Note: Descriptions are shown in the official language in which they were submitted.
Wo 9~29967 r~ J, c 1~
21 89023
--1--
- IIG~IT lSODULATI~G DEVICE IlAVIliG
A VINYI, E~l!IIER-'`' qrn l~A~RIX
R l,:l.[l, uu~,d of the Invention
Field of the Invention
This invention relates to a light modulating
device (e.g., a polymer-dispersed liquid crystal device
hereinafter referred to as a "PDLC device") and, more
10 particularly, to a light modulating device which is
based on an; uv~d matrix that is the reaction
product of a vinyl ether.
DescriDtion of the Related Art
Various types of light modulating devices are
15 known. One type is the so-called PDLC device that
includes an electrically responsive liquid crystal
layer in which liquid crystal droplets are dispersed
tl.~ ùu~uu~ a polymer matrix. One way to prepare the
liquid crystal layer is by combining the liquid crystal
20 material with a polymerizable matrix ~LeCUL~U~ and then
subjecting the mixture to polymerization conditions.
Polymerization causes phase separation of the liquid
crystal material, resulting in tlne formation of liquid
crystal droplets dispersed L}l~uù~l~uuL the polymerized
25 matrix.
PDLC devices are trAn~ nt in the absence of an
electric field due to light scattering and become
transparent upon application of the f ield . Reverse
mode PDLC devices are also known. These devices are
30 LL~ ale"L in the absence of an electric field and
become trAn~ nt upon application of the f ield .
Various PDLC matrices are known. They include the
polymerization products of epoxy, isocyanate, and
certain photo-curable vinyl ~. (e.g., acrylates
35 or the reaction product of a ~lti-functional thiol
W095l29967 21 & q ~2 ~ r~ c8
with a multi-functional acrylate or a multi-functional
allyl) .
Sllr--rv of the Invention
In a first aspect, the invention ~eatures an
5 optically responsive f ilm that includes liquid crystal
dispersed in a crosslinked polymer matrix that includes
the reaction product of an isotropic polymerizable
mixture that includes at least one vinyl ether and at
least one multi-functional reactant other than a vinyl
10 ether.
A "vinyl ether" is a reactant having one or more
polymerizable carbon ~.vLL~OlI double bonds linked via a
single bond to an oxygen atom where the other group
bonded to the oxygen atom is not a carbonyl group nor a
15 llydL uy-:ll atom .
A "multi-functional" reactant is a reactant
containing two or more groups that participate in the
polymerisation reaction by reacting with the carbon-
carbon double bond(s) of the vinyl ether. A "mono-
20 functional" reactant, in contrast, contains only onesuch group.
An "ene" is a reactant having a polymerizable
carbon-carbon double bond.
By "isotropic" it is meant that the polymerizable
25 mixture does not exhibit a liquid crystalline ~ e
at the polymerization t~ UL'' prior to
poly-merization to prepare the optically respûnsive
film.
In some preferred ~ Ls, the vinyl ether may
30 be a multi-functional vinyl ether, mono-functional
vinyl ether, or combination thereof . Specif ic examples
of preferred vinyl ethers include 1~YdLV~Y~VU~Y1 vinyl
ether or esters thereof (e.g., an ester of a
dicarboxylic acid such as isophthalic acid, glutaric
35 acid, or s~ cin;~ acid); butanediol divinyl ether; 1,4-
cy~l~)h~Y~nF.~Ii Lhanol monovinyl ether or esters thereof
Wo 9s/29967 r~
21 ~9023
--3--
(e.g., an ester of a dicarboYylic acid such as
isophthalic acid, glutaric acid, or succinic acid);
1,4-cyclnh~YAn~ thanol divinyl ether; the propenyl
ether of propylene ~ bul.ate; triethylene glycol
5 divinyl ether; vinyl ether-functional urethane
oligomer6; and f luorinated vinyl ethers .
The multi-functional reactant may be a thiol,
ene, silicon hydride, alcohol, epoxy, or combination
thereof. In one preferred ~ ''ir L, the multi-
10 functional reactant is a multi-f~nrt i nnA 1 thiol . Such
thiols preferably have the general formula
Z[OCO(CH2)"SH]m where Z is a polyvalent organic moiety
which i8 a CHo3 ~LUII~ cu..~aining nucleus of a tri- or
tetravalent alcohol of the type of glycerol or
15 pentaerythritol, m is 3 or 4, and n is an integer
between 1 and 5, inclusive. Examples of preferred
thiols include trimethylolpropane tris(3-
mercaptopropionate), pentaerythritol tetra(3-
mercaptopropionate), and combinations thereof.
A second example of a preferred multi-functional
reactant is a multi-fllnt~tinnAl ene. Preferred multi-
functional enes include multi-fl7nt~tinnAl allyls,
acrylates, methacrylates, acrylamides, methacrylamides,
vinyl silanes, and combinations thereof.
In other preferred ~ , the optically
responsive film is the reaction product of the vinyl
ether, the multi-functional reactant, and at least one
mono-flln--t i nnA 1 reactant other than a vinyl ether .
Examples of preferred mono-functlonal reactants include
30 mono-fl-nrtinnAl enes (e.g., allyls, acrylates,
methacrylates, acrylamides, methacrylamides, vinyl
silanes, maleates, fumarates, and combinations
thereof), thiols, silicon hydrides, alcohols, epoxies,
and combinations thereof. "Naleate" refers to a mono-
35 and/or di-ester of maleic acid and/or maleic anhydride.
"Fumarate" refers to a mono- and/or di-ester of fumaric
wo gs/29967 P~ C ~
21 89023
--4--
acid and/or fumaric anhydride. Maleic acid and fumaric
~cid are the cis and trans forms, respectively, of
butenedioic acid.
The optically responsive film may also be the
5 reaction product of the vinyl ether, the multi-
~unctional reactant, and at least one acid reactant.
An "acid reactant" refers to a copolymerizable species
provided with one or more groups classified as Lewis
acids. Examples of preferred acid reactants include
10 acrylic acid, methacrylic acid, and combinations
thereof .
Specific examples of preferred optically
responsive f ilms include those f ilms which are the
reaction product of:
(1) a vinyl ether, a multi-functional allyl, and
a multi-functional thiol;
(2) a vinyl ether, a multi-functional acrylate,
and a multi-f-~nrtion~l thiol;
(3) a vinyl ether, a multi-functional allyl, a
20 multi-functional acrylate, and a multi-functional
thiol;
(4) a vinyl ether, a multi-functional allyl, a
multi-functional thiol, and at least one mono-
functional acrylate, methacrylate, or co~bination
25 thereof;
(5) a vinyl ether, a multi-functional allyl, a
multi-functional acrylate, a multi-functional thiol,
and at least one mono-functional acrylate,
methacrylate , or combination thereof;
(6) a vinyl ether, a multi-functional thiol, and
~t least one mono-functional acrylate, methacrylate, or
combination thereof;
(7) a vinyl ether, a multi-functional allyl, and
at least one mono-functional acrylate, methacrylate, or
35 combination thereof;
Wo 9s/29967 2 8 9 ~ 2 3 ~ c :~8
(8) a vinyl ether, a multi-f~lnctjnn~l thiol, a
multi-functional acrylate, and at least one mono-
functional acrylate, methacrylate, or combination
thereof;
t9) a vinyl ether, a multi-functional acrylate,
and at least one mono-functional acrylate,
methacrylate, or combination thereof;
(10) a vinyl ether, a multi-functional allyl, and
a multi-functional acrylate; ~nd
lo (11) a vinyl ether, a multi-f~lnctionAl allyl, a
multi-functional acrylate, and at least one mono-
functional acrylate, methacrylate, or combination
thereof .
The invention further features a light modulating
15 device that includes the above-described optically
responsive films to which an electric field is applied
through a pair of ele~LL~es.
In a ~c-econd aspect, the invention features an
optically responsive f ilm that i nr~ c liquid crystal
20 dispersed in a crosslinked polymer matrix that in~ c
the reaction product of an isotropic polymerizable
mixture that includes at least one multi-functional
vinyl ether.
In preferred ~ho~ c of ~he second aspect of
25 the invention, the film is the reaction product of (a)
the multi-functional vinyl ether and at lea6t one mono-
fllnrti nnAl vinyl ether or (b) the multi-functional
vinyl ether ~nd at least one multi-flln~t~nnAl reactant
other than a vinyl ether which may be a thiol, ene,
30 cilicon hydride, alcohol, epoxy, or combination
thereof. Examples of preferred multi-functional vinyl
ethers include 1~4-cy~lnhc~y~n~ hAnnl divinyl ether,
triethylene glycol divinyl ether, and vinyl ether-
functional urethane oligomers .
The optically responsive film may be the reaction
product of the multi-functional vinyl ether and at
W09s/29967 2 1 8 9 023 ~ o~
--6--
least one mono-functional reactant other than a vinyl
ether. r ~ Of mono-fllnrtinnAl reactants include
mono-functional thiols, enes (e.g., a mono-functional
allyl, acrylate, methacrylate, acrylamide,
5 methacrylamide, vinyl silane, or combination thereof),
silicon hydride, alcohol, epoxy, or combination
thereof ~ . The f ilm may also be the reaction product of
the multi-functional vinyl ether and at least one acid
reactant (e.g., acrylic acid, methacrylic acid, or
lO combination thereof ) or the reaction product of the
multi-functional vinyl ether and a maleate, fumarate,
or combination thereof.
The invention further features a light modulating
device that includes the above-described optically
15 responsive films to which an electric field i5 applied
through a pair of electrodes.
In a third aspect, the invention features a method
of preparing an optically respon8ive film that in~ A~-c
the steps of:
(a) ' in;n~ liguid crystal and at ieast one
vinyl ether to form a substantially ~ , -o~
isotropic mixture; and
(b) polymerizing the vinyl ether in the presence
of the liquid crystal under reaction conditions
25 sel ~rte~l to cause phase separation of the liquid
crystal to form an optically responsive film that
includes liguid crystal dispersed in a croc,:l ink~
polymer matrix.
