Note: Descriptions are shown in the official language in which they were submitted.
~ woss/24483 2 1 8 5 ~ i 6 ~ u~ ~C
RECOMBINANT HUMANIZED ANTI-FB5 ANTIBODIE5.
Field of the Invention
The present invention is related to the field of
molecular biology, and more particularly to humanized
antibodies .
Back~round
The present invention relates to the generation, by
recombinant DNA methods, of novel recombinant
immunoglobulins specif ic ior the human FB5
(endosialin) cancer antigen. The invention also
discloses methods f or the production of these
recombinant antibodies, for the diagnosis and
treatment of certain human cancers.
Transformation of a normal cell to a malignant cell is
often ar~n~An;ed by a change in the expression of
cell surface antigens . These dif ferent phenotypes can
be detected using monoclonal Ant;ho~ specific for
such antigens. In this way, different cancer cells
can be detected and characteri~ed (Lloyd, K.O. (1983)
"Human Tumour Antigens: Detection and CharAct~r;7~tion
with Monoclonal Antibodies" in R.B. Herberman, ed.,
Ba3ic and Clinical Tumour Immunology, pp 159-214,
Martinus Ni jhoff, Boston) . These cell surface
antigens are cl~ruu' iate targets for tumour
immunotherapy and ~ n~
Of particular value for the ~l;A~n~7s;~ and therapy o~ a
broad range of cancers would be the identif ication of
an antigen associated with a broad range of cancers.
Tumour stromas are potential sites for the location of
such antigens. One such antigen (F19) is expressed on
the surface of reactive stromal fibroblasts associated
with ~gO~ of epithelial cancers (Garin-Chesa, P. et
al. (1990) Proc. Natl Acad. Sci. 87, 7235-7239;
~1851 1~
W0 95l24483
-- 2 --
with ~909~ of epithelial cancers (Garin-Chesa, P. et
al. (1990) Proc. Natl Acad. Sci. 87, 7235-7239;
Rettig, W.J. et al. (1988) Proc. Natl Acad. Sci. 85,
3110-3114). In clinical trials, a monoclonal antibody
specific for the F19 antigen accumulated at tumour
sites successfully locating hepatic metastases from
colorectal carcinomas (Welt, S. et al. (1992) Proc.
Am. Assoc. Cancer Res. 33, 319) . This illustrates the
diagno6tic potential of monoclonal antibodies specif ic
for tumour stromal antigens. Another tumour stromal
antigen (FB5 or ~endosialin~) has been i~l~ntif;ed and
partially characterized (Rettig, W. J . et al . (1992 )
Proc. Natl Acad. Sci. 89, 10832-10836). A murine
monoclonal antibody (m~bFB5) has been raised against
the FB5 antigen. This antibody has been used to show
that the FB5 antigen is expressed on the luminal
surface of vascular endothelial cells of a wide range
of malignant tumours . Specif ically, in
~ h; .c~torh~m; rill analyses of vascular endothelial
cells of human tumours, FB5 expression was found in 26
of 36 carcinomas, 18 of 25 neuroectodermal tumours and
41 of 61 sarcomas. In contrast, it could not be
detected in any of a wide range of normal adult
tissues including tissues of the following organ
systems: breast, cardiovascular, connective tissues,
digestive tract, endocrine, haematopoietic, lymphoid,
reproductive, skin and urinary. Similary, FB5 was not
expressed in cultured human malignant cells (excepting
a subset of sarcomas ), and stromal f ibroblasts of only
a small proportion of epithelial cancers exhibited FB5
expres s ion .
The specificity of the FB5 murine antibody makes it a
powerful tool for the detection of human cancers 1n
vitro. = For~a number of reasons the location of the
FB5 antigen on the luminal surface of tumour vascular
Woss/z4483 21 851 1 6 r~ c/o~oqs
-- 3
endothelial cells makes the antigen an ideal target
for tumour immunotherapy and diagno3is in vivo.
Firstly, a wide range of cancer types may be diagnosed
and treated by the FB5 antibody (or antibody
conjugate). Secondly, the endothelial cell surface is
readily accessible to antibodies that are circulating
in the blood stream. Thirdly, antibody-targeted
destruction of tumour blood vessels could lead to
widespread necrosis in solid tumours. Finally, on
binding to FB5 - expressing cells the m?~bFB5 is
rapidly internalized raising the possibility that the
antibody could be used for the specific delivery of
cytoxic agents for the destruction of tumour blood
vessels. However, the in vivo use of murine
antibodies as agents for the diagnosis and treatment
of human diæeases in severely curtailed by a number of
factors. Specifically, the human body recognises
murine antibodies as f oreign . This recognition of
the murine antibodies can elicit a human anti-mouse
antibody (HAMA) response (Schroff, R. et al. (1985~ -
Cancer Res. 45, 879-885) which results in rapid
clearance of the antibody from the circ~ t;~n
Furthermore, the Fc portion of a murine antibody is
not as efficacious as the human Fc at stimulating
human complement or cell-mediated cytotoxicity. For
the Ln vivo use of murine ~n~;horl;es in diagnosis and
therapy, these problems must be circumvented.
EP120694 (Celltech) and EP125023 (Genentech) disclose
the development of ' chimaeric~ antibodies using
recombinant DNA methods. Such ~nt;horl;es comprise the
variable regions from one species (eg mouse) and the
constant region6 from another species (eg human).
Such chimaeric antibodies would have the advantage
that they retain the specif icity of the murine
antibody but can also stimulate human Fc dependent
Wo 95n4483 2 1 8 5 ~ 1 6 P~ ogs
-- 4
complement fixation and cell-mediated cytotoxicity.
However, the murine variable regions can still elicit
a HAMA response (Bruggemann, M. ~. (1989) J. Exp.
Med. 170, 2153-Zl57) thereby limiting the value of
chimaeric antibodies as diagnostic and therapeutic
agents .
British Patent Application Number GB2188638A (Winter)
discloses a process whereby recombinant antibodies can
be generated by substitution of only the variable
region CDRs of one antibody with those from another.
Typically, this ' CDR-grafting' technology has been
applied to the generation of recombinant,
pharmaceutical antibodies consisting of murine CDRs,
human variable region Ll ~lhs and human constant
regions (eg Riechmann, L. et al, (1988) Nature, 332,
323-327). Such 'reshaped' or 'humanized' antibodies
have less murine content than chimaeric antibodies and
retain the human constant regiDns necessary for the
stimulation of human Fc dependent effector functions.
In consequence, CDR grafted antibodies are less likely
than chimaeric antibodies to evoke a HAMA response
when administered to humans, their half-life in
circulation should approach that of natural human
antibodies and their diagnostic and therapeutic value
is enhanced.
