Note: Descriptions are shown in the official language in which they were submitted.
21 88~20
NON-FOOD CROP PLANT BIOREACTOR
The present invention relates to the use of a non-food crop plant as a
bioreactor. More specifically this invention relates to the expression of transgenes
S of interest for oral a~lmini~tration using non-food crop plants. An example of a
suitable non-food crop plant that can be used as a bioreactor is nicotine-free tobacco.
BACKGROUND OF THE INVENTION
Full citations for references appear at the end of the examples section.
Numerous plants have proven themselves to be amenable to transformation
with heterologous genes and for some time tobacco has been the model system for
plant transformation. Despite the fact that crop-protection focused biotechnologies
15 have not found application in non-food crop plant production, a major role does
remain for such plants as bioreactors. An example of a non-food crop plant is
tobacco, which is capable of producing high levels of soluble protein (fraction 1
protein, FlP; Woodleif et al 1981) and pilot systems have been developed to purify
this fraction for use as a high protein dietary supplement (MollL~nali et al 1993).
Expression of m~mm~ n genes in several plants including tobacco and
Arabidopsis has been recognized as efficient, low cost, non-sterile bioreactors for the
production of proleills valuable to both medicine and industry (Ma and Hein 1995).
Recently evidence was presented that demonstrated that the four chains of the
25 secretory immlmoglobulin were properly expressed and assembled in plants and that
the antibody was fully functional (Ma et al 1995). Furthermore, bacterial (Haq et al
1995), and viral (Mason et al 1996) antigens produced in transgenic tobacco and
potato effectively illlllllll~i~P~l mice when the transgenic potato was a(lmini~tered
orally. However, prior to the atlmini~tration of plant tissue obtained from transgenic
30 tobacco, the proLeills had to be partially purified. Clearly steps involving processing
are undesirable if ease of oral ~-lmini~tration is to be m~ximi~e~l as well as
minimi~ing any associated costs for production.
2 1 88~20
From both a regulatory and public safety stand point non-food crop plants are
ideal species for the transgenic production of biologically active prolcills. Non-food
crop plants minimi7e the risk of accidental leakage of transgenic plant materialexpressing genes for biologically active proteins into the human food chain. Other
5 plant bioreactor systems based on canola (Rooijen et al. 1995), potato (Manson et al
1996), rice and cassava (Ma and Hein 1995) do not offer this advantage.
Furthermore, non-food crop plants can be selected so that production in areas where
there are no naturally occurring wild species further minimi7es the risk of geneleakage to the local flora, an example of this would be to grow tobacco in regions
10 where tobacco does not ovcl~illler, such as Canada. With any non-food crop plant,
transgenic ploLcills can be produced using any tissue or organ of the plant. However
if protein production is based on leaves, not seeds or tubers, and when coupled with
the fact that the leaves are harvested before flowering there is virtually no risk of
uncontrolled bioreactor plants occurring in future crop seasons.
Thus there is a need to provide a non-food crop plant capable of being used
to prepa~c transgenic proteins of interest suitable for oral ~t1mini~tration.
SUMl\~ARY OF THE INVENTION
The present invention relates to the production of a protein of interest using
a non-food crop plant.
According to the present invention there is provided a method for the
25 plcpa~alion of a protein of interest suitable for oral ~imini.ctration within a non-food
crop plant comprising, transforming the non-food crop plant with a suitable vector
cont~ining a gene of interest and appropliate regulatory regions to ensure expression
of the gene of interest within the non-food crop plant, such that the non-food crop
plant is characteri_ed as being non-toxic, non-addictive, palatable, and requiring little
30 or no processing prior to oral ~(lmini~tration.
21 ~220
This invention further relates to the above method wherein the non-food plant
is characteri_ed in having a low aLkaloid level. This invention also includes methods
l1tili7ing a plant characteri_ed in having a low nicotine level.
This invention also relates to the above method wherein the protein of interest
includes ph~rm~celltic2lly active proteins, such as growth regulators, insulin,
inlelrerol1 and related compounds, interleukins, growth hormone, ely~opoietin, G-
CSF, GM-CSF, hPG-CSF, M-CSF, Factor VIII, Factor IX, tPA, antibodies, antigens
and any combinations and derivatives thereof.
Furthermore, this invention includes the above method wherein a suitable
vector contains a gene of interest along with applopliate regulatory regions to ensure
expression of the gene of interest within desired plant tissues.
This invention also provides for compositions comprising a tissue from a non-
food crop cont~ining a transgenic protein of interest.
Another aspect of an embodiment of this invention is a method for the
treatment of a medical aliment by ~tlmini~tering a suitable amount of the above
composition comprising a tissue from a non-food crop plant cont~ining as a
ph~rm~re~ltically active component a transgenic protein of interest.
Although the present invention is exemplified by the preparation of transgenic
ploteills of interest using tobacco, in practice any non-food crop plant may be used.
