Note: Descriptions are shown in the official language in which they were submitted.
2191002
Background of The Invention:
The present invention relates to methods and formulae for attaching poly-
carbohydrate hydrocolloids to cellulose fibers in order to prepare what are
known as gum-fiber composites or gumfers, and the use of such composites
in preparing building products.
Gumfers are reactive with inorganic cementing agents including Portlandcement, magnesium or aluminium cements, lime, silicates, gypsum plaster,
and clay, which are used to prepare building products including for example:
cement or plaster based castings; tiles; roofing shingles; non-fired
bricks and building blocks; wall sheathing; sub-flooring; mortars; filling
and patching compounds; and dry cementing mixes. Said gum-fiber composites
may be used as filling or as reinforcing agents for cementing agents, the
incorporation of which results in improved working properties of wet acqueous
admixtures and in improved physical properties of the building products
formed thereof, including for example: low density, high strength to
weight ratio, and increased volumetric yields.
The methods involve the attachment of hydrocolloids to cellulose and tohemi-cellulose fibers in high weight ratio by inducing hydrogen bonding
at pH values less than 7.0, and by co-precipitating with mineral salts,
including for example: salts of calcium, magnesium, aluminium, iron, and
salts of oxides, carbonates, silicates, sulphates, phosphates, or borates.
By polycarbohydrate hydrocolloid is meant all water soluble or water
dispersible forms of complex carbohydrates including those starchy or
gummy substances found in plants, algae, fungae, or bacteria, which are
sugar polymers and are chiefly carbohydrate in nature. Said carbohydrates
are generally composed of repeating units of simple sugars such as for
example, glucose, galactose, mannose, arabinose, rhamnose, or gulose,
together with randomly or regularly repeating units of uronic acid sugar
units such as for example, glucuronic, galacturonic, mannuronic, or
guluronic acids, and also with randomly or regularly repeating units
of sulphated or phosphorylated sugar units including for example, glucose-
2191002
6-phosphate, or galactose-2,4, or 6-sulphate. Examples of such gums
include: sodium alginates, agar, carrageenin, gellan gum, xanthan gum,
starch phosphates, and pectins.
By cellulose is meant alpha-cellulose, micro-crystalline cellulose, or
hemi-cellulose, or all such water insoluble polycarbohydrates, chiefly
composed of repeating glucose or xylose units in linear beta 1,4 linkage,
which are found in plant cell walls.
According to prior art methods, the addition of fibers to cementing agents
as fillers or reinforcing agents in making building products is well known
and includes not only the use of cellulose fibers but also of glass, metal,
mineral, and synthetic polymer fibers as well. To give only one example
of prior art use of cellulose fibers in a building product formulation, the
technique of adding straw to mud or clay in order to prepare sun-dried
bricks was practised by early civilizations. Normally, only a small pro-
portion of fibers may be incorporated into a wet acqueous mixture contain-
ing cementing agents without causing deleterious effects on either the wet
mixture thus formed or on the final building product itself, and in the
case of cellulose fibers, the maximum amount of cellulous material which
may be added seldom exceeds 10% by weight of the cementing agent employed,
or a ratio of 1 parts fiber to 10 parts cementing agent. In the case of
cements, lime, gypsum plasters, and clays, water is an essential component
to be added to all such materials, not only to achieve workability, but
also to react with inorganic cementing agents in order to form a solid,
crystalline structure of good strength bearing properties, in which the
added fiber is imbedded after hardening and curing.
Physical and chemical properties of the fiber and its reactivity towards
the cementing agent are important as concerns the reinforcing effect of
fiber on tensile or compressive strength of the building product, and the
effect of its inclusion on other properties such as th~rm~l expansion, and
density. For example, particle size, fiber length, surface area, density,
water absorption capacity, thermal coefficient of expansion, elasticity or
-- 2 --
2191002
brittleness, tensile strength, and chemical or physical reactivity of the
fiber compared to the cementing agent, all bear measurable effect on final
properties of the building product formed. To give several examples of
effects af properties of the fiber on building products; if surface area of
fiber is large for exa~ple, then an excess of liquid water may be required
to wet the fiber as well as to saturate the cementing agent and other
additives, thereby contributing to weeping of water from acqueous
mixtures or to a reduction in final bond strength in the building product
after curing; if fiber density is comparable to that of other ingredients,
lightness or high strength to weight ratios in the product may not be
achieved; if physical or chemical attraction for cementing agent is
poor, then low adhesion to cohesion ratios may result so that the fiber is
insufficiently bonded to the agent, and physical separation from the
product results on impact or stress; if thermal coefficient of expansion
of fiber differs greatly from that of the cementing agent and other
additives after curing, then the fiber may separate from the product when
subjected to extremes of either temperature or moisture or both, especially
so and also if bonding of fiber to the product is poor.
When preparing products using inorganic cementing agents, the compoundsusually being in the form of dry, free-flowing powders, or in the form of
a wet cake in the case of clay, are first wetted with water in order to
form a slurry or paste, and various fillers including, sand, stone, gravel,
pazzolanic materials, ignaceous materials such as perlite, micaceous
materials such as vermiculite, and other additives including accelerating
or retarding agents, curing and bon~ing agents, and colorants are added in
various proportions. Where a fiber is to be used, it is usually added in a
manner similar to that employed for other fillers. Wet mixtures are then
paured or cast into forms or molds, or may be used as mortars, grouting
compounds, or as slips in order to coat surfaces, or to repair cracks and
fill crevices. Often, and before castings begin to set, the surfaces of
such mixtures are spread over or worked in with a trowel, in order to pack
the mixture firmly into the mold or crevice and to obtain a smooth surface
finish once the product has been hardened and cured; therefore, wet mixtures
2191002
must be of suitable consistency to permit workability, and the desired
consistency is usually obtained by varying the proportion of water or other
additives added to the mixture. Next, the mixtures must be allowed to
remain undisturbed while chemical reaction with added water takes place and
before the mixtures have begun to set and harden in order not to weaken
final bond strength which it is desired to create. Castings are at this
point usually covered over to prevent rapid evaporation of moisture from
surfaces which could lead to shrinkage cracking, or improper curing.
Finally, after sufficient hardening, casting frames are carefully removed,
and the product is allowed to dry thoroughly by natural or forced convection,
which completes the curing stage, allowing the product to develop its
ultimate bond strength.
According to prior art methods, when fibers containing a high cellulosecontent are added to building products, there generally results the need
to use an excess of water beyond that normally required in order to form a
manageable slurry or paste. Cellulose fibers generally have a high water
absorption capacity compared to other cementing agents or additives, there-
fore an amount of water in excess of that normally required must be added
to the mixtures. Cellulose fibers also possess a feathery nature,
consequently fibers tend to nest or interlock, making spreading, casting,
and surface finishing difficult. In attempts to eliminate nesting,
additional quantities of water are again often added in order to promote
lubrication. Cellulose fibers are also chemically inert, so that any bond-
ing which does take place is purely physical.
These factors, coupled together with the need to use excess water, lead to
many disadvantages to the use of cellulose fibers in building products.
For example, wet mixtures tend to separate on st~n~;nq due to attraction
of cellulose fibers for one another. Gross separation of components,
shrinkage and cracking, weeping of water from mixtures, and spalling of
cn~ron~nts from casting surfaces often occurs. Wet mixtures have longer
hold-out periods before setting, and require longer periods of time to dry.
The final bond strength in the cured articles is often weakened, both due to
the excess of moisture which has been added and also due to the inertness of
21qlO02
cellulose.
Particularly when cellulose fibers in the form of coarse wood chips, sawdust,
or splinters is used, these materials often contain substantial quantities of
water soluble substances or of insoluble oils or resins such as lignin. Such
impurities may effect final bond strength similarly to the use of excess water,
since the soluble impurities interfere with setting, and the oils and resins
are water repellant.
In order to improve the degree of chemical and physical bonding betweencellulose and cementing agent, thereby improving ultimate bond strength,
building products are often formed using a pressure technique in order to
express both trapped air and water and to compact the wet mixtures. The
pressed material may then also be heated or accelerating agents may also be
added in order to accelerate the rate of setting and curing in the products.
These methods, although resulting in an impL~v~-l~nt in bonding, also result
in greater density so that little or no advantage of the low density of
cellulose fibers and the ability of such fibers to create air poc]~ets in the
products once excess moisture has been expressed or removed by evaporation
during curing and drying, is to be obtained. For example, pure cellulose
possesses a pycnometer density of 1.4 g/cc whereas cementing agents and
other additives possess densities between 2.3 and 3.0 g/cc. Expanded cellulose
fibers containing air can achieve bulk densities as low as 0.1 to 0.2 g/cc, and
hence possess superior heat insulating value compared to other building
materials. When mixtures are compressed, the potential insulating quality of
cellulose is lost. The use of such methods also necessitates an unusual
expenditure of energy and equipment beyond that normally required.
Since both the moisture expansion coefficient and also the thermal coefficient
of expansion of cellulose fibers often differs significantly from that of
other c~ r~ s in a building mixture, and also since binding of fibers
within the matrix of the building product is often poor for reasons
previously given, the products often have a tendency to shrink and crack
while drying, and also when subjected to extremes in either moisture or
2191002
temperature, and the products are, therefore, in many cases unsuitable for
exterior construction purposes in particular.
It can be seen from the preceding that prior art processes in which cellulose
fibers are employed are seldom successful in achieving significant advantages
in working properties, strength or impact resistance, lightnessl or durability,
which permits wider use of the products in a variety of applications,
particularly as concerns exterior uses. Building products containing cellulose
fibers are, therefore, limited to interior uses such as for example:
patching compounds or wall panelling, the latter of which is often prepared
using pressure techniques and consequently offer no impLo~t,.~nt in either
strength or density over existing products made without the use of cellulose
fibers. Attempts at purification of fibers by alkaline bleaching to remove
impurities, or by grinding to finer particle size, do not seem to alter the
properties of cellulose to any significant extent to be of greater benefit
when used in building product formulations.
In view of the failure of prior art technology to provide either methods or
forms of cellulose suitable for use in building products to be of beneficial
advantage, it is an object of this invention to provide methods for reacting
such fibers with hydrocolloids or gums in order to prepare gum-fiber
composites or gumfers. Said gumfers exhibit increased chemical and physical
reactivity with cementing agents, resulting in improved properties of
building formulations and the products obtained thereof. Said composites
are convenient to use when added to building formulations, and do not result
in products which exhibit many of the preceding disadvantages of prior art
methods.
Gumfers prepared according to the methods of this invention possess superior
physical and chemical properties compared to untreated forms of cellulose,
including for example: elasticity, lubricity and ability to chemically
react with cementing agents and physically bind with aggregate fillers to a
controlled and variable degree according to their manner of preparation.
When used in building product formulations, gumfers may be used at
-- 6 --
21 91 002
concentrations exceeding 10% by weight of the cementing agents employed and up
to 25~/o by weight of the cementing agents employed, or a ratio of 2.5 parts
gumfer to 1.0 parts cementing agent. Gumfers hold moisture in acqueous
mixtures and prevent weeping, permit good workability, do not interfere with
setting or hardening, result in products which dry and cure rapidly, do not
exhibit shrinkage or cracking or spalling of components from surfaces after
curing, have a smooth surface finish, possess low density, greater durability,
and increased strength and impact resistance compared to prior art products
made using conventional cellulose fibers.
