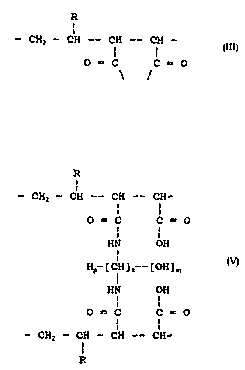Note: Descriptions are shown in the official language in which they were submitted.
1~ W095/25132 185331 Pcr~US94/02742
WATER INSOLUBLE CROSS-LINKED POLYCARBONIC ACID ~' ' J;~ S
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
Removal of proteins from aqueous media by p,l:c;~,iLd~iun.
DISCUSSION OF THE PRIOR ART
Proteins have traditionally been removed from aqueous media through use
of reagents or heat. Certain solvents (chloroform, urea~ denature protein and
cause its precipitation. Similarly, raising the electrolyte level of a medium to a
high degree by addition of salts also precipitate the protein. Further, heat not10 only denatures but generally cQa~ tes protein, thus separating it from aqueous
media.
There are three major drawbacks to these methods of protein removal.
First, the material removed from the medium is not purely protein. Instead, when15 the medium contains a complex mixture of chemicals as when the medium is a
cell Iysate, use of these methods may cause removal of other materials in addition
to protein. Second, the protein removed by these methods is generally
irreversibly denatured or denaturable only by time consuming procedures such as
dialysis. Third, either isolation or purification of the protein ~,ddi~iunally involved
20 use of somewhat toxic solvents (phenol and/or chloroform).
A polymeric material described in U.S. Patent 4,421,653 attempted to
overcome these problems. That material, a polyethylene maleic anhydride
derivative, cross-linked with diamine, however requires up to 9 volumes in
25 solution, in order to d~plu~i.ld~ a single volume of blood serum. This great
excess of the "deu,u~ i,l,q agent" reveals its low efficiency and renders it
unsuitably expensive for commercial processes. Furthermore, this matrix will
pl~:C;,Ui~d~ proteins in the alkaline range (i.e., pH 10), thus rendering desorption
WO 9S/2~132 2 ~ 8 5 3 3 ~f PcrNss4/02742 1~1
of the desired protein from the matrix very difficult under mild alkaline conditions.
SUMMARY OF THE INVENTION
There is provided a group of water insoluble cross-linked polyhydroxy
5 polycarboxylic acid compositions obtained by cross-linkin~ a polymer of the
formula:
R
(- CH2 - CH -- CH -- CH -
O = C C = ) q ( I )
0
wherein R is hydro~en or lower alkylene or lower alkoxy of 1-4 carbon atoms, or
phenyl,
q is an integer of 7 to 10,000,
20 with an alpha, omega diaminohydroxy alkane of the formula:
H2N~I(H)p(CH)~(OH)m] NH2 (Il)
wherein z is an integer of 1 - 4,
p is O or an integer up to z - 1,
m is 1 or an integer up to z,
25 and hydrolyzing the unreacted anhydride groups, wherein the ratio of the initial
amount of diaminohydroxy alkane to the initial amount of poly (alkylene malelc
anhydride) is between about 1 and about 200 to 1 mol/mol.
These cross-linked polyhydroxy polycarboxylic acid compositions have at30 least two strands each having a strand skeleton of the formula
R
- CHl - CH --- CH -- CH -- (III)
1 l
o = C C = o
wherein one carbonyl group of at least one inter-strand maleoyl moiety thereof in
40 each strand is covalently linked to a
2 1 8533 ~
- WO 95125132 P~, I I ~J,.. 1 ' . '12
-HN.[(H)p(CH),.(OH)ml.NH- moiety (IV~
to provide the presence therein of at least one inter-strand cross linkin~ moiety
of the formula:
5 R
- CH2 -- CH ---- CH -- CH-
0 = C C = O (V)
HN OH
Hp~[CH]~~~[OH~n~
HN OH
O = C C = O
- CHI - CH - CH -- CH-
R
wherein R is hydrogen or lower alkylene or lower alkoxy of 1-4 carbon atoms, or
25 phenyl,
z is an integer of 1 - 4,
p is O or an integer up to z - 1,
m is 1 or an inte~er up to z,
wherein the ratio of cross-links to poly (alkylene carbonic acid~ strands is between
30 about 1 and about 200 to 2.
Also present in the product of the reaction of the polymer of formula I
with the hydroxydiamine of formula ll is the correspondino "poly maleic ester"
of presumed to be of the formula Vl:
2 1 8533
WO 95/25132 PCT/IJS94/02742 ~1
R R
- CHz -CH -- CH -- CH- (- CH - CH ---- CH -- CH-
, , 2
O = C C = O O = C C= O )y
HN OH O OH
10Hp--[CH]~--rOH]~y
HN OH (VI )
O = C C = O
- CH2 - CH - CH -- CH-
R
20 wherein y is an integer up to m and the other values are as above.