21 ~220
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features of the invention will become more appalclll from the
following description in which lcferellce is made to the appended drawings wherein:
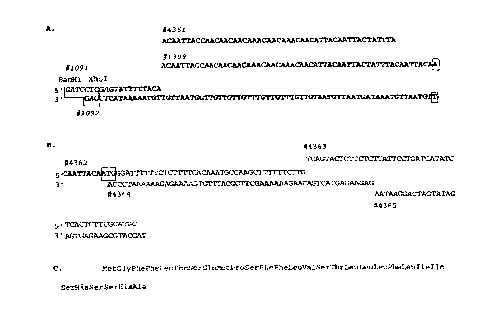
FIGURE 1 shows the oligonucleotide cassettes for integration of TMV 5'-
untr~n.cl~te~l region and PR-lb transit peptide sequences into transgene
constructs. Figure l(A) the TMV leader sequence. Figure l(B) the PR-lb
signal peptide sequence. Figure l(C) the amino acid translation of encoded
signal peptide. Identity, sequence, and position of each oligonucloeitde within
the cacsettes are indicated. TMV sequence is marked in bold, PR-lb signal
peptide sequence in plain letters and additional sequence in italics.
Nucleotides co~ g all or part of the initiation codon are present within
boxes. Oligonucleotides #1091, #1092 and #1093 represent the TMV oligo
set. Oligonucelotids #1091, #1092, and #4361 through #4365 represent the
TMV-PR oligo set.
FIGURE 2 displays the map of the T-DNA region of pCDX-TL-MAFPII
FIGURE 3 displays the map of the T-DNA region of pMON-TL-MAFPII
FIGURE 4 is a Western blot showing Type II AFP accllmlll~tion in field plants.
Western blot analysis of total soluble protein (2.5 ~g) extracts from Rl
generation Type II AFP transgenic plants in which the AFP was targeted to
accllml-l~te in the extracellular space. Samples are decign~te~l by number to
indicate the plot from which it was collected and by letter to indicate the
transformed parent plant from which the Rl plants were descended. Records
at the Delhi station inflie~te that A plants were descended from parent II4c2-
#10, B plants from II4c2-#l, C from pII3a-#7, and D plants were wild-type
controls (4D). Total soluble protein extract from a field-grown wild type
plant was included in the analysis as a negative control and mixed with mature
Type II AFP (25 ng) purified from sea raven sera to generate the positive
control (AFP).
21 ~220
DESCRIPTION OF PREFERRED EMBODIMENT
Even though non-food crop plants are ideal species for use as bioreactors from
both a regulatory and public safety point of view, there a several obstacles that must
5 be overcome prior to their use as bioreactors. A major problem with the use of non-
food crop plants, for example tobacco, as bioreactors is that these plants may contain
undesirable secondary plant products. Secondary plant products are constituents that
are generally toxic or reduce the palatability of the plant tissue or that are addictive
in nature. This is a significant concern since one of the benefits of plcpaling proleills
10 of interest within a plant is that large q~l~ntiti~s of protein may be required for
a~1mini~tration (Ma and Hein, 1995), therefore any toxic, addictive, or otherwise non-
desirable products should be avoided within the plant tissue. For example, tobacco
plants contain high levels of secondary plant products such as nicotine and related
alkaloids, making the plant tissue unsuitable for the direct oral ~(lmini~tration.
15 Earlier studies have described the ~t1mini.~tration of tobacco-derived proteins to mice,
however, the ploteills were in a partially purified form. For example the study by
Mason et al (1996) involved the direct oral ~(lmini.~tration of viral antigens expressed
in potato tuber and tobacco. The potato tuber samples were directly fed to mice, yet
the antigen, when obtained from tobacco, had to be partially purified using sucrose
20 gradients prior to ~mini~tration to mice.
A non-food crop plant host to be used as a bioreactor for the production of
ploteins suitable for direct oral a~lmini~tration of transgenic-expressed proteins of
interest should comprise the following properties: that it
25 a) is non-toxic
b) requires little or no processing prior to oral ~mini~tration
c) is characterized in having low levels of non-desirable secondary plant products
such as alkaloids and/or nicotine so that the medicinal matrix is palatable and
not addictive.
Use of the terms "low nicotine" and "low alkaloid" with Icl~erellce to plants,
such as tobacco, means a plant cont~ining less than about 0. 35 % total alkaloids. Such
21 88220
plants may be obtained through conventional breeding programs (e.g. Chaplin, 1977),
through selective down regulation of undesired genes (e.g. U.S. 5,260,205, issued
November 9, 1993 and U.S. 5,369,023, issued November 29, 1994; inventors
Nakatani and Malik), or by any other means e.g. metagenesis followed by selection
5 of desired traits. However, as is known to one of skill in the art, the llltim~te
nicotine or alkaloid level may still depend upon the envilolllllent under which the
plant is grown.
By "suitable vector" it is meant a vector comprising a gene of interest that is
10 capable of being expressed within plant tissue. Such a vector may also include
ubiquitous or tissue specific promoters and other 5' and 3' regulatory elements as
would be known to one of skill in the art. Other elements that may be included with
this vector include sequences for ~arge~ing the protein of interest to the cytosol or
secretory pathway such as the C-terminal KDEL sequence, an endoplasmic reticulum15 retention motif (Schouten et al 1966). Furthermore, such a vector may includemarker genes for the detection of expression within the transgenic plant.
