Note: Descriptions are shown in the official language in which they were submitted.
WO 95/30661 PCT/EP95101507
Le A 30 388-PC 1 21$9456
FILt:, t~tt~'1ia THIS A ~~~ c
ffi(i"TRANSLATIOfd
SUBSTITUTED AROMATIC THIOCARBOXAMIDES AND THEIR USE AS
I~~RBICIDFS
The invention relates to novel substituted aromatic thiocarboxamides, to
processes for.
their preparation and to their use as herbicides.
It is ali-eady known that certain aromatic carbothioamides, for example 2,6-
dichloro-
benzothioamide ("chlorthiamid"), possess herbicidal properties (cf. GB-B
987253).
However, the activity of this previously known compound, especially at low
application
rates and concentrations, is not entirely satisfactory in all areas of
application.
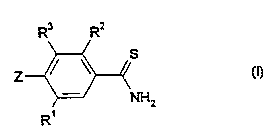
The novel substituted aromatic thiocarboxamides have now been found of the
general
formula (I)
R3 Rx
(4
NHz
R'
in which
R' represents hydrogen or halogen,
Rz represents the following group
-A'-Az-A'
in which
A' represents a single bond, or represents oxygen, sulphur, -SO-, -SOz , -CO-
or the group -N-A'-, in which A' represents hydrogen, hydroxyl, alkyl,
alkenyl, alkinyl, alkoxy, aryl, alkylsulphonyl or arylsulphonyl,
-.
WO 95/30661 PCT/EP95/01507
-2_
21~R456
A' additionally represents in each case optionally substituted alkanediyl,
alkenediyl, alkinediyl, cycloalkanediyl, cycloalkenediyl or arenediyl,
A2 represents a single bond, or represents oxygen, sulphur, -SO-, -SOZ , -CO
or the group -N-A"-, in which A" represents hydrogen, hydroxyl, alkyl,
alkenyl, alkinyl, aryl, alkoxy, alkylsulphonyl or arylsulphonyl,
A2 additionally represents in each case optionally substituted alkanediyl,
alkenediyl, alkinediyl, cycloalkanediyl, cycloalkenediyl or arenediyl,
A3 represents hydrogen, hydroxyl, amino, cyano, isocyano, thiocyanato, vitro,
carboxyl, carbamoyl, thiocarbamoyl, sulpho, chlorosulphonyl, halogen or
represents in each case optionally substituted alkyl, alkoxy, alkylthio,
alkylsulphinyl, alkylsulphonyl, alkylamino, dialkylamino, alkoxycarbonyl,
dialkoxy(thio)phosphoryl, alkenyl, alkenyloxy, alkenylamino,
alkylideneamino, alkenyloxycarbonyl, alkinyl, alltinyloxy, alkinylamino,
alkinyloxycarbonyl, cycloalkyl, cycloalkyloxy, cycloalkylalkyl,
cycloalkylalkoxy,cycloalkylideneamino,cycloalkyloxycarbonyl,cycloalkyl-
alkoxycarbonyl, aryl, aryloxy, arylalkyl, arylalkoxy, aryloxycarbonyl,
arylalkoxycarbonyl, heterocyclyl, heterocyclylalkyl, heterocyclylalkoxy or
heterocyclylalkoxycarbonyl,
R' represents hydrogen or halogen or together with R= represents an alkanediyl
or
an alkenediyl group which optionally contains at the beginning (or end) or
within the hydrocarbon chain an oxygen atom, a sulphur atom, an SOz group,
an NH group, an N-alkyl group, a carbonyl group and/or a thiocarbonyl group,
and
Z represents in each case optionally substituted monocyclic or bicyclic,
saturated
or unsaturated heterocyclyl, heterocyclylamino or heterocyclylimino.
The novel substituted aromatic thiocarboxamides of the general formula (I) are
obtained
WO 95/30661 PCT/EP95101507
- ', ~ -3- 2189456
if substituted aromatic nitriles of the general formula (II)
R3 R~
Z ~ ~ CN
R'
in which
R', Rz, R' and Z have the meanings given above
are reacted with hydrogen sulphide (HZS) or with thioacetamide, optionally in
the
presence of a reaction auxiliary and optionally in the presence of a diluent.
The novel substituted aromatic thiocarboxamides of the general formula (I) are
notable
for strong and selective herbicidal activity.
In the definitions, the saturated or unsaturated hydrocarbon chains, such as
alkyl,
alkanediyl, alkenyl or alkinyl - alone or in conjunction with heteroatoms,
such as in
alkoxy, alkylthio or alkylamino - are each straight-chain or branched.
Halogen generally represents fluorine, chlorine, bromine or iodine, preferably
fluorine,
chlorine or bromine, especially fluorine or chlorine.
The invention preferably relates to compounds of the formula (I) in which
R~ represents hydrogen, fluorine, chlorine or bromine,
R= represents the following group
-A~-Az-As
in which
A' represents a single bond, or represents oxygen, sulphur, -SO-, -SOz-, -CO-
- WO 95/30661 PCT/EP95/01507
'. 1 -4- 2189456
or the group -N-A''-, in which A'' represents hydrogen, hydroxyl, C,-Ca
alkyl, C; C,-alkenyl, C3 CQ alkinyl, C,-C4 alkoxy, phenyl, C,-C;
alkylsulphonyl or phenylsulphonyl,
A' additionally represents in each case optionally fluorine- or chlorine-
substi
tuted C,-C6 alkanediyl, C.~-C6 alkenediyl, CZ C~alkinediyl, C; C6
cycloalkanediyl, C; C6 cycloalkenediyl or phenylene,
A2 represents a single bond, or represents oxygen, sulphur, -SO-, -SOZ-, -CO
or the group -N-A"-, in which A' represents hydrogen, hydroxyl, C,-C4
alkyl, C3 C; alkenyl, C3-C4 alkinyl, C,-CQ alkoxy, phenyl, C; C;
alkylsulphonyl or phenylsulphonyl,
A~ additionally represents in each case optionally fluorine- or chlorine-
substi-
tuted C,-C6 alkanediyl, Ca C6-alkenediyl, CZ C6 alkinediyl, C; C6
cycloalkanediyl, C3 C~ cycloalkenediyl or phenylene,
A' represents hydrogen, hydroxyl, amino, cyano, isocyano, thiocyanato, nitro,
carboxyl, carbamoyl, thiocarbamoyl, sulpho, chlorosulphonyl, halogen, or
represents in each case optionally halogen- or C,-C, alkoxy-substituted
alkyl, alkoxy, alkylthio, alkylsulphinyl, alkylsulphonyl, alkylamino,
dialkylamino, alkoxycarbonyl or dialkoxy(thio)phosphoryl having in each
case 1 to 6 carbon atoms in the alkyl groups, or represents in each case
optionally halogen-substituted alkenyl, alkenyloxy, alkenylamino,
alkylideneamino, alkenyloxycarbonyl, alkinyl, alkinyloxy, alkinylamino or
alkinyloxycarbonyl having in each case 2 to 6 carbon atoms in the alkenyl,
alkylidene or alkinyl groups, or represents in each case optionally halogen-,
cyano-, carboxyl-, C,-C4 alkyl- and/or C,-C; alkoxy-carbonyl-substituted
cycloalkyl, cycloalkyloxy, cycloalkylalkyl, cycloalkylalkoxy,
cycloalkylideneamino, cycloalkyloxycarbonyl or cycloalkylalkoxycarbonyl
having in each case 3 to 6 carbon atoms in the cycloalkyl groups and
optionally 1 to 4 carbon atoms in the alkyl groups, or represents in each
WO 95/30661 PCT/EP95/01507
. _5_ 2189456
- .
case optionally nitro-, cyano-, carboxyl-, halogen-, C,-Ca alkyl-, C,-Cq
halogenoalkyl-, C,-C4 alkyloxy-, C,-C4 halogenoalkyloxy- and/or C,-C4
alkoxy-carbonyl-substituted phenyl, phenyloxy, phenyl-C,-Cg alkyl, phenyl-
C,-C4 alkoxy, phenyloxycarbonyl or phenyl-C,-C4 alkoxycarbonyl, (in each
case optionally totally or partially hydrogenated) pyrrolyl, pyrawlyl,
imidazolyl, triawlyl, furyl, thienyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, oxadiazolyl, thiadiawlyl, pyridinyl, pyrimidinyl, triazinyl,
pyrazolyl-C,-C; alkyl, furyl-C,-C, alkyl, thienyl-C,-C; alkyl, oxawlyl-'
C,-C4 alkyl, isoxazole-C; C4 alkyl, thiawle-C,-C4 alkyl, pyridinyl-C,-C4
alkyl, pyrimidinyl-C,-C; alkyl, pyrazolylmethoxy or furylmethoxy, or
represents perhydropyrenylmethoxy or pyridylmethoxy,
R' represents hydrogen, fluorine, chlorine or bromine or together with RZ
represents
an allcanediyl or alkenediyl group having in each case up to 4 carbon atoms
which optionally contains at the beginning (or end) or within the hydrocarbon
chain an oxygen atom, a sulphur atom, an SOz group, an NH group, an
N-C,-C4 alkyl group, a carbonyl group and/or a thiocarbonyl group, and
Z represents in each case monocyclic or bicyclic, saturated or unsaturated
hetero-
cyclyl, heterocyclylamino or heterocyclylimino having in each case 2 to 6
carbon atoms and 1 to 4 nitrogen atoms in the heterocyclic ring system, which
optionally additionally contains an oxygen atom or sulphur atom and/or
optionally up to three groups from the series -CO-, -CS-, -SO- andlor S02 ,
and
which is optionally substituted by one or more groups from the series nitro,
hydroxyl, amino, cyano, carboxyl, carbamoyl, thiocarbamoyl, halogen, C,-C6
alkyl (which is optionally substituted by halogen or C,-C4 alkoxy), Cz C6
alkenyl or Cz C6 alkinyl (which are in each case optionally substituted by
halogen), C,-C6 allcoxy or C,-C6 alkoxy-carbonyl (which are in each case
optionally substituted.by halogen or C,-C; alkoxy), Cz C6 alkenyloxy or C,-C6
alkinyloxy (which are in each case optionally substituted by halogen), C,-C6
alkylthio, Cz C6 alkenylthio or Cz C6 alkinylthio (which are in each case
optionally substituted by halogen), C,-C6 alkylamino or di-(C,-C4 alkyl)-
amino,
WO 95/30661 PCT/EP95/01507
6
_ 6 _ 2189456
C3 C6-cycloalkyl or C3 C6 cycIoalkyl-C; C~ alkyl (which are in each case
optionally substituted by halogen and/or C,-C; alkyl), phenyl, phenoxy,
phenylthio, phenylsulphinyl, phenylsulphonyl or phenylamino (which are in
each case optionally substituted by nitro, cyano, halogen, C,-C° alkyl,
C,-CQ
halogenoalkyl, C,-C4 alkyloxy, C,-C° halogenoalkyloxy and/or C,-C4
alkoxy-
carbonyl).
