Note: Descriptions are shown in the official language in which they were submitted.
WO 95127767 P~
218fi848 - 1 -
LI~BRICATED N~T~T~ 'lY~ D J~El~OD
, 5 This invention relates to lubricated metal
workpieces, particularly of steel and aluminium, used
in the production of press-formed components, and in
particular to a method of using such workpieces to make
structures of shaped components.
There is current interest in techniques for
producing adhesively bonded structures of shaped
aluminium cmnrnnl~ntq for use in the automotive
industry. Such a technique is described for example in
EPA 127343. The technique of converting a coil of
aluminium metal sheet into a structure of shaped
components f or use in the automotive industry may
typically involve the following steps:-
- The metal surface is pre-treated to
provide a strongly bonded layer thereon which acts as a
base for subsequently applied adhesive.
- A lubricant is applied to the treated
metal coil. The coil may then be stored or
transported, with the lubricant serving to protect the
treated metal surface, and is cut up into pieces ready
2 5 f or press - f orming .
- The pieces of metal sheet are press-formed
into ~ ^nt,~ of desired shape. This and subsequent
operations are all performed on an automobile
product ion l ine .
3 - Adhesive is applied to selected areas of
the shaped components, without first removing the
lubricant .
- The I ,~nn~ntc are assembled into the
shape of the desired structure, and may be spot welded
or otherwise fixed to hold the structure together until
the adhesive is cured.
W0 9~127767 21 8 ~ ~ ~ 8 I ~
_ ~ _
- The a&esive is cured at elevated ;~
temperature .
- The metal surfaces of the structure are -
subjected to an aqueous ;~lk~linP cleaner which removes
5the lubricant. ~-
- The structure is painted.
Alternatively, the presg-formed c~r~mPntq
may be secured together to form the structure by
mechanical means, e.g. by rivets or spot-welds, either
10in ~ n to or instead of adhesive bonding.
A lubricant f or use in such a technique needs
to fulfil several requirements:
a) The lubricant must, obviously, have suitable
lubricating properties for the press-forming operation.
5b) The lubricant should be solid at likely metal
storage temperatures in order to prevent stacked sheets
from sticking together. Furthermore, a film of
lubricant that is liquid or sticky is prone to smear
and to pick up dust and dirt.
20c) Since it is not practicable in a production
line to remove lubricant prior to application of
adhesive, the lubricant needs to be compatible with an
adhesive if one i8 to be used.
d) After the a&esive has been applied and
25cured, the lubricant must be readily removable by an
aqueous ~lk~l;nP cleaner of the type convPn~ n~lly
used to prepare metal surfaces for r~;ntin~
The lubricants of EPA 227360 are designed to
be useful, not only for the technique described above,
3but also for other forming and shaping operations
perf ormed on a variety of metals .
In one aspect, EPA 227360 provides a
lubricating composition for press forming consisting of
a lubricant dissolved or dispersed in a volatile liquid
35medium, wherein the lubricant comprises at least one
ester of a polyhydric alcohol having two or three
~ Wo 95/27767 ~ 1 8 6 8 ~ 8 p I .,~
hydroxyl groups of which one or two are esterified with
a long chain carboxylic acid and has a melting point
above ambient temperature but low enough to permit
removal f rom a metal surf ace by an as~ueous A 1 kA~ l; n,A
5 cleaner.
EPA 227360 mentions that mixtures of esters
may be used and may be advantageous; and that the
lubricant may contain a minor proportion up to 50~ of
one or more other lubricating compounds such as long-
10 chain carboxylic acids. The lubricants exemplified
are: diethylene glycol monostearate in solution in
xylene; and diethylene glycol distearate in solution
in xylene.
Although the lubricants described in BPA
227360 are generally successful at meeting re~uirements
c) and d), they are sometimes less successful at
meeting re~{uirements a) and b) . It is sur~risingly
found that lubricants of this kind are ineffective, so
far as aluminium forming operations are cnArArn~d, at
20 temperatures above their liyuidus. For good aluminium
lubricating properties, in ester lubricants of this
kind, it appears necessary that some, , An~ be
present in the solid state, so that the lubricant is
solid or at least mushy or viscous, at the forming
25 ten~perature which may be as high as 35C or 40C or
even higher.
It might appear a simple matter to solve this
isolated problem by using a different ester with a
higher melting point. A difficulty with this strategy
3 is that higher melting esters tend to be relatively
hard at low and ambient temperatures, to the extent
that they readily spall and flake off metal surface to
which they are applied. Metal forming at 15 or 20C
cannot satisfactorily be performed under conditions
35 where the lubricant flakes off the metal workpiece.
