Note: Descriptions are shown in the official language in which they were submitted.
BASF Aktiengesellschaft 940389 O.Z. 0050/44965
2193128
Fungicidal mixtures
The present invention relates to a fungicidal mixture which con-
tains f
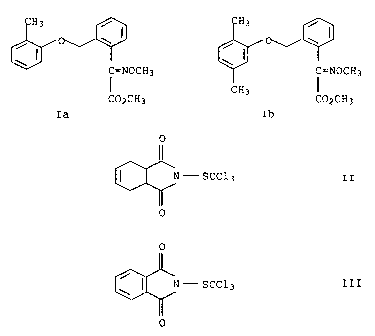
a) an oxime ether carboxylic acid ester of the formula I
/ I
0 CH3 / I CH3
C=NOCH3 C=NOCH3
I CH3 I
CO2CH3 C02CH3
Ia Ib
and
b) a phthalimide derivative selected from the group of
compounds II and III
0
C-scc13 II 0
0
~ N - SCC13 III
0
in a synergistically active amount.
The invention additionally relates to methods of controlling
harmful fungi using mixtures of the compounds I (i.e., Ia or Ib)
and II or of the compounds I and III and the use of the compound
I, the compound II and the compound III for the production of
mixtures of this type.
The compounds of the formula I, their preparation and their ac-
tion against harmful fungi are disclosed in the literature
(EP-A 253 213).
BASF Aktiengesellschaft 940389 O.Z. 0050/44965
2 2193128
The phthalimide derivatives II and III (US-A 2,553,770;
2,553,771; 2,553,776), their preparation and their action against
harmful fungi are likewise known.
With respect to a decrease in the application rates and an
improvement of the spectrum of action of known compounds, the
present invention is based on mixtures which, with a reduced
total amount of applied active compounds, have an improved action
against harmful fungi (synergistic mixtures).
Accordingly, the mixtures defined at the beginning have been
found. It has additionally been found that on simultaneous joint
or separate application of the compound I and the compound II or
the compound III or on application of the compound I and the com-
pound II or the compound III successively harmful fungi can be
controlled better than with the individual compounds.
The compounds of the formula I can be present in the E or the Z
configuration with respect to the C=X double bond (with respect
to the carboxylic acid function group). Accordingly, they can be
used in the mixture according to the invention in each case ei-
ther as the pure E or Z isomer or as an E/Z isomer mixture. The
E/Z isomer mixture or the E isomer is preferably used, the E iso-
mer being particularly preferred.
Preferably, the pure active compounds I and II or III are
employed in the preparation of the mixtures, to which, if
required, further'active compounds against harmful fungi or other
pests such as insects, arachnids or nematodes, or alter~atively
herbicidal or growth-regulating active compounds or fertilizers,
can be admixed.
The mixtures of the compounds I and II or I and III and the
simultaneous joint or separate use of the compounds I and II or I
and III are distinguished by an outstanding action against a wide
spectrum of phytopathogenic fungi, in particular from the Ascomy-
cetes and Basidiomycetes class. In some cases they are systemi-
cally active and can therefore also be employed as foliar and
soil fungicides.
They have particular importance for the control of a multiplicity
of fungi on various crop plants such as cotton, vegetable plants
(eg. cucumbers, beans and cucurbits), barley, grass, oats, cof-
fee, maize, fruit plants, rice, rye, soybean, grape, wheat, deco-
rative plants, sugar cane and a multiplicity of seeds.
f
BASF Aktiengesellschaft 940389 O.Z. 0050/44965
2193128
In particular, they are suitable for the control of the following
phytopathogenic fungi: Erysiphe graminis (powdery mildew) on
cereals, Erysiphe cichoracearum and Sphaerotheca fuliginea on
cucurbits, Podosphaera leucotricha on apples, Puccinia species on
cereals, Rhizoctonia species on cotton and lawns, Ustilago spe-
cies on cereals and sugar cane, Venturia inaequalis (scab) on
apples, Helminthosporium species on cereals, Septoria nodorum on
wheat, Botrytis cinerea (gray mold) on strawberries and vines,
Cercospora arachidicola on groundnuts, Pseudocercosporella herpo-
trichoides on wheat and barley, Pyricularia oryzae on rice, Phy-
tophthora infestans on potatoes and tomatoes, Plasmopara viticola
on vines, Alternaria species on vegetables and fruit and also
Fusarium and Verticillium species.