The invention particularly relates to compounds of the formula (I) in which
R' represents hydrogen, fluorine or chlorine,
R' represents the following group
-A~-Az-As
in which
A' represents a single bond, or represents oxygen, sulphur, -SO-, -SOz-, -CO-
or the group -N-A°-, in which A° represents hydrogen, hydroxyl,
methyl,
ethyl, n- or i-propyl, methoxy, ethoxy, n- or i-propoxy, methylsulphonyl or
ethylsulphonyl,
A' additionally represents methylene, ethane-1,1-diyl, ethane-1,2-diyl,
propane-I,1-diyl, propane-1,2-diyl, propane-1,3-diyl, ethene-1,2-diyl,
propene-1,2-diyl, propene-1,3-diyl, ethine-1,2-diyl, propine-1,2-diyl or
propine-1,3-diyl,
Az represents a single bond, or represents oxygen, sulphur, -SO-, -SOZ , -CO-
or the group -N-A°-, in which A° represents hydrogen, hydroxyl,
methyl,
ethyl, n- or i-propyl, methoxy, ethoxy, n- or i-propoxy, methylsulphonyl,
ethylsulphonyl, n- or i-propylsulphonyl or phenylsulphonyl,
Az additionally represents methylene, ethane-1,1-diyl, ethane-1,2-diyl,
propane-I,1-diyl, propane-1,2-diyl, propane-1,3-diyl, ethene-1,2-diyl,
WO 95/30661 PCT/EP95/01507
-7_ 2189456
propene-1,2-diyl, propene-1,3-diyl, ethine-1,2-diyl, propine-1,2-diyl or
propine-1,3-diyl,
A; represents hydrogen, hydroxyl, amino, cyano, nitro, carboxyl, carbamoyl,
sulpho, fluorine, chlorine, bromine, or represents in each case optionally
fluorine-, chlorine-, methoxy- or ethoxy-substituted methyl, ethyl, n- or
i-propyl, n-, i-, s- or t-butyl, n-, i-, s- or t-pentyl, methoxy, ethoxy, n-
or
i-propoxy, n-, i-, s- or t-butoxy, n-, i-, s- or t-pentyloxy, methylthio,
ethyl=
thio, n- or i-propylthio, n-, i-, s- or t-butylthio, methylsulphinyl, ethyl-
sulphinyl, n- or i-propylsulphinyl, methylsulphonyl, ethylsulphonyl, n- or
10, i-propylsulphonyl, methylamino, ethylamino, n- or i-propylamino, n-, i-, s-
or t-butylamino, dimethylamino, diethylamino, methoxycarbonyl,
ethoxycarbonyl, n- or i-propoxycarbonyl, dimethoxyphosphoryl,
diethoxyphosphoryl, dipropoxyphosphoryl or diisopropoxyphosphoryl, or
represents in each case optionally fluorine- or chlorine-substituted propenyl,
butenyl, propenyloxy, butenyloxy, propenylamino, butenylamino,
propylideneamino, butylideneamino, propenyloxycarbonyl,
butenyloxycarbonyl, propinyl, butinyl, propinyloxy, butinyloxy,
propinylamino, butinylamino, propinyloxycarbonyl or butinyloxycarbonyl,
or represents in each case optionally fluorine-, chlorine-, cyano-, carboxyl-,
methyl-, ethyl-, n- or i-propyl-, methoxycarbonyl- or ethoxycarbonyl-
substituted cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropyl-
oxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, cyclopropylmethyl,
cyclobutylmethyl, cyclopentylmethyl, cyclohexylmethyl, cyclopropyl-
methoxy, cyclobutylmethoxy, cyclopentylmethoxy, cyclohexylmethoxy,
cyclopentylideneamino, cyclohexylideneamino, cyclopentyloxycarbonyl,
cyclohexyloxycarbonyl, cyclopentylmethoxycarbonyl or cyclohexyl-
methoxycarbonyl, or represents in each case optionally nitro-, cyano-,
carboxyl-, fluorine-, chlorine-, bromine-, methyl-, ethyl-, n- or i-propyl-,
trifluoromethyl-, methoxy-, ethoxy-, n- or i-propoxy-, difluoromethoxy-,
trifluoromethoxy-, methoxycarbonyl- and/or ethoxycarbonyl-substituted
phenyl, phenyloxy, benzyl, phenylethyl, benzyloxy, phenyloxycarbonyl,
S
- WO 95/30661 PCT/EP95101507
~ -8- 218945b
benzyloxycarbonyl, (in each case optionally completely or partially hydro-
genated) pyrrolyl, pyrazolyl, imidazolyl, triazolyl, furyl, thienyl, oxazolyl,
isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, pyridinyl,
pyrimidinyl, triazinyl, pyrawlylmethyl, furylmethyl, thienylmethyl,
oxazolylmethyl, isoxawlemethyl, thiawlmethyl, pyridinylmethyl,
pyrimidinylmethyl, pyrazolylmethoxy, furylinethoxy or pyridylmethoxy,
R' represents hydrogen, fluorine or chlorine or together with Rz represents an
alkanediyl or alkenediyl group having in each case 1 to 3 carbon atoms which
optionally contains at the beginning (or end) or within the hydrocarbon chain
an
oxygen atom, a sulphur atom, an NH group, an N-methyl group, a carbonyl
group and/or a thiocarbonyl group, and
Z represents in each case monocyclic or bicyclic, saturated or unsaturated
hetero-
cyclyl, heterocyclylamino or heterocyclylimino having in each case 2 to 5
carbon atoms and 1 to 3 nitrogen atoms in the heterocyclic ring system, which
optionally additionally contains an oxygen atom or sulphur atom and/or
optionally up to two groups from the series -CO-, -CS-, -SO- and/or SO= , and
which is optionally substituted by one or more groups from the series vitro,
hydroxyl, amino, cyano, carboxyl, carbamoyl, thiocarbamoyl, fluorine,
chlorine,
bromine; methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, (which are
optionally
substituted by fluorine, chlorine, methoxy or ethoxy); propenyl, butenyl,
propinyl or butinyl (which are in each case optionally substituted by fluorine
or
chlorine); methoxy, ethoxy, n- or i-propoxy, n-, i-, s- or t-butoxy, methoxy-
carbonyl or ethoxycarbonyl (which are in each case optionally substituted by
fluorine, chlorine, methoxy or ethoxy); propenyloxy, butenyloxy, propinyloxy
or butinyloxy (which are optionally substituted by fluorine or chlorine);
methyl-
thio, ethylthio-, n- or i-propylthio, n-, i-, s- or t-butylthio, propenylthio,
butenyl-
thio, propinylthio or butinylthio (which are in each case optionally
substituted
by fluorine or chlorine); methylamino, ethylamino, n- or i-propylamino, n-, i-
,
s- or t-butylamino, dimethylamino or diethylamino; cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclobutylmethyl, cyclopentyl-
S
- WO 95/30661 218 9 4 5 ~ P~~5~01507
~ _9_
methyl or cyclohexylmethyl (which are in each case optionally substituted by
fluorine, chlorine, methyl, ethyl, n- or i-propyl), phenyl, phenoxy,
phenylthio,
phenylsulphinyl, phenylsulphonyl, or phenylamino (which are in each case
optionally substituted by vitro, cyano, fluorine; chlorine, bromine, methyl,
ethyl,
n- or i-propyl, trifluoromethyl, methoxy, ethoxy, n- or i-propoxy, difluoro-
methoxy, trifluoromethoxy, methoxycarbonyl or ethoxycarbonyl).
Very particularly preferred groups of compounds of the formula (I) are the
compounds
of the formulae (Ia), (Ib) and (Ic) drawn below
R2
S
Z
N~ (la)
R'
(/b)
rt
R4
pc)
i
Hz
R'
where
R', Rz and Z have the meanings indicated above as particularly preferred,
R" and R' are identical or different and independently of one another in each
case indi-
vidually represent hydrogen, fluorine, chlorine, methyl or ethyl - or in the
formula (Ib) can also together represent oxygen or sulphur - and
WO 95/30661 PCT/EP95/01507
-lo- X189456
Q represents oxygen, sulphur, N-methyl or N-ethyl.
