Note: Descriptions are shown in the official language in which they were submitted.
2~ 8~8~6
WO 96/12956 PCTIUS95/14041
DIR~CT FLuoR~ F~cE-coNJuGATEn Tl\~MuNoAssAy
FO12 PLATFT.FT ACTIVATION
Back~rou ' of th~
P-selectin, also known as granule membrane protein-140 (GMP-140),
or PADGEM protein, is an integral membrane glJ~,u~ t~;., found in secretory
granules of both platelets and endothelial cells. See E.I.B. Peerschke, Am J, ~lin
10 ~h~ , 455 (1992). After activation of these cells by agonists such as thrombin,
it is rapidly, ~ to the cell surface during l ~ P-selectin belongs
to the cell surface during ~ \ P-selectin belongs to the selectin family of
vascular cell surface receptors that shate sequence similarity and overall domain
ul~ul;~liul~. See G.I. Johnston et al, ~11. ~i. 1033 (1989). The other known
15 selectins are ELAM- I, a cytokine-inducible endothelial cell receptor for neutrQphils,
and a leukocyte surface structure which plays a role in directing the homing of
Iylll~l,v~t~ to high endothelial venules of peripheral Iymph nodes. It has recently
been shown by J.-G Geng et al, BloQd, 1~. 65a (1989), that human neutrQphils
bind in a Ca2+-dependent manner to purified P-selectin ;, . ", .. .1.;1;, ~ on plastic.
20 ru.~..,....u.c, adhesion of neutrophils to r --~ stimulated with ra~pid activators
such as histamine is mediated at least in part by P-selectin P-selectin is also involYed
in binding of activated platelets to monocytes and neutrophils. See, S.A. Harnburger
et al., Blood, ~, 55Q (1990) and E. Larsen, Ç~ 2, 305 ( 1989)
Because platelet activation , a number of vascular disorders
25 such as unstable angina, peripheral vascular disease, stroke, and prQcedures such as
.u.~iù,ul~ly and corQnary IIIIUIIIIJUI~D;D~ effort has been exerted during
the last two decades to develop more sensitive and specific methods to detect
activated, circulating platelets. See, for example, C.W. Hamrn et al., J. Am Coll.
Ç~iQL 10, 998 (1987); D.J. Fitzgerald et al., Cirr~ tirn 77, 142 (1988) and A.H.30 Gershlick, Cire-~ .n 81, 128 (1991). The most reliable markers of in vivo platelet
activation have been substances released from platelets after activation, which can be
measured in the plasma or urine: platelet factor 4 (PF4), B-ll..U,, ~1 ~O~ i-- (B-TG),
and ,... ~ Jl;t~ _ of tlllUIIIbù7. Ul~ A2. These markers have not achieved widespread
clinical ~rC~pt^~~, however, because of technical limitations pertaining to sample
35 collection, processing, and analysis.
wo 96/12956 2 t ~ ~ $ ~ ~ PCT/US95/14041
Severdl changes in surface membrane ~IJ~.U~ ,t.,~ expxssion can be
detected during platelet activation with specific murirJe ~ ' ' antibodies. For
example, as reported by SJ. Sh2ttil, in ~ , lQ, 3û7 (1987), and C.S. Abrams et
al., ~ , 128 (1990), changes in the conversion of the GP~ma complex to a
5 functional fibrinogen receptor can be detected. J.N. George et al., J, I'lin Tnv~c~
:Z~, 340 (Ig86) reported that plat~let activation with ~ , J,..~ alpha granule
release can be ascertained by examtning P-selectin expression. Thus, assays havebeen designed that combine the use of activation-specific .,.. ~.1. 1 antibodies with
flow cytometry. See, for example, R.E. Scharf et al., A~ al '
10 Thr~.mh- cic 1~. 1475 (1992). These assays can be performed on whole blood and
can facilitate the detection of platelet ~ that are ll.,tu~ u~ with
respect to their activation status. However, flow-cytometry requires expensive
;..~1, . . ,. ~l ;.... complex data processing and is not practical either to process large
numbers of samples ~.. ~ .. , . ,. ~11~ or to derive results within the timefrdme required to
affect clinical outcomes in acute situations such as those mentioned above.
Therefore, a need exists for a sensitive, simple and rapid in vitro assay
to detect the extent of p~ateleL activation.
S of ' I
The present invention provides a method to deter~nine the extent of
",_""" 1: , platelet activation. In commonly assigned U.S. Patent Application Serial
No. 08/142,766, filed October 26, 1993, an assay for platelet activation was
disclosed which invol~ed pre-isolation of platelets in vitro, i.e., in platelet-rich
plasma. Although this method greatly increased the accuracy and rapidity of the assay
for activated platelets, the method of the present invention constitutes an i~ll~l~J
over this method in tbat it may be practiced on whole blood without a pre-isolation
step, thus further reducing the processing time. ru. ll,.... , - cell loss that may result
due to pre-washing and ~ . ~, ;r. .~ ;, .. . iS eliminated.
In the method of the present invention, a sample of whole blood is
30 obtained from a patient whose level of platelet activation is to be determined and
divided into two portions. One control sample is treated with an activation agonist to
maximally activate the platelets contained therein, employing a suitable agonist such as
ADP, while the other sample is not treated with exogenous activation agonists. Next,
both samples are treated with a prefixing solution, such as ~. - . f .... l~ ' 1- hJ ~, and
wo sc/~2ss6 ~ ~ 8 ~ ~ PCT/US95/14041
allowed to incubate for a period of time sufficient to partially fix the platelcts. As used
herein, the terrn 'partiaDy fixed" indicatea a state in which the platelets containec in
said samples will not be further activated or damaged by vortexing irl the subsequent
step, while the red blood cells in the sample will maintain their ability to react with the
5 Iyticagent ~ . 'JL employed. Wllile_ ~, gvortexing~an~yllllv~yLl~lytic
agent is added to the samples. After allowir~g a suff~cient time for the Iysis of the
el ~ iu u _ ~a to occur, a leukocyte stabilizer is added to stop the Iysis reaction.
