Note: Descriptions are shown in the official language in which they were submitted.
STA 108-Foreign Countries / St/ngb/S-P
_1_
Ultrafine cobalt metal powder, process for the production thereof and use of
the cobalt metal powder and of cobalt carbonate
S Background of the Invention
The present invention relates to ultrafine cobalt metal powder consisting of
fine
crystallites, wherein the crystallites exhibit a habit ranging from rice-grain
shaped
to spherical and more than 90% of the crystallites have a diameter in the
range of
from 0.5 pm to 2 pm, a process for the production of the cobalt metal powder
via
the intermediate stage of the cobalt carbonate, and methods for the use of the
cobalt metal powder and of the cobalt carbonate.
The main fields of application of ultrafine cobalt metal powder are the
production
of hard metals and of diamond tools. The two applications place different
demands
on the cobalt metal powder. For use in hard metals, a very low content of
impuri-
ties such as sodium, calcium and sulphur is particularly important. It is also
im-
portant that the content of oxygen and carbon is not too high. The particle
size
and particle shape are of secondary importance.
To produce hard metals, mainly mixtures of tungsten carbide and of about 6 to
15% of cobalt metal powder are sintered at temperatures of about 1350 to
1450°C.
This is a liquid phase sintering of the cobalt metal powder, during which part
of
the tungsten carbide dissolves in the cobalt. On cooling, recrystallization
processes
take place in the course of which small quantities of impurities such as
sodium
(Na), calcium (Ca) and sulphur (S) contained in the starting materials are
already
deposited preferentially at the grain boundaries of the tungsten carbide
crystals.
This can lead to a local reduction in strength and hence to a decrease in
bending
strength (12th International Plansee Seminar '89, Vol. 2 (pages 421-428)). In
the
case of very fine hard metal parts such as, for example, microbores, this
effect
results in the tools readily breaking at the positions of decreased strength.
It is also important that the content of oxygen and carbon is not too high,
with
values to a total of up to 0.9 wt% being acceptable. Both an increased oxygen
content and an increased carbon content can influence the carbon balance
during
the sintering process, so that the development of embrittlement through
etaphasis
or through formation of C-porosity owing to carbon esters may possibly result.
The two effects also distinctly impair the quality of the hard metal.
STA 108-Foreien Countries
~I~88i5
-2-
In the production of diamond tools based on cobalt metal powder, which tools
consist mainly of cobalt metal powder, synthetic diamonds and other powdered
substances, for example, copper, tin, iron, nickel, etc. the influence of the
physical
properties such as particle size and particle shape quite definitely
dominates.
S Although the chemical impurities in the above-mentioned elements can give
rise to
a microporosity, this is only a minor factor. The reason for this lies in the
temperature range of 700 to 950°C conventionally employed in the
production of
diamond tools. In contrast to the production of hard metals, at these
temperatures
solid phase sintering occurs, so that the properties of the initial powders
are
predominantly preserved.
Experimentally it is observed that a decrease in the particle size gives rise
to an
increase in the hardness of hot-pressed cobalt segments. In general the Hall-
Petch
equation states that the hardness is reciprocal to the square root of the
medium
particle diameter.
IS This relation can be explained theoretically by the fact that the hardness
is
influenced by the specific proportion of grain boundaries per unit of volume,
since
the grain boundaries impede the propagation of dislocations. As the hardness
correlates with the cutting properties of the segments, an increase in
hardness
frequently results in tools having a longer useful life and is therefore of
great
importance. In order to increase the specific proportion of grain boundaries
per
unit of volume, the primary particle size of the powders can be decreased (J.
Konstanty and A. Busch in PMI, Vol. 23, No. 6, (1991)). Another possible
method
of increasing the specific proportion of grain boundaries per unit of volume
consists however, at an identical or similar particle size, in altering the
particle
shape in such a way that the primary crystals have more of a rounded habit.
There are a number of different ultrafine cobalt metal powders which to
varying
degrees fulfil the requirements of manufacturers of hard metals or of diamond
tools.
EP-A 0 113 281, owned by the firm Eurotungstene, Grenoble, France, describes
the production of cobalt metal powder by the polyol process, whereby different
cobalt compounds are reduced by polyols at 85°C. This cobalt metal
powder may
contain up to 3 wt.% of carbon and oxygen, so that at the otherwise high
chemical
purity and the high specific proportion of grain boundaries per unit of
volume,
23189-8007
CA 02188815 2004-06-17
effected by the particle shape, an adverse influence on the
hard metal properties cannot be excluded.