The methods and products of this invention do not require special equipment
or energy intensive methods in order to improve binding between fiber and
cementing agent. Consequently, the products of this invention may be made
to exhibit superior heat insulating qualities, strength, and lightness in
weight compared to existing products.
Since the products of this invention do not possess a susceptibility to decay
and disintegration as do other products made using conv~n~ional forms of
cellulose, they may find use in a wide variety of applications both exterior
as well as interior including for example: cement or clay roofing shingles,
or exterior or interior siding or sheathing, especially when such products
are also waterproofed after being formed and cured using c~-'v~ntional prior
art waterproofing techniques.
The methods of this invention are not limited to the use of only purified
forms of cellulose, but may be demonstrated on a wide variety of cellulose
substrates including for example: bleached or non-bleached paper pulp,
sawdust, wood chips, recycled newsprint and boxboard, or by-products from
agriculture or food processing. Th2 methods of this invention can in many
instances overcome the deleterious effects of water soluble impurities and
oils or resins present in these materials.
Since the preservation of forests is rapidly bec~m;ng a growing envi.ol~.~ntal
issue, the use of by-product cellulose fiber sources .such as recycled newsprint,
-- 7 --
2191002
agricultural wastes, or forestry slash, to prepare building products which are
composites containing a high percentage of cellulose fibers which can replace
existing wood products, will be a trend of the future. The methods of this
invention will allow for the preparation of building products containing high
percentages of cellulose which can replace existing wood products, thereby
having the potential to reduce environmental impact caused by deforestation.
The methods of this invention result in dry, powdered, free-flowing forms of
gum-fiber composites which are suitable for use in building formulations in
order to prepare dry pre-mixed compounds such as mortar mixes, patching or
grouting ~"L~o~lds, or mixes suitable for casting applications. Alternatively,
the methods of this invention allow for the preparation of moist gumfers which
are not dried before use and may therefore be used directly at site of the
building product application, by adding various cementing agents and fillers
to the moist gumfer, and using the mixtures thus formed to produce the final
building product.
Summary of The Invention:
m e inventive idea which this invention embodies is to suspend a material
containing a relatively high proportion of cellulose fibers in an acqueous
hydrocolloid solution at a temperature of between 5~ and 25~C, to which is
then added a mineral salt and an acidulant.
m e acidulant reacts with the mineral salt and also with the hydrocolloid,
releasing cations of the mineral salt into the medium. m e cations of
the mineral salt and the anions of the acidulant interchange for those cations
and anions which are associated with uronic acid groups of the hydrocolloid,
thereby causing the hydrocolloid to precipitate or to gel. The acidulant is
added in sufficient quantity to both neutralize the hydrocolloid and the
mineral salt as well as any other alkaline substances which may be present or
added, and also to reduce pH in the suspension to a value less than 7.0 but
greater than 2Ø me reduction in pH promotes not only an exchange of
cations with the hydrocolloid, but also attachment of the hydrocolloid to
21 91 002
cellulose by means of hydrogen bonding. The cations and anions provided by the
mineral salt and the acidulant, the hydrocolloid, and the cellulose fibers are
subsequently all together precipitated out of solution.
The cation-anion reacted hydrocolloid coats the cellulose fibers, forming a
gum-fiber composite or gumfer which is insoluble in water. The solution which
remains as a supernatent fluid is non-gelatinous and non-adhesive, has a water-
like consistency and may therefore be easily separated from the gumfer, the
hydrocolloid having been removed from solution by co-precipitation.
The gum-fiber composite may be rinsed or washed with water to remove residual
salts and acid, and then also drained or filtered, and pressed to a moisture
content of 50~/0 or less. The gumfer may then be dried to a moisture content of10% or less and reduced to any desired particle size by grinding, using
conventional methods. The gumfer is then suitable for use in building product
formulations containing inorganic cementing agents. Alternatively, after most
of the water has been removed by draining or filtration and the gumfer has beenwashed, the gumfern~ed not be pressed or dried, but is instead left in a moist
state in the form of an acqueous suspension or cake, to which may then be added
cementing agents and other additives in order to obtain a wet formulation
suitable for the preparation of building products. It is found that when
gumfers are added to building product formulations, the cation and anion reacted sugar
residues of the gumfer are reactive with cementing agents used in such
formulations. Water used in the process, after having been recovered from
the gumfer by straining or pressing, may be recycled to the beginning of the
process in order to prepare new quantities of gum-fiber composites.
The cellulose fiber source used to prepare gumfers, may be dervied from any
suitable plant source and used in any suitable particle size which permits
agitation of the hydrocolloid suspension, including for example: wood chips,
sawdust, splinters, or bark from woods, plant stalks or straws, forestry
slash, tree stumps, seed hulls, fruit fiber, sugar cane bagasse, sugar beet
pulp, soya fiber, and other wastes from forestry, agriculture, or food
2191002
processing operations; bleached or non-bleached paper pulp; newsprint or box-
board; purified forms of alpha-cellulose, microcrystalline cellulose, or hemi-
cellulose; grass clippings, leaves; or seaweeds and other algae. Since the
cellulose source may also contain substantial quantities of impurities such as
oils or lignin, therefore, the cellulose source should also contain at least -
50~/O by weight as cellulose or hemi-cellulose to be of practical use for the
purpose of this invention.
As it is preferable to suspend the cellulose source in a gum solution using as
high a concentration as is physically possible in order to permit agitation and reduce
water consumed in the process, the cellulose source is~preferrably-first reduced in
particle size using co~lv~-tional methods, in order to permit the use of
higher concentrations and lesser energy requirements for agitation.
As to the types of hydrocolloid which may be used, it is found that not all
hydrocolloids are suitable for purposes of this invention, although many
hydrocolloids are known which will associate with cellulose through hydrogen
bonding. In general, only those gums with a mostly linear backbone and a
m;n;mllm degree of side-branching, which possess structural characteristics
similar to cellulose, are useful for purposes of this invention. Such gums
mUst also possess a relatively high proportion of acid groups including for
example: carboxyl, sulphate, or phosphyl, attached to the linear backbone of
the gum chiefly by means of carbon atoms 2,3, or 6 of repeating sugar units.
Other specific structural characteristics also appear to be necessary as for
example, the types and repetitive nature of sugar units in the backbone, and
the manner in which these units are linked together, as for example via beta
1,4; beta 1,6; or beta 3,6 linkages. In general it is found that the more
closely the gum resembles cellulose in structure and in composition, the
lesser the degree of steric hindrance imposed by side chains or acid groups,
and the greater degree of the reactivity of the gum particularly with multi-
valent mineral cations such as Ca, Mg, Fe, Al, then the greater will be
the affinity of the gum for cellulose through hydrogen hon~;ng and co-
precipitation, and the greater the degree of bond;ng between gum and cellulose,
resulting in a more permanent and durable, and not readily dissociated form
of gum-fiber composite, which is ~ st suitable for purposes of this invention.
-- 10 --
2191002
Gums which are most suitable for purposes of this invention which may be used
alone or together in combination include the following:
Alginates, derivedfrom seaweedsFomposed of linear segments of D-mannuronic
acid and L-guluronic acid, and alternating residues of these acids in beta
1,4 linkage, preferably when used in a sodium salt form,
; carrageenin, also derived from seaweeds, composed of nearly linear repeating
galactose and 3,6-anhydrogalactose units, both sulphated and non-sulphated at
carbon positions 2,4 or 6, and preferably when used in kappa or iota forms,
these forms being more sensitive to monovalent ions K, MH4, and also to
multivalent mineral ions, including Ca, Mg,
; agar, also from seaweeds, a similarly linear and sulphated polygalactose
ester,
; gum ghatti, a mixed polymer of L-arabinose, D-galactose, D-mannose,
D-xylose, and D-glucuronic acid, with linear backbone mostly of galactose,
mannose and galacturonic residues, with acid labile side chains, preferably
when used in a most linear form with side chains removed by acid treatment,
; pectins from fruits, which are linear polymers of galacturonic acid, both
methylated and non-methylated, the pectic acid monovalent ion salt form,
or the de-methylated form being most preferred,
; dextran, composed of linear chains of 1,6-isomaltose linked glucose,
preferably when used in sulphated form as dextran sulphate,
; gellan gum, of bacterial origin from pseud~on~ elodea, composed of linear
chains of glucose, glucuronic acid, and rhamnose, acylated with acetate and
glycerate, preferably when used in a low acyl or de-acylated form,
; xanthan gum, from the bacterium xanth~n~ campestris, composed of linear
chain backbone of repeating glucose units linked beta 1,4 similarly to
cellulose, and trisaccharide side chains comprised of a glucuronic acid
residue be~ two mannose residues, the furthest from the chain carrying
a pyruvate group, the one closest being acetylated, and especially being
used whenever mineral salts cont~;ning ferric or iron (III) cations are
used in the process,
; carboxy-methyl cellulose, a cellulose polymer with carboxy-methyl group
substitution on carbon positions 2,3 or 6 of repeating glucose units linked
beta 1,4, preferably when used in a low degree of substitution form, and
21 91 002
especially when mineral salts containing tri-valent ions including ferric or
aluminium ions are used in the process,
; starch phosphate monoesters or oxidized starch, preferably linear amylose
fractions with repeating sugar units phosphorylated or carboxylated at carbon
positions 2,3 or 6, and preferably when used in a pre-gelatinized form,
; konjac flour, welan gum, guar gum, rhamsan gum, locust bean gum, and cereal
beta glucans, especially when used in a form which is either phosphorylated,
sulphated, or carboxylated at carbon positions 2,3 or 6, of sugar residues.
Gums which are less preferred or are unsuitable for purposes of this invention
for the following reasons include:
; gums which are mostly linear, hence do associate with cellulose through
hydrogen bonding, but which do not possess uronic acid groups, hence do not
gel or precipitate with mineral salts, including, guar gum, locust bean gum,
dextran, konjac flour or mannan, welan, rhamsan, cereal ~-glucans, dextrins
or amylose in their natural or unmodified forms. Guar gum and locust bean
may be induced to gel with borate ions at a pH greater than 7.0, therefore,
the use of these gums is limited to a specific variation of the invention,
; Gums which do exhibit a limited degree of association with cellulose
through hydrogen ~on~;n~ and also possess free acid groups, but also possess
side chains or are highly branched and, therefore, do not gel or precipitate
with mineral salts, including: gum arabic, gum ghatti in natural form,
tragacanth, karaya, amylopectin, polydextrose, and xanthan. Xanthan gum
will gel only in the presence of ferric ion at the pH of this invention,
hence is only useful when iron (III) salts are used as precipitants.
; Gums which are mostly linear, but contain a high proportion of uronic acid
groups affording steric hindrance, hence exhibit limited association with
cellulose and limited gelling capacity with mineral salts, including:
carboxymethyl cellulose, or lambda carrageenin,
; Gums which are mostly linear but contain side chains offering steric
hindrance and no uronic acid groups, including: methyl, hydroxy-methyl,
hydroxy-ethyl, and hydroxy-propyl cellulose, or mixed esters of these.
Other gums and hydrocolloids which do associate with cellulose to a limited
2191002
degree and do exhibit gelling or precipitation with mineral salts~ but are
proteinaceous in nature, and are hence less preferred because of their
greater susceptibility to bacterial and fungal decay compared to hydrocolloids,
including: casein, albumin, gelatine, and gluten.
