Note: Descriptions are shown in the official language in which they were submitted.
W095/24945 2 1 8 4 2 5 3 PCT/GB95/00552
(i)
TREATMENT OF UNDESIRABLE HALOGENATED ORGANIC COMPOUNDS
THIS INVENTION relates to the treatment of undesirable
halogenated organic compounds. It relates in particular to
a process and installation for treating such a compound.
According to a first aspect of the invention, there is
provided a process for treating an undesirable halogenated
organic compound, which process comprises
heating a reactor wall by means of electrical
induction or resistance heating;
maintaining the reactor wall at a predetermined0 reaction temperature of at least 1500C;
allowing heat to radiate from the reactor wall into a
reaction zone adjacent to, and in contact with, the reactor
wall;
feeding a substantially solids-free gaseous feedstock
comprising an undesirable halogenated organic compound,
into the reaction zone;
maintaining a non-oxidizing substantially solids-free
gaseous atmosphere in the reaction zone;
directly heating up the compound by means of the heat
radiated by the reactor wall, with the compound being
heated sufficiently for it to pyrolyse and thus be
transformed into more desirable components; and
withdrawing a gaseous product comprising the more
desirable components, from the reaction zone.
W095/24945 2 1 8 4 2 5 3 PcT/Gsg5/00552
(2)
The Applicant is aware that halogenated organic compounds
are produced in industry as hazardous waste products. It
is required to destroy such hazardous or toxic waste
products, and the process of the present invention provides
a means of effectively destroying such halogenated
compounds, with the production of at least one more
desirable compound. By 'effectively destroying~ is meant
converting the halogenated compound substantially entirely
into the more desirable compound, with at most an
acceptable limit of the original halogenated compound
remaining and/or at most an acceptable limit of another non
desirable compound being produced.
It will be appreciated that, while reference has been made
to a feedstock comprising a single undesirable halogenated
compound, the process can equally be applied to a process
comprising a mixture of undesirable halogenated compounds.
The undesirable halogenated organic compound may typically
be one of the following, or mixtures of two or more
thereof: PCB (PolyChloroBiphenyl), TCB (TriChloroBenzene),
lindane (HexaChloroCycloHexane). DDT, TCDD
(TetraChloroDiphenylDioxine), SF6 (HexaFluoroSulphide),
F5SSF5 (DecaFluoroDisulphide), PCP (PentaChloroPhenol),
chloroform, dieldrin, perchlorinated aliphatics and RFCl
(Chlorinated Fluoro Hydrocarbons, or Freons). The process
of the invention is'thus suitable for destroying a range or
family of undesirable halogenated compounds.
The process may include preheating the feedstock, prior to
feeding it into the reaction zone, to remove contam-nAnts
therefrom and/or to preheat it. Thus, in one embodiment of
the invention, the halogenated compound may be available
as, or as part of, a gaseous waste stream or product. If
the concentration of the halogenated compound in the waste
w095/2494s 2 1 ~ 4 2 5 3 PcT/Gsgsl00ss2
(3)
stream lS sufficiently high, the waste stream can be used
directly as the feedstock. However, the gaseous waste
stream can be pretreated, eg by subjecting it to suitable
absorption for the halogenated compound, if its
concentration in the waste stream is too low, to produce
the feedstock.
In another embodiment, the waste stream, and hence the
halogenated compound, may be in liquid form. The process
may then include pretreating the waste stream to remove
either the halogenated compound or the other liquids,
depending on which is the major component. Such
pretreatment may comprise leaching or the like. The liquid
stream may then be heated and vaporised, to form the
feedstock.
lS In yet another embodiment of the invention, the waste
product may be in solid form. Pretreatment to extract the
halogenated compound may comprise leaching or the like, or
heating the solid waste to sublime or vaporize the
halogenated compound. The solid waste product may also be
dissolved in a suitable solvent prior to ~aporisation to
form the feedstock. The solvent may itself be a
halogenated organic waste product or stream.
When the halogenated compound is not in gaseous form, the
method thus includes vaporizing or gasifying it prior to
feeding it into the reaction zone as the feedstock.
The process may, however, also include preheating the
~aporized feedstock, if necessary. Thus, the feedstock may
be superheated, ie heated to above the condensation
temperature of all components present therein. Thus, the
feedstock may typically be preheated to a temperature in
the range 400C-700C, but ensuring that destruction of
woss/2494s 2 1 8 ~ 2 5 3 PcT/Gsgs/00ss2
(4)
feeds~ock does not yet commence, ie the feedstock is
preheated to below the incipient pyrolysis temperature.
