Note: Descriptions are shown in the official language in which they were submitted.
wo 95/35103 2 1 9 3 3 9 6
Title: A ~hal 1 l làceutical co" "Jo~;lion for the r revention and/or treatment of
viral infections and optionallv ill~lallllllalions as well as a method for the
treatment thereof
Field of the Invention
5 The present invention relates to a pharmaceutical colll,oG~;lion for the
prevention and treatment of viral infections and optionally illrldllllllalions
accon",d"ying viral infections. The invention relates more specifically to
pha""aceutical compositions co"~ i"g ~-lupeol as the antivirally active
substance. The invention relates fulll,erll,ol~ to a method of preventing
10 and treating viral infections and optionally illrlallllllaLions by oral
aL~ dliul~ of the plla""dceutical composition to a person with a need
thereof.
Backaround Art
Until now, it has been impossible to provide an efficient ~""~.o~ilion for
15 preventing and/ortreating viral infections caused by cold virus etc, such as
influenza virus, Rhino virus, Corona virus etc. or other viruses in the upper
respiratory passages. Plal;lically all humans suffer from infections in the
upper respiratory passages from time to time, such as cold and flu. The
symptoms of these infections include a sore throat and earache (otitis), a
20 runny nose, itchy eyes, and a pain in the muscles and the joints. The
infections are caused by a variety of different viruses which together are
referred to as "cold virus". Although vaccines are available for a restrjcted
number of influenza strains, no efficient methods are known for preventing
or treating most of the infections in the upper respiratory passages. Such
25 viral infections, e.g. caused by Rhino virus, which is responsible for
- a~Jpl ."d" laLc:l~r 50% of all viral infections in the upper respiratory passages,
are wide-spread and can cause ili health or be directly potentially lethal for
susceptible groups, such as children, elderly people, and persons suffering
wo gS/35103 2 ~ 9 3 3 9 6
from a deficient immunity, such as AlDS-patients, cancer patients otc. A
method of treating these symptoms and the underlying infections would
be of immense i""~o, Lance.
GB Pstent Application No. 2,198,041 A discloses compositions which i.a.
5 contain lupeol. The Cbul,uOa;LiOI-a are stated to have an effect on alcohol
addiction, but it does not appear that this effect can be ascribed to lupeol.
EP-A-0 287 000 discloses a process for the pl~,ualaLion of plant extracts,
which i.a. may contain lupeol. These extracts are stated to be applicable
by the treatment of prostatic hy~.~. L~ uphy, but it does not appear whether
10 the effect can be ascribed to lupeol.
WO 90/14764 discloses a number of Lt l~u~llozonideD having an antiviral
effect. These compounds differ, however, e~,~.lLk~l!y from ,B-lupeol, as
they contain three oxygen atoms to form a trioxycyuloptlllLalle ring. The
antiviral effect is ascribed to this trioxycyclopellLalle ring system.
15 Aqueous, unpurified extracts of bitter ginseng orally adlllill;_L~ d have
been used for many years in China against chronic hepatitis. The chemical
compound or compounds active by the above treatment are, however, not
known. Thus it could not be foreseen that a specific fraction can be
extracted from bitter ginseng, viz. ~- lupeol, which has the unexpected
20 useful effect described in the present s,ueuiriuaLion.
Brief Des~,,iv~ion of the Invention.
The invention relates according to a first aspect to a pl,a""Dce,Jtical
cOlll,uOaiLiOll for the prevention and/or treatment of viral infections, said
CUIIIIUOD;L;On being ~.h~ uL~::Iiadcl by ~,o"",,ising
25 one or more ,~-lupeol derivatives of the formula
wo 95/35103 2 1 ~ 3 3 ~ 6 PCTIDK95100256
_6~CH~
H3C
CH~I CH- I--CH3
I
. CH3
RO~ '><
H3C CH3
where R ~ n~,s~lL~ a hydrogen atom, a straight-chained or branched
aliphatic C1 6-hydrocarbyl group, which may be saturated or may contain
one or more unsaturated bonds selected from double and triple bonds, a
C1 6-acyl group, which may be straight-chained or branched and may
5 contain one or more unsaturated bonds selected from double and triple
bonds, or a group, which is easily decoi",uosed under the con,liLion~
prevailing in the human or animal body to release the ,B- lupeol derivative,
as well as conventional phallllaceutically acc~,uLable adjuvants, additives,
and carriers.
10 The aliphatic C1 6-hydrocarbyl group includes methyl, ethyl, branched and
Ul Ibl ancl1ed propyl, butyl, pentyl and hexyl, ethenyl, branched and
~",L"a"ched propenyl, butenyl, pentenyl and hexenyl, ethynyl, branched
and Ul ,k, ancl-ed propynyl, butynyl and hexynyl and co~ ,uondi"g
compounds ~.or,L.A;..;"g two or more double or triple bonds.