A method disclosed herein of making the cross-linked polyhydroxy
polycarboxylic acid composition entails cross-linking a polymer of Formula I with
an alpha omega diaminohydroxy alkane of Formula 11 and hydrolyzing the
25 unreacted anhydride groups with acid to yield a mixture of compounds of
formulae V and Vl.
This polymaleic ester (Vl) may be readily hydrolysed back to the parent
polyol (V) by mild treatment with a stron~q base, suitably dilute aqueous alkali at
30 ambient temperature for several hours, preferably at least overnight. Alkaline
hydrolysis of the mixture yields sul,sla"lidlly pure formula V.
Both of these cross-linked polyhydroxy polycarboxylic acid co~ ,iLions
provide a method of precipitatin,q a protein from an aqueous medium uo"L..;";"~q35 the same which comprises adding thereto an effective amount of such a cross-
linked polyhydroxy polycarboxylic acid cOl~lpOaiLiull to provide a protein/poly-hydroxy polycarboxylic acid composition matrix. Compounds of formula V are
however s~l"Lanlially more efficient than the mixtures containing the
corresponding esters. Suitably, the amount of polyhydroxy polycarboxylic acid
40 composition utilized, by weight, is at least equal to the amount of protein
_ _ _ . . . . . .
2 1 8533 1
WO 95/2513Z PCTNS94/02742
estimated to be contained in the aqueous medium co~ 9 same. Fu~ l"o~t:
it is desirable that the polyhydroxy polycarboxylic acid composition is utilized in
an aqueous medium. In this p,~ipi~dLion step, it has been found useful to provide
that the concenI~dIions by weight of the protein and of the polyhydroxy poly-
5 carboxylic acid composition in their respective aqueous media have a ratio ofbetween about 3:1 to about 1:3, most suitably when the conce"I,dIioi,s by
weight of the protein and of the cross-linked poiyhydroxy polycarboxylic acid
composition in their respective aqueous media are SUIJ~Ldl ' 'Iy equal.
The actual precipitation process is somewhat pH dependent, within fairly
broad ranges, however. Thus where R is hydrogen, it is desirable that the pH of
the cross-linked polyhydroxy polycarboxylic acid composition containing medium
is between about 3 to about 6.2 to provide a medium after mixture of the
components which does not exceed about pH 6.5. Similarly, where R is phenyl,
15 it is desirable that the pH of the polyhydroxy polycarboxylic aid containing
medium be between about 5.5 to about 7.5 to provide a medium after mixture of
the components which does not exceed about pH 7.5.
After the precipitation reaction has occurred it is desirable to centrifuge the
20 reaction mixture to recover the matrix therefrom as a pellet.
Separating the protein from the matrix without denaturing said protein may
be carried out by treating said matrix with a buffer at about pH 8.6 to about 9.5.
Suitably there are used about 1 to about 5 volumes of buffer, at about pH 8.6 to25 about 9.5, per volume of pellet of the matrix. While the invention is not limited
thereto, superior results have been obtained where the buffer is a Tris buffer.
The process is also especially useful for the sepaldIi~n of a nucleic acid
from fluids containing both protein and nucleic acid. In such cases the source of
30 the nucleic acids or mixtures thereof is often a cell Iysate suspended in aqueous
guanidinium thiocyanate.
WO95/25132 2 1 8 5 3 3 1 PCTNS94/02742 1~
Alternatively, one may recover the cross-linked polyhydroxy polycarboxylic
acid composition from the matrix to recover it by treating the matrix with an
aqueous solution of sodium lauryl sulfate and then, desirably, centrifu~ino the
reaction mixture and recoverin~q the polyhydroxy polycarboxylic acid from or as,5 the residual pellet.
In this process, in which the protein is denatured, there are used about 1
to about 3 volumes of sodium lauryl sulfate of a concentration of between about
0.5 and about 2 % wlw per volume of matrix residual pellet. Thereafter, it is
10 desirable to include the further step of washin~ the recovered polyhydroxy poly-
carboxylic acid residue and resuspendin,q it in a buffer where R = phenVI,
phosphate buffered saline, suitably at pH 7.1-7.5 is used. Alternatively, where
R = H, acetate buffer at pH 4-4.5 is used.
~ESCRIPTIQN OF THE PREFFRPFn EMBODIMENTS
The Cross-Linked Polvhvdroxv Dolvcarboxvlic Acid Comr~osition
The present invention e~co",passe~ a co"~po~ilion of matter formed by
cross-linkin~q a polymer of formula I
R
(- CH2 - CH --- CH --- CH -
O = C C = O )q (I)
o
30 with an alpha, ome~a diaminohydroxy alkane of formula ll:
H2N.I(H)p~CH)~(OH)~n] NH2 (Il)
The symbols of the atoms shown in the brackets of formula I represent the
repeatin~q unit of the polymer, and q l~p~e~ the number of such united in the
polymer before cross-linkin~ the polymer with diaminohydroxy alkane. The units
35 as ic~,r~sellLed by q may vary from 7 to 10,000.
2 1, 8533 l
WO 95/25132 PCT/U594/02742
R is hydrogen or lower alkylene or lower alkoxy of 1-4 carbon atoms, or
phenyl .