The non-oxidizing atmosphere may be a neutral, ie a
non-reducing, atmosphere in the reaction zone. Instead, a
reducing atmosphere can be maintained in the reaction zone.
This may be effected by maintaining a slight hydrogen
excess in the reaction zone. Thus, the process may include
adding hydrogen or a hydrogen donating compound such as
methane, to the reaction zone.
The heat radiated from the reactor wall into the reaction
zone is thus in the form of electromagnetic waves, covering
the entire electromagnetic spectrum, but with the infrared
portion thereof predominating. The transformation of the
undesirable halogenated compound can comprise one or more
of the following steps: exciting the halogenated compound
sufficiently by means of the radiated heat, and in
particular by means of infrared radiation, to dissociate it
into a halogen radical as well as a further acceptable
radical ('radical A')i exchanging the halogen radical in
the halogenated compound with a further radical ('radical
B') to form a non hazardous or more acceptable compound;
combining the halogen radical with another radical
('radical C') to form a more acceptable compound; and
breaking down radical A into more acceptable smaller
radicals or components.
More particularly, the reaction mechanism is believed to
involve allowing the compound to absorb sufficient infrared
radiation or energy from the radiated heat to heat it up to
its pyrolysis temperature, at which temperature thermal
decomposition of the compound into the radicals takes
place. In other words, the carbon-halogen bonds in the
molecules of the compound break, and optionally react with
woss/24945 ~1 ~ 4 ~ 5 3 PCT/GBg5/00552
(s)
hydrogen radicals present in the reaction zone, to form the
more desirable compounds. Thus, molecules containing
asymmetric components, such as carbon-halogen components,
are grey or opaque to infrared radiation, and absorb
radiation heat until sufficient heat has been absorbed for
them to become excited, as described above; the resultant
fine carbon dust and radicals which are formed, or the
resultant products after reaction with the further
radicals, as hereinbefore described, can be symmetrical, in
which case they will be transparent to infrared radiation,
and not absorb further radiant heat, with such heat thus
being available to heat up other or residual asymmetric
molecules until substantially all asymmetric molecules have
been converted to symmetric molecules. The heat content of
the asymmetric molecules, ie the heat absorbed, is utilized
in the endothermic splitting reactions, with the resultant
sy-.lmetric products being, as stated, transparent to radiant
heat and thus not being heat absorbant, so that the product
gas exits the reaction zone at low temperature, usually at
less than 100C, and typically at about 40C-60C.
The process may thus include adding a secondary reactant to
provide radical C. The secondary reactant may contain
radical C in relatively pure form, or it may be in
molecular form.` When in molecular form, the process may
include exciting the molecular reactant to dissociate it
into radical form. i This excitation may then be effected
prior to adding the secondary reactant to the reaction
zone. Alternatively, the secondary reactant can be added
in non excited form to the reaction zone so that it and the
halogenated compound are excited simultaneously. Radical C
can thus be a hydrogen radical; however, it can instead be
any other suitable radical to provide a desired end
product. The secondary reactant can thus be methane or
woss/24945 2 1 8 4 2 5 3 PcTlGss5loo552
(6)
hydrogen, as hereinbefore described, or a
silicon-containing compound.
The reactor wall is thus maintained at a suitable elevated
temperature of at least 1500C, normally above 1600C, eg
about 2000-3000C, to ensure the desired pyrolysis and
transformation of the halogenated compound. The
temperature is thus dictated by the temperature at which
the particular compound dissociates into its component
atoms or radicals. Where a mixture of such compounds is
used, the pyrolysis temperature will be determined by that
compound which has the highest transformation temperature.
The heating of the reactor wall by resistance or induction
heating results in high thermal efficiencies. In
particular, substantial heat losses associated with heating
lS means located externally of the reactor wall, such as
radiation coupling, are avoided or at least substantially
reduced. For example, with externally located heating
means, some heat is reflected off the outer surface of the
reactor wall, and uneven temperature distributions often
occur.
Heating of the feedstock in the reaction zone is thus only,
or primarily, effected by means of the heat radiated from
the reactor wall, which heat heats up the feedstock
directly. Thus, indirect heating of the feedstock, eg by
means of contact thereof with elements, which are heated up
by, for example, induction or by the heat radiated from the
reactor wall, and which are located in the reaction zone,
as the only or the primary heating of the feedstock in the
reaction zone, is avoided. However, if desired, additional
secondary heating may be provided in the reaction zone.
This secondary heating may be provided by locating direct
heated graphite heating elements in the reaction zone, or
w0~sl2494~ 2 ~ 8 4 2 5 3 PCT/GBg5/00552
(7)
by DC/AC or RF plasma, a heating arc, additional infrared
radiation, micro waves or eximer laser energy, or laser
radiation in, or directed into the reaction zone. The
secondary heating, when present, only constitutes a minor
proportion of the heat supplied to the reaction zone, with
the major proportion being provided by the heat radiated
from the wall.