In pref erred ~mho~ of the third aspect of
30 the invention, the vinyl ether is a multi-functional
vinyl ether, a mono-fl~nrtinnAl vinyl ether, or
combination thereof. The reactant mixture (i.e., the
mixture of li~uid crystal and vinyl ether) may further
include a multi-functional reactant other than a vinyl
35 ether, e.g., a thiol, ene, silicon hydride, alcohol,
epoxy, or combination thereof. The reactant mixture
W0 9~29967 2 t 8 9 0 2 3 ~ , Q I~CR
.
--7--
may also further include at least one mono-functional
reactant other than a vinyl ether (e.g., a mono-
fllnction;~l thiol, ene, silicon hydride, alcohol, epoxy,
or combination thereof). Moreover, the reactant
5 mixture may further include at least one acid reactant
(e.g., acrylic acid, methacrylic acid, or combination
thereof ) . The reactant may also further include a
maleate, fumarate, or combination thereof.
The vinyl ether-based optically responsive f ilms
10 according to the invention offer several advantages.
For example, the vinyl ethers can be cured either
thPr~-l ly or by ~ O~ULe: to radiation (e.g.,
ultraviolet or electron beam radiation). In addition,
the polymerization reaction can be initiated and/or
15 catalysed cationically, free radically, or through a
combination of both. The vinyl ethers cure rapidly but
( in the case of cationic initiation) are not inhibited
by ~ ric oxygen. Moreover, a variety of
materials can be co-reacted with the vinyl ether,
20 ~ hl ing the properties of the polymer matrix to be
tailored for a particular application. The vinyl
ethers are also less toxic than, e.g., acrylates.
Other features and advantages will be apparent
from ~he following description of the preferred
25 ~ s thereof and from the claims.
Brief Descril~tion of the Drawinqs
The invention will be more fully understood with
reference to the following drawings in which:
FIG. 1 is a schematic view, partially in cro6s-
30 section, of a light modulating device according to the
invention .
FIG.2 is a ~:Lo~ ional view of an extrusion
die useful in preparing films according to the
invention .
FIG. 3 is an enlarged ~:Losi-s~ct~clnAl view the
extrusion die shown in FIG. 2.
WO 95129967 -a~ cx
FIG. 4 is a cross-~pct i c nA 1 view of an alternative
extru6ion die useful in preparing films according to
the invention.
Descril~tion of the Preferred F ~
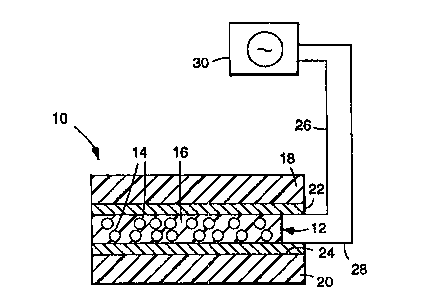
Referring to FIG. 1, there is shown a light
modulating device 10 comprising an optically responsive
film 12 having a multiplicity of discrete liquid
crystal droplets 14 having ~li Prs in the range from
about 0.1 to 10 times the wavelength of light to be
lO scattered dispersed in a crosslinked polymeric matrix
16 that is the polymerization product of one or more
vinyl ethers.
The vinyl ethers may be mono-functional, multi-
functional (i.e., having two or more polymerizable
15 vinyl ether moieties), or a combination thereof.
Examples of suitable vinyl ethers include hydroxy butyl
vinyl ether (HBVE, commercially available from
International Specialty Products, Wayne, NJ); 1,4-
cyrl nhPYAnP~ t~-anol divinyl ether (CHVE, commercially
20 available from International Specialty Products, Wayne,
NJ); propenyl ether of propylene carbonate (PEPC,
commercially available from International Specialty
Products, Wayne, NJ); triethylene glycol divinyl ether
(DVE-3, commercially available from International
25 Specialty Products, Wayne, NJ); butanediol divinyl
ether (commercially available from BASF, Parsippany,
NJ); vinyl ethers commercially available from Allied-
Signal Corp., Morristown, NJ under the tradename
"Vectomer" (e.g., Vectomer 2010, 2020, 4010, and 4020);
30 vinyl ether-maleate mixtures commercially available
from DSM Resins U. S ., Inc., E lgin, IL under the
trAdename "Uralac" (e.g., Uralac 3004-102 and 3004-
300); and fluorinated vinyl ethers (e.g.,
C~F~S02N(C2H5)CH2CH20CH=CH2 prepared according to the
35 ~L~C dUL~ described in U.S. Patent No. 3,078,245).
W0 95/29967 2 1 8 9 0 2 3 ~ R
.
_g_
The vinyl ethers may be u6ed alone or, more
preferably, co-reacted with one or more multi-
functional reactants and/or one or more mono-functional
reactants. Examples of materials with which the vinyl
5 ethers can be polymerized to form matrix 16 fall into
the following classes.
(1) Class I includes mono-functional and multi-
fllnr~inn;ll non-vinyl ether enes such as acrylates,
methacrylates, allyls, acrylamides, methacrylamides,
10 vinyl silanes, maleates, fumarates, or combinations
thereof .
Examples o~ mono-functional acrylates and
methacrylates include acrylate and methacrylate esters
of non-tertiary alkyl alcohols, the molecules of which
15 have from 1 to about 14 carbon atoms. Included within
this class of matrix reactants, are, for example,
isooctyl acrylate, isononyl acrylate, 2-ethylhexyl
acrylate, decyl acrylate, dodecyl acrylate, n-butyl
acrylate, hexyl acrylate, isooctyl methacrylate, and
20 lauryl methacrylate.
Examples of multi-functional acrylates and
methacrylates include 1,6-h--YAn~ ioldiacrylate,
trimethylpropane triacrylate, propylene glycol
n;- ^-rylate, pentaerythritol tetraacrylate, and 1,2-
25 ethylene glycol diacrylate.
Examples of mono- and multi-functional allyls
include mono-, di-, and triallyl '- and allyl
a - cnnt:~ i n i n~ an hydroxyl group reacted with a
mono- or multi-fllnrtinn~l isocyanate, e.g., triallyl
30 iso~y~ ul-te~ trimethylolpropane diallyl ether, allyl
benzene, allylcyclnh~y~ne~ diallyldiphenylsilane, and
allyl-functional nl i~, =i such as 9460 commercially
available from Mnn- -Polymer & Dajac Laboratories,
Inc., Trevose, PA.
Examples of mono-functional acrylamides and
methacrylamides include N,N-dimethylacrylamide, N,N-
Wo95129967 2 1 89Q23
--10--
diethylacrylamide, N-dodecylmethacrylamide, and N-
ethylmethacrylamide .
EYamples of multi-functional acrylamides and
methacrylamides include 1,6-hexamethylenebisacrylamide,
5 N,N' o.;i thylene-bisacrylamide, 1,6-
h~YA- yl~-nPb;~ ~hacrylamide, N,N-iso-valerylidene-
bis ~ hAcrylamide~ and m-xylene-bisacrylamide.
Examples of mono-functional vinyl silanes include
vinyltrimethylsilane, vinyltrimethoxysilane,
10 vinyltris(trimethylsiloxy)silane, and sil~lYAn~ such as
that commercially available from HUls America under the
trade designation "PS408. "
Examples of multi-functional vinyl silanes include
trivinylmethylsilane, 1, 3-divinylte LL ~hyldisiloxane,
15 1, 3-divinyl-1, 3-diphenyl-dimethyldisiloxane,
divinyldimethylsilane, divinyldiphenylsilane, 1,1,3,3-
tetravinyldimethyldisiloxane, tetravinylsilane, and
1, 3, 5, 7 -tetravinyltetramethylcyclotetrA e:i 1 oYAnp
Also suitable are ene-functional si l~YAn-~C such as
20 acryloyl-functional s;lnYAn~ (e-g-, 1,3-bist(P-
~crylu~y ~lly1)phenethyl]t~ ~L ~ hyldisiloxane);
methacryloyl-functional silnyAn~ (e.g., 1,3-bis(3-
methacrylu..y~- u~yl) tC~ yldisiloxane and e:; 1 oYAn~
such as that commercially available from HUls America
Z5 under the trade designation "PS406"); allyl-functional
siloxanes (e . g., the hydrolysis product of
allyldimethylchlorosilane); vinyl-functional ~iloyAn~s
(e.g., 1,3-divinylte~L hyldisiloxane, 1,3-divinyl-
1, 3-diphenyl-dimethyldisiloxane, 1, 1, 3, 3-
30 tetravinyl~ yldisiloxane~ and 1,3,5,7-
tetravinyltetramethylcyclotetrasiloxane); and hexenyl-
f uncti ona 1 s i 1 o Y~ nc~ ~: ( e . g ., 1, 3 -bis ( 6 -hex- 1-
enyl) t~ yldisiloxane, which is the hydrolysis
product of 6-hex-1-enyl~1i ylchlorosilane).
Also useful are allyl or (meth) acrylated oligomers
of polyurethanes, polyesters, polyols, alkylene oxides,
WO 95~29967 1~~ K l~c~
2 1 ~ 3
--11--
polybutadienes, or epoxies. An example of a suitable
acrylated polybutadiene is SARTOMER CD 5000
(commercially available from Sartomer Co. ) . A useful
acrylated polyester i6 SARTONER 609 (from Sartomer Co. )
5 and a suitable acrylated polyurethane is SARTONER 9610
(Sartomer Co. ) . Other useful acryl ~ 1 i 3 ~ include
those sold under the trade name "Ebecryl" by Radcure
Specialties and the trade name "Photomer" from Diamond
Shamrock .
