Note: Descriptions are shown in the official language in which they were submitted.
A-20666/A/C H M 85 21918 3 2
-1 -
Block ol~omers containing 2.2.6.6-tetramethyl-4-piperidyl groups as
stabilizers for organic
materials
The present invention relates to single specific block oligomers containing
2,2,6,6-
tetramethyl-4-piperidyl groups, to their use as light stabilizers, heat
stabilizers and oxidation
stabilizers for organic materials, particularly synthetic polymers, and to the
organic materials
thus stabilized. Furthermore, the present invention relates to a mixture with
a narrow
molecular weight distribution, containing at least three different block
oligomers, and to a
method of the preparation thereof.
The stabilization of synthetic polymers with derivatives of 2,2,6,6-
tetramethylpiperidine has
been described for example in US-A-4 086 204, US-A-4 331 586, US-A-4 335 242,
US-A-4 234 707, EP-A-357 223 and EP-A-377 324.
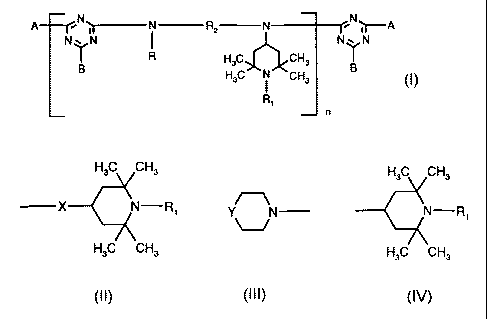
The present invention relates in particular to a compound of the formula (I)
A i ~ N R2 N i
N'\'N I N'''N
~R
)
Fi3C ~ CH3
R~
n
in which the polydispersity Mw/Mn is 1;
n is 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 or 15;
the radicals R, are independently of one another hydrogen, C,-CBalkyl, C2-
CBhydroxyalkyl,
-CH2CN, C3-Csalkenyl, C3-Csalkynyl, C,-C9phenylalkyl which is unsubstituted or
substituted
on the phenyl by 1, 2 or 3 C,-C4alkyl; or C,-CBacyl;
R2 is C2-C,2alkylene, Ca-C,2alkenylene, Cs-C,cycloalkylene, Cs-C,cycloalkylene-
di(C,-Caalkylene), C,-C4alkylenedi(C5-C~cycloalkylene), phenylenedi(C,-
C4alkylene) or
C4-C,2alkylene interrupted by 1,4-piperazinediyl, -O- or >N-X, with X, being
C,-C,2acyl or
(C,-C,2alkoxy)carbonyl or having one of the definitions of R4 given below
except hydrogen;
or R2 is a group of the formula (a), (b) or (c);
2191832
-2-
H3C CH3 H3C CH3
N-(CH2)m N (a)
v
H3C CH3 H3C CH3
-CH2 i H-CH2 (b)
O
C=O
O O
t~)
0 0
with m being 2 or 3,
X2 being C,-C,Balkyl, C5-C,2cycloalkyi which is unsubstituted or substituted
by 1, 2 or 3
C,-C4alkyl; phenyl which is unsubstituted or substituted by 1, 2 or 3 C,-
C4alkyl or
C,-C4alkoxy; C,-Csphenylalkyl which is unsubstituted or substituted on the
phenyl by 1, 2 or
3 C,-C4alkyl; and
the radicals X3 being independently of one another C2-C,2alkylene;
the radicals A are independently of one another -OR3, -N(R4)(R5) or a group of
the
formula (II);
H3C CH3
X N-R, (II)
H3C CH3
R3, R4 and R5, which are identical or different, are hydrogen, C,-C,Balkyl, CS-
C,2cycloalkyl
which is unsubstituted or substituted by 1, 2 or 3 C,-C4alkyl; C3-C,Balkenyl,
phenyl which is
unsubstituted or substituted by 1, 2 or 3 C,-C4alkyl or C,-C4alkoxy; C,-
C9phenylalkyl which is
unsubstituted or substituted on the phenyl by 1, 2 or 3 C,-C4alkyl;
tetrahydrofurfuryl or
2191832
-3-
C2-C4afkyl which is substituted in the 2, 3 or 4 position by -OH, C,-CBalkoxy,
di(C,-Caalkyl)amino or a group of the formula (III);
(III)
with Y being -O-, -CH2-, -CH2CH2- or >N-CH3,
or -N(R4)(R5) is additionally a group of the formula (III);
X is -O- or >N-Rs;
R6 is hydrogen, C,-C,Balkyl, C3-C,salkenyl, C~-C,2cycloalkyl which is-
unsubstituted or
substituted by 1, 2 or 3 C,-C4alkyl; C,-C9phenylalkyl which is unsubstituted
or substituted on
the phenyl by 1, 2 or 3 C,-C4alkyl; tetrahydrofurfuryl, a group of the formula
(IV),
H3C CH3
N-R, (IV)
H3C CH3
or C2-C4alkyl which is substituted in the 2, 3 or 4 position by -OH, C,-
Caalkoxy,
di(C,-Caalkyl)amino or a group of the formula (III);
R has one of the definitions given for R6; and
the radicals B have independently of one another one of the definitions given
for A;
with the proviso that in the individual recurrent units of the formula (I),
each of the radicals
B, R, R, and R2 has the same or a different meaning.
H3C CH3
In the repeating units of the formula (I), the radical R and the radical N-R,
H3C CH3
can have a random distribution or a block distribution.
Examples of alkyl containing not more than 18 carbon atoms are methyl, ethyl,
propyl,
isopropyl, butyl, 2-butyl, isobutyl, t-butyl, pentyl, 2-pentyl, hexyl, heptyl,
octyl, 2-ethylhexyl,
t-octyl, nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, hexadecyl and
octadecyl.
2191832
-4-
An example of C2-Cahydroxyalkyl and of C2-C4alkyl substituted by -OH is 2-
hydroxyethyl.
Examples of C2-C4alkyl substituted by C,-CBalkoxy, preferably by C,-C4alkoxy,
in particular
methoxy or ethoxy, are 2-methoxyethyl, 2-ethoxyethyl, 3-methoxypropyl, 3-
ethoxypropyl, 3-
butoxypropyl, 3-octoxypropyl and 4-methoxybutyl.
Examples of C2-C4alkyl substituted by di(C,-C4alkyl)amino, preferably by
dimethylamino or
diethylamino, are 2-dimethylaminoethyl, 2-diethylaminoethyl, 3-
dimethylaminopropyl, 3-
diethylaminopropyl, 3-dibutylaminopropyl and 4-diethylaminobutyl.
The group of the formula (111) is preferably -N
U
Preferred examples of C2-C4alkyl substituted by a group of the formula (III)
are groups of
the formula ~N (cH2)2~ . The group ~N IcH2)Zy is particularly
preferred.
Examples of alkoxy containing not more than 8 carbon atoms are methoxy,
ethoxy,
propoxy, isopropoxy, butoxy, isobutoxy, pentoxy, isopentoxy, hexoxy, heptoxy
or octoxy.
Examples of Cs-C,2cycloalkyl which is unsubstituted or substituted by 1, 2 or
3 C,-C4alkyl
are cyctopentyl, methylcyclopentyl, dimethylcyclopentyl, cyclohexyl,
methylcyclohexyl,
dimethylcyclohexyl, trimethylcyclohexyl, t-butylcyclohexyl, cyclooctyl,
cyclodecyl and
cyclododecyl. Unsubstituted or substituted cyclohexyl is preferred.
Examples of alkenyl containing not more than 18 carbon atoms are allyl, 2-
methylallyl,
butenyl, hexenyl, undecenyl and octadecenyl. Alkenyls in which the carbon atom
in the 1-
position is saturated are preferred, and allyl is particularly preferred.
An example of C3-Csalkynyl is 2-butynyl.
2191832
-5-
Examples of phenyl substituted by 1, 2 or 3 C,-C4alkyl or C,-C4alkoxy are
methylphenyl,
dimethylphenyl, trimethylphenyl, t-butylphenyl, di-t-butylphenyl, 3,5-di-t-
butyl-4-
methylphenyl, methoxyphenyl, ethoxyphenyl and butoxyphenyl.
Examples of C,-C9phenylalkyl which is unsubstituted or substituted on the
phenyl by 1, 2 or
3 C,-C4alkyl are benzyl, methylbenzyl, dimethylbenzyl, trimethylbenzyl, t-
butylbenzyl and 2-
phenylethyl. Benzyl is preferred.
Examples of acyl (aliphatic, cycloaliphatic or aromatic) containing not more
than 12 carbon
atoms are formyl, acetyl, propionyl, butyryl, pentanoyl, hexanoyl, heptanoyl,
octanoyl and
benzoyl. C,-CaAlkanoyl and benzoyl are preferred. Acetyl is especially
preferred.
Examples of (C,-C,zalkoxy)carbonyl are methoxycarbonyl, ethoxycarbonyl,
propoxycarbonyl, butoxycarbonyl, pentoxycarbonyl, hexoxycarbonyl,
heptoxycarbonyl,
octoxycarbonyl, nonyloxycarbonyl, decyloxycarbonyl, undecyloxycarbonyl and
dodecyloxycarbonyl.
Examples of alkylene containing not more than 12 carbon atoms are ethylene,
propylene,
trimethylene, tetramethylene, pentamethylene, hexamethylene, octamethylene,
decamethylene and dodecamethylene. R2 is for example C2-CBalkylene or C4-
CBalkylene, in
particular C2-Csalkylene, preferably hexamethylene.
An example of C4-C,2alkenylene is 3-hexenylene.
An example of CS-C,cycloalkylene is cyclohexylene.
Examples of C4-C,2alkylene interrupted by 1,4-piperazinediyl are
-CH2CH2 ~N-CH2CH2 or -CH2CH2CH2 NON-CH2CHZCH2
Examples of C4-C,2alkylene interrupted by -O-, e.g. 1, 2 or 3 -O-, are 3-
oxapentane-1,5-diyl,
4-oxaheptane-1,7-diyl, 3,6-dioxaoctane-1,8-diyl, 4,7-dioxadecane-1,10-diyl,
4,9-dioxadodecane-1,12-diyl, 3,6,9-trioxaundecane-1,11-diyl and 4,7,10-
trioxatridecane-
1,13-diyl.
2191832
-6-
Examples of C4-C,2alkylene interrupted by >N-X, are
-CH2CH2CH2-N(X,)-CH2CH2-N(X,)-CH2CH2CH2-, in particular
-CH2CH2CH2-N(CH3)-CH2CH2-N(CH3)-CH2CH2CH2-.
An example of C5-C,cycloalkylenedi(C,-C4alkylene) is cyclohexylenedimethylene.
Examples of C,-C4alkylenedi(C5-C~cycloalkylene) are methylenedicyclohexylene
and
isopropylidenedicyclohexylene.
An example of phenylenedi(C,-C4alkylene) is phenylenedimethylene.
R is preferably hydrogen, C,-C,oalkyl, cyclohexyl or a group of the formula
(IV), in particular
a group of the formula (IV).
The radicals R, are preferably independently of one another hydrogen, C,-
C4alkyl, allyl,
benzyl or acetyl. Hydrogen and methyl are particularly preferred.
The radical B is preferably N-(2,2,6,6-tetramethylpiperidin-4-yl)butylamino, N-
(1,2,2,6,6-
pentamethylpiperidin-4-yl)butylamino, dibutylamino, 1,1,3,3-
tetramethylbutylamino or 4-
morpholinyl.
The variable n is preferably 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 or 13, for
example 3, 4, 5, 6, 7, 8,
9, 10 or 11 as well as 3, 4, 5, 6, 7, 8 or 9, in particular 3, 5 or 7.
Polydispersity indicates the molecular-weight distribution of a polymeric
compound. In the
present application, the polydispersity is the ratio of weight-average (Mw)
and number-
average (Mn) molecular weights. A value of Mw/Mn equal to 1 means that the
compound
is monodispers and has only one molecular weight and no molecular weight
distribution.
A preferred embodiment of the instant invention relates to a compound of the
formula (I)
wherein
n is 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 or 13;
2191832
,-
the radicals R, are independently of one another hydrogen, C,-CBalkyl, -CH2CN,
C3-Csalkenyl, C,-C9phenylalkyl which is unsubstituted or substituted on the
phenyl by 1, 2 or
3 C,-C4alkyl; or C,-Ceacyl;
R2 is C2-C,zalkylene, C5-C,cycloalkylene, C5-C,cycloalkylenedi{C,-C4alkylene),
C,-C4-alkylenedi(Cs-C,cycloalkylene) or phenylenedi(C,-C4aikylene);
R6 is hydrogen, C,-C,Balkyl, C5-C,2cycloalkyl which is unsubstituted or
substituted by 1, 2 or
3 C,-C4alkyl; C,-C9phenylalkyl which is unsubstituted or substituted on the
phenyl by 1, 2 or
3 C,-C4alkyl; tetrahydrofurfuryl, a group of the formula (IV) or C2-C4alkyl
which is substituted
in the 2, 3 or 4 position by -OH, C,-CBalkoxy, di(C,-C4alkyl)amino or a group
of the formula
(III); and
R is a group of the formula (IV).
A preferred compound of the formula (I) is that wherein
R2 is C2-C,oalkylene, cyclohexylene, cyclohexylenedi(C,-C4alkylene), C,-
C4alkylene-
dicyclohexylene or phenylenedi(C,-C4alkylene);
R3, R4 and R5, which are identical or different, are hydrogen, C,-C,2alkyl, C5-
C,cycloalkyl
which is unsubstituted or substituted by 1, 2 or 3 C,-C4alkyl; C3-C,2alkenyl,
phenyl which is
unsubstituted or substituted by 1, 2 or 3 C,-C4alkyl; benryl which is
unsubstituted or
substituted on the phenyl by C,-C4alkyl; tetrahydrofurfuryl or C2-C3alkyl
which is substituted
in the 2 or 3 position by -OH, C,-C4alkoxy, di(C,-C4alkyl)amino or a group of
the formula (III);
or
-N(R4)(R5) is additionally a group of the formula (III); and
Rs is hydrogen, C,-C,2alkyl, C5-C~cycloalkyl which is unsubstituted or
substituted by 1, 2 or 3
C,-C4alkyl; benryl which is unsubstituted or substituted on the phenyl by 1, 2
or 3
C,-C4alkyl; tetrahydrofurfuryl, a group of the formula (IV) or C2-Csalkyl
which is substituted in
the 2 or 3 position by -OH, C,-C4alkoxy, di(C,-C4alkyl)amino or a group of the
formula (III).