S. .l .~ ly~ an amount of a cell membrane fixative is added to fully fix and to
stabilize the âamples. Aa used herein, the term "stabilize" indicates a state in which
10 the samples can be stored for up to at least about 72 hours before r' " g the analysis.
Anti-P-selectin antibody is then added to each sample in an amount
effective to bind to the activated platelets in each sample. The antibody-activated
platelet complexes in each sample are determined nuulu~ .tl;~,al y, by means of a
15 fluorescent label that is attachcd to the anti-P-selectin antibody, or by addition to the
complexes of a fluorescent label which binds to a binding site on the bound antibody.
A ratio of the n.. ,~.. ~ of the complexed activated platelets in the sample not
y activated to the n - .~ , of the maximally activated platelets provides
a measure of the extent of platelet activation in the ' donor of the platelets.
~û Thus, the present invention provides a nuul~ ,c-coniugated
;,."".... ~,i,:l;....... ~ assay (FC~3A), for measuring ~ b~ platelet acrivation
~""`1"'-' ~v
(a) obtaining a sample of whole blood from a patient whose platelet
activation is to be determined and dividing it into a first sample and a
second sampl~;
(b) adding an amount of an activation agonist, such as adenosine 5'-
1'. L , ' ' (ADP), to said first sample for a period of time effective
to maximally activate the activatable platdets in said first sample; while
g the second sample for an equivalent period of time;
(c) adding an amount of a solution effective to stop the platdet
activation reaction, such as 1~ ~r -, ...~ Jc, to said first sample for
a period of timc effective to partially fix the activated platelets in said
first sample, while v the second sample for an equivalent
period of time;
2l 84g~6
WO 96/129S6 PCI/US9~/14041
(d) Vortexing the samples. and s~..~.~ti~ adding:
(i) an amount of an cly~blul,~u~, Iytic agenl cffective to Iyse the red
bloodL cdls in tbe samples;
(u) an amount of a leukocyte stabilizer effective to stop the action
of the Iytic agent; and
(iii) an amourLt of a cerL membrane fixative effective to completely
fix stabili7e the platclets;
(e) forming bina~y labelled complcxes with the activated platelets in each
sample by sdding to each sample an amount of
(i) an anti-P-sdcctin antibody conjugated to a fluorescent label; or
(ii) an anti-P-selcctin antibody conjugated to a binding site for a
detcctable fluorescent label followed by a detectable ffuorescent
label which specifically binds to said binding site; and
(fl ~ e the n -- /~ - r of the binary labelled complexes in each
sample, wherein a ratio of the n~ of said second sample to
said first sa~nple provides a measure of the extent of a platelet
activation in said second sample.
As used herein, the phrase ". - ln~,.. ~ platelet activation" is defined
ZO to mean that the activation measured is due to in vivo activation and is ~not due to the
addition of exogenous activating agents to the sample in vitro. In a preferred
"1 ,o.l; .. 1 of the invention, step (d) is performed by placing the samples in a Q-
Prep mac~Line (Coulter~!9 Corporation, Hialeah, FL) and performing a 30 sccond
cycle. The Q-Prep instrument consists of a matched, three reagent system that
25 provides a gentle, no-wash erythrocyte Iysing and fLxing system which maintains
leukocyte l.lu~I.olo~;~ and cell surface integrity. Previous to the d~,~.lu,ull~ of this
assay, the Q-prep machine had been used primarily to prepare white blood cells for
ffow cytometry. Surp~isingly, although the treatment of the platelets in the Q-prep
machine fLxes them so that the platelets are stable for an extendcd period of tLme, the
30 platelets are stirL able lo react with at~tibodies. The use of whole blood processed in
this manner offers an altemative to the use of PRP, since the results obtained using
whole blood that has been treated in the Q-prep machine correlate to those obtained
using PRP. See ExarrLples 7 and ~.
_ _
2 ~ 848~
WO 96112956 PC /IJS9S/14041
To develop the assays. p~atelet samples were activated with various
doses of ADP and fixed platelets were incubated with a n ~ -conjugated anti-
P-selectin antibody in wells of microtiter plates. The n .. ,~ . ., A~' intensity was read
on a R~ analyzer. Once the platelet samples were fixed, the
5 data ~ A;A procedures can be completed in less than two hours. The
intra-assay coefficient of variation (CV) was 6.97%, the time-based inter-assay CV
was 6.17%. The present assay ~' an excellent correlation (r = 0.936) with
flow cytometry in the .... A- r ,l of expressed P-selectin in platelets of 20 normal
donors.
U~ A~ JIY~ the i ' ~ of P-selectin in platelets in response to
increasing doses of ADP occurred in a dose-dependent manner and correlated
positively with ADP-induced platelet ~ L~ in the ~ on the basis of
both ~ doses of ADP (r = 0.99) and on the basis of time intervals (r = 0.92).
The amount of fibrinogen detected on the surface of the platelets was
15 also increased in response to ADP, whereas the intensiy of bound antibodies to the
GP IIb-ma complex underwent little alteration. In activated platelets, the intensity of
fibrinogen antibody binding was correlated with the intensity of P-selectin antibody
binding (r = 0.85). AlAhus, in another aspect of the present invention, the . ". A~l .. ~., ...:
of the available sites for fibrinogen binding on platelets may be used to determine the
20 extent of ." ~ platelet activation. This analysis can be carried ou~t on platelets
isolated from a tissue or ~ ya;ulo~;~ fluid such as human blood, e.g., the first and
second samples may comprise whole blood or platelet-rich plasma.
These results ~ that the present invention provides a rapid
and u..~ assay for platelet activation via ~ - of surface antigens
25 present or activated human platelets, and that P-selectin is a more sensitive and
specific marker than the GP Ilb-ma complex or than fibrinogen for platelet activation.