(2) Class II includes multi- and mono-fllnrtir~
thiols. Examples of suitable mono-functional thiols
include isooctyl 3 ~ opropionate. Preferred
multi-functional thiols have the general formula
Z[OCO(CH2)nSH]nn where Z is a polyvalent organic moiety
15 which is a CHo3 yLvu~ cvllLaining nucleus of a tri- or
tetravalent alcohol of the type of glycerol or
pentaerythritol, m is 3 or 4, and n is an integer
between 1 and 5, inclusive. Spe~; f ir examples include
trimethylolpropane tris(3 ~ ~Lopropionate) and
20 pentaerythritol tetra (3 v~Lopropionate) .
Al60 useful are mercapto-f-lnr~;-,n;-l E~ Y~nC~c
(e.g., poly(3 - ~d~opropylmethylsiloxane), or
ol;~ ci or copolymers thereof; 1,1,3,3-t~L thyl-
1,3-bis(3 .cl~Lopropyl)tl;~;loY~n~; and siloxanes such
25 as that commercially available from Hiils America under
the trade designation "PS405n).
The thiols may be ;nrll-A~A as part of W
polymerizable systems based on thiol-ene chemistry in
which one or more multi- or mono-fllnrt jr~n~l enes (e.g.,
30 a mono- or multi-fllnrt;~n~l allyl, acrylate,
methacrylate, or combination thereof ) reacts with the
thiol. Commercially available materials based upon
thiol-ene chemistry include NOA 65 and NOA 68, each of
which includes a photoinitiator and is available from
35 Norland Products, Inc. New Brunswick, New Jersey, and
compositions commercially available under the trade
WO 95~9967 r~ C I~'iR
21 89az3 - ~
--12--
designation RCC-15C, RCC-lSD, RCP-611, and NCC-2B from
W. R. Grace & Co ., Atlanta , GA .
(3) Class III includes multi- and mono-functional
silicon hydrides. Examples of suitable mono-functional
5 silicon hydrides include trimethylsilane and
dimethylphenylsilane. Examples of suitable multi-
functional silicon hydrides include dimethylsilane,
diphenylsilane, and methylphenylsilane. Also suitable
are hydrosiloxanes (e.g., 1,1,3,3-
10 tetramethyldisiloxane; 1,3,5,7,9-
pentamethylcyclopentasiloxane;
phenyltris ( dimethylsi loxy ) s i lane; and 1, 3, 5, 7 -
tetramethylcyclotetrasiloxane) .
(4) Class IV includes multi- and mono-functional
15 alcohols. Examples of suitable multi-functional
alcohols include those having molecular weights between
200 and 3000 g/mol, e.g., polyethylene oxide diols
commercially available from Aldrich Co., Milwaukee, WI ;
diols commercially available under the trade
20 designation "Terathane" from E. I . du Pont de Nemours &
Co., Wilmington, DE; and "Tone 0201" commercially
available from Union Carbide, Danbury, CT. r l~c of
suitable mono-fllnr~t;nnAl alcohols include 1-octanol, 1-
decanol, and 1 Aocle~ ol. Also useful are carbinol-
25 fl]n~tinnll silny~n~c (e.g., 1,3--bis(4-
l~ydLu~yLuLyl)t~:LL LhylAi~ilnY~n~ and 1,3-
bis (l~ydlu~Ly~Lùpyl) tetramethyldisiloxane) .
(5) Class V innlllA~c epoxie6. Examples of
suitable epoxies include Bostik 7575 commercially
30 available from Emhart rh~,~iC~Al Group and Epon 828
commercially available from Shell Oil Co. Also useful
are epoxy-functional SilnY~nl~c (e.g., 1,3-
bis(glycidc,,.y~Lùpyl) tetramethyldisiloxane) .
To enhance the T-peel adhesion of optically
35 responsive film 12, one or more copolymerizable acid
reactants may copolymerized with the vinyl ether.
WO95l29967 2 1 8 9023 ~ 8
--13--
Examples of suitable acid reactants include unsaturated
carboxylic acids (e.g., acrylic acid, methacrylic acid,
crotonic acid, vinyl acetic acid, itaconic acid, maleic
acid, fumaric acid, allylacetic acid, rin ;r acid,
5 and ullsclLuL~Led acid-terminated polyester ,,l i5 ~);
mono-esters of unsaturated dicarboxylic acids (e . g.,
mono-esters of maleic and fumaric acid); hydroxy-
functi~n~l;70~ carboxylic acid6 te.g., 4 ~lydLu~yb~llzoic
acid); mercapto-functionAl i 90~1 carboxylic acids (e.g.,
10 3 c.pLopropionic acid); and sulfonic acids (e.g.,
l~ydLu~ y~ollsonp~ulfonic acid and sulfanilic acid) . The
amount of acid reactant preferably is not so high as to
yield a brittle inf lexible f ilm with a relatively low
T-peel strength and/or a f ilm with a high degree of
15 memory (as defined below).
Matrix reactants falling within any particular
class may be used in combination with each other or in
combination with materials in the other classes. The
particular choice of matrix reactant (or combination
20 thereof ) will depend upon the desired physical
characteristics of the final film. For example, the
matrix reactants may be chosen such that the ref ractive
index of the polymerized matrix (inrl~ ;n~ dissolved
liquid crystal) matches the ordinary index of
25 refraction (n") of the liquid crystal material. In
addition, if the vinyl ether reactant(s) is co-reacted
with any of the aforementioned classes of matrix
rPArtAnt~:~ e.g., alcohols, then other _ '~ that
react only with the co-reactant, e.g., isocyanates, may
3 0 be used .
In choosing the amounts and identities of matrix
reactants, several criteria generally apply. First, it
is desirable to choose matrix reactants to adjust
polymerization rate (and thereby optimize, e.g., haze,
35 switching voltage, and droplet ~LLU~:LUL? of the PDLC
film 12, as well as allow the use of lower liquid
WO 95/29967 2 ~ 89023 r~I/u.. c '~R
--14--
crystal contents). For example, allyls, vinyl silanes,
vinyl ethers without maleates, and methacrylates tend
to homopolymerize free-radically very slowly and
therefore should preferably be used in combination with
5 co-reactants that sustain and increase the
polymerization rate. Such a combination will allow a
high degree of conversion to be reached in a reAconAhle
length of time. Examples of such co-reactants include
acrylates, acrylamides, vinyl ether/maleate mixtures,
10 and thiols. Ilowever, the amount of thiol is preferably
limited (e.g., not to exceed about 2096 by weight)
21nd/or the functionality of the thiol is preferably
high to avoid production of a relatively low molecular
weight matrix and/or a matrix having a relatively low
15 degree of crosslinking. This is because thiols are
chain transfer agents that can terminate propagating
polymer chains; t:~V~:l, thiols do not readily
homopolymerize. In addition, when a relatively high
percentage of slow-reacting reactants (e.g., allyls,
20 vinyl silanes, or methacrylates) is used, the slow-
reacting reactants preferably should have relatively
large equivalent weights (e.g., oligomers) and the rate
su6taining co-reactants should be of relatively low
equivalent weight (e.g. ' D) .
A second criterion relates to the functionality of
the reactants. Specifically, it is desirable that at
least some of the vinyl ether reactants and/or some of
the ene reactants are multifunctional in order to
produce a crosslinked matrix. Crr~ccl inkinrJ increases
30 the resistance to damage caused by extreme temperatures
and further reduces "memory. " "Memory" refers to the
change in zero-volt opacities before and after the
device has been powered. Generally, the opacity
dif f erence ( and thus the contrast between the on- and
35 off-states) is greatest the first time the PDLC device
is operated. On the other hand, too high a
WO 9!i/29967 r~ cx
~ 2~ 89023
--15--
crsCcl inl~in~ level i5 undesirable because it shifts the
switching voltage to higher voltages. The amount of
multi-functional reactant(s) required will depend upon
the ~LU~;~ULe and functionality of the particular
5 reactant (s) . Low molecular weight and/or high
functionality (i.e., low equivalent weight) reactants
(e.g., hoY~np~ l diacrylate) and reactants with more
rigid backbones between functional groups (e.g., 1,4-
cycl~hoY;~no~i h;~n~l divinyl ether) are preferably
10 used at lower levels than flexible and/or high
equivalent weight reactants (e.g., triethylene glycol
divinyl ether). In addition, polar mono-flln~ti~n~l
reactants such as acrylic and methacrylic acid act as
wea~ crosslinkers through llyd~ly~ll bonding.
It has also been found that optical properties
such as haze can be minimized by optimizing the
refractive index of the matrix reactants relative to
that of the liquid crystal material. For example, it
has been found that optimizing the relative levels of
20 isooctyl acrylate and 2-phen~ y~:~l-yl acrylate (i.e.,
replacing some of the isooctyl acrylate with 2-
pheno~ye~-yl acrylate, or vice-versa), minimil~oc haze
in the powered PDLC device.
The following combinations are specific examples
25 of useful matrix combinations:
(a) RCC-15C obtained without initiator and with
50~ less thiol (W.R. Grace & Co. ), isooctyl acrylate,
acrylic acid, triethylene glycol divinyl ether, and 2-
phenoxyethyl acrylate;
(b) isooctyl acrylate, Vectomer 2010 (Allied-
Signal Corp.; vinyl ether oligomer), Uralac 3004-102,
acrylic acid, and trimethylolpropane tris (3-
mercaptopropionate);
(c) isooctyl acrylate, Vecto~er 2020 (Allied-
35 Signal Corp.; vinyl ether ~l i; ), acrylic acid,
W095129967 r~ o~3~
2t89(~23 - ~
--16--
Uralac 3004-102, 2-phenoxyethyl acrylate, and
trimethylolpropane tris (3 ~ ~ opropionate);
(d) isooctyl acrylate, Vectomer 2020, acrylic
acid, 1,4-cyclohexane dimeth~nnl-iivinyl ether, 2-
5 phenoxyethyl acrylate, and trimethylolpropane tris (3-
mercaptopropionate);
(e) isooctyl acrylate, Uralac 3004-300 (DSM Resins
USA, Inc.; vinyl ether/maleate oligomer), acrylic acid,
Uralac 3004-102, and trimethylolpropane tris(3-
10 mercaptopropionate);
(f) isooctyl acrylate, Uralac 3004-300, acrylic
acid, 2 phenu.sy~Lhyl acrylate, and Uralac 3004-102;
(g) isooctyl acrylate, Vectomer 4010 (Allied-
Signal Corp.; vinyl ether monomer), methacrylic acid,
2 phe~ yetl~yl acrylate, and Uralac 3004-102;
(h) isooctyl acrylate, lauryl methacrylate,
Vectomer 4020 (Allied-Signal Corp.; vinyl ether
monomer), methacrylic acid, 2-phenoxyethyl acrylate,
zlnd Uralac 3004-102;
(i) isooctyl acrylate, 9460 (~ ~r -Polymer &
Dajac Laboratories, Inc.; allyl-functional oligomer),
acrylic acid, 2 phellG..y~:L~-yl acrylate, and Uralac 3004-
102; and
(j) isooctyl acrylate, Vectomer 2020, acrylic
25 acid, 2-phenoxyethyl acrylate, Uralac 3004-102, and
diethyl fumarate.