The reactor wall may be of graphite, or may be graphite
lined. This will ensure that the high reaction
temperatures required can be handled by the wall. In
addition, graphite provides the required combination of
temperature resistance and chemical resistance;
furthermore, its electrical conductivity can be utilized
for resistance heating of the wall.
The feedstock must then, however, contain substantially no
chemical component capable of releasing reactive oxygen
which can react with the graphite wall or lining to an
appreciable extent. By 'reactive oxygen' is meant oxygen
which is released, at the pyrolysis temperature, in the
form of a radical, such as the hydroxyl radical, which can
react with graphite. The pretreatment can thus comprise
removing such components from the feedstock, if necessary.
The pretreating may also include removing other substances,
such as sulphur and phosphorus, which are capable of
reacting to form substances which are harmful to the
graphite, from the feedstock.
If desired, an inert carrier gas, such as argon, for the
halogenated compound, can be used, with the feedstock then
comprising the halogenated compound and the carrier gas.
w095/24945 2 1 8 4 2 5 3 pcTlGss~looss2
(8)
The velocity of the feedstock through the reaction zone may
be such that there is l~m' n~r flow in the reaction zone, at
the pyrolysis temperature.
The reactor wall may be of vertical cylindrical form, with
the reaction zone being provided inside the cylindrical
wall. The vertical location of the wall ensures that
settling of carbon dust on the wall is minimized. The
reaction zone may comprise a preheating section and a
pyrolysis section located adjacent the preheating section,
with the feedstock entering the preheating section and the
product being withdrawn from the pyrolysis section. The
feedstock will thus be preheated up to the required
pyrolysis temperature in the preheating section, with
pyrolysis being effected in the pyrolysis section. The
reaction wall in at least the pyrolysis section, and
optionally also the preheating section, may be
substantially non-porous. Since the reactor wall in the
preheating section must be clean for good heat radiation
and since radicals already start forming as the feedstock
is superheated in the preheating section with such radicals
tending to polymerise, forming tars, soot, and the like,
due to the temperature being below the pyrolysis
temperature, and which tend to deposit on the wall as a
layer which is opa~ue to radiation, the process may include
periodically cleaning the reactor wall in the preheating
section.
According to a second aspect of the invention, there is
provided an installation for treating an undesirable
halogenated organic compound, which installation comprises
a reactor comprising reactor wall defining a reaction
zone adjacent thereto and in contact therewith;
W095/24945 2 1 8 4 2 5 3 PCT/GBg5/00552
(9)
electrical induction or resistance heating means for
heating the reactor wall to a predetermined reaction
temperature;
feed means for feeding a gaseous feedstock comprising an
undesirable halogenated organic compound lnto the reaction
zone so that the feedstock is in contact with, and passes
along the reaction wall;
means for maintaining a non-oxidizing atmosphere in the
reaction zone, with the reactor being adapted to heat up
the feedstock in the reaction zone sufficiently by means of
the heat radiated by the reactor wall for it to pyrolyse
into more desirable components; and-
withdrawal means for withdrawing a gaseous product
comprising the more desirable components from the reaction
zone.
The reactor wall may, as hereinbefore described, be of
vertical cylindrical form with the reaction zone being
provided on the inside of the tube, and comprising a
preheating section in which the feed can be further
preheated, and a pyrolysis section adjacent the preheating
section, and with the reactor wall in at least the
pyrolysis section being substantially non-porous at the
reaction temperature.
The installation may include cleaning means for cleaning
the reac~or wall in the preheating section, or for ensuring
that th- reactor wall in the preheating section remains
clean.
The cleaning means may comprise means for applying a film,
blanket or envelope of inert gas, hydrogen or recycled
product gas against the reactor wall, in order to preclude
the feedstock from contacting the reactor wall. Instead,
the cleaning means may comprise means for periodically
PcT/Gs95loo5~2
W095/24945 2 1 ~ 4 2 5 ~
(10)
blowing graphite granules or the like against the reactor
wall to remove the deposits therefrom. Instead, the
cleaning means may comprise a mechanically operable
cleaning tool, eg a drill, which may be of carbide,
alumina, zirconia or other suitable ceramic material. In
yet another embodiment of the invention, the cleaning means
may comprise means for imparting pressure pulses or shocks
to the reactor wall, thereby to remove any deposits.
The invention will now be described by way of example with
reference to the accompanying drawings.