It is found that preferred gums such as those precedingly described may also be
used in combination, and that certain combinations where the gums are synergistic
with one another and one of the gums is less preferred, or is a non-preferred
gum may also be used. For éxample, a mixture of carrageenin which is preferred
and konjac flour which is not preferred, or, a mixture of alginates which are
preferred and pregelatinized amylose starch which is not preferred, may be used
to prepare gumfers.
The mineral salt may be a soluble or insoluble salt in hydrous or anhydrous
form, cont~;n;ng any of the following mineral element cations, used alone or
together in combination: Li; K; NH4; Ca, Mg, Be, Sr, or Ba: Ti, or Zr; V, Cr,
or Mo; Mn; Fe; Co; Ni, Pd, or Pt; Cu, Ag, or Au; Zn; Pb; Cd; Hg; Al, *r Ga; Si,
Ge, or Sn; Ce; or Bi, and for purposes of this invention is most preferably
a salt of relatively low solubility in water cont~;n;ng the cations Ca, Mg, Ba,
~e, Al, Zn, NH4 , or K.
The anionic group of the mineral salt may consist of any of the following,
used alone or together in combination; oxide; hydroxide; chloride; bicarbonate
or carbonate; phosphate; orthophosphate; pyrophosphate; hydrcapatite;
phosphonate; phosphite, metaborate,tetraborate; flourite; aluminate;
aluminosilicate; fluosilicate; magnesium silicate; silicate; silicide;
metasilicate; orthosilicate; sulphate; sulphite; bisulphate, thiosulphate;
sulphide; selenate; tungstate; pyrate; arsenate; nitrates;or,an organic acid
anion consisting of: acetate; lactate; citrate; gluconate; tartrate; malate;
fumarate; maleate; stearate; adipate; succinate; benzoate; phthalate, but
for purposes of this invention is most preferably an oxide, carbonate, sulphate,phosphate including meta, ortho, and pyro forms, fluorite, fluosilicate, and
other silicates including meta or ortho forms, or mixed metal forms of
silicates. The mineral salt may also consist of a natural or synthetic
21~1002
.
mineral substance including for example: Portland cement, clay, talc, lim~stone,
silicate, gypsum, pulverized concrete or brick, hydroapatite, hydroboracite,
hydrohaematite, hydromagnesite, or hydrophite, all of which are governed by the
preceding. For example, Portland cement is a mixture of calcium silicates,
calcium hydroxide, and calcium sulphate.
The acidulant may be a salt which produces an acidic reaction when dissolved in
water, or an acid itself, either highly soluble or of low solubility in water
including: acidic salts of Na, K, NH4, Ca, or Mg, for example, monophosphates:
soluble mineral acids including: hydrochloric; sulphuric; nitric; phosphoric,
phosphorous, phosphonic, phosphinic, and organo derivates of these: boric;
sulphonic; or organic acids including: acetic, lactic, citric, gluconic,
malic, malonic, tartaric, carbon dioxide, or carbonic acid; or organic acids
and anhydrides of low solubility in water, including: maleic, fumaric, adipic,
succinic, stearic, benzoic, phthalic, aspartic, or glutamic; or organic
substances which revert to an acid form when dissolved in water including:
glucono-delta-lactone, or carbon dioxide. For purposes of this invention,
acidic substances which are most preferred include: monophosphates, hydrochloric
acid, sulphuric acid, phosphoric acid, and organo-derivates of phosphoric,
phosphorous, phosphonic, or phosphinic acids, boric acid, sulphonic acids,
organic anhydrides or acids,and carbonic acid or carbon dioxide.
Now, as concerns optimum conditions and variables of the process, including
for example, water hardness, pH, temperature, ingredient concentration, and
as to the choice of mineral salt, acidulant, hydrocolloid, or cellulose source
used, and the effect of these choices on methods of preparation and on properties
of gum-fiber composites and their use in building products, reasons for
preferred choices are given as follows.
According to methods of this invention, it is found that when a hydrocolloid
of preferred characteristics as previously described is dissolved in water,
a gum-like solution of viscous and adhesive nature is formed. When cellulose
fibers or materials of relatively high cellulose content are added to the
solution, the cellulose fibers being insoluble, are observed to swell due to
absorption of water from the solution, but remain suspended. No reaction
21 91 002
between cellulose and the hydrocolloid is evident since the solutîon within
the suspension remains viscous and adhesive and no reduction in viscosity is
apparent. If, however, either a highly soluble mineral salt or a salt of low
solubility containing the cations K, NH4, Ca, Mg, Al, or Fe for example, is
added, then depending on the type of gum used, the solution containing the
hydrocolloid will gel, and if the pH is also lowered to a value between 2.0
and 7.0 the suspension will im~ediately thin and the gum precipitate out into
the cellulose fibers, leaving behind a clear supernatent fluid of water-like
consistency, free of precipitate, which fluid and the precipitate itself are
non-gummy and non-adhesive in nature. It is also found, that if an acid-
ulent alone is used without added mineral salt, that a certain degree of
flocculation or precipitation also takes place, which is not as prominent
as when a mineral salt is also added, and that afterwards the solution still
remains somewhat gum-like or adhesive in nature.
From these observations it may be deduced that the hydrocolloid attaches
itself to cellulose fibers by means of hydrogen bonding, and is associated
with cellulose in a hypothetical helix structure held in place by the
mineral cations and anions contained by the mineral salt and acidulant which
have been added and are now part of the gum-fiber composite itself. This
deduction is confirmed by either heating the gumfer suspension or the moist
precipitate, or by raising the pH to a value greater than pH 7.0, in which
case it is observed that the suspension or the precipitate become gel-like
and adhesive in nature once again, indicating that hydrogen bonding between
gum and fiber has been disrupted and that the gum has been released back
into solution once again. This disruption in hydrogen hon~ing is not
observed when the gumfer has been dried to a moisture content of 10% or
less, and only occurs when the gumfer is remoistened and adjusted to an
alkaline pH value greater than 7.0, or is reheated to a temperature above
30 C. The hypothetical hon~;n~ between cellulose and hydrocolloid together
with mineral cations and anions is similar to that which occurs in plants,
between the cellulose of cell walls and the gum matrix into which the cells
are imbedded. For example, in plants, calcium and silica are often found
in significant amounts and are believed to act as cementing agents, holding
- 15 -
21 9 1 002
hydrocolloid and cellulose in place.
If the mineral salt is highly soluble,then immediate gelling of hydrocolloid
without addition of acidulant occurs, so that it may be difficult to add
acidulants to the mixture in order to induce hydrogen bonding and co-
precipitation of the gumfer, due to swelling of the mixture, the mixture
now being unstirrable. Therefore, it is most preferable to use a mineral
salt of low acqueous solubility such as previously described, including for
example: oxides, carbonates, di- and tri-phosphates, silicates, or sulphates,
of Ca, M~, Fe, or Al, so that cations of these salts are more slowly released,
controlling the rate of gelling and precipitation of the hydrocolloid. The
gelling rate may also be similarly controlled when the acidulant also
possesses a low solubility or a low rate of conversion to an acid form. The
mineral salts and acidic substances may be added in the form of liquid
solutions, or dry, granular, or crystalline materials, but are most preferably
first pre-dispersed, pre-dissolved, or pre-diluted in water before being
added to ensure their complete dispersion or dissolution in the suspension
and to prevent pocket reaction from taking place. Hence, it may be seen that
the choice of mineral salt and acidulant can be widely varied in order to
control both rates of gelling and precipitation to ~esiredadvantage.
It is also found that the preferred temperature of the suspension is less
than 30~C and is preferably between 5~ and 25~C, in order to prevent gumfers
from dissociating. The final pH in the suspension may be between 2.0 and
7.0, but is preferably between pH 3.0 and 5.0, which is effective for purposes
of gelling and co-precipitation and in order to conserve the amount of
acidulant to be added, or to limit the amounts of soluble impurities which
may have to be later removed from the precipitate by washing or pressing.
If the final pH is less than 3.0, then excess acidity may strip metal cations
from the hydLocolloid~ in which case the gumfer will be obtained in a free
acid form, which form may not be entirely suitable for purposes of this
invention.
As concerns the nature of cations and anions used, it is found that certain
hydrocolloids may be ;nduce~ to gel using mineral salts cont~ining sodium,
- 16 -
21 9 1 002
potassium or am~onium cations, for example, gellan gum or carrageenin. As
will be seen from the disclosure which follows~that except for ammonium or
potassium in certain cases, these particular cations are not entirely
desirable for inclusion in a building product formulation.
For example, where the gumfer is to be used in a formulation containingportland cement powder which is a mixture of calcium silicates, it is most
preferable to use a calcium salt such as calcium silicate or cement powder
itself as the mineral salt used to precipitate the gumfer. Also, since
phosphates, phosphonates, sulphates, sulphonates, borates, and carbonates
have a proven and beneficial effect on bonding and curing properties of
portland cements, it is preferable to use either phosphoric, phosphonic,
sulphuric, sulphonic, boric, or carbonic acids, or suitable combinations
of these acids as acidulants, since anions and cations of these salts and
acids when attached to the gumfer, together with residual amounts of ions
left over unreacted in the solution, will then be incorporated into the build-
ing product when the gumfer is added, thereby providing a beneficial effect.
Similarly, where gumfers are designed to be used in a building product
formulation which contains gypsum, plaster of Paris, or partially or fully
hydrated calcium sulphate, then it is most desirable to employ either
calcium sulphate, calcium carbonate, calcium oxide, calcium hydroxide or
calcium silicates or cement powder, or combinations of these for example
as the mineral salt, and also potassium salts as well, and to add sulphuric
acid as the acidulant, which results in the formation of calcium and
potassium cations and sulphate anions in the suspension, which will then be
incorporated into the gumfer. The gumfer will then display greater reactivity
with gypsum or plast~rbased formulations. For example, potassium salts are
known accelerators for setting of Plaster of Paris formulations. Similarly,
when using gumfers to prepare lime based mortars or mortar mixes, it is
preferable to precipitate gumfers using calcium oxide or calcium hydroxide
as the mineral salt, and to add carbonic acid or carbon dioxide as the
acidulant. Finally, when preparing
21 9 1 002
clay based products as for example non-fired ceramic castings, clay tiles or
bricks, it may be desirable to use clay, talc, or other aluminium, iron or
magnesium silicates as the mineral salt, and to use carbon dioxide, hydro-
chloric acid, or acetic acid as the acidulant. Obviously, a wide range of
formulations is possible.
