Note: Descriptions are shown in the official language in which they were submitted.
2lsas73
-2-
METHOD AND APPARATUS FOR DELIGNIFYING CHEMICAL PULP
The present invention relates to a method and apparatus for delignifying
chemical pulp. In particular, the invention relates to a method and apparatus
for delignifying pulp under alkaline conditions with oxygen in such a way that
5 preferably unoxidized white liquor is used as the source of alkali. Especiallypreferably, the method and apparatus according to the invention are applicable
to oxygen delignification of so called Kraft pulp.
It is known from the prior art that pulp from a digester - a batch or a
continuous digester - may be treated with oxygen to decrease the kappa
10 number, which means, in practice, removing at a desired effectiveness the
lignin acting as a binding agent of the fibres and being also partly inside the
fibres. Oxygen delignification like this is also performed in a known manner
under alkaline conditions, the pH being typically at the beginning of the
treatment in the order of 10 - 11, and at the end of the treatment close to
15 neutral, i.e. about 7 - 8. Presently, oxidized green or white liquor is used as the
source of alkali almost exclusively. Oxidization has been considered necessary,
since the liquors contain sulphide, which, while oxidizing, turns thiosulphate
and partly also sulphite. Thiosulphate, and also sulphite, for their part, turn
exothermic sulphate, especially in the presence of the fibres, whereby the
20 exothermic reaction is generally believed to lead to the deterioration of the pulp
quality, mainly by splitting up of cellulose chains. This phenomenon has, for
instance, been discussed in Coetzee's article " Review of Operating and
Technical Experience in 2 1/2 Years of Continuous Sapoxal Bleaching" in the
TAPPI International Bleaching Conference, Vancouver, 1973, as well as in
25 Baczynska's article "Use of White and Green Liquors as Alkalis in the Oxygen
Stage of Kraft Pulp Bleaching" in a Polish Journal "Przeglad Papier", September
1979, Vol. 35, no. 9, pp 309-313. High temperatures up to about 140 ~C
generally used in oxygen delignification increase the negative effect of the
exothermic reaction on the pulp quality. The oxidization of green and white
30 liquor requires a process department of its own, where the liquors are treated
either with air, as previously, or with pure oxygen, as has been done after
2190573
recent development, in such a way that the sulphide can be oxidized to
sulphate form as well as possible. In the same oxidization process, gaseous
compounds, for example carbonaceous and sulphuric compounds, are
generated, which compounds may be removed from the process to the
5 treatment of gases.
In a few publications from the 1970's and 1980's (Baczynska's presentation
"Use of Oxidized White Liquor in the Oxygen Stage of Pulp Bleaching" in the
EUCEPA Conference (Vienna), October 1977, and Charina et al.'s article
"Unoxidized White Liquor in Oxygen-Alkali Bleaching of Kraft Pulp" in a Russian
10 Journal "Bumazh. Prom.", Nov. 1983, no.11, pp. 12-13), a possibility is
discussed according to which white or green liquor would be used as a source
of alkali directly without oxidization in oxygen delignification. However,
prejudice has been created by, for instance, Chudakov et al.'s article "Use of
Oxidized White Liquor during Refining of Prehydrolysis Kraft Pulp", in a Russian15 Journal "Khim. Drev." (Riga), May/June 1986, no. 3, pp.28-30, in which it is
stated that oxidized white liquor is more effective than unoxidized
white liquor leading to increased pulp viscosity and alpha cellulose content.
Due to both this prejudice and performed mill experiments, unoxidized white
liquor has never been used as the source of alkali in oxygen delignification. One
20 natural barrier to the use of green or white liquor is the above-mentioned
tendency of the liquors to generate gas while oxidizing. As the oxygen
delignification is conventionally performed with a relatively long retention time,
the delignification result cannot be uniform when large gas bubbles are
generated in the pulp, which gas bubbles prevent the oxygen from
25 encountering with all fibres.
Further, the risk that the temperature-raising effect of the exothermic
oxidization reaction deteriorates the quality of the fibres has been taken into
account, and to a great extent for that reason unoxidized white or green liquor
has not been industrially utilized as the source of alkali in delignification. In
30 fact, it is required of Finnish pulp mills that the alkali used as the alkali source
has to be almost entirely oxidized. The required oxidation degree is 90 %,
2lsas73
-4
which means that at least 90 % of the sulphide present in the liquor has to be
oxidized .