In the drawings
FIGURE 1 shows a simplified flow diagram of a process
according to the invention for treating an undesirable
halogenated compound; and
FIGURE 2 shows a flow diagram of a pilot laboratory scale
simulation of the process of Figure 1.
Referring to Figure 1, reference numeral 10 generally
indicates a process according to the invention for treating
an undesirable halogenated organic compound.
The process 10 includes an optional pretreatment or
concentration stage 12 for pretreating a waste product
stream which contains an undesirable halogenated organic
compound to be destroyed. The stage 12 is linked, by means
of a flow line 16, to a vaporization/gasification stage 14.
The stage 14 is linked, by means of a flow line 18, to a
reactor or pyrolysis stage 20. An additional or secondary
reactant feed line 22 leads into the stage 20. The stage
20 is in turn linked, by means of a flow line 26, to a
by-product collection stage 24.
W095/2494a 2 1 8 4 2 5 3 PCTtGBgS/00552
( 1 1 )
The stage 12 will be dispensed with if the waste product
stream contains a sufficiently high concentration of the
undesirable halogenated compound, eg is a liquid stream
consisting of the undesirable halogenated compound only.
Furthermore, if the waste product stream is in gaseous form
and contains a sufficiently high concentration of
halogenated compound, then the stage 12, and indeed the
gasification stage 14, can be dispensed with. Such would
typically be the case if the waste product stream emanates
from a vinyl chloride manufacturing process.
If the waste product stream contains a relatively low
concentration of the undesirable halogenated compound, eg
if it is a ventilation air stream or liquid effluent
stream, then concentration and extraction of the
halogenated compound will be effected in the stage 12.
If the waste product stream is aqueous based, then the
halogenated compound must be removed therefrom, eg leached
therefrom using a suitable solvent such as a halogenated
solvent, in the stage 12. If the liquid stream is organic
based but is contaminated with water, then such water
should be substantially removed, eg by suitable leaching,
since the rem~;n~er of the process 10 is not tolerant to
water. Any other non hazardous components present in the
waste stream should then also be removed in the stage 12,
eg by suitable leaching.
If the liquid waste product stream contains a sufficiently
high concentration of the undesirable halogenated compound
and contains substantially no compounds undesirable to the
pyrolysis stage 20, it is routed through the stage 14 to
the stage 20. Thus, the liquid stream passes through the
stage 14 only to gasify or vaporize it.
woss/24945 2 ~ ~ 4 2 5 3 PcT/Gsg5/00552
(12)
The flow line 16 may be a conduit, conveyor, or the like
depending on the physical form of the stream or product
being transferred from the stage 12 to the stage 14.
In the stage 14, the halogenated compound is vaporized or
gasified, and superheated, while keeping it at as high a
concentration as possible. The heating can be effected in
an oven which can be heated by any convenient method, for
example, in an induction or ohmic heated electric oven.
The gaseous stream or feedstock from the stage 14 then
passes, by means of the flow line 18, which is typically a
conduit, to the reaction stage 20. The pyrolysis stage 20
can be in the form of a pyrolysis furnace. The conduit 18
transfers the product from stage 14 directly to the stage
20.
The nature, type and construction of the stage 20 normally
depends on the specific halogenated compound to be treated
therein. Where a mixture of halogenated compounds is to be
treated, then the reaction or wall temperature, and hence
the construction of the stage 20, will be dictated by that
component which requires the highest pyrolysing or
transformation temperature. However, the temperature is
also related to the residence time. The structure will
thus be optimized according to the reaction temperature
required, and the specific reaction volume required at a
specific temperature to give a desired residence time.
Furthermore, the construction material also depends on the
halogenated compound(s) and the temperature to be applied.
Halogenated compounds generally dictate that a pyrolysing
temperature in the region of 1500C to 3000C is required.
The stage 20 therefore has a substantially non-porous
graphite reactor tube or a substantially non-porous reactor
w095l24945 2 1 8 4 2 5 3 PcT/Gsg5/00552
(13)
tube lined with graphite. The furnace is heated by direct
ohmic or resistance heating. Utilizing a graphite lined
furnace for the high pyrolysis temperatures which are
required gives, as has been demonstrated on laboratory
scale, good results in view of the properties of graphite,
such as excellent thermal shock resistance, large thermal
gradients obtainable across the tube or lining, good
electrical conductance, and increasing mechanical strength
as temperature increases. An essentially similar pyrolysis
furnace design based on induction heating would also
provide the same functionality and can hence in principal
be used. The reactor tube has a preheating section and a
pyrolysing section as hereinbefore described.