It is preferred that the symbol R in formula I is hydrogen. Such a polymer,
5 wherein q is from 120 to about 250 can be obtained from-Monsanto Chemical
Co., St. Louis, Missouri, U.S.A, under the name ethylene-maleic acid anhydride
copolymer (EMAI. Also preferred is a polymer of formula I wherein R is methoxy.
Such a polymer wherein q is from about 100 to about 600, can be obtained under
the name Gantrez AN from GAF Corp., Chemical Division, Wayne, New Jersey.
10 Also preferred is a polymer of formula I wherein R is methenyl, ethenyl, methoxy
or ethoxy.
In Formula ll, z is an integer of 1-4, p is 0 or an integer up to z-1, and m
is 1 or an integer up to z. It is understood each lCH) group in formula ll has either
15 one or no hydroxyl groups attached thereto. The overall cross-linking moiety has
at least one hydroxyl group and may have up to one hydroxyl group per ~CH~
group in the cross-linking chain, i.e, up t o z hydroxyl groups between the two
amide groups.
Alpha, omega diaminohydroxy alkanes such as those of formula ll are
commercially available, e.g., 1,3-diamino-2-hydroxy-propane lAldrich Chemical
Co., Milwaukee, Wl).
Any anhydride groups remaining in the water insoluble cross-linked
25 polyhydroxy polycarboxylic acid are hydrolyzed.
In the water insoluble cross-linked polyhydroxy polycarboxylic acid
co" I,uosiLiun, the ratio of the initially charged diaminohydroxy alkane to the initially
charged polylalkylene maleic anhydride) is between about 1 and about 200 to 1
30 mol/mol.
WO 95/25132 2 1 8 5 3 3 1 PCIIUS94~02742 !~
In a further embodiment, the water insoluble cross-linked polyhydroxy
polycarboxylic acid hss at least two strands, each havin~ a strand skeleton of
formula lll:
R
- CH2 - CH -- CH -- CH - (III)
10 0 = c c S o
wherein one carbonyl oroup of at least one maleoyl moiety thereof in each strandis covalently linked to a alpha, ome~a diaminohydroxy alkyl of formula IV:
15 -HN.[(H)plCH),. (OH)ml NH- moiety (IV)
This provides the presence therein of at least one cross-linkin~ moiety of
the formula V:
20 R
- CH2 -- CH ---- CH --- CH--
O = C C = O (V)
HN OH
Hp--rCH]z--[OH]~
30 HN OH
O = C C = O
- CHI - CH - CH --- CH-
The symbols R, p, z and m have the same values as in formulae I and ll
40 above respectively. The ratio of cross-links to poly(alkylene carbonic acid) strands
in formula V is between about 1 and about 200 to 2 (1:2 throu~h 200:2).
Method of MakinQ Cross-l inked PolYhvdroxv Do)Ycarboxvlic Acid Comr osition
In a further ~",bodi",e"l of the invention, the method of makin~ the water
45 insoluble cross-linked polyhydroxy polycarboxylic acid ~o,,,~,o~iliun entails cross-
2 1 8 533 ~ 2742
WO 9512~132 PCI/US94/0
linking a polymer of formula I with an alpha, omega diaminohydroxy alkane of
formula ll, and hydrolyzing the unreacted anhydride groups. A volume of poly-
- (alkylene maleic anhydride) conforming to formula I is added to a reacting vessel.
A volume of alpha, omega diaminohydroxyalkane coll~u"ll;.,g to formula ll is also
5 added to the reacting vessel. The ratio of the initially charged diaminohydroxy
alkane to the initially charged poly~alkylene maleic anhydride) is between about1 and about 200 to 1 mollmol.
This is performed typically by mixing the polymer of formula I with an
10 alpha, omega diaminohydroxy alkane in water or in an organic solvent such as
acetone for 1-5 hours followed by 0-24 hours during which the reaction mixture
is allowed to stand at room temperature. The reaction may be carried out at
atmospheric pressure at room temperature or elevated temperature. The diamino-
hydroxy alkane converts by the cross-linking reaction the anhydride groups of the
15 polymer of formula I into carboxy and amide groups. Contemporaneously, in
amounts depending on the reaction col1diLions utilized, some of the hydroxyl
groups in the linkin~q hydroxydiamido chain are esterified by further reaction with
anhydride to form the correspondins "poly maleic ester" IVI) . At some time during
or after this reaction, unreacted anhydride groups are converted into carboxy
20 groups by hydrolysis in an aqueous medium (as by addition an acid solution tolower pH). While the mixture containing the esterified moieties (Vl) is operative
for the removal of proteins, it is preferred to hydrolyse these ester moieties by
,ii~e:.lions in aqueous alkali, suitably dilute alkali for example 0.05 to 0.5 Naqueous sodium hydroxide, suitably at ambient temperature for from about 12 to
25 about 36 hours to yield the pure polyhydroxy compound (V).