By "protein of interest" it is meant any protein that is to be expressed in a
transformed plant. Such proteins may include, but are not limited to,
20 ph~rm~r,eutic~lly active proteil~, for example growth factors, growth regulators,
antibodies, antigens, their derivatives and the like.
By "oral ~(lmini~tration~ it is meant the a~h~ lion of a tissue or organ of
a non-food crop plant, for example the leaf, root, fruit etc with minim~l or no prior
25 processing. Such tissues or organs may be provided in the form of a salad or the like
along with other ph~rm~çeutir~l ingredients, or ingredients to increase palatability,
if desired. It is also contemplated that minim~l proces~ing of the tissue or organ may
take place prior to a~mini~tration of the extract. For example, ~)loteills secreted
within the extracelluar space of leaf tissues could be readily obtained using vacuum
30 or centrifugal extraction, or tissues could be extracted under pres~ule by passage
through rollers or grinding or the like to squeeze or liberate the protein free from
within the extracelluar space. Minimal processing could also involve preparation of
21 88~20
crude extracts of soluble proteins, since these plel)al~lions would have negligible
cont~min~tion from secondary plant products.
By "medical aliment" it is meant a defined medical condition that can be
5 treated a specific ph~rm~eutical suitable for the treatment of the condition.
Examples of such medical ailments and their corresponding suitable ph~rm~e~ltical
include, but are not limited to: diabetes and the ~lmini~tration of IL-4 or insulin or
a combination thereof; conditions related to blood clotting and the a~lmini.ctration of
Factor VIII, Factor IX or tPA or combinations thereof; m~flir~l conditions requiring
10 the stimul~tion of progenitor cells to monocytes/macrophages and the ~lmini~tration
of G-CSF, GM-CSF, hPG-CSF, M-CSF or combinations thereof; viral infections and
the ~tlmini~tration of inlelrerolls e.g. hllelrel~ , hllelrelo~ , inlelreloll-~.
Several methods have been proposed for the removal of nicotine from tobacco,
15 however, these processes typically involve the treatment of post harvest-tissue. For
example the use of solvents (EP 10,665, published May 14, 1980; inventors Kur7h~l~
and Hubert), or potassium metabisulphite, potassium sulphate and nitrate (U.S.
4,183,364, issued January 8, 1980; inventor Gllmu~h~n) have been proposed as
methods for removing nicotine from tobacco leaves. All of these treatments are
20 designed to m~int~in the flavour and aroma of the tobacco, and these methods involve
extensive post-harvest processing and are therefore not suitable for the preparation of
products as described in this invention.
Recently, specific alteration of nicotine levels within tobacco, by either over
25 expression (i.e. increasing) or antisense expression (decreasing) of putrescine N-
methyltransferase, a rate limiting enzyme involved in the nicotine biosynthetic
pathway, has been suggested (U.S. 5,260,205, issued November 9, 1993 and U.S.
5,369,023, issued November 29, 1994; hlvellLol~ Nakatani and Malik). However,
these methods are directed to the alteration of nicotine levels without modifying the
30 levels of other alkaloids that affect the flavours and aroma of tobacco. No nicotine
free plants were actually produced, nor was the use of these transgenically modified
tobacco plants, as a bioreactor for the synthesis of pfoleills of interest, suggested.
21 ~220
-- 8 -
However, such a transgenic tobacco plant, if produced, may be useful as a bioreactor
for the synthesis of proteins of interest as contemplated by this invention.
The breeding of low alkaloid-cont~ining tobacco plants has been reported
5 (Chaplin 1977), however, use of such a plant as a bioreactor has not been suggested.
Due to reduced levels of undesirable secondary plant products within suitable
non-food crop plants, it is contemplated that transgenic pl~teins produced within such
plants could also be rapidly processed for subsequent ~1mini~tration~ Such rapid10 processing could involve such methods as those employed for the plcpaldtion of FlP
as disclosed in Woodleif et al (1981). These include the aqueous extraction of soluble
protein from green tobacco leaves by precipitation with KHSO4, following removalof chloroplastic debris.
In order to establish the efficacy of transgene expression in nicotine-free
tobacco, and ensure soluble protein production a Type II antifreeze protein (AFP) was
used to prepare transgenic tobacco plants.
As a further example to establish the efficacy of the use of a non-food crop
20 plant as a bioreactor, the preparation of low-nicotine tobacco plants expressing
interleukin-4 (IL-4) is disclosed. IL-4 is a growth and dirrelcll~iation factor for T-
cells. In particular, IL-4 promotes the development of the subset of T helper (Th2)
cells from native T cells upon antigen stim~ tion. IL-4 producing Th2 cells
generally protect against the onset of many organ-specific autoimmlln~ diseases,25 including type 1 diabetes. Thus the availability of an abundant source of IL-4 may
prove invaluable for the immunotherapy of diabetes. Transgenic low-nicotine tobacco
plants can be used in direct feeding studies to determine the efficacy of the
recombinantly produced protein.