15 The C1 6-acyl group includes methanoyl, ethanoyl, branched and
u,,b,c,,,-.hed propanoyl, butanoyl, pentanoyl and hexanoyl, ethenoyl,
branched and u"L"a"cl-ed propenoyl, butenoyl, pentenoyl and hexenoyl,
butynoyl, branched and unbranched propynoyl, butynoyl, pentynoyl and
hexynoyl and con~ cl-d;"s compounds cGIlL.;.,;,,s two or more double or
20 triple bonds.
It should be understood that a group which is easily decomposed under
woss/3sl03 21 9339~
the conditions prevaiiing in the human or animal body includes any group
that can be Llall~rulllled into the ~-lupeol derivative under physiological
con iiLions.
According to a particularly preferred ~,,ILodi.,,~,,L of the invention, R is
5 hydrogen.
In addition, it has been found that the presence of ammonium ions
providcs an antiviral effect against a number of laboratory viruses, such as
VSV ( = Vesicular Stomatitis Virus) and Semliki virus as well as against
for instance Rhino virus. The most probable ",eul,a";..", of the antiviral
10 effect mediated through ammonium ions is con~; ie~ I:d to be related to the
fact that ammonium ions interfere with the binding of ammonium-sensitive
viruses to virus receptors on the target cell and therefore improve the
capacity of the host or the env;~u~ a~L of el;alillaLillg the virus via
non-specific cell processes, or via neutralization by means of suitable
15 alllibOd; s. Such viruses include for instance HlV-virus, hepatitis virus
usual cold viruses lsuch as Rhino virus, infiuenza virus etc.) or other
infectious ammoniumion-sensitive viruses.
E-iased on ,u~ ~.I; a l;l lal y a~,Uc:l ;1 U C:l I L~ it appears that ammonium ions have an
effectexclusivelyonthereceptorlevelthroughlllelllLlalle-likeillL~lauliolls~
20 as said ammonium ions must be constantly present at the time when the
virus is introduced in the cell cultures in order to provide an optimum
antiviral effect.
Therefore another aspect of the invention is to provide a phalllla~ ~utical
composition comprising a ~-lupeol derivative of the above formula I as well
25 as an ammonium ion releasing compound.
The ammonium ions are preferably derived from a salt of a pharma-
ceutically accel~laLI~ inorganic or organic acid. Any ,ullall"aceutically
wo 9S/35103 2 1 9 3 3 9 6 I~
acce,uLable acid can be used, and examples thereof are h~dluchlolic acid,
sulphuric acid, phosphoric acid, carbonic acid, acetic acid, and tartaric
acid. Ammonium chloride, ammonium sulphate, ammonium hydrogen
- carbonate or ,~onoallllll~ ;um dihydrogen phosphate are preferably used.
5 The ammonium ions may f~"Ll,e""o,~ be derived from a compound of the
general formula 11
X
I
X4- N~- X2 ye 11
x3
where X1-X4, which may be identical or different, are selected from
hydrogen; C1 6alkyl, which may be straight-chained or branched, saturated
or unsaturated and may optionaily contain one or more substituents
15 selected from halogen, hydroxy, C14-alkoxy or amino; aryl, which is
optionally substituted with C1 4alkyl, haiogen, hydroxy, C14-alkoxy or
amino, and
Y is a phy~;olo~i.,..:!y acc~l~Lable salt-forming anion, preferably selected
from F-, Cl-, Br~ and 1-.
It has been found that the COIlliJ;llaLiOl~ of ,B-lupeol and ammonium ions
provides a synergistic antiviral effect against a number of viruses, such as
VSV, Rhino virus and probably also influenza virus.
A third aspect of the invention relates to a pha. . - .aceutical col l l,uG ~; Lioll as
~ 25 defined above and further cu~ ;"s one or more mono or polysulphated
mono, oligo or polysac~,ha~;des or analogues and/or derivatives thereof,
including compounds with heparin or heparan structure, which do not
possess essential anti-coaguiant properties.
WO95/35103 2 ! 93396
Viral infections are known to produce ;llrlal~ alions which are probably
mediated via neutrophilic granulocytes accumulated in the affected area
and causing further ill~lallllllalion through the release of various
substances, such as cytokines and other mediators. Furthermore, it is
5 thought that cationic protein complexes adjacent to or situated in the
neutrophilic granulocytes play an important role as they promote the
i"rla"""dlury reactions causing the known cold symptoms Isore throat,
pain in the joints, fever, etc.). P" ' llillaly experiments indicate that the
mere presence of a highly anionic substance related to the heparin
10 structure, but without the anti-coagulant effect of heparin, such as the
sodium salt of sucrose octasulphate ISOS), or another SOS-like
component, can counteract this process because the latter may optionally
"neutralize" the charge of the cationic proteins present in the accumulated
neutrophilic granulocytes. The latter granulocytes are bound to the
15 virus-infected cells through ICAM-1-markers with the result that the usual
illrlallllllaLuryreactionsarecullsidelabl~lreducedorcompletelysuppressed~
It is known to use sulphated sugars including the aluminum complex of
sucroseoctasulphate,sucralphate,inthetreatmentofillrlaul,~,dLiu,,sinthe
ya~LI uiuL~Liual region or for topical ,, ~i n on the skin for prophylaxis
20 or treatment of illrlaulllldLiull~ cf. for instance DK printed accepted
No.165,357 and DK-PS No.169,018. Furthermore, EP Patent
Application No. 0 230 023 A2 discloses l~hallllaceutical co"".o:,iLions
comprising sulphated ~ ' ,, ' ~c" ides including sucrose octasulphate, for
promoting ulcer healing. Thus it is assumed that SOS together with local
25 growth factors form a bioloyi~.ally active complex which initiates and
stabilizes, respectively, the growth factors resulting in accelerated ulcer
healing processes.