In the preheating section, the feedstock is heated to the
pyrolysis temperature of 1500C-3000C, while in the
pyrolysis section, the halogenated organic molecules are
exposed to a specific infrared radiation in the high
temperature environment, and in an atmosphere essentially
devoid of oxygen or an oxygen-donating compound such as H2O
or CO2, ie in a non-oxidizing atmosphere. Preferably, a
reducing or hydrogen atmosphere is used. The absorbed
energy is converted to chemical energy, resulting in the
formation of radicals, especially hydrocarbon and halogen
radicals. Sufficient energy must be available to complete
the splitting reaction; if insufficient energy is available
then partial splitting occurs, and the formed radicals can
back react, thus rejoining the carbon halogen bonds. If
sufficient energy is available, further splitting of the
carbon hydrogen bonds will occur. The hydrogen and halogen
radicals join to form a lower energy species, ie a hydrogen
halide. Depending on the available energy, the pyrolysis
may be continued until only elemental carbon radicals
remain. These carbon radicals may react with others,
forming hexagonal radicals, which may further join to form
W095/24945 2 1 8 4 2 5 3 PCT/GB9Sl00552
(14)
graphite nuclei, or amorphic carbon dust. With the process
of this invention, complete destruction of halogenated
organic compounds to elemen~al carbon radicals is achieved,
and therefore the temperatures utilised are significantly
hi~her than in known processes employing pyrolysis of
chlorinated organics.
Since the halogen atoms are present as radicals in the
furnace, they are highly reactive, and these 'radicalized~
halogen nuclei may react with other radicals present,
resulting in formation of the lowest chemical energy
species in equilibrium. These species become lower in
energy with hydrogen radicals than with carbon or oxygen
radicals, thereby promoting destruction of the halogenated
compounds. Where there is sufficient excess, on a molar
basis, of free hydrogen radicals available compared to
halogen radicals, the graphite nuclei can be expected to be
free of halogens. It is essential, therefore, in the
process of the invention, that sufficient hydrogen is
available for the complete reaction of all halogen nuclei
with hydrogen.
If the feed to the pyrolysis reactor 20 contains
non-reactive oxygen containing molecules, the halogen
radicals will be split off first. The oxygen atoms will
initially remain with the mother carbon atoms, which will
finally be split off as carbon monoxide which is very inert
in the reducing atmosphere in the furnace. Minor amounts
of water and carbon dioxide will form carbon monoxide and
hydrogen where applicable, in the furnace, thus consuming
some carbon radicals. The thermodynamics prevalent under
the pyrolysis conditions indicate that formation of
diox;neq, furanes and phosgene are energetically
unfavourable; if such substances are present in the
feedstream, they will also be destroyed.
W095/24945 2 1 8 4 2 5 3 PcT/Gsg5/oo552
(15)
Heat transfer into the organic media in the pyrolysis
reactor is by radiation heat transfer. At higher applied
temperatures, the non symmetric organic molecules,
especially halogenated organics, effectively absorb
radiation heat. The molecules are transformed into
radicals with hydrogen and chlorine being split off as
radicals. Components which do not contain halogen have as
a rule high negative Gibbs Helmholz free energy. Once a
halogen radical has been split off, the remaining radical
becomes less 'grey' for absorption of thermal radiation.
Finally, the remaining free elemental carbon, hydrogen, and
halogen radicals are transparent to thermal radiation, and
the radiation heat can thus pass through the gas layers to
reach all halogenated molecules and radicals. The result
of this is that energy will be absorbed by the media, until
all the molecules and radicals have been destroyed or
transformed to single radicals. The heat energy absorbed
by the compounds is consumed in the highly endothermic
splitting reactions and, as the materials produced by this
reaction are transparent to the infrared radiation in the
reaction zone, these products only gain heat by con~ection
and therefore remain at relatively low temperatures. Argon
carrier gas utilized in the reactor and secondary reactants
such as hydrogen are transparent to infrared radiation and
therefore are heated only slightly by convection in the
reaction tube. These phenomena ensure that the exit
temperature of the product stream remains relatively low,
eg typically at 40C-60C.
In the pyrolysis section, any non-symmetric agglomerated
carbon radicals; forming fine carbon dust, will effectively
absorb and further radiate heat, completing the reaction.
Since the heating energy enters through the walls of the
furnace, the formation of tar and fouling of the heating
surfaces at cold spots will not readily occur. The carbon
wos~l2494s 2 1 8 4 2 5 3 pcTlGs95loos52
(16)
dust which is formed has an extremely fine particle size,
and does not settle readily. Furthermore, the carbon
particles, being conductive, do not readily attach to the
walls by means of electrostatic forces.
