Note: Descriptions are shown in the official language in which they were submitted.
WO 95/23386 PCTIUS95/01997
, f.
= ~~0~~4~':
IMPROVED METHOD AND APPARATUS FOR
MICROSCOPIC SCREENING OF CYTOLOGICAL SAMPLES
Background of the Invention
The present invention relates to the methods
and apparatus for microscopic screening of cytological
samples, and more particularly to an improved
computerized method and apparatus for more reliably
examining and screening Pap smears and other cytological
samples for abnormal cells (atypia).
Typically, the screening of microscopic samples
on a slide is accomplished by a cytotechnologist manually
moving a microscope stage on which the slide is mounted
so as to scan and view, through a microscope, each of the
approximately sixty-thousand cells that are contained on
the slide. In a typical laboratory environment the
cytotechnologist is expected to review over ten such
slides per hour. Thorough examination is required since
a single abnormal cell (atypium) in the approximately
sixty-thousand cells, may be present. Importantly, the
single atypium may be sufficient to achieve early
detection of, e.g., an incipient carcinoma or symptom of
cervical cancer. Unfortunately, the false negative rate
in commercial screenings, such as Pap smear screenings,
is typically 10% or worse, even though a rate of 1% to 2%
is medically achievable.
After the cytotechnologist has scanned the
slide, each suspected positive, that is those slides
containing possible atypia, and a portion of those slides
that the cytotechnologists have determined contain no
abnormal cells, are rescreened by a pathologist for a
full-diagnosis. To facilitate atypia relocation, the
cytotechnologist physically marks the slides with a p,tint
dot placed near the abnormal cell or cells. The
pathologist can then restrict his or her rescreening zo
searching in the vicinity of the paint dots for the
W0 95123386 PCT/US95101997
218308f. =
-2-
suspected abnormal cell or cells found by the
cytotechnologist. Unfortunately, because of inexactness
in the location of such paint dots and the size of the
paint dots relative to the suspected abnormal cell or
cells, considerable time is still required for the
pathologist to relocate the suspected abnormal cells and
to fully diagnose each rescreened slide.
Thus, in practice, much of the time spent by
the pathologist, and by the cytotechnologist, is
unfortunately spent performing non-diagnostic functions
such as relocating suspected abnormal cells. Because such
non-diagnostic tunctions distract from more important
diagnostic functions, they contribute to the above-
mentioned high false negative rate. Furthermore, the
additional time that is spent screening each slide
significantly increases the costs associated with routine
medical screenings, such as Pap smears.
One prior apparatus and method which addresses
the foregoing is described in U.S. Patent No. 3,851,972
('972 patent), issued December 3, 1974. In the method of
the '972 patent, individual cells are relocated through
computer memorization of coordinate signals
representative of a specific cell's location on the
slide. Cell looation is memorized as stage x and y
coordinates, (as taken from step counters) referenced to
the instrument's "Home" position, that is the position of
the stage when the slide is loaded onto the stage. When
the slide is reloaded onto the microscope stage, a
mechanical assembly returns the slide to its original
position based upon the memorized coordinates.
Unfortunately, such operation requires highly repeatable
and precise repositioning relative to the stage's home
position, and relative to the instrument's optical axis.
In practice these requirements result in excessively
demanding tolerances on mechanical components and
WO 95/23386 PCT/US95/01997
~ 21830 81
-3-
electrical drive circuitry, with correspondingly high
hardware cost.
In other instruments unrelated to automated
microscope systems, such as in numerical-controlled
machine tools, and in some research automated microscope
system employed in scientific research, closed-loop
positioning systems (positioning servos) incorporating
linear encoders or similar sensors are used to provide
precise positioning (sub-micron accuracy) of a controlled
element. Such research automated microscope systems
utilize a specialized precision stage in order to achieve
such precise positioning. While the degree of precision
permitted by such systems may satisfy the requirements
for some microscopy applications, the hardware cost is
much too high to permit their use in commercial automated
microscope systems.
Another problem in screening microscopic
samples is that the microscope used by the
cytotechnologist may be a different microscope than that
used by=the pathologist to later relocate the possible
atypia. This is common since a pathologist will usually
work with and rescreen the slides of a number of
cytotechnologists. Unfortunately, instruments and
systems such as those described above, which record the
location of atypia, or other features, using stage-
dependent x and y coordinates relative to the home
position of particular microscope stages, are not useful
unless the pathologist is using a microscope that is
identical to the microscope used by the cytotechnologist.
This is because the location of the possible atypia are
recorded relative to the home position on a particular
stage of a particular microscope. Both the locations of
the home position, and the measured location of the
atypia relative to the home position, are highly
dependent on the mechanical structure and precision of
the microscope stage. Furthermore, the automated
WO 95123386 PCT/US95/01997
21.83081 ~
-4-
equipment or automated positioning system used to
reposition the microscope to the exact location of the
possible atypia must be extremely precise, and must
perform identically in each of the microscopes unless
positioning servos are utilized so as to compensate for
differences in movement patterns of, e.g., the stages of
the two microscopes. In either case, such equipment is
very expensive.
Even such prohibitively expensive microscope
systems do not-offer a complete solution to the problems
created by the use of different microscopes during
screening and rescreening. As such microscopes are used,
and thus, as the stage and positioning equipment wears,
such systems become out of tolerance. As a result,
accurate repositioning of a microscope, based on position
information obtained from another microscope, becomes
increasingly difficult, and eventually impossible.
Thus, a further problem with the system of the
'972 patent arises because the '972 system uses
coordinates that are-measured relative to a particular
stage. Specifically, both (i) the discrete motor steps
along the x and y axes that are counted and used to
identify locations on the slide and (ii) the angular
relationship between the x and y axes, are dependent on
the particularautomated positioning equipment used to
position the stage of the '972 system. In other words,
the '972 system uses a microscope-dependent coordinate
system to identify the location of the features on the
slide. Thus, microscopes other than the microscope used
to locate the features (or an identical-microscope) are
not able to accurately relocate the features. Therefore,
the '972 system is not suitable for use in a multiple
microscope environment.
The present invention advantageously addresses
and overcomes the forgoing problems and shortcomings of
prior microscope systems.