After the reaction is completed, an aqueous phase may be added to the
mixture, the organic phase removed conventionally as by evaporation under
- vacuum, and the residue dried at room temperature to provide the water insoluble
30 cross-linked polvhydroxy polycarboxylic acid.
WO95/25132 2 1 8 5 3 3 1 PCT~Sg4/02742
~o
Method of Protein Removal usina the Water Insoluble Cross-l inked PolYhvdroxY
polvcarboxvlic Acid ComDosition
A further e" IbG`di. / lent of the invention is a method of precipitating a protein
from an aqueous medium conlai"i"~ the same which cor"~., ises adding thereto an
5 effective amount of the cross-linked polyhydroxy polycarboxylic acid composition
to provide a protein/polyhydroxy polvcarboxylic acid Co"lposiLion matrix.
The aqueous medium may be a diluted or undiluted biolo0ical fluid
containin0 protein desired to be removed and includes such fluids as whole blood,
10 plasma, sera, Iymph, bile, urine, liquid, spinal fluid, sputum, sweat and the like,
as well as stool excretions. It is possible also to use fluid p,~pa,dlions of human
or other animal tissue such as skeletal muscle, heart, kidney, lun0s, brain,
includin0 cell culture extracts or milk or microbiological culture fluids or plant
extracts. The preferred biological fluids are human blood and bacterial cell
1 5 Iysates.
The water insoluble cross-linked polyhydroxy polycarboxylic acid
composition may be added to the aqueous medium containin0 protein in the form
of an emulsion, a suspension, a solution or a dry powder.
The ratio of the cross-linked polyhydroxy polycarboxylic acid composition
tothebiologicalfluidcanvaryaccordin0tothedegreeofdepluLdi,li~dliondesired.
The optimum ratio is, however, preferably d~L~rl";"ed in each case havin0 regardto the concentration of proteins, the nature and the concentration of the
25 substance to be purified, the temperature, the pH value and the ion conGe"lldlion.
The temperature and the pH value are, in principle, not critical. However, the
temperature 0enerally lies between 0 and 100C, preferably between 4C., but
not above 60C as substantial irreversible protein denaturation occurs above this
temperature .
It is noted that the efficiency of protein precipitation by the cross-linked
polyhydroxy polycarboxylic acid composition appears to increase at hi0her
2 1 ~533 1
WO 95125132 PCIIUS94102742
11
temperatures. In other words, less cross linked polyhydroxy polycarboxylic acid
con,~.o ,;lion is required to remove 90% of protein from a sample solution at 60CC
then from an otherwise identical protein solution at 30C.
The pH value in the aqueous medium containing protein, after addition of
the water insoluble cross-linked polyhydroxy polycarboxylic acid does not exceedabout pH 7.5, or preferably about pH 6.5.
The conct:~L~dliuns by wei~ht of the protein and of polyhydroxy poly-
10 carboxylic acid and their respective aqueous media suitably have a ratio of
between about 3:1 to about 1:3.
The amount of cross-linked polyhydroxy polycarboxylic acid composition
utilized, by wei~ht is generally at least equal to the amount of protein estimated
15 to be contained in the aqueous medium containing same.
When the water insoluble cross-linked polyhydroxy polycarboxylic acid
composition is suspended in its own aqueous medium prior to being added to the
protein-containing aqueous medium, and R is H, the pH of the polyhydroxy poly-
20 carboxylic acid composition containing medium is between about 3 to about 5,to provided a medium after mixture of the components which does not exceed
sbout pH 6.5.
Alternatively, when R is phenyl, the pH of the cross-linked polyhydroxy
25 polycarboxylicacid composition containin~q medium is between about 5.5 to about
7.5 to provide a medium after mixture of the components which does not exceed
about pH 7.5.
- The degree of the dep,ol~;"i~lion of the aqueous medium depends on the
30 density of the reactive ~qroups in the cross-linked polyhydroxy polycarboxylic acid
composition a~ent. The density of the reactive groups is not critical for the
WO 95125132 2 1 8 5 3 3 I P~ s94,~2742 1~
12
operability of the invention provided that an adequate quantity thereof is present
in order to guarantee the bonding of a sufficient quantity.
Typically, the cross-linked polyhydroxy polycarboxylic acid composition5 agent is added to the biolo~ical fluid and after a fixed time (generally 5 to 15
minutes) of intensive contact (e.g. by stirring or inversion followed by standin~).
The resultin~q water insoluble phase comprisin~ a matrix of cross-linked poly-
hydroxy polycarboxylic acid c~" Iposilio, 1 and protein which has asso~,idL~d with
the protein is removed. This removal can be carried out by any conventional
10 method customary for phase separation (e.g. centrifu~ation, filtration or
sedimentation). The removal of the water insoluble phase provides, thereby, a
deproteinized supernatant.
Where the removal of the water insoluble phase is by centrifugation,
15 centrifugation should be performed at from about 5 to 100,000 o's for from 0.2
to 10 hours or settlin~q under unit ~qravity. Ultracentrifugation speeds may be used
advantageously because the resulting pellet is so ti~htly packed no fines are lost
when the supernatan~ is decanted.