- Thus, the present assay can be used to evaluate, monitor and stage
platelet activation-related events associated with acute coronary syndromes, and in
restenosisfollowingp.,. ~ coronary ~ (PTCA). With
30 respect to the role of circulating activated platelets in these states, see, for example, I.
Weinberger et al., Am J. Card.. 70, 981 (1992); E. Minar et al., ~a~, 170, 767
(lg89); R.S. SchwartA,. et al., J. Am Cnl Car~l 19. 267 (1992) and D. Tshoepe etal., 5~, 88. 37 (1993).
wo 96112956 2 ~ 8 4 8 2 6 PCT/US95114041
Br~ef ~ J~- of the Fi~lres
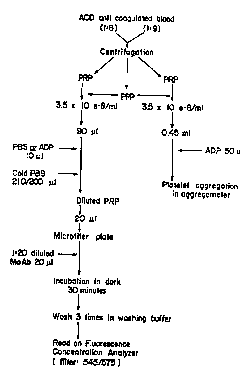
hgure I is a schematic depiction of one e 1 ~A ~ of the present
assay.
Flgure 2 is a graph depicting the n, -- -.. r intensity of P-selectin in
S resting platelets detected in the present assay using various dilutcd anti-P-selectin
antibody (CD62) and IgGI (n = 2) ~ PlateleB from two normal donors
werc sampled and tested in ~he assay using various dilutions of CD62 as indicated
Circles represent llu.L,~,~.,;r.,, binding (IgGI), whereas circles in bold represent
specific binding (CD62). n,.o-cac~.. cc intensity was IOg ,.. ~r.. ,. A
Figure 3 is a graph depicting the 1;~ intensity in diluted
resting platelets detected in the present assay using anti-P-selectin antibody (n = 2),
wherein samples from two normal donors were tested using CD62 diluted at 1:20 involume. n ~ intensity was log-l r ~ and the coefficient r was
computed using linear regression.
Figute 4 is a graph depicting n"~ intensity in resting platelets
detected in the present assay using various dilutions of CD62 (n = 2) Platelets from
two normal donors were sampled and tested by the present assay~ Log-l, ~ r " ".. ,1
nUUlGa~ G intensity was used in the ~ ,. of the coefficient r using linear
regression.
20 Figure S is a graph depicting the n, .. ,~ ~ intensity i~n ADP-
stimulated plateles in plasma detected using the present assay (n = 2). Platelets were
sampled from two Dormal donors and tested by the assay. Both ~lly~u~.yill~ (PE)-conjugated and I ; ~ ' CD62 were diluted to the same ~ Closed
circies represent the ll.,...r~,,, 1~ intensities detected using PE-conju~ated CD62,
25 while triangles represent the values detected using I ; ~ ' CD62, and the open
circles represent the levels of fl, I~ intensity detected by using
CD62 and PE-conjugated CD62.
Figure 6 is a graphic correlation of the present assay with flow
cytometricanalysisinAIl,.,,,;,,~;.. ,~ofP-selectininplateletsinplatelet- (n
30 = 20). Platelets were sampled from 20 normal donors' platelet . using the
present assay and flow cytometric analysis ~;,., ~1l -,,. ., `1~. A value of r = 0.936 was
obtained from thc linear regression, indicating the positive association between the
two assays (p < 0.001).
2 1 84~26
WO 96/12956 ' PCT/US95/14041
Flgure 7 is a graph depicting the plate]et 9LL~ I slopcs with
stimulating doses of ADP as measured in an _c L. r~ (n = 3). Platelet
9-L~C'' ~ in plasma w s performed in triplicate by ~ y. The slopes were
the mcans of the stecpcst slopcs of ~ c ~ ; , trlcing curvcs in rcsponse to each5 dose of ADP.
Figure 8 is a graph ~I n ~ association bctween the expression
of P-selectin and stimulating ,- .. . - . ..~ A~ of ADP assayed in the present assay (n =
3). Final - of ADP in plate~ets in plasma were used.
Figure 9 is a depiction of the time course change of platelet reacrivity to
10 ADP as measured by ~,L''~ in ~ L~L~ I and by expression of P-selectin as
detected by the present assay (n = 3). Platelets from three normal donors were
sampled and assayed by &LL.rL."--~ and by the present assay cim~ nrr~ y The
hour indicated is the storage time of platelets after blood was drawn. The steepest
slopes Of 9-LLI` L~ ,I)-tracing curves and ]og~ r~ " .... ~ lluulc~cc~ e intensity were
15 used.
Figure 10 depicts the correlation of platelet ~ by
~66.~6~ lY with the expression of P-selectin as deterrnined by the present assay in
ADP-stimulated platelets in plasma (n = 3). Platelets from three normal donors were
assayed in triplicate by: LL~ rL~ Ily and by the present assay ~ y. The
20 doses of ADP used arc as indicated. Coefficient r was computed using linear
regression.
- hgure 11 depicts the correlation of exprcssion of P-selectin by the
present assay with 9 i C/ ~ Lrl ;~ ~ by ~ y in ADP-stimulated platelets in plasma
at time intervals (n = 3). A dose of 2~ llM of ADP was used in the .' of
25 a66lc60,1ion by ..~,6.e~;ull.~ly and by the present assay in platelets in plasma sampled
from three normal donors. Values are the means i standard deviation (SD) of three
samples in triplicate. Coefficient r was computed using linear regression.
Figure 12 depicts expression of GPIlb-ma complex and fibrinogen on
the surface of ADP-stimulated platelets in plasma (n = 3). ADP-stimulated platelets in
30 plasma from three normal donors were sampled and assayed by tbe present assay.
Values are the means i SD of three samples in triplicate.