In practice, for the generation of efficacious
humanized antibodies retaining the specificity of the
original murine antibody, it is not usually sufficient
simply to substitute CPRs. In addition there is a
requirement for the inclusion of a small number of
critical murine antibody residues in the human
variable region. The identity of these residues
depends on the structure of both the original murine
antibody and the acceptor human antibody. British
~ wo gsl~i483 2 1 8 5 1 1 6 . ~
Patent Application Number 9019812_8 discloses a method
for identifying a minimal number of substitutions of
foreign residues sufficient to promote efficacious
antigen binding.
The present invention provides novel, humanized
monoclonal Ant;ho~l;es specific for the human FB5
cancer antigen. This has been achieved by the
conversion of the murine FB5 monoclonal antibody to
hllr~n; 7Prl antibodies by utilising CDR-grafting
technologies . The invention also provides methods f or
the production of these humanized Ant;ho~lies to be
used in the diagnosis and treatment of certain human
cancers. Prior to the work of the inventors, it was
not known that FB5 or any other non-human antibody
specific for the the EB5 antigen could be hllr~n; ze~ 80
as to retain useful binding specificity.
Brief descri~tion of the f iqure8
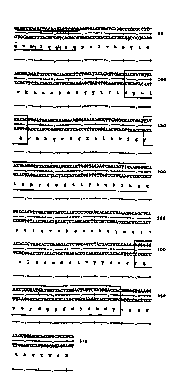
Fiqure 1 Shows the DNA sequence and corresponding
amino acid sequence of the murine FB5 heavy chain
variable region (VH). The CDRs are boxed. Underlined
nucleotides and amino acid residues are derived from
the oligonucleotide primers used. A backslash is used
to indicate the result obtained by the use of
consensus primers.
Fiqure 2 Shows the DN~A sequence and corrP~:r~-n~ling
amino acid sequence of the murine FB5 light chain
variable region (VK). The CDRs are boxed. Underlined
nucleotides and amino ~acid residues are derived from
the oligonucleotide primers used.
Woss/24483 ~ b .~ .. ;Q~045
-- 6
Fiqure 3 Shows the vector pSVq~t for the expressioli
of chimaeric or bumanized heavy chains in mammalian
cells .
Fiaure 4 Shows the vector pSVhva for the expression
of chimaeric or humanized light chains in mammalian
cells .
Fi~ureæ 5-10 These figure provide graphical data of
ELISA results demonstrating the binding properties of
humanized FB5 specific antibodies.
3. Su~mary o~ the Invention
One aspect of the invention is to provide humanized
antibodies specif ic f or the FB5 antigen .
Another aspect of the invention is to provide
polynucleotides ~n~o~;n~ humanized antibodies specific
for the FB5 antigen. Various expression vectors
comprising polynuclePtides encoding h~ ni 7F.ri FB5
antibodies j oined to promoter sequences are also
provided. Similarly, another aspect of the invention
is host cells transformed with expression vectors for
the expression of humanized FB5 specif ic antibodies .
Another aspect of the invention is to provide -
humanized anti-FB5 antibodies that are labeled with a
deteGtable label or a therapeutic label.
Another aspect of the invention is to provide
methods for treating and/or diagnosing cancer by
administering a composition comprising a-humanized FB5
specific antibody. One method of detecting cancer
cells involves the steps of administering a labeled
antibody ~detectable label) to a patient and
subse~uently detecting where in the body the labeled
antibody has bound.
4. Detailed Description o~ the Specific ~ ' '; t~
wossl24483 2 1 ~ 5 1 1 6 ~ "'~q~
As used herein, the term "humanized~ antibody
refers to a molecule that has its CDRs
(complementarily determining regions) derived from a
non-human species immunoglobulin and the , . ; n~ of
the antibody molecule derived mainly from a human
immunoglobulin. The.term "antibody~ as used herein,
unless indicated otherwise, is used broadly to refer
to both antibody molecules and a variety of antibody
derived molecules. Such antibody derived molecules
comprise at least one variable region (either a heavy
chain of light chain variable region) and include
molecules such as Fab fragments, Fab' fragments,
F(ab' )2 fragments, Fd fragments, Fabc fragments, Sc
antibodies (single chain antibodies), diabodies,
individual antibody light chains, individual antibody
heavy chains, chimeric fusions between antibody chains
and other molecules, and the like.
The term " conventional molecular biology methods "
refers to techniques ~ for manipulating polynucleotides
that are well known to the person of ordinary skill in
the art of molecular ~ biology . Examples of such well
known techniques can~be found in Molecular Cloninq: A
Laboratorv Manual 2nd Edition, Sambrook et al, Cold
Spring Harbor, NY (1989). Examples of Convf~n~;nnr~l
molecular biology techniques include, but are not
limited to, n vitro ligation~ restriction
r-n~nnllcl ease digestion, PCR, cellular transformation,
hybridization, electrophoresis, DNA sequencing, cell
culture, and the like.
The term ~variable region~ as used herein in
reference to immunoglobulin molecules has the ordinary
meaning given to the; term by the person of ordinary
skill in the act of immunology. Both antibody heavy
- chains and antibody light chains may be divided into a
"variable region" and a "constant region". The point
of division between a variable region and a heavy
wo ssn4483 2 l 8 5 1 ~ ng~ ~
region may readlly be det ~rm1 n~ ~ by the person of
ordinary skill in the art by reference to standard
texts descrlbing antibody structure , e . g ., Kabat et al
"Sequences of Proteins of Immunological Interest: 5th
Edition" U.S. Department of ~Health and ~uman
Services, U.S. G.,v, t Printing Office (1991).
The present invention p~ovides humanized antibody
molecules specific for FB5 antigen in which at least
parts of the CDRs of the heavy and/or light chain
variable regions of a human antibody lthe receptor
antibody) have been substituted by analogous parts of
CDRs of a murine monoclonal antibody and the humanized
antibody can specifically bind to the same as the FB5
antibody . In a pref erred embodiment of the sub; ect
invention, the CDR regions of the humanized FB5
specif ic antibody are derived f rom the murine antibody
FB~. Some of the humanized antibodies described
herein contain some alterations of the acceptor
antibody, i . e ., human, heavy and/or light chain
variable domain framework regions that are necessary
f or retaining binding specif icity of the donor
monoclonal antibody. In other words, the fL .J~J~k
region of some ~ nt~ the humanized antibodies
described herein does not necessarily consist of the
precise amino acid sequence of the f ramework region of
a natural occurring human antibody variable region,
but contains various substitutions that improve the
binding properties of a humanized antibody region that
is specific for the same target as the murine FB5
specif ic antibody . A minimal number of substitutions
are made to the f ramework region in order to avoid
large-scale introductions of non-human framework
residues and to ensure minimal immunogenicity of the
humanized antibody in humans. The donor monoclonal
antibody of the present invention is the FB5 murine
wogsli4483 2 1 85 1 1 6 P ~ 5,~?~0q~
g
antibody, which is specific for the human FB5 cancer
ant igen .