The present method of protein removal may also be used to extract a
substance, which is precipitated by the cross-linked polyhydroxy polycarboxylic
acid composition or is precipitated therewith by a suitable treatment such as, for
example, by the use of special buffer solutions or other extraction agents such as
surfactants. Removal of this substance may be for preparative or analytic
25 purposes. If buffer solutions are used to separate the protein from the matrix, it
is accomplished by stirring, grinding and/agitatin~q said matrix with a buffer at
about pH 8.6 to about 9.5 for from 10-60 minutes. There sre used sbout 1 to
sbout 5 volumes of buffer, at sbout pH 8.6 to about 9.5, per volume of pellet ofthe matrix. The buffer may suitsbly be Tris buffer. When the matrix is trested
30 a surfsctant extraction agent, Isuitably sodium lauryl sulfste) there may be used
sbout 1 to sbout 3 volumes of sodium lauryl sulfste of a concentration at
between sbout 0.5 snd about 2% w/w per volume of matrix residual pellet.
2 1 8 5 3 3 1 ,~,oS94/02742
* WO 95125132 P
13
When one performs the above steps and recovers the water insoluble cross-
linked polyhydroxy polycarboxylic acid co",posiLiol~ from the precipitated matrix,
the polyhyd roxy polycarboxylic acid composition may be washed and resuspended
in phosphate buffered saline of pH 7.1-7.5. Thus, a further embodiment of the
5 method of p~ecipitating a protein is to perform the further step of washing the
said recovered cross-linked polyhydroxy polycarboxylic acid composition and
resuspending same in phosphate buffered saline, preferably having pH 7.1-7.5.
The method of precipitating protein is especially useful when the protein
10 is present in an aqueous medium with a nucleic acid or mixtures thereof. This is
frequently the case when the source of nucleic acid or mixtures thereof is a cell
Iysate suspended in aqueous guanidium thiocyanate.
The deproteinized su~JellldIa"L (the deproteinized fluid remaining behind
15 after deproteinization) can be further proce:,sed in any manner. For preparative
purposes (e.g. for the purification of peptides, glycoproteins, steroids, lipoids,
nucleic acids, enzymes, hormones, vitamins, viruses, polysaccharides or alkaloids)
further purification steps can, for example, be carried out. In this case, there are
suitable, in particular, chromatography (e.g. ion exchange, Sephadex, affinity or
20 adsorption chromatography), filtration, ~e.g. LlLldrillld~ion)l electrophoresis (e.g.
block, disc or carrier-free electrophoresis), isoelectric focusing and selectiveprecipitation .
Without in any way restricting the scope of present invention, Applicant
25 wishestostatehisundel~La~1dillgofthepresentinvention,namelythelllechall;~
by which the cross-linked polyhydroxy polycarboxylic acid composition removes
protein from an aqueous medium. Precipitability is a function of solubility in an
aqueous medium. Solubility in turn is a function at least in pârt of the degree of
a protein's hydrophobicity. All proteins have at least some hydrophobic portions30 of their surface exposed to the aqueous medium. Applicant believes his cross-linked polyhydroxy polycarboxylic acid composition permits the hydrophobic
portions of different protein molecules to approach one another and aggregate to
WO 9S/2S132 2 1 8 5 3 3 1 PCT/US94/027~12
14
such a degree that the proteins eventually pl~c;lJildl~. (This appears to be
co,lubo,dl~d by the increased protein removal efficiency of the cross-linked
polyhydroxy polycarboxylic acid composition from solutions at higher
temperatures. By contrast, where protein precipitation is caused by other
5 phenomena, e.g., association/dissociation, protein precipitation is seen to fall off
as temperature rises).
Before this can happen, the cross-linked polyhydroxy polycarboxylic acid
composition associates with one or more protein molecules by non-covalent
10 interaction, such as electrical charge attraction. IThe cross-linked polyhydroxy
polycarboxylic acid composition has numerous negative charges which can
interact with the partial positive charges present in several points in all protein
molecules, e.g. at arginine residues). The local ordering of water imposed by the
surface hydrophobic groups is thermodynamically unfavored. Bound water may
15 be released when these hydrophobic groups which are apolar, interact with oneanother and aggregate. Thus, when two or more proteins which have interacted
with the flexible cross-linked polyhydroxy polycarboxylic acid composition like
beads on a string, the composition-string can then enfold such apolar portions of
different protein-beads may aggregate. When the number or size of aggregated
20 protein moiecules is large enough, the protein-composition complex pl~ ,iLdL~s.
The following examples are meant to illustrate the present invention and do
not restrict the invention in any respect.
W095/2!jl32 2 1 8533 1 p,~S94/02742
EXAMPLE 1
PrerJarationoFawater-lnsolubleFl~ciuiLdLillaAqent(Mixtureofuartiallvesterified
material ~Vl) and Deesterified mat~ri~l (V)
One hundred grams (0.063 moles) of styrene maleic anhydride copolymer
5 (SAM~Resin 1 000A) obtained from Atochem Inc., Malvern, PA is dissolved in 1 Lof acetone. To this solution is added a second solution containing 17.5 ,q. (0,194
moles~ofl,3-diamino-2hvdroxypropane(AldrichChemicalCompany,Milwaukee,
Wisconsin) in 1 L of acetone at a rate of 5.0 ml/min. with constant stirring for a
period of 3.5 h.
