Note: Descriptions are shown in the official language in which they were submitted.
2194128
METHOD OF INCREASING COAGULATION EFFICIENCY
DURING ELECTROCOAGULATION PRINTING
s The present invention pertains to improvements
in the field of electrocoagulation printing. More
particularly, the invention relates to a method of
increasing coagulation efficiency during electrocoagula-
tion printing.
~o In US Patent No. 4,895,E~29 of January 23, 1990,
Applicant has described a high-speed electrocoagulation
printing method and apparatus in which use is made of a
positive electrode in the form of a revolving cylinder
having a passivated surface onto which dots of colored,
coagulated colloid representative of an image are
produced. These dots of colored, coagulated colloid are
thereafter contacted with a substrate such as paper to
cause transfer of the colored, coagulated colloid onto
the substrate and thereby imprint the substrate with the
zo image. As explained in this patent, the positive
electrode is coated with a dispersion containing an
olefinic substance and a metal oxide prior to electrical
energization of the negative electrodes in order to
weaken the adherence of the dots of coagulated colloid to
z5 the positive electrode and also to prevent an
uncontrolled corrosion of the l?ositive electrode. In
addition, gas generated as a result of electrolysis upon
energizing the negative electrodes is consumed by
reaction with the olefinic substance so that there is no
so gas accumulation between the negative and positive
electrodes.
The electrocoagulation printing ink which is
injected into the gap defined between the positive and
negative electrodes consists essentially of a liquid
- 1 -
::.~s
J
219428
colloidal dispersion containing an electrolytically
coagulable colloid, a dispersing medium, a soluble
electrolyte and a coloring agent.. Where the coloring
agent used is a pigment, a dispersing agent is added for
uniformly dispersing the pigment into the ink. After
coagulation of the colloid, any rE:maining non-coagulated
colloid is removed from the surface of the positive
electrode, for example, by scraping the surface with a
soft rubber squeegee, so as to ful~~Ly uncover the colored,
coagulated colloid which is thereafter transferred onto
the substrate. The surface of the positive electrode is
thereafter cleaned by means of a plurality of rotating
brushes and a cleaning liquid to remove any residual
coagulated colloid adhered to the surface of the positive
electrode.
When a polychromic image is desired, the
negative and positive electrodes, the positive electrode
coating device, ink injector, rubber squeegee and
positive electrode cleaning device are arranged to define
a printing unit and several printing units each using a
coloring agent of different color are disposed in tandem
relation to produce several differs=_ntly colored images of
coagulated colloid which are transferred at respective
transfer stations onto the substrate in superimposed
relation to provide the desirE:d polychromic image.
Alternatively, the printing units can be arranged around
a single roller adapted to bring the substrate into
contact with the dots of colored, coagulated colloid
produced by each printing unit, and the substrate which
is in the form of a continuous wE:b is partially wrapped
around the roller and passed through the respective
transfer stations for being imprinted with the
differently colored images in superimposed relation.
- 2 -
219128
The electrocoagulation printing method described
in the aforementioned US Patent No. 4,895,629 is carried
out at room temperature which is generally about 25-30°C.
Applicant has observed that the maximum optical density
of the dots of colored, coagulated colloid formed on the
positive electrode active surface, that could be reached
with an ink having a temperature of 30°C and with a
voltage of 55 volts applied for 4 microseconds between
the negative and positive electrodes, was 1.60. By
increasing the voltage to 60 volts under the same
conditions, there was no valuable i:r~crease in the optical
density of the coagulated colloid, but rather an
undesirable gas generation between -the electrodes. If the
concentration of the electrolyte in the ink was reduced
to control the gas generation, a reduction in the optical
density of the coagulated colloid was observed.
It is therefore an object of the present invention
to overcome the above drawbacks and to increase the effi-
ciency of coagulation during electrocoagulation printing.