Gene constructs were transformed into N.tabacum cv. Xanthi and 81V9-4
tobacco strains (available from Agricuture and Agri-Food Canada, Pest ManagementResearch Center, Delhi Farm, Genetics Section) . 81V9-4 is a flue-cured tobacco line
2 1 ~3822(~
cont~ining only trace amounts of alkaloids and is an ideal component for tobacco-
based molecular f~rming applications. Gene constructs were built for production of
pro and mature forms of a Type II antifreeze protein (AFP), or IL-4, to be
accllm~ tecl in the cell cytosol and extracelluar space.
IgG expression in plants lends support to the idea that passage through the
endoplasmic reticulum may enhance protein accllmlll~tion. In plants bred to
simlllt~n~-ously express mouse IgG light and heavy chain p~ eills targeted to the
extracelluar space, the amount of each protein increased up to 60-fold over that seen
10 when expressed individually into the same colllpalllllent (Hiatt et al 1989).Expression of both protein components into the cytosol, however, did not affect their
level of accumulation. IgG processing and assembly in lymphocytes occurs throughthe action of heavy-chain binding proteins present in the endoplasmic reticulum (ER).
The observed increase in yield when both proteills were targeted to the extracelluar
15 space has been attributed to an enh~n~e~ stability contingent on IgG assembly.
Consistent with this hypothesis, active antibody complexes were observed when the
plOlt~inS were targeted to the extracelluar space but not when targeted to the cytosol.
To further maximize expression levels and transgene protein production the
20 gene encoding the protein of interest, the codon usage within the non-crop plant of
interest should be dele~ d. Also, it has been shown that endoplasmic reti~llhlm
retention signals can dramatically increase transgene protein levels, for example the
KDEL motif (Schouten et al 1996). Replacing any secretory signal sequence with aplant secretory signal will also ensure talg~lillg to the endoplasmic reticulum
25 (Denecke et al 1990).
~,Y~np'~-~
Gene Construction
To m~imi7e potential AFP production at the translational level, the native 5'-
untr~n~l~te~ region of the fish cDNA was replaced with the 5'-untr~n~l~te-1 leader
21 ~38220
- 10 -
region of the tobacco mosaic virus (TMV) (Richards et al 1977 & 1978, Sleat et al
1988). The fish signal peptide sequence was also replaced with that of the tobacco
pathogenesis-related protein lb (PR-lb) (Cornelissen et al 1986, Sijmons et al 1990,
Denecke et al 1990). Oligonucleotide c~settes were designed: three oligonucleotides,
#1091, #1092 and #1309, which would hybridize to generate the complete TMV
leader sequence (TMV CasseKe: Figure lA); and five oligonucleotides #4361-#4365
which in concert with oligonucleotides #1091 and #1092 would hybridize to generate
a linked TMV leader and PR-lb signal peptide DNA sequence (TMV-PR CasseKe:
Figure lB). The 5' end of these c~settes corresponded to a BamH I sticky end
restriction site. The 3' end of the TMV casseKe was blunt and incorporated an
additional adenine nucleotide intended to form the first nucleotide of the start codon
while the 3' end of the TMV-PR casseKe was compatible with Nde I-cut DNA but
would not be able to re-gen~ the restriction site following ligation.
To facilitate addition of the TMV and TMV-PR cassettes, sea raven Type II
AFP cDNA was site-specifically mllt~t~ according to the method described by
Kunkel (1985) to incorporate an Nde I restriction site at either bp 157 or 207. These
mutations also introduced applol)liate in-frame methionine start codons at the
junctions between sequences encoding pre and pro, or pro and mature portions of the
AFP. To build the gene construct for ~cllm~ tion of AFP into the cytosol, plasmids
cont~ining the mllt~te~ Type II AFP cDNAs were initially cut with NdeI and made
blunt-ended with mung bean nuclease. The cDNA fragments were released by
cleavage with Sal I, were isolated and directionally ligated into a BamH IISal I-cut
pTZ18 vector together with annealed TMV oligonucleotides #1091, #1092 and #1309.The TMV-proAFP and TMV-mAFP constructs were then cut with BamH I and Hinc
II, and the isolated AFP gene fragments ligated separately into pMON 893. For gene
constructs targeting AFP to the extracelluar space, the mllt~tç~l Type II AFP cDNAs
were initially subcloned from their pTZ 19 vectors into Hind III/Sal I-cut pBluescript
vectors to position a BamH I restriction site 5' to the AFP coding sequence. ThepBluescript-AFP clones were cut with BamH I and Nde I restriction enzymes to
remove the sea raven signal peptide sequence or signal peptide and pro-region DNA.
The plasmid portions of these digests were isolated and ligated with armealed
2~ 88220
oligonucleotides #1091, #1092, and #4361 through #4365. The gene constructs werethen cut with Xba I and R~7n I, and separately cloned into pCDX-l.
Cloning of the Type II AFP gene constructs into pMON 893 or pCDX-l
5 oriented the gene cassette into the vector between the double CaMV 35S promoter
(Kay et al 1987) and the NOS polyadenylation sequence. AFP encoded by the
transgene constructs was identical to the pro and mature AFP forms present in fish
with the exception of one additional methionine at the N-terminal end of the proteins.