A particularly preferred compound of the formula (I) is that wherein
R2 is C2-CBalkylene;
R3, R4 and R5, which are identical or different, are hydrogen, C,-Cealkyl,
cyclohexyl which is
unsubstituted or substituted by methyl; C3-CBalkenyl, phenyl which is
unsubstituted or
substituted by methyl; benzyl, tetrahydrofurfuryl or C2-C3alkyl substituted in
the 2 or 3
position by -OH, C,-C4alkoxy, dimethylamino, diethylamino or 4-morpholinyl; or
-N(R4)(RS) is
additionally 4-morpholinyl; and
2191832
_8_
Rs is hydrogen, C,-CBalkyl, cyclohexyl which is unsubstituted or substituted
by methyl;
benryl, tetrahydrofurfuryl, a group of the formula (I~ or C2-C3alkyl
substituted in the 2 or 3
position by -OH, C,-C4alkoxy, dimethylamino, diethylamino or 4-morpholinyl.
A compound of the formula (I) of special interest is that wherein
n is 3, 5 or 7;
the radicals R, are independently of one another hydrogen or methyl;
R2 is C2-Csalkylene;
the radicals A are independently of one another -N(R4)(RS) or a group of the
formula (II);
R4 and R5, which are identical or different, are hydrogen, C,-CBalkyl, 2-
hydroxyethyl or
2-methoxyethyl or -N(R4)(R5) is additionally 4-morpholinyl;
X is >NRs;
R6 is C,-C4alkyl; and
the radicals B have independently of one another one of the definitions given
for A.
A further compound of the formula (I) which is of special interest is a
compound of the
formula (X)
A~N~-.-N Rz-N N '~--N Rz-N N~A (X)
Nw N I
N'''N N w N
R ~C ~ a"~ ~ R
B~ ~C ~ ~ g ~C ~ ~ B.
n -1
wherein n, A, B, R, R, and R2 are as defined above and B* has one of the
definitions given
for B;
with the provisos that (1 ) B* is different from B and (2) each of the
radicals 8, R, R, and R2
has the same meaning in the individual recurring units of the formula.
A preferred compound of the formula (X) is that wherein
n is 3, 5 or 7;
the radicals R, are independently of one another hydrogen or methyl;
R2 is C2-Csalkylene;
2191832
_9_
A and B', which are identical, are -N(R4)(RS);
R4 and R5, which are identical or different, are hydrogen, C,-Csalkyl, 2-
hydroxyethyl or
2-methoxyethyl or -N(R4)(RS) is additionally 4-morpholinyl;
B is a group of the formula (II) with R, being as defined above;
X is >NRs;
R6 is C,-C4allryl; and
R is a group of the formula (IV) with R, being as defined above;
with the proviso that each of the radicals B, R, R, and R2 has the same
meaning in the
individual recurring units of the formula.
A further embodiment of the instant invention is a mixture containing at least
three different
compounds of the formula (I), preferably of the formula (X), which vary only
by the variable
n, said mixture having a polydispersity Mw/Mn of 1.1 to 1.7, for example 1.1
to 1.65, 1.1 to
1.6, 1.1 to 1.55, 1.1 to 1.5, preferably 1.1 to 1.45 or 1.1 to 1.40, in
particular 1.1 to 1.35.
Further examples for the polydispersity Mw/Mn are 1.15 to 1.7, for example
1.15 to 1.65,
1.15 to 1.6, 1.15 to 1.55, 1.15 to 1.5, preferably 1.15 to 1.45 or 1.15 to
1.40, in particular
1.15 to 1.35.
A preferred mixture contains
a) a compound of the formula (la),
A i ~ N Rz N i A (la)
N~ N I N~ N
H3C CH3
B
CH3
3
b) a compound of the formula (Ib) and
2191832
-10-
A i ~N RZ N i ~A (Ib)
i
N'''N I N'''N
R H3C ~ CH3 ~'B
H3C ~ CH3
R' 5
c) a compound of the formula (Ic)
A i (~----~ N RZ N i 1--- A (IC)
N~ N N~ N
R H3C CH3
B
H3C N ~ CH3
R~
7
wherein A, B, R, R, and R2 are in the formulae (la), (Ib) and (Ic) identical
and are as defined
above, and the ratio of the compounds of the formula (la) to (Ib) to (Ic) is
2:1.5:1 to
2:0.5:0.05, in particular 2:1:0.5 to 2:0.5:0.08 or 2:0.75:0.3 to 2:0.5:0.08.
The compound of the formula (la) corresponds preferably to a compound of the
formula
(Xa),
N
--N Rz-N N~-N Rz N N1--A
i s
N~ N I N~ N ( N~ N
R H3C a"~ ~ R H3C
B' n
H3 CH3 B H3C ~ N ~ ~ CH3 B'
C I
R, R,
2
(Xa)
the compound of the formula (Ib) corresponds preferably to a compound of the
formula (Xb)
2191832
-11 -
A~N~N RZ N N~N ~ N N~--A (Xb)
N~ N I N~ N I N~'1N
R H3C a"~ ~ R
B~ ~C y ~~ B ~C y ~ y'ia B.
4
and the compound of the formula (Ic) corresponds preferably to a compound of
the formula
(Xc).
A~/N~---N-RZ N N~N RZ N N~--A (XC)
N~ N N~ N I N~ ~~N
R ~C ~'~a ~ R ~C a'ia
B. ~ ~ i ~~~ B H3C ~N~ ~~ B
R,
6
The indicated mixtures can additionally contain a compound of the formula
(Id), for example
a compound of the formula (Xd),
A~N~--N RZ N --~~N~--A (Id)
N~ N I N~ N
R H3C CH3
B
H3C N ~ CH3 B
R~
A'-~ N ~ N R2 N ~ ~~---A (Xd)
N~ N ( N~ N
R H3C CH3
H3C N ~ CH3 B
R~
and/or a compound of the formula (le)
2191832
-12-
R~
H3C N CH3
HsC ~ ~CH3
N R2 N
N ~N
B~~ ~N N~ ~~B
N ~ N (le)
N ~
H3C ~ CH3 R
H3C i CH3
R~
These compounds which are known from US-A-4,108,829 and US-A-4,442,250 may be
present in the mixtures in an amount from 30 % to 0.5 %, preferably 20 % to
0.5 % or 8
to 0.5 % with regard to the weight of the total mixture.
The preferred embodiments indicated above for the compounds of the formula (I)
also
relate to the mixtures thereof.
A particularly preferred mixture is one containing a compound of the formula
(la), a
compound of the formula (Ib) and a compound of the formula (Ic) wherein
the radicals R, are independently of one another hydrogen or methyl;
R2 is C2-Csalkylene;
A and B, which are identical or different, are -N(R4)(R5) or a group of the
formula (II) with R,
being as defined above;
R4 and R5, which are identical or different, are hydrogen, C,-CBalkyl, 2-
hydroxyethyl or
2-methoxyethyl or -N(R4)(RS) is additionally 4-morpholinyl;
X is >NRs;
Rs is C,-C4alkyl; and
R is a group of the formula (IV) with R, being as defined above.
Another particularly preferred mixture is one wherein the three different
compounds of the
formula (I) correspond to compounds of the formulae (Xa), (Xb) and (Xc)
2191832
-13-
wherein
the radicals R, are independently of one another hydrogen or methyl;
R2 is C2-Csalkylene;
A and B*, which are identical or different, are -N(R4)(R5) or a group of the
formula (II);
R4 and R5, which are identical or different, are hydrogen, C,-CBalkyl, 2-
hydroxyethyl or
2-methoxyethyl or-N(R4)(RS) is additionally 4-morpholinyl;
B is a group of the formula (II) with R, being as defined above;
X is >NRs;
Rs is C,-C4alkyl; and
R is a group of the formula (IV) with R, being as defined above;
with the proviso that each of the radicals B, R, R, and R2 has the same
meaning in the
individual recurring units of the fornulae.
A and B*, which are identical or different, are preferably -N(C,-Cealkyl)2 or
a group
HsC CHs HsC CHs
v v
N-H or i N CH3
(C~-C4alkyl) ~ (C~-C4alkyl)
H3C CH3 H3C CH3
A and B* are in particular identical and are -N(C,-Cealkyl)2.
A further embodiment of the instant invention is a method for preparing a
mixture having the
polydispersity indicated above and containing at least three different
compounds of the
formula (I), which comprises
1 ) reacting a compound of the formula {A)
cyN~l --ci {A)
N \'N
~I'B
with a compound of the formula (B)
2191832
-14-
H ~ R2 N H (B)
R HsC / \ CH3
H3C N CH3
R~
in a stoichiometric ratio to obtain a compound of the formula (C);
N N
CI~ ~ ~ ~ N / ~CI (C)
N~ N R N~ N
HsC ~ CHs
H3C N CH3
R~
2) reacting a compound of the formula (C) with a compound of the formula (B)
in a ratio of
1:2 to 1:3, preferably 1:2 to 1:2.5, in particular in a ratio of 1:2, to
obtain a mixture of at least
three different compounds of the formula (D) with n being 3, 4, 5, 6, 7, 8, 9,
10, 11, 12, 13,
14 or 15 or being 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 or 13, preferably 3, 4, 5,
6, 7, 8, 9, 10 or 11
or preferably 3, 4, 5, 6, 7, 8 or 9, in particular 3, 5 and 7;
H-N-Rx N N~N Rx N H (D)
N~ N
HaC CHa ~ R HaC CHa
H3C i CH3 B H3C ~ CH3
R~ R' n -1
3) reacting the mixture obtained in 2) with a compound of the formula (E)
CI~N~A (E)
N \'N
~B
in about a stoichiometric ratio to obtain the desired mixture; the reactions 1
) to 3) being
carried out in an organic solvent in the presence of an inorganic base.
A particularly preferred embodiment of the instant invention relates to a
method for
preparing a mixture having the polydispersity indicated above and containing
at least three
2191832
-15-
different compounds of the formula (X), which comprises the above reactions 1
) to 3) with
the proviso that a compound of the formula (E*)
CI~N~A (E*)
N \'N
~B*
is applied instead of a compound of the formula {E).
Examples for suitable organic solvents are toluene, xylene, trimethylbenzene,
isopropylbenzene, diisopropylbenzene and essentially water-insoluble organic
ketones such
as for example methyl ethyl ketone and methyl isobutyl ketone. Xylene is
preferred.
Examples for an inorganic base are sodium hydroxide, potassium hydroxide,
sodium
carbonate and potassium carbonate. Sodium hydroxide is preferred.
The reaction 1 ) is carried out, for example, at a temperature of 40°C
to 70°C,
preferably 50°C to 60°C.
The reaction 2) is carried out, for example, at a temperature of 110° C
to 180°C,
preferably 140°C to 160°C.
The reaction 3) is carried out, for example, at a temperature of 110°C
to 180°C,
preferably 140°C to 160°C.
Possible by-products are the above shown compounds of the formulae (Id) and
(le).
The compound of the formula (A) can be prepared, for example, by reacting
cyanuric
chloride with a compound B-H in a stoichiometric ratio in the presence of an
organic solvent
and an inorganic base.
Furthermore, the compound of the formula (E) or (E*) can be prepared, for
example, by
reacting cyanuric chloride with compounds of the formulae A-H and B-H or B*-H
in a
stoichiometric ratio in the presence of an organic solvent and an inorganic
base.
2191832
-16-
It is appropriate to use for the preparation of the compounds of the formulae
(A) and (E) or
(E*) the same solvent and the same inorganic base than in the above indicated
reactions 1 )
to 3).
The starting materials used in the above process are known. In the case that
they are not
commercially available, they can be prepared analogously to known methods. For
example,
some starting materials of the formula (B) are described in WO-A-95/21157,
US-A-4,316,837 and US-A-4,743,688.
An embodiment of the instant invention is also a mixture obtainable by the
above indicated
method.
The intermediates of the formula (D) are novel and are a further embodiment of
the instant
invention. In addition, the instant invention relates to a mixture containing
at least three
different compounds of the formula (D) which vary only by the variable n, said
mixture
having a polydispersity Mw/Mn of 1.1 to 1.7.
The preferred embodiments of the variable n and the radicals R, R,, R2 and B
indicated
above for the compounds of the formula (I) also relate to the intermediates of
the formula
(D).
A compound of the formula (I) or (D) with a polydispersity Mw/Mn of 1 may be
prepared
by building up said compound step by step. Some representative examples for
such a
procedure are shown below.
I) A compound of the formula (I) wherein R is a group of the formula (IV) and
n is 3 may
conveniently be prepared by reacting a compound of the formula (E) with a
large excess of
a compound of the formula (B) to obtain a compound of the fomula (F) according
to
Scheme I-1. The molar ratio of the compound of the formula (E) to the compound
of the
formula (B) may be for example 1:4.
2191832
-17
Scheme I-1:
N
~CI + HN R2 NH
N'\'N
H3C 1 'CH3 H3C CH3
'~(\B
N N
H3C I CH3 H3C ~ CH3
R~ R~
(E) (B)
~N~N R2 NH
N ~ ~ ~N
H3C CH3 H3C CH3
HsC ~ ~CH3 HaC ~ ~CH3
R, R,
(F)
Subsequently, the compound of the formula (F) may be reacted with the compound
of the
formula (C) in a stoichiometric ratio to obtain the desired compound as shown
in Scheme
I-2.
2191832
-18-
Scheme I-2:
2 ~N~N R2 NH +
N ~ I'N
H3C CH3 H3C CH
B H3C NCH H C N~CH3
3
R~ R~
(F)
CI~N~N R2 N i ~CI
N ~ ~N~ N ~ I IN
H3C CH3 H3C CH3
HsC _ ~ ~ \CH3 HsC w ~ i \CH3
R~ R~
tC)
A % ~ N R2 N i II A
N~ N N~ N
H3C CH3 H3C CH3
N~ ~ ~N~ ~
HsC ( CH3 H3C I CH3
R~ R~
II) A compound of the formula (I) wherein R is a group of the formula {IV) and
n is 4 may
conveniently be prepared by reacting a compound of the formula (F) with a
compound of
the formula (A) in a stoichiometric ratio to obtain a compound of the formula
{G) according
to Scheme II-1.