They are additionally applicable in the protection of materials
(eg. wood preservation), for example against Paecilomyces
variotii.
The compounds I and II or I and III can be applied simultaneously
jointly or separately, or successively, the sequence in the case
of separate application in general having no effect on the con-
trol success.
The compounds I and II or I and III are customarily applied in a
weight ratio of from 1:1 to 1:1000, preferably from 1:1 to 1:500,
in particular from 1:3 to 1:300 (1:11 or III).
Depending on thetype of effect desired, the application rates of
the mixtures according to the invention are from 0.02 tjo 5 kg/ha,
preferably from 0.05 to 3.5 kg/ha, in particular from 0.1 to
3.5 kg/ha. The application rates here for the compound I are from
0.005 to 0.5 kg/ha, preferably from 0.01 to 0.5 kg/ha, in parti-
cular from 0.01 to 0.3 kg/ha. The application rates for the com-
pound II or the compound III are correspondingly from 0.1 to
5 kg/ha, preferably from 0.1 to 3.5 kg/ha.
In the treatment of seed, application rates of mixture of from
0.001 to 50 g/kg of seed, preferably from 0.01 to 10 g/kg, in
particular from 0.01 to 5 g/kg, are in general used.
If harmful fungi which are pathogenic for plants are to be con-
trolled, the separate or joint application of the compounds I and
II or I and III or of the mixtures of the compounds I and II or
I and III is carried out by spraying or dusting the seeds, the
plants or the soils before or after sowing of the plants or
before or after emergence of the plants.
f
BASF Aktiengesellschaft 940389 O.Z. 0050/44965
4 2193128
The fungicidal synergistic mixtures and the compounds I and II or
I and III according to the invention can be prepared, for exam-
ple, in the form of directly sprayable solutions, powders and
suspensions or in the form of high-percentage aqueous, oily or
other suspensions, dispersions, emulsions, oil dispersions,
pastes, dusting compositions, broadcasting compositions or gran-
ules and applied by spraying, atomizing, dusting, broadcasting or
watering. The application form is dependent on the intended use;
it should in each case guarantee a dispersion of the mixture
according to the invention which is as fine and uniform as
possible. -
The formulations are prepared in a manner known per se, eg. by
addition of solvents and/or carriers. Inert additives such as
emulsifiers or dispersants are customarily admixed to the
formulations.
Suitable surface-active substances are the alkali metal, alkaline
earth metal or ammonium salts of aromatic sulfonic acids, eg.
lignosulfonic acid, phenolsulfonic acid, naphthalenesulfonic acid
and dibutylnaphthalenesulfonic acid, as well as of fatty acids,
alkyl- and alkylarylsulfonates, alkyl-, lauryl ether and fatty
alcohol sulfates, and also salts of sulfated hexa-, hepta- and
octadecanols or fatty alcohol glycol ethers, condensation pro-
ducts of sulfonated naphthalene and its derivatives with formal-
dehyde, condensation products of naphthalene or of naphthalene-
sulfonic acids with phenol and formaldehyde, polyoxyethylene
octylphenol ethers, ethoxylated isooctyl-, octyl- or nonylphenol,
alkylphenol or tributylphenyl polyglycol ethers, alkyl~aryl poly-
ether alcohols, isotridecyl alcohol, fatty alcohol-ethylene oxide
condensates, ethoxylated castor oil, polyoxyethylene or polyoxy-
propylene alkyl ethers, lauryl alcohol polyglycol ether acetate,
sorbitol esters, lignin-sulfite waste liquors or methylcellulose.