-- 7
-
2t 84826
WO 96/12956 _ PCT/US95/14041
Detailed D~ ;V~i~,.. of the lnvention
~ , ,~1., 1 antibodies and polyclonal antibody 1~ - tl ;~
comprising fluorescent labels or binding sites for ligands comprising fluorescent
labels are ,..., .... ~ available, availablc to the art or preparable by art ._~,U6....
5 procedures. I.. l., r- ~ murine arlti-P-selectin antibodies are listed in Table l,
below.
~h QI
Anti-P-
selectin kabel Reference
Antibodv
S 12 Fl or R E. Scharf et al., A; t~,~ ;v~ul~lu~;~
v~.r~ tl~l Thmmhl-~ic ~, 1475 (1992);
R.P. McEver et al, L~iQL
.,~2. 9799 (1984).
- - - P.E. Stenberg et al., J. C~ll Bi- l
~11, 880 (1985).
KC4 --- S.C. Hsu-Lin et al., l,l~
Chem.. 259, 9121 (1984); R.
Bonfanti et al., ~l~i, 1~, 1109
(1989).
AC1.2; 1-18, 2- - - - E. Larsen et al., ~, 59, 305
15, 2-17 (1989).
CD62 rl,ru.,lrLl,li.. ornolabel Becton-Dickinson
Gl - - - S.A. H.,.l,~u,~s.,. et al., ~lood~, 75,
550 (1990).
Unlabelled antibodies carl be conjugated to fluorescent labels such as
10 fluorescein isocyanate (FITC) by starldard techniques. See, for example, S.J. Shattil
et al., Blood, 70, 307 (1987) and J.W. Goding et al., M~ oclu,.~,l An~ihn~ c
Prinrinl~c ~n~l Practice - Production ~nrl A~tlir~ n of Monoclonal ~ntihrtrii~c in Cell
Biolo~v. B;o~ , y and 1" .. ~ . Acadernic Press. London (1986) at pages
255-280. Alternatb/ely, the antibodies catl be prepared as biuLillylLLt~,d conjugates and
.. . ... ...... , _ _ _ _ _
2 1 ~4826
WO 96112956 PCTNS95/14041
reacted with ~ . yLlui.. ~I-clJlhY;Lll as taught by Goding, ibid., McEver et al.,
ibid., and S.J. Shattil et al., J. Biol. rh~m ~Q, 11107 (1985). Polyclonal anti-P-
selectin antibody 1 ~ can be prepared and detected as taught by P.E.
Stenberg, ~. rrll Bin~ lQl, 880 (1985).
Although ADP is a preferred platelet activation a~ ,~.c~Liu~. agonists,
other useful agents for platelet acdvatdon include thrombin, serotonin, collagen and
lluubu~u~c~ as well as bioactdve subunit ~I,Y~Lidcs thereof.
The .,lyihlu~,~Li~, Iydc agent of step d(i) of the present invendon is
preferably forrnic acid. The Iydc agent may also contain a stabilizer that serves to
10 increase the shelf life of the agent.
In a preferred r 1 ~u~ of the invendon, the leukocyte stabilizer is a
, . .., ll .;, ~ ,, ." of sodium carbonate, sodium sulfate and sodium chloride. Preferably,
the three ~ are in aqueous soludon. The leukocyte stabilizer may further
comprise a stabilizing agent which serves to extend the shelf life of the soludon.
1~ Preferably, the cell membrane fixadve employed in step d(iii) is
' ' ' Jde. Although y ~r", l, 1-l- hJie is the preferred fixative, several othersell membrane fixatdves are known to those of skill in the art. The cell membrane
fixatdve may further comprise a buffer solution.
Preferably, step (d) of the assay is carried out using automated
20 ;II~Llul~.~...L~lLiu l which vortexes the samples and rapidly adds the recite~d reagents.
The use of such automated i~LI shonens the u~cy~u~Liu~l time of samples
and thus allows a more rapid response in urgent clinical situations. One example of
such an instrument is the Q-Prep machine ~ u~uLuLu~ by Coulter~) Corporation,
Hialeah, FL. The Q-Prep instrument consists of a matched, three reagent system that
25 provides a gentle, no-wash e. .yLIUL,Y ~ Iysing and fixing system which maintains
leukocyte II-U.lJI.UlV~Y and cell surface integrity. The use of platelets prepared in this
manner offers an altemative to the use of PRP, since the results obtained using whole
blood that has been treated in the Q-prep machine correlate to those obtained using
PRP. See Examples 7 and 8.
-30 The invention will be funher described by referencc to the following
detailed examples, wherein adenosine ~ ' (ADP, Catalog No. 88~-3),
p~u~ hyde (Catalog No. 62Hû174) and other chemicals were obtained from
Sigma (Sigma Chemical Co., St. Louis, MO). N.,Y.,O.,I ~Lluill (PE)-conjugated
(Catalog No. 348107) and pure (Catalog No. 348100) murine .l.. ~ anti-human
wo 96/12956 ~ 1 ~3 4 8 2 6 PCT/US95114041
platclet P-selectin antibodies (CD62) and PE-conjugated isotype specific mouse IgG I
(Catalog No. 340013) were piirchased from Becton-Dickinson (Mountain View, CA).
- FlTC-conjugated murine ~ 3, . ~i anti-human platelet GP~ma aDtibody
(CD41a) (Catalog No. 0i349) was obtained frorn AMAC (Westbrook, ME). FITC-
S conjugated sheep anti-human fibrinogen antibody (Catalog No. K90056I ) was
obtained from BIO-DESIGI~ (K~ , ME). Antibodies were diluted UsiDg
1% fetal calf serum l ' ~,j ' buffered saline (PBS) solution.