2191832
-19-
Scheme II-1:
~N~N R2 NH + CI~N~CI
N ~ ~ ~N N ~ ' IN
H3C CH3 H3C CH3
H3C ~ ~ ~CH3 H3C ~ i ~ NCH
3
R~ R~
(F) (A)
A--~N1--N R2 N i ~CI
N~ N N~ N
H3C CH3 H3C CH3
H3C i ~ \CH3 H3C ' i ~ NCH
3
R, R,
(G)
Then, the compound of the formula (G) may be reacted with a large excess of a
compound
of the formula (B) to obtain a compound of the formula (H) as shown in Scheme
II-2. The
molar ratio of the compound of the formula (G) to the compound of the formula
(B) may be
for example 1:4.
2191832
-20-
Scheme II-2:
A~N~N R2 N i ~CI +
N~ ~N~ N~ N
H3C CH3 H3C CH3
H3C i ~ ~CH3 H3C ~ i ~ NCH
3
R~ R~
(G)
HN R2 NH '
H3C CH3 H3C CH3
H3C ~ CH3 H3C ~ CH3
R, R,
(B)
A~N~I ---N R2 N ~ ~~ --N R2 NH
N~ IIN N~ N
H3C CH3 H3C CH3 ~ H3C CH3 H3C CH3
H3C N~CH3 H3C N~CH3 H3C N~CH3 HsC NCH
3
R' R~ R' R,
{H)
Subsequently, the compound of the formula (H) may be reacted with a compound
of the
formula (A) in a stoichiometric ratio to obtain a compound of the formula (K),
following the
Scheme II-3.
2191832
-21 -
Scheme II-3:
2 A~N~I --N R2 N~N~N R2 NH
N~ N N~ ''N
H3C CH3 H3C CH3 ~ H3C CH3 H3C CH3
H3C I 'CH3 H3C '~' 'CH3 H3C 'I' 'CH' H'C \N~CH
3
R, R, R, R,
(H)
+ CI~N~---CI
N\/N
~B
(A)
A % ( N R2 N i ~A
N~ N N~ ~~N
H3C CH3 H3C CH3
N~ ~ ~N~ ~
HsC ( CH3 H3C ~ CH3
R~ R~
d
(K)
III) A compound of the formula (I) wherein R is a group of the formula (IV)
and n is 5 may
conveniently be prepared by reacting a compound of the formula (H) with a
compound of
the formula (C) in a stoichiometric ratio to obtain a compound of the formula
(l_)
2191832
- 22 -
2 A~N~N R2 N~N~N RZ NH
N~ N N~ ''N
H3C CH3 H3C CH3 ~ H3C CH3 H3C CH3
H3C ~ ~CH3 H3C ' ~ ~ ~C~ H3C ' ~ ~ ~CH3 H3C ~N~CH3
R~ R~ R~
(H)
+ CI ~ N ~ N R2 N i ~ CI
N~ N N~ N
H3C CH3 H3C CH3
H3C _ ~ , wCH3 H3C ~ ~ ~ wCH3
R, R,
(C)
A i I N R2 N i ~A
N N w ' IN
H3C CH3 H3C CH3
HsC _ ~ ~ wCH3 HsC ~ ~ ~ wCH3
R~ R~
(L)
The reactions I) to III) are carried out, for example, in an organic solvent
such as toluene,
xylene, trimethylbenzene in the presence of an inorganic base such as sodium
hydroxide at
a temperature of 110°C to 180°C, preferably 140°C to
160°C.
When the compound of the formula (I) corresponds to a compound of the formula
(X), the
corresponding compounds of the formulae (Xa), (Xb) and (Xc) may be prepared in
analogy
to the above schemes by using a compound of the formula (E*) instead of a
compound of
the formula (E).
2191832
-23-
The intermediate of the formula (D) wherein n is for instance 3 and which has
a
polydispersity Mw/Mn of 1 may be prepared, for example, by reacting a compound
of the
formula (C) with a compound of the formula (B) in a ratio of 1:10 to 1:50,
preferably 1:20 to
1:40, in particular 1:20 to 1:35. The reaction may be carried out e.g. in an
organic solvent or
neat in the presence of an inorganic base. The solvent and/or the excess of
the reactant of
the formula (B) can be eliminated by distillation at the appropriate
conditions. Examples for
an organic solvent are toluene, xylene, trimethylbenzene, isopropylbenzene and
diisopropylbenzene. Xylene is preferred. Examples for an inorganic base are
sodium
hydroxide, potassium hydroxide, sodium carbonate and potassium carbonate.
Sodium
hydroxide is preferred. The reaction is carried out at a temperature of, for
example, 110°C
to 180°C, preferably 140°C to 160°C.
The compounds of the formula (I) as well as the described mixtures with a
narrow molecular
weight distribution are very effective in improving the light, heat and
oxidation resistance of
organic materials, especially synthetic polymers and copolymers. In
particular, a low
pigment interaction as well as a very good colour is observed in
polypropylene, especially
polypropylene fibres.
Examples of organic materials which can be stabilized are:
1. Polymers of monoolefins and diolefins, for example polypropylene,
polyisobutylene,
polybut-1-ene, poly-4-methylpent-1-ene, polyisoprene or polybutadiene, as well
as polymers
of cycloolefins, for instance of cyclopentene or norbornene, polyethylene
(which optionally
can be crosslinked), for example high density polyethylene (HDPE), high
density and high
molecular weight polyethylene (HDPE-HMW), high density and ultrahigh molecular
weight
polyethylene (HDPE-UHMW), medium density polyethylene (MDPE), low density
polyethy-
lene (LDPE), linear low density polyethylene (LLDPE), branched low density
polyethylene
(BLDPE).
Polyolefins, i.e. the polymers of monoolefins exemplified in the preceding
paragraph, prefe-
rably polyethylene and polypropylene, can be prepared by different, and
especially by the
following, methods:
a) radical polymerisation (normally under high pressure and at elevated
temperature).
CA 02191832 2005-03-14
29276-600
- 24 -
b) catalytic polymerisation using a catalyst that normally contains one or
more than
one metal of groups IVb, Vb, Vlb or VIII of the Periodic Table. These metals
usually
have one or more than one Ifgand, typically oxides, halides, alcoholates,
esters,
ethers, amines, alkyls, alkenyls and/or aryls that may be either n- or Q-
coordinated.
These metal complexes may be in the free form or fixed on substrates,
typically on
activated magnesium chloride, titanium(III) chloride, alumina or silicon
oxide. These
catalysts may be soluble or insoluble in the polymerisation medium. The
catalysts
can be used by themselves in the polymerisation or further activators may be
used,
typically metal alkyls, metal hydrides, metal alkyl halides, metal alkyl
oxides or me-
tal alkyloxanes, said metals being elements of groups la, Ila and/or Ilia of
the
Periodic Table. The activators may be modified conveniently with further
ester,
ether, amine or silyi ether groin. These catalyst systems are usually termed
Phillips; Standard Oil Indiana, Ziegier (-Natta), TNZ (DuPoM), metallocene or
single
site catalysts (SSC).
2. Mixtures of the polymers mentioned under 1 ), for example mixtures of
polypropylene
with polyisobutylene, polypropylene with polyethylene (for example PPIHDPE,
PPILDPE)
and mixtures of different types of polyethylene (for example LDPElHDPE).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl
monomers,
for example ethylene/propylene copolymers, linear low density polyethylene
(LLDPE) and
mixtures thereof with low density polyethylene (LDPE), propylene/but-1-ene
copolymers,
propylene/isobutylene copolymers, ethylene/but-1-ene copolymers,
ethylenelhexene copo-
lymers, ethylenelmethylpentene copolymers, ethylene/heptene copolymers,
ethylene%c-
tene cropolymers, propylene/butadiene copolymers, isobutylenersoprene
copolymers, ethy-
lene/alkyl acrylate copolymers, ethylene/alkyl methacrylate copolymers;
ethylene/vinyl
acetate copolymers and their copolymers with carbon monoxide or
ethylene/acrylic acid
copolymers and their salts (ionomers) as well as terpolymers of ethylene with
propylene and
a diene such as hexadiene, dicyclopentadiene or ethylidene-norbomene; and
mixtures of
such copolymers with one another and with polymers mentioned in 1 ) above, for
example
polypropylene/ethylene-propylene copolymers, LDPE/ethylene-vinyl acetate
copolymers
(EVA), LDPE/ethylene-acrylic acid copolymers (EAA), LLDPE/EVA, LLDPE/EAA and
alter
*Trade-mark
2191832
-25-
nating or random polyalkylene/carbon monoxide copolymers and mixtures thereof
with other
polymers, for example polyamides.
4. Hydrocarbon resins (for example C5-C9) including hydrogenated modifications
thereof
(e.g. tackifiers) and mixtures of polyalkylenes and starch.
5. Polystyrene, polyp-methylstyrene), poly(a-methylstyrene).
6. Copolymers of styrene or a-methylstyrene with dienes or acrylic
derivatives, for example
styrene/butadiene, styrene/acrylonitrile, styrene/alkyl methacrylate,
styrene/butadiene/alkyl
acrylate, styrene/butadiene/alkyl methacrylate, styrene/maleic anhydride,
styrene/acrylo-
nitrile/methyl acrylate; mixtures of high impact strength of styrene
copolymers and another
polymer, for example a polyacrylate, a diene polymer or an
ethylene/propylene/diene terpo-
lymer; and block copolymers of styrene such as styrene/butadiene/styrene,
styrene/iso-
prene/styrene, styrene/ethylene/butylene/styrene or
styrene/ethylene/propylene/ styrene.
7. Graft copolymers of styrene or a-methylstyrene, for example styrene on
polybutadiene,
styrene on polybutadiene-styrene or polybutadiene-acrylonitrile copolymers;
styrene and
acrylonitrile (or methacrylonitrile) on polybutadiene; styrene, acrylonitrile
and methyl meth-
acrylate on polybutadiene; styrene and malefic anhydride on polybutadiene;
styrene, acrylo-
nitrile and malefic anhydride or maleimide on polybutadiene; styrene and
maleimide on poly-
butadiene; styrene and alkyl acrylates or methacrylates on polybutadiene;
styrene and
acrylonitrile on ethylene/propylene/diene terpolymers; styrene and
acrylonitrile on polyalkyl
acrylates or polyalkyl methacrylates, styrene and acrylonitrile on
acrylate/butadiene copoly-
mers, as well as mixtures thereof with the copolymers listed under 6), for
example the
copolymer mixtures known as ABS, MBS, ASA or AES polymers.
8. Halogen-containing polymers such as polychloroprene, chlorinated rubbers,
chlorinated
and brominated copolymer of isobutylene-isoprene (halobutyl rubber),
chlorinated or sulfo-
chlorinated polyethylene, copolymers of ethylene and chlorinated ethylene,
epichlorohydrin
homo- and copolymers, especially polymers of halogen-containing vinyl
compounds, for
example polyvinyl chloride, polyvinylidene chloride, polyvinyl fluoride,
polyvinylidene fluo-
2191a32
-26-
ride, as well as copolymers thereof such as vinyl chloride/vinylidene
chloride, vinyl chlo-
ride/vinyl acetate or vinylidene chloride/vinyl acetate copolymers.
9. Polymers derived from a,~i-unsaturated acids and derivatives thereof such
as polyacry-
lates and polymethacrylates; polymethyl methacrylates, polyacrylamides and
polyacryloni-
triles, impact-modified with butyl acrylate.
10. Copolymers of the monomers mentioned under 9) with each other or with
other unsatu-
rated monomers, for example acrylonitrile/ butadiene copolymers,
acrylonitrile/alkyl acrylate
copolymers, acrylonitrile/alkoxyalkyl acrylate or acrylonitrile/vinyl halide
copolymers or
acrylonitrile/ alkyl methacrylate/butadiene terpolymers.
11. Polymers derived from unsaturated alcohols and amines or the acyl
derivatives or
acetals thereof, for example polyvinyl alcohol, polyvinyl acetate, polyvinyl
stearate, polyvinyl
benzoate, polyvinyl maleate, polyvinyl butyral, polyallyl phthalate or
polyallyl melamine; as
well as their copolymers with olefins mentioned in 1 ) above.
12. Homopolymers and copolymers of cyclic ethers such as polyalkylene glycols,
polyethy-
lene oxide, polypropylene oxide or copolymers thereof with bisglycidyl ethers.
13. Polyacetals such as polyoxymethylene and those polyoxymethylenes which
contain
ethylene oxide as a comonomer; polyacetals modified with thermoplastic
polyurethanes,
acrylates or MBS.
14. Polyphenylene oxides and sulfides, and mixtures of polyphenylene oxides
with styrene
polymers or polyamides.
15. Polyurethanes derived from hydroxyl-terminated polyethers, polyesters or
polybuta-
dienes on the one hand and aliphatic or aromatic polyisocyanates on the other,
as well as
precursors thereof.
16. Polyamides and copolyamides derived from diamines and dicarboxylic acids
and/or
from aminocarboxylic acids or the corresponding lactams, for example polyamide
4, poly-
amide 6, polyamide 6/6, 6/10, 6/9, 6/12, 4/6, 12/12, polyamide 11, polyamide
12, aromatic
2191832
-27-
polyamides starting from m-xylene diamine and adipic acid; polyamides prepared
from
hexamethylenediamine and isophthalic or/and terephthalic acid and with or
without an
elastomer as modifier, for example poly-2,4,4,-trimethylhexamethylene
terephthalamide or
poly-m-phenylene isophthalamide; and also block copolymers of the
aforementioned poly-
amides with polyolefins, olefin copolymers, ionomers or chemically bonded or
grafted
elastomers; or with polyethers, e.g. with polyethylene glycol, polypropylene
glycol or poly
tetramethylene glycol; as well as polyamides or copolyamides modified with
EPDM or ABS;
and polyamides condensed during processing (RIM polyamide systems).
17. Polyureas, polyimides, polyamide-imides, polyetherimids, polyesterimids,
polyhydanto-
ins and polybenzimidazoles.
18. Polyesters derived from dicarboxylic acids and diols and/or from
hydroxycarboxylic
acids or the corresponding lactones, for example polyethylene terephthalate,
polybutylene
terephthalate, poly-1,4-dimethylolcyclohexane terephthalate and
polyhydroxybenzoates, as
well as block copolyether esters derived from hydroxyl-terminated polyethers;
and also poly-
esters modified with polycarbonates or MBS.