The choice of mineral salt and acidulant is also pre-determined by the types
of cations and anions which are residual in the supernatent solution after
reaction,some of which will be residual in the gumfer, especially so if the
gumfer is not washed after precipitation. For example, most hydrocolloids
are available for use in a dry, powdered, sodium salt form, where Na cations
are attached to anionic carboxyl, phosphyl, or sulphate groups of the
hydrocolloid. When an acidulant is added, the acid formed reacts with the
mineral salt to release cations of the salt which are P~ nged for sodium
cations attached to the hydrocolloid. After reaction, sodium ions donated
by the gum, and anions of the acidulant remain in solution. For example,
if calcium lactate is added as the mineral salt, and hydrochloric acid is
added as the acidulant, then after reaction, sodium, chloride, and lactate
ions all reside within the solution so that in essence the solution contains
both sodium chloride and sodium lactate. If these salts are not adequately
removed by washing and pressing techniques after precipitation, then when the
gumfer is used in a portland cement based formulation for example, such salts
may interfere with setting of the cement and may effloresce to the surface
of the product after curing. In this example, it would be more desirable and
especially when the gumfer is not washed or pressed after precipitation, to
employ either phosphates, carbonates, silicates,or borates as the mineral
salt, and phosphoric acid, carbonic acid, or boric acid as the acidulant,
since residual salts cont~;n;ng sodium ions which are formed in these cases willconsist of either sodium phosphate, sodium carbonate, sodium silicate, or
sodium borate, any of which may have a more beneficial effect on setting
and curing properties of cement based formulations when included together with
the added gumfer, washing of the gumfer to remove trace impurities after
preparation prior to its use in a building product formulation thus being
less critical.
2191002
Thus, it can be seen that the mineral salt and acidulant may be selected from
a wide variety of sources, the choice of which is not only based on the ability
of such substances to react with hydrocolloid and induce precipitation, but is
also based on a sound knowledge of cementing agent chemistry, whether the gumferis to be used in cement, plaster, lime, or clay based formulations, The
chemistry of such cementing agents is well-known to anyone trained in the
science of using such materials.
When preparing gumfers using a mineral salt, it is desirable to include lesser
quantities of salts of ammonium, Ba, Cd, Cu, Ag, Zn, Pb, or Hg, and also
silicates, phosphates, borates, or sulphonates, for the following reasons.
Ammonium salts and borates when residual in gumfers and thus in the building
product forumation itself, particularly when phosphate anions are also
present, will confer properties of fire-retardancy on gumfers and also on the
building product, and the use of mono-, di-, tri- or poly ammonium phosphates
for this purpose is already well-known. Copper, cadmium, silver, zinc, lead,
or mercury salts for example, are toxic to microorganisms, and their use will
help prolong life of gumfers and also the building product, when exposed to
moist, humid conditions, as for example in exterior use. As previously
described, phosphates, silicates, and borates improve ultimate bond strength.
Zinc, barium, and silicates confer properties of water repellancy on concrete
products, and silicates also accelerate setting and curing. It may be
advantageous to include water soluble silicates such as sodium or potassium
silicates together with insoluble salts of Ca, Mg, Fe, Al, in order to
produce mixed silicates such as aluminium ferrosilicate, calcium magnesium
silicate or calcium aluminosilicate when preparing gumfers for example
since certain combinations of these may be beneficial for cement or clay
formulations.
The prece~;ng gumfer chemistry may be illustrated by means of the following
reaction s~m~:
-- 19 --
2191002
Example Gum Na Salt + Mineral + Acidulant = Gelled Gum + Salts
Salt Residual in
Supernatent
1 Gum-C02Na + MgC12 +HCl = Gum-C02(MgCl) + -NaCl, HCl
2 Gum-C02Na + Ca(HP04) + H3P04 = Gum-C02Ca(H2PO4) + Na2HPO4'
Na(H2P04),
H3P04.
3 Gum-C02Na + CaSiO3H20 + H2S 4 = Gum-co2casio4H3 + Na2so4~ H2 4'
Gum-C02Ca(HS04) Na2SiO3,H2SiO3,
sio2 .
4 Gum-C~2Na + CuS04 3 2 = Gum-C02Cu(Acetate) +Na Acetate,
(acetic acid) Cu(Acetate)2,
Na2S04,
Acetic acid.
Gum-C02Na + CaC03 + H02CRC02H = Gum-C02Ca- + C02, Ca(HC03),
(maleic acid) -02CRC02Ca(HC03) Na Maleate.
6 Gum-C02Na + ZnO+ C02 = Gum-C02Zn(HC03) + Zn(HC03),
Na2C03,
N2(HC03)
H2C03 .
Hydrogen bonding of gelled gum to cellulose occurs by means
of hydroxyl groups of both gum and cellulose, on carbon positions
2,3,or 6 of sugar residues, and is reinforced by Hydrogen ions
in solution, or at pH values less than 7Ø
- 20 -
2191002
As relates to concentrations and ratios of ingredients to be employed, the
hydrocolloid is usually employed at a concentration of between 1% and 2~o
of an acqueous solution. Since most hydrocolloids yield solutions which
are highly viscous at low concentration, it is desirable to use the lowest
viscosity forms of these gums wherever possible to permit higher gum loadings
and greater batch yields, so that in some cases 5% to 8% gum solutions are
possible, for example where low viscosity grades of alginates are available
for use.
The cellulous material may be used at any concentration between 5~/0 and 20%
by weight of the hydrocolloid solution, which permits convenient agitation.
Hence, the ratio of hydrocolloid to cellulose fiber may be infinitely varied
between O : 1 and 1 : 1. For practical purposes of this invention however,
gum : fiber ratios are varied between 1 parts gum to 20 parts cellulose
and 1 parts gum to 2 parts cellulose. By means of such variation in gum
: cellulose ratio, the amounts of gum added to cellulose fiber hence the
degree of reactivity towards cementing agents may be controlled and varied
up to and including some practical maximum, which is determined by the types
of mineral salt, acidulant, hydrocolloid, and cellulose source used to prepare
gumfers.
The mineral salt may be used in any concentration however, for practical
purposes, the mineral salt is used in an amount nearly stoichiomentric with
that required to react all uronic acid groups of the hydLocolloid, plus
uronic acid groups of the cellulose source due to uronic acid hemicellulose
content. For example, in the case where an alginate is used, the typical
equivalent weight of a repeating sugar unit in alginic acid is given as
between 176 and 194 daltons. Since each sugar residue in an alginate molecule
contains at least one uronic acid group on average, the amount of mineral
salt to be added ~o react with alginate is to be det~rm;ne~ as follows:
Weiqht of sodium alginate in formula x Combining weiqht of mineral salt used
Combining weight of sugar residue cont~;n;ng one free acid group in Na salt form
= Weight mineral salt to be added.
Generally, the mineral salt is used at a c~-~ntration of be~ n 25% and lOO~o
by weight of the hydrocolloid added.
- 21 -
2191002
The acidulant is added in an amount stoichiometric with that necessary to
displace all cations attached to uronic acid sugar units of the hydrocolloid,
and therefore in an amount stoichiometric with the mineral salt added, plus
an amount sufficient to neutralize other alkaline substances which may have
been added or are already present, plus an amount sufficient to achieve
a final pH of between 2.0 and 7.0, and most preferably, a final pH of between
3.0 and 5.0 in the suspension after reaction.
As relates to other additives beneficial to the process or to methods of
combining ingredients in order to permit proper dispersion and dissolution
of the hydrocolloid in water, it is found that in some cases it is desirable
to add sequestering agents to the water in order to chelate metal ions which
might interfere with hydration and dissolution of the gum. Agents which
are suitable for this purpose, which may be added in any amount up to 0.5%
of the gum solution by weight include the following, used alone or together
in combination: sodium or potassium salts of organic acids, phosphates,
polyphosphates, metaphosphates, or silicates. Many of these agents create
an alkaline reaction, raising the pH to a value greater than 7.0, which
is beneficial for hydration and dissolution of the gum. In other cases,
cellulose fibers may also first be treated with an alkali or alkaline substance
in addition to the preceding, including for example: sodium or potassium
carbonates or h~dLu~ides, while also adding detergents or sulphonates in
order to pre-soften fibers and to remove acidic impurities, oils or lignin,
which might interfere with gumfer formation, or might contaminate the building
product formulation to which the gumfer is added. To ensure proper dispersion
and dissolution of the hydrocolloid, it is prefer~ble to use powdered or
granular forms of the hydro~olloid together with sufficient agitation, or to
premix the gum with all or a portion of the fiber source and then add the
pre-mixture to water used in the ~rucess, while simultaneously providing a
suitable means of agitation of both gum and fiber. Such previously described
techniques are known to prior art.
As a specific variation of the process and especially whenever wood fibers
cont~in;ng soluble red~l~;n~ sugars including glucose are added to the process,
upon addition of mineral salt,an oxidase enzyme such as glucose oxidase,
together with a catalase enzyme may be added to convert glucose to gluconic
acid. The catalase enzyme decomposes hydrogen peroxide formed during the
_ 22 -
2191002
conversion reaction, which might otherwise poison the oxidase enzyme. Since
oxygen is also required for the conversion reaction, it may continuoUsly be
added by means of bubbling air through the reaction mixture. The gluconic acid
thus being formed, reacts with the mineral salt and hydrocolloid, releasing
cations and anions while simultaneously reducing pH in the reaction mixture,
causing ions and hydrocolloid to co-precipitate onto cellulose fibers thereby
forming a gumfer. In this manner, the addition of acidulant to the process
may be avoided as it is provided in a latent form by means of the reducing
sugars contained in the cellulose source.
The methods of this invention may be carried out using any suitable tank or
vessel of appropriate construction to withstand repeated exposure to the
contents of the reaction mixture, and also provided with a suitable means of
agitation. The process of this invention may also be carried out in a
continuous or semi-continuous fashion, as for example by adding ingredients
to a continuous mixer in order of use and in ratios and at rates at which
they are needed. After precipitation, gumfers may be transported by means
of pumping or gravity flow, and the supernatent fluids may be separated
by draining, filtration, and pressing techniques. Gumfers may be washed,
dried to a ~ isture content of 10% or less, and reduced in particle size
using ~nv~ional methods and equipment known to prior art. Depending
on the cellulose source used, and once neutralized to a pH of 7.0 or greater,
water exhausted from the process by draining, pressing, or washing, may
be recycled to the beginning of the process. Where a natural wood fiber
has been used, soluble impurities such as sugars extracted during the process,
may be removed by draining, washing, or pressing, or converted to sugar acids
using the precedingly described technique.
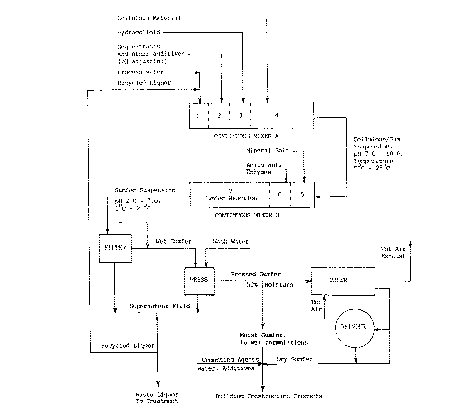
A continuous method of making gumfers is illustrated by means of Figure I.
With reference to Figure I, in the upper part of the drawing is shown
a continuous mixer A and B, to which ingredients charged to the process
are added in amounts and in order of use to form a gumfer. The sections
of A and B in which individual reactions take place are indicated by
means of the dashed lines, and are subsequently numbered in the direction
of flow from one section to another. Process water and recycled water
are added to section 1 and are mixed uniformly using appropriate mechanical
means, such as for ex~mple by means of a bladed shaft which passes through
the centre of the mixer, to form a mixture having a temperature of less
2191002
than 30~C. This mixture then flows into section 2 to which a sequestering
agent is then added. The sequestrant being properly dispersed and dissolved
in the water mixture, the mixture thus formed having a pH of 7.0 or greater,
then flows into section 3. To section 3 is then added a hydrocolloid
or a mixture of hydrocolloid and cellulose source, which is similarly
dispersed and dissolved. The hydrocolloid solution then enters section
4, and the h~l~nce of the cellulose source is added and similarly dispersed.