Ten aspirin-free normal donors (age: 24~1, mai'e: 5, female: 5) were
recruited through the healthy donor center at Mayo Clinic. Blood was drawn in a 21
10 gauge butterfly necdle and a plastic syringe and collected using 15 ml ~ul,~,u,ul.jl.,..c
centrifuge tubes (Coming Inc.) containing 1:6 (for platelet activation) and 1:9 (for
platelect ~ ~r,~ ) Yolumes of acid citrate dextrose (ACD). Blood samples were
centrifuged at 250 xg for 10 minutes at 15 C iD a Mistral 3000-i centrifuge with rate of
S for bri~ke and Al ~ 1 l settings, I~S,U..Li ~ ..ly, tO obtain platelet-rich pla~ma
1~ (PRP). Platelet-poorplasma(PPP)waspreparedbyfurther~, 1; r, IVA~ ofthe
remaining blood at 1500 xg for 10 minutes. Platelet counts were performed on theCoulter Counter (Coulter Electronics, Inc.) and PRP was adjusted with PPP to a
constant count of 3.0 x 10 e-8/rnl.
Platelet a~ ;n~ studies were performed at 37-C on a dual channel
20 dc,~;1.,~5....l.,t~,l (Dayton Dual Channel Aggregation Module), at a stirring speed of 900
rpm. Optical density for PRP and PPP was set at 10% and 90%, IC ~t~ y
Adenosine 1' ~ ' , ' (ADP) (0.05 ml) was added to 0.45 ml of stirred suspension
of PRP up to the finai ~, .... ~ ..~ . Al i~ .11 as shown. The maximal or steepest slope of the
tracing curve was measurcd.
The maximal slope of the platelet A~".~ - tracing curYe was
computed using the equation: Dfi = f(ti)-f(ti-l)/ti-t(i-l) = h max(cm)/t(min), where h
max is the height of the steepest slope of the curve in ~ , and t is the time ofthe steepest slope of the curYe in minutes. Cocfficient (r) values were computed using
linearregression. Insome~ ,c logarithm-~ r..,.,~ ~idatawereused.
The lluulcsc~ .c ". A~ ' were obtairled using the IDEXX
n.. ,S~.. , r-.. ~ l Analyzer (FCA) machine (IDEXX I tl~n~rnn~c, Inc.,
Westbrook, ME). This instrument uses a specially designcd (96-2311) 2 mm
diamet~r filter membrane-bottomed plate (I:luoricon assay plate) that separates
antibody-bound cdls from non-bouDd anibody in solution by applying a Yacuum (0-
_ _
2 1 84826
WO 96/12956 PCTIUS95/14041
25 mmHg) from below the membrane. The total ~.,II/O..iil,~dy-bound lluc..~..,.."c is
determined by front-surface fluorimetly. The instrument has a nuu ~ t~, capable of
exciting and reading at several ~ .-2;LI~ (400/450 nm-590/620 nm).
F. '- 1.
FluorPc~Pnce-coniu~ated Immunobindirl~ Assav
for plntPIP~ ~cti~ation
Ten ~11 of phosphate buffer saline tPBS, 0.01 M, pH 7.4, as baseline)
or ADP in a series of (10 11M~25 ~M, 50 ~M, 100 IlM and 150
10 were added to a 90 ,Lll sample of PRP in round-bottomed ~uly~Lyl~,.lc tubes and
allowed to incubate at room ~ for five minutes. Eighty 111 of mixed sample
was i~u~ ~ly added to a 1 ml final c~ of 1% of I ' ' ' ' ,~.1~ PBS
solution in 1.5 ml vials, and the samples were incubated at 4-C for four hours. The
fixed samples were washed twice by .- ~l - i r ~ in a .. i~ , . r. .c,. . using PBS
15 solution. The washed platelets were diluted to 8.5 x 10 e-7/ml in PBS solution.
A 20 111 aliquot of diluted samples was placed in 96 well plates. A 20
,L~LI aliquot of 1:20 diluted CD62, or 1:40 diluted CD4 1 a, or 1:50 diluted ,. "l; r;~ . ..",. . .
Ab was added and the samples were incubated in the dark at room ~ Lu.c for 30
minutes. The cells in the Fluoricon assay plates were ' and washed three
20 times in washing buffer (1% Tween PBS) by applying a vacuum memb~ rane from
below the plates of 25 rnm Hg in the Pandex FCA machine. This step removed any
unbound antibody and free nuu.c~..,...,c marker from the c~mrl~y~ Antibody- - ~
bound n . ,. ., ,~. . . ,1 ~ was determined by reading plates at a gain of I for CD41a and
.-- -; r;l .. ;",,~. .. Ab and of 10 for CD62 on the FCA instrument at the appropriate
25 wavdength. Data was recorded as relative nuu. c~u~ cc units tFU), after subtracting
the blank.
In r~ designed to determine the optimal dilution of PRP and
of lluu,cac~ c-conjugated antibodies, the same total volume, witn varying dilutions
of PRP and the antibodies, was used. For the ,i. ~, . ., .:,, -~ ;. .., of the time course
30 change of platelet reactivity to ADP as measured by ~c~ rC~ 1ll and by expression of
P-selectin, PRP was stored at room a,...~ .Lu~c in the centrifuge tubes after being
diluted to a constant . . . - ; . ,.l ;. .., with PPP. At each time point, PRP waS
withdrawn from the stock tubes and added to test tubes and the activation and
~ cc~ assays performed as described above. For the c~ of the present
w096l12956 21 8482~ PCrlUS95/14r41
assay to the flow cytometric analysis, samples were prepared as described in flow
cytometric analysis example, and 100 ~ of the samples were placed in Fluoricon
plates and read on tnc FCA instrument.