Z in the general formulae (I) and ((Ia), (Ib) and (Ic) represents in
particular the
heterocyclic groups listed below
R'
R, ~Q~ i s Qi 'N_Q,
N N R N~ Rs N-
N Q, Q,
Rs Rs Rs
(Z~) (~ (Z~
R'
, ~ ,
Qz.Q~N~ R N N\ R~N~Q~N~
~N N-S N=N
Rs
m
Rs Rs
,
Q~.S~N\ R Q.Ni Rs / Ni
N-N Q? Q' -N
R' ~R' Rs
(Z') (~ (~)
,
R~N~Q ~ R~N~N~ Rs
,
Rs Rs Rs R~ Rs
(Z,o) (Zn) (Zm)
, ~ ,
R~N~Q~Ni Q2Q'N~ s Q'
R~ N-
N-Q, Q,
Ri Rs Rs
~") (Z,s)
WO 95/30661 ~ ~ ~ ~ ~ ~ ~ PCT/EP95/01507
_ 11 _
R' R'
Q? Q' 'N-Q' 'N-Q'
R N R°-C\ /~ R \ N
Q' N-N N-Q'
R6
~17)
Rs R~
R ~ QN- Q'_Q 'N\ R N QN-
a '
-N R°~Q,
R° R° R° R
Rs
iz,~ (~ (z~')
Re R'
f z
a~ N~
N/ N~ R '1 1I N1 Y
=N N-N a~-N
R6 R
Q'
R R Q /N
N-N N--
cz~5) cue)
where in each case
Q' represents a group from the series -CO-, -CS-, -CH2 , -CH(OH)-, -CHCI-,
-CHBr-, -C(=CHz)-, -C(=CHF)-, -C(=CFZ)-, -C(=CHCI)-, -C(=CHBr)-,
-C(=CHOCHF~-, -C(=CHOCF3)-, -C(=CFIOCI-IZCF3)-,
Qz represents oxygen, sulphur or a group from the series -CO-, -CS-, -CH2 , -
CHF-,
-CF= , -CHCI-, -CHBr-, -CHOCHFZ , -CHOCF; , -CHOCHzCF3 ,
WO 95/30661 PCT/EP95/01507
_12_ 2189456
R6 represents hydrogen, amino, nitro, cyano, carboxyl, carbamoyl, fluorine,
chlorine, bromine, methyl, ethyl, n- or i-propyl, cyclopropyl, difluoromethyl,
trifluoromethyl, chlorodifluoromethyl, methoxy, ethoxy, n- or i-propoxy,
difluoromethoxy, trifluoromethoxy, chlorodifluoromethoxy, methylthio,
ethylthio, n- or i-propylthio, difluoromethylthio, trifluoromethylthio, chloro-
difluoromethylthio, methylamino, ethylamino, n- or i-propylamino, dimethyl-
amino, diethylamino, methoxycarbonyl or ethoxycarbonyl, and
R' represents hydrogen, hydroxyl, amino, cyano, methyl, ethyl, n- or i-propyl,
difluoromethyl, methoxy, ethoxy, n- or i-propoxy,
or where optionally two adjacent groups - R6 and R6 or R' and R' or R6 and R'
together represent in each case optionally fluorine-, chlorine-, bromine-,
methyl-, ethyl-,
n- or i-propyl-substituted alkanediyl or alkenediyl having in each case up to
4 carbon
atoms which is optionally interrupted by oxygen, sulphur or a group from the
series
-SO-, SOZ-, -N(CH3)- or N(CZHS}- at the beginning (or at the end) or within
the
hydrocarbon chain.
The definitions of radicals listed above, indicated in general or in ranges of
preference,
apply both to the end products of the formula (I) and, correspondingly, to the
respective
starting materials and intermediates required for preparation. These radical
definitions
can be combined as desired with one another, which therefore includes any
desired
combinations between the indicated ranges of preferred compounds.
Examples of the compounds of the formula (I) according to the invention are
listed in
the groups below.
WO 95/30661 PCT/EP95/01507
_. ~
-13- 2189456
Group 1
O R3 RZ
HzN~N~ / \ S
N
CF~ N t NHz
R
(IA I)
In this formula, RZ and
R', R' have
the meanings
indicated
in the
following
list:
Synthesis
Ex. No. R' R= R;
1 H F H
2 H C1 H
3 H CI CL
4 C1 F H
5 F F H
6 F F C1
7 F CH3 H
8 F C2H5 H
9 F -CH2C1 H
F F F
1I F -NHC2H5 H
12 F -CH2CN _ H
13 ' F -N(CH3)S02C2H5 H
14 CI -N(CH3)S02C2H5 H
WO 95/30661 218 9 4 5 6 P~'~~S~olso7
-14-
Ex. No. R' Rz R3
15 CL ~ -N(CH3)S02C2H5 CI
16 F -NH-COCFg H
17 F -OH H
18 Ci -OH H
19 F -CFi(CHg~ H
20 F NH-S02-CH3 H
21 F -S02-CH3 H
22 F -S02-0-CH3 H
23 F -S02-NH-CH3 H
24 F -COOCH3 H
25 F -CO-NH-CHg H
26 Cl -COOCH3 CI
27 C( -COOC2H5 H
28 F _0_C2H5 H
29 F -N(C2H5)S02C2H5 H
30 F -N(S02CH3)2 H
31 F -CO-N(CH3)2 H
32 . F -S-CH2-C~CH H
33 Cl -S-CH2-C=CH F
WO 95/30661 PCf/EP95/01507
-15- 2?89456
.
Ex. No. R' R= R'
34 F -S-GH2-C~CH Cl
35 F -O-CH(CH3)-C~CH H
36 F -S-CH2-COOCH3 H
37 F -O-CH2CH2-OCH3 H
38 F -O(CH2CH20~CHg H
39 F -O-GH2-CH~2 H
40 F -O-CH2-C~CH H
41 F -SH H
42 F -S-CH3 H
43 F -S-C2Hg H
44 F -S-CH{CH3)2 H
45 F -O-CH2-CF3 H
46 F -O-CH(CH2F)2 H
-OC~HCOOCZHS
47 F CHI H
-OCHCOOCHzC=CH
48 F CHI H
WO 95/30661 PCT/EP95101507
y ~ -16- 218946
Ex. No. R' Rz R'
49 F -NH-S02CZH5 H
50 C1 -NH-S02C2H5 H
51 F -NH-S02C2H5 Cl
52 F -NH-S02CH(CH3)2 H
53 F NH-S02C4Hg H
54 F NCH-OC2H5 H
55 F N~(CH3)OC2H5 H
56 F -N~(OCH3~ H
57 F -N~Fi-N(CHg~, H
58 F -SCN H
59 F -S02C1 H
60 F -O-CS-N(CH3)2 H
61 F -S-CO-N(CH3)2 H
62 F -NH-P(O)(CH3)OC2H5 H
63 F -NH-P(O)(OC2Hg~ H
64 F -NH-COC2H5 H
65 F -N(CH3)COCF3 H
66 F -NH-COCH(CH3)2 H
67 F -NH-CO-CO-C(CH3)3 H
WO 95/30661
- 1 _ " _ 218 9 4 5 6 P~'~'9siu1s07
Ex. No. R' R= R3
68 F -NH-CO-Nfi2 H
69 F -NH-CO-NHCH3 H
70 F NH-CO N(CH3)2 H
71 F -N(COCH3~ H
72 F -NfI COCH(CH3)Cl H
73 F -S-CH2-CH=CH2 H
74 CI -S-CH2-CEi=CH2 H
75 F -S-CH(CH3)C~CH H
76 F -S-CH(CH3)COOC2H5 H
77 F -S(O)-CH3 H
-S COOCH~
78 F H
-S-CH2 ~
79 F H
-S_ 'COOCH3
80 F ~ H
WO 95/30661 P(.'T/EP95/01507
_,8_ 2189456
Ex. No. R' R= R'
_O-CO
81 F
/O~
-O-CHz
82 F ~ g
~O
83 F H
84 F -O-CH2-CH H
85 F -O-S02CH3 H
86 F -OCH2-CH(C1~CH2 H
87 F -O-CHZ-COOCH3 H
88 F -O-CHFZ H
89 F -OCOOCHZCH2C1 H
90 F -OCHZP(O)(OC2H5)2 H
91 Cl -O-CH(CHg~P(O)(OCZHS)2 H
WO 95/30661 PCT/EP95/01507
. -, ~ -19- 2189456
Ex. No. R' R= R3
HP(07(~z~slz
~0
92 F g
-O-N=C I
93 F ~,J g
94 F -O-N(C2H5~ H
95 F H
96 F -NH-SO--a H
97 F -NH-SO--a CI
98 C~ -NH-SO~ H
WO 95/30661 ~ ~ ~ ~ ~ ~ PCT/EP95/01507
-20-
. .,
Ex. No. R' RZ R'
-NH-SO~
99 F F
-NH-5~
100 F g
-NH-S
101 F H
-NH-SOz CH2 ~ /
102 F H
103 F -NCH(CH3)2S02C2H5 H
104 F -N(CH3)S02CH(CH3)2 H
105 H -N(CH3)S02C2H5 CI
106 C1 -N(CH3)S02C4Hg H
107 F -N(CH3)SOZC2H5 H
108 ' F -N(CH3)S02CH3 H
109 F -N(S02C2H5)2 H
WO 95130661 2 1 8 9 4 5 6 P~~5101507
._ _21 _
Ex. No. R' R= R'
110 F -N(S02CH3)S02C2H5 H
N-S02CZH5
111 F H
112 F N(CH3)2 H
113 F -NH2 H
114 CI -NH2 H
115 Cl -O-CH(CH3~ H
116 F -O-CH(CH3~ H
117 F ~ H
118 C1 H
119 F -O-CH2-COOC2Hg H
120 . F -S-CH2-COOCH3 H
t21 F -S-CH2-COOC2H5 H
WO 95/30661 PCTIEP95/01507
., ~ _2~_ 2189456
Ex_ No. R' R= R'
122 Cl -S-CH2-COOC2H5 H
123 F -CH2-CH(CI)COOCH3 H
124 F -GH2-CH(CI)COOC2H5 H
125 F -CH2-CH(Cl)COIQHC2H5 H
126 CI -CH2-CH(CI)CONHC2H5 H
-CH=~HCONHCH(CH~2
127 C1 C~ H
-CHz~HCONHCH(CH~i
128 F C~ H
129 F -COOC3H7-i H
Group 2
O
HZN. N ~ 3
N
CHFZ N NN2
(~-2)
In this formula, R', R= and R; have, for example, the meanings indicated above
in
Group 1.