The h~ ni z~d antibodies of the present invention
include complete antibody molecules having full length
heavy and light chains, or any fragment thereof, such
as the Fab or (Fab' ), fragments, a heavy chain and
light chain dimer, or~ any minimal fragment thereof
such as a Fv, an SCA ~(single chain antibody), and the
like, specific for the FB5 antigen molecule.
In addition to providing for humanized FB5
specific antibodies, the subject invention provides
for polynucleotides encoding humanized FB5 specific
antibodies . The subj ect polynucleotides may have a
wide variety of sequences because of the degeneracy of
the genetic code. A person of ordinary skill in the
art may readily change a given polynucleotide sequence
encoding a humanized FB5 specif ic antibody into a
different polynucIeotide encoding the same hllm~n;7~
FB5 specific antibody ~m~or~ir -. The polynucleotide
sequence encoding the; antibody may be varied to take
into account factors affecting expression such as
codon frequency, RNA sec~n~l~ry structure, and the
1 ike .
The humanized antibodies of the subject invention
may be produced by a variety of methods useful for the
production of polypeptides, e.g. in vitro synthesis,
recombinant DNA production, and the like. Preferably,
the hllr-n; ~,1 antibodies are produced by recombinant
DNA technology.
The humanized FB5 specific Ant;horl;es of the
invention may be produced using recombinant
immunoglobulin expression technology. The recombinant
production of immunoglobulin molecules, including
humanized antibodies ~are described in U. S . patent
4,816,397 (Boss ~ ~L), U.S. patent ~,816,567 (Cabilly
et al) U.K. patent GB 2,188,638 (Winter et ~), and
Wossn4483 21 8 51 1 6 P~ 095
-- 10 -
U.K. patent GB 2,209,757. Techniques for the
recombinant expression of immunoglobulins, including
humanized immunoglobullns, can also be found, among
other places in Goeddel et al, Geng Ex~ression
TerhnnlQqY Methods ; n EnzymolQqY Vol . 185 Academic =
Press (1991), and Borreback, AntibodY Enqineerinq,
W.E~. Freeman (1992) . Additional information
rnnr~rning the generation, design and expression of
recombinant antibodies can be found in Mayforth,
Desiqninq Antibodies, Academ1c Press, San Diego
( 1993 ) .
The re~ ` ,; nAnt humanized anti-FB5 antibodies of
the invention may be produced by the f ollowing process
or other recombinant protein expression methods:
a. Constructing, by conventional molecular
biology methods, an expression vector
com~rising an operon that encodes an
antibody heavy chain in which the CDRs and a
minimal portion of the variable region
LL ~.~JLk. that are required to retain donor
antibody binding specif icity are derived
from a non-human immunoglobulin, such as the
murine FB5 monoclonal antibody, and the
r~ ;nrler of the antibody is derived from a
human immunoglobulin, thereby producing a
vector for the expression of a humanized
antibody heavy chain.
b. Constructing, by conventional molecular
biology methods, an expression vector
comprising an operon that encodes an
antibody light chain in which the CDRs and a
minimal portion of the variable region
f ramework that are required to retain dono~-
antibody binding specif icity are derived
from a non-human immunoglobulin, such as the
WO9Sf24483 2 ~ 8 51 1 6 r~ r~ogs
- 11 -
murine FB5 monoclonal antibody, and the
1- ; n~l~r of the antibody is derived from a
human; ~1 obulin, thereby producing a
vector for the expression of humanized
antibody light chain.
c. Transferring the expression vectors to a
host cell by conventional molecular biology
methodæ to produce a transfected host cell
for the expression of humanized anti-FB5
antibodies .
d. Culturing the transfected cell by
conventional cell culture techniques so as
to produce humanized anti-FB5 antibodies.
Host cells may be cotransfected with two
expression vectors of ~ the invention, the first vector
cnnt;l;ning an operon encoding a heavy chain derived
polypeptide and the second ~ntAin;ns an operon
encoding a light chain derived polypeptide. The two
vectors may contain different selectable markers but,
with the exception of the heavy and light chain coding
sequences, are preferably identical. This procedure
provides for equal expression of heavy and light chain
polypeptides. Alternatively, a single vector may be
used which encodes both heavy and light chain
polypeptides . The coding sequences f or the heavy and
light chains may comprise cDNA or genomic DNA or both.
The host cell used to express the
recombinant antibody of the invention may be either a
bacterial cell such as Escheri~ coli, or preferably
a eukaryotic cell. Preferably a l; An cell such
as a chinese hamster ovary cell, may be used. The
choice of expression vector is dependent upon the
choice of host cell, and may be selected so as to have
the desired expression and regulatory characteristics
in the selected host cell.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Woss/24483 21 85 1 1 6 ,~ 1,,,5~
-- 12 -
The general methods for construction of the
vector of the invention, transfection of cells to
produce the host cell of the i~vention, culture of
cells to produce the antibody of the invention are all
conventional molecular biology methods. Likewise,
once produced, the r,~ ' ;n~nt antibodies of the
invention may be purified by standard procedures of
the art, including cross-flow filtration, ammonium
sulphate precipitation, affinity column
chromatography, gel electrophoresis and the like.
The humanized FB5 3pecific antibodies of the
present invention may be used in conjunction with, or
attached to other antibodies (or parts thereof ~ such
as human or humanized monoclonal antibodies. These
other antibodies may be reactive with other markers
(epitopes) characteristic for the disease against
which the antibodies of the invention are directed or
may have different specificities chosen, for example,
to recruit molecules or cells of the human immune
system to the diseased cells. The antibodies of the
invention (or parts thereof ) may be administered with
such antibodies (or parts thereof ) as separately
administered compositions or as a single composition
with the two agents linked by conv~nti- n~l chemical or
by molecular biological methods. Additionally the
diagnostic and therapeutic value of the antibodies of
the invention may be augmented by l;-hPl 1 ;n~ the
humanized antibodies with labels that produce a
detectable signal (either n vitro or=;L~, v vo) or with
a label having a therapeutic~property. Some labels,
e . g . radionucleotides may produce a detectable signal
and have a therapeutic property. Examples of
radionuclide labels include 12sI, l31I, l4C. Examples of
other detectable labels include a fluorescent
chromophore such as fluorescein, phycobiliprotein or
tetraethyl rhf~min~ for fluorescence microscopy, an
wo 95li4483 2 1 8 5 1 1 6 r~
- 13 -
enzy~ne which produces a f luorescent or colored product
for ~te~t;~n by fluorescence, absorbance, viæible
color or agglutination, which produces an electron
dense product for demonstration by electron
microscopy; or an electron dense molecule such as
f erritin, peroxidase or gold beads f or direct or
indirect electron microscopic v;s~Al;7~t;-~n. ~abels
having therapeutic properties include drugs for the
treatment of cancer, such as methotrexate and the
l ike .
The subject invention also provides for a variety
of methods for treating and/or detecting cancer cells.