Powder, broadcasting and dusting compositions can be prepared by
mixing or joint grinding of the compounds I and II or I and III
or the mixture of the compounds I and II or I and III with a
solid carrier.
Granules (eg. coated, impregnated or homogeneous granules) are
customarily prepared by binding the active compound or the active
compounds to a solid carrier.
Fillers or solid carriers used are, for example, mineral earths
such as silica gel, silicic acids, silicates, talc, kaolin, lime-
stone, lime, chalk, bole, loess, clay, dolomite, diatomaceous
earth, calcium and magnesium sulfate, magnesium oxide, ground
t
BASF Aktiengesellschaft 940389 O.Z. 0050/44965
2193128
~
plastics, and fertilizers such as ammonium sulfate, ammonium
phosphate, ammonium nitrate, ureas and vegetable products such as
cereal flour, tree bark meal, wood meal and nutshell meal,
cellulose powder or other solid carriers.
5
The formulations in general contain from 0.1 to 95 % by weight,
preferably from 0.5 to 90 % by weight, of one of the compounds I
and II or I and III or the mixture of the compounds I and II or I
and III. The active compounds are in this case employed in a
purity of from 90 % to 100 %, preferably from 95 % to 100 %
(according to NMR spectrum or HPLC).
The compounds I and II or I and III and the mixtures or the cor-
responding formulations are applied by treating the harmful fungi
or the plants, seeds, soils, surfaces, materials or spaces to be
kept free from them with a fungicidally active amount of the mix-
ture, or of the compounds I and II or I and III in the case of
separate application. Application can be carried out before or
after attack by the harmful fungi.
Examples illustrating the synergistic action of the compounds ac-
cording to the invention on harmful fungi.
The fungicidal action of the compounds and mixtures thereof is
illustrated in the following experiments:
The active ingredients were formulated as 20% strength emulsions
in a mixture of 70 wt% of cyclohexanone, 20 wt% of Nekanil LN
(Lutensol AP6, a spreader-sticker having an emulsifying and dis-
persing action and based on ethoxylated alkylphenols) and 10 wt%
of Emulphor EL (Emulan EL, an emulsifier based on ethoxylated
fatty alcohols), and diluted to the desired concentration with
water.
The leaf area under fungus attack was then assessed in percent.
These figures were then converted into degrees of action. The ex-
pected degrees of action of the active ingredient composition
were determined in accordance with the Colby formula (Colby,
S.R., "Calculating synergistic and antagonistic responses of her-
bicide combinations", Weeds, la, pp. 20-22, 1967) and compared
with the degrees of action observed.
Colby formula E= x + y - x y
100
f
BASF Aktiengesellschaft 940389 O.Z. 0050/44965
6 2193128
E = expected degree of action, expressed in % of the untreated
control, when active ingredients A and B are used in concentra-
tions of m and n
x = degree of action, expressed in % of the untreated control,
when active ingredient A is used in a concentration of m
y = degree of action, expressed in % of the untreated control,
when active ingredient B is used in a concentration of n
With a degree of action of 0, the leaf attack of the treated
plants corresponds to that of the untreated control plants; a de-
gree of action of 100 means that the treated plants exhibit no
leaf attack.
Action on Botrytis cinera
Paprika seedlings of the "Neusiedler Ideal Elite" variety having
4 to 5 leaves were sprayed to runoff with the active ingredient
formulations. After the sprayed-on layer had dried, the plants
were treated with a conidial suspension of the fungus Botrytis
cinera, and kept for 5 days at 22-24 C and high humidity. Leaf at-
tack was then assessed visually.
Active ingredient Application rate Degree of action
[ppm]
observed calculated
-/- ' -/- 0
Ia 125 15
III 125 80
Ia + III 125 + 125 95 83
40