For use in various parts of the world, there is a need
WO95/27767
21868~8
-- 4
for a single lubricant system which meets both these
high- and low-temperature criteria. It is an object of
this invention to meet that need. '` '-
In one aspect the invention provides
lubricated metal, wherein a surface of the metal '-
carries a film of a lubricant which
a) consists essentially of at least one full
ester of a di- or poly-hydroxy compound with a C8 - C18
saturated carboxylic acid optionally in admixture with
a minor amount of at least one long-chain carboxylic
acid and/or a minor amount of at least one partial
ester of a di- or poly-hydroxy compound with a C8 - C1
saturated carboxylic acid, and
b) has a hardness in the range 0 . 2 - lO N/mm at
all temperatures in the range 15 - 30C, preferably in
the range 0.1 - lO N/mm at all temperatures in the
range 15 - 3 5 C .
In another aspect, the present invention
provides a method of making a structure of shaped
aluminium ~ ~ AntS starting from lubricated ~ m;n;llm
metal sheet as defined, comprising the steps:
- forming pieces of the sheet into
components,
- bringing the ,~ _ -ntR together in the
shape of the desired structure,
- and securing the ~l AntA together by
-'hAn; ~-Al and/or adhesive means .
Hardness of the lubricant is measured by a
techni~ue whereby a block of the uncoated lubricant is
3 equilibrated at a given temperature and is penetrated
by a steel needle. ~he test procedure used eRsAnt;~lly
involves driving a pointed 12 mm diameter needle into
the lubricant at a 5peed of 20 mm/minute, achieved with
the use of materials testing machine such as an
35 Instron, and recording the load as a function of the
needle penetration into the lubricant. Separate tests
_ _ . , _ . , = ~ . _ . , . .. . . _ _ _ _ _ . _
~ Wo 9~l27767 2 1 8 6 8 ~ o
-- 5 --
are conducted at various temperatures to derive the
full curves. The hardness value ~uoted i9 then found
as the slope of the graph of penetration load versus
penetration distance.
Although forming, e.g. press-forming, of
metal sheet is generally performed at temperatures in
the range 15 - 30C or 35C, in some tropical l~^,r~ti. nA
tt",~eld~ures in the press may rise to 40C or even
45C. It is therefore preferred that lubricant films
of this invention have specif ied hardness values at
temperatures within the range 15 - 40C, in the case of
particularly preferred lubricants, within the range
15 - 45C.
If the lubricant film is too hard, it is
likely to be brittle and have poor frictional
characteristics during forming e.g. press-forming.
If the lubricant film is too soft, then again the
lubricating characteristics are inferior.
Preferably, the lubricant film has a hardness in the
range 0.1 - 5 N/mm at all temperatures within the
range specified at which forming e.g. press-forming
is likely to take place in different parts of the
world. It is surprising that the hardness of the
lubricant film has useful predictive value for its
lul~ricating characteristics.
The major c, ^^t of the lubricant film is
a Eull ester of a polyhydric alcohol with a long-chain
carboxylic acid. Dihydric or trihydric alcohols having
2 - 6 carbon atoms are suitable, for example ethylene
3 glycol, propylene glycol, diethylene glycol and
glycerol. The long chain carboxylic acid is preferably
a saturated straight-chain monocarboxylic acid having
from 12 to 18 carbon atoms in the chain, such as
lauric, palmitic or stearic acid. Mixtures of esters
may be used and may be advantageous. Particularly
preferred esters are ethylene glycol di-laurate (~GD~)
Wo gSl27767 21 8~8 4 8 - 6 ~ c ~- ~
and propylene glycol di-6tearate.
The full ester or esters may be used
optionally in ~' Yt~lre wit~ a minor ,amount of at least
one long-chain carboxylic acid, preferably a saturated
S straight-chain monocarboxylic acid having from 14 - 20 -
carbon atoms in the chain. This optional minor
^nt iS present in an amount of less than 509~ most
usually 5 - 2096, by weight on the weight of the
mixture. A particularly preferred fatty acid is
10 stearic acid.
The full ester or esters may be used
preferably in A~' Ytllre with a minor amount of at least
one partial ester of a polyhydric alcohol with a long-
chain carboxylic acid. Dihydric or trihydric alcohols
15 are suitable, f or example ethylene glycol, propylene
glycol, diethylene glycol and glycerol. The long chaincarboxylic acid is preferably a saturated straight-
chain monocarboxylic acid having from 12-18 carbon
atoms in the chain, such as lauric, palmitic or stearic
20 acid. Mixtures of partial esters may be used and may
be advantageous. Particularly preferred partial esters
are ethylene glycol -1 ~lrate IEGMT~ and propylene
glycol monostearate.