These methods involve the administration to of
humanized FB5 specific antibodies, either labelled or
l Ah,ol 1 ed, to a patient . One method of detecting
cancer cells in a human involves the step of
administering a labeled humanized FB5 specific
antibody (labelled with a detectable label) to a human
and subsequently detecting bound labeled antibody by
the presence of the label.
The recl ;nAnt antibodies of this invention may
also be used for the selection and/or isolation of
human monoclonal antibodies, and the design and
synthesis of peptide or non-peptide compounds
(mimetics) which would be useful for the same
diagnostic and therapeutic applications as the
antibodies (e.g. Saragovi et al., (1991) Science
253 :792-795) .
When the humanized FB5 specif ic antibodies of the
invention are used n vitro, the antibodies are
typically administered in a composition comprising a
pharmaceutical carrier. A pharmaceutical carrier can
be any compatible, non-toxic substance suitable for
delivery of the monoclonal antibodies to the patient,
Sterile water, alcohol, fats, waxes, and inert solids
may be included in the carrier. Pharmaceutically
.
W09~/24483 2 1~5 1 l 6 r_"u~s~o30g5
- 14 -
accepted adjuvants (buffering agents, dispersing
agent) may also be incorporated into the
pharmaceutical composition.
The humanized antibodies compositions of the
invention may be administered to a patient in a
variety of ways. Preferably, the pharmaceutical
compositions may be administered parenterally, i.e.,
subcutaneously, intramuscularly or intravenously.
Thus, this invention provides compositions for
parenteral administration which comprise a solution of
the human monoclonal antibody or a cocktail thereof
dissolved in an acceptable carrier, preferably an
aqueous carrier.- A variety of aqueous carriers can be
used, e.g., water, buffered water, 0.4gi saline, 0.39~
glycine and the like. These solutions are sterile and
generally free of particulate matter. These
compositions may be sterilized by conventional, well
known sterilization techniques. The compositions may
contain pharmaceutically acceptable auxiliary
substances as required to approximate physiological
conditions such as pH adjusting and buffering agents,
toxicity adjusting agents and the like, for example
sodium acetate, sodium chloride, potassium chloride,
calcium chloride, sodium lactate, etc. The
concentration of antibody in these ~ormulations can
vary widely, e.g., from less than about 0.5~c, usually
at or at least about 196 to as much ae 15 or 209~ by
weight and will be selected primarily based on fluid
volumeæ , viscosities , etc ., in accordance with the
particular mode of administration selected.
Actual methods for preparing parenterally
administrable compositions and adjustments necessary
for administration to subjects will be known or
apparent to those skilled in the art and are described
in more detail in, for example, F~emington's
Pharmaceutical Science, 15th ~d., Mack Publishing
~ wog~i4483 2~ 1 6 I~ 95
Company, Easton, Pa (1980), which is incorporated
herein by reference.
The subj ect invention provide numerous humanized
antibodies specific for the FB5 antigen based on the
discovery that the CDR regions of the murine
monoclonal antibody could be spliced into a human
acceptor fL .~VLh so as to produce a h1~r~n;7~rl
recombinant antibody speciic or the FB5 antigen.
Preferred humanized FB5 specific antibodies contain
additional changes~ in the 1d.~ /Lh region (or in
other regions ) to increase binding f or FB5 antigen .
Particularly preferred embodiments of the invention
are the exemplified humanized antibody molecules that
have superior binding properties for FB5.
The following examples are offered by way of
illustration of the invention, and should not be
interpreted as a limitation of the invention.
E~A~pT.~.q
In the ollowing examples all nl~c.~Ary restriction
and modif ication enzymes, plasmids and other reagents
and materials were obtained from commercial sources
unless otherwise indicated.
Unless otherwise indicated, all general recombinant
DNA methodology was performed as described in
"Molecular Cloning, A Laboratory Manual" (1989) Eds J.
Sambrook et al., published by Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, New York.
In the following examples these abbreviations may be
employed:
dCTP deoxycytidine triphosphate
_ _ _ _ _ _
WO 95t24483 2 1 8 5 1 1 6 P~ L 'iq'~O95
-- 16 --
dATP deoxyadenosine triphosphate
dGTP deoxyguanosine triphosphate
dTTP deoxythymidine triphosphate
DTT dithiothreitol
C cytosine~
A adenine
G guanine
T thymine
PBS phosphate buffered saline
PBSB phosphate buffered saline
cnnt~;n;n~ 0.5~6 (~/v) bovine serum
albumin
PBST phosphate buffered saline
,-nnt~;n;ng 0.05~ (v/v) Tween -20
3XAMP~E 1 PR~L)~ LLUN OF ~ T5~n A~ uLI~sS SPECIFIC
FOR l~T~ FB5 }~NTIGEN
The source of the donor CDRs used to prepare these
recombinant ~nt;ho~1;es wa~ a~murine monoclonal
antibody, mAbFs5, which is specific for the FB5
antigen of certain human cancers (Rettig, W.J. ~L-
(1992) Proc. Natl Acad. Sci.:89, 10832-10836) . The
FB5 monoclonal antibody was produced by immunisation
of (BALB/c x ~) F~ mice with human f ibroblasts and
wo ss/i44s3 ~ r~
-- 17 --
subsequent production and screening of hybridoma
cells. Cytoplasmic RNA was prepared from the m~b FB5
hybridoma cell line by the method of Favoloro, J. et
al., (1980), Methods in Enzymology, 65, 718-749) .
cDNA was synthesised using Ig variable region primers
as follows: for the Ig heavy chain variable region
(VH), the primer CG2aFOR (5 '
GGAAGCTTAGACCGATGGGG~I~iLL~~ , 3' ) (SEQ ID NO:l); for
the light chain variable region (VK), the primer
CK2 FOR
(5' GGAAGCTTGAAGATG~ T~ TTGGTGCAGC 3') (SEQ ID
NO:2) . cDNA synthesis reactions consisted of 4~1g RNA,
25pmol CG2aFOR or CK2FOR, 250~M each o~ dATP, dCTP,
dGTP and dTTP, 100mM TrisXCl pH8.3, 140mM KCl, 10mM
DTT, 10mM MgC12 and 31. 5 units of RNase inhibitor
(Pharmacia, Milton Keynes, U.K. ) in a total volume of
50,u1. Samples were heated to 70C for 10 minutes
(min) then slowly cooled to 42C over a period of 30
min. 100 units of Moloney Murine Leukaemia virus (M-
MLV) reverse transcriptase (Life Technologies Ltd,
Paisley, U.K. ) was added and incubation at 42C
,-nn~;nllPrl for 1 hour.