~ The presence of sulphated sd-,-,l,a,ides in or around the upper respiratory
passages is thought to be advantageous in that these substances can
30 accel~dl~ the ulcer healing/curing in the throat or the oral cavity during
., . -- -- -- -- . _ _ _ _ . . .. .. . .. . .. .. ... = = ... _ . . .. _ ... _ _ . . . . ..
WO95/35103 21 ~33 9~ r~llJ~ ~
minor microbial infections, especially during virus infections causing
allllllaliunst e.g. by the presence of cationic substances. The sulphated
saccha~ides will be retained in the illflallllllaluly areas and thereby reduce
the illrlallallaLuly processes in the affected area.
5 A particular aspect of the present invention is therefore sulphated
sac~.llalides for use as an anti-illrlallllllaLuly substance in the oral cavity
and the Iymphatic ring, respectively, around the lower respiratory passages
(below the nasopharynx), as well as a method of treating illrlal 1 ll llaLiOns i this area.
10 According to an embodiment of the present invention, the Sdccl,a,ide is a
mono or polysulphated mono, di, tri or L~:LIa~ ,hàlid~. According to a
particular e",Lo-lialal ~L, the saccharide is a monosacchal ide selected from
xylose, fructose and glucose or a ~ i~e~,cl,aride selected from sucrose,
lactose, maltose and cP~Iobiose
15 In a preferred ~IL~ L the sacchdlide forms a complex or a salt with
ammonium ions or with a metal selected from Al, Na, K, Ca, Mg, Ba, Zn,
Cu, Zr, Ti, Bi, Mn, and Os, or with an amino acid.
According to a preferred e",l,c' Il~L of the present invention the
sulphated d;aac~lla,ide is sucrose octasulphate or a complex or a salt of
20 sucrose octasulphate with ammonium ions or with a metai selected from
Al, Na, K, Ca, Mg, Ba, Zn, Cu, Zr, Ti, Bi, Mn and Os, or a salt of sucrose
octasulphate with an amino acid.
Among these sucrose octasulphate or the sodium, potassium or NH4+ salt
thereof or the aluminum complex of sucrose octâsulphate, sucralphate, are
25 preferred.
Interferons usually present under ordinary virus infections, especially in
wo 9S/35103 P~l/~.~_ l
~T 93396
connection with colds, have been shown to intensify the antiviral effect of
~- lupeol and ammonium ions. Thus it has been found that i~lLelre~uns in
relatively low conce"LIaLiu"s of 0.1-2 units/ml intensify the antiviral effect.
Fu,ll,e,,,~o,e:~ it can be advantageous as a further ingredient of the
5 pha""aceutical composition to use human or non-human immunoglo-
bulines directed towards the substances contributing to intensify colds,
such as ",ic,ooryq";D"~o (virus) etc.
According to a particular embodiment of the invention the phal ll~ac~:utica
cor,,,uooiLions comprise therefore as a further ingredient human or
10 non-human immunoglobulines.
The plla,l"aceutical COIll,uOailiOll iS preferably in the form of chewing
gums, lozenges, chewing tablets, leooriL~luLo, drops, troches, gels, mouth
ointments, solutions or in form of mucoadl,~,~.hrc formulations, preferably
in the form of depot ple:~ualdLiuns~ By depot ~ntlpalaLions is in this
15 uor",eu~io" to be ulldeloluod IJl_l~alaLiulls and formulations with a
controlled, sustained release of active ingredients.