Liquid crystal materials useful in forming the
droplets 14 may be nematic or cholesteric.
Furthermore, they may have either positive or negative
30 dielectric anisotropy. Particularly preferred (in the
case of light modulating devices for automotive and
architectural applications) are nematic liquid crystal
materials having positive dielectric anisotropy.
Commercially useful examples of such liquid crystal
35 materials include LICRISTAL E7, BL006, BL009, ML1005,
NL1008, 17151, 17153, 1731S, 17722 (5~ i - available
Wo 95l29967 ~ ~IJ~J.. 'C '~R
~ 21 8qO23
--17--
under the trade designation BLo38), and 17723
(= -t;r-- available under the trade designation
BLo36), all of which are available from EM Industries,
Hawthorne, New York. Mixtures of these liquid crystal
5 materia~s may also be used. Low birefringence liquid
crystal mixtures may be used as well , e. g., to provide
a wider viewing angle.
Formation of an optically responsive film
according to the invention i6 typically carried out in
10 a phase separation process. Polymerization induced-
phase separation has been found to be useful when the
uncured polymer matrix material is mi ~C; h~ ~ with a low
molecular weight liquid crystal material. Liquid
crystal droplets form when the 501llh; 1 ;ty of the liquid
15 crystal material in the polymer matrix material
decreases as a result of an increase in the molecular
weight of the matrix material that occurs when the
matrix material polymerizes to f orm a continuous phase .
As the solubility of the liquid crystal material
20 decreases, it phase separates from the polymer matrix
material and forms droplets. The droplets increase in
size and/or purity until the polymer matrix material
locks in the f inal droplet morphology. The
polymerization is carried out in the presence of the
25 liquid crystal material, thereby ~n5~hl; n~ tailoring of
the polymer matrix in terms of molecular weight,
crosslink density, liquid crystal compatibility, and/or
~lhPs;~
Although many polymer matrix material/liquid
30 crystal combinations according to the invention form
m;c:c~;hl~- mixtures at room t c~LuLe~ in others it may
be n~c~55~ry to heat the combination slightly to form a
solution and prevent pL ~ tUL ~ phase
separation .
Matrix 16 can be prepared by thermal-initiated
polymerization of the polymer matrix material or, more
W0 95/29967 F ~ - / L ~. 'C ~ iR
21 89023
--18--
preferably, by photo-initiated polymerization of the
polymer matrix material using low intensity W
radiation. Generally, the amount of photoinitiator is
from about 0 . 01 part to about 10 parts per 100 parts of
5 polymer matrix material by weight. Useful
photoinitiators and/or catalysts may be of the free
radical or cationic type. Examples of suitable free
radical photoinitiators include the benzoin ethers,
substituted benzoin ethers such as benzoin methyl ether
10 or benzoin isopropyl ether, substituted A~etorhPnnnQ~:
such as 2,2-diethoxy-acetoFh~n~n~, and 2,2-~ Yy-2-
phenyl-acetorh~nonF~, substituted alpha-ketols such as
2-methyl-2 ~IydLu~y~Luuiorh~n-~n~ aromatic sulphonyl
chlorides such as 2-naphthalene sulphonyl chloride, and
15 photoactive oximes such as l-phenyl-1, 1-yLu~anedione-2-
(O-ethoxycarbonyl) oxime. Other suitable free radical
polymerization initiating systems which may be used to
effect the polymerization include 2, 4-bistrichloro-
methyl-6-substituted-s-triazines, and ~--n70~h~nnne with
20 an amine, for example, h~n7orh~nr~nP and p-(N,N-
diethylamino) ethyl benzoate. r 1~ of cationic
photocatalysts for effecting polymerization include
' onium sa lts ( e . g ., dipheny l i~l on i ~m
hexafluuLu~hA~Lh~te and triphenyl sulfonium
25 hexafluoroantimonate) and Lewis acid catalysts (e.g.,
cyclopentadienyl iron xylene hexafluororhnsrh~te).
Sensitizers such as phenanthrene may be used in
conjunction with the photocatalysts and/or
photoinitiators as well.
Low inten6ity W lamps with different spectral
L~1J " ~"8 are commercially available and may be used.
The lamp should be selected such that the maximum
output of the lamp is near the maximum absorption of
the initiator. Fluorescent lamps (e.g., F40T12-350BL
35 lamps commercially available from Osram Sylvania,
Danvers, MA) in which the intensity of each lamp bank
W09~29967 P~~ R
~ 21 89023
19
i6 in the range of about 0 . 25 to 10 mW/cm2 (more
preferably in the range of about 0 . 5 to 5 mW/cm2) are
suitable for this application. The total radiation to
which the polymer matrix material is exposed preferably
5 is in the range of about 100 to 1500 mJ/cm2. The
particular radiation intensity and total energy
e:A~ODuL~ requirements will vary ri~rPn~in~ on the liquid
crystal, initiator, and polymer matrix materials.
Preferably, the liquid crystal material and the
10 polymer matrix material are provided in approximately
equal parts by weight, although the parts by weight of
the liquid crystal material can vary from 10-90% by
weight, even more preferably from 25-75% by weight.
The optimum liquid crystal content is within 5% by
15 weight of the 10ilc~,lL, ~Ition in which a further 5% by
weight increase in liquid crystal content would yield a
film in which the color of transmitted white light
would change from slightly red to white.
Referring again to FIG. 1, although the optically
20 responsive film 12 may be provided in free-standing
form, in many applications it will be desirable to
provide a sandwichlike construction in which the film
12 is interposed between a pair of f irst and second
substrates 18 and 20, respectively. The thit~kn~ s of
25 the film preferably ranges from about 5 to 25 microns,
more preferably in the range of about 10 to 25 microns,
and most preferably in the range of about 15 to 21
microns. It will be understood that the device 10 may
be provided with only a single substrate if, for
30 example, the device is to be applied to a motor vehicle
sunroof or an architectural window in which case the
sunroof or the window have a function analogous to that
of the second substrate.
At least one of the substrates 18 and 20 is at
35 least partially tran~alel.t to allow incident visible
light to pass therethrough. One of the substrates
Wo9s/29967 21 89023 ~ o t~8
--20--
(preferably the one which light first impinges) may be
modif ied to have selective light transmission
characteristics, for example, to selectively transmit
light of a wavelength ~UL~ l;n~ to a certain color
5 of the visible ~e~iL,u.l~, ultraviolet light, or infrared
light. Materials suitable for the substrates 18 and 20
include gla6s (which may be tempered) and plastics such
a5 polyethylene tt~ IL~lalate, polyethylene
naphthalate, or other polyester or copolyester
o materials, polyethersulfone, polyimide, poly(methyl
methacrylate), and poly~ .ate. The substrates may
be treated 80 as to enhance their abrasion and scratch
resistance. The sub~L~tes are typically about 25 to
50 microns thick for flexible, durable constructions,
15 although they may range in thickness from 1 microns to
greater than 250 microns. If glass is employed for at
least one of the substrates, the thinknpcs may be in
excess of 250 microns.
With continued reference to FIG. 1, in order to
20 induce a change in the orientation of the liquid
crystal droplets BO as to cause the optically
re6ponsive film 12 to switch between the translucent
off-state and the transparent on-state, it is necessary
to apply an electric f ield acrsss the f ilm 12 (the f ilm
25 12 may also be switched by applying a magnetic f ield
across the same or by raising the t~ elLuLc: of the
film above the clearing point t~ c.Lu,~: of the
~nn~rClllated liquid crystal). Accordingly, the device
10 may further comprise first and second ele.:~,udes 22
30 and 24, respectively, which are positioned intermediate
the substrates 18 and 20 and the optically responsive
film 12. The electrodes 22 and 24 are cnnnPrtPC~ to,
respectively, first and second leads 26 and 28 (e.g.,
using the cnnnPntnr described in PCT International
35 application No. PCT/US93/12128, entitled "Electrical
CnnnPctor", which, in turn, are electrically connected
W0 95129967 1~
~ 21 89023
--21--
to a variable power supply 30, preferably of the
alternating current type (e.g., a zero-cross power
supply). Preferably, the rL~UUI:111y of the alternating
field 6hould be in the range of 40 to 100 Hz. The
5 f ield 6hould alternate suf f iciently rapidly 60 that a
human obsel~ CL of the device cannot perceive
flickering. Thu6, upon application of an electric
field across the film 12, the optic axes of the liquid
cry6tal droplets become aligned. If the refractive
lo indices of the liquid crystal material and the polymer
matrix have been closely matched, the film 12 will
switch between the tr~n~ lc~nt off-state and the
transparent on-state.