The full esters should be present in the
25 lubricant mixture at a rrnrPntration of 40 - lO0 wt~.
Preferably the lubricant consists of 50 - 85 wt~ of a
full ester such as EGDL, 10 - 30 wt~ of a partial ester
such as EGML and up to 20~ e.g. 5 - 20 wt~ of a fatty
acid such as stearic acid. Lubricant composition can
3 drift during storage, resulting in a somewhat differellt
composition on the lubricated metal surf ace, and these
f igures ref er to the lubricant when f reshly made .
Proportions herein are determined by analysis e.g. by
standard technir~ues involving gas chromatography, mass
35 spectrometry and IR spectrometry; they do not
n~.r~-cs~rily correspond closely to manufacturers ' stated
_ _ _ _ ,, , , , _ .. . . . . _ . ... .
Wo 95l2776,7 2 1 8 6 8 4 8 P~l,. . 7
- 7 -
proportions in commercially available materials.
To the best of our belief, there is no single
ester of commercial purity which meets the above- stated
hardness requirement. Suitable l1~hrirAntq may be
5 achieved in one or both of two ways . The f irst is by
blending two or more, ~n~ ~ together . The second
is by using purer material.
These esters are not easy to purify. But to
the best of our knowledge and belief, EGDL has a
10 melting point of about 50C; and EGML has a melting
point in the range 23 - 25C; and mixtures of the two
have melting ranges intermediate these two f igures .
Full esters and partial esters such as EGDL
and EGML can have impurities arising from two main
15 Sources:-
a) The nominal fatty acid, e.g. lauric acid, isin fact a mixture of saturated long-chain
monocarboxylic acids, typically cnntAin;n~ more than
30~1; of acids other than the nominated one. An effect
20 of these cnnr~m; nAting acid3 is to depress the melting
point of the ester.
b~ The ester is derived from a fatty acid
mi~:ture which cnn~;nA ethylenically unsaturated
acids. Such impurities make the lubricant less
25 adhesive-compatible and less easy to remove from the
metal surface, and are therefore preferably absent or
present in amounts below 5~ by weight.
As noted above, the lubricating
characteristics of the lubricant film on lubricated
3 metal according to this invention fall off at both
excessively high and excessively low temperature. We
have developed a test, which is described below, for
measuring lubricating characteristics in terms of a
frictional coefficient (mu). This frictional
35 coefficient is preferably below about 0.1 at all
temperature9 within the range of interest, that is to
Wo 95/2~76~ 2 1 8 6 8 ~ 8 P~ IAID~ S~
-- 8
say 15C up to 30C or 35C or 40C or 45~C. As
noted above, the hardness of the lubric~t film at
any temperature is predictive of its frictional
coPf f; ripnt,
Depending on its intended use, the lubricant
may need to be compatible with subsequently applied
adhesive. In general, the esters described herein are
compatible as a result of being either absorbed or
displaced by subsequently applied adhesive without
grossly impairing the adhesive bond strength
obtainable. By contrast, resinous lubricants and metal
soap lubricants are generally not adhesive compatible
in this sense.
The lubricant has a melting point above
ambient temperature, preferably of at least 30C, more
preferably at least 40C. This ensure6 that the
lubricant is present as a solid f ilm on the metal
substrate, which avoids problems with 6mearing and
blocking during coiling, decoiling, slitting and
cutting. The use of such a lubricant avoids
~l~ntAminAtion of the metal surface with a possible
adhesive-incompatible oil or contæm;nAnt and prevents
local build up of lubricant to an undesirably thick
Iayer .
2~ The lubricant melts at a temperature low
enough to permit its removal from a metal surface by an
aqueous AlkAl ;nP cleaner, such as is used in automotive
production lines to prepare metal parts for F~;ntin~
The highest practicable temperature for aqueous
30 alkaline cleaners in such circumstances is about 70C.
Lubricants melting below 70C and preferably below 65C
can thus be removed by aqueous A 1 kA l; n~ cleaners .