WO 95/23386 PCT/US95101997
= 2183081
-5-
Summary of the Invention
The present invention overcomes such problems
and shortcomings by providing an improved and simplified
automated microscope positioning system and method for
relocating features within a sample. The system operates
independently of the particular microscope on which the
features are initially located, and/or relocated.
In operation, a microscope system is used to
relocate at least one featuire within the sample. The
sample has been previously located, and the location has
been stored in a memory device using a microscope-
independent coordinate system.
To relocate the at least one feature using the
microscope system, the sample is mounted against a stage,
which moves within a microscope-dependent coordinate
system and relative to a lens assembly of the microscope.
The location of the at least one feature is recalled from
the memory device, and transformed from the microscope-
independent coordinate system into the microscope-
dependent coordinate system. A field of view, which is
defined by the lens assembly, is moved by moving the
stage relative to the lens assembly so as to position the
field of view on the location of the at least one feature
on the sample. Thus, the at least one feature is
relocated within the sample using the microscope.
In accordance with one embodiment of the
invention, a first calibration slide is mounted on the
stage of the first microscope. The calibration slide
contains at least one fiducial marking that may consist
of three fiducial points that are at non-collinear points
on the first calibration slide, or may consist of any
other marking or set of markings that are sufficient to
define a two dimensional coordinate system (for example,
two points and an angle, in the case of an oblique
coordinate system).
WO 95/23386 s PCT/US95101997
-6-
Note that instead of the first calibration
slide, many other calibration means are contemplated
within the scope of this invention. For example, the
fiducial marking may be imprinted directly onto the
stage, or onto the sample. The calibration means need
only contain fiducials that (1) define a coordinate
system, and (2) can be fixed in a position relative to
the stage during calibration of the stage which consists
of storing the locations of the fiducial markings.
Through the first microscope, a portion of the
slide is viewed. A first "field of vi-ew" is defined by
the first microscope and is most commonly equated with
that portion or area of the slide that is viewed.
Alternatively, the first field of view may be some lesser
area that is identified by a box or circle, by cross-
hairs, or the like. The field-of view may also be a point
on the portion of the slide that is viewed.
After mounting the calibration slide on the
stage, the first microscope positioning system is
calibrated by reading and storing in a memory device the
location of the at least one fiducial marking using a
first microscope-dependent coordinate system. The
microscope-dependent coordinate system may be an oblique
or Cartesian coordinate system, having a "Home" position
(or an origin), an x-axis (along which an abscissa is
measured), and a y-axis (along which an ordinate is
measured). Note that as used herein the term oblique
coordinate system means any coordinate system using two
straight line non-parallel axis, such axes may be
orthogonal or non-orthogonal. The first microscope-
dependent coordinate system need not, however, be
orthogonal, i.e., the x-axis need not be normal to
(rotated 90 relative to) the y-axis, but may not be
parallel to the y-axis. Alternatively, the microscope-
dependent coordinate system may be a polar coordinate
system, an elliptical coordinate system, a parabolic
WO 95/23386 PCT/US95/01997 -7-
coordinate system, a hyperbolic coordinate system, or the
like.
Next, a sample is mounted against the stage of
the first microscope. The sample, e.g., blood cells, that
contains one or more features that are abnormal or
atypical cells (or atypia). The sample may also be a
crystalline material, such as a metal, and the features
may be discontinuities or other flaws in the structure of
the crystal. In any case, the field of view is moved to
the location of one of the features, where the location
is defined in the first microscope-dependent coordinate
system. The location of the at least one feature (defined
in the microscope-dependent coordinate system) is then
transformed into a microscope-independent coordinate
system. Such transformation is achieved using the
previously stored location of the at least one fiducial
marking on the calibration slide as the basis for a new,
fiducial-based coordinate system independent of the
microscope in which the sample is mounted. Preferably,
the transformation is from the above-mentioned oblique
coordinate system, to another oblique or Cartesian
coordinate system that is independent of the microscope.
The location of the feature is stored in the
memory device using the microscope-independent coordinate
system, and the sample is removed from the microscope
stage.
Preferably using a second microscope, a second
calibration slide is mounted against the stage of the
second microscope. The second calibration slide may be
the same as or substantially identical to the calibration
slide used in the calibration of the first mentioned
microscope (tolerance being dependent on the particular
application of the present invention--based on the size
of the features to be relocated, how difficult the
features are to manually relocate once the field of view
is moved to a location near the features, and generally,
WO 95/23386 PCT/iTS95/01997
2183 as1 =
-8-
the precision desired for the particular application).
The second calibration slide contains the at least one
fiducial marking located in substantially the same
location as the at least one fiducial marking on the
first mentioned calibration slide.
A portion of the second calibration slide is
viewed, and a second field of view is defined by the
second microscope. The second field of view may be of
any of the above-mentioned types. Then, using the
calibration method, the second microscope is calibrated
using the at least one fiducial marking and a second
microscope-dependent coordinate system, based on the
second microscope.
Next, the sample containing the features to be
relocated is mounted against the stage of the second
microscope. The stored feature location is then read
from the memory device, and transformed from the
microscope-independent coordinate system to the second
microscope-dependent coordinate system.
= Finally, the feature locating information in
the second microscope-dependent coordinate system is
utilized to move the second field of view to the feature
to be relocated in the sample. As a result, the feature
is relocated within the sample independent of the
particular microscope used to locate or relocate the
feature.
The invention also may be characterized as an
apparatus for carrying out the above-described method.
The at least one feature within the sample is previously
located, and the location is stored in the memory device
using the microscope-independent coordinate system.
The apparatus has (1) means for mounting the
sample against the stage of the microscope. A field of
view is defined by the lens assembly of the microscope.
The apparatus also includes (2) means for recalling from
the memory device the location of the at least one
WO 95/23386 PCT/US95/01997
= !
-9-
feature, and (3) means for transforming the location of
the at least one feature from the microscope-independent
coordinate system into the microscope-dependent
coordinate system. Finally, the apparatus has (4) means
for moving the field of view by moving the stage relative
to the lens assembly so as to position the field of view
on the location of the at least one feature in the
sample. Thus, an apparatus is provided for relocating the
at least one feature withinthe sample.
