Note: Descriptions are shown in the official language in which they were submitted.
2183641
WO 95/22532 ~.~ 9 ~ 95/00466
SUB~1I1U 1~ IR~ NONES AND ~R USE AS HE~IC IDES
Ihe inventionrelates to newsubstit~ triazolin-n~, toprocesses fortheirp~ lion,
and to their use æ herbicides.
It hæ been disclosed that certain s~liLul~d triazolim-n~, such æ, for example, the
S compound 2-(~cyano-2,5-difluoro-phenyl)-5-methyl 4 propargyl-2,~dihydro-3H-
1,2,~triazol-3-one, have herbicidal l~lo~lLies (cf. EP-A 370332).
However, the activity of these known compounds is not s~ti~f~ ty in all respects.
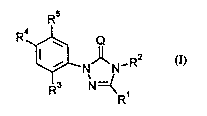
Ihere have now been found the new substituted triazolinones ofthe general formula (I)
R4~?``N' =~R1R2 (1)
in which
Q represents o~ n or slllphur,
Rl represents halogenoallyl,
R2 repreients hydrogen, arnino, cyano, alkyl, alkenyl, aLkinyl, halogenoalkyl,
halog~nn~lkenyl, halo~no~lkinyl, alkoxyalkyl, allylid~neimino or in each case
optionally substill-t~l cycloalkyl or cycloallylalkyl,
R3 represents hydrogen or haloge~l,
` 2~83641
W~ 9S/22532 P~17~;tg5/00466
R4 represents cyano or nitro, and
R5 represents isocyano, thiocyanato, sulpho, halo~enoslllrhonyl, aLkylaminooxy,
dialkylaminooxy, alkylid~ minooxy, cycloalkyli~ ";~ooxy, or in each
case optionally substituted cycloalkenyloxy or heterocyclyloxy, or represents
S one of the following groups which are bonded via nitrogen or oxygen
-NR6R7, -N=CR8R9, -aCO-RI, -aCS-RI, -O-CHR~I-P(OXORI2)2, where
R6 represents hydrogen, or represents alkyl, alkenyl or alkinyl, each of
which is optionally substituted by halogen, or represents the group
-Co-RI3 in which
Rl3 represents hydrogen, or represents alkyl or alkoxy, each of which is
optionally ~l~hsti~ l by halogen or alkoxy, or represents amino,
alkylamino or dialkylamino, or represents in each case optionally
substituted cycloalkyl, cycloalkylalkyl, aryl, arylalkyl or heterocyclyl-
alkyl,
R7 represents the group -(Co)n-RI3 in which
Rl3 has the abovementioned m~ning and
n represents the numbers 1 or 2,
R8 represents hydrogen, alkyl or alkoxy,
R9 represents alkoxy, alkylamino or dialkylamino,
Rl represents alkyl which is optionally s~-ksti~ by halogen, or represents
~ 1 8 36 4 1
.
WO 95/22532 P( 11~95100466
alkoxy, or represents alkylamino, or represents dialkylamino or
æ~ optionally s~-bsti~lt~ aryl,
R'l represents alkyl or cycloalkyl, each of which is optionally s~lbstihlt~l by
halogen, or represents in each case optionally sl-bstitl~ aryl or
S heterocyclyl, and
Rl2 represents alkyl.
Furtll~rm~lre~ it has been found that the new substih~i triazolinones of the general
formula (I) are obtained when
(a) ~ no~ryltriazolinones of the general formula (II)
NHR6
R4~--N,~N-R2 (Il)
R3 N =(
in which
Q Rl, R2, R3, R4 and R6 have the abovementioned m~nin~
are reacted with acid halides of the general formula (m)
X'{Co)n-R'3 (III)
in which
n and Rl3 have the abovementioned m~ning and
- 3 -
2183641
VVO 95/22532 P~ ;t g5/00466
Xl represents halogen,
or with ortho esters of the general formula aV)
R8-C(OR9)3 av)
in which
S R8 and R9 have the abovementioned m~nin~,
if a~r~ iate in the presence of a reaction auxiliary and if ~ liate in the presence
of a diluent,
or when
(b) hydroxyaryltriazolinones of the general formula (V)
OH
R4~--N, N_R (V)
R3 N=~
R
in which
Q, Rl, RZ, R3 and R4 have the abovementioned mt~nin~;
are reacted with acid halides of the general formula (VI)
XZ-C~RI (VI)
15 inwhich
~1 83641
VVO 95/22532 ~17~;t'95/00466
Rl has the abovernentioned m~ning and
X2 represents halogen,
if ~lo~ in the presence of an acid ~cceptor and if a~r~li~ in the presence of
a diluent,
S or when
(c) halogenoaryltriazolinones of the general fnrmlll~ (VII)
R4~--NJ~ N - R2 (Vll)
R3 N =~R'
in which
Q, Rl, R2, R3 and R4 have the abovementioned m~nin~ and
10 X3 represents halogen,
are reacted with hydroxyalkylphosphonic esters of the general formula (VIII)
HO-CHRIl-P(o)(oRl2)2 (Vm)
in which
Rll and Rl2 have the abovementioned m~nin~,
15 if ~ liate in the presence of an acid acceptor and if al)~r~liate in the presence of
2 1 8364 1
WO 95/22532 P~/EW5/00466
a diluent.
Finally, it has been found ~at the new substibltpA ~iazolinones of the gene~l
formula (I) have illtelc~ti,lg herbicidal ~lo~lties.
Surprisingly, the substituted triazolinones of the general fc)nn~ (I) according to the
S invention show a considerably better herbicidal activity against problem weeds in
~ lp~ on with the substituted triazolinones known from the prior art, such as, for
example, the compound 2{4-cyano-2,5-difluoro-phenyl~5-methyl~
2,4-dihydro-3H-1,2,4-triazol-3-one.