VH and VK cDNAs were then amplified using the
polymerase chain reaction (PCR) as described by Saiki,
R.K. et al., (1988), Science, 239, 487-491. The
primers used were:
CG2aFOR ( 5 ' GGAAGCTTAGACCGATGGGG~ 3 ' )
( SEQ ID NO: I )
CK2FOR (5 ' GGAAGCTTGAAGATGGATACAGTTGGTGCAGC 3 ' )
( SEQ ID NO: 2 )
VHlBACK (5 ' AGGTSMARCTGCAGSAGTCWGG 3 ' )
(SEQ ID NO:3)
WO9c/24483 2~ 851 1 ~ P~ ,,s.~r~o4c
- 18 -
SK2BACK ~ 5 ' ACTAGTCGACATGGRCTTHMAGRTGSAG 3 ' )
(SEQ ID NO:4)
where M = C or A, H = not G, R = A or G, S = C or G,
and W = A or T. Such primeræ and their use in the PCR
amplification of mou~e Ig DNA are described by
Orlandi, R. et al., (1989), Proc. Natl Acad. Sci. USA,
86, 3833-3837. For PCR amplification of VH, 5~11
RNA/cDNA hybrid was mixed with 25pmol CG2aFOR and
VHIBACK primer~. For PCR amplification of VK, 5~1
RNA/cDNA hybrid was mixed with 25pmol CK2FOR and
SK2BACK primers. To these mixtures was added 200~M
each of dATP, dCTP, dGTP and dTTP, 67mM TrisHC1 pH8.8,
17mM (NHj),SO~, 10mM MgC1~, 0.02~(w/v) gelatin and 2.5
unit6 of AmpliTa~ DNA polymerase (Perkin Elmer Ltd,
Beaconsfield, U.K. ) in a total volume of 50~L1. These
were then subjected to 25 thermal cycles of PCR at
94C, 30s; 50C, 405; 72C, 30s; ending with 5 min at
72C. For cloning and sequencing, amplified DNA was
purified by electrophoresis in a low melting point
agarose gel and by Elutip-d column chromatography
(Schleicher and Schuell, Dussel, Germany). Amplified
VH DNA was cut with HindIII ~and PstI and cloned into
M13mpl8 or M13mpl9 cut with HindIII and PstI (Life
Technologies Ltd, Paisley, U.K.) . Amplified VK DNA
was cut with ~dIII and ~I and cloned into ~dIII
and ~I cut M13mpl8 or M13mpl9 (Life Technologies
Ltd, Paisley, U.K, ) .
The resulting clones were sequenced by the dideoxy
method (Sanger, F. et al., (1977), Proc. Natl Acad.
Sci. USA, 74, 5463-5467) using Sequenase (United
States Biochemical, Cleveland, Ohio, USA) . The DNA
and protein sequences of the FB5 VH and VK domains are
shown in Figures 1 and 2. The location of the CDRs
was determined with reference to Kabat, E. A. et al.
(1987) "Sequences of Protein of Immunological
W09~114483 21 8 5 1 1 6 P~llu~
Interest ", US Department of Health and E~uman Services ,
US Governme~t Prillting Office, and utilising computer
assisted alignment with other VH and VK sequence6.
The transfer of the murine CDRs to human frameworks
was achieved by oligonucleotide site-directed
mutagenesis, based on the method of Nakamye, K. and
Eckstein, F. (19~6) Nucleic Acids Res. 1g, 9679-9698.
The human f, JLh regions chosen to receive the
transplanted CDRs were NEWM and REI for the heavy and
light chains respectively. The structures of these
proteins have been solved crystallographically. The
templates for mutagenesis were human framework region
genes ct~nt~;n;ng irrelevant CDRs and consisted of
synthetic D~As cloned into M13 phage (Riechmann, ~. et
al. ~l988) Nature, 332, 323-327) . The
oligonucleotides used ~were:
VHCDR1 5 '
CGTCCAGGTGGCTGTCTCACCCAGTGT~Tl~i~rATAr-TCAGTGAA
GG TGTAGCCAGACGCGGTGCAGGTCAGGCTC 3 '
( SEQ ID NO: 5 )
VHCDR2 5 ' TTGTCACTCTGCCCTTGAA~L~ l~lAGGTAGTATCAT
CATrAT~Arr~TTA~T~T~TCCAATCCACTCAAG 3 '
( SEQ ID NO: 6 )
VHCDR3 5'ccTr~r~r~Lr~ArGGTr~Arr~r~A~ lC~ GCCCCAGTAGTCC
ATAGAGTAGTcA~AGTAACcATr~TArr~ATTccc~ LL~:LL~ic
~rAATAATAr.
(SEQ ID NO:7
WO 95/24483 21 8 5 1 1 6 I~l/~J,.,~309~ ~
- 20 -
VKCDR1 5 ' GGAGCCTTACCTGGGGTCTGCTGGTACCAGGCTACAGCAGTA
CCCACATTCTGGCTGGCTCTACAGGTG 3 '
(SEQ ID NO:8)
VKCDR2 5 '
CTGCTTGGr~r~rrZ~r.TGTACCGATTCGATGCCGAGTAGATCAG
CAGC 3 '
(SEQ ID NO:9)
VKCDR3 5 ' CTACTCACGTTTGATTTGCA~ ilc~ ~GCCGAACGT
GTACATGGGATAGTTGGTATATTGCTGGCAGTAGTAGGTGG 3 '
(SEQ ID NO:10)
A number of additional, murine residues were
introduced into the variable region fLC~ hS by
using a separate oligonucleotide or by extension of
the CDR primers. Specifically:
NEWM V(24) changed to A~ (NEWM VHCDR1
oligonucleotide)
NEWM S(27) changed to Y: (NEWM VHCDR1
oligonucleotide)
NEWM S (30) changed to T (NEWM VHCDR1
oligonucleotide)
NEWM K(75), QFS(77-79), A(85) to S,TAY,E
(olignucleotide : 5 '
CG~ C~ i(iCTGTCACGCTGCTGAGTCTCAGGTAGG~
GAG~ bL~iLi:lACC 3') (SEQ ID NO:11)
These residues that have been changed are believed to
be important for retaining original antigen
specificity. Although the invention is not dependent
upon any particular explanation for the results
obtained by making the additional residue changes,
some possible explanations for their significance are
as f ol lows:
~ W095/24483 2 1 85 1 1 6 ~"~
-- 21 -
The change of residues NEWM V (24 ) to the smaller A
facilitates the accommodation of the heterlogous CDR1
loop. The NEWM S ~27) to Y change was made because
S ~27) is an unusual re6idue in subgroup II human heavy
chains (Riechmann et al. (1988) Nature 332, 323-327) .
Amino acids VH(27-30), are residues of the 'vernier
zones' as defined by Foote and Winter (Foote, J. and
Winter G. (1992) J. Mol. Biol. 224, 487-499. These
zones are important for adjusting CDR structures to
promote antigen binding. This explanation Arrmln~
for the changes NEWM S (27) to Y and NEWM S (30) to T.