In another embodiment, the invention may be
characterized as a microscope system including: (1) a
first microscope having a first stage; (2) a first
calibration slide containing at least one fiducial
marking; (3) means for mounting the first calibration
slide against the first stage; (4) means for viewing a
portion of the first calibration slide with the first
microscope and defining a first field of view; (5) means
for calibrating the first microscope by recording the
location of the at least one fiducial marking using a
first microscope-dependent coordinate system; (6) means
for mounting a sample containing slide against the first
stage, wherein the sample contains at least one feature;
(7) means for moving the first field of view to the
location of the at least one feature, wherein the
location of the at least one feature is defined in the
first microscope-dependent coordinate system; (8) means
for transforming the location of the at least one feature
to a microscope-independent coordinate system, wherein
the means for transforming is responsive to the means for
calibrating; (9) means for storing the location of the at
least one feature using the microscope-independent
coordinate system; (10) a second microscope having a
second stage; (11) a second calibration slide that is the
same as or substantially identical to the first
calibration slide, and having at least one fiducial
marking that is in a substantially similar location on
WO 95123356 PCT/US95/01997
2183081
-10-
the second calibration slide as the at least one fiducial
marking on the first calibration slide; (12) means for
mounting the second calibration slide against the second
stage of the second microscope; (13) means for viewing a
portion of the second calibration slide wherein a second
field of view is defined by the second microscope; (14)
means for calibrating the second microscope by recording
the location of the at least one fiducial marking using a
second microscope-dependent coordinate system; (15) means
for mounting the sample against the second stage; (16)
means for reading from storage the location of the at
least one feature in the microscope independent
coordinate system and for transforming the at least one
feature location to the second microscope-dependent
coordinate system; and (17) means responsive to the
location of the at least one feature in the second
microscope-dependent coordinate system for moving the
second field of view to the location of the at least one
feature on the sample.
Brief Descrintion of the Drawings
The above and other aspects, features and
advantages of the present invention will be more apparent
from the following more particular description thereof,
presented in conjunction with the following drawings
wherein:
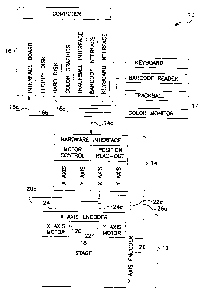
FIG. 1 is block diagram of a microscopic
screening apparatus made in accordance with the present
invention;
FIG. 2 is a top view of a calibration slide
made in accordance with the invention and suitable for
use in conjunction with the microscopic screening
apparatus of FIG. 1;
FIGS. 3A and 3B are side and front views
respectively of a microscope than includes first and
second stepper motors and first and second linear
WO 95/23386 PCT/US95/01997
= 218~08i'
-11-
encoders for use in the microscopic screening apparatus
of FIG. 1;
FIG. 4 is a flow diagram showing steps
traversed by the microscopic screening apparatus of FIG.
1 during a setup phase;
FIG. 5 is a flow diagram showing steps
traversed by the microscopic screening apparatus of FIG.
1 during a storage phase;
FIG. 6 is a flow diagram showing steps
traversed by the microscopic screening apparatus of FIG.
1 during a retrieval (or relocating) phase; and
FIG. 7 is a flow diagram showing steps
traversed during the storage phase of FIG. 5, including
additional steps that comprise a scanning routine.
Corresponding reference characters indicate
corresponding components throughout the several views of
the drawings.
Detailed Description of the Invention
= The following description of the presently
contemplated best mode of practicing the invention is not
to be taken in a limiting sense, but is made merely for
the purpose of describing the general principles of the
invention. The scope of the invention should be
determined with reference to the claims.
Referring first to FIG. 1, a block diagram is
shown of a microscopic screening apparatus 10 made in
accordance with the present invention. The screening
apparatus includes (1) a microscope 12; (2) an interface
unit 14; and (3) a processor 16.
The microscope 12 includes a stage 18 that is
preferably coupled to an x-axis stepper motor 20, and a
y-axis stepper motor 22. The stepper motors 20, 22 are
used to position the stage along substantially linear x
and y axes, respectively, which need not be orthogonal
(rotated 90 relative to one another), but must not be
CA 02183081 2002-05-16
-12-
parallel. A suitable stepper motor and driver system is
available as model CT 32-39 from Compumotor of
California. The stepper motors 20, 22 position the stage
in response to the x and y positioning signals 20a and
22a, respectively, that are received from the interface
unit 14.
Also coupled to the stage 18 are an x-axis
encoder 24 and a y-axis encoder 26. The encoders 24, 26
*
are preferably linear encoders such as model number MSA
001 available from RSF Elektronik of Germany. In
operation, the linear encoders 24, 26 produce one
electrical pulse for each unit, e.g., micron of travel by
the stage along the x or y axes. Note that the encoders
described herein are accurate to within two to three
microns, which is adequate for viewing animal or plant
cells. As the stage moves in one direction along the x
axis, for example, an x-axis counter included whether the
encoder 24 counts (or accumulates) the electrical pulses
generated by the x-axis encoder 24. When the stage moves
in the other direction along the x axis, the counter
reduces the number of electrical pulses accumulated in
the counter. As a result, the counter provides an
accurate indication as to the precise position of the
stage along the x-axis relative to an origin. In the
event the stage reverses its direction, the electrical
pulses are assigned a negative sign, and are therefore
subtracted from the number of electrical pulses counted
by the x-axis counter. The y-axis encoder 26 operates
similarly along the y-axis of the stage using a y-axis
counter included within the y-axis encoder. Thus, by
reading the number of counted electrical pulses from x
and y-axis counters, the microscopic screening system is
able to precisely determine the location of the stage 18
(relative to an origin at some position along the x and y
axes). The x and y-axis counters included in the x and y
axis encoders, generate x and y-axis position signals 24a
*Trade-mark
CA 02183081 2002-05-16
-13-
and 26a, respectively, that are coupled to the interface
unit 14.
The interface unit 14 is coupled to the
processor 16, and is preferably a model PC-23 motion
controller available from Compumotor of California. The
interface unit 14 receives a control signal 14a rrom the
processor 16 that is used to generate the x and y-axis
positioning signals 20a and 22a that control the stepper
motors 20, 22. The processor receives a location signal
16a from the interface unit 14 that is generated in
response to the x and y-axis position signals generated
by the linear encoders 24, 26. Therefore, the location
signal, which is preferably a digital signal, is
indicative of the location of the stage 18.