The commercial cobalt metal powder product Co OF
from the firm Eurotungstene, according to technical
information supplied by the company, is manufactured from
cobalt hydroxides. This product is distinguished by a
relatively high specific proportion of grain boundaries per
unit of volume. However, the increased content of sodium
and sulphur can be disadvantageous.
A completely different technical procedure is
disclosed in US-A 5 246 481, owned by the firm Sherritt
Gordon, Alberta, Canada. Here the production of this powder
is carried out through the reduction of cobaltamine sulphate
solutions, to which have been added soluble silver salts as
nucleating agents. The doping with silver salts can lead to
exceptionally high contents of silver, typically of up to
3,600 ppm, in the cobalt metal powder. Furthermore the
carbon content, which according to information from the
company is about 1,750 ppm, is remarkable.
Summary of the Invention
There has now been found a cobalt metal powder
which possess the required properties. The present
invention provides an ultrafine cobalt metal powder
consisting of fine crystallites, wherein the crystallites
exhibit a habit ranging from rice-grain shaped to spherical
and more than 90~ of the crystallites have a diameter in the
range of from 0.5 ~m to 2 Vim, characterised in that it has a
sodium content of less than 100 ppm and a carbon content of
less than 500 ppm.
In one aspect, the invention provides a method of
preparing cobalt metal powder, comprising: (a) reacting a
3
CA 02188815 2004-06-17
23189-8007
soluble cobalt salt with a solution or suspension of an
alkali metal carbonate or hydrogen carbonate, an alkaline
earth metal carbonate or hydrogen carbonate, cobalt
carbonate or hydrogen carbonate, or ammonium carbonate or
hydrogen carbonate in a pH range of 5.5 to 6.8 and at a
temperature below 35°C; (b) separating the cobalt carbonate
precipitated product of step (a) and washing with water the
separated cobalt carbonate precipitated product until the
required purity is obtained and drying the washed cobalt
carbonate; and (c) reducing the cobalt carbonate product of
step (b) to cobalt metal powder.
Preferably the content of sodium is less than
50 ppm and that of calcium and sulphur respectively is less
than 30 ppm.
In an additional preferred embodiment, more than
900 of the crystallites have a length to width ratio in the
range of from 1:1 to 5:1, while the diameter of the
crystallites is preferably from 0.7 ~m to 1.1 Vim. The
particle size of the crystallites, measured in accordance
with ASTM B 330, is preferably from 0.7 ~m to 0.95 Vim.
3a
STA 108-Foreign Countries
~~8~°l7
-4-
Table 1 below provides a survey of the ultrafine cobalt metal powder according
to
the invention compared with various commercial products.
Table l:
Survey of various ultrafine cobalt metal powders
Manu- HCS1' H.C. StarckEuro- Euro- SherrittSumitomo
fact~.uer GmbH tun stenetungsteneGordon
ProductProduct Co IV Co OF Co ex Co OF
C
accordingCommercial Polyol
to the product
invention
Particle0.7 - > 0.95 0.9 0.5 0.7 0.8 -
0.95 - 0.9 1.9
size
FSSS/pm
Habit rice-grainellipsoidatspheroidalsphericalsphericaloblong
to sphericalhabit mth crystals
habit rounded
crystal
surfaces
Typical
impurities
Na 50 60 240 5 20 - 130 -
90 150
(ppln)
I S Ca (ppm)30 40 8 6 6 70 -
80
S (ppm)30 35 140 20 50 **
C (ppm)< 500 < 500 < 500 2000 1750 **
C+OZ 0.8 0.8 0.9 3 - 4 0.9 **
(art
%)
* Due to the production process, this grade of Co has Ag contents of up to
3,600 ppm, which is exceptionally high for Co metal powders.
** The relevant information is not given in the patent specification.
Compared with the commercial product Co IV C from HOST (Hermann C. Starck,
GmbH & Co. KG, Goslar) the new product according to the invention exhibits a
further increase in purity and again an increased specific proportion of grain
boundaries per unit of volume due to a rice-grain shaped to spherical habit.
In
addition, in a preferred embodiment the product according to the invention has
particle sizes of from 0.7 to 0.95 ltm, measured in accordance with ASTM B
330,
whereas in the commercial product Co IV C these are larger than 0.95 ~tm.