The cellulose-hydrocolloid suspension thus formed then flows into section
5 of continuous mixer B. For all intensive purposes, A and B are the
same mixer, so that section 5 is merely a continuation of mixer A. To
section 5 is then added a mineral salt which is similarly dispersed and
dissolved, and the suspension then enters section 6. To section 6 is
added an acidulant in order to achieve a pH of between 2.0 and 7.0, and
preferably between 3.0 and 5.0 in the final mixture, and the entire suspension
contain;ng water, sequestrant, hydrocolloid, cellulose source, mineral
salt, and acidulant is thoroughly mixed in section 7 to ensure complete
gelling and co-precipitation of ingredients and formation of a gumfer.
A suitable means of agitation and an appropriate residence time within
section 7 is provided by means of controlling the rate of flow so that
complete reaction may be allowed to take place. The precipitated gumfer
suspension, now being at a temperature of less than 30~C and a pH of
between 2.0 and 7.0, is subsequently filtered, pressed, washed, dried,
and reduced in particle size using appropriate means, as shown in the
lower part of Figure I.
As relates to the use of gumfers in building product formulations, whether
used in high moisture content form as obtained directly from the process,
or whether used in dry, particulate, and particle size reduced form as
obtained by drying and size reduction, the gumfers of this invention
may be added to building product formulations cont~in;ng cementing agents
such as: portland cement, lime, silicates, g-ypsum, or clay, in order
to prepare building products using methods precedingly described.
In gumfers prepared according to methods of this invention, cations andanions donated by the mineral salt and acidulant are attached to uronic
acid or substituted groups of the hyd~ocolloid, such as phosphyl, sulphate,
or carboxyl groups, the hy~r~colloid itself being attached to cellulose
- 24 -
2191002
fibers by means of hydrogen bonding. When such gumfers are added to
building product formulations, the uronic acid sugar residues or the
substituted sugar groups, and the cations and anions which are attached
to these groups, react with the cementing agent, thereby bonding the
hydrocolloid directly to cementing agents used in the formulation,
Since the hydrocolloid is itself indirectly bonded to cellulose fibers,
the fibers are themselves indirectly bonded to the cementing agent, and
in this manner the entire gumfer heco~Qs an integral part of the building
product. Since the pH of some cementing agents is greater than 7.0 when
mixed with water, for example portland cement or lime, some dissociation
of hydrocolloid from the gumfer may occur on mixing gumfer with the cementing
agent using water, however this dissociation is localized unless the
mixture is excessively agitated. Therefore it is desirable when using
gumfers to prepare acqueous cementing mixes, to employ a limited degree
of mixing, a temperature of less than 30~C, and to add acidulants to
the mixture whenever possible.
It is found that gumfers may be added to building product formulations
in an amount up to and including 250% by weight of the cementing agents
used, based on dry weight of gumfers cont~;n;ng 10% moisture. The actual
amount of gumfer to be employed in any given formulation is dependant
on the types of hydrocolloid and cellulose fiber source used, on the
ratio of these two ingredients, on the types of cementing agents used,
and on particle size of the gumfer and its ability to retain moisture.
When used in dry, powdered, or particulate form, gumfers may be pre-mixed
with dry, powdered cementing agents including other fillers and additives
as precedingly described, in order to prepare dry building mixes suitable
for use as mortars or as patching compounds, or as mixes suitable for
casting purposes when rehydrated with water. Alternatively, cementing
agents may be added to gumfers which are in a moist state as obtained
directly from the ~Lo~Ss, together with water and other additives as
previously described, in order to prepare wet mixtures suitable for casting
purposes which are designed for on site construction use.
Through the use of other prior art additives as previously described,
wet mixtures thus formed may be made more suitable for the preparation
-- 25 ~
21 9 1 002
of various construction products including for example: cement or plaster
castings; cement or plaster wallboard or sheathing panels; cement or
non-fired clay roofing shingles or tiles; non-fired clay bricks or building
blocks; wall-fill, sub-flooring; or acqueous and non-acqueous cement
or lime based mortar mixes and grouting compounds. Where it is desired
to use such products in exterior and interior applications, they may
be suitably water-proofed and fire-proofed using appropriate prior art
means such as for example, by coating finished surfaces with plastic
resins or with silicate solutions, and by including ammonium phosphates
in formulations.
It is found that the formulations and building products made using gumfers
prepared according to methods of this invention, possess all those desired
imp~uv~.~lts in properties previously described including: workability
of wet mixtures; high elasticity and lubricity of fibers; ability of
fibers to bind with cementing agents to a controlled and variable degree
dependant on hydrocolloid to cellulose ratio, on hydrocolloid type, and
on ratio of gumfer to cementing agent; non-weeping of moisture from acqueous
mixtures; non-interference with casting, setting, drying, and curing
properties; non-shrinkage and non-cracking; no spalling after curing;
and also, low density, high strength, and increased impact resistance
of the products compared to products made using prior art methods.
Particularly as concerns workability, gumfers are somewhatlubrous sincethe cellulose fiber has been coated with a gum of highly elastic or rubbery
nature. Consequently, when gumfers are added to building product formulations
and the mixtures spread by troweling, gumfers do not nest and unlike
untreated cellulose fibers, the lubrous nature of gumfers offers little
or no resistance to shear.
In cases where hydrocolloids alone are added directly to formulations
cont~;n;ng esperi~lly portland cement or gypsum for purposes of attempting
to improve the working properties of such mixtures, it is found that
setting, drying, and curing properties of such mixtures are severely
affected and that often such mixtures will not set or cure for days or
even weeks. This does not occur when gumfers are used, and the hydrocolloid
has first been precipitated onto cellulose fibers. In other words, except
- 26 -
2191002
when the alkalinity of cementing agents is high and when gumfers are
used at high gum:fiber ratios exceeding 1 : 2, and in relatively high
amounts compared to cementing agents used, hydrocolloids do not appear
to dissociate from gumfers when added to building formulations, and do
not affect setting or curing properties of the mixtures.
The process for preparing gumfers is a low energy process since the methods
may be carried out at ambient temperatures between 5~C and 25~C. The
process can utilize many waste forms of cellulose including bleached
paper pulp or wood chips for example, to which energy has already been
added in terms of particle size reduction, or in terms of prior extraction
of solubles and impurities in the case of recycled paper pulp. Since
gumfers may be used in wet acqueous form as obtained directly from the
process, the addition of energy to evaporate residual moisture is not
an absolute requirement. Energy requirements for the process are instead
in the form of energy necessary to refine ingredients before use in the
process including hydrocolloid, mineral salt, and acidulant; however,
as may be seen from the disclosure, alternative, unpurified ingredient
sources may be substituted and utilized, as for example by using the
hydrocolloid in acqueous solution form as obtained during a particular
stage of its extraction from natural sources, rather than in a dry, powdered
form to which it has been necpss~ry to add additional energy in order
to obtain the hydrocolloid in a refined state. Or to give one further
example, by using either pulverized, recycled concrete, gypsum, or clay
products as the mineral salt as the case may apply, in place of using
refined cement powder, plaster of paris, or refined clays, to which energy
has been added in order to prepare these ingredients in a refined, hence
reactive form.
Since cellulose fibers and hydrocolloids are to be found throughout nature,
and are readily extractable and renewable resources, there are few restrictions
to the use of such materials in the present invention as long as plant
forms continue to proliferate. In fact, these substances are to found
together with cementing agents calcium and silicate in practically all
plant forms. Therefore, as a refinement of the present invention, it
may be stated that the most optimum ratio of ~lydLo~olloid to cellulose
to be employed in the invention is that ratio in which these substances
- 27 -
2191002
are found to occur naturally throughout nature. By the use of such ratios,
neither ingredient will be depleted from the environment in an uneven
amount compared to the other, and both ingredients would therefore be
entirely renewable, thus ensuring continuous supply and availability.
Since both the type of hydrocolloid and cellulose source used, the particle
size of the cellulose fiber, the ratio of gum to cellulose, as well as
the types and quantities of mineral cations and anions which may be attached
to the gumfer can all vary, it is obvious that a wide range in building
product formulations is possible. By virtue of such variability, it
is possible to obtain a wide range of desirable properties in building
formulations and products, and that the use of gumfers in building products
leads to an adv~n~ nt in the art of their preparation, incomparable
to existing prior art methods. It is believed that the present invention
will lead to adv~n~mPnts in the art of preparing building products which
are fiber composites, useful for construction purposes.
In the following are given examples of gumfer preparation, illustratingthe variety of ingredient types, concentrations and ratios, processing
methods and conditions which may be employed, and also including examples
of the use of gumfers in preparing building construction products:
- 28 -
2191002
Example 1 Newsprint gumfer using konjac-carrageenin blend, or kappa-
carrageenin.
9.0 g of newsprint is cut into ~" squares and added to 325 ml water in
a blender. The newsprint is chopped until fine and smooth. ~ g of a
proprietary mixture of konjac-carrageenin believed to be in a ratio of
1 : 1 is added with stirring. After several minutes, the mixture is
almost gel-like, slimy, and viscous. When 0.2 g of citric acid is added
with stirring, the .~l;m;ness and gel-like character of the suspension
disappears, the newsprint pulp flocculates and settles out, and the solution
surrounding the pulp is clear and water-thin, and is easily decanted,
strained, or expressed from the pulp. The expressed pulp feels somewhat
rubbery or elastic. Metal ions in the water are believed to assist in
precipitation of the gumfer. When the moist gumfer is heated to a temperature
of 35 C, the gumfer becomes slimy and gel-like in texture once again,
indicating that gum dissociates from fiber. When cooled, the gumfer
reverts to a non-slimy, non-gelatinous condition, and the rubbery and
elastic nature of the gumfer is restored.
The experiment is repeated using 20.0 g of micro-crystalline cellulose
of average particle size 8 microns suspended in 200 ml water. In one
case, 1.0 g of kappa-carrageenin without added konjac flour is added,
and in another 1.0 g of proprietary mixture of konjac-carrageenin is
used instead. When citric acid is added, similar results to the preceding
are obtained.
The experiment is repeated using 20.0 g bleached paper pulp of average
particle size 35 microns and 5.0 g of kappa-carrageenin suspended and
dissolved in 400 ml hard water containing 0.8 g of 60% sodium lactate
solution added as a sequestrant. When either potassium or ammonium lactate
are added to give O.lN, followed by lactic acid to give 0.05N, similar
results to the preceding are obtained. When calcium lactate pentahydrate
is used instead, a gumfer is not formed.
The conclusions which may be reached are that either mixtures of konjac-
carrageenin or kappa-carrageenin alone are capable of forming gumfers
in the presence of either K or NH4 but not Ca , when also in the presence
- 29 -
2191002
of an acidulant. Other forms of carrageenin including iota forms are
expected to react similarly, and also with calcium ion as well, according
to their known properties.
Example 2: No gumfer formation with xanthan gum, xanthan-locust bean
mixture, welan gum, rhamsan gum, flax gum, carboxymethyl cellulose, or
methyl celluloseusing bleached paper pulp.