In response to the location signal 16a, the
processor 16 determines the location of the stage 18
after it has been positioned by a first operator, for
example a cytotechnologist or other person responsible
for locating features in the sample. (The term
cytotechnologist is used herein as an example of one
possible first operator, however it is to be understood
that the first operator need not be a cytotechnologist.
For example, a pathologist can serve as the first
operator.) In addition, the processor 16 can position the
stage 18 using a positioning servo in conjunction with
the control signal 16a as an output signal and the
location signal 14a as a feedback signal. The positioning
servo may be implemented in a software servo routine such
as the software servo routines commonly coded into
interface units such as the PC-23 interface unit, or
included in documentation that accompanies such interface
units. See, e.g., "PC-23 Indexer Users' Guide."
Note however, that some adjustment must be made
to the software servo routines in order to use the linear
encoders 24, 26 in conjunction with the stepper motors
20, 22 described above. This is because the linear
*Trade-mark
WO 95/23386 PCT1US95/01997
-14-
encoders 24, 26 are more accurate than the stepper
motors. That is, the encoders have more "steps" (or
possible distinguishable positions) than the stepper
motors 20, 22. As a result, the processor 16 can direct
the interface unit 14 to position the stage 18 at a
prescribed encoder position, to which the stepper motors
20, 22 are incapable of moving the stage 18. Unless
appropriate modifications are made to the software servo
routines provided in the PC-23 or other interface unit
documentation, the stage may "jitter" between two stepper
motor positions that bracket the desired encoder
position. That is, the encoder 24 and/or 26 will
oscillate between indicating that the stepper motor 20,
22 has moved past the desired location and indicating
that the stepper motor has not yet reached its desired
location. In order to modify the servo routines, a
tolerance must be built into the x and y-axis position
signals 24a and 26a. For example, if "110" is the
desired x-axis position signal (indicating x-axis encoder
position 110), the servo is implemented so as to indicate
that stepper motor 20, 22 has reached the location of the
feature whenever the encoder is within 3 of 110. Such
adjustments to the software servo routine can easily be
made by one skilled in the art and are dependent on the
exact hardware used to implement the present invention.
The processor 16 performs the above-described
functions in response to a control program, which is
preferably a control program made in accordance with the
flow diagrams described below.
Referring next to FIG. 2, a top view is shown
of a calibration slide 28 made in accordance with a
preferred embodiment of the present invention. The
calibration slide 28 contains a first fiducial 30, a
second fiducial 32 and a third fiducial 34, which are at
non-collinear points on the calibration slide 28.
CA 02183081 2002-05-16
-15-
Referring next to FIGS. 3A and 3B, side and
front views, respectively, are shown of a microscope 36
that includes the x-axis stepper motor-20 (or first
stepper motor), and the y-axis stepper motor 22 (or
second stepper motor). Also shown are the x-axis encoder
24 (or first linear encoder) and the y-axis encoder 26
(or second linear encoder). The microscope 36 may be of
conventional * design. A suitable microscope is available
as model BHT from Olympus of New York. The stepper motors
20, 22 are mounted beneath the stage 18 and move the
stage 18 as described above. The encoders 24, 26 are
mounted along adjacent edges of the stage 18, and also
operate as described above. A suitable microscope stage
18, capable of being modified with the encoders 24, 26
and stepper motors 20, 22, is available as model BH2-SVR
*
or BH2-SVL available from Olympus of New York.
Also shown as a part of the microscope 36 is a
lens assembly 38 through which the operator can view a
magnified image of a portion of a slide that is mounted
on the stage 18. As the stage 18 is moved by the stepper
motors, the image viewed by the lens assembly 38 also is
moved so as to view another portion of the slide 18.
Generally, the portion of the slide 18 that is viewed
constitutes a field of view, however the field of view
may be some lesser portion of the slide such as a lesser
portion identified by cross-hairs, a circle or square, or
the like.
Because the location of the stage 18 is
precisely indicated by the x and y-axis position signals
24a and 26a as the stage 18, and therefore the field of
view, is moved, the precise position of the field of view
is also represented by the x and y-axis position signals.
Referring next to FIG. 4 a flow diagram is
shown of steps traversed by the microscopic screening
apparatus during a setup phase. In operation, a setup
routine of the control program is executed within the
*Trade-mark
WO 95/23386 PCT/US95101997
h~Q~~f1i ~
-16-
processor 16 during the setup phase. Before beginning
the setup process, however, the calibration slide 28 is
mounted against the stage 18 by the first operator (e.g.,
the cytotechnologist or pathologist). -
The setup routine is initialized (Block 1000)
by setting a counter c(or second counter) equal to one.
Note that the second counter may be a location in a
memory device, or may be a separate integrated circuit
counter. It is to be understood, however that the counter
a need not be a integrated circuit capable of executing
counting function, but can be merely location in the
memory device that is accessed by the processor so as to
perform functions of an integrated circuit counter. Next,
the processor 16 instructs the interface unit 14 using
the control signal 14a (which is preferably a command
signal to the PC-23 interface unit generated in response
to the control program) to move the stage 18 to an
estimated location of the first fiducial 30. The
estimated location of the first fiducial is preprogrammed
into the processor. In response to the control signal
14a, the interface unit generates the x and y-axis
positioning signals 20a and 22a, and in response to the x
and y-axis positioning signals, the stepper motors 20 and
22 move (Block 1002) the stage 18 so that the estimated
location of the first fiducial is at, or within the field
of view.
When the processor has positioned the field of
view on the estimated location, the cytotechnologist
centers (Block 1004) the fiducial 30, ifnecessary. This
may be accomplished by the cytotechnologist operating a
digitizing device such as a joy stick, mouse, or
trackball 17 that is coupled to the processor 16. Such
digitizing devices are of conventional design and are
well known in the art. When the first fiducial 30 is thus
centered, the cytotechnologist indicates to the processor
that the location of the first fiducial is now ready to
W 0 95/23386 PCT/US95101997
. ~j
-17-
be recorded. Such indication may be accomplished by the
cytotechnologist pressing or "clicking" a button (Block
1006), as is known in the art. In response to the
indication from the cytotechnologist, the location of the
stage, as indicated by the location signal 16a, is read
and recorded (Block 1008) in the memory device within the
processor. The memory device may be a conventional
integrated circuit memory device,or may be a magnetic
disk (floppy disk 16b qrhard disk 16c) or tape, a punch
card, an optical disk, orany other volatile or non-
volatile memory or the like. As mentioned above, the
location of the stage 18 is determined in response to the
location signal 16a, which is generated by the interface
unit in response to the x and y-axis position signals 24a
and 26a that are generated by the encoders 24, 26.