Lubricants melting above 70C may or may not be
removable depending on whether they have chemical
35 groups, e.g. hydroxyl groups, which can react with the
alkali to assist removal from the metal surface. Thus
_ _ _ _ _ . . .. . . . . . . .. . .. . _ _ ~ _
Wo gs/27767 21 8 6~ ~ 8
g
fo~- example, a commercially available wax having a
melting point of 85C and an acid number of 135 to
155 by DIN 53402, was found not to be removable by
aqueous ;~kAl inf~ cleaners. A lubricant is deemed
removable by aqueous ~ l k:~ 1; n~ cleaners if it can be
removed by treatment for 2 minutes at 70C with a 15~
by weight aqueous solution of Ridolene 160 (a silicate-
based proprietary cleaner marketed by I . C . I . plc . )
A further aspect of this invention involves
applying the lubricant to the metal in the absence of
any volatile solvent or diluent. This avoids the need
to evaporate volatile liquid from the lubricant film,
and avoids t~e need to include any surface active agent
in the lubricant. It is found that the molten
lubricants have satisfactory viscosity for spraying or
for application by roller coat. To ensure rapid
soli-~; ficatio~ of the lubricant film, the metal may be
pre-cooled. To ensure good adhesion of a uniform
f ilm, the metal may be pre -heated .
Alternatively the lubricant may be dissolved
in a volatile solvent for application to the metal.
Indeed, very thin films can only be applied from
solution. The use of solution permits control of
lubricant film thickness to within less than 0.5 g/m2.
The lubricant may be applied to steel or
other metals, but is likely to be pr;n~ y used on
aluminium, which term is used to cover the pure metal
and alloys in which Al is the maj or, ~~ ^n~ . A metal
surface may carry a strongly-bonded inorganic and/or
3 organic pretreatment or primer layer, on the top of
which the lubricant is present. Such non-metallic
layers are well known, and may be provided for example
as chemical conversion coatings or deposited coatings
of the no-rinse type, based on chromium, titanium or
35 zirconium; or may be an anodic oxide layer (on Al or
Ti) or a siloxane layer. The metal may be in sheet
WO 95/27767 2 ~ ~ 6 8 ~ 8 P~ ~ 5~
- 10 -
form. The rate of application of lubricant will depend
on the intended use, but may typically be ~in the range
of 0.1 - 10 g/m2, e.g. 0.25 - 8 g/m', particularly 1 -
4 g/m', for aluminium coil to be formed into
5 adhesively bonded structures.
Reference is directed to the AC ~ ying
drawings in which:
- Figure 1 is a schematic view of a strip-
draw apparatus used for testing lubricated metal;
- Figure 2 is a perspective view of a
modif ied strip-draw apparatus;
- Figure 3 is a graph of hardness against
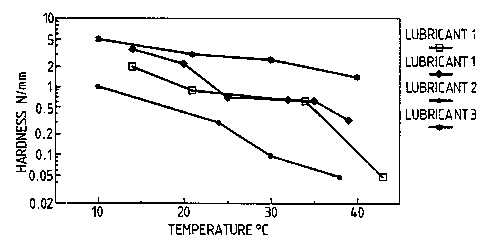
temperature for several lubricants.
- Figures 4 and 6 are Bar Charts showing
frictional coefficients of two lubricants at different
temperatures and different rates of application.
Figure 4 is for lubricant 2. Figure 6 is for
lubricant 1.
- Figure 5 is a bar chart showing lubricant
residues after different bakes followed by cleaning.
~ purpose built strip-draw rig was designed
and constructed with reference to ASTM 4173-82 for
testing sheet metal forming lubricants. The apparatus
is shown in Figure5 1 and 2. The die set shown in
Figure 1 was designed to simulate material flowing
between pressurised binder surfaces ~ ntA;nin~ a draw
bead a ~ L ~ t . The die set of Figure 2 was designed
to simulate flow between parallel binder surfaces 50 as
to allow conventional frictional values to be obtained.
3 Referring to Figures 1 and 2, one die 10 of
each tool set is mounted on a load cell 12. The other
die 14 of the tool set is mounted on a hydraulic
cylinder 16. Elat strips 18, hydraulically pressurised
between the two dies, can then be pulled through a
particular tool set while the clamp load is measured.
The draw load is also measured using a second load cell
., .. , _ . _ . ... _ _ _ .. , , . , .. ,, _ _ _ _ _ . .
~ W09S12776'7 21 8 ~8~ 8
-- 11
Z0 mounted between a testing machine gripping jaw 22
and a cross head 24. Thus, when used in conjunction
with the f lat parallel platen set of Figure 2, a
conventional~ frictional value is obtained.
The strip draw rig is designed to be mounted
on either a press s;r-llAtQr or a standard te~sile
testing f rame, depending on the variables under
investigation .
Lubricated strips of material, 50 mm wide,
were placed between the two f aces of the f lat tool set
of Figure 2 and hydraulically pressurised to a
particular load. The strips were then drawn through
the die set of Figure 1 for a distance of approximately
250 mm, the draw and clamp forces being recorded as a
function of time/displacement of the drawn strip.
Results presented in the form of a graph (draw force/2)
versus clamp load have a slope eciual to the
conv,onti~AnAl friction coefficient.