The T-DNA portion of the final constructs are depicted in Figures 2 and 3. Cleavage
10 of the signal peptide components from the expressed AFPs was predicted (von Heijne
1986) to occur immediately prior to the added methionine in both the pro and mature
AFP gene constructs so the AFP accllmul~ting in the cytosol and extracelluar space
should be identical.
Gene constructs were transformed into N.tabacum cv. Xanthi and 81V9-4
tobacco strains according to the method described by Horsch et al (1988). AKempts
to gelleldte plants carrying transgenes for cytosolic accllmlll~tion of the AFP were
made inl~llllillellLly over a period of several years. Discs infected withA.tumefaciens
carrying these gene constructs showed little tendency to form callus and seemed more
20 subject to bacterial infection than comparably treated discs transformed with other
transgene constructs. Eventually six transgenic plants were regenerated: one proAFP
transgenic in each tobacco strain, three mature AFP transgenics in Xanthi and one
mature AFP transgenic in 81V9-4. By contrast, within six months, eight transgenic
plants were regenerated carrying gene constructs for AFP ~ccllmlll~tion into the25 extracellular space: two proAFP transgenics in each strain and four mature AFP
transgenics in 81V-9. Tldl~rolllled and regelleldt~d plants were initially selected for
kanamycin resistance. Transgenic status was subsequently determined by direct PCR
screening for the AFP transgene using primers #3339
S'-TATTTTTTACAACAATTACCAACAAC-3' and #4708
30 5'-CAGCAGTCATCTGCATACAGCAC-3' which hybridize to the TMV leader and
at the 3' end of the sea raven AFP coding sequence, respectively. Type II AFP gene
constructs were amplified in 30 cycles of dendluldlion for 1 min at 95~C, ~nn~ling
' 21~38220
for 1 min at 55~C, and elongation for 2 min at 72~C. This generated amplification
products of 440 and 388 bp from the gene constructs for cytosolic accllm~ tion of
the pro and mature AFPs, and 532 and 480 bp fragments from gene constructs
designed for accllmlll~tion of the same AFPs into the extracellular space.
Analysis of Transgene Expression
Plants were removed from the growth chamber and m~int~in~d at room
telllpel~Lul~ under a grow light for a minimllm 48 h prior to RNA or protein
10 extraction. RNA was prepared by selective precipitation according to the method of
Palmiter (1974). Total RNA (40 ,ug) was analysed by Northern blots according to
the method of Lehrach et al (1977) and probed with [a-32P]-labelled sea raven AFP
cDNA sequence.
Total soluble protein extracts were prepared from three to four leaves taken
from the upper half of a healthy plant according to the method described by
Gengenheimer (1990). Extracts were dialysed at 4~C against 0.1%, 0.01% and
0.001% ascorbic acid in SpectraPorl #3 dialysis tubing (MWCO 3.5 kDa) and
centrifuged for 15 min at 12,000xg to remove precipitate formed during dialysis prior
20 to lyophilization. Samples were resuspended in Millipore~-filtered water and their
protein concentrations determined by Bradford assay (16) relative to a BSA standard.
To extract protein present in the appoplast by vacuum infiltration (Sijmones
et al 1990), leaves were cut longillldin~lly into 4 cm x 0.8 cm strips, and rolled
25 lengthwise in 5 cm x 1.8 cm strips of Parafilrn~ so that the bottom of the leaf was
exposed to view. The leaf strip was exposed to vacuum derived from a water
aspirator twice for 2 min or until the exposed leaf surface was dark green. Eachtreated leaf strip yielded 10 + 2.5 ul of extract. Larger volume samples were too
dilute for use and were discarded. Samples which contained green pellets were also
30 discarded to reduce the possibility of cytosolic protein co~ ion. All rem~ining
extracts from a given plant were pooled and the amount of protein present determined
21 ~8220
- 13 -
by Bradford (1976) protein assay relative to a BSA standard. Extract concentrations
were generally between 0.1-0.2 llg/,ul.
Western Analysis
Lyophilized protein was resuspended directly in loading buffer (60 mM Tris-
HCl, pH 6.8/10% glycerol/5% ,B-mercaptoethanol/2% (w/v) SDS/ 1.3 x 10-3% (w/v)
bromophenol blue), and were electrophoresed through a 17% polyacrylamide/0.1 M
sodium phosphate, pH 6.8/4 M Urea/0.1% SDS gel using 0.1 M sodium phosphate,
pH 6.8/0.1% SDS running buffer. Electrophoresis was conducted at 50 V for 3-4 h
or until the 6 kDa pre-stained marker was at the bottom of the gel. Western blots
were prepared according to the method described by Burnette et al (1981) and probed
with polyclonal antibody to the mature sea raven AFP. A chemiluminescence
detection kit (Amersham) for probing of Western blots was used to detect bound first
15 antibody. All reactions were carried out according to the m~nllfa~tllrer's directions.
FY~nrl~ 1: AFP Gene Expression
Protein Acc -m~ tion
Western blot analysis of total soluble protein extracted from plants carrying
genes for cytosolic AFP expression did not find evidence of either pro or mature AFP
acc lm~ tion even when up to 80 ,ug of protein extract were analysed. In contrast,
a protein which co-migrated with mature AFP from sea raven and cross-reacted with
25 antibody to Type II AFP was ~letecte~l in as little as 2.5 ~g total soluble protein
extMct of plants in which the AFP was targeted to the extracellular space (Figure 4).