~ g of 60~/o sodium lactate solution is added to 200 g water at 15~C as
a sequestrant. 1.0 g of proprietary mixture of xanthan-locust bean gum
believed to be in a ratio of 3 : 2, is mixed with 10.0 g of bleached
paper pulp of average particle size 110 microns, and the mixture added
to the solution with stirring. A slimy and viscous suspension is formed.
When K lactate is added to give O.lN, followed by lactic acid to give
O.lN, a slight thickening effect is noticed, but no precipitation is
observed and the suspension remains viscous and slimy. The ratio of
gum to fiber employed is 1 : 10.
When the preceding experiment is repeated using xanthan gum only at a
ratio of 1 parts gum to 4 parts fiber, and when similar concentrations
of either Ca lactate pentahydrate, NH4 lactate, Al lactate, Zn lactate,
or Ferrous (II) lactate are added, together with either lactic, glycolic,
malic, or phosphoric acid as acidulants, no gelling or precipitation
is observed, and similar results are obtained.
The same experiment repeated using either welan gum, rhamsan gum, ~lax
gum, carboxymethyl cellulose, or methyl cellulose, with the preceding
mineral salts and acidulants used at similar concentrations and ratios,
does not produce a gumfer precipitate with either K, Ca, NH4, Fe ,Al,
or Zn, and similar results to the prece~; n~ are obtained.
The conclusions which may be reached are that none of the prece~lng gums
produces a gumfer with either K ions or multivalent metal cations, excepting
xanthan gum which is known to produce a precipitate in the presence of
ferric (III) ions at pH values less than 7.0, and ca.~o~y..~hyl cellulose,
which is known to precipitate in the presence of either ferric (III)
ion or aluminium ion at pH values less than 7Ø
- 30 -
2191002
-
Example 3: Gumfers prepared using gellan gum.
2.5 g of de-acylated gellan gum is added to ~00 ml water at 5~C containing
0.8 g of 60% Na lactate solution, with stirring in a blender. A clear,
adhesive, gUm-liike solution of high viscosity is obtained. 10.0 g of
bleached paper pulp of average particle size 110 microns is added and
thoroughly dispersed by stirring, to yield a suspension containing 1
parts gum and 4 parts fiber. The suspension remains adhesive and viscous.
7.2 g of 60~~ K lactate to give 0.112N, followed by 6.0 g citric acid
to give 0.062N are added with stirring. Upon addition of K salt and
acid, flocculation is noticed, and fiber begins to separate from the
mixture. After several minutes, the fiber is settled and a clear, non-
viscous, non-adhesive supernatent fluid remains apart from the precipitate.
This fluid is water-thin and is easily separated from the fibrous precipitate
by filtration. The pulp which is recovered by straining is elastic and
rubbery in nature. The precipitate does not retain liquid and is easily
expressed to remove free-flowing moisture.
The conclusion which may be reached is that gellan gum forms a gumfer
with cellulose fibers in the presence of potassium ions at pH values
less than 7Ø The gum is removed from solution and co-precipitated
with cellulose fibers by means of hydrogen bonding, presumably also being
held in place by potassium ions in a helix type structure, characteristic
of gums which gel using either K or Ca ions, including gellan gum,
carrageenin, alginates, or agar. The pulpy precipitate is dried to a
moisture content of lO.O~o or less and may be ground to a fine particle
size. According to the preceding and also according to its known properties,
gellan gum is also expected to form gumfers with multivalent metal ion
including Ca, Mg, Ba, Fe(II), Fe(III), Al, or Zn.
2191002
Example 4: Gellan gumfers made using bleached paper pulp.
Repeats of example 3 are made employing 5.0 g gellan gum and 20.0 g bleached
paper pulp, or a gum:fiber ratio of 1:4, dissolved and suspended in 800 ml
water at 20~C, containing 0.8 g 60~o Na lactate, and also employing different
variables in three separate trials:
Trial 1: K lactate followed by lactic acid are added to give O.lN ofeach substance. The suspension thins immediately on addition of lactic
acid, and flocculation of fiber is noticed. Calculation of theoretical pH
using the Henderson-Hasselbach equation gives pH 3.08. The supernatent fluid
is clear, thin, non-adhesive, and non-viscous in nature. The gum-fiber
composite is removed by straining to yield 510 ml of supernatent, and when
the gumfer is also pressed, a total of 760 ml expressed fluid is obtained,
representing 95% of the original water used in the process. ~ of this fluid
or 400 ml is recycled to the subsequent trial.
Trial 2: To 400 ml of make-up water plus 400 ml recycled fluid from
trial 1 to give a total of 800 ml fluid at a temperature of 18~C, are added
similar amounts of gellan gum and paper pulp as used in trial 1.
K lactate to give 0.05N, followed by 2.0 g of sodium silicate solution
cont~;n;n~ 37.6% solids consisting of SiO2:Na20 in a ratio of 3.22:1; are
added with stirring, followed by lactic acid with stirring to give 0.05N.
Coarse flooculation results on addition of lactic acid. Results are similar
to trial 1, and a gumfer is formed. The supernatent fluid is easily
recovered from the precipitate by straining and pressing, and 400 ml of
supernatent is recycled to a subsequent trial.
Trial 3: 400 ml recycled fluid from trial 2 is added to 400 ml make-up water to give 800 ml total liquid at 20~C. Na lactate, gellan gum,
and paper pulp are used at levels as in trial 2. 4.0 g of calcium lactate
pentahydrate is added, followed by 5.0 g of 85% lactic acid with stirring.
An extremely coarse flocculate and precipitate is formed which is easily
strained and pressed. The expressed liquor is clear, non-adhesive, and
non-viscous in nature.
- 32 -
2191002
The conclusions which may be reached are that gellan gum forms gumfers in the
presence of either K or Ca ions at high H ion concentration or at pH
values less than 7Ø
Example 5: Gellan gumfers and construction products made using portland
cement and various fiber sources.
Trial 1: The following are added to a blender with stirring, in order of
use: 900 ml water at 20~C/ 6.0 g Na silicate solution, 5.0 g gellan gum,
25.0 g bleached paper pulp of average particle size 100 microns to give a
gum:fiber ratio of 1:5~ followed by 10.0 g portland cement powder as the
mineral salt, pre-blended with 6.0 g fumaric acid as the acidulant.
Approximately 5-10 minutes after addition of cement-fumaric acid blend,
gelling is noticed. After several additional minutes stirring, gel texture
brea]cs down and gross flocculation is noticed. The supernatent is clear,
non-viscous, and non-adhesive in nature. An additional 10.0 g cement powder
is added to the suspension with stirring, and the mixture is strained and
pressed to yield a cake ~" deep by 4" square, containing 34% solids. The
cake surface is coarse and fibrous, and difficult to smooth. The cake is
allowed to dry and harden. When sufficiently dry, a lightweight board is
obtained which is suitable to be used as a wall covering material. The
solids content of this board is approximately 65 parts gumfer per 100 parts,
of which approximately 52 parts is gum plus cellulose, 3 parts is silica,
and 5 to 10 parts is fumaric acid. 35 parts of the total solids content in
the cake is cement powder, hence the ratio of gumfer to cement powder in the
product is 1.86:1~ or 186~o by weight of the cementing agent added.
Trial 2: A repeat of trial 1 is made using similar amounts of water,gellan, Na silicate, cellulose, except that a fiber particle size of 35
microns is used instead. A mixture containing 20.0 g cement powder and
6.0 g fumaric acid is added, followed by 40.0 g dry sand. A precipitate
forms, and the suspension is drained and pressed into a wet cake weighing
190 g cont~;n;ng 48.6% solids by weight. The cake is easier to shape and
to smooth than in trial 1, and when sufficiently dry, is suitable for use
as a wall covering material. The solids content of the board is approximately
38~ parts gumfer per 100 parts, of which approximately 30.7 parts is gum
- 33 ~
2191002
plus cellulose, 1.7 parts is silica, and 6 parts is fumaric acid. 20~ parts
is cement powder, and 41 parts is sand, hence, the ratio of gumfer to
cementing agent in the product is 1.93:1, or 193% of the cementing agent used.
Trial 3: A repeat of trial 2 is made, employing similar amounts of gum,
silicate, and fiber, but lesser amounts of cement and sand. Microcrystalline
cellulose is used as the fiber source, with average particle size 20 microns.
Similar results are obtained, except that the dried cake contains approxi-
mately 45.3 parts gumfer per 100 parts, of which approximately 38~ parts is
gum plus fiber, ~ parts is silica, and 5~ parts is fumaric acid. 18 parts
of the solids content is cement powder, and 36~ parts is sand, therefore,
the ratio of gumfer to cementing agent used is nearly 2.5:1~ or 250% by
weight of the cementing agent employed.
Example 6: Alginate gumfer
400 ml of water at 20~C containing 0.8 g of 60~o Na lactate is charged to a
blender. 5.0 g of a grade of sodium alginate, a 2% solution of which produces
a viscosity of 2~000 centistokes per second, is premixed with 20.0 g of
bleached paper pulp of average particle size 35 microns, and the mixture is
added with stirring, to give a gum:fiber ratio of 1:4. The addition of K
lactate, followed by lactic acid, to give O.lN of each substance with
stirring, does not yield a flocculate or precipitate. When the experiment
is repeated using Ca lactate pentahydrate instead to give O.lN with
stirring, an immediately gelling effect is noticed. When lactic acid is
subsequently added with stirring to give O.lN, gelling is initially enhanced
followed by th;nning of the suspension and flocculation of fiber. On
stirring, gel texture breaks do~n, and a precipitate is formed. The super-
natent fluid is clear, non-viscous and non-adhesive in nature. The preci-
pitated gumfer is easily strained and pressed, and may be dried to a moisture
content of 10% or less, then finely ground to a free-flowing powder.
The conclusion which may be reached is that sodium alginates form gumfers
with cellulose fibers in the presence of calcium ions but not potassium
ions, when also in the presence of high H ion concentration, or pH values
- 34 -
2191002
less than 7Ø Alginates are, therefore, also expected to form gumfers in the
presence of other multivalent ions, including Mg, Al, Fe, Zn, but not with
monovalent ions according to their known properties.
Example 7: Alginate gumfer m--ade using K silicate, Ca carbonate, and NH4
phosphate as mineral salts.
To 900 ml water at 20 C is added with stirring, 12.0 g of K silicate solution
containing 30~/O solids in a SiO2:Na2O ratio of 2.5:1, and 100 g of a mixture
of 80 g microcrystalline cellulose and 20 g of a low viscosity grade of
sodium alginate in a ratio of 1 parts gum to 4 parts cellulose fiber. The
acqueous mixture thus formed is stirred for several minutes to yield a
viscous and adhesive suspension. To the mixture is then added with stirring,
12g finely powdered calcium carbonate, followed by 7 g ammonium
monophosphate predissolved in 50 ml water, followed by 32 g of 75% phosphoric
acid solution prediluted in 50 ml water. Upon addition of acidulants,
immediate gelling is noticed, followed by t,~;nn;ng, flocculation, and
precipitation of a gumfer. The precipitate may be recovered by straining
and pressing, and may be dried to a moisture content of 10% or less, then
finely ground to a free-flowing powder. It is noticed that this particular
gumfer is fracture-resistant and more difficult to grind than gumfers formed
using other types of mineral salts.
Example 8: Alginate gumfer and construction product made using portland
cement and Na silicate as mineral salts.