In accordance with the present invention, there is
provided an improved electrocoagulation printing method
comprising the steps of:
a) providing a positive electrolytically inert
electrode capable of releasing trivalent metal ions and
having a continuous passivated surface moving at substan
tially constant speed along a prE~determined path, the
passivated surface defining a positive electrode active
surface;
b) forming on the positive electrode active
surface a plurality of dots of colored, coagulated
colloid representative of a desired. image, by electroco
agulation of an electrolytically coagulable colloid
present in an electrocoagulation printing ink comprising
a liquid colloidal dispersion containing the electrolyti
- 3 -
294 X28
cally coagulable colloid, a dispersing medium, a soluble
electrolyte and a coloring agent; and
c) bringing a substrate: into contact with the
dots of colored, coagulated colloid to cause transfer of
s the colored, coagulated colloid from the positive
electrode active surface onto the substrate and thereby
imprint the substrate with the image;
the improvement which comprises maintaining the positive
electrode active surface and the ink at a temperature of
~o about 35°C to about 60°C to increase the conductivity of
the ink and the release of trivalent metal ions from the
passivated surface into the ink in step (b) so that the
trivalent metal ions initiate coagulation of the
electrolytically coagulable colloid and are released in a
15 quantity sufficient to increase the optical density of
the coagulated colloid, thereby increasing coagulation
efficiency in step (b).
As explained in Applica.nt's copending Canadian
patent application No. 2,138,190 filed December 15, 1994,
zo a breakdown of passive oxide film: occurs in the presence
of electrolyte anions, such as Cl', Br' and I', there
being a gradual oxygen displacement from the passive film
by the halide anions and a displacement of adsorbed
oxygen from the metal surface by the halide anions. The
z5 velocity of passive film breakdown, once started,
increases explosively in the presence of an applied
electric field. There is thus :Formation of a soluble
metal halide at the metal surface. In other words, a
local dissolution of the passive oxide film occurs at the
3o breakdown sites, which releases metal ions into the
electrolyte solution. Where a positive electrode made
of stainless steel or aluminum is utilized in
Applicant's electrocoagulation printing method,
dissolution of the passive oxide film on such an
i''~. . ~,
2194128
electrode generates Fe3+ or A13+ ions. These trivalent
ions then initiate coagulation of t:he colloid.
It has surprisingly been found, according to
the invention, that by increasing the temperature of the
positive electrode active surface as well as the
temperature of the ink to within the range of from about
35°C to about 60°C, and preferably to about 40°C, not
only is there an increase in the conductivity of the ink
which assists electrocoagulation, but there is also an
increase in the rate of local dissolution of the passive
oxide film on the positive electrode, which causes a
greater quantity of trivalent metal ions to be released
into the ink. If the temperature of the positive
electrode active surface is below 35°C, the quantity of
trivalent metal ions released into the ink is insuffi-
cient for obtaining the desired increase in the optical
density of the coagulated colloid. At a temperature above
60°C, problems such as condensation of water vapor on the
equipment are encountered.
When operating with an ink having a temperature
of about 40°C and a reduced electrolyte concentration and
with a voltage of 60 volts, the method according to the
invention enables one to obtain dots of colored,
coagulated colloid having an optica:L density of 1.70.
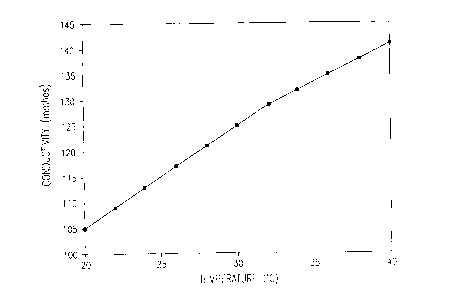
In the accompanying drawings, Figure 1 is a
graph showing the variation of the ink conductivity as a
function of its temperature. As shown, the conductivity
of the electrocoagulation printing ink according to the
invention increases as the temperature thereof increases.
Where a polychromic image is desired, steps (b)
and (c) of the above electrocoagulation printing method
are repeated several times to dEsfine a corresponding
number of printing stages arranged at predetermined
locations along the aforesaid path and each using a
- 5 -
2194128
coloring agent of different color, and to thereby produce
several differently colored images of coagulated colloid
which are transferred at the respective transfer
positions onto the substrate in superimposed relation to
provide a polychromic image.
The positive electrode u:~ed can be in the form
of a moving endless belt as described in Applicant's US
Patent No. 4,661,222, or in the form of a revolving
cylinder as described in the aforementioned US Patent
No. 4,895,629 or in Applicant's US Patent No. 5,538,601.