The pha""aceuticai composition is preferably in the form of a chewing
yum, which per piece of chewing gum having a weight of 500 to 3000
mg, preferably of approximately 1000 mg, Cullludouo.
a) 0.01 to 2000, preferablyO.15-1000, particularlypreferred 1-800,
such as 20-600 ~9 of a ~-lupeol derivative/piece, cal~ ted as ,6-
lupeol,
- b) O to 100, preferably 1-50, particularly preferred 2 to 40, such as 5-
30 mg of NH4 +-ions/piece, calculated as ammonium chloride,
WO 9S/35103 2 l ~ 3 3 ~ 6 r~
c) 0 to 1000, preferably 10-500, particularly preferred 25-250 mg of
a sulphated saccha~ elpiece~ c~lcl l'atPd as SOS,
as well as conventional chewing gum inyltd;cnl~
The chewing gum is prepared by means of conventional chewing gum
5 bases and conventional chewing gum additives, such as 5w~ n6.,:"
flavours, colorants, softeners, and texturizing substances. It may
fu,Ll,~""o,~ be necessary to use solubilizers or other release-controlling
measures in order to release the ,~I,a""~colo~: ~lly active substances
disclosed herein from the chewing gum. A further illustration of sol~
10 can for instance be found in EP-0 486 563 B1, in which a general mention
of the plepalalion of chewing gum is found together with examples of
applicable chewing gum illylcd;~..ll~.
The invention relates fulll,~,lllor~ to the use of one or more ,~-lupeol
derivatives of the general formula I
_oCH~
H3C "
RO ~CH3
H3C CH3
15 in which R lI:pll~51~ " a hydrogen atom, a straight-chained or branched
aliphatic Cl 6-hydrocarbyl group, which may be saturated or may contajn
.one or more unsaturated bonds selected from double and triple bonds, a
Cl 6-acyl group, which may be straight-chained or branched and may
contain one or more unsaturated bonds selected from double and triple
wo ss~3s103 2 ~ 9 3 3 9 ~ r~
bonds, or a group which is easily decu~.~,uosed under the uoudiLiu"s
prevailing in the human or animal body to release the ~-lupeol derivative
forthe,u,e,ud,t,Liollofalln;d;~ llLforthepreventionand/ortreatmentof
viral infections.
5 Fu,Ll,~:""or~, the invention relates to the use of one or more,l~-lupeol
derivatives of the general formula I
_~:H~
RO~CH3
H3C CH3
in which R ,~ .lL~ a hydrogen atom, a straight-chained or branched
aliphatic C1 6-hydrocarbyl group, which may be saturated or may contain
10 one or more unsaturated bonds selected from double and triple bonds, a
C1 ~-acyl group, which may be straight-chained or branched and may
contain one or more unsaturated bonds selected from double and triple
bonds, or a group which is easily decomposed under the condiLio"s
prevailing in the human or animal body to release the ,~-lupeol derivative,
15 as well as one or more ammonium ion releasing compounds for the
pld,udldLioll of a l"ed;~.dll,~ "L for the prevention and/or treatment of viral
infections.
Furthermore the invention relates to the use of one or more ,B-lupeol
derivatives of the formula l
WO 95/35103 PCI/DK95/00256
2 1 9 ~
11
_6~CH
H3C "
~CH3
CH3
RO~ ><
H3C CH3
in which R leple~e~ a hydrogen atom, a straight-chained or branched
aliphatic C1 6-hydrocarbyl group, which may be saturated or may contain
one or more unsaturated bonds selected from double and triple bonds, a
C1 6-acyl group, which may be straight-chained or branched and may
5 contain one or more unsaturated bonds selected from double and triple
bonds, or a group which is easily decor,,,uossd under the condiLiol.s
prevailing in the human or animal body to release the ,~-lupeol derivative,
as well as one or more mono or polysulphated mono, oligo, or
polysdc.,h~l ides or analogues or derivatives thereof, for the pl e~ul c~ Lio~ ~ of
10 a ",e.l;.;a~e~lL for the prevention and/or treatment of viral infections and
a:~aOciaLt:d i~rlcl,,,,,,c,Lions.
Finally, the invention relates to the use of one or more ~-lupeol derivatives
of the formula I
~CH
H3C
~ '
~ --CH3
RO ><~
H3C CH3
in which R lel le~ellL~ a hydrogen atom, a straight-chained or branched
WO 9S/35103 r~
2~ 93396
aliphatic C1 6-hydrocarbyl group, which may be saturated or may contain
one or more unsaturated bonds selected from double and triple bonds, a
C1 6-acyl group, which may be strai9ht-chained or branched and may
contain one or more unsaturated bonds selected from double and triple
5 bonds, or a group which is easiiy decor",uosed under the conditions
prevailing in the human or animal body to release the ~-lupeol derivative,
one or more ammonium ion releasing compounds, as well as one or more
mono or polysulphated mono, oligo, or polysscc hal ides for the preparation
of a 1 ~ di~.a~ L for the prevention and/or treatment of viral infections and
10 ~.co~ id~ llllllldLilJIl:~,
Thus the invention is particularly useful in treating infections in the upper
respiratory passages, especially cold viruses, such as Rhino virus, influenza
virus, enterovirus, Coxsackie virus and other cold viruses.
In addition, the invention allows the use of one or more of the above
15 mentioned active ingredients for treating HIV, hepatitis virus, cytomegalo
virus, herpes virus and other viral infections as well as for treating
dLl,t~ ;la,usis as well as for suppressing tumour cell growth.