Ihe invention ~lcr~ly relates to compounds of the forrnula (I) in which
10 Q represents oxygen or sulphur,
Rl represents straight-chain or branched halo~Pno~lkyl having 1 to 6 carbon atoms
and 1 to 13 identical or dirre~cll halogen atoms, in particular flnl~nnP, chlorine,
bromine or iodine,
R2 represents hydrogen, amino, cyano, straight-chain or branched alkyl having 1 to
8 carbon atoms, in each case straight-chain or branched alkenyl or alkinyl, eachof which hæ 2 to 6 carbon atoms, straight-chain or branched halo~eno~lkyl
having 1 to 6 carbon atoms and 1 to 13 identical or dirr~llt halogen atoms, in
particular fluorine, chlorine, bromine or iodine, or represents in each case
straight-chain or branched halogPnoalkPnyl or halogPno~lkinyl, each of which
hæ 2 to 6 carbon atoms and 1 to 11 identical or dirr~l~llt halogen atoms, in
particular fluorine, chlorine, bromine or iodine, or represents straight-chain or
branched alkoxyalkyl, each of which has 1 to 4 carbon atoms in the individual
alkyl moieties, or represents straight-chain or branched alkylidPnPimino having
1 to 8 carbon atorns, or represents cycloalkyl or cycloalkylalkyl, each of whichhæ 3 to 8 carbon atoms in the cycloalkyl moiety and, if a~ liate, 1 to
~183641
WO 95/22532 P~ 5/00466
4 carbon atoms in the straight-chain or branched alkyl moiety and each of
which is optionally mon~ substiblted or polys~kstitl~ in the cycloalkyl moiety
by identical or di~lcllt halogen substihl~t~ in particular fllloline, chlorine,
~.l,e andlor iodine,
5 R3 represents hydrogen, flucnin~ chlorine, b~ e or iodine,
R4 represents cyano or nitro, and
R5 represents isocyano, thiocyanato, sulpho, halogenosulphonyl,
Cl-C6-alkylarninooxy, di(CI-C4-alkyl~aminooxy, Cl-C6-alkylid~.,~.";.,- oxy or
Cs-C6-cycloalkylid~ minooxy,orrepresentsCs-C6-cycloalkenyloxy,perhydro-
furanyloxy or perhydropyranyloxy, each of which is optionally s~lbstit~lt~1 by
halogen, Cl-C4-alkyl or Cl-C4-alkoxy, or represents one ofthe following groups
which are bonded via nitrogen or oxygen
-NR6R7, -N=CR8R9, -O-CO-RI, -O-CS-RI, -O-CHRIl-P(oxoRl2)2~ where
R6 represents hydrogen, or represents alkyl, alkenyl or alkinyl, each of
which has up to 6 carbon atoms and each of which is optionally substi-
tuted by flll. rin~ and/or chlorine, or represents the group -Co-RI3 in
which
Rl3 represents hydrogen, or represents alkyl or alkoxy, each of which
has 1 to 6 carbon atoms and each of which is optionally
substituted by flll-)rin~, chlorine or Cl-C4-alkoxy, or represents
amino, or represents alkylamino or dialkylamino, each of which
has 1 to 6 carbon atoms in the alkyl moieties, or represents in
each case optionally substituted C3-C6-cycloalkyl,
C3-C6-cycloalkyl-CI-C4-alkyl, phenyl, na~lllhyl, phenyl-
2183~4~
VVO 95/22532 P~ ;t'95/00466
Cl-C4-allyl, furyl, thienyl or pyridyl, suitable substi~t~ in each
case being: -
halogen, cyano, nitro, in each case straight-chain or branched
allyl, alkoxy, alkylthio, alky~ phinyl or alkyl~llphonyl, each of
which has 1 to 4 carbon atoms, in each case shaight-chain or
branched halo~ lkyl, halo~no~lkoxy, halo~no~lkylthio,
halogenoalkylsulphinyl or halog~no~lkyl~llphc)nyl, each of which
has 1 to 4 carbon atoms and 1 to 9 identical or di~ halogen
atoms, in each case straight-chain or branched aL~oxy~l~llyl or
alkoxyimin(l~lkyl, each of which has 1 to 4 carbon atoms in the
individual alkyl moieties, and phenyl which is optionally
monos~lhstitllt~1 or polys~lbstibltel by identical or di~e~
sllbsti~ nt~ from the series c~n~icting of halogen and/or straight-
chain or branched alkyl or alkoxy, each of which has 1 to
4 carbon atoms, and/or straight-chain or branched halo~en- ~lkyl
or halogçn- ~lkoxy, each of which has 1 to 4 carbon atoms and
- 1 to 9 identical or difr~c;[l~ halogen atoms;
R7 represents the group ~Co)n-RI3 in which
Rl3 has the mP~ning given above as being ~ef~ d and
n represents the numbers 1 or 2,
R3 represents hydrogen, or represents alkyl or alkoxy, each of which has 1
to 6 carbon atoms,
R9 represents alkoxy, alkylamino or dialkylamino, each of which has 1 to
6 carbon atoms in the alkyl groups,
2 i ~36~ 1
WO 95/22532 PCI/EP95/00466
R~ represents alkyl having 1 to 6 carbon atoms which is optionally
substituted by fluorine and/or chlorine, or lcplcsellt~ alkoxy or
alkylamino or dialkylamino, each of which has 1 to 6 carbon atoms in
the allcyl groups, or represents optionally sllhstitllte~ phenyl, ~lcr~llcd
S phenyl sllbstit~nt~ being, again, those which have been mentioned
above for R6 as being ~ ,f~llcd,
Rl~ lC~)lCSt;llt~ alkyl having 1 to 6 carbon atoms or cycloalkyl having 3 to
6 carbon atoms, in each case optionally sl-hstit~lt~l by flllcrin~ and/or
chlorine, or represents in each case optionally substi~t~1 phenyl,
naphthyl, furyl, thienyl or pyridyl, pler~llcd phenyl s~lkstit~l~nt~ being,
again, those which have been mentioned above for R6 as being
~lcr~llcd, and
Rl2 represents alkyl having 1 to 6 carbon atoms.
In particular, the invention relates to compounds of the formula O in which
15 Q represents oxygen or sulphur,
R~ represents strai~ht-chain or branched halogeno~lkyl having 1 to 4 carbon atoms
and 1 to 9 identical or dirr~lcllt halogen atoms, in particular fluorine, chlorine,
bromine or iodine,
R2 represents hydrogen, amino, cyano, straight-chain or branched alkyl having 1 to
4 carbon atoms, in each case straight-chain or branched alkenyl or alkinyl, eachof which has 3 to 4 carbon atoms, straight-chain or branched halog~noalkyl
having 1 to 4 carbon atoms and 1 to 9 identical or dirr~ lt halogen atoms, in
particular fluorine or chlorine, in each case straight-chain or branched
halog~ lkenyl or halogenoalkinyl, each of which has 3 to 4 carbon atoms and
21 83641
VVIO 95/22532 P~ 95/00466
1 to 5 identical or di~ t halogen atoms, in particular fluorine or chlorine, or
represents straight-chain or branched alkoxyalkyl having in each case 1 to 3
carbon atoms in the individual alkyl moieties, or repl~s~llt~ straight-chain or
branched aLkylid~ ;"~ n having 1 to 4 carbon atoms, or re~l~s~ cycloalkyl
S or cycloalkylalkyl, each of which has 3 to 6 carbon atoms in the cycloalkyl
moiety and, if a~ liate, 1 to 3 carbon atoms in the straight-chain or branched
aLkyl moiety and each of which is optionally mnm~s~lhstit~t~l or polysubstitutedin the cycloaLtcyl moiety by identical or di~el~ halogen sllbst~ nt~ in
particular fluorine or chlorine,
10 R3 represents hydrogen, fluorine or chlorine,
R4 represents cyano or nitro, and
Rs represents isocyano, thiocyanato, sulpho, chlorc)s .lph~ nyl, Cl-C4-allcyl-
aminooxy, di-(CI-C3-alkyl}aminooxy, Cl-C4-alkylidene-aminooxy, Cs-C6-cyclo-
alkylideneaminooxy, Cs-C6-cycloalkenyloxy, tetrahydrofuranyloxy,
perhydropyranyloxy or one of the following groups which are bonded via
nitrogen or oxygen
-NR6R7, -N=CR8R9, -O-CO-RI, -O-CS-R~, -O-CHRIl-P(oxoRl2)2 where
R6 represents hydrogen, or represents alkyl having 1 to 4 carbon atoms
which is optionally substituted by flllcrinP, or represents the group