In addition, clarifiers have previously been used to clarify liquors, which
clarifiers have not been able to remove all removable substances detrimental
5 to delignification reactions, e.g. carbon and metals, from the liquors. Recently
taken into use, disk and drum filters or other types of pressure filters used infiltration of liquor have improved the situation. The liquors from these kinds of
filters are much purer compared with the liquors from clarifiers.
Unexpectedly, it has been observed in connection with our experiments that
10 green or white liquor can readily be used as the source of alkali in a multi-step
oxygen delignification process developed by us. Since in our multi-step process
additional oxygen is mixed into the pulp preferably several times by means of
an efficient mixer, the gas generated in the pulp is also uniformly mixed into
the pulp, whereby generation of large gas accumulations becomes impossible.
15 On the other hand, the process developed by us also comprises a preferred
alternative according to which it is possible to remove gas after one or more
oxygen delignification steps in such a way that all gaseous products having
been generated during the oxidization of the liquor can be removed from the
pulp.
20 Further, the delignification process according to our invention may be
intensified by arranging a first delignification step to be run at a slightly lower
temperature than the following steps. This procedure prevents the deteriorating
effect of the exothermic oxidization reaction of the liquors on the cellulose
quality.
25 By means of further treatment of the liquors in a filter-type cleaner it is possible
to ensure that the substances detrimental to delignification reactions, e.g. themetals, may be removed from the liquors.
- 2lsas73
_ --5--
An advantage in the method according to our invention is that a cellulose mill
does not have to invest on the relatively expensive oxidization department of
the liquors and that it is possible to directly use white or green liquor comingfrom the process in the adjustment of the pH.
5 Other characterizing features of the method and apparatus according to the
invention become apparent from the appended claims.
In the following, the method and apparatus according to the invention are
explained in detail, with reference to the appended figures, in which
Fig. 1 illustrates a process according to a preferred embodiment of the
10 invention,
Fig. 2 illustrates a process according to a second preferred embodiment
of the invention,
Fig. 3 illustrates a process according to a third preferred embodiment of
the invention,
Fig. 4 illustrates a process according to a fourth preferred embodiment
of the invention.
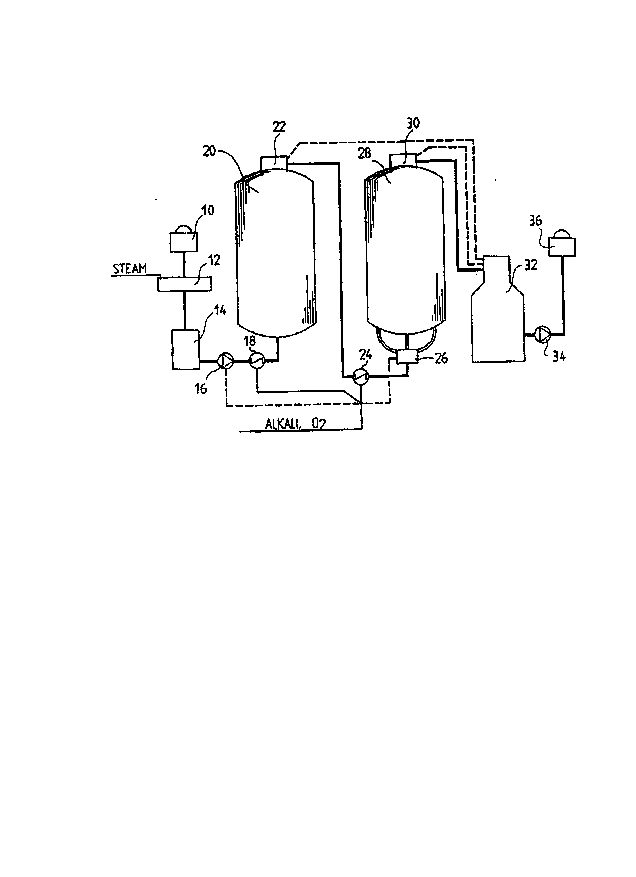
Figure 1 illustrates an apparatus applying a method according to a preferred
embodiment of the invention. The apparatus comprises process means
preceding the delignification stage, which process means may include for
20 example a brown stock washer 10, a steam feeding device/mixing device 12,
an intermediate tank 14 and a pump 16. Following said apparatus, the actual
process according to the invention comprises a mixer 18, a first delignificationtower 20, a first discharge apparatus/gas separator 22, a second mixer 24, a
feeding device 26, a second delignification tower 28, and a second discharge
25 apparatus/gas separator 30. The discharge device 22 or 30 can be of the type
described in US Patent 5,462,585 and is therefore not described in detail
herein. The described process may be followed by an intermediate tank 32
and/or a washer 36 with a pump 34. The process, the means of which were
described above, functions in such a way that oxygen and alkali are mixed into
30 the pulp to be delignified, flowing at a temperature of at least about 70~C, by
2lsas73
--6--
means of a mixer 18, the alkali being unoxidized white or green liquor, meaning
a liquor having less than 90 %, more preferably less than 50 % and most
preferably less than 10 % of its sulphides oxidized. It is also possible to effect
the mixing of said chemicals by means of a feed pump (shown by a broken
5 line), if the pump is designed particularly for this purpose, whereby, of course,