19. Polycarbonates and polyester carbonates.
20. Polysulfones, polyether sulfones and polyether ketones.
21. Crosslinked polymers derived from aldehydes on the one hand and phenols,
ureas and
melamines on the other hand, such as phenoUformaldehyde resins,
urea/formaldehyde
resins and melamine/formaldehyde resins.
22. Drying and non-drying alkyd resins.
23. Unsaturated polyester resins derived from copolyesters of saturated and
unsaturated
dicarboxylic acids with polyhydric alcohols and vinyl compounds as
crosslinking agents, and
also halogen-containing modifications thereof of low flammability.
24. Crosslinkable acrylic resins derived from substituted acrylates, for
example epoxy acry-
lates, urethane acrylates or polyester acrylates.
2191832
-28-
25. Alkyd resins, polyester resins and acrylate resins crosslinked with
melamine resins,
urea resins, isocyanates, isocyanurates, polyisocyanates or epoxy resins.
26. Crosslinked epoxy resins derived from aliphatic, cycloaliphatic,
heterocyclic or aromatic
glycidyl compounds, e.g. products of diglycidyl ethers of bisphenol A and
bisphenolF, which
are crosslinked with customary hardeners such as anhydrides or amines, with or
without
accelerators.
27. Natural polymers such as cellulose, rubber, gelatin and chemically
modified homolo-
gous derivatives thereof, for example cellulose acetates, cellulose
propionates and cellu-
lose butyrates, or the cellulose ethers such as methyl cellulose; as well as
rosins and their
derivatives.
28. Blends of the aforementioned polymers (polyblends), for example PP/EPDM,
Poly-
amide/EPDM or ABS, PVC/EVA, PVC/ABS, PVC/MBS, PC/ABS, PBTP/ABS, PC/ASA,
PC/PBT, PVC/CPE, PVC/acrylates, POM/thermoplastic PUR, PC/thermoplastic PUR,
POM/acrylate, POM/MBS, PPO/HIPS, PPO/PA 6.6 and copolymers, PA/HDPE, PA/PP,
PA/PPO, PBT/PC/ABS or PBT/PET/PC.
29. Naturally occurring and synthetic organic materials which are pure
monomeric com-
pounds or mixtures of such compounds, for example mineral oils, animal and
vegetable
fats, oil and waxes, or oils, fats and waxes based on synthetic esters (e.g.
phthalates, adi-
pates, phosphates or trimellitates) and also mixtures of synthetic esters with
mineral oils in
any weight ratios, typically those used as spinning compositions, as well as
aqueous emul-
sions of such materials.
30. Aqueous emulsions of natural or synthetic rubber, e.g. natural latex or
latices of carbo-
xylated styrene/butadiene copolymers.
The invention thus also relates to a composition comprising an organic
material susceptible
to degradation induced by light, heat or oxidation and at least one compound
of the formula
(I), preferably formula (X), with a polydispersity Mw/Mn = 1, with the proviso
that the totality
of the compounds of the formula (I) being present in the composition has a
polydispersity
2191832
- 29 -
Mw/Mn of 1 to 1.7, for example 1 to 1.65, 1 to 1.6, 1 to 1.55, 1 to 1.5, 1 to
1.45, 1 to 1.4 or
1 to 1.35.
The invention further relates to a composition comprising an organic material
susceptible to
degradation induced by light, heat or oxidation and a mixture containing at
least three
different compounds of the formula (I), preferably formula (X), which vary
only by the
variable n, said mixture having a polydispersity Mw/Mn of 1.1 to 1.7 or 1.1 to
1.5, with the
proviso that the totality of the compounds of the formula (I) being present in
the composition
has a polydispersity Mw/Mn of 1.1 to 1.7 or 1.1 to 1.5.
The organic material is preferably a synthetic polymer, more particularly one
selected from
the aforementioned groups. Polyolefins are preferred and polyethylene and
polypropylene
are particularly preferred.
A further embodiment of the instant invention is a method for stabilizing an
organic material
against degradation induced by light, heat or oxidation, which comprises
incorporating into
said organic material at least one compound of the formula (I), preferably
formula (X), with a
polydispersity Mw/Mn =1, with the proviso that the totality of the compounds
of the formula
(I) being present in the composition has a polydispersity Mw/Mn of 1 to 1.7,
preferably 1 to
1.5 or 1 to 1.4, in particular 1 to 1.35.
The compounds of the formula (I) or a mixture thereof can be used in various
proportions
depending on the nature of the material to be stabilized, on the end use and
on the
presence of other additives.
In general, it is appropriate to use, for example, 0.01 to 5 % by weight of
the compounds of
the formula (I) or the mixture thereof, relative to the weight of the material
to be stabilized,
preferably 0.05 to 1 %.
The compounds of the formula (I) or the mixture thereof can be added, for
example, to the
polymeric materials before, during or after the polymerization or crosslinking
of the said
materials. Furthermore, they can be incorporated in the polymeric materials in
the pure form
or encapsulated in waxes, oils or polymers.
2191832
-30-
In general, the compounds of the formula (I) or the mixture thereof can be
incorporated in
the polymeric materials by various processes, such as dry mixing in the form
of powder, or
wet mixing in the form of solutions or suspensions or also in the form of a
masterbatch; in
such operations, the polymer can be used in the form of powder, granules,
solutions,
suspensions or in the form of latices.
The materials stabilized with the compounds of the formula (I) or the mixture
thereof can be
used for the production of mouldings, films, tapes, monofilaments, fibres,
surface coatings
and the like.
If desired, other conventional additives for synthetic polymers, such as
antioxidants, UV
absorbers, nickel stabilizers, pigments, fillers, plasticizers, corrosion
inhibitors and metal
deactivators, can be added to the organic materials containing the compounds
of the
formula (I) or mixtures thereof.
Particular examples of said conventional additives are:
1. Antioxidants
1.1. Alkylated monophenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-
tert-butyl-4,6-
dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-
butylphenol, 2,6-di-tert-
butyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol, 2-(a-
methylcyclohexyl)-4,6-di-
methylphenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tricyclohexylphenol, 2,6-
di-tert-butyl-4-
methoxymethylphenol, nonylphenols which are linear or branched in the side
chains, for
example, 2,6-di-nonyl-4-methylphenol, 2,4-dimethyl-6-(1'-methylundec-1'-
yl)phenol, 2,4-di-
methyl-6-(1'-methylheptadec-1'-yl)phenol, 2,4-dimethyl-6-(1'-methyltridec-1'-
yl)phenol and
mixtures thereof.
1.2. Alkylthiomethylphenols, for example 2,4-dioctylthiomethyl-6-tert-
butylphenol, 2,4-di-
octylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-di-
dodecylthio-
methyl-4-nonylphenol.
2191832
-31 -
1.3. Hy~droquinones and alkyrlated hydroquinones, for example 2,6-di-tert-
butyl-4-methoxy-
phenol, 2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-
Biphenyl-4-octa-
decyloxyphenol, 2,6-di-tert-butylhydroquinone, 2,5-di-tert-butyl-4-
hydroxyanisole, 3,5-di-tert-
butyrl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyphenyl stearate, bis-(3,5-
di-tert-butyl-4-
hydroxyphenyl) adipate.
1.4. Tocopherols, for example a-tocopherol, [i-tocopherol, ~tocopherol, S-
tocopherol and
mixtures thereof (Vitamin E).
1.5. Hyrdroxyrlated thiodipheny~l ethers, for example 2,2'-thiobis(6-tert-
butyl-4-methylphenol),
2,2'-thiobis(4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol), 4,4'-
thiobis(6-tent-butyl-
2-methylphenol), 4,4'-thiobis-(3,6-di-sec-amylphenol), 4,4'-bis-(2,6-dimethyl-
4-hydroxyphe-
nyl) disulfide.
1.6. Alkyrlidenebisphenols, for example 2,2'-methylenebis(6-tert-butyl-4-
methylphenol), 2,2'-
methylenebis(6-tert-butyl-4-ethylphenol), 2,2'-methylenebis[4-methyl-6-(a-
methylcyclohexyl}-
phenol], 2,2'-methylenebis(4-methyl-6-cyclohexylphenol), 2,2'-methylenebis{6-
nonyl-4-
methylphenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), 2,2'-
ethylidenebis(4,6-di-tert-
butylphenol), 2,2'-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2'-
methylenebis[6-(a-
methylbenzyl)-4-nonylphenol], 2,2'-methylenebis(6-(a,a-dimethylbenryl)-4-
nonylphenol],
4,4'-methylenebis(2,6-di-tert-butylphenol}, 4,4'-methylenebis(6-tert-butyl-2-
methylphenol),
1,1-bis(5-tert-butyl-4-hydroxy-2-methylphenyl)butane, 2,6-bis(3-tert-butyl-5-
methyl-2-
hydroxybenzyl}-4-methylphenol, 1,1,3-tris{5-tert-butyl-4-hydroxy-2-
methylphenyl)butane,
1,1-bis(5-tert-butyl-4-hydroxy-2-methyl-phenyl)-3-n-dodecylmercaptobutane,
ethylene glycol
bis[3,3-bis(3'-tert-butyl-4'-hydroxyphenyl)butyrate], bis(3-tert-butyl-4-
hydroxy-5-methyl-
phenyl}dicyclopentadiene, bis[2-(3'-tert-butyl-2'-hydroxy-5'-methylbenryl)-6-
tert-butyl-4-
methylphenyl]terephthalate, 1,1-bis-(3,5-dimethyl-2-hydroxyphenyl)butane, 2,2-
bis-(3,5-di-
tert-butyl-4-hydroxyphenyl)propane, 2,2-bis-{5-tert-butyl-4-hydroxy2-
methylphenyl)-4-n-
dodecylmercaptobutane, 1,1,5,5-tetra-(5-tert-butyl-4-hydroxy2-
methylphenyl)pentane.
1.7. O-. N- and S-benzyrl compounds, for example 3,5,3',5'-tetra-tert-butyl-
4,4'-dihydroxydi-
benryl ether, octadecyl-4-hydroxy-3,5-dimethylbenzylmercaptoacetate, tridecyl-
4-hydroxy-
3,5-di-tert-butylbenrylmercaptoacetate, tris(3,5-di-tert-butyl-4-
hydroxybenzyl)amine, bis(4-
2191832
-32-
tert-butyl-3-hydroxy-2,6-dimethylbenzyl)dithioterephthalate, bis(3,5-di-tert-
butyl-4-hydroxy-
benzyl)sulfide, isooctyl-3,5di-tert-butyl-4-hydroxybenzylmercaptoacetate.
1.8. Hyrdroxyrbenzyrlated malonates, for example dioctadecyl-2,2-bis-(3,5-di-
tert-butyl-2-
hydroxybenzyl)-malonate, di-octadecyl-2-(3-tert-butyl-4-hydroxy-5-
methylbenzyl)-malonate,
di-dodecylmercaptoethyl-2,2-bis-(3,5-di-tert-butyl-4-hydroxybenzyl)malonate,
bis[4-(1,1,3,3-
tetramethylbutyl)phenylJ-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate.
1.9. Aromatic hydrox5rbenzyrl compounds, for example 1,3,5-tris-(3,5-di-tert-
butyl-4-hydroxy-
benzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-
2,3,5,6-tetra-
methylbenzene, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)phenol.
1.10. Triazine Compounds, for example 2,4-bis(octylmercapto)-6-(3,5-di-tert-
butyl-4-
hydroxyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-
hydroxyanilino)-
1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-
1,3,5-triazine,
2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris-(3,5-
di-tert-butyl-4-
hydroxybenzyl)isocyanurate, 1,3,5-tris(4-tent-butyl-3-hydroxy-2,6-
dimethylbenzyl)-
isocyanurate, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenylethyl)-1,3,5-
triazine, 1,3,5-tris(3,5-
di-tert-butyl-4-hydroxyphenylpropionyl)-hexahydro-1,3,5-triazine, 1,3,5-
tris(3,5-dicyclohexyl-
4-hydroxybenzyl)isocyanurate.
1.11. Benzy~lphosphonates, for example dimethyl-2,5-di-tert-butyl-4-
hydroxybenzylphospho-
pate, diethyl-3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl3,5-di-
tert-butyl-4-
hydroxybenzylphosphonate, dioctadecyl-5-tert-butyl-4-hydroxy3-
methylbenzylphosphonate,
the calcium salt of the monoethyl ester of 3,5-di-tert-butyl-4-
hydroxybenzylphosphonic acid.
1.12. Acylaminophenols, for example 4-hydroxylauranilide, 4-
hydroxystearanilide, octyl N-
(3,5-di-tert-butyl-4-hydroxyphenyl)carbamate.
1.13. Esters of [i-y3.5-di-tert-butyl-4-hyrdrox)~phenyrl)propionic acid with
mono- or polyhydric
alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-
hexanediol, 1,9-
nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene
glycol, diethy-
lene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)
isocyanurate, N,N'-bis(hy-
2191832
-33-
droxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol,
trimethylol-
propane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1 14 Esters of~i-(5-tert-butyrl-4-hyrdroxyr-3-methylphenyrl)propionic acid
with mono- or poly-
hydric alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol,
octadecanol, 1,6-hexane-
diol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol,
thiodiethylene
glycol, diethylene glycol, triethylene glycol, pentaerythritol,
tris(hydroxyethyl) isocyanurate,
N,N'-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol,
trimethylhexanediol,
trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-
trioxabicyclo[2.2.2]octane.
1 15 Esters of j3-(3 5-dirt cl~ ohex) I-~ 4-h~ dr roxlrphenyrl)propionic acid
with mono- or polyhydric
alcohols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol,
1,9-nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N'-
bis(hydroxyethyl)ox-
amide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol,
trimethylolpropane, 4-
hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1 16. Esters of 3.5-di-tert-butyrl-4-hydroxyrphenyrl acetic acid with mono- or
polyhydric alco-
hols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-
nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N'-
bis(hydroxyethyl)-
oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol,
trimethylolpropane, 4-
hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1 17 Amides of i~-(3 5-di-tert-butyl-4-hyrdroxyphenyl)propionic acid e.g. N,N'-
bis(3,5-di-tert--
butyl-4-hydroxyphenytpropionyl)hexamethylenediamine, N,N'-bis(3,5-di-tert-
butyl-4-hydroxy
phenylpropionyl)trimethylenediamine, N,N'-bis(3,5-di-tert-butyl-4-
hydroxyphenylpropionyl)
hydrazine.