-C~RI3 in which
Rl3 represents hydrogen, or represents alkyl or alkoxy, each of which
has 1 to 4 carbon atoms and each of which is optionally
substituted by fluorine, chlorine, methoxy or ethoxy, or
represents amino, or represents alkylamino or dialkylamino, each
- 10-
~ ~ 8364 1
WO 95/22532 K 1~95/00466
of which has 1 to 4 carbon atoms in the alkyl moieties, or
e~l~s~llt~ in eaeh case optionally subst~ cyclopropyl,
cyclopentyl eyclohexyl, cyelopropylmethyl, eyelopentylmethyl,
eyelohexylmethyl, phenyl, benzyl, phenylethyl, furyl, thienyl or
pyridyl, suitable substi~ ntc in each case being
fluorine, ehlorine, bromine, eyano, nitro, in each case straight-
ehain or branched alkyl, alkoxy, alkylthio, alkylculrhinyl or
alkyl.culphonyl, each of which has 1 to 4 carbon atoms, in each
case s~aight-ehain or branched halo~en~lkyl~ halo~noalkoxy,
halo~no~lkylthio, halo~no~lkylclllphinyl or halogenoalkyl-
sulphonyl, each of which has 1 to 4 car~on atoms and 1 to
9 identical or diLre~ halogen atoms, in particular fluorine
and/or chlorine atoms, in each case straight-ehain or branched
aLtcoxyc~l,ollyl or alkoxyimino~lkyl, each of which has 1 to
4 earbon atoms in the individual alkyl moieties, and phenyl
which is optionally mnnos~lkstitllted or polys~lb~liL~(~ by
ntie~l or dirrelc;llt substit~l~ntc from the series cnn~ictin~ of
fluorine, chlorine, bromine and/or straight-ehain or branched
alkyl or alkoxy, each of which has 1 to 4 carbon atoms, and/or
straight-chainorbranchedhalo~no~lkyl orhalo~enn~lkl xy, each
of which has 1 to 4 carbon atoms and 1 to 9 identical or
dirrel~lll halogen atoms, in particular fluorine and/or ehlorine
atoms;
R7 represents the group -(Co)n-R'3 in which
Rl3 has the mP~ning given above as being particularly plcr~ d and
n represents the numbers 1 or 2,
2 1 ~364 ~
WO 95/22532 P~ 95/00466
R8 lC~lC~ hydrogen, or lc~)lcS~ i alkyl or alkoxy, each of which has 1
to 4 carbon atoms,
R9 represents alkoxy, alkylamino or dialkylamino, each of which has 1 to
4 carbon atoms in the alkyl groups,
S Rl represents alkyl having 1 to 4 carbon atoms which is optionally
sllkstitllt~1 by fllmrin~ and/or chlorine, or represents alkoxy or
alkylamino or dialkylamino, each of which has 1 to 4 carbon atoms in
~e alkyl groups, or represents optionally s~lkstit~ 1 phenyl, ~lcr~llcd
phenyl sllkstitll~nt~ being, again, ~ose which have been mentioned
above for R6 as being particularly plcLled,
Rll represents alkyl having 1 to 4 carbon atoms or cycloalkyl having 3 to
6 carbon atoms, in each case optionally substituted by flllorin~ andlor
chlorine, or represents in each case optionally sllbstihltel phenyl,
naphthyl, furyl, thienyl or pyridyl, ~l~r~lled phenyl sukstitl~nt~ being,
again, those which have been mentioned above for R6 as being
particularly ~ r~lled, and
Rl2 represents alkyl having 1 to 4 carbon atoms.
The abovementioned definitions of radicals, in general or where pler~ d ranges are
given, apply to the end products of the formula (I) and, analogously to the starting
20 m~t~ri~L~ or int~rmP~ tes required in each case for the pl~ lion.
These definitions of radicals can be combined with each other as desired, that is to say
collll)il~lions between the abovementioned ranges of ~l~r~ d compounds are also
possible.
- `- 2183641
V~)95/22532 ~1~;tg5/00466
If, for example, 2{5-amino~cyano-2-fluoro-phenyl~S-difluol~n~ l~methyl-
2,~dihydro-3H-1,2,~triazol-3-one and dichloroacetyl chloride are used as starting
s ~ Ps for carrying out process (a) accorLlg to ~e invention, the course of the
reaction can be outlined by the following equation
NH2 o ~
NC~--NJ~N--CH3 , Cl CHCI2 , ~--NJ~N_CH3
CHF2 F N=~
CHF2
If, for example, 2-(2-chloro~cyano-S-hydroxy-phenyl)~ethyl-S-trifluorome~yl-
2,~dihydro-3H-1,2,~triazole-3-thione and ethyl chlol~fo~ e are used as starting
substances for carrying out process (b) according to the invention, the course of the
reaction can be outlined by the following equation
OH L~
NC~ + Cl OC2Hs ~ C H
CF3 Cl N =~
If, for example, 2{2,5-difluoro~nitro-phenyl}S-dichloromethyl~difluoromethyl-
2,4 dihydro-3H-1,2,~triazol-3-one and diethyl hydroxym~ phcsphnnate are used
as starting substances for carrying out process (c) according to the invention, the course
of the reaction can be outlined by the following equation:
2 ~ 8364 1
V~O 95/22532 PCr/EP95/00466
02~N,~ o
F N ~ CHF2 HO-CH2-P(O)(Oc2H5)2
CHCI2
O-CH2-P(O)(oc2H5)2
- HF ~1 N N--CHF2
CHCI2
Formula (II) provides a general definition of the ~min~ ~ryltriazolinones to be used as
starting s~lkst~n~s in process (a) according to the invention for the ~lel)a.alion of the
compounds of the general formula (I). In formula (II), Q Rl, R2, R3, R4 and R6
S pl~r~l~ly, or in particular, have those m~nin~ which have already been mentioned
above in cc nn~ion with the ~les~rirtion of the con~u"ds of the formula (I) as being
r~"ed, or particularly ~l~re,l~d, for Q R~, R2, R3, R4 and R6.
lhe starting s -bst~n~Ps of the formula (II) were hitherto not known from the literature;
however, they are the subject-matter of earlier, non-prior-published patent applications.
10 Ihe ~minoaryltriazolinones of the general formula (II) are obtained when
halogenoaryltriazolinones of the general formula (Vm) - above - are reacted with~mmoni~ at temperatures between 0C and 150C, if a~ liate in the presence of a
diluent, such as, for example, dimethyl sulphoxide (cf. the ~ lion ~x~l4)1cs).
Formula aII) provides a general definition of the acid halides fur~rnore optionally
15 to be used as starting S~.IJ~LSIj~CeS in process (a) according to the invention. In
formula iO, n and R~3 pler~lal~ly, or in particular, have those mP~nin~ which have
already been mentioned above in cr-nn~tion with the description of the compounds of
- 14-
2 1 8364 t
WO 95/22532 P~ ;tgS/00466
the fc)rm~ (I) as being ~lcr~ d, or particularly pl~r~ d, for n and R~3.
Xl ~lcrcl~ly lc~læc~ fluorinr, chlorine or l~ e, in particular chlorine.
Ihe starting sukst~nr~s of the forrnula (III) are known rh~nir~l.c for organic synthesis.
Formula aV) provides a general definition of the ortho esters furth~rmore optionally to
5 be used as starting sl~b~ rP~ in process (a) according to the invention. In
formula (IV~, Rg and R9 l~lcr~l~ly, or in particular, have those m~nin~ which have
already been mentioned above in col-l-~ion with the (l~rrirtion of the compounds of
the formlll~ (I) as being plcr~lled, or particularly pl~f~lled, for R8 and R9.
Ihe starting s~lbst~nr~ of the formlll~ aV) are known chemicals for organic synth~i.c.
10 Formula (V) provides a general definition of the hydroxyaryltriazolinones to be used
as starting sul~l~,-r~ in process (b) according to the invention for the ~lc~lion of
the compounds of the general formula (I). In formula (V~, Q Rl, R2, R3 and R4
cr~l~ly, or in particular, have those mr~nin~ which have already been mentioned
above in connection with the description of the compounds of the formula (I) as being
15 plcr~ d, or particularly ~rcr~llcd, for Q R~, R2, R3 and R4.