To select the optimal conditions for the assay, various dilutions of
5 antibody and antigen (platelcts) were tested in the present assay. The selection of
optimal conditions vas based on selecting the ,~ . . tl..1 ;"" of the antibody that was
on the steepest slope of the dilution curve and from which the most specific and the
least non-specific reactions were obtained. Optimal conditions were obtained using
anti-P-selectin antibody at a 20-fold dilution in volume or at an all~il u 1~ T ' ratio
10 of 0.04 llgll.S x 10 e-6 cells. At this dilution, the specific reaction was greatest while
.,...~1.,.1;. ~ the lowest non-specific reaction (F;gure 2) and a wide range of
of P-selectin was detectable in a dose-dependent manner (Flgure 3).
rul~ ulc, it was in the middle of a linear dilution curve (Figure 4), which makes
the assay specific and sensitive. When the same amount of Ull., ; ~ ~ ,." . .~1. ."~
lS antibody to P-selectin was added to platelets in plasma followed by trne addition of
nuulcs~.. e-conju~ated P-selectin antibody, n 1l~ f intensity of the platelets
was decreased by 50%, indicating a satisfactory ~:ulll~.diLiv= inhibition of binding
(Figure 5).
Expression of P-selectin in four fixed platelet aliquots (mean levels:
20 56.00 to 60.00 FUII S x 10 e-g cells) from the same hear~thy subject w~ere determined
in ten replicates (for intra-assay variability) and in triplicates (for inter-assay
variability) in mub~iple separate assays. The intra-aasay CVs for the means of ten
replicates ranged from 3.45% to 10.78% ~mean: 6.97%). The time-based interassay
CVs for the means of triplicates ranged from 5.93% to 12.39% (mean: 8~11%). The
~5 sample-based inter-assay c~,rS for the means of triplicates ranged from 2.82% to
13.99% (mean: o.l7%).
After incubation with CD62 for 30 minutes, a drop of platelets in
plasma was transferred to a slide. Platelets were then evaluated by ll~l~lua~u,u~.
ADP-activated platelets bec~tme larger, developed protrusions, and changed to a
30 spherical shape under light llfil,lua~u~u~. These activated platelets ~' ' red
., .. r under n~ luaCulJ~
12
2 1 84826
1-- wo 96112956 PCINS9S/14041
F.
Flow C ' Ar~o~vs;c
PJatelet ~ were obtained from the Mayo Clinic blood bank
within 24 hours of collection of blood from volunteer donors. Platelet concentrate
5 (PC) was prepared by collecting blood (450 + 45 ml) from random donors in 63 ml of
CPD in a pyrogen-free (Fenwal T ~hn7-Atr~ n~ Morton Grove, IL) quad blood
collection pack with an attached satellite bag containing 100 rnl of ADSOL solution.
After blood collection, whole b~ood was rr r~tnfilg~ri for 5.2 minutes at 1400 g's at
20-24-C. The platelet-rich plasma was pressed into an empty satellite bag, leaving
10 ~ 'y 50 ml of PC The PC was left ~ d for l hour, was
..... 1. d on a platelet rotator, and stored on a horizontal flatbed shaker. Twenty
individual PC units were sampled after 24 hours of storage.
Samples were prepared for analysis by fixing 100 111 of platelets with I
ml cold 1% ~ -- A r .. ~Alrirhydl, for I hour at 4-C. The platelets were washed (x2) with
15 l ' l ' -buffered salinelEDTA (PBS/EDTA), the pellet was Ir~llyh .~.'il ~i in I ml
PBS/EDTA and stored at 4 C in the dark. The following day (within 24 hours) 50 111
of the ~ ..ir .l platelets were labeled with 10 ,ul of .~ antibody CD41
(AMAC, Inc., Westbrook, ME). After a 10-minute incubation in the dark at rraom
h~ Lul~ (RT), 20 111 of MoA6 (Becton-Dickinson, San Jose, CA) waS added and
incubated 20 more minutes in the dark at 25-C. The sample was then ~washed (xl)
with PBS/EDTA and the pellet waS . ~ ir ~l in 1 ml of cold 1% ~, - A r~ ., ., .Al.l. h ~
and stored at 4-C in the dark for flow cytometric analysis. All samples were anaiyzed
within 6 hours of labeling. Samples were analyzed on a flow cytometer (FACScan,
Becton-Dickinson, Mountain View, CA) within 6 hours of labeling. The percentage
of platelets expressing P-selectin (the percentage of activated platelets) was determined
as described by R. Funbeer et al., Tr~ncfusirm 3Q, 20 (1990). The mean GPIIb-ma
surface densiy was determined for the subsets of P-selectin-negative platelets and P-
selectin-positive platelets. See, H.M. Rinder et al., Tr~lcfilcinn ~, 409 (1991);
Anrcthr~ri~)lo~y~ 963 (1991).
Quantitative expression of P-selectin as lluu._sc~ intensity or as the
ratio of P-selectin positively stained platelets in one-day stored platelets in platelet
--- from 20 normal donors were ddermined ~ -- v ~ by the present
assay and by flow cytometric analysis, lC~ Linear regression analysis of the
data showed that the log-tr~Ancf~ r ri n r~ intensiy of P-selectin as
13
WO 96112956 2 t ~ ~ 8 ~ ~ PCT/t~S95114041 1
deterrnined in FC~3A was correlated with the raio of P-selectin positively stained
platelets expressed as a log-~, r _ r " . ~ ratio as measured in flOw cytometric analysis
(r = 0936, p ~ 0.0vl) (Figure 6).
1; `- 3.
T duc~ion of F~y~ c ~ . of P-selec~jn
: ' A in Pl~telets by Al)P
Platelets in plasma from three healthy subjects aggregated in the
a~ Sv~ ~. in response to ADP in a dose-dependent manner (Fig~re 7), and the
steepest slopes of the ~LC~ tracing curves were correlated wivh increasing
simulating doses of ADP (Flgure 7). P-selectin in platelets in plasma from the same
-~- three healthy subjects was also i " ' to the plasma membrane of platelets in
response to ADP in a dose-dependent manner as measured by ~ u~ L~
lluulGa~clluG with increasing stimulating doses of ADP, in accord with Example 2(Flgure 8).