In the later case, the printing stages are arranged
around the positive cylindrical Electrode. Preferably,
the positive electrode active surface and the ink are
maintained at a temperature of about 35-60°C by heating
the positive electrode active sur:Eace and applying the
ink on the heated electrode surface to cause a transfer
of heat therefrom to the ink.
When use is made of a positive electrode of
cylindrical configuration rotating at substantially
constant speed about its central longitudinal axis, step
(b) of the above electrocoagulation printing method is
carried out by:
i) providing a plurality of negative electro
lytically inert electrodes electrically insulated from
one another and arranged in reci~ilinear alignment to
define a series of corresponding negative electrode
active surfaces disposed in a p:Lane parallel to the
longitudinal axis of the positive electrode and spaced
from the positive electrode active surface by a constant
predetermined gap, the negative electrodes being spaced
from one another by a distance at least equal to the
electrode gap;
ii) coating the positive electrode active
surface with an olefinic substance and a metal oxide to
- 6 -
,~ . Z 19 41 ~~ ~
form on the surface micro-droplets of olefinic substance
containing the metal oxide;
iii) filling the electrode gap with the afore-
said electrocoagulation printing ink;
iv) electrically energizing selected ones of
the negative electrodes to cause point-by-point selective
coagulation and adherence of the colloid onto the olefin
and metal oxide-coated positive electrode active surface
opposite the electrode active surfaces of the energized
negative electrodes while the positive electrode is
rotating, thereby forming the dots of colored, coagulated
colloid; and
v) removing any remaining non-coagulated
colloid from the positive electrode active surface.
As explained in US Patent No. 4,895,029,
spacing of the negative electrodes from one another by a
distance which is equal to or greater than the electrode
gap prevents the negative electrodes from undergoing edge
corrosion. On the other hand, coating of the positive
electrode with an olefinic substance and a metal oxide
prior to electrical energization of the negative
electrodes weakens the adherence of the dots of
coagulated colloid to the positive electrode and also
prevents ,an uncontrolled corrosion of the positive
electrode. In addition, gas generated as a result of
electrolysis upon energizing the negative electrodes is
consumed by reaction with the olefinic substance so that
there is no gas accumulation between the negative and
positive electrodes.
Examples of suitable electrolytically inert
metals from which the positive and negative electrodes
can be made are stainless steel, platinum, chromium,
nickel and aluminum. The positive Electrode is preferably
made of stainless steel, aluminum or tin so that upon
-
,* z~9~~za
electrical energization of the negative electrodes,
dissolution of the passive oxide film on such an
electrode generates trivalent ions which then initiate
coagulation of the colloid.
The gap which is defined between the positive
and negative electrodes can range from about 50 a to
about 100 u, the smaller the elecarode gap the sharper
are the dots of coagulated colloid produced. Where the
electrode gap is of the order of 50 u, the negative
electrodes are the preferably spaced from one another by
a distance of about 75 u.
Examples of suitable olefinic substances which
may be used to coat the surface of the positive electrode
in step (b)(ii) include unsaturated fatty acids such as
arachidonic acid, linoleic acid, linolenic acid, oleic
acid and palmitoleic acid and unsaturated vegetable oils
such as corn oil, linseed oil, clive oil, peanut oil,
soybean oil and sunflower oil. The olefinic substance is
advantageously applied onto the positive electrode active
surface in the form of an oily dispersion containing the
metal oxide as dispersed phase. Examples of suitable
metal oxides include aluminum oxide, ceric oxide,
chromium oxide, cupric oxide, magnesium oxide, manganese
oxide, titanium dioxide and zinc o:~ide~ chromium oxide is
the preferred metal oxide. Depending on the type of metal
oxide used, the amount of metal oxide may range from
about 15 to about 40% by weight:, based on the total
weight of the dispersion. A particularly preferred
dispersion contains about 75 wt.% of oleic acid or
linoleic acid and about 25 wt.o of chromium oxide.
Operating at a temperature of about 35-60°C enables one
to lower the concentration of metal oxide in the oily
dispersion and thus to reduce wear of the positive
electrode active surface.