Ihe starting substances of the formula (V) were hitherto not known from the literature;
however, they are subject-matter of an earlier, non-prior-published patent application
(cf. D~P 4238125/LeA 29445 dated 12.11.1992).
The hydroxyaryltriazolinones of the general formula (V) are obtained when
20 alkoxyaryltriazolinones of the general forrnula (IX)
- 2183641
VV~ 95/22532 P~ ;t'95/00466
OR
R4`~l--N J~ N - R2 (IX)
R3 N =(
in which
Q Rl, R2, R3 and R4 have the abovementioned m~nin~ and
R represents alkyl (I~lcr~l~ly methyl or e~yl)
5 are reacted with with a dealkylating agent, such as, for example, boron~[II) bromide, at
te~ res of between 0C and 50C in the presence of a diluent, such as, for
exam~le, methylene chloride, and the product is worked up in the cl]~tom~ry n~
(cf. the pl~al~lion examples).
Formula (VI) provides a general definition of the acid halides fur~hP~n-)re to be used
10 as starting substances in process (b) according to the invention. In formula (VI), Rl
er~l~ly, or in particular, has the mP~ning which has already been mentioned above
in c~)nn~tion with the description of the compounds of the formula a) as being
plcr~lled, or particularly pl~r~ d, for R~;
~2 ~ul~r~l~bly represents fluorine, chlorine or bromine, in particular chlorine.
15 lhe starting substances ofthe formula (VI) are known chemicals for organic synthesis.
Formula (VII) provides a general definition of the halogenoaryltriazolinones to be used
as starting substances in process (c) according to the invention for the ~ lion of
the compounds of the general formula (I). In formula (VII), Q Rl, R2, R3 and R4
cr~,l~ly, or in particular, have those m~nin~ which have already been mentioned
- 16-
2 1 33~4 ~
VVO 95/22532 K~/EP95/00466
above in c~ -~ion with the description of the compounds of the f~rm~ (I) as being
~,~ft;l,~, or particularly l)lc;r~llcd, for Q Rl, R2, R3 and R4.
X3 ~r~"~bly represents fluorine or chlorine, in particular flllc)rin~.
The starting substances of the formula (VII) were hitherto not known from the
S literature; however, they are the subject-matter of an earlier, non-prior-published patent
application.
The halogenoaryltria~olinones of the general fcrml~l~ (VII) are obtained when
triazolinones of the general formula (~)
H_NJJ~N_R2
N ~( (X)
R
10 in which
Q R' and R2 have the abovementioned m~nin~
are reacted with halogenoarenes of the general formula (X[)
R4~
(Xl)
R3
in which
lS R3, R4 and Rs have the abovementioned m~nin~ and
`- 218364~
V~TO 95/22532 P~ l7~;t'95/00466
X~ represents h~logen, ~ r~l~ly flll-)rin~ or chlorine, in particular flll()rin~.,
at tel~ res b~lw~ell 0C and 150C in the presence of a diluent, such as, for
example, dimethyl sulphoxide and in the presence of an acid acceptor, such as, for
example, po~.~ilm~ carbonate, and the products are worked up in the ~ .y
S manner (cf. the ~ lion examples).
Formula (Vm) provides a general definition of the hydroxyalkylph-)sph-nic estersfurth~rmore to be used as starting s~ ces in process (c) according to the invention.
- In formula (Vm), R~ and Rl2 ~el~bly, or in particular, have those m~nin~; which
have already been mentioned above in c-nn~ction with the description of the
10 compounds of the formula (I) as being pl~lled, or particularly ple~led, for R~ and
R12,
Ihe starting substances of the formula(Vm) are known chemicals for organic
syntll~si.c
Suitable diluents for carrying out processes (a), (b) and (c) according to the invention
15 are the cll.stcm~ry organic solvents. Ihese in~l~l(le, in particular, aliphatic, alicyclic or
aromatic, optionally halogen~te 1 hydrocarbons, such as, for example, benine, bt n7~nt .,
toluene, xylene, chlorob~v~ ., dichloroben7Pne; petroleum ether, hexane, cyclohexane,
dichloromPth~ne, chloroform, tetrachl~ ." ,~h~l~e; ethers, such as diethyl ether,
diisopropyl ether, dioxane, tetrahydrofuran, ethylene glycol dimethyl ether or ethylene
20 glycol diethyl ether; ket-~n~, such as acetone, b~ non~ or methyl isobutyl ketone;
nitriles, such as a~etc)nitlile, propionitrile or be~,~r,l,ill;le; amides, such æ
N,N-dimethylformamide, N,N-dimethylacetamide, N-methylformanilide,
N-methylpyrrolidone or hexamethylI-hc)sI~horic triamide; esters, such as methyl acetate
or ethyl acetate, sulphoxides, such æ dimethyl sulphoxide, alcohols, such as m~th~nol,
25 ethanol, n- or i-propanol, ethylene glycol monomPthyl ether, ethylene glycol monoethyl
ether, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether, their
- 18-
- - 21 836~ 1
WO 95/22532 P~ ;t'95/00466
mixtures with water, or pure water.
Process (a) according t~o the invention is l~lcr~ly canied out in the presence of a
suitable reaction auxiliary. Reaction auxiliaries which are suitable for the reaction wit~h
the acid halides ofthe formula (III) are all ~ o",~l y inorganic or organic bases. Ihese
S in~lude, for example, the hydrides, hydroxides, ~mi(l~, alcoholates, ~ t~t~, carbonates
or hydrogen C~ eS of ~lk~lin~ earth metals or allcali metals, such as, for example,
sodium hydride, sodium amide, sodium methylate, sodium ethylate, pot~ m tert-
butylate, sodium hydroxide, pot~ m hydroxide, ~mmonium hydroxide, sodium
acetate, pot~sil-m acetate, calcium acetate, ~""~ ";llm acetate, sodium carbonate,
10 potassium C~ubOl)ille, pot~i-lm hydrogen c~l~ol~e, sodium hydrogen carbonate or
~mmnnillm carbonate, and also bæic organic nitrogen compounds such æ
l~lylamine~ triethylamine, tributylamine, N,N dirnethyl~nilin~, pyridine,
N-methylpiperidine, N,N-dimethylaminopyridine, diæabicyclooctane (DABCO),
diazabicyclonl)n~ne (DBN) or diazabicyclolm~l~n~ (I)BU).
15 Reaction auxiliaries which are generally employed for the reaction with the ortho esters
of the formula (IV) are acidic catalysts. Suitable catalysts are, plcr~lably, strong
protonic acids, such æ, for example, hydrochloric acid or hydrogen chloride, sulphllric
acid, m~th~n~lllphonic acid, b~ phonic acid and ~tolu~n~lllphonic acid.
When carrying out process (a) according to the invention, the reaction tell4~l~lres can
20 be varied within a substantial range. In general, the process is carried out at
temp~s between 0C and 150C, pl~f~ly at tempe~es between 10C and
100C.
Process (a) according to the invention is generally carried out under atmospheric
pressure. However, it is also possible to carry out the process under elevated or reduced
25 pressure, in gerl~l between 0.1 bar and 10 bar.
- 19-
2 1 83 6 4 1
VVO 95/22532 ~11~;t 95/00466
To caITy out process (a) according to the invention, the starting s~b~ u~ quired in
each case are generally employed in a~ ~ely equimolar amounts. However, it is
also possible to use a larger excess of one of the two components employed in each
case. In general, the reactions are carried out in a suitable diluent in the presence of a
S reaction auxiliary, and the reaction mixture is stirred for several hours at the
ten~ure required in each case. Workingup in the process according to the
invention is carried out in each case by c~ k~".~.y methods (cf. the l)l~ion
exarnples).