Antivirally active substances may function in various ways:
(i) either as a substance capable of pluL~.,Lillg the target cells provided it
20 is present simultaneously with the virus. If the latter is a condition for
producing the antiviral activity, it is very likely that the antiviral effects
involve a direct binding of the antiviral substance either to the virus or the
receptor thereof or a cOlllLillaLiull thereof. Many plant extracts will show
this type of "non-specific', receptor-dependent antiviral activity. Most
25 frequently, it is only possible to produce this type of antiviral activity
provided the substance is present at all times, especially from the time the
virus is added,
Wo 95/35103 2 1 9 3 3 ~ S
(ii) or as a substance which is capable of showing an effect without being
present during the actual virus infection, such as in connection with a
previous contact with the target cell, or by being present after the virus
infection, but before a substantial production of viruses has taken place.
5 It is very likely that through this type of antiviral substances more
fulldalllellLcl changes inside the cells are produced via the synthesis of
intracellularproteins/enzymes,whichsecondalilycausearelativelyspecific
inhibition of the Llall~c,i,uLiun and/orthe Llarl~laLion of the virus in such a
manner that the new intracellular proteins result in a so-called "antiviral
10 state" of the cell. When the antiviral state has been produced in the cell,
the substance need no longer be present in principie as the cells are
protected for a certain period of time, although the pruLeuLion must be
expected to decrease gradually over time.
Ammonium ions are thought to belong to type (i) in the effect ,,,echc, ,i..""
15 although a certain, but weaker antiviral activity can be measured in cell
cultures by the addition of NH4+ 2 to 4 hours after the infection.
B-lupeol is thought to belong to type (ii) in the effect l"eul,a"k"".
~-lupeol is present in many plants, such as in the shell of lupin seeds, in
chiccle rubber, in latex from figs and rubber plants, and in vârious
20 medicinal plants, such as in extracts from bitter ginseng. ,t~-lupeol is
CO u ll l lel ciclly available and may be obtained e.g. from the company Sigma.
The scope of . ,c ' ' ' :y of the invention will appear from the following
with reference to the drawings and the examples. It should, however, be
understood that the detailed description and the specific examples are
~ 25 merely included to illustrate preferred e",L,od;."e"L~, and that various
- alterations and IllodiricaLions within the scope of pluLeuLiOII will be obvious
to persons skilled in the art on the basis of the detailed desu,i,uLiom
WO 95/35103 2 1 9 3 3 9 6 PCTII)K95100256
14
Brief De~ ioll of the Drawinqs
The invention is explained in greater detail with reference to the drawings,
in which
Fig. 1 illustrates the antiviral activity of ~-lupeol (also called B1-g) against5 Rhino virus,
Fig. 2 the antiviral activity of interferon-a (HulFN-a) against Rhino virus,
Fis. 3 the antiviral activity of B1-a against EMC virus,
Fig. 4A the antiviral activity of B1-g against Rhino virus at various
dilutions,
10 Fig. 4B the antiviral activity of B1-g + interferon-a against Rhino virus,
Fig. 5 the antiviral activity oF NH4+ ions against VSV, Semliki virus and
EMCIII virus,
Fig. 6 the antiviral activity of NH4CI against Rhino virus,
Fig. 7A the antiviral activity of B1-g, B1-g + NH4CI as well as B1-g,
15 NH4CI + SOS against Rhino virus at an SOS dilution of 1:100 relative to
a 20% SOS stock solution in water,
Fig. 7B the same at an SOS dilution of 1:200,
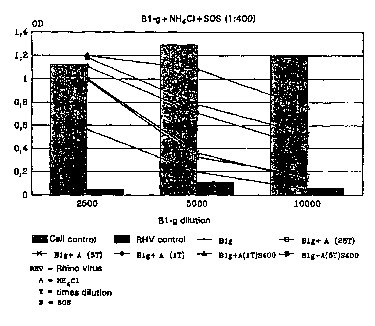
Fig. 7C the same at an SOS dilution of 1:400,
-
Fig. 8 the antiviral activity of B1-g, NH4CI, SOS and interferon-a against
20 Rhino virus, and
WO 95/35103 2 1 9 3 3 9 6 1 "~
Fig. 9 the kinetics for the induction of an antiviral state.
Detailed Des-.,iuLion of the Invention
The method used for d~ l lg antiviral activity is described below.
The cell cultures employed are VERO cells, WISH cells, MDBK cells and
5 HEP cells which are common laboratory cell cultures and which are
described in greater detail in Berg, K.: Purification and ulla~aul~ aLion of
murine and human i,lL~r~,uns. A review of the literature of the 1970s
(thesis). Acta Pathol. Microbiol. Scand., Sec. C., Suppl.279.: page 1-136,
1982. The viruses employed are VSV, EMC, Semliki virus, influenza virus
10 and Rhino virus.