The electrodes 22 and 24 may be formed of various
15 materials ;nt lllAin~ chromium, indium oxide, tin oxide,
stainless steel, indium tin oxide, gold, silver,
copper, aluminum, titanium, cadmium stannate,
transition metal oxides, and mixtures and alloy6
thereof. With the use of sY;~;7~hle electrode
20 materials (e.g., silver) it may be desirable to
environmentally protect the same with a thin
passivating dielectric layer. The use of such a
protective layer may enhance the ability of the
electrode to resi6t thermal, r~.h~ moi6ture and/or
25 ultraviolet-induced degradation 6uch as i6 tl;~clos-~A in
PCT International application No. PCT/US92/10332,
entitled "Light Modulating Devices Inc~u~oLc~ting an
T ~ .d Electrode~. The electrode6 must be capable of
receiving an electrical input from the leads 26 and 28
30 and transmitting the same so as to apply an electric
field across the film 12. Preferably the electrodes 22
and 24 are positioned adjacent to opposite sides or
surfaces of the film 12 and extend over, across and
parallel to the same.
At least one of the electrodes 22 and 24
preferably is at least partially transparent to visible
Wo 95~9967 P~ C l~
21 89023
--22--
light, although electrodes which provide preferential
light transmi6sion characteristics, such as color tint
or ultr~violet or infrared ~ilter, may be u6ed. The
electrodes 22 and 24 need not be equally LL~I.Dl,a~c:..L.
5 At least one of the ele~ ~Lodes should provide a visible
light transmission of at least 1~, preferably at least
10%, and more preferably at least 50%. The electrode
coating should have a conductivity greater than 0. 001
mhos per 6quare. The electrode material may be coated
10 or otherwise applied to the f irst and second sub6trates
18 and 20. Where only one of the substrates and one of
the ele~ L,~,des is transparent, the transparent
substrate and transparent electrode should be on the
same side of the device.
In operation, a user of the device 10 applies an
electric ~ield across the f ilm 12 using power supplied
by power supply 30, thereby rendering the device
transmissive to light.
Whether the light modulating device is supplied as
20 a free-standing film, with one substrate, or with two
substrates, the device may be applied to a sur~ace such
as a motor vehicle sunroof, a motor vehicle side
window, or an architectural window with, for example,
suitable adhesive; preferably, the adhesive i8
25 optically transparent. As the device switches between
the translucent off-state and the transparent on-state
( in the case of nematic liquid crystal material having
positive dielectric anisotropy), the device preferably
has a uniform, even appearance.
The invention will be more fully understood with
references to the following examples which are not to
be construed as limiting the scope of the invention.
E~aMPLE~
The following examples describe the preparation of
35 light modulating devices based upon optically
responsive PDLC films. In Examples 12-23 the device
WO95/299C7 P~
2 ~ 8~023
--23--
was prepared by first ~gA~sinlJ an unpolymerized
composition of matrix reactant(s) and liguid crystal
and then pumping the composition to a coating die
through which the compo6ition was extruded onto the
5 electrode side of an approximately 51 micron thick
indium-tin oxide (IT0)-coated polyester film (90/10
indium/tin, 80 ohms/sguare, commercially available from
Southwall Terhnnl ogies, Palo Alto, CA) according to the
process described in greater detail in PCT
10 International application No.
(Attorney's Docket No. 50778PCT5A) entitled "Precision
Coating Process for Preparing Polymerizable Films"
filed C~aICULLC~ 1Y with, and Acsj~n~ to the same
~ccignee as, the present application.
The coating die 40 is shown in Figure 2. The
unpolymerized composition 44 was supplied by a pump 46
to the die 40 for application in the form of a
continuous coating bead to the moving IT0-coated
polyester film 48, supported by a backup roll 50. The
20 backup roll 50 was a pacer roll driven by a Torquer
TArh~ teL precision motor (available from Inland Motor
Division, Bradford, VA). The temperatures of the die
and backup roll were controlled by circulating a
t~ a~uL~ controlled fluid through them. Where
25 indicated in the examples, vacuum was applied to vaccum
chamber 42 to stabilize the coating bead. The
unpolymerized composition 44 was supplied through a
channel 52 to a manifold 54 for distribution through a
slot 56 and coating onto the moving film 48. The
30 height of slot 56 was controlled by means of a U-shaped
shim 41 (typically made of brass or stAinl~Cc steel).
Referring to Figure 3, die 40 consisted of an
upstream bar 64 and a d~ ~L~am bar 66. The lip of
the u~ al~ bar was formed as a curved land 68 and the
35 lip of the Leam bar was formed as a substantially
straight sharp edge 70 having an edge radius n~ greater
W095/29967 ~ J. s/ol~
21 ~qo23
--24--
than 10 microns. The radius of the curved land 68 was
equal to the radius of the backup roll 50 plus a
minimal, and non-critical, 0.13 mm allowance for
coating gap and f ilm th i rknP~:5 .
The length L~ of the curved land 68 on the u~=~-Lehlu
bar 64 was 12 . 7 mm and the length L2 Of land 82 was 12 . 7
mm. The edge angle A~ of the downstream bar 66 was 50-
60O. The die attack angle A2 between the ~' ,. LLeam bar
66 surface of the coating slot 56 and the tangent plane
10 P through a line on the film 48 surface parallel to,
and directly opposite, the sharp edge 70 was 95O.
The coating gap G~ is the distance between the
sharp edge 70 and the film 48. Slot height H is the
distance between u~LLe~ l bar 64 and downstream bar 66,
15 and was controlled by controlling the thickness of shim
41. The slot height u6ed in the examples was 0.152 mm.
Overbite O i6 a positioning of the sharp edge 70 of the
` ...._LLea~l, bar 66, with respect to the downstream edge
72 of the curved land 68 on the u~LL~.IIu bar 64, in a
20 direction toward the film 48.
Cu..veLyel~ce C is a counterclockwise, as shown in
Figure 3, positioning of the curved land 68 away from a
location parallel to the f ilm 48, with the downstream
edge 72 being the center of rotation. In the examples,
25 cullveLye~ce was 0 . 57 .
Vacuum land gap G2 was 152 microns.
Figure 4 is ~_LUSs s~_Lional view of the extrusion
die used to prepare f ilms according to the invention
and shows an alternate conf iguration where the vacuum
30 bar 74 is isolated from the bottom die bar 65 by a
fleYible metal 6eal 88. This configuration allows
adjustment of the coating gap G~ and cu.lveLy~ e C
without affecting the vacuum land gap G2-
The width of the coating produced by a given die
35 was reduced where indicated by ~ rkl ;ng~ the die andthe vacuum chamber by Cull~ ULLellLly incorporating a)
Wo 9~29967 P~~ l0 l'~
2 ~ 89023
--25--
shaped plugs to reduce the widths of the die cavity
manifold 54 and vacuum chamber 42 to the ~Prkl inq width
and b) a shim into the die that has a shim slot width
CC~L L ~ ; nq to the ~Qr~kl i nq width .
A second ITO-coated polyester f ilm was unwound
from a second unwind roll and passed around a 2 . 54 cm
diameter sintered metal laminator bar where the second
film was laminated to the coated face of the first film
according to the ~- oce~uLe described in PCT
10 International application No.
(Attorney's Docket No. 50777PCT7A), entitled
"Lamination Process for Coating" filed ac-l-uurLe~ ly
with, and assigned to the same assignee as the present
application. The laminator bar was located
15 approximately 12 cm downstream from the backup roll
such that the coated f ilm was not in contact with the
backup roll or other idler or takeup rolls at the point
of lamination, and positioned so that the uncoated
f irst substrate was d~:~L assed below the plane def ined
20 by the first film as it passed over the backup roll and
the idler roll; the extent of depression is hereinafter
referred to as "interference. " Air PL~S~U1~
(approximately 2.4 bar) through the air bar laminator
was adjusted to provide a cushion of air between the
25 air bar laminator and the second film.
The uncured laminate cull~LLu~;~ion was cured by
passing the cc,l,;,LLu.:Lion through a cooled curing
chamber constructed of ultraviolet trar.~al~,llL
AcrylitelM OP-4 (available from Cyro Industries, Mt.
30 Arlington, NJ), extending approximately 61 cm (2 feet)
into a cure chamber es~uipped with two banks of
fluoLesc.=l.L black lights (F20T12-350BL, available from
Osram Sylvania , Danvers , MA), one bank positioned on
each side of the laminate. Air t~ clLUL-~ in the
35 cooling chamber was monitored by a thr- _ le mounted
in the chamber under the second f lu~l e scel~t bulb and
WO 95/299~7 ~ c'C l~x
21 ~9023 ~ ~
--26--
controlled at the indicated t~ cltUL~ by introducing
temperature controlled air. Each 6ide of the laminate
construction was expo6ed to approximately 250-600 mJ/cm2
of radiation calculated from light intensities measured
5 through a conductive electrode using a WIBRITE
radiometer (model number UBM365M0, available from
Electronic InaL~, ~ation and Technology, Inc.,
Sterling, VA) equipped with a gla6s filter responsive
between 300 and 400 nm, with a maximum trAnF~ )n at
10 365 nm. The rA~ Pr was sp-~iAlly calibrated to
read in absolute intensity.
In the case of Examples 1-9, the devices were
prepared using a modified version of the ~LuceduLe:
descrlbed in PCT International application No.
15 PCT/US92/00173. A puddle of unpolymerized liquid
crystal/matrix composition was placed on the moving
~urface of an IT0-coated polyester film measuring 51
microns thick just prior to the nip gap of the
precision coater, where a second IT0-coated PET film
20 entered to form a laminate in which the IT0-coated
surfaces were in a facing relationship. The
t~ ~LuLe of the nip rolls was maintained at 27C by
circulating a cooling solution from a ~:OI~aL~llL
t~ ~LUL~ bath through the rolls. The nip gap was
25 typically set between 0.11 - 0.14 mm to ~ ` Le the
1-h i c~l~n~ of the electrode materials and to allow f or
the desired PDLC matrix thickness.