Processes (b) and (c) according to the invention are l~lcfelal)ly calried out in the
10 presence of a suitable acid acceptor. Suitable acid ac~tc,~ are all cl~loln~y inorganic
or organic bases. These include, for exarnple, the hydrides, hydroxides, amides,alcoholates, acet~tç~, carbonates or hydrogen C~hlOl~S of alk~lin~ earth metals or
allcali metals, such as, for example, sodium hydride, sodium amide, sodium methylate,
sodium ethylate, potassium tert-butylate, sodium hydroxide, potassium hydroxide,15 ~mm-~nium hydroxide, sodium acetate, pot~ -m acetate, calciurn acetate, arnm~nillm
acetate, sodium c~bol~e, potassium C~ ilte, potassium hydrogen c~bol~le, sodium
hydrogen carbonate or ~mm-)nium carbonate, and also basic organic nitrogen
compounds such as trimethylamine, triethylamine, tributylamine, N,N-dimethyl~nilin~
pyridine, N-methylpiperidine, N,N-dimethylaminopyridine, diazabicyclooctane
20 (DABCO), diazabicyclo~on~n~ (DBN) or diazabicyclolm~lp~n~ (DBU).
When calTying out processes (b) and (c) according to ~e invention, the reaction
te~ res can be varied within a substantial range. In general, the processes are
carried out at lelll~l~ lres between 0C and 100C, ~ bly at le~ res between
10C and 80C.
25 Processes (b) and (c) according to the invention are generally carried out under normal
pressure-~owever, it is also possible to carry out the processes under elevated or
reduced pressure, in general between 0.1 bar and 10 bar.
- 20 -
- , 2183~1
WO 95/22532 P~ ;t'95/00466
To carry out processes (b) and (c) according to the invention, the starting s~kst~n
required in each case are generally ernployed in a~l)~x;",~l~ly equimolar amounts.
However, it is also possible to use one of the two co,l~ "~ employed in each case
in a larger excess. The reactions are generally carried out in a suitable diluent in the
S presence of an acid acce~tol, and the reaction mixture is stirred for several hours at the
ten~ah~ required in each case. Working-up in the process according to the
invention is carried out in each case by customary methods (cf. the pl~ion
examples).
The active compounds according to the invention can be used as defoliants, desicc~nt~,
10 agents for destroying broad-leaved plants and, especially, as weed-killers. By weeds, in
the broadest sense, there are to be lm~l~tood all plants which grow in locations where
they are undesired. Whether the substances according to the invention act as total or
selective herbicides depends ~ss~nti~lly on the amount used.
The active compounds according to the invention can be used, for exarnple, in
15 conn~ction with the following plants:
Dicotyledon weeds of the ~ ra: Sinapis, Lepidium, Galium, Ste~ Matricaria,
Antll~n i~, Galinsoga, Chenopodium, Urtica, Senecio, A~ us, Portulaca,
X~nthillm, Convolvulus, Ipomoea, Polygonum, Sesb~ni~ Ambrosia, Cirsium, Carduus,Sonchus, Solanum, Rorippa, Rotala, Tin~ni~ Larnium, Veronica, Abutilon, Emex,
20 Da~ura, Viola, Galeopsis, Papaver, Centaurea, Trifolium, Ranunculus and Taraxacum.
Dicotyledon cultures of the ~nera: Gossypium, Glycine, Beta, Daucus, Phaseolus,
Pisum, Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis, Brassica,
T ~ct~lc~ Cucumis and Cucurbita
Monocotyledon weeds of the g~ra: Echinochloa, Setaria, Panicum, Digit~ri~
25 Phleum, Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus,
- 21 -
2i 83641
W~ 95/22532 P~ 5/00466
Sorghurn, Agropyron, Cynodon, Monochoria, Fiml)li~lylis, S~gitt~ria, Eleoch~ris,Scirpus, p~p~ tn, T~r3l~rmlml, Sph~nocle~ Dactyloct~nil~nn, Agrostis, Alopecurus and
Apera
Monocotyledon cultures ofthe ~nrra: ~yza, Zea, Triticlml, Hordeurn, Avena, Secale,
S Sorghum, Panicurn, ~Crll~lm, Ananas, Asparagus and Allium.
However, the use of the active compounds according to the invention is in no wayrestricted to these genera, but also extends in the same n~l~l to other plants.
Ihe cornpounds are suitable, depending on the conrlntration, for the total c~"~ g
of weeds, for ~ le on industrial terrain and rail tracks, and on paths and squares
10 with or without tree pl~ntin~ Equally, the compounds can be employed for co",l.~ g
weeds in ~l~ ial cultures, for exarnple ~ulæ~ions, decorative tree pl~ntin~,
o~ s, vineyards, citrus groves, nut or~ ~, banana plantations, coffee plantations,
tea plantations, rubber plantations, oil palm plantations, cocoa plantations, soft fruit
pl~ntin~ and hopfields, in lawns, turf and pasture-land, and for the selective co~ g
15 of weeds in annual cultures.
Ihe compounds a) according to the invention are particularly suitable for selectively
col~ g monocotyledon and dicotyledon weed~s in monocotyledon and dicotyledon
cultures, such as, for exarnple, in wheat and barley, by the pre~ lgellce and also the
post-emergence method.
20 To a certain extent, the compounds of the formula a) also have a fungicidal activity,
for example against pyricularia oryzae, phytophthora i~ s and venturia in~e~
Ihe active compounds can be converted into the cll~tom~ry forrmll~tions, such assolutions, emulsions, wettable powders, suspensions, powders, dusting agents, pastes,
soluble p~wders, granules, suspension-emulsion c n.-P.,I.~es, natural and synthetic
- 2 ~ 83~ ~
W~ 95/22532 PCr/E~95/00466
m~t~ impre~t~l with active compound, and very fine capsules in polymeric
substances.
Ihese form~ tions are produced in a known manner, for exarnple by mixing the active
compounds with ext~n~, that is liquid solvents and/or solid carriers, optionally with
5 the use of surface-active agents, that is emulsifying agents and/or dispersing agents
and/or foam-fo~ning agents.
In the case of the use of water as an extender, organic solvents can, for example, also
be used as auxiliary solvents. As liquid solvents, there are suitable in the rnain:
aromatics, such as xylene, toluene or alkyl~ h~lenes, chl~rin~te~ aromatics and
10 chlo~ d aliphatic hydrocarbons, such æ chlolubG"~ ~, chloroethylenes or
methylene chloride, aliphatic hydrocarbons, such æ cyclnhPx~n~ or ~ lS, for
example petroleum fractions, mineral and vegetable oils, alcohols, such æ butanol or
glycol æ well æ their ethers and esters, keton~, such æ ~etc)n~, methyl ethyl ketone,
methyl isobutyl ketone or cyclohexanone, strongly polar solvents, such as
15 dimethylr~lmall~ide and dimethyl sulphoxide, æ well æ water.
As solid carriers there are suitable:
for example ~."",o"illln salts and ground natural minerals, such as kaolins, clays, talc,
chalk quartz, attapulgite, montmorillonite or diaton~eous earth, and ground synthetic
minerals, such æ highly disperse silica, alumina and silicates, æ solid carriers for
20 granules there are suitable: for example crushed and fractionated natural rocks such as
calcite, marble, pumice, sepiolite and dolomite, as well as synthetic granules of
inorganic and organic meals, and granules of organic material such æ sawdust, coconut
shells, maize cobs and tobacco stalks; as emulsifying and/or foam-forrning agents there
are suitable: for exarnple non-ionic and anionic em~ fiers~ such æ polyoxyethylene
25 fatty acid esters, polyoxyethylene fatty alcohol ethers, for example alkylaryl polyglycol
ethers, alkylsulphonates, alkyl sl~lph~t~ arylslllrhon~tPs as well æ albumen hydrolysis
- 23 -
- 21 83641
WO 95/22532 ~17~g5/00466
products; as di~ illg agents there are suitable: for example lignin-s~lrhite waste
liquors and methylcellulose.