E:XANPI,E 1
A lubricant f ormulation according to the
invention had the composition, in wt5c:-
61~f ethylene glycol dilaurate (EGDL).
19% ethylene glycol monolaurate (EGML)
11% stearic acid
9~ other ester species
The identity of the ~ Ant ,A, was tl_t~Arml nAd
by standard gas chromatography/mass spectrometry
3 techni~ues. This formulation is hereinafter called
lubricant
Another lubricant f ormulation according to
the invention had the composition, in wt~:-
70% ethylene glycol dilaurate
21. 596 ethylene glycol monolaurate
8 . 5% other ester species
W09sl27767 21 8fi8 ~ 8 .~
- 12 -
The identity of these components also was
determined by ætandard gas chromatography/ma;ss
spectrometry techniques. The fn l~t;nn is
hereinaf ter called lubricant 3 .
A formulation called lubricant 2 was made up
for comparison. Lubricant 2 nnnt~;nq commercially
available EGML 90~ and 5tearic acid 10~. This
lubricant falls outside the scope of the present
invention, and is included for comparison purposes
1 0 onlY.
This commercially supplied ethylene glycol
monolaurate has been analysed by us and f ound to
contain seven different acids in proportions as
follows: caprylic (C8) 3.9~; capric (C10) 5.89f;
lauric (C12) 339~; myristic (C14) 16.89~;
palmitic (C16~ 11.996; oleic and stearic (C18) 28~.
Lubricants 1, 2 and 3 were applied by
spraying on to aluminium alloy sheets which had been
preheated to 50C. By this means, uniform films could
be applied at controlled thickness. The hardness of
the lubricants was measured (by the method described
above) and the results are recorded in Figure 3.
Lubricants 1 and 2 were further tested in the
strip draw rig illustrated in Figures 1 and 2. In each
case, tests were performed at different t~ ~ dLL-r ~s in
the range 0 - 50C; and at five different rates of
lubricant application ranging from 1 - 6 g/m~. The
results of these tests are shown in Figure 4 (for
lubricant 2) and Figure 6 (for lubricant 1 batch 2, see
3 below)-
~MPLE 2
Lubricants 1 and 3 from Example 1 were
evaluated. Lubricant 2 from Example 1 was used for
comparative purposes.
wo95l27767 ~ 8
-- 13 --
E~erimental P~ ~cel.. e
The experimental ~ork described below was
carried out on 1. 6 mm gauge 5754 material.
5 2.1 Application of Lubricant to AlllTn;n;llm Sheets
The procedure for lubricant application
consisted of pre-heating a reservoir of the new
lubricant to 70C, and applying this onto sheets using
air-assisted airiess spray nozzles. Lubricant was
10 applied to sheets which were held at both room
temperature (20C), and preheated to 60C. These
sheets were then placed in stacks. In the case of the
pre-heated material, the sheets were placed in a stack
when the lubricant had solidified.
2 . 2 Adhe6ive Co:npati~ility
The standard test method for adhesive
COlllpatibility i8 to assemble standard lap shear j oints
wi th a 10 mm overlap, using lubricated 1. 6 mm
20 pretreated coupons and a standard adhesive. A string
of six such joints are then exposed to c ' ;n~d
stress/humidity testing under a constant load. The
time to failure of the first three joints in a set of
six joints is then noted. Individual lap shear joints
25 are also exposed to salt spray for given periods of
time, and then tested for static strength retention.
Tests were carried out on j oints manuf actured
with the luoricant 1 on their surfaces prior to
bonding. Two lubricant weight levels were evaluated,
3 namely 2 . 0 g/m' and 5 . 5 g/m' .
2.3 Lubriclnt Softening as a Functio~ of Temperature
The Wax Penetration Test, was used to
determine the softening response as a function of
35 temperature. The test procedure used essentially
in~olves driving a pointed 12 mm diameter needle into
WO 95l27767 ~18 6 8 ~ 8 r~ .s/c. ~
- 14 -
the lubricant at a speed of 20 mm/minute, àchieved with
the use of materials testinq machine sùch as an
Instron, and recording the load as a function of the
needle penetration into the lubrican~. Separate tests
are conducted at various temperatures to derive the
full curves. The hardness value quoted is then found
as the slope of the graph of penetration load versus
penetration distance.
2.4 Strip Draw Evaluation
Lubricated sheets were produced with 3 g/m~
of different lubricants via the pre-heated blank route,
as indicated in section 2.1. These 3heets were
guillotined into strips 50 mm wide and then drawn
through the strip draw rig, using the described
procedure, to allow friction values to be determined at
temperatures of 10, 20, 30, 40 and 50C.