Next, the counter a is tested (Block 1010) to
see whether it has reached three, and then incremented
(Block 1012). The processor 16 causes the stepper motors
to move (Block 1002) the stage 18 to the location of the
second fiducial 32 in the manner described above and
again the cytotechnologist centers the fiducial (Block
1004). The cytotechnologist indicates that the second
fiducial 32 is positioned (Block 1006), and the location
of the second fiducial is determined and stored (Block
1008).
The counter a is again tested (Block 1010), and
incremented (Block 1012). The processor 16 moves the
stage 18 (Block 1002) to the position of the third
fiducial 34 and the cytotechnologist centers the fiducial
(Block 1004). The cytotechnologist indicates that the
third fiducial is positioned (Block 1006), and the
location of the third fiducial is determined (Block 1008)
and stored. At this point the calibration of the
microscope system is complete.
The counter a is tested again (Block 1010), and
because it has now reached three, a first transformation
2183Q81
WO 95123386 PCT/US95/01997
-18-
matrix D-1 is generated (Block 1014). The transformation
matrix D'l is defined as follows:
fi-f2 f2-fi
1_ 1
D det D~-f3 f3 2
wherein
det D = L1f3-f2/(fi-f2)-(f1-f2)1f3f211,
and wherein coordinates fi,f2
I represent the location of
said first fiducial, coordinates f2,f2 represent the
location of the second fiducial, and coordinates f3,f3
represent the location of the third fiducial (as
determined based on the location signal from the
cytotechnologist's microscopic screening apparatus). The
stored locations of the fiducials are also used to adjust
the preprogrammed locations in the processor 16 of the
fiducials (Block 1016), thereby improving the estimated
locations of the fiducials utilized during subsequent
calibrations of the first microscope system.
Finally, the cytotechnologist selects a scan
area on the sample containing slide by, e.g., moving the
field of view to the upper left corner of a rectangle
that defines the scan area, and then moving the field of
view to the lower right corner of the rectangle. The scan
area, i.e., the rectangle, defines limits that will be
utilized by the processor 16 during execution of a
storage phase, described below, to determine the area of
the sample containing slide over which scanning of the
WO 95/23386 PCTIUS95/01997
= ~18.3081
-19-
sample containing slide will occur. Note that instead of
a single rectangular area, the scan area may be of any
geometric shape, and may consist of multiple
discontinuous areas of the sample-containing slide. The
cytotechnologist also selects a scan velocity for the
scan, or a step size and a step delay; selects a scan
overlap; and selects a starting point for the scan. Such
selections are discussed more completely below.
Referring next to FIG. 5, a flow diagram is
shown of steps traversed by the microscopic screening
apparatus during a storage phase. The storage phase may
be carried out by the processor 16 in response to the
control program. Generally, before the storage phase
begins, the cytotechnologist removes the calibration
slide 28 from the stage 18 and mounts a sample containing
slide against the stage. The sample containing slide
contains a sample, which may consist of human cells, and
which may contain one or more possible abnormal cells
(atypia).
= The cytotechnologist scans (Block 2000) the
sample containing slide for such atypia by moving the
stage, and therefore the field of view, across the sample
(Block 2000). This may be done manually by the
cytotechnologist using the digitizing device (e.g., 17),
as described above, or may be done in response to the
processor 16 executing a scanning subroutine. In response
to the scanning subroutine, the processor 16 generates a
control signal 14a that causes the stepper motors 20, 22
to move the stage 18 in a prescribed scan pattern such
that the field of view is moved across the entire scan
area (or all scan areas) in a prescribed manner.
Specifically, the processor (16) generates the
control signal 14a and instructs the interface unit 14 to
position the field of view at, e.g., the upper left
corner of the rectangle that is the scan area. This is
accomplished by the processor 16 generating a"GOTO"
WO 95/23386 PCT/US95/01997
2193 1 081
-20-
command for specific x-axis and y-axis coordinates of the
upper left corner of the rectangle. The positioning
servos are used to assure that the field of view is
properly positioned at the specific x and y-axis
coordinates. Next, the processor 16 generates a
"VELOCITY" command wherein a direction of travel and a
speed for the field of view to scan across the sample-
containing slide are specified. In response to the
"VELOCITY" command, the interface unit 14 scans the field
of view toward the upper right corner of the scan area at
the scan velocity (set by the cytotechnologist during
execution of the setup routine) by moving the field of
view along a straight line. When the field of view
reaches the upper right corner of the scan area, as
detected by the linear encoders 24, 26 and indicated by
the location signal 16a in response to the x and y-axis
position signals 24a, 26a, the processor 16 steps the
field of view toward the lower right corner by a
prescribed step. The prescribed step positions the field
of view=to scan back across the slide from right to left
and provides for the scan overlap between successive
scans. (The scan overlap is set by the cytotechnologist
during execution of the setup routine.) The scan overlap
is an area along the lower edge of a previous scan that
is rescanned at the top edge of the current scan. The
scan overlap assures that the entire scan area is
scanned, and helps to assure that no atypia are missed
because they are too near to the edge of a scan to be
clearly viewed by cytotechnologist.
In response to another "VELOCITY" command from
the processor 16, the field of view is again scanned
across the sample at the scan velocity. This subsequent
scan follows a straight line parallel to that of the
previous scan, but is toward the left edge of the scan
area, i.e., right to left. The field of view scans across
the scan area until it reaches the right edge of the scan
WO 95123386 PCI'/US95/01997
~ 2183081
-21-
area, at which time it steps toward the lower left edge
of the scan area by the prescribed step.