From the stage 20, the secondary products produced therein
pass along the flow line 26 to the post treatment or by
product collection stage 24. The post treatment applied
will depend on the secondary products in question.
Thus, for example, if the feedstock to the process
comprises chlorinated hydrocarbons, and methane or
hydrogen, preheated to an adequate temperature to preclude
condensation of the feedstock to any appreciable extent, is
introduced along the flow line 22, then the secondary
products obtained from the stage 20 will comprise
essentially carbon dust, hydrochloric acid, and hydrogen.
The stage 24 will then comprise HCl scrubbers, carbon dust
filters and waste gas recycling.
In the event that PCB or TriChlorBenzene is the halogenated
compound, a suitable preheated silicon bearing compound may
be introduced into the stage 20 along the flow line 22.
The secondary products from the stage 20 will then include
silicon carbide. In addition, when the secondary products
include silicon chloride (SiCl4), this can be rerouted back
to the stage 20 for excitation therein, as hereinbefore
described, together with additional HC1, H2, Si or C,
depending on the stoichiometric requirements. The fine
silicon carbide powder thus obtained can be used to make
highly homogeneous silicon carbide elements utilizing
sintering techniques.
Similarly, when the secondary products which pass from the
stage 20 along the flow line 26 include boron chloride
woss/24945 2 1 8 4 2 5 3 PcTlGs95loo552
(17)
(BC13) or boron hydride (BH3), these can be rerouted back
from the stage 24 to the stage 2Q where they are vaporized
and excited, and the resultant radicals allowed to react
with carbon radicals, to form boron carbide (C3B4) dust,
which can also be used in hard metal applications.
In the event that it is not desired to react any of the
secondary products obtained from the stage 20 to produce
further products, the off-gas from the stage 20 must be
treated. The gas has a high calorific value, and can be
used as a fuel. The gas will contain HC1 which can be
collected in a series of wet scrubbers or by a dry recovery
process.
The process 10 was simulated on pilot laboratory scale,
utilizing the layout of Figure 2.
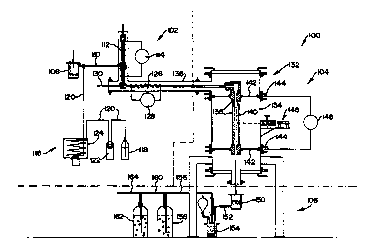
In Figure 2, reference numeral 100 generally indicates the
pilot laboratory scale apparatus used. The apparatus 100
has a section 102 corresponding to stage 14 of Figure 1; a
section 104 corresponding to stage 20 of Figure 1; and a
section 106 corresponding to stage 24 of Figure 1.
The section 102 comprises a batch solids feeder 108, with
a conduit 110 leading from the feeder 108 to an
electrically operable evaporator or vaporizer 112. The
evaporator 112 includes a Variac (trade name) 2kW heating
arrangement 114.
The section 102 also includes a continuous liquid stream
feeder, generally indicated by reference numeral 116.
The continuous liquid stream feeder 116 includes a
container 118 containing a supply of the unwanted liquid
hydrocarbon product to be destroyed, with a conduit 120
W095/24945 2 1 8 4 2 5 3 pcTlGs95loo552
(18)
leading from the container 118 to a peristaltic pump 122.
The conduit 120 leads ~rom the peristaltic pump to a hot
oil bath and stirrer arrangement 124, and then ties into
the conduit 110.
The evaporator 112 is integral with a superheater 126
fitted with a Variac (trade name) 2kW heating arrangement
128. A conduit 130, through which argon carrier gas, and
methane or hydrogen can be introduced, leads into the
superheater 126.
The section 104 comprises an upright cylindrical or tubular
reactor, generally indicated by reference numeral 132. The
reactor 132 comprises an outer cylindrical shell 134 to
which is connected a tubular component 136 forming part of
the superheater 126. Inside the outer shell 134 is mounted
an outer graphite tube 138 along the inside of which
extends an inner graphite reaction tube 140, which is
operatively connected to the superheater 126. The upper
and lower ends of the outer graphite tube are mounted in
graphite limpets 142, while the limpets are mounted to oil
cooled aluminium contact rings 142 located around the
outside of the outer casing 134. The tube 140 was isolated
by means of graphite and alumina wool.
A pyrometer 146 is also mounted to the outer casing, and is
operatively connected to the inner tube 140. A Variac
(trade name) 44kW heating arrangement 148 is connected, by
means of seven cables, to each of the aluminium contact
rings 144 so that the graphite limpets, and hence the inner
graphite tube 140, can thereby be heated up to the required
pyrolysis temperature.
.