2 . 5 Pre6~ For:ning Evaluation o~ the Lubric~mt 1
Press forming testa were carried out on a
press simulator to evaluate the lubricant. Two
distinct trials were used, namely:
(a) Square pan depth to failure.
(b) Pressed dome height to failure.
The above trials were carried out under
standard conditions on a 275 mm square tooling without
the draw bead sections.
Sheets of AA5754-0 were pressed with 3 g/m'
of both lubricants 1 and 2 to allow the comparative
3 performance to be assessed.
2 . 6 Simulation of Po~6ible Thermal Cycle6 of Pre-
Lubricated Stacks
In order to simulate possible thermal cycles
35 which may be experienced by pre-lubricated material,
lubricated stacks were produced by applying the
_ _ _ _ _ . . .. _ ... . , , .. . _
~ W0951277fi7 ?~ ~l 8~8 A ~
- 15 -
lubricant to pre-heated blanks, as described in
section 2.1.
Four stacks were produced l-nnt~;n;n~ some
thirty sheets, each 500 x 500 mm, with a nominal 3 g/m2
5 lubricant weight. These stacks were heated to four
different temperatures in an oven at, 30, 35, 40 and
45C respectively. After removal from the oven, each
stack was le~t to cool with a centrally applied weight
of 18.1 kg. After destacking, coupons were removed
10 from a number of adjacent sheets to quantify any
lubricant transfer observed.
2 . 7 Cleaning, Oven E:vaporation and ~
Two distinct tests were carried out in this
15 section, namely different oven bakes followed by a
cleaning stage, including no bake, and a typical bonded
stL-ucture route with the adhesive cure cycle included.
For the first series of tests, pretreated
strips of aluminium were lubricated with lubricant 2
20 (3 4 g/m2 ) and lubricant 1 (3 . 8 g/m2 ), and given the
following treatment:
a ) 2 0 minutes at 17 0 C
b) 20 minutes at 180C
c) 20 minutes at 190C
d) 20 minutes at 200C
e ) No oven bake .
All strips were then cleaned in stirred
20 g/litre solutions of Chemkleen C~t165 at a
temperature of 60C for 3 minutes. After drying,
3 organic ~ ~rt~m;n~tion on the strips was measured, as
carbon, by analysis at 600OC.
For the second series of tests, clean sheets
of aluminium were coated with lubricant 1 and lubricant
2 at a coating weight of approximately 4.5 g/m'. The
35 sheets were then subjected to a cumulative oven-bake
and alkali-clean cycle. This consisted of:
WO 95l27767 218 6 8 4 8 r~ r ~
- 16 -
a) 10 mins at 145C
b) 20 mins at 190C ; - '
c) 20 mins at 190C
d) 30 secs alkali clean, (stirred 2.59~ w/w
Ridolene 336 at 60C).
Final coat weights were measured after the
cleaning stage using gravimetric det~inat;on~
3. RESOT~TS
3.1 Applic~tlon of the Lubricant to the Sheet~
Satisfactory results were obtained by
spraying the lubricant 1 onto sheets held at both room
temperature, approximately 20C, and sheets pre-heated
to 60C. The lubricant solidified upon contact with
the sheets held at ambient temperature. However, the
latter condition allowed the lubricant to remain liquid
on the sheets f or a short time period .
The lubricant itself passed through the spray
nozzles without any additional problems to those
encountered with the lubricant 2.
3.2 Adhe~ive Co~pati~ility
The results of stress-humidity and salt spray
testing on joints produced with lubricant 1 on their
surfaces are presented in Table 1. This Table shows a
good strength retention after 20 weeks salt spray, and
a testing duration in exces~ of 100 days during
stress/humidity with a 5 MPa applied stress.
3.3 Lubrica~t Softening a~ a Punctiorl of TenLperature
The results of the Wax Penetration Testing
carried out on lubricants 1, 2 and 3 are presented in
Figure 3.
Two batches of lubricant 1 were made on
separate occasions. Batch 1 is shown by filled
_ _ _ _ _ _ _ _ _ , . , . . . _ . .. . , .. , ..... , . . . . . _ _
o 95/27767 ~ 8
q j oined by a solid line . Batch 2 is shown by
shaded squares joined by a dotted line. Both materials
fall within the scope of the invention, as does
lubricant 3, shown by stars.
Lubricant 2 is shown for comparison. The
hardness was relatively low at all temperatures.
3.4 Strip Draw Evaluation
Table 2 shows the comparative performance of
the lubricants l, 2 and 3 over the measured temperature
range for a given lubricant weight of 3 g/m'. These
f igures show an improved perf ormance of the lubricant
batch 1 at temperatures of 30, 40 and 50C. They also
indicate a similar performance at 20C.