This protein was unique to the transgenic plants and was absent in extracts of a wild-
type plant and a plant, pII3a-#9, which had been transformed and regenerated butdet~llnilled to be non-transgenic by PCR. Insufficient resolution was obtained with
30 these extracts to dirrerelltiate size differences between the AFPs produced by the
plants carrying transgenes for production of pro or mature AFPs. This was probably
due to the viscosity of the total soluble protein extracts which often caused distortion
2 1 88220
- 14 -
of samples during the running of gels. For this reason, positive control Type II AFP
was mixed with total soluble protein extract from a lab-grown wild-type plant togenerate a suitable size standard. Wild-type protein extract was not mixed with the
molecular weight markers, so the size of plant-produced AFP could not accurately5 estimated from these extracts. AFP accllmlll~tion varied between plants and appeared
slightly higher in plants II4c~-#1 and 10 which carried genes for mature AFP
production. Based on Western blot comparison with a known amount of Type II AFP,the amount of AFP produced by the plants has been estim~ted to be approximately
0.5-1% of the total soluble protein.
mRNA Transcription from Transgenes for Cytosolic AFP Accumulation
RNA extracts of plants transgenic with gene constructs for cytosolic AFP
accumul~tion were analysed by Northern blot to determine if the AFP transgene was
transcribed. Two plants, Xanthi TmSR #1 and 81V9-4 TmSR #l, carrying transgenes
for AFP accumulation into the cell cytosol were found to produce a 0.96 kb RNA
transcript which hybridized with the AFP cDNA used as a probe: on longer exposure
of the blots, the same RNA transcript was also seen in extract from Xanthi TmSR #2
and slightly larger one of approximately 1.02 kb was detected in extract from 81V9-4
20 TpSR #3. TldllsC~ip~S from pro and mature AFP transgene constructs were expected
to differ by 51 nucleotides. Both plant-produced AFP gene transcripts were both
considerably larger than a PCR-gel1eLated control sample estim~ted to be 0. 36 kb and
representing the size of the mature AFP transgene between the TMV leader and the3' end of the coding sequence. This was consistent with the size dirrclcllce expected
25 based on the additional coding sequence and 3'-untr~n~l~t~d region (155 nt totdl)
present in the transgene construct, and with the use of the NOS polyadenylation
sequence to obtain polyadenylated transcripts. Both transcripts were smaller than an
equivalent gene ~ldnsclil)l of 1.06 kb produced in a plant carrying the transgene for
targeted mature AFP ~ccllm~ tion into the extracellular space. This size dirrel~llce
30 was consistent with the addition of the 90 nt signal peptide coding sequence to the
transgene construct for secretion of the AFP. Based on EtBr staining, more RNA
was loaded onto the gel from the plant producing AFP for accllmlll~tion into the
21 88220
extracellular space compared to the amount of RNA from the plants Lalg~ g the AFP
to the cytosol. No comparison could be made regarding the relative amounts of AFP
mRNA transcribed from these constructs. However, similar loadings were achieved
between the plants L~lgelillg the AFP to the cytosol. The dirrelel1ce in amount of
5 transcript observed, therefore, suggests that this transgene construct was being
expressed at variable levels in the dirrer~lll plants.
AFP Present in the Apoplast
Vacuum infiltration extracts were prepared from AFP-producing plants to
determine if the ~ressed AFP was present in the extracellular space. These extracts
were not subject to the viscosity and sample distortion associated with total soluble
protein extracts, so were also considered more suitable to estim~te the sizes of the
plant AFPs. Western blot analysis confirmed the presence of AFP. AFP was detected
15 in plants pII3a-#7 and II4c2-#l which carried mature and proAFP transgenes
respectively, as a diffuse band of approximately 14.7 kDa which migrated at a similar
rate to mature AFP isolated from fish sera. A small mobility dirrelcllce was observed
between the AFPs produced by the two plants, however, proAFP isolated from fish
sera was distinctly larger than either plant-produced AFP.
Having established the presence of AFP in vacuum infiltration extracts of
those plants carrying transgenes for extracellular AFP ~ccllm~ tion, the extracts were
concentrated ten-fold and tested for thermal hy~Lelesis activity and effects on ice
crystal morphology using the nanolitre osmometer. Extracts from plants expressing
25 gene constructs for either the pro or mature AFP both showed evidence of AFP
activity. Ice crystals grown in these plant extracts were seen as the characteristic eye-
shaped bi~yl~llids produced in the presence of native Type II AFP. Thermal
hy~Lelesis activity was measured and based on comparison with a standard activity
curve the amount of active AFP present in the extracellular space was estim~te-l as
30 2% of the total protein present in the colllpalLlnent.
21 88220
- 16 -
RNA analysis of the transgenic plants has shown that they are able to
transcribe the integrated transgene constructs, and that the size of the plant AFP
mRNA from both construct types is consistent with that expected for a full-length,
polyadenylated ll~nsc~ . mRNA produced from the transgene for AFP a~cllmlll~tion5 into the extracellular space was obviously tr~n~l~t~ble.