_ g _
21!94128
The oily dispersion containing the olefinic
substance and the metal oxide is advantageously applied
onto the positive electrode active surface by providing a
distribution roller extending parallel to the positive
cylindrical electrode and having a peripheral coating
comprising an oxide ceramic material, applying the oily
dispersion onto the ceramic coating to form on a surface
thereof a film of the oily dispersion uniformly covering
the surface of the ceramic coating, the film of oily
dispersion breaking down into micro-droplets containing
the olefinic substance in admixture with the metal oxide
and having substantially uniform ~oize and distribution,
and transferring the micro-droplets from the ceramic
coating onto the positive electrode active surface. As
explained in Applicant's US Patent No. 5,449,392 of
September 12, 1995, the use of a distribution roller
having a ceramic coating comprising an oxide ceramic
material enables one to form on a surface of such a
coating a film of the oily dispersion which uniformly
covers the surface of the ceramic coating and thereafter
breaks down into micro-droplets containing the olefinic
substance in admixture with the metal oxide and having
substantially uniform size and distribution. The micro-
droplets formed on the surface of the ceramic coating and
transferred onto the positive electrode active surface
generally have a size ranging from about 1 to about 5 u.
A particularly preferred oxide ceramic material
forming the aforesaid ceramic coating comprises a fused
mixture alumina and titania. Such a mixture may comprise
about 60 to about 90 weight o of alumina and about 10 to
about 40 weight o of titanic.
According to a preferred embodiment of the
invention, the oily dispersion :is applied onto the
_ g _
219~1~8
ceramic coating by disposing an applicator roller
parallel to the distribution ro:Ller and in pressure
contact engagement therewith to form a first nip, and
rotating the applicator roller and the distribution
roller in register while feeding the oily dispersion into
the first nip, whereby the oily dispersion upon passing
through the first nip forms a film uniformly covering the
surface of the ceramic coating. The micro-droplets are
advantageously transferred from the distribution roller
to the positive electrode by disposing a transfer roller
parallel to the distribution roller and in contact
engagement therewith to form a sE:cond nip, positioning
the transfer roller in pressure contact engagement with
the positive electrode to form a third nip, and rotating
the transfer roller and the positive electrode in
register for transferring the micro-droplets from the
distribution roller to the transfer roller at the second
nip and thereafter transferring the micro-droplets from
the transfer roller to the posit=ive electrode at the
third nip. Such an arrangement of rollers is described in
the aforementioned US Patent No. 5,449,392.
Preferably, the applicator roller and the
transfer roller are each provided with a peripheral
covering of a resilient material which is resistant to
attack by the olefinic substance, such as a synthetic
rubber material. For example, u:~e can be made of a
polyurethane having a Shore A hardness of about 50 to
about 70 in the case of the applicator roller, or a Shore
A hardness of about 60 to about 80 in the case of the
transfer roller.
In some instances, depending on the type of
olefinic substance used, Applicant has noted that the
film of oily dispersion only partially breaks down on the
surface of the ceramic coating into the desired micro-
- 10 -
. . 2194128
droplets. Thus, in order to ensure that the film of oily
dispersion substantially completely breaks on the ceramic
coating into micro-droplets of olefinic substance
containing the metal oxide and having substantially
uniform size and distribution, step (b)(ii) of the
electrocoagulation printing method of the invention is
preferably carried out by providing first and second
distribution rollers extending parallel to the positive
cylindrical electrode and each having a peripheral
coating comprising an oxide ceramic material, applying
the oily dispersion onto the ceramic coating of the first
distribution roller to form on a ~;urface thereof a film
of the oily dispersion uniformly covering the surface of
the ceramic coating, the film of oily dispersion at least
partially breaking down into micro-droplets containing
the olefinic substance in admixture with the metal oxide
and having substantially uniform ~~ize and distribution,
transferring the at least partially broken film from the
first distribution roller to they second distribution
roller so as to cause the film to substantially
completely break on the ceramic coating of the second
distribution roller into the desired micro-droplets
having substantially uniform size and distribution, and
transferring the micro-droplets frc>m the ceramic coating
of the second distribution rollE:r onto the positive
electrode active surface. PrefE~rably, the ceramic
coatings of the first distribution roller and the second
distribution roller comprise the same oxide ceramic
material. Such an arrangement of rollers is described in
Applicant's US Patent No. 5,538,601 of July 23, 1996.
