Note: Descriptions are shown in the official language in which they were submitted.
- 1 2 1 93783
TITLE OF THE lNv~NllON
Dry chlorination of PGM-bearing chromite ores
or concentrates.
FIELD OF THE INVENTION
The present invention pertains to a process for
recovering PGM from PGM-bearing chromite ores or
concentrates.
BACKGROUND OF THE lNv~NllON
The term "Platinum Group Metals" (PGM)
designates the six platinoids, namely, ruthenium,
rhodium, palladium, osmium, iridium and platinum. These
metals are often found together in variable proportions,
and platinum normally predominates.
PGM are generally associated with the presence
of ultramafic rocks containing agents such as nickel or
chromium compounds, either sulfides or oxides. In fact,
chromite (Fe0-Cr203) has been observed to be associated
with sulfides of the PGM in some important geological
formations, such as the Bushveld Complex found in South
Africa (Merensky Reef and UG2 Chromitite).
The current technology used for the recovery of
PGM from these chromites ores calls for the grinding of
the ore to about 75 microns (-200 mesh) followed in
certain cases by gravity concentration and then by
flotation. The concentrate of the sulfides containing
the PGM and other sulfides, such as iron copper and
nickel sulfides, is then smelted so as to produce a matte
2~ 93783
- 2 -
rich in PGM~. The acid digestion of the matte leads to
the individual components of the PGM after rather
elaborate chemical operations.
The froth flotation process is a technique of
general use in sulfides processing. It allows the
production of base metal concentrates from ores with
relatively low sulfides content. Copper and nickel
productions, for example, depend upon enrichment by
flotation to achieve high yield from ores having sulfide
contents at the level of a few percents.
In the case of the PGM, the froth collected
from the flotation of the chromite ores has a PGM content
of 100 to 400 ppm and incorporates copper sulfide and
nickel sulfide at the percent level.
Because of a number of factors, the flotation
of the sulfides from chromite ore is rather delicate and
much influenced by variations in ore composition induced
by oxidation, occlusion in chromite (coarse grinding) and
other variables responsible for the modifications of the
surface of the particles. Although nominal recovery of
the order of 80~ are reported for the flotation of the
PGM and associated sulfides from chromite ores, the
actual practice can lead to significantly lower figures,
and constant adjustment of the operational parameters are
required. This situation and the fact that some types of
PGM-bearing minerals cannot be floated readily makes
_ _ 3 2~ 93 78 3
desirable the investigation of alternate methods for the
recovery of PGM from chromite ores.
The analytical procedures for the determination
of PGM as generally practiced call for the oxidation of
the substrate in the presence of aqua regia, a mixture of
hydrochloric and nitric acids, the presence of a free
halogen, such as chlorine, being required for the more
refractory elements of the group (see J.C. Van Loon and
R.R. Barefoot, Determination of the Precious Metals, pp.
55,101, John Wiley & sons, 1991). Another approach calls
for a fusion with nickel sulfide followed by acid
digestion of the melt under oxidizing conditions. Such
procedures are deemed fairly efficient in converting all
of the PGM in the form of soluble complexed chlorides
that can be determined in solution by instrumental
analysis.
It will be obvious that such approaches,
although appropriate for analytical purposes, cannot be
retained for industrial production. The amount of
chemicals required for the total digestion of the PGM ore
would render the cost of the operation absolutely
prohibitive. Chromite ores contains typically from 20 to
30 percent chromite (FeO-Cr203) associated with 3-4 ppm
of PGM. Since the standard oxidizing agents cited above
are reported as strong enough to oxidize readily the iron
oxide and trivalent chromium oxide of the chromite, the
consumption of oxidizer per unit weight of PGM would
~ 21 ~3783
become so high as to remove any profitability from the
process.
The examination of the chemical literature
concerning the oxidation of ferrous oxide or trivalent
chromium oxide is quite convincing to the effect that
oxidizing agents strong enough to oxidize PGM will very
readily oxidize ferrous oxide or trivalent chromium oxide
to ferric oxide and hexavalent chromium oxide.