no separate mixer is needed. 3 - 15 kg/ADMT, preferably 5 - 10 kg/ADMT, and
most suitably about 7 kg/ADMT of liquor calculated as NaOH is fed into the
pulp. Correspondingly, 3 - 20 kg/ADMT, preferably 5 - 15 kg/ADMT, and most
suitably about 9 kg/ADMT of oxygen is fed into the pulp. After the mixing of
10 the chemicals, the pulp is led, through a steam mixer or other
temperature-raising apparatus if required, into the tower 20, where the
temperature of the pulp is in the order of 80 - 1 10 ~C, the pH at the beginningof the treatment in the range of 10 - 13, the pressure about 2 - 10, preferably
about 6 - 8 bar, and the treatment time about 0.1 - 1 hours, preferably about
15 0.2 - 0.75 hours. The ordinary dimensioning of towers would allow the use
of even higher temperatures, most often up to about 120 ~C, but when the
temperature exceeds 100 ~C, the pulp quality begins to deteriorate too much
and the quality of the pulps treated at temperatures exceeding 110 ~C can
hardly be considered acceptable. As the sulphides of white or green liquor
20 oxidize, the temperature of the pulp rises to
some extent. However, the initial temperature of the delignification is
correspondingly adjusted somewhat lower than what is normal, so that the
quality of the pulp cannot deteriorate significantly. At the same time, the pH
decreasing as the lignin in the pulp is separated into the liquid, some
25 carbonaceous and sulphuric gases are generated.
Preferably, if not necessarily, the pulp is removed from the tower 20 by means
of a discharge means 22. By means of said discharge means, gases generated
in the pulp in both delignification reactions and the oxidization of the liquor
may be separated. It is also to be observed that at the same time the inert gas
30 having got into the pulp along with oxygen may be removed, as the oxygen
presently used in the delignification is not pure but contains approximately 5
- 10 % impurities. As the oxygen is consumed in both the delignification and
21 qa 5~3
--7--
the oxidization of the liquor, these impurities already form a large part of thetotal amount of the gas. If the gases are not removed, they are uniformly
mixed into the pulp prior to leading the pulp into a second tower 28. The
mixing is performed by means of the same mixer 24, by which fresh oxygen
5 and alkali are fed into the pulp, which alkali may be unoxidized white or green
liquor. Through a second mixer, 5 - 15 kg/ADMT, preferably 5 - 10 kg/ADMT,
and most suitably about 7 kg/ADMT of liquor calculated as NaOH is fed into
the pulp. Correspondingly, 5 - 20 kg/ADMT, preferably 5 - 15 kg/ADMT, and
most suitably about 9 kg/ADMT of oxygen is fed into the pulp. The feeding of
10 the pulp into the second tower, through a steam mixer or other
temperature-raising apparatus if required, is effected by means of a special
dividing device 26, by means of which the pulp to be fed is divided into more
than one partial flows which are directed through the bottom of the tower at
several locations in such a way that it is ensured that the pulp column rises
15 uniformly in the tower. An embodiment of the dividing device is described, for
instance, in Finnish Patent Application laid open for opposition Fl-B-94442. If
desired, the feeding of the alkali and oxygen could also be performed by said
dividing device, as shown in Fig.1 by a broken line. Naturally, a corresponding
feeding device 26 could also be used in the feed of the tower 20. In the tower
20 28, the treatment temperature is in the order of 80 - 110 ~ C, pH at the
beginning of the treatment 10 - 13, the pressure about 1 - 10 bar, typically
about 2 - 7 bar, and the treatment time about 0.2 - 1 hours, preferably about
0.5 - 0.75 hours.
When discharging the pulp from the second tower, gas is again preferably, if
25 not necessarily, removed therefrom prior to leading the pulp to the next
treatment stage.
In connection with performed experiments, the following has been observed:
The quality of the pulp has been discovered within the limits of measurement
to remain the same as when performing the delignification with the reference
30 pulp alkalized with oxidized white liquor. No substantial difference in the
oxygen consumption was observed compared with conventional delignification.