The method of examples 6 and 7 is repeated, using the following quantities
of ingredients added to 1 litre of water at 20~C in order of use: 6.0 g
Na silicate solution; 6.0 g of high viscosity Na alginate preblended with
30.0 g bleached paper pulp of average particle size 100 microns; 4.0 g
portland cement powder preblended with 6.0 g fum~ric acid or gluco-delta-
lactone is added with stirring. Within 30 seconds after addition of
mineral salt acidulant mixture, gelling is noticed and gel texture reaches
a m~ m after approximately 5 minutes. On continued agitation, gel
texture is noticed to break down, and within an additional 10 minutes, the
mixture berom~s thin and slurry-like, and coarse flocculation and
21 ~ 1 002
precipitation of fiber is noticed. To the mixture is then added 54 g portland
cement powder and 120 g dry sand. The mixture is strained to remove super-
natent which is clear, non-viscous, and non-adhesive. The recovered pulp is
pressed to yield 430 g of cake containing 52% solids. To the cake is then
added an additional 20 ml water and 5 g cement powder preblended with 1.0 g
xanthan gum, and all are mixed to a desired consistency. The resulting cake
is then formed into slabs ~" thick by 41' square, and allo~ed to set, harden,
and dry. After drying, the slabs contain approximately l9. 6 parts alginate
gumfer per 100 parts, of which approximately 13~ parts is cellulose, 2.7
parts is gum, 0.8 parts is silica, and 2.7 parts is fumarates or gluconates.
The cement content of the cake is 26. 8 parts and the sand content 53.6 parts.
Hence, the ratio of gumfer to cementing agent used is 0.73:1~ or 73% of the
cementing agent used. The dried slabs are suitable for use as panelling
materials. The ratio of gu~ to cellulose in the alginate gumfer is 1:5.
Example 9: Gellan gumfers used in portland cement castings.
1) 20.0 g of dry powdered gellan gumfer prepared according to methods of
example 5~ and containing approximately 60% bleached paper pulp of particle
size 100 microns, 15% gellan gum, 12% portland cement powder, 11% fumarates,
and 3% silica, and in a gum to fiber ratio of 1:4, is preblended with 40 g
of portland cement powder and 120 g dry sand, to yield 180 g of a dry
building product premix. To this premix is then added 150 g water with
stirring, to yield 330 g of a paste containing 541~o/o solids. The mixture is
of suitable consistency to permit casting into molds. The mixture is tamped
down into a tube to form a cylinder 2" in diameter by 2" high, and the
casting is allowed to set, harden, and dry. During setting, 5.0 g of excess
moisture weeps from the surface of the casting and is decanted, leaving a
solids content of 55~% in the mixture. The density of the casting when dry
is 1.08 g/cc, compared to a similar casting of ordinary portland cement
made using only 75 g water added to 180 g cement powder without additives,
of density 1.73 g/cc. As the gumfer casting contains 11.1% gumfer,
22.2% cement powder, and 66.6% sand, the ratio of gumfer to cementing agent
used is 0.5:1, or 50~O by weight of the cementing agent used. The casting
is hard, fracture resistant, and durable. No spalling of components from
- 36 -
2191002
the surface of the casting is evident.
2) The preceding experiment is repeated using the same cellulose fiber
source, but left untreated, using the same proportions of fiber:cement:sand.
To 180 g of premix it is found necessary to add 180 g water to form a wet
mixture of comparable consistency, hence an excess of 30 g water compared to
the preceding example. The mixture, totalling 360 g conta;ning 50~/O solids
is similarly molded and allowed to set, harden, and dry. ~uring setting,
60 g of water weeps from the surface of the casting and is decanted. After
drying, the casting has a density of only 1.03 g/cc, but is not as hard and
fracture resistant, and spalling of components from the surface is noticed.
In comparing the two castings, the gellan gumfer casting is judged to be
superior both in strength and appearance, and also possesses a comparable
density which is much lesser than that of portland cement or cement-sand-
castings which do not contain cellulose fibers.
Example 10: Cement products prepared using alginate gumfer.
An alginate gumfer of gum to fiber ratio 1 to 5 is prepared, according to
methods of preceding examples. Ingredients are added in order of use, in
the following proportions: 3.6 litres water at 20~C; 3.2 g of 60% Na
lactate solution; 160 g of microcrystalline cellulose of average particle
size 50 microns; and 32.0 g of high viscosity sodium alginate gum. To the
suspension is then added 24.0 g of portland cement powder with stirring,
follo~Jed by 560 ml of vinegar solution cont~;n;ng 5% acetic acid by volume.
After flocculation and precipitation of gumfer, the gumfer is strained
and pressed to yield a cake conta;n;ng 50~/O moisture. The cake is crumbled
and spread out to dry. After drying, the gumfer is finely ground to a free-
flowing powder and a faint odour of vinegar is detected. The dried gumfer
powder is used to prepare cement castings in the following manner:
A dry premix of each of the following formulas is made:
- 37 -
21qlO02
-
Formula 1 2 3 4
Dry powdered gumfer 10 20 30 40
Perlite 30 20 10 nil
Welan gum 0.5 0.8 1.1 1.4
Portland cement powder 60 60 60 60
Total parts dry mix: 100.5 100.8 101.1 101.4
by weight
Welan gum is added to give a desired consistency to acqueous mixtures, in
order to retain shape and texture when foamed or aerated.
Then, to each mixture is added with stirring, suitable quantities of water,
6% hydrogen peroxide solution, and a catalyst to decompose the peroxide
solution into oxygen and water.
Water 110 110 120 140
6% Hydrogen peroxide 10 10 10 10
Catalyst 0.5 0.5 0.5 0.5
Total parts by weight: 221.0 221.3 231.6 252.5
Upon addition of catalyst, foaming results, and the mixtures are observed to
expand greatly in volume. The foamed and expanded mixtures are cast into the
form of cylinders 4" in diameter by 2" high, and allowed to set, harden, and
dry. Mixtures are observed to retain their shape and volume after setting.
Following are the comparative results obtained:
Formula 1 2 3 4
% Solids in wet mix 45~ 45~ 43~ 40
Ratio of gumfer to cement0.17:1 0.33:1 0.5:1 0.67:1
Density of Wet Mix, g/cc: 0.98 0.83 0.87 0.83
Dry Density of Castings, g/cc: 0.49 0.42 0.42 0.37
All castings after being suitably dried, are resistant to fracture,
possess desirable surface hardness, and do not exhibit spalling. The
casting of formula 1 has a tendency to shrink and crack while still
moist and after setting. Formula 4 requires a longer period of time
to set and dry. Formula 3 is judged to be superior in terms of density,
setting and drying properties, and in strength and appearance.
_ 38 -
21 91 002
It is observed that the final densities of products made according to the
preceding methods are substantially lower than those obtained using portland
cement or concrete castings without added cellulose fibers. For example, thel
density of a portland cement casting is determined to be 1.73 g/cc. Hence the
castings of this example are only 25% or ~ that of cement castings, and all
examples are lighter than water. When sealed with epoxy resin, gumfer
castings of this example are observed to float on water, and may be used as
flotation devices or as boat construction materials.
Example 11: Wood fiber/algin gumfer, made with mineral salts: potassium
silicate, tri-calcium phosphate, and ammonium monophosphate. Used to prepare
cement construction products.
1) Gumfer preparation:
Lodge Pole Pine fiber is obtained from forestry cutting and mill waste in
the form of coarse splinters. The fiber is sifted to remove large splinters
and to obtain a material of more uniform fiber length approximately 1/8" long.
40.0 g of sifted fiber is pre-mixed with 10.0 g of a low viscosity grade sodium
alginate to give a premix cont~;n;ng 1 parts gum to 4 parts fiber. The premix
is then added to 750 ml water at 20~C containing 6.0 g of a K silicate
solution, with stirring. The pH of the suspension after 15 minutes is
determined to be 9.8. To the suspension is then added 6.0 g tricalcium
phosphate powder. The pH after 5 minutes stirring is determined to be 9.7.
3.0 g of ammonium monophosphate is next added with stirring, and after 5
minutes, the pH is determined as 6.6. The suspension remains viscous and
gum-like, and no flocculation or precipitation is noticed. 6.0 g of 75%
phosphoric acid solution is then added with stirring, and immediate gelling,
followed by flocculation is noticed. After 5 minutes additional stirring,
a supernatent fluid is obtained which is clear, non-viscous, and non-adhesive
in nature. The pH of this fluid is determined to be 3.3.
The wood fiber-algin gumfer is recovered by straining and pressing, and the
gumfer is elastic and rubbery in nature. The gumfer is dried to a moisture
content of lO~o or less and is ground to a free-flowing solid of fiber length
comparable to that of the original material before precipitation. The dried
gumfer is used to prepare a cement product in the following manner.
- 39 -
21 9 1 002
2) Preparation of Cement Castings using wood fiber gumfer:
10 parts of dry, free-flowing wood fiber gumfer is preblended with 60 parts
portland cement powder to yield 70 parts premix. 50 parts of water is then
added to the premix with stirring to form 120 parts of a paste of suitable
consistency to be spread or to be packed into molds. The wet mixture contains
58.~/o solids, of which approximately 14.3 parts per 100 parts is wood fiber
gumfer and 85 . 7 parts is cement, hence the ratio of gumfer to cementing agent
in the product is 1:6, or 16.7% of the cementing agent used. The acqueous
mixtures are cast into the shape of this discs, 4" in diameter by ~" thick,
and allowed to set, harden, and dry.
3) Preparation of cement castings using untreated wood fiber:
A comparison of gumfer castings with castings made using untreated fiber, is
carried out in the following manner:
10 parts of untreated sifted Lodge Pole Pine fiber is pre-mixed with 60 parts
portland cement powder, and to the premix is then added 50 parts of water with
stirring. The wet mixture, therefore, contains a similar proportion of
ingredients, except that it has a slightly higher cellulose content. This
mixture is similarly cast and allowed to set, harden, and dry. 7 parts of
water are observed to weep from the surface of the casting during setting and
are subsequently decanted.
4) Portland cement castings without added fiber:
A comparison with portland cement castings without added gumfer or wood fiber
is made in the following manner:
To 120 parts of portland cement powder is added 50 parts water with stirring
to form a paste of comparable consistency to the preceding examples, contain-
ing 70~~/O solids by weight. This mixture is similarly cast and allowed to set,harden, and dry.
A comparison of all three castings yields the following results:
- 40 -
2191002
Casting Example 1 2 3
Wood fiber gumfer - Untreated Wood Portland Cement
fiber without additives
Property Observed
Setting Time 12-24 hours 48 hours 12-24 hours
Workability viscous, spreads viscous, fibers thin, pours,
well, casts well i nest, more easy to spread
difficult to and also to
spread and cast cast
Moisture retention no weeping weeps some weeping
Spalling none severe none
Surface finish mostly smooth irregular, excellent,
chipped smooth
Dry density of
casting, g/cc: 1.15 1.03 1.73
When the castings of examples 1 and 2 are soaked under water for several
weeks, no change in the gumfer casting is apparent; however, the casting
containing untreated wood fiber of example 2 is noticed to disintegrate,
and water insoluble impurities float to the water surface.
It is seen from these results, that gumfer castings are comparable to cement
castings, with the exception of density which is al ~ st 40~0 less, and are
superior to castings made using untreated wood fibers.