Adhesives such æ carboxymethylcellulose and nat~al and synthetic polymers in theform of powders, granules or latexes, such as gurn arabic, polyvinyl alcohol andS polyvinyl acetate, as well as natural phosrholipids, such as ceph~lin~ and le~ithin.c, and
synthetic rhosph- lipids, can be used in the formlll~tions. Further additives can be
mineral and vegetable oils.
It is possible to use colorants such as inorganic pi~n~nt~, for exarnple iron oxide,
titanium oxide, Prussian Blue, and organic dyestuff~;, such as ~li7Arin dyestuffs, a7Jo
dyestuffs and metal phthalocyanine dyestuffs, and trace nutrients such as salts of iron,
m~n~nesP; boron, copper, cobalt, molybdenum and zinc.
Ihe formlll~tions in general comprise l~lw~ O.l and 95 per cent by weight of active
compound, l~lcr~l~ly b~lw~ll 0.5 and 90%.
For co"~ ;"g weeds, the active compounds according to the invention, as such or in
the form of their f~rmlll~tions, can also be used as mixtures with known herbicides,
finished formlll~tions or tank mixes being possible.
Suitable herbicides for the mixtures are known herbicides, for example anilides such as,
for example, diflufenican and propanil; arylcarboxylic acids such as, for example,
dichloropicolinic acid, dicamba and picloram; aryloxyalkanoic acids such as, forexample, 2,4 D, 2,4 DB, 2,4 DP, fluroxypyr, MCPA, MCPP and triclopyr; aIyloxy-
phenoxy-alkanoic esters such æ, for example, diclofop-methyl, fenox~l~ethyl,
fluazifop-butyl, haloxyfop-methyl and qui~lofop-ethyl; ~7.inones such as, for example,
chloridazon and n.orflurazon; c~ba~les such as, for example, chl~ ro~l~..,
~m~1irh~m, ph~nm~lirh~m and ~1u~ ; chloro~ nilides such as, for example,
25 alachlor, acetochlor, butachlor, m~t~7Arlllor, metolachlor, pretilachlor and propachlor;
- 24 -
VVO 95/22532 2 ~ 8 3 6 4 1 P~1~95/00466
dinitro~nilin~ such as, for example, oryzalin, p~ntlim~ lin and ~ifluralin; diphenyl
ethers such as, for ~ 4)1c, ~ciflllnrf~ bifenox, fluoroglycofen, r(J, ~rPI " halosafen,
lactofen and oxyfll-c~rfen; ureas such æ, for exarnple, chlortoluron, diuron, fl~lomPtl-ron,
isoproturon, linuron and ~ vlhl~7~ron; hydroxyl~mines such as, for example,
5 alloxydim~ cl~im, cycloxydim~ sethoxydim and ~alkoxydim; imi~7~1inones such
æ, for ~ le, il~ lh~l~yr, ;~ h~be~ r and im~7~-r1in; nitriles such
æ, for example, bromoxynil, dichlobenil and ioxynil; oxy~ rnides such æ, for
example, mefenacet; s -lphr)nylureas such æ, for example, amidosulfuron, bPn.~ulfi-ron-
methyl, chlorimuron-ethyl, chlorsulfi~ron, cin~slllfilron~ m~slllfilron-methyl,
10 nicosulfuron, primisulfuron, pyr~7ls~1filron~thyl, ~hif~n~lllfilron-methyl, triasulfuron
and tribenuron-methyl; thioc~ ",i1~ such æ, for ~ le, butylate, cycloate, di-
allate, EPrC, esprocarb, molinate, prosulfocarb, thiob~ll~b and tri-allate; triazines
such æ, for example, a~azine, cy~n~7in~, sil--~ le, simetryn, terbutryn and
terbutyla_ine; tri~7inl n~s suchæ, forexample, he~7inone""~ln;l.~llandmetribuzin;
15 others such æ, for example, aminotria_ole, b~ate, l~l,~ , ci~ clhylin~
cloln~ ltq, clopyralid, difenzoquat, dithiopyr, ethofilm~t~" fluorochloridone, glufo-
sinate, glyphns~te, isoxaben, pyridate, quinchlorac, qllinm~rac, s -lphos~t~ andtridiphane.
Mixtures with other known active compounds, such æ fungicides, insecticides,
20 acaricides, nematicides, bird repellants, plant nutrients and agents which improve soil
structure, are also possible.
The active compounds can be used æ such, in the form of their fnrmlll~tions or in the
use forms ~lc~alcd th~lc~olll by further dilution, such æ ready-to-use solutions,
suspensions, emulsions, powders, pastes and granules. Ihey are used in the cll~tom~ry
25 manner, for example by watering, spraying, atomizing or scattering.
The active compounds according to the invention can be applied either before or after
emergence of the plants. They can also be incorporated into the soil before sowing.
- 25 -
21 ~36~ 1
Wl~ 95/22532 P~ ;t 95/00466
Ihe amount of active con~ulld used can vary within a s lksta~ti~l range. It depends
eS~Sfntis~lly on the nature of ~e desired effect. In general, dle amounts used are between
10 g and lO kg of active compound per hectare of soil sllr~, p1~,f~bly between
SO g and S kg per ha.
S Ihe ~1e~lion and use of ~e active compounds according to ~e invention can be
seen from the following examples.
- 26 -
- -- 2 ~ 836~ 3
VVO 95/22532 P~ ;t'95/00466
lion Fxamples:
Exan~le 1
N~ C(CH3)3
~ O
N ~ C2H5
F N =~
(Process (a))
S 1.01 g (0.01 mol) of triethylamine together with 3.15 g (0.01 mol) of 2~2-fluoro-
4-cyano-5-amino-phenyl)~ethyl-5-trifluoromethyl-2,4-dihydro-3H-1,2,4-tria~o1-3-one
in 50 ml of ~cetl-nitrile are introduced into the reaction vessel, and the stirred mixture
is ~eatedwith 1.21 g (0.01 mol) oftrimethylacetyl chloride, stirred for 5 hours at room
tempe~re (20C) ~and conc~ d. Ihe residue is stirred with water, filtered offwith
suction and purified over a silica gel [lacuna] (eluent: cyclohexane~ethyl acetate 3:1).
1.7 g (43 % of theory) of 2-[2-fluoro~cyano-5~tert-butyl-carbonylalnino}phenyl]-4-ethyl-5-trifluoromethyl-2,4-dihydro-3H-1,2,4-tria~o1-3-one of meltingpoint 149C are
obtained.
Example 2
N~OC2H5
C~ O
~J~N,C2HS
F N ~
- -- 2183b4~
wo 95n2s32 P~ ;t'95/00466
(Process (a))
3.15 g (0.01 mol) of 2~2-fluoro~cyan~5-amino-phenyl)~ethyl-5-trifluor~ yl-
2,4 dihydro-3H-1,2,4-triazol-3-one and 0.01 g of p-toln~nPslllrh( nic acid in 20 ml of
triethyl orthof~)""i1le are stirred for 20 mimltes at reflux t~ll4~l~l~e. When cold, the
S solution is concell~ d and recryst~lli7~1 from a sm~ll arnount of iso~l~anol.