WO 95130661 PCT/EP95/01507
-. ~ -23- 2189456
Group 3
CH3
H~N~N
N
CF~ N HZ
(IA-3)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group I.
rou 4
CH3 O
Hl~~ N
' N
CHF N !HZ
(1,a,-'1)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group I.
Group 5
(CH3)ZN\ O
N
N
CHFZ N IHZ
(IA-5)
In this formula, R', Rr and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCT/EP95/01507
-24_ 2x89456
Group 6
0
H,
N
N
CFF~ N HZ
In this formula, R', Rz and R' have, for example, the meanings indicated above
iri
Group I.
Group 7
(CH3)ZN\ 0
N
N
CF3 N
(i~._7)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group I.
Grou
(CH3)ZN\ O
N
N
CI~ N IHZ
(IA-s)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCTIEP95/01507
~ -25- 2189456
Groun 9
(CH3)ZN\ O
N
N
,
Br N
~_9)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group 1.
Groun 10
F
H2
(IA-10)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
Grou I
IHZ
(IA-I 1)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 218 9 4 ~ b ~~~5/01507
..
-26-
Group 12
~~H3)3~
0 ~H2
(IA 12)
In this formula, R', R2 and R' have, for example, the meanings indicated above
in .
Group 1.
ou 13
0
N
N
N IHZ
(IA-I3)
In this formula, R', Rz and R; have, for example, the meanings indicated above
in
Group 1.
Group 14
O
N
~~ N N Hz
(IA-14)
In this formula, R', ~Rz and R' have, for example, the meanings indicated
above in
Group 1.
WO 95/30661 PCT/EP95/01507
-27- 2189456
Groun 1 S
S R' R2
(CHs)z~H ~ S
N N / \
C ~ N NH2
Rt
(IA-IS)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
Group 16
S
N
I N
CF3 N IHZ
~tA-I6)
In this formula, R', Rr and R' have, for example, the meanings indicated above
in
Group I.
Group 17
O
~N~
SOZ t N
~N~ IHz
O (IA-17)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCTIEP95/01507
-28- 2189456
Group 18
O R' Rz
S
I N~N
NH
z
_ .. (1p, 18)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
rou I9
O
O ~N
H
CHa--~O z
CHa
(IA 19)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group I.
Grou 2
N
I I Hz
O
O (IA-20}
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCT/EP95101507
-, ~ -29- 2189456
Group 21
CH3 ~0
~N~
~N
Hz
OF
(I~.-z1)
In this formula, R', R' and R' have, for example, the meanings indicated above
in-
Group 1.
Gro ~ 22
O
N ~N
MHz
0 (/A-22)
In this formula, R', R~ and R' have, for example, the meanings indicated above
in
Group 1.
Group 23
C
CH3
~,e,_23)
In this formula, R', Rr and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCf/EP95101507
-3~- 2189456
24
C ~3
Hz
CH3
(IA 24}
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group 1.
Crroup 25
HZ
., (IA-25)
In this formula, R', R= and R3 have, for example, the meanings indicated above
in
Group I.
rou 26
l
CH3
IH2
CH3 (IA 26)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCT/EP95/01507
-31- 2189456
Group 27
H2
(IA-27)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
Grou 2
CHF
N
r
Ch
(ta 2s)
In this formula, Rt, R= and R' have, for example, the meanings indicated above
in
Group I.
Gr a 2
Cf
(IA-29)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group I.
WO 95130661 PCT/EP95101507
32
2189456
Group 30
f
CH3
~CN
3
CH3
(IA-30)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
Group 31
O
N
O
(IA-31)
In this formula, R', Rr and R' have, for example, the meanings indicated above
in
Group 1.
rou 32
O
N
CN N
HZ
(IA-32)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group 1.
' WO 95130661 PCT/EP95/01507
'' ~ -33-
2~89456
Group 33
HZ
(IA-33)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
Group 34
C
NOz
-N
CH3
(IA~34)
In this formula, R', R~ and R' have, for example, the meanings indicated above
in
Group 1.
Grou 3
\ S
CH3 ~ ,
N NHz
CH3 R'
(IA-3 5)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCl'/EP95/01507
._ ~ -34- 2189456
Gr a
S R' RZ
N~ ~ ~ S
N
C ~ N NH2
R'
(IA-36)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
Group '37
O
CF3~N
N ~ Hs
H O
In this formula, R', R~ and R' have, for example, the meanings indicated above
in
Group I.
Group 38
0
CF3~N
N-~ IHz
H S (IA-38)
In this formula, R', Rr and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCT/EP95/01507
_ -2188456
Grou
0
CF3~N
N
CZHS S
(IA-39)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group 1.
Group 40
O
CHFz~N
N-
CH3 O
(IA-4o)
In this formula, R', Rr and R' have, for example, the meanings indicated above
in
Group I.
Group 41
O
CHFz~N
~N ~ Hz
CH3 S
(IA-41)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCT/EP95/01507
-, -36-
2189456
Group 42
R' R'
S
iJ
// N
I1 ~S R'
N
(IA-42)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
Group 43
CHFa
J~N
I N
CF3 N IH2
(IA-43)
In this formula, R', Rz and R; have, for example, the meanings indicated.
above in
Group 1.
a 44
F-(CHZ\3 ~ Rs Rz
N ~ ~ S
N
fi~ N NHZ
R,
(~-~)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCTIEP95/01507
_37_
2189456
Group 45
CF3
iN~ IHz
CH3
(IA-45)
In this formula, R', R' and R' have, for example, the meanings indicated above
in
Group I.
Group 46
CFi3
~N~
CH N~N
3
(IA-46)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group I.
Group 47
CHFZ S
Hz
(1A-a7)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCC/EP95/01507
38 2189456
Group 48
CF-,
YI
N, ~H
(IA-48)
In this formula, R', Rz and R3 have, for example, the meanings indicated above
iri
Group I.
rou 49
O
C
(IA-49)
S In this formula, R', R2 and R' have, for example, the meanings indicated
above in
Group I.
Group 50
CH3 O R" R'
H ~ ~ S
C 3~~ /
N-N NHZ
(IA-50)
In this formula, R', ~RZ and R' have, for example, the meanings indicated
above in
Group 1.
P(.'T/EP95/01507
W0 95/30661 218 9 4 ~ 6
-39-
Group SI _
NHz
(IA-51)
In this formula, R', Rz and R' have, for example, the meanings indicated above
iri
Group I.
Group 52 _
F-(CHz'3
N
N, N H
z
(IA-52)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
Group 53
CH3
w J~ 3
N
N
O ~ N VHz
3
(IA-53)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCT/EP95/01507
:~
0_ z~ s~4~6
Group 54
CH3 0
~\/N
O~ N
N-~ HZ
CH3 O
(Ir1-54)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group I.
Group 55
CH
N
H2
O
(IA-ss)
In this formula, R', R' and R' have, for example, the meanings indicated above
in
Group I.
Group 56
O
T
~N~N
N 'H
z
(IA-56)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCT/EP95/01507
-. ~ -41- 2 ~ 89456
Group 57
O
J NH2
0 R' (IA-57)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group 1.
Group 58
O R° R'
/ N / \
0-~ NHZ
0 R'
(IA-ss)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group I.
Group 59
CHF, O
\/N
O~ N
N IHZ
(IA-59)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group I.
r
WO 95130661 PCf/EP95101507
., ~ -42- 2~ 89456
Group 60
N-
CH3 O ..
(IA-so)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group 1.
Group 61
S
N ~ ~~ _ v
N ~ NH2
CH3 y
(IA-61)
In this formula, R', Rv and R' have, for example, the meanings indicated above
in
Group 1.
Grouu 62
CH3 0
N
N
i
CFZ N IHZ
(IA-62)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
, WO 95/30661 PCT/EP95101507
- 43 -
2189456
Group 63
O
CFg~N
N--~
CH3 O
(IA-63)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
Group 64
O
CF3~N
N-
NHz O ~~)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
Group 65
O
CF3~N
N Hz
NHCH3 O (Ip,_65)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 ' PCTIEP95/01507
-44-
21894~b
Grog
CF
(IA-66)
In this formula, R', R~ and R' have, for example, the meanings indicated above
in
Group I.
Grog 67
O
CHFZ~N
~N ~ VHZ
NHZ O
(IA-67)
5 In this formula, R', Rz and R' have, for example, the meanings indicated
above in
Group I.
Group 68
0
CF3~N
N ~ Hz
0
NHCH(CH3)z
(IA-68)
In this formula, R', R2 and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCT/F,P95/01507
- 45
2189456
Group 69
0
N
CF3-{~
N ~ HZ
CIi3 O
(IA-69)
In this formula, R', Rz and R' have, for example, the meanings indicated above
iri
Group 1.
ro 7
O
N
CH30-~~
N ~ Hi
CHI O
(IA-7o)
In this formula, R', Rz and R; have, for example, the meanings indicated above
in
Group 1.
rpu 71
,O
~~(/N
N
CZH50-~/
CH3 O
(IA-71)
In this foimula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
WO 95130661 PCT/EP95/01507
-46-
._
2189456
Group 72
S
N--~
H
CH3 0
(IA-72}
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group 1.