Briefly a ,i~yle laycr cell cuiture is r~LaL~ l,ed in microtrays.A certain
amount of the antivirally active substance in a suitable dilution is added to
the cell culture together with or followed by a suitable amount of virus
("challenge virus"). A control culture receives nothing but challenge virus.
15 The virus infected cultures are incubated until the production of virus is
distinct in the virus control culture (4 to 5 days as far as Rhino virus is
concerned).An MTS/PMS solution comprising 1.0 ml MTS stock solution
(110~9 MTS + 39.2 ml PBS, pH-value 5.6 kept at +4~C in the dark), 2.3
ml medium and 30 ~11 PMS stock solution (13 mg PMS (Sigma, H5004, Lot
20 13, P.9625) + 6.5 ml distilled water, kept at 4 C in the dark with a layer
of paraffin oil on the top) is added, and based on OD(optical density)-
readings in an OD-scanner the relative protection of the cells against viral
attacks can be calculated. A high OD-reading indicates that the cells are
protected against virus, and a low OD-value indicates that the cells have
25 been killed by virus. Thus, the virus control cultures will typicall.y have an
OD-value of < 0.100, while non-infected control cell cultures will have an
OD-value > 1.000. An antivirally active substance is thus a substance
being capable in the presence of medium and challenge virus to provide
WO 95/35103 2 1 9 3 3 9 ~ ~
16
ul u~ on against the test virus in a cell culture.
As far as the MTS-methods are concerned, reference is furthermore made
to Berg, K., B. H. Simonsen, M. B. Hansen, and S. Nielsen: A method for
analysing a sample for the presence of a biological substance, especially
5 a virus, use of the method for quantitative d~L~nll;llalion of biological
substances and agents for use as novel substances detected by the
method. PCT/DKI89/00010. 1 to 32, 1989.
Hansen M. B., S. E. Nielsen, and K. Berg: Fe-ex~""i,ldLiù" and further
development of a precise and rapid dye method for measuring cell
10 growth/cell kill. J. Immunol. Methods. 119: 203 to 210, 1989.
Berg, K., M. B. Hansen and S. E. Nielsen: A sensitive bioassay for precise
quc"~Lir;ualiùl- of interferon activity as measured via the Illiluullolldlial
deh~/-JIuy~nase function in cells ~MTT-method). AMPIS 98: 156 to 162,
1 990.
EXAMPLES
Examole 1
Antiviral activitv of ~-luoeol measured bv means of the MTS-svstem
500 to 1000 WlSH-cells in 100 ~I medium were seeded in wells in a
microtray and incubated for 24 hours at 34 C in an dLIllo~,ul,e:,~ containing
20 5~~c COz. The medium was replaced by fresh medium CollLai"i"g dilutions
of ,~-lupeol (25 to 1.6,ug/ml, cf. Fig.1) and incubated for further 24 hours
at34ocinanallllo~ull~l~collLaillilly5%co2~Thefollowingdaychallenge
Rhino virus was added and after 4 to 5 days at 34 C in an aLIl~o.,uhe~:
containing 5% C02 MTS was added over 2 hours, wl,e,t:c,rl,:r the
~ 25 microtray was measured in an OD-scanner. A total protection against
WO 95/35103 2 1 9 3 3 9 6 PCT/I)K95/00256
17
Rhino virus was obtained at 3 ~g/ml ~-lupeol ( = B1 -g). However, at high
conce"L,~Iiol,s of ~-lupeol a de,~ ;.,9 cell number appears which must
be ascribed to some toxicity of ,6-lupeol at such conce"L,llLiol1s.
ExamDle 2
5 Antiviral activitv of interferon-a irHulFN-a-2b, "intron A") aqainst Rhino
virus
10,000 WISH cells were seeded in a microtray, and the following morning
the medium was replaced by two-fold dilutions of HulFN-a-2b ("intron A"~
in fresh medium co"l_:.l;"g 2% serum (cf. Fig. 2). On the following
10 morning the medium was replaced by fresh medium cor,l_;.,;"g Rhino
virus. The results in Fig. 2 clearly show that Rhino virus is relatively
sensitive to HulFN-a-2b, and that a protection of cl,u~uluXilll~L~ / 90% is
achieved at ~I,u~ulu~dlll~hly 8 units IFN/ml. Fulll,~"",o~, the toxicity of
intron A appears to be negligible.
15 ExamDle 3
Antiviral activitv of B1-q
10,000 WISH cells were seeded and incubated at 37~C for 24 hours as
described in Example 2, and dilutions of B1-g were added to the cultures
in dilutions co" ~,uul IJ;.,g to the cc."c~"L~ ~Lion range indicated in Example
20 1. After 24 hours the medium was replaced by challenge virus in fresh
medium while simultaneously growing challenge virus control cultures and
non-infected control cultures. 24 hours later, the cultures were incubated
with MTS for 2 hours at 37 C, and the tray was scanned as described
above .