The presence of plant-produced AFPs in vacuum infiltration extracts in~ t~s
that the plant recognized the PR-lb signal peptide component and correctly targeted
the attached AFPs to the extracellular space. The predicted size of the AFPs forexport to the extracellular space is 18.7 or 17 kDa with the signal peptide component
attached and 15.8 or 14.1 kDa without the signal peptide for the pro and mature
AFPs, respectively. Western blot analysis of the plant vacuum infiltration extracts
showed that the AFPs produced from the proAFP and mature AFP transgene
constructs and present in the extracellular space both co-migrated with mature Type
15 II AFP and were approximately 14.7 kDa. This suggests that not only is the plant
capable of cleaving the signal peptide from the expressed AFP but that it can remove
most or all of the pro-region of the AFP.
F,~q...l le 2: Interleukin-4 expression
Full length murine IL-4 cDNA is 585 base pairs long (Lee et al 1988) and
codes for a 140 amino acid prepfoleill with a 40 amino acid secretory signal (Otsuka
et al 1987). This gene sequence encoding the 40 amino acid secretory signal was
replaced with a plant secretory signal obtained from the tobacco PRl-b secretory25 signal (Denecke et al 1990) to ensure ~lg~lhlg to the endoplasmic reticulum. The
reslll~nt vector was introduced into low-nicotine tobacco plants using the methods
described above.
In order to determine the biological activity of plant recombinant IL-4, in
30 vitro,
the stim~ tion of growth of murine IL-4 dependent CT.4S cell line (Rapoport et al
1993) is dete~ illed. Applopliate dilutions of purified plant recombinant IL-4 is
2 1 88220
added to cultures cont~ining 5x103 CT.4S cells in flat bottom 96-well plates in a final
volume of 100 ml for 48 hr. CT.4S cell proliferation is assessed by addition of 1
mCi/well of [3H]thymidine 18 hr before termination of culture, and [3H] thymidine
incorporation is de~e~ ed by liquid scintillation counting.
The immllnoreactivity of plant recombinant IL-4 is determined by solid phase
sandwich ELISA (Abrams et al 1992). Briefly, the BVD4-lDll anti-IL-4 mAB is
used as a capture antibody and is paired with the biotinylated BVD6-24G2 antiIL-4
mAB as the detecting antibody. Mouse rIL-4 (Ph~rmingen) is used a standard.
In vivo activity of plant produced IL-4 is delellllilled in NOD mice. NOD and
control strain (in~lliti~- and diabetes-free) female mice (20 mice/group) are fed either
transgenic low nicotine tobacco leaves expressing recombhlall~ly produced IL-4. At
various times during the 4 week treatment, circ~ ting serum levels of plant
15 recombinant IL-4-fed and un-fed NOD and control mice are analyzed by ELISA asdescribed above. Both NOD and control mice are monitored for any potential toxiceffects arising from the plant recombinant IL-4 feeding, and their serum
concentrations of IgE and IgG quantified by ELISA. Increase in serum IgE levels
indicate that recipient mice are more susceptible to allergic responses.
Feeding of plant recombinant IL-4 to protect against the onset of in~llliti~
and/or Type 1 diabetes in NOD mice is also determin~d. Neonatal female NOD mice
(2-3 weeks of age) fed or not fed with plant recombinant IL-4 are m~int~in~d in a
specific pathogen free animal facility. Mice are monitored weekly for their blood
25 glucose levels (BGL), and mice that are hyperglycemic (i.e. BGL > 11.1 mmol/L)
for two consecutive weeks are diagnosed as Type 1 diabetic. The onset of in~llliti~
is monitored by immllnohistoch~ l analysis of pancreatic tissue by sacrificing
mice at various times (2 weeks to 2 months) after plant recombinant IL-4-feeding is
initi~tç~l .
All scientific publications and patent documents are incorporated herein by lererellce.
2~ 8~220
- 18 -
The present invention has been described with regard to prefelled embodiments.
However, it will be obvious to persons skilled in the art that a number of variations
and modifications can be made without departing from the scope of the invention as
described in the following claims.
s
21 ~8220
- 19 -
References
Abrams, J.S., M.G. Roncarolo, H. Yasel, U. Anderson, G. Gleich, J. Silver (1992)Strategies of anti-cytokine monoclonal antibody development. Tmmllnol. Rev.
127, 5-24.
Bradford M.M. (1976) A rapid and sensitive method for the qll~ntit~tion of
microgram qu~ntiti~s of protein utili7ing the principle of protein dye binding.
Anal. Biochem. 72, 248-254.
Burnette W.H. (1981) Western blotting: electrophoretic transfer of proteills from
SDS-polyacrylamide gels to unmodified nitrocellulose and radio-graphic
detections with antibody and radioiodinated protein A. Anal. Biochem. 112,
195-203.
Chaplin J.F. (1977) Breeding for varying levels of nicotine in tobacco. In: Proc Amer Chem Soc, 173rd meeting, New Orleans.