The reaction mixture is Ihen allowed to stand for 12 hours at room
temperature. After completion of the reaction, 3 L of water is added with stirrin~
and then the polymer is allowed to settle under unit gravity. The aqueous organic
phase is removed by decantation. The cross-linked polymer is suspended in 1 L
15 of H20 and ground for 1 minute using a Gifford Wood homogenizer (medium
settinJI. The pH of the suspension is then adjusted to 1.5 by the addition of
hydrochloric acid. After 1 hour, the pH is adjusted to 9.0 with sodium hydroxideand the mixture stirred for 30 minutes. The pH is then adjusted to 7.0 and the
supernatant is discarded. The polymer is then washed with phosphate, buffered
20 ssline 0.01 M, - pH 7.2 and finally suspended in this buffer to yield at 5.0% W/V
suspension. This yields a mixture of the poly hydroxy material (V) and the
polymaleic ester material (Vl).
Polyhydroxy polycarboxylic acid compositions where R = H, lower alkylene
25 or lower alkoxy with 1 to 4 carbon atoms may be made according to the above
steps, except the styrene maleic anhydride polymer is replaced with ethylene
maleic anhydride polymer (e.g., "EMA-21" from l~1L ~ lLO Chemical Co., St.
Louis, MO); alpha-methyl-ethylene maleic anhydride polymer; or alpha-methoxy-
ethylene maleic anhydride polymer, respectively.
Polyhydroxy po)yca, L~o~ylic acid co~ ,osiLiolls having a cross linking moiety
with two carbon atoms may be formed according to the above steps, except that
W09S125132 2185331 p~US94/02742 4
16
the 1,3-diamino-2-hydroxypropaneisreplacedwithadiaminohydroxyethane,such
as 1 ,2-diamino-1 -hydroxyethane. Polyhydroxy polycarboxylic acid compositions
in which the cross linking moiety has multiple hydroxyl groups may be formed
according to the above steps by replacing the 1,3-diamino-2-hydroxy propane
5 with 1,2-diamino-1,2-dihydroxyethane.
Polyhydroxy polycarboxylic acid compositions having a cross linking moiety
with three carbon atoms having multiple hydroxyl groups may be formed
according to the above steps, except that the 1,3-diamino-2-hydroxypropane is
10 replaced with a 1,3-diamino-di- or 1,3-diamino-tri-hydroxypropane such as 1,3-
diamino-1,2-dihydroxy-propane or 1 ,3-diamino-1 ,2,3-dihydroxypropane.
Polyhydroxy polycarboxylic acid compositions having a cross linking moiety
with four carbon atoms may be formed according to the above steps, except that
15 the 1,3-diamino-2-hydroxypropane is replaced with an alpha, omega-diamino-
mono-hydroxy-n-butane,suchas 1 ,4-diamino-3-hydroxybutaneor 1,4-diamino-1-
hydroxybutane. Polyhydroxy polycarboxylic acid compositions in which the cross
linking moiety has more than one hydroxyl group may be formed by the above
steps, except the alpha, omega-diamino-mono-hydroxy-n-butane is replaced with
20 a 1,4-diamino-di-, 1,4-diamino-tri- or 1,4-diamino-tetra-hydroxybutane, such as
1,4-diamino-2,3-dihydroxybutane or 1,4-dismino-1-2-dihydroxybutane; 1,4-
diamino-1 ,2,3-trihydroxybutane; and 1 ,4-diamino-1 ,2,3,4-tetrahydroxybutane.
EXAMPLE 2
25 Peesterification of Water-lnsoluble r,e-,iuildli~a Agent (Mixture of rJartiallv
esterified material (Vl) and Dee~ iried material IV).
The polyhydroxy polycarbonyl mixed composition formed in Example 1
suspended in the buffer at a 5.0% weight/volume suspension, was centrifuged
at 3000 x 9 for 10 minutes and the suspending buffer discarded. The pellet was
30 then dispersed in deionized water to yield a 5.0% w/v suspension. An equal
volume of 0.2N aqueous sodium hydroxide solution was slowly added to the
polymer suspension with stirring. The alkaline mixture was then allowed to stand
.. .. : . : : . . _ _ .. _ . _ _ _ _ _ .. . .
wo gsnsl32 2 ~ 8 5 3 3 ~ PCI/US94102742
at room temperature for 24 hours. Free base was then removed (in the
supernate) by repeated centrifugation and washing with deionized water. The
polymer was then equilibrated with 0.01M phosphate buffer pH 7.2 to vield a
5.0% w/v polymer suspension. This yields the polyhydroxy material ~Vl free of
5 the polymaleic ester material (Vl).