Ferrous oxide is readily oxidized by air in the
presence of acids such as nitric acid or hydrochloric
acid (see S.M. Latimer, The oxidation state of the
elements, 2nd ed., p. 224, Prentice-Hall, 1952). Even
chromite itself, FeO-Cr203, is reported oxidized by air,
which is a much milder oxidizer than chlorine or nitric
acid. This reaction is the industrial method of
preparation of sodium chromate:
2 FeO-Cr203 + 4Na2CO3 + 3-5~2 ~ 4Na2CrO4 + 4CO2 + Fe203
(see H. Remy, Treatise on Inorganic Chemistry, Vol. II,
p. 155, Elsevier Publishing Company, 1956).
STATEMENT OF THE INVENTION
While attempting to purify PGM-bearing
chromites from the base metal components, the inventors
have found that, under appropriate conditions, chlorine
could react with the PGM in chromites without significant
attack on chromite itself. This result, unexpected from
the statements of the chemical literature as indicated
_ 5 2~937~3
above, opens the way to a new approach to the recovery of
PGM from chromite ores.
The present invention therefore relates to a
process for the recovery of PGM from PGM bearing chromite
ores or concentrate which comprises the steps of:
- dry chlorinating the ores or concentrates
to produce chlorinated ores or concentrates;
- digesting the chlorinated ores or
concentrates with hydrochloric acid to produce soluble
PGM salts;
- filtering residual insoluble chromite ores
or concentrates to provide substantially unaltered
chromites and a pregnant solution containing PGM and a
small quantity of other metals; and
- recovering PGM from the pregnant solution.
If chlorine is present in the digesting step,
the reaction is facilitated.
In one form of the invention, the dry
chlorinating step is carried out at temperatures from
350~C to 800~C.
In another form of the invention, the dry
chlorinating step is conducted in the presence of sodium
chloride.
In another form of the invention, the ores or
concentrates are heated prior to the dry chlorinating
- 6 - 2193783
step if the heat of reaction of chlorine with the
substrate is not sufficient to promote the reaction.
The inventors have found that if the treated
material is a concentrate containing relatively large
amounts of sulfides and PGM as compared to the starting
ore, the reaction of chlorine with said concentrate is so
exothermic as to generate on site most or all of the
energy required to initiate the reaction. Under such
conditions, the initial temperature can be much lower
than 350~C, some reaction being observed on the material
at room temperature.
The invention presents several advantages over
the pyrometallurgical approach currently used for the
recovery of PGM from chromite ores, namely:
- the preparation of a concentrate by
flotation is not required (although a concentrate could
still be used, if available, with the present invention).
This step, which relies on physical properties of the ore
(surface characteristics and size of sulfide particles),
calls for constant adjustments for the flotation, and
some ores are not amendable to it;
- the percentage of recovery of PGM is from
10 to 20~ higher than what is obtained in practice with
the pyrometallurgical approach;
- the osmium which is almost completely lost
in the pyrometallurgical approach is largely recovered in
the condenser of this process.
_ 7 _ 2 l 9 3 78 3
- the circuit of the present invention is
less elaborate than the pyrometallurgical circuit, no
recycling of slag or chemical digestion of matte being
required to reach the stage of dissolved PGM; and
- the present invention can be operated on a
modular basis and is less capital incentive than the
pyrometallurgical approach.
Other objects and further scope of
applicability of the present invention will become
apparent from the detailed description given hereinafter.
It should be understood, however, that this detailed
description, while indicating preferred embodiments of
the invention, is given by way of illustration only,
since various changes and modifications within the spirit
and scope of the invention will become apparent to those
skilled in the art.
IN THE DRAWINGS
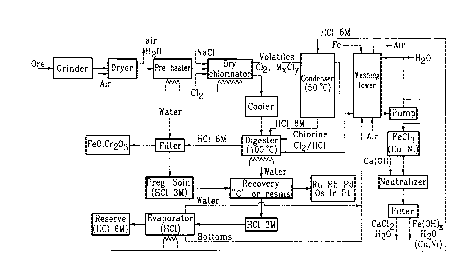
Figure 1 is a block diagram illustrating the
various steps of one embodiment of a dry chlorination of
chromite ores made in accordance with present invention.
DESCRIPTION OF PREFERRED EMBODIMENTS
First, the ore is reduced to an appropriate
size. It will be readily understood that too coarse a
material will prevent access to the values inside the
particles and will slow down the reaction. On the other
_ - 8 - 2 l ~ 3 78 3
hand, very fine grinding involves significant cost. A
value of particles from 75 microns to 45 microns appears
as a practical compromise between reactivity and cost of
grinding.