After exiting the nip rolls, the sandwichlike
C~JII LLu~;Lion was cured by transporting it into a
30 temperature-controlled cure chamber where it was
irradiated with long wavelength W light for
approximately 3 minutes. The intensity of the W light
was measured by a EIT WIBRITE rA~ er model number
UBM3 65M0 a6 described above .
The resulting light modulating devices prepared
according to either of the two methods were
WO 95/299C7 ~ 0 1~C8
21 89~3
--27--
characterized by measuring the ele-:Ll~, .,~Lical response
(Test Pl~lcedu~e A) and haze (Test P~oc~-lul~ B).
Test E~ e A
The ele~.~Lv ~p~ical ~ Of the PDLC devices
5 were characterized using a computer-_u..-~ ~ lled test
6tand consisting of an IBM personal computer interfaced
with Kepco 125-lKVA-3T power supply, a Dyn-Optics
optical Monitor 590, and a Valhalla Scientific 2300
Series Digital Power Analyzer. The optics of the Dyn-
10 Optics Optical Nonitor were adjusted such that thespec~ r tr~n¢"~i ccin~ of photopically-filter light at
an approximate 6 collection half angle was measured
relative to an open beam.
A sample of a PDLC film/electrode sandwich
15 measuring several square centimeters was attached to
the leads of the power supply using a connector such as
that described in the aforementioned Engfer et al.
application. A 60 Hz voltage ranging from zero to 120
volts AC (VAC) was applied to the sample in 5 VAC 0 increments and the spec~ r transmission recorded .
Test PLOI ~.1.., e B
The haze o~ the powered (120 VAC, 60 Hz) PDLC
devices was measured using a Pacif ic Scientif ic Gardner
XL-835 Colorimeter according to the manufacturer's5 instructions.
r le 1
A PDLC device was ~LeyaIed using the "modifed
Miller p~ duL~ described above from a fluid
containing (a) 50 parts BL036 liquid crystal mixture
30 (EM Industries, Hawthorne, NY) and (b) 50 parts of the
following mixture; 2.5 wt.S Esacure KB-l photoinitiator
(Sartomer, West Chester, PA), 20. 0 wt. % Vectomer 2020
(Allied Signal Inc., Morristown, NJ), 5.0 wt.% acrylic
acid (Aldrich, Milwaukee, WI), 25 . 0 wt. % isooctyl
35 acrylate, 15 . 0 wt. % 2-phenoxyethyl acrylate (Sartomer,
West Chester, PA), 10.0 wt.96 trimethylolpropane tris(3-
WO 95/299~7 ~ c~
21 8~023 ~
--28--
mercaptopropionate) (Aldrich, Nilwaukee, WI), 11.2 wt.%
Uralac 3004-100 tDSM Resins, U.S., Inc., Elgin, IL),
and 11.2 wt.% cynlnh~YAn~ ~;r ' hAnol divinyl ether
tInternational Specialty Products, Wayne, NJ). The
5 laminate was cured by 6~ JODuLe: to W light tintensity
approximately 3.0 mW/cm2) at about 23C to produce a
PDLC film approximately 21 microns thick.
The PDLC device exhibited on- and of f -6tate
tr;~nFm;cclnnf: of 72.5% and 0.9% respectively and a haze
lO of 6. 4% .
le 2
A PDLC device was prepared as described in Example
1 from a fluid containing ta) 55 parts BL036 liquid
crystal mixture and tb) 45 parts of the following
15 mixture; 2.5 wt.% Esacure KB-l photoinitiator, 30.1
wt.% 4010-T1995 tmade from 0.09 grams KB-l, 2.82 grams
T1995 1,1,3,3-teLL yl-1,3-bis(3-
mercaptopropyl)disiloxane (H~lls America, Piscataway,
NJ), 7 . 24 grams Vectomer 4010 (Allied Signal Inc.,
20 Morristown, NJ), cured approximately 5 minutes at
approximately 3 mW/c* 365 nm W light), 7.5 wt.%
acrylic acid, 30 . 0 wt. % isooctyl acrylate, 10 . 0 wt. %
trimethylolpropane tris(3 ~ Lopropionate), and 20.0
wt.% h~yAn~ ol diacrylate (Sartomer, West Chester,
25 PA). The laminate was cured by ~ oDu~e to W light
(intensity approximately 2.0 mW/cm2) at about 23C to
produce a PDLC f ilm approximately 19 microns thick .
The PDLC device exhibited on- zmd of f -state
trAn=m;CRinn~: o~ 74.0% and 1.2% respectively.
r lo 3
A PDLC device was prepared as described in Example
1 from a fluid containing (a) 60 parts BL036 liquid
crystal mixture and (b) 40 parts of the following
mixture; 0.5 wt.% Esacure KB-1 photoinitiator, 0.5 wt.%
35 diphenyl iodonium hexafluor~phocrhAte~ 44.6 wt.%
Vectomer 4010, 44.6 wt.% hydroxy butyl vinyl ether
WO 95n99G7 r~ o
~ 2189(~2~
--29--
(International Specialty Products, Wayne, NJ), and 10. 0
wt.% B2405.5 1,3--bis(4-
lly-lLu,cyLuLyl)tetramethyldisiloxane (Hi~ls America,
Piscataway, NJ). The laminate was cured by exposure to
5 W light (intensity approximately 2. 0 mW/cm2) at about
24C to produce a PDLC film approximately 15 microns
thick .
The PDLC device exhibited on- and of f -state
transmissions of 75.1~6 and 9.3% respectively.
E2~plo
A PDLC device was prepared as described in Example
1 from a fluid containing (a) 60 parts BL036 liquid
crystal mixture and (b) 40 parts of the following
mixture; 1.8 wt.% Esacure KB-1 photoinitiator, 27.0
wt.% 2-phenoxyethyl acrylate, 10.0 wt.% Uralac 3004-101
(DSM Resins , U . S ., Inc ., Elgin , IL), 52 . 2 wt . % Uralac
3004-102 (DSN Resins, U.S., Inc., Elgin, IL), and 9.0
wt.% Uralac 3004-109 (DSM Re6ins, U.S., Inc., Elgin,
IL). The laminate wa6 cured by e~ JO~UL~ to W light
(intensity approximately 2.0 mW/cm2) at about 24C to
produce a PDLC film approximately 21 microns thick.
The PDLC device exhibited on- and of f -state
transmis6ion6 of 64.7% and 1.9% respectively.
~ mDle 5
A PDLC device was ~ c:~ared as described in Example
1 from a fluid containing (a) 80.8 part6 BL036 liquid
crystal mixture and (b) 49.8 parts of the following
mixture; 0.5 wt.% Esacure KB-l photoinitiator, 0.4 wt.%
diphenyl iodonium hexafluu, ,~ LIhAte, 62.4 wt.%
Vectomer 4010, 7.0 wt.% butyl vinyl ether (Aldrich,
Milwaukee, NI), and 29.8 wt.~6 octadecyl vinyl ether
(BASF Corporation, Par6ippany, NJ). The laminate wa6
cured by ~ JOZ:~UL~: to W light (intensity approximately
2.0 mW/cm2) at about 24C to produce a PDLC film
35 approximately 18 microns thick.
Wo ss/29967 r~ o ~
21 89~23 ~ ~ ~
--30--
The PDLC device exhibited on- and of f -state
transmissions of 7 . 2% and 1.1% respectively.
-- 1~ C
A PDLC device was prepared as described in Example
5 1 from a fluid containing (a) 45 parts BL036 liquid
crystal mixture and (b) 55 parts of the following
mixture; 1.0 wt.% Esacure KB-l photoinitiator, 1.0 wt.%
triphenyl sulfonium hexafluoroantimonate, 44.4 wt.%
Vectomer 2020, 38.7 wt.% divinyl ether of triethylene
10 glycol (International Specialty Products, Wayne, NJ),
and 14 . 9 wt. % 4-propenyloxymethyl-l, 3-2-dioYn1 Anon~
(PEPC, International Specialty Products, Wayne, NJ).
The laminate was cured by t:~CpO:.UL e to W light
(intensity approximately 2.0 mW/c*) at about 22C to
15 produce a PDLC f ilm approximately 18 microns thick .
The PDLC device exhibited on- and of f -state
transmis6ions of 51. 8% and 4 .1% respectively.
1~ 7
A PDLC device was prepared as described in Example
20 1 from a fluid containing (a) 45 parts 8L036 liquid
crystal mixture and (b) 55 parts of the following
mixture; 2.5 wt.9~ Esacure KB-l photoinitiator, 25.0%
Vectomer 2010 (Allied Signal Inc., Morristown, NJ), 7.5
wt.% acrylic acid, 15.0 wt.% isooctyl acrylate, 10.0
25 wt. % trimethylolpropane tris (3 - ~ Lopropionate), and
39 . 9 wt. % Uralac 3004-102 . The laminate was cured by
~ o.,u.~ to W light (intensity approximately 2.0
mW/cm2) at about 23C to produce a PDLC film
approximately 2 0 microns thick .
3 0 The PDLC device exhibited on- and of f -state
trAn~ i ons of 73 .1% and 1. 4% respectively .
r ln 8
A PDLC device was ~Le:~a~ed as described in Example
1 from a mixture containing (a) 60 parts BL036 liquid
35 crystal mixture and (b) 40 parts of the following
mixture; 2.5 wt.% ~Ssacure KB-l photoinitiator, 7.5 wt.%
WO 95/29967 r~l,.,, 'C ~x
~ 2189~23
--31--
acrylic acid, 20.0 wt.% isooctyl acrylate, and 70.0
wt. % Vectomer 4020 (Allied Signal Inc., Morristown,
NJ). The laminate was cured by t~O~UJ.t~ to W light
(intensity approximately 2.0 mW/cm2) at about 23C to
5 produce a PDLC f ilm approximately 15 microns thick .
The PDLC device exhibited on- and of f -state
tr;-n¢~ ion~: of 73.2% and 2.8% respectively.