1.18. Ascorbic acid (vitamin C)
1.19. Aminic antioxidants, for example N,N'-di-isopropyl-p-phenylenediamine,
N,N'-di-sec
butyl-p-phenylenediamine, N,N'-bis(1,4-dimethylpentyl)-p-phenylenediamine,
N,N'-bis(1
2191832
ethyl-3-methylpentyl)-p-phenylenediamine, N,N'-bis(1-methylheptyl)-p-
phenylenediamine,
N,N'-dicyclohexyl-p-phenylenediamine, N,N'-diphenyl-p-phenylenediamine, N,N'-
bis(2-naph-
thyl)-p-phenylenediamine, N-isopropyl-N'-phenyl-p-phenylenediamine, N-(1,3-
dimethylbutyl)-
N'-phenyl-p-phenylenediamine, N-(1-methylheptyl)-N'-phenyl-p-phenylenediamine,
N-cyclo-
hexyl-N'-phenyl-p-phenylenediamine, 4-(p-toluenesulfamoyl)diphenylamine, N,N'-
dimethyl-
N,N'-di-sec-butyl-p-phenylenediamine, diphenylamine, N-allyldiphenylamine, 4-
isopropoxy-
diphenylamine, N-phenyl-1-naphthylamine, N-(4-tert-octylphenyl)-1-
naphthylamine, N-phe-
nyl-2-naphthylamine, octylated diphenylamine, for example p,p'-di-tert-
octyldiphenylamine,
4-n-butylaminophenol, 4-butyrylaminophenol, 4-nonanoylamino-phenol, 4-
dodecanoyl-
aminophenol, 4-octadecanoylaminophenol, bis(4-methoxyphenyl)amine, 2,6-di-tert-
butyl-4-
dimethylaminomethylphenol, 2,4'-diaminodiphenylmethane, 4,4'-
diaminodiphenylmethane,
N,N,N',N'-tetramethyl-4,4'-diaminodiphenylmethane, 1,2-bis[(2-
methylphenyl)amino]ethane,
1,2-bis(phenylamino)propane, (o-tolyl)biguanide, Bis[4-(1',3'-
dimethylbutyl)phenyl]amine,
tert-octylated N-phenyl-1-naphthylamine, a mixture of mono- and dialkylated
tert-butyUtert-
octyldiphenylamines, a mixture of mono- and dialkylated nonyldiphenylamines, a
mixture of
mono- and dialkylated dodecyldiphenylamines, a mixture of mono- and
dialkylated isopro-
pyVisohexyldiphenylamines, a mixture of mono- and dialkylated tert-
butyldiphenylamines,
2,3-dihydro-3,3-dimethyl-4H-1,4-benzothiazine, phenothiazine, a mixture of
mono- and dial-
kylated tert-butyUtert-octylphenothiazines, a mixture of mono- and dialkylated
tert-octyl-phe-
nothiazines, N-allylphenothiazin, N,N,N',N'-tetraphenyl-1,4-diaminobut-2-ene,
N,N-bis-
(2,2,6,6-tetramethyl-piperid-4-yl-hexamethylenediamine, bis(2,2,6,6-
tetramethylpiperid-4-
yl)sebacate, 2,2,6,6-tetramethylpiperidin-4-one, 2,2,6,6-tetramethylpiperidin-
4-ol.
2. UV absorbers and light stabilisers
2.1. 2-(2'-~droxyrphenyrl)~benzotriazoles, for example 2-(2'-hydroxy-5'-
methylphenyl)-benzo-
triazole, 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)benzotriazole, 2-(5'-tert-
butyl-2'-hydroxyphe-
nyl)benzotriazole, 2-(2'-hydroxy-5'-(1,1,3,3-
tetramethylbutyl)phenyl)benzotriazole, 2-(3',5'-di-
tert-butyl-2'-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl- 2'-
hydroxy-5'-methyl-
phenyl)-5-chloro-benzotriazole, 2-(3'-sec-butyl-5'-tert-butyl-2'-
hydroxyphenyl)benzotriazole,
2-(2'-hydroxy-4'-octyloxyphenyl)benzotriazole, 2-(3',5'-di-tert-amyl-2'-
hydroxyphenyl)benzo-
triazole, 2-(3',5'-bis-(a,a-dimethylbenzyl)-2'-hydroxyphenyl)benzotriazole,
mixture of 2-(3'-
tert-butyl-2'-hydroxy-5'-(2-octyloxycarbonylethyl)phenyl)-5-chloro-
benzotriazole, 2-(3'-tert-
butyl-5'-[2-(2-ethylhexyloxy)-carbonylethyl]-2'-hydroxyphenyl)-5-chloro-
benzotriazole, 2-(3'-
2191832
tert-butyl-2'-hydroxy-5'-(2-methoxycarbonylethyl)phenyl)-5-chloro-
benzotriazole, 2-(3'-tert-
butyl-2'-hydroxy-5'-(2-methoxycarbonylethyl)phenyl)benzotriazole, 2-(3'-tert-
butyl-2'-
hydroxy-5'-(2-octyloxycarbonylethyl)phenyl)benzotriazole, 2-(3'-tert-butyl-5'-
[2-(2-ethylhexyl-
oxy)carbonylethyl]-2'-hydroxyphenyl)benzotriazole, 2-(3'-dodecyl-2'-hydroxy-5'-
methylphe-
nyl)benzotriazole, and 2-(3'-tert-butyl-2'-hydroxy-5'-(2-
isooctyloxycarbonylethyl)phenylben-
zotriazole, 2,2'-methylene-bis[4-(1,1,3,3-tetramethylbutyl)-6-benzotriazole-2-
ylphenol]; the
transesterification product of 2-[3'-tert-butyl-5'-(2-methoxycarbonylethyl)-2'-
hydroxyphenyl]-
2H-benzotriazole with polyethylene glyco1300;, where R = 3'-tert-butyl-4'-
hydroxy-5'-2H-ben-
zotriazol-2-ylphenyl.
2.2. 2-Hydroxyrbenzophenones, for example the 4-hydroxy, 4-methoxy, 4-
octyloxy, 4-decyl-
oxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-
dimethoxy derivatives.
2 3 Esters of substituted and unsubstituted benzoic acids, as for example 4-
tertbutyl-phenyl
salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoyl resorcinol,
bis(4-tert-butylben-
zoyl) resorcinol, benzoyl resorcinol, 2,4-di-tertbutylphenyl 3,5-di-tert-butyl-
4-hydroxybenzo-
ate, hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, octadecyl 3,5-di-tert-
butyl-4-hydroxy-
benzoate, 2-methyl-4,6-di-tert-butylphenyl 3,5-di-tert-butyl-4-
hydroxybenzoate.
2.4. Acryrlates, for example ethyl a-cyano-[i,[i-diphenylacrylate, isooctyl a-
cyano-[i,[3-di-
phenylacrylate, methyl a-carbomethoxycinnamate, methyl a-cyano-[i-methyl-p-
methoxy-
cinnamate, butyl a-cyano-[i-methyl-p-methoxy-cinnamate, methyl a-carbomethoxy-
p-
methoxycinnamate and N-([i-carbomethoxy-[i-cyanovinyl)-2-methylindoline.
2.5. Nickel compounds, for example nickel complexes of 2,2'-thio-bis-[4-
(1,1,3,3-tetra-
methylbutyl)phenol], such as the 1:1 or 1:2 complex, with or without
additional ligands such
as n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel
dibutyldithiocar-
bamate, nickel salts of the monoalkyl esters, e.g. the methyl or ethyl ester,
of 4-hydroxy-3,5-
di-tert-butylbenzylphosphonic acid, nickel complexes of ketoximes, e.g. of 2-
hydroxy-4-
methylphenyl undecylketoxime, nickel complexes of 1-phenyl-4-lauroyl-5-
hydroxypyrazole,
with or without additional ligands.
2191832
-36-
2.6. Sterically hindered amines, for example bis(2,2,6,6-tetramethyl-4-
piperidyl)sebacate,
bis(2,2,6,6-tetramethyl-4-piperidyl)succinate, bis(1,2,2,6,6-pentamethyl-4-
piperidyl)seba-
cate, bis(1-octyloxy-2,2,6,6-tetramethyl-4-piperidyl}sebacate, bis(1,2,2,6,6-
pentamethyl-4-pi-
peridyl} n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate, the condensate of
1-(2-hydroxy-
ethyl}-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, the
condensate of N,N'-
bis(2,2,6,6-tetramethyl-4-piperidyl}hexamethylenediamine and 4-tert-octylamino-
2,6-di-
chloro-1,3,5-triazine, tris(2,2,6,6-tetramethyl-4-piperidyl)nitrilotriacetate,
tetrakis(2,2,6,6-
tetramethyl-4-piperidyl)-1,2,3,4-butane-tetracarboxylate, 1,1'-(1,2-
ethanediyl)bis(3,3,5,5-
tetramethylpiperazinone), 4-benzoyl-2,2,6, 6-tetramethylpiperidine, 4-
stearyloxy-2,2,6,6-
tetramethylpiperidine, bis(1,2,2,6,6-pentamethylpiperidyl)-2-n-butyl-2-(2-
hydroxy-3,5-di-tert-
butylbenzyl)malonate, 3-n-octyl-7,7,9,9-tetramethyl-1,3,8-
triazaspiro[4.5]decan-2,4-dion,
bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)sebacate, bis(1-ociyloxy-2,2,6,6-
tetramethyl-
piperidyl)succinate, the condensate of N,N'-bis-(2,2,6,6-tetramethyl-4-
piperidyl)hexamethy-
lenediamine and 4-morpholino-2,6-dichloro-1,3,5-triazine, the condensate of 2-
chloro-4,6-
bis(4-n-butylamino-2,2,6,6-tetramethylpiperidyl )-1,3,5-triazine and 1,2-bis(3-
aminopropyl-
amino)ethane, the condensate of 2-chloro-4,6-di-(4-n-butylamino-i,2,2,6,6-
pentamethyl-
piperidyl)-1,3,5-triazine and 1,2-bis-(3-aminopropylamino}ethane, 8-acetyl-3-
dodecyl-
7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, 3-dodecyl-1-
(2,2,6,6-tetramethyl-
4-piperidyl)pyrrolidin-2,5-dione, 3-dodecyl-1-(1,2,2,6,6-pentamethyl-4-
piperidyl)pyrrolidine-
2,5-dione, a mixture of 4-hexadecyloxy- and 4-stearyloxy-2,2,6,6-
tetramethylpiperidine, a
condensation product of N,N'-bis(2,2,6,6-tetramethyl-4-
piperidyl)hexamethylenediamine and
4-cyclohexylamino-2,6-dichloro-1,3,5-triazine, a condensation product of 1,2-
bis(3-amino-
propylamino)ethane and 2,4,6-trichloro-1,3,5-triazine as well as 4-butylamino-
2,2,6,6-tetra-
methylpiperidine (CAS Reg. No. [136504-96-6]); N-(2,2,6,6-tetramethyl-4-
piperidyl)-n-do-
decylsuccinimid, N-(1,2,2,6,6-pentamethyl-4-piperidyl)-n-dodecylsuccinimid, 2-
undecyl-
7,7,9,9-tetramethyl-1-oxa-3,8-diaza-4-oxo-spiro[4,5]decane, a reaction product
of 7,7,9,9-
tetramethyl-2-cycloundecyl-1-oxa-3,8-diaza-4-oxospiro [4,5]decane and
epichlorohydrin.
2.7. Oxamides, for example 4,4'-dioctyloxyoxanilide, 2,2'-diethoxyoxanilide,
2,2'-dioctyloxy-
5,5'-di-tert-butoxanilide, 2,2'-didodecyloxy-5,5'-di-tert-butoxanilide, 2-
ethoxy-2'-ethyloxani-
lide, N,N'-bis(3-dimethylaminopropyl)oxamide, 2-ethoxy-5-tert-butyl-2'-
ethoxanilide and its
mixture with 2-ethoxy-2'-ethyl-5,4'-di-tert-butoxanilide and mixtures of ortho-
and para-
methoxy-disubstituted oxanilides and mixtures of o- and p-ethoxy-disubstituted
oxanilides.
2191832
- 37 -
2.8. 2-(2-Hydroxyphenyrl)~-1.3.5-triazines, for example 2,4,6-tris(2-hydroxy-4-
octyloxyphenyl)-
1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bas(2,4-dimethylphenyl)-
1,3,5-triazine, 2-
(2,4-dihydroxyphenyl)-4,6-bas(2,4-dimethylphenyl)-1,3,5-triazine, 2,4-bis(2-
hydroxy-4-propyl-
oxyphenyl)-6-(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-
octyloxyphenyl)-4,6-bis(4-
methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-dodecyloxyphenyl)-4,6-bis(2,4-
dimethylphenyl)-
1,3,5-triazine, 2-(2-hydroxy-4-tridecyloxyphenyl)-4,6-bas(2,4-dimethylphenyl)-
1,3,5-triazine,
2-[2-hydroxy-4-(2-hydroxy-3-butyloxy-propoxy)phenyl]-4,6-bas(2,4-dimethyl)-
1,3,5-triazine, 2-
[2-hydroxy-4-(2-hydroxy-3-octyloxy-propyloxy)phenyl]-4,6-bas(2,4-dimethyl)-
1,3,5-triazine, 2-
[4-(dodecyloxy/tridecyloxy-2-hydroxypropoxy)-2-hydroxy-phenyl]-4,6-bis(2,4-
dimethylphe-
nyl)-1,3,5-triazine, 2-[2-hydroxy-4-(2-hydroxy-3-dodecyloxy-propoxy)phenyl]-
4,6-bis(2,4-di-
methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-hexyloxy)phenyl-4,6-Biphenyl-
1,3,5-triazine, 2-
(2-hydroxy-4-methoxyphenyl)-4,6-Biphenyl-1,3,5-triazine, 2,4,6-tris[2-hydroxy-
4-(3-butoxy-2-
hydroxy-propoxy)phenyl]-1,3,5-triazine, 2-(2-hydroxyphenyl)-4-(4-
methoxyphenyl)-6-phenyl-
1,3,5-triazine.
3. Metal deactivators, for example N,N'-diphenyloxamide, N-salicylal-N'-
salicyloyl hydrazine,
N,N'-bis(salicyloyl) hydrazine, N,N'-bas(3,5-di-tert-butyl-4-
hydroxyphenylpropionyl) hydrazine
3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalyl dihydrazide,
oxanilide, isophthaloyl
dihydrazide, sebacoyl bisphenylhydrazide, N,N'-diacetyladipoyl dihydrazide,
N,N'-bis(salicyl-
oyl)oxalyl dihydrazide, N,N'-bis(salicyloyl)thiopropionyl dihydrazide.