This finely ground material is then dried to
less than 0.1~ of free water and heated up to the
appropriate temperature for the dry chlorine treatment.
This temperature of dry chlorination has been found to be
variable depending on the refractoriness of the ore under
treatment. With certain ores, a temperature of 450~C has
been sufficient to recover more than 90~ of the PGM. In
other instances, higher temperatures (up to 700~C) have
been required to achieve similar recovery. The
temperature of dry chlorination is an important factor in
the capacity of treatment of a given apparatus. This
temperature must be high enough to ensure the desired
reactions with the PGM, while too high a temperature may
prove detrimental to the equipment and may induce
undesirable reactions with chromite. If the variations
of reactivity of the ores are taken into account, it has
been found that a range of temperature from 350~C to
800~C allows to obtain good reactivity, leading to high
percentage of extraction of PGM while limiting to
acceptable values the side reactions with chromite The
ground ore can be heated to the selected temperature
prior to contacting with chlorine or heated up in the
chlorinator itself. Both procedures have been tried but
the preheating approach turned out to be simpler, a
rotary kiln or a fluidized bed being favored.
- 9 21 ~3783
The hot ore is then introduced in the dry
chlorinator, which is kept at the selected temperature,
and a slow stream of chlorine is circulated through the
mass at constant temperature. It must be noted that the
amount of chlorine required is very small and amounts
from 1.0 to 1.5 times the combined chlorine in the course
of the dry chlorination. The amount of chlorine consumed
by the PGM is relatively negligible, the consumption
being related to the amount of reacting chromite and base
metals, such as iron, nickel and copper present in the
starting ore. As an indicative estimate, it can be said
that some of the base metals (Cu, Ni) at most a few
percents of the iron in the chromite and less than one-
tenth of one percent of the chromium in the chromite will
be converted into the corresponding soluble chlorides.
In order to ensure an homogeneous distribution of the
chlorine through the reacting mass, the chlorine stream
can be diluted by a carrier gas, such as nitrogen for
example. The dry chlorination can be achieved on a
continuous basis or batch-wise by simple percolation
through the mass of the reacting gas or by the
fluidization of the reacting bed or by renewal of the
surfaces in a rotating kiln. The contacting time
required is relatively long, of the order of half an hour
to two hours, depending on the nature of the ore, the
temperature used and, to some extent, on the granulometry
of the ore. For sake of simplicity of an apparatus which
operates under rather aggressive conditions (chlorine at
600~C for example), a static bed of appropriate thickness
has been found convenient. Since the material to be
- lo 2 1 93783
treated is to be loaded hot, the heating of the dry
chlorination reactor has to be just enough to compensate
for heat losses of the system.
The volatiles leaving the dry chlorinator are
directed to a condensing tower and quenched by a spray of
hydrochloric acid at a concentration of 6M. The
temperature in this tower is of the order of 50~C and the
condensable are collected and carried down by the acid
spray.
The solid left in the dry chlorinator after
treatment is dumped after cooling in a digester
containing the acid from the spray in the condensing
tower, and is further digested, at 100~C, for one to
several hours, the stirring of the mass being ensured by
recirculation of the off gases of the condensation tower
through the slurry. The off gases from the digester,
mostly nitrogen, hydrochloric acid vapors, chlorine and
traces of sulfur compounds, are directed back to a gas
treatment system after recycling condensed hydrochloric
acid to the digester.
The temperature and duration of digestion in
hydrochloric acid in the presence of chlorine must be
adjusted in such a fashion as to ensure the complete
solution of the PGM. The heat increases the rate of such
reactions, which would proceed nevertheless at room
temperature but at a slower rate.
2 ~ q3783
For sake of completeness of dissolution as
readily as possible of the PGM, hot treatment is
preferred over cold treatment and a one to several hours
of contacting is retained.