WO 9~35103 1 ~
21 93396
The results (Fig. 3) show that B1-g has a moderate antiviral sctivity
against EMC virus. Similar results were obtained against VSV and Semliki
virus. The addition of small amounts of interferon-ail,L~"a;rie,i the antiviral
activity considerably. Thus, as iittle as 0.5 units of interferon resulted in
5 almost 80% protection compared to 30% prul~uLiu" without interferon. It
should be noted, that very often interferon is present in these amounts
(0.2 to 0.6 units/ml) in patients suffering from moderate viral infections,
such as ordinary cold and the like.
Examole 4
10 Anti-Rhino virus activitv of B1-q
A co"~ -l;"g ~,uelilue:lll as described above was performed with Rhino
virus. As illustrated in Fig. 4A, Rhino virus appears to be much more
sensitive to B1-g at a dilution of 1:100 - 1:200 than for instance VSV and
EMC (from a 1 mg/ml stock solution of B1-g), as it is able to suppress the
15 viral infection by more than 80 to 90%. Con~.uond;.,g results must be
expected with influenza virus. Thus it appears that B1-g has a very stron~3
activity towards Rhino virus compared to the effect towards VSV and
EMC. This difference could not be foreseen.
The addition of 0.5 units of interferon-a/ml i~ ":,;t;ed the antiviral activity
20 to a s;~ ,itic~..,L extent, cf. Fig. 4B.
Examole 5
Antiviral activitv of NH1
~ 10,000 WISH cells were seeded in wells in a microtray for 24 hours and
incubated for 24 hours at 37 C in an ~l~. o~,ol)e,~ containing 5% C02.
25 5uhse~ ently, the medium was replaced by fresh medium containing
WO 95/35103 2 1 ~ 3 3 9 ~ PCT/DK9S/0025~
19
dilutions of NH4+ and virus, and after incubation for 24 hours at 37~C in
an dllllo~ le co"L~ Ig 5% CO2, MTS was added over 2 hours at 37~C
and 5% CO2, whereafter the microtray was measured in a OD-scanner
As iilustrated in Fig. 5, NH4+ ions are capable of inhibiting VSV, and to a
5 minor extent Semliki virus, whereas no protection appears against EMC.
Examr le 6
Anti-Rhino virus activitv of NH1 +
As described in Example 5, the antiviral activity of NH4+ towards Rhino
virus was tested, and after incubation for 24 hours at 37~C in an
10 atmosphere cOIlLailli~lg 5~h CO2, MTS was added over 2 hours at 37~C
and 5% CO2, wl,t:,~arl~l the microtray was measured in an OD-scanner.
As it appears from the results in Fig. 6, a strong antiviral effect is obtained
by a dilution of a saturated NH4CI-solution of 1:900. In contrast a toxic
effect appears at higher conce~ ILI ~lions of NH4 + for the laboratory culture
15 employed. The toxicity in vivo for humans is, however, as it is well-
known, negligible ammonium chloride being an ingredient of inter alia
liquorice.
As it appears from Figs. 5 and 6, NH4+ posses.as a varying antiviral
strength towards different viruses, and flJIlllelfnol~ the NH4+
20 con1~,,L,c,lion varies which in each particular case provides the optimum
antiviral effect.
~ Examole 7
Antiviral activitv of B1-a. B1-a + NH~CI as well as B1-q + NH~CI + SOS
towards Rhino virus
WO 95/35103 2 1 9 3 3 9 6 PCT/DK95/00256
Tests were carried out as described in Example 5, whereby, however, the
temperature was 34 C and the incubation was carried out for 4 to 5 days.
The results appear from Figs. 7A, 7B and 7C.
Neither the use of SOS alone in the dilutions of 1:100, 1:200 or 1:400,
5 NH4CI alone at the dilutions of 1:100() or 1:2000 nor NH4CI in
combination with SOS have any Siy~iriCclllL antiviral effect.
The use of B1-g alone reveals a good effect being illLell~iFied by the
simultaneous use of NH4CI, which alone at 34~C only provides a very low
protection. Nevertheiess, an increasing effect is obtained with an
10 increasing NH4+ concc,,LlaLiom The additional use of SOS in the dilution
of 1:100 provides only & moderate increaso of the effect.
When comparing Figs. 7A, 7B and 7C it appears that the favourable effect
of the cu~ aLion of B1-g, NH4CI and SOS is most si~"irica"l at an SOS-
dilution of 1:400 (Fig. 7C), where a protection of almost 95% is found
15 collea~olldillg to a B1-g co"ce"L,dLion of siy,lirica"Lly less than 1 ,ug/ml. The fact that the most favourable effect is obtained at the lowest
cu"ce"i~dLiol- of SOS tested is probably due to some toxicity of SOS
towards the laboratory cells used. SOS is, however, known to be
cOlll~leLely non-toxic to humans in all collce:llLlaLions relevant in practice.