2 g (54 % of theory) of 2-(2-fluoro~cyano-5-ethoxymethylPn~rnin-)phenyl)~ethyl-
S-trifluolv~ hyl-2,4-dihydro-3H-1,2,4-triazol-3-one of mP.lting point 91C are
obtained.
Example 3
O OC2H5
NC~ N'CH3
F N~
CHF2
(Process (b))
2 g (0.007 mol) of 2-(2-fluoro~cyano-5-hydroxy-phenyl)~methyl-5-difluoromethyl-
2,4-dihydro-3H-1,2,4-triazol-3-one in 50 ml of ~cet-)nitrile are treated with 0.23 g
(0.0077 mol) of sodium hydride (80 % in p~lll), and ~e mixture is stirred for
20 minl~ at 20C; 0.84 g (0.0077 mol) of ethyl chlor~f~lll~le is s~1bsPqllPntly added,
and the mi~ure is stirred for 8 hours at room lell~~ re (20C). After concentration,
the mixture is stirred with water, acidified with concentrated hydrochloric acid, and
extracted three times using dichlol~m~hane, and the organic phase is dried over
sodium s~llph~tP and c~-n~ L~d. For purification, the mixture is cllr~ ographed
20 over silica gel (eluent: cyclohexane/ethyl acetate 1:1).
- 28 -
- ` 2 1 3364 1
WO 95/22532 ~1~;t'95/00466
0.8 g (32 % of theory) of 2-[2-fluoro~cyano-5~ethoxy-carbonyloxy~phenyl]-
~methyl-S-difluor~ hyl-2,4-dihydr~3H-1,2,4-triazol-3-one is obtained æ an oil.
~H NMR (CDCl3): 3.50; 4.35-4.43; 7.55-7.58 ppm
Example 4
CH(cH3)2
O e(OC2H5)2
S ~ O
I~NJ~N ,C2H5
F N=~
CF3
(Process (c))
6.3 g (0.03 mol) of diethyl 1-hydroxy-iso-butyl-rhos~l-o~ in 100 ml of ~cet~-ni~ile
are treated with 0.9 g (0.03 mol) of sodium hydride (80 % in p~lll) and the mixture
is stirred for 20 minllt~ at 20C. Af~er 3.15 g (0.01 mol) of 2-(2,5-difluoro-
4-cyanophenyl}4 ethyl-5-trifluon,lll~lhyl-2,4-dihydro-3H-1,2,4-triazol-3-onehavebeen
added, the mixture is stirred for 12 hours at room tel~ re (20C). Ihe mixture is
stirred with water and the product which hæ precipitated is filtered off with suction
and recryst~lli7~ from cyclohexane.
3 g (59 % of theory) of 2-[2-fluoro~cyano-5-(1-diethoxyphosphoryl-iso-butoxy-
phenyl]~ethyl-5-trifluoromethyl-2,4-dihydro-3H-1,2,~triazol-3-one of m~lting point
113C are obtained.
Other examples of compounds of the formula (I) which can be prepared analogouslyto Examples 1 to 4 and following the general description of the plc~lion processaccording to the invention are those listed in Table 1 below.
- 29 -
- - 2 1 8364 1
wo gsn2532 PC,1~;t'95/00466
R4~--NJ~N-R2 (1)
R3 N =~
Table 1: Examples of ~e compounds ofthe fic)tTmll~ a)
E~Rl R2 R3 R4 Rs Q Physical
No. properties
5 CF3 CH3 F CN NH-CO-C2H5 O ~p.: 132C
6 CF3 C2H5 F - CN -- -- O r~p.: 107C
N CO `~3 _ 2 (decomp.)
7 CF3 CH3 F CN N~CH3 0 nLp.: 123C
COCF3
8 CF3 C2Hs F CN NH-COCF3 O nLp.: 146C
9 CF3 CH3 F CN NH-COCH3 O r~p.: 155C
10CF3 CH3 F CN NH-COCH(CH3)2 O 'H NMR (CDCl3,
d): = 1.14; 2.67;
3.36; 7.81; 8.15;
10.27
- 30 -
- 2 1 8364 ~
WO95n2532 ~17~ 95/00466
Ex Rl R2 R3 R4 Rs Q Physical
No. propelties
11CF3 C2H5 F CN NH-COCOC( CH3)3 O m.p.: 176C
12CF3 C2Hs F CN NH-CO-NH2 O mp.: >250C
S 13CF3 CH3 F CN ~CH3 S IH NMR (CDCI3,
N~ d): 3.36. 3.70
COCF3
14CF3 CH3 F CN N(COCH3)2 0 IH NMR (CDCl3,
d): 2.27; 3.38;
8.06; 8.38
15CF3 C2H5 F CN NH-COCH3 O m.p.: 169C
16CF3 C2Hs F CN ~CH3 0 IH NMR (CDCl3,
NH-CO~H d): 1.85-1.8; 3.90-
Cl 3.98; 8.93-8.9
17CF3 C2H5 F CN NH-COC(CH3)2CH2Cl O mp.: 149C
18CF3 C2Hs F CN NH-COCH2-Cl O ~p.: 121C
19CF3 C2H5 F CN N(COCH3)2 O mp.: 112C
(de~omp.)
CHF2 C2H5 Cl CN ,~ S
NH-CO~CI
21 CHF2 CH3 F CN NH-CO-NH2 O
æ CHF2 CH3 F NO2 N(COCH3)2
23 CF3 CH3 F NO2 NH-COC(CH3)3 O
- - 2 1 8364 1
W095/22532 P~ ;tg5/00466
E~. Rl R2 R3 R4 Rs Q Physical
No. properties
24 CHF2CH3 F CN N=CH-OC2H5 O m.p.: 149C
CF3C2Hs F CN ~CH3 0 IH NMR (CDCl3,
N=C~ d~: 1.95; 3.90-3.96;
OC2H5 7.12-7.15
26 CF3C2H5 F CN N~(OCH3)2 0 mp.: 139C
27 CF3C2Hs F CN N-CH-N(CH3)2
28 CF3CH3 Cl CN N~(OCH3)2 0
29 CF3CH3 F CN N~(OC2Hs)2 S
CF3C2Hs F NO2 N=CH-OC2H5 O
31 CHF2CH3 F CN ~CH3 S
N=C~
OC2Hs
32 CF3C2Hs F CN -OCOOC2H5 O IH NMR (CDCl3,
d): 3.90-4.00;
4.35-4.45; 7.58-
7.60
33 CF3CH3 F CN -OCOOCH2-CH2-Cl O
34 CHF2 CH3 Cl CN -OCOOC2H5 O
CF3 CH3 F NO2 -OCOO C2Hs
36 CF3 C2Hs F CN -OCOOC2H5 S
37 CF3 C2Hs F CN -OCH2-PO(OC2H5)2 O mp.: 89C
- 32 -
VVO 95/22532 2 i 8 ~ 6 4 ~ ;tg5/00466
Ex Rl R2 R3 R4 Rs Q Physical
No. p~ ellies
38 CF3CH3 F CN -OCH2-PO(OC2Hs)2
39 CF3CH3 F CN 4fH-Po(OC2Hs)2 o
CH3
S 40 CHF2CH3 F CN -ocH-po(oc2H5)2 0
CH3
41 CF3C2Hs F CN -OfH-Po(OC2Hs)2 0 ~p.: 90C
CH3
42 CF3C2Hs F CN -ocH-Po(OC2Hs)2 o
43 CF3 C2Hs F CN of H-PO(OC2H5)2 o
44 CF3C2Hs F CN -OIcH-PO(Oc2H5)2 0
~o
CF3C2Hs F CN -ocH-Po(OC2Hs)2 o
~, N 2
46 CF3CH3 F CN -N~-CO-C2H5 0
47 CF3C2Hs F CN N=CH-N(CH3)2 0 nLp.: 155C
- 33 -
21 3364~
WO 95/22532 P~ ;t'95/00466
Ex. R~ R2 R3 R4 Rs Q Physical
No. properties
48 CF3 C2Hs F CN NH-COOC2Hs O m.p.: 112C
49 CF3 C2Hs F CN NH-COCOOC2Hs O m.p.: 133C
CF3 C2Hs F CN NH-CHO O m.p.: 156C
51 CF3 CH3 F NO2 NH-COCF3 O
52 CHF2 CH3 Cl CN N(COCH3)2 O
53 CF3 CH3 F CN ~ O
54 CF3 CH3 F CN -SCN O n~= 1.5221