In accordance with the above procedure, the polymaleic ester material (Vl)
mixed with the polyhydroxy material ~V~ can be purified. This may be done with
materials from the following sources.
Polyhydroxypolycarboxylicacidco~po~ilio~lswhereR = H,loweralkvlene
or lower alkoxy with 1 to 4 carbon atoms may be made according to the above
steps, except the styrene maleic anhydride polymer is replaced with ethylene
maleic anhydride polymer le.~., EMA-21" from Monsanto Chemical Co., St.
15 Louis, M0); alpha-methyl-ethylene maleic anhydride polymer; or alpha-methoxy- ethylene maleic anhydride polymer, respectively.
Polyhydroxy polycarboxylic acid compositions having a cross linking moiety
with one carbon atom may be formed according to the above steps except 1,3-
20 diamino-2-hydroxy-propane is replaced with a diaminohydroxymethane.
Polyhydroxy polycarboxylic acid compositions having a cross linking moiety
with two carbon atoms may be formed according to the above steps, except that
the1,3-diamino-2-hydroxypropaneisreplacedwithadiaminohydroxyethane,such
25 as 1, 2-diamino-1 -hydroxyethane. Polyhydroxy polycarboxylic acid r o" ".osilions
in which the cross linking moiety has multiple hydroxyl ~roups may be formed
according to the above steps by replacing the 1,3-diamino-2-hydroxy propane
with 1,2-diamino-1,2-dihydroxyethane.
Polyhydroxy polycarboxylic acid compositions having a cross linking moiety
with three carbon atoms having multiple hydroxyl groups may be formed
according to the above steps, except that the 1 ,3-diamino-2-hydroxypropane is
2 1 8 533 1
WO 95/25132 PCT/US94/02742
18
replaced with a 1,3-diamino-di- or 1,3-diamino-tri-hydroxypropane such as 1,3-
diamino-1 ,2-dihydroxy-propane or 1 ,3-diamino-1,2,3-dihydroxyptopane.
Polyhydroxy polycarboxylic acid compositions havin~ a cross linking moiety
5 with four carbon stoms may be formed accordin~ to the above steps, except thatthe 1,3-diamino-2-hydroxypropane is replaced with an alpha, ome~a-diamino-
mono-hydroxy-n-butane, such as 1 ,4-diamino-3-hydroxybutane or 1 ,4-diamino-1-
hydroxybutane. Polyhydroxy polycarboxylic acid compositions in which the cross
linking moiety has more than one hydroxyl group may be formed by the above
10 steps, except the alpha, omega-diamino-mono-hydroxy-n-butane is replaced witha 1,4-diamino-di-, 1,4-diamino-tri- or 1,4-diamino-tetra-hydroxybutane, such as
1,4-diamino-2,3-dihydroxybutane or 1,4-diamino-1-2-dihydroxybutane; 1,4-
diamino-1,2,3-trihydroxybutane; and 1,4-diamino-1,2,3,4-tetrahydroxybutane.
EXAMPLE 3
The polyhydroxy polycarboxylic composition made accordin~ to Example
1 (V and Vl) is evaluated for its ability to pl~U;,l~i~d~t~ the diverse materials listed
in Table 1 below. All the materials listed in Table 1 (from human serum albumin
throush plasmid DNA) are obtained in powder or particulate form from the Si~ma
20 Chemical Company, St. Louis, Missouri.
Human serum albumin (HSA) is dissolved in 0.01 M sodium phosphate
buffered 0.9% saline, having a pH 7.3-7.5 at a concentration of 33 m~/ml. lThe
remainin~ compounds; human gamma ~lobulins through plasmid DNA, are
25 similarly dissolved in an identical phosphate buffered saline at the conc~"~,d~iu"s
indicated in Table 1. The isoel~, ic point (pl) as well as the percent carbohydrate
(/0 Carb.) of each protein is indicated in Table 1.
A 5% wei~ht/volume solution of the polyhydroxy polycarboxylic acid
30 co"" osi~ion made in Example 1 is also made usin~ the sodium phosphate
buffered saline solution.
2 1 8533 1
PCI/US94/~)2742
WO 9~/2'il32
19
One volume of the cross-linked polyhydroxy polycarboxylic acid
~.o~"posiLion solution l"depru~i"i~ q sgent") is combined with 1, 2 or 4 equal
volumes of the sample solution. Each co" ' :.,dLion of solutions is mixed by
inversion then allowed to stand at room temperature for 5-15 minutes. Each
5 solution is then centrifuE~ed at 2,000 x G for 10 minutes to remove the protein-
polyhydroxy polycarboxylic acid co",po~iLion matrix. The percent of protein
removal from each remaining supernatant is measured by ultraviolet absorption
(at 280 nm) or colorimetric d~ Le" ";"dLion ~usin~ the "BCA Protein Assay Rea~ent"
from the Pierce Company, Rockville, IL). The pe~e"Ld~ of protein removal
10 ("% Removal"~ is indicated in Table 1 for each sample at all 3 volume
co"lb;"dtions.
