CH(i-Pr)CO2Et H H H H 'H-NMR (CDCI3),
= 0.94 (6H);
1.21 (3H), 2.35
(IH); 3.31 (IH);
4.03 (2H); 7.12-
7 39 (4H)-
56 -CH(i-Pr)CONHCH(CH3)Ph H H H H m.p.: 156.9C
57 -CH(CH3)CO2lBu H H H H m.p.: 98C
0 58 -(CH ) CO,'Bu H H H H 'H-NMR (CDCI3),
2 3 _ ~ = 1.43 (s,9H);
2.48 (t,2H); 3.16
(t,2H); 7.14-7.36
(4H) .
LeA3D 141 -37-
~A21 82699
.~
59 -CH2-CO2'Bu H H H H 'H-NMR (CDCi3),
~i = 1.47 (s.9H):
3.66 (s,2H); 7.15-
7.36 (m,4H).
60 -CH(CH3)CO2CH, H H H H 'H-NMR (CDCI3),
~i = 1.37 (d,3H);
3.60 (q,lH); 3.70
(5,3H); 7.13-7,34
(4H) .
61 -CH(CH2-CH(CH3)3)CO2CH3 H H H H 'H-NMR (CDCI3),
~i = 0.86 (6H);
1.53 (IH); 1,67
(2H) 3.50 (IH);
3.55 (3H); 7.13-
7,34 (4H)-
62 -CH(i-ijr)CO,'Bu H H H H 'H-NMR (CDCI3),
~i = 0.96 (6H);
1.39 (9H); 3.19
(IH) 7.1Z-7.32
(4H),
S63 -C(CH3)2-CH2-C(CH3)3 H H H H 'H-NMR (CDCI3),
~i = 1.00 (9H):
1.04 (6H); 1.58
(2H); 7.05-7.28
(4H).
64 CH3~--\ CH3 H H H H m.p.: 178C
~< ~CH3
\-- CH3
65 -C(CH3),-(CH2)5C(CH3)3 H H H H 'H-NMR (CDCI3),
~5 = 0.60-1.60
(25H); 7.08 (2H);
7.25 (2H).
Le A 3Q 141 - 38 - -
~ ~21 82699
Use E~ample:
In order to l'~ the activity against fungi, the minimum inhibitory
w..,~"~ ."~ (MlCs) of .~ ;l"..l~ according to the invention are ,~ "....,~l
An agar prepared usmg malt extract is t~ed with active C~nlro~n~1~ acwrding to
S the invention in ~llll-. .,i-.. l;--.,~ of f~om 0.1 mg/l to 5000 mg/l. A~er ~.. 1.. 1;~,";/.. , Of
the agar, it is ~ ~ with pure cultures of the test org~nisms listed in Table 1.
Afterstorageat28Cand60to70%relativeA~ humidityfor2weeks,theMlCis
determined The MIC is the lowest ~ \ of active t~ll~ml1 ~ which the species of
microbe used shows no grow~, and is mdicated in ~able 3 below.
10 T~le 3
Co ound MIC/ MICIppm MIC/
mp ppm ~h~nmillm Aspergillus
brevicule globosum niger
>600 >100 >600
--C8H17
~600 <100 <600
T~A30 141 ~39~
.. . ..