Group 73
cc K
S~N / \ S
CF N NHz
3
(IA 73)
In this formula, R', R~ and R' have, for example, the meanings indicated above
in
Group 1.
rou 74
CHFz-5
HOC Hz
K
(IA-74)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCT/EP95/01507
~, _47_
2189456
Group 75
O R' R1
~ S
I ~~N
NH
z
O R'
(IA_75)
In this formula, R', Rz and R; have, for example, the meanings indicated above
iri
Group 1.
rou 76
O R" R'
~/ ~ ~ S
N' \N
N NH
z
O R~ (IA-76)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group 1.
Grou 7 __
O R' R'
JJ ~ S
N' \N
CN NH
z
O R' (/A_.77)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group 1.
WO 95/30661 PCT/EP95/01507
-48-
2189456
ou 78
Hz
(IA-7a)
In this formula, R', R~ and R' have, for example, the meanings indicated above
in
Group I.
Group 79
N
i
V
(IA 79)
In this formula, R', Rr and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCT/EP95/01507
_49-
2189456
Table 80
Hz
CH3 ~~-80)
In this formula, R', R' and R' have, for example, the meanings indicated above
in
Group 1.
Group 81
N/ 'N / \ S
NHz
" .. {1A 81)
In this formula, R', Rz and R' have, for example, the meanings indicated above
in
Group 1.
Group 82
N
H' N
V
N
H3C
S
(IA-82)
In this formula, R', R= and R' have, for example, the meanings indicated above
in
Group I.
WO 95/30661 PCf/EP95/01507
°- 2189456
Group 83
0 R3 RZ
HsC N~ ~ ~ S
I .
N NHz
CF3~~ (Ip-83)
In this formula, R', RZ and R' have, for example, the meanings indicated above
in
Group I.
rou 84
0
F2Cii ~ S
N
I
N NHs
CH3R'
(Ia-s4)
In this formula, R', R2 and R' have, for example, the meanings indicated above
in
Group 1.
Group 85
0
CI-t3~ NI
CF3 N N
t~-I)
In this formula, R', R° and R' have the meanings indicated in the
following list:
< <
WO 95/30661 PCT/EP95/01507
-51-
~18945~
Ex. No. R' R4 RS
1 F CHg CH
2 Cl CHg ~3
3 H CHg CH3
F C1
3
F C1 CL
6 F C2H5 CH3
rou 86
Rs R<
O wN,C~a
CH3~N
/ \
N
CF~ N _ N
In this formula, R', R' and RS have, for example, the meanings indicated above
in
Group 85.
WO 95/30661 PCT/EP95/01507
-. _52_
2189456
Group 87
CH
CH3~NI
CF3 N
In this formula, R', R° and RS have, for example, the meanings
indicated above in
Group 85.
Group 88
N,CH(CH3)C=CH
CH~N~ / \ S
I \N
CF~ N _ NH2
" (IC-1)
In this formula, R', R° and RS have, for example, the meanings
indicated above in
Group 85.
Group 89
O
CH3~N
CF~ N N
(IC-2)
In this formula, R', R4 and RS have, for example, the meanings indicated above
in
Group 85.
WO 95/30661 PCT/EP95/01507
.' -53-
2189456
Using, for example, 2-(2-fluoro-4-cyano-5-methoxy-phenyl)-4-methy l-5-
difluoromethyl-
2,4-dihydro-3H-1,2,4-triazol-3-one and hydrogen sulphide as starting
materials, the
course of reaction of the process according to the invention can be
illustrated by the
following equation:
O
HzC ~ O-CHI ~C O O-CHI
N ~ ~ ~ Hz~ ~NI~
FzC~N ~N N
F FzCH ' NHz
F
A general definition of the substituted aromatic nitrites to be used as
starting materials
in the process according to the invention for the preparation of the compounds
of the
general formula (I) is given by the formula (II). In the formula (II), R', R=,
R' and Z
preferably or in particular have those meanings which have already been
indicated
above, in connection with the description of the compounds of the formula (I),
as
preferred or, respectively, as particularly preferred for R', R=, R' and Z.
The starting materials of the formula (II) are known and/or can be prepared by
known
processes (cf. EP-A 370332; DE-A 4238125; DE-A 4303376; US-P 5084084; Prepara-
tion Examples).
Suitable diluents for carrying out the process according to the invention are
the custom-
ary organic solvents. These include, in particular, aliphatic, alicyclic or
aromatic,
optionally halogenated hydrocarbons, for example benzine, benzene, toluene,
xylene,
chlorobenzene, dichlorobenzene, petroleum ether, hexane, cyclohexane,
dichloromethane, chloroform, tetrachloromethane; ethers, such as diethyl
ether,
diisopropyl ether, dioxane, tetrahydrofuran or ethylene glycol dimethyl or
diethyl ether;
ketones, such as acetone, butanone or methyl isobutyl ketone; nitrites, such
as
acetonitrile, propionitrile or benzonitrile; amides, such as N,N-
dimethylformamide,
N,N-dimethylacetamide, N-methylformanilide, N-methylpyrrolidone or hexamethyl-
phosphoric triamide; esters, such as methyl acetate or ethyl acetate,
sulphoxides, such
as dimethyl sulphoxide, azines, such as pyridine, alcohols, such as methanol,
ethanol,
n- or i-propanol, ethylene glycol monomethyl ether, ethylene glycol monoethyl
ether,
WO 95/30661 PCT/EP95/01507
-54_
2189456
diethylene glycol monomethyl ether, diethylene glycol monoethyl ether,
mixtures
thereof with water, or pure water.
The process according to the invention is preferably carried out in the
presence of a
suitable reaction auxiliary. Suitable such auxiliaries are all customary
inorganic or
organic bases. These include, for example, alkaline earth metal or alkali
metal hydrides,
hydroxides, amides, alcoholates, acetates, carbonates or hydrogen carbonates,
for
example sodium hydride, sodium amide, sodium methylate, sodium ethylate,
potassium
tent-butylate, sodium hydroxide, potassium hydroxide, ammonium hydroxide,
sodium
acetate, potassium acetate, calcium acetate, ammonium acetate, sodium
carbonate,
potassium carbonate, potassium hydrogen carbonate, sodium hydrogen carbonate
or
ammonium carbonate and also basic organic nitrogen compounds, such as
trimethylamine, triethylamine, tributylamine, N,N-dimethylaniline, pyridine, N-
methylpiperidine, N,N-dimethylaminopyridine, diazabicyclooctane (DABCO), diaza-
bicyclononene (DBN) or diazabicycloundecene (DBU).
The reaction temperatures when carrying out the process according to the
invention can
be varied within a relatively large range. It is generally carried out at
temperatures
between 0°C and 100°C, preferably at temperatures between
10°C and 80°C.
The process according to the invention is generally carried out under
atmospheric
pressure. However, it is also possible to operate under elevated or reduced
pressure,
generally between 0.1 bar and 10 bar.
To carry out the process according to the invention the starting materials of
the formula
(II) are introduced, generally in a suitable diluent in the presence of a
reaction
auxiliary, and the hydrogen sulphide or the thioacetamide is slowly metered
in. The
hydrogen sulphide or the thioacetamide are preferably employed in a relatively
large
excess. The reaction mixture is stirred for a number of hours at the
particular
temperature required. Working up in the process according to the invention is
effected
in each case in accordance with customary methods (c~ the Preparation
Examples).
WO 95/30661 PCT/EP95/01507
-55- 2189456
The active compounds according to the invention can be used as defoliants,
desiccants,
haulm killers and, especially, as weedkillers. By weeds, in the broadest
sense, there are
to be understood all plants which grow in locations where they are not wanted.
Whether the substances according to the invention act as total or selective
herbicides
depends essentially on the amount used.
The active compounds according to the invention can be used, for example, in
connec-
tion with the following plants:
D_icotvledon weeds of the een ra~ Sinapis, Lepidium, Galium, Stellaria,
Matricaria,
Anthemis, Galinsoga, Chenopodium, Urtica, Senecio, Amaranthus, Portulaca,
Xanthium,
Convolvulus, Ipomoea, Polygonum, Sesbania, Ambrosia, Cirsium, Carduus,
Sonchus,
Solanum, Rorippa, Rotala, Lindernia, Lamium, Veronica, Abutilon, Emex, Datura,
Viola, Galeopsis, Papaver, Centaurea, Trifolium, Ranunculus and Taraxacum.
Dicotvledon crops of the eenera: Gossypium, Glycine, Beta, Daucus, Phaseolus;
Pisum,
Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis, Brassica,
Lactuca,
Cucumis and Cucurbita.
Monocotyledon weeds of the eenera~ Echinochloa, Setaria, Panicum, Digitaria,
Phleum,
Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus, Sorghum,
Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria, Eleocharis, Scirpus,
Paspalum, Ischaemum, Sphenoclea, Dactyloctenium, Agrostis, Alopecurus and
Apera.
Monocotyledon cro s of the eenera~ Oryza, Zea, Triticum, Hordeum, Avena,
Secale,
Sorghum, Panicum, Saccharum, Ananas, Asparagus and AILium.
However, the use of the active compounds according to the invention is in no
way
restricted to these genera, but also extends in the same manner to other
plants.
Depending on the concentration, the compounds are suitable for total weed
control, for
example on industrial terrain and rail tracks, and on paths and areas with or
without
WO 95/30661 PC17EP95/01507
~ -56- 21 89456
tree stands. Equally, the compounds can be employed for controlling weeds in
peren
nial crops, for example forests, ornamental tree plantings, orchards,
vineyards, citrus
groves, nut orchards, banana plantations, coffee plantations, tea plantations,
rubber
plantations, oil palm plantations, cocoa plantations, soft fruit plantings and
hopfields,
in lawns, turf and pastures, and for selective weed control in annual crops.