CF3 CH3 F CN -SO2Cl O mp.: 205C
56 CF3 CH3 F CN ~ O mp.: 53C
-O-N =~
57 CF3 CH3 F CN -~N(C2Hs)2 S (amorphous)
58 CF3 CH3 F CN -O-N(C2Hs)2 O mp.: 78C
59 CF3 CH3 F CN ~ S IH NMR (CDCl3,
~~ ~): 1.5-2.3; 3.70;
5.0; 5.85; 6.05;
7.75; 8.15
CF3 CH3 F CN A O m.p.: 66C
-o~
61 CF3 CH3 F CN r O mp.: 86C
-o~
- 34 -
WO 95/22532 ~ 95/00466
ExR' R2 R3 R4 Rs Q Physical
No. ~ ies
62CF3 CH3 F CN ~ S IH N~ (CDCl3,
d): 1.65; 2.0; 3.5;
3.70; 3.85; 4 75;
7.75; 8.2
63CF3 CH3 F CN ~ O ~p.: 128C
o~ o
64CF3 CH3 F CN -OCS-N(CH3)2 0 'H NMR (CDCl3,
~): 3.40; 3.45;
3.50;
7.52-7.60 ppm;
65CF3 C2Hs F CN /a~ O 'H NMR (CDCI3,
-~ ~): 1.95; 3.87;
3.90-3.98 ppm
- - - 2 1 8364 1
WO 95/22532 P~ ;t g5/00466
Starti~ substances of th~ formula (T~):
Example (II~
NH2
O
, C2HS
F N =~
9.54 g (0.03 mol) of 2-(2,5-difluoro~cyano-phenyl)4 ethyl-5-trifluoromethyl-
2,4-&ydro-3H-1,2,4-triazol-3-one in 150 m1 of dimethyl sulphoxide are heated to
120C, and ~mmc)ni~ gas is passed in for 20 hours. When cold, the solution is stirred
on ice-water, the product which has ~l~;cipiL~Ied is filtered offwi~ suction and washed
with water, and the residue is recryst~lli7~1 from isopLu~ ol.
5.8 g (61 % of theory) of 2-(2-fluoro-4-cyano-5-aminophenyl)-4-ethyl-
5-~ifluoromethyl-2,~dihydro-3H-1,2,4-triazol-3-one of melting point 193C are
obtained.
Startin~ substances of the formula ~V):
Example (V-1)
OH
O
I~NJ~N,C2H5
F N=~
CF3
4.95 g (0.015 mol) of 2~2-fluoro~cyano-5-me~oxy-phenyl)~yl-5-~i~uorome~yl-
2,4-dihydro-3H-1,2,4-triazol-3-one in 200 ml of methylene chloride are in~oduced into
- 36 -
`- 2 1 ~364 1
WO 95/22532 ~ 95/00466
~e reaction vessel at 10C, and a one-molar solution of 45 g (0.045 mol) of boron(m)
bromide in me~ylene chloride is added dropwise. Ihe reaction mixture is stirred for
12 hours at 20C and then treated wi~ 100 ml of water. Af~er the mixture has been
stirred for ten mimltes, the organic phase is separated off, washed with water, dried
5 using sodium s llrh~te and filter~i Ihe solvent is carefully removed from ~e filtrate
by ~ till~tion under a water pump vacuur~L
3.1 g (65 % of theory) of 2{2-fluoro~cyano-S-hydroxy-phenyl)~ethyl-
S-trifluorum~lhyl-2,4-dihydro-3H-1,2,4-triazol-3-one of m~lting point 195C are
obtained.
10 Starting substances of the formula (VII~:
Example (VII-1)
~ O
N N~a~
a `N-
~
a-3
5.3 g (0.038 mol) of potassium C~bOl~ t; are added at room ten~ure to 5.3 g
(0.032 mol) of 4-methyl-S-trifluorome~yl-2,~dihydro-3H-1,2,4-triazol-3-one (cf. for
example, US 3.780.052) and S.S g (0.032 mol) of S-chloro-2,4-difluorob~v~)"il~ile in
100 ml of dimethyl sulphoxide and ~e mixture is sllbsequ~ntly heated at 100C for
36 hours. For working-up, the cooled reaction mixture is poured into water, the pH is
brought to 2 using dilute hydrochloric acid~ and the mixture is extracted repeatedly
using dichloromPth~n~ The combined organic phases are dried over sodiurn sulphate
20 and concentrated in vacuum. lhe residue is cl~ n~lographed over silica gel (eluent:
dichlorom~th~ne) .
- - 2 1 ~36~ 1
WO 95/22532 ~l~;t'95/00466
1.8 g (18 % of theory) of 2~2-chloro~cyano-5-fluoro-phenyl~4 methyl-
5-trifluo~ Lhyl-2,4-dihydro-3H-1,2,4-triazolin-3-one of m~l~ng point 105C are
obt~in~l
Use Exam~les:
5 In the use exarnples, the cornpound (A) below is used as co~p~ on subst~n~e-
\~ O
,CH2C--CH (A)
F N=~
CH3
2-(4-cyano-2,5-difluoro-phenyl)-5-methyl-4-propargyl-2,4-dihydro-3H-
1,2,4-triazol-3-one (disclosed in EP-A 370332).
~np'e A
10 Pre-e~ gellce test
Solvent: 5 parts by weight of acetone
Fmlll~ifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable p~c~lion of active cornpound, 1 part by weight of active
compound is mixed with the stated arnount of solvent, the stated amount of eln--l~ifier
15 is added and the concentrate is diluted with water to the desired con~ntration.
Seeds of the test plants are sown in normal soil and, after 24 hours, watered with the
pl~al~lion of the active compound It is exl~edient to keep constant the amount of
water per unit area. The concentration of the active cornpound in th~ ~le~lion is of
- 38 -
WO 95n2532 ;~ ~ ~ 3 6 ~ 1
no importance, only the amount of active compound applied per unit area being
decisive. A~ter three weeks, the degree of ~ ge to the plants is rated in % damage
in cn, "l'~' ;.con to the development of the ~ltl~d control. Ihe figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, clearly superior activity compared with the prior art in barley (0-10 /O) and
wheat (0 /O) crops is shown, for example, by the compounds of P~e~ion
Examples 4, 13, 17, 25, 26, 37 and 41 against weeds such as Digitaria (8~95 /O),
Abutilon (100 /O), Chenopodium (95-100 /O), Galinsoga (70-100 /O), Matricaria(60-100 /O), Portulaca (70-100 /O), Solanurn (80-100 /O) and ~lola (80-100 /O).
- 39 -