F. '- 4.
Ti - Course l'hsn~e of Platelet Rea~tivitv to AnP
Reactivity of platelets in plasma to ADP as measured by C~;~lG2gCLiUII or
by expression of P-selectin from three healthy donors were measured ,both by an
aE;~ ;ull~ l and by the procedure of l~xample 1, at time intervals ranging from 1.5
to 10 hours after blood was drawn. Platelets in plasma aggregated in response toincreasing stimulating doses of ADP in parallel with the expression of P-selectin at
time intervals (Figure 9). The reactivity of platelets to ADP was increased at time
intervals. and reached similar peak levels at the seventh hour (Flgure 9).
FY~n~
RP~ of Anp-induced A ` ' EY~
of p_~PIP~'~in jn Platelets
Data from Examples 34 were analyzed using linear regression. This
showed that the expression of P-selectin in platelets in plasma as measured by the
present assay was correlated positively with platelet L .~ dl ;- - . of platelets in plasma
as determined by a~ G~vl.l..L, y in response to ADP both on the basis of stimulating
doses of ADP (Flgure 10) and on the basis of time intervals (Flgure 11).
-
1~
2 1 84826
96112956 PCTtUS95tl4041
~x~m~le 6.
12. ' '- of E of p,~plPr~in ~ ~ R re of
GP nh.TTTs ' Fi' jn r~ r to ~np
To determine the alterations in the amount of GPI[b-ma complex and
5 P-selectin expressed on the platelet surface and in levels of fibrinogen bound to
plaKlets or exposed on platelets in response to the stimulation of ADP, platelets in
plasma from two healthy subjects were sampled and tested in the assay of Example I
A . vl ~ y by using mnnnrlnnAI antibodies to GPIIb-ma as well as with
--- ."~ antibodies to P-selectin, and a po~yclonal antibody to fibrinogen.
10 Detectable GPnb-ma complex in platelets showed little change in response to
stimulation with ADP (Figure 12). In contrast, the levels of fibrinogen bound toplatelets or exposed on the surface of platelets in response to increasing doses of ADP
was increased and reached a peak level at a dose od 5.0 llM of ADP (Figure 12). In
platelets in plasma, the changes in the amount of detectable G~b-ma complex
15 correlated weakly with alteration in the levels of bound or exposed fibrinogen in
response to the stimulating doses of ADP ranging from 0.0 to 10.0 IIM (y = 3~5.64 +
3.29 X, r = 0.45, p > 0.05), and also correlated only weaWy with the alteration in
levels of expressed P-selectin (y = 269.47 + 170.44 * log (X), } = 0.663, p ~ 0.05).
The alteration to levels of fibrinogen bound to platelets or exposed on the surface of
20 platelets was correlated more strongly with changes in levels of P-selec~tin expressed
in response to the incre_sing stimulating doses of ADP ranging from 0.0 to 10.0 IlM
(y = 167.3 + 0.44 X, r = 0.858, P < 0.05), suggesting the ~Ccoris~inn of P-selectin
with the binding or exposure of fibrinogen on ADP-stimulated platelets.
Detection of P-selectin ~ ;. . to the platelet surface as
25 determined by the present method is a sensitive and specific measure of platelet
activation, which correlates well with more complex traditional measures of this~l l.... ,. l ,.... ~ ~ Although simpler cells could be employed for nuulu~ y~ the
using microtiter plates with filters and the . "~ of front-surface
nuv...~ y to measure this ~ perrnits .~ to be made in 96 wells
30 as a single ~ procedure. For further expansion of the analytic capacity, a
n. " ,. r~ analyzer with the ability to read 10 plates (960 wells) as an even more
automated procedure (screen machines, IDEXX, Portland, ME) is also available.
These features make the present method very practical for the study of the dynamics
of platelet activation in the clinical disease states discussed above.
WO96/129 6 2 ~ 8 4 8 2 6
S PCIIUS95/14041
F - '- 7.
C~ o~ 12. ` T' - Whole Blood ' PRP ~ ci~ CP62
7.C R ~ ~ of E" ' ' ' Al' ''
A 6 ml sample of whole blood WRS isolated, divided into twenty 100
S ILI samples. and added to round-bottomed ~I~ ,.... tubes. ~llirry 111 of phosphate
buffered saline (PBS, 0.01 M, pH 7.4, as baseline) or ADP (2.5 ,uM) wcre added to
each sample and all were allowed to incubate at room ~ for five minutes.
One hundred l,11 of 1% of ~ r .",. ~ J~ PBS solution was added to each tube,
arld th~ samples were incubatcd at room t ~ r for fifteen minutes. Following
10 tbis incubation, each sample was placed in a Q-Prep machine (Coultera9 rnrpnr~sinn~
Hialeah, FL) for a 30-second cycle. During this cycle, the sample was vortexed while
a series of three reagents was added. The first reagent added was a solution of formic
acid at a c .... ~ , ..) of 1.2 mlJL. Next, a solution of sodium carbonate (6.0 glL),
sodium chloride (14.5 g/L) and sodium sulfate (31.3 glL) was added. Fmally, a
solution of p~-.. r.. ,~. hyl~ was added (10.0 g/L). The fixed samples were then
washed twice by ~ ., r, ~C~ in a ~ u~ iru~,., using PBS solution. The washed
platelets were diluted to 8.5 x 10 e-1/ml in PBS solution. All samples were analyzed
within 72 hours of fixing.
A 20 111 aliquot of each of the diluted samples was placed in 96 well
20 plates. A 20 ~11 aliquot of l :20 di]uted CD62 Ab was added and the sa~mples were
incubated in the dark at room lc,l.~,du.c for 30 minutes. The cells in the Fluoricon
assay plates were ~ L ' and washed three times in washing buffer ~1% Tween
PBS) by applying a vacuum membrane from below the plates of 25 mm Hg in the
Pandex FCA machine. This step removed any unbound antibody and free
lluu-ci~.,.-,c marker from the complexes. Antibody-bound ILule~ ,c was
daermined by reading plates $ a gain of 10 for CD62 on the FCA instrument at theappropriate w~ . Data was recorded as relative nu~JIc~c.,.lce units (E~U), aftersubtracting the blank and is shown in Table II along with the results obtained from 20
samples of PRP that were analyzed according to the method of Example 2.