The compounds of the formula (I) according to the invention are particularly
suitable
for selective control of monocotyledon and dicotyledon weeds in monocotyledon
and
dicotyledon crops, both pre- and post-emergence.
To a certain extent, the compounds of the formula (I) also show a fungicidal
action, for
example against Pyricularia oryzae in rice.
The active compounds can be converted into the customary formulations, such as
solutions, emulsions, wettable powders, suspensions, powders, dusts, pastes,
soluble
powders, granules, suspoemulsion concentrates, natural and synthetic materials
impreg-
nated with active compound, and microencapsulations in polymeric substances.
These formulations are produced in a known manner, for example by mixing the
active
compounds with extenders, that is liquid solvents and/or solid carriers,
optionally with
the use of surfactants, that is emulsifiers and/or dispersants and/or foam-
formers.
If water is used as an extender, organic solvents can, for example, also be
used as
auxiliary solvents. Liquid solvents which are mainly suitable are: aromatics
such as
xylene, toluene or alkylnaphthalenes, chlorinated aromatics and chlorinated
aliphatic
hydrocarbons such as chlorobenzenes, chloroethylenes or methylene chloride,
aliphatic
hydrocarbons such as cyclohexane or paraffms, for example petroleum fractions,
mineral and vegetable oils, alcohols such as butanol or glycol as well as
their ethers
and esters, ketones such as acetone, methyl ethyl ketone, methyl isobutyl
ketone or
cyclohexanone, strongly polar solvents such as dimethylfotrnamide and dimethyl
sulphoxide, and water.
WO 95/30661 PCCIEP95/01507
7- 2189456
Suitable solid carriers are:
for example ammonium salts and ground natural minerals such as kaolins, clays,
talc,
chalk, quartz, attapulgite, montmorillonite or diatomaceous earth, and ground
synthetic
minerals such as highly disperse silica, alumina and silicates; suitable solid
carriers for
granules are: for example crushed and fractionated natural rocks such as
calcite, marble,
pumice, sepiolite and dolomite, or else synthetic granules of inorganic and
organic
meals, and granules of organic material such as sawdust, coconut shells, maize
cobs
and tobacco stalks; suitable emulsifiers and/or foam-formers are: for example
non-ionic
and anionic emulsifiers, such as polyoxyethylene fatty acid esters,
polyoxyethylene
fatty alcohol ethers, for example alkylaryl polyglycol ethers,
alkylsulphonates, alkyl
sulphates, arylsulphonates and protein hydrolysates; suitable dispersants are:
for
example lignin-sulphite waste liquors and methylcellulose.
Adhesives such as carboxymethylcellulose and natural and synthetic polymers in
the
form of powders, granules or latexes such as gum arabic, polyvinyl alcohol and
polyvinyl acetate, or else natural phospholipids such as cephalins and
lecithins, and
synthetic phospholipids can be used in the formulations. Further additives can
be
mineral and vegetable oils.
It is possible to use colourants such as inorganic pigments, for example iron
oxide,
titanium oxide and Prussian Blue, and organic dyes such as alizarin dyes, axo
dyes and
metal phthalocyanine dyes, and trace nutrients such as salts of iron,
manganese, boron,
copper, cobalt, molybdenum and zinc.
The formulations generally comprise between 0.1 and 95 per cent by weight of
active
compound, preferably between 0.5 and 90%.
For controlling weeds, the active compounds according to the invention, as
such or in
the form of their formulations, can also be used as mixtures with known
herbicides,
finished formulations or tank mixes being possible.
Possible components for the mixtures are known herbicides, examples being
anilides,
WO 95/30661 PCTIEP95/01507
2189456
for example, diflufenican and propanil; arylcarboxylic acids, for example
dichloropicoIinic acid, dicamba and picloram; aryloxyalkanoic acids, for
example 2,4
D, 2,4 DB, 2,4 DP, fluroxypyr, MCPA, MCPP and triclopyr; aryloxy-phenoxy-
alkanoic
esters, for example diclofop(-methyl), fenoxaprop(-ethyl), fluazifop(-butyl),
haloxyfop(-
methyl) and quizalofop(-ethyl); azinones, for example chloridawn and
norflurazon;
carabamates, for example chlorpropham, desmedipham, phenmaedipham and propham;
chloroacetanilides, for example alachlor, acetochlor, butachlor, metazachlor,
metolachlor, pretilachlor and propachlor; dinitroanilines, for example
oryzalin,
pendimethalin and trifluralin; diphenyl ethers, for example acifluorfen,
bifenox,
chlormethoxynil (X-52), chlornitrofen, fluoroglycofen, fomesafen, halosafen,
lactofen,
nitrofen and oxyfluorfen; areas, for example chlortoluron, cumyluron (JC-940),
diuron,
dymron (daimuron), fluormeturon, isoproturon, linuron and methabenzthiazuton;
hyroxylamines, for example alloxydim, clethodim, cycloxydim, sethoxydim and
tralkoxydim; imidazolinones, for example imazethapyr, imazamethabenz, imazapyr
and
imazaquin; nitriles, for example bromoxynil, dichlobenil and ioxyrul;
oxyacetamides,
for example mefenacet; sulphonylureas, for example AC-014 (AC-322140),
amidosulfuron, bensulfuron(-methyl), chlorimuron(-ethyl), chlorsulfuron,
cinosulfuron,
DPX-47, HOE-404, imazosulfuron, metsulfuron(-methyl), nicosulfuron,
primisulfuron,
pyrazosulfuron(-ethyl), thifensulfuron(-methyl), triasulfuron and tribenuron(-
methyl);
thiocarbamates, for example butylate, cycloate, diallate, dimepiperate, EPTC,
esprocarb,
molinate, prosulphocarb, thiobencarb (benthiocarb) and triallate; triazines,
for example
atrazine, cyanazine, dimethametryn, prometryne, simazin, simetryne, terbutryne
and
terbutylazin; triazinones, for example hexazinon, metamitron and metribuzin;
others, for
example aminotriazole, benfuresate, bensulide, bentazone, benzofenap,
bromobutide,
butamifos, cafenstrole (CH-900), cinmethylin, clomazone, clomeprop,
clopyralid,
DEH-112, difenzoquat, dimethenamid, dithiopyr, ethofumesate, flumetsulam,
fluorochloridone, glufosinate, glyphosate, amiprophos(-methyl), anilofos,
etobenzanid
(HW-52), isoxaben, KPP-314, KUH-833, KUH-911, KUH-920, MK-243, naproanilide,
NSK-850, oxadiazon, piperophos, propanil, pyrazolate, pyrazoxyfen,
pyributicarb,
pyridate, quinchlorac, quinmerac, sulphosate and tridiphane.
Mixtures with other known active compounds, such as fungicides, insecticides,
i ~i i I
CA 02189456 2005-02-08
30517-69
- 59 -
acaricides, nematicides, bird repellants, plant nutrients
and soil conditioners, are also possible.
The active compounds can be used as such, in the
form of their formulations or in the use forms prepared
therefrom by further dilution, such as ready-to-use
solutions, suspensions, emulsion's, powders, pastes and
granules. They are used in the customary manner, for
example by watering, spraying, atomizing or spreading.
The invention also provides a method of combatting
unwanted plants, wherein a compound or composition of the
invention is caused to act on the unwanted plants, their
habitat or both.
The active compounds according to the invention can
be applied either before or after emergence of the plants.
They can also be incorporated into the soil before sowing.
The amount of active compound used can vary within
a substantial range. It depends essentially on the nature
of the desired effect. In general, the amounts used are
between 10 g and 10 kg of active compound per hectare of
soil surface, preferably between 50 g and 5 kg per ha.
The preparation and use of the active compounds
according to the invention can be seen from the examples
which follow.
Preparation examples:
Example 1
O NH-S02-C2Hs
N- 'N C j-
I
N NHz
F
l I
CA 02189456 2005-02-08
30517-69
- 59a -
Hydrogen sulphide is passed at from 50°C to 60°C
to saturation point into a mixture of 5.5 g (15 mmol) of
2-(4-cyano-2-fluoro-5-ethylsulphcnylamino-phenyl)-5,6,7,8-
tetrahydro-1,2,4-triazolo[4,3-a]pyridin-3(2H)-one, 5 ml of
triethylamine and 50 ml of pyridine and the mixture is
stirred at 60°C for 30 minutes more. It is then
concentrated in vacuo, the residue is stirred with 2 N
hydrochloric acid and the solids are filtered
WO 95/30661 PCT/EP95/01507
-6~- 2189456
off. The solid product is recrystallized from isopropanol.
4.8 g (80% of theory) of 2-(2-fluoro-5-ethylsulphonylamino-4-thiocarbamoyl-
phenyl)-
5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyridin-3(2H)-one are obtained of
melting point
220°C.
Example 2
p NHZ
Hsr.2\N~N ~S
\ / CAN
FCC ~-= p
4.04 g (0.04 mol) of triethylamine are added to 6.3 g (0.02 mol) of 2-(2-
fluoro-4-
cyano-5-amino-phenyl)-4-ethyl-5-trifluoromethyl-2,4-dihydro-3H-1,2,4-triazol-3-
one in
100 ml of acetone. Hydrogen sulphide is passed in rapidly at 23°C, and
the internal
temperature rises to 33°C. The reaction is complete after 1 hour. The
solution is
concentrated on a rotary evaporator and the residue is recrystallized from
isopropanol.