For site directed mutagenesis the VH and VK
oligonucleotides encoding the murine CDRs and NEWM
K, QFS, A change were phosphorylated with T4 Rinase
(Life Technologie3 Ltd, Paisley, U. K. ) . A 25 fold
molar excess of each ~of the three VH (plus NEWM
R,QFS,A primer) or VK primers were added to 0.5~g of
d~ ,~L iate VH or VK single stranded template DNA in
M13 (NEWM VH: M13VHPCR1; REI: M13VKPCR2) in 40mM
Tris HCl pH7.5, 20mM MgC1" 50mM NaC1 and annealed by
heating to 90C for a few minutes and slowly cooling
to 37C. The annealed DNA was f~t~n~l~A with 2.5 units
of T7 DNA polymerase (cloned, United States
Biochemical, Cleveland, Ohio, USA) in a reaction
mixture rrn~A;nlng 0.5 units of T4 DNA ligase (Life
Technologies Ltd, Paisley, IJ.K.), 0.25mM of each of
dATP, dGTP, dTTP, and dCTP (Pharmacia, Milton Keynes,
U.K.), 40mM Tris HCl pH7.5, 20mM MgC1" 50mM NaCl,
6.5mM DTT and lmM ATP~ in a total volume of 30~L1. The
mixture was incubated at room temperature for lh~ A
1~1 aliquot of this extension/ligation mixture was
then used in an asymmetric PCR f or the specif ic
amplification of the newly synthesized strand. The
reaction crntAl n~d 1L1 extension/ligation mixture,
_ _ _ ~ _ _ . . . . . .. .. _ .. , .. _ _
WOgs/24483 21 ~51 ~ 6 r~ lQ~o~)s
- 22 --
2S0~M of each of dATP, dGTP, dTTP and dCTP, 67mM Tris
HCl pH8.8, 17mM (NH~) ,SO~, 10mM MgCl~, 0.02~ Iw/v)
gelatin, 2 . 5 Units of AmpliTaq DNA polymerase and
25pmol of appropriate oligonucleotide primer (5'
AACAGCTATGACCATG 3' (SEQ ID NO:12) for NEWM VH; 5'
CTCTCTCAGGGCCAGGCGGTGA 3' (SEQ ID NO:13) for REI VK)
in a total volume of 50111. The reaction mixtures were
subjected to 30 thermal cycles of PCR at 94C, 30 s;
55C, 30 s; 72C, 1 min ending with 72C, 5 min. The
newly synthesized strand was then amplified by adding
20 pmol of appropriate oligonucleotide primer (5 '
GTA~AACGACGGCC~GT 3' ~SEQ ID NO:14) for NEWM VH and 5'
GCGGGCCTCTTCGCTATTACGC 3' (SEQ ID NO:15) for REI VK)
and adjusting the reaction mixture to include a
further 5 nmols of each of dATP, dGTP, dTTP and dCTP
and 2 . ~ Units of AmpliTaq. The raactions were
subjected to a further 20 PCR cycles as above. The
amplified VH and VK DNAs were-purified from 1.59~ w/v
low melting point agarose gels by elutip-d column
chromatography . Purif ied DNA was digested with
ldIII and Bam~II plus RsaI (for VHs) or BstXI (for
VKs) (all reaction enzymes from l-ife Technologies ~td,
Paisley, U.K. ) . There is an RsaI site in the parental
VHPCRl and a ~XI site in the parental VKPCR2 but
these sites are deleted during mutagenesis. These
digestions therefore select for newly synthesized DNA.
The HindIII/BamHI digested VH and VK DNAs were ligated
into ~dIII/BamHI cut M13mpl8 or M13mpl9 (both from
Pharmacia, Milton Keynes, U.K. ) and transformed into
competant E. coli TGl (Amersham International plc,
Amersham, U. K . ) . Single stranded DNA was prepared
f rom individual ' plaques ' and sequenced by the dideoxy
method using Sequenase (United States Biochemical,
Cleveland, Ohio, USA) according to Manufacturer' s
instructions. Triple CDR mutants were identified in
~ W0 9~/24483 2 1 ~ r~ Q~nss
this way and selected~ for construction of VX and VK
expression vectors.
The expression vectors for the humanized VE. and VK
genes, pSVql~t and pSV~ivq are shown in Figures 3 and 4.
The hl~r ni7ed VH genes, together with the
immunoglobulin heavy chain promoter, appropriate
splice sites and signal peptide sequences were excised
from the M13 clones with HindIII and ~Lm~I and
ligated into the heavy chain expression vector,
pSVqPt. This vector rnn~inc the murine heavy chain
nrl obulin c~nh:~nr~r~ the ~t gene under control of
the SV~0 promoter/~-nhz~nr~r or selection in mammalian
cells, the human IgG1 ~constant region domain and
sequences for replication and selection in E. CQli.
The humanized VK gene was cloned into the light chain
e~pression vector pSVhw in the same way. All
f eatures of pSV~[ are the same as in pSVr~t except
that the q~st gene is replaced by the gene for
hygromycin resistance ~(hw~ and a human kappa constant
region is included instead of the IgGl constant
region .
For transfection into r-rr~ n cells lO~Lg of the
heavy chain expression vector DNA and 20~g of the
light chain vector DNA were linearized by digestion
with PvuI (Life Technologies Ltd, Paisley, U.K. ),
coprecipitated with ethanol and redissolved in 20/11 of
water. The recipient cell line was NSO, a non-
immunoglobulin producing mouse myeloma, obtained f rom
the European collection of Animal Cell Cultures,
Porton, U.K., ECAC No. 85110505 cells were grow~l in
Dulbecco's Modified Eagle's Medium supplemented with
10~ foetal calf serum and antibiotics (DMEM~ (Life
Technologies Ltd, Paisley, U.K. ) . Approximately 10'
NSO cells were ha:rvested by centrifugation and
Wogsl24483 21 851 1 6 r~ 4~ ~
-- 24 --
resuspended in 0 . 5ml DMEM, the digested DNA was added
and the cells transferred to a cuvette and placed on
ice for 5 min. A single pulse of 170 volts, 96011 -
farads was administered (Genepulser, BioRad, R;~l rl,
California, U.S.A. ) . After a further 3Q min on ice
the cells were replaced in a flask in 20ml DMEM and
allowed to recover for 24 hours. After this time the
cells were distributed into a 24 well plate in
selective medium (DMEM with 0 . 811g/ml mycophenolic acid
and 250~1g/ml xanthine). After 3 to 4 days the medium
was changed for fresh selective medium. Colonies of
transfected cells were visible after 10 to 14 days.
The production of human antibody in the wells
r-~nt~; n; n~ transfected clones was measured by ELISA.