3 . 5 Pre~s Forming E~aluation of the Lubricant 1
The results of the press forming trial8 are
sho~Am in Table 3. This Table indicates that both
lubricants give a similar performance during 8tretch
20 forming, but an improved performance is obtained during
square pan forming wi~h the lubricant 1. The values
quoted are the average of five tests in each case. The
tests themselves were carried out in ambient conditions
of around 22-24C.
3 . 6 Simulation of Pos~ible Thennal Cycles of Pre-
Lubricated Stacks
Stacks of lubricated sheets, having lubricant
on their surf ace, which were heated to 3 0, 3 5 and
30 40C and subsequently cooled with a centrally applied
weight showed no evidence of de-stacking ~roblems or
lubricant transfer between adjacent sheets. The
corr~qp~n-l;n~ stack heated to 45C was more difficult
to separate, showing clear evidence of a "patchy~
35 appearance, and slight lubricant transfer between
adj acent sheets . With the lubricant 2, a similar
_ _ _ . . . .
W095/27767 ~1 8~848 .
- 18 -
effect was seen at a temperature of 35C.
3.7 C'le~;n~, Oven ~vaporzltion and R~
The results of the different oven bakes
5 followed by a cleaning stage, including no bake
followed by a cleaning stage, are given in Figure 5.
This figure shows that high oven bakes contribute to
the surface cl~nl ;n~s.
The results of the oven evaporation trials
10 show that lubricant l evaporates almost totally
compared to lubricant 2, 0.03 g/m2 and 0.35 g/m'
residue respectively. Final coat weights were also
measured after the final alkali clean using gravimetric
determination. The results of both lubricants fell to
5 between -O.Q1 and -0.03 g/m~ indicating that the
cleaning stage is removing all of the residues.
4 DIS~- S
20 4.1 Applicatio~ of the Lubricant to the Sheets
No difficulties were experienced with the
spray applicatio~ of lubricant l. Adherence to the
metal surface was improved by pre-heating the sheets,
although a satis~actory appearance was obtained with
25 room temperature metal.
4 . 2 A&esive Compatibility
The results of the-stress/humidity testing
show that joints manufactured with lubricant 1 at a
3 level of 2 g/m2 are still on test, with l90 days
achieved to date for all stress levels tested.
Salt spray data after 20 weeks exposure
shows P~r~Pl 1 ~n~ strength retention at both lubricant
weight levels, out -perf ormlng the lubricant 2 .
~w09sl27767 2186848 ~- r~
- 19 -
4.3 Lubricant Softeni~g as a Function of ~remperature
Results of the Wax Penetration te8t,
presented in Figure 3, show the hardness i~ Luvl '
over the temperature range ~ m; nPd . Earlier work had
5 suggested that the hardness value 6hould be m~; nti~ i nPd
between 0.1 and 1. 0 N/mm ûn the vertical logarithmic
axis. Significantly ~Y~ee~;n~ the higher hardness
values at lower temperatures will produce a wax which
is brittle and has iimited value in terms of press die
10 forming. At the higher temperatures, hardness values
less than 0 . 1 N/mm correspûnd to the melting range of
the wax. Figure 3 shows that the hardness of
lubricant 1 falls to 0.1 N/mm around 41-42C,
corresponding to its melting range. This is
approximately 10-12C higher than lubricant 2. Thus
the upper melting range has been significantly
increased without producing a brittle wax at the lower
temperature range. ~ubricant 3 has a hardness that is
strikingly ind ~ .deL,t of temperature.
4.4 Strip Draw Evaluation
In terms of frictional performance versus
temperature for a lubricant weight of 3 . o g/m2, Table
2, the lubricant 1 demonstrated a signif icant
25 il~l~JLUV~ t in the frictional coefficient at 30, 40 and
50C, whilst having a similar performance at 20C.
4 . 5 Pres~ Forming Evaluation of the Lubricant 1
The results of the press forming evaluation
3 indicate that the lubricant 1 formulation has an
equal performance during stretch forming, but a
somewhat better performance during square pan pressing.
this should give an advantage to c- ~ U~ S such as
door rings and door inner pressings, where deep corner
35 features are required having small corner sweep radii.
Thus the performance; ~uv, ^nt will be beneficial.