4. Phosphates and phosphonites, for example triphenyl phosphate, Biphenyl
alkyl phosphates,
phenyl dialkyl phosphates, tris(nonylphenyl) phosphate, trilauryl phosphate,
trioctadecyl phos-
phate, distearyl pentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl)
phosphate, diisodecyl
pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl) pentaerythritol
diphosphite, bis(2,6-
di-tert-butyl-4-methylphenyl)-pentaerythritol diphosphite,
diisodecyloxypentaerythritol di-
phosphite, bas(2,4-di-tert-butyl-6-methylphenyl)pentaerythritol diphosphite,
bis(2,4,6-tris(tert-
butylphenyl)pentaerythritol diphosphite, tristearyl sorbitol triphosphite,
tetrakis(2,4-di-tert-
butylphenyl) 4,4'-biphenylene diphosphonite, 6-isooctyloxy-2,4,8,10-tetra-tert-
butyl-12H-di-
benz[d,g]-1,3,2-dioxaphosphocin, 6-fluoro-2,4,8,10-tetra-tert-butyl-12-methyl-
dibenz(d,g]-
1,3,2-dioxaphosphocin, bas(2,4-di-tert-butyl-6-methylphenyl)methylphosphite,
bis(2,4-di-tert-
butyl-6-methylphenyl)ethylphosphite.
2191832
-38-
5. Hydroxylamines, for example, N,N-dibenzylhydroxylamine, N,N-
diethylhydroxylamine,
N,N-dioctylhydroxylamine, N,N-dilaurylhydroxylamine, N,N-
ditetradecylhydroxylamine, N,N-
dihexadecylhydroxylamine, N,N-dioctadecylhydroxylamine, N-hexadecyl-N-
octadecyl-
hydroxylamine, N-heptadecyl-N-octadecylhydroxylamine, N,N-dialkylhydroxylamine
derived
from hydrogenated tallow amine.
6. Nitrones, for example, N-benzyl-alpha-phenyl-nitrone, N-ethyl-alpha-methyl-
nitrone, N-oc-
tyl-alpha-heptyl-nitrone, N-lauryl-alpha-undecyl-nitrone, N-tetradecyl-alpha-
tridecyl-nitrone,
N-hexadecyl-alpha-pentadecyl-nitrone, N-octadecyl-alpha-heptadecyl-nitrone, N-
hexadecyl-
alpha-heptadecyl-nitrone, N-ocatadecyl-alpha-pentadecyl-nitrone, N-heptadecyl-
alpha-hep-
tadecyl-nitrone, N-octadecyl-alpha-hexadecyl-nitrone, nitrone derived from N,N-
dialkyl-
hydroxylamine derived from hydrogenated tallow amine.
7. Thiosynergists, for example, dilauryl thiodipropionate or distearyl
thiodipropionate.
8. Peroxide scavengers, for example esters of p-thiodipropionic acid, for
example the lauryl,
stearyl, myristyl or tridecyl esters, men;aptobenzimidazole or the zinc salt
of 2-mercapto-
benzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide,
pentaerythritol tetrakis(~i-
dodecylmercapto)propionate.
9. Polyamide stabilisers, for example, copper salts in combination with
iodides and/or phos-
phorus compounds and salts of divalent manganese.
10. Basic co-stabilisers, for example, melamine, polyvinylpyrrolidone,
dicyandiamide, triallyl
cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides,
polyurethanes,
alkali metal salts and alkaline earth metal salts of higher fatty acids for
example calcium
stearate, zinc stearate, magnesium behenate, magnesium stearate, sodium
ricinoleate and
potassium palmitate, antimony pyrocatecholate or tin pyrocatecholate.
11. Nucleating agents, for example, inorganic substances such as talcum, metal
oxides
such as titanium dioxide or magnesium oxide, phosphates, carbonates or
sulfates of, prefe-
rably, alkaline earth metals; organic compounds such as mono- or
polycarboxylic acids and
2191832
-39-
the salts thereof, e.g. 4-tert-butylbenzoic acid, adipic acid, diphenylacetic
acid, sodium suc
cinate or sodium benzoate; polymeric compounds such as ionic copolymers
("ionomers").
12. Fillers and reinforcing agents, for example, calcium carbonate, silicates,
glass fibres,
glass bulbs, asbestos, talc, kaolin, mica, barium sulfate, metal oxides and
hydroxides, car-
bon black, graphite, wood flour and flours or fibers of other natural
products, synthetic fi-
hers.
13. Other additives, for example, plasticisers, lubricants, emulsifiers,
pigments, fieology
additives, catalysts, flow-control agents, optical brighteners, flameproofing
agents, antistatic
agents and blowing agents.
14. Benzofuranones and indolinones, for example those disclosed in US-A-
4325863, US-A-
4338244, US-A-5175312, US-A-5216052, US-A-5252643, DE-A-4316611, DE-A-4316622,
DE-A-4316876, EP-A-0589839 or EP-A-0591102 or 3-[4-(2-acetoxyethoxy)phenyl]-
5,7-di-
tert-butyl-benzofuran-2-one, 5,7-di-tert-butyl-3-(4-(2-
stearoyloxyethoxy)phenyl]benzofuran-
2-one, 3,3'-his[5,7-di-tert-butyl-3-(4-[2-hydroxyethoxy]phenyl)benzofuran-2-
one], 5,7-di-tert-
butyl-3-(4-ethoxyphenyl)benzofuran-2-one, 3-(4-acetoxy-3,5-dimethylphenyl)-5,7-
di-tert-but-
yl-benzofuran-2-one, 3-(3,5-dimethyl-4-pivaloyloxyphenyl)-5,7-di-tert-butyl-
benzofuran-2-
one.
The compounds of the formula (I) or the mixtures thereof can also be used as
stabilizers,
especially as light stabilizers, for almost all materials known in the art of
photographic
reproduction and other reproduction techniques as e.g. described in Research
Disclosure
1990, 31429 (pages 474 to 480).
The weight ratio of the compounds of the formula (I) or the mixture thereof to
the
conventional additives may be 1:0.5 to 1:5.
The compounds of the formula (I) or mixtures thereof are particularly useful
for stabilizing
pigmented polyolefins, in particular polypropylene.
2191832
-40-
The explanations and comments given above for the compounds of the formula (I)
and
mixtures thereof with regard to the stabilization of organic materials are
also applicable to
the intermediates of the formula (D) and mixtures thereof.
The invention is illustrated in more detail by the following Examples. All
percentages are by
weight, unless otherwise indicated.
GPC (Gel Permeation Chromatography) is used as an analytical procedure for
separating molecules by their difference in size and to obtain molecular
weight
averages(Mw, Mn) or information on the molecular weight distribution of
polymers.
The technique is well known and described, for instance, in "Modem Size -
Exclusion Liquid Chromatography" by W.W.Yan et al., edited by J.Wiley & Sons,
N.Y., USA, 1979, pages 4-8, 249-283 and 315-340.
A narrow molecular weight distribution is characterized by a polydispersity
(Mw~Mn)
close to 1.
The GPC analyses shown in the following Examples are carried out with a GPC
chromatograph OPerkin-Elmer LC 250 equipped with ~Perkin-Elmer RI detector
LC 30 and with APerkin-Elmer oven LC 101.
All the analyses are carried out at 45°C by using three columns
PLGEL 3 p.m
Mixed E 300 mm lenght x 7.5 mm i.d.(from Polymers Laboratories Ltd.
Shropshire,
U.K).
Tetrahydrofurane is used as eluant (flow 0.40 ml/min) and the samples are
dissolved in tetrahydrofurane (2%) (% w/v).
In the structural formulae of the following examples, n' indicates that there
are
repetitive units in the molecules and the products obtained are not uniform.
These
2191832
-41 -
products are characterized by the number average molecular weight Mn and the
polydispersity Mw/Mn
The products described in Examples 1, 2, 5, 6 and 10, in particular Example
10,
relate to a preferred embodiment of the instant invention.
EXAMPLE 1 : Preparation of the compound of the formula
Hac\ % ~ C<H9
-~1 mv--(CH
w
H3C CH3
H3C ~CH3
H9C'
H3C ~ CH3
H3C ~ H 1131i!~~'~W t73
HaC ~ CH3 H3C \\''~~~'I CH3
H H
A solution of 74.3 g (0.35 moles) of N-(2,2,6,6-tetramethyl-4-piperidinyl)-n-
butylamine in 50 ml of water is added slowly, at 0°C to a solution of
64.5 g
(0.35 moles) of cyanuric chloride in 500 ml of xylene, keeping the temperature
during the addition and for further 1 hour.
After 2 hours at room temperature the mixture is cooled to 0°C and an
aqueous
solution of 14.7 g (0.368 moles) of sodium hydroxide in 50 ml of water is
added.
After ~/2 hour at 0°C and further 2 hours at room temperature, the
aqueous solution
is separated off and 69.2 g (0.175 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-
piperidinyl)-1,6-hexanediamine are added.
The mixture is heated to 50°C for 1 hour and 48.4 g (0.35 moles) of
ground
potassium carbonate are added and heated to 60°C for 4 hours.
After washing with water, the organic phase is concentrated under vacuum at
60-70°C/10 mbar, being 250 ml of xylene recovered.
2191832
-42-
138.1 g (0.35 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-
hexanediamine are added and the mixture is heated to 150°C for 2 hours,
cooled
again and added with 14 g (0.35 moles) of ground sodium hydroxide.
The mixture is heated to 140°C for further 4 hours, being the residual
water of
reaction eliminated off azeotropically and for further 4 hours at
160°C.
After cooling to 60°C, the mixture is diluted with 300 ml of xylene,
filtered and
washed three times with 100 ml of ethylene glycol.
After concentrating under vacuum at 60°C/10 mbar 78.7 g (0.147
moles) of 2-
chloro-4,6-bis[N-(2,2,6,6-tetramethyl-4-piperidinyl)-n-butylamino)-1,3,5-
triazine are
added.
The mixture is heated to 140°C for 3 hours and 5.9 g (0.147 moles) of
ground
sodium hydroxide are added, being the mixture heated to reflux and being the
reaction water eliminated off azeotropically.
The mixture is heated to 160°C for 4 hours, added with further 5.9 g
(0.147 moles)
of ground sodium hydroxide and heated again to 160°C for 2 hours.
After cooling to 60°C, the mixture is diluted with 300 ml of xylene,
filtered and
concentrated under vacuum at 140°C/1 mbar.
A solid is obtained with m.p. (melting point) =166-170°C after
drying.
Mn (by GPC) = 3360 g/mol
Mw/Mn = 1.18
The GPC analysis shows a chromatogram as in Figure 1.
2191832
- 43 -
EXAMPLE 2 : Preparation of the compound of the formula
c~
c -, . .,-.
n'
~c c~
c~ u
C4"9
H,C ~ CFIa ~a~
~'~aC ~C~ ~'' ~ H3C Chl3
Hac ~ ~a Hac ~ ctl~
Clad
A mixture of 6.6 g (0.143 moles) of formic acid and of a solution obtained by
dissolving 4.3 g (0.143 moles) of paraformaldehyde in 16 ml of 2% (w/v)
aqueous
NaOH solution is added slowly to a solution of 11 g of the compound of the
Example 1 in 50 ml of xylene and heated to 110°C, the water added and
the water
of reaction simultaneously being separated off azeotropically.
The mixture is then cooled to 70-80°C and a solution of 4 g of sodium
hydroxide in
20 ml of water is added at 30-80°C.
The aqueous layer is separated off and the mixture is dehydrated, separating.
off
the water azeotropically.
After evaporation in vacuum (140°C/1 mbar) a product with m.p. 184-
190°C is
obtained.
Mn (by GPC) = 3650 g/mol
Mw/Mn = 1.20
The GPC analysis shows a chromatogram as in Figure 2.
2191832
EXAMPLE 3-6:
Following the procedure described in Example 1, under the same reaction
conditions and using the appropriate reagents, the following compounds of the
formula (I) are prepared.
A i i N R2 N N~A
NYN N'''N ( )
I
B NaC ~ ~ ~ B
I"'~C ~ a"~ I-~C N
n'
219132
- 45 -
Example A B R~ Rz Mn Mw/Mn m~P~(°C)
(GPC
analysis)
3 I ~ -H -(CHz)s- 2450 1.21 152-159
N N (Figure 3)
C~ C~
0 0
4 ~ ( -CH3 -(CHz)s- 2770 1.20 180-187
N N (Figure 4)
C~ C~
0 0
(C4H9)zN- (C4H9)zN- -H -(CHz)6- 3870 1.16 85-95
(Figure 5)
6 (C4H9)zN- (C4H9)zN- -CH3 -(CHz)6- 4060 1.16 98-106
(Figure 6)
6lbis - ~ -" - ~ -" -H -(CHz)s- 3340 1.18 115-125
"3~- j -~ "~°- j -~"~ (Figure _
I "Z I "z 6/bls)
CHI CH3
2191832
-46-
EXAMPLE 7: Preparation of the compound of the formula
Ca~ C4~
HyC CHa
HaC CHs
H-N N ~N~N (CHZ)6 N ~ ~N 'N-H
N ~ N N'''N
HaC Clia HaC CHa HaC CHa ~ HyC CHa
~ N Ca~
~C4-N HaC I - 'CHs HsC ~ ~CHa
H H HsC ~ CHa
HsC ~ CHa HsC i CHa
H9C ~ CHa H
H
a) Preparation of N,N'-dibutyl-N,N',N"-tris-(2,2,6,6-tetramethyl-4-
piperidinyl)-N"-[6-
(2,2,6,6-tetramethyl-4-piperidinylamino)-hexyl]-[1,3,5]-triazine-2,4,6-
triamine.
A solution of 53.5 g (0.1 mole) of 2-chloro-4,6-bis[N-(2,2,6,6-tetramethyl-4-
piperidinyl)-n-butylamino]-1,3,5-triazine in 250 ml of xylene is added slowly
at
reflux temperature to a solution of 157.9 g (0.4 moles) of N,N'-bis(2,2,6,6-
tetramethyl-4-piperidinyl)-1,6-hexane diamine in 250 ml of xylene.