16
~ WO 96tl2956 2 ~ ~ 4 ~ ~ 6 PCTtUS95/14041
Table II.~ , of Resulb Using Whole Blood and PRP and Using CD62
as a Marker of Platelet A.Li~
Whole Blood PRP
ADP-activated PBS Control Anp Acbvated PBS ('.~ nl
3326 0 2000 0
2 1704 0 1262 0
3 3228 0 5206 0
4 4158 0 3874 0
7870 0 6044 0
6 3792 0 3284 0
7 4594 0 3122 0
8 6588 0 4244 0
9 7024 0 9052 0
3762 0 6750 0
Data is recorded as absolute ~ ,. r~ units (FU).
As can be seen from Table II, the degree of pla~elet activation achieved
utilizing whole blood correlates well with the degree of activation achieved when
utilizing platelet-rich plasma. r Il.. ~.. ~ there was no activation observed in the
control samples of either whole blood or PRP.
F ' 8
C~ ' of 12. ' of Tlcin~ Whole Blood ' PRP and TT
FITC Labeled Fibrino~en ns a Marker for Platelet Activation
A 6 ml sample of whole blood was isolated, divided into twelve 100 ,ul
30 samples, and added to round-bottomed p~ y~ c tubes. Thirty Ill of fibrinogen,labeled with FITC (1:100 dilution) were added to each sample. S ~ ly, 30
of either ADP (2.5 ~lM) or phosphate buffer saline (PBS, 0.01 M, pH 7.4, as
baselme)was added to each tube and the samples were allowed to incubate at room
r. ~ . . . c for five minutes. One hundred ~LI of 1% of ~ hyde PBS
35 solution was added to each tube, and the samples were incubated at room t~ a~h,c
for fifteen minutes. Following this incubation, each sample was placed in a Q-Prep
machine (Coulter~ Corporation, Hialeah, FL) for a 30-second cycle. During this
17
W096/12956 ~i8~B2~ P~rllJS9~14041 ~
cycle, the samplc was vortexed while a series of three reagents was added. The first
reagent added was a solution of fomlic acid at a ~ of 1.2 mLtL. Next,;a
solution of sodium carbonate (6.0 g~L), sodium chloride (14.5 glL) and sodium
sulfate (31.3 glL) was added. Finally, a solution of I ' I ' ' ,1~C was added
5 (lO.O g/L). The fixed samples were then Washed tWice by ~ ; rl ~;rl ~ in a
u~ (lirL~, using PBS solution. The washed platelets werc diluted to 8.5 x 10 e-
~/ml in PBS soludon. All satnples were analyzed within 72 hours of fixing.
A 20 ~1 aliquot of diluted samples was placed in 96 well plates, A 20
111 aliquot of 1:20 diluted CD62 Ab was added and the samples were incubated in the
10 dark at room ~ for 30 minutes. The cells in the Fluoricon assay plates were
' and washcd three times in washing buffer (1% Tween PBS) by applying
a vacuum membrane from below the plates of 25 mm Hg in the Pandex FCA
machine. This step removed any unbound antibody and free r~ marker
from the complexes. Antibody-bound lluu c~ c was determined by reading plates
15 at a gain of 10 for CD62 on the FCA instrument at the appropriate wavelength, Data
was recorded as relative n~ units (F[l), after subtracting the blar~k and is
shown in Table m along with the results obtained from Example 6 wherein PRP was
used. Table IV shows the data that was obtained by following the above protocol
employing FITC labded fibrinogen as a 1:50 dilution,
Ta' ` TTI
C ' of Results Using Whole Blood and PRP and Using FlTC
Labeled Fibrinogen at a l:100 Dilution as a Marker of Platelet
Activatjon.2
Sample I (PRP) 4226 0
Sample 2 (PRP) 3972 0
Sample 3 (PRP) 4208 12
Sample 4 (Whole Blood) 3624 0
Sample 5 (Whole Blood) 3454 0
Sample 6 (Whole Blood) 3640 0
Data recorded as absolute nl,~les,.,..,e units (FU).
18
21~826
WO 96112956 PCT/US95/14041
- As d ~ ~ ~ ' by the data on Table m, fibrinogen binding is quite
obvious and readily detectable. Although the numbers for plat~let-rich plasma (Pr~P~
are somewhat higher than that obtained using whole blood, the use of whole bloodprovided an equally suitable indication of platelet activation. As can be seen by
5 comparing the data contained in Tables m and IV (below), the utilization of a higher
",.. ,1.. 1;.. ~ ~ of FlTC labeled fibrinogen is.not necessa~y to elicit a detectable
response from either the whole blood samples or the platelet rich plasma samples.
Table IV.
C~ : of Results of Whole Blood and PRP Methods Using FITC
Labeled Fibrinogen at a 1:50 Dilution as a Marker of Platelet
Activation.3
PBS
Sample 1 (PRP) 11838 150
Sample 2 (PRP) 12184 80
20 Sample 3 (PRP) 11862 98
Sample 4 (Whole blood) 3772 172
Sample 5 (Whole blood) 7238 216
Sample 6 (Whole blood) 6238 1~0
25 3 Data recorded as absolute n -, ,- ~ ~ units (I:U).
l;r~ patent3 and patent documents are; ~ 1 by
reference herein. as though individually i..~u.r ' by reference. The invention has
been described with reference to various specific and preferred e~..b- and
30 techniques. However, it should be understood that many variations and . ,.l;
may be made while remaining within the spirit and scope of the inventi~n.
19