_, . . .. ,, _ _ _ _ _ . , ,
WO 95/27767 '~1 868q 8
- 20 -
4 . 6 Simulation of Po~sible Thermal Cycle,~ of Pre-
,ubricated Stack~ ,
Results of thermal cycleæ applied to pre- .
lubricated stacks have shown no evidence of lubricant
5transfer at 40C, and only slight evidence of lubricant -
transfer when the stack was heated to 45C and then
cooled . Thus, problems of lubricant transf er with the
lubricant l will now become apparent at temperatures
between 40 and 45C. This performance is much better
10than the lubricant 2 where no evidence of transf er was
vislble when the stack was heated to 30C, but evidence
was visible when the stack was heated to 35C. Hence a
10C temperatur- i _LiV. ~ in terms of h=inr~1 ;n~
perf ormance has been achieved .
4 . 7 Cleaning, Oven Evaporation and ~
Trials to assess the cleaning of the surf ace
after either various oven bakes or no bake, Figure 5,
have shown that ~ n solution does clean the
20surfaces reasonably effectively. High oven bake
conditions, 190C and 200C, definitely contribute to
surface ~-1eAn1 ini~Sq in the case of lubricant l, which
evaporates very cleanly from the aluminium surfaces.
Trial8 to determine the relative evaporation
25and ,~ ;n;ng residues of lubricant l versus lubricant
2 have shown that the lubricant l evaporates almost
completely prior to the cleaning 6tage. Further, lower
levels of carbon residuals are obtained.
3o
~ W0 9512M67 218 6 8 ~ 8
- 21 -
Tal~le 1: Stress/~Iunidity and Salt 5pray Dat~ for the
Lubricant 1
.. S
Lubri~ant Stress/};umidity Salt Spray Re5ults
Weight g/m' Days to Failure ~MPa~
3 MPa 4 MPa 5 MPa O wks 8 wks 20 wks
102.0 lso~ lso+ lso+ 27.8 2s.s (92~) 25.1 (9o~)
s.s lso+ lso+ 137 28.4 2s.2 (89~) 23.6 (83s)
15 Table 2
Friction Value at temperature C
Lubricant 10 20 30 40 50
0 . 017 0 . 012 0 . 046 0 . 069
2 0 . 020 0 . 036 0 . 096 0 . 107
30 . 092 0 . 020 0 . 043 nd nd
nd - not ~et~r~n~ nf~d
Tal~le 3: ~ffect of Lu~ricant on Press Fn~-h; 1; ty
L~hricant Coat Weight Ss~uare Pan Dome ~Ieight
(g/m2 ) Depth mm mm
23.0 59.1 51.5
35 1 3.0 74.3 52.0
WO 9S127767 ~1 8 68 ~ 8
- 22 -
~Y~MPLE 3
Propylene-1, Z-glycol distearate (PG12DS) was
made as f ollows:
,.
Material~:
Stearic Acid MW 284.47 mp 69 - 70C
Propane-1,2-diol: MW 76.09 bp 188.2C
Xylene bp 137 - 140C
~quip:ne~ t:
Heated 5 l reaction vessel fitted with
th~ -ter, Dean and Stark, and reflux condenser.
Procedure:
Stearic acid (1500 g) and propane-1, 2-diol
(200 g) were placed in the reaction vessel.
Approximately 1. 5 l of xylene was added, and
the mixture stirred.
The vessel was heated to boiling and reaction
continued under reflux for apprn~ tPly 10 hours
(bp_135C) .
Azeotropic li~luid gathered in the Dean and
Stark was removed at f requent intervals . A tDtal of
25 apprnYir~tely 100 ml being collected. Two additions of
10 ml of propane-1, 2-diol were made during the course
of reaction to c~ ,-nC~te for 1088 of diol in the
azeotrope .
Once the boiling point of the mixture had
3 risen to 137C, xylene was removed by distill~t;on.
Distillation was stopped as the boiling point reached
142 C .
The reaction product was cooled, placed in an
open flat tray and r-;nt~in,od at 75C in an explosion
35 proof forced air oven for a further two hours to remove
any residual solvent (rotary évaporation would normally
. _ _ _ _ . . ... . _ , . ,, .. , _ . .
~ wo s5/27767 2 t 8 6 8 ~ 8 r~ Y
- 23 -
be a preferred method).
Blends of this product with different
proportions of commercial purity EGML ~see Example 1)
were made. The hardness of these luoricant
5 forr-~11At;nn~ at varioug temperatures were determined.
Ester A B C D E
10 PG12DS 100 90 70 40 20
EGML 0 10 30 60 80
Hardness N/mm
11C 3.8 1.5 1.5 1.1 0.95
15 20C 2.5 1.8 1.4 0.85 0.55
35C 0.51 0.75 0.47 0.33 liquid
Lubricants A, B, C and D but not E are in
20 accordance with the invention.
3o