2.9 g (42% of theory) of 2-(2-fluoro-4-thiocarbamoyl-5-amino-phenyl)-4-ethyl-5-
trifluoromethyl-2,4-dihydro-3H-1,2,4-triazol-3-one are obtained of melting
point 161°C.
Example 3
S Nf+SOz-CzHs
S
\ / ~N
N
FzCH~ F
11 g (0.0276 mol) of 2-(2-fluoro-4-cyano-5-ethylsulphonylaminophenyl)-4-methyl-
5-
difluoromethyl-2,4-dihydro-3H-1,2,4-triazole-3-thione are stirred at
70°C for 4.5 hours
in 100 ml of pyridine while passing in hydrogen sulphide. The solution is
concentrated
on a rotary evaporator, the residue is stirred in water, the mixture is
acidified with
concentrated hydrochloric acid, and precipitated product is filtered off,
washed with
WO 95/30661 PCC/EP95/01507
-61- 2189456
water and recrystallized from isopropanol.
9 g (77% of theory) of 2-(2-fluoro-4-thiocarbamoyl-5-
ethylsulphonylaminophenyl)-4-
methyl-5-difluoromethyl-2,4-dihydro-3H-1,2,4-triazole-3-thione are obtained of
melting
point 183°C.
In analogy to Preparation Examples 1, 2 and 3 and in accordance with the
general
description of the preparation process according to the invention it is also
possible, fow
example, to prepare the compounds of the formula (I) listed in Table 1 below.
R' Rz
S
z ~ ~ cn
NHz
R'
Table 1: Examples of the compounds of the formula (I)
Ex. No. R' Rz R' Z Melting
point (°C)
4 F F H ~ 110
HsCW N ~ N~
_ I
N
FJC
S F -NH-S02-C2H5 H ~ 143
HsCW N ~ N~
_ I
N
F3C
6 F -0-CH-C-CH H S 162
CHI HOC ~N~ N~
_ I
N
F=CH
WO 95/30661 PCT/EP95/01507
! 62 2189456
Ex. No. R' R' R' Z Melting
point (°C)
7 F -NH-S02-C2H5 H ~ 237
HOC-NHS ~~ (.lriethyl-
N
ammoaium
salt)
8 F F H ~ \N S N/ 220
J~C
_ t
N
F=CH
9 F -NH-S02-C2H5 H ~'' 218
H~CwN~N~
_ I
N
FZCH
F NH-S02-C2H5 H N~N~ 185
N
F3C
I1 F F H ~ z18
~N~
N
12 F -OC2H3 H 202
~N~
N
WO 95/30661 PCT/EP95/01507
-63-
2189456
Ex. No. R' R= R' Z Melting
point (°C)
13 F I~IH-S02-C2H5 H 2I0
14 F NH-S02-C2Hg H ~'I 203
HaCi~N~N/
_ I
~''~ N
F2CH'
15 F F H o
I~ 185
~CwN~N/
_ I
N
F=CH
16 F i -soz-C=Hs H
170
CHz HsCz ~ N
_ f
N
FCC
17 F -OCH(CH3)2 H o 206
N~N~
I
N
18 F OH H o 250
N~N~
I
N
WO 95/30661 PCT/EP95/01507
. ..
-64- 21894(
Ex. No. R' R= R' Z Melting
point (°C)
19 F -N-SOz-C=H5 H O 98
Cl'~a FaC ~N-
N
20 F -Nfi-S02-C2Hg H O 208
FaC~N-
N--
H3C~ \'O
21 F -NH-S02-C2H5 H O 55
FaC~N-
N-.
HOC \\S
22 F -IQH-S02-C2H5 H H3C [ O (amorphous)
N~
H3C
O
23 F -ISH-S02-C2H5 H O 183
[ I
N~
O
24 F -N-SOz CH3 H O 167
CHI F C N-
N-
H~C~ O
WO 95/30661 PGT/EP95/01507
. -, -65-
2189456
Ex. No. R' R- R' Z Melting
point (°C)
25 F -NH-S02-CH3 H O 130
FzC~N
N
H,C~ O
26 F -NH-S02-CH3 H 243
27 F F H ~ I99
F3C N-
O
28 F -NH-S02-C2H5 H O 202
H~C~N~N/
_ I
N
FCC
29 F -NH-S02-CH3 H O'I 200
H~C~N~N/
_ I
N
FCC
30 F -NH-S02-CH(CH3)2 H OII 204
HaCwN~N~
_ I
N
FCC
WO 95/30661 PCT/EP95/01507
-, ~ -66- 2~ g945b
Ex. No. R' R= R' Z Melting
point
(C)
31 F -L1H-S02-C2H5 H S 195
H~C~N~N~
_ 1
~'~'N
FzC
32 F -~'CH-C-CH H 122
C ~C~
Hs N
_ I
N
F$C
33 F -~Hi C-CH H C'I 190
H~C~N~N/
_ 1
N
F'C
34 F NH-S02-CH3 H sI' 178
H3C~N~N/
_ I
N
F5C
35 F NH-S02-C3H7 H ~II 203
HjC~N~N/
_ I
N
F3C
36 F -Nfi-S02-C2H5 H ~ 199
(CH~zCH w
~ N/
N
_ I
N
HrC~
WO 95130661 PCT/EP95101507
., ~' -67- 2189456
Ex. No. R' R= R' Z Melting
point
(C)
37 F. -NH-S02-C2H5 H ~ 153
HsCz~N N/
I
N
38 F -NH-S02-C2H5 H ~ 206
~~zCH w N ~ N/
_ I
~''~N
(CH~zCH~
39 F -NH-S02-C2H5 H ~I( 167
~CswN~N/
N
40 F F H ~ 130
HaCwN~N/
_ I
N
~
FsC
.
41 F -OCH2CF3 H 173
HsC~N N/
_ I
N
FsC
42 F -OC2H40CH3 H ~I! 148
HaCwN~N/
_ I
N
FsC
WO 95/30661 PCT/EP95/01507
.., ~ -6g- z~s94~6
Ex. No. R' R= R' Z Melting
point (°C)
43 F -OC2H5 H ~I 155
i N/
I
-N
44 F -OC3H7 H CI 130
i N/
I
- N
45 F F H CI 131
i N/
i
-N
g ~ 202
46 F -OCH(CH3)2 ~
N- _N/
~~O
47 F -OC2H5 H ~ 185
N~N/
~~O
48 F -NH-S02-C2H5 H ~I 111
i N/
-N
49 H -NH-S02-C2H5 H ~I 118
N/
I
- N
" , WO 95/30661 PCT/EP95/01507
~ 69 2189456
Ex. No. R' R= R' Z Melting
point (C)
50 F -NH-SOZ-C3H~_n H Ct 143
i N~
t
-N
51 F -OCZHqOCZHqOCHg H 168
FiCH ~
N
_ I
N
H3C
52 F -NH-S02-C3H7-n H CHa (amorphous)
FaC N ~S
~N~
~
O
53 F -NH-S02-C3H~_a H CHa 232
FaC~! N ~O
~N~
11
ff
~IO~I
54 F -NH-SOZ-C3H7_i H CHa 226
FaC N O
O
55 F -NH-S02-C3H7_i H O 187
~C~.N~N~
I I
J= N
FaC
WO 95/30661 PCT/EP95101507
y -70-
2189456
Ex. No. R' R- R' Z Melting
point (°C)
56 F -NH-S02-CgH7-i H 236
~N/
~~~//'''~~ I
N
i7 F -NH-S02-C3H7_i H 252
~N/
[~ N
58 F -NH-S02-C3H7-i H C 109
HsC tJ~N/
I
N
FzCH
59 F -NH-S02-C3H7-i H C ~ 207
Hs =~N N/
I
N
F=CH
2I5
60 F -NH-S02-C3H7-i H ht3C
/
N
-N
61 F -N(CH3}-S02C2H5 H ~'I 102
HaC N~N/
I I
J=N
F3C
WO 95130661 PCT/EP95/01507
-71-
2189455
Ex. No. R' R= R' Z Melting
point (°C)
62 F -NH-S02-C2H5 H ~II 185
~C N~N/
I
N
lisC
63 Cl -NH-S02-C2H5 H OII 121
~'~aC N~~N/
F ~N
a
64 F F H O 157
FiCH
~N N/
I I
.'~N
Fi~C
65 F -lYH-S02-C2H5 H O 195
N/
O
66 F -OH H S
~C N~N~ 193 (decomp)
DBU salt
N
FiCH
WO 95/30661 PCT/F,P95/01507
~ -72- 2189456
Preparation of the st~rtine comnounds~
Example II-1:
F3C~ CFi3
/ N
N ~
~ N"O
F
i
F
CN
5.8 g (0.042 mol) of potassium carbonate are added at room temperature to 6.3
g
(0.034 mol) of 4-methyl-3-trifluoromethyl-1,2,4-triazolin-5-one (cf. e.g. US
3,780,052)
and 5.4 g (0.034 mol) of 2,4,5-trifluorobenzonitrile (cf. e.g. EP 191181) in
150 ml of
dimethyl sulphoxide and the mixture is subsequently heated at 100°C for
14 hours. For
working up, the cooled reaction mixture is placed in water, adjusted to a pH
of 2 with
dilute hydrochloric acid and subjected several times to extraction with
dichloromethane.
The combined organic phases are dried over sodium sulphate and concentrated in
vacuo. The residue is chromatographed over silica gel (eluent:
dichloromethane).
6.2 g (60% of theory) of 1-(4-cyano-2,5-difluorophenyl)-4-methyl-3-
trifluoromethyl-
1,2,4-triawlin-5-one are obtained of melting point 74°C.