This process is repeated until the entire scan
area has been scanned by the cytotechnologist. As
alternatives, the cytotechnologist can select scan
patterns that scan bottom to top, left to right or right
to left stepping between successive scans, with right or
left, top to bottom or bottom to top scanning,
respectively. Furthermore, instead of using the
"VELOCITY" command to realize the above-described
velocity-type motion profile, the "GOTO" command, in
conjunction with the step size and step delay (defined
during the setup routine), can be utilized to realize a
frame-type motion profile. Furthermore, in any of these
motion profiles, the cytotechnologist can vary the scan
velocity at any time during the scan using the digitizing
device.
When using the frame type motion profile, the
processor 16 generates the control signal 14a and
instructs the interface unit 14 to position the field of
view at, e.g., the upper left corner of the rectangle
that is the scan area. This is accomplished by the
processor 16 generating the "GOTO" command for the
specific x-axis and y-axis coordinate of the upper left
corner of the rectangle that is the scan area. The
positioning servos can be used to assure that the field
of view is properly positioned at the specific x and y-
axis coordinates. Next, the processor 16 waits for a
period defined by the scan delay and then generates the
"GOTO" command. This "GOTO" command instructs the
interface unit 14 to move the field of view toward the
upper right corner of the scan area to a subsequent area,
or frame, adjacent to the portion of the sample that is
viewed when the field of view is in the upper left corner
of the scan area. The area viewed when the field of view
is in the upper left corner overlaps with the
CA 02183081 2005-12-29
-22-
subsequently viewed area by the scan overlap in order to
help assure that the entire slide is thoroughly scanned.
Next, the processor 16 again waits for the scan delay,
and another 'GOTO" command is issued by the processor 16
in order to move the field of view again toward the upper
right corner of the scan area to the next frame. This
process of waiting and moving is repeated until the field
of view reaches the right edge of the scan area. Then,
the processor 16 steps the field of.view toward the lower
right corner of the scan area by the prescribed step and
waits for the period defined by the scan delay. The
process of waiting and moving is again repeated so as to
move the field of view frame-by-frame back toward left
edge of the scan area. In this way, the frame-type motion
profile is realized, as an alternative to the velocity-
type motion profile.
The scanning routine also allows the
cytotechnologist to manually deviate from-the selected
scan pattern by operating the digitizing device (e.g.,
17). Thus, the cytotechnologist is able to position
groups of..cells within the portion of the slide that is
viewed, even though the scan pattern would otherwise
bring only part of the group into the portion of the
slide that is viewed (during the current scan, and the
remainder of the group into the portion of the slide that
is viewed on the subsequent scan). This operation is
described more completely below.
In any case, when the cytotechnologist locates
a possible atypium (and centers the possible atypium if
necessary using the digitizing device) he or she
indicates that a possible atypium has been located by
pressing the button or "clicking" (Block 2001). In
response to this indication, the processor 16 reads the
location signal 16a (Block 2002) and temporarily stores
such signals (Block 2004). The location signal 16a is
indicative of.the x and y-axis position signals 24a, 26a
tl: .
~ . i
WO 95123386 PCT/US95/01997
=
-23-
that are generated by the linear encoders 24, 26, and
therefore the location signal 16a indicates stage
location while the field of view is on the possible
atypium.
In accordance with the present'invention, the
stage location is translated from the encoder-based
coordinates (microscope-dependent coordinates), as
indicated by the location signal 16a, into a microscope-
independent coordinate system (Block 2006). such
transformation may be achieved as follows:
(1) Read the location of the possible atypium
pa, p.2 which is indicated by the location signal 16a;
(2) Calculate (pt-fs), where p. is a vector
representation of the location of the possible atypia pa,
pa, and E. is a vector representation of the location of
the second fiducial f2,f2; and
(3) Calculate D-1(pe-fs), and where pg-fz is as
defined above and wherein
f?-f2 f2
1 -fi
D_1=
det D f2
2 3 _f2 f3-f2
wherein
det D = f3-f21(f1-f2)-(f1-f2)1f3-f2
as described above.
Note that fa is the origin of the microscope-
independent coordinate system. Thus, ps-f2 represents a
translation of the origin of a first microscope-dependent
- - ---------
WO 95/23386 PCTIUS95/01997
2183081
i
-24-
coordinate system to the microscope-independent
coordinate system (independent of the encoders). After
the origin is translated, the transformation matrix
accounts for magnitudinal (i.e., scale) differences, and
for angular differences between the microscope-
independent coordinate system and the first microscope-
dependent coordinate system. Note that the angular
differences include potentially independent angular
differences in the x and y-axes. Thus, not only is
rotation possible between the two coordinate systems, but
the axes of the coordinate systems may be rotated,
relative to one another, by differing amounts.
Note that the above transformation transforms
the location of the possible atypia in terms of the
encoders p' in the first microscope-dependent coordinate
system into a location in terms of the three fiducials L.
in the microscope-independent coordinate system.
The translated location of the possible atypium
in the microscope-independent coordinate system is then
stored in the memory device of the processor (Block
2008). Next, a feature counter n (or first counter),
which indicates the number of features stored, is
incremented (Block 2010). The feature counter n may be a
location in the memory device or an integrated circuit
counter, as with the counter a. The cytotechnologist then
indicates whether he or she wants to remain in search
mode (Block 2012). In the event the cytotechnologist does
want to remain in search mode (Block 2012), the processor
allows the cytotechnologist to continue searching the
sample (Block 2000) using the digitizing device for
position control. In the event the cytotechnologist does
not want to continue searching the sample (Block 2012),
he or she so indicates by, e.g., pressing the "END" key
on the keyboard (Block 2014), and the storage phase
terminates. In this way, the locations of possible
WO 95/23386 PCT/US95/01997
21830~81.
-25-
atypia within a sample containing slide are located and
stored in the memory device.
As an alternative embodiment of the above
described process, the location of the feature in the
first microscope-dependent coordinate system, instead of
being stored temporarily, may be stored in the memory
device along with the locations of the fiducials in the
first microscope-dependent coordinate system. The above
described transformation of the location of the at least
one feature from the first microscope-dependent
coordinate system into the microscope-independent
coordinate system, in this alternative embodiment, is
performed during execution of a relocating program
(described below) immediately before transformation of
the location of the at least one feature from the
microscope-independent coordinate system into the second
microscope-dependent coordinate system. In this
alternative embodiment, after the above-described
coordinate transformation (from the first microscope-
dependent coordinate system to the microscope-independent
coordinate system) is performed, the location of the at
least one feature is stored temporarily using the
microscope-independent coordinate system, before being
transformed to the second microscope-dependent coordinate
system.