STA 108-Foreign Countries
-5-
The present invention also provides a process for the production of the new
cobalt
metal powder. This is a process for the production of cobalt metal powder in
which a soluble cobalt salt is reacted with a liquefied form such as a
solution
and/or suspension, of alkali carbonate, alkaline-earth carbonate, cobalt
carbonate
and/or ammonium carbonate and/or the respective hydrogen carbonates, in the pH
range of from 5.5 to 6.8, the precipitate formed is separated off, washed with
water until the required purity is attained, dried and the cobalt carbonate
thus
obtained is reduced to the cobalt metal powder.
A process for the production of cobalt metal powder via the intermediate stage
of
the cobalt carbonate is also disclosed in the Japanese patent application JP-A
78 123 722, owned by the firm Sumitomo, Japan. Owing to the processing
conditions thereof, the said process leads to powders of a completely
different
morphology compared with that of the powders obtained by the process according
to the invention.
The crystals obtained according to JP-A 78 123 722 preferably have a diameter
of
1 to 2 pm on average and a length equal to 10 to 20 times the diameter. This
indicates, however, a low specific proportion of grain boundaries per unit of
volume and consequently a lower hardness as well.
In the process according to JP-A 78 123 722, the reaction of the cobalt salts
to
form cobalt carbonate is carried out at pH values in the range of from 7.0 to
7.4.
In reactions within this pH range the Co ions present in the solution are
precipitated quantitatively. A disadvantage, however, is that there is a
decline in
the effectiveness with which the precipitate can be washed, which leads to the
increased contents of Na, Ca and S in the end product.
In contrast, the process according to the invention is carried out with the pH
value
being adjusted to less than 6.8, preferably to 6.0 to 6.7. By this means a
consider-
ably more effective washing of the precipitate is achieved and hence the
impurity
content is definitely lowered. However, precipitation at a pH value of less
than 6.8
is inconsistent with the requirement that the Co ions be completely reacted.
The
reaction solution flowing off is still pink-coloured, that is, it contains
considerable
quantities of unreacted Co ions, which would also enter the waste water. This
portends on the one hand environmental problems and on the other hand, because
of the high price of Co raw materials, an indefensible economic loss. In the
STA 108-Foreign Countries
? 1888 f
process according to the invention, the precipitation is nevertheless carried
out at a
pH of less than 6.8. The grave disadvantages of this condition for the
precipitation
are avoided according to the invention by recirculating the cobalt contained
in the
reaction solution flowing off and by adding it to the CoCI,, solution prior to
the
reaction, if necessary with readjustment to a suitable pH value. There are a
large
number of feasible technical procedures which can be carried out in order to
separate off cobalt as a sparingly soluble compound. These include:
- precipitation of the cobalt as cobalt hydroxide by the addition of sodium
hydroxide
- precipitation of the cobalt as basic cobalt hydroxide by alkaline oxidation
with hydrogen peroxide
- precipitation of the cobalt as cobalt carbonate by the addition of alkali
carbonates and/or alkaline earth carbonates or the respective hydrogen
carbonates at suitable pH values
- precipitation of the cobalt as basic cobalt carbonate by the blowing in of
carbon dioxide.
By carrying out the precipitation according to the invention, the formation of
a
precipitate containing a particularly fine-grained cobalt is ensured.
Measurements
taken by means of the Malvern Master Sizer instrument on samples withdrawn
from the precipitate-containing suspension showed that the D9o value is at
most
90 pm, particularly preferably at most 40 gm. If the precipitations are
carried out
as in the known prior art, the d9~ value is 130 pm.
The reaction product is advantageously washed with water until the required
purity
is attained. Washing is carried out preferably in several stages, with the
water
temperatures selected being first of all in the range of from 0°C to
35°C and
finally in the range of from 35°C up to boiling temperature. The cobalt
carbonate
is then dried, preferably in a moving bed. finally the product is converted to
cobalt oxide. The latter is reduced, preferably using hydrogen, to ultrafine,
very
pure cobalt metal powder.
STA 108-Foreign Countries
2188~1~
It is particularly preferred that between the drying and reducing stages a
calcination be carried out at temperatures of from 500°C to
800°C. The actual
reduction is advantageously carried out at temperatures of between
400°C and
550°C.