WO 95130661 PCT/EP95/01507
-73- 21894.56
Example II-2
F
0.83 g (0.006 mol) of potassium carbonate is added at room temperature to 1.52
g
(0.005 mol) of I-(4-cyano-2,5-difluorophenyl)-4-methyl-3-trifluoromethyl-1,2,4-
triazolin-5-one and 0.48 g (0.005 mol) of methanesulphonamide in SO ml of
dimethyl
sulphoxide and the mixture is subsequently heated at 120°C for 12
hours. For working
up, the cooled reaction mixture is placed in water, adjusted to a pH of 2 with
dilute
hydrochloric acid and subjected several times to extraction with
dichloromethane. The
combined organic phases are dried over sodium sulphate and concentrated in
vacuo.
The residue is chromatographed over silica gel (eluent:
dichloromethane/methanol
20:1).
0.55 g (28% of theory) of 1-(4-cyano-2-fluoro-5-methylsulphonylaminophenyl)-4-
methyl-3-trifluoromethyl-1,2,4-triazolin-5-one is obtained of melting point
67°C.
Examule II-3
FCC
H
CN
0.3 g (10 mmol) of sodium hydride (80%) is added at from 0°C to
5°C to an initial
charge of 1.8 g (10 mmol) of ethyl 3-amino-4,4,4-trifluoro-crotonate in 30 ml
of
F~C~ CHI
WO 95/30661 PCT/EP95/01507
-74- 2189456
1
dimethylformamide and 2 ml of toluene. The mixture is stirred at from
0°C to 5°C for
30 minutes. After the mixture has been cooled to -70°C, 0.9 g (5 mmol)
of 4-cyano-
2,5-difluoro-phenyl isocyanate - dissolved in 10 ml of toluene - is added and
the
mixture is stirred at from -60°C to -70°C for I50 minutes. After
the cooling bath has
been removed, 2 ml of acetic acid are added. The mixture is then diluted with
water to
about twice the volume and subjected to extraction with ethyl acetate. The
organic
phase is concentrated and the residue is crystallized with diisopropyl ether.
1.1 g (69% of theory) of I-(4-cyano-2,5-difluoro-phenyl)-3,6-dihydro-2,6-dioxo-
4-
trifluoromethyl-1-(2H)-pyrimidine are obtained of melting point 194°C.
Example II-4
CFiz
A mixture of 0.83 g (3 mmol) of 1-(4-cyano-2,5-difluorophenyl)-3,6-dihydro-2,6-
dioxo-
3,4-dimethyl-1(2H)-pyrimidine, 0.32 g (3 mmol) of methane sulphonamide, 0.6 g
of
potassium carbonate and 10 m of dimethyl sulphoxide is heated at 120°C
for 10 hours.
After cooling, the mixture is poured into ice-water and acidified with 2 N
hydrochloric
IS acid. It is then subjected to extraction with ethyl acetate and the organic
phase is
washed with water, dried with sodium sulphate and filtered. The solvent is
carefully
removed from the filtrate by distillation under a water-pump vacuum.
0.8 g (76% of theory) of 1-(4-cyano-2-fluoro-5-methylsulphonylaminophenyl)-3,6-
dihydro-2,6-dioxo-3,4-dimethyl-I(2H)-pyrimidine is obtained as crystalline
residue
(melting point >250°C).
NHS02Cti~
WO 95/30661 PCT/EP95/01507
2?89456
-,5
-, ~
Use Examples:
Example A
Pre-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent, the stated amount of
emulsifier
is added and the concentrate is diluted with water to the desired
concentration.
Seeds of the test plants are sown in normal soil and, after 24 hours, watered
with the
preparation of active compound. It is expedient to keep constant the amount of
water
per unit area. The concentration of the active compound in the preparation is
of no
importance, only the amount of active compound applied per unit area being
decisive.
After three weeks, the degree of damage to the plants is rated in % damage in
compari-
son to the development of the untreated control. The figures denote:
0% = no action (like untreated control)
100% = total destruction
WO 95/30661 PCT/F,P95101507
.. ~
-7 6- 2?9456
Tablc
Pre-emergence test/greenhouse
Active Applica-BarleyMaize Amaran-Cheno- Matri-Portu-Sola-
compound lion thus podium curialaca num
(Synthesis rate
Example (glha)
Number)
(3) 125 0 0 100 100 100 100 100
(5) 125 0 0 100 100 90 90 100
(6) 125 0 30 100 I00 93 100 100
(7) I25 30 0 100 100 95 100 95
WO 95/30661 PCT/EP95101507
-77- 2189456
Table B
Pre-emergence test/greenhouse
Active Applica-Wheat Abu- Amatan-Cheno-Matri-Portu-Sola-
Maize
compound lion thilonthus podiumcaria laca num
(Synthesisrate
Example (g/ha)
Number)
19 60 10 0 100 95 100 100 100 100
20 60 20 0 100 100 100 100 100 100
.
ZI 60 0 0 I00 100 100 100 100 100
22 250 0 20 lOD 100 100 100 95 100
23 60 0 0 95 70 95 100 95 70
24 30 0 20 100 95 100 100 100 100
25 30 0 0 100 100 9D 100 100 100
26 60 0 0 100 80 100 100 100 90
4 250 0 10 80 50 70 95 90 70
5 125 0 0 10 100 100 70 90 100
3 60 0 0 100 100 100 100 100 100
6 60 20 30 100 100 100 100 - 95
100
7 125 50 0 95 100 100 95 100 95
8 60 40 0 100 100 100 95 95 100
9 60 0 0 100 100 100 95 90 100
1 125 0 0 100 100 100 95 100 100
12 60 20 0 70 70 100 95 95 70
13 60 0 0 100 t00 100 70 90 100
16 b0 10 0 95 20 100 90 80 80
I7 30 0 0 100 100 IOD 100 100 100
- WO 95/30661 PCTIEP95/01507
--.
-7g- 2189456
Table B (continued)
Pre-emergence testJgreenhouse
Active Applica- Wheat Abu- Amaran-Cheno-Matri-Portu-Sola-
Maize
compound lion thilonthus podiumcarialaca num
(Synthesis rate
Example (glha)
Number)
28 60 0 20 100 100 100 100 100 100
29 60 0 0 I00 100 100 I00 100 100
30 60 0 0 100 100 100 90 100 100
31 60 0 0 100 100 100 100 100 100
32 30 10 0 - 100 100 100 95 100
33 30 10 10 95 95 100 100 100 100
34 60 0 0 100 100 100 100 100 100
35 60 0 0 100 100 100 90 95 100
40 250 20 0 30 40 100 95 100 80
41 60 20 30 100 I00 100 100 100 100
45 125 0 0 95 100 100 100 90 95
46 60 0 70 100 100 100 100 '100 100
47 30 0 0 lOD 95 100 IOD 100 100
48 60 0 0 100 100 100 100 100 100
51 30 0 20 100 - 100 95 100 100
WO 95/30661 P(.'f/EP95/01507
'~ 79 2189456
Example B
Post-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol ether
To produce a suitable preparation of active compound, 1 part by weight of
active
compound is mixed with the stated amount of solvent, the stated amount of
emulsifier
is added and the concentrate is diluted with water to the desired
concentration.
Test plants which have a height of 5 - 15 cm are sprayed with the preparation
of active
compound in such a way as to apply the particular amounts of active compound
desired
per unit area. After three weeks, the degree of damage to the plants is rated
in
damage in comparison to the development of the untreated control.
The figures denote:
0% = no action (like untreated control)
100% = total destruction
- WO 95/30661 PCT/EP95/01507
°- 2189456
-1
Table C
Post-emergence
test/greenhouse
Active Applica-Wheat Maiu Abu- Amaran-Cheno- Sola-Vero-
compound tion thilonthus podium num nica
(Synthesisrate
Example (g/ha)
Number)
19 4 S 20 95 95 95 100 100
20 4 0 15 100 95 95 100 95
21 4 5 60 90 100 70 100 100
22 30 15 0 100 100 40 100 20
23 30 0 50 95 - 90 100 I00
24 30 IS 70 100 100 100 100 100
25 15 0 50 100 I00 100 100 100
26 15 0 30 50 90 50 50 100
IS 10 20 100 I00 100 100 100
3 30 10 30 100 100 100 lOD 100
6 8 30 50 100 100 100 100 95
7 60 10 50 100 100 95 100 95
~
8 60 10 30 100 100 100 100 100
60 10 30 100 100 I00 100 100
I IS 10 50 100 95 95 100 100
12 8 10 30 100 100 95 100 100
13 15 0 30 95 100 80 100 90
.
16 60 20 60 95 100 100 100 100
17 8 10 10 100 100 95 !00 100
WO 95/30661 PCT/EP95/01507
-$' - 2189456
Table (continued)
Post-emergence test/greenhouse
Active Applica-Wheat Maize Abu- Amaran- Cheno-Sola-Vero-
compound tion thilonthus podiumnum nica
(Synthesisrate
Example (g/ha)
Number)
28 30 0 30 100 100 90 100 100
29 8 S 0 70 100 90 100 95
30 8 0 SO 100 100 95 100 70
31 8 0 70 100 100 90 100 100
32 4 1S 30 100 100 95 100 100
33 4 20 60 100 100 I00 100 100
34 15 0 30 100 100 100 100 100'
3S 30 0 25 100 100 90 100 9S
40 125 IO S SO 70 100 100 90
41 15 20 50 100 80 90 100 9S
43 15 0 50 100 95 100 100 100
44 8 0 IS 100 100 100 9S 100
4S 8 IO 40 9S 95 95 100 -
46 8 1S 20 100 100 9S 100 100
47 8 10 10 100 100 95 100 100
48 8 5 60 100 100 80 100 100
49 8 0 60 60 100 10 100 100
50 15 5. 70 100 95 80 100 95
S1 ~ 1S 1S 60 100 100 70 100 100