The dry chlorination, followed by digestion,
has now transformed more than 90~ of the PGM into soluble
chlorinated entities. The addition of some sodium
chloride in the system has produced soluble chlorides
with some of the PGM that would otherwise give insoluble
chlorides. In the case of iridium, for example, this
metal gives a trichloride with chlorine at 500~C, but
this salt is not water-soluble. However, in the presence
of sodium chloride, at 600~C, the action of chlorine on
Ir leads to sodium hexachloroiridate Na2IrCl6, a soluble
salt. A similar behavior is noted with rhodium and
ruthenium (see J.C. Van Loon et al, cited above).
The pregnant solution is then separated from
the insoluble chromite ore by filtration and the cake is
submitted to several washings in order to minimize the
retention of the valuable species. After the rinsing
operations, the acid concentration in the pregnant
solution is of the order of 3 molar.
The recovery of valuable species from this
solution can be achieved by a variety of techniques, such
as precipitation over activated carbon, or circulation
over ion-exchange resins. The resulting depleted
solution is directed to a distillation line in order to
- 12 _ 2 1 ~3783
recycle the hydrochloric acid at a concentration of 6M
which corresponds to the azeotropic composition of the
HCl-H20 system. The bottoms of this distillation
contain the iron, the chromium and the base metals (Ni,
Cu, etc...) that have been dissolved in the course of the
digestion of the dry-chlorinated ore. This material is
directed to the gas treatment system which also receives
the vapors of the digester.
The entities found at the inlet of the
purification system are oxidizing by nature (Cl2) and
acidic. They also include all the ions liberated by the
digestion. This mixture is reduced by percolation over
iron filling, and the acidic lixiviate is neutralized
with limestone. Under alkaline conditions, base metal
oxides are precipitated as hydroxides; hexavalent
chromium, after reduction, is precipitated by the
relatively large amounts of trivalent iron present, and
the chlorine ends up as calcium chloride. The resulting
slurry can be filtered and calcium chloride eliminated as
solid after saturation of the circuit with this salt.
Referring to TABLE 1, six examples are given to
illustrate the implementation of the invention. The PGM-
bearing chromite ore (100 g) is reduced to the
appropriate size, heated up at an appropriate temperature
with or without addition of salt, in the presence of
chlorine, and then digested in 6M hydrochloric acid for
a determined period of time. The reacting mass is then
filtered and rinsed; the filtrate, after removal of
~ 21 93783
excess hydrochloric acid, is contacted with either
activated carbon or ion-exchange resins to recover PGM.
The analyses are done by ICP-MS technique.
In the case of Example 2, the pregnant solution
after filtration of the insolubles contained 30 ppm of
chromium and 50,000 ppm of iron. The acid was
fractionated by distillation and the bottoms treated by
a slight excess of limes so as to give a neutral filtrate
and a solid cake where ferric hydroxide predominates.
In another embodiment of the present invention,
the finely ground and dried material to be treated is a
concentrate containing relatively large amounts of
sulfides and PGM as compared to the starting ore. When
the chlorine is added to this concentrate, the reaction
is so exothermic as to generate on side most or all of
the energy required to initiate reaction. Accordingly
the concentrate can be preheated to a temperature which
is much lower than 350~C. Indeed under certain
circumstances, there can be reaction on the material at
room temperature.
Although the invention has been described above
with respect with one specific form, it will be evident
to a person skilled in the art that it may be modified
and refined in various ways. It is therefore wished to
have it understood that the present invention should not
be limited in scope, except by the terms of the following
claims.
21 93783
~3 ~
~t ~~ Ul ~ W ~ oZ 3
,_
O P~ 1 0 ~) O
n : m
~ t (t t ~t t ~t t ~t ~ (1) C
~ ~ ~ (D (D 3
,.. _ ,,. _ ,.. _ ,.. _ ,,, o
t ~ Q O ~ t H
pJ ~t p~ t ~ ~t ~) t (D
.. ,. ,. ~. ~ O
PJ ; _ --- _
3 3 3 3
~a
3 Q~
I'-
n 1~ ~t
O (D
~3
a~ ~ ~ w ~P w
o o o o o Q ~ ~ ~P
_ 1~ 1~ ~ 1-- 1-- ~ ~ ~
~n ~n o o ul o
O O O O ~,
o o o o o o Q 3
o o o o o o ~ tl
1'-
t
.
W ~ ~P Ul W ~ ~
~ H
~ ~ SU
1--
Q
o\O ~ ~
~ n
C
~1 a~ o~ o o~ ~ <