~ 9
A PDLC device was prepared as described in Example
1 from a fluid containing (a) 27.2 parts BL036 liquid
crystal mixture and (b) 25.0 parts of the following
mixture; 2.5 wt.% Esacure K8-1 photoinitiator, 22.4
wt. % isooctyl acrylate, 19 . 7 wt. % 2-phenoxyethyl
acrylate, 10 . 5 wt . % C~F"S02N (C2H5) CH2CH20CH=CH2, 14 . 9 wt.
Uralac 3004-102, and 30.0 wt.% Uralac 3004-300 (DSM
Resins, U.S., Inc., Elgin, IL). The laminate was cured
by ~,spo~u,~ to W light (intensity approximately 2.0
mW/cm2) at about 24C to produce a PDLC film
approximately 11 microns thick.
The PDLC device exhibited on- and off-state
transmissions of 75 . 4% and 2 . 0% respectively.
r 1~ 10
A PDLC device was ~L~ aIed by placing a few drops
of an unpolymerized fluid containing (a) 22.7 parts
8L036 liquid crystal mixture and (b) 23.4 parts of the
following mixture; 0.2 wt.% Esacure K8-1
photoinitiator, 2.9 wt.% isooctyl acrylate, 42.7 wt.%
PS927 poly(mercaptopropyl)methylsiloxane (H~ils America,
Piscataway, NJ), and 54 . 2 wt. % Vectomer 4010 near the
30 center of the conr~ tive side of a piece of IT0-coated
glass measuring approximately 5 cm x 6. 4 cm. A square
ring of PET film measuring approximately 5 cm x 5 cm x
0.6 cm (yltc~a,tcd by first cutting a piece of PET
measuring 5 cm x 5 cm from a 0.0025 cm thick piece of
35 PET, followed by cutting a 3 . 8 x 3 . 8 square from the
center of the 5 cm x 5 cm piece) was placed on top of
Wo 95l29967
?1 ~90~3
--32--
the IT0-coated glas6. A second piece of IT0-coated
glas6 with the conductive side facing the uncured
matrix/liquid crystal mixture was laid on top of the
first IT0-coated glass piece at an approximately 90
5 orientation angle to the first piece. Manual pLe6,juL
was applied to distribute the unpolymerized mixture
between the glass pieces. The laminate was then cured
by t'X~O~ L~ to W light (intensity approximately 0.5
mW/cm2) at room temperature to produce a PDLC device.
The PDLC device exhibited on- and off-state
transmissions of 46 .1% and 0. 8% respectively.
r l~ 11
A PDLC device was prepared according to Example lO
from a fluid containing (a) 27.7 parts BL036 liquid
15 crystal mixture and (b) 26 . 0 parts of the following
mixture; 0 . 6 wt. % Esacure KB-1 photoinitiator, 57 . 3
wt.% trimethylolpropane tri6(3 ~ uaptopropionate), and
42 .1 wt. % cy~ h~YAne dimethanol divinyl ether. The
laminate was cured by exposure to W light (intensity
20 approximately 0 . 5 mW/cm2) at room temperature to produce
a PDLC device.
The PDLC device exhibited on- and of f -state
tr~n-micclt nc of 60.0% and 1.6% respectively.
r lt~ 12
A PDLC device was pL.:~altd as described in the
precision coating method above from a fluid containing
(a) 55 parts of a mixture consisting of 30.0 wt.% RCC-
15C curable matrix mixture obtained without initiator
and with 50% less thiol (W.R. Grace, Atlanta, GA), 7.5
wt.% acrylic acid, 30.0 wt.% isooctyl acrylate, 15.0
wt.% 2-pht-1.o,Ly.~hyl acrylate, 15.0 wt.% divinyl ether
of triethylene glycol, and 2 . 5 wt . % KB-1
photoinitiator, and (b) 45 parts BL036 liquid crystal
mixture having a solution viscosity of 42 cps (measured
35 on a Brookfield viscometer using a ~3 spindle operating
at 60 rpm). The fluid, which was tleqAcct~tl under vacuum
WO95/299C7 2 8q023 F~l~o. ''01~8
for approximately 2 minutes at ambient t~ ~LUL~, was
applied as a 15 . 2 cm ( 6 inch) wide strip to the
electrode surf ace of an ITO-coated polyester f ilm
t90/10 indium/tin ratio, 80 ohm6/square, 51 microns (2
5 mil) thick PET, available from Southwall TP~-hn~l ogies,
Palo Alto, CA) at a rate of approximately 152.4 cm/min
(5 ft/minute) using an 88.9 cm die similar to that
illustrated in Figure 4 which was deckled to produce a
narrower coating and conf igured with a 152 micron shim,
10 a coating land having a length (Ll) of 12 . 7 mm, a vacuum
land having a length L2 of 12.7 mm, a 0.57
a 33 micron overbite, a vacuum land gap G2 f 0.152 mm,
a die attack angle A2 f 95, and a coating gap of 102
microns. The v--ve:r~nce of the vacuum bar was 0 and
15 no vacuum was applied to the vacuum chamber during
coating. Both the die and back-up roll were temperature
controlled at 21C. A ~LasDuLa of 1.7 bar was
maintained to the sintered metal bar during lamination
and the lamination bar was adjusted to provide an
20 interference of 3 . 6 mm. The resulting laminate was
cured by O:~L~ODUL e to UV light ( intensity approximately
1.1 mW/cm2) at a~out 21C to produce a PDLC film
approximately 24+1 microns thick.
The PDLC device had on- and of f -state
25 transmissions of 73.196 and 1.2%, respectively, and a
haze of 5. 8% .
Ex~l~le 13
A PDLC device was prepared as described in Example
12 except that the fluid contained (a) 125 parts of
30 BL036 liquid crystal mixture and (b) 125 parts of the
following mixture; 2.5 wt.~ Esacure KB-l
photoinitiator, 7 . 5 wt. % methacrylic acid (Aldrich,
Milwaukee, WI), 10 . o wt. % isooctyl acrylate, 15. 0 wt. %
lauryl methacrylate (Rohm Tech, Inc., Malden, MA), 20. 0
35 wt.% 2 pl.env~y~:Lhyl acrylate, 15.0 wt.% Uralac 3004-
102, and 30.0 wt.% Vectomer 4020. The die was
W09s/29967 ~ 01~8
2~ ~9023
--34--
configured with an overbite of 48 microns. An air
pLeS~ULdl of 2.4 bar was maintained to the lamination
bar which was adjusted to provide an interference of
4 .1 mm . The resulting laminate was cured by ~L~or~uL e
5 to W light (intensity approximately 2.02 mW/cm2) at
about 22C to produce a PDLC film approximately 22-23
microns thick.
The PDLC device had on- and of f -state
trAnFm;ccions of 72.2% and 1.2%, respectively, and a
10 haze of 7.1%.
r 1~
A PDLC device was prepared as described in Example
12 except that the fluid contained (a) 112.5 parts of
BL036 liquid crystal mixture and (b) 137 . 5 parts of the
15 following mixture; 2.5 wt.% Esacure RB-1
photoinitiator, 5.0 wt.% acrylic acid, 22.5 wt.%
isooctyl acrylate, 10 . 0 wt. % trimethylolpropane tris (3-
mercaptopropionate), 30.0 wt.% Uralac 3004-102, and
30.0 wt.% Uralac 3004-300. The die was configured with
20 an overbite of 43 microns and a vacuum of 1. 9 mm Hg
was applied to the vacuum chamber during coating. An
air ~L~5 UL~ of 2.4 bar was maintained to the laminator
bar which was adjusted to provide an interference of
4.1 mm. The resulting laminate was cured by ~ JO~UL'~
25 to W light ( intensity approximately 2 . 02 mW/cm2) at
about 23C to produce a PDLC film approximately 33
microns thick.
The PDLC device had on- and off-state
transmissions of 72.9% and 1.5%, respectively, and a
30 haze of 6. 6%.
- 1~ 15
A PDLC device was prepared as described in Example
12 except that the fluid contained (a) 150 parts of
BL036 liquid crystal mixture and (b) loo parts of the
35 following mixture; 2 . 5 wt. % Esacure KB-l
photoinitiator, 7 . 5 wt. % ~ethacrylic acid, 30 . O wt. %
WO 95129967 ~ CIC '~C8
2~ 89023
--35--
isooctyl acrylate, 15.0 wt.% 2-phenoxyethyl acrylate,
15.0 wt.% Uralac 3004-102, and 30.0 wt.% Vectomer 4010.
The die was conf igured with an overbite of 18 microns
and a vacuum of 3.7 mm Hg was r-;ntAin~ to the vacuum
5 chamber during coating. An air ~JLL~c.uLe of 2.4 bar was
maintained to the laminator bar which was adjusted to
provide and interference of 4.1 mm. The resulting
laminate was cured by ~A~O'-uL e to W light ( intensity
approximately 1. 99 mW/cm2) at about 21C to produce a
10 PDLC f ilm 18 microns thick .
The PDLC device had on- and off-state
transmissions of 71.1% and 1.7%, respectively, and a
haze of 7 . 9% .
le 16
A PDLC device was prepared as described in Example
12 except that the fluid contained (a) 135 parts of
BL036 liquid crystal mixture and (b) 165 parts of the
following mixture; 2 . 5 wt. % Esacure KB-1
photoinitiator, 25.0 wt.% Vectomer 2010, 7.5 wt.%
20 acrylic acid, 15.0 wt.% isooctyl acrylate, lo.O wt.%
trimethylolpropane tris (3 ~d~.Lopropionate), and 40 . 0
wt.% Uralac 3004-102. The die was configured with an
overbite of 41 microns and a coating gap of 71 microns.
A vacuum of 4 . 3 mm Hg was applied to the vacuum chamber
25 during coating which was carried out at 29 oc and a
speed of approximately 0 . 9 meters per minute. An air
p~ UL -~ of 2 . 4 bar was maintained to the laminator bar
which was adjusted to provide an interference of 3.1
mm. The resulting laminate was cured by eA~JO_UL'2 to W
30 light (intensity approximately 2 . 01 mW/cm2) at about
21C to produce a PDLC film approximately 30 microns
thick .