According to a preferred embodiment, the oily
dispersion is applied onto the ceramic coating of the
first distribution roller by disposing an applicator
- 11 -
~.,,
xy
,S .'.i~
2 i 941.?8
roller parallel to the first distribution roller and in
pressure contact engagement therewith to form a first
nip, and rotating the applicator roller and the first
distribution roller in register while feeding the oily
dispersion into the first nip, whereby the oily
dispersion upon passing through the first nip forms a
film uniformly covering the surface of the ceramic
coating.
According to another preferred embodiment, the
at least partially broken film of oily dispersion is
transferred from the first distribution roller to the
second distribution roller and the micro-droplets are
transferred from the second distribution roller to the
positive electrode by disposing a first transfer roller
between the first distribution roller and the second
distribution roller in parallel relation thereto,
positioning the first transfer roller in pressure contact
engagement with the first distribution roller to form a
second nip and in contact engagement with the second
distribution roller to form a third nip, rotating the
first distribution roller and the first transfer roller
in register for transferring the at least partially
broken film from the first distribution roller to the
first transfer roller at the second nip, disposing a
second transfer roller parallel t:o the second distri-
bution roller and in pressure: contact engagement
therewith to form a fourth nip, positioning the second
transfer roller in pressure contaca engagement with the
positive electrode to form a fifth nip, and rotating the
second distribution roller, the second transfer roller
and the positive electrode in register for transferring
the at least partially broken film from the first
transfer roller to the second distribution roller at the
third nip, then transferring the micro-droplets from the
- 12 -
219 4128
second distribution roller to the second transfer roller
at the fourth nip and thereafter transferring the micro-
droplets from the second transfer .roller to the positive
electrode at the fifth nip. Such an arrangement of
rollers is also described in the aforementioned U S
Patent No. 5,538,601. Preferably, i=he applicator roller,
first transfer roller and second transfer roller are each
provided with a peripheral covering of a resilient
material which is resistant to attack by the olefinic
substance.
The olefin and metal oxide-coated positive
active surface is preferably polished to increase the
adherence of the micro-droplets onto the positive
electrode active surface, prior to step (b) (iii). For
example, use can be made of a rotating brush provided
with a plurality of radially extending bristles made of
horsehair and having extremities contacting the surface
of the positive electrode. The friction caused by the
bristles contacting the surface upon rotation of the
brush has been found to increase the adherence of the
micro-droplets onto the positive electrode active
surface.
Where the positive cylindrical electrode
extends vertically, step (b)(iii) of the above electro-
coagulation printing method is advantageously carried out
by continuously discharging the ink onto the positive
electrode active surface from a fluid discharge means
disposed adjacent the electrode gap at a predetermined
height relative to the positive electrode and allowing
the ink to flow downwardly along t:he positive electrode
active surface, the ink being thus carried by the
positive electrode upon rotation thereof to the electrode
gap to fill same. Preferably, excess ink flowing
downwardly off the positive electrode active surface is
- 13 -
A
219412
collected and the collected ink is recirculated back to
the fluid discharge means.
The colloid generally uscsd is a linear colloid
of high molecular weight, that is, one having a molecular
weight comprised between about: 10,000 and about
1,000,000, preferably between 100,000 and 600,000.
Examples of suitable colloids include natural polymers
such as albumin, gelatin, casein and agar, and synthetic
polymers such as polyacrylic acid, polyacrylamide and
polyvinyl alcohol. A particularly preferred colloid is an
anionic copolymer of acrylamide ancL acrylic acid having a
molecular weight of about 250,000 and sold by Cyanamid
Inc. under the trade mark ACCOSTRED1GTH 86. The colloid is
preferably used in an amount of abc>ut 6.5 to about 12~ by
weight, and more preferably in an amount of about 7o by
weight, based on the total weight of the colloidal
dispersion. Water is preferably used as the medium for
dispersing the colloid to provide the desired colloidal
dispersion.