Capture antibody, goat anti-human IgG, gamma chain
specific (Sera-Lab Ltd, Crawley Down, U.K.) was
diluted to 5~g/ml in 50mM carbonate buffer pH9.6, and
used to coat polystyrene ELISA plates (Dynatech
Immulon 1), 200/11 per well, overnight at 4C. After
washing 3 times with PBST, 50-100~11 of the culture
medium to be screened was added to the wells and
incubated at 37C for 60 min. The wells were washed
again with PBST and the reporter antibody, peroxidase-
conjugated goat anti-human IgG, gamma chain specific
(Sera-Lab Ltd, Crawley Down, U.K. ) or peroxidase- ~
conjugated goat anti-human kappa chain (Sera-Lab Ltd,
Crawley Down, U.K) was added at lOOng per well and the
plate incubated for a further 60 min. The plate was
washed as bef ore then the colour was developed .
Substrate buffer was prepared by mixing lOOmM citric
acid and lOOmM disodium l-yd~ ~c~ phosphate to pH5 . O .
25mg of o-phenyl~n~ m;n~ was dissolved in 50ml and
5~1 of 30~6 hydrogen peroxide added just before use.
200 111 was dispensed per well and incubated at room
temperature in the dark. The reaction was stopped by
wo gs/24483 2 1 8 5 1 1 S P~ '0~095
-- 25 --
addition of 50~11 per :well of 12.59~ sulphuric acid and
the absorbances were read at 492nm.
Positive cell clones were P~n~ for antibody
purification. For the final expansion to production
volume the cells were diluted in DME:M cont~;n;n~ 1096
IgG-free fetal calf serum. For small scale
purification 500ml of~ conditioned medium from static
f lask or spinner cultures was harvested by
centrifugation and 0 . l volumes of ~. OM TrisHCl pH8 . 0
and 0.5 to l.0ml of Protein A-agarose (Boehringer
Mannheim, Lewes, U.K. ) were added. ~his was stirred
overnight at room temperature then collected on a
dispo6able column. This was washed with l0 column
volumes of 0 . lM TrisHCl pH8 . 0, l0 column volumes of
0 . 0lM TrisHCl pH8 . 0 and eluted with 0 . lM glycine
buffer, pH3 . 0 . 1. Oml fraction~ were collected into
tubes c~nt;~1n;n~ 100111 of 1.0M TrisHC1, pH8Ø
Fractions ~-~nt~;n;n~ antibody were pooled and dialysed
against PBS. The concentrations of the antibody
preparations were cl~-t~rm;n~d using a Micro BCA Protein
Assay Reagent Kit (Pierce, Rockford, USA) . Samples
were checked by running on l096 SDS-polyacrylamide
gel5 .
Additional changes to the variable region were
introduced in order to improve the af f inity of the
reshaped antibody, FB5HuVH/HuVK for FB5. The
chimeric FB5 antibody, in which the murine constant
region domains of the heavy and light chains had been
replaced by the human constant regions used in the
humanized antibody, was constructed as described by
Orlandi ~L-, (1989) Two hybrid chimeric/humanized
antibodies were constructed consisting of the chimeric
heavy chain with the humanized light chain and the
humanized heavy chain with the chimeric light chain.
.
Wo ss/Z4483 ~ 1 8 5 1 1 6 ~ Q~04s
- 26 -
Both of these antibodies showed binding to the LA1-5s
target cells with similar efficacies to the chimaeric
and murine antibodies (within 3 fold). Despite these
efficacies, further attention was directed towards the
heavy and light chains in order to improve the
affinity of the humanized antibody.
Three further versions of the FB5HuVH and the HuVK,
were designed. The amino acid sequences of these VHs
and VKs are shown in table 1_
Table l shows the variable region sequences of
FB5HuVH, FB5HuVHK, FB5HuVHQA, LT, VK,
FB5HuVHK,Q,A,LT,VK, FB5HuVK and FB5HuVKF. Murine
framework residues are shown in lower ca~e. Residue
REI L(104) and T(107) are unusual residues for human
subgroup I kappa chains; these residues have been
replaced by the more common, V and K residues
(underlined in table 1) .
The additional changes to the HuVH and HuVK
constructs are shown below (numbering according to
Kabat ~L-, ~
FB5HuVHK (38), FB5HuVHQA,LT,VK (66-67, 69-70, 72-73),
FB5HuVHK,QA,LT,VK (38, 66-67, 69-70, 72-73), and
FB5HuVKF (71) . Two variations of the HuVKs shown in
table 5 were constructed by inclusion of a Y residue
at position 3 6 .
These new versions were constructed by mutagenesis of
the original reshaped heavy chain M13 single stranded
DNA clone. The method of Higuchi, R. et al. (1988)
Nucleic Acids Res. 16, 7351- 7367, which utilizes
overlapping PCR amplification with mutagenic primers,
was employed. The modified variable regions were
cloned into the expression vector pSVq~t as before and
cotransfected with the MuVK plasmid into NS0 cells.
Antibody producing cell clones were selected, f~Yp~n~
.. .. . _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
~ W095/24483 2 1 8 5 1 1 6 P~luo,.5~09ci
and purified for testing. Subsequent to this, fully
humanized version antibodies consisting of the
modif ied HuVHs and HuVKs were prepared in the same
way .
Table 1
FB5HuVH: QVQLQESGPGLVRPSQTLSLTCTaSGyTFtDYVI
HwvRQppGRGLEwI~ylN~y~ yN~?K~K(iKv
TMLVDTSsNtayLRLSSVTAeDTAVYyCARRGNS
YDGYFDYSMDYWGQGSLVTVSS
(SEQ ID N0:16)
FB5HuVHK: QVQLQESGPGLVRPSQTLSLTCTaSGyTFtDYVI
HWVkQPPGRGLEWI(iYlN~y~ l yN(~2KFKGRv
~MLVDTSsNtayLRLsSVTAeDTAVYYCARRGNS
YD(iY~lJY~ -L)Yw~s~GsLvTvss
(SEQ ID No:i7)
FB5HuVHQA, LT, VK: QVQLQESGpGLVRPSQTLSLTCTaSGyTFtDYVI
HwvRQppGRGLEwI(iylN~yl)LJDl 1 YN5~)K~'K~qa
TltVvkSsNtayLRLSSVTAeDTAVYYCARRGNS
YDGYFDYSMDYWGQGSLVTVSS
(SEQ ID N0:18)
FB5HuVHK, QA, LT,VK: QVQLQESGPGLVRPSQTLSLTCTaSGyTFtDYVI
HwvkQppGRGLEwI~iylN~yL~L~Lrl l yN~KFKGqa
TltVvkSsNtayLRLSSVTAeDTAVYYCARRGNS
YDGYFDYSMDYWGQGSLVTVSS
(SEQ ID N0:19)
FB5HuVK: DIQMTQSPSSLSA~v~L)~\,lllo~ASQNVGTAVA
WLQQTPGKAPKLLIYSA~;N~Y l~iv~SRFSGSGSG
TDYTFTISSLQPEDIATYYCQi,2Ylr~lYlllY'l~ G
TKyQ IK
(SEQ ID N0:20)
W095/24483 2l 851 1 6 r~ c
- 2a -
FB5HuVKF: DIQMTQSPSSlJSA~v~Jl~v~ ASQNVGTAVA
WLQQTPGKAPKI,LIYSA~N~ l~iV~SRFSGSGSG
TDETFTISSI.QPEDIAl Y Y~,'UUY'l'N Y~YTFGQG
TKVQI_
~SEQ ID N0:21)
W09sl24483 2 1 8 5 i 1 6 r~l,u. ~Q~Qg~
- 29 --
EXAMPLE 2 ~ C BINDIN~; OF r~T~MANT7:~r) FB5 A~ UL~
TO rAl?~'TN~lMA t~F~T.r..c:,
Recombinant antibodies ~chimaeric and h~ n; 7ed) were
tested in mixed hemad60rption (MHA) rosettillg assays
(Rettig et al. (1987) ~. Immunol. 138, 4484-4489;
Rettig ~. (1985) Cancer Res. 45, 815-821) for
their ability to bind~to LAl-5s target cells (human
neuroblastoma cells). The cells were grown in 60 well
Terasaki plates to form cf~nfl~.ont monolayers. ~ells
were washed twice with PBSB and 1O~L1 of antibody added
to the cells (antibodies diluted in DMEM without fetal
calf serum, serially diluted two fold. Incubation
with test antibody was cr~nt;nllP~l at room temperature
for 1 hour after which cells were washed three times
with PBSB and incubated with human red blood cells
(type O +) conjugated to protein A (Pierce, Illinois,
USA) diluted in PBSB. Incubation with the indicator
cells was ~-r7nt;nl1~d at room temperature for 30 min
af ter which unbound PA - RBCs were removed by washing
twice with PBSB. The percentage rosetting for each
dilution of antibody waæ detexmined and the
concentration of antibody required for 50~ rosetting
calculated. This data for the recombinant antibodies
is presented below.