The device had on- and off-state trAn--niccionC of
72.6% and 1.2%, respectively, and a haze of 5.8%.
WO 9~129967 r~"~J~, 5 ~4~
--36--
Ex~m~l~ 17
A PDLC device was prepared as described in Example
12 except that the fluid contained (a) 135 parts of
BL036 liquid crystal mixture and tb) 165 parts of the
5 following mixture; 2.5 wt.% Esacure RB-1
photoinitiator, 10.0 wt.% Vectomer 2020, 7.5 wt.~
acrylic acid, 17 . 5 wt. % isooctyl acrylate, 12 . 5 wt. % 2-
phGn~.~.yGL~Iyl acrylate, 10.0 wt.% trimethylolpropane
tris(3-mercaptopropionate), and 40.0 wt.% Uralac 3004-
10 102 . The die was conf igured with an overbite of 25microns and a coating gap of 76 microns. The die
temperature was maintained at 26.4C and a vacuum of
0 . 9 mm Hg was applied to the vacuum chamber during
coating. An air ~LG6~ULG of 2.4 bar was maintained to
15 the laminator bar which was adjusted to provide an
interference of 3 . l mm. The resulting laminate was
cured by G~O~jULG to UV light (intensity approximately
2.0 mW/cm2) at about 25C to produce a PDLC film
approximately 28-29 microns thick.
The PDLC device had on- and off-state
transmissions of 73.9% and 1.2%, respectively, and a
haze of 5%.
~SX mpl~ 18
A PDLC device was ~LGpa~ed as described in Example
25 12 except that thG coating fluid had the following
Composition: (a) 50 parts of a mixture consisting of
20.0 wt.% Vectomer 2020, 5.0 wt.% acrylic acid, 25.0
wt.% isooctyl acrylate, 15.0 wt.% 2-phG~ G-~Iyl
acrylate, 10 wt. % trimethylolpropane tris (3-
30 mercaptopropionate), 22.5 wt.% cyrlnh~y~n~ dimethanoldivinyl ether, and 2.5 wt.% Escacure RB-1, and (b) 50
parts BL036 liquid crystal mixture. The viscosity of
the coating fluid was 134 cps (measured on a Bronkf;~
viscometer using a ~3 spindle operating at 60 rpm).
35 The coating temperature was 21C and during lamination
an air ~Le5:~uLG of 2.4 bar was maintained to the
W095l29967 2 1 8 9023 1~"~ c ~
--37--
laminator bar which was adjusted to provide an
interference of 3.8 mm. The fluid was applied a6 a
15.2 cm (6 inch) wide strip to the electrode surface of
an ITO-coated polyester film at a rate of approximately
5 152.4 cm/min (5 ft/minute) using the precision coating
process described in Example 7 except that a 4 6 micron
overbite, a coating gap of 102 microns, and a vacuum of
1. 9 mm Hg ( 1 inch of water) was used to apply the
solution at 22C. The film was cured at 21C by
10 exposing each side to approximately 530 mJ/cm2 at an
intensity of 1. 0 mW/cm2 to produce a PDLC film with a
th; rl-n-~cc of 23+1 miCronS.
The PDLC device had on- and of f -state
trln~~niC:si~nc of 71.9% and 1.1%, respectively, and a5 haze of 4 . 8S .
r le 19
A PDLC device was prepared as described in Example
12 except that the fluid contained (a) 220 parts of
BL036 liguid crystal mixture and (b) 180 parts of the
20 following mixture; 2.5 wt.% Esacure KB-l
photoinitiator, 30.0 wt.% 9460 allyl aliphatic urethane
(Ml~nl -Folymer & Dajac, Trevose, PA), 7.5 wt.S
acrylic acid, 25 . O wt. % isooctyl acrylate, 20 . O wt. 9~ 2-
phenoxyethyl acrylate, and 15 . 0 wt. S Uralac 3004-102 .
25 The die was configured with an overbite of 51 microns
and a coating gap set at 76 microns. A vacuum of 0.9
mm Hg was applied to the vacuum chamber during coating.
An air ~L_~'`ULa of 1.7 bar was maintained to the
laminator bar which was adjusted to provide an
30 interference of 3 . 8 mm. The resulting laminate was
cured by e~O_UL~ to W light (intensity approximately
1.9 mW/cm2) at about 22C to produce a PDLC film
~pproximately 13-14 microns thick.
The PDLC device had on- and of f -state
35 tr~n~~iccir,nc of 73.8% and 1.2%, respectively, and a
haze of 4 . 8%.
W0 9~il29967 . I ~, I I u.,. ,. _ ' ~
21 8~023
--38--
r l~ 20
A PDLC device was y~ey~-Led as described in Example
12 except that the fluid contained (a) 333 parts of
BL036 liquid crystal mixture and (b) 267 parts of the
5 following mixture; 2.5 wt.% Esacure KB-l
photoinitiator, 20.0 wt.% 9460 allyl aliphatic
urethane, 5.0 wt.% acrylic acid, 30.0 wt.% isooctyl
acrylate, 20.0 wt.% 2-phenoxyethyl acrylate, and 22.5
wt.% Uralac 3004-102. The die wa3 configured with an
lo overbite of 41 microns. A vacuum of 1. 9 mm Hg was
applied to the vacuum chamber during coating. An air
yrasDuL ~ of 3.4 bar was maintained to the laminator bar
which was adjusted to provide an interference of 3.8
mm. The re6ulting laminate was cured by t~yO~iUL-~ to W
15 light (intensity approximately 1.8 mW/cm2) at about 21C
to produce a PDLC f ilm approximately 15 microns thick .
The PDLC device had on- and off-state
tr:~n~ filt~nc of 74.8% and 1.2%, respectively, and a
haze of 4.7%.
r l~ 21
A PDLC device was prepared as described in Example
12 except that the f luid aontained (a) 655 parts of
BL036 liquid crystal mixture and (b) 516 parts of the
following mixture; 2.5 wt.% Esacure KB-l
25 photoinitiator, 20.0 wt.% Vectomer 2020, 5.0 wt.%
~crylic acid, 35.0 wt.% isooctyl acrylate, 15.0 wt.% 2-
phenoxyethyl acrylate, 5.0 wt.% diethyl fumarate
(Aldrich, Milwaukee, WI), and 17.5 wt.% Uralac 3004-
102 . The die was conf igured to coat an 88 . 9 cm wide
30 strip, with an overbite of 41 microns and a vacuum of
1. 9 mm Hg was applied to the vacuum chamber during
coating. An air ~Lt:C ~ULe, of 1.7 bar was maintained to
the laminator bar which was adjusted to provide an
interference of 6.35 mm. The resulting laminate was
35 cured by ~,-yo:,uL~ to W light (intensity approximately
WO95l29967 2 1 ~ ~023
--39--
1.54 mW/cm2) at about 200C to produce a PDLC film
approximately 14-15 microns thick.
The PDLC device had on- and of f -state
transmissions of 73.4% and 1.1%, respectively, and a
5 haze of 4 . 5%.
2 2
A PDLC device was prepared as described in Example
21 except that the fluid contained 500 parts of BL036
liquid crystal mixture and 333 parts of a mixture
10 having the composition of 2 . 5 wt. % Esacure RB-l
photoinitiator, 7.5 wt.% acrylic acid, 30.0 wt.%
isooctyl acrylate, 15. 0 wt. % 2-phenoxyethyl acrylate
15.0 wt.% Uralac 3004-102, and 30.0 wt.% Uralac 3004-
300. The die was configured with an overbite of 43
15 microns, a vacuum land gap G2 of 24 . 5 mm and a vacuum of
1. 9 mm Hg was applied to the vacuum chamber during
coating. The IT0-coated polyester film used for the
ele~;LL~,des was approximately 130 microns (5 mils)
thick. An air pressure of 3 . 4 bar was maintained to
20 the laminator bar which was adjusted to provide an
interference of 6.4 mm. The resulting laminate was
exposed to W light having an average intensity of
approximately 1. 68 mW/c* at about 23 C to produce a
PDLC f ilm approximately 18 microns ~hick .
The PDLC device had on- and of f -state
transmissions of 73.4% an~51 1.7%, respectively, and a
haze of 5 . 3% .
lSx~ 23
A PDLC device was yL eL,a~e~ as described in Example
30 12 using a fluid containing (a) 45 parts of a mixture
consisting of 20.0 wt.% of the oligomer contained in
RCC-15C (W.R. Grace, Atlanta, GA), 2.5 wt.% acrylic
~cid, 40.0 wt.% isooctyl acrylate, 25.0 wt.% 2-
phen~...y.~ yl acrylate, 10.0 wt.% Uralac 3004-102, and
35 2.5 wt.% KB-l photoinitiator, and (b) 55 parts BL036
liquid crystal mixture having a solution viscosity of
Wo 95/29967 PCr/US951043~8
21 8qO23
--40--
42 cps (measured on a Brookf ield viscometer using a ~3
spindle operating at 60 rpm). The fluid was applied to
the electrode substrate at a rate of approximately 4 . 6
m/min using a die configured with a 3.8 micron
5 overbite. A vacuum of 3 . 7 mm Hg was applied to the
vacuum chamber during coating. Both the die and back-up
roll were temperature controlled at 20C. A ~L~8ZiU- e:
of 3 . 4 bar was maintained to the sintQred metal bar
during lamination and the lamination bar was adjusted
10 to provide an interference of 3 . 8 mm. The laminate wa6
cured at 21C by exposure to 244 mJ/cm2 W light at an
average intensity of approximately 2.0 mW/cm2to produce
a PDLC ~ilm approximately 19 microns thick.
The PDLC deYice had on- and off-state
15 transmissions of 74.3% and 1.0%, respectively, and a
haze of 4 . 0% .
Other ~ c are within the f ollowing claims .