The ink also contains a :>oluble electrolyte and
a coloring agent. Preferred electrolytes include alkali
metal halides and alkaline earth metal halides, such as
lithium chloride, sodium chloride, potassium chloride and
calcium chloride. Potassium chloride is particularly
preferred. The electrolyte is preferably used in an
amount of about 4 . 5 to about 6 o by weight, based on the
total weight of the dispersion. Thus, less electrolyte is
required at a temperature of about. 35-60°C than at room
temperature in order to counterbalance the increase in
the ink conductivity at 35-60°C. 'The coloring agent can
be a dye or a pigment. Examples of suitable dyes which
may be used to color the colloid are the water soluble
dyes available from HOECHST such a Duasyn Acid Black for
coloring in black and Duasyn Acid Blue for coloring in
- 14 -
2194' 2~
cyan, or those available from RIEDE:L-DEHAEN such as Anti-
Halo Dye Blue T. Pina for coloring in cyan, Anti-Halo Dye
AC Magenta Extra VOl Pina for coloring in magenta and
Anti-Halo Dye Oxonol Yellow N. Pina for coloring in
yellow. When using a pigment as a coloring agent, use can
be made of the pigments which arE: available from CABOT
CORP. such as Carbon Black Monarch~ 120 for coloring in
black, or those available from HOECHST such as Hostaperm
Blue B2G or B3G for coloring in cyan, Permanent Rubine
F6B or L6B for coloring in magenta and Permanent Yellow
DGR or DHG for coloring in yellow. A dispersing agent is
added for uniformly dispersing the pigment into the ink.
Examples of suitable dispersing agents include the non-
ionic dispersing agent sold by ICI Canada Inc. under the
trade mark SOLSPERSE 27000. The pigment is preferably
used in an amount of about 6.5 to about 12o by weight,
and the dispersing agent in an amount of about 0.4 to
about 6% by weight, based on the total weight of the ink.
After coagulation of the colloid, any remaining
non-coagulated r_olloid is removE:d from the positive
electrode active surface, for example, by scraping the
surface with a soft rubber squeegee, so as to fully
uncover the colored, coagulated colloid. Preferably, the
non-coagulated colloid thus removed is collected and
mixed with the collected ink, and the collected non-
coagulated colloid in admixture with the collected ink is
recirculated back to the aforesaid fluid discharge means.
The optical density of the dots of colored,
coagulated colloid may be varied by varying the voltage
and/or pulse duration of the pulse-modulated signals
applied to the negative electrodes.
According to a preferred embodiment, the
substrate is in the form of a continuous web which is
passed through the respective transfer positions for
- 15 -
219 1 ~?8
being imprinted with the colored _Lmages at the printing
stages. Step (c) is preferably carried out by providing
at each transfer position a pressure roller extending
parallel to the positive cylindrical electrode and in
pressure contact engagement therewith to form a nip and
permit the pressure roller to be driven by the positive
electrode upon rotation thereof, and guiding the web so
as to pass through the nip.
Preferably, the pressure roller is provided
with a peripheral covering of a synthetic rubber material
such as a polyurethane having a Shore A hardness of about
95. A polyurethane covering with such a hardness has been
found to improve transfer of the colored, coagulated
colloid from the positive electrocLe active surface onto
the substrate. The pressure exerted between the positive
electrode and the pressure roller preferably ranges from
about 50 to about 100 kg/cm2.
After step (c), the pos_Ltive electrode active
surface is generally cleaned to remove therefrom any
remaining coagulated colloid. According to a preferred
embodiment, the positive electrode is rotatable in a
predetermined direction and any remaining coagulated
colloid is removed from said positive electrode active
surface by providing an elongated rotatable brush
extending parallel to the longitudinal axis of the
positive electrode, the brush bE:ing provided with a
plurality of radially extending bristles made of
horsehair and having extremities contacting said positive
electrode active surface, rotating the brush in a
direction opposite to the direction of rotation of the
positive electrode so as to cause said bristles to
fractionally engage the positive electrode active
surface, and directing jets of cleaning liquid under
pressure against the positive elecarode active surface,
- 16 -
2 ~ '~ 412 ~3
from either side of the brush. In ~~uch an embodiment, the
positive electrode active surface and the ink are
preferably maintained at a temperature of about 35-60°C
by heating the cleaning liquid to thereby heat the
positive electrode active surface upon contacting same
and applying the ink on the heated electrode surface to
cause a transfer of heat therefrom to the ink.
- 17 -