AntibQdv Co~centxatiorl of antibod~r for
509~ rQsett;nq (nq/ml)
Murine FB5 - -- 3
Chimaeric FB5 - 3
FB5HuVH/HuVK 8 0
FB5HuVHK/HuVK 1 6
FB5HuVHK, QA, LT r VK/HuVK 16
Wo gs/244~3 2 1 8 5 1 1 6 PCTIUS95/03095
-- 30 -
Appropriate negative controls were included in
experiment s
These and other recombinant antibodies have been
tested in ELISAs using the I~1-5s target cells. The
E~ISA method used is as follows:-
LAl-5s cells are diluted to 1.5 x 105 - 2.5 x 105
cells/ml in DMEM, 1096 FCS and 200/11 (ie 3-5 x 10~
cells ) added to each well . Cells are grown until
nearly confluent (about 2 days). Plates are washed 2
x with PBS and 100~11 antibody (diluted in DMEM) added.
Incubation is carried out at 4C for 1 hour. The
wells are washed 3 x with PBS and 100~1 of appropriate
reporter antibody added, ie goat anti-human IgGl, HRPO
conjugate (Sera-lab, 0.4 mg/ml, diluted in 1: 500 in
DMEM); incubation is carried out at 4C for 1 hour.
Wells are washed 3 x with PBS and bound reporter
antibody detectea using H2O2 and o-
phenyl~n~ ; Am; T~ ; hydrochloride and the OD 492nm
measured .
The E~ISA data presented in the attached graphs,
together with the rosetting data, indicate that
humanized antibodies of the Examples bind the FB5
antigen. In particular the FB5HuVHK/HuVK antibody
exhibits binding affinities close to the murine and
chimaeric FB5 antibodies. Such recombinant antibodies
(o~ which these are o~ 'R) therefore provide for
novel, recombinant antibody molecules for the
diagnosis and therapy of human cancers characterized
by the expression of the FB5 antigen in the tumor
stroma .
~ wos~/24483 2 1 ~ 5 i 1 6 l~
Biolocrical DePosi~
On March ll, 1994 Applicants have deposited with
the American Type Culture Collection, Rockville , Md .,
USA (ATCC) an NSO cell lines producing humanized
antibody ~B5 HuVHK/HuVK, under ATCC accession no. CRL
11575. This deposit was made under the provisions of
the Budapest Treaty on the International Recognition
of the Deposit of Microorganisms for the purposes of
patent procedure and the Regulations thereunder
(Budapest Treaty). This assures m~l;n~n;~n~.~o of a
viable culture for 30~ years from date of deposit. The
organisms will be made available by ATCC under the
terms of the Budapest Treaty, and subject to an
agreement between Applicants and ATCC which assures
unrestricted availability upon issuance of the
pertinent U. S . patent Availability of the deposited
strains is not to be construed as a license to
practice the invention in contravention of the rights
granted under the authority of any ~,v~ L in
accordance with its patent laws.
Incor~orRtion bY reference
All patents, patents applications, and
publications cited are incorporated herein by
ref erence .
~cuivalents ~ ;
The f oregoing written specif ication is considered
to be suf f icient to enable one skilled in the art to
practice the invention. Indeed, various modifications
of the above-described makes for carrying out the
invention which are obvious to those skilled in the
field of molecular biology or related fields are
intended to be within the scope of the following
claims .
WO 95/24483 2 1 8 5 1 1 6 . ~I/u~ 3 f 3ù3~
- 31 1 -
Internadonal Application No: PCT/
MICROORGANISMS
Opllon~l Shoot in connr,clion with tho microoro~nism rofcrred to on p~go 3t, line~ t-3C o~ tht~ description
A IDENTIFICATION OF DEPOSIT
Funher dopo~it~ ~ro id-nti~ied on n ~ddltion~l hoet
Nrtne of d~posittry institution
Attter~cttrt Ty~pe Cudtt~Te Collscttor
Address of depositary institution ~including postal codo and country~
12301 P-rkd~wnDriv~
Rockville, MD 20852
US
Dateofdeposit ~ ^h t1 19Yg AooessionNumber CRL11575
8 ADDITIONAL INDICATIONS ~ (l ne bb~ i~w~ PDli bl.~ Tbb ir~crmulon ucrDu=rt .~ n~ .c~
C DESlGNATEDSTATESFORWHlCHlNDlCATlONSAREMADE ~ .n e
D SEPARATE FURNISHING OF INDICATIONS t~. b~ i~ w~ cppli Ibl~)
Tho Indlc~tion~ Iir,~e~ below wlll b- ubmi~-d ~o ~h- In~rne~lcn-l Bur.eu l~cr ~SD-CilV ~ht o-ner~ tul~ o~ ~h- Ino o- icnr o
~cc --lo~ Numc-r or D~W~
E ~ This sheet w~s n ccived with the hntenttdontd tpplic~tion when filed (D be chcclced by the ncceivb~ Office)
(Authorited Offced
[1 The dttc of n ceipt (fnom the applicrmt) by the hn~emaùonal 3unct~u
w~ts
(Authori7ed Of ficer)
Form t~_I/ttU8134 (~anur~ry 1981~