After the addition, 8 g (0.2 moles) of sodium hydroxide are added and the
mixture is heated to reflux for 8 hours.
The mixture is then filtered and the solution is concentrated under vacuum
(140°C/1 mbar) and the excess of N,N'-bis(2,2,6,6-tetramethyl-4-
piperidinyl)-
1,6-hexane is eliminated off under vacuum (190°C/0.2 mbar).
The solid so obtained is dissolved in 200 ml of xylene and washed four times
with water (50 ml) and dried on Na2S04.
After filtration the xylene solution is evaporated under vacuum
2191832
-47-
(140°C/10 mbar) and, after drying, a product with m.p. 67-72°C
is obtained.
Analysis for C~ H,~ N":
Calculated : C=71.17%; H=11.61 %; N=17.22%
Found : C=70.47%; H=11.49%; N=17.09%
b) Preparation of N,N'-bis[4-[(2,2,6,6-tetramethyl-4-piperidinyl)-butylamino]-
6
chloro-[1,3,5]-triazin-2-yl]-N, N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6
hexanediamine.
To a solution of 36.03 g (0.1 mole) of 2,4-dichloro-6-[(2,2,6,6-tetramethyl-4-
piperidinyl)-butylamino]-[1,3,5]-triazine in 200 ml of xylene, 19.7 g (0.05
moles)
of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-hexanediamine are added.
The mixture is heated to 50°C for 1 hour and 15.2 g (0.11 moles) of
ground
potassium carbonate are added and heated to 60°C for 4 hours.
The mixture is cooled, filtered and washed twice with 50 ml of water.
The organic phase is dried on sodium sulfate, filtered and evaporated under
vacuum (100°C/10 mbar).
After drying a solid is obtained with m.p. 100-103°C.
Analysis for organic chlorine:
Calculated: 6.80%
Found : 6.78%
c) Preparation of the compound of the above shown formula.
A solution of 35.7 g (0.04 moles) of the compound prepared as in a) and
20.8 g (0.02 moles) of the compound prepared as in b) in 200 ml of xylene is
heated to reflux for 3 hours.
2191832
-48-
The mixture is added to 3.2 g (0.08 moles) of ground sodium hydroxide and
heated to reflux being the reaction water eliminated off azeotropically.
The mixture is heated to 190°C in a closed vessel for 14 hours,
cooled and
filtered.
The organic solution is washed three times with water (50 ml) dried on sodium
sulfate, filtered and evaporated under vacuum (140°C/1 mbar).
After drying a product is obtained with m.p. 150-155°C.
Analysis for C,62H~H~:
Calculated: C=70.54%; H=11,18%; N=18.28%
Found : C=70.34%; H=11.10%; N=18.06%
EXAMPLE 8: Preparation of the compound of the formula
~.~
H3C CH3
H~ N ~N~N (~)6-N ~ ~N N-H
N ~ N NYN
HOC CH3 H3C CH3 H3C CH3 H3C CH3
u N C4H9
N H3C i CHI H9C ~ CH3
H H H3C CH3
H3C 1 _CH3 H3C N CH3
H3C I CH3 H
H
2191832
- 49 -
a) Preparation of the compound of the formula
H3C CH3 C4Hs
H-N N ~ ~ N (CH2)6 N H
N~ N
H3C CH3 ~ H3C CH3 H3C CH3
HsC4- N HsC _ N w CHs HsC _ N _ . CHs
H H
H3C ~ CH3
H3C j CH3
H 2
A solution of 20 g (0.022 moles) of the compound prepared as described in
example 7 a) and 8.1 g (0.022 moles) of 2,4-dichloro-6-[(2,2,6,6-tetramethyl-4-
piperidinyl)-butylamino]-1,3,5-triazine in 100 ml of xylene is heated to
40°C for
1 hour.
3.1 g (0.022 moles) of ground potassium carbonate is added and the mixture is
heated to 60°C for 2 hours, to 80°C for 1 hour and cooled to
room
tem peratu re.
34.7 g (0.088 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-
hexanediamine and 0.88 g (0.022 moles) of ground sodium hydroxide are
added.
The mixture is heated to reflux for 15 hours, being the reaction water
eliminated off azeotropically.
After cooling, the mixture is filtered and the organic solution washed three
times with ethylene glycol (50 mi) and three times with water (50 ml).
2191832
-50-
The organic solution is then dried under sodium sulfate, filtered and
evaporated under vacuum (140°C/0.1 mbar).
A solid product is obtained with m.p. 110-115°C.
Analysis for Cs~ H"a N~:
Calculated:C=70.85%; H=11.38%; N=17.77%
Found: C=70.34%; H=11.26%; N=17.52%
b) Preparation of the compound of the above shown formula.
A solution of 10 g (0.0063 moles) of the compound prepared as in a) and 3.3 g
(0.00315 moles) of the compound prepared as in Example 7 b} in 100 ml of
trimethylbenzene is heated to reflux for 3 hours and added with 1.75 g (0.013
moles) of ground potassium carbonate.
The mixture is heated to reflux for 24 hours, being the reaction water
eliminated off azeotropically.
The mixture is cooled, filtered and evaporated under vacuum
(140°C/0.1 mbar). A solid product is obtained with m.p. 176-
183°C.
Analysis for C242 H4~ N~:
Calculated: C=70.50%; H=11.15%; N=18.35%
Found: C=70.46%; H=11.17%; N=18.21
2191832
-51 -
EXAMPLE 9: Preparation of the compound of the formula
C4~-(9 C4"9
H3C CH3 ~ ~C CH3
H-N N ~ N C -N
( I-h)6 I N N H
N~ N N''N
H,c c~ H3c cH3 IY Hac cH3
4 N ~C ; C~ ~C i CH3 N-C<Hv
H H
~C ~C~ ~~aC ~C~
H3C ( CFIa H3C
3
H 7 H
a) Preparation of the compound of the formula
H3C CH3 C H9
14
N
H - N N ~ ~ N (CHz)6 N H
N~ N
H3C CH3 ~ H3C CH3 H3C CH3
~C - N w ~ ~ w
H3C ~ CH3 H3C ~ CH3
H N
CH3
CH3
A solution of 8 g (0.005 moles) of the compound prepared as in Example 8 a)
and 1.83 g (0.005 moles) of 2,4-dichloro-6-[(2,2,6,6-tetramethyl-4-
piperidinyl)-
butylamino]-(1,3,5]-triazine in 100 ml of xylene is heated to 40°C for
1 hour.
After addition of 1.4 g (0.01 mole) of ground potassium carbonate, the mixture
is heated to 60°C for 2 hours and to 80°C for 1 hour.
2191832
-52-
7.9 g (0.02 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-
hexanediamine and 0.4 g (0.01 mole) of ground sodium hydroxide are added
and the mixture is heated to reflux for 4 hours being the reaction water
eliminated off azeotropically.
The mixture is then filtered and the organic solution washed three times with
ethylene glycol (30 ml} and with water (50 ml).
After drying on sodium sulfate and filtration, the organic solution is
concentrated under vacuum (140°C/1 mbar).
After drying, a solid product with m.p. 143-147°C is obtained.
Analysis for C,~ H~ N~:
Calculated : C=70.73%; H=11.29%; N=17.98%
Found: C=70.68%; H=11.25%; N=17.88%
b) Preparation of the above shown compound.
A solution of 9.5 g (0.0042 moles) of the compound prepared as in a) and
2.2 g (0.0021 moles) of the compound prepared as in Example 7 b) in 100 ml
of trimethylbenzene is heated to reflux for 1 hour.
1.2 g (0.0084 moles) of ground potassium carbonate are added and the
mixture is heated to reflux for 16 hours, being the reaction water eliminated
off
azeotropically.
The mixture is then concentrated to 50 ml raising the temperature up to
180°C
fvr further 10 hours.
Subsequently, the mixture is cooled, washed three times with water (30 ml)
and dried on sodium sulfate.
2191832
-53-
After filtration, the organic solution is concentrated under vacuum
(140°C/0.1 mbar).
After drying a solid product with m.p. 180-184°C is obtained.
Analysis for C~ H~ N,2:
Calculated: C=70.49%; H=11.13%; N=18.38%
Found: C=70.03%; H=11.01 %; N=18.21
EXAMPLE 10: Preparation of the compound of the formula
C4' 9 ~"bC4
N N -N N N -N N
y)s ~ ~~)s
N~ N N~ N n~ N~ N
C ~ ~C d"~a ~'~aC ~ ~Cs
~"~a H3C i CH3 ~C ~ ~ ~C i ~'~a
H H
N-Ca~"~ I-bCa N H H HaCa-N
C4' 9
C
H
A solution of 74.3 g (0.35 moles) of N-(2,2,6,6-tetramethyl-4-piperidinyl)-n-
butylamine in
50 ml of water is added slowly at 0°C to a solution of 64.5 g (0.35
moles) of cyanuric
chloride in 500 ml of xylene, keeping the temperature during the addition and
for further
1 hour.
After 2 hours at room temperature, the mixture is cooled to 0°C and an
aqueous solution of
14.7 g (0.368 moles) of sodium hydroxide in 50 ml of water is added.
After 1/2 hour at 0°C and for further 2 hours at room temperature, the
aqueous solution is
separated off and 69.2 g (0.175 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-
piperidinyl)-1,6-
hexanediamine are added.
2191832
-54-
The mixture is heated to 50°C for 1 hour and 48.4 g (0.35 moles) of
ground potassium
carbonate are added and heated to 60°C for 4 hours.
After washing with water, the organic phase is concentrated under vacuum at
60-70°C/10 mbar, being 250 ml of xylene recovered.
138.1 g (0.35 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-
hexanediamine are
added and the mixture is heated to 150°C for 2 hours, cooled again and
added with 14 g
(0.35 moles) of ground sodium hydroxide.
The mixture is heated to 140°C for further 4 hours, being the residual
water of reaction
eliminated off azeotropically and for further 4 hours at 160°C.
After cooling to 60°C, the mixture is diluted with 300 ml of xylene,
filtered and washed three
times with 100 ml of ethylene glycol.
After concentrating under vacuum at 60°C/10 mbar, 54.4 g (0.147 moles)
of 2-chloro-4,6-
bis-(dibutylamino)-1,3,5-triazine are added.
The mixture is heated to 140°C for 3 hours and 20.3 g (0.147 moles) of
ground potassium
carbonate are added, being the mixture heated to reflux and being the reaction
water
eliminated off azeotropically.
The mixture is heated to 160°C for 4 hours, added to further 20.3 g
(0.147 moles) of ground
potassium carbonate and heated again to 160°C for 2 hours. After
cooling to 60°C, the
mixture is diluted with 300 ml of xylene, filtered and concentrated under
vacuum at
140°C/1 mbar.
A solid is obtained with m.p. =130-136°C after drying.
Mn (by GPC) = 2830 g/mol
Mw/Mn = 1.22
219132
-55-
The GPC analysis shows a chromatogram as in Figure 7.
EXAMPLE 11: Preparation of the compound of the formula
N~N~N y)6-N N~N y)6 N N~N
N~ N N~ N n~ N~ N
a"~ ~C
I ~ H3C ~ ~ H3C I ~'is
H H H H
N Q~Q-OOH ~c~ N HOC~~(~-I2 N
~2~ ~C k ~ ~2~z
~CH20H ~C H ~ H0C~~2C~12
A) Synthesis of 2,4-bis-(bis-(2-hydroxyethyl)-amino]-6-chloro-[1,3,5]-
triazine.
Keeping the temperature at 0-5°C, 92.2 g (0.5 moles) of cyanuric
chloride are slowly added
to a solution of 100 ml of acetone in 920 ml of water, cooled to 0°C.
Subsequently, 105.1 g
(1 mole) of diethanolamine are slowly added to the reaction mixture while
maintaining the
temperature at about 5°C.
The solution is stirred for 1/2 hour at 5-10°C and a solution of 63.6 g
(0.6 moles) of Na2C03
in 700 ml of water is added slowly, being the solution heated to 45°C
and maintained at this
temperature for 4 hours.
The mixture is then filtered and the solid so obtained is washed twice with
water and dried
in an oven under vacuum (100°C/1 mbar). After drying, the product is a
white solid with a
melting point of 146-147°C.
Analysis for C"H~N504CI:
Calculated: CI=11.02%
Found: CI=11.00%
2191832
-56-
B) At 0°C, a solution of 74.3 g (0.35 moles) of N-(2,2,6,6-tetramethyl-
4-piperidinyl)-n-
butylamine in 50 ml of water is slowly added to a solution of 64.5 g (0.35
moles) of cyanuric
chloride in 500 ml of xylene, keeping the temperature during the addition and
for further
1 hour.
After 2 hours at room temperature, the mixture is cooled to 0°C and an
aqueous solution of
14.7 g (0.368 moles) of sodium hydroxide in 50 ml of water is added.
After 1/2 hour at 0°C and further 2 hours at room temperature, the
aqueous solution is
separated off and 69.2 g (0.175 moles) of N,N'-bis-[2,2,6,6-tetramethyl-4-
piperidinyl]-1,6-
hexanediamine are added. The mixture is heated to 50°C for 1 hour and
48.4 g
(0.35 moles) of ground potassium carbonate are added and heated to 60°C
for 4 hours.
After washing with water, the organic phase is concentrated under vacuum at
60-70°C/10 mbar, being 250 ml of xylene recovered.
138.1 g (0.35 moles) of N,N'-bis-[2,2,6,6-tetramethyl-4-piperidinyl]-1,6-
hexanediamine are
added and the mixture is heated to 150°C for 2 hours, cooled again and
added with 14 g
(0.35 moles) of ground sodium hydroxide.
The mixture is heated to 140°C for 4 hours, being the residual water of
reaction eliminated
off azeotropically, and subsequently, the mixture is kept at 160°C for
further 4 hours.
After cooling to 60°C, the mixture is diluted with 300 ml of xylene,
filtered and washed three
times with 100 ml of ethylene glycol. After concentrating under vacuum at
60°C/10 mbar,
47.3 g (0.147 moles) of 2,4-bis-[bis-(2-hydroxyethyl)-amino]-6-chloro-[1,3,5]-
triazine are
added.
The mixture is heated to 140°C for 10 hours, cooled to room temperature
and added with
14 g (0.35 moles) of sodium hydroxide in 50 ml of water. The mixture is then
heated to 95°C
for 4 hours, cooled to room temperature and the water phase is separated off.
2191832
-57-
The organic phase is washed once with water, being the residual water
eliminated off
azeotropically.