The present invention also provides the use of the cobalt metal powder
according
to the invention as a binding material for diamond tools, hard metals and
abrasive
component parts.
This invention further provides the use of the cobalt carbonate obtainable by
the
process according to the invention for the production of cobalt oxide of the
general formula Co01_\ for use in batteries. This cobalt carbonate is also
eminently suitable as a doping agent for electroceramics.
The invention is illustrated below by the following non-limiting Examples.
STA 108-Foreign Countries
?1a~38i5
Example 1
2 split streams (a) and (b) below were reacted in a continous operation in a
stirred-tank reactor to produce a suspended precipitate (suspension):
a) a cobalt chloride solution (concentration of the Co ions 105 g/1) having a
S volumetric flow rate of 4.3 1/h and
b) a sodium hydrogen carbonate solution (concentration 90 g/1) having a
volumetric flow rate of 14.3 1/h. A pH value of 6.5 was measured in the
reaction suspension flowing out and a d9~ value of 38 pm, measured using
a Malvern Master Sizer, was determined for the suspended precipitate. The
suspension was filtered. The filtrate, which still contained cobalt, was
treated with sodium hydroxide. The cobalt hydroxide precipitated and was
filtered off and was dissolved in HCl and added to the reaction solution
(a). The filtration residue from the reaction suspension was washed in
portions, first of all nine times with cold water at 28°C, subsequently
with
hot water at a temperature of 76°C. After the product had been dried at
580°C in a spray dryer, it was decomposed at 520°C to cobalt
oxide which
then, under hydrogen at 490°C, was reduced to cobalt metal powder. It
was
possible to obtain a cobalt metal powder having a particle size of 0.88 pm,
measured by the Fisher Sub Sieve Method.
A chemical analysis of the metal yielded the following impurities:
Na: 30 ppm
Ca: 10 ppm
S : < 10 ppm
STA 108-Foreign Countries
~18881~
Example 2
2 split streams (a) and (b) below were reacted in a continous operation in a
static
mixer to produce a suspension:
a) a cobalt chloride solution (concentration of the Co ions 82 g/1) having a
volumetric flow rate of 3.8 1/h and
b) an ammonium hydrogen carbonate solution (concentration 101.7 g/1) having
a volumetric flow rate of 8.9 1/h. A pH value of 6.4 was measured in the
suspension flowing out and a d9o value of 37 pm, measured using a
Malvern Master Sizer, was determined for the suspended precipitate. The
reaction suspension was filtered. The filtrate, which still contained cobalt,
was treated with magnesium carbonate. The cobalt carbonate precipitated
and was filtered off and was dissolved in HCl and added to the reaction
solution a). The filtration residue from the reaction suspension was washed
in portions, first of all with ice water, subsequently with hot water at a
temperature of 67°C. After the product had been dried at 590°C,
it was
decomposed at 600°C to cobalt oxide which then, under hydrogen at
500°C, was reduced to cobalt metal powder. It was possible to obtain a
cobalt metal powder having a particle size of 0.90 pm, measured by the
Fisher Sub Sieve Method.
A chemical analysis of the metal yielded the following impurities:
Na: 3 S ppm
Ca: 8 ppm
S : < 10 ppm
STA 108-Foreign Countries
-1°- ?? ~~u 1'~
Example 3
2 split streams (a) and (b) below were reacted in a continous operation in a
packed
column to produce a suspension:
a) a cobalt chloride solution having a volumetric flow rate of 43.2 1/h
(concentration of the Co ions 98 g/1) and
b) a sodium hydrogen carbonate solution (concentration 92 g/1) having a
volumetric flow rate of 155 1/h. A pH value of 6.6 was measured in the
reaction suspension flowing out and a d9~ value of 40 Vim, measured using
a Malvern Master Sizer, was determined for the suspended precipitate. The
reaction suspension was filtered. The filtrate, which still contained cobalt,
was treated with sodium carbonate. The cobalt hydroxide precipitated and
filtered off was added in portions to the reaction solution a). The filtration
residue from the reaction suspension was washed in portions, first of all
with cold water, subsequently with hot water at a temperature of 83°C.
After the product had been dried at 560°C, it was decomposed at
680°C to
cobalt oxide which then, under hydrogen at 460°C, was reduced to cobalt
metal powder. It was possible to obtain a cobalt metal powder having a
particle size of 0.93 pm, measured by the Fisher Sub Sieve Method.