21 ~Q~ 73
--8--
ln other words, the total consumption of oxygen at the experiment mill
remained the same when comparing the oxygen consumption of the process
according to the invention with the use in oxygen delignification of white liquor
oxidized separately with oxygen in a conventional manner. In some cases, the
5 total consumption of oxygen may even be expected to decrease when starting
the use of unoxidized white or green liquor.
Example
The mill-scale experiments were effected in a two-step oxygen delignification
stage. The hardwood pulp was treated in the two-step delignification process
10 shown in the figures in such a way that the treatment time in the first tower was 15 minutes and in the second tower 45 minutes. At the beginning,
oxidized white liquor was used in the delignification, after which unoxidized
white liquor was added into the liquor to be fed in such a way that the amount
of the liquor calculated as NaOH remained constant. During an experiment
15 period of several weeks, we were able to adjust the running parameters in such
a way that when using totally unoxidized white liquor, where almost none of
the sulphides of the white liquor have been oxidized, for the adjustment of the
pH in the delignification the viscosity constantly remained over 900 dm3/kg
during the last week, which has to be considered a sufficient target value for
20 hardwood pulp delignified with oxygen. Correspondingly, the viscosity after the
whole bleaching process remained over 850 dm3/kg, which is, in turn, a
sufficient target value for bleached pulp. On the basis of the experiments, it
can be concluded that if the running parameters are adjusted appropriately, the
use of unoxidized white liquor results in as good pulp quality as the use of
25 oxidized white liquor.
The performed experiments were limited to two periods. During the first
experiment period higher temperatures were used, i.e. 99 ~C in the first tower
and 104 ~C in the second tower. Also, larger amounts of chemicals were used,
i.e. 9 kg/ADMT/tower of liquor calculated as NaOH, as well as 9
30 kg/ADMT/tower of oxygen. During the second experiment period, treatment
temperatures were lowered in both towers, so that they were 95 ~C in the first
2lsas73
- - 9-
tower and 100 ~C in the second tower. When lowering the treatment
temperature, also the feed of the liquor (calculated as NaOH) to both towers
was decreased, at first to 7.5 kg and subsequently to 6.5 kg/ADMT/tower.
Also decreasing the feeding of the oxygen was tested by decreasing it from 9
5 kg to 8 kg/ADMT/tower.
Decreasing of neither the liquor nor the oxygen did cause any drastic changes
in the viscosity of the pulp or in the kappa number. The decrease in the kappa
number varied sporadically from about six to about eight during the whole
experiment period. It can be mentioned, though, that with larger feeding
10 amounts of oxygen and liquor the decrease in the kappa number remained
clearly above six kappa units, whereas with smaller amounts the kappa number
decreased sporadically less than six units.
Fig. 2 shows a process according to a second preferred embodiment of the
invention, in which an illustration of pre-treatment of the liquor coming to the15 delignification process is added to the process of Fig. 1. As noted earlier, in the
prior art the liquors were usually treated by means of clarifiers, in which a great
amount of impurities were left in the liquors, for example in the form of carbon,
metals and other undissolved material. Impurities like this either interfere with
the following delignification and bleaching reactions, consume the used
20 chemicals, or deteriorate the pulp quality. By disposing for example a disk or
drum filter 40 in the feed line of the liquor in such a way that the liquor coming
to the delignification is filtered in the filter 40 it is possible to remove most of
the impurities, and at the same it can be ensured that the delignification is
optimized in this respect.
25 The filter 40 can be, for instance of the type discussed in US Patent
5,149,448 issued to the present applicant, or in EP-A-0,692,994 or EP-A-
0,708,676. The separation efficiency of the filter is in the range of from at
least 95 to 99 percent. It should be mentioned in this context that, with
clarifiers, the separation efficiency is normally about 90 percent, under ideal
30 conditions up to about 95 percent. As is known, however, clarifiers are very
~ 2lsas73
-10-
sensitive to changes in operating conditions which may easily decrease.
Another filter suitable for use as the filter 40 is described in PCT/FI94/00485
of the present applicant. A commercially available, so-called X-
FILTER, which is a cross-flow filter, manufactured and sold by the present
5 applicant, is another possibility for the filter 40.
The mixing of the liquor into the pulp may be performed either by means of a
pump, mixer, injection into the pulp between the pump 16 and the tower 20,
or dividing feeding device (corresponding to the apparatus 26 of Fig. 1)
attached to the conduits of the bottom of the tower 20.