After concentration under vacuum at 200°C/12.3 mbar, a yellowish
product is obtained with
a melting point of 155 -160°C.
Mn (by GPC) = 2852 g/mol
Mw/Mn = 1.48
The GPC analysis shows a chromatogram as in Figure 8.
EXAMPLE 12: Preparation of the compound of the formula
Z
N--~N~--N (Q~)6-N N~N-(Q.~)6 N N~--N
N. N N~ N n' N~ N
~ HaC
I ~ ~C I
H H H H
N ~~~ ~C4 N ~~~ N
~Z~1~3 ~C k ~ H3~2~2
H3~2~2
A) Synthesis of 2,4-bis-[bis-(2-methoxyethyl)-amino]-6-chloro-[1,3,5]-
triazine.
Following the procedure described in Example 11 under A}, 92.2 g (0.5 moles)
of cyanuric
chloride are reacted with 133.2 g (1 mole) of bis-[2-methoxyethyl]-amine in a
solution of
100 ml of acetone in 920 ml of water.
After evaporation of the acetone/water mixture, a resinous compound is
obtained which is
crystallized by ethanol. The product is a white solid with a melting point of
40-44°C.
2191832
-58-
Analysis for C,5H28N504CI:
Calculated: CI = 9.38%
Found: CI = 9.42%
B) Following the procedure described in Example 11 under B) and using the
appropriate
amounts of the suitable reactants, the desired product is obtained as a white
solid with a
melting point of 138-153°C.
Mn (by GPC) = 3017 g/mol
Mw/Mn = 1.35
The GPC analysis shows a chromatogram as in Figure 9.
EXAMPLE D: Preparation of the compound of the formula
H --_ N (CHZ)6 _-- N N ~ N (C~)6 N H
N~ N
H3C CH3 H3C ~ CH3 ~C4- N H3C ~ CH3 H3C ~ CH3
H3C ~ CH3 H3C ~ CH3 H3C i CH3 H3C i CH3
H H H3C ~ CH3 H H
H3C ~ CH3
H 2
At 0°C, a solution of 74.3 g (0.35 moles) of N-(2,2,6,6-tetramethyl-4-
piperidinyl)-n-
butylamine in 50 ml of water is added slowly and under stirring to a solution
of 64.5 g (0.35
moles) of cyanuric chloride in 500 ml of xylene. The mixture is then stirred
during 2 hours at
room temperature and, after cooling to 0°C, an aqueous solution of 14.7
g (0.368 moles) of
sodium hydroxide in 50 ml of water is added. Subsequently, the aqueous
solution is
separated off and 69.2 g (0.175 moles) of N,N'-bis-[2,2,6,6-tetramethyl-4-
piperidinyl]-1,6-
hexanediamine are added. The mixture is heated to 50°C for 1 hour.
Then, 48.4 g (0.35
moles) of ground anhydrous potassium carbonate are added and the mixture is
heated to
60°C for 4 hours. After washing with water, the organic phase is
concentrated a little
2191832
- 59 -
(250 ml of xylene are recovered) and 1381 g (3.5 moles) of N,N'-bis-[2,2,6,6-
tetramethyl-4-
piperidinyl]-1,6-hexanediamine are added. After heating the mixture to
140°C for 2 hours,
28 g (0.70 moles) of ground sodium hydroxide are added and the mixture is
heated to reflux
for 8 hours, being the water of the reaction distilled off azeotropically. 250
ml of xylene are
added and the mixture is then filtered. The solution is concentrated under
vacuum
(140°C/1 mbar) and the excess of N,N'-bis-(2,2,6,6-tetramethyl-4-
piperidinyl]-1,6-
hexanediamine is eliminated off under vacuum (190°C/0.2 mbar). The
solid so obtained is
dissolved in xylene, washed four times with water (50 ml) and dried on Na2S04.
After
filtration, the xylene solution is evaporated under vacuum (140°C/10
mbar) and, after
drying, a product with a melting point of 130-138°C is obtained.
Analysis for C,~H~N~:
Calculated: C=71.02%; H=11.46%; N=17.52%
Found: C=70.95%; H=11.48%; N=17.54%
EXAMPLE D-1: Preparation of the compound of the formula
H - N (CHz)6 - N N 11---- N (Cj~)6 N H
N~ N
C CH' H'C CH' C ~ H'C CH' H3C CH
x ~4 k ~
H'C ~ CH' H'C ~ CH' H3C i CH' H3C i "CH3
H H H3C ~ CH' H H
H'C ~ CH'
H n'
At 0°C, a solution of 74.3 g (0.35 moles) of N-(2,2,6,6-tetramethyl-4-
piperidinyl)-n-
butylamine in 50 ml of water is added slowly and under stirring to a solution
of 64.5 g
(0.35 moles) of cyanuric chloride in 500 ml of xylene. The mixture is then
stirred 2 hours at
room temperature and, after cooling to 0°C, added with an aqueous
solution of 14.7 g
(0.368 moles) of sodium hydroxide in 50 ml of water. Subsequently, the aqueous
solution is
separated off and 69.2 g (0.175 moles) of N,N'-bis-[2,2,6,6-tetramethyl-4-
piperidinyl]-1,6-
hexanediamine are added. The mixture is heated to 50°C for 1 hour.
Then, 48.4 g
2191832
-60-
(0.35 moles} of ground anhydrous potassium carbonate are added and the mixture
is
heated to 60°C for 4 hours.
After washing with water, the organic phase is concentrated a little (250 ml
of xylene are
recovered) and 138.1 g (0.35 moles) of N,N'-bis-[2,2,6,6-tetramethyl-4-
piperidinyl]-1,6-
hexanediamine are added.
After heating the mixture to 140°C for 2 hours, 28 g (0.70 moles) of
ground sodium
hydroxide are added and the mixture is heated to reflux for 8 hours, being the
water of the
reaction distilled off azeotropically.
The mixture is then cooled to 60°C, diluted with 300 ml of xylene and
filtered. Subsequently,
the solution is washed three times with 100 ml of ethylene glycol and
concentrated under
vacuum at 140°C/1 mbar. After drying, the solid obtained has a melting
point of 138-143°C.
Mn (by GPC) = 2555 g/mol
Mw/Mn = ~ .25
The GPC analysis shows a chromatogram as in Figure D-1.
EXAMPLES D-2 to D-5: Following the process described in Example D-1 and using
the
respective reagents in the appropriate molar ratios, the following compounds
of formula
H N RZ N N1' ----N R2 N H
N~ N
HaC CHa HaC ~CH3 ~ HaC CH3 H3C CH3
H3C i CH3 H3C i CH3 B H3C ~ CH3 H3C ~ CH3
R' R' R, R'
n'
are prepared.
2191832
-61 -
Example R, R2 B melting
Mn Mw/Mn
point (C)
CH3 CH3
D-2 -H -(CH2)s-H3C-C-CHZ C-NH- 97-105 2631 1.28
CH3 CH3
CH3
D-3 -H -(CH2)s-H3C-C-NH- 121-128 2475 1.34
CH3
D-4 -H -(CH2)s-~ 125-130 2340 1.24
D-5 -H -(CH2)s--N(C4H9)2 68-72 2773 1.20
The GPC analysis of Examples D-2 to D-5 shows a chromatogram as in figures D-2
to D-5.
EXAMPLE 1: Light-stabilizing action in polypropylene fibres.
2.5 g of the stabilizer shown in Table 1, 1 g of tris(2,4-di-t-butylphenyl)
phosphite,
1 g of calcium monoethyl 3,5-di-t-butyl-4-hydroxybenzyl-phosphonate, 1 g of
calcium stearate and 2.5 g of titanium dioxide are mixed in a slow mixer with
1000 g of polypropylene powder having a melt index =12 g/10 min (measured at
230°C and 2.16 kg).
The mixtures are extruded at 200-230°C to obtain polymer granules which
are then
converted into fibres using a pilot-type apparatus (~Leonard-Sumirago(VA),
Italy)
and operating under the following conditions:
Extruder temperature: 230-245°C
Head temperature: 255-260°C
Draw ratio : 1:3.5
Linear density: 11 dtex per filament
2191832
- 62 -
The fibres prepared in this way are exposed, after mounting on white
cardboard, in
a 65WR Weather-O-Meter (ASTM D2565-85) with a black panel temperature of
63°C.
For samples taken after various times of exposure to the light, the residual
tenacity
is measured using a constant-speed tensometer, and the exposure time in hours
needed to halve the initial tenacity (T~) is then calculated.
For purposes of comparison, fibres prepared under the same conditions as
stated
above, but without adding the stabilizers of the present invention, are also
exposed.
The results are shown in Table 1.
TABLE 1
Stabilizer T~ (hours)
without stabilizer 250
compound of Example 1 2100
compound of Example 2 1950
compound of Example 3 1990
compound of Example 4 1820
compound of Example 5 1900
compound of Example 10 2210
EXAMPLE II: Pigment interaction in polypropylene plaques.
5.625 g of the stabilizer shown in Table 2, 13.500 g of Pigment Blue 15
"Flush" (50%
mixture in polyethylene) and 25.875 g of polypropylene powder (having a melt
index of
approximately 14 measured at 230°C and 2.16 Kg) are added to fill a
~Haake internal mixer
at room temperature (Haake Buchler Rheochord System 40 using a 60 cc 3 piece
Rheomixer with cam blades). The cam blades are rotating at 5 RPM (revolutions
per
2191832
-63-
minute). A ram closed the bowl under a weight of 5 kg. The temperature is
increased to
180°C and held at 180°C. The total time is 30 minutes.
The mixture is removed while at 180°C after 30 minutes and cooled down
to room
temperature. The mixture so obtained - called the "concentrate" - will be used
again.
0.900 g of this concentrate, 3.600 g of titanium dioxide "Flush" (50% mixture
in
polyethylene), and 40.500 g of polypropylene powder (having a melt index of
approximately
14 measured at 230°C and 2.16 Kg) are added to a ~HAAKE mixer bowl at
160°C. The cam
blades are rotating at 20 RPM. A ram closes the bowl under a weight of 5 kg.
The
temperature is increased to 170°C and the RPM is increased to 125. The
total time is 30
minutes.
The molten mixture is removed at 170°C, transferred to a hand held tool
at room
temperature and transformed into a round plaque 1 mm x 25 mm in diameter. The
mixture
now so obtained is called the "letdown" and the plaque the "letdown plaque."
Color difference, delta E (CIE color difference equation), of sample letdown
plaque
containing the stabilizer indicated in Table 2 versus control letdown plaque
without the
stabilizer are measured. The measurement is done using an Applied Color
Systems
Spectrophotometer Model CS-5 (USA). The measurement parameters used are
400 - 700 nm - scan, small area view, reflectance, illuminate D65, 10 degree
observer.
The above processing conditions are designed to simulate the manufacture of
concentrates
(masterbatches) of pigments and stabilizers and the subsequent let-down
(dilution) into
finished plastic articles.
A high delta E indicates pigment agglomeration and poor dispersion. A delta E
of 0.5 or less
will not be seen as different by the eye.
2191832
TABLE 2
Stabilizer Delta E
compound of Example 1 0.3
compound of Example 10 0.4
EXAMPLE III: Light-stabilizing action in polypropylene tapes.
1 g of each of the compounds listed in Table 3, 1 g of tris[2,4-di-tert-
butylphenyl] phosphite,
0.5 g of pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-
hydroxyphenyl)propionate] and 1 g of
calcium stearate are mixed in a turbomixer with 1000 g of polypropylene powder
having a
melt index of 2.1 (measured at 230°C and 2.16 Kg).
The mixtures are extruded at 200-220°C to give polymer granules which
are subsequently
converted to stretched tapes of 50 N.m thickness and 2.5 mm width, using a
semi-industrial
type of apparatus (~Leonard-Sumirago (VA) - Italy) and working under the
following
conditions:
Extruder temperature: 210-230°C
Head temperature: 240-260°C
Stretch ratio: 1:6
The tapes thus prepared are mounted on a white card and exposed in a Weather-O-
Meter
65 WR (ASTM D 2565-85) with a black panel temperature of 63°C.
The residual tenacity is measured, by means of a constant velocity tensometer,
on a
sample taken after various light exposure times; from this, the exposure time
(in hours)
required to halve the initial tenacity (T~) is measured.
By way of comparison, tapes prepared under the same conditions as indicated
above, but
without the addition of the stabilizers of the present invention, are exposed.
2191832
- 65 -
The results obtained are shown in Table 3.
TABLE 3
Stabilizer T~ (hours)
without stabilizer 500
compound of Example 1 2920
compound of Example 10 2600
EXAMPLE IV: Antioxidant action in polypropylene plaques.
1 g of each of the compounds listed in Table 4 and 1 g of calcium stearate are
mixed in a
slow mixer with 1000 g of polypropylene powder having a melt index of 4.3
(measured at
230°C and 2.16 Kg).
The mixtures are extruded twice at 200-220°C to give polymer granules
which are then
converted into plaques of 1 mm thickness by compression-moulding at
230°C for 6 minutes.
The plaques are then punched using a DIN 53451 mould and the specimens
obtained are
exposed in a forced circulation air oven maintained at a temperature of
135°C.
The specimens are checked at regular intervals by folding them by 180°
in order to
determine the time (in hours) required for fracturing them.
The results obtained are given in Table 4.
2191832
TABLE 4
Stabilizer Time to fracture (hours)
without stabilizer 250
compound of Example 1 1560
compound of Example 10 1490
EXAMPLE V: Color of polypropylene plaques after oven ageing.
g of each of the compounds listed in Table 5, 1 g of tris[2,4-di-tert-
butylphenyl] phosphate,
1 g of pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)
propionate] and 1 g of
calcium stearate are mixed in a slow mixer with 1000 g of polypropylene powder
having a
melt index of 2.1 (measured at 230°C and 2.16 Kg).
The mixtures are extruded twice at 200-220°C to give polymer granules
which are then
converted into plaques of 1 mm thickness by compression-moulding at
230°C for 6 minutes.
The plaques are then exposed for seven days in a forced circulation air oven
maintained at
120°C. After the oven exposure, the yellowness index (YI) of the
plaques is measured
according to ASTM D 1925 by ~MINOLTA CR 210 chromometer (MINOLTA - Japan). The
results obtained are shown in Table 5.
TABLE 5
Stabilizer YI
compound of Example 1 22.10
compound of Example 10 21.10