The inner or central graphite tube had a 22mm ID and an
effective length of 2m. During the pilot scale test it was
w095/24945 2 1 8 4 2 5 3 PcTlGs9sloo5s2
(19)
heated up to 2660C, by using it as the resistance element
in the high current AC heating arrangement or circuit 148.
The section 106 comprises a dry filter or trap 150 for
carbon, with the lower end of the inner tube 140 leading
into the dry filter 150. A conduit 152 connects the dry
filter 150 to a wet filter 154. A conduit 156 connects the
wet filter 154 to a first hydrochloric acid scrubber 158,
with a conduit 160 connecting the scrubber 158 to a second
hydrochloric acid scrubber 162. A conduit 164 leads from
the scrubber 162 to a vacuum pump (not shown).
Thermal insulation materials used throughout, eg. inside
the shell 134 were carbon fibre felt and high alumina felt.
Temperatures were measured by means of an optical
pyrometer, and the measuring gate was mounted in an argon
atmosphere containing steel case.
Tests were conducted on the pilot laboratory scale
apparatus of Figure 2, as follows:
EXAMPLE 1
The product gas from the reactor 132 was analyzed and found
to consist essentially of hydrogen and HCl. The solid
carbon product from the reactor 13 2 was analyzed by
leaching it with n-hexane according to the standard ASTM
method to isolate any residual chlorinated organic
material. In the test program chloroform, TCB and PCB were
used as the undesired halogenated compound, with methane as
a hydrogen donor (secondary reactant) and argon as an inert
carrier. For analysis, a lOg sample of the carbon produced
was leached twice for 20 hours and the leachate
concentrated to lme. This sample was analyzed in a gas
chromatograph mass spectrometer (GC-MS). GC-MS analysis
sensitivity allows a detection of lOpg (pico grams) from
w095/24945 2 1 8 4 2 5 3 PcT/Gs95loo552
(20)
1+1 feed, ie. five 9's. In the analyses 5 - 6 significant
figures were obtained. Traces of the original feedstock
were detected at between 100 and 1 ppm - equivalent to
between 99.99~ and 99.9999~ respectively destruction of the
feedstock. The efficiency of destruction was found to be
directly related to the feed rate and therefore more
complete destruction can be anticipated with longer
residence times at the pyrolysis temperature. A longer
graphite reaction tube 140 will thus lead to increase
destruction and enhanced energy efficiency.
EXAMPLE 2
A number of test runs were conducted, utilizing solid,
liquid and gaseous feeds. Liquid feeds were passed through
a falling film evaporator, and the vapours then superheated
in a graphite heater to 400C-700C, depending on the
feedstock. Solid feeds were evaporated or sublimed in a
batch pot, and the vapours conducted through heated
conductors into the reactor. Gaseous feeds, such as freons
and halothanes were superheated, as was done for the
vapours from the liquid feeds.
The product gas from the reactor was in all cases merely
warm to touch, and tolerable by hand, indicating a
temperature only slightly abo~e ambient, eg about 40C.
Fine carbon dust was filtered from the product gas using a
glass-wool filter, ~and the hydro-halogen products were
absorbed in two large alkaline absorbers. The carbon yield
removed from the wool was 80~-95~. Some of the dust was
thus caught in the wool, while the rest passed through the
wool into the absorbers.
Product gas was in each case sampled in a liquid nitrogen
trap, as well as in a quartz cell for IR spectrophotometric
analysis. Carbon was leached twice, for 24h periods in
wogs/2494s 2 1 8 4 2 5 3 PCT/GB95/00552
(21)
each case, using cyclohexane, in a soxhlet apparatus, to a
leached concentrate of less than lm2. The leachate was
analysed by injecting l~e into GC-MS. 50m fused silica
column was used. The sensitivity of the MS was claimed to
be better than 0,4pg.
No halogenated feedstock or halogenated daughter compounds
could be detected in the product gases. This gave a
destruction efficiency better than seven 9's.
Standard Test conditions:
Feedstock lg/min
H donor CH4 or H2 in stoichiometric ratio:
Cl:H = 1:2
Main reactor,
ie wall temperature 2100C
Evaporator 420C
Preheater 550C
w095/24945 2 1 8 4 2 5 3 PCT/GB95/00552
(22)
Summary of Results
Reactor
Wall
Test Feed Feed Temp. Dest.