Note that in this alternative embodiment, the
locations of the first, second and third fiducials must
be stored in the memory device along with the location of
the at least one feature in the first microscope-
dependent coordinate system. In the preferred embodiment,
wherein the location of the at least one feature is
translated into the microscope independent coordinate
system during the storage phase, only the location of the
at least one feature in the microscope-independent
coordinate system needs to be stored (more than
temporarily) in the memory device.
WO 95123386 PCT/US95/01997
'~i~lf~~~~ =
-26-
Referring next to FIG. 6, a flow diagram is
shown of steps traversed by the microscopic screening
apparatus during a retrieval (or relocating) phase.
The relocating phase may be carried out by the
processor 16 in response to a relocating program.
Typically, the relocation will be done by a second
operator (e.g., the pathologist) responsible for
reviewing the possible atypia located by the
cytotechnologist. If the pathologist is using a
different microscope and stage (also having a pair of
encoders, pair_of stepper motors, an interface unit and a
processor) than the cytotechnologist, as is typically the
case, the pathologist will first need to calibrate his or
her microscope by traversing calibration steps (Blocks
1000 through 1012) of the setup phase as described above
using a second calibration slide. The pathologist may use
the same calibration slide (the first calibration slide)
to calibrate his or her microscope and stage, or may use
a second calibration slide that contains fourth, fifth
and sixth fiducials. The fourth, fifth, and sixth
fiducials are in substantially the same positions on the
second calibration slide as the first, second and third
fiducials are in on the first calibration slide,
respectively. The tolerance between the first, second and
third fiducials, and the fourth, fifth and sixth
fiducials, respectively, is dependent on the particular
application of the present invention--based on the size
of the features to be relocated, how difficult the
features are to manually relocate once the field of view
is moved to a location near the features, and generally,
the precision desired for the particular application.
During the execution of the calibration steps,
a second transformation matrix will be generated as
follows:
WO 95/23386 PGTIUS95/01997
~ ~~.8308i=
-27-
wherein
fl1-fl1 fl1-fll
D' 3 2 1 2
=
f/2-f/2 f/2-f/2
3 2 1 2
wherein coordinates fl2,f~1 represent the location of the
fourth fiducial, coordinates fl2,f'2 represent the
location of the fifth fiducial, and coordinates fl3,fl3
represent the location of the sixth fiducial. Note that
the prime "I" indicates that the second transformation
matrix and the three fiducial locations immediately above
are determined using the location signal generated by the
pathologist's interface unit, whereas the first
transformation matrix and previously mentioned fiducial
locations are determined using the cytotechnologists
microscopic screening apparatus.
Next, the pathologist removes the calibration
slide and mounts the sample containing slide containing
the previously located possible atypia. The pathologist
instructs the microscopic screening apparatus to locate
the first atypia by pressing the button or "clicking".
In response to such instruction, the retrieval phase
begins by setting a first flag n equal to the number of
feature locations stored in the memory device (Block
3000) and setting a second flag a equal to "1" (Block
3002). Next, the stored location of the first atypium is
read from the memory device (Block 3004). Note that the
memory device that stores the location of the first
atypium is shared between the cytotechnologist's
microscope system and the pathologist's microscope
system. Such sharing may be achieved by using a common
WO 95/23386 PCT/US95/01997
2183Q81
-28-
integrated circuit memory device, a removable magnetic or
optical disk or tape, a removable punch card, a local or
wide area network, a telecommunications network (and a
modem for each microscope system in the case of an analog
telecommunications network), a wireless communications
network, or the like. This type of memory sharing is well
known in the art.
Next, the stored location is transformed from
the microscope-independent coordinate system to an
encoder-based coordinate system (Block 3006) for the
pathologist's microscopic screening apparatus (that is, a
second microscope-dependent coordinate system), as
follows:
(1) Calculate [DiLQ], where D' is defined above,
and wherein La is the stored location of the possible
atypium using the microscope-independent coordinate
system; and
(2) Calculate [DILa] +fs , where [D/La] is as
calculated above, and wherein f; is a vector
representation of the location of the second fiducial as
stored using the pathologist's microscopic screening
apparatus (using the second microscope-dependent
coordinate system).
Note that fs is the origin of the microscope-
independent coordinatesystem. Thus, this calculation
represents a translation from the origin of the
microscope-independent coordinate system to the origin of
the second microscope-dependent coordinate system.
In response to the transformation, the
processor 16 causes the stage 18 to move to the stored
location of the first (of possibly several) possible
atypium using the stepper motors 20, 22 and the encoders
24, 26 as the positioning servo (Block 3008), described
above. Once the stage 18, and therefore the field of
WO 95123386 PCTIUS95f01997
V
-29-
view, is positioned at the first possible atypium, the
pathologist can make a determination as to whether or not
a particular possible atypium is in fact an abnormal
cell, such as a cancerous cell.
If after completing his or her analysis of the
first possible atypium (feature) the pathologist
indicates that his or her analysis is complete by
pressing the button or "clicking" (Block 3010), then the
microscope system tests whether the pathologist pressed a
right arrow key or a down arrow key on a keyboard that is
coupled to the processor, or a secondary button on the
digitizing device (Block 1012). Keyboards coupled to
processors such as the one used to implement the present
invention are commonly used and known in the art, as are
right and down arrow keys on the keyboard, and secondary
buttons of trackballs. If the pathologist has pressed the
right or down arrow key or the secondary button (Block
1012), then the microscope system tests whether there are
additional fiducials stored in the memory device (Block
3014). I=f there are additional features stored in the
memory device, i.e. the second flag a does not equal the
first flag n, (Block 3014), the second flag a is
incremented (Block 3016) and the location of feature is
read from the memory device (Block 3004) and transformed
(Block 3006), and the stage is moved (Block 3008), etc.,
as described above. If there are no additional features
stored in the memory device, i.e., the second flag a
equals the first flag n, (Block 3014), the second flag is
set to one (Block 3018) (which corresponds to the first
feature stored in the memory device) and execution
continues with the reading of the first feature location
from the memory device (Block 3004).