A chemical analysis of the metal yielded the following impurities:
Na: 36 ppm
Ca: 9 ppm
S : 11 ppm
CA 02188815 2004-06-17
23189-8007
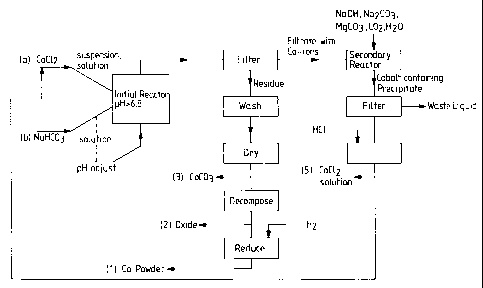
The foregoing is graphically illustrated in the
single figure of drawing showing initial feed streams of
components for the initial reactor, namely, (a) liquefied
cobalt chloride in solution or suspension form with
adjustment of it directly or by a separate pH adjusting
fluid (acidic) to establish a pH under 6.8 in the initial
reactor. As mentioned above, this is entirely contrary to
industry practice and expectation for reasons stated above.
The initial reactor, which can be of a batch or continuous
flow type, produces a muddy precipitate that is filtered to
yield a filtrate with cobalt ions and a solid residue that
is washed and dried as described above, then decomposed to
produce cobalt-oxide, which is in turn reduced by added
hydrogen or other reducing agent to produce a high purity,
ultrafine cobalt powder. While this purified and sized
cobalt powder is the principal end product (1), other
possible off-takes from the process are useful per se, e.g.
cobalt oxide (2) useful in battery electrodes; and cobalt
carbonate (3) useful as a dopant for electro-ceramics.
Reference is made to certain U.S. patents and copending
patent applications of common assignment with this
application showing how these cobalt carbonate and cobalt
oxide products may be used:
U.S. Patent 5,718,844 (Krynitz et al/H.C. Starck
GmbH & Co. KG) shows the utility of cobalt metal powder
cobalt oxide, cobalt hydroxide and/or CoX(Co0)1_X as active
constituents of nickel electrodes in nickel/cadmium and
nickel/metal hydride secondary batteries. See also,
references cited therein at pp. 1-3 and the corresponding
published European patent application of Starck, 94-111967.9
filed Jan. 4, 1995 and references cited in the search report.
published therewith.
11
CA 02188815 2004-06-17
23189-8007
--Electro-ceramics include perovskite structure
compounds of the general formula X (B'B")03 where X can be,
inter alia, lead (Pb), B" is Ta or Nb and B' can be
inter alia,,cobalt (Co). Ta and Nb can be provided as
relatively,phase pure tantalates and niobates, i.e B'
(II)B"206 or B' (III)B' 204. Cobalt as a B' choice can be
provided in -II or -III valence states and can be
incorporated as cobalt oxide, cobalt carbonate or cobalt
carbonate hydroxide. The preparation of the niobates and
tantalates is shown in the U.S. Patent 5,721,182 of Reichert:
et al. and EP 580 974 Al of Reichert et al, both of common
assignment with this application and references cited in the
specifications of said applications. See, also, U.S. Patent;
No. 5,288,474 granted to Reichert et al. and references
cited therein.
lla
STA 108-Foreign Countries
- 12-
Coming back to the filtrate of product from the initial reactor, the filtrate
can be
fed to a secondary reactor and then combined with alkali (e.g. sodium)
hydroxide,
alkali carbonate or alkaline earth (e.g. magnesium) carbonate to produce an
output
wet mass with a filterable precipitate (cobalt hydroxide) therein. This mass,
a
useful product per (4) can be filtered to produce a spongy cobalt hydroxide
that
can be recovered in several ways:
(I) reaction with NaOH to produce cobalt hydroxide (4')
(II) reaction with hydrogen peroxide to produce a basic cobalt hydroxide (4')
(III) reaction with a carbonate to produce cobalt carbonate
(IV) reaction with carbon dioxide to produce a basic cobalt carbonate (3")
(V) dissolution in hydrochloric acid to produce a cobalt chloride solution
that
can be fed back to feed stream (a).
It will now be apparent to those skilled in the art that other embodiments,
improvements, details, and uses can be made consistent with the letter and
spirit
1 S of the foregoing disclosure and within the scope of this patent, which is
limited
only by the following claims, construed in accordance with the patent law,
including the doctrine of equivalents.