Run Feed Stock H-Donor Method Rate C. Eff. %
PCB CH4 Liquid 1 g/m 2100 99,9999
2 PCB CH4 Liquid 1 g/m 2100 99,9999
3 PCB CH4 Liquid 2 g/m 2100 99,9999
4 PCB CH4 Liquid 4 g/m 2100 99,9999
PCB CH4 Liquid 1 g/m 2500 99,9999
6 PCB H2 Liquid 1 g/m 2100 99,999
7 Chlolofo.lll H2 Liquid 1 g/m 2100 99,99+
8 Tetra H2 Liquid 1 g/m 2100 99,99+
chloride
9 T .in-l~n.o H2 E~a~)Olàt~ - 2100 99,9999
Hexes H2 Ev~u-a~e - 2100
11 Tar H2 E~/~ulate - 2100 99,99+
12 DDT H2 Liquid 1 g/m 2100 99,999
13 Dieldrin H2 Liquid 1 glm 2100 99,99+
14 CFC H2 Gas 1 g/m 2100
The carbon products were obtained as a loose powder and as
dense agglomerated pieces.
20 It is often difficult to obtain desired maximum limits of
super poisons, such as TCDD, in the flue gas emanating from
incineration. Typically, the upper limit of such poisons
can be as high as lppb (ie 0,OOlppm), but it can be as low
as 0,lppb. These limits are difficult to detect due to the
25 fact that, in fossil fuel incinerators generally used, the
hazardous waste stream is diluted with a support fuel,
typically by a factor of about 2 0 . Furthermore, the
WO 9~/24945 2 1 8 4 2 5 3 PCTIGB9~/00552
(23 j
support fuel requires a substantial volume of air for
combustion and, since the upper limits of super poisons are
based on the total volume of flue gas produced, substantial
masses of such super poisons will still be emitted to the
atmosphere during such incineration.
The Applicant thus believes that in the process of the
present invention, wherein super poisons such as TCDD are
not produced in view of the complete absence of oxygen
which prevails, and wherein there is no dilution of the
feedstock by means of a support fuel and/or air, these
problems are to a large extent overcome.
Th- Applicant also believes that the process of the present
in ention provides a convenient means for effectively
handling hazardous chemical wastes containing halogenated
compounds, and in particular halogenated hydrocarbons,
which are difficult to destroy with conventional methods or
where there is a danger of producing toxic secondary waste
materials which may then possibly be even more hazardous
than the halogenated compounds.
The Applicant more particularly believes that with the
process 10, problems associated with known methods of
destruction of hazardous wastes containing chlorinated
hydrocarbons, are at least alleviated. Presently, such
hazardous wastes are destroyed by means of incineration, at
applied temperatures of 1100 to 1200C. The incineration
can be effected in two stages, with hazardous components
being gasified in the first stage by applying temperatures
of around 700C in an oxidizing atmosphere. In the second
stage a high oxygen content is required to prevent
formation of phosgene, and this stage utilizes a
temperature of 1100 to 1200C. The high oxygen atmosphere
is effected by utilizing burners combusting oxygen or
W095/24945 21 ~ 4 2 5 3 PCT/GB95/00552
(24)
oxygen enriched air, and different forms of nitrogen oxides
are generated. If desired, the combustion air to the
burners can be heated by applying a DC plasma thereto.
Another known halogenated organic destruction system
utilises a plasma furnace with controlled oxygen levels to
achieve destruction. This system operates at temperatures
above 5000C, is capital and energy intensive, and is
substantially inflexible for by product production.
The process of the invention is thus characterized thereby
that the reaction zone contains substantially no solid
material, elements or particles either introduced with the
feedstock of inherently in the reaction zone, eg to heat or
assist in heating the feedstock, with the only solid
material being any solid product which is formed. Such
exclusive gaseous phase operation promotes simplicity of
construction and generation of the installation. For
example, fouling of the reactor wall is m; ni mi zed.
The process is further characterized thereby that the
feedstock occupies the entire reaction zone, and is even in
contact with the reactor wall. Since the feedstock is in
gaseous form cont~ining little or no solids, other than
possibly some solid reaction products, and bearing in mind
the reaction mechanism as hereinbefore described, little or
no fouling of the reactor wall in the pyrolysis region
occurs. Thus, the use of wall cleaning means in the
pyrolysis region, such as providing an envelope of inert
gas against the wall or blanket can largely, if not
entirely, be avoided. This results in a simpler
construction, and lower capital and operating costs. Such
inert gas envelopes or blankets are also used to protect
reactor walls against high reactor temperatures in cases
where heating means other than resistance or induction
W095/24945 2 1 8 4 2 5 3 PCT/GBg5/00552
(25)
heating of the wall are used, and are clearly not required
for this purpose in the present instance, thereby also
avoiding potential problems associated therewith, such as
reduction in heating efficiency of the feedstock if the
inert blanket admixes turbulence with the feedstock
adjacent the wall causing carbon dust clouds which shield
radiation heat transfer.