10 Fig. 3 illustrates a process according to a third preferred embodiment of theinvention, in which a change has been made to the process according to Fig.
1 or 2 (in Fig. 2 this is shown by broken lines as the part of the process is not
necessary but only a preferred embodiment). The change is about the feed of
oxygen to the process. In the embodiment of Fig. 3, at least part of the oxygen
15 is mixed into the liquor to be fed to the delignification stage by means of amixer 42 or the like mixing device, e.g. a pump, whereby there is sufficiently
time for the sulphides of the liquor to oxidize at least partly into thiosulphate
already before the liquor is fed into the delignification tower 20. Instead of
oxygen, an oxygen-containing gas, such as air, may naturally be used. It is
20 preferable to feed the liquor pre-oxidized in the described manner into the pulp
by means of a mixing member, preferably by means of a dividing mixer/feeding
device (in the figures the apparatus 26 in connection with the tower 28) which
is preferably attached to the conduits at the bottom of the tower, although alsothe pump 16 or the mixers 18 and 24 may be used. The reason why the
25 mixing is so preferable is that the temperature of the liquor rises to about 150
~C in the oxidization, whereby it is worthwhile to mix the hot liquor into the
pulp as uniformly as possible to prevent deterioration in parts of the pulp.
According to a further embodiment, the whole of the used liquor is fed in the
oxidized form to a first delignification step, as shown in Fig. 3. According to
30 a second further embodiment, liquor is oxidized as shown in Fig. 3, but it is divided in given proportion, preferably evenly, between the different
219a573
delignification towers. Further, according to a third embodiment, the whole of
the oxygen used in the delignification is mixed into the liquor, although it is
preferable to feed only that amount of oxygen which is required for the
oxidization of the liquor in the flow channel. Where the oxygen is fed
5 somewhere else than into the pulp in the embodiment of Fig. 3, a separate
mixer may be used, as shown in Fig. 1, or alternatively, a dividing feeding
device of the tower may also be used for the mixing of the oxygen.
One alternative embodiment may also be a process in which the liquor required
in the delignification is fed to the first delignification step, the temperature of
10 which is relatively low, preferably in the order of 80 - 90 ~C. If one wished to
achieve lower temperatures, pulp coming from the brown stock washing would
have to be cooled. In the first delignification step, oxidization of the liquor
primarily takes place, while in the second step it is possible to perform the
actual delignification at a higher temperature, partly utilizing heat generated in
1 5 the oxidization, and also utilizing additional oxygen preferably fed to the second
step. The temperature being low in the first step, the pulp does not deterioratein spite of a slight overdose of liquor. Naturally, a treatment process with more
steps would be possible, whereby the oxidization of the liquor would primarily
take place in the first tower and the actual delignification in the second and
20 third towers.
Fig. 4 illustrates yet one process according to a fourth preferred embodiment
of the invention. The starting point for the progressive process according to
Fig. 4 was the fact that previously the source of alkali in the delignification has
been liquor which has been oxidized in some way or another, and that in
25 addition, fresh liquor, NaOH, has been used for the adjustment of the pH in the
bleaching. The use of oxidized liquor does not change the liquor balance of the
mill at all, since the liquor is taken from the circulation of liquor. By contrast,
fresh liquor brought to the bleaching means that an addition is made to the
circulation of the liquor, whereby the liquor balance is disturbed. The method
30 according to our invention brings a solution to this problem, too, since the
modern filtering technology enables the purification of the green or white liquor
- 219~573
-12-
taken from the liquor circulation so efficiently that the purified liquor may also
be fed to the bleaching to raise the pH. One of the many alternatives is the
peroxide bleaching stage of TCF (totally chlorine free bleaching) or ECF
(elemental chlorine free bleaching), the pH of which may now be adjusted
5 according to the method of our invention by using liquor from the liquor
circulation. Previously this would have been out of the question, since the
liquor from the circulation would have contained too great an amount of metals
that decompose peroxide.
As may be observed on the basis of the above description, a delignification
10 process has been developed which breaks the previous prejudices and biases
and brings about significant savings for the chemical pulp mill as regards both
investments and consumption of chemicals. However, it has to be taken into
account that the above description only concerns a few preferred embodiments
of the invention, which embodiments are not, by any means, intended to be
15 interpreted as limiting the invention. In other words, the use of unoxidized
white liquor according to the invention may also be performed in a one-step
delignification stage, even if a process with two or more steps does definitely
bring some advantages as regards the process technology. Hence, the
appended claims alone define the actual scope of the invention.