The proteins human serum albumin, human ~qamma ~qlobulins, hemoglobin
~ransferrin and Cytochrome C are all removed at levels of 90% or above by the
15 cross-linked polyhydroxy polycarboxylic acid co,,,uosiLion~ These proteins have
uniformly low levels of carbohydrate A~sociAt~ld with them. By contrast, A1 acid~qlycoprotein ho,~ad;;.h p~u~ddase and Fetuin each have substantial amounts
of carbohydrate and therefore inferior "percent removal". Finally as is seen with
both DNA sample in Table 1, non-proteinaceous materials are hardly precipitated
20 a~ ~11 by ~he c~0s6 linked polyhyd~oxy polycarboxylic acid compos ~lon.
WO 95/2~132 2 1 8 533 I PCrNsg4/02742 ~1
Table 1
Reactive Compounds
Vol. of Dep,ul~ ;"~
A~ent (Vl) to Vol. of Sample
(/0 Removal)
S a m p I e pl %Carbo 1/1 1/2 1/4
C o n c . hydrate.
lmglml)
Human 33 4.9 > 99 99 go
Serum
1 5 Albumin
Human 25 5-7 2-3 >99 95 91
Gamma
Globulins
Hemoglobin 10 6.8 >99 99
Transferrin 1 5.9 6 95 92
25 Cyto- 2 10.6 >99 99
chrome C
Unreactive Compounds
30 a 1 - A c i d 1 2.7 41 60 2
Glycoprotein
Horseradish 1 8.8 22 23 2
Pe, u~cidase
Fetuin 1 3.5 22 55
Calf Thymus A250 = 2 . < 1 < 1 < 1
DNA 0
P I a s m i d A260=0.
DNA 5
Note - These values were taken from isolated systems. For h~L~lu~el~eous
45 systems selectivity will increase due to a competitive environment.
2 1 8533 1
WO 95/Z513Z PCI'IUS94101742
21
EXAMPLE 4
The tests of Example 3 were repeated at a concentration of 2.5% wt/vol
suspension of the partially esterified polymer ~Vl) and dee~ ,iried polymer ~V)
prepared in dcco,da"ce with Example 2.
Table 2
Reactive Compounds
Vol. of Deproteinizing
Agent to Vol. of Sample
(% Removal)
Part~ Y esterified ~Vl) + (v) Deesterified ~V) only
Sample C o n c . 1/l 1/2 1/4 1/1 1/2 1/4
~mg/ml)
Human 33 ~99 85 60 >99 >99 96
Serum
Albumin
20 Human 25 ~95 88 50 >99 95 90
Gamma
Globulins
Hemo~lobin 10 >99 99 82 >99 >99 ~99
Transferrin 1 95 92 58 ~ 99 98 90
Cyto- 2 > 99 > 99 90 ~ 99 ~ 99 ~ 99
chrome C
Unreactive Compounds
a 1 - A c i d 1 60 2 0.5 65 4
Glycoprotein
35 Horseradish 1 20 2 0.5 24 2
Peroxidase
Fetuin 1 45 1 0.5 51
40 Calf Thymus AZGO=2.0 <1 <1 <1 <1 <1 <1
DNA
Piasmid DNA A2r~0=0.5 <1 <1 <1 <1 <1 <1
EXAMPLE 5
WO 95/25132 2 1 8 5 3 3 1 PCTNS94102742
22
Two solutions each of human serum albumin and human gamma globulins
are made according to Example 3 (i.e., at 33 and 25 mg/ml. respectively, in
0.01M sodium ,ullGsphdl~ buffered 0.9% saline at pH 7.3-7.5). There is also
prepared a 5% weight/volume solution of cross-linked polyhydroxy polycarboxylic
5 acid composition made according to Example 1.
The first human serum albumin and human gamma globulin solutions are
maintained at room temperature (approximately 2oocl and the second of each of
these solutions are heated to and kept at 60'C. The cross-linked polyhydroxy
10 polycarboxylic acid composition solution is then added to each of the four protein
solutions in a 1/4 volume of "deproteinizing agent" to volume of protein sample.
The results of the protein removal efficiency measured as in Example 2, appear below in Table 3.
1 5 :[~
~Lm~ % Removal
Human Serum Albumin - 20C 90
Human Serum Albumin - 60C ~reater than 99
Human Gamma globulin- 20GC 91
20 Human Gamma globulin - 60C greater than 99
EXamDle 6
The experiment of the foregoing Example 5 was repeated using the
deesterified a~ent (V) produced in Example 2 at a polymer concerll,dlion of 2.5%25 wt/vol. The polymer to sample ratio was 1/5. The results are S~ dli~d in
Table 4 beiow.
~ 218533~
PCI/US94/02742
-- WO 95/25132
23
~k~
Removal
Human Serum Albumin - 20C 80
Human Serum Albumin - 30C 90
5 Human Serum Albumin - 60C greater than 99
Human Gamma Globulin- 20C 82
Human Gamma globulin - 30C 94
Human Gamma globulin - 60'C grealer than 99
.