- 41 -
,,j~
21qlO02
Example 12: Wood fiber-algin gumfer and its use in preparing cement
based construction products.
Lodge Pole Pine wood fiber which is a mixture of fine sawdust and coarse
splinters up to 1/8" in diameter by 1" long, is used to prepare a gum-
fiber composite as follows:
To 15L water is added 10 g of 30~~O potassium silicate solution as a sequestrant.
The pH of the solution is measured as 9.8. 85 g of a dry powdered sodium
alginate, a 2~o solution of which has a viscosity of 500 cps, is dry blended
with 65 g of a sodium alginate, a 2% solution of which has a viscosity
of 160 cps, to yield 150 g of a mixture of sodium alginates. The mixture
is then blended with 150g wood fiber and the resulting mixture added
to the solution with stirring. 600 g of additional wood fiber is then
added with stirring, to yield a suspension cont~;n;ng 750 g wood fiber
and 150 g alginates, or a gum : fiber ratio of 1 : 5. The suspension
is stirred for an additional 15 minutes to ensure complete hydration
of gum and wood fiber. The pH after 15 minutes is determined to be 7.7,
and the suspension is viscous and adhesive in nature.
100 g of portland cement powder slurried in 200 ml water, and serving
as the mineral salt, is added to the suspension with stirring. After
stirring for 20 minutes to ensure hydration of the powder and partial
reaction with alginates, the pH is measured as 11.7, and the suspension
is nearly gel-like. 140 g of 75% phosphoric acid solution diluted in
1 liter water is then added slowly with stirring, and the pH is determined
after various time periods. The initial pH after addition of acidulant
is 5.0; 5 minutes later, the pH is 4.7; after 10 minutes, the pH is 5.4;
at 15 minutes, 5.8; and at 20 minutes, 6Ø
Upon addition of acid, the gel texture of the suspension breaks, the
suspension thins, and the wood fiber is noticed to flocculate but remains
suspended and settles slowly. It is concluded that the acid reacts with
the cement powder and alginate to produce a solution containing sodium
and calcium phosphates which are water soluble, hence act as buffering
agents. The supernatent fluid is non-viscous and non-adhesive in nature,
and is easily separated from the gumfer by draining. The gumfer is pressed
to a moisture content of approximately 50% by weight, and almost all
- 42 -
2191002
the original water added may be recovered as a liquor. m e expressed
gumfer is dried to a ~ isture content of less than l~/o by weight, and
is not reduced in particle size before use in preparing a construction
product.
The preceding experiment is repeated using an additional 750 g wood fiber
and 150 g mixed alginates, to yield a total of 1955 g of dried wood fiber
gumfer, or a yield of 88.5% based on total solids and acidulant charged
to the process. The loss in yield is presumed to be due to water soluble
impurities in the wood fiber such as sugars, which are lost in th~ process
water after expression. The dried gumfer is used to prepare cement based
construction products as follows:
Trial A: 1,200 g of dried wood fiber gumfer is blended with 6,800 g
of portland cement powder. To the mixture is added 5 L water with stirring
to form 13,000 g of a smooth paste of barely pourable consistency. This
mixture is noticed to spread well, and may be cast. 11,000 g of this
mixture is tamped down into a mold having dimensions characteristic of
a typical concrete building block, and the casting is allowed to set
and harden. The casting is notice; to set or gel within 6 hours, and
may be stripped from the mold after 12 hours. After stripping and drying,
the casting has a density of only l.l5 g/cccompared to concrete products
which have densities of 2.5 to 3.0 g/cc, and weighs only 16 lb or 7.3
Kg, compared to an ordinary building block made of concrete which weighs
45 to 50 lbs, or 20 to 23 Kg. The gumfer castingappears strong and durable,
is fracture resistant and water-proof, and the surface finish is smooth
and no spalling is apparent.
Trial B: Cement castings in the shape of small discs approximately 4"
in diameter, containing wood fiber gumfer are prepared using the pre~eding
method, and D unts of water added, dry densities, and other characteristics
are determined.
- 43 -
21~1002
Results of cement-fiber castings, trial B:
Test # Gumfer Cement Total Water Total Cement to Water to Casting
Solids Added Wet Mix Gumfer Ratio Solid Ratio Volume Density
(g) (g) (g) (g) (g) (cc) (g/cc)
.
1 10 90 100 50 150 9 : 1 0.5 : 1 102 1.2
2 20 80 100 74 174 4 : 1 0.74 : 1 121 0.98
3 30 70 100 100 200 2.33 : 1 1 : 1 163 0.72
4 40 60 100 110 210 1.5 : 1 1.1 : 1 193 0.61
100 134 234 1 : 1 1.34 : 1 217 0.54
6 62.5 37.5 100 112.5 212.5 0.6: 1 1.125: 1 257 0.45
7 20 80 100 75 175 4 1 0.75 1 123 0.94
All mixtures containing 20% gumfer or less are pourable and spread well,
including the casting of trial A at 15% loading. All castings set within 6
hours and may be stripped after 12 - 24 hours. Castings containing up to 30%
gumfer have a mostly smooth surface finish of hardened cement. All casting
mixtures cont~;n;ng 30% or more gumfer are difficult to spread and must be
pressed into molds and require longer periods of time to dry. At 30% or
greater gumfer loading, fibers are visible on surfaces after hardening.
Castings containing up to 40~/0 gumfer are strong, durable, fracture resistant,
and water-proof. At 50~0 and higher gumfer loading, castings are more fragile
but integral, and fibers can be dislodged from surfaces. A loading of 62.5%
appears to be the maximum permissable in which fibers are still coated with
cement powder and the casting has a reasonable degree of integrity, without
falling apart. Example 6 could be used as a wall fill material, which owing
to its low density, would have superior heat insulation qualities. Examples 1
and 2 can be used for load bearing applications, for example to replace concretebuilding blocks. & ch pLU~I S Will similarly ~oss~s low densities and high
heat insulation ~alues. Casting example 7, containing untreated wood fiber,
when compared to example 2, exhibits a longer period of time before setting and
hardening, weeping of ~ isture from wet mixtures, sp~ n~ of surface c~on~nt
a lesser degree of moisture resistance, and a greater degree of susceptibility
to fracture on impact.
-- 44 --
2191002
Example 13: Use of gumfers in gypsum plaster based castings.
A variety of gumfers prepared according to preceding examples and obtained
as dry, free-flowing particulates, are blended with plaster of paris in various
ratios. The mixtures are sifted into a pre-determined quantity of water
and allowed to soak for 2 minutes. All samples are then mixed by hand for
3 minutes, then cast into containers in the shape of flat discs approximately
4~ round by ~" to 1" thick. The castings are allowed to set and harden,
and are stripped from the molds and allowed to dry. A comparison of various
characteristics of wet mixtures and properties of castings is made.
Test # Gumfer Type Plaster Gumfer Total Water Water to
(g) (g) Solids Added Solids Ratio
(g) (g)
1 Kappa-carrageenin, 60 10 70 62 0.886
precipitated onto
35 micron alpha-
c*llulose, using
K and lactic acid
2-1 Alginate, precipitated 60 10 70 50 0.714
onto 50 micron micro-
crystalline cellulose,
2-2 using cement and acetic 55 15 70 50 0.714
acid-
3 Gellan, precipitated 60 10 70 70 1.0
onto 100 micron alpha-
cellulose, using cement
and fumaric acid.
4-1 Gellan, precipitated 60 10 70 50 0.714
onto 35 micron alpha-
4-2 cellulose, using 55 15 70 60 0.86
calcium sulphate,
4-3 sodium silicate, and 15 5 20 30 1.5
fumaric acid.
5-1 Gellan, precipitated 60 10 70 62 0.89
onto wood fiber, using
5-2 K lactate, Ca sulphate, 52 18 70 80 1.14
and fumaric acid.
- 45 -
2191002
Table, continued:
6 Gellan, precipitated 55 15 70 76 1.09
onto 35 micron alpha-
cellulose, using
Ca lactate, lactic acid.
7 Alginate, precipitated 60 10 70 50 0.714
onto 35micron alpha-
cellulose, using
Ca lactate, lactic acid.
The preceding are compared with plaster castings made using untreated
alpha-cellulose fibers and also with plaster castings containing no additives.
8 35 micron alpha- 44 12 56 56 1.0
cellulose only.
9 100 micron alpha- 46 7~ 53.5 60 1.15
cellulose.
plaster only 100 - 100 60 0.6
11 plaster only 70 - 70 40 0.57
12 plaster only 70 - 70 50 0.714
Mixtures are compared for workability, weeping, and setting time.
Castings are compared for shrinkage, cracking, hardness, surface finish,
and dry density.
- 46 -
2191002
Casting Workability Setting Weeping Shringage Surface Hardness* Dry Density
Example Time Finish
(minutes) (g/cc)
_ 1 spreads well 7 none nonegood, no VS 0.82
stiffens on spalling
mixing,
pourable.
2-1 very good,20 slight none as above H 0.94
casts similarly
to plaster.
2-2 as above 13 slight none as above H 0.85
3 stiff paste, 8 none none as above S 0.77
foam-like,
casts well
4-1 as above 11 slight none as above H 0.96
4-2 as above 15 slight none as above MH 0.75
4-3 as above 15 none none as above VS 0.57
(strips first)
5-1 as ahove,17 none none as above S 0.68
spreads well,
casts well.
5-2 stiff, foamy, 20 none none as above S 0.54
difficult to
cast, but spreads
well.
6 Stiff paste, 6 none slight as above S 0.66
non-pourable
7 Smooth paste, 10 slight none as above H 0.88
casts well
8 stiff, nests 10 yes, slight chalky VS 0.77
difficult to pour badly.
g very stiff, 6 as above yesv. chalky VS 0.68
as above
slurry, 7 yes none excellent H 1.10
casts well
11 as above 7 yes none excellent VH 1.15
12 as above 7 yes none excellent H 0.94
* Hardness: V = very; S = soft; M = moderately; H = hard, according to fingernail
scratch test. - 47 -
2191002
In comparing results of these examples, it can be seen that in some cases
gumfers do not affect setting time, workabilty is good to excellent,
and little or no weeping and shrinkage occur. It is noticed that the
integrity of gumfer-plaster castings is better that of castings containing
untreated cellulose fibers, and that the surface finish and hardness
are in some cases comparable to that of plaster castings, and that the
final densities are considerably lower. Most gumfer castings
can be clean fractured by scoring with a knife, and allow penetration of
fasteners without fracture.Hence, many of the preceding gumfer castings
can be used as wall boards or sheathing materials. It is obvious from
the preceding results, that a wide range in properties of gumfer castings
may be obtained, by varying the types of hydrocolloid, cellulose source,
mineral salt, acidulant, and the amounts of gumfer used in any particular
formula.
It is noticed that in cases where gumfers are prepared using potassium salts,
that setting and hardening of plaster mixtures is in some cases accelerated.
In cases where fumaric acid or organic acids are used as acidulants in
gumfer preparation, residual amounts of these are noticed to contribute
to foaming and aeration of the plaster mixtures, due to reaction with
calcium and magnesium carbonates which are also present in the dry plaster
powders. This effect is of course beneficial to reducing final density, and
to obta;n;ng light weight castings of superior insulating value.
- 48 -