In the event the pathologist does not press the
right or down arrow or the secondary trackball button
(Block (3012), the second counter a is tested to see
whether it is equal to one (Block 3020). In the event the
CA 02183081 2005-12-29
-30-
second counter a is equal to one (Block 3020), the second
counter a is set equal to the first counter n (Block
3022), and in the event the second counter a is not equal
to one (Block 3020), the second counter a is decremented
by one (Block 3024).
If after completing his or her analysis of the
first possible atypium (feature) the pathologist does not
indicate that his or her analysis is complete by pressing
the button or "clicking" (Block 3010), but instead
presses a number key or keys on the keyboard (Block
3026), then the value corresponding to the number key(s)
is loaded into a third counter d (Block 3028). The third
counter may be a location in the memory device or an
integrated circuit counter, as with the counter a, and
the feature counter n. Number keys such as are used to
implement the present invention are commonly used and
known in the art, and preferably include number keys
corresponding to the digits zero through nine. If the
value in the third counter d is greater or equal to
one, but less than or equal to the first (or feature)
counter n (Block 3030), the second counter a is set equal
to the third counter d (Block 3032), and execution
continues with reading the feature location from the
memory device (Block 3004). In the event the third
counter d is not greater than or equal to one and less
than or equal to the first counter n (Block 3026), the
pathologist is given another chance to press a number
key(s) (Block 3026), and may receive an error message
(not shown) indicating that the number key(s) pressed is
out of range, i.e., greater or equal- to one, but less
than or equal to the first counter n.
If after completing his or her analysis of the
first possible atypium (feature) the pathologist has not
pressed the trackball button or an arrow key (Block 3010)
and has not pressed a number key(s) (Block 3026), but
instead presses an "END" key on the keyboard (Block
WO 95/23386 PCd'/US95/01997
-31-
3034), the retrieval phase terminates. Note that the
"END" key may be a key marked "End" or any other key on
the keyboard other than the number keys and the arrow
keys that are used to indicate that the pathologist
wishes to terminate the retrieval phase. In the event the
pathologist has not pressed the trackball button or an
arrow key (Block 3010), has not pressed a number key(s)
(Block 3026), and does not press the "END" key (Block
3034), execution continues with testing whether the
button or the arrow key has been pressed (Block 3010), as
described above.
Thus, a pathologist is able to quickly relocate
possible atypia identified by a cytotechnologist using an
automated microscopic screening apparatus.
Referring next FIG. 7, a flow diagram is shown
of the steps traversed during the storage phase,
including steps that comprise a scanning subroutine and a
searching subroutine. Upon initializing the scanning
routine, the stage begins to scan the slide as described
above, using one of the above-described scanning patterns
and motion profiles (Blocks 4000 and 4002). Upon viewing
a possible atypium within the portion of the slide that
is viewed (Block 4003), the cytotechnologist indicates
that a possible atypium has been viewed by pressing or
"clicking" the button (Block 4004). The scanning routine
then enters a search mode (Block 4006) wherein the
cytotechnologist manually locates the stage using the
digitizing device (e.g., 17). In the event the
cytotechnologist wishes to record the location of the
possible atypium (Block 4008), the cytotechnologist
centers the atypium (Block 4010) within or at the field
of view and presses the button twice quickly (Block
4012), or "double-clicks". The term "double-click" is
commonly used and well known in the art. The location of
the atypium is read from the encoders (Block 4014) and
stored (Block 4016) using the microscope independent
WO 95/23386 PCT/US95/01997
2183081 =
-32-
coordinate system as described above. The microscopic
screening apparatus then resumes the search mode (Block
4006) wherein the cytotechnologist manually locates the
stage using the digitizing device.
When the cytotechnologist wishes to leave the
search mode and resume scanning of the slide, or in the
event the cytotechnologist does not wish to record the
location of the possible atypium, the cytotechnologist
presses or "clicks" the button (Block 4018). In response
to the pressing of the button, the stage 18 is returned
to the location at which the scan was interrupted and
resumes scanning the slide (Block 4020). The
cytotechnologist may stop the scan at any time and enter
the search mode by again pressing or "clicking" the
button (Blocks 4003 and 4004).
After the stage has moved from, for example,
left to right across the entire slide (Block 4022), the
encoders 24, 26 are read and the actual position of the
stage 18 is compared with the expected position of the
stage by the positioning servo software (Block 4024) In
the event the actual and expected positions differ (Block
4026), the position of the stage 18 is adjusted (Block
4028) and the scan mode continues by moving the stage
downward for the next scan pass, which is from right to
left across the stage (Block 4002).
After the entire area of the slide has been
scanned (Block -4022), the scanning routine terminates
(Block 4030). Thus, the sample contained on the slide is
scanned for possible atypia. When the possible atypia are
located, the cytotechnologist interrupts the scan, and
enters a searching routine. After confirming the location
of the possible atypium, the invention records its
location using the microscope-independent coordinate
system.
Whila the invention herein disclosed has been
described by means of specific embodiments and
WO 95/23386 PCT/US95/01997
-33-
applications thereof, numerous modifications and
variations could be made thereto by those skilled in the
art without departing from the scope of the invention set
forth in the claims.
In particular, while the first microscope
system of above embodiment stores the location of the at
least one feature using the microscope-independent
coordinate system, and the second microscope system
recalls such location, it is to be understood that either
(a) storage by the first microscope system of the
fiducial locations and the location of the at least one
feature using the first microscope-dependent coordinate
system and recalling by the second microscope system, or
(b) storage of the fiducial locations using the second
microscope system using the second microscope-dependent
coordinate system, and subsequently the location of the
at least one feature using the first microscope system
using the second microscope-dependent coordinate system
(transformation having been accomplished within the first
microscope system), are possible within the scope of the
present invention depending on which of the two
microscope systems (which may be the same microscope
system) performs the above-described translational,
angular, and magnitudinal transformations.
Furthermore, while the present invention has
been described hereinabove as utilizing particular scan
patterns and motion profiles, many possible scan patterns
and/or motion profiles are contemplated within the scope
of this invention. Selection of such scan patters and/or
motion profiles will depend on the particular application
of the present invention and personal preferences of its
operators.