Cornelissen B.J.C, R.A.M Hooft van Huijsduijnen, L.D.Van Loon & J.F.Bol (1986)
Molecular chara ;t~ alion of messenger mRNAs for "pathogenesis-related"
protehls la, lb and lc, influcecl by TMV infection of tobacco. EMBO J. 5,
37-40.
Denecke J., J.Botterman & R.Deblaere (1990) Protein secretion in plant cells can occur via a default pathway. Plant Cell 2, 51-59.
Fourney R.M., J.Miyakoshi, R.S.Day III & M.C.Paterson (1988) Northern blotting:
efficient RNA staining and transfer. Focus 10:1, 5-7.
~0 Gengenheimer P (1990) Pl~al~ion of Extracts from Plants. Methods of Enzymology
182, 184-185.
21 88220
- 20 -
Haq T.A., H.S. Mason, J.D. Clements, C.J. Artzen (1995) Oral i~ ni~ion with
a recombinant bacterial antigen produced in plants. Science 268, 714-716.
Hiatt A., R.Cafferkey & K.Bowdish (1989) Production of antibodies in transgenic
5plants. Nature 342, 76-78.
Horsch R.B., J.Fry, N.Hofmann, J.Neidermeyer, S.G.Rogers & R.T.Fraley. (1988)
Leaf disc transformation. Plant Molecular Biology Manual A5, 1-9. Ed.
S.B.Gelvin, R.A.Schilperoort, D.P.S.Verma. Kluwer Academic Publishers.
10Dordrecht/Boston/London.
Kay R., A. Chan, M . Daly & J.McPherson (1987) Duplication of CaMV 35S promoter
sequences creates a strong enhancer for plant genes. Science 236, 1299-1302.
15Kunkel T.A. (1985) Rapid and efficient site-specific mutagenesis without phenotypic
selection. Proc. Natl. Acad. Sci. USA 82, 488-492.
Lehrach H., D.Diamond, J.M.Wozney & H. Boedtker. (1977) RNA molecular
weight determinations by gel electrophoresis underdenaLu~ g conditions; a
20critical reex~min~tion. Biochemistry 16, 4743.
Ma J.K.C., M. Hein (1995) Tmmum~therapeutic potential of antibodies produced in
plants. TibTech 13, 522-527.
25Mason H.S., J.M. Ball, J-J. Shi, X. Jiang, M.K. Estes, C.J. Arntzen (1996)
Expression of Norwalk virus capsid protein in transgenic tobacco and potato
and its oral immlln(lgenicity in mice. Proc. Nat. Acad. Sci. 93, 5335-5340.
MollL~l~i L., P. Fantozzi, S. Pedone (1993) Tobacco fraction 1 (FlP utili7~tion of
30oral feeding and enteral feeding of patients. 1 Heavy Metal evaluation. Food
SAci. Tech. 26, 259-263.
21 88220
Otsuka T., D. Vallaret, T. Yokota, Y. Takebe, F. Lee, N. Arai, K. Arai (1987)
Structural analysis of the mouse chromosomal gene encoding interleukin-4
which expresses B cell, T cell and mast cell stim~ ting activities. Nucl. Acid
Res. 15, 333-334
s
Palmiter R.D. (1974) Magnesium precipitation of ribonucleopll~teill complexes:
Expedient techniques for the isolation of undegraded polysomes and messenger
ribonucleic acid. Biochemistry 13, 3606-3615.
~0 Rapoport M.J., D. Zipris, A.H. Lazarus, A. Jaramillo, D.V. Serreze, E.H. Leiter,
P. Cyopick, J.S. Danska, T.L. Delovitch (1993) IL-4 reverses thymic T cell
proliferative unresponsiveness and prevents diabetes in NOD mice. J. Exp.
Med. 178, 87-99.
Richards K., H.Guilley, G.Jonard & G.Keith (1977) Leader sequence of 71
nucleotides devoid of G in tobacco mosaic virus RNA. Nature 267, 548-550.
Richards K., H.Guilley, G.Jonard & L.Hirth (1978) Nucleotide sequence at the 5'
extremity of tobacco mosaic virus RNA. Eur. J. Biochem. 84, 513-519.
Schouten A et al. (1966) The C-terminal KDEL sequence increases the expression
level of a single-chain antibody designed to be targeted to both the cytosol andthe secretory pathway in transgenic tobacco. Plant Molec. Biol. 30, 781-793.
Sleat D.E., R.Jull, P.C.Turner & T.M.A.Wilson (1988) On the mechanism of
translational enhancement by the 5'-leader sequence of tobacco mosaic virus
RNA. Eur. J. Biochem. 175, 75-86.
Sijmons P.C., B.M.M.Dekker, B.Schrammeijer, T.C.Verwoerd, P.J.M.van den
Elzen & A.Hoekema (1990) Production of correctly processed human serum
albumin in transgenic plants. Bio/Technology 8, 217-221.
2~ 81322U
von Heijne G. (1986) A new method for predicting signal cleavage sites. Nucl.
Acids Res. 14, 4683-4690.
Woodleif W.G., J.F. Chaplin, C.R. Campbell, D.W. DeJong (1981) Effect of
variety and harvest treatments on protein yield of close grown tobacco
Tobacco Sci. 25, 83-86.