20 ExamPle 8
Anti-Rhino virus activitv of B1-a, NH1CI, SOS and interferon-a
Tests were carried out as described in Example 5, whereby all the
~ substances were added simultaneously with the virus. The results appear
from Fig. 8. As it appears, interferon-a in an amount of 0.5 unitslml
25 intensifies further the favourable effect obtained by a cor"i i"aLion of
_ _ . . _ . .. _ _ . ... .. ~ ... , ~ _ _ .
wo 95/35103 ~ ~ q ~ r~
NH4CI, B1-g and SOS, whereby an almost total l~uL~.Iion is obtained by
the use of B1-g, NH4+ ions and interferon-a.
Accordingly, the natural presence of interferon in a human during an
infection must be expected to have an intensifying effect on B1-g and
5 NH4+. Analogous results appear with SOS in the dilutions of 1:200 and
1:400 (not shown). Similar results are obtained with 0.25 and 0.125 units
of interferon/ml.
ExamPle 9
Antiviral activitv of B1-a, NH~+ ions. SOS, i"L~rr~,uns and combinations
1 0 thereof
The antiviral activity was measured according to the above method. Four
different viruses (EMC, VSV, Semliki Forest virus as well as Rhino virus)
and three different cell lines (A-549, WISH, VER0) were used for the
tests. The results appear from the Table below.
TABLE 1
Antiviral ctivity against dif erent virusea on di-ferent cell lines.
Target cell WISH cell A-549 cell VERO cell WISH
cell
Virus VSV Semliki EMC VSV Semliki EMC Influenza A Influenza B Rhino
Antiviral
component
SOS-- -- + ---- +
(NH4)2SO4 + + - + + _ + + +
NH4Cl + _ _ + _ +
10 SOS+(NH4)2sO4 + ND ND +
HuIFN-a ++ ++ +++ ++ ++ ++ ND ND ++
B1-g + -/+ + + + + ND ND ++
B1-g+HuIFN-a + + + + + + ND ND +++
B1-g+NH4Cl ND ND ND ND ND ND ND ND +++
15 B1-g+SOS ND ND ND ND ND ND ND ND ++
B1-g+NH4Cl+SOS ND ND ND ND ND ND ND ++++
B1-g+NH4Cl+HuIFN-a ND ND ND ND ND ND ND ++++
VSV virus, Semliki Forest virus and influenza viru8 belong to the enveloped viruses; EMC and
Rhino virus belong to the non-enveloped virusec.
20 ND = not determined.
wo 95/35l03 2 1 ~ 3 3 ~ ~ PCTmK
23
As it appears from the above, Rhino virus is inhibited by ammonium ions
and by B1-g as well as by interferon-a. Influenza virus is also assumed to
be inhibited by ammonium ions. SOS appears to have some antiviral effect
towards EMC, but no dc ~ antiviral activity towards Rhino virus. It
5 appears clearly that Rhino virus (which e,.d",,uliries a cold virus) is inhibited
by the co"~L,i"aLion of B1-g, NH4+, interferon ~ SOS.
ExamPle 10
Kinetic tests
A test was p~,~u~ued to examine possible dir~,d"ces in the antiviral
10 effect as a function of the time for the initiation of the antiviral treatment
relative to the e~LaLl;..l""~"L of the viral infection.
500 to 1000 WISH cells were seeded on day -1 in wells in microtrays and
divided into 3 groups. To one group of cells (group -24h~ was added B1-g
in various cunct:llLldLions in the range of 25 to 1.6 ,ug/ml, ~r.hele:a~L~l all of
15 the cells were incubated for 24 hours at 37 C in an dL",Gs,ul,e,t: containing 5% CO2. On day 0 Rhino-challenge virus was added to all of the wells,
and simultaneously B1-g was added to another group of cells (group Oh).
The incubation was continued for 24 hours at 34~C and 5~~6 CO2.
Sl Ihserlu~rltlyt the third group of cells received B1 -g (group + 24h), and all20 of the cells were further incubated for 4 to 5 days at 34~C and 5% CO2
followed by an MTS treatment and measuring in an OD-scanner as
described above.The results appear from Fig. 9.
As it appears, the antiviral effect is almost the same Idyaldldss whether
the B1-g addition is carried out 24 hours before the viral infection or
25 simultaneously with said viral infection.
Ful~ ,,ll,ol~ it is seen that even if the B1-g treatment is not initiated until
WO 9~/35103 r~ u~
~1 933~ ~
24
24 hours after the viral infection, i.e. at the time where the viral infection
has ~ lir~:~Lt:d itself, a distinct antivirai effect is obtained.
While the invention has been described with reference to specific
ernho~' "e"b thereof, it is obvious that it can be varied in many ways.
5 Such variations are not to be ~,onside, ed a deviation from the scope of the
invention, and all such ",odiric~,Lions which are obvious to persons skilled
in the art are also to be con aid(:l ed comprised by the